Abstract
Aims:
Gulf War Illness (GWI) is manifested as multiple chronic symptoms, including chronic pain, chronic fatigue, sleep problems, neuropsychiatric disorders, respiratory, gastrointestinal, and skin problems. No single target tissue or unifying pathogenic process has been identified that accounts for this variety of symptoms. The brainstem has been suspected to contribute to this multiple symptomatology. The aim of this study was to assess the role of the brainstem in chronic sleep problems and pain in GWI veterans.
Materials and methods:
We enrolled 90 veterans (Age = 50 ± 5, 87% Male) who were deployed to the 1990–91 Gulf War and presented with GWI symptoms. Sleep quality was evaluated using the global Pittsburgh Sleep Quality Index. Pain intensities were obtained with the Brief Pain Inventory sum score. Volumes in cortical, subcortical, brainstem, and brainstem subregions and diffusion tensor metrics in 10 bilateral brainstem tracts were tested for correlations with symptom measures.
Key findings:
Poorer sleep quality was significantly correlated with atrophy of the whole brainstem and brainstem subregions (including midbrain, pons, medulla). Poorer sleep quality also significantly correlated with lower fractional anisotropy in the nigrostriatal tract, medial forebrain tract, and the dorsal longitudinal fasciculus. There was a significant correlation between increased pain intensity and decreased fractional anisotropy in the dorsal longitudinal fasciculus. These correlations were not altered after controlling for age, sex, total intracranial volumes, or additional factors, e.g., depression and neurological conditions.
Significance:
These findings suggest that the brainstem plays an important role in the aberrant neuromodulation of sleep and pain symptoms in GWI.
Keywords: Gulf War Illness, Magnetic resonance imaging, Diffusion tensor imaging, Sleep, Pain, Brainstem
1. Introduction
About 30% of veterans who served in the 1990–91 Persian Gulf War suffer from Gulf War Illness (GWI) [1,2], a chronic condition that is still not well understood and for which adequate treatment is lacking. GWI manifests with multiple chronic symptoms, including pain, fatigue, sleep problems, neurological, gastrointestinal (irritable bowel syndrome, IBS), respiratory, and skin problems, which have usually first appeared within a year of return from the Gulf though some having onset considerably later [2–4]. Symptoms of GWI persist or get progressively worse over time, for the majority of affected veterans, with few experiencing improvement or recovery [5–7]. Previous research has sought to identify the epidemiology, the conditions in theater that may have caused GWI, nervous system dysfunctions, pathophysiological mechanisms underlying GWI, and experimental models of GWI and its causation [2]. So far, no single target tissue or pathogenic process has been identified that is capable of uniting the wide range of symptoms reported. However, the condition may be best explained as a disorder of the nervous system. Identifying potential nervous system damage could be essential to facilitate the understanding of the pathophysiologic mechanisms and provide more effective treatments for GWI.
Damage to the human brain, particularly the brainstem, has been suspected to contribute to GWI-related symptomatology. Recent studies using automated volumetric measures from structural magnetic resonance imaging (MRI) suggest that veterans with GWI have greater overall atrophy in subcortical regions [8], as well as the brainstem [9], thalamus, and the cerebellum than controls. Another MRI measure, diffusion tensor imaging (DTI) [10,11], is a MRI sequence method that detects brain damages at a microstructural level. Several microstructural indices can be obtained from DTI: fractional anisotropy (FA), a commonly used index of microstructural directionality, to probe nerve fiber arrangements, axonal integrity, and the degree of axonal myelination [12]; mean diffusivity (MD, s/mm2), a measure of magnitude of microstructural water diffusion, primarily indicates broad cellular damage including edema and necrosis [13]; axial (AD, s/mm2) and radial (RD, s/mm2) diffusivities each represent diffusion levels along or perpendicular to the principal axis, respectively, and in some cases may detect pathological axonal or myelin states [14]. Recent DTI investigations of microstructural integrity or structural connectivity levels of brainstem fiber tracts suggested these tracts could be associated with pain control [15,16], sleep-wake cycles [17], dopaminergic deficiency [18], and serotonergic dysfunction [19]. In this context, identifying brainstem damages via macrostructural volumes and the microstructural integrity levels of brainstem fiber tracts may provide insight into structural/functional dysregulations related to chronic pain, sleep difficulties, stress disorders, and chronic fatigue in GWI, and thereby advance our understanding of GWI pathology.
The aim of this study was to investigate the role of the brainstem in regulating pain and sleep in GWI using advanced brain imaging (volumetric MRI and DTI tractography) and psychophysical measurements. The results may contribute to the development and refinement of pharmacological or neuro-modulatory approaches to augment descending modulation pathways.
2. Methods
2.1. Subjects
Data from 90 veterans were collected at the War Related Illness and Injury Study Center (WRIISC) at the Veterans Affairs (VA) Hospital in Palo Alto, CA, USA. The study protocol was approved by the Stanford University Institutional Review Board. All veterans provided written informed consent to participate in the study and share their data in accordance with the Declaration of Helsinki. Inclusion criteria included: 1) deployment to the Operation Desert Shield/Desert Storm (ODS/S) between August 1990 and May 1991 (the initial time-period of the Gulf War) in Saudi Arabia, Kuwait or Iraq; and 2) had completed MRI scans, self-report questionnaires, and clinical reports including diagnosis and screening, neuropsychological assessments reported by a WRIISC psychologist, neurologist, and psychiatrist. Exclusion criteria included: 1) poor MRI quality, 2) incomplete, insufficient information from any self-report or evaluations by clinicians; or 3) serious neurological or neuropsychiatric conditions e.g., multiple sclerosis, Parkinson’s disease, epilepsy, stroke, dementia, schizophrenia, or prior neurosurgical interventions in the brain.
2.2. Clinical assessments
All 90 veterans presented with chronic symptoms lasting more than six months and met both CDC/CMI [3] and Kansas [4] case definitions of GWI. Table 1 shows criteria for the diagnosis or screening of the major symptoms of GWI. According to VA and Department of Defense Clinical Practice Guidelines for Management of Concussion and mTBI [20], a positive TBI was defined by structural brain injury or physiological disruption of brain function, as a result of traumatic events such as head striking/struck by object or blast. Psychiatric status including PTSD and depression was diagnosed in accordance to DSM-IV-TR Axis I Disorders. Fatigue screening was determined based on the CDC CFS (chronic fatigue syndrome) Symptom Inventory [21], where a positive screen should consist of at least 3 out of the 19-item being endorsed chronically (≥6 months), and fatigue limiting daily activities by more than 50% over the past 6 months. Sleep disturbance including poor sleep quality and probable clinical insomnia were determined based on the Pittsburgh Sleep Quality Index (PSQI) [5] global scores (PSQI-GLOB). The presence of chronic pain was determined by positive self-reports of muscle and joint pains present for 6 months or more in the CFS inventory. Neurological problems were determined based on presentations of at least 3 positive symptom items out of the 22-item Neurobehavioral Symptom Inventory (NSI) [22] assessment. Presence of IBS was defined by Rome III criteria [23], and breathing difficulties were defined by the shortness of breath (SOB) items in the CDC CFS, supplemented by a difficulty breathing item under the review of systems (ROS). Obstructive sleep apnea (OSA) was defined as the number of apneas and hypopneas being greater or equal to 5 events per hour of sleep, according to VA and Department of Defense Clinical Practice Guidelines for Management of Chronic Insomnia Disorder and Obstructive Sleep Apnea [24].
