Abstract
Background
Legg-Calvé-Perthes disease (LCPD) progresses through 4 stages characterized by unique radiographic features, and stage duration is recognized as an important prognostic factor. Newer perfusion magnetic resonance imaging (pMRI) allows for the evaluation of vascularity early in the disease process. This study aims to describe the relationship between global and regional perfusion patterns on early pMRI and the duration of Waldenström stages. A secondary aim was to verify the relationship between hypoperfusion and subsequent lateral pillar class.
Methods
Through a prospectively collected multicenter international cohort, patients with early LCPD (Waldenström Stage I) and pMRI were followed with serial radiographs at 3-month intervals for a minimum of 2 years. Epiphyseal hypoperfusion was quantified by HipVasc Software for the entire epiphysis and regional thirds of the femoral head. Waldenström stages and lateral pillar class were determined by mode assessments from 3 pediatric orthopedic surgeons. Duration of the stage was defined as the interval between the first radiograph demonstrating features of stage IIa and stage IIIa for fragmentation and between IIIa and IV for reossification.
Results
One-hundred and seven patients (88.8% male, median age 8.0 years) met the study criteria. The average global hypoperfusion was 73.7%. Poorer global perfusion was predictive of a longer duration of fragmentation (rho = 0.34, P < .001) and of reossification (rho = 0.38, P = .003). The average regional hypoperfusion of the medial, central, and lateral third of the femoral head was 65.3%, 83.7%, and 61.3% respectively, and was similarly related to the duration of fragmentation (rho = 0.26, 0.24, and 0.31, respectively) and of reossification (rho = 0.31, 0.43, and 0.39, respectively) (P < .05 for all). Similar to previous studies, we found a significant positive association between hypoperfusion in the lateral third of the femoral head and lateral pillar class (P = .037).
Conclusions
The degree of both global and regional hypoperfusion correlated with the duration of fragmentation and reossification stages in LCPD. Lateral epiphyseal hypoperfusion is predictive of lateral pillar class. Taken together, the information provided by perfusion magnetic resonance imaging can provide crucial prognostic information for children with LCPD.
Key Concepts
-
1.
Amount of hypoperfusion both globally and regionally on perfusion MRI correlates with the duration of fragmentation and reossification stages in LCPD.
-
2.
Lateral epiphyseal hypoperfusion correlates with lateral pillar class.
-
3.
Perfusion information offered by contrast MRI can offer crucial prognostic information in children with LCP.
Level of Evidence
II Prognostic Study
Keywords: Legg-Calve-Perthes, Perthes, Perfusion MRI, Duration
Introduction
Legg-Calve-Perthes disease (LCPD) is an idiopathic hip disorder characterized by avascular necrosis of the femoral capital epiphysis [1], [2]. The disease is self-limited, but the resulting degenerative process can undermine the integrity of the hip joint, and potentially lead to stiffness, pain, and premature osteoarthritis [1], [3], [4]. LCPD almost always progresses through 4 stages [3], [5]. The initial, fragmentation, reossification, and residual stages are characterized by unique radiographic features, first described by Waldenström [5], [6], [7] and later modified by Joseph et al. [5]. The severity of the disease course in LCPD and the ultimate clinical outcome are notoriously variable among patients. Despite significant research efforts, determining the prognosis for an individual patient in the early stages of the disease remains a challenge. Based on the current knowledge, the overall prognosis seems dependent on several factors, including the age at disease onset, the severity of femoral head deformity, congruency of the femoral head and acetabulum, and height of the lateral pillar of the femoral epiphysis, among others [1], [8], [9], [10] [4].
The duration of each stage of active disease is also recognized as a critical prognostic factor [10]. During the fragmentation stage (Waldenström Stage II), the femoral head is at greatest risk for deformation which in turn increases long-term morbidity [3], [5], [10]. As a result, the longer the duration of fragmentation, the greater the risk of significant femoral head deformity. Prognosis and treatment recommendations have historically been based on radiographic classification systems [3]. Advanced magnetic resonance imaging (MRI) techniques, however, allow for more accurate assessment in earlier stages of the disease process, prior to the development of irreversible morphologic changes [1], [11]. Specifically, gadolinium-enhanced MRI, or perfusion MRI (pMRI), has been shown to be an effective and reliable way to evaluate epiphyseal revascularization and perfusion patterns [11], [12], [13], [14], [15], [16].
