Abstract
Introduction:
Autoimmune bullous diseases (AIBDs) are a group of illnesses characterized by autoantibodies targeting adhesion molecules in the skin and mucosa. Accurate diagnosis of the specific subtype of AIBD is crucial for effective management and predicting prognosis, especially in cases with an increased risk of malignancy. However, differentiating between subtypes can be challenging due to overlapping symptoms.
Overview of diagnostic tests:
Direct immunofluorescence microscopy (DIF) detects in vivo bound antibodies in perilesional tissue biopsies and provides details about the probable site of autoantibody deposition within the skin/mucosae, immunoglobulin type, and pattern of antibody deposition. Indirect immunofluorescence (IIF) microscopy with organ substrate is a minimally invasive serological test that detects circulating autoantibodies. Enzyme-linked immunosorbent assay (ELISA) quantifies serum autoantibodies against specific autoantigens. Quantitative ELISA is useful for diagnosis, monitoring therapy, and assessing disease activity. Commercially available ELISA kits, including the multi-variant ones, can detect antibodies associated with AIBDs. BIOCHIP is a technique based on IIF that offers a sensitive and specific diagnostic alternative to ELISA. It uses microarrays with multiple antigenic substrates to simultaneously screen common AIBDs. The BIOCHIP slides contain different substrates, allowing the identification of multiple types of autoantibodies in a single test.
Indian context:
While these diagnostic tests offer valuable insights into target antigens, antibody patterns, and disease subtypes, it is important to note that the availability of these tests is limited in most centers across India. This limitation can be attributed to factors such as the relatively higher cost of these investigations, challenges related to the stability of immuno-reactants, and a shortage of trained personnel capable of performing such tests.
Conclusion:
This review discusses the diagnosis of AIBDs based on resources available in India, as of today. It also provides with practically applicable diagnostic algorithms for pragmatic diagnosis of AIBDs in Indian scenario.
Keywords: Autoimmune blistering disease, BIOCHIP mosaic, commercially available tests, ELISA, immunofluorescence
Introduction
Autoimmune bullous diseases (AIBDs) comprise disorders characterized by autoantibodies targeted against adhesion molecules present in the skin and mucosa (oral, conjunctival, nasal, pharyngeal, laryngeal, upper esophageal, genital, and perianal mucosa). These diseases can be categorized into intraepidermal and subepidermal disorders. In intraepidermal disorders, autoantibodies are directed against adhesion molecules called desmosomes that connect adjacent keratinocytes, therefore causing an intra-epidermal split that is a split located above the basement membrane zone [Figure 1]. The subepidermal group of disorders is characterized by the presence of autoantibodies that target the structural molecules found in the dermal-epidermal junction (also known as the basement membrane zone), leading to the subepidermal split. In dermatitis herpetiformis, the autoantibodies target the tissue and epidermal transglutaminase. Due to the overlap of symptoms and relative rarity of many of these diseases, it becomes clinically challenging to identify the exact subtype of AIBDs. Few of the AIBDs such as laminin 332 mucous membrane pemphigoid (MMP) and paraneoplastic pemphigus (PNP) have an increased risk of malignancy, therefore identifying the precise disease subtype is essential for the management and prognostication of these patients.[1] Table 1 discusses the characteristics of different AIBDs.
Figure 1.
Schematic diagram for the representation of the structure of the basement membrane zone (BMZ) or dermo-epidermal junction (DEJ). The basic components include basal keratinocytes, keratinocyte trans-membrane proteins, desmosomal plaque, hemidesmosome complex, lamina lucida, lamina densa, and sublamina densa with major proteins highlighted in grey boxes. The yellow dashed line represents the level of split induced by salt-splitting for IIF that is at the level of lamina lucida, the weakest zone of the basement membrane zone
Table 1.
Characteristics of different AIBDs
| Disease | Ig type | Target antigen | Relevant clinical findings | Investigations |
|---|---|---|---|---|
| Pemphigus disorders | ||||
| Pemphigus vulgaris | IgG | Dsg 1, 3 | Flaccid blisters/erosions of the skin and/or mucosa | HPE- Supra-basal split, acantholytic cells, a row of tombstones |
| DIF- IC-IMF | ||||
| IIF- Human skin substrate: IC-IMF | ||||
| ELISA: Dsg3±Dsg1 | ||||
| Pemphigus foliaceus | IgG | Dsg 1 | Scaly papules, crusted erosions in the seborrheic distribution or erythroderma, mucosa spared | HPE- Sub-corneal split, acantholytic cells |
| DIF- IC-IMF | ||||
| IIF- Human skin substrate: IC-IMF | ||||
| ELISA: Dsg1 | ||||
| IgA pemphigus | IgA | Dsc 1, 2, 3 Dsg 1, 3 |
Flaccid pustules, annular or circinate arrangement, pustules mainly in intertriginous areas, face and scalp. Associated with IgA monoclonal gammopathy | HPE- Intraepidermal blister, neutrophilic infiltrate |
| DIF- IC-IMF | ||||
| IIF- Human skin substrate: IC-IMF | ||||
| ELISA: Dsc1, 2, 3 Dsg1, Dsg3 | ||||
| Paraneoplastic pemphigus | IgG | Plakin family proteins: desmoplakin 1 and 2, plectin, BP230, microtubule-actin cross-linking factor 1, envoplakin, periplakin, and epiplakin . | Polymorphous bullae, erosions, ‘target lesions’ Predominant involvement of oral mucosa, lips, palms, and soles. Associated with hematological malignancies, thymoma, Castleman’s disease. |
HPE-Epidermal acantholysis, variable levels of cleft formation, dyskeratotic keratinocytes, interface dermatitis |
| DIF- IC-IMF+IMF at the dermo-epidermal junction | ||||
| IIF- Human skin substrate: IC-IMF+IMF at the dermo-epidermal junction | ||||
| ELISA: Dsg1, Dsg3, BP230, envoplakin | ||||
| Pemphigoid disorders | ||||
| Bullous pemphigoid | IgG | BP180, BP230 | Tense blisters, erosions, erythema, urticarial plaques, intense pruritus, old age; no predominant mucosal involvement | HPE- Subepidermal split±eosinophils |
| DIF- IMF at the dermo-epidermal junction, n-serration | ||||
| IIF SSS: IMF at blister roof | ||||
| ELISA: BP180 NC16a, BP230 | ||||
| Mucous membrane pemphigoid | IgG/IgA | BP180, laminin 332, BP230, α6β4 integrin, laminin 311 | Predominant mucosal involvement and scarring (oral>conjunctival>nasal) | HPE- Subepidermal split±inflammatory infiltrate |
| DIF- IMF at the dermo-epidermal junction, n-serration | ||||
| IIF SSS: IMF at blister roof and/or floor | ||||
| ELISA: BP180, BP230, type VII collagen | ||||
