Abstract
Most recent large-scale global analyses on transcatheter aortic valve implantation (TAVI) for aortic stenosis (AS) showed favorable survival outcomes in patients with high body mass index (BMI). We performed a meta-analysis pooling all clinical studies to assess the validity of improved post-TAVI prognosis in obese patients. MEDLINE and Scopus were queried till January 2023 to identify studies comparing AS patients with BMI≥30 kg/m2, and BMI 18.5 to <25 undergoing TAVI. Data were analyzed using a random-effects model to derive odds ratios (ORs) for all outcomes, and hazard ratios (HRs) for long-term overall survival with 95% confidence intervals. The primary outcomes of interest included 30-day all-cause mortality and long-term overall survival while secondary outcomes consisted of myocardial infarction (MI), major bleeding, major vascular events and acute kidney injury (AKI). A total of 24 studies comprising 38,743 patients were included in this meta-analysis. All-cause mortality at 30-days was significantly reduced in patients with BMI>30 kg/m2 (OR 0.71, P<0.0001) vs. normal BMI. Every 1 kg/m2 increase in BMI was associated with better overall survival (HR 0.96, P<0.0001). Obese patients had greater long-term overall survival (HR 0.87, P<0.00001) compared with non-obese patients. No significant differences in MI (OR 0.84, 95% CI 0.52-1.34), major bleeding (OR 0.94, 95% CI 0.72-1.21), major vascular events (OR 1.18, 95% CI 0.97-1.43) and AKI (OR 1.17, 95% CI 0.87-1.56) were observed between the two groups. Obese AS patients might have similar procedural complications, but reduced mortality, and increased overall survival in comparison with normal weight individuals.
Keywords: Obesity, transcatheter aortic valve implantation, aortic stenosis
Introduction
Aortic stenosis (AS) comprises the most frequent degenerative valvular heart disorders associated with significant disease burden owing to poor prognosis and reduced heart function [1-3]. The prevalence of severe AS increases with age, affecting around 4% of the elderly population (>75 years). Conventional surgical valve replacement, including cardiopulmonary bypass (C.B.P.) and sternotomy, has been the standard therapy. However, these techniques are met with post-operative complications such as stroke, acute respiratory distress syndrome, heart block and multisystem-organ failure in high-risk patients [4,5]. The overall high complication rates associated with conventional treatments has led to the introduction of alternate, minimally invasive techniques such as transcatheter aortic valve implantation (TAVI) which involves the implantation of a valve percutaneously via the trans-femoral (TF) or transapical (TA) route [6]. TAVI is recommended and considered a viable option mainly for treatment in severe AS patients deemed to be inoperable or at high-risk for surgical aortic valve replacement (SAVR) [7,8]. Furthermore, between 2011 and 2014, there was a 25-fold rise in the rate of obese individuals undergoing TAVI in the United States, highlighting the rapidly growing indications for the procedure in patient profiles ranging from low to high surgical risk groups [9,10]. Obese individuals, particularly morbidly obese (MO) patients (Body mass index (BMI) ≥40 kg/m2), are underrepresented in clinical trials, and there is a lack of data regarding long-term results following TAVI in this specific population. MO patients present a distinct combination of complications, such as metabolic syndrome-related comorbidities, as well as respiratory and mobility issues, which may complicate their periprocedural recovery.
However, the validity of an “obesity paradox” in cardiovascular illness is still being contested, albeit this paradigm has lately been questioned in MO patients [11,12]. Peri-procedural and long-term outcomes following TAVI in obese individuals have showcased contradictory results [13-15]. Single-center studies with small sample sizes, notably in the MO cohort, and a variety of BMI cutoff points utilized to determine patient groups further complicates the interpretation of these results [16]. Obesity is regarded as a significant and modifiable risk factor for cardiovascular morbidity and mortality, in both the general population as well as patients with cardiovascular disease (CVD) [17]. Despite their negative impact on overall health, intermediate and high-weight individuals proved to be protected throughout many cardiovascular procedures. This disparity was also discovered in individuals with severe AS who underwent TAVI [15,18]. A previous meta-analysis conducted by Lv and colleagues [19] investigated the obesity paradox in patients undergoing TAVI. Since then, newer cohort studies with larger sample sizes comparing clinical outcomes in patients with vs. without obesity have shown inconsistent and conflicting results. A prospective cohort study [20] demonstrated no difference in overall-mortality between normal and obese patients, however, overweight patients had significantly lower mortality rates on multivariable analysis. Sharma and coworkers [13] evaluated the relationship between BMI and baseline and procedural characteristics from the Society of thoracic surgeons-American college of cardiology transcatheter valve therapy (STS/ACC TVT) Registry. They demonstrated overweight patients, and those with class I and class II obesity to have a decreased risk of one-year mortality compared with normal weight patients. Furthermore, among those with BMI≤30 kg/m2, each 1-kg/m2 increase was associated with a 2% and 4% decrease in the risk of 30-day and one-year mortality, respectively; for B.M.I.≥30 kg/m2, a 1-kg/m2 increase was associated with a 3% increased risk of 30-day mortality but not with one-year mortality. These conflicting findings in recently published clinical data warrants the conduction of an updated meta-analysis to showcase a comprehensive clinical evaluation of TAVI in obese individuals, and to confirm the validity of the improved outcomes with its therapy.
Methods
Our meta-analysis was conducted in accordance to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guideline [21].
Data sources and search strategy
Two investigators (FY and AM) independently searched through the electronic databases PubMed (MEDLINE) and Scopus for relevant articles. All studies published from inception till January 2023 were reviewed. No restrictions were implemented based on geographical location, year of publication, publication type, and language of publication. The following keywords were used ‘body mass index’, ‘BMI’, ‘obesity’, ‘transcatheter aortic valve replacement’, ‘transcatheter aortic valve implantation’ and ‘TAVI’ alongside their MeSH (medical subject headings) terms incorporated with Boolean Operators “OR” and “AND”. The detailed search strategy utilized for each database is given in Supplementary Table 1. Further, we manually searched through reference lists of review articles, pertinent editorials and original publications. Google scholar, medrix.org, and ClinicalTrials.gov were also searched to identify grey literature and preprints. We excluded case studies, reviews, conference abstracts, preclinical experiments and letters from this analysis.