Table 1.
List of multiple chronic symptoms present in GWI veterans.
| Category | Diagnostic criteria | Definition of positive |
|---|---|---|
| Sleep | Pittsburgh Sleep Quality Index Global Scores (PSQI-GLOB) | Poor sleep quality: PSQI-GLOB >5. Probable clinical insomnia: PSQI-GLOB >10 |
| Pain | CDC Chronic Fatigue Symptom Inventory (CFS) | Positive self-report from 2 CFS items: muscle pains and joint pains present for 6 months or more. |
| Fatigue | CDC Chronic Fatigue Syndrome Inventory | Have more than 3 symptom items (present for ≥6 months) and, fatigue over the past 6 months has limited >50% degree of daily activity. |
| Neurologic | Neurobehavioral Symptom Inventory (NSI) | More than 3 positive symptom items out of the 22-item NSI self-report |
| PTSD | DSM-IV-TR Axis I Disorders | DSM-IV-TR Axis I Disorders |
| Depression | DSM-IV-TR Axis I Disorders | Major Depressive Disorder or Depressive Disorder reported in DSM-IV-TR Axis I Disorders |
| IBS | Rome III criteria | Positive irritable bowel syndrome (IBS) screen was determined by Rome III criteria applied on 10-item self-report measure |
| Breath Difficulties | CFS and review of systems (ROS) | Positive self-report from 1 ROS item: difficulty breathing, and 2 CFS items: shortness of breath (frequency and present for ≥6 months) |
| Obstructive Sleep Apnea (OSA) | VA/DoD Clinical Practice Guideline | The number of obstructive events (apneas and hypopneas) ≥5 events per hour on actigraphy or polysomnography |
| TBI | VA/DoD Clinical Practice Guideline | Events of brain injury from head striking or head blast. |
Table 2 shows definition of symptom severities according to neuropsychological assessments. Specifically, mild, moderate, or severe TBI were determined according to the reviews of patients’ medical records in accordance with the VA and Department of Defense Clinical Practice Guidelines for Management of Concussion and mTBI [20]. PTSD severity was assessed by the PTSD Checklist for the DSM-5 (PCL-5) [25] and depression severity was assessed by the Geriatric Depression Score (GDS) score [26]. Severity of sleep difficulties was assessed using PSQI-GLOB. Severity of chronic pain was primarily measured by the Brief Pain Inventory (BPI) [27] short form as a summary score of item 3–6. Fatigue severity was evaluated by the self-reported degrees about how much fatigue limited daily activity over the past 6 months. Neurological symptom severity was assessed based on the NSI scoring.
Table 2.
Clinical assessments of GWI-related symptoms and definition of severity levels.
| Category | Scoring criteria | Category or range | Severity levels | Description |
|---|---|---|---|---|
| Sleep | PSQI-global (PSQI-GLOB) | 0 (none)–21 (worst) | Approximate cut-offsc: 0 = none, 1–5 = mild, 6–10 = moderate, 11–16 = severe, 16–21 = extreme | A 19-item, self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval |
| Pain | Brief Pain Inventory (BPI) Short Form sum score (BPI-sum) | 0 (none)–40 (worst) | Averaged score: 0 = none, 1–3 = mild, 4–6 = moderate, 7–8 = severe, 9–10 = extreme | Summary of 4 pain items (each 0–10): pain now, usual pain, best and worst pain during last week |
| Fatigue | CDC Chronic Fatigue Syndrome Inventory | 0 (none)–4 (worst) | 0 = 0% normal overall activity: no limitations, 1 = 1%–25%, 1 = 26%–49%, 2 = 50% activity level reduced to 50% of normal, 3 = 51%–75%, 3 = 76%–99%, 4 = 100% totally disabled bedridden constantly. | Self-report: to what degree has your fatigue limited your daily activity over the past 6 months? |
| Neurologic | Neurobehavioral Symptom Inventory (NSI) | 0 (none)–88 (worst) | Averaged score: 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = extreme | 22-item self-report measure of post-concussive symptoms |
| PTSD | Checklist for DSM-5 (PCL-5)a | 0 (none)–80 (worst) | Approximate cut-offsc: 0–6 = none; 7–20 = mild; 21–48 = moderate; 49–65 = severe; 66–80 = extreme | The severity levels were determined by psychiatrist based on PCL-5. |
| Depression | Geriatric Depression Scale (GDS)b | 0 (none)–15 (worst) | 0–4 = none; 5–8 = mild; 9–11 = moderate; 12–15 = severe | Consists of 15-item questions indicate the presence of depression |
| TBI | VA/DoD Clinical Practice Guideline | None: no event of TBI. Mild: TBI with normal brain scan and 0–30 min loss of consciousness (LOC). Moderate: TBI with normal or abnormal brain scan and LOC up to 24 h. |
Positive TBI: objective criteria for brain injury or head blast. |
In a few (N = 11) veterans who did not obtain a PCL-5 evaluation, PTSD severity level was determined by Clinician-Administered PTSD Scale (CAPS).
In a few (N = 13) veterans who did not obtain a GDS evaluation, depression severity level was determined by Beck Depression Inventory-II (BDI-II) scores.
The PCL-5 and PSQI-global has not to date been studied in terms of severity (no, mild, moderate, severe, extreme) cut-off scores.
2.3. MRI acquisition
MRI imaging data were acquired at the Palo Alto VA using a 3 T General Electric (GE) Discovery MR750 scanner with an eight channel, GE head coil. As part of the WRIISC clinical protocol, structural and diffusion MRI sequences were acquired for each subject: 1) high-resolution structural T1-weighted volume using a 3-dimensional spoiled-gradient recalled acquisition (3D-SPGR) in steady state (136 sagittal slices, TR/TE = 7.3/3.0 ms; flip angle = 11°; field of view = 250 mm; slice thickness = 1.2 mm with 0.6 between slices; acquisition matrix = 256 × 256; number of excitations = 1.0; resolution = 1.05 mm × 1.05 mm × 0.60 mm); 2) 57 axial slices of diffusion weighted images (DTI) were acquired using a 2D single-shot EPI sequence with TR/TE = 6600/84.1 ms, 1 × 1 × 2.5 mm3 resolution. Eight volumes without diffusion gradients (b0) and 2 scans using 30-directional diffusion-weighting gradients (b-value = 1000s/mm2) were obtained and combined for a 60-directional sequence. Subsequent tensor fitting produced maps of FA, MD, RD and AD.
2.4. MRI processing and ROI extraction
The acquired 3-dimensional T1WI data were processed with cortical reconstruction, noise and distortion correction, volume segmentation, intensity normalization, and parcellations using the latest released FreeSurfer stable version 6.0.0 software package (http://surfer.nmr.mgh.harvard.edu/) [28,29]. A FreeSurfer standard parcellation atlas (the Desikan-Killiany atlas) with anatomically labeled regions of interest (ROIs) was assigned to each individual T1WI to extract quantitative volumes in parcellated brain regions. For volumetric measures, the estimated total intracranial volume (eTIV), total cortical and subcortical volumes, individual subcortical volumes (hippocampus, amygdala, accumbens area, thalamus, caudate, putamen, pallidum, cerebellar cortex) (Fig. 1A), the whole brainstem volume, and volume of three brainstem subregions (midbrain, pons, medulla) were extracted (Fig. 1B).
