Abstract
Abstract Fragile X syndrome (FXS) is caused by mutations in the fragile X mental retardation 1 gene, characterized by low plasma cholesterol levels. Considering the essential role of brain cholesterol in signaling and synaptogenesis, it is important to screen for brain cholesterol abnormalities in FXS and explore their link with neuropsychological profiles. Brain cholesterol is synthesized in situ, and the excess is primarily converted to 24(S)-hydroxycholesterol (24(S)-OHC). 27-hydroxycholesterol (27-OHC) is the major cholesterol oxidation metabolite that crosses the blood-brain barrier from peripheral circulation into the brain. Plasma levels of 24(S)-OHC and 27-OHC were quantified in FXS and control individuals. The FXS group underwent transcranial magnetic stimulation to evaluate corticospinal excitability and inhibition. The clinical profile was assessed using questionnaires evaluating specific symptoms related to autism, aberrant behaviors, and anxiety. Study results show a significant decrease in plasma levels of 24(S)-OHC in FXS as compared to controls (78.48 nM ± 20.90 vs. 99.53 nM ± 32.30; P = 0.006). Moreover, a negative correlation was observed between plasma levels of 24(S)-OHC and motor evoked potential (rs = −0.57; P = 0.05) in FXS. Similarly, a negative correlation was also found between plasma levels of 24(S)-OHC and the total score of the Social Communication Questionnaire (rs = −0.72; P = 0.002) and the Anxiety Depression and Mood Scale (rs = −0.61; P = 0.02). The 24(S)-OHC is associated with specific neurophysiological and behavioral characteristics in individuals with FXS. Larger studies are warranted to confirm the potential of 24(S)-OHC as a reliable biomarker for FXS.
Supplementary key words: fragile X messenger ribonucleoprotein 1, cholesterol, oxysterols, 24(S)-hydroxycholesterol, 27-hydroxycholesterol, transcranial magnetic stimulation, clinical profile
Fragile X syndrome (FXS) is the most frequent monogenetic cause of autism spectrum disorder (ASD) and inherited intellectual disability (ID) (1). FXS is caused by mutations in the fragile X messenger ribonucleoprotein 1 gene (FMR1), located on chromosome X (2). As a result, there is a reduction or a total loss of its encoded protein called fragile X messenger ribonucleoprotein (FMRP). FMRP is an RNA-binding protein (3) that controls the translation of many targets messenger RNAs (4) which potentially encode for proteins involved in lipid biosynthesis and catabolism (5). Metabolic anomalies have been reported in FXS, including obesity (6) and hypocholesterolemia (7, 8).
Hypocholesterolemia is defined as a decrease of plasma total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels below the fifth percentile of a normalized population, adjusted for age, gender, and ethnicity (9). Berry-Kravis et al. (7) showed a significant decrease of plasma TC, LDL-C, and high-density lipoprotein cholesterol (HDL-C) among males with FXS compared to healthy controls. Moreover, Çaku et al. (8) showed that 30% of individuals with FXS meet the criteria of hypocholesterolemia. They also reported an inverse association between plasma levels of apolipoprotein B and apolipoprotein A1 with aberrant behavior and adaptive capacities, suggesting a potential alteration of cholesterol-related metabolic pathways that may affect brain function in FXS.
The brain is the dominant pool of cholesterol, containing about 25% of the body's cholesterol (10). Moreover, it produces its own cholesterol. In fact, astrocytes synthesize cholesterol and release it as cholesterol-rich apolipoprotein E lipoprotein complex into the extracellular medium. Neurons capture these lipoproteins through their LDL receptor and LDL receptor-related protein1 (11). Subsequently, apolipoprotein E undergoes recycling, when cholesterol levels rise above a threshold, the acyl-CoA cholesterol acyltransferase 1 removes a portion by converting it into cholesterol esters which constitute ∼1% of the TC pool in the adult brain and exist as lipid droplets (12, 13). The brain cholesterol issued to maintain cell membrane integrity and function, as well as the synthesis of myelin, which is crucial for efficient nerve signal transmission. The excess cholesterol is oxidized to 24(S)-hydroxycholesterol (24(S)-OHC) via cytochrome P450-46A1 (CYP46A1), which is almost exclusively expressed in neural cells (14). 24(S)-OHC crosses the blood-brain barrier, diffuses into the systemic circulation, and then is eliminated by the liver. This mechanism enables the primary pathway of cholesterol elimination from the brain (10, 15).
In contrast, the 27-hydroxycholesterol (27-OHC) is the most abundant peripheral derivative of cholesterol. It is ubiquitously synthesized in hepatic and extrahepatic tissues, released into the blood circulation, and transported into the brain through blood-brain barrier (16, 17, 18). There is thus a crosstalk between both oxysterols where 24(S)-OHC is the major metabolite of the brain cholesterol, while 27-OHC is the major one of the peripheral cholesterols. Brain cholesterol plays a crucial role in neural growth, synaptogenesis, biosynthesis of neurosteroids (19), and cell signaling via the modulation of the synaptic transmission (20). Depletion of cholesterol results in an alteration of different neuron receptors, specifically the N-methyl-D-aspartate receptor (NMDAR) (21). Sensory hypersensitivity (22), epileptic seizures (23, 24), and abnormal electroencephalograms (25) in FXS individuals and FMR1 KO animal models have been reported confirming neuronal network hyperexcitability. Precisely, an imbalance between excitatory and inhibitory receptors (NMDAR) and gamma-aminobutyric acid type A (GABAA) has been reported in the FMR1 KO mouse (26) and individuals with FXS (27). Transcranial magnetic stimulation (TMS) studies in FXS observed cortical hyperexcitability (28, 29) when the corticospinal tract was stimulated through the human motor cortex. Reduced plasma levels of 24(S)-OHC and 27-OHC have been reported in neurocognitive diseases including Alzheimer's (30), Huntington's (31), and Parkinson’s disease (32). All these conditions are characterized by synaptic dysfunction. Despite the evidence supporting the implication of cholesterol in synaptic function and the documented cholesterol alteration in FXS (7, 8), no study has investigated brain cholesterol metabolism in FXS. We hypothesized that individuals with FXS have an alteration in brain cholesterol metabolism that is associated with specific neurophysiological and clinical traits. In the present work, we compared plasma levels of 24(S)-OHC and 27-OHC between individuals with FXS and healthy controls and correlated them with the neurophysiological and clinical profile as evaluated by TMS and questionnaires, respectively.