Previous studies have established early perfusion of the femoral head as a predictor of later radiographic outcomes following nonoperative and proximal femoral osteotomy (PFO) treatment. Others have briefly assessed the relationship between initial perfusion and duration of fragmentation. These previous studies have been limited by small patient cohorts, various LCPD disease stages with short-term follow-up, or only moderate interobserver variability [14], [15], [17], [18]. Using a large, prospective, multicenter cohort of patients, the purpose of this study was to investigate the relationship between both global and regional perfusion patterns seen on early pMRI and the duration of both Waldenström stages II and III (fragmentation and reossification). A secondary purpose was to confirm the correlation between perfusion index on early pMRI and subsequent lateral pillar class based on plain radiographs.
Materials and methods
Patient selection
After institutional review board approval, the prospectively collected data from a multicenter study group was screened for patients with a minimum of two-year radiographic follow-up. Inclusion criteria for this study were as follows: diagnosis of LCPD, ages between 6 and 11 years at disease onset, Waldenström Stage I at the initial visit, and pMRI performed during Stage 1 [7]. Because this specific study intended to correlate global and regional epiphyseal perfusion with stage duration, we only included patients whose MRIs were eligible for quantitative interpretation by HipVasc software [15]. Patients were excluded from the study if they had <2 years of follow-up and incomplete radiographic data, defined as <7 radiographs within the first 2.5 years.
Data collection
For patients that met the inclusion criteria, the following demographic and clinical data were collected from the study database: age at disease onset, sex, laterality of disease, surgery type if applicable, and timing of follow-up. The extent of epiphyseal hypoperfusion on early pMRI was quantified by HipVasc software as previously described (Figure 1, Figure 2) [12], [15]. For all patients, 2 or 3 independent pediatric orthopedic surgeons assessed the global hypoperfusion of the entire epiphysis using HipVasc. The regional epiphyseal hypoperfusion indexes of the lateral, central, and medial thirds of the femoral head were calculated by the program. Final perfusion indices were calculated by averaging the scores from all readers.
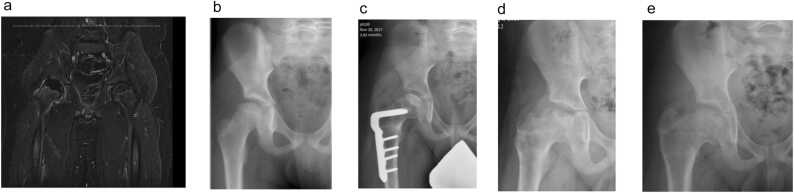
Figure 1.
a. Perfusion MRI in a 9 + 4 month old male demonstrates 50.5% global hypoperfusion of the right hip based on HipVasc analysis (Lat: 52.4%, Central: 75.8%, Medial: 35.8%). b. Anteroposterior (AP) radiograph of the right hip at initial visit demonstrates Waldenström Stage 1B. c. Immediate postoperative AP hip radiograph after varus femoral osteotomy demonstrates Waldenström stage 2A (early fragmentation). d. AP hip radiograph at approximately 3-month follow-up demonstrates early healing stage (Waldenström stage 3A). The duration of fragmentation was therefore deemed to be 63 days. e. AP hip radiograph at Waldenström stage 4 indicates that the duration of the healing stage was approximately 550 days.
Figure 2.
a. Perfusion MRI in a 7 + 11-month-old male demonstrates 83.9% global hypoperfusion of the right hip based on hipVasc analysis. (Lat: 65.6%, Central: 94.4%, Medial: 87.2%). b. AP radiograph of the right hip at the initial visit demonstrates Waldenstrom Stage 1B. c. AP hip radiograph at 3-month follow-up after varus femoral osteotomy demonstrates Waldenstrom stage 2A. d. AP hip radiograph demonstrates healing (Waldenstrom 3A) after 259 days of fragmentation. E. AP hip radiograph at Waldenstrom stage 4 indicates that the duration of the healing stage was approximately 665 days.
