Abstract
Objectives: Black and Hispanic American patients have seen an increase in heart failure (HF) rates, with higher rates of hospitalizations and age-adjusted mortality. Our study aims to examine the associations between Social Determinants of Health (SDoH), difficulties associated with the workload assigned to the patients by healthcare providers/healthcare system measured as Burden of Treatment (BoT), and Quality of Life (QoL) in a predominantly minority, low income population of patients with heart failure in the South Bronx. Methods: We included 265 patients hospitalized for HF decompensation. They were administered questionnaires to evaluate SDoH, QoL (EQ-5D), and BoT (Patient Experience with Treatment and Self-management-PETS questionnaire) at baseline. We fitted 10 zero-inflated negative binomial models to determine associations between total SDOH and total QoL with each BoT domain. We modelled the likelihood that a patient reports no burden on a given domain as well as the severity of the burden among patients who report burden. Results: The mean age of our cohort was 63.7 years, with 66% male, 50% Hispanic ethnicity and 48% Black. Spanish was the predominant primary language of communication. Their mean Charlson Comorbidity Index was 5.32 (SD = 2.6). Heart failure with reduced ejection fraction (HFrEF) was present in 72% of our participating patients. The mean composite SDoH score was 3.4 (SD = 1.9), with 31% of the cohort reporting problems paying their bills, 28% with food insecurity, and 35% requiring public assistance. Among the 5 domains measured by EQ-5D for evaluating QoL, moderate to severe difficulty was experienced by 88% of our cohort in at least one of the five domains, and severe difficulty in at least one of the five domains was reported in 23% of our patients. Of the ten domains evaluated for Burden of Treatment (BoT), the highest median scores obtained from our cohort were for difficulty with medical expenses, role and social activity limitations, difficulty with accessing healthcare services, difficulty with medical information, and physical and mental exhaustion due to self-care. Zero-inflated models identified a significant association between higher SDoH scores and having some burden of treatment in 6 of the 10 domains, particularly in the domains of difficulty with healthcare expenses and difficulty with self-care interfering with social/daily activities. Additionally, high SDoH scores were also associated with greater severity of burden in 7 of the 10 domains, particularly relating to understanding medical information and difficulty with healthcare expenses. Poor QoL was associated with increased BoT in 6 of the 10 domains. QoL was strongly associated with the burdens of physical and mental exhaustion and difficulty with medical appointments. Conclusions: Our findings highlight the interplay of SDoH, QoL and BoT in driving health disparities in heart failure patients. The positive correlation between SDoH and BoT demonstrates the impact that SDoH has on the treatment experiences of HF patients and their ability to manage their illness. Difficulty with healthcare expenses, understanding medical information, keeping track of medical appointments, self-care responsibilities affecting work, family and daily activities, and increased physical and mental fatigue due to self-care were the predominant domains affecting our study population. These findings signal the need for healthcare systems to identify at-risk individuals and implement individualized strategies to reduce the burden that treatment places on patients. Minimally Disruptive Medicine, which emphasizes understanding the patient’s perspectives and tailoring treatment to the patient’s daily life, may be an important tool in providing equitable care to these at-risk populations.
Keywords: Heart failure, social determinants of health, burden of treatment, quality of life, patient-centered care, minimally disruptive medicine
Introduction
About one in four people will develop heart failure (HF) in their lifetime due to the rising prevalence of heart failure (HF) with age [1]. In spite of advances in HF treatment, including increased use of Goal-Directed Medical Therapy (GDMT), the morbidity and mortality from HF has continued to remain high [1]. One crucial reason for this is that HF requires patients to manage their symptoms, comply with a strict treatment regimen, including medications, diet, and lifestyle changes, and follow prescribed care transitions after hospitalization requiring a complex interplay between healthcare providers/their organizations, as well as their support systems [2]. Physician-prescribed treatment protocols usually include multiple follow-up appointments for provider visits, diagnostic tests, and modification of patient behavior or lifestyle which creates hardship to the patient [2-5]. “Burden of Treatment” (BoT) is defined as burdens associated with the workload assigned to the patient by healthcare providers, including their health system, and the ramifications this workload has on a patient’s capacity for self-care. For patients undergoing HF treatment, the challenges include understanding their condition, following complex medication regimens, and adjusting to lifestyle changes [2].
Black and Hispanic American patients in particular have seen an increase in HF rates, with higher HF hospitalizations and age-adjusted mortality rates [1,6]. Increased incidence of modifiable risk factors like hypertension, diabetes and obesity, unrecognized structural heart disease and younger age of heart failure onset in this population could contribute to the higher HF rates [7,8]. Appropriate and optimum dosage of GDMT prescriptions, access to newer therapeutic modalities (such as cardiac resynchronization therapies, cardiac transplant, and referrals for adjunctive therapies like cardiac rehabilitation) remains low in this population, leading to higher HF hospitalizations and mortality [6,9]. In addition, these minority populations experience environmental and socioeconomic challenges that contribute to higher incidence rates and worse outcomes related to heart failure [10]. Health inequities usually reflected by higher Social Determinants of Health (SDoH) are also important risk factors in at-risk populations [1]. Social Determinants of Health (SDoH) are defined as conditions people are born into, grow, work, live and age in, and reflect a patient’s life and affect their ability to access care [11,12]. Patients with higher SDoH scores have an increased risk of 90-day mortality following HF hospitalization [13,14]. Quality of Life (QoL) is a patient reported measure of perception about their general health status and allows comparison within the population and disease categories [15]. Patients with HF on average have lower QoL than healthy patients and those with chronic illnesses. This is due to limitations secondary to their symptoms, mobility challenges and associated depression/anxiety. Women and patients with heart failure with Reduced Ejection Fraction (HFrEF) have been observed in previous studies to have poorer QoL than men [16]. In addition, QoL is an independent predictor of health outcomes including hospitalization and mortality in HF patients [16].