Study selection and eligibility criteria
All relevant articles identified via systematic search were exported to EndNote X9 Reference Manager (Clarivate Analytics, Philadelphia, Pennsylvania) using which duplicates were removed among various online databases. Two independent investigators (FY and AM) performed initial screening of the remaining articles based on title and abstract to ensure they met the required eligibility criteria. Finally, full texts were evaluated for relevance. Any discrepancies were resolved by discussion with the third investigator (FZ). The search was restricted to the following inclusion criteria: observational retrospective and prospective cohort studies of patients with aortic stenosis aged ≥18 years undergoing transcatheter aortic valve implantation, studies with an experimental group (obese patients) and a control group (normal patients), and studies that reported at least one of the outcomes of interest including 30-day all-cause mortality, long-term overall survival, myocardial infarction (MI), major bleeding, major vascular events, and acute kidney injury (AKI) as primary or secondary endpoints. Clinical outcomes and complications were recorded according to the Valve Academic Research Consortium-2 consensus definitions (VARC2) [22]. According to the definitions set by the World Health Organization (WHO), B.M.I. (kg/m2) was categorized as underweight (<18.5), normal (18.5 to <25), overweight (25 to <30) and obese (≥30). In our study, the patients with B.M.I.≥30 kg/m2 were labeled as obese, and the patients with 25-30 kg/m2 were labeled as overweight.
Data extraction and study quality assessment
Two investigators (FY and AM) independently extracted data from the shortlisted articles using pre-specified collection forms. In addition to the population, and study characteristics, data on primary and secondary outcomes were also extracted. The data extracted included: study data including first author’s last name, publication year, country of origin; experimental data including study design, study period, follow-up duration, cut offs of B.M.I., type of TAVI procedure, primary (30-day all-cause mortality and long-term overall survival) and secondary outcomes (myocardial infarction, major bleeding, major vascular events, and acute kidney injury); demographic data including total sample size, number of patients in the experimental and control groups, and major patient characteristics comprising mean age, male/female percentage, comorbidities, and statistical data including outcome statistics for the estimate of relative risk (R.R.s), odds ratio (O.R.s), and hazard ratios (H.R.s) and statistical method. Long-term overall survival was defined as survival recorded across at least one-year follow-up. The quality of each non-randomized study was assessed using the Newcastle-Ottawa Quality Assessment Scale (N.O.S.) [23]. The N.O.S utilizes a star scoring system comprising of eight items divided into three domains; selection, comparability and outcome or exposure. Each study was assigned a score from 0 to 9. Studies scoring seven or above were considered to be of high quality and thus at a low risk of bias whereas those scoring five or less were considered to be of poor quality.
Statistical analysis
We performed the statistical analysis using Review Manager (RevMan) V.5.3 Cochrane Collaboration, London, United Kingdom. A random-effects model was utilized to pool odds ratios (O.R.s) with 95% confidence intervals (C.I.s) that served as the appropriate estimate for evaluation of 30-day mortality and procedural complications extrapolated from either the demographic data or directly reported in the original studies. It was our first priority to integrate the OR statistics derived from multivariate analysis because of the adequate eliminations for confounding factors. Regarding the long-term overall survival, we used hazard ratios (H.R.s) with 95% CI as the summarized estimates, because the H.R. was the only appropriate statistic compatible for both censoring and time to event analysis. Similarly, incorporating the multivariate H.R. statistics would be considered first. In instances of a lack of multivariate statistic reporting, the H.R. value was extrapolated using published survival data with the log-rank p value as proposed by Tierney and coworkers [24]. Publication bias was ascertained for all clinical outcomes by generating a funnel plot and confirming the bias by Egger’s regression test. The Higgins I-squared (I2) statistical model was used to assess heterogeneity in the clinical outcomes of the included studies whereby a value of I2=25%-50% was considered mild, 50%-75% as moderate, and greater than 75% as severe heterogeneity [25]. A p value of <0.05 was considered as statistically significant.
Results
Study characteristics and baseline demographics
The PRISMA flow chart summarizes the search and study selection process in Figure 1. The initial search retrieved a total of 844 relevant articles. After reviewing the titles and abstracts, 560 were removed due to duplication and irrelevance. From the finalized studies, 174 were assessed for eligibility on the basis of their full text. A total of 24 clinical studies were then included in this meta-analysis [13,15-16,20,26-45]. From the included studies, n=10 reported the outcome of 30-day mortality, n=23 reported long-term overall survival as an outcome, and n=13 reported procedural complications. Six studies were prospective observational studies [15,20,27-29,44] whereas 18 studies [13,16,26,30-43,45] were retrospective observational studies. A total of 38,743 patients who underwent TAVI were included in our study. 47.7% of the included participants were male, and the mean age of the included patients was 82.1 years. The follow-up period of the eligible studies ranged from 30 days to three years. The baseline characteristics of the included studies are presented in Table 1.
Figure 1.
PRISMA flowchart diagram.
Table 1.