| Linear IgA disease | IgA | LAD-1, BP230 | Tense blisters, erosions; no predominant mucosal involvement | HPE- Subepidermal split±neutrophilic infiltrate |
| DIF- IMF (IgA) at the dermo-epidermal junction, n-serration | ||||
| IIF SSS: blister roof | ||||
| Pemphigoid gestationis | IgG | BP180, BP230 | Erythema, urticarial papules, and plaques, rarely vesicles, intense pruritus; predominant in the periumbilical area, pregnancy, or postpartum period | HPE- Subepidermal split±eosinophils |
| DIF- IMF at the dermo-epidermal junction, n-serration | ||||
| IIF SSS: IMF at blister roof | ||||
| ELISA: BP180, BP230 | ||||
| Epidermolysis bullosa acquisita | IgG | Type VII collagen | Mechano-bullous (like epidermolysis bullosa) and inflammatory variant (like BP or MMP) with tense blisters and erosion over skin and mucosa | HPE- subepidermal split±inflammatory infiltrate |
| DIF- IMF at the dermo-epidermal junction, u-serration | ||||
| IIF SSS: blister floor | ||||
| ELISA: Type VII collagen | ||||
| Anti-p200/laminin γ1 pemphigoid | IgG | p200 antigen laminin γ1 | Tense blisters, erosions, no predominant mucosal involvement like BP | HPE- Subepidermal split±inflammatory infiltrate |
| DIF- IMF at the dermo-epidermal junction, n-serration | ||||
| IIF SSS: blister floor | ||||
| Dermatitis herpetiformis | IgA | Epidermal transglutaminase 3, endomysium | Intensely pruritic, grouped vesicles and excoriations, predominantly affect extensor surfaces associated with gluten-sensitive enteropathy | HPE- Subepidermal split±neutrophilic abscess in dermal papillary tips |
| DIF granular or fibrillar pattern IMF in dermal papillae |
DIF - Direct immunofluorescence; Dsg - Desmoglein; HPE - Histopathological examination; IC - Intercellular; Ig - Immunoglobin; IIF - Indirect immunofluorescence; IMF - Immunofluorescence; SSS - Salt split skin
A simple bedside test from the base of the blister such as Tzanck smear may be used to differentiate the pemphigus from the pemphigoid group. Tzanck smear from the base of a pemphigus erosion but not from a pemphigoid lesion will show acantholytic cells. The sensitivity of the Tzanck smear in pemphigus is almost 100% and the specificity is 43.4%.[2] However, it is important to note that acantholytic cells can also be observed in other disorders, such as staphylococcal scalded skin syndrome, bullous impetigo, herpetic infections, varicella, and Hailey-Hailey disease. Therefore, the presence of acantholytic cells in the Tzanck smear is not diagnostic of pemphigus in isolation. Histopathological evaluation of a blister reveals the location of split formation along with inflammatory infiltrate. It can also show acantholytic cells in the epidermis/epithelium in pemphigus patients. In PNP, interface changes and inflammatory cells can be seen in the upper dermis/sub-epithelium along with acantholytic and dyskeratotic cells in the epidermis/epithelium. In dermatitis herpetiformis, there may be neutrophilic micro-abscesses at the papillary tips of the dermis. However, histopathology findings are non-specific on many occasions and do not pinpoint the precise diagnosis in most cases. Tests that detect tissue-bound or circulating antibodies are needed to establish the autoimmune nature of the disease. These tests comprise immunofluorescence (IF) microscopy (direct IF, DIF; Indirect IF, IIF), enzyme-linked immunosorbent assay (ELISA), BIOCHIP, and immunoblot (IB).[3] However, such facilities are unavailable at a majority of centers in India. The issues related to the stability of immuno-reactants during sample transport to the referral laboratory, high cost, and limited availability of technical expertise remain hindrances in their common usage. In this article, we attempt to overview the approach to diagnosing AIBDs using tests currently available in India.
Direct immunofluorescence (DIF)
Direct immunofluorescence (DIF) remains the gold standard for the detection of the autoimmune nature of AIBDs. The method detects in vivo bound antibodies in the perilesional tissue biopsy samples of skin or mucosa taken from patients of AIBD. Details about the site of deposit of antibody in the skin, immunoglobulin type, and pattern of staining are gathered from DIF. However, DIF does not provide any information pertaining to the target antigen against which antibodies are formed.[4]
The biopsy sample is collected from the non-blistered skin/mucosa adjacent to the new lesion. Blistered tissue can give false-negative outcomes resulting from degradation or loss of deposited immuno-reactants. The biopsy sample can be immediately frozen in liquid nitrogen. Otherwise, the samples can be kept in Michel’s transport media or 0.9% normal saline for up to 24 h till a frozen section is prepared.[5] However, transporting biopsy in normal saline at room temperature has the advantage of improved exposure of epitopes, reduced background staining, and improved appearance on DIF.[6] The use of honey as a transport medium for skin biopsy samples intended for DIF has been explored. Honey is known for its preservative properties and ability to prevent autolysis and putrefaction. However, the viscosity of honey may limit its penetration into deeper tissues, potentially leading to immune complex deterioration over time. The sensitivity of honey as a transport medium for skin biopsy specimens was found to be 100% at week 1, 92.6% at week 2, and 53.8% at week 4.[7]
DIF shows intercellular deposition of IgG ± C3 in the pemphigus group of diseases such as pemphigus vulgaris, pemphigus foliaceus (IgA pemphigus shows deposition of IgA along with C3), and PNP (intercellular and basement membrane staining).[8] The sensitivity of DIF ranges from 82 to 91% and specificity is around 98% for the pemphigus group of diseases.[9]
Hair DIF demonstrates potential as a replacement for skin or mucosal DIF in pemphigus patients, especially when utilizing anagen hair follicles. This technique involves identifying IgG depositions with a fish-net pattern on the outer root sheath (ORS) of anagen scalp hairs in pemphigus patients. [Figure 2] A subset of anagen hair DIF cases can also exhibit C3 depositions, albeit with slightly reduced fluorescence intensity. Various studies highlight DIF’s sensitivity to ORS for pemphigus, ranging from 85% to 100%. Notably, pemphigus patients yielded negative DIF outcomes with telogen hairs.[10,11,12] To ensure reliable outcomes, it is crucial that hair samples include hair follicle-ORS structures and are promptly processed to prevent sample degradation. The authors of this article have found ORS DIF to correlate with skin biopsy DIF and positive ORS DIF predicting clinical relapse in pemphigus.[13]
Figure 2.