Fig. 1.
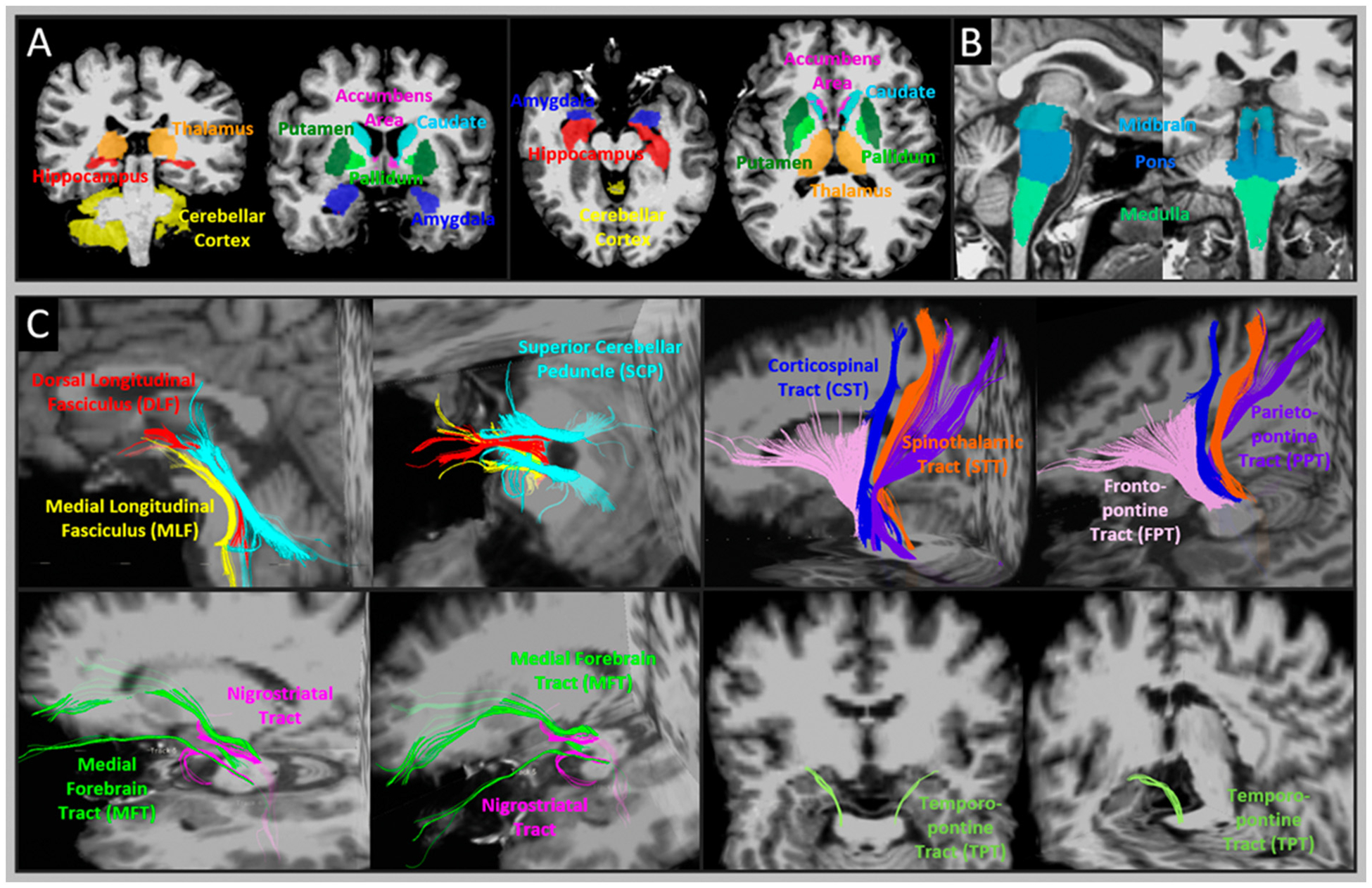
Illustration of ROI where the volumes of the brainstem nuclei and diffusion metrics were measured. A: example of 8 subcortical ROIs (hippocampus, amygdala, accumbens area, thalamus, caudate, putamen, pallidum, cerebellar cortex) and B: 3 brainstem ROIs (medulla, pons and midbrain) that volumetric measures were taken. C: example of 10 bilateral pairs of brainstem tract-of-interest, including dorsal longitudinal fasciculus (DLF), medial longitudinal fasciculus (MLF), superior cerebellar peduncle (SCP), nigrostriatal tract (NST), medial forebrain tract (MFT), corticospinal tract (CST), spinothalamic tract (STT), frontopontine tract (FPT), parietopontine tract (PPT), and temporopontine tract (TPT).
The DTI data was processed using a recently developed approach [15] for automatic diffusion tensor tractography and microstructural measures of brainstem tracts. This approach includes: (1) an advanced image processing pipeline that rigorously resolves the distortions which occur particularly in the brainstem area and improves accuracy of transferring individual maps of FA and diffusivities to the Montreal Neurological Institute (MNI) common space. (2) An atlas (template) of 10 bilateral pairs of the major brainstem tracts was built in MNI space. The atlas was generated by averaging the data of 17 veterans whose brainstem tracts were tracked manually in individual space and transformed to MNI space. Thereafter, the averaged tract map was thresholded at a level of 0.6, corresponding to at least 60% of probability (i.e., the probability of the tractographic streamlines) of sharing the same anatomy. The resultant binary tracts are referred to here as the brainstem tract atlas.
In this study, each individual DTI volume was non-linearly registered to MNI space through our in-lab image processing pipeline, and the transformation parameters were recorded. Thereafter, the binary atlas of the 10-pairs of the major brainstem tracts was transformed from MNI space to individual’s native space applying inverse transformation parameters. In individual spaces, binary maps of the following tracts were determined as tract-of-interest (Fig. 1C): the medial longitudinal fasciculus (MLF), dorsal longitudinal fasciculus (DLF), superior longitudinal fasciculus (SCP), medial forebrain tract (MFT), nigrostriatal tract (NST), corticospinal tract (CST), spinothalamic tract (STT), frontopontine tract (FPT), parietopontine tract (PPT), and temporopontine tract (TPT). According to our previous study [15], the anatomy of these fiber tracts is largely consistent with population-based human brainstem connectome atlases of similar brainstem tracts [17,30]. Diffusion microstructural indices, including FA, MD, RD and AD, were extracted from each pair of bilateral tracts-of-interest.
