Abstract
Objective
To update evidence‐based medicine recommendations for treating motor fluctuations of Parkinson's disease (PD).
Background
The International Parkinson and Movement Disorder Society (MDS) Evidence Based Medicine in Movement Disorders Committee recommendations for the treatments of PD were first published in 2002 and regularly updated. The current review uses a new methodology, including the Cochrane Risk of Bias tool and a modified version of GRADE (Grading of Recommendations, Assessment, Development, and Evaluations).
Methods
On January 1, 2023, a literature search was conducted without date limit in the MEDLINE, Embase, and Cochrane databases using the following search terms: Parkinson disease, levodopa and, for the Embase database, randomized controlled trial (RCT). The inclusion criteria for studies were: patients with PD, on oral levodopa therapy, experiencing motor fluctuations, investigating an intervention that was (commercially) available in at least one country, study design RCT, and with a follow‐up duration of at least 3 months.
Results
A total of 102 studies were included. Levodopa extended release, pramipexole immediate release and extended release, ropinirole immediate release, rotigotine, opicapone, safinamide, and bilateral subthalamic nucleus deep brain stimulation (DBS) were assessed as efficacious, and continuous intestinal levodopa infusion, continuous subcutaneous levodopa, continuous subcutaneous apomorphine, ropinirole prolonged release, ropinirole patch, entacapone, rasagiline, istradefylline, amantadine extended release, zonisamide, bilateral globus pallidus DBS, and pallidotomy were assessed as likely efficacious for the treatment of motor fluctuations in people with PD who are already being treated with levodopa.
Conclusions
There are several treatment options that can improve motor fluctuations in PD. These recommendations will assist physicians and patients in determining which intervention to use. © 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease, systematic review, randomized controlled trial, treatments, motor response fluctuations
The number of interventions for treating motor symptoms in Parkinson's disease (PD) continues to expand. Evidence‐based medicine (EBM) recommendations are designed to assist a treating physician in deciding which intervention to use in an individual PD patient. The International Parkinson and Movement Disorder Society (MDS) Evidence Based Medicine in Movement Disorders Committee has published EBM reviews on PD treatments using a systematic review approach since 2002. 1 The most recent update reviewed studies to December 31, 2016. 2
Following the literature searches using electronic databases and the selection of studies fulfilling eligibility criteria, each study was rated by at least two investigators using the Rating Scale for Quality of Evidence. After assessment of the available data, each intervention was then assigned an efficacy conclusion, safety assessment, and a statement on overall implications for clinical practice classified as clinically useful, possibly useful, unlikely useful, not useful, or investigational. 2
Due to improved techniques in EBM reviews, the MDS has subsequently revised the method used for this new updated EBM review and has adopted the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) framework. GRADE has several advantages: it is based on robust methodological principles, it has a linear and transparent process, including in cases of conflicting evidence, and it is by far the most commonly used methodology across medicine. 3 Because MDS is an international society, recommendations rather than guidelines have been published to date. Because we anticipate there are few to no possibilities of conducting meta‐analyses given the nature of the available literature, which are a requirement in the GRADE system, a modified version of GRADE was used. Therefore, the team pursued the production of systematic reviews, using the Cochrane Risk of Bias tool and a less complex, modified version of GRADE, with a narrative of the conclusions as the default. 4 This initiative, including the training of all raters, was carried out in collaboration with Cochrane Movement Disorders (author J.C.).
1. Methods
1.1. Population, Intervention, Comparator, Outcomes, Timepoints
For the systematic review, the MDS EBM Committee developed a basic PICOT (Population, Intervention, Comparator, Outcomes, Timepoints) for the treatment of motor fluctuations in PD. For the PICOT, the population includes individuals with PD who are using levodopa and experiencing motor fluctuations, whether early fluctuations or fluctuations that persist despite adjustments to the oral medication. Possible comparators were placebo or an active comparator, including best medical treatment (BMT). Outcomes included the duration of OFF‐time, duration of ON‐time, severity of PD symptoms in the off‐medication state, disability, health‐related quality of life (HR‐QoL), and adverse effects. Follow‐up measurements had to occur at least 3 months after initiation of the study treatment. Hence, for each intervention the following PICOT question was formulated: Does an intervention improve motor fluctuations in PD patients on oral levodopa therapy who are experiencing early motor fluctuations or persistent motor fluctuations despite (attempted) optimal oral therapy compared with placebo or an active comparator after a follow‐up duration of at least 3 months, based on results from randomized controlled trials (RCTs)?