Few studies have evaluated the associations between SDoH, QoL, and BoT among patients with HF. We therefore sought to evaluate the impact of SDoH and QoL on the various domains of Burden of Treatment to identify patients with greater hardships with treatment and care in our population, which is predominantly minority and low income. Additionally, we used models to understand the likelihood that a patient reported no burden on the evaluated domains and the severity of the burden among those patients who did report a burden. We evaluated the correlation of burden with social determinants, quality of life, and other demographics variables. Understanding these associations and addressing this knowledge deficit would help providers and systems serving at-risk communities design customized population-based interventions to reduce disparities in HF treatment.
Methods
The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines were followed during the preparation of this manuscript. This study protocol was reviewed and approved by New York City Health and Hospitals/Lincoln Institutional Review Board. Formal informed consent was obtained from the patients at enrollment.
Design
We are conducting an ongoing prospective longitudinal cohort study, focusing on adult patients hospitalized for HF decompensation. All consenting adult hospitalized patients ≥ 18 years of age admitted with a primary diagnosis of CHF decompensation were included in the study. Patients with heart transplant, mechanical support (Left Ventricular Assist Device/CRT device), moderate-severe dementia, with insufficient data for analysis including those who left Against Medical Advice, and those in hospice care were excluded. Participants were administered surveys assessing SDoH, QoL (EQ-5D), and BoT (Patient Experience with Treatment and Self-management (PETS) survey). The initial survey was conducted in person during hospitalization, and follow-up surveys are being conducted via phone at 6 months and one-year post discharge. Here, we present an analysis of the initial baseline data.
Quality of Life (QoL)
QoL was measured using the EQ-5D questionnaire with permission from the EuroQoL group. The EQ-5D questionnaire evaluates 5 dimensions - mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Responses are recorded on a 3 point scale, with a response of “1”, meaning no difficulty and a response of “3” meaning severe difficulty. A visual analog scale from 0-100 was used to assess the patient’s perception of their health, with a response of 0 being the worst possible health and a response of 100 being the best possible health.
Social Determinants of Health (SDoH)
Our survey assesses patients’ Social Determinants of Health (SDoH), including needs such as health insurance, medical bills, medication costs, financial assistance, education, language, and job training. It also measures food insecurity, housing issues (worries about losing housing, problems with living conditions, homelessness), daycare needs, immigration support, and social isolation. The responses were recorded as a “Yes” or “No” for all the questions.
Burden of Treatment (BoT)
The PETS instrument, featuring 10 domains, measured BoT, and was administered to participating patients [26]. Responses, captured on 5-point Likert scale, span from “strongly agree” to “strongly disagree” OR “very difficult” to “very easy”, and were aggregated and scaled to a 0-100 range for analysis.
The domains include: 1. Medical Information: Challenges in understanding health issues, dietary choices, medication usage, treatment changes, and medical advice. 2. Medications: Difficulties in managing and adhering to medication regimens, and coordinating these with daily activities. 3. Medication Bother: Problems with managing side effects of medications. 4. Medical Appointments: Issues with scheduling, tracking, and maintaining appointments, including coordinating hospital visits. 5. Monitoring Health: Struggles with tracking health behaviors, such as exercise, weight, blood pressure, etc. 6. Relationships with Others: Dependence on others for health care needs, reminders for medication/appointments. 7. Health Care Expenses: financial burden associated with paying for medications, food, and navigating insurance. 8. Difficulty with Health Care Services: Problems with provider communication, specialist scheduling, etc. 9. Role and Social Activity Limitations: How self-care impacts work, family and daily activities. 10. Physical and Mental Fatigue: Frequency of feelings of anger, depression, exhaustion, and frustration due to self-care.
Statistical analysis
The statistical analyses aim to evaluate whether QoL and SDoH are associated with the BoT at baseline. Descriptive statistics were used to summarize baseline characteristics, SDoH, QoL, and BoT. These characteristics were compared between patients with heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced Ejection Fraction (HFrEF). We analyzed each burden domain independently to retain their unique constructs. Due to the high presence of reported zeros among the BoT scores, we fit 10 zero-inflated negative binomial models to determine associations between total SDOH and total QoL with each BoT domain. The use of zero-inflated models allows us to jointly model the likelihood that a patient reports no burden on a given domain as well as the severity of the burden among patients who report burden. We present these components separately through visualizations of coefficients and their respective confidence intervals. All analyses are adjusted for age, gender, race, substance abuse, heart failure type, education, and income. Interaction effects between SDOH and QoL are also tested.
Results
Study population
The study population consists of 265 consenting patients who were hospitalized from March 2022 to September 2023. Of the 294 screened participants, 265 agreed to participate in the study (response rate of 90%). The baseline characteristics of the study cohort are summarized in Table 1. Their mean age was 63.7 years, with 28% younger than 55 years, 31% between 56 to 64 years, 29% between 65 to 79 years, and 12% more than 80 years. Our cohort was 66% male, 50% Hispanic ethnicity, and 48% Black. Spanish was the predominant primary language of communication. Of the sample, 45% had a high school or lower level of education. While 19% of the study cohort lived alone, 83% were not currently employed and 82% had a mean household income of less than $27,000/year. Hypertension was the most common comorbidity (90%) in our cohort, followed by diabetes mellitus (52%), and chronic kidney disease (40%). The mean Charlson Comorbidity Index was 5.32 (SD = 2.6). Heart failure with reduced ejection fraction (HFrEF) was present in 72% of our participating patients.
Table 1.