Materials and Methods
Study population and design
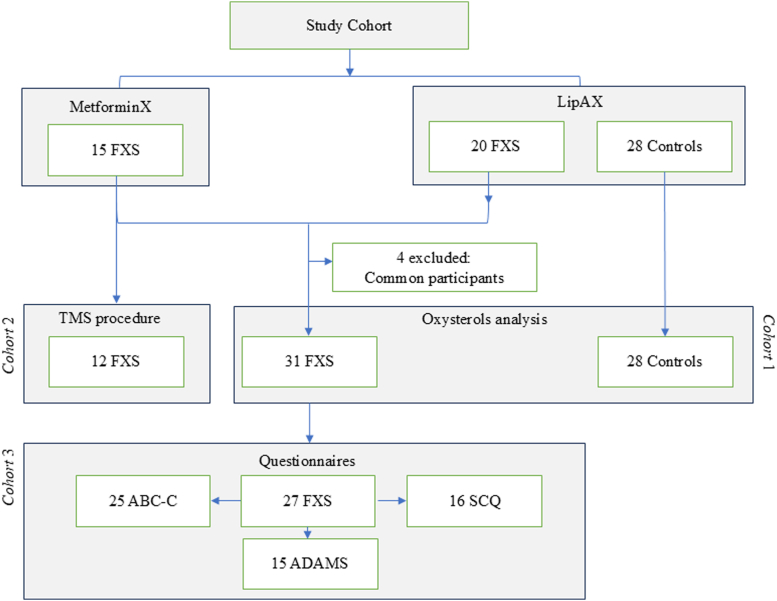
The study population included participants from the MetforminX and LipAX cohorts, comprising a total of 31 individuals with FXS and 28 healthy controls (Fig. 1). The MetforminX trial has been previously described (33). Data obtained from pretreatment visits of 15 individuals with FXS through the MetforminX study were included in the present study. A total of 20 individuals with FXS and 28 controls were recruited through the LipAX study. Both studies were approved by the Ethics Board of the Research Center of the CIUSSS de l’Estrie-CHUS, Canada, and were performed in accordance with the Declaration of Helsinki.
Fig. 1.
Study design.
Participants with FXS were recruited at the FXS clinic located at CIUSSS de l’Estrie-CHUS, while controls were recruited from the local community. The inclusion criteria consisted of males and females, aged between 12 and 45 years old, with a confirmed molecular diagnosis of FXS for the FXS group (Southern blot and polymerase chain reaction). For both FXS and control groups, the exclusion criteria were as follows: the use of medications affecting cholesterol metabolism such as hypolipidemic treatments, retinoids, corticosteroids; liver/renal dysfunction; uncontrolled hypothyroidism or hyperthyroidism; malabsorption/malnutrition; malignancy or any acute condition; diabetes; genetic dyslipidemia; and specific for controls; and any neurodevelopmental, neurocognitive, or psychiatric disorder.
There was a single study visit including a blood collection followed by an evaluation of the clinical profile in FXS participants conducted through questionnaires completed by their parents. Participants of the MetforminX cohort underwent the TMS procedure. Blood samples were collected in K2EDTA tubes (BD Vacutainer®). Plasma was recovered following successive centrifugations (300 g for 10 min and 2,400 g for 15 min to remove all cellular components) and stored at −80°C. FMRP dosage was performed on whole cell extracts from platelets (pg/106 platelets) by Western blotting as previously described (34).
Quantification of plasma lipids and oxysterols: 24(S)-OHC and 27-OHC
TC, HDL-C, and triglycerides were measured by enzymatic methods (Modular Roche P800), while LDL-C was calculated using the Friedewald formula. Apolipoprotein B, apolipoprotein A1, and lipoprotein (a) were measured by immunoturbidimetric assays (Roche Diagnostics, Cobas 501 analyzer). Measurement of 24(S)-OHC and 27-OHC was performed as previously described (35). Briefly, a volume of 200 μl of human EDTA-K2 plasma was supplemented with 20 μl of internal standards (24(R/S)-OHC-d7 and 27-OHC-d6 at 24.5 μM (10 ng/μl)). The extraction of oxysterols involved a lipid extraction step using 3 ml of DCM: MeOH (1:1) with 50 μg/ml of BHT. The alkaline hydrolysis step was performed using 10 N potassium hydroxide. Following hydrolysis, 3 ml of Dulbecco's phosphate-buffer saline was added to the extraction tube, vortexed for 30 s, and centrifuged at 2,200 g for 5 min. The organic layer was carefully transferred to a clean glass tube. The extracted oxysterols were then lyophilized and then reconstituted in 200 μl of MeOH containing 5 mM ammonium formate. After incubation at 30°C, the samples were transferred to HPLC autosampler vials and stored at 4°C until instrumental analysis. Oxysterol analysis was performed using ultra-performance liquid chromatography (1,290 Infinity, Agilent, Santa Clara, CA) coupled with mass spectrometry (MS) on a triple quadrupole mass spectrometer instrument equipped with an electrospray ionization source (6460 MS/MS with electrospray ionization jet stream, Agilent, Santa Clara, CA) (LC-MS/MS). The chromatographic column employed was a Luna Omega Polar C18 (2.1 × 100 mm, particle size 1.6 μm, Phenomenex, Torrance, CA). The total run time for quantifying 24(S)-OHC and 27-OHC was 17.5 min.
TMS procedure
The TMS protocols used in the present study have been previously described in Proteau-Lemieux et al. (33) and Morin-Parent et al. (28). Measurements obtained by TMS are known to reflect specific neuronal mechanisms, as detailed in Table 1. Briefly, a figure-of-eight coil of 70 mm diameter connected to a BiStim Magstim TMS apparatus was placed tangentially to the midline (45°) on the optimal spot of the left hemisphere to induce a motor response in the contralateral first-dorsal interosseus muscle. Resulting motor contractions were recorded using electromyography with a tendon belly montage using self-adhesive circular electrodes, resulting in a motor-evoked potential (MEP) used to quantify the corticospinal excitability. Resting motor threshold (rMT) was established according to the guidelines of the International Federation of Clinical Neurophysiology (36). Test stimulus intensity for baseline MEP and paired-pulse measures were obtained at 125% of rMT. Conditioning stimulus intensity for short interval intracortical inhibition (SICI) and intracortical facilitation was set to 80% of rMT, while 125% of rMT was used for the long intracortical inhibition (LICI). The corticospinal silent period was obtained by administering a TS while participants performed an isometric contraction corresponding to 20% of the maximal force of the first-dorsal interosseus muscle as measured with a dynamic pressure gauge.