Direct immunofluorescence of plucked hair showing linear IgG deposition in the intercellular junction in the outer root sheath
DIF of Tzanck smears has emerged as another alternative to skin or mucosal DIF for early diagnosis of pemphigus vulgaris. It determines IgG deposits on acantholytic cell surfaces. Positive DIF reveals ring-shaped IgG deposition on acantholytic cells or fishnet pattern fluorescence in cell clumps. Air-dried Tzanck smears stored at room temperature for up to 10 days maintain their DIF positivity.[14] The sensitivity of Tzanck smear DIF in pemphigus patients varies from 40% to 100%, with a specificity of 100%. Positive immune deposits were found in Tzanck smears of pemphigus cases but not in other bullous diseases.[15,16] Considering the cost-effectiveness and non-invasiveness of Tzanck smear DIF, it has been proposed as an initial diagnostic approach in cases with challenging biopsies such as oral mucosa, and in children.[17] This may also be true for ORS DIF in cases of pemphigus vulgaris.
Pemphigoid disorders show linear binding of IgG ± C3 at the dermo-epidermal junction. Broad differentiation between pemphigoid diseases can be done on the serration pattern (u-serration vs n-serration). The “u-serrated” pattern is termed when the arch is formed at the bottom, while the “n-serrated” pattern is termed when the arch is formed at the top. The “u-serrated” pattern is pathognomonic of antibodies binding against type VII collagen antigen in epidermolysis bullosa acquisita and bullous systemic lupus erythematosus, while the “n-serrated” pattern is seen in all other pemphigoid diseases[18,19] [Figures 3 and 4]. The sensitivity and specificity of DIF for bullous pemphigoid have been reported to be 90.8% and 98%, respectively.[9] Dermatitis herpetiformis shows granular IgA deposits at the tips of the papillary dermis and along the basement membrane zone.[20] The chronic bullous disease of childhood/linear IgA bullous disease (LAD) shows a linear deposit of IgA with/without C3 along DEJ. To achieve a more distinct delineation of the target antigens, serological analysis with well-characterized antigenic protein or its epitope as substrate is necessary.
Figure 3.
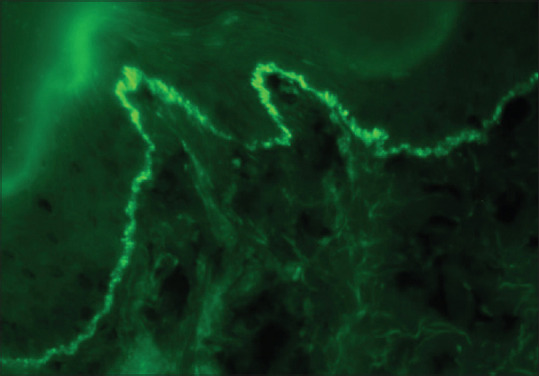
Direct immunofluorescence showing “u”- serrated pattern on IgG antibody in a case of Epidermolysis Bullosa Acquisita (fluorescein isothiocyanate, ×400)
Figure 4.

Direct immunofluorescence showing “n”- serrated pattern on IgG antibody in a case of Bullous Pemphigoid (fluorescein isothiocyanate, ×630)
Indirect immunofluorescence (IIF) with organ substrate
Indirect immunofluorescence (IIF) detects circulating autoantibodies directed against target antigens involved in AIBDs. Due to its minimally invasive nature, serology proves highly beneficial in situations where acquiring biopsy specimens poses challenges, such as children and adults not willing to biopsy. The patient’s serum containing primary antibodies is put on organ substrates and anti-human immunoglobulin antibodies conjugated with a fluorescent dye such as fluorescein isothiocyanate are applied, which bind to the primary antibody. The dye is excited to emit a wavelength that can be observed under a fluorescent microscope.[21]
Human skin is used as a substrate for most practical purposes. For research, monkey and guinea pig esophagus have been used for pemphigus vulgaris and foliaceous, respectively. Rat bladder urothelium rich in plakins is used to perform IIF with 74% sensitivity and 99–100% specificity for identifying PNP.[4,22]
Linear staining at the basement membrane zone is a feature of pemphigoid disorders, that are further delineated by salt split technique on human skin substrate using 1 M NaCl with a sensitivity of 44.6%–97.56% and a specificity of 98.9%–99.75%.[23,24] Autoantibodies against BP180, BP230, and α6β4 integrin typically bind to the blister roof. These proteins are key components of the hemidesmosome complex and anchor the epidermis to the DEJ. In contrast, autoantibodies targeting the p200 antigen and type VII collagen bind to the floor of the blister since these proteins are present in deeper layers and connect DEJ to the dermis [Figure 5]. Antibodies in MMP patients bind to the epidermal side of the split. Patients with a subset of MMP with exclusive anti-laminin 332 antibodies (anti-laminin 332 type MMP) stain the dermal side of the split, while epidermal binding in such patients is attributed to co-existing anti-BP180 autoantibodies.[25,26] IIF should be ideally done with IgA along with IgG, for diagnosis of IgA pemphigus, LAD, and MMP.
