Abstract
Activation of dermal mast cells through the Mas-related G-protein-coupled receptor B2 receptor (MrgprB2 in mice; MrgprX2 in humans) is a key component of numerous inflammatory skin diseases including dermatitis and rosacea. Sensory neurons actively suppress mast cell activation through the regulated release of glutamate, resulting in reduced expression of Mrgprb2 as well as genes associated with proteins found in mast cell granules. To determine whether exogenous glutamate receptor agonism could suppress mast cell function, we determined that mast cells have relatively selective expression of the glutamate receptor ionotropic, kainate 2 (GluK2). A GluK2-specific agonist, SYM2081, effectively inhibited mast cell degranulation in response to MrgprB2 agonism in both murine mast cells and human skin explants in vitro as well as in vivo following both intradermal and topical administration of SYM2081 to mice. Analyses of transcriptomic datasets from SYM2081-treated mast cells using standard differential expression approaches as well as an interpretable machine learning technique revealed a previously unrecognized cellular program coordinately regulated by GluK2 agonism. GluK2 agonism suppressed the expression of Mrgprb2 and genes associated with mast cell proliferation. Suppression of mast cell proliferation by SYM2081 exposure was confirmed based on reduced Ki-67 expression and BrdU incorporation in vitro and in vivo. Finally, pretreatment with SYM2081 reduced skin inflammation in murine models of dermatitis and rosacea. Thus, agonism of GluK2 represents a promising approach to suppress mast cell activation and may prove beneficial as therapy for inflammatory diseases in which mast cell activation is pathogenic.
One Sentence Summary:
Small molecule agonism of GluK2 reduces dermal mast cell responses to MrgprB2 ligation and suppresses skin inflammation.
INTRODUCTION
Mast cells are granulocytic cells situated in barrier tissues where they integrate innate and adaptive immunity, provide host defense, and promote allergic diseases (1–5). In mice, dermal mast cells originate from yolk sac-derived progenitors but are gradually replaced by definitive hematopoietic progenitors. Under steady-state conditions, dermal mast cells self-renew in situ without the contribution of progenitors from the bone marrow (6–8). Mast cells can be activated when antigen binds to IgE prebound to the high-affinity Fcε receptor (FcεRI) expressed on the surface of mast cells. Connective tissue-type mast cells found in the dermis and peritoneal cavity can also be activated by ligating the Mas-related G-protein-coupled receptor B2 (MrgprB2) (9–11). MrgprB2 (or its human orthologue MrgprX2) can be activated by the neuropeptide Substance P (12), a cleavage product of human cathelicidin LL-37 (13, 14), β-defensins (15, 16), bacterial quorum sensing proteins (5), and several Food and Drug Administration (FDA)-approved medications associated with pseudoallergy (9). Mast cell activation results in the release of numerous preformed mediators from granules as well as the synthesis of cytokines, chemokines, and other soluble factors.
Activation of dermal mast cells by Substance P released from pain-sensing neurons (nociceptors) has been recognized as a common element in the pathogenesis of several murine skin disease models. In mice patch-immunized with house dust mite extract, a model of atopic dermatitis, Type 2 allergic inflammation requires activation of transient receptor potential vanilloid 1 (Trpv1)-expressing nociceptors and Substance P- and MrgprB2-mediated mast cell activation (17, 18). Moreover, patients with atopic dermatitis show increased density of mast cells in lesional skin, high serum concentrations of Substance P, and increased expression of MrgprX2 in lesional skin (18–22). Similarly, croton-oil-mediated dermatitis, a model of irritant dermatitis, requires Trpv1-expressing neurons, Substance P, and MrgprB2 for the development of inflammation (23, 24). Allergic contact dermatitis, as modeled by hapten-mediated contact hypersensitivity, and rosacea, as modeled by dermal administration of a cleavage product of cathelicidin, LL-37, both involve MrgprB2-mediated mast cell activation (2, 25–29). Additionally, common triggers of rosacea flares are known to activate Trpv1-expressing nociceptors, potentially contributing to mast cell activation (30). Thus, activation of mast cells through MrgprB2 (mouse) or MrgprX2 (human) appears to be common to multiple skin inflammatory disease processes and represents a promising pathway to target for anti-inflammatory therapy (31–33).
We recently reported that a subset of nonpepetidergic sensory neurons identified based on the expression of Mas-related G-protein coupled receptor member D (MrgprD) tonically release the neurotransmitter glutamate that acts on dermal mast cells and alters their capacity to respond to MrgprB2 ligands (23). In the absence of MrgprD-expressing neurons, or when the capacity of these neurons to release glutamate is reduced, dermal mast cells became hyperresponsive to MrgprB2 activation at least in part due to increased expression of Mrgprb2. In contrast, agonism of MrgprD increased neuronal release of glutamate, rendering dermal mast cells hyporesponsive to direct activation by MrgprB2 agonism. Agonism of MrgprD also suppressed inflammation in irritant dermatitis, allergic dermatitis, and rosacea models. Based on inhibitor studies, neuron-derived glutamate appeared to act through the GluK2 glutamate receptor expressed on mast cells. Based on these findings and given the importance of MrgprB2/X2-mediated mast cell activation in the pathogenesis of multiple cutaneous diseases, we hypothesize that direct agonism of the GluK2 glutamate receptor on mast cells could be employed to suppress dermal mast cell activation with the goal of providing therapeutic benefit.
RESULTS
Dermal and peritoneal mast cells express GluK2
Based on ligand binding specificity, ionotropic glutamate receptors are classified as NMDA, AMPA, kainate, or GluD receptors (34). RNA expression profiling of peritoneal and dermal mast cells showed that these cell types preferentially expressed genes for the kainite receptors GluK2 (Grik2) and GluK5 (Grik5) (23, 35). GluK2 and GluK5 receptors are thought to signal as a heterodimer, and GluK2 is required for signal transduction as GluK5 cannot signal as a homodimer (34). To investigate glutamate receptor expression by mast cells from multiple tissues and in other immune cell types, we reanalyzed publicly available transcriptional data collected by the Immgen consortium (36) (Fig. 1A). Mast cells isolated from skin, peritoneal cavity, tongue, heart, palate, and fat expressed Grik2, encoding GluK2. Mast cells isolated from the spleen, lung, and bone marrow showed negligible Grik2 expression. Somewhat unexpectedly, the expression of ionotropic glutamate receptors appears to be relatively restricted to mast cells.
Fig. 1. Peritoneal cavity mast cells and dermal mast cells express GluK2.

(A) Heatmap generated with data from Immgen Consortium showing the expression of ionotropic glutamate receptors on immune cell subsets. (B) Representative images of murine peritoneal cavity mast cells (PCMCs), transverse ear skin sections, and dorsal root ganglia (DRG) sections with immunofluorescence visualization of GluK2 (red) and mast cells (MC; Avidin, green). Nuclei are shown in blue. Scale bar, 50 μm. FMO, fluorescence minus one control. (C) Percentages of GluK2+ cells within total mast cells (defined as avidin+) in PCMC cultures and skin. Results are representative of 3 independent experiments.
To validate the transcriptomic data, we evaluated the expression of GluK2 in peritoneal cavity mast cell (PCMC) cultures and sections of skin isolated from unmanipulated mice by immunofluorescent microscopic visualization. Tissues were labeled with a GluK2/3 specific monoclonal antibody and avidin, which binds to abundant negatively charged proteoglycans found in mast cell granules, thereby allowing for easy identification of these cells (37). Expression of GluK2/3 was evident in PCMCs. In the skin, GluK2 expression was specific for avidin+ dermal mast cells and was not observed in other cell types. We also observed expression of GluK2/3 expression in the dorsal root ganglia (DRG) (Fig. 1B). Around 90% of avidin-positive mast cells in primary PCMC cultures and nearly all avidin-positive dermal mast cells expressed GluK2/3 (Fig. 1C). Thus, in the skin, expression of GluK2 is restricted to dermal mast cells, and within the immune system, expression is likely restricted primarily to mast cells found in specific tissues.
GluK2 agonists suppress peritoneal cavity mast cell degranulation
To determine whether direct agonism of GluK2 could suppress mast cell function, we selected 3 agonists with increasing specificity for GluK2. Glutamate broadly activates all glutamate receptors. Kainic acid agonizes kainate receptors but can activate some AMPA receptors. 4-methyl-glutamate (SYM2081) binds with high specificity to GluK2 at the nanomolar to micromolar concentrations (38). PCMCs were incubated with the indicated concentration of GluK2 agonist for 48 hours followed by activation by the synthetic MrgprB2 agonist compound 48/80 (9) (Fig. 2A and B). The extent of mast cell activation was determined by the percent of mast cells that degranulated based on surface expression of the lysosomal-associated membrane protein 1 (LAMP-1, CD107a) and avidin binding, which both occur following mast cell degranulation (39, 40). Incubation with kainic acid showed a dose-dependent reduction in degranulation to compound 48/80 which approached the degree of suppression observed with the positive control, glutamate (6 mM). SYM2081 also displayed a dose-dependent suppression of PCMC degranulation with a half-maximal effective concentration (EC50) of 2.271 nM and a maximum suppression of approximately 60% at concentrations above 100 nM (Fig. 2C to E). SYM2081 also suppressed degranulation mediated by two other well-characterized MrgprB2 ligands, Substance P and PAMP9–20 (9, 41) (fig. S1A). The ability of GluK2 agonism to suppress mast cell degranulation is consistent with our earlier observations that antagonism of GluK2 exaggerated mast cell degranulation (23). Incubation with SYM2081 at 1600 nM or glutamate at 6 mM for 48 hours did not affect PCMC viability (fig. S1B and C). The capacity of SYM2081 to suppress MrgprB2-mediated mast cell degranulation was confirmed by measuring the amount of β-hexosaminidase released from granules in response to compound 48/80 (Fig. 2F). Incubation with SYM2081 had no effect on FcεRI-mediated mast cell degranulation, as assayed by flow cytometry and release of β-hexosaminidase (Fig. 2G and H).