Table 1.
TMS measurements and respective involved neuronal mechanism
| TMS Measurements | Neuronal Mechanism |
|---|---|
| Resting motor threshold (rMT) | Cortical excitability mediated by Glu and NMDAR |
| Short interval intracortical inhibition (SICI) | Intracortical inhibition mediated by GABAA |
| Long intracortical inhibition (LICI) | Intracortical inhibition mediated by GABAB |
| Intracortical facilitation (ICF) | Cortical facilitation mediated by Glu |
| Cortical silent period (CSP) | Spinal and cortical inhibition mediated by GABAA+B |
Clinical evaluation
The Social Communication Questionnaire (SCQ), presented in a YES/NO questionnaire format, is composed of 40 items that screen symptoms associated with autism, including reciprocal social interaction, communication, repetitive behaviors, and stereotyped behaviors (37).
Social Responsiveness Scale, a 65-item questionnaire, designed to assess the severity of autism symptoms with a primary focus on social impairment, as well as common autistic behaviors such as language difficulties and stereotypy (38). The latter is composed of five domains of social awareness, social cognition, social motivation, social communication, restricted interests, and repetitive behavior, rated from 1 (not true) to 4 (almost always true) (39).
Anxiety Depression and Mood Scale (ADAMS) is a 29-item questionnaire used to assess mood disorders in persons with ID. The questionnaire evaluates five subdomains: hyperactive behavior, depressed mood, social avoidance, general anxiety, and obsessive/compulsive behavior. Each item is rated on a 4-point scale ranging from 0 (not a problem) to 3 (severe problem) (40).
Repetitive Behavior Scale-Revised is a 43-item questionnaire designed to assess the spectrum of repetitive behaviors in individuals with ASD, encompassing children, adolescents, and adults. It consists of six subscales: Stereotyped Behavior, Self-injurious Behavior, Compulsive Behavior, Routine Behavior, Sameness Behavior, and Restricted Behavior. Each item is rated on a 4-point scale: 0 (Behavior does not occur), 1 (Behavior occurs and is a mild problem), 2 (Behavior occurs and is a moderate problem), and 3 (Behavior occurs and is a severe problem) (41).
Aberrant Behavior Checklist-Community, Fragile-X version is a 58-item questionnaire used to assess aberrant behavior in individuals with ID and is specifically validated for the FXS population (42). Aberrant Behavior Checklist-Community, Fragile-X version evaluates six dimensions of behavior including irritability, hyperactivity, social unresponsiveness, social avoidance, stereotypy, and inappropriate speech. Each item is rated on a scale from 0 (not at all a problem) to 3 (severe problem) (43).
Statistical analyses
Statistical differences between the groups were tested using student’s t test for quantitative variables (reported as mean ± standard deviation) and Fisher tests for categorical variables. Qualitative data were shown as a percentage. Spearman’s rank correlation was utilized to evaluate the association between oxysterols levels and the clinical profile. For all statistical tests, a P-value < 0.05 was considered significant. Analyses were performed using GraphPad Prism 8.
Results
Study population
Plasma oxysterols and plasma lipids were measured in 31 FXS and 28 healthy controls (Cohort 1) (Fig. 1). TMS results were available in a subgroup of 12 FXS participants from the MetforminX trial at the pretreatment visit (Cohort 2) (Fig. 1). Clinical data including score results of questionnaires were obtained from 27 FXS participants of MetforminX and LipaX studies (Cohort 3) (Fig. 1). The characteristics of each cohort are shown in Table 2. A total of 31 participants with FXS and 28 healthy controls were recruited, including 84% and 75% males, respectively. The median age of FXS and control groups as well as male-FXS and full mutation subgroups were comparable (supplemental Table S1). The BMI ranged from 16.3 to 43 (kg/m2) for the participants with FXS and from 17.3 to 37 (kg/m2) for the controls. The age range and BMIs were comparable with no statistical differences between cohorts. 74% of the FXS group had a full mutation diagnosis. The majority of FXS individuals with detectable FMRP were mosaics (4.6–42.9 pg/106 platelets). Hypocholesterolemia was observed in 36% of participants with FXS. Half of the FXS group was medicated: 26% were taking antipsychotics and/or antidepressants, while the majority (48%) were on other types of medications such as vitamins, contraceptives, and thyroid medication.
Table 2.
Characteristics of the study cohorts
| Cohorts | Controls | 1-Oxysterol |
2-TMS |
3-Questionnaires |
|---|---|---|---|---|
| FXS | FXS | FXS | ||
| N | 28 | 31 | 12 | 27 |
| Males N (%)a | 21 (75) | 26 (84) | 10 (83) | 22 (81) |
| Age, median (range)b | 26.50 (13–42) | 28 (12–44) | 28 (17–44) | 28 (14–44) |
| BMI median (range)b | 26.5 (17.3–37) | 26.4 (16.3–43) | 29.37 (19.30–42.27) | 26.30 (16.30–42.95) |
| Type of mutation N (%) | ||||
| Full mutation | - | 23 (74) | 9 (75) | 21 (77.78) |
| Mosaicism | - | 8 (26) | 3 (25) | 6 (22.22) |
| FMRP (pg/106) N (%) | ||||
| Not detectable | - | 16 (52) | 6 (50) | 15 (56) |
| Detectable | - | 11 (35) | 6 (50) | 10 (37) |
| Not measured | - | 4 (13) | - | 2 (7) |
| Lipid profile | ||||
| ˂5th percentile N (%) | ||||
| TC | 1 (4) | 11 (36) | 3 (25) | 10 (37) |
| HDL-C | 1 (4) | 4 (13) | 2 (17) | 2 (7) |
| LDL-C | 3 (11) | 9 (29) | 2 (17) | 8 (30) |
| Triglycerides | 1 (4) | 2 (6.4) | 1 (8) | 1 (4) |
| Medication | ||||
| Medicated N (%) | 8 (29) | 16 (52) | 5 (42) | 15 (56) |
| Antipsychotic | 0 | 8 (26) | 2 (17) | 8 (30) |
| Antidepressant | 1 (4) | 8 (26) | 3 (25) | 8 (30) |
| Stimulant | 0 | 2 (6) | 0 | 2 (7) |
| Other | 7 (25) | 15 (48) | 8 (67) | 12 (44) |
| Not medicated N (%) | 20 (71) | 15 (48) | 7 (58) | 12 (44) |
FMRP, fragile X messenger ribonucleoprotein; FXS, Fragile X syndrome; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TMS, transcranial magnetic stimulation.