Figure 5.

In-house salt-split skin indirect immunofluorescence showing roof binding of IgG in a case of Bullous Pemphigoid
In addition to aiding in diagnosis, monitoring titers over time can provide valuable insights into disease progression and treatment response. A notable reduction in titers in IIF could signal a positive response to therapy in pemphigus, while an increase might indicate disease relapse or inadequate response. Titers can serve as objective measures in clinical trials, enabling the evaluation of the efficacy and safety of emerging treatments. Nevertheless, it is important to note that the assessment of these antibodies via IIF is inherently subjective, offering only semiquantitative data. This evaluation is based on the dilution of serum samples and relies on the specific substrate used, which can introduce variability in antigen expression levels.[27]
For human salt split preparation, viable skin is taken from the sole, either from a cadaver or an amputated limb. The subcutaneous fat is removed, and the skin is cut into smaller (1 × 1 cm) pieces. The piece of skin is incubated in 1M NaCl for 12–16 h at room temperature using a magnetic stirrer. After incubation, one piece is removed from the solution and a frozen section is obtained and stained with rapid hematoxylin and eosin stain. The section is examined under a light microscope for split at the dermo-epidermal junction. Once split is achieved, multiple frozen sections are cut (4–5 microns thick), preferably on coated glass slides, and stored at −20o C for future use. During the test, the salt-split skin slides are incubated with the patient’s serum, followed by fluorescence isothiocyanate-coated anti-human IgG/IgA. Staining is observed on the roof or floor side. It can occur on both floor and roof in uncommon situations i.i., in MMP.
BIOCHIP
BIOCHIP is a relatively newer technique based on IIF. It has been established as a diagnostic substitute to the ELISA for screening the disease. BIOCHIP uses a single serum sample and can identify autoantibodies directed against multiple recombinant target antigenic substrates in a solitary test site (microarrays), therefore simultaneously screening common AIBDs.[28] Antigenic substrates are mechanically cut into millimeter-sized pieces and pasted on thin glass slides. A typical BIOCHIP slide has ten incubation fields containing six different types of antigenic substrates, which include (i) frozen tissue section of monkey esophagus, (ii) 1M NaCl human split skin, (iii) human embryonic kidney (HEK293) cells transfected with Dsg1 protein ectodomain, (iv) HEK293 cells transfected with Dsg3 protein ectodomain, (v) micro-drops of NC16A-4X BP180 antigen, and (vi) HEK293 cells transfected with C-terminal globular domain of the BP230[29,30] [Figures 6 and 7]. BIOCHIP with five incubation fields is sold in India. An extended BIOCHIP mosaic containing 12 antigenic substrates is not available in India.
Figure 6.

BIOCHIP mosaic indirect immunofluorescence showing (a) positivity for recombinant BP180-NC16A (b) positivity for C-terminal globular domain of BP230
Figure 7.

BIOCHIP salt-split skin indirect immunofluorescence showing roof binding of IgG in a case of Bullous Pemphigoid
Euroimmun BIOCHIP employs “Titerplane Technology,” in which a reagent tray made of glass is used which has a hydrophilic reaction field area and hydrophobic peripheral area, therefore limiting the spillage of reagent fluid and its mixing with the adjacent reaction field of the reagent tray. The reagent tray is placed facing upward, and serum samples are poured over the reaction fields using a pipette. After that, the BIOCHIP slides are placed facing downward over the reagent tray and incubated for 30 min. After washing with phosphate buffer saline, they are re-incubated with fluorescein isothiocyanate-conjugated anti-human antibodies for another 30 min in the reagent tray. With this technique, all reaction fields are exposed to the serum samples concomitantly, and individual reaction begins at the same moment for every serum sample, preventing the variation in the intensity of immunofluorescence due to variance in incubation time. This method requires a minimal serum sample. A humidity chamber is not needed as the procedure is performed in a closed space. Another advantage is that the crystals and particles collect over the reagent tray and not BIOCHIPs due to the gravitational effect helping in optimal fluorescent imaging which is assessed by using a fluorescent microscope.[31,32]
BIOCHIP has shown a 95–100% specificity for the BP180 antigen and 98.3–100% for the BP230 antigen. Sensitivity is 83–100% for BP180 antigen in comparison to low sensitivity of 24.3–66.7% for BP230 antigens.[9,33,34] The newer BIOCHIP mosaics have been expanded to include rat bladder (detects anti-plakin antibodies of PNP) and recombinant coeliac-specific parts of gliadin (detects anti-endomysial antibodies) substrates. This BIOCHIP mosaic has a sensitivity of 90%, 98%, and 100%, for desmoglein-1, desmoglein-3, and Collagen XVII NC16A, respectively. The sensitivity of BIOCHIP developed for the detection of laminin 332 antibodies had a sensitivity range of 75–85% and a specificity of almost 100%.[35]
BIOCHIP technology can analyze various serum samples concomitantly resulting in a higher throughput and requires a short incubation period of 60–100 min. Moreover, all incubation steps are carried out at room temperature and require low reagent consumption. As a drawback, BIOCHIP techniques do not provide a quantitative value, unlike ELISA.[31]
Enzyme-linked immunosorbent assay (ELISA)
ELISA is used to identify and quantify serum autoantibodies directed against specific autoantigens; hence can be used for diagnosis and monitoring of therapy. The principles of uni-parametric (single-parameter) and multivariant profile ELISAs are fundamentally similar because they are both based on the same basic ELISA technique. This process entails the immobilization of a target antigen (uni-parametric) or several antigens (multi-parametric) onto a microplate, followed by blocking and incubation with diluted serum samples. After washing to remove unbound components, a labeled secondary antibody, which targets the primary antibodies, is applied. Subsequently, after another wash, a substrate is introduced, which the enzyme-linked secondary antibody converts into a quantifiable signal. The signal intensity correlates with the presence and concentration of specific antibodies in the serum, measurable using a microplate reader. ELISA is used for diagnosis as well as for monitoring of therapy. Reduction in titers of ELISA with anti-Dsg1, anti-Dsg3, anti-BP180, and anti-type VII collagen antibodies has been shown to correlate with treatment response, while an increase may imply relapse.[36,37,38] However, the reactivity of anti-BP230 antibodies appears to remain relatively stable in relation to changes in the clinical state of patients with bullous pemphigoid, except for a small subset of cases.[39] The key differences between uni-parametric and multi-parametric ELISA lie in their setup and execution due to the measurement of one versus multiple analytes (antibodies) against the antigen(s).