Baseline characteristics of included studies
| Author, Year | Type of study | Country | Study Period | Sample size | Gender (male ratio) | Age (years) | Outcome reporting HRa/ORb | Type of TAVI procedure | Follow-up period, months | Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Abawi, 2017 [15] | Prospective observational | Netherlands | 2008-2016 | 549 | HR | Transfemoral, Transapical, Direct aortic | 12 | 30-day mortality, long-term overall survival, procedural complication | ||
| Abramowitz, 2016 [26] | Retrospective observational | U.S.A. | 2012-2015 | 805 | 484 (60.1%) | 82.0 ± 8.8 | H.R., OR | Transfemoral, Transapical, Transaortic, Subclavian | 12 | 30-day mortality, long-term overall survival, procedural complication |
| Aung, 2013 [30] | Retrospective observational | U.K. | 2007-2012 | 175 | 86 (49.1%) | 83.0 ± 7.0 | H.R. | Transfemoral, Transapical | 12 | Long-term overall survival |
| Beohar, 2014 [31] | Retrospective observational | U.S.A. | 2007-2009 | 485 | 261 (53.8%) | N.A. | H.R. | Transfemoral, Transapical | 12 | Long-term overall survival |
| Boukhris, 2021 [43] | Retrospective observational | Canada | 2009-2019 | 412 | HR | Transfemoral, non-transfemoral | 12 | 30-day mortality, long-term overall survival, procedural complication | ||
| Corcione, 2021 [27] | Prospective observational | Italy | 2008-2018 | 3,705 | HR | NA | NA | 30-day mortality, long-term overall survival, procedural complication | ||
| Dvir, 2014 [32] | Retrospective observational | U.S.A. | 2007-2009 | 1,108 | 603 (54.4%) | 82.7 ± 7.2 | H.R. | Transfemoral, Transapical | 12 | Long-term overall survival |
| Gonzalez-Ferreiro, 2017 [16] | Retrospective observational | Spain | 2008-2015 | 770 | 332 (43.1%) | 80.7 ± 6.3 | H.R. | Transfemoral, Trans-axillary | 36 | Long-term overall survival, procedural complication |
| Iung, 2014 [33] | Retrospective observational | France | 2010-2011 | 2,552 | 1288 (50.5%) | 82.9 ± 7.2 | OR | Transfemoral, Transapical, Subclavian | 1 | 30-day mortality |
| Kamga, 2013 [29] | Prospective observational | Belgium | 2009-2011 | 30 | 16 (53.3%) | 86.0 ± 3.0 | H.R. | Transfemoral | 12 | Long-term overall survival |
| Kische, 2016 [39] | Retrospective observational | Germany | 2013-2015 | 172 | 100 (58.1%) | 81.0 ± 5.5 | OR | Transfemoral | 12 | 30-day mortality, long-term overall survival |
| Koifman, 2016 [34] | Retrospective observational | U.S.A. | 2007-2014 | 448 | 233 (52.0%) | 82.9 ± 8.3 | H.R. | Transfemoral | 12 | Long-term overall survival, procedural complication |
| Konigstein, 2015 [45] | Retrospective observational | Israel | 2009-2013 | 409 | 173 (42.3%) | 82.0 ± 5.7 | H.R., OR | Transfemoral | 12 | 30-day mortality, long-term overall survival, procedural complication |
| Marzo, 2021 [28] | Prospective observational | Italy | 2014-2019 | 645 | OR | Transfemoral | 6 | 30-day mortality, long-term overall survival, procedural complication | ||
| Mok, 2013 [36] | Retrospective observational | Canada | 2005-2009 | 319 | 147 (46.1%) | 80.0 ± 8.0 | H.R. | Transfemoral, Transapical, Transaortic | 12 | Long-term overall survival |
| Nieuwkerk, 2021 [41] | Prospective observational | U.S.A., Brazil, Israel, European countries | 2007-2018 | 12,381 | HR, OR | Transfemoral | 12 | 30-day mortality, long-term overall survival | ||
| Quine, 2020 [20] | Prospective observational | U.S.A., Australia | 2008-2019 | 634 | HR | Transfemoral, Transapical, Subclavian, Direct aortic, Subclavian and Transfemoral | 12 | 30-day mortality, long-term overall survival, procedural complication | ||
| Salizzoni, 2016 [38] | Retrospective observational | Italy | 2007-2012 | 1,904 | 757 (39.8%) | 81.7 ± 6.2 | H.R. | Transfemoral, Transapical, Transaortic, Trans axillary | 24 | Long-term overall survival |
| Seiffert, 2014 [37] | Retrospective observational | Germany | 2008-2011 | 845 | 413 (48.9%) | 80.9 ± 6.5 | H.R. | Transfemoral, Transapical, Subclavian | 12 | Long-term overall survival |
| Sharma, 2020 [13] | Retrospective observational | U.S.A. | 2011-2015 | 31,929 | HR | Transfemoral, Transapical, Transaortic, Subclavian | 12 | 30-day mortality, long-term overall survival, procedural complication | ||
| Tokarek, 2019 [35] | Retrospective observational | Finland | 2008-2015 | 148 | HR | Transfemoral, Transapical, Transaortic, Subclavian | 12 | 30-day mortality, long-term overall survival, procedural complication | ||
| Van der Boon, 2013 [41] | Retrospective observational | Netherlands | 2005-2011 | 940 | 506 (53.8%) | 81.0 ± 7.0 | H.R., OR | Transfemoral, Transaortic | 12 | 30-day mortality, long-term overall survival, procedural complication |
| Yamamoto, 2013 [42] | Retrospective observational | France | 2010-2011 | 3,072 | 1564 (50.9%) | 82.8 ± 7.2 | H.R., OR | Transfemoral, Transapical, Subclavian | 12 | 30-day mortality, long-term overall survival, procedural complication |
| Yoon, 2016 [40] | Retrospective observational | Korea | 2010-2014 | 848 | 396 (46.7%) | 81.8 ± 6.6 | H.R. | Transfemoral, Transapical, Transaortic, Subclavian | 24 | Long-term overall survival |
Hazards ratio;
Odds ratios.
Quality assessment and publication bias
According to the Newcastle Ottawa Scale, seven studies were assigned the score seven, 14 studies were assigned the score eight, and three studies were assigned the score nine. Overall, all of the included studies were of good quality owing to their robust methodology as shown in Supplementary Table 2. Funnel plots were created for 30-day mortality, A.K.I., major bleeding, vascular complications, and long-term overall survival as shown in Supplementary Figures 1, 2, 3, 4, 5, 6. All of the aforementioned outcomes were subjected to some degree of asymmetry. Egger’s regression test was used to confirm the risk of publication bias. Upon confirmation, only overall survival with 1 kg/m2 increase in B.M.I. had some degree of publication bias as shown in Supplementary Table 3.
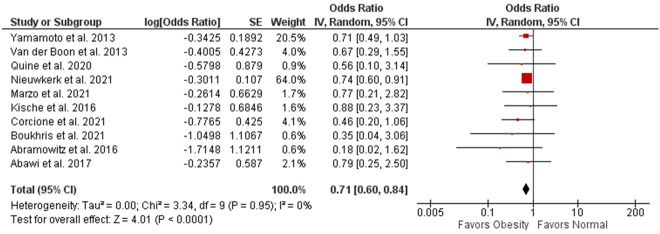
30-day mortality
10 studies compared 30-day mortality between obese patients and normal patients. Upon analysis, a significant decrease could be observed in 30-day mortality among obese patients (OR 0.71, 95% CI 0.60-0.84, P<0.0001; I2=0%) as shown in Figure 2.
