Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) has a markedly deleterious impact on a patient’s quality of life. It manifests as pain, paresthesia, numbness, and weakness, particularly in the context of cisplatin (CDDP), a widely utilised chemotherapeutic agent renowned for its pronounced peripheral nerve toxicity. Trichosanthes kirilowii Maxim. (Cucurbitaceae, TK) and cucurbitacin D(CucD), its bioactive compound, have been demonstrated to possess anti-tumour, anti-inflammatory, and antioxidant properties. However, their potential to alleviate CIPN has not been fully exploredyet. The present study evaluated effectiveness of TK and CucD in mitigating CDDP-induced neuropathic pain using both cellular and animal models. CDDP, TK extracts (TKD and TKE), and CucD dose-dependently reduced viability and apoptosis of PC12 cells. Conversely, pre-treatment with TKD, TKE, and CucD exhibited significant protective effects against CDDP-induced cytotoxicity, preserving cell viability and morphology while enhancing neurite outgrowth. In vivo, administration of CDDP resulted in the development of mechanical allodynia and thermalhyperalgesia in rats. However, treatment with TKD and TKE led to a notable improvement in pain threshold and a reduction in hyperalgesia, while CucD demonstrated less pronounced effects. Although body weight was reduced in the CDDP-treated group, it was not significantly mitigated bytreatments. In conclusion, results of this study indicate that TKD, TKE, and CucD have the potential to alleviate CDDP-induced neuropathic pain by protecting against cell damage, promoting neuriteregeneration, and improving pain responses in animal models. Further investigation into TK and CucD as therapeutic options for managing CIPN is warranted.
Keywords: chemotherapy-induced peripheral neuropathy, cisplatin, Trichosanthes kirilowii maxim., cucurbitacin D
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a significant clinical condition marked by intense pain, tingling, numbness, and weakness that can severely impair a patient’s quality of life.1 -4 It results from various chemotherapy agents such as platinum compounds, taxanes, plant alkaloids, and proteasome inhibitors known to trigger diverse neuropathological damage.5 -8
Cisplatin (CDDP) is a prominent anti-tumor agent used to treat cancers such as advanced ovarian, testicular, gastric, and breast cancers.9 -13 CDDP is known to be the most potent peripheral neurotoxic agent. However, issues such as nephrotoxicity and bone marrow suppression are commonly observed.14 -16 Neuropathy induced by CDDP typically occurs starting from a dose of 350 mg/m². It has been reported that 92% of patients are affected by CDDP at doses of 500 to 600 mg/m².17 -19 The majority of CDDP models of CIPN exhibit a chronic nature. However, it has been shown that a single dose of CDDP can induce mechanical allodynia and heat hyperalgesia, while failing to elicit cold allodynia. 20 In the case of multiple doses, animals consistently exhibit mechanical allodynia, although effects on cold allodynia and heat hyperalgesia vary. 21 Neurotoxicity induced by platinum compounds is known to primarily damage neurons in the dorsal root ganglia (DRG).22,23 CDDP can damage peripheral neurons through accumulation of DNA-CDDP adducts, which can lead to apoptosis in DRG neurons. 12
Trichosanthes kirilowii Maxim. (Cucurbitaceae, TK) has seen widespread use in traditional medicine due to its anti-tumor,24 -26 anti-inflammatory27,28, antioxidant,29,30 and anti-diabetic 31 properties. An ethanol extract of TK has been shown to possess protective effects against CDDP-induced nephrotoxicity, 32 underscoring its potential for addressing adverse effects associated with chemotherapeutic agents. Bioactive compounds of TK are known as cucurbitacins with a total of 17 major molecules documented, ranging from cucurbitacin A to cucurbitacin T. They are predominantly present in root tubers. Among cucurbitacins identified, cucurbitacin B (approximately 250 µg/g) and CucD (approximately 50 µg/g) exhibit the highest concentrations. 33 CucD is recognized for its robust anticancer properties.34 -36 However, it has also been observed to mitigate cold and mechanical allodynia induced by paclitaxel, a finding consistent with that observed for TK. 37 Although Cucurbitacin B was also investigated in the study of Lee et al, 37 it was excluded due to its dose-dependent cytotoxic effect in non-cancerous RAW 264.7 cells.
While existing research has explored analgesic effects of TK and CucD, studies focusing on CIPN are limited with a notable exception of paclitaxel, a taxane class drug. 37 Therefore, this study aimed to explore the efficacy of TK and CucD in relieving CDDP-induced neuropathic pain in both in vitro and in vivo contexts.
Materials and Methods
Drugs
Trichosanthes kirilowii Maxim. (TK) was provided by Sanyacho Village (Jangheung, Republic of Korea) and extracted using distilled water (TKD) or 70% ethanol (TKE) by FORUSGEN (Chungju, Republic of Korea). Cucurbitacin D (Cat# CFN90209, CucD) with a purity of over 98% was sourced from ChemFaces (Wuhan, China) and prepared in dimethyl sulfoxide (DMSO, Sigma-Aldrich). Cisplatin (Cat# 1134357, CDDP) was acquired from Sigma Aldrich (St. Louis, MO, USA). Pregabalin (Cat# PRGB-192B08S, PRG) was obtained from HL Genomics (Yongin, Republic of Korea).
Cell Culture
PC12 cells (Cat# CRL-1721, RRID: CVCL_0481) were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). They were cultured in RPMI medium (Welgene, Gyeongsan, Republic of Korea) supplemented with 10% heat-inactivated horse serum (Gibco™, Waltham, Massachusetts, USA), 5% fetal bovine serum (FBS, J R Scientific, Woodland, CA, USA), and 1% penicillin-streptomycin (Lonza, Basel, Switzerland). These cells were maintained in a 37ºC humidified atmosphere containing 5% CO2.
Cell Viability Assay
Cell viability was assessed using the MTT assay. Cells were plated into a 96-well plate at a density of 1 × 104 cells/well and treated with various concentrations of CDDP, TKD, TKE, or CucD for 48 hours. Pre-treatment with TKD, TKE, or CucD was conducted 2 hours before administering CDDP. Subsequently, aqueous MTT reagent (5 mg/ml, Sigma-Aldrich) was added to each well followed by incubation at 37ºC for 2 hours. After the supernatant was removed, 100 μl of DMSO (Duksan, Seoul, Republic of Korea) was added to dissolve formazan crystals and the absorbance was measured at 570 nm using a VersaMax microplate reader (Molecular Devices, Winnersh, UK).
Neurite Outgrowth Assay Using PC12 Cells
For attachment of PC12 cells, solutions of poly-l-lysine (PLL, Sigma Aldrich) and fibronectin (Sigma-Aldrich) were employed. Fibronectin powder was dissolved in distilled water (D.W.) to a final concentration of 40 μg/ml. PLL was applied in each well of microplates for 2 hours at RT. Plates with the fibronectin solution were stored at 4ºC overnight. Solutions were then removed and plates were washed 3 times (5 minutes for each wash) with 200 μl of PBS containing CaCl2 and MgCl2 (Gibco™). After being coated with PLL and fibronectin, plates were stored at 4ºC. Before plating cells, plates were exposed to UV light for 30 minutes.