Most patients underwent surgical containment in the form of PFO. A smaller additional cohort of patients treated nonoperatively or with other surgical approaches was also included. The type of treatment was left to the discretion of the study site and respective treating surgeon. Patients were then prospectively followed with serial anteroposterior and frog-view pelvis radiographs at 3-month intervals for 2 years postoperatively, and then at 6-month intervals thereafter (Figure 1, Figure 2). Each set of radiographs was reviewed independently by 3 separate pediatric orthopedic surgeons who assigned a Waldenström stage for each x-ray and an overall lateral pillar classification for each affected hip [10]. A final Waldenström stage for each radiograph was determined based on the mode of the 3 individual observations. In the rare cases where there was no clear mode value from the individual ratings, a consensus rating was agreed upon through open discussion. The duration of each stage was then calculated as the interval between the first radiograph demonstrating the features of 1 stage and the first radiograph showing features of the next stage. For example, the duration of fragmentation was defined as the time interval between the first radiograph that showed Waldenström stage IIa and the next radiograph that showed stage IIIa. Similarly, we defined the duration of reossification as the time interval between the first radiograph that showed Waldenström stage IIIa and stage IV.
Data analysis
Shapiro-Wilk tests were performed to determine the normality of data for continuous variables, and appropriate descriptive statistics were calculated to characterize the overall patient population, radiographic data, and disease duration. Median and interquartile range (IQR) were used for non-normally distributed data versus mean and standard deviation for normally distributed data. Spearman’s rho correlation, coefficient of determination, and simple linear regression were carried out to determine the extent to which average decreased perfusion on initial MRI is correlated with and can predict the duration of fragmentation and duration of reossification. Correlation coefficients between 0 and 0.3 were considered weak, 0.31 to 0.70 moderate, and 0.71 to 1.0 strong. Bivariate analysis using Kruskal-Wallis test was used to determine the association between average decreased perfusion on initial MRI and lateral pillar class. This analysis was repeated with regional pMRI data. Additional subanalyses were performed on patients who were surgically treated, and those who specifically had a femoral varus osteotomy. All statistical analysis was performed using Statistical Package for Social Sciences Statistics Version 28 (IBM) and P-values less than .05 were considered statistically significant [19].
Results
Global perfusion and stage duration
After applying our inclusion and exclusion criteria, 107 patients were identified for this study with an average 9.9 radiographs for evaluation (range: 7-15 radiographs). In this sample, 95 (88.8%) were male and 56 (52.3%) were right-sided. The median age at diagnosis was 8.0 (IQR: 2.3 years; range: 6.1-11.9 years).
Three patients bypassed fragmentation (Table 1). Of the patients who did not bypass fragmentation (n = 104), the mean duration of fragmentation was 232.5 ± 97.3 days (range: 48.0-518.0 days) and the median global hypoperfusion was 73.7% (IQR: 26.1%) (Table 2). A positive but weak correlation was found between the global degree of hypoperfusion and duration of fragmentation (rho = 0.34). The regression model predicted 11.6% of the variance, and the model was a good fit for the data (F = 15.4, P < .001) (Table 2). Based on our model equation, for every 10% decrease in perfusion, the duration of fragmentation increases by approximately 18 days.
Table 1.
Demographic and clinical data of 3 patients who bypassed the fragmentation stage.
| Age at diagnosis (years) | Sex | Affected side | Average global% hypoperfusion | Lateral third % hypoperfusion | Central third % hypoperfusion | Medial third% hypoperfusion | Lateral pillar class |
|---|---|---|---|---|---|---|---|
| 7.4 | Male | Left | 80.2 | 94.6 | 91.3 | 44.8 | A |
| 8.9 | Male | Left | 9.0 | 19.9 | 4.9 | 4.9 | A |
| 6.7 | Male | Right | 68.6 | 90.3 | 85.9 | 72.1 | A |
Table 2.
Spearman rho correlation, coefficient of determination (R2), analysis of variance, and coefficient estimate for regression models predicting duration of fragmentation from global and regional (lateral, central, and medial) HipVasc hypoperfusion percent.