Figure 2.
Forest plot of 30-day mortality and its association with obesity.
Long-term overall survival
11 studies compared the long-term overall survival between obese and normal patients, and their pooled estimates based on a random-effects model (HR 0.87, 95% CI 0.82-0.93, P<0.00001; I2=5%) indicated that obesity could be predictive of better prognosis among TAVI patients (Figure 3). Comparably, a significant improvement could be seen for long-term overall survival with every 1 kg/m2 increase in BMI (HR 0.96, 95% CI 0.94-0.98, P<0.0001; I2=45%) (Figure 4).
Figure 3.
Forest plot of long-term overall survival and its association with obesity.
Figure 4.
Forest plot of long-term overall survival and its association with B.M.I.
Procedural complications
Seven studies reported an incidence of MI. No significant difference was observed between obese and normal patients in MI (OR 0.84, 95% CI 0.52-1.34, P=0.66; I2=0%) as given in Figure 5. Similarly, pooled data of 11 studies showed no significant difference for major bleeding (OR 0.94, 95% CI 0.72-1.21, P=0.02; I2=54%) and major vascular events (OR 1.18, 95% CI 0.97-1.43, P=0.51; I2=0%) (Figures 6 and 7). Moreover, analysis of 10 studies revealed no difference between obese and normal patients suffering from A.K.I. following TAVI (OR 1.17, 95% CI 0.87-1.56, P=0.12; I2=36%) as shown in Figure 8.
Figure 5.
Forest plot of myocardial infarction and its association with obesity.
Figure 6.
Forest plot of major bleeding and its association with obesity.
Figure 7.
Forest plot of major vascular complications and its association with obesity.
Figure 8.
Forest plot of A.K.I. and its association with obesity.
Discussion
Our meta-analysis evaluated the existence of the obesity paradox in clinical outcomes among patients undergoing TAVI. The results of our analysis revealed obesity to be significantly associated with reduced 30-day mortality, and better overall long-term survival with no significant differences in the incidence of MI, major bleeding, A.K.I. and major vascular events in either of the two groups. Obesity has a strong correlation with metabolic diseases, hypertension and dyslipidemia, making it a strong risk factor for the occurrence of major cardiovascular events. However, recent evidence has highlighted that obese individuals may have a better prognosis following cardiovascular surgical interventions. A meta-analysis of 26,842 heart failure patients conducted by Khan and coworkers [46] showed that obese individuals (BMI≥30 kg/m) receiving left ventricular assist device (LVAD) implantation had significantly improved short-term (≤1 year) survival rates when compared with non-obese patients. A retrospective study including 865 candidates undergoing aortic valve replacement for AS found that a BMI in the lower 30 s resulted in lower risks of 30-day and long-term mortality [47]. Furthermore, another investigation revealed significantly reduced bleeding complications and incidence of stroke in obese patients compared with their counterpart (P<0.001 and P<0.03, respectively) [48].
The results of our analysis can be validated with findings from several other investigations. A meta-analysis of 12,330 patients conducted by Lv and coworkers [19] revealed a favorable prognosis in terms of 30-day and long-term mortality (P=0.024 and P<0.001, respectively) in obese individuals in comparison with non-obese patients. Furthermore, similar to our findings, they also observed that with every increase of 1 kg/m2 in BMI the rates of 30-day mortality significantly reduced. Similarly, another investigation sought to analyze 221,000 patients from the National Inpatient Sample Database found obese (P<0.001) and MO (P<0.001) TAVI patients to be significantly associated with reduced in-hospital mortality when compared with normal-weight individuals [49], further substantiating our results. Gupta and coworkers [50] assessed the obesity paradox in a total of 99,829 participants and ascertained the obese group to be significantly corelated with a lower risk of short and long-term mortality. However, unlike our results, they determined that obese patients were at significantly higher odds of developing AKI (P=0.01) compared to their normal-weight counterparts. It is important to emphasize that certain studies reported no significant differences in mortality rates across varying BMI categories [39,41]. For example, Abramowitz and coworkers [26] included a total of 805 patients and upon multivariate analysis found mortality rates to be comparable in both the cohorts. However, most of these investigations were limited by their small sample sizes which may account for the discrepancies with our findings.
The incidence of AS is generally more prevalent in obese patients than in non-obese patient [51]. Several factors that may elucidate the reasoning behind positive surgical outcomes in obese patients undergoing interventions for cardiovascular diseases should be discussed. According to Hayashida and coworkers [52] major vascular complications, which included access site related injuries leading to death, blood transfusions or surgical interventions, were found to be predictors of 30-day mortality in patients who underwent TAVI (P=0.049). Perhaps having a higher body surface area (BSA) and a larger vasculature serves to protect obese patients in acquiring vascular injuries during procedures. Zafrir and coworkers [53] analyzed data from the heart failure long-term registry of the Heart Failure Association of the European Society of Cardiology and found a progressive inverse relationship between all-cause mortality and BSA levels. Although our analysis stated no significant differences in bleeding and major vascular complications between the two cohorts, this may be attributed to the limited number of studies reporting on these outcomes.
Furthermore, age should also be considered a vital confounder in establishing the relationship between B.M.I. and mortality in TAVI patients. Older patients tend to be weaker and leaner compared to younger individuals and are associated with several accompanying comorbidities [54]. A study of 7099 patients revealed increasing age to be significantly associated with poor 30-day mortality outcomes in participants undergoing TAVI [55]. Obesity has become increasingly prevalent in the younger populations [56] and these individuals are also diagnosed earlier than their regular weight counterparts. Hence, it can be concluded that timely diagnosis, increased tolerance to surgical procedures and aggressive pharmacological intervention for several comorbidities may put obese patients at an advantage and likely result in fewer mortality rates, as observed in our analysis.