Fisher exact test.
Mann-Whitney test.
Oxysterol levels in individuals with FXS and controls
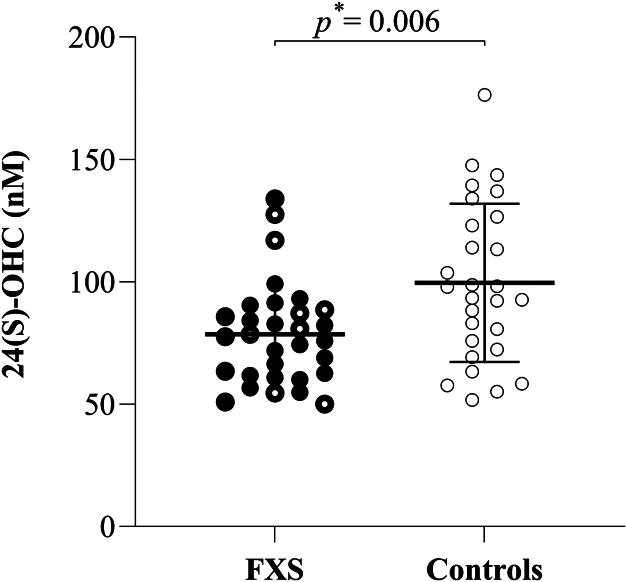
Plasma levels of 24(S)-OHC were significantly lower in the FXS group compared to the control group (78.48 nM ± 20.90 vs. 99.53 ± 32.30 nM; P = 0.006), as shown in Fig. 2. The subgroup of individuals with FXS carrying full mutation were even lower as compared to controls (76.03 nM ± 18.48 vs. 99.53 nM ± 32.30; P = 0.005). Similar results were obtained when comparing males only (74.80 nM ± 18.74 vs. 91.98 nM ± 28.66; P = 0.027) (supplemental Table S2). Plasma levels of 27-OHC were also lower in the FXS group compared to the control group, although the difference did not reach statistical significance (254.5 nM ± 77.01 vs. 282.2 nM ± 116.3; P = 0.693) (supplemental Table S2).
Fig. 2.
Plasma levels of total 24(S)OHC, quantified following saponification, in individuals with FXS (n = 31) and healthy controls (n = 28).  Individuals with FXS,
Individuals with FXS,  Individuals with FXS carrying full mutation,
Individuals with FXS carrying full mutation,  Controls, ∗ Mann-Whitney test. The bar represents the mean, and the whiskers represent the standard deviation.
Controls, ∗ Mann-Whitney test. The bar represents the mean, and the whiskers represent the standard deviation.
Plasma levels of 24(S)-OHC correlated positively with plasma TC (rs = 0.66; p ˂ 0.0001) and LDL-C (rs = 0.45; P = 0.01). Comparable results were obtained for plasma 27-OHC when correlated with TC (rs = 0.43; P = 0.01) and LDL-C (rs = 0.42; P = 0.02). No association was found between BMI and plasma levels of 24(S)-OHC and 27-OHC.
Association of plasma oxysterols with TMS measurements
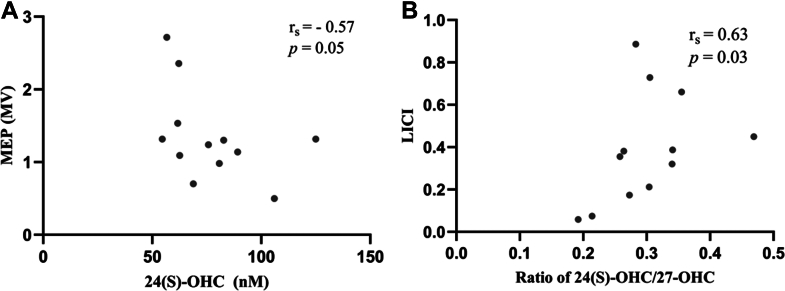
Spearman correlation conducted between TMS measurements and oxysterols in 12 individuals with FXS revealed a negative association between plasma levels of 24(S)-OHC and 27-OHC with the MEP (rs = −0.57, P = 0.05; rs = −0.68, and P = 0.018, respectively), as shown in Fig. 3A. A significant positive correlation was obtained between ratio of 24(S)-OHC/27-OHC and LICI (rs = 0.63, P = 0.03) (Fig. 3B). Correlation results between oxysterols and TMS variables in FXS group and controls are shown in supplemental Tables S3 and S4, respectively.
Fig. 3.
Correlation between 24(S)-OHC and ratio of 24(S)-OHC/27-OHC and TMS measurements in individuals with FXS (n = 12). (A) Correlation between 24(S)-OHC and MEP; (B) correlation between ratio of 24(S)-OHC/27-OHC and LICI. MV, millivolts.
Association of plasma oxysterols with clinical profile in FXS
There was a statistically significant negative association between plasma levels of 24(S)-OHC and the total scores of SCQ (rs = −0.72, P = 0.002) and ADAMS (rs = −0.67, P = 0.02), in individuals with FXS as shown in Fig. 4.
Fig. 4.
Correlation between 24(S)-OHC and clinical profile in individuals with FXS. (A) Negative correlation between plasma levels of 24(S)-OHC and the total score of SCQ (n = 16). (B) Negative correlation between 24(S)-OHC and total score of ADAMS (n = 15).
Discussion
This study remains the first to investigate brain cholesterol in humans with FXS. Overall, our results show that the major metabolites of brain and peripheral cholesterol, 24(S)-OHC and 27-OHC, respectively are decreased in FXS. Furthermore, 24(S)-OHC plasma levels are associated with specific traits of neurophysiological and clinical profile in this population.