Fig. 2. GluK2 agonism suppresses MrgprB2-mediated murine PCMC activation.

(A) Representative flow cytometric plots of PCMCs after 48-hour incubation with vehicle, 100 μM kainic acid (KA), or 6 mM glutamate (Glu) and challenged with 20 μg/ml compound 48/80 for 20 min. (B) Summary of the percentage of degranulated LAMP1+Avidin+ mast cells detected by flow cytometry as in (A). (C) Representative flow plots of PCMCs after 48-hour incubation with vehicle or SYM2081 (1600 nM) followed by challenge with compound 48/80 (20 μg/ml). (D) Summary of the percentage of degranulated LAMP1+Avidin+ mast cells detected by flow cytometry as in (C). (E) Dose-response relationship between the concentrations of SYM2081 and its inhibitory effect on compound 48/80-mediated PCMC degranulation shown in (C). (F) The percentage of β-hexosaminidase released from PCMCs incubated with vehicle, SYM2081, or 6 mM glutamate for 48 hours and challenged with compound 48/80 (20 μg/ml). (G and H) Percentage of degranulated mast cells as assayed by flow cytometry (G) or β-hexosaminidase released from PCMC cultures (H) incubated with either vehicle or SYM2081 sensitized with anti-DNP IgE and challenged with DNP-HSA (1 ng/ml). Results are representative of at least 3 independent experiments, each with primary murine PCMC cultures pooled from n=2 mice. Data in (B, D, and F) were analyzed by one-way ANOVA with Dunnett’s multiple comparison test, and each column is compared with the vehicle-treated, compound 48/80-challenged positive control. **p<0.01, ***p<0.001, ****p<0.0001.
Acute glutamate exposure has been reported to trigger degranulation in mast cells from bone marrow (42). To exclude the possibility that suppressed degranulation reflected a reduction in total granule contents, we labeled total intracellular granules by incubation with fluorescein isothiocyanate (FITC)-avidin for 5 days followed by washes to remove excess avidin from the medium. To validate this approach, cells were activated with compound 48/80. Cells that had not degranulated based on the absence of surface LAMP-1 showed labeling with FITC-avidin indicating successful incorporation of the label into granules (fig. S1D). Degranulated LAMP-1+ cells showed a reduction in FITC-avidin, consistent with the release of some avidin-bound granules. We repeated this experiment and incubated FITC-avidin-labeled cells with 1600 nM SYM2081 or 6 mM glutamate for 48 hours. We also labelled cells with Lysotracker to evaluate total granule volume. Neither glutamate nor SYM2018 reduced intracellular avidin or Lysotracker values, demonstrating that total granule content was unaffected by glutamate or SYM2081 (fig. S1E and F). Similarly, amounts of total intracellular β-hexosaminidase were unaffected by incubation with SYM2081 or glutamate (fig. S1G). Together, these data demonstrate that SYM2081 efficiently suppressed MrgprB2- but not FcεRI-mediated mast cell degranulation without impacting cell viability or inducing spontaneous degranulation.
GluK2 agonist suppresses dermal mast cell degranulation in vivo
To determine whether SYM2081 could suppress mast cell degranulation in vivo, cohorts of C57BL/6 mice (wildtype) were given intradermal (i.d.) injections with 1600 nM SYM2081 or vehicle twice daily. After 2 days, mice were intravenously (i.v.) injected with Evans blue dye and challenged by i.d. injection of either phosphate-buffered saline (PBS) or compound 48/80. The extent of mast cell degranulation was visually assessed based on the degree of extravasation of Evans blue dye into the dermis, which was then quantified by optical absorbance (Fig. 3A and B). SYM2081 treatment decreased the amount of Evans blue dye in the dermis, consistent with reduced mast cell degranulation. We confirmed that fewer mast cells degranulated in SYM2081-treated skin by direct visualization of histologic sections stained with toluidine blue to highlight mast cells (Fig. 3C and D). This was corroborated by microscopic immunofluorescent visualization of avidin-labeled mast cells (fig. S2A and B). Treatment with SYM2081 did not affect mast cell degranulation in response to FcεRI-mediated mast cell activation in a passive cutaneous anaphylaxis assay (Fig. 3E).
Fig. 3. GluK2 agonism suppresses murine and human dermal mast cell degranulation.

(A to D) Flank skin was injected with either vehicle or 1600 nM SYM2081 twice daily for 2 days. Mice were treated with Evans blue (i.v.), then locally injected with PBS or 10 μg/ml compound 48/80. (A) Representative images showing Evans blue dye extravasation 20 minutes after PBS or compound 48/80 challenge. (B) The quantification of dye extracted from punch biopsies of skin injection sites is shown. (C and D) Shown are the quantification (C) and representative microscopic images of degranulated mast cells (D). Black arrowheads indicate non-degranulated mast cells. Red arrowheads indicate degranulated mast cells. (E) Ears were pretreated with either vehicle or 1600 nM SYM2081, sensitized with 20 ng/20 μl anti-DNP IgE and challenged with 100 μg intravenous DNP-HSA. The amount of Evans blue dye extravasation is shown. (F) Wildtype flank skin was treated with either topical vehicle or SYM2081 twice daily for 2 days followed by intradermal challenge with either PBS or 10 μg/ml compound 48/80. The amount of Evans blue dye extravasation is shown. Mrgprb2−/− were challenged but not given topical treatment and “unmanipulated” mice received no treatments. (G) Human skin explants were incubated with either vehicle or 1600 nM SYM2081 for 2 days then challenged with 240 μg/ml compound 48/80 for 1 hour. The percentage of degranulated mast cells based on Toluidine blue staining of skin section is shown (n=6). Scale bar, 50 μm. For (B, C, E, and F), results are pooled from at least 3 independent experiments, each with at least n=3. Vehicle and SYM2081 were delivered to different skin sites of the same animal (B, C, and E) or topically applied to different groups of animals (F). Data were analyzed by two-way ANOVA with Šídák’s multiple comparison tests. *p<0.5, **p<0.01, ****p<0.0001. ns, not significant.
A potential concern was that repeated needle sticks required for intradermal administration of SYM2081 could activate mast cells and potentially confound our results. To control for this, we formulated SYM2081 into a topical cream at a concentration of 100 μM. Cohorts of wildtype and Mrgprb2−/− mice were treated with vehicle or topical SYM2081 twice daily for 2 days, then administered Evans blue dye (i.v.) and challenged with either PBS or compound 48/80 (i.d.). As a further control, we included an “unmanipulated” group of wildtype mice. Topical application of SYM2081 reduced Evans blue dye extravasation in response to compound 48/80, indicative of reduced mast cell degranulation, but did not affect the number of dermal mast cells (Fig. 3F, fig. S2C to E). Mast cell degranulation was elevated in the PBS-challenged mice compared with unmanipulated and Mrgprb2−/− mice. Thus, injection of PBS alone is sufficient to activate some mast cells through a Mrgprb2-dependent mechanism. We speculate that the injection itself, which is a noxious stimulus associated with pain, could be sufficient to trigger release of Substance P (a ligand for MrgprB2) from Trpv1-expressing neurons. Alternatively, the introduction of a bolus of PBS could induce a small amount of cell damage that is sufficient to degranulate mast cells. However, mast cell degranulation in response to both PBS injection and the direct effect of compound 48/80 was suppressed by topical application of SYM2081.
Finally, to determine whether SYM2081 could suppress mast cell degranulation in human skin, fresh human skin explants were incubated for 48 hours in 1600 nM SYM2081 or vehicle. Compound 48/80 was then added to the culture media for 60 minutes. The number of degranulated mast cells, as assessed by visualization of toluidine blue stained histology sections, was reduced in tissue incubated in SYM2081 without altering the total number of dermal mast cells (Fig. 3G, fig. S2F and G, table S1). Thus, SYM2081 suppressed MrgprB2-mediated mast cell degranulation in vivo in mice and MrgprX2-mediated mast cell degranulation in human skin explants.