Moreover, adipokine physiology plays a crucial role in the improved survival rates of obese patients. An increased fat distribution in obesity offers a more extensive energy reserve to compensate for the increased catabolic demands in patients with cardiovascular disease [57]. Increased levels of adipocytes and lipoproteins might be beneficial as they bind to and neutralize bacterial toxin [58]. An elevated adiponectin level and a more rigorous catecholamine response to stress are also reported in patients with lower BMI [59] which may provide plausible reasonings for our results. Lastly, amongst the varying BMI categories reported in most studies, underweight patients carry the greatest risk of the mortality burden following TAVI. Studies suggest that cachexia is strongly interrelated with neuroendocrine and immunologic abnormalities [60] leading to adverse outcomes post-surgical procedures [61].
Body Mass Index (BMI) is widely utilized for assessing adiposity and categorizing individuals by weight classifications, encompassing underweight, normal weight, overweight, and obese categories. Nonetheless, its use as the sole measure of obesity has been questioned due to inherent limitations. BMI relies solely on weight and height, failing to distinguish between lean mass (muscle, bone) and fat mass. Moreover, BMI does not indicate fat distribution across the body, distinguishing between central (abdominal) and peripheral fat deposition [62]. Central obesity is particularly linked to heightened metabolic risks compared to peripheral fat. To address these limitations, alternative tools offer improved assessments of obesity. Waist circumference and waist-to-hip ratio measure abdominal fat distribution, which is metabolically active and strongly associated with obesity-related health risks [63]. Furthermore, advanced techniques such as dual-energy X-ray absorptiometry (DXA), bioelectrical impedance analysis (BIA), and underwater weighing provide more precise evaluations of body fat percentage and distribution than BMI [64]. These alternative measures contribute to a more comprehensive understanding of adiposity and its implications for health.
Our study comprises a few limitations that should be discussed. Firstly, most of the studies included in this analysis are retrospective, resulting in potential recall bias. Our meta-analysis could not conduct a subgroup analysis concerning factors such as TAVI access sites, race differences and severity of AS, as most investigators did not report on these parameters. Future investigations are encouraged to assess the impact of these factors to produce more concrete results. Secondly, our analysis comprised some studies that utilized univariate O.R.s to report their results, which might diminish the accuracy of our outcome. Lastly, since the incidence of comorbidities like hypertension, diabetes, and coronary heart disease is greater in obese patients compared to non-obese patients, it is imperative to adjust these factors when analyzing the overall effect of obesity on outcomes like mortality and complications associated with TAVI.
Conclusion
The results of this meta-analysis verify the concept of the ‘obesity paradox’ in the context of interventions such as TAVR in patients suffering from AS. Obesity and a high BMI significantly improved short and long-term overall mortality in patients of TAVI. However, no significant decrease was observed when analyzing complications of major bleeding, major vascular events, MI and AKI in the obese group.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based stud. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744–756. doi: 10.1056/NEJMra1313875. [DOI] [PubMed] [Google Scholar]
- 4.Thourani VH, Ailawadi G, Szeto WY, Dewey TM, Guyton RA, Mack MJ, Kron IL, Kilgo P, Bavaria JE. Outcomes of surgical aortic valve replacement in high-risk patients: a multiinstitutional study. Ann Thorac Surg. 2011;9:49–56. doi: 10.1016/j.athoracsur.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Li X, Xie W, Wang Y, Xu Z, Bai YX, Zhou Q, Wu Q. Risk factors and short-term outcomes of postoperative pulmonary complications in elderly patients after cardiopulmonary bypass. Clin Interv Aging. 2024;19:31–9. doi: 10.2147/CIA.S439601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 7.Mesnier J, Panagides V, Nuche J, Rodés-Cabau J. Evolving indications of transcatheter aortic valve replacement-where are we now, and where are we going. J Clin Med. 2022;11:3090. doi: 10.3390/jcm11113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckmann E, Martens A, Alhadi FA, Ius F, Koigeldiyev N, Fleissner F, Stiefel P, Kaufeld T, Haverich A, Shrestha M. Is bentall procedure still the gold standard for acute aortic dissection with aortic root involvement? Thorac Cardiovasc Surg. 2016;64:116–123. doi: 10.1055/s-0035-1552580. [DOI] [PubMed] [Google Scholar]
- 9.Ando T, Akintoye E, Trehan N, Telila T, Briasoulis A, Takagi H, Grines CL, Afonso L. Comparison of in-hospital outcomes of transcatheter aortic valve implantation versus surgical aortic valve replacement in obese (Body Mass Index ≥ 30 Kg/M2) Patient. Am J Cardiol. 2017;120:1858–1862. doi: 10.1016/j.amjcard.2017.07.098. [DOI] [PubMed] [Google Scholar]
- 10.Siontis GC, Praz F, Pilgrim T, Mavridis D, Verma S, Salanti G, Søndergaard L, Jüni P, Windecker S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J. 2016;37:3503–3512. doi: 10.1093/eurheartj/ehw225. [DOI] [PubMed] [Google Scholar]