Neurite outgrowth was evaluated using a Neurite Outgrowth Staining Kit (Cat# A15001, Invitrogen™, Thermo Fisher Scientific, Carlsbad, CA, USA) according to the manufacturer’s protocol. Briefly, cells were seeded onto 96-well black/clear bottom plates (SPL Life Sciences, Pocheon, Republic of Korea) in a growth medium containing 100 ng/ml Nerve Growth Factor (NGF, Sigma-Aldrich) followed by incubation for 48 hours. Subsequently, cells were washed with PBS and stained with 1× working Stain Solution for 20 minutes at RT. After staining, 1× working Background Suppression Dye was added to each well. A fluorescence microplate reader (Synergy H1, Biotek, Seoul, Republic of Korea) was then set to standard TRITC settings.
Detection of Apoptosis Induction
Apoptosis induction was assessed using Annexin-V/7-AAD analysis. Treated PC12 cells were harvested via centrifugation at 225 RCF for 8 minutes at RT, washed with PBS, stained with Annexin-V-FITC (Cat# 556570, RRID: AB_2869085, BD Biosciences, Franklin Lakes, New Jersey, USA) for 15 minutes, then supplemented with 7-AAD Ready Made Solution (Cat# SML1633, Sigma-Aldrich) for 5 minutes in the dark. Following staining, cells were analyzed using a FACS flow cytometer (Beckman Colter, Brea, CA, USA).
Western Blotting
Cells were washed twice with PBS, lysed with RIPA buffer (Biosesang, Yongin, Republic of Korea), and incubated on ice for 30 minutes. Cellular debris was removed by centrifugation at 13 000 rpm for 20 minutes at 4ºC. Protein concentrations were determined using a Bio-Rad protein assay dye (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein were separated on SDS-PAGE gels and meticulously transferred onto nitrocellulose (NC) membranes (Cytiva, Little Chalfont, Buckinghamshire, UK) at 85 V for 90 minutes. Blots were incubated with primary antibodies overnight at 4ºC, washed with PBS-T (0.1% Tween-20 in PBS), and developed using an ECL detection kit (DoGEN, Seoul, Korea). The following primary antibodies were used: PARP (Cat# 9542, RRID: AB_2160739, Cell signaling Technology, Danvers, Massachusetts, USA), cleaved caspase-3 (Cat# 9661, RRID: AB_2341188, Cell signaling Technology), p-p53 (Cat# 9284, RRID: AB_331464, Cell signaling Technology), p53 (Cat# sc-126, RRID: AB_628082, Santa cruz), p-H2A.X (Cat# 2577, RRID: AB_2118010, Cell signaling Technology), and H2A.X (Cat# 2595, RRID: AB_10694556, Cell signaling Technology).
Measurement of Intracellular ROS Levels
Cellular ROS generation was assessed using a DCFDA/H2DCFDA Assay Kit (Cat# ab113851, Abcam). Cells were incubated with DCFDA solution at 37ºC for 30 minutes in the dark. These cells were then washed with 1× Buffer and treated with different drugs for 48 h. Fluorescence was observed and recorded using a fluorescence microplate reader at Ex/Em = 485/535 nm. All values of ROS level (%) were normalized to the control group.
Animals
Male Sprague Dawley rats (RRID_RGD_734476, 5 weeks old) were obtained from YoungBio (Seongnam, Republic of Korea). These animals were transferred to a specific pathogen-free animal center and housed in cages under standard experimental conditions (temperature: 23°C ± 2°C, humidity: 65 ± 5%, and a 12-h light and 12-h dark cycle). They were maintained on a standard diet with freely available water. This animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Rudacure Co., LTD (Incheon, Republic of Korea; Permit No. 23-RCI-R-051).
Establishment of CDDP-Induced Peripheral Neuropathy
CDDP 20 mg was dissolved in 400 µl of DMSO and then diluted with 99.6 ml of saline to achieve a final concentration of 0.2 mg/ml. A solution of 2 ml was administered intraperitoneally. CDDP (2 mg/kg) was administered once a day for 4 consecutive days, with a 24-h interval between each administration to induce pain. Dose and duration of CDDP were chosen based on previously reported studies.38 -40 On the seventh day following CDDP administration, allodynia responses and thermal nociceptive responses were quantified to confirm the induction of pain. The success of pain induction was indicated by a reduction in baseline pain response measurement of more than 40%.
Animal Study
Rats were randomly divided into 2 groups: a control group (n = 6) and a CIPN group (n = 36). Rats in the CIPN group were further divided into 6 subgroups: (1) 0.9% normal saline (Saline), (2) PRG (30 mg/kg), (3) TKD (500 mg/kg), (4) TKE (500 mg/kg), (5) CucD low (5 μg/kg), and (6) CucD high (25 μg/kg). TK and CucD doses were chosen based on previous studies.31 -33,37 TKD, TKE, and PRG were suspended in Saline. CucD (400 µg) was dissolved in 1% (0.16 ml) DMSO, followed by sequential addition of 9% (1.44 ml) DMSO, 40% (6.4 ml) PEG400 (Duksan), 5% (0.8 ml) Tween80 (Duksan), and 45% (7.2 ml) saline. This resulted in a solubility of 25 µg/ml CucD. Following the 7th day of CDDP administration, the presence of neuropathy was confirmed. Thereafter, saline and CucD were administered via intraperitoneal injection, while TKD and TKE were administered orally. Mechanical allodynia was assessed using the Von Frey test. Thermal hyperalgesia was evaluated using the Hargreaves test before CDDP administration and at 7, 8, 9, 10, 14, and 21 days after CDDP administration. Body weights of rats were recorded on the day of CDDP injection (day 0) and on days 1, 2, 3, 7, 8, 9, 10, 14, and 21.
Assessment of Serum TNF-α, IL-1β, and IL-6 Levels
Serum TNF-α, IL-1β, and IL-6 levels were measured using ELISA kits (TNF-α: Cat# DY510-05; IL-1β: Cat# DY501-05; and IL-6: Cat# DY506-05, R&D systems, Minneapolis, MN, USA) according to the manufacturer’s protocol.
Statistical Analysis
Quantitative data are expressed as mean ± standard deviation (SD) or mean ± standard error of the mean (SEM). Significance was assessed via one-way analysis of variance (ANOVA) or two-way ANOVA according to the experimental design. All statistical analyses were performed using GraphPad Prism (RRID: SCR_002798, version 8.0. GraphPad Software Inc., USA). A P-value of less than .05 was considered statistically significant. Statistically significant differences are marked by asterisks (*P < .05, **P < .01, ***P < .001, and ****P < .0001).