Transcriptomic analyses reveal distinct gene modules regulated by GluK2
To characterize transcriptional changes in mast cells following GluK2 agonism, we performed bulk RNA sequencing on PCMCs incubated with vehicle, 6 mM glutamate, or 1600 nM SYM2081 for 48 hours. GluK2 agonism largely reduced gene expression which is consistent with its inhibitory effects on mast cell functions (fig. S3A and B). Visualization using principal component analysis (PCA) demonstrated that the reduction in gene expression (relative to untreated cells) was more profound in glutamate-treated cells compared with those treated with SYM2081 which had an intermediate phenotype (Fig. 4A). Overrepresentation analyses of differentially expressed genes (DEGs) (data file S1) across Reactome pathways demonstrated that genes with reduced expression were enriched in virtually identical pathways in both glutamate- and SYM2081-treated cells. Most of the 10 most significantly different pathways were related to a reduction in genes associated with the cell cycle, suggesting that GluK2 agonism may suppress proliferation (Fig. 4B and C). These results demonstrate that although glutamate has a larger effect, both agonists resulted in the same transcriptional alterations at a pathway level.
Fig. 4. Transcriptomic analyses reveal distinct gene modules in murine mast cells regulated by GluK2.

(A) Shown is principal component analysis (PCA) of DEGs from PCMCs treated with vehicle, SYM2081, or glutamate for 48 hours. (B and C) Pathways associated with genes that had reduced expression in PCMCs following glutamate (B) or SYM2081treatment (C) are shown. Numbers in the bar represent gene enrichment/number of genes in pathway. The total number of genes defining each pathway is in data file S1. (D and E) Shown are pathways (D) and a heatmap of genes with reduced expression following glutamate and SYM2081 treatment and increased expression in mast cells isolated from DT-treated MrgprDDTR mice (E). (F) Performance of the SLIDE model in a 4-fold cross-validation framework with permutation testing (negative/random control) in distinguishing sample labels (agonism, control, antagonism). Central line represents median of the correlation. Top and bottom lines represent the third and first quartile of the correlation, respectively. (G) Shown is a correlation network representation of the most discriminative latent factor. Blue nodes have lower expression in SYM2081- and glutamate-treated PCMCs and higher in MrgprDDTR mast cells, whereas red nodes have higher expression in SYM2081- and glutamate-treated PCMCs and lower expression in MrgprDDTR mast cells. Edge thickness corresponds to strength of correlation (thicker = higher correlation). All correlations were positive in this network.
Since PCMCs are cultured in growth factors, the strong reduction in genes associated with proliferation could be restricted to mast cells grown in vitro. We previously reported that mast cells in MrgprdDTR mice that lack MrgprD-expressing neurons after treatment with diphtheria toxin (DT) are hyperresponsive to MrgprB2 ligands due to the loss of a neuronal source of glutamate (23). To focus our analysis on those genes that are coordinately regulated by GluK2 agonism both in vitro and in vivo, we looked for overlaps of DEGs that had at least two-fold reduced expression in SYM2081- and glutamate-treated PCMCs with those genes that had at least two-fold increased expression comparing dermal mast cells isolated from MrgprdDTR and littermate control mice after DT treatment and sorted by fluorescence activated cell sorting (FACS). We identified a set of 33 coordinately regulated genes (Fig. 4D and E, data file S1). Altered pathways were primarily related to the cell cycle and replication, similar to those observed in PCMCs, further supporting the possibility that GluK2 agonism suppresses mast cell proliferation.
Identifying groups of similarly altered genes by overlapping individual sets of DEGs is an effective approach, but is unable to identify coordinately regulated genes that fall below arbitrary univariate fold-change or significance value cut-offs. Further, overrepresentation analyses using DEGs identified at univariate significance thresholds against known pathway databases cannot identify context-specific cellular programs (co-expression modules) as we interrogate these DEGs against pre-defined gene sets. To address these, we used Significant Latent Factor Interaction Discovery and Exploration (SLIDE), an interpretable machine learning approach that we recently developed. Using a latent factor regression approach with a range of rigorous statistical guarantees, SLIDE allows for the identification of a necessary and sufficient set of context-specific co-expression modules or latent factors that provide both discrimination between groups and inference of de-novo cellular programs to capture the transcriptional impact of GluK2 agonism (43). Using this approach, we identified 2 latent factors that could discriminate treated and untreated samples as measured in a k-fold cross-validation framework with permutation testing (Fig. 4F and G, table S2 and S3). The most discriminatory latent factor contained a set of very highly coordinately expressed genes, most with reduced expression (shown in blue) in SYM2081- and glutamate-treated PCMCs and increased expression in dermal mast cells isolated from MrgprdDTR mice. Notable within this set of genes are Mrgprb2 and Cma1, which were also identified through DEG analysis, as well as Mcpt4 and other MRGPR family genes such as Mrgprb1 and Mrgprx2. We validated that GluK2 agonism reduced the expression of Mrgprb2 and Cma1 in PCMCs, mouse skin, and human skin explants (fig. S3C to E). Thus, by combining standard DEG and pathway overrepresentation analyses with an interpretable machine learning approach, we have identified a cellular program in mast cells regulated by GluK2 agonism that suggests that GluK2 agonism may suppress mast cell proliferation.
GluK2 agonism suppresses mast cell proliferation in vitro and in vivo
To determine whether GluK2 agonism reduces mast cell proliferation, PCMCs were cultured in vehicle, 1600 nM SYM2081, or 6 mM glutamate along with 10 μM 5-Bromo-2’-deoxyuridine (BrdU) for 24 hours. Cell proliferation, as measured based on the incorporation of BrdU and expression of Ki-67, was assayed by flow cytometry. SYM2081 and glutamate both reduced BrdU incorporation and expression of Ki-67, indicative of reduced proliferation (Fig. 5A to C, fig. S4A). SYM2081 treatment also decreased PCMC numbers in vitro (fig. S4B). To test whether GluK2 agonism suppresses mast cell proliferation in vivo, topical SYM2081 was applied on the flanks of wildtype mice twice daily for 9 days. After 2 days, mice were given a single intraperitoneal injection of 1 mg BrdU, and the mice were placed on water supplemented with 0.8 mg/ml BrdU for an additional 7 days. Topical application of SYM2081 lowered BrdU incorporation and reduced Ki-67 expression in mast cells indicative of reduced proliferation (Fig. 5D to F, fig. S4C). However, it did not decrease dermal mast cell number, at least in this treatment schedule (fig. S4D), which might only be evident with a longer treatment. The antiproliferative effect of SYM2081 was not observed in macrophages (Fig. 5E and F, fig. S4E and F), which also undergo self-renewal within the dermis during homeostasis but do not express GluK2 (44, 45). This BrdU treatment schedule labeled approximately 16% of dermal mast cells. This degree of mast cell proliferation does not result from hair shaving or application of a topical cream, as mast cells in unmanipulated and vehicle-treated mice showed similar BrdU incorporation. In addition, the proliferation of dermal mast cells is unlikely the result of recruitment of CD117+integrinβ7+ mast cell precursors, as they do not contribute to the maintenance of mast cell clonal territories under steady state and we observed only negligible numbers of these cells in the dermis (fig. S4G) (6). Collectively, these data confirm our transcriptomic analyses and indicate that a functional outcome of GluK2 agonism includes suppression of dermal mast cell proliferation.
Fig. 5. GluK2 agonist suppresses murine mast cell proliferation in vitro and in vivo.

(A to C) PCMCs were incubated with vehicle, 1600 nM SYM2081 or 6 mM glutamate for 48 hours. BrdU (10 μM) was added for the final 24 hours. (A and B) Representative flow cytometric plots (A) and quantification (B) of BrdU+ mast cells. (C) The percentage of Ki-67+ mast cells as determined by flow cytometry is shown. (D to F) Cohorts of mice were treated twice daily with topical vehicle or SYM2081 on flank skin. BrdU was administered by i.p. injection (1 mg) at the start of treatment and was present in drinking water (0.8 mg/ml) for a total of 7 days. (D) Representative flow plots show BrdU incorporation by dermal mast cells. (E and F) Quantification of the percentage of BrdU+ (E) and Ki-67+ (F) dermal mast cells or macrophages is shown. Data in (A to C) are representative of at least 3 experiments, each with murine PCMC cultures pooled from at least n=2 mice. Data in (E) are pooled from 4 independent experiments, each with at least n=3. Each dot represents the average of all the animals in one experiment. Data in (F) are pooled from 3 independent experiments, each with at least n=3. (B and C) Data were analyzed by one-way ANOVA with Dunnett’s multiple comparison test, and each column is compared with the vehicle-treated positive control. (E) Data were analyzed using paired-t test. (F) Data were analyzed using unpaired t-test. FMO, fluorescence minus one control. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
GluK2 agonist ameliorates mast cell-dependent inflammatory skin diseases
To determine whether SYM2081 can suppress cutaneous inflammatory responses, we tested its efficacy in mouse models known to require mast cells for pathogenesis. To determine whether SYM2081 could suppress inflammation in contact hypersensitivity, a model of allergic contact dermatitis, topical SYM2081 or vehicle was applied twice daily for 2 days on the abdominal skin of wildtype mice followed by sensitization with 0.5% 1-Fluoro-2,4-dinitrobenzene (DNFB). The mice were challenged 5 days later with 0.2% DNFB on one ear and vehicle on the other (Fig. 6A). SYM2081 treatment during sensitization ameliorated ear swelling at the challenge phase by about 50% (Fig. 6B), comparable to the degree of reduction seen in mast cell-deficient mice (2). Microscopically, the SYM2081 treatment group had reduced overall skin thickness and inflammatory infiltrate (Fig. 6C and D). Flow cytometry analyses of the cellular components revealed a reduction in CD45+ immune cells, macrophages, neutrophils, and CD8+ T cells and a slight increase in γδ T cells (Fig. E). Cytokine concentrations analyzed from whole skin indicated a reduction of CCL2 and interleukin (IL)-27 in SYM2081 treated skin (Fig. 6F).