Commercially available multivariant ELISA kits that rely on recombinant target antigens are gaining usage in the serologic diagnosis of AIBDs.[36] They process the diagnostically most relevant antigens in adjoining wells of a microplate simultaneously. The two commercial multivariant systems include MBL (expressed on Baculovirus, Nagoya, Japan; (*multivariant system is no longer available on MBL website)) comprising five different antigens, BP180 NC16A, BP230, desmoglein 1, desmoglein 3, and type VII collagen and Euroimmun (expressed in HEK293 cells, Luebeck, Germany) that includes envoplakin antigen in addition.[36,40,41] Multivariant ELISA gives semi-quantitative results. As of now, the titers determined may not correlate with actual titers of serum antibodies.
The multivariant profile ELISA system developed by MBL had an 88% consistency with results derived from conventional multi-step individual ELISA systems with sensitivities of 92% and 93% for anti-Dsg1 and anti-Dsg3 antibodies, respectively. Sensitivities for anti-BP180 and anti-BP230 antibodies were 66% and 62%, respectively, whereas anti-type VII collagen antibodies had a sensitivity of 81%. Specificities for all antibodies ranged from 98 to 100%.[42,43] Multivariant ELISA system (Euroimmun, Luebeck, Germany) had antibody sensitivities of 95% for anti-Dsg1, 100% for anti-Dsg3, 95% for anti-BP180, 60% for anti-BP230, 93% for anti-type VII collagen, and 86% for anti-envoplakin antibodies. Specificities for different antibodies ranged between 97 and 100%. These results had a consistency of 87% with individual ELISA systems.[44,45] A recently conducted retrospective study in Indian AIBD cohort found good agreement between multivariant ELISA and the gold-standard diagnosis (clinical and histopathologic+DIF), particularly for the pemphigus group of disorders and bullous pemphigoid.[46]
Profile ELISA is a standardized objective automated, reproducible method that allows the processing of multiple samples at a time. Results may be falsely negative when recombinant antigenic proteins utilized do not have all of the epitopes present in vivo, or there is a complete absence of target antigen in the microplate.[47]
ELISA with substrates other than serum
The diagnostic potential of salivary anti-Dsg1 and anti-Dsg3 ELISA for pemphigus vulgaris has been explored, with a focus on comparing these methods with serum-based assays. Saliva, as a biofluid, offers simplicity and repeatability in sample collection. It is hypothesized that serum components like autoantibodies could traverse the capillary walls of salivary glands to be present in saliva.[48] It is a safe, non-invasive modality of diagnosis when a blood sample cannot be obtained. It has a high specificity of around 98.9% for diagnosis.[48] It can also be used for monitoring of the disease activity. Salivary Dsg3 and Dsg1 titers have shown to have a positive correlation with their corresponding serum titers, but may not correlate with the ABSIS scores/disease activity.[49] Reports have also explored the use of blister fluid as a substrate for ELISA, BIOCHIP, and IIF methods in the context of pemphigus and subepidermal AIBDs like bullous pemphigoid.[50,51,52,53]
Immunoblot
These techniques are based on the molecular weight of the target antigens in AIBDs. The antigens are recombinant molecular proteins or extracts of the dermis, epidermis, bovine gingiva, amnion membrane, or cultured keratinocytes.[54] The proteins (antigens) are segregated as per the molecular weight using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the gel is then moved to nitrocellulose paper. Sera containing autoantibodies is added to nitrocellulose paper to observe for band formation at a particular molecular weight, indicating the target antigen. The results are deduced after matching with a control. Sensitivity remains low due to denaturation of antigenic proteins during electrophoresis, especially with desmoglein 1–3.[55,56] Though the authors’ lab is developing an immunoblot for the diagnosis of AIBDs, it is not available for AIBD diagnosis in India.
Table 2 provides a concise overview of investigations and their characteristics for AIBDs.[1,9,20]
Table 2.
Investigations to precisely identify the disease subtype of AIBDs
| Test | Sample required | Substrate | What is detected? | Location of deposit | Specific antigen identified? | Antibody titer determined? | Diagnostic or prognostic value | Equipment required | Drawbacks |
|---|---|---|---|---|---|---|---|---|---|
| Direct immunofluorescence (DIF) | Patient skin | - | Antibody/complement bound to patient skin | ICS/DEJ/Papillary dermis | No | No | Diagnostic, may aid in treatment withdrawal | IF microscope and microtome | |
| Serration pattern analysis in DIF | Patient skin | - | Antibody/complement bound to patient skin | Serration pattern specific for deposit at DEJ | No. Helps in assuming the antigen, if it is a ‘u’ serration pattern | No | Diagnostic- differentiates EBA/BSLE (u serration pattern) from all other sub-epidermal AIBDs (n serration pattern) | IF microscope and microtome | Perceived to be a complicated procedure, requiring profound technical expertise |
| Indirect immunofluorescence (IIF) | Patient serum | Human skin/monkey esophagus/others | Antibody in patient serum | ICS/DEJ | No | Yes | Diagnostic. IIF titer may correlate with disease severity | IF microscope and microtome | Difficulty in procuring the substrates |
| Salt split IIF | Patient serum | Salt split human skin/monkey skin | Antibody in patient serum | ICS/DEJ with binding at the floor or roof of artificially induced split | No. Helps in assuming the antigen in case of floor binding | - | Aids in diagnosis. In the case of floor binding, possibilities are limited | IF microscope and microtome | Difficulty in procuring the substrates Perceived to be a complicated procedure, requiring profound technical expertise |
| BIOCHIP IIF | Patient serum | Tissue (salt split skin/monkey esophagus), recombinant proteins, proteins expressed by transfected cells | Antibody in patient serum | Yes | No | Diagnostic | IF microscope | Cost | |
| Single parameter ELISA | Patient serum | Antigenic epitopes of proteins coated on wells | Antibody in patient serum | Yes | Yes | Both diagnostic and prognostic | ELISA plate reader | Antibodies against epitopes other than the one coated can give false negative results. The cost of individual ELISA is high Have to have a high pre-test probability The bulk of samples required to run the test may prohibit timely assay and reporting. | |
| Multi-parameter (profile) ELISA | Patient serum | Antigenic epitopes of proteins coated on wells | Antibody in patient serum | Yes | No | Diagnostic | ELISA plate reader | Cost |
ICS – Intercellular space; DEJ - dermo-epidermal junction; IF - Immunofluorescence; EBA - Epidermolysis bullosa; BSLE - Bullous systemic bullous erythematosus
Conclusion
Identifying the specific disease subtype of AIBDs is crucial for prognostication and management. Confirmation of AIBDs is a step-ladder approach as per recommendation, including the clinical presentation, simple bedside Tzanck test, a histopathological examination from the lesional skin, and DIF examination from perilesional skin and serology. The serological examination includes IIF, ELISA, and BIOCHIP. Commercially available multivariant profile ELISA and BIOCHIP mosaic-based IIF allow the identification of common AIBDs simultaneously from a single serum sample. As they have only the common recombinant antigens in the microplate, they are unable to identify the rare subtypes of AIBDs whose antigenic epitopes or complete antigens are absent in the microplate. Multivariant assays process many samples simultaneously making it cost-effective and allowing timely diagnosis, thereby reducing morbidity and mortality. Diagnostic algorithms that can be followed in India using available resources as of date for intra-epidermal and sub-epidermal blistering diseases have been depicted in Figures 8 and 9.