The plasma levels of 24(S)-OHC were significantly reduced in the FXS individuals, with an increased difference observed in FXS carrying full mutation. Similar results were previously reported in other neurodegenerative diseases such as Alzheimer’s disease (30), multiple sclerosis (44), and Huntington’s disease (45). In FXS, studies on the brain cholesterol remain limited. Specifically, Parente et al. (46) reported an increase of brain cholesterol content levels in the amygdala of a Fmr1-Δexon 8 rat model of FXS. A significant decrease of CYP46A1, the enzyme that metabolizes brain cholesterol into 24(S)-OHC, was also observed along with a decrease of the sterol regulatory binding protein 2. The latter is responsible for maintaining brain cholesterol homeostasis by controlling the transcriptional machinery of genes involved in cholesterol synthesis depending on cholesterol cellular levels (14). Moreover, Talvio et al. (47) reported a decrease of ATP-binding cassette transporter A1 (ABCA1) (cholesterol membrane transporter to the extracellular environment) in both human and mouse iPSC-derived astrocyte model of FXS. This alteration was associated with an accumulation of cholesterol and its precursors in FXS mouse astrocytes. ABCA1 expression is primarily regulated by LXR activity, which is influenced by cholesterol levels (48). However, when cholesterol levels are elevated, increased production of oxysterols activates LXRs and leads to enhanced ABCA1 expression (49). These observations suggest a potential decrease of the CYP46A1 enzyme activity in FXS that might explain a reduction of plasma 24(S)-OHC observed in our study. Our results are consistent with these two reports. These results altogether suggest a decrease in brain cholesterol degradation into 24(S)-OHC and a consecutive increase of cholesterol content. The latter leads to a decrease of cleaved sterol regulatory binding protein 2 and ABCA1 as a result of the classical homeostatic response to intracellular cholesterol accumulation (46). Alternatively, Ren et al. (50) observed a reduction in enzymes involved in cholesterol biosynthesis pathway (Cyp51A1 and Sc4mol (MSM01)), as well as a decrease of cholesterol content in astrocytes from human FXS-derived induced pluripotent stem cells (hiPSCs). The authors reported that human FXS-derived induced pluripotent stem cell–derived astrocytes shared more similarities with fetal astrocytes than with mature human astrocytes, which may explain the immaturity in expression of cholesterol biosynthetic enzymes. We should note that the regulation of cholesterol synthesis and degradation is complex, and the underlying cause of the alteration of brain cholesterol metabolism in FXS remains unclear. However, considering the further reduction of 24(S)-OHC in FXS individuals with full mutation as well as the role of FMRP in the regulation of expression of numerous brain proteins (51), we hypothesize that FMRP might control the expression and/or activity of CYP46A1. Further, in vitro studies are warranted to explore the underlying mechanism and validate this hypothesis.
Several factors might influence plasma levels of 24(S)-OHC including, age and sex. There is an age-dependent variation of plasma levels of 24(S)-OHC (52, 53). The highest levels are reported in the first and second year of life and then decline to reach adult levels around the age of 12–15 years (52). In the current study, cohorts were limited to the individuals aged 12 years and older. Since only two participants were under 15 years old, the potential effect of age as a variable affecting plasma levels of 24(S)-OHC was minimal. Furthermore, we conducted a correlation analysis to assess age-related variation in plasma levels of 24(S)-OHC. Our result showed no significant association in either FXS or control group. In addition, sex has a specific effect on cholesterol metabolism (54, 55): females display higher levels of 24(S)-OHC as compared to males, both in healthy controls (55) or individuals with mental disorder (55). In accordance with previous reports, we observed higher plasma levels of 24(S)-OHC in female controls as compared to male controls. However, considering the limited number of females, we could not conclude about sex-based differences of plasma 24(S)-OHC. However, the number of males and females in FXS and control groups was not statistically different to potentially influence the levels of 24(S)-OHC.
This study represents a first attempt to screen the potential association between brain cholesterol levels and neurophysiologic profile of individuals with FXS. An inverse relationship was observed between plasma 24(S)-OHC and MEP: individuals with lower plasma 24(S)-OHC displayed higher corticospinal excitability. An increase in MEP amplitude obtained at comparable intensities can be interpreted as a sign of heightened excitability and/or conductivity of cortical neurons (56). Furthermore, a positive, statistically significant association was observed between the ratio of 24(S)-OHC/27-OHC and LICI measurement. LICI describes the suppression of neuronal activity following double-pulse TMS and reflects intracortical inhibition mediated by the GABAergic system, specifically through postsynaptic GABAB receptors (57). Taken together, these results suggest that individuals with FXS displaying lower plasma levels of 24(S)-OHC showed higher cortical excitability and lower GABAB-mediated intracortical inhibition compared to those with higher cholesterol levels. Our findings align with previous studies that have reported an imbalance between cortical excitation and inhibition in FXS resulting in cortical hyperexcitability. For instance, we previously reported a reduction of GABAA-mediated inhibition and an increase of GABAB-mediated inhibition, as shown respectively by an increase of SICI and a decrease of LICI in individuals with FXS as compared to healthy controls (28).
In the present study, no significant association between oxysterols and GABAA-mediated inhibition was observed, suggesting that in FXS, brain cholesterol alterations may primarily impact GABAB-mediated inhibition. The link between brain cholesterol and the GABAA receptors has been reported. Both, cholesterol depletion and enrichment can affect their function, suggesting that an optimal level of brain cholesterol is essential for optimal receptor activity (58). Although, there is limited research about the link between cholesterol and GABAA receptors, similar mechanisms might apply to GABAB receptors. The present results suggest a link between brain cholesterol degradation and GABAB receptors; however, further research is warranted to establish this relationship.
Our results revealed a significant negative association between plasma levels of 24(S)-OHC and the total score of SCQ and ADAMS in FXS, indicating a link between brain cholesterol catabolism and autistic behaviors and anxiety, respectively. A similar relationship between SCQ and cholesterol content in lipid rafts isolated from platelets was reported in individuals with FXS (59). We should note that ASD is a common feature in FXS, with 30%–54% of FXS males meeting diagnostic criteria for ASD (60). Hypocholesterolemia has been reported in 23% of individuals with ASD (61); however, only one study investigated brain cholesterol. Specifically, Graya et al. (62) found an increase in plasma levels of 24(S)-OHC in 36 children with ASD as compared to 38 healthy controls matched for age and sex. Considering the limited research on brain cholesterol in individuals affected with ASD and/or FXS, other studies including the measurement of other brain-derived oxysterols are important to further understand alterations of brain cholesterol in living subjects. Anxiety is also a common condition observed in many neurodevelopmental and mental disorders. Up to 86% of males and 77% of females with FXS meet diagnostic criteria of anxiety disorder (40). To our knowledge, this is a first report in FXS showing an association between brain cholesterol catabolism and anxiety. Nevertheless, Guidara et al. (63) have shown a significant decrease of 24(S)-OHC plasma levels in patients with bipolar disorder, the underlying mechanism remains unknown.