Sample characteristics of the study population (N = 265)
| Characteristic | n (%), 1Mean (SD) |
|---|---|
| Age (years old)1 | 63.66 (7.77) |
| < 55 | 74 (28%) |
| 56-64 | 82 (31%) |
| 65-79 | 77 (29%) |
| > 80 | 32 (12%) |
| Sex | |
| Male | 174 (66%) |
| Female | 91 (34%) |
| Race | |
| African American | 126 (48%) |
| Hispanic/Latinx | 133 (50%) |
| Other | 6 (2.3%) |
| Living Condition | |
| Living alone | 50 (19%) |
| Living with family | 215 (81%) |
| Education | |
| Less than high school | 154 (58%) |
| High school graduate or equivalent (GED) | 69 (26%) |
| College/university/bachelor’s degree | 42 (16%) |
| Currently Employed | |
| Yes | 46 (17%) |
| No | 219 (83%) |
| Substance Abuse | |
| Non-Abuser | 170 (64%) |
| Abuser | 95 (36%) |
| Type of HF | |
| HFrEF | 191 (72%) |
| HFpEF | 74 (28%) |
| Comorbidity | |
| Yes | 87 (70%) |
| No | 38 (30%) |
| Comorbidity Conditions | |
| Hypertension | 238 (90%) |
| Diabetes Mellitus | 137 (52%) |
| CAD | 83 (31%) |
| AICD | 21 (31%) |
| AFib | 71 (27%) |
| Asthma | 59 (22%) |
| COPD | 60 (23%) |
| OSA | 29 (11%) |
| CKD | 105 (40%) |
| ESRD | 24 (9%) |
| CVA | 27 (10%) |
| PAD | 23 (9%) |
| Cirrhosis | 11 (4%) |
| Cancer | 31 (12%) |
| Social Determinants of Health (SDoH)1 | 3.35 (1.90) |
| Quality of Life (QoL)1 | 8.03 (2.14) |
denotes Mean (SD).
Social Determinants of Health (SDoH)
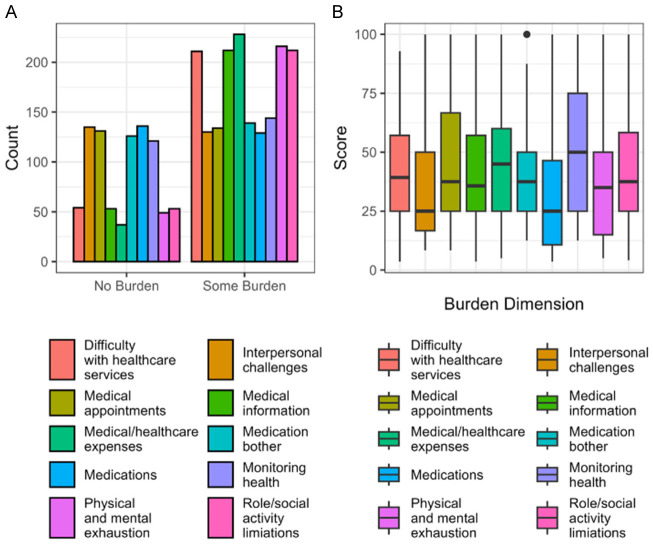
With respect to SDoH (Figure 1B), 31% of the cohort reported problems paying their bills, 28% reported food insecurity, and 35% needed help to receive public assistance/social security income/social security disability. Housing insecurity was present in 24% of the sample, with 15% being street homeless. Social isolation was present in 19% of our study population. The mean composite social determinants of health score was 3.4 (SD = 1.9).
Figure 1.
Distributions of the quality of life and social determinants scores in the study population.
Quality of Life (QoL)
Quality of Life measured by EQ-5D responses are displayed in Figure 1A. In the mobility domain 59% had moderate difficulty while 5% had severe difficulty. Difficulties with self-care were moderate in 34% and severe in 8% of the cohort. Moderate difficulty with usual activities was experienced by 51%, while 15% experienced severe difficulty. Pain causing discomfort was moderate in 43% of the cohort. while 31% admitted to having moderate anxiety and depression. Moderate to severe difficulty was experienced by 88% of our cohort in at least one of the five domains. Severe difficulty in at least one of the five domains was reported in 23% of our patients. The baseline mean visual analog score is 55. The mean QoL composite score was 8.03 (SD = 2.14).
Burden of Treatment (BoT)
Of the ten domains evaluated, the highest median scores obtained from our cohort were for the difficulty with medical expenses domain, role and social activity limitations domain, difficulty with healthcare services domain, difficulty with medical information domain, and physical and mental exhaustion domain. The medical expenses domain includes difficulties with paying for food, medical expenses and understanding insurance coverages and has a median score of 35 (IQR = (25, 55)). The role/social activity domain includes burden from role and social activity limitations due to interference with work, family responsibilities, daily and leisure activities and has a median score of 33.33 (IQR = (12.5, 58.33)). The difficulty with healthcare services domain includes burden due to seeing too many different specialists, lack of communication between providers, difficulty getting appointments at convenient times, and waiting too long at doctor’s office/pharmacy and has a median score of 25 (IQR = (11, 54)). The medical information domain includes difficulties with learning about health problems, diet, medications, changes to their treatment plan, rationale for medications, finding trustworthy sources of medical information, and understanding advice from providers and has a median score of 25 (IQR = (7, 50)). The physical and mental exhaustion category includes feeling of anger, frustration and depression due to self-care responsibilities and has a median score of 25 (IQR = (10, 45)).
The lowest median burden scores arose were present in the interpersonal challenges domain which includes the effect of treatment on social relationships (Median = 0, IQR = (0, 25)) and the burden of managing medications domain (Median = 0, IQR = (0, 25)).
Comparison of HFpEF and HFrEF
HFrEF patients were significantly younger (62 vs 66 years), had higher rate of substance use (P = 0.003), and were more likely to be male (P < 0.001) compared to those with HFpEF. In both cohorts, the majority of patients had a high school or lower level of education and annual household income less than $27,000/year. The number of SDoH was also comparable in both groups. Comparison of BoT domains confirmed higher burden due to self-care causing role and social activity limitations, difficulty with medical/healthcare expenses, difficulty interpreting medical information, and difficulty with healthcare services in patients with HFpEF, compared to patients with HFrEF, though differences are not statistically significant. Additionally burden due to scheduling, tracking, and maintaining appointments, including coordinating hospital visits, difficulties in managing and adhering to medication regimens, and coordinating these with daily activities and self-care impacting work, family and daily activities were higher in patients with HFrEF, though these differences were not statistically significant.