Figure 8.
Algorithm for diagnosis of pemphigus in Indian context. *Clinical diagnosis of pemphigus is considered in the context of recurrent flaccid erosions on skin and/or mucosal blistering. ** DIF can also be performed on the plucked hair as an alternative to perilesional skin. +Pemphigus vegetans is considered in patients presenting with vegetative plaques in flexural distribution. ++PNP is considered in patients with recalcitrant mucositis and polymorphic skin lesions, including lichenoid lesions
Figure 9.
Algorithm for diagnosis of subepidermal AIBDs in the Indian context. The serration pattern analysis and immunostaining on the roof/floor on salt split IIF can help in the interpretation here since specific antibody testing is not always available. *Clinical diagnosis of subepidermal AIBDs can be considered classically in the context of tense blisters (pemphigoid group) or grouped papule-vesicles on extensors (DH)
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil.
References
- 1.Witte M, Zillikens D, Schmidt E. Diagnosis of autoimmune blistering diseases. Front Med (Lausanne) 2018;5:296. doi: 10.3389/fmed.2018.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958–64. doi: 10.1016/j.jaad.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Costan VV, Popa C, Hâncu MF, Porumb-Andrese E, Toader MP. Comprehensive review on the pathophysiology, clinical variants and management of pemphigus (Review) Exp Ther Med. 2021;22:1335. doi: 10.3892/etm.2021.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giurdanella F, Diercks GFH, Jonkman MF, Pas HH. Laboratory diagnosis of pemphigus: Direct immunofluorescence remains the gold standard. Br J Dermatol. 2016;175:185–6. doi: 10.1111/bjd.14408. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra S, Minz RW, Saikia B. Immunofluorescence in dermatology. Indian J Dermatol Venereol Leprol. 2012;78:677–91. doi: 10.4103/0378-6323.102355. [DOI] [PubMed] [Google Scholar]
- 6.Vodegel RM, de Jong MC, Meijer HJ, Weytingh MB, Pas HH, Jonkman MF. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10. doi: 10.1186/1471-5945-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumudhini S, Pai S, Rao C, Rao R. A comparative study of Michel's medium versus honey as a transport medium for skin specimens prior to direct immunofluorescence microscopy and antigen mapping. J Cutan Pathol. 2019;46:729–35. doi: 10.1111/cup.13494. [DOI] [PubMed] [Google Scholar]
- 8.Porro AM, Caetano Lde VN, Maehara L de SN, Enokihara MM dos S. Non-classical forms of pemphigus:pemphigus herpetiformis, IgA pemphigus, paraneoplastic pemphigus and IgG/IgA pemphigus. An Bras Dermatol. 2014;89:96–106. doi: 10.1590/abd1806-4841.20142459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saschenbrecker S, Karl I, Komorowski L, Probst C, Dähnrich C, Fechner K, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974. doi: 10.3389/fimmu.2019.01974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao R, Shenoi SD, Balachandran C. Demonstration of pemphigus specific immunofluorescence pattern by direct immunofluorescence of plucked hair. J Am Acad Dermatol. 2008;58:AB85. doi: 10.1111/j.1365-4632.2009.04153.x. [DOI] [PubMed] [Google Scholar]
- 11.Kumaresan M, Rai R, Sandhya V. Immunofluorescence of the outer root sheath: An aid to diagnosis in pemphigus. Clin Exp Dermatol. 2011;36:298–301. doi: 10.1111/j.1365-2230.2010.03981.x. [DOI] [PubMed] [Google Scholar]
- 12.Alexandru A, Zurac S, Salavastru CM, Andrei R, Tebeica T, Staniceanu F, et al. Direct immunofluorescence on hair follicles--present and future perspectives. Am J Dermatopathol. 2013;35:472–6. doi: 10.1097/DAD.0b013e31827747b2. [DOI] [PubMed] [Google Scholar]
- 13.Malhi K, Chatterjee D, Mahajan R, Handa S, De D. Direct immunofluorescence on plucked hair outer root sheath can predict relapse in pemphigus vulgaris. Int J Dermatol. 2024 doi: 10.1111/ijd.17405. Epub ahead of print. PMID:39086016. [DOI] [PubMed] [Google Scholar]
- 14.Verma KK, Khaitan BK, Singh MK. Antibody deposits in Tzanck smears in pemphigus vulgaris. J Cutan Pathol. 1993;20:317–9. doi: 10.1111/j.1600-0560.1993.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 15.Shahidi Dadras M, Asadi Kani Z, Hamedani B, Dadkhahfar S, Rastgoo N, Mozafari N. Can immunofluorescence on skin/mucosal scraping smear for pemphigus diagnosis substitute direct immunofluorescence on skin biopsy? Dermatol Pract Concept. 2022;12:e2022101. doi: 10.5826/dpc.1203a101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aithal V, Kini U, Jayaseelan E. Role of direct immunofluorescence on Tzanck smears in pemphigus vulgaris. Diagn Cytopathol. 2007;35:403–7. doi: 10.1002/dc.20657. [DOI] [PubMed] [Google Scholar]
- 17.Yavuzekinci Ü, Özcan D, Seçkin D. Non-oral mucosal involvement of pemphigus vulgaris: Tzanck smear test as a handy diagnostic tool. Int J Dermatol. 2015;54:e325–6. doi: 10.1111/ijd.12872. [DOI] [PubMed] [Google Scholar]
- 18.Inchara YK, Rajalakshmi T. Direct immunofluorescence in cutaneous vesiculobullous lesions. Indian J Pathol Microbiol. 2007;50:730–2. [PubMed] [Google Scholar]