2.5. Statistics
All statistics were performed using SPSS (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). Generally, age, sex and eTIV are known to affect volumetric and diffusion measures. In addition, previous studies [31,32] demonstrated that depression or PTSD also significantly affects DTI measures. Thus, key confounding factors that have major effects on imaging measures as well as those interacting with imaging and sleep/pain correlations need to be adjusted in statistical analyses. Prior to testing for any imaging and clinical correlations, a multivariate analysis of variance (MANOVA) model (see Supplementary Table S1) was used for testing 18 confounding factors to determine the key factors (except for sleep and pain) that significantly affect MRI measures and the relationships between MRI and sleep/pain symptomatology. In this MANOVA model, volumes of 11 bilateral subcortical regions (see Fig. 1B and C), or diffusion metrics of the 10 bilateral brainstem tracts (see Fig. 1A) were the multiple dependent variables, each clinical diagnosis and severity scoring, and their interaction with sleep or pain was the independent variable. Independent variables that appeared to have a significant effect in the MANOVA model were used as confounding factors and were necessary to be controlled. Relationships between each MRI measure and sleep/pain severity were tested using Pearson’s correlation model. In addition, partial correlation models were used to control for the prior determined confounding factors. For all tests (maximum test No. = 330), the uncorrected significance was p < 0.05, and the critical significance was p < 0.025 adjusting with the Benjamini-Hochberg [33] false discovery rate (FDR).
3. Results
3.1. Demographic and clinical characteristics
Table 3 listed the demographic and clinical diagnoses or screening results of the 90 GWI veterans. All veterans met the CDC diagnostic criteria of GWI, while 49% of the veterans also met the Kansas exclusion criterion. The majority of the veterans presented three major symptoms, including poor sleep quality (99%), chronic pain (99%), neurological impairments (97%). A large amount of the veterans also presented other combat-related chronic symptoms or illnesses, such as chronic fatigue (70%), IBS (70%), breathing difficulties (71%), PTSD (71%), depression (62%), and combat-related TBI (68%). Furthermore, veterans with TBI had marginally higher prevalence of PTSD (F = 3.91, p = 0.05), were mostly male (F = 3.97, p = 0.05), and had more sleep difficulty (F = 3.67, p = 0.06).
Table 3.
Demographic and clinical diagnosis/screening characteristics of the 90 GWI veterans.
| Number of positive | Mean (±SD) or percent | |
|---|---|---|
| Age (y) | 90 | 50.2 ± 5.3 |
| Gender | ||
| Male | 78 | 87% |
| Female | 12 | 13% |
| Years of education (y) | 90 | 14.2 ± 2.0 |
| Ethnicity | ||
| Caucasian | 67 | 74% |
| non-Caucasian | 14 | 16% |
| Mixed (multiracial) | 9 | 10% |
| Combat duration (m) | 90 | 6.5 ± 3.1 |
| Combat expose scale | 90 | 10.2 ± 7 |
| GWI diagnosis | ||
| Meet CDC/CMI criteria | 90 | 100% |
| Meet Kansas criteria | 90 | 100% |
| Meet Kansas exclusion | 44 | 49% |
| TBI/Head Blast | 61 | 68% |
| PTSD | 64 | 71% |
| Depression | 56 | 62% |
| Chronic fatigue | 63 | 70% |
| Sleep difficulty | ||
| Poor sleep quality | 89 | 99% |
| Probable clinical insomnia | 67 | 74% |
| Obstructive sleep apnea (OSA) | 56 | 60% |
| Pain symptoms | 89 | 99% |
| Neurologic symptoms | 87 | 97% |
| Positive IBS screens | 63 | 70% |
| Breathing difficulties | 65 | 72% |
3.2. GWI symptom severities
Table 4 lists the proportion of the GWI veterans with each symptom severity. The majority of the GWI veterans presented with severe or greater level of poor sleep quality, moderate to severe levels of pain, and fatigue and PTSD, mild to moderate levels of neurological deficits and depression, and mostly mild TBI. In addition, severities of the 6 GWI symptoms significantly correlated with each other. Specifically, greater sleep difficulty was significantly associated with more severe pain, fatigue, neurological problems, PTSD and depression. Increased pain was significantly associated with poorer sleep quality and more severe neurological problems. Table 5 presents the correlations between the severity of each featured GWI symptom.
Table 4.
Symptomatic severities of the 90 GWI veterans.
| Symptom severity levels | |||||
|---|---|---|---|---|---|
| % of none | % of mild | % of moderate | % of severe | % of extreme | |
| Sleep | 0% | 6% | 19% | 44% | 31% |
| Pain | 0% | 14% | 46% | 39% | 1% |
| Fatigue | 1% | 18% | 10% | 68% | 2% |
| Neurologic | 2% | 38% | 44% | 15% | 1% |
| PTSD | 13% | 12% | 40% | 22% | 12% |
| Depression | 21% | 32% | 23% | 23% | N/A |
| TBI | 32% | 60% | 8% | 0% | N/A |
Table 5.
Partial correlation among GWI symptom severity (partial correlation controlling for age and sex).
| Pearson’s correlation | Sleep | Pain r (p) |
Fatigue r (p) |
Neurologic r (p) |
PTSD r (p) |
Depression r (p) |
|---|---|---|---|---|---|---|
| Sleep | – | 0.252 (0.021) | 0.300 (0.006) | 0.632 (0.000) | 0.395 (0.000) | 0.422 (0.000) |
| Pain | – | 0.114 (0.302) | 0.329 (0.002) | 0.110 (0.319) | 0.028 (0.797) | |
| Fatigue | – | 0.408 (0.000) | 0.301 (0.005) | 0.314 (0.004) | ||
| Neurologic | – | 0.515 (0.000) | 0.469 (0.000) | |||
| PTSD | – | 0.426 (0.000) | ||||
| Depression | – |
Bold: significant after adjusting with False Discovery Rate (FDR).
TBI severity was not included in the correlation test because its score ranges very little.
The relationship between each confounding factor and ROI measures is summarized in Appendix (Supplementary Table S1). Age, and eTIV had significant effects on both volumetric and diffusion measures, and sex difference significantly affected diffusion measures. Six confounding factors, including age, sex, eTIV, years of education, severity of neurological symptoms, and depression, were identified that may affect the imaging measures and imaging correlations with sleep or pain. In brief, age, eTIV and sex had significant effects on brain imaging measures. Age also significantly affected the sleep-imaging relationship. Years of education, neurological severity, depression severity and eTIV interacted with the pain-imaging relationship. Considering these confounding factors, the correlation tests between sleep/pain and MRI measures were performed in the following three steps: 1) Pearson’s correlation coefficient without controlling for confounding factors. 2) Partial correlation tests after controlling for age, sex and eTIV. 3) Partial correlation tests between sleep and each MRI measure after controlling for age, sex, eTIV, years of education, severities of neurological and depressive symptoms.
3.3. Relationship between sleep/pain symptoms and MRI measures
The 3-step correlation tests were performed between MRI measures (including volumetric measures in 15 bilateral ROIs and microstructural measures in 10 bilateral brainstem tracts) and GWI symptom severity. Table 6 shows the relationships between sleep/pain severity and MRI measures in ROIs where significant correlations were found. Increased sleep difficulty significantly correlated (1st - step) with greater volume loss in the pallidum (r = −0.316, p = 0.003) and the whole brainstem (r = −0.298, p = 0.005), including midbrain (r = −0.294, p = 0.005), pons (r = −0.280, p = 0.008) and medulla (r = −0.260, p = 0.014). Increased sleep difficulty also significantly correlated with reduced FA in the NST (r = −0.390, p = 0.000), MFT (r = −0.317, p = 0.002) and DLF (r = −0.267, p = 0.011). All statistics remained significant after controlling for age, sex and eTIV (2nd - step). FA reduction of the NST and MFT remained the largest correlation with sleep difficulties after controlling for all potential confounding factors (3rd - step). On the other hand, increased pain intensity significantly correlated (1st - step) with reduced FA in the DLF (r = −0.302, p = 0.004) and NST (r = −0.242, p = 0.022). After controlling for age, sex and eTIV (2nd - step), and controlling for all potential confounding factors (3rd - step), only correlations between pain severity and FA of the DLF remained significant. Fig. 2 depicts scatter plots of the most robust relationships: Fig. 2A: PSQI-GLOB and volumes of the pallidum and the brainstem subregions; Fig. 2B: PSQI-GLOB and FA of the NST, MFT and DLF; Fig. 2C: BPI-sum and FA of the DLF and NST.