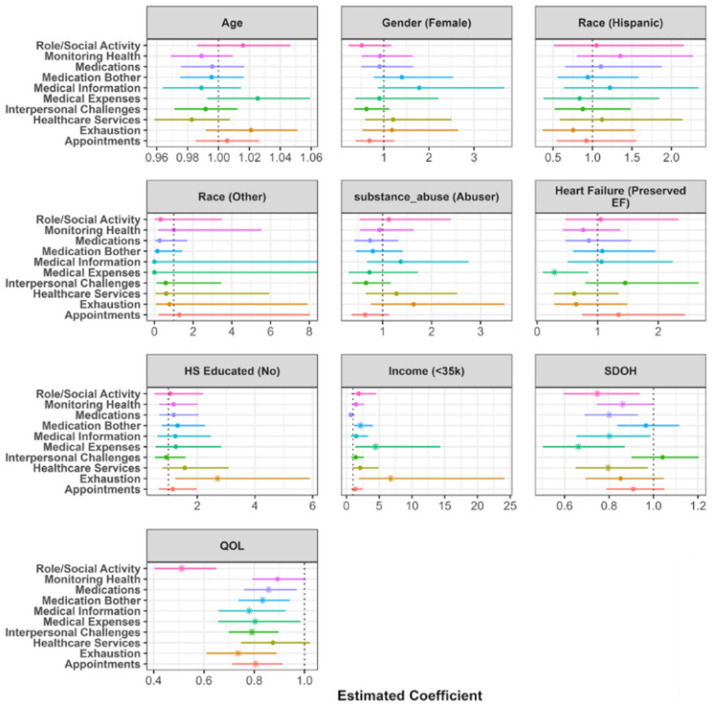
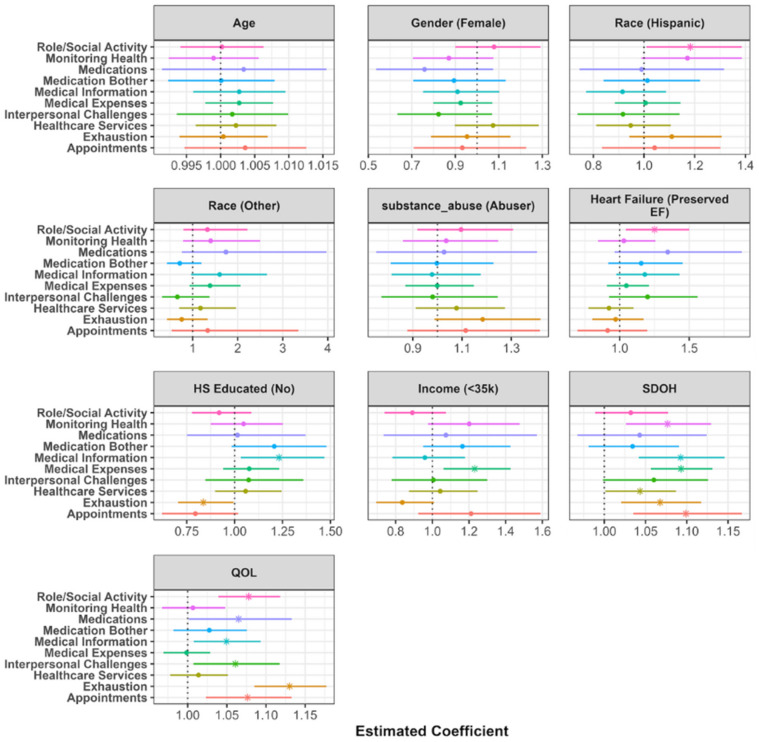
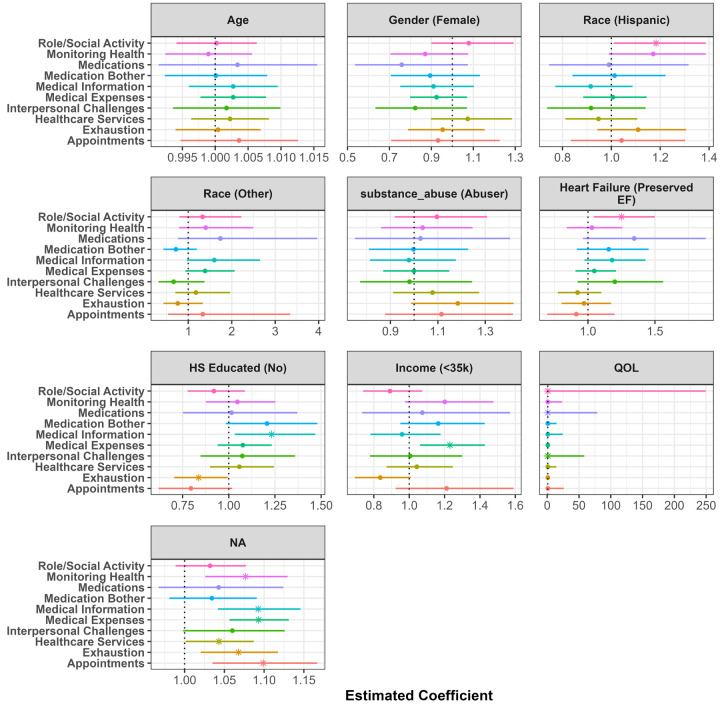
However, after controlling for demographics, SDoH, and QoL domains, as shown in Figure 3, the odds of having burden due to medical expenses are significantly higher in HFpEF patients compared to HFrEF patients. Additionally, as shown in Figure 4, the severity of burden relating to self-care affecting work, family and daily activities was higher in HFpEF patients when controlling for the aforementioned items.
Figure 3.
Visualization of the zero inflated component of the zero inflated negative binomial linear model. Estimates represent the odds ratio of endorsing any item for burden compared to endorsing no burden items. The point represents the point estimate, and the horizontal line represents a 95% confidence interval. A value less than 1 symbolizes higher odds of endorsing items among this respective demographic group compared to reference group. Values greater than 1 symbolize lower odds of endorsing any items among this respective demographic group compared to reference group.
Figure 4.