- 19.Sood R, Chatterjee D, De D, Saikia UN, Mahajan R, Handa S, et al. Accuracy of serration pattern analysis by direct immunofluorescence in subepidermal autoimmune blistering diseases. Indian J Dermatol Venereol Leprol. 2024;90:336–41. doi: 10.25259/IJDVL_20_2023. doi:10.25259/IJDVL_20_2023. [DOI] [PubMed] [Google Scholar]
- 20.De D, Khullar G, Handa S, Saikia UN, Radotra BD, Saikia B, et al. Clinical, demographic and immunopathological spectrum of subepidermal autoimmune bullous diseases at a tertiary center: A 1-year audit. Indian J Dermatol Venereol Leprol. 2016;82:358. doi: 10.4103/0378-6323.175928. [DOI] [PubMed] [Google Scholar]
- 21.Shetty VM, Subramaniam K, Rao R. Utility of immunofluorescence in dermatology. Indian Dermatol Online J. 2017;8:1–8. doi: 10.4103/2229-5178.198774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poot AM, Diercks GF, Kramer D, Schepens I, Klunder G, Hashimoto T, et al. Laboratory diagnosis of paraneoplastic pemphigus. Br J Dermatol. 2013;169:1016–24. doi: 10.1111/bjd.12479. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Xiang R, Jing K, Li Z, Wang Y, Zhang H, et al. Diagnostic values of indirect immunofluorescence using salt-split skin, direct immunofluorescence and BP180 NC16A ELISA on bullous pemphigoid. Chin Med J (Engl) 2022;135:1379–80. doi: 10.1097/CM9.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sárdy M, Kostaki D, Varga R, Peris K, Ruzicka T. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748–53. doi: 10.1016/j.jaad.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, Li X, Qian H. Anti-Laminin 332-type mucous membrane pemphigoid. Biomolecules. 2022;12:1461. doi: 10.3390/biom12101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau I, Goletz S, Holtsche MM, Zillikens D, Fechner K, Schmidt E. Anti-p200 pemphigoid is the most common pemphigoid disease with serum antibodies against the dermal side by indirect immunofluorescence microscopy on human salt-split skin. J Am Acad Dermatol. 2019;81:1195–7. doi: 10.1016/j.jaad.2019.03.077. [DOI] [PubMed] [Google Scholar]
- 27.Ng PP, Thng ST, Mohamed K, Tan SH. Comparison of desmoglein ELISA and indirect immunofluorescence using two substrates (monkey oesophagus and normal human skin) in the diagnosis of pemphigus. Australas J Dermatol. 2005;46:239–41. doi: 10.1111/j.1440-0960.2005.00191.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Li F, Wang X, Wang R, Chen T, Zhao J, et al. BIOCHIP mosaic for the diagnosis of autoimmune bullous diseases in Chinese patients. Eur J Dermatol. 2020;30:338–44. doi: 10.1684/ejd.2020.3839. [DOI] [PubMed] [Google Scholar]
- 29.Yang A, Xuan R, Melbourne W, Tran K, Murrell DF. Validation of the BIOCHIP test for the diagnosis of bullous pemphigoid, pemphigus vulgaris and pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2020;34:153–60. doi: 10.1111/jdv.15770. [DOI] [PubMed] [Google Scholar]
- 30.Xuan RR, Yang A, Murrell DF. New biochip immunofluorescence test for the serological diagnosis of pemphigus vulgaris and foliaceus: A review of the literature. Int J Womens Dermatol. 2018;4:102–8. doi: 10.1016/j.ijwd.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EUROIMMUN AG. IFA. 2023. [Last accessed on 2023 Jun 19]. Available from:https://www.euroimmun.com/products/techniques/ifa/
- 32.Clinical Laboratory int. From BIOCHIPs to artificial intelligence –defining new horizons in IFA. [Last accessed on 2023 Jun 19]. Available from:https://clinlabint.com/from-biochips-to-artificial-intelligence-defining-new-horizons-in-ifa/
- 33.Gornowicz-Porowska J, Seraszek-Jaros A, Bowszyc-Dmochowska M, Kaczmarek E, Pietkiewicz P, Bartkiewicz P, et al. Accuracy of molecular diagnostics in pemphigus and bullous pemphigoid: Comparison of commercial and modified mosaic indirect immunofluorescence tests as well as enzyme-linked immunosorbent assays. Postepy Dermatol Alergol. 2017;34:21–7. doi: 10.5114/ada.2017.65617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prüßmann W, Prüßmann J, Koga H, Recke A, Iwata H, Juhl D, et al. Prevalence of pemphigus and pemphigoid autoantibodies in the general population. Orphanet J Rare Dis. 2015;10:63. doi: 10.1186/s13023-015-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goletz S, Giurdanella F, Holtsche MM, Nijenhuis M, Horvath B, Diercks GFH, et al. Comparison of two diagnostic assays for anti-laminin 332 mucous membrane pemphigoid. Front Immunol. 2021;12:773720. doi: 10.3389/fimmu.2021.773720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt E, Dahnrich C, Rosemann A, Probst C, Komorowski L, Saschenbrecker S, et al. Novel ELISA systems for antibodies to desmoglein 1 and 3: Correlation of disease activity with serum autoantibody levels in individual pemphigus patients. Exp Dermatol. 2010;19:458–63. doi: 10.1111/j.1600-0625.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- 37.Amagai M, Komai A, Hashimoto T, Shirakata Y, Hashimoto K, Yamada T, et al. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–7. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- 38.Marzano AV, Cozzani E, Fanoni D, De PO, Vassallo C, Berti E, et al. Diagnosis and disease severity assessment of epidermolysis bullosa acquisita by ELISA for anti-type VII collagen autoantibodies: An Italian multicentre study. Br J Dermatol. 