- 11.Wolny R, Maehara A, Liu Y, Zhang Z, Mintz GS, Redfors B, Madhavan MV, Smits PC, Von Birgelen C, Serruys PW, Mehran R, Leon MB, Stone GW. The obesity paradox revisited: body mass index and -long-term outcomes after PCI from a large pooled patient-level database. EuroIntervention. 2020;15:1199–1208. doi: 10.4244/EIJ-D-19-00467. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Vallakati A, Einstein AJ, Lavie CJ, Arbab-Zadeh A, Lopez-Jimenez F, Mukherjee D, Lichstein E. Relationship of body mass index with total mortality, cardiovascular mortality, and myocardial infarction after coronary revascularization: evidence from a meta-analysis. Mayo Clin Proc. 2014;89:1080–1100. doi: 10.1016/j.mayocp.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A, Lavie CJ, Elmariah S, Borer JS, Sharma SK, Vemulapalli S, Yerokun BA, Li Z, Matsouaka RA, Marmur JD. Relationship of body mass index with outcomes after transcatheter aortic valve replacement: results from the national cardiovascular data-STS/ACC TVT registry. Mayo Clin Proc. 2020;95:57–68. doi: 10.1016/j.mayocp.2019.09.027. [DOI] [PubMed] [Google Scholar]
- 14.González-Ferreiro R, Muñoz-García AJ, López-Otero D, Avanzas P, Pascual I, Alonso-Briales JH, Trillo-Nouche R, Pun F, Jiménez-Navarro MF, Hernández-García JM, Morís C, González Juanatey JR. Prognostic value of body mass index in transcatheter aortic valve implantation: a “J”-shaped curve. Int J Cardiol. 2017;232:342–347. doi: 10.1016/j.ijcard.2016.12.051. [DOI] [PubMed] [Google Scholar]
- 15.Abawi M, Rozemeijer R, Agostoni P, van Jaarsveld RC, van Dongen CS, Voskuil M, Kraaijeveld AO, Doevendans PAFM, Stella PR. Effect of body mass index on clinical outcome and all-cause mortality in patients undergoing transcatheter aortic valve implantation. Neth Heart J. 2017;25:498–509. doi: 10.1007/s12471-017-1003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González Ferreiro R, López Otero D, Álvarez Rodríguez L, Iglesias Álvarez D, Cid Menéndez A, Antúnez Muiños PJ, Cid Álvarez B, Sanmartín Pena XC, Cruz-González I, Trillo Nouche R, González Juanatey JR. Transcatheter aortic valve replacement in patients with morbid obesity: procedure outcomes and medium-term follow-up. JACC Cardiovasc Interv. 2019;12:1192–1193. doi: 10.1016/j.jcin.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Das SR, Alexander KP, Chen AY, Powell-Wiley TM, Diercks DB, Peterson ED, Roe MT, De Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-segment elevation myocardial infarction results from the NCDR (national cardiovascular data registry) J Am Coll Cardiol. 2011;58:2642–2650. doi: 10.1016/j.jacc.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bundhun PK, Li N, Chen MH. Does an obesity paradox really exist after cardiovascular intervention?: a systematic review and meta-analysis of randomized controlled trials and observational studies. Medicine (Baltimore) 2015;94:e1910. doi: 10.1097/MD.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv W, Li S, Liao Y, Zhao Z, Che G, Chen M, Feng Y. The “obesity Paradox” does exist in patients undergoing transcatheter aortic valve implantation for aortic stenosis: a systematic review and meta-analysis. Interac Cardiovas Thora Surg. 2017;25:633–642. doi: 10.1093/icvts/ivx191. [DOI] [PubMed] [Google Scholar]
- 20.Quine EJ, Dagan M, William J, Nanayakkara S, Dawson LP, Duffy SJ, Stehli J, Dick RJ, Htun NM, Stub D, Walton AS. Long-term outcomes stratified by body mass index in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2020;137:77–82. doi: 10.1016/j.amjcard.2020.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic review. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB Valve Academic Research Consortium (VARC)-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2) Eur J Cardiothorac Surg. 2012;42:S45–60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O’Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 January 2022)
- 24.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Available online: www.cochrane-handbook.org.
- 26.Abramowitz Y, Chakravarty T, JilaihawI H, Cox J, Sharma RP, Mangat G, Nakamura M, Cheng W, Makkar RR. Impact of body mass index on the outcomes following transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2016;88:127–134. doi: 10.1002/ccd.26394. [DOI] [PubMed] [Google Scholar]
- 27.Corcione N, Testa A, Ferraro P, Morello A, Cimmino M, Albanese M, Giordano S, Bedogni F, Iadanza A, Berti S, Regazzoli D, Trani C, Pepe M, Frati G, Biondi Zoccai G, Giordano A Registro Italiano GISE sull’impianto di Valvola Aortica Percutanea (RISPEVA) Study Investigators. Baseline, procedural and outcome features of patients undergoing transcatheter aortic valve implantation according to different body mass index categories. Minerva Med. 2021;112:474–482. doi: 10.23736/S0026-4806.21.07379-1. [DOI] [PubMed] [Google Scholar]
- 28.De Marzo V, Crimi G, Benenati S, Buscaglia A, Pescetelli F, Vercellino M, Della Bona R, Sarocchi M, Canepa M, Ameri P, Balbi M, Porto I. BMI and acute kidney injury post transcatheter aortic valve replacement: unveiling the obesity paradox. J Cardiovasc Med (Hagerstown) 2021;22:579–585. doi: 10.2459/JCM.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 29.Kamga M, Boland B, Cornette P, Beeckmans M, De Meester C, Chenu P, Gurné O, Renkin J, Kefer J. Impact of frailty scores on outcome of octogenarian patients undergoing transcatheter aortic valve implantation. Acta Cardiol. 2013;68:599–606. doi: 10.1080/ac.68.6.8000007. [DOI] [PubMed] [Google Scholar]
- 30.Aung N, Dworakowski R, Byrne J, Alcock E, Deshpande R, Rajagopal K, Brickham B, Monaghan MJ, Okonko DO, Wendler O, Maccarthy PA. Progressive rise in red cell distribution width is associated with poor outcome after transcatheter aortic valve implantation. Heart. 2013;99:1261–1266. doi: 10.1136/heartjnl-2013-303910. [DOI] [PubMed] [Google Scholar]