Results
Effects of CDDP, TKD, TKE, and CucD on Viability of PC12 Cells
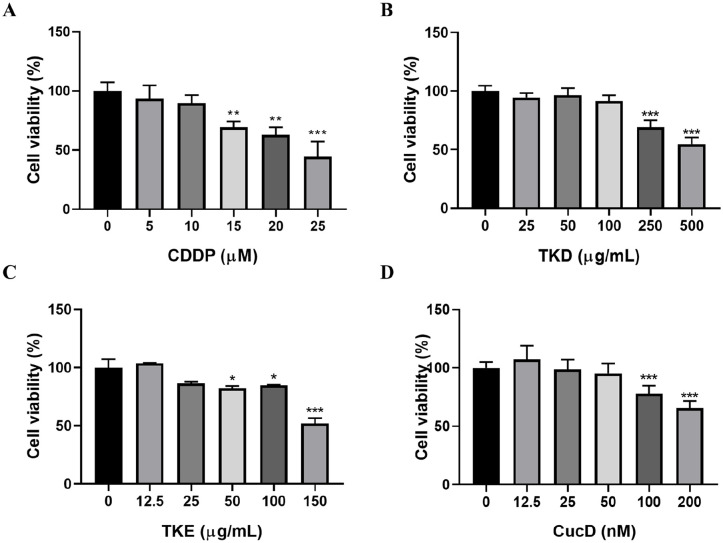
To evaluate the impact of CDDP on neuropathy, PC12 cells were subjected to treatment of CDDP with increasing concentrations and cell viability was evaluated at 48 hours after treatment. CDDP at concentrations starting from 15 μM and higher diminished cell viability in a dose-dependent manner (Figure 1A). Treatment with CDDP decreased cell viability to 69.3% at 15 µM, 62.9% at 20 µM, and 44.6% at 25 µM. Non-toxic concentrations of TKD, TKE, and CucD for PC12 cells were identified. As shown in Figure 1B to D, TKD at concentrations up to 100 μg/ml, TKE at concentrations up to 25 μg/ml, and CucD at concentrations up to 50 nM did not significantly decrease viabilities of PC12 cells.
Figure 1.
Effects of CDDP and drugs on cell viability in PC12 cells. Cells were treated with different concentrations of (A) CDDP, (B) TKD, (C) TKE, and (D) CucD for 48 hours. Data are means ± SD (n = 3). *P < .05, **P < .01, ***P < .001 versus control group.
TKD, TKE, and CucD Protect PC12 Cells Against CDDP-Induced Cytotoxicity
To assess protective effects of TKD, TKE, and CucD against CDDP-induced cytotoxicity, PC12 cells were pre-treated with TKD, TKE, and CucD for 2 hours, followed by exposure to CDDP for 48 hours. Pre-treatment with TKD, TKE, and CucD significantly attenuated the reduction in cell viability induced by CDDP. In the absence of TKD pretreatment, CDDP treatment resulted in a 60% decrease in cell viability. Conversely, TKD pretreatment led to a significant increase in cell viability to 87.2% at 25 µg/ml compared to the control without TKD pretreatment (Figure 2A). In TKE pretreated groups, cell viability showed marked increases (90.1%, 85.8%, and 88.9% at 6.25, 12.5, and 25 µg/ml, respectively) at all 3 concentrations used for TKE compared to the control without TKE pretreatment (CDDP treatment alone; Figure 2B). Pre-treatment with CucD offered protection against CDDP-induced cellular damage, increasing cell viability to 99.2% at 25 nM (Figure 2C). While TKD, TKE, and CucD alone did not affect cell viability, they effectively mitigated toxic effects of CDDP on PC12 cells. CDDP treatment alone resulted in cells exhibiting slightly pointed morphologies. However, co-treatment with TKD, TKE, or CucD led to cell aggregation and a rounded shape in cell morphology analyses (Figure 2D).
Figure 2.
Effects of drugs on CDDP-induced cytotoxicity in PC12 cells. Cells were co-treated with the CDDP (20 μm) and indicated concentrations of (A) TKD (0-100 μg/ml), (B) TKE (0-25 μg/ml), or (C) CucD (0-50 nM) for 48 hours. Data are presented as the means ± SD (n = 3). *P < .05, **P < .01, ***P < .001 versus control group; #P < .05, ##P < .01, ###P < .001 versus CDDP group. (D) At the end of treatment, Morphological changes of PC12 cells were photographed under an inverted microscope (200× magnification).
TKD, TKE, and CucD Protect PC12 Cells Against CDDP-Induced Neurodegeneration
To examine effects of TKD, TKE, and CucD on neurite outgrowth in PC12 cells, we initially determined if CDDP could inhibit neurite extension. NGF is a neurotrophic factor that can promote neurite extension and differentiation in developing neurons. First, PC12 cells were cultured with NGF. Upon exposure to CDDP alone, significant neurite atrophy was induced. Treatment with TKD, TKE, or CucD promoted neurite extension in PC12 cells at 48 hours post-treatment (Figure 3A). In comparison with the control group, CDDP alone resulted in an average decrease of 0.73-fold in neurite outgrowth. However, when TKD was used for pretreatment, neurite outgrowth was restored to 0.93-fold at 6.3 µg/ml, 0.89-fold at 12.5 µg/ml, and 1.09-fold at 25 µg/ml. After pre-treatment with TKE, neurite outgrowth was restored to 0.93-fold at 6.3 µg/ml, 0.89-fold at 12.5 µg/ml, and 1.09-fold at 25 µg/ml. Pre-treatment with CucD resulted in neurite outgrowth being restored to 1.06-fold at 25 nM. These results were significantly higher than those observed in cells treated with CDDP alone (Figure 3B-D).
Figure 3.
Protective effects of drugs on CDDP-induced neurite atrophy in PC12 cells. To stimulate neurite outgrowth, NGF (100 ng/ml) was used to pre-treat the cells for 2 days. (A) Representative images of co-treatment with CDDP and TKD, TKE, or CucD in the presence of NGF (200× magnification). (B-D) Neurite-bearing cells were quantitatively analyzed as described in the neurite outgrowth assay. Values are the means ± SD of 3 independent experiments (n = 3). *P < .05, **P < .01, ***P < .001 versus NGF group; #P < .05, ##P < .01, ###P < .001 versus CDDP group.
TKD, TKE, and CucD Attenuate CDDP-Induced Apoptosis in PC12 Cells
To explore mitigating effects of TKD, TKE, and CucD on CDDP-induced apoptosis, we conducted FACS analysis and assessed expression levels of apoptosis-related proteins in PC12 cells. As illustrated in Figure 4A, treatment with CDDP alone promoted apoptotic cell death. However, such cell death was significantly diminished following treatment with TKD, TKE, and CucD. Treatment with CDDP alone increased apoptotic cell population by approximately 20%, whereas pre-treatment with TKD decreased it in a dose-dependent manner. Pretreatment with TKD at 50 and 100 µg/ml decreased apoptotic cell populations to 15.3% and 11.3%, respectively (Figure 4B). Pre-treatment with TKE significantly decreased the apoptosis rate, reaching 13% at a concentration of 12.5 µg/ml (Figure 4C). Pre-treatment with CucD also decreased apoptotic cell death to 17.6% at 25 nM (Figure 4D). Statistical analysis of apoptotic cells further corroborated protective effects of TKD, TKE, and CucD. Furthermore, treatment with CDDP alone increased cleavage of PARP and caspase-3. However, treatment with TKD, TKE, or CucD attenuated such increases of caspase-3 and cleavage of PARP (Figure 5).