Visualization of the conditional component of the zero inflated negative binomial model. Estimates represent the rate ratio of endorsing an additional item for burden among those who report some burden. The point represents the point estimate, and the horizontal line represents a 95% confidence interval. A value less than 1 symbolizes a lower rate of endorsing an additional item among this respective demographic group (compared to reference group). A value greater than 1 symbolizes a higher odds of endorsing an additional item among this respective demographic group (compared to reference group).
Burden of treatment in zero-inflated model
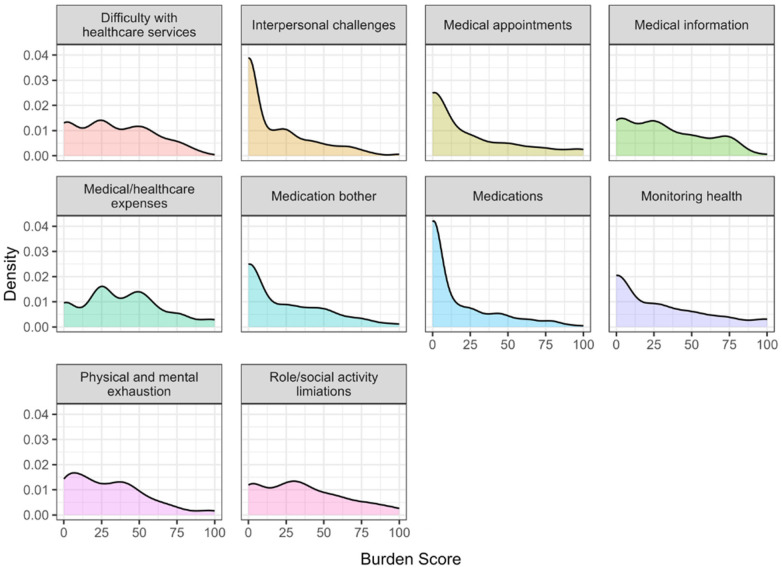
The observed distributions of the different burden of treatment domains are displayed in Figure 2. We particularly note how several of the domains have zero-inflated distributions, representing that a meaningful proportion of participants report no burden (Figure 8). To account for this in analyses, we use the zero-inflated negative binomial model. The BoT scores for the ten domains are described in Figure 2. Figure 2A displays the number of zero and non-zero responses across each burden dimension, with non-zero responses denoting the presence of any burden. Some measures, such as medical and health care expenses and physical and mental exhaustion have a significantly higher proportion of non-zero responses compared to the reported scores of zero. Items such as interpersonal challenges and medications, however, have approximately equal numbers of zero and non-zero responses. This suggests that the data is heavily zero-inflated. Figure 2B shows the distributions of each burden dimension excluding zero responses. For those who reported some level of burden, monitoring health (reflected by struggles with tracking health behaviors, such as exercise, weight, blood pressure, and financial burden associated with paying for medications, food, and navigating insurance) elicited the highest-burden scores.
Figure 2.
Visualizations of the distribution of burden across the different dimensions. (A) shows the proportion of patients reporting no and some burden in each domain. The high proportion of patients report no burden suggest that the data is zero inflated. (B) shows the distribution of burden scores among individuals who report some burden for that category. The zero-inflated negative binomial models consider these populations distinctly by jointly modeling the probability of reporting a zero burden score and the probability of reporting a higher burden score given a nonzero score.
Figure 8.
Density plots showing the zero-inflated distributions of BoT domains.
Presence of burden analysis results
Results of the nine adjusted models evaluating the relationship between SDoH and QoL for each burden of treatment domain are displayed in Figures 3 and 4. Figure 3 shows the odds ratios (OR) arising from the zero-inflated component of the negative binomial models. The odds ratios represent the odds of reporting zero burden in a given domain compared to reporting some amount of burden, controlling for the included demographics, SDoH, and QoL items. Odds ratios and their corresponding confidence intervals for each predictor are plotted together to visualize the variation in predictive power across each burden domain. The majority of burden domains were not significantly associated with age, gender, race, education, or income. One exception is the physical and mental exhaustion domain; patients with less than high school education and an income < $27,000 were more likely to not report feelings of anger, depression, exhaustion, and frustration due to self-care than those with at least a high school education and income > $27,000 (Education: OR = 2.71, 95% CI = (1.24, 5.91), p-value = 0.013; Income: OR = 6.75, 95% CI = (1.89, 24.12), p-value = 0.003). HFpEF patients were more likely to report a burden associated with financial burden associated with paying for medications, food, and navigating insurance (OR = 0.29, 95% CI = (0.1, 0.85), p-value = 0.024). No other burden domains were associated with HF type.
A higher SDoH total score was significantly associated with a higher presence of non-zero burden levels in six of the ten domains. The strongest effects were seen between SDoH and the burden of paying for medications, food, and navigating insurance (OR = 0.66, 95% CI = (0.5, 0.87), p-value = 0.003) and the burden of self-care impacting work, family and daily activities (OR = 0.75, 95% CI = (0.6, 0.94), p-value = 0.012). Worse QoL was significantly associated with a higher presence of burden levels in nine of the ten domains. The strongest association was also between QoL and difficulty due to self-care affecting work, family and daily activities (OR = 0.51, 95% CI = (0.4, 0.65), p-value < 0.001) followed by difficulty due to feelings of anger, depression, exhaustion, and frustration due to self-care (OR = 0.74, 95% CI = (0.61, 0.89), p-value = 0.001).
Severity of burden analysis results
Figure 4 displays the results of the conditional component of the zero-inflated models. These rate ratios (RR) represent the likelihood of reporting a higher burden among patients who report some level of burden in each respective domain, adjusting for the included demographic, SDoH, and QoL variables. Age, gender, race, and substance abuse were not significantly associated with the severity of burden in this population. Patients with HFpEF were more likely to report higher counts of burden associated with self-care impacting work, family and daily activities compared to those with HFrEF (RR = 1.25, 95% CI = (1.04, 1.5), p-value = 0.016). Patients with less than a high school education reported higher levels of burden associated with challenges in understanding health issues, dietary choices, medication usage, treatment changes, and medical advice (RR = 1.23, 95% CI = (1.03, 1.47), p-value = 0.02). Additionally, patients earning less than $27, 000 annually reported higher financial burden associated with paying for medications, food, and navigating insurance (RR = 1.23, 95% CI = (1.37, 14.36), p-value = 0.006).