The results of the present study should be interpreted in the light of some limitations. First, levels of brain cholesterol metabolites were quantified using peripheral blood samples rather than cerebrospinal fluid. This approach may not accurately reflect the state of central lipids. Second, the number of participants was limited to conclude about alterations of brain cholesterol metabolite in FXS. Although the present findings suggest the presence of such perturbations, further studies including a larger sample size as well as the measurement of other brain-derived oxysterols are needed to explore and potentially understand the metabolism of brain cholesterol in living subjects. Third, this study did not include magnetic resonance imaging data, which could have provided quantitative measurements of GABA neurotransmitter levels in the brain and helped to better understand the correlation between brain cholesterol metabolites and these specific neurochemical profiles.
In conclusion, this study represents a significant advancement in our understanding of FXS, providing the first evidence of reduced brain cholesterol catabolism in individuals with this condition. Our findings demonstrate a novel association between plasma levels of 24(S)-OHC, a brain-specific cholesterol metabolite, and key neurophysiological and clinical features of FXS. Moreover, our results identify 24(S)-OHC as a promising biomarker candidate to predict distinct neurophysiological and clinical profiles in FXS. Future studies should aim to replicate these findings in larger cohorts, investigate the mechanistic links between cholesterol metabolism and FXS symptoms, and explore the potential of cholesterol-targeted therapies in FXS treatment.
Data availability
The fundamental data of this study are included in the article. However, additional datasets (raw data) are available from the corresponding author on reasonable request.
Supplemental data
This article contains supplemental data.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgment
We would like to express our sincere gratitude to the Canadian Institutes of Health Research (CIHR) and Fonds de Recherche du Québec - Santé (FRQS) for their financial support, which was vital for the success of this project. Additionally, we thank Centre de recherche du CHUS for their research support, which truly helped us in the advancement of this work.
Author contributions
F. C., P.-L. M., A. A., R. P., S. B., L. G., and J.-F. L. writing–review & editing; F. C., L. G., M. P.-L., and J.-F. L. visualization; F. C. and P.-L. M. validation; A. A. project administration; R. P. and M. P.-L. resources; R. P., S. B., and S. Bo. formal analysis; L. G. conceptualization; D. R. and M. L. methodology; A. Ç. and J.-F. L. supervision; A. C. investigation; A. L. writing–original draft; M. L. data curation.
Supplemental data
References
- 1.Hunter J., Rivero-Arias O., Angelov A., Kim E., Fotheringham I., Leal J. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am. J. Med. Genet. A. 2014;164:1648–1658. doi: 10.1002/ajmg.a.36511. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan L.M., Phillips M.A., Forster-Gibson C.J., Beckett J., Partington M.W., Simpson N.E., et al. Genetic mapping of DNA segments relative to the locus for the fragile-X syndrome at Xq27.3. Am. J. Hum. Genet. 1985;37:463–472. [PMC free article] [PubMed] [Google Scholar]
- 3.Zang J.B., Nosyreva E.D., Spencer C.M., Volk L.J., Musunuru K., Zhong R., et al. A mouse model of the human fragile x syndrome I304n mutation. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darnell J.C., Van Driesche S.J., Zhang C., Hung K.Y.S., Mele A., Fraser C.E., et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leboucher A., Pisani D.F., Martinez-Gili L., Chilloux J., Bermudez-Martin P., Van Dijck A., et al. The translational regulator FMRP controls lipid and glucose metabolism in mice and humans. Mol. Metab. 2019;21:22–35. doi: 10.1016/j.molmet.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raspa M., Bailey D.B., Jr., Bishop E., Holiday D., Olmsted M. Obesity, food selectivity, and physical activity in individuals with fragile X syndrome. Am. J. Intellect. Dev. Disabilities. 2010;115:482–495. doi: 10.1352/1944-7558-115.6.482. [DOI] [PubMed] [Google Scholar]
- 7.Berry-Kravis E., Levin R., Shah H., Mathur S., Darnell J.C., Ouyang B. Cholesterol levels in fragile X syndrome. Am. J. Med. Genet. A. 2015;167A:379–384. doi: 10.1002/ajmg.a.36850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Çaku A., Seidah N.G., Lortie A., Gagné N., Perron P., Dubé J., et al. New insights of altered lipid profile in fragile X syndrome. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moutzouri E., Elisaf M., Liberopoulos E.N. Hypocholesterolemia. Curr. Vasc. Pharmacol. 2011;9:200–212. doi: 10.2174/157016111794519354. [DOI] [PubMed] [Google Scholar]
- 10.Kotti T.J., Ramirez D.M.O., Pfeiffer B.E., Huber K.M., Russell D.W. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3869–3874. doi: 10.1073/pnas.0600316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrov A.M., Pikuleva I.A. Cholesterol 24-hydroxylation by CYP46A1: benefits of modulation for brain diseases. Neurotherapeutics. 2019;16:635–648. doi: 10.1007/s13311-019-00731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibuya K., Watanabe T., Urano Y., Takabe W., Noguchi N., Kitagishi H. Synthesis of 24(S)-hydroxycholesterol esters responsible for the induction of neuronal cell death. Bioorg. Med. Chem. 2016;24:2559–2566. doi: 10.1016/j.bmc.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Muneton S., Sjövall J., Jovanovic J.N., Griffiths W.J. The effect of 24S-hydroxycholesterol on cholesterol homeostasis in neurons: quantitative changes to the cortical neuron proteome. J. Proteome Res. 2008;7:1606–1614. doi: 10.1021/pr7006076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heverin M., Bogdanovic N., Lütjohann D., Bayer T., Pikuleva I., Bretillon L., et al. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J. Lipid Res. 2004;45:186–193. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Heverin M., Meaney S., Lütjohann D., Diczfalusy U., Wahren J., Björkhem I. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 2005;46:1047–1052. doi: 10.1194/jlr.M500024-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Wu M., Zhai Y., Liang X., Chen W., Lin R., Ma L., et al. Connecting the dots between hypercholesterolemia and Alzheimer’s disease: a potential mechanism based on 27-hydroxycholesterol. Front. Neurosci. 2022;16:842814. doi: 10.3389/fnins.2022.842814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulig W., Cwiklik L., Jurkiewicz P., Rog T., Vattulainen I. Cholesterol oxidation products and their biological importance. Chem. Phys. Lipids. 2016;199:144–160. doi: 10.1016/j.chemphyslip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Craig M., Yarrarapu S.N.S., Dimri M. StatPearls Publishing; Treasure Island (FL): 2021. Biochemistry, Cholesterol. in StatPearls. [PubMed] [Google Scholar]