| Regression model | HipVasc predictor | Spearman rho correlation | R2 | F statistic | Coefficient estimate | P |
|---|---|---|---|---|---|---|
| Duration of Fragmentation: All* (N = 104) | Global | 0.34 | 0.116 | 15.4 | 1.85 | <.001 |
| Lateral | 0.31 | 0.096 | 14.7 | 1.62 | <.001 | |
| Central | 0.24 | 0.058 | 7.1 | 1.37 | .009 | |
| Medial | 0.26 | 0.068 | 11.2 | 1.27 | .001 | |
| Duration of Fragmentation: Surgical* (N = 92) | Average | 0.33 | 0.109 | 15.7 | 2.14 | <.001 |
| Lateral | 0.31 | 0.096 | 11.2 | 1.65 | .001 | |
| Central | 0.24 | 0.058 | 9.1 | 1.90 | .003 | |
| Medial | 0.29 | 0.084 | 11.9 | 1.40 | <.001 | |
| Duration of Fragmentation: PFO (N = 88) | Average | 0.29 | 0.084 | 12.8 | 2.08 | <.001 |
| Lateral | 0.22 | 0.048 | 5.1 | 1.22 | .026 | |
| Central | 0.19 | 0.036 | 8.3 | 1.78 | .005 | |
| Medial | 0.23 | 0.053 | 8.7 | 1.31 | .004 | |
| Duration of Fragmentation: Nonsurgical (N = 12) | Average | 0.57 | 0.325 | 2.0 | 1.48 | .192 |
| Lateral | 0.64 | 0.410 | 9.1 | 2.30 | .013 | |
| Central | 0.49 | 0.240 | 0.6 | 0.738 | .472 | |
| Medial | 0.17 | 0.028 | 0.3 | 0.628 | .607 | |
| Duration of Healing: All (N = 37) | Average | 0.38 | 0.144 | 10.2 | 9.16 | .003 |
| Lateral | 0.39 | 0.152 | 6.4 | 7.15 | .016 | |
| Central | 0.43 | 0.185 | 12.6 | 9.36 | .001 | |
| Medial | 0.31 | 0.096 | 5.1 | 5.80 | .030 |
PFO, proximal femoral osteotomy.
Bold values indicate statistical signficance
Except those who bypass fragmentation (N = 3).
Thirty-seven patients had follow-up through Waldenström stage IV. The median duration of reossification stage for these patients was 700.0 days (IQR: 538.0 days; range: 252.0-2317.0 days). There was a moderate correlation between global hypoperfusion and the duration of reossification (rho = 0.38), and the model was able to predict 14.4% of the variance (F = 10.2, P = .003) (Table 2). From the model equation, for every 10% decrease in perfusion, the duration of reossification increases by approximately 90 days (Figure 1, Figure 2).
Regional hypoperfusion and stage duration
Most patients had the lowest perfusion in the central region (88.8%, 95/107) versus the lateral (4.7%, 5/107) or the medial region (6.5%, 7/107) of the femoral head. The median regional hypoperfusion of the medial, central, and lateral third of the femoral head was 65.3%, 83.7%, and 61.3%, respectively (Table 2). Similar to global hypoperfusion, there was a positive but weak correlation between the regional hypoperfusion indices and the duration of fragmentation. In each model, the regional hypoperfusion predicted that for every 10% decrease in perfusion, the duration of fragmentation increased by 12 to 16 days, and each model was statistically significant (Table 2).
Of the patients with follow-up through stage IV, there was a consistent positive correlation between the amount of regional hypoperfusion and the duration of reossification. For each correlation, 9.6% to 18.5% of the variance in the duration of reossification could be explained by the percent hypoperfusion and all models were statistically significant (Table 2).
Global and regional perfusion and lateral pillar class
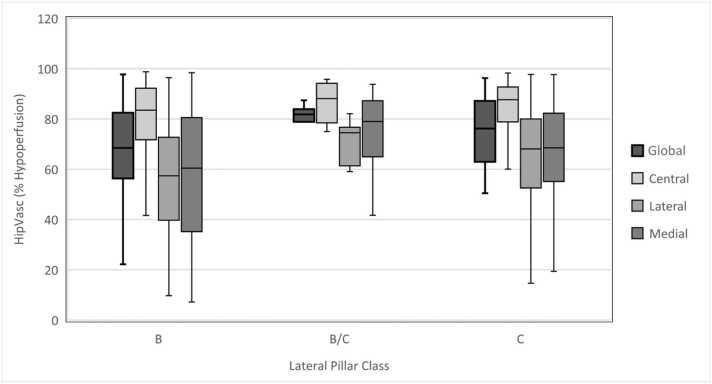
The 3 patients that bypassed fragmentation were all graded as Lateral Pillar Class A (Table 1). Of the patients who did not bypass fragmentation, most were Lateral Pillar B (62.1%, 64/103) versus B/C border (6.8%, 7/103) or C (32.0%, 33/103). Across all patients, we did not find a significant association between global hypoperfusion and lateral pillar class (P = .170). However, there was a statistically significant association between the percent hypoperfusion in the lateral third of the head and lateral pillar class (P = .037) (Fig. 3).