- 31.Beohar N, Zajarias A, Thourani VH, Herrmann HC, Mack M, Kapadia S, Green P, Arnold SV, Cohen DJ, Généreux P, Xu K, Leon MB, Kirtane AJ. Analysis of early out-of hospital mortality after transcatheter aortic valve implantation among patients with aortic stenosis successfully discharged from the hospital and alive at 30 days (from the Placement of Aortic Transcatheter Valves Trial) Am J Cardiol. 2014;114:1550–1555. doi: 10.1016/j.amjcard.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dvir D, Waksman R, Barbash IM, Kodali SK, Svensson LG, Tuzcu EM, Xu K, Minha S, Alu MC, Szeto WY, Thourani VH, Makkar R, Kapadia S, Satler LF, Webb JG, Leon MB, Pichard AD. Outcomes of patients with chronic lung disease and severe aortic stenosis treated with transcatheter versus surgical aortic valve replacement or standard therapy: insights from the PARTNER trial (Placement of AoRTic TraNscathetER Valve) J Am Coll Cardiol. 2014;63:269–279. doi: 10.1016/j.jacc.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Iung B, Laouénan C, Himbert D, Eltchaninoff H, Chevreul K, Donzeau-Gouge P, Fajadet J, Leprince P, Leguerrier A, Lièvre M, Prat A, Teiger E, Laskar M, Vahanian A, Gilard M FRANCE 2 Investigators. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100:1016–1023. doi: 10.1136/heartjnl-2013-305314. [DOI] [PubMed] [Google Scholar]
- 34.Koifman E, Kiramijyan S, Negi SI, Didier R, Escarcega RO, Minha S, Gai J, Torguson R, Okubagzi P, Ben-Dor I, Satler LF, Pichard AD, Waksman R. Body mass index association with survival in severe aortic stenosis patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2016;88:118–124. doi: 10.1002/ccd.26377. [DOI] [PubMed] [Google Scholar]
- 35.Tokarek TA, Dziewierz A, Sorysz D, Bagieński M, Rzeszutko Ł, Krawczyk-Ożóg A, Dudek D, Kleczyński P. The obesity paradox in patients undergoing transcatheter aortic valve implantation: is there any effect of body mass index on survival? Kardiol Pol. 2019;77:190–197. doi: 10.5603/KP.a2018.0243. [DOI] [PubMed] [Google Scholar]
- 36.Mok M, Nombela-Franco L, Dumont E, Urena M, Delarochellière R, Doyle D, Villeneuve J, Côté M, Ribeiro HB, Allende R, Laflamme J, DeLarochellière H, Laflamme L, Amat-Santos I, Pibarot P, Maltais F, Rodés-Cabau J. Chronic obstructive pulmonary disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes, prognostic markers, and functional status change. JACC Cardiovasc Interv. 2013;6:1072–1084. doi: 10.1016/j.jcin.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Seiffert M, Sinning JM, Meyer A, Wilde S, Conradi L, Vasa-Nicotera M, Ghanem A, Kempfert J, Hammerstingl C, Ojeda FM, Kim WK, Koschyk DH, Schirmer J, Baldus S, Grube E, Möllmann H, Reichenspurner H, Nickenig G, Blankenberg S, Diemert P, Treede H, Walther T, Werner N, Schnabel RB. Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol. 2014;103:631–640. doi: 10.1007/s00392-014-0692-4. [DOI] [PubMed] [Google Scholar]
- 38.Salizzoni S, D’Onofrio A, Agrifoglio M, Colombo A, Chieffo A, Cioni M, Besola L, Regesta T, Rapetto F, Tarantini G, Napodano M, Gabbieri D, Saia F, Tamburino C, Ribichini F, Cugola D, Aiello M, Sanna F, Iadanza A, Pompei E, Stefàno P, Cappai A, Minati A, Cassese M, Martinelli GL, Agostinelli A, Fiorilli R, Casilli F, Reale M, Bedogni F, Petronio AS, Mozzillo RA, Bonmassari R, Briguori C, Liso A, Sardella G, Bruschi G, Fiorina C, Filippini C, Moretti C, D’Amico M, La Torre M, Conrotto F, Di Bartolomeo R, Gerosa G, Rinaldi M TAVI Team. Early and mid-term outcomes of 1904 patients undergoing transcatheter balloon-expandable valve implantation in italy: results from the italian transcatheter balloon-expandable valve implantation registry (ITER) Eur J Cardiothorac Surg. 2016;50:1139–1148. doi: 10.1093/ejcts/ezw218. [DOI] [PubMed] [Google Scholar]
- 39.Kische S, D’Ancona G, Agma HU, El-Achkar G, Dißmann M, Ortak J, Öner A, Ketterer U, Bärisch A, Levenson B, Ince H. Transcatheter aortic valve implantation in obese patients: overcoming technical challenges and maintaining adequate hemodynamic performance using new generation prostheses. Int J Cardiol. 2016;220:909–913. doi: 10.1016/j.ijcard.2016.06.155. [DOI] [PubMed] [Google Scholar]
- 40.Yoon SH, Ahn JM, Hayashida K, Watanabe Y, Shirai S, Kao HL, Yin WH, Lee MK, Tay E, Araki M, Yamanaka F, Arai T, Lin MS, Park JB, Park DW, Kang SJ, Lee SW, Kim YH, Lee CW, Park SW, Muramatsu T, Hanyu M, Kozuma K, Kim HS, Saito S, Park SJ Asian TAVR Investigators. Clinical outcomes following transcatheter aortic valve replacement in Asian population. JACC Cardiovasc Interv. 2016;9:926–933. doi: 10.1016/j.jcin.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 41.van Der Boon RM, Chieffo A, Dumonteil N, Tchetche D, Van Mieghem NM, Buchanan GL, Vahdat O, Marcheix B, Serruys PW, Fajadet J, Colombo A, Carrié D, van Domburg RT, de Jaegere PP PRAGMATIC-Plus Researchers. Effect of body mass index on short- and long-term outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2013;111:231–236. doi: 10.1016/j.amjcard.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto M, Mouillet G, Oguri A, Gilard M, Laskar M, Eltchaninoff H, Fajadet J, Iung B, Donzeau-Gouge P, Leprince P, Leuguerrier A, Prat A, Lievre M, Chevreul K, Dubois-Rande JL, Teiger E FRANCE 2 Registry Investigators. Effect of body mass index on 30- and 365-day complication and survival rates of transcatheter aortic valve implantation (from the FRench Aortic National CoreValve and Edwards 2 [FRANCE 2] registry) Am J Cardiol. 2013;112:1932–1937. doi: 10.1016/j.amjcard.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 43.Boukhris M, Forcillo J, Potvin J, Noiseux N, Stevens LM, Badreddine M, Gobeil JF, Masson JB. Does “obesity paradox” apply for patients undergoing transcatheter aortic valve replacement? Cardiovasc Revasc Med. 2022;38:1–8. doi: 10.1016/j.carrev.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 44.van Nieuwkerk AC, Santos RB, Sartori S, Regueiro A, Tchétché D, Mehran R, Delewi R CENTER collaboration. Impact of body mass index on outcomes in patients undergoing transfemoral transcatheter aortic valve implantation. JTCVS Open. 2021;6:26–36. doi: 10.1016/j.xjon.