1.2. Search
On January 1, 2023, a literature search was conducted without date limit in Ovid MEDLINE, Ovid Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) (https://crso.cochrane.org/) using search terms for Parkinson disease and levodopa. Additionally search filters to capture RCT studies were employed for MEDLINE 5 and Embase 6 but not for Cochrane CENTRAL as this is limited to RCT studies. 7 Conference abstracts from Embase and CENTRAL were included in the final search results (see Supporting Information: Data S1 for the detailed search terms). Duplicate records were removed using dedupendnote.nl. 8
1.3. Selection of Studies
All articles that were identified through the search were uploaded to the Rayyan web tool (www.rayyan.ai) and screened for selection, based on title and abstract, according to the following inclusion criteria: patients with PD, on oral levodopa therapy, experiencing motor fluctuations, investigating an intervention that was (commercially) available in at least one country, the study design was an RCT, with a follow‐up duration of at least 3 months, written in English, and reporting on at least one of the following outcomes: OFF‐time, ON‐time, motor impairment in the off‐medication state (eg, Unified Parkinson's Disease Rating Scale [UPDRS]‐Part III and International Parkinson and Movement Disorder Society sponsored revision of the UPDRS [MDS‐UPDRS]‐Part III Motor Examination), disability (eg, UPDRS‐Part II and MDS‐UPDRS‐Part II Motor Aspects of Experiences of Daily Living), HR‐QoL (eg, Parkinson's Disease Questionnaire‐39 [PDQ‐39]), and adverse events. In cases of uncertainty, the article was included in the next phase of the selection process. Screening was carried out by 16 team members, with the articles to be screened divided in such a way that each article was reviewed by two team members independently. If these two team members did not agree, a third person screened, and this was decisive. For the remaining articles, the full text was obtained and uploaded to the Rayyan web tool. Subsequently each of these articles was screened for eligibility by two reviewers independently using inclusion and exclusion criteria.
1.4. Data Extraction
Of the included articles, the following data were extracted by two team members: outcomes, intervention details, first author, publication year, study population, characteristics of the intervention (eg, daily dose), number of patients assigned to the intervention, characteristics of the control intervention, number of patients assigned to the control group, outcome measurements, timing of outcome assessment, type of analysis (eg, intention‐to‐treat), how the results were reported (eg, mean change, number of patients experiencing an event), and the outcomes themselves. The outcome characteristics varied among the different studies and an attempt was made to extract data regarding all available outcomes in the individual articles. These could include differences between groups, changes from baseline for groups, or the number of patients experiencing an event in each group, together with the reported characteristics of the outcome such as mean values, median values, confidence interval (CI), and P‐value.
1.5. Risk of Bias Assessment
For each suitable outcome in each included article, two team members assessed the risk of bias, first separately and then collaboratively, in terms of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and any potential other biases. Each of these eight items was categorized as either low risk, high risk, or an unclear risk of bias. 9
1.6. Efficacy Conclusion
An efficacy conclusion was formulated for each intervention, similar to the GRADE framework, using two axes: (1) the clinical relevance of the outcome and (2) the quality of the evidence (Table 1). To determine the clinical relevance of the outcome, minimal clinically important differences (MCID) on the respective scale were used if these data were available. For changes in duration of ON‐time, OFF‐time, and time without dyskinesia, MCID was 1 h per day. 10 For the UPDRS‐Part III and the MDS‐UPDRS‐Part III, MCID was 4 points. 11 For the UPDRS and MDS‐UPDRS‐Part II, MCID was 3 points, 12 and for the PDQ‐39, MCID was 5 points. 13 Using a modified GRADE approach, the quality of evidence for the effectiveness of each intervention was assessed based on risk of bias, imprecision, inconsistency, indirectness, and likelihood of publication bias. For each of these domains, two reviewers consistently determined whether there were “no serious concerns”, “serious concerns”, or “very serious concerns”, in order to prepare the efficacy conclusion. Subsequently, possible efficacy conclusions were: Efficacious, Likely Efficacious, Not Efficacious, and Insufficient Evidence. The primary differences between the new MDS EBM approach and the original GRADE recommendations for drawing conclusions are that the importance of benefit is determined by the clinical relevance of the outcomes rather than the effect size, and that both low and very low levels of evidence result in a classification of Insufficient Evidence. These efficacy conclusions are presented in a table. For each intervention, a brief summary is provided specifying the articles and outcomes on which the efficacy conclusion is based, together with, to the best extent possible, an indication of effect size.