Table 6.
Correlations between sleep/pain severities and MRI measures with and without controlling for confounding factors.
| ROI | Measure | Sleep (PSQI-GLOB) | Pain (BPI-sum) | ||||
|---|---|---|---|---|---|---|---|
| Control for none | Control for 3-factorsa | Control for 6-factorsb | Control for none | Control for 3-factorsa | Control for 6-factorsb | ||
| Total subcortices | Volume | −0.226* | −0.262* | −0.172 | −0.137 | −0.144 | −0.109 |
| Hippocampus | Volume | −0.211* | −0.192 | −0.114 | −0.080 | −0.049 | −0.026 |
| Amygdala | Volume | −0.213* | −0.181 | −0.159 | −0.019 | 0.017 | 0.060 |
| Pallidum | Volume | −0.316** | −0.312** | −0.233* | −0.156 | −0.146 | −0.083 |
| Total Brainstem | Volume | −0.298** | −0.325** | −0.191 | −0.147 | −0.149 | −0.106 |
| Midbrain | Volume | −0.294** | −0.330** | −0.202 | −0.190 | −0.232* | −0.201 |
| Pons | Volume | −0.280** | −0.285** | −0.176 | −0.114 | −0.093 | −0.070 |
| Medulla | Volume | −0.260* | −0.245* | −0.142 | −0.154 | −0.151 | −0.133 |
| DLF | FA | −0.267* | −0.268* | −0.194 | −0.302** | −0.290** | −0.252* |
| SCP | FA | −0.230* | −0.198 | −0.116 | −0.236* | −0.236* | −0.204 |
| NST | FA | −0.390** | −0.400*** | −0.303** | −0.242* | −0.224* | −0.194 |
| MFT | FA | −0.317** | −0.304** | −0.298** | −0.169 | −0.154 | −0.155 |
Bold: significant after adjusting with False Discovery Rate (FDR).
Abbreviations: MLF = medial longitudinal fasciculus, DLF = dorsal longitudinal fasciculus, SCP=superior cerebellar peduncle, NST = Nigrostriatal tracts, MFT = medial forebrain tract.
Significant correlations:
p < 0.05.
p < 0.01.
p < 0.001.
Partial correlation controlling for 3-factors: age, sex and eTIV.
Partial correlation tests between sleep and each MRI measures after controlling for 6-factors: age, sex, eTIV, years of education, severities of neurological and depressive symptoms.
Fig. 2.

Scatter plots of relationships between standardized Z score of MRI measures and sleep/pain severities that were statistically significant. A) Significant correlations between PSQI-GLOB and volumes of the pallidum and 3 brainstem ROIs (midbrain, pons and medulla). B) Significant correlations between PSQI-GLOB and FA of the nigrostriatal tract (NST), medial forebrain tract (MFT) and the dorsal longitudinal fasciculus (DLF). C) Significant correlations between BPI-sum and FA of the DLF and NST. Each neuroimaging measure is presented as standardized Z score to be comparable with different measurement scalars. The r and p values indicate the significance of partial correlation after controlling for age, sex and eTIV.
In addition, no significant correlation was observed between sleep/pain severity and volumetric measures in other brain regions, nor significant correlations between sleep/pain severity and microstructural/non-FA measures in other tracts including MLF, CST, STT, FPT, PPT and TTP.
Complementary results detailing partial correlations between every GWI-related symptomatic severity and each MRI measure, after controlling for age, sex and eTIV, are shown in Appendix (Supplementary Table S2).
4. Discussion
We investigated the correlations between the severities of two common GWI symptoms (sleep disturbances and pain intensity) and MRI measures of brainstem volumes and white matter tract integrity in veterans deployed in the 1990–1991 initial time-period of the Persian Gulf War. We found that in veterans with GWI, severity of poor sleep quality significantly correlated with atrophy of the brainstem (including midbrain, pons, and medulla) and the pallidum, and reduced fractional anisotropy in the nigrostriatal, medial forebrain and the dorsal longitudinal tracts. By contrast, pain intensity was significantly correlated with fractional anisotropy primarily in the dorsal longitudinal fasciculus of the brainstem. These findings, which are suggestive of brainstem tissue injury, are located in brain regions thought to underly the sleep-wake cycles and pain control mechanisms, suggesting a key role of brainstem damage in GWI symptomatology.
4.1. Brainstem imaging and sleep difficulties
Poor sleep difficulties and insomnia are frequently reported symptoms in GWI. There is evidence that GWI is associated with atrophy of the brainstem [8,9]. Results from the present study and other MRI studies extend evidence for GWI-specific brainstem damage by linking poor sleep quality with structural abnormality of the brainstem. In other relevant areas, volumetric MRI studies report that significant volume loss in the brainstem region is associated with sleep disturbances in patients with Alzheimer’s disease [34], and obstructive sleep apnea [35,36]. The mechanisms underlying the relationship between brainstem volume and subjective sleep quality could be explained by loss of neurons of aminergic and/or cholinergic nuclei that are crucial for sleep regulation [37]. Structural integrity (i.e., volume) of these brainstem neurons, such as the dorsal raphe (serotonin), locus coeruleus (noradrenaline), ventral tegmental area (dopamine), and dorsolateral tegmentum (acetylcholine), have been strongly implicated in sleep quality [38]. In additional studies, lesions in the brainstem were found to be associated with broad sleep difficulties or insomnia [39,40]. Further, brainstem volume loss is pronounced in civilians with OSA [35,41], which could also be explained by the role of brainstem structures in maintaining the patency of the upper airway. Taken together, these findings suggest that the macrostructure of the brainstem may play a critical role in sleep maintenance and quality.
We also found that sleep quality was negatively correlated with atrophy in the pallidum. Although the involvement of the globus pallidus in sleep regulation has not been fully explored, a few recent studies have suggested that pallidal atrophy or lesions might be involved in the control of sleep -wake cycle [42,43]. Furthermore, recent evidence demonstrates relationships between pallidal atrophy and sleep behavior disorders and prodromal Parkinson’s diseases [44,45]. Together with the present study, this evidence suggests that the globus pallidus could also be important in the mechanisms of sleep–wake regulation through the adenosine and dopamine transmitter systems [46].