Figure 3.
Global and Regional Hypoperfusion Percent of Total Sample* and by Lateral Pillar Class. * Except those who bypassed fragmentation (N = 3).
Surgical versus nonsurgical
Of the 107 patients in the sample, 95 (88.8%) patients underwent surgical containment. Almost all of these patients (92.6%, 88/95) underwent a PFO. The others had an epiphyseal drilling or tunneling (n = 5) or an adductor tenotomy with an arthrogram and Petrie cast application (n = 2). For all surgical patients and for those who specifically had a PFO, there was a positive and significant correlation between global and regional percent hypoperfusion and duration of fragmentation (Table 2).
In the nonoperative patients (n = 12), the lateral degree of hypoperfusion had a moderate correlation with the duration of fragmentation (rho = 0.64) and the model was able to explain 47.7% of the variance (P = .013). Although the numbers were limited, a similar positive correlation was seen between the global and central percent hypoperfusion and duration of fragmentation in the nonoperative patients but this was non-significant (Table 2).
Discussion
Historically, plain radiographs have been used to grade the severity of the disease and guide indications for intervention in LCPD [3]. Unfortunately, employing this “wait to classify” approach allows femoral head collapse to occur when the goal of treatment is to prevent this collapse from occurring in the first place. As a result, there is great clinical and research interest in earlier prognosticators, such as pMRI, which can be obtained before the development of irreversible deformity [1], [11], [13], [14]. Initial studies on pMRI have demonstrated a moderate correlation between the degree of hypoperfusion and later femoral head deformity [15]. However, additional studies with larger numbers are necessary to further explore the relationship between initial hypoperfusion in the early stage and the duration of Waldenstrom stages II and III (fragmentation and reossification), which has been shown to be an important prognostic factor [1], [15], [20]. The current study demonstrates the predictive value of pMRI for the duration of both fragmentation and reossification stages (Waldenstrom II and III) and provides novel information about regional hypoperfusion patterns in LCPD.
During the fragmentation stage, extrusion of the femoral epiphysis and lateral pillar collapse occur which strongly correlate with the severity of disease and the ultimate radiographic outcome. As such, the duration of fragmentation and severity of collapse are considered key prognostic indicators in LCPD [3], [4], [17]. In all our models (while the strength varied) there was a consistent positive correlation between percent hypoperfusion on pMRI and the duration of fragmentation. This data agrees with previous preliminary data by Sankar et al., which found that patients with lower preoperative perfusion had less bypass of the fragmentation stage and longer duration of fragmentation stage than patients with higher preoperative perfusion [17]. The weak to moderate correlation in our study, however, suggests that other factors are likely involved, such as age of onset, surgical treatment, or degree of extrusion [4], [17], [18], [21], [22]. Nonetheless, there was a consistent correlation between the percent hypoperfusion on MRI, both globally and regionally, and the duration of fragmentation.
These findings about the prognostic value of pMRI are important given evolving concerns about the use of gadolinium-type contrast in pediatric patients, as well as reservations amongst some about the generalizability of pMRI data since it only provides a “snapshot” of femoral head perfusion at the time of the scan [12], [20], [23]. The consistency of our correlations highlights the value of this single “snapshot” pMRI to predict the duration of stages and lateral pillar class, both of which are important prognostic factors [16], [24], [25]. Furthermore, the same positive correlation between percent hypoperfusion and duration of fragmentation was upheld for the duration of Stage III (reossification), and the correlation was even stronger for the global, lateral, and central hypoperfusion indices. Each regression model showed statistical significance as well, revealing not only the positive relationship but also the predictive value of pMRI for the duration of stage in LCPD. As longer stage duration correlates with worse disease, these results support the prognostic value of pMRI and rationalizes its use despite previous concerns.
This study also adds to the literature about regional hypoperfusion in LCPD. The central region had the highest average degree of hypoperfusion initially (83.71% vs 61.33% laterally and 65.33%, medially). Atsumi et al. found that the blood supply of the lateral epiphyseal arteries is impaired at the origin in LCPD and revascularization occurs from ingrowths of small vessels into the femoral epiphysis [26]. Similarly, Kim et al. found a crescent-shaped revascularization pattern in serial perfusion MRIs of LCPD patients, with more perfusion laterally, medially, and posteriorly versus centrally and anteriorly [12]. Thus, differences in regional perfusion are likely due to revascularization patterns after an ischemic event [1], [2]. In the current study, each region of avascularity (similar to the global hypoperfusion of the entire epiphysis), was predictive of the duration of fragmentation and duration of reossification. The 3 regions were equally predictive of the duration of fragmentation, while the central region was the most predictive region for the duration of reossification.