Fig. 6. GluK2 agonist treatment ameliorates DNFB-induced contact hypersensitivity in mice.
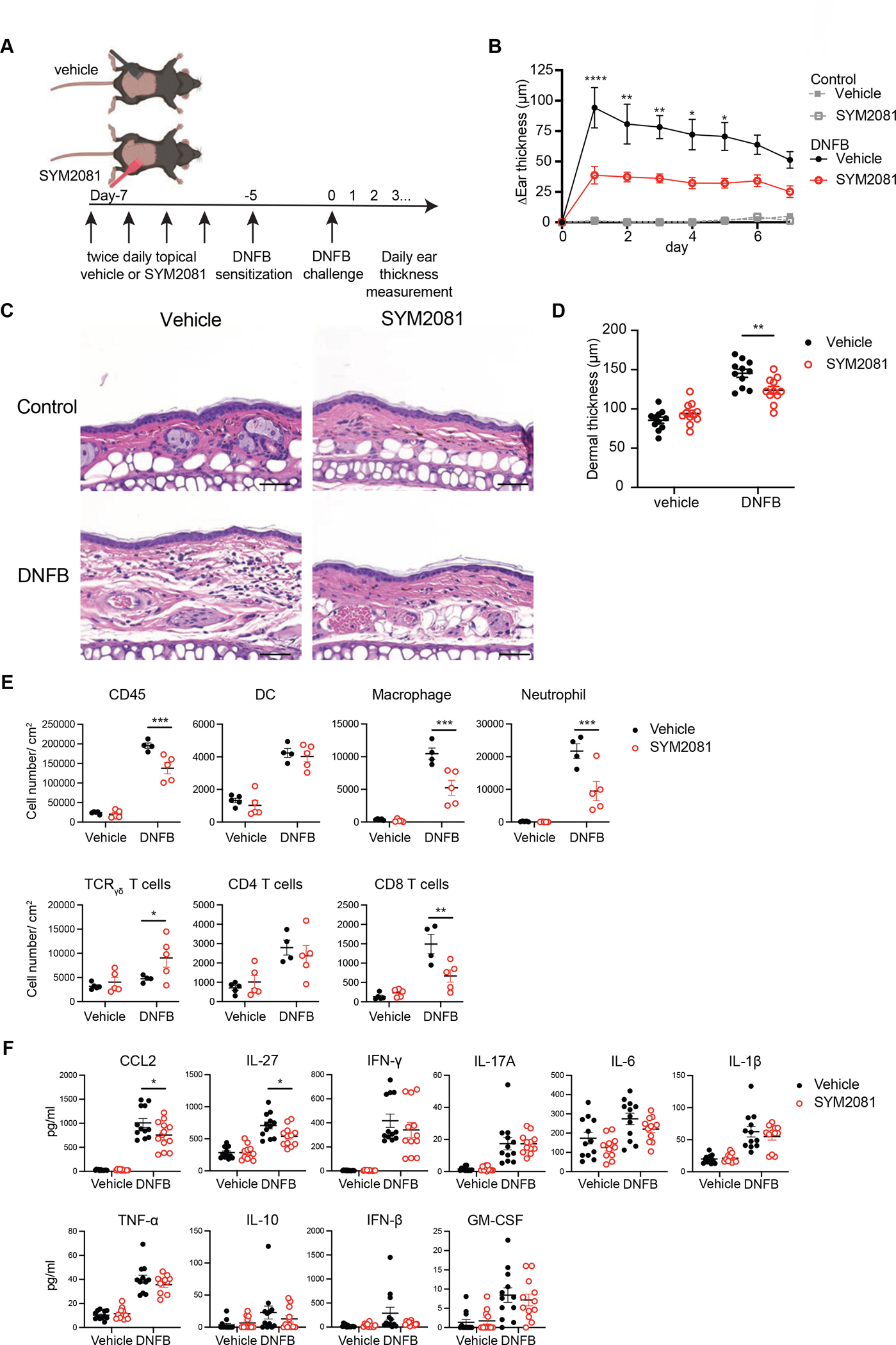
(A) Diagram showing the experimental design. Abdominal skin was treated with topical vehicle or SYM2081 twice daily for 2 days before sensitization with 0.5% DNFB. Five days later, ears were challenged with 0.2% DNFB or vehicle and ear thickness changes were measured. (B to D) (B) Daily ear thickness measurements after DNFB challenge, (C) representative H&E-stained images of ears 1 day post challenge, and (D) dermal thickness measurements 1 day post challenge are shown. Scale bar, 50 μm. (E and F) Ears were taken 1 day post challenge for flow cytometry and cytokine quantification. (E) Shown are total cell numbers of CD45+ immune cells, dendritic cells (MHCII+CD11c+CD64−), macrophages (MHCII+CD64+CD11b+Gr-1−), neutrophils (MHCII−CD11b+Gr-1hi), γδ T cells change (CD3+TCRγδ+), CD4+ T cells (CD3+TCRβ+CD4+), and CD8+ T cells (CD3+TCRβ+CD8+). (F) Shown are concentrations of key cytokines. Measurable induction of IL-12, IL-1α or IL-23 was not observed. Results are representative of at least 2 independent experiments. Data in (B, and D to F) were analyzed by two-way ANOVA with Šídák’s multiple comparison test. *p<0.5, **p<0.01, ***p<0.001, ****p<0.0001. MHCII, major histocompatibility complex class II; IFN, interferon; TNF, tumor necrosis factor; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Topical application of croton oil to murine skin models irritant contact dermatitis and involves substance P- and Mrgprb2-dependent mast cell activation (23, 46, 47). Treatment of wildtype mice with SYM2081 twice daily for 2 days prior to application of croton oil resulted in reduced ear swelling at the peak time point 6 hours post application (Fig. 7A and B). Evaluation of H&E-stained histologic sections confirmed reduced edema and overall skin thickness in SYM2081-treated skin (Fig. 7C and D). Histology sections stained with toluidine blue confirmed a reduced percentage of degranulated mast cells, but the total number of mast cells was unaffected (Fig. 7E and F, fig. S5A). The effect of SYM2081 in this acute inflammation model seems to be largely restricted to the degree of mast cell degranulation and tissue edema, as there was minimal change in either cellular infiltrate (fig. S5B) or cytokine concentrations (fig. S5C) with SYM2081 pretreatment.
Fig. 7. GluK2 agonist treatment attenuates mast cell-dependent inflammatory skin diseases in mice.

(A to F) Ears were treated with intradermal (i.d.) injection of vehicle or 1600 nM SYM2081 twice daily for 2 days. 1% croton oil or vehicle was topically applied to both sides of the ears. (A) Diagram showing the experimental design. (B to F) Ear thickness changes (B), H&E-stained images (C), dermal thickness measurements (D), toluidine blue staining (E), and quantification of degranulated mast cells (F) are shown at 6 hours following croton oil or vehicle (control) application. Scale bar, 50 μm. (G to J) 320 μM LL-37 mixed with vehicle or 1600 nM SYM2081 was injected into flank skin of wildtype mice twice daily for 2 days. (G) Diagram showing the experimental design. (H) Representative images showing the development of erythema 72 hours after the first injection. Scale bar, 5 mm. (I and J) Quantification of the lesion size (I) and erythema scores (J) are shown. Data in (B, I, and J) Results are pooled from 3 independent experiments, each with at least n=3. (D and F) Results are pooled from 2 independent experiments, each with at least n=4. Data in (B) and (J) were analyzed by two-way ANOVA with Šídák’s multiple comparison test. Unpaired Student’s t-test was performed for data in (D) and (F). Mann-Whitney test was performed for (I). *p<0.5, **p<0.01, ****p<0.0001.
Finally, mice were given intradermal injections with cathelicidin (LL-37) mixed with 1600 nM SYM2081 or vehicle twice daily for 2 days (Fig. 7G). Dermal injection of LL-37 is a well-established approach to induce a rosacea-like inflammation clinically characterized by erythema. This process is mast cell- and MrgprB2-dependent (13, 28, 29, 48, 49). At 72 hours after the first injection, the size of the erythematous lesions was evaluated, and the injection site was harvested for mRNA analysis. SYM2081 reduced lesion size and erythema (Fig. 7H to J) as well as expression of rosacea-associated biomarkers including Klk5, Mmp9, and Il1b (fig. S5D). Collectively, these data demonstrated that administration of SYM2081 either by intradermal injection or topical application can effectively suppress the development of inflammation in disease models in which MrgprB2-mediated mast cell activation is a required component of pathogenesis.