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konigstein M, Havakuk O, Arbel Y, Finkelstein A, Ben-Assa E, Leshem Rubinow E, Abramowitz Y, Keren G, Banai S. The obesity paradox in patients undergoing transcatheter aortic valve implantation. Clin Cardiol. 2015;38:76–81. doi: 10.1002/clc.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan MS, Yuzefpolskaya M, Memon MM, Usman MS, Yamani N, Garan AR, Demmer RT, Colombo PC. Outcomes associated with obesity in patients undergoing left ventricular assist device implantation: a systematic review and meta-analysis. ASAIO J. 2020;66:401–408. doi: 10.1097/MAT.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 47.Roberts WC, Roberts CC, Vowels TJ, Ko JM, Filardo G, Hamman BL, Matter GJ, Henry AC, Hebeler RF Jr. Effect of body mass index on survival in patients having aortic valve replacement for aortic stenosis with or without concomitant coronary artery bypass grafting. Am J Cardiol. 2011;108:1767–1771. doi: 10.1016/j.amjcard.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Einarsdóttir FH, Gunnarsdóttir ELT, Gunnarsdóttir SLX, Jensen EM, Viktorsson SA, Ingvarsdóttir IL, Larusdottir KJ, Heitmann LA, Gudbjartsson T. The effect of obesity on the outcome of surgical aortic valve replacement for aortic stenosis. Laeknabladid. 2023;109:235–42. doi: 10.17992/lbl.2023.05.743. [DOI] [PubMed] [Google Scholar]
- 49.Patel E, Varghese JJ, Garg M, Yacob O, Sánchez JS, Garcia-Garcia HM. Comparison of body mass index (four categories) to in-hospital outcomes in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2023;192:190–5. doi: 10.1016/j.amjcard.2023.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Gupta R, Mahmoudi E, Behnoush AH, Khalaji A, Malik AH, Sood A, Bandyopadhyay D, Zaid S, Goel A, Sreenivasan J, Patel C, Vyas AV, Lavie CJ, Patel NC. Effect of BMI on patients undergoing transcatheter aortic valve implantation: a systematic review and meta-analysis. Prog Cardiovasc Dis. 2023;78:58–66. doi: 10.1016/j.pcad.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Larsson SC, Wolk A, Hakansson N, Back M. Overall and abdominal obesity and incident aortic valve stenosis: two prospective cohort studies. Eur Heart J. 2017;38:2192–2197. doi: 10.1093/eurheartj/ehx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayashida K, Lefevre T, Chevalier B, Hovasse T, Romano M, Garot P, Mylotte D, Uribe J, Farge A, Donzeau-Gouge P, Bouvier E, Cormier B, Morice MC. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4:851–858. doi: 10.1016/j.jcin.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Zafrir B, Salman N, Crespo-Leiro MG, Anker SD, Coats AJ, Ferrari R, Filippatos G, Maggioni AP, Mebazaa A, Piepoli MF, Ruschitzka F, Paniagua-Martin MJ, Segovia J, Laroche C, Amir O Heart Failure Long-Term Registry Investigators. Body surface area as a prognostic marker in chronic heart failure patients: results from the heart failure registry of the heart failure association of the european society of cardiology. Eur J Heart Fail. 2016;18:859–868. doi: 10.1002/ejhf.551. [DOI] [PubMed] [Google Scholar]
- 54.Gurm HS, Whitlow PL, Kip K BARI Investigators. The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (BARI) J Am Coll Cardiol. 2002;39:834–840. doi: 10.1016/s0735-1097(02)01687-x. [DOI] [PubMed] [Google Scholar]
- 55.Attinger-Toller A, Ferrari E, Tueller D, Templin C, Muller O, Nietlispach F, Toggweiler S, Noble S, Roffi M, Jeger R, Huber C, Carrel T, Pilgrim T, Wenaweser P, Togni M, Cook S, Heg D, Windecker S, Goy JJ, Stortecky S. Age-related outcomes after transcatheter aortic valve replacement: Insights From the SwissTAVI Registry. JACC Cardiovasc Interv. 2021;14:952–60. doi: 10.1016/j.jcin.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 56.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 57.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M ADHERE Scientific Advisory Committee and Investigators. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the acute decompensated heart failure national registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Csige I, Ujvárosy D, Szabó Z, Lorincz I, Paragh G, Harangi M, Somodi S. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:3407306. doi: 10.1155/2018/3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber MA, Neutel JM, Smith DHG. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol. 2001;37:169–174. doi: 10.1016/s0735-1097(00)01103-7. [DOI] [PubMed] [Google Scholar]
- 60.Mano A, Fujita K, Uenomachi K, Kazama K, Katabuchi M, Wada K, Terakawa N, Arai K, Hori Y, Hashimoto S, Nakatani T, Kitamura S. Body mass index is a useful predictor of prognosis after left ventricular assist system implantation. J Heart Lung Transplant. 2009;28:428–433. doi: 10.1016/j.healun.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Wang Z, Huang J, Fan J, Du H, Liu L, Che G. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the ‘obesity paradox’ really exist? Eur J Cardiothorac Surg. 2017;51:817–828. doi: 10.1093/ejcts/ezw386. [DOI] [PubMed] [Google Scholar]
- 62.Etchison WC, Bloodgood EA, Minton CP, Thompson NJ, Collins MA, Hunter SC, Dai H. Body mass index and percentage of body fat as indicators for obesity in an adolescent athletic population. Sports Health. 2011;3:249–252. doi: 10.1177/1941738111404655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravensbergen HR, Lear SA, Claydon VE. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. J Neurotrauma. 2014;31:292–300. doi: 10.1089/neu.2013.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duren DL, Sherwood RJ, Czerwinski SA, Lee M, Choh AC, Siervogel RM, Cameron Chumlea W. Body composition methods: comparisons and interpretation. J Diabetes Sci Technol. 2008;2:1139–1146. doi: 10.1177/193229680800200623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.