Higher levels of SDoH were significantly associated with increased burden of treatment levels in seven of the ten domains. The association between SDoH and the challenges in understanding health issues, dietary choices, medication usage, treatment changes, and medical advice as well as financial burden associated with paying for medications, food, and navigating insurance was the strongest (information: RR = 1.09, 95% CI = (1.04, 1.15), p-value < 0.001; expenses: RR = 1.09, 95% CI = (1.06, 1.13), p-value < 0.001). Worse QoL was associated with increased BoT in six of the ten domains. Specifically, quality of life is strongly associated with the increased frequency of feelings of anger, depression, exhaustion, and frustration due to self-care and issues with scheduling, tracking, and maintaining appointments, including coordinating hospital visits (exhaustion: RR = 1.13, 95% CI = (1.09, 1.18), p-value < 0.001; appointments: RR = 1.08, 95% CI = (1.02, 1.13), p-value = 0.004).
Although Variance inflation factor (VIF) values indicate a lack of collinearity in the models, high correlation was present between SDoH and demographic variables such as gender, race, education, and income (Figure 5). To validate our findings, we refit the ten models without the total SDoH score (Figures 6 and 7). Excluding SDoH did not significantly change the results, suggesting that SDoH explained additional variability in the burden domains beyond the demographic variables. No interaction effects were present in the models.
Figure 5.
Correlation between study variables.
Figure 6.
Visualization of the zero inflated component of the zero inflated negative binomial linear model excluding SDoH. Estimates represent the odds ratio of endorsing any item for burden compared to endorsing no burden items. The point represents the point estimate, and the horizontal line represents a 95% confidence interval. A value less than 1 symbolizes higher odds of endorsing items among this respective demographic group compared to reference group. Values greater than 1 symbolize lower odds of endorsing any items among this respective demographic group compared to reference group.
Figure 7.
Visualization of the conditional component of the zero inflated negative binomial model excluding SDoH. Estimates represent the rate ratio of endorsing an additional item for burden among those who report some burden. The point represents the point estimate, and the horizontal line represents a 95% confidence interval. A value less than 1 symbolizes a lower rate of endorsing an additional item among this respective demographic group (compared to reference group). A value greater than 1 symbolizes a higher rate of endorsing an additional item among this respective demographic group (compared to reference group).
Discussion
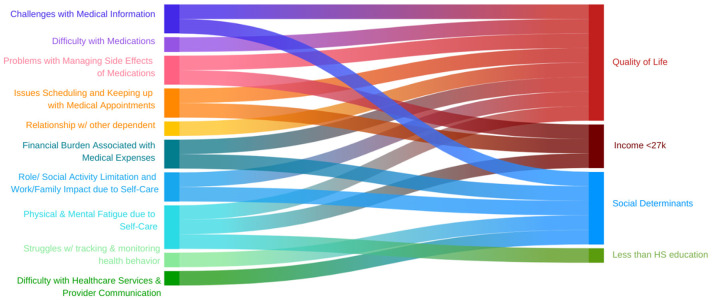
Our study aimed to evaluate the relationship between SDoH, QoL and BoT associated with heart failure. Our primary findings confirmed that a higher number of SDoH was associated with an increased presence of BoT in six of the evaluated ten domains, with the greatest burden being difficulty with medical and healthcare expenses and difficulty with self-care interfering with work, family responsibilities, daily activities and social commitments. Similarly, among those with any burden, the severity of their burden in 7 out of the 10 domains was greater if they had higher SDoH. The highest impacts were in difficulty with understanding medical information including treatment plans and advice from different providers, followed by increased financial burden associated with paying for medications, food, and navigating insurance. Our cohort had poor QoL with 88% having moderate to severe difficulty in at least one of the five domains. Like with SDoH, 9 out of the 10 BoT domains were present in patients with poorer QoL, led by self-care affecting work, family and daily activities, and increased frequency of feelings of anger, depression, exhaustion, and frustration secondary to self-care (Figure 9). HFpEF patients with BoT had greater difficulty with self-care limiting work, family and social responsibilities compared to patients with HFrEF. This is an important consideration as majority of our HFpEF cohort were women. Among patients with HFpEF, those with BoT had more difficulty due to the financial burden associated with paying for medications, food, and navigating insurance. compared to those who did not have any BoT.
Figure 9.
Association of Quality of Life, Income < 27k, Social Determinants of Health and Less than HS education with different domains of Burden of Treatment.
Health systems in the US have begun to recognize the importance of SDoH and follow the CMS and Joint Commission established framework to address disparities in care [17,18]. When collected appropriately, SDoH information allows a unique understanding of the patient, their living conditions, surroundings, and their socioeconomic milieu [1]. Individual as well as multiple within person SDoH have been known to be associated with mortality in patients with heart failure [14,19]. Our results shed light on the crucial role SDoH plays in contributing to the BoT in patients with HF. Based on data showing that education and literacy helps patients understand the complexity of medical information [20], we can infer that patients with less education will have increased difficulty handling and processing the medical information delivered to them at all points of their healthcare contact. That being said, while level of education remains an inadequate surrogate to health literacy, methods to identify patients with low health literacy needs to be standardized and simplified so that appropriate interventions to improve patients’ health related activities outside of the hospital can implemented (e.g. adhering to disease management instructions, compliance with medications and treatment strategies). Improving provider-patient interaction and refining patients’ ability to participate effectively in shared decision making process can all help to improve their outcomes [21].