DISCUSSION
In this study, we demonstrated that GluK2 is expressed in murine skin mast cells and that mast cells are the primary murine immune cell type that expresses GluK2. Small molecule agonism of GluK2 using SYM2081 suppressed mast cell degranulation in vitro and in vivo in response to MrgprB2 ligands and acute skin injury (e.g., hypodermic injections). SYM2081 was also capable of suppressing mast cell degranulation in human skin explants. The agonism of GluK2 reduced the expression of the activating receptor Mrgprb2 and key granule proteases such as Cma1 while also suppressing mast cell proliferation. Finally, in the MrgprB2-dependent inflammatory skin disease models of irritant dermatitis, contact dermatitis, and rosacea, SYM2081 reduced the development of skin inflammation. Our data suggests that GluK2 agonism may represent a potentially beneficial approach to suppress mast cell activation for the prevention of inflammatory cutaneous diseases.
Current approaches under clinical development to suppress mast cells include antagonism of MrgprX2 to prevent mast cell activation or the c-kit receptor tyrosine kinase to suppress mast cell proliferation. GluK2 agonism appears to suppress both pathways simultaneously. GluK2 agonism reduced the expression of Mrgprb2. This demonstrates that the expression of Mrgprb2 is a regulated process and its reduced expression was likely responsible for the observed reduction in mast cell activation following GluK2 agonism. We also found that GluK2 agonism suppressed genes associated with cellular proliferation which was validated both in vivo and in vitro. Notably, Mrpgrb2 and genes associated with cell proliferation were part of a larger, highly related set of coordinately regulated genes with reduced expression following GluK2 agonism that also included the protease Cma1 and other members of the MRG family. Thus, mast cell function may be altered beyond the parameters that we have examined. For example, GluK2 agonism did not affect FcεRI-mediated mast cell degranulation. However, reduced expression of mast cell proteases (e.g. Cma1) could still impact skin pathology independent of the rate of acute degranulation (18, 50–53).
A prediction of the antiproliferative effect of GluK2 agonism is that the number of skin mast cells should decline over time. Although mast cell numbers were reduced by SYM2081 in vitro, we did not observe a reduced number of dermal mast cell numbers in vivo following 2 days or 9 days of SYM2081 treatment. Given the modest reduction in BrdU incorporation observed, we speculate that a longer treatment duration might be required to detect this effect. It has been reported that glutamate can trigger mast cell degranulation in bone-marrow-derived mast cell cultures and mast cells in tendons (42). In our studies of PCMCs and dermal mast cells we found that glutamate or SYM2081 did not induce mast cell degranulation or affect granule volume. This discrepancy may result from intrinsic functional differences between mast cells found in different tissues. This observation is further supported by the heterogeneous expression of Grik2 by mast cells isolated from different tissues.
Our study has some limitations. We have focused exclusively on agonism of GluK2 and not other glutamate receptors based on the prior work indicating that Grik2 is a key gene associated with dermal mast cells (35) and data from Immgen indicating selective expression of Grik2 on mast cells. Grik5 (GluK5) is also expressed by dermal mast cells (23). We have not examined the function of GluK5 because it is thought to function only as an obligate heterodimer with GluK2 and the paucity of selective agents to target this receptor subunit (34). Although we did not observe expression of GluK2 in the skin other than by mast cells, GluK2 is widely expressed throughout the nervous system including DRG. Thus, administration of SYM2081 in vivo, could potentially mediate some of its effect indirectly through modulation neuronal function. We think this is unlikely as our results in vitro with PCMCs phenocopy our findings in vivo. In addition, glutamate is actively excluded from the CNS by the blood-brain barrier (54, 55), and we did not observe gross abnormalities in mice treated with SYM2081 administered by intraperitoneal or intradermal injection. Nevertheless, we cannot exclude that administration of a GluK2 agonist could have effects on cells of the nervous system. Finally, though we have demonstrated reciprocal transcriptional and functional mast cell modulation using GluK2 antagonism (23) and agonism, we have not formally demonstrated a genetic requirement for Grik2 in mast cell biology.
In sum, our results have shown that selective agonism of the GluK2 glutamate receptor can suppress cutaneous mast cell function. Using an integrative approach combining functional and transcriptomic profiling and interpretable machine learning, we have revealed a previously under-appreciated role of glutamate in regulating cutaneous mast cell biology. Moreover, selective agonism of this pathway may represent a beneficial approach for the prevention of allergic and inflammatory diseases where hyperactive mast cells represent a key component of pathogenesis.
MATERIALS AND METHODS
Study design
The goal of this study was to determine the glutamate receptor expression on mast cells, and if glutamate receptor agonism could modulate mast cell activation and skin inflammation. We analyzed publicly available datasets to examine the expression of mast cell glutamate receptors and validated GluK2 expression in dermal mast cells with immunofluorescence. We chose three GluK2 agonists and investigated their effects on mast cell degranulation in vitro. We moved on with the selective GluK2 agonist SYM2081 for in vivo experiments in mice through either intradermal injection or topical application and ex vivo in human skin explants. To characterize the transcriptomic changes within mast cells following GluK2 modulation, we performed bulk RNA sequencing on PCMCs treated with vehicle, glutamate, and SYM2081 as well as sorted dermal mast cells from MrgprDDTR mice. We used Significant Latent Factor Interaction Discovery and Exploration to uncover the interactive network of the core genes underlying the observed effect of GluK2 agonism or antagonism. Then, we validated the antiproliferative effect of GluK2 agonists using BrdU incorporation assay and intranuclear Ki-67 staining, both in vitro and in vivo. In murine disease models, we delivered SYM2081 either before or at the same time as the disease-inducing agents to investigate the effect of SYM2081 on skin inflammation. We used gross and histological evaluation, and quantitative reverse transcription polymerase chain reaction (qRT-PCR) of disease biomarkers to assess disease severity in models of irritant and contact dermatitis, and rosacea. Animals were randomized to different groups and treated in parallel with matched animal age and sex. Animal experiments were performed and analyzed by investigators not blinded to group assignments. Human skin explant histology samples were evaluated by investigators blinded to group assignments. All animal results represent three or more independent experiments with three biological replicates for each experiment, unless otherwise specified. All mice were maintained under specific-pathogen-free conditions and all animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Research animals
6- to 12-week-old C57BL/6J female mice purchased from The Jackson Laboratory (000664) were used for PCMC cultures. 6- to 12-week-old C57BL/6J wild-type male and female mice were used for inflammatory skin disease models. Specifically, we used both male and female mice for Croton-oil irritant dermatitis model, male mice for DNFB contact hypersensitivity model, and female mice for rosacea model. Mrgprb2−/− mice were kindly provided by B. McNeil (Northwestern University, Chicago).
Human skin explants
Excess normal skin was obtained through our institutional tissue bank with informed consent from healthy female donors (age 10 to 59-years old) undergoing surgical procedures at the University of Pittsburgh Medical Center (UPMC). A list of patient information can be found in table S1. The de-identified tissue specimens were acquired through the Pitt Biospecimen Core with Institutional Review Board approval (IRB #0501119). Skin tissues were processed to remove subcutaneous adipose tissues, cut into 1 cm × 2 cm squares, and cultured in AIM-V medium (Thermo Fisher Scientific, 12055083). The dermal side of the explants was in contact with the medium and the epidermal side was exposed to air.
Analysis of glutamate receptor expression
Expression values of ionotropic glutamate receptors in immune cells were obtained from Immgen Gene Skyline using the ImmGen ULI RNASeq Data Group. Expression values were normalized using DESeq2. Data were visualized using GraphPad Prism software.
Immunofluorescent microscopy
For PCMCs, 1×105 PCMCs in 200 μl culture media were added to poly-D-lysine coated coverslips (Corning, 354087) and incubated at 37 °C for 30 minutes before being washed twice with PBS and fixed in 1% paraformaldehyde (PFA) for 10 minutes at room temperature. For sectioned tissues, ear or flank skin and DRG were fixed in 1% PFA overnight and dehydrated in 30% (w/v) sucrose in PBS. Tissues were then embedded in O.C.T. compound and frozen. The frozen tissues were cut at 6 μm and mounted onto Superfrost plus slides before immunolabeling as previously described (56). Primary antibodies and fluorescent agents used include rabbit anti-GluR6/7 (Sigma-Aldrich, clone NL904, 04–921) at the concentration of 1:50, and Avidin-FITC (Thermo Fisher Scientific, 21221) at 1:100. The secondary antibody used was goat anti-rabbit IgG (H+L)-Alexa Fluor 555 (Thermo Fisher Scientific, A-21428) at the concentration of 1:200. Tissues were mounted with ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific, P36935). Antibodies were diluted in antibody-diluting buffer (1% bovine serum albumin, 0.1% Tween20, 0.1% NaN3 in PBS). Images were captured with a KEYENCE fluorescence microscope (Keyence corporation).