Figure 4.
Inhibitory effects of drugs on CDDP-induced apoptosis in PC12 cells. (A) PC12 cells were untreated (control) or treated with CDDP alone or CDDP + drugs (TKD, TKE, CucD), stained with Annexin V-FITC/7-AAD and cell apoptosis measured by flow cytometry. Quantitative analysis of apoptosis rate of PC12 cells treated with (B) TKD, (C) TKE, or (D) CucD in the presence or absence of CDDP. Data are analyzed by one-way ANOVA and expressed as means ± SD (n = 3). ***P < .001 versus control group; #P < .05 versus CDDP group.
Figure 5.
Western blot analysis of the expression levels of PARP and cleaved caspase-3 in PC12 cells treated with TKD (A), TKE (B), or CucD (C). GAPDH was used as a loading control. Full-length blots are presented in Supplemental Figure S1.
TKD, TKE, and CucD Prevent CDDP-Induced ROS Generation in PC12 Cells
As illustrated in Figure 6A, CDDP treatment alone resulted in a 60% increase in ROS level compared to the control (no CDDP treatment). In the group that received TKD pre-treatment, ROS levels exhibited a dose-dependent decrease, reaching 85% and 78% of control levels after pre-treatment with TKD at 50 and 100 µg/ml, respectively. In a similar manner, pre-treatment with TKE decreased ROS levels in a dose-dependent manner, with reductions of 111.7% and 108.9% after pre-treatment with TKE at 12.5 and 25 µg/ml, respectively. Pre-treatment with CucD also attenuated the production of ROS induced by CDDP, reaching 115% at 25 nM. However, the ROS inhibitory effect of CucD was more pronounced at 25 nM than at a higher concentration of 50 nM. While pre-treatment with CucD led to a substantial reduction in ROS level when compared to the CDDP-alone group, ROS levels after pre-treatment with CucD remained significantly elevated, exhibiting a 32% increase compared to that in the control group. These findings imply that while CucD alone exerts no effect on ROS levels, it appears to induce ROS when it is applied concomitantly with CDDP. Induction of DNA damage by platinum has been shown to result in an increase in phosphorylation of H2A.X and activation of the pro-apoptotic protein p53.41,42 As illustrated in Figure 6B to D, phosphorylation levels of H2A.X and p53 were substantially elevated following treatment with CDDP alone. However, pre-treatment with TKD, TKE, or CucD significantly inhibited the ratio of phospho- to total-form of H2A.X and p53 phosphorylation. These findings suggest that these drugs might be able to block CDDP-induced DNA damage. Collectively, these observations provide considerable evidence that TKD, TKE, and CucD can effectively mitigate CDDP-induced cellular toxicity and apoptosis by suppressing ROS-mediated DNA damage.
Figure 6.
Suppressive effects of drugs on CDDP-induced ROS production in PC12 cells. (A) PC12 cells exposed to CDDP for 48 hours with drugs followed by staining cells with H2DCFDA. Values are analyzed by one-way ANOVA and expressed as means ± SD (n = 3). ***P < .001 versus control group; #P < .05, ###P < 0.001 versus CDDP group. (B–D) Western blot analysis of p-p53, p53, γ-H2A.X, and H2A.X in cells treated with TKD (B), TKE (C), or CucD (D). GAPDH was used as a loading control. Full-length blots are presented in Supplemental Figure S2.
CDDP Induces Peripheral Neuropathy
To model CIPN, CDDP was administered intraperitoneally, followed by pain behavior assessments according to the timeline shown in Figure 7A. Repeated CDDP injections from Day 0 to Day 3 resulted in a significant decrease in 50% mechanical withdrawal threshold, indicative of mechanical allodynia. The CDDP-treated group exhibited a reduction of 84.85% in the threshold by Day 7, with significant pain responses persisting until Day 21 (Figure 7B). Additionally, paw withdrawal latency (PWL) to heat exposure significantly decreased after CDDP injections, suggesting thermal hyperalgesia. The CDDP-administered group showed a 20.76% reduction in PWL by Day 7. Such reduction continued until Day 21 (Figure 7C). On Day 7 following CDDP injection, the threshold was reduced by 14.75%. However, such reduction was not statistically significant.
Figure 7.
In vivo experimental design and CIPN rat model establishment. (A) A schematic representation summarizing the establishment of the CIPN model through intraperitoneal injection of CDDP (2 mg/kg for 4 days), drugs administration, and evaluation parameters. (B) 50% PWT of hind paw of control and CDDP group rats. ***P < .001, ****P < .0001 versus control group (n = 6). (C) PWL of hind paw of control and CDDP group rats. **P < .01, ****P < .0001 versus control group (n = 6). Two-way ANOVA test was used for statistical analysis in panels (B and C).
TKD, TKE, and CucD Alleviates Symptoms of CIPN in In Vivo Models
Next, we evaluated effects of TKD, TKE, and CucD in mitigating CIPN in rats. After inducing CIPN with repeated CDDP treatment for 4 days, TKD, TKE, or CucD was administered consecutively for 4 days (D7-D10; Figure 7A).
Administration of CDDP reduced the mechanical withdrawal threshold by nearly 80% by Day 7 in all groups except the control group, which did not receive CDDP. Groups treated with TKD, TKE, CucD high, and PRG exhibited significant increases in pain threshold compared to the negative control group (NC; Saline) on D7-D10 (Figure 8A). On D7, increases in pain threshold after treatment with TKD, TKE, CucD high, and PRG were 82.10%, 82.85%, 83.62%, and 79.82%, respectively. On D8, these increases were 85.92%, 84.65%, 84.98%, and 84.98%, respectively. On D9, these increases were 91.46%, 90.82%, 91.46%, and 91.59%, respectively. On D10, these increases were 92.46%, 92.21%, and 94.39%, respectively. However, the CucD low (5 μg) group exhibited threshold increases of 74.61%, 72.32%, 83.15%, and 84.32% on D7, D8, D9, and D10, respectively. These increases did not reach statistical significance.
Figure 8.