TABLE 1.
International Parkinson and Movement Disorder Society evidence‐based medicine modified Grading of Recommendations Assessment, Development, and Evaluation (GRADE) efficacy conclusions
| Level of evidence in favor of the intervention | Important benefit (clinically relevant outcome) | Benefit of intermediate importance (clinical relevance of the outcome uncertain) | No important benefit (outcomes without clinical relevance) |
| High | Efficacious | Likely efficacious | Not efficacious |
| Moderate | Likely efficacious | Likely efficacious | Unlikely efficacious |
| Low or very low | Insufficient evidence | Insufficient evidence | Insufficient evidence |
1.7. Adverse Effects
A different setup of the search and selection procedures would be required for a systematic literature review of adverse effects; for example, including not only RCTs but also cohort studies, data from registries, and phase 4 studies. Therefore, we mention only significant and notable side effects and refer readers to the Summary of Product Characteristics (SmPC) for further information on side effects.
2. Results
The literature search yielded a total of 15,485 articles. After title and abstract selection, 917 articles remained; and, ultimately, after screening based on the full articles, 102 studies were included. A flowchart of the search and screening procedures is shown in Fig. 1. Efficacy conclusions are summarized in Table 2 and provided separately for each intervention, followed by a brief summary justifying the efficacy conclusion.
FIG. 1.
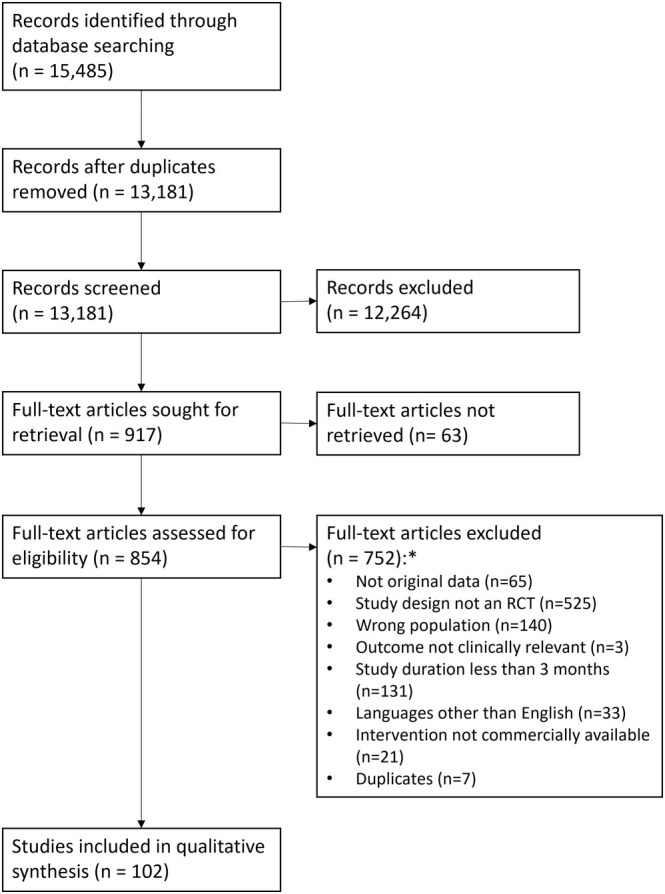
Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) search and selection flowchart. *The numbers add up to more than 755 because, for some articles, more than one reason was identified.