PCMC culture
Peritoneal cells from 6- to 12-week-old C57BL/6J female mice were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Atlas, F-0500-A), 20 mM HEPES (Sigma-Aldrich, H0887), 1 mM Sodium pyruvate (Gibco, 11360070), 1x Non-essential amino acids (Corning, MT25–025-CI), 50 μM 2-mercaptoethanol (Sigma-Aldrich, M3148), 50 μg/ml penicillin/streptomycin (Gibco, 15140122), 4 mM L-glutamine (Corning, MT25–005-CI), and 25 ng/ml recombinant mouse IL-3 (PeproTech, 213–13) and 15 ng/ml stem cell factor (PeproTech, 250–03) or X-VIVO 15 medium (Lonza, 02–053Q) supplemented with the same amount of recombinant mouse IL-3 and stem cell factor. The cells were maintained at a concentration of 0.5 to 1×106 cells/ml with weekly medium changes. After 3 weeks of culturing, the cell population purity was confirmed by flow cytometry, with more than 95% of cells being mast cells.
For GluK2 agonist supplementation, different concentrations of kainic acid (Tocris, 0222), glutamic acid (Sigma-Aldrich, G1251), and SYM2081 (Enzo, ALX-550–186-M005) were added to the media with daily refreshment. For glutamic acid, the medium was titrated with sodium hydroxide solution (Fisher Scientific, SS256–500) to ensure the pH was unchanged. For cell counting, 1×105 cells were plated in each well in a 96-well plate, supplemented with either vehicle or 1600 nM SYM2081. After indicated periods of time, cell numbers were determined using a hemocytometer (Hausser Scientific, 3120) according to manufacturer’s instructions.
PCMC degranulation assay
PCMCs were incubated in Tyrode’s buffer at 37 °C for 20 minutes before adding compound 48/80 (Sigma-Aldrich, C2313), substance P (Tocris/Fisher, NC2226616), or PAMP9–20 (Tocris/Fisher, 65–511). The final concentrations were 20 μg/ml for compound 48/80, 25 μg/ml for substance P, and 6.25 μM for PAMP9–20. For flow cytometry, cells were incubated at 37 °C for 20 minutes before being washed and stained in FACS buffer (2% fetal bovine serum, 5 mM EDTA, 0.04% Azide in PBS) at 4°C for 30 minutes. After debris and doublet exclusion, mast cells were defined as FcεRIα+ CD117+. Within the mast cell population, degranulated mast cells were defined as LAMP1+ Avidin+. For the β-hexosaminidase (β-hex) release assay, cells were incubated at 37 °C for 45 minutes before proceeding as described (57). The optical density (OD) was read at 405 nm using a spectrophotometer. The percentage of β-hex release was calculated by the following formula: . For IgE-mediated degranulation, PCMCs were treated with 1 ng/ml anti-DNP-IgE (Sigma-Aldrich, D8406) 16 hours before adaptation in Tyrode’s buffer at 37 °C for 20 minutes. Tyrode’s buffer containing DNP-HSA (Sigma-Aldrich, A6661) was added for a final concentration of 1 ng/ml. For flow cytometry, cells were then incubated at 37 °C for 30 minutes before being washed and stained in FACS buffer as described above. For the β-hexosaminidase release assay, cells are then incubated at 37 °C for 45 minutes before proceeding as described (57).
In vivo SYM2081 treatment
For intradermal injection, 1600 nM SYM2081 in sterile PBS was injected twice daily with PBS as vehicle controls. For topical treatment, 100 μM SYM2081 lotion was created by adding 1% (v/w) 10 mM SYM2081 solution in sterile PBS to Aveeno daily moisturizing body lotion and mixing thoroughly with a laboratory blender (VWR, 58977–169). SYM2081 lotion was applied on the shaved back skin of the mice topically twice daily. For SYM2081 supplementation in human skin explant cultures, SYM2081 was added to culture media for a final concentration of 1600 nM with daily refreshment.
Evans blue extravasation
Evans blue extravasation was carried out as previously described (9, 23). Briefly, for compound 48/80, 50 μl 12.5 mg/ml Evans blue solution in sterile PBS was intravenously injected into mice under isoflurane-induced anesthesia. 10 minutes later, 20 μl 10 μg/ml compound 48/80 in sterile PBS was injected intradermally. 20 minutes later, skin injection sites were harvested with a punch biopsy (Fisher Scientific, 12–460-413). For the IgE-induced passive cutaneous anaphylaxis, 20 μl 1 μg/ml anti-DNP-IgE was intradermally injected into the ear pinnae of the mice. The next day, 50 μl 12.5 mg/ml Evans blue solution containing 100 μg DNP-HSA was intravenously injected. Thirty minutes later, ears were harvested for Evans Blue measurement. Evans blue dye was extracted in formamide and OD at 620 nm was measured with a spectrophotometer. The amount of Evans blue was normalized to the size of the skin biopsy.
RNA sequencing and analysis
For in vitro datasets, total mRNA from PCMCs was prepared using the QIAGEN RNeasy kit (QIAGEN, 74104). The mRNA libraries were generated using Illumina Truseq Stranded mRNA Library Prep kit, followed by 58bp single indexed sequencing on an Illumina NextSeq500 to obtain 50 million reads per sample. For in vivo datasets, four-week-old MrgprdDTR or MrgprdCre control mice were given 300 ng DT (i.p.) twice a week for 3–4 weeks. Subsequently, ear skin samples from MrgprdDTR or MrgprdCre mice (n=4 each) were digested and stained as detailed below using FACS buffer without azide (2% fetal bovine serum, 5 mM EDTA in PBS). Mast cells were sorted into lysis buffer in a 96-well plate using FACS Aria™ cell sorter (Becton Dickinson). Reads were aligned using kallisto (v0.46.1) (58) to the GRCm38/mm10 genome assembly and quantified using bootstraps. Subsequently, sleuth was used for differential expression analyses. DEGs were defined using q (p from a Wald test adjusted for multiple comparisons) < 0.05. Overrepresentation analyses were performed using the R cluster profiler package (59) against the Reactome pathway database. A significance threshold of q (p from a hypergeometric test adjusted for multiple comparisons) < 0.05 was used to define overrepresentation in a pathway.
Identifying context-specific cellular programs of mast cell agonism using SLIDE
SLIDE was used to identify latent factors (context-specific co-expression modules) necessary and sufficient to discriminate between transcriptomic profiles derived from mast cells from mice treated with glutamate and SYM2081, untreated mice and MrgprDDTR mice. Specifically, we had 20 samples with 4 mice each for glutamate, SYM2081, in vitro controls, MrgprDDTR mice, and littermate controls. Inputs to SLIDE were mean normalized expression values for the 1,000 most variable genes (measured in an unsupervised setting) across these 20 samples. For group labels, agonists were encoded as 1, controls as 0 and antagonists as −1. Hyperparameter optimization was performed as previously described (43). Model performance was measured across 10,000 replicates of leave-one-out cross validation framework as previously described (43). Briefly, for each cross validation run, samples were randomly divided into 20 subsets, such that for each fold, 19 subsets served as the training set, and the 20th one served as the test set, with each subset serving as the test set once. Model performance using Spearman correlations was measured on the held-out sets across folds. Model significance was measured using permutation testing (sample label permutations in a matched cross-validation framework) as previously described (43). For each latent factor, the top 20 features were used in the corresponding correlation network visualizations.
In vitro and in vivo BrdU treatment
For in vitro BrdU treatment, BrdU (Sigma, B5002) was supplemented in the culture media for a final concentration of 10 μM. 24 hours later, cells were collected. BrdU incorporation was read with flow cytometry per the manufacturer’s instructions. For in vivo BrdU treatment, mice were given an intraperitoneal injection of 1 mg BrdU in sterile PBS. Mice were then provided with drinking water containing 0.8 mg/ml BrdU and 1% (w/v) glucose for 7 days. BrdU incorporation was read with flow cytometry per the manufacturer’s instructions.
Mouse model of contact dermatitis
For the irritant dermatitis model, mice were directly challenged with 1% Croton oil in acetone: olive oil (4:1; 20 μl to both sides of the ear). Ears were measured with a micrometer (Mitutoyo) before and 6 hours after the challenge. Data are expressed as the ear thickness 6 hours after the challenge minus the baseline thickness. The whole ear was fixed in 10% buffered formalin and processed for H&E and toluidine blue staining as previously described (23, 25, 60). Dermal ear thickness measurement and mast cell degranulation quantification were carried out. For dermal ear thickness measurement, at least 6 random sites were chosen on both sides of the ear, and the distance between the ear cartilage epidermis was measured using ImageJ. For mast cell quantification, all mast cells from at least 3 skin sections were examined for an individual sample. A degranulated mast cell is defined as a mast cell with pale metachromatic staining, a non-intact border, and visible surrounding granules.