In addition to brainstem atrophy, the current study showed that the FA of two major brainstem tracts (NST and MFT) is strongly correlated with sleep quality. There have been other reports of sleep difficulties being negatively correlated with microstructural alterations in connections/circuits linking the brainstem and basal ganglia to the medial frontal area [40,47]. These findings are supported by the functional linkages between the dorsal brainstem pathways and sleep-wake status. For example, FA reduction of the NST (fiber connection linking the ventral tegmental area – substantia nigra – basal ganglia) and MFT (fiber connection linking the PAG – thalamus – ventromedial prefrontal cortex) may suggest a disturbance of dopaminergic and GABAergic pathways participating in the ascending reticular activating system (ARAS) [48]. FA reduction in DLF also correlated (but markedly less than NST and MFT) with PSQI. The DLF (fiber connection links dorsal spinal cord – locus coeruleus – periaqueductal gray area – hypothalamus), which includes primarily pathways involving the pedunculopontine nuclei (acetylcholine system), locus coeruleus (noradrenaline system), and raphe nuclei (serotonin system), as a part of ARAS, which all join the dopaminergic and GABAergic pathways, also seem to play a function in modulating a normal sleep-wake cycle [49,50]. Together, these findings suggest that damage or degeneration of the fiber projections in the brainstem-frontal pathways may lead to global sleep difficulties in GWI.
4.2. Brainstem imaging and pain intensity
In the present study, no macrostructural volume change was related to pain intensity, whereas a robust correlation between chronic overall pain (i.e., BPI-sum) and imaging was observed with the FA reduction in the DLF. This result confirmed by our previous findings in 17 veterans showing a significant relationship between lower FA of the DLF and pain intensities including current pain and overall pain in the past month [15]. A recent study showed that FA reduction in brain fibers connecting through the periaqueductal gray area is associated with higher pain sensitivity after heat stimuli [16]. The DLF tract is an anatomical fiber connection beginning in the hypothalamus, which projects through the periaqueductal gray matter down to the dorsal spinal cord. This fiber tract is believed to represent a descending analgesic pathway [51,52] – the descending pain system, which plays a critical role in modulating the perception and experience of pain [53–55]. In the context of GWI, the chronic pain may result from structural deficits of the descending pain pathways (primarily fitting with the anatomy of the DLF). In summary, defining the relationship between DLF and chronic pain in these studies could substantially advance our understanding of the mechanisms underlying pain and provide investigators a range of possible targets to treat GWI.
4.3. Brainstem imaging and GWI symptomatology
A noteworthy clinical feature in our GWI cohort was that the severity of sleep problems was associated with the degree of chronic pain, chronic fatigue, chronic neurological deficits, PTSD and depression in parallel, consistent with reports from veterans and civilians [56–58]. Observations have [59], at least partly, attributed this psychological, somatic, and cognitive symptom complex to the problems of tissue injury or damage of the central neuron system, where the cholinergic (cognitive/attention/mnemonic regulation), serotonergic (stress/vegetative regulation), dopaminergic (energy expenditure regulation), and noradrenergic (attention, mood, arousal, and stress related) neurotransmitter systems are dysfunctional. The neural mechanisms through which all of these neurotransmitter systems are managed involve brainstem structures, including the reticular nuclei (raphe nuclei, locus coeruleus, periaqueductal gray area, hypothalamus, ventral tegmental area, substantia nigra, accumbens nuclei, pedunculopontine nuclei, medial lemniscus, etc.) and related pathways (e.g., MLF, DLF, NST, MFT, SCP) that carry the axons with these neurotransmitters from reticular nuclei to their corresponding subcortical and cortical projection areas such as basal ganglia, hippocampus and amygdala, and thalamus, as well as medial and orbitofrontal cortex. This neuroanatomy, together with the findings of the current study, suggest that the GWI-related symptom complex may be due to tissue injury in specific brainstem structures.
Interestingly, pair-wise correlations between each individual GWI symptom and neuroimaging measures suggested that macrostructural volume loss in the brainstem, pallidum and hippocampus were involved in sleep and neurocognitive psychiatric dysfunctions, whereas microstructural damages in brainstem tracts, including NST, MFT, DLF and FPT were mainly involved in sleep, pain and fatigue problems. These patterns of involvement in different tissue-types suggests that some GWI symptoms result from neuronal damage in the brainstem nuclei centers, while others may be caused by injury to brainstem circuits.
4.4. Limitations
A number of limitations of this study need to be mentioned: first, this study lacks of comprehensive sleep assessment. The global PSQI is a broad measure which indicates poor sleep quality but does not identify the source or specific nature of sleep disturbance. Thus, our study cannot provide information about the specific dimensions of sleep-wake which are related to neuroimaging (e.g., sleep duration, sleep efficiency, excessive daytime sleepiness) nor whether GWI veterans meet criteria for specific sleep-wake disorders (e.g., insomnia disorder, obstructive sleep apnea, circadian rhythm sleep-wake disorder, nightmare disorder, REM sleep behavior problems). Second, this study did not investigate specific cortical regions, since brainstem and subcortical structures are thought to have the most important roles in regulating sleep/pain functioning. Future imaging analyses are needed to examine possible damage of other cerebral structural changes in relation to GWI symptomatology. Lastly, the approach using a-priori parcellations or predetermined tracts may not be sensitive to regional findings that do not correspond to ROI boundaries. Whole-brain or voxel-wise analysis is an alternative option to examine the possibility of missed foci of volume loss, cortical thinning, and microstructural breakdowns. However, advanced image processing procedures that overcome post-processing errors, as well as brainstem-specific voxel-wise analysis algorithms need to be developed for this purpose.
5. Conclusion
This study suggests that the combination of poor sleep quality and chronic pain in GWI is related to specific microstructural and macrostructural brainstem deficits. The brainstem mechanisms modulating GWI symptomatology may further our understanding of the pathophysiology of GWI and lead to discovery of etiological factors underlying this condition. This may promote the development of effective treatment strategies, including behavioral [60] and pharmacologic interventions that target sleep pathways originating in the brainstem [61], and descending pain modulation pathways [62]. The present findings demonstrate a potential set of biomarkers in GWI for monitoring syndrome progression and assessing treatment effects.
Supplementary Material
Acknowledgement
The authors would like to thank all veterans for volunteering to participate in this project.
Without their generous support this research would not have been possible. The authors also thank Ms. Stacy Moeder for administrating the California War Related Illness and Injury Study Center (WRIISC-CA) research programs and Dr. Miguel T. Robinson for managing the WRIISC-CA database of veterans’ self-report questionnaires, Ms. Halee N. Staggs for coordinating the study.
Funding
This study is supported by the Department of Veterans Affairs, Office of Academic Affiliations, California War Related Illness and Injury Study Center (WRIISC-CA) fellowship program. This study is also supported by the VA Clinical Science Research and Development (CSR&D) grant entitled “The role of the brain stem in GWVI pathology” (1 I01 CX002182-01). This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Palo Alto Health Care System.
Footnotes
CRediT authorship contribution statement
Yu Zhang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Andrei A. Vakhtin: Software, Validation, Investigation, Writing – review & editing. Jessica Dietch: Validation, Investigation, Writing – review & editing. Jennifer S. Jennings: Investigation, Resources, Writing – review & editing. Jerome A. Yesavage: Investigation, Resources, Writing – review & editing. J. David Clark: Investigation, Resources, Writing – review & editing. Peter J. Bayley: Conceptualization, Supervision, Writing – review & editing. J. Wesson Ashford: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. Ansgar J. Furst: Conceptualization, Methodology, Software, Data curation, Writing – review & editing, Supervision, Funding acquisition, Project administration.
Declaration of competing interest
The authors declare no financial or other conflicts of interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lfs.2021.119724.