TABLE 2.
Efficacy conclusions of interventions for the treatment of motor fluctuations
| Class | Intervention | Efficacy conclusion | Safety a |
|---|---|---|---|
| Levodopa/PDI | Controlled release | Insufficient evidence | |
| IPX066, ER | Efficacious | ||
| Intestinal infusion | Likely efficacious | Acceptable risk with specialized monitoring | |
| Subcutaneous infusion | Likely efficacious | ||
| Dopamine agonists | Pramipexole IR | Efficacious | |
| Pramipexole ER | Efficacious | ||
| Ropinirole IR | Efficacious | ||
| Ropinirole PR | Likely efficacious | ||
| Ropinirole patch | Likely efficacious | ||
| Rotigotine | Efficacious | ||
| Apomorphine continuous | Likely efficacious | Acceptable risk with specialized monitoring | |
| Apomorphine intermittent | Insufficient evidence | ||
| COMT inhibitors | Entacapone | Likely efficacious | |
| Opicapone | Efficacious | ||
| MAO‐B inhibitors | Rasagiline | Likely efficacious | |
| Selegiline | Insufficient evidence | ||
| MAO‐B inhibitor plus channel blockers | Safinamide | Efficacious | |
| Others | Istradefylline | Likely efficacious | |
| Amantadine IR | Insufficient evidence | ||
| Amantadine CR | Likely efficacious | ||
| Terguride | Insufficient evidence | ||
| Nicotine | Insufficient evidence | ||
| Zonisamide | Likely efficacious | ||
| Perampanel | Insufficient evidence | ||
| Helicobacter pylori eradication | Insufficient evidence | ||
| Coenzyme Q10 | Not efficacious | ||
| Exenatide | Insufficient evidence | ||
| Surgery | Bilateral STN DBS | Efficacious | Acceptable risk with specialized monitoring |
| Bilateral GPi DBS | Likely efficacious | ||
| Unilateral pallidotomy | Likely efficacious | ||
| Subthalamotomy | Insufficient evidence | ||
| Bilateral zona incerta DBS | Insufficient evidence | ||
| GDNF | Insufficient evidence |
Unless otherwise stated, the conclusion for safety is acceptable risk without specialized monitoring.
Abbreviations: PDI, peripheral decarboxylase inhibitor; ER, extended release; IR, immediate release; PR, prolonged release; COMT, catechol‐O‐methyltransferase; MAO‐B, monoamine oxidase type B; CR, controlled release; STN, subthalamic nucleus; DBS, deep brain stimulation; GPi, globus pallidus; GNDF, glial cell‐derived neurotrophic factor.
2.1. Levodopa Carbidopa Controlled Release
2.1.1. Efficacy Conclusion
Three trials met the eligibility criteria for this question. 14 , 15 , 16 There is insufficient evidence to support the efficacy of levodopa carbidopa controlled release to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.1.2. Summary
Three trials evaluated the use of levodopa carbidopa controlled release for reducing OFF‐time compared with levodopa carbidopa immediate release. 14 , 15 , 16 One trial found no difference between the active treatment and placebo, 16 while the other two showed a mild effect on reducing OFF‐time. 14 , 15 However, all studies had significant design limitations and raised serious concerns regarding risk of bias. Additionally, these trials showed no differences between levodopa carbidopa controlled release and placebo in terms of increasing ON‐time or improving disability scores.
2.2. IPX066 (Levodopa Carbidopa Extended Release)
2.2.1. Efficacy Conclusion
One trial met the eligibility criteria for this question. 17 IPX066 is efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.2.2. Summary
One trial evaluated the use of IPX066 for reducing OFF‐time compared with levodopa carbidopa immediate release, showing a difference between groups of 1.2 h (P < 0.0001) in favor of IPX066. 17 The same study showed an increase in ON‐time without troublesome dyskinesia (0.9 h, P < 0.001) and ON‐time without any dyskinesia (0.7 h, P < 0.05) compared with the control group. Mean dosing frequency was 3.6 doses per day (SD 0.7 doses) for IPX066 versus 5.0 doses per day (SD 1.2 doses) for levodopa carbidopa immediate release (P < 0.0001). IPX066 was not effective in improving disability, with differences in UPDRS‐II of −0.9 points (P < 0.003) during ON‐medication state and −0.8 points P < 0.005) during off‐medication state, both of which were not considered clinically relevant. Additionally, IPX066 was unlikely to improve quality of life as the difference in PDQ‐39 scores between IPX066 and levodopa carbidopa immediate release was −2.5 points (P < 0.05), which did not reach clinical relevance. Comparisons using SF‐36 and EQ‐5D (EuroQol 5 Dimension) showed no significant differences between the groups.