For the contact hypersensitivity model, mice were sensitized on day 0 by epicutaneous application of 25 μl of 0.5% DNFB (Sigma, D1529) in acetone: olive oil (4:1) onto dry shaven abdominal skin. On day 5, baseline ear thickness was measured with a micrometer followed by a challenge with 10 μl of 0.2% DNFB to both sides of one ear and vehicle to the other ear. Ear thickness was measured at the respective time points. For histology, ears were harvested on day one post-challenge, and processed for H&E staining and dermal ear thickness measurement.
Mouse model of rosacea
The skin rosacea model was performed as previously described (28, 49, 61). Briefly, shaved back skin was intradermally injected with 50 μl 320 μM cathelicidin LL-37 (AnaSpec Inc, AS-61302) twice a day for 2 days. 1600 nM SYM2081 or vehicle was coinjected with LL-37 solutions. 72 hours later, photos of the skin were taken. The size of erythema lesions was measured, and redness scores (from 1 to 5, with 1 being the least red and 5 being the reddest) were assigned to each image using ImageJ by a blinded investigator as previously described (62). The skin was also harvested for RNA extraction.
Flow cytometry
Single-cell suspensions were obtained as previously described (63). The skin was minced finely with scissors and resuspended in RPMI-1640 with 1x glutamine (Corning, MT10040CV) with 2.5 mg/mL collagenase XI (Sigma-Aldrich, C7657), 0.25 mg/mL hyaluronidase (Sigma-Aldrich, H3884), 0.1 mg/mL DNase (Sigma-Aldrich, H5025), 0.01 M HEPES (Sigma-Aldrich, H0887), and 10% fetal bovine serum followed by incubation in a shaking incubator for 1 hour at 37°C at 250 rpm. The resulting cells were filtered through a 40 mm cell strainer (Thermo Fisher Scientific, 22–363-547). A list of antibodies used can be found in table S4. For extracellular staining, antibodies were diluted in FACS buffer (2% fetal bovine serum, 5 mM EDTA, 0.04% Azide in PBS) at 4°C for 30 minutes. For intranuclear staining of Ki-67, a Foxp3 staining kit (Invitrogen, 00–5523-00) was used per the manufacturer’s instructions. Samples were analyzed on LSRFortessa flow cytometers (Becton Dickinson). Data were analyzed using FlowJo software (TreeStar). After gating on live singlets, each cell type was defined based on expression of markers noted in the figure legends.
Cytokine quantification
Tissue was homogenized in 500 mL Cell extraction buffer (Invitrogen, FNN0011) supplemented with phenylmethylsulfonyl fluoride (PMSF, Sigma-Aldrich, 52332) and protease inhibitor (Sigma-Aldrich, P2714) according to the manufacturer’s recommendation. Tissue homogenates were centrifuged for 10 minutes at 7500 rpm. Cytokines in the supernatants were quantified using a bead-based LEGENDplex immunoassay (BioLegend, 740446) according to the manufacturer’s protocol. Samples were analyzed on LSRFortessa flow cytometers (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed using LEGENDplex analysis software.
qRT-PCR
Skin tissue samples were homogenized using the Navy RINO Lysis Kit (Next Advance, NAVYR5), then RNA was isolated using Trizol (Sigma-Aldrich, T9424) following the manufacturer’s protocol. RNA to cDNA conversion was performed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368813). cDNA was analyzed using TaqMan Gene Expression assays. A complete list of TaqMan Gene Expression primers used can be found in table S5. 2−ΔCT values were calculated relative to Hprt expression for murine samples and ACTB for human samples. Data are presented as relative fold change compared with their respective controls.
Statistical analysis
Statistical tests were performed and visualized using GraphPad Prism software. All data are presented as means ± standard error of mean (SEM) unless otherwise specified. All samples that underwent successful procedures or treatments were included in the analyses. To determine normality, a Shapiro-Wilk test was performed on each dataset. When P>0.05, the dataset was considered to be normally distributed. For normally distributed data, unpaired or paired two-tailed Student’s t-tests were performed for two groups and analysis-of-variance (ANOVA) tests with multiple comparison tests were used for more than two groups. When P<0.05, a Mann-Whitney test was performed. To characterize the dose-response relationship between SYM2081 concentrations and its inhibition on PCMC degranulation, a non-linear regression was performed. For in vitro experiments, each dot represents a replicate from a particular batch of primary cell culture. For in vivo experiments, each dot represents an animal unless otherwise specified. For human skin explant experiments, each dot represents a patient.
Supplementary Material
Acknowledgments:
We thank B. McNeil of Northwestern University for providing Mrgprb2−/− mice; C. Donohue Carey, L. Cevhertas, and J. Zhang of the University of Pittsburgh for assistance in obtaining and processing human skin explants; and T. Edwards of the University of Pittsburgh for helpful discussions. We also thank the Division of Laboratory Animal Resources, Unified Flow Core of the University of Pittsburgh, and UPMC dermatopathology service. Work performed in the Pitt Biospecimen Core (RRID:SCR_025229) and services and instruments used in this project were supported, in part, by the University of Pittsburgh, the Office of the Senior Vice Chancellor for Health Sciences.
Funding:
This work was supported by the National Institute for Health (NIH) grants R01AR071720 (to DHK), and R01 AR077341 (to DHK); R01AR079233 and R01AR074285 (to LDF and TLS); DP2AI164325, U01EY034711 (to JD); and T32AI089443 (to CSC). This research was also supported in part by the University of Pittsburgh Center for Research Computing and the HTC cluster, which is supported by NIH award number S10OD028483.
Footnotes
Competing interests: A patent with D.H.K as inventor has been filed by the University of Pittsburgh describing the use of GluK2 agonism to suppress mast cell function.
Data availability:
All data associated with this study are present in the paper or the Supplementary Materials. All code and data are available at https://github.com/jishnu-lab/GluK2_MC. The Zenode DOI is https://doi.org/10.5281/zenodo.14060440. Sequencing data has been deposited at the NCBI Gene Expression Omnibus (GSE268986).
References and notes
- 1.Krystel-Whittemore M, Dileepan KN, Wood JG, Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 6, 620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Köhler A, Peschke K, Vöhringer D, Waskow C, Krieg T, Müller W, Waisman A, Hartmann K, Gunzer M, Roers A, Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 34, 973–984 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Starkl P, Watzenboeck ML, Popov LM, Zahalka S, Hladik A, Lakovits K, Radhouani M, Haschemi A, Marichal T, Reber LL, Gaudenzio N, Sibilano R, Stulik L, Fontaine F, Mueller AC, Amieva MR, Galli SJ, Knapp S, IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcus aureus. Immunity. 53, 793–804.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arifuzzaman M, Mobley YR, Choi HW, Bist P, Salinas CA, Brown ZD, Chen SL, Staats HF, Abraham SN, MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 5, eaav0216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pundir P, Liu R, Vasavda C, Serhan N, Limjunyawong N, Yee R, Zhan Y, Dong X, Wu X, Zhang Y, Snyder SH, Gaudenzio N, Vidal JE, Dong X, A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity. Cell Host Microbe. 26, 114–122.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitzmann A, Naumann R, Dudeck A, Zerjatke T, Gerbaulet A, Roers A, Mast Cells Occupy Stable Clonal Territories in Adult Steady-State Skin. J. Invest. Dermatol. 140, 2433–2441.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Kitamura Y, Shimada M, Hatanaka K, Miyano Y, Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 268, 442–443 (1977). [DOI] [PubMed] [Google Scholar]
- 8.Gentek R, Ghigo C, Hoeffel G, Bulle MJ, Msallam R, Gautier G, Launay P, Chen J, Ginhoux F, Bajénoff M, Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity. 48, 1160–1171.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 9.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X, Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 519, 237–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, McNeil BD, Dong X, Hernandez JD, Sagi-Eisenberg R, Hammel I, Roers A, Valitutti S, Tsai M, Espinosa E, Galli SJ, Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Invest. 126, 3981–3998 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tauber M, Basso L, Martin J, Bostan L, Pinto MM, Thierry GR, Houmadi R, Serhan N, Loste A, Blériot C, Kamphuis JBJ, Grujic M, Kjellén L, Pejler G, Paul C, Dong X, Galli SJ, Reber LL, Ginhoux F, Bajenoff M, Gentek R, Gaudenzio N, Landscape of mast cell populations across organs in mice and humans. J. Exp. Med. 220 (2023), doi: 10.1084/jem.20230570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X, A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron. 101, 412–420.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian H, Gupta K, Guo Q, Price R, Ali H, Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J. Biol. Chem. 286, 44739–44749 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Zhang Y, Zhang Y, Lai Y, Chen W, Xiao Z, Zhang W, Jin M, Yu B, LL-37-induced human mast cell activation through G protein-coupled receptor MrgX2. Int. Immunopharmacol. 49, 6–12 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, McNeil BD, Beta-defensins are proinflammatory pruritogens that activate Mrgprs. J. Allergy Clin. Immunol. 143, 1960–1962.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H, β-Defensins activate human mast cells via Mas-related gene X2. J. Immunol. 191, 345–352 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, Reber LL, Marichal T, Starkl P, Cenac N, Dong X, Tsai M, Galli SJ, Gaudenzio N, House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 20, 1435–1443 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia T, Che D, Zheng Y, Zhang H, Li Y, Zhou T, Peng B, Du X, Zhu L, An J, Geng S, Mast Cells Initiate Type 2 Inflammation through Tryptase Released by MRGPRX2/MRGPRB2 Activation in Atopic Dermatitis. J. Invest. Dermatol. 144, 53–62.e2 (2024). [DOI] [PubMed] [Google Scholar]