2013;168:80–4. doi: 10.1111/bjd.12011. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, et al. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci. 2006;41:21–30. doi: 10.1016/j.jdermsci.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Gornowicz-Porowska J, Seraszek-Jaros A, Bowszyc-Dmochowska M, Bartkiewicz P, Kaczmarek E, Dmochowski M. Clinical evaluation of a multiparametric ELISA as a rapid tool for routinely diagnosing IgG-mediated autoimmune blistering dermatoses in ethnic Slavs. J Clin Lab Anal. 2017;32:e22336. doi: 10.1002/jcla.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Beek N, Rentzsch K, Probst C, Komorowski L, Kasperkiewicz M, Fechner K, et al. Serological diagnosis of autoimmune bullous skin diseases: Prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49. doi: 10.1186/1750-1172-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horváth ON, Varga R, Kaneda M, Schmidt E, Ruzicka T, Sárdy M. Diagnostic performance of the “MESACUP anti-Skin profile TEST.”. Eur J Dermatol. 2016;26:56–63. doi: 10.1684/ejd.2015.2692. [DOI] [PubMed] [Google Scholar]
- 43.Tampoia M, Giavarina D, Di Giorgio C, Bizzaro N. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering skin diseases: A systematic review and meta-analysis. Autoimmun Rev. 2012;12:121–6. doi: 10.1016/j.autrev.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Damoiseaux J, van Rijsingen M, Warnemünde N, Dähnrich C, Fechner K, Tervaert JWC. Autoantibody detection in bullous pemphigoid: Clinical evaluation of the EUROPLUS™ Dermatology Mosaic. J Immunol Methods. 2012;382:76–80. doi: 10.1016/j.jim.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 45.van Beek N, Dähnrich C, Johannsen N, Lemcke S, Goletz S, Hübner F, et al. Prospective studies on the routine use of a novel multivariant enzyme-linked immunosorbent assay for the diagnosis of autoimmune bullous diseases. J Am Acad Dermatol. 2017;76:889–94. doi: 10.1016/j.jaad.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Kumar V, De D, Gupta S, Narayan R V, Mahajan R, Chatterjee D, et al. Use of multivariant enzyme-linked immunosorbent assay (ELISA) in the diagnosis of autoimmune bullous disorders: Perspective of a resource-limited setting. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1195_2023. doi:10.25259/IJDVL_1195_2023. [DOI] [PubMed] [Google Scholar]
- 47.Tighe PJ, Ryder RR, Todd I, Fairclough LC. ELISA in the multiplex era: Potentials and pitfalls. Proteomics Clin Appl. 2015;9:406–22. doi: 10.1002/prca.201400130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortazavi H, Khatami A, Seyedin Z, Vasheghani Farahani I, Daneshpazhooh M. Salivary desmoglein enzyme-linked immunosorbent assay for diagnosis of pemphigus vulgaris: A noninvasive alternative test to serum assessment. Biomed Res Int. 2015;2015:698310. doi: 10.1155/2015/698310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De D, Khullar G, Handa S, Joshi N, Saikia B, Minz RW. Correlation between salivary and serum anti-desmoglein 1 and 3 antibody titres using ELISA and between anti-desmoglein levels and disease severity in pemphigus vulgaris. Clin Exp Dermatol. 2017;42:648–50. doi: 10.1111/ced.13124. [DOI] [PubMed] [Google Scholar]
- 50.Shanmugasekar C, Ram Ganesh VR, Jayaraman A, Srinivas CR. Blister fluid immunofluorescence in a case of pemphigus vulgaris. Indian J Dermatol. 2010;55:188–9. doi: 10.4103/0019-5154.62755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surya R, Tejasvi B, Shenoi SD, Pai S, Rao C, Rao R. Detection of anti-basement membrane zone antibodies in the blister fluid in subepidermal autoimmune bullous diseases. Indian J Dermatol. 2017;62:649–53. doi: 10.4103/ijd.IJD_403_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sernicola A, Russo I, Saponeri A, Alaibac M. Biochip detection of BP180 autoantibodies in blister fluid for the serodiagnosis of bullous pemphigoid: A pilot study. Medicine (Baltimore) 2019;98:e14514. doi: 10.1097/MD.0000000000014514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu CK, Huang HY, Chen WR, Nishie W, Ujiie H, Natsuga K, et al. Paper-based ELISA for the detection of autoimmune antibodies in body fluid-the case of bullous pemphigoid. Anal Chem. 2014;86:4605–10. doi: 10.1021/ac500835k. [DOI] [PubMed] [Google Scholar]
- 54.Grootenboer-Mignot S, Descamps V, Picard-Dahan C, Nicaise-Roland P, Prost-Squarcioni C, Leroux-Villet C, et al. Place of human amniotic membrane immunoblotting in the diagnosis of autoimmune bullous dermatoses. Br J Dermatol. 2010;162:743–50. doi: 10.1111/j.1365-2133.2009.09566.x. [DOI] [PubMed] [Google Scholar]
- 55.Mihai S, Sitaru C. Immunopathology and molecular diagnosis of autoimmune bullous diseases. J Cell Mol Med. 2007;11:462–81. doi: 10.1111/j.1582-4934.2007.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurien BT, Danda D, Bachmann MP, Scofield RH. SDS-PAGE to immunoblot in one hour. Methods Mol Biol. 2015;1312:449–54. doi: 10.1007/978-1-4939-2694-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]