References
- [1].Kerr KJ, Gulf War illness: an overview of events, most prevalent health outcomes, exposures, and clues as to pathogenesis, Rev. Environ. Health 30 (4) (2015) 273–286, 10.1515/reveh-2015-0032. [DOI] [PubMed] [Google Scholar]
- [2].White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, et al. , Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment, Cortex 74 (2016) 449–475, 10.1016/j.cortex.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaldt BL, et al. , Chronic multisymptom illness affecting Air Force veterans of the Gulf War, JAMA 280 (11) (1998) 981–988, 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- [4].Steele L, Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service, Am. J. Epidemiol 152 (10) (2000) 992–1002, 10.1093/aje/152.10.992. [DOI] [PubMed] [Google Scholar]
- [5].Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ, The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research, Psychiatry Res 28 (2) (1989) 193–213, 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [6].Buskila D, Fibromyalgia, chronic fatigue syndrome, and myofascial pain syndrome, Curr. Opin. Rheumatol 13 (2) (2001) 117–127, 10.1097/00002281-200103000-00005. [DOI] [PubMed] [Google Scholar]
- [7].Escalante A, Fischbach M, Musculoskeletal manifestations, pain, and quality of life in Persian Gulf War veterans referred for rheumatologic evaluation, J. Rheumatol 25 (11) (1998) 2228–2235. [PubMed] [Google Scholar]
- [8].Christova P, James LM, Engdahl BE, Lewis SM, Carpenter AF, Georgopoulos AP, Subcortical brain atrophy in Gulf War Illness, Exp. Brain Res 235 (9) (2017) 2777–2786, 10.1007/s00221-017-5010-8. [DOI] [PubMed] [Google Scholar]
- [9].Zhang Y, Avery T, Vakhtin AA, Mathersul DC, Tranvinh E, Wintermark M, Massaband P, Ashford JW, Bayley PJ, Furst AJ, Brainstem atrophy in Gulf War Illness, Neurotoxicology 78 (2020) 71–79, 10.1016/j.neuro.2020.02.006. [DOI] [PubMed] [Google Scholar]
- [10].Basser PJ, Pierpaoli C, Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996, J. Magn. Reson 213 (2) (2011) 560–570, 10.1016/j.jmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- [11].O’Donnell LJ, Westin CF, An introduction to diffusion tensor image analysis, Neurosurg. Clin. N. Am 22 (2) (2011) 185–196, viii, 10.1016/j.nec.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Basser PJ, Jones DK, Diffusion-tensor MRI: theory, experimental design and data analysis a technical review, NMR Biomed 15 (7–8) (2002) 456–467, 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- [13].Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G, Diffusion tensor MR imaging of the human brain, Radiology 201 (3) (1996) 637–648, 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- [14].Wheeler-Kingshott CA, Cercignani M, About “axial” and “radial” diffusivities, Magn. Reson. Med 61 (5) (2009) 1255–1260, 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Y, Vakhtin AA, Jennings JS, Massaband P, Wintermark M, Craig PL, Ashford JW, Clark JD, Furst AJ, Diffusion tensor tractography of brainstem fibers and its application in pain, PLoS One 15 (2) (2020), e0213952, 10.1371/journal.pone.0213952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Geisler M, Rizzoni E, Makris N, Pasternak O, Rathi Y, Bouix S, Herbsleb M, Bar KJ, Weiss T, Kikinis Z, Microstructural alterations in medial forebrain bundle are associated with interindividual pain sensitivity, Hum. Brain Mapp (2020), 10.1002/hbm.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Edlow BL, Takahashi E, Wu O, Benner T, Dai G, Bu L, Grant PE, Greer DM, Greenberg SM, Kinney HC, et al. , Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders, J. Neuropathol. Exp. Neurol 71 (6) (2012) 531–546, 10.1002/hbm.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Y, Wu IW, Buckley S, Coffey CS, Foster E, Mendick S, Seibyl J, Schuff N, Diffusion tensor imaging of the nigrostriatal fibers in Parkinson’s disease, Mov. Disord 30 (9) (2015) 1229–1236, 10.1002/mds.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pillai RLI, Huang C, LaBella A, Zhang M, Yang J, Trivedi M, Weissman M, McGrath P, Fava M, Kurian B, et al. , Examining raphe-amygdala structural connectivity as a biological predictor of SSRI response, J. Affect. Disord 256 (2019) 8–16, 10.1016/j.jad.2019.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Management of Concussion/m, TBIWG: VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury, J. Rehabil. Res. Dev 46 (6) (2009) CP1–68. [PubMed] [Google Scholar]
- [21].Wagner D, Nisenbaum R, Heim C, Jones JF, Unger ER, Reeves WC, Psychometric properties of the CDC Symptom Inventory for assessment of chronic fatigue syndrome, Popul. Health Metrics 3 (2005), 8, 10.1186/1478-7954-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cicerone KD, Kalmar K, Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury, J. Head Trauma Rehabil 10 (3) (1995) 1–17. [Google Scholar]
- [23].Dang J, Ardila-Hani A, Amichai MM, Chua K, Pimentel M, Systematic review of diagnostic criteria for IBS demonstrates poor validity and utilization of Rome III, Neurogastroenterol. Motil 24 (9) (2012) 853–e397, 10.1111/j.1365-2982.2012.01943.x. [DOI] [PubMed] [Google Scholar]
- [24].Group TMoCIDaOSAW, VA/DoD Clinical Practice Guideline for Management of Chronic Insomnia Disorder and Obstructive Sleep Apnea, 2019, 10.7326/M19-3575. [DOI] [Google Scholar]
- [25].Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL, The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation, J. Trauma. Stress 28 (6) (2015) 489–498, 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- [26].Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO, Development and validation of a geriatric depression screening scale: a preliminary report, J. Psychiatr. Res 17 (1) (1982) 37–49, 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- [27].Cleeland CS, Ryan KM, Pain assessment: global use of the Brief Pain Inventory, Ann. Acad. Med. Singap 23 (2) (1994) 129–138. [PubMed] [Google Scholar]
- [28].Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM, Sequence-independent segmentation of magnetic resonance images, Neuroimage 23 (Suppl. 1) (2004) S69–S84, 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- [29].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. , Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain, Neuron 33 (3) (2002) 341–355, 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- [30].Meola A, Yeh FC, Fellows-Mayle W, Weed J, Fernandez-Miranda JC, Human connectome-based tractographic atlas of the brainstem connections and surgical approaches, Neurosurgery 79 (3) (2016) 437–455, 10.1227/NEU.0000000000001224. [DOI] [PubMed] [Google Scholar]
- [31].van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, Baune BT, Brak IV, Carballedo A, Connolly CG, et al. , White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group, Mol. Psychiatry 25 (7) (2020) 1511–1525, 10.1038/s41380-019-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ju Y, Ou W, Su J, Averill CL, Liu J, Wang M, Wang Z, Zhang Y, Liu B, Li L, et al. , White matter microstructural alterations in posttraumatic stress disorder: an ROI and whole-brain based meta-analysis, J. Affect. Disord 266 (2020) 655–670, 10.1016/j.jad.2020.01.047. [DOI] [PubMed] [Google Scholar]