- 19.Tominaga M, Takamori K, Itch and nerve fibers with special reference to atopic dermatitis: therapeutic implications. J. Dermatol. 41, 205–212 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y, Mast cells in atopic dermatitis. Curr. Opin. Immunol. 21, 666–678 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salomon J, Baran E, The role of selected neuropeptides in pathogenesis of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 22, 223–228 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M, Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Dermatol. 147, 71–79 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Edwards TN, Chaudhri VK, Wu J, Cohen JA, Hirai T, Rittenhouse N, Schmitz EG, Zhou PY, McNeil BD, Yang Y, Koerber HR, Sumpter TL, Poholek AC, Davis BM, Albers KM, Singh H, Kaplan DH, Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell. 184, 2151–2166.e16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ständer S, Ständer H, Seeliger S, Luger TA, Steinhoff M, Topical pimecrolimus and tacrolimus transiently induce neuropeptide release and mast cell degranulation in murine skin. Br. J. Dermatol. 156, 1020–1026 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Dudeck J, Ghouse SM, Lehmann CHK, Hoppe A, Schubert N, Nedospasov SA, Dudziak D, Dudeck A, Mast-Cell-Derived TNF Amplifies CD8(+) Dendritic Cell Functionality and CD8(+) T Cell Priming. Cell Rep. 13, 399–411 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Gaudenzio N, Marichal T, Galli SJ, Reber LL, Genetic and Imaging Approaches Reveal Pro-Inflammatory and Immunoregulatory Roles of Mast Cells in Contact Hypersensitivity. Front. Immunol. 9, 1275 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ, Mast cell-associated TNF promotes dendritic cell migration. J. Immunol. 176, 4102–4112 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, Morhenn VB, Gallo RL, Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 13, 975–980 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Muto Y, Wang Z, Vanderberghe M, Two A, Gallo RL, Di Nardo A, Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J. Invest. Dermatol. 134, 2728–2736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Two AM, Wu W, Gallo RL, Hata TR, Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J. Am. Acad. Dermatol. 72, 749–58; quiz 759 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Wollam J, Solomon M, Villescaz C, Charlot B, Napora J, Vest A, Cavarlez C, Dvorak L, Nevarez A, Viswanath V, Boehm M, 42364 MRPGRX2 antagonist EP262 prevents inflammation and disease in a mouse model of atopic dermatitis. Journal of the American Academy of Dermatology. 89, AB66 (2023). [Google Scholar]
- 32.Evommune | Chronic Inflammation | MRGPRX2 Antagonist EVO756, (available at https://www.evommune.com/mrgprx2-antagonist/).
- 33.Benet Z, Luu T, Brock E, Sanchez R, McEwen L, Coyle K, Chang K, Leung J, Schanin J, Youngblood B, An Agonistic Monoclonal Antibody Against Siglec-6 Broadly Inhibits Mast Cell Activation in Transgenic Mice. Journal of Allergy and Clinical Immunology. 151, AB168 (2023). [Google Scholar]
- 34.Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, Sobolevsky AI, Swanson GT, Swanger SA, Greger IH, Nakagawa T, McBain CJ, Jayaraman V, Low C-M, Dell’Acqua ML, Diamond JS, Camp CR, Perszyk RE, Yuan H, Traynelis SF, Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacol. Rev. 73, 298–487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dwyer DF, Barrett NA, Austen KF, Immunological Genome Project Consortium, Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 17, 878–887 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heng TSP, Painter MW, Immunological Genome Project Consortium, The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Tharp MD, Seelig LL, Tigelaar RE, Bergstresser PR, Conjugated avidin binds to mast cell granules. J. Histochem. Cytochem. 33, 27–32 (1985). [DOI] [PubMed] [Google Scholar]
- 38.Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D, Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology. 46, 793–806 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Joulia R, L’Faqihi F-E, Valitutti S, Espinosa E, IL-33 fine tunes mast cell degranulation and chemokine production at the single-cell level. J. Allergy Clin. Immunol. 140, 497–509.e10 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Folkerts J, Gaudenzio N, Maurer M, Hendriks RW, Stadhouders R, Tam S-Y, Galli SJ, Rapid identification of human mast cell degranulation regulators using functional genomics coupled to high-resolution confocal microscopy. Nat. Protoc. 15, 1285–1310 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, Oetjen LK, Wang F, Kim BS, Dong X, Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity. 50, 1163–1171.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alim MA, Grujic M, Ackerman PW, Kristiansson P, Eliasson P, Peterson M, Pejler G, Glutamate triggers the expression of functional ionotropic and metabotropic glutamate receptors in mast cells. Cell. Mol. Immunol. 18, 2383–2392 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahimikollu J, Xiao H, Rosengart A, Rosen ABI, Tabib T, Zdinak PM, He K, Bing X, Bunea F, Wegkamp M, Poholek AC, Joglekar AV, Lafyatis RA, Das J, SLIDE: Significant Latent Factor Interaction Discovery and Exploration across biological domains. Nat. Methods. 21, 835–845 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sieweke MH, Allen JE, Beyond stem cells: self-renewal of differentiated macrophages. Science. 342, 1242974 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK, New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 17, 34–40 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Gábor M, Models of acute inflammation in the ear. Methods Mol. Biol. 225, 129–137 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Negi P, Agarwal S, Garg P, Ali A, Kulshrestha S, in Recent Developments in Anti-Inflammatory Therapy (Elsevier, 2023), pp. 315–330. [Google Scholar]
- 48.Roy S, Chompunud Na Ayudhya C, Thapaliya M, Deepak V, Ali H, Multifaceted MRGPRX2: New insight into the role of mast cells in health and disease. J. Allergy Clin. Immunol. 148, 293–308 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi JE, Werbel T, Wang Z, Wu CC, Yaksh TL, Di Nardo A, Botulinum toxin blocks mast cells and prevents rosacea like inflammation. J. Dermatol. Sci. 93, 58–64 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Redhu D, Franke K, Aparicio-Soto M, Kumari V, Pazur K, Illerhaus A, Hartmann K, Worm M, Babina M, Mast cells instruct keratinocytes to produce thymic stromal lymphopoietin: Relevance of the tryptase/protease-activated receptor 2 axis. J. Allergy Clin. Immunol. 149, 2053–2061.e6 (2022). [DOI] [PubMed] [Google Scholar]
- 51.Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M, Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J. Neurosci. 23, 6176–6180 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubinstein I, Nadel JA, Graf PD, Caughey GH, Mast cell chymase potentiates histamine-induced wheal formation in the skin of ragweed-allergic dogs. J. Clin. Invest. 86, 555–559 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe N, Tomimori Y, Terakawa M, Ishiwata K, Wada A, Muto T, Tanaka T, Maruoka H, Nagahira K, Nakatsuka T, Fukuda Y, Oral administration of chymase inhibitor improves dermatitis in NC/Nga mice. J. Invest. Dermatol. 127, 971–973 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Hawkins RA, The blood-brain barrier and glutamate. Am. J. Clin. Nutr. 90, 867S-874S (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith QR, Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 130, 1016S–22S (2000). [DOI] [PubMed] [Google Scholar]
- 56.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ, Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 23, 611–620 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Malbec O, Roget K, Schiffer C, Iannascoli B, Dumas AR, Arock M, Daëron M, Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J. Immunol. 178, 6465–6475 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Bray NL, Pimentel H, Melsted P, Pachter L, Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Yu G, Wang L-G, Han Y, He Q-Y, clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, Wu J, Li Y, Zhang S, Ho J, Davis BM, Albers KM, Kaplan DH, Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell. 178, 919–932.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon S-H, Hwang I, Lee E, Cho H-J, Ryu JH, Kim T-G, Yu J-W, Antimicrobial Peptide LL-37 Drives Rosacea-Like Skin Inflammation in an NLRP3-Dependent Manner. J. Invest. Dermatol. 141, 2885–2894.e5 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Kim M, Kim K-E, Jung HY, Jo H, Jeong S-W, Lee J, Kim CH, Kim H, Cho D, Park HJ, Recombinant erythroid differentiation regulator 1 inhibits both inflammation and angiogenesis in a mouse model of rosacea. Exp. Dermatol. 24, 680–685 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH, Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 43, 515–526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. All code and data are available at https://github.com/jishnu-lab/GluK2_MC. The Zenode DOI is https://doi.org/10.5281/zenodo.14060440. Sequencing data has been deposited at the NCBI Gene Expression Omnibus (GSE268986).