Effects of drugs administration on CDDP-induced mechanical allodynia and thermal hyperalgesia in rats. Modulation of various pain response behaviors by TKD, TKE, or CucD treatments was assessed using the Von Frey (A) and Hargreaves (B) tests, with the results depicted in individual graphs for each test. Data points are mean ± SEM (n = 6) and were analyzed by two-way ANOVA with Sidak’s multiple comparisons test. *P < .05, **P < .01, ***P < .001, ****P < .0001 (A); Data are presented as the mean ± SEM (n = 6) and were analyzed by two-way ANOVA with Newmam-Keuls multiple comparisons test. *P < .05, **P < .01 (B). (C) Body weights of the rats in different subgroups receiving different treatments were monitored for 21 days. Observation was presented as @P < .05, @@@P < .001, @@@@P < .0001 versus Cisplatin + Saline group.
When assessing heat nociceptive response, groups treated with TKD, TKE, CucD high, and PRG showed significant increases (26.43%, 28.53%, 23.99%, and 25.85% increases, respectively) in pain threshold compared to the negative control group (saline) on D7. On D9, groups treated with TKD, TKE, CucD low, CucD high, and PRG exhibited threshold increases of 27.95%, 34.16%, 26.44%, 23.30%, and 29.56%, respectively. On D10, TKD and TKE groups exhibited threshold increases of 32.78% and 33.93%, respectively, in comparison with the negative control group (saline), demonstrating statistically significant effects of TKD and TKE on pain threshold (Figure 8B). Additionally, administration of CDDP alone led to significant weight loss compared to the control group, whereas no weight gain was observed in drug-treated groups compared to the normal control (NC, saline) group (Figure 8C).
TKD, TKE, and CucD do not Affect Secretion of Pro-inflammatory Cytokines
Sensory disturbances commonly associated with chemotherapeutic agents might have originated from a shared mechanism, which is likely to be independent of their anti-cancer effects. Inflammatory cytokines might play a pivotal role in the development of CIPN. They might also be responsible for painful paresthesias commonly observed in patients undergoing chemotherapy. 43 These pro-inflammatory cytokines not only contribute to axonal damage, but also modulate nociceptor sensitivity and activity, thereby further exacerbating the pain.44 -46 Serum was isolated from blood sample collected on the 21st day following the initial administration of CDDP. We assessed changes in inflammatory cytokines in the blood and analyzed them with ELISA. We measured levels of representative pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in the serum and found no significant differences between control, NC, and drug-treated groups (Figure 9).
Figure 9.
Effects of drugs administration on the levels of pro-inflammatory cytokines in the serum of rats with CIPN. No significant difference was observed between the effects of control, CDDP, and all drugs. Data are expressed as mean ± SEM and were analyzed by one-way ANOVA.
Discussion
This study explored the potential of Trichosanthes kirilowii Maxim. (TK), its extracts TKD (an aqueous extract) and TKE (a 70% ethanol extract), and Cucurbitacin D (CucD), a prominent bioactive compound derived from TK, in alleviating chemotherapy-induced peripheral neuropathy (CIPN) using both in vitro and in vivo models. CDDP has been proven to be an effective chemotherapeutic agent for treating solid tumors.9 -13 However, it is important to note that CDDP-induced neuropathy has a high incidence, which can have a significant impact on various factors related to chemotherapy treatment, including drug dose, treatment duration, and patient compliance. The lack of effective treatment options for this condition can be a major concern as it can hinder treatment effectiveness and pose a challenge to the patient’s well-being and compliance with the treatment regimen. The development of neuropathy is associated with damage inflicted upon cell bodies of sensory neurons located within the peripheral nervous system by CDDP. In vitro results indicated that CDDP substantially reduced viability of PC12 cells in a dose-dependent manner, confirming its neurotoxic effects. Conversely, TKD, TKE, and CucD did not exhibit significant cytotoxicity at specific concentrations, indicating their potential safety for subsequent studies. Crucially, pre-treatment with TKD, TKE, or CucD effectively shielded PC12 cells from CDDP-induced cytotoxicity. This protective effect manifested as a reduction in cell viability loss and preservation of cell morphology, with treated cells maintaining a rounded shape in contrast to a pointed morphology seen for CDDP-treated cells.
CDDP exposure can decrease lengths of neurites (defined as projections from the cell body that include axons and dendrites). This decrease in length is attributable to the neurotoxic impact of CDDP on sensory neurons. A number of studies have examined whether regulating neurite outgrowth is a potential therapeutic approach for peripheral neuropathy. 47 TKD, TKE, and CucD enhanced neurite extension in PC12 cells, reversed CDDP-induced neurite atrophy, and restored the percentage of neurite-bearing cells to control levels. Neurite retraction is a key event in the initial phase of neuronal cell death. 48 Activation of the caspase family of proteins is essential for both initiation and execution of the apoptotic process. 49 Caspase-3, a key protease in apoptosis, holds significant importance in neurons. 50 Caspase-3 is the primary rate-limiting caspase responsible for cleaving PARP, ultimately leading to apoptotic cell death. 51 Our study demonstrated that co-exposure to TKD, TKE, or CucD and CDDP could suppress CDDP-induced apoptosis pathway in PC12 cells by blocking the cleavage of caspase-3 and PARP. These findings suggest that these compounds could safeguard against cell death and promote neurite regeneration, underscoring their potential to alleviate CDDP-induced neurodegenerative effects.
Reactive oxygen species (ROS) have a pivotal role in the pathogenesis of CDDP-induced peripheral neuropathy.52,53 Accumulation of CDDP within mitochondria can trigger an oxidative stress response, which has the potential to damage mitochondrial DNA and trigger ROS production.54,55 Antioxidant properties of both TK extract and CucD have been previously documented, suggesting a potential protective effect against ROS induction by CDDP, which might contribute to a reduction in apoptosis. Pre-treatment with TKD, TKE, or CucD led to a significant reduction in ROS level increased by CDDP, achieving levels comparable to those in the control group. CDDP can induce apoptosis through processes involving ROS, γ-H2AX, and p53.56,57 The γ-H2AX, also known as phosphorylated H2A.X, is a critical regulatory step that ensures cell survival by either promoting DNA repair or directing cells toward apoptosis.42,58 Treatment with CDDP has been shown to cause generation of ROS known to cause DNA damage. 59 This in turn results in an increase in γ-H2AX, while the tumor suppressor protein p53 is stabilized and activated. This sequence of events leads to the initiation of a process known as apoptosis. Phosphorylation of p53 and the ratio of γ-H2A.X to H2A.X were found to be downregulated in cells pretreated with TKD, TKE, or CucD to a greater extent than in the control group without such pretreatment (CDDP treatment alone). Therefore, suppression of ROS might be one mechanism by which TKD, TKE, and CucD can protect against CDDP-induced apoptosis.
In animal models, CDDP administration resulted in significant decreases in mechanical and thermal pain thresholds, indicative of the onset of CIPN. All groups pre-treated with TKD, TKE, or CucD except the CucD low group exhibited substantial improvements in mechanical thresholds and pain responses relative to the control group without pretreatment (CDDP treatment alone). While allodynia and heat nociceptive responses showed no significant differences at later stages, TKD and TKE treatment groups showed less degeneration in pain responses, suggesting their potential therapeutic advantage.