Previous work by Kim et al. showed that in 29 patients, pMRI indices of the total epiphysis and its lateral third were predictive of lateral pillar involvement at the mid-fragmentation stage [15]. With a larger sample and more regional datapoints, our study reinforces that lateral epiphyseal hypoperfusion is predictive of lateral pillar class (Table 2). The current study, however, did not find global hypoperfusion to be associated with lateral pillar class as observed by Kim et al. One explanation for this difference could be that a different statistical analysis was performed. Kim et al. used a one-way Analysis of variance test to compare groups, while this study used a Kruskall-Wallis Test, the nonparametric test equivalent, to compare hypoperfusion between groups based on the non-normal distribution of the data. Another explanation may be from the different sample sizes, which could affect the distribution of the data and the higher proportion of patients in our sample treated with a PFO in the early stages of the disease. Although not entirely replicated, both studies demonstrate the general value of pMRI data in predicting lateral pillar class. Furthermore, the findings of this study also support the additional value of regional perfusion data and strengthen the relationship between lateral hypoperfusion and lateral pillar class. This association is important, given the known role of lateral pillar class in predicting disease severity [27], [28].
It should be noted that the majority of the sample (88.8%) underwent surgical treatment, of which 92.6% had a PFO performed; this may raise concerns about the generalizability of our results beyond those that had a PFO. Joseph et al. and Sankar et al. previously found that surgical treatment with a PFO may shorten the fragmentation stage and minimize collapse [17], [22]. However, subanalyses of all surgical patients, PFO patients alone, and nonoperative patients did not change the trend of a positive correlation between global and regional hypoperfusion and duration of fragmentation. All the models predicting the duration of fragmentation from hypoperfusion remained significant as well.
This study has many notable strengths, including its prospective study design, large patient sample, and novelty of additional regional hypoperfusion data. In addition, this is the first study to correlate early-stage hypoperfusion in LCPD with the duration of reossification. However, there are limitations to this study that must be considered. First, determining the exact duration of LCPD stages based on periodic radiographs is inherently flawed. While the regular three-month intervals between serial radiographs (collected prospectively) provided the best possible estimate for disease duration, the features that characterize each stage may have preceded the date of each radiograph, resulting in an overestimation of stage duration. Second, only a small cohort of our sample had radiographic follow-up through stage IV for reossification duration, decreasing our sample size for analysis of this stage length. Notably, reossification is a much longer process than the duration of fragmentation, and follow-up through this phase was not an inclusion criterion for this study. Still, we found a similar trend for the duration of reossification as we did with our larger sample for the duration of fragmentation where more hypoperfusion was correlated with longer stage duration. Third, this study does not assess the radiographic or functional outcomes of treatment. Previous studies show that duration of fragmentation is prognostic, with better outcomes associated with shorter disease duration [5]. However, assessment of radiographic or functional outcomes was not the aim of this study. Future study group investigations will evaluate whether patients with lower perfusion index on initial MRI have worse outcomes at the time of healing and at skeletal maturity as well as the effects of surgical intervention [4].
Although it represents just a single snapshot in time, pMRI offers important prognostic information in LCPD based on the results of this study. Global and regional hypoperfusion clearly correlated with the duration of both fragmentation and reossification stages, and lateral hypoperfusion correlated with lateral pillar classification. This information is valuable to stratify disease severity, determine which patients may benefit most from early intervention, and counsel families about the expected disease course. Further investigations should assess the role of both global and regional perfusion data in the response to treatment and treatment outcome.