- 20.Korinek M., Gonzalez-Gonzalez I.M., Smejkalova T., Hajdukovic D., Skrenkova K., Krusek J., et al. Cholesterol modulates presynaptic and postsynaptic properties of excitatory synaptic transmission. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-69454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korinek M., Vyklicky V., Borovska J., Lichnerova K., Kaniakova M., Krausova B., et al. Cholesterol modulates open probability and desensitization of NMDA receptors. J. Physiol. 2015;593:2279–2293. doi: 10.1113/jphysiol.2014.288209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musumeci S.A., Bosco P., Calabrese G., Bakker C., De Sarro G.B., Elia M., et al. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 23.El Idrissi A., Ding X.H., Scalia J., Trenkner E., Brown W.T., Dobkin C. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci. Lett. 2005;377:141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- 24.Berry-Kravis E. Epilepsy in fragile X syndrome. Dev. Med. Child Neurol. 2002;44:724–728. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- 25.Ethridge L.E., De Stefano L.A., Schmitt L.M., Woodruff N.E., Brown K.L., Tran M., et al. Auditory EEG biomarkers in fragile X syndrome: clinical relevance. Front. Integr. Neurosci. 2019;13:60. doi: 10.3389/fnint.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ethridge L.E., White S.P., Mosconi M.W., Wang J., Pedapati E.V., Erickson C.A., et al. Neural synchronization deficits linked to cortical hyper-excitability and auditory hypersensitivity in fragile X syndrome. Mol. Autism. 2017;8:22. doi: 10.1186/s13229-017-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brighi C., Salaris F., Soloperto A., Cordella F., Ghirga S., de Turris V., et al. Novel fragile X syndrome 2D and 3D brain models based on human isogenic FMRP-KO iPSCs. Cell Death Dis. 2021;12:498. doi: 10.1038/s41419-021-03776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morin-Parent F., Champigny C., Lacroix A., Corbin F., Lepage J.-F. Hyperexcitability and impaired intracortical inhibition in patients with fragile-X syndrome. Transl. Psychiatry. 2019;9:312. doi: 10.1038/s41398-019-0650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberman L., Ifert-Miller F., Najib U., Bashir S., Woollacott I., Gonzalez-Heydrich J., et al. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile X syndrome and autism spectrum disorder. Front. Synaptic Neurosci. 2010;2:26. doi: 10.3389/fnsyn.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varma V.R., Büşra Lüleci H., Oommen A.M., Varma S., Blackshear C.T., Griswold M.E., et al. Abnormal brain cholesterol homeostasis in Alzheimer's disease—a targeted metabolomic and transcriptomic study. Npj Aging Mech. Dis. 2021;7:11. doi: 10.1038/s41514-021-00064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leoni V., Long J.D., Mills J.A., Di Donato S., Paulsen J.S., PREDICT-HD study group Plasma 24S-hydroxycholesterol correlation with markers of Huntington disease progression. Neurobiol. Dis. 2013;55:37–43. doi: 10.1016/j.nbd.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leoni V., Masterman T., Diczfalusy U., De Luca G., Hillert J., Björkhem I. Changes in human plasma levels of the brain specific oxysterol 24S-hydroxycholesterol during progression of multiple sclerosis. Neurosci. Lett. 2002;331:163–166. doi: 10.1016/s0304-3940(02)00887-x. [DOI] [PubMed] [Google Scholar]
- 33.Proteauux-Lemie M., Lacroix A., Galarneau L., Corbin F., Lepage J.F., Çaku A. The safety and efficacy of metformin in fragile X syndrome: an open-label study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;110 doi: 10.1016/j.pnpbp.2021.110307. [DOI] [PubMed] [Google Scholar]
- 34.Lessard M., Chouiali A., Drouin R., Sébire G., Corbin F. Quantitative measurement of FMRP in blood platelets as a new screening test for fragile X syndrome. Clin. Genet. 2012;82:472–477. doi: 10.1111/j.1399-0004.2011.01798.x. [DOI] [PubMed] [Google Scholar]
- 35.Rojas D., Benachenhou S., Laroui A., Aden A.A., Abolghasemi A., Galarneau L., et al. Development and validation of a liquid chromatography-tandem mass spectrometry assay to quantify plasma 24(S)-hydroxycholesterol and 27-hydroxycholesterol: a new approach integrating the concept of ion ratio. J. Steroid Biochem. Mol. Biol. 2023;235 doi: 10.1016/j.jsbmb.2023.106408. [DOI] [PubMed] [Google Scholar]
- 36.Rossi S., Antal A., Bestmann S., Bikson M., Brewer C., Brockmöller J., et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert Guidelines. Clin. Neurophysiol. 2021;132:269–306. doi: 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rynkiewicz A., Szura M., Bernaciak D., Kozak A., Karwowska M. Polish adaptation of the social communication questionnaire (SCQ) and female autism phenotype: an investigation of potentially sex-biased items in the screening assessment and their impact on scores. Brain Sci. 2021;11:682. doi: 10.3390/brainsci11060682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prigge M.B.D., Bigler E.D., Travers B.G., Froehlich A., Abildskov T., Anderson J.S., et al. Social Responsiveness Scale (SRS) in relation to longitudinal cortical thickness changes in autism spectrum disorder. J. Autism Dev. Disord. 2018;48:3319–3329. doi: 10.1007/s10803-018-3566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan W., Smith L.E., Hong J., Greenberg J.S., Mailick M.R. Validating the social responsiveness scale for adults with autism. Autism Res. 2017;10:1663–1671. doi: 10.1002/aur.