This study has some limitations. First, detailed mechanistic insights into how TKD, TKE, and CucD exerted their protective effects are unknown, which hinders a deeper understanding of their exact mechanisms of action. Specifically, in vitro results demonstrated that higher concentrations of CucD did not have a significant effect on CDDP-induced neurodegeneration or apoptosis. This observation could be attributed to the ability of CucD to protect against DNA damage induced by ROS by inhibiting γ-H2A.X and p53 while simultaneously activating mitogen-activated protein kinases (MAPKs) signaling induced by ROS. 60 The MAPK signaling pathway is a complex network of protein kinases that regulate various cellular processes, including cell proliferation, differentiation, survival, death, and inflammation. 61 ROS are signaling molecules produced in response to various forms of stress, functioning as messengers that act either upstream or downstream in the MAPK cascade. 62 CDDP exerts its effects by damaging DNA, leading to cell cycle arrest as cells attempt to repair the damage. If the repair process fails, cells can transition into mitosis and subsequently undergo apoptosis. During this process, ROS are generated, which can activate the p38-MAPK pathway, further enhancing cellular responses to damage. 63 Therefore, it can be hypothesized that co-exposure to CucD and CDDP might have induced ROS, which could potentially mitigate the protective effect of CucD against CDDP-induced neurodegeneration and apoptosis. Furthermore, a brief duration of in vivo experiments might not adequately reflect these treatments’ long-term effects or potential side effects. Additionally, cytokine ELISA experiments performed on day 21 post-euthanasia of all rats revealed no significant changes in pro-inflammatory cytokines (IL-6, IL-1β, TNF-α). This absence of notable results could be attributed to the chosen timing of sacrifice, which might not have been ideal for assessing cytokine levels. Future studies should address these limitations by delving into the molecular mechanisms involved, conducting extended efficacy and safety evaluations, and optimizing experimental timing to capture therapeutic benefits of these compounds more effectively.
Overall, these findings highlight the potential of TKD, TKE, and CucD in counteracting CIPN, presenting valuable opportunities for further research and development of therapeutic strategies to manage chemotherapy-induced neurotoxicity.
Conclusions
The study indicates that Trichosanthes kirilowii Maxim. extracts (TKD and TKE) and Cucurbitacin D (CucD) might help alleviate chemotherapy-induced peripheral neuropathy (CIPN) induced by cisplatin (CDDP). In cell tests, these compounds could improve cell viability, increase neurite outgrowth, and reduce ROS and apoptosis. In animal models, TKD and TKE significantly reduced pain symptoms, while CucD was less effective at lower doses. Further research is needed to understand their mechanisms of action, long-term effects, and optimal dosing for clinical use.
Supplemental Material
Supplemental material, sj-tif-1-ict-10.1177_15347354251339121 for Trichosanthes kirilowii Maxim. and Bioactive Compound Cucurbitacin D Alleviate Cisplatin-Induced Peripheral Neuropathy In Vitro and In Vivo by Sooyeon Kang, Gaeun Choi, Daeun Kim, Hogeol Kim, Chunhoo Cheon and Seong-Gyu Ko in Integrative Cancer Therapies
Supplemental material, sj-tif-2-ict-10.1177_15347354251339121 for Trichosanthes kirilowii Maxim. and Bioactive Compound Cucurbitacin D Alleviate Cisplatin-Induced Peripheral Neuropathy In Vitro and In Vivo by Sooyeon Kang, Gaeun Choi, Daeun Kim, Hogeol Kim, Chunhoo Cheon and Seong-Gyu Ko in Integrative Cancer Therapies
Supplemental material, sj-tif-3-ict-10.1177_15347354251339121 for Trichosanthes kirilowii Maxim. and Bioactive Compound Cucurbitacin D Alleviate Cisplatin-Induced Peripheral Neuropathy In Vitro and In Vivo by Sooyeon Kang, Gaeun Choi, Daeun Kim, Hogeol Kim, Chunhoo Cheon and Seong-Gyu Ko in Integrative Cancer Therapies
Acknowledgments
Not applicable.
Footnotes
ORCID iDs: Sooyeon Kang  https://orcid.org/0000-0003-2745-9463
https://orcid.org/0000-0003-2745-9463
Chunhoo Cheon  https://orcid.org/0000-0002-7078-0079
https://orcid.org/0000-0002-7078-0079
Seong-Gyu Ko  https://orcid.org/0000-0002-2345-430X
https://orcid.org/0000-0002-2345-430X
Ethical Considerations: The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Rudacure Co., LTD (Incheon, Republic of Korea; Permit No. 23-RCI-R-051).
Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Author Contributions: SK was responsible for conceptualization, data curation, investigation, methodology development, validation, visualization, and contributed to both the original draft writing and the review and editing of the manuscript. GC and DK performed the experiments and contributed to data collection. HK contributed to the development of methodology. CC contributed to the review and editing of the manuscript. SK supervised the overall project, administered the project, and secured funding.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government (MSIT; No. 2020R1A5A2019413).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data Availability Statement: All data generated or analyzed during this study are included in this published article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Beijers AJ, Mols F, Tjan-Heijnen VC, et al. Peripheral neuropathy in colorectal cancer survivors: the influence of oxaliplatin administration. Results from the population-based PROFILES registry. Acta Oncol. 2015;54(4):463-469. doi: 10.3109/0284186x.2014.980912 [DOI] [PubMed] [Google Scholar]
- 2. Kolb NA, Smith AG, Singleton JR, et al. The Association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016;73(7):860-866. doi: 10.1001/jamaneurol.2016.0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maihöfner C, Diel I, Tesch H, Quandel T, Baron R. Chemotherapy-induced peripheral neuropathy (CIPN): current therapies and topical treatment option with high-concentration capsaicin. Support Care Cancer. 2021;29(8):4223-4238. doi: 10.1007/s00520-021-06042-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hung HW, Liu CY, Chen HF, Chang CC, Chen SC. Impact of chemotherapy-induced peripheral neuropathy on quality of life in patients with advanced lung cancer receiving platinum-based chemotherapy. Int J Environ Res Public Health. 2021;18(11). doi: 10.3390/ijerph18115677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thibault K, Rivals I, M'Dahoma S, et al. Structural and molecular alterations of primary afferent fibres in the spinal dorsal horn in vincristine-induced neuropathy in rat. J Mol Neurosci. 2013;51(3):880-892. doi: 10.1007/s12031-013-0095-4 [DOI] [PubMed] [Google Scholar]