While our results are consistent with previous studies demonstrating the higher BoT among patients with HF [22-25], their association with SDoH is striking and significant. Living with SDoH increases levels of stress in patients, and when they have illnesses such as HF which require a lot of self-care, managing multiple medications, frequent hospital visits, specialist appointments, etc., it increases their burden of care, and affects their ability to comply with any/all of their medical instructions [27]. Similarly, the increase in loneliness and lack of social support in our communities impairs patients’ emotional and psychological wellbeing and their ability to self-care, thereby adversely affecting their short- and long-term outcomes [28]. SDoH domains like poverty and unemployment affect HF patients’ ability to manage their illness including eating healthy, access to stable housing, and access to care [29]. Better identification of individual SDoH by implementing standardized screening processes, and aligning tools which provide patients’ assistance to understand their health insurance including ensuring they are not paying more than they need out of pocket, facilitating linkage to medication assistance programs, community service society resources to help with post enrollment health insurance assistance, are a few examples of ways to reduce patients’ financial burden. Organizations like The Coalition of the Homeless in New York City provide city and statewide resources for at-risk populations to assist with housing and food insecurity.
Patients overwhelmed by the physical symptoms of HF and demands of their everyday life have limited capacity to cope while meeting their obligations to themselves and their families, and choose in most cases to avoid their healthcare needs [30]. Health professionals impose more demands on these patients thereby exacerbating a vicious cycle stretching the patient’s capacity to self-care, leading to poor outcomes [25,31]. Hence healthcare providers must consider patients’ symptoms as not only an indicator of disease progression but also as a hindrance to self-care. The perceived increased workload affects the patient’s ability to engage emotionally and socially, further contributing to their disease burden [32]. Our results are similar to other published studies demonstrating a positive correlation of a greater burden of treatment and worse QoL [22]. Improving provider awareness of the signals and implications of the burdens experienced by patients will help in improving the provider-patient relationship and allow providers to effectively tailor their treatment recommendations for improved patient compliance. Community health workers with strong ties to the community are a great resource for health care system to provide culturally appropriate health education and medical information, assist patients in transitions of care, guide them on health behaviors and advocate for their needs.
Disparities in HF burden and care persist among racial and ethnic minorities. Our study of Black and Hispanic HF patients highlights the trifecta of higher SDoH with poor quality of life leading to higher and more severe BoT. With burgeoning costs and lagging value of care, existing healthcare systems and frameworks are not designed to adapt care to our growing population with increased comorbidities needing complex care [33]. The disconnect between patients and their healthcare systems in recognizing the “work” that is thrust upon patients by clinicians/health care systems is due to a lack of adequate tools to identify the at-risk population and implement strategies to overcome them. When a patient’s workload increases, their ability to handle the burden decreases and this creates an imbalance that makes it difficult for them to manage their healthcare needs along with the demands in their life causing disruption leading them to either choose to prioritize life demands over healthcare needs or vice versa, both resulting in affecting quality of life [16]. Minimally Disruptive Medicine (MDM) tailors treatment to the patient’s daily life, and reduces their BoT. It incorporates evidence-based care combined with shared decision-making with patients to improve compliance with treatments by giving patients the tools including education to recognize the importance and relevance of the various treatments and influence them to be invested in their care [33].
Implementing MDM is crucial for healthcare systems aiming to enhance health equity. Key strategies include developing validated tools for assessing the BoT, enhancing patient-centered care coordination, improving care transition models post-hospitalization, and integrating social support networks involving family and friends. Emphasizing the patient’s perspective and ensuring care plans are tailored to individual and community needs are essential. Educating patients to communicate their challenges and preferences enables a collaborative decision-making process with healthcare providers.
Our study has several strengths. We provide insight to an understudied high-risk population. We further the understanding of the relationship between BoT and SDoH and QoL in the context of heart failure. Limitations include the inherent issues related to the observational design including only hospitalized patients with HF, suggesting inaccurate generalizability of results. Another limitation is our modest sample size, which could impact power in testing additional associations.
Conclusion
Evaluating the BoT is a vital part of the clinical care of patients with heart failure. Our study demonstrated the effects of SDoH and QoL on BoT. Future studies involving a larger patient sample from diverse care settings could validate our results while allowing testing of additional associations. With the advances in HF management, it is critical for healthcare providers to recognize the BoT and SDoH of their patients, incorporate the patient’s perspective through shared decision making to address their burden of treatment, and determine the best plan of care to deliver minimally disruptive medicine, and thereby provide patient-centered equitable healthcare.
Disclosure of conflict of interest
None.
References
- 1.Bozkurt B, Ahmad T, Alexander KM, Baker WL, Bosak K, Breathett K, Fonarow GC, Heidenreich P, Ho JE, Hsich E, Ibrahim NE, Jones LM, Khan SS, Khazanie P, Koelling T, Krumholz HM, Khush KK, Lee C, Morris AA, Page RL 2nd, Pandey A, Piano MR, Stehlik J, Stevenson LW, Teerlink JR, Vaduganathan M, Ziaeian B Writing Committee Members. Heart failure epidemiology and outcomes statistics: a report of the heart failure society of America. J Card Fail. 2023;29:1412–1451. doi: 10.1016/j.cardfail.2023.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallacher K, May CR, Montori VM, Mair FS. Understanding patients’ experiences of treatment burden in chronic heart failure using normalization process theory. Ann Fam Med. 2011;9:235–243. doi: 10.1370/afm.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stange KC. The generalist approach. Ann Fam Med. 2009;7:198–203. doi: 10.1370/afm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]