Author contributions
Sankar Wudbhav: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Lee Julianna: Data curation, Formal analysis, Methodology, Writing – original draft. Chong David: Data curation, Formal analysis, Writing – review & editing. Hailer Yasmin: Data curation, Formal analysis, Writing – review & editing. Agrizzi de Angeli Luis: Data curation, Writing – review & editing. Yang Scott: Data curation, Writing – review & editing. Laine Jennifer: Conceptualization, Data curation, Formal analysis, Writing – review & editing. Kim Harry: Conceptualization, Data curation, Investigation, Supervision, Writing – review & editing.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Royalties from Wolters Kluwer Health for edited textbook, consulting OrthoPediatrics, consulting Siemens—W.S. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kim H.K. Legg-Calvé-Perthes disease. J Am Acad Orthop Surg. 2010;18(11):676–686. doi: 10.5435/00124635-201011000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Pavone V., Chisari E., Vescio A., Lizzio C., Sessa G., Testa G. Aetiology of Legg-Calvé-Perthes disease: a systematic review. World J Orthop. 2019;10(3):145–165. doi: 10.5312/wjo.v10.i3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catterall A., Pringle J., Byers P.D., Fulford G.E., Kemp H.B., Dolman C.L., et al. A review of the morphology of Perthes' disease. J Bone Jt Surg Br. 1982;64(3):269–275. doi: 10.1302/0301-620x.64b3.6807991. [DOI] [PubMed] [Google Scholar]
- 4.Joseph B. Prognostic factors and outcome measures in Perthes disease. Orthop Clin North Am. 2011;42(3):303–315. doi: 10.1016/j.ocl.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Joseph B., Varghese G., Mulpuri K., Narasimha Rao K., Nair N.S. Natural evolution of Perthes disease: a study of 610 children under 12 years of age at disease onset. J Pedia Orthop. 2003;23(5):590–600. doi: 10.1097/00004694-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Waldenström H. The definite form of the coxa plana. Acta Radio. 2016;57(7):79–94. doi: 10.1177/0284185116642923. [DOI] [PubMed] [Google Scholar]
- 7.Hyman J.E., Trupia E.P., Wright M.L., Matsumoto H., Jo C-H, Mulpuri K., et al. Interobserver and intraobserver reliability of the modified Waldenström classification system for staging of Legg-Calvé-Perthes disease. J Bone Jt Surg Am. 2015;97(8):643–650. doi: 10.2106/jbjs.n.00887. [DOI] [PubMed] [Google Scholar]
- 8.Wiig O., Terjesen T., Svenningsen S. Prognostic factors and outcome of treatment in Perthes' disease: a prospective study of 368 patients with five-year follow-up. J Bone Jt Surg Br. 2008;90(10):1364–1371. doi: 10.1302/0301-620x.90b10.20649. [DOI] [PubMed] [Google Scholar]
- 9.Stulberg S.D., Cooperman D.R., Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Jt Surg Am. 1981;63(7):1095–1108. [PubMed] [Google Scholar]
- 10.Herring J.A., Kim H.T., Browne R. Legg-Calve-Perthes disease. Part I: classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Jt Surg Am. 2004;86(10):2103–2120. [PubMed] [Google Scholar]
- 11.Sebag G., Ducou Le Pointe H., Klein I., Maiza D., Mazda K., Bensahel H., et al. Dynamic gadolinium-enhanced subtraction MR imaging—a simple technique for the early diagnosis of Legg-Calvé-Perthes disease: preliminary results. Pedia Radio. 1997;27(3):216–220. doi: 10.1007/s002470050104. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.K., Burgess J., Thoveson A., Gudmundsson P., Dempsey M., Jo C.H. Assessment of femoral head revascularization in Legg-Calvé-Perthes disease using serial perfusion MRI. J Bone Jt Surg Am. 2016;98(22):1897–1904. doi: 10.2106/jbjs.15.01477. [DOI] [PubMed] [Google Scholar]
- 13.Lamer S., Dorgeret S., Khairouni A., Mazda K., Brillet P-Y, Bacheville E., et al. Femoral head vascularisation in Legg-Calvé-Perthes disease: comparison of dynamic gadolinium-enhanced subtraction MRI with bone scintigraphy. Pedia Radio. 2002;32(8):580–585. doi: 10.1007/s00247-002-0732-5. [DOI] [PubMed] [Google Scholar]