- 6. Staff NP, Cavaletti G, Islam B, et al. Platinum-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst. 2019;24 Suppl 2(Suppl 2):S26-S39. doi: 10.1111/jns.12335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamamoto S, Egashira N. Pathological mechanisms of bortezomib-induced peripheral neuropathy. Int J Mol Sci. 2021;22(2). doi: 10.3390/ijms22020888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mo H, Yan X, Zhao F, et al. Association of taxane type with patient-reported chemotherapy-induced peripheral neuropathy among patients with breast cancer. JAMA Netw Open. 2022;5(11):e2239788. doi: 10.1001/jamanetworkopen.2022.39788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Einhorn LH. Treatment of testicular cancer: a new and improved model. J Clin Oncol. 1990;8(11):1777-1781. doi: 10.1200/JCO.1990.8.11.1777 [DOI] [PubMed] [Google Scholar]
- 10. Sohn JH, Kim YT, Rha SY, et al. Paclitaxel and cisplatin combination chemotherapy in pretreated breast cancer. Cancer Res Treat. 2003;35(3):267-273. doi: 10.4143/crt.2003.35.3.267 [DOI] [PubMed] [Google Scholar]
- 11. Shin SJ, Chun SH, Kim KO, et al. The efficacy of paclitaxel and cisplatin combination chemotherapy for the treatment of metastatic or recurrent gastric cancer: a multicenter phase II study. Korean J Intern Med. 2005;20(2):135-140. doi: 10.3904/kjim.2005.20.2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364-378. doi: 10.1016/j.ejphar.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019;88:102925. doi: 10.1016/j.bioorg.2019.102925 [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury-mechanisms of induction and implication for therapy. Curr Cancer Ther Rev. 2006;2(3):271-279. doi: 10.2174/157339406777934717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490-2518. doi: 10.3390/toxins2112490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Volarevic V, Djokovic B, Jankovic MG, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019/March/13;26(1):25. doi: 10.1186/s12929-019-0518-9. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H, Rørth M, Krarup C. Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain. 2007;130(Pt 4):1076-1088. doi: 10.1093/brain/awl356 [DOI] [PubMed] [Google Scholar]
- 18. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461-2470. doi: 10.1016/j.pain.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 19. Ezzi MS, Othieno-Abinya NA, Amayo E, et al. Prevalence and predictors of cisplatin-induced peripheral neuropathy at the Kenyatta National Hospital. J Glob Oncol. 2019;5(5):1-6. doi: 10.1200/jgo.19.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joseph EK, Levine JD. Comparison of oxaliplatin- and cisplatin-induced painful peripheral neuropathy in the rat. J Pain. 2009;10(5):534-541. doi: 10.1016/j.jpain.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hopkins HL, Duggett NA, Flatters SJL. Chemotherapy-induced painful neuropathy: pain-like behaviours in rodent models and their response to commonly used analgesics. Curr Opin Support Palliat Care. 2016;10(2):119-128. doi: 10.1097/spc.0000000000000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis. 2002;9(2):220-233. doi: 10.1006/nbdi.2001.0468 [DOI] [PubMed] [Google Scholar]
- 23. Podratz JL, Knight AM, Ta LE, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41(3):661-668. doi: 10.1016/j.nbd.2010.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SR, Seo HS, Choi H-S, et al. Trichosanthes kirilowii ethanol extract and cucurbitacin D inhibit cell growth and induce apoptosis through inhibition of STAT3 activity in breast cancer cells. Evid Based Complement Alternat Med. 2013;2013(1):975350. doi: 10.1155/2013/975350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park SM, Jeon SK, Kim OH, et al. Anti-tumor effects of the ethanolic extract of Trichosanthes kirilowii seeds in colorectal cancer. Chin Med. 2019;14:43. doi: 10.1186/s13020-019-0263-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park HJ, Park SH. The ethanolic extract of Trichosanthes kirilowii root exerts anti-cancer effects in human non-small cell lung cancer cells resistant to EGFR TKI. Nutr Cancer. 2023;75(1):376-387. doi: 10.1080/01635581.2022.2114509 [DOI] [PubMed] [Google Scholar]
- 27. Ozaki Y, Xing L, Satake M. Antiinflammatory effect of Trichosanthes kirilowii Maxim, and its effective parts. Biol Pharm Bull. 1996;19(8):1046-1048. doi: 10.1248/bpb.19.1046 [DOI] [PubMed] [Google Scholar]
- 28. Kim H-Y, Seol I-C, Yoo H-R, Kim Y-S. The effect of Trichosanthes kirilowii Maximowicz extract and Trichosanthes kirilowii Maximowicz cheonghyeol plus on anti-inflammatory factor expression in human umbilical vein endothelial cells (HUVECs). J Intern Korean Med. 2022;43(4):514-528. doi: 10.22246/jikm.2022.43.4.514 [DOI] [Google Scholar]
- 29. Hou Z, Zhu L, Meng R, Wang B. Hypolipidemic and antioxidant activities of Trichosanthes kirilowii maxim seed oil and flavonoids in mice fed with a high-fat diet. J Food Biochem. 2020/August/01;44(8):e13272. doi: 10.1111/jfbc.13272 [DOI] [PubMed] [Google Scholar]
- 30. Wang W, Li S, Zhu Y, et al. Antioxidant and neuroprotective effects of seed oils from Trichosanthes kirilowii and T. laceribractea in Caenorhabditis elegans: a comparative analysis and mechanism study. Antioxidants. 2024;13(7):861. doi:)10.3390/antiox13070861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lo HY, Li TC, Yang TY, et al. Hypoglycemic effects of Trichosanthes kirilowii and its protein constituent in diabetic mice: the involvement of insulin receptor pathway. BMC Complement Altern Med. 2017;17(1):53. doi: 10.1186/s12906-017-1578-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seo CS, Kim TW, Kim YJ, et al. Trichosanthes kirilowii ameliorates cisplatin-induced nephrotoxicity in both in vitro and in vivo. Nat Prod Res. 2015;29(6):554-557. doi: 10.1080/14786419.2014.952229 [DOI] [PubMed] [Google Scholar]
- 33. Zhang HQ, Liu P, Duan JA, et al. Hierarchical extraction and simultaneous determination of flavones and triterpenes in different parts of Trichosanthes kirilowii Maxim. by ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. J Pharm Biomed Anal. 2019;167:114-122. doi: 10.1016/j.jpba.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 34. Sikander M, Hafeez BB, Malik S, et al. Cucurbitacin D exhibits potent anti-cancer activity in cervical cancer. Sci Rep. 2016/November/08;6(1):36594. doi: 10.1038/srep36594. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sikander M, Malik S, Khan S, et al. Novel mechanistic insight into the anticancer activity of cucurbitacin D against pancreatic cancer (Cuc D attenuates pancreatic cancer). Cells. 