2.3. Levodopa Carbidopa Intestinal Gel
2.3.1. Efficacy Conclusion
Two trials met the eligibility criteria for this PICOT. 18 , 19 Levodopa carbidopa intestinal gel is likely efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.3.2. Summary
One placebo‐controlled study evaluated the use of levodopa carbidopa intestinal gel (~16 h/day) for reducing OFF‐time compared with levodopa carbidopa immediate release, showing a mean difference of −1.9 h (P < 0.01) between groups. 18 The same study showed an increase in ON‐time without troublesome dyskinesia (1.9 h, P < 0.01) and in ON‐time without any dyskinesia (2.3 h, P = 0.01) compared with the control group. Levodopa carbidopa intestinal gel also showed improvement in disability, with a difference of 3 points in UPDRS‐II (P < 0.001), and in quality of life, with a 7‐point difference in PDQ‐39 scores (P < 0.05). A second trial, an open‐label comparison against BMT, showed a significantly greater improvement in disability with levodopa carbidopa intestinal gel (~16 h/day) compared with BMT (changes in UPDRS‐II: −2.3 vs. 0.5 points, P = 0.006). 19 However, there were no significant differences in motor impairment or HR‐QoL. In summary, levodopa carbidopa intestinal gel is considered likely efficacious based on one relatively small study with a follow‐up duration of only 3 months and concerns regarding risk of bias. 18 Additionally, the open‐label study provides insufficient evidence to definitively contribute to the efficacy statement at this point. 19
2.4. Continuous Subcutaneous Foslevodopa Foscarbidopa
2.4.1. Efficacy Conclusion
One trial met the eligibility criteria for this PICOT. 20 Continuous subcutaneous foslevodopa foscarbidopa is likely efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.4.2. Summary
One placebo‐controlled trial assessed whether the use of continuous subcutaneous foslevodopa foscarbidopa (24 h/day) reduces OFF‐time compared with levodopa carbidopa immediate release, showing a difference of −1.8 h (P = 0.002) between the two groups. 20 In the same study, an increase in ON‐time without troublesome dyskinesia was observed (1.8 h, P < 0.01) in the continuous subcutaneous foslevodopa foscarbidopa group compared with the control group. There is insufficient evidence regarding the effects of continuous subcutaneous foslevodopa foscarbidopa on disability and quality of life.
2.5. Pramipexole
2.5.1. Efficacy Conclusion
Six trials met the eligibility criteria for this question. 21 , 22 , 23 , 24 , 25 , 26 Pramipexole immediate release and extended release are efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.5.2. Summary
Five trials evaluated the use of pramipexole for reducing OFF‐time compared with placebo. 21 , 22 , 23 , 24 , 25 These studies showed that patients using pramipexol experience a statistically significant and clinically relevant reduction in OFF‐time 22 , 24 , 25 and an improvement of the MDS‐UPDRS‐II score when in off‐medication state. 21 , 23 , 25 In one study, 24 it was found that pramipexole, compared with placebo, provides a clinically meaningful increase in ON‐time without troublesome dyskinesia and in quality of life, as assessed with the PDQ‐39. 24 However, another study did not find a difference in quality of life between pramipexole and placebo. 25 Two trials 25 , 26 tested pramipexole extended release and pramipexol immediate release against placebo: both pramipexol formulations provided a clinically meaningful improvement of OFF‐time duration, 25 , 26 off‐medication state MDS‐UPDRS‐II, 25 and total PDQ‐39. 26 The efficacy of pramipexole was tested against an active comparator in two trials: rotigotine was not inferior to pramipexole in terms of the number of discontinuations and reduction in OFF‐time, 24 and improvement of MDS‐UPDRS‐II (average of off‐medication and on‐medication states) was comparable for pramipexole and bromocriptine. 21
2.6. Ropinirole
2.6.1. Efficacy Conclusion
Fourteen trials met the eligibility criteria for this question. 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 Ropinirole immediate release is efficacious to treat motor fluctuations in PD patients on oral levodopa therapy. Ropinirole prolonged release and ropinirole patch are likely efficacious to treat motor fluctuations in PD patients on oral levodopa therapy.