- 5.Hart E. System induced setbacks in stroke recovery. Sociol Health Illn. 2001;23:101–123. [Google Scholar]
- 6.Lewsey SC, Breathett K. Racial and ethnic disparities in heart failure: current state and future directions. Curr Opin Cardiol. 2021;36:320–328. doi: 10.1097/HCO.0000000000000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the american heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 8.Vivo RP, Krim SR, Cevik C, Witteles RM. Heart failure in Hispanics. J Am Coll Cardiol. 2009;53:1167–75. doi: 10.1016/j.jacc.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Lewsey SC, Breathett K. Racial and ethnic disparities in heart failure: current state and future directions. Curr Opin Cardiol. 2021;36:320–328. doi: 10.1097/HCO.0000000000000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piña IL, Jimenez S, Lewis EF, Morris AA, Onwuanyi A, Tam E, Ventura HO. Race and ethnicity in heart failure: JACC focus seminar 8/9. J Am Coll Cardiol. 2021;78:2589–2598. doi: 10.1016/j.jacc.2021.06.058. [DOI] [PubMed] [Google Scholar]
- 11.Meyers AG, Salanitro A, Wallston KA, Cawthon C, Vasilevskis EE, Goggins KM, Davis CM, Rothman RL, Castel LD, Donato KM, Schnelle JF, Bell SP, Schildcrout JS, Osborn CY, Harrell FE, Kripalani S. Determinants of health after hospital discharge: rationale and design of the vanderbilt inpatient cohort study (VICS) BMC Health Serv Res. 2014;14:10. doi: 10.1186/1472-6963-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel H, Bornstein SS, Kane GC Health and Public Policy Committee of the American College of Physicians. Carney JK, Gantzer HE, Henry TL, Lenchus JD, Li JM, McCandless BM, Nalitt BR, Viswanathan L, Murphy CJ, Azah AM, Marks L. Addressing social determinants to improve patient care and promote health equity: an american college of physicians position paper. Ann Intern Med. 2018;168:577–578. doi: 10.7326/M17-2441. [DOI] [PubMed] [Google Scholar]
- 13.Sterling MR, Ringel JB, Pinheiro LC, Safford MM, Levitan EB, Phillips E, Brown TM, Goyal P. Social determinants of health and 90-day mortality after hospitalization for heart failure in the REGARDS study. J Am Heart Assoc. 2020;9:e014836. doi: 10.1161/JAHA.119.014836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroff P, Gamboa CM, Durant RW, Oikeh A, Richman JS, Safford MM. Vulnerabilities to health disparities and statin use in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. J Am Heart Assoc. 2017;6:e005449. doi: 10.1161/JAHA.116.005449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27:7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravera A, Santema BT, Sama IE, Meyer S, Lombardi CM, Carubelli V, Ferreira JP, Lang CC, Dickstein K, Anker SD, Samani NJ, Zannad F, van Veldhuisen DJ, Teerlink JR, Metra M, Voors AA. Quality of life in men and women with heart failure: association with outcome, and comparison between the kansas city cardiomyopathy questionnaire and the EuroQol 5 dimensions questionnaire. Eur J Heart Fail. 2021;23:567–577. doi: 10.1002/ejhf.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIver DL. CMS Framework for Health Equity 2022-2032 [Google Scholar]
- 18.Gottlieb LM, Wing H, Adler NE. A systematic review of interventions on patients’ social and economic needs. Am J Prev Med. 2017;53:719–729. doi: 10.1016/j.amepre.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Calvillo-King L, Arnold D, Eubank KJ, Lo M, Yunyongying P, Stieglitz H, Halm EA. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28:269–282. doi: 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNaughton CD, Cawthon C, Kripalani S, Liu D, Storrow AB, Roumie CL. Health literacy and mortality: a cohort study of patients hospitalized for acute heart failure. J Am Heart Assoc. 2015;4:e001799. doi: 10.1161/JAHA.115.001799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson PN, Shetterly SM, Clarke CL, Bekelman DB, Chan PS, Allen LA, Matlock DD, Magid DJ, Masoudi FA. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305:1695–701. doi: 10.1001/jama.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordfonn OK, Morken IM, Bru LE, Larsen AI, Husebø AML. Burden of treatment in patients with chronic heart failure - A cross-sectional study. Heart Lung J Crit Care. 2021;50:369–374. doi: 10.1016/j.hrtlng.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Nordfonn OK, Morken IM, Lunde Husebø AM. A qualitative study of living with the burden from heart failure treatment: exploring the patient capacity for self-care. Nurs Open. 2020;7:804–813. doi: 10.1002/nop2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehan OC, Leff B, Ritchie CS, Garrigues SK, Li L, Saliba D, Fathi R, Boyd CM. A systematic literature review of the assessment of treatment burden experienced by patients and their caregivers. BMC Geriatr. 2019;19:262. doi: 10.1186/s12877-019-1222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eton DT, Yost KJ, Lai JS, Ridgeway JL, Egginton JS, Rosedahl JK, Linzer M, Boehm DH, Thakur A, Poplau S, Odell L, Montori VM, May CR, Anderson RT. Development and validation of the patient experience with treatment and self-management (PETS): a patient-reported measure of treatment burden. Qual Life Res. 2017;26:489–503. doi: 10.1007/s11136-016-1397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers EA, Yost KJ, Rosedahl JK, Linzer M, Boehm DH, Thakur A, Poplau S, Anderson RT, Eton DT. Validating the Patient Experience with Treatment and Self-Management (PETS), a patient-reported measure of treatment burden, in people with diabetes. Patient Relat Outcome Meas. 2017;8:143–156. doi: 10.2147/PROM.S140851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Gurvitz MZ, Havranek EP, Lee CS, Lindenfeld J, Peterson PN, Pressler SJ, Schocken DD, Whellan DJ. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 28.Cené CW, Loehr L, Lin FC, Hammond WP, Foraker RE, Rose K, Mosley T, Corbie-Smith G. Social isolation, vital exhaustion, and incident heart failure: findings from the atherosclerosis risk in communities study. Eur J Heart Fail. 2012;14:748–753. doi: 10.1093/eurjhf/hfs064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Social Determinants of Health - Healthy People 2030 | health.gov. Accessed March 15, 2024. https://health.gov/healthypeople/priority-areas/social-determinants-health.
- 30.Welstand J, Carson A, Rutherford P. Living with heart failure: an integrative review. Int J Nurs Stud. 2009;46:1374–1385. doi: 10.1016/j.ijnurstu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65:1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Austin RC, Schoonhoven L, Clancy M, Richardson A, Kalra PR, May CR. Do chronic heart failure symptoms interact with burden of treatment? Qualitative literature systematic review. BMJ Open. 2021;11:e047060. doi: 10.1136/bmjopen-2020-047060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. doi: 10.1136/bmj.b2803. [DOI] [PubMed] [Google Scholar]