2019;9(1):103. doi: 10.3390/cells9010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varela C, Melim C, Neves BG, et al. Cucurbitacins as potential anticancer agents: new insights on molecular mechanisms. J Transl Med. 2022/December/31;20(1):630. doi: 10.1186/s12967-022-03828-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee JH, Kim B, Ko SG, Kim W. Analgesic effect of SH003 and Trichosanthes kirilowii Maximowicz in paclitaxel-induced neuropathic pain in mice. Curr Issues Mol Biol. 2022;44(2):718-730. doi: 10.3390/cimb44020050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cata JP, Weng HR, Dougherty PM. Behavioral and electrophysiological studies in rats with cisplatin-induced chemoneuropathy. Brain Res. 2008;1230:91-98. doi: 10.1016/j.brainres.2008.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin H, Heo BH, Yoon MH. A new rat model of cisplatin-induced neuropathic pain. Korean J Pain. 2015;28(4):236-243. doi: 10.3344/kjp.2015.28.4.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Kim WM, Kim SH, et al. Prostaglandin D(2) contributes to cisplatin-induced neuropathic pain in rats via DP2 receptor in the spinal cord. Korean J Pain. 2021;34(1):27-34. doi: 10.3344/kjp.2021.34.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krüger K, Thomale J, Stojanović N, et al. Platinum-induced kidney damage: unraveling the DNA damage response (DDR) of renal tubular epithelial and glomerular endothelial cells following platinum injury. Biochim Biophys Acta. 2015;1853(3):685-698. doi: 10.1016/j.bbamcr.2014.12.033 [DOI] [PubMed] [Google Scholar]
- 42. Prabhu KS, Kuttikrishnan S, Ahmad N, et al. H2AX: a key player in DNA damage response and a promising target for cancer therapy. Biomed Pharmacother. 2024;175:116663. doi: 10.1016/j.biopha.2024.116663 [DOI] [PubMed] [Google Scholar]
- 43. Brandolini L, d'Angelo M, Antonosante A, Allegretti M, Cimini A. Chemokine signaling in chemotherapy-induced neuropathic pain. Int J Mol Sci. 2019;20(12). doi: 10.3390/ijms20122904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59(1):3-9. doi: 10.1016/j.cyto.2012.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fumagalli G, Monza L, Cavaletti G, Rigolio R, Meregalli C. Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Front Immunol. 2020;11:626687. doi: 10.3389/fimmu.2020.626687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhol NK, Bhanjadeo MM, Singh AK, et al. The interplay between cytokines, inflammation, and antioxidants: mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed Pharmacother. 2024;178:117177. doi: 10.1016/j.biopha.2024.117177 [DOI] [PubMed] [Google Scholar]
- 47. Quarta S, Baeumer BE, Scherbakov N, et al. Peripheral nerve regeneration and NGF-dependent neurite outgrowth of adult sensory neurons converge on STAT3 phosphorylation downstream of neuropoietic cytokine receptor gp130. J Neurosci. 2014;34(39):13222-13233. doi: 10.1523/jneurosci.1209-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Unsain N, Higgins JM, Parker KN, Johnstone AD, Barker PA. XIAP regulates caspase activity in degenerating axons. Cell Rep. 2013;4(4):751-763. doi: 10.1016/j.celrep.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 49. Cullen SP, Martin SJ. Caspase activation pathways: some recent progress. Cell Death Differ. 2009/July/01;16(7):935-938. doi: 10.1038/cdd.2009.59 [DOI] [PubMed] [Google Scholar]
- 50. D'Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010/July/01;17(7):1104-1114. doi: 10.1038/cdd.2009.180 [DOI] [PubMed] [Google Scholar]
- 51. Sairanen T, Szepesi R, Karjalainen-Lindsberg ML, et al. Neuronal caspase-3 and PARP-1 correlate differentially with apoptosis and necrosis in ischemic human stroke. Acta Neuropathol. 2009;118(4):541-552. doi: 10.1007/s00401-009-0559-3 [DOI] [PubMed] [Google Scholar]
- 52. Hu X, Jiang Z, Teng L, et al. Platinum-induced peripheral neuropathy (PIPN): ROS-related mechanism, therapeutic agents, and Nanosystems. Front Mol Biosci. 2021;8:770808. doi: 10.3389/fmolb.2021.770808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Katanić Stanković JS, Selaković D, Rosić G. Oxidative damage as a fundament of systemic toxicities induced by cisplatin—the crucial limitation or potential therapeutic target? Int J Mol Sci. 2023;24(19). doi: 10.3390/ijms241914574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marullo R, Werner E, Degtyareva N, et al. Cisplatin induces a Mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One. 2013;8(11):e81162. doi: 10.1371/journal.pone.0081162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kleih M, Böpple K, Dong M, et al. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019/November/07;10(11):851. doi: 10.1038/s41419-019-2081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bañuelos CA, Banáth JP, Kim JY, Aquino-Parsons C, Olive PL. Gammah2ax expression in tumors exposed to cisplatin and fractionated irradiation. Clin Cancer Res. 2009;15(10):3344-3353. doi: 10.1158/1078-0432.ccr-08-3114 [DOI] [PubMed] [Google Scholar]
- 57. Olive PL, Banáth JP. Kinetics of H2AX phosphorylation after exposure to cisplatin. Cytometry B Clin Cytom. 2009/March/01;76B(2):79-90. doi: 10.1002/cyto.b.20450 [DOI] [PubMed] [Google Scholar]
- 58. Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275(13):9390-9395. doi: 10.1074/jbc.275.13.9390 [DOI] [PubMed] [Google Scholar]
- 59. Kiss RC, Xia F, Acklin S. Targeting DNA damage response and repair to enhance therapeutic index in cisplatin-based cancer treatment. Int J Mol Sci. 2021;22(15). doi: 10.3390/ijms22158199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Son Y, Cheong YK, Kim NH, et al. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Braicu C, Buse M, Busuioc C, et al. A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers. 2019;11(10). doi: 10.3390/cancers11101618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jalmi SK, Sinha AK. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Review. Front Plant Sci. 2015;6:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dolado I, Swat A, Ajenjo N, et al. P38α MAP Kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11(2):191-205. doi: 10.1016/j.ccr.2006.12.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-ict-10.1177_15347354251339121 for Trichosanthes kirilowii Maxim. and Bioactive Compound Cucurbitacin D Alleviate Cisplatin-Induced Peripheral Neuropathy In Vitro and In Vivo by Sooyeon Kang, Gaeun Choi, Daeun Kim, Hogeol Kim, Chunhoo Cheon and Seong-Gyu Ko in Integrative Cancer Therapies
Supplemental material, sj-tif-2-ict-10.1177_15347354251339121 for Trichosanthes kirilowii Maxim. and Bioactive Compound Cucurbitacin D Alleviate Cisplatin-Induced Peripheral Neuropathy In Vitro and In Vivo by Sooyeon Kang, Gaeun Choi, Daeun Kim, Hogeol Kim, Chunhoo Cheon and Seong-Gyu Ko in Integrative Cancer Therapies
Supplemental material, sj-tif-3-ict-10.1177_15347354251339121 for Trichosanthes kirilowii Maxim. and Bioactive Compound Cucurbitacin D Alleviate Cisplatin-Induced Peripheral Neuropathy In Vitro and In Vivo by Sooyeon Kang, Gaeun Choi, Daeun Kim, Hogeol Kim, Chunhoo Cheon and Seong-Gyu Ko in Integrative Cancer Therapies