2.6.2. Summary
Four trials evaluated the use of ropinirole immediate release for reducing OFF‐time compared with placebo and showed a statistically significant borderline clinically meaningful reduction of OFF‐time. 27 , 28 , 33 , 34 In three trials, ropinirole prolonged release was found to reduce OFF‐time, but due to high risk of bias in two of the three studies the conclusion is likely efficacious. 35 , 36 , 37 A single study on ropinirole immediate release showed no relevant improvement of ON‐time compared with placebo. 33 Three studies showed that ropinirole PR is likely efficacious for increase in ON‐time compared with placebo based on a relevant improvement in all, but high risk of bias in two studies. 35 , 37 , 38 Four trials showed a non‐relevant change in disability for ropinirole immediate release 33 , 34 and ropinirole prolonged release 35 , 37 compared with placebo. A single study reported on ropinirole patch and showed a clinically relevant reduction in OFF‐time compared with placebo patch (likely efficacious), but no relevant change in ON‐time duration and disability. 39 One trial compared ropinirole immediate release and ropinirole prolonged release, and showed no relevant difference between the two formulations regarding OFF‐time, ON‐time, disability, and HR‐QoL (insufficient evidence). 40 Ropinirole's efficacy was assessed in four trials against active comparators. Improvement in OFF‐time was comparable between ropinirole immediate release and both rotigotine and bromocriptine. 29 , 30 , 31 Furthermore, ropinirole prolonged release was not different compared with additional levodopa in terms of effects on disability and quality of life. 32
2.7. Rotigotine
2.7.1. Efficacy Conclusion
Six trials met the eligibility criteria for this question. 24 , 29 , 41 , 42 , 43 , 44 Rotigotine is efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.7.2. Summary
Six trials evaluated the use of rotigotine for reducing OFF‐time compared with placebo. 24 , 29 , 41 , 42 , 43 , 44 They found a statistically significant and clinically meaningful reduction of OFF‐time by 0.9–1.8 h compared with placebo. The treatment difference compared with placebo was more than 1 h per day in five studies, 24 , 29 , 41 , 42 , 44 but was only 0.9 h/day in one study, 43 in which a large placebo effect was seen (1.5 h/day OFF‐time reduction in the placebo group vs. 2.4 h/day in rotigotine 8 mg/day group). Regarding disability, all six studies demonstrated improvement, with statistical significance observed in four studies. 24 , 29 , 41 , 42 However, the remaining two studies, 43 , 44 one of which was relatively large (406 participants randomized to rotigotine), 43 did not show a statistically significant improvement. Two studies evaluated quality of life using the PDQ‐39 24 and PDQ‐8 44 with improvements reported in the rotigotine group, statistically significant in only one study. 24 Consequently, the evidence for the effects of rotigotine on disability and quality of life was deemed insufficient.
2.8. Apomorphine
2.8.1. Efficacy Conclusion
Three trials met the eligibility criteria for this research question. 45 , 46 , 47 Continuous subcutaneous apomorphine infusion is likely efficacious for the treatment of motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy. There is insufficient evidence to support the use of apomorphine sublingual film and apomorphine subcutaneous pen (intermittent) as a rescue medication for off‐medication episodes in patients with PD.