- [33].Benjamini Y, Hochberg Y, Controlling the false discovery rate: a practical and powerful approach to multiple testing, J. R. Stat. Soc. Ser. B Methodol 57 (1) (1995) 289–300. [Google Scholar]
- [34].Lee JH, Jung WS, Choi WH, Lim HK, Aberrant brain stem morphometry associated with sleep disturbance in drug-naive subjects with Alzheimer’s disease, Neuropsychiatr. Dis. Treat 12 (2016) 2089–2093, 10.2147/NDT.S114383 (eCollection 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Licis A, Davis G, Eisenstein SA, Lugar HM, Hershey T, Sleep disturbances in Wolfram syndrome, Orphanet. J. Rare Dis 14 (1) (2019), 188, 10.1186/s13023-019-1160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Celle S, Peyron R, Faillenot I, Pichot V, Alabdullah M, Gaspoz JM, Laurent B, Barthelemy JC, Roche F, Undiagnosed sleep-related breathing disorders are associated with focal brainstem atrophy in the elderly, Hum. Brain Mapp 30 (7) (2009) 2090–2097, 10.1002/hbm.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Grinberg LT, Rueb U, Heinsen H, Brainstem: neglected locus in neurodegenerative diseases, Front. Neurol 2 (2011) 42, 10.3389/fneur.2011.00042 (eCollection 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hauw JJ, Hausser-Hauw C, De Girolami U, Hasboun D, Seilhean D, Neuropathology of sleep disorders: a review, J. Neuropathol. Exp. Neurol 70 (4) (2011) 243–252, 10.1097/NEN.0b013e318211488e. [DOI] [PubMed] [Google Scholar]
- [39].Salih F, Klingebiel R, Zschenderlein R, Grosse P, Acoustic sleep starts with sleep-onset insomnia related to a brainstem lesion, Neurology 70 (20) (2008) 1935–1937, 10.1212/01.wnl.0000312336.92028.9b. [DOI] [PubMed] [Google Scholar]
- [40].Kocevska D, Cremers LGM, Lysen TS, Luik AI, Ikram MA, Vernooij MW, Tiemeier H, Sleep complaints and cerebral white matter: a prospective bidirectional study, J. Psychiatr. Res 112 (2019) 77–82, 10.1016/j.jpsychires.2019.02.002. [DOI] [PubMed] [Google Scholar]
- [41].Bahia C, Pereira JS, Obstructive sleep apnea and neurodegenerative diseases: a bidirectional relation, Dement. Neuropsychol 9 (1) (2015) 9–15, 10.1590/S1980-57642015DN91000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lutkenhoff ES, Johnson MA, Casarotto S, Massimini M, Monti MM, Subcortical atrophy correlates with the perturbational complexity index in patients with disorders of consciousness, Brain Stimul 13 (5) (2020) 1426–1435, 10.1016/j.brs.2020.07.012. [DOI] [PubMed] [Google Scholar]
- [43].Qiu MH, Vetrivelan R, Fuller PM, Lu J, Basal ganglia control of sleep-wake behavior and cortical activation, Eur. J. Neurosci 31 (3) (2010) 499–507, 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rahayel S, Gaubert M, Postuma RB, Montplaisir J, Carrier J, Monchi O, Remillard-Pelchat D, Bourgouin PA, Panisset M, Chouinard S, et al. , Brain atrophy in Parkinson’s disease with polysomnography-confirmed REM sleep behavior disorder, Sleep 42 (6) (2019), 10.1093/sleep/zsz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rahayel S, Postuma RB, Montplaisir J, Bedetti C, Brambati S, Carrier J, Monchi O, Bourgouin PA, Gaubert M, Gagnon JF, Abnormal gray matter shape, thickness, and volume in the motor cortico-subcortical loop in idiopathic rapid eye movement sleep behavior disorder: association with clinical and motor features, Cereb. Cortex 28 (2) (2018) 658–671, 10.1093/cercor/bhx137. [DOI] [PubMed] [Google Scholar]
- [46].Lazarus M, Chen JF, Urade Y, Huang ZL, Role of the basal ganglia in the control of sleep and wakefulness, Curr. Opin. Neurobiol 23 (5) (2013) 780–785, 10.1016/j.conb.2013.02.001 (Epub 2013 Apr 24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Duning T, Deppe M, Brand E, Stypmann J, Becht C, Heidbreder A, Young P, Brainstem involvement as a cause of central sleep apnea: pattern of microstructural cerebral damage in patients with cerebral microangiopathy, PLoS One 8 (4) (2013), e60304, 10.1371/journal.pone.0060304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW, Control of sleep and wakefulness, Physiol. Rev 92 (3) (2012) 1087–1187, 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sakurai T, The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness, Nat. Rev. Neurosci 8 (3) (2007) 171–181, 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- [50].Schwartz MD, Kilduff TS, The neurobiology of sleep and wakefulness, Psychiatr. Clin. North Am 38 (4) (2015) 615–644, 10.1016/j.psc.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D, Neuroimaging of the periaqueductal gray: state of the field, Neuroimage 60 (1) (2012) 505–522, 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Willis WD, Westlund KN, Neuroanatomy of the pain system and of the pathways that modulate pain, J. Clin. Neurophysiol 14 (1) (1997) 2–31, 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL, Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study, J. Neurosci 26 (16) (2006) 4437–4443, 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ossipov MH, Dussor GO, Porreca F, Central modulation of pain, J. Clin. Invest 120 (11) (2010) 3779–3787, 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tracey I, Mantyh PW, The cerebral signature for pain perception and its modulation, Neuron 55 (3) (2007) 377–391, 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- [56].Plumb TR, Peachey JT, Zelman DC, Sleep disturbance is common among servicemembers and veterans of Operations Enduring Freedom and Iraqi Freedom, Psychol. Serv 11 (2) (2014) 209–219, 10.1037/a0034958. [DOI] [PubMed] [Google Scholar]
- [57].Aaron R, Noel M, Dudeney J, Wilson A, Holley A, Palermo T, The role of sleep quality on the relationship between posttraumatic stress symptoms and pain in women, J. Behav. Med 42 (5) (2019) 924–933, 10.1007/s10865-019-00016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Finan PH, Goodin BR, Smith MT, The association of sleep and pain: an update and a path forward, J. Pain 14 (12) (2013) 1539–1552, 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tillman GD, Calley CS, Green TA, Buhl VI, Biggs MM, Spence JS, Briggs RW, Haley RW, Hart J Jr., Kraut MA, Event-related potential patterns associated with hyperarousal in Gulf War illness syndrome groups, Neurotoxicology 33 (5) (2012) 1096–1105, 10.1016/j.neuro.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mathersul DC, Eising CM, DeSouza DD, Spiegel D, Bayley PJ, Brain and physiological markers of autonomic function are associated with treatment-related improvements in self-reported autonomic dysfunction in veterans with gulf war illness: an exploratory pilot study, Glob. Adv. Health Med 9 (2020), 2164956120922812, 10.1177/2164956120922812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dujardin S, Pijpers A, Pevernagie D, Prescription drugs used in insomnia, Sleep Med. Clin 13 (2) (2018) 169–182, 10.1016/j.jsmc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- [62].Obata H, Analgesic mechanisms of antidepressants for neuropathic pain, Int. J. Mol. Sci 18 (11) (2017), 10.3390/ijms18112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