- 14.Du J., Lu A., Dempsey M., Herring J.A., Kim H.K. MR perfusion index as a quantitative method of evaluating epiphyseal perfusion in Legg-Calve-Perthes disease and correlation with short-term radiographic outcome: a preliminary study. J Pedia Orthop. 2013;33(7):707–713. doi: 10.1097/BPO.0b013e3182a05dc1. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.K., Wiesman K.D., Kulkarni V., Burgess J., Chen E., Brabham C., et al. Perfusion MRI in early stage of Legg-Calvé-Perthes disease to predict lateral pillar involvement: a preliminary study. J Bone Jt Surg Am. 2014;96(14):1152–1160. doi: 10.2106/jbjs.m.01221. [DOI] [PubMed] [Google Scholar]
- 16.Sankar W.N., Thomas S., Castañeda P., Hong T., Shore B.J., Kim H.K. Feasibility and safety of perfusion MRI for Legg-Calvé-Perthes disease. J Pedia Orthop. 2014;34(7):679–682. doi: 10.1097/bpo.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 17.Sankar W.N., Lavalva S.M., McGuire M.F., Jo C., Laine J.C., Kim H.K.W. Does early proximal femoral varus osteotomy shorten the duration of fragmentation in Perthes disease? Lessons from a prospective multicenter cohort. J Pedia Orthop. 2020;40(5):322–328. doi: 10.1097/BPO.0000000000001451. [DOI] [PubMed] [Google Scholar]
- 18.Yoo W.J., Choi I.H., Cho T.J., Jang W., Chung C.Y., Park M.S., et al. Risk factors for femoral head deformity in the early stage of Legg-Calvé-Perthes disease: MR contrast enhancement and diffusion indexes. Radiology. 2016;279(2):562–570. doi: 10.1148/radiol.2015151105. [DOI] [PubMed] [Google Scholar]
- 19.IBM SPSS Statistics for Macintosh. Version 28.0. IBM Corp; 2021.
- 20.Schoenecker P.L. Do We Need Another Gold Standard to Assess Acute Legg-Calvé-Perthes Disease? Commentary on an article by Harry K.W. Kim, MD, MS, et al.: "Perfusion MRI in Early Stage of Legg-Calvé-Perthes Disease to Predict Lateral Pillar Involvement. A Preliminary Study". J Bone Joint Surg Am. Jul 16 2014;96(14):e125. doi:10.2106/jbjs.n.00353. [DOI] [PubMed]
- 21.Yoo W.J., Kim Y.J., Menezes N.M., Cheon J.E., Jaramillo D. Diffusion-weighted MRI reveals epiphyseal and metaphyseal abnormalities in Legg-Calvé-Perthes disease: a pilot study. Clin Orthop Relat Res. 2011;469(10):2881–2888. doi: 10.1007/s11999-011-1931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph B., Rao N., Mulpuri K., Varghese G., Nair S. How does a femoral varus osteotomy alter the natural evolution of Perthes' disease? J Pedia Orthop B. 2005;14(1):10–15. doi: 10.1097/01202412-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Castañeda P. Can We Solve Legg-Calvé-Perthes Disease with Better Imaging Technology? Commentary on an article by Harry K.W. Kim, MD, MS, et al.: "Assessment of Femoral Head Revascularization in Legg-Calvé-Perthes Disease Using Serial Perfusion MRI". J Bone Joint Surg Am. Nov 16 2016;98(22):e103. doi:10.2106/jbjs.16.00833. [DOI] [PubMed]
- 24.Essig M., Shiroishi M.S., Nguyen T.B., Saake M., Saake J.M., Enterline D., et al. Perfusion MRI: the five most frequently asked technical questions. AJR Am J Roentgenol. 2013;200(1):24–34. doi: 10.2214/ajr.12.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sujlana P., Skrok J., Fayad L.M. Review of dynamic contrast-enhanced MRI: technical aspects and applications in the musculoskeletal system. J Magn Reson Imaging. 2018;47(4):875–890. doi: 10.1002/jmri.25810. [DOI] [PubMed] [Google Scholar]
- 26.Atsumi T., Yamano K., Muraki M., Yoshihara S., Kajihara T. The blood supply of the lateral epiphyseal arteries in Perthes' disease. J Bone Jt Surg Br. 2000;82(3):392–398. doi: 10.1302/0301-620x.82b3.10193. [DOI] [PubMed] [Google Scholar]
- 27.Herring J.A., Neustadt J.B., Williams J.J., Early J.S., Browne R.H. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pedia Orthop. 1992;12(2):143–150. doi: 10.1097/01241398-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Herring J.A., Kim H.T., Browne R. Legg-Calve-Perthes disease. Part II: prospective multicenter study of the effect of treatment on outcome. J Bone Jt Surg Am. 2004;86(10):2121–2134. [PubMed] [Google Scholar]