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordeiro L., Ballinger E., Hagerman R., Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. J. Neurodevelop Disord. 2011;3:57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooker J.L., Dow D., Morgan L., Schatschneider C., Wetherby A.M. Psychometric analysis of the repetitive behavior scale-revised using confirmatory factor analysis in children with autism. Autism Res. 2019;12:1399–1410. doi: 10.1002/aur.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sansone S.M., Widaman K.F., Hall S.S., Reiss A.L., Lightbody A., Kaufmann W.E., et al. Psychometric study of the aberrant behavior checklist in fragile X syndrome and implications for targeted treatment. J. Autism Dev. Disord. 2012;42:1377–1392. doi: 10.1007/s10803-011-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aman M.G., Singh N.N., Stewart A.W., Field C.J. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am. J. Ment. Defic. 1985;89:485–491. [PubMed] [Google Scholar]
- 44.Mukhopadhyay S., Fellows K., Browne R.W., Khare P., Krishnan Radhakrishnan S., Hagemeier J., et al. Interdependence of oxysterols with cholesterol profiles in multiple sclerosis. Mult. Scler. 2017;23:792–801. doi: 10.1177/1352458516666187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray S.M., Dai J., Smith A.C., Beckley J.T., Rahmati N., Lewis M.C., et al. Changes in 24(s)-hydroxycholesterol are associated with cognitive performance in early huntington's disease: data from the TRACK and ENROLL HD cohorts. J. Huntingtons Dis. 2024;13:449–465. doi: 10.3233/JHD-240030. [DOI] [PubMed] [Google Scholar]
- 46.Parente M., Tonini C., Buzzelli V., Carbone E., Trezza V., Pallottini V. Brain cholesterol biosynthetic pathway is altered in a preclinical model of fragile X syndrome. Int. J. Mol. Sci. 2022;23:3408. doi: 10.3390/ijms23063408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talvio K., Wagner V.A., Minkeviciene R., Kirkwood J.S., Kulinich A.O., Umemori J., et al. An iPSC-derived astrocyte model of fragile X syndrome exhibits dysregulated cholesterol homeostasis. Commun. Biol. 2023;6:789. doi: 10.1038/s42003-023-05147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang B., Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018;14:452–463. doi: 10.1038/s41574-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mouzat K., Chudinova A., Polge A., Kantar J., Camu W., Raoul C., et al. Regulation of brain cholesterol: what role do liver x receptors play in neurodegenerative diseases? Int. J. Mol. Sci. 2019;20:3858. doi: 10.3390/ijms20163858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren B., Burkovetskaya M., Jung Y., Bergdolt L., Totusek S., Martinez-Cerdeno V., et al. Dysregulated cholesterol metabolism, aberrant excitability and altered cell cycle of astrocytes in fragile X syndrome. Glia. 2023;71:1176–1196. doi: 10.1002/glia.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richter J.D., Zhao X. The molecular biology of fmrp: new insights into fragile X syndrome. Nat. Rev. Neurosci. 2021;22:209–222. doi: 10.1038/s41583-021-00432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J. Intern. Med. 2006;260:493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 53.Bretillon L., Lütjohann D., Ståhle L., Widhe T., Bindl L., Eggertsen G., et al. Plasma levels of 24S-hydroxycholesterol reflect the balance between cerebral production and hepatic metabolism and are inversely related to body surface. J. Lipid Res. 2000;41:840–845. [PubMed] [Google Scholar]
- 54.Mittendorfer B. Sexual dimorphism in human lipid metabolism. J. Nutr. 2005;135:681–686. doi: 10.1093/jn/135.4.681. [DOI] [PubMed] [Google Scholar]
- 55.Chiappelli J., Quinton M.S., Volfson D., Cwik M., Marshall W., Bruce H., et al. Assessment of brain cholesterol metabolism biomarker 24S-hydroxycholesterol in schizophrenia. NPJ Schizophr. 2020;6:34. doi: 10.1038/s41537-020-00121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Badawy R.A.B., Loetscher T., Macdonell R.A.L., Brodtmann A. Cortical excitability and neurology: insights into the pathophysiology. Funct. Neurol. 2012;27:131–145. [PMC free article] [PubMed] [Google Scholar]
- 57.Müller-Dahlhaus J.F.M., Liu Y., Ziemann U., Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex – a pharmacological TMS study. J. Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sooksawate T., Simmonds M.A. Influence of membrane cholesterol on modulation of the GABA(A) receptor by neuroactive steroids and other potentiators. Br. J. Pharmacol. 2001;134:1303–1311. doi: 10.1038/sj.bjp.0704360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toupin A., Benachenhou S., Abolghasemi A., Laroui A., Galarneau L., Fülöp T., et al. Association of lipid rafts cholesterol with clinical profile in fragile X syndrome. Sci. Rep. 2022;12:2936. doi: 10.1038/s41598-022-07064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaufmann W.E., Kidd S.A., Andrews H.F., Budimirovic D.B., Esler A., Haas-Givler B., et al. Autism spectrum disorder in fragile X syndrome: cooccurring conditions and current treatment. Pediatrics. 2017;139:S194–S206. doi: 10.1542/peds.2016-1159F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benachenhou S., Etcheverry A., Galarneau L., Dubé J., Çaku A. Implication of hypocholesterolemia in autism spectrum disorder and its associated comorbidities: a retrospective case-control study. Autism Res. 2019 doi: 10.1002/aur.2183. [DOI] [PubMed] [Google Scholar]
- 62.Grayaa S., Zerbinati C., Messedi M., HadjKacem I., Chtourou M., Ben Touhemi D., et al. Plasma oxysterol profiling in children reveals 24-hydroxycholesterol as a potential marker for Autism Spectrum Disorders. Biochimie. 2018;153:80–85. doi: 10.1016/j.biochi.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 63.Guidara W., Messedi M., Maalej M., Naifar M., Khrouf W., Grayaa S., et al. Plasma oxysterols: Altered level of plasma 24-hydroxycholesterol in patients with bipolar disorder. J. Steroid Biochem. Mol. Biol. 2021;211 doi: 10.1016/j.jsbmb.2021.105902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The fundamental data of this study are included in the article. However, additional datasets (raw data) are available from the corresponding author on reasonable request.