2.8.2. Summary
One trial evaluated the use of continuous subcutaneous apomorphine infusion (~16 h/day) for long‐term improvement of motor fluctuations in PD patients in comparison with placebo. 46 A clinically meaningful difference between the two groups in the reduction of OFF‐time was observed, with a decrease of 1.9 h per day in favor of apomorphine (p = 0.0025). This was accompanied by a significant improvement of 2 h in ON‐time without troublesome dyskinesia compared with placebo (P = 0.0008). 46 Apomorphine sublingual film 47 and subcutaneous injection 45 were assessed as rescue medication for off‐medication states (intermittent apomorphine). Both treatments improved the off‐medication state to a good on‐medication state within 15–40 min, as measured by the MDS‐UPDRS‐III. MDS‐UPDRS‐II scores did not change during the 12‐week observation period with the use of apomorphine sublingual film. 47 Neither trial showed a significant or clinically meaningful improvement in quality of life as assessed by the PDQ‐8 and PDQ‐39. 45 , 46 , 47 Therefore, continuous subcutaneous apomorphine infusion is considered likely efficacious based on a single study, with some concerns about potential bias, including unblinding caused by the occurrence of skin nodules. The apomorphine rescue treatments are rated as having insufficient evidence, primarily because no convincing sustained improvement was observed throughout the 12‐week treatment period.
2.9. Entacapone
2.9.1. Efficacy Conclusion
Six trials met the eligibility criteria for the comparison with placebo. 48 , 49 , 50 , 51 , 52 , 53 Entacapone is likely efficacious for the treatment of motor fluctuations in patients with PD in comparison with placebo. Two trials investigated the efficacy of entacapone in comparison with an active control. There is insufficient evidence to suggest that entacapone is more efficacious than cabergoline for treating motor fluctuations in PD patients. There is insufficient evidence to suggest that an immediate switch to levodopa‐carbidopa‐entacapone is more effective for managing motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy compared with a delayed switch to levodopa‐carbidopa‐entacapone.
2.9.2. Summary
Six RCTs evaluated the efficacy of entacapone for treating motor fluctuations in patients with PD compared with a placebo. 48 , 49 , 50 , 51 , 52 , 53 These studies assessed potential changes in ON‐time duration, with five also examining changes in OFF‐time duration. Concerns regarding allocation concealment led to a moderate level of evidence. The impact of entacapone on disability was evaluated in six trials compared with a placebo, 48 , 49 , 50 , 51 , 52 , 53 with concerns about allocation concealment, imprecision, and inconsistency of results resulting in very low evidence for its efficacy. Only two studies assessed the effect of entacapone on quality of life, 49 , 53 with similar concerns about risk of bias also resulting in very low evidence supporting its efficacy. One study compared the efficacy of entacapone with cabergoline. 54 Due to the small study size and lack of additional studies, it was impossible to determine the certainty of the evidence, resulting in insufficient evidence for this comparison. 54 Another study used an immediate versus delayed switch design in an open‐label format, 55 assessing quality of life at 16 weeks. However, concerns about risk of bias and imprecision led to insufficient evidence for efficacy.
2.10. Opicapone
2.10.1. Efficacy Conclusion
Three trials met the eligibility criteria for this question. 56 , 57 , 58 Opicapone is efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy. 56 , 57 , 58
2.10.2. Summary
Three trials evaluated the efficacy of opicapone in reducing OFF‐time compared with placebo. 56 , 57 , 58 These studies found a statistically significant and clinically meaningful reduction in OFF‐time duration. In two trials, 56 , 57 the reduction was around 120 min, with a corresponding increase in ON‐time of ~60 min more than placebo. 56 , 57 However, the third trial reported a smaller benefit in OFF‐time duration reduction, with only about a 0.7 h difference from placebo. Despite these improvements in ON‐ and OFF‐times, none of the trials showed a significant improvement in quality of life, as measured by the PDQ‐39. 56 , 57 , 58 Two studies assessed the efficacy of opicapone in improving disability (UPDRS‐II); one found no significant improvement, 57 while the other reported statistically significant but minimal improvements in the off‐medication state, with a difference of about 1 point compared with placebo. 58
2.11. Rasagiline
2.11.1. Efficacy Conclusion
Six trials met the eligibility criteria for this question. 59 , 60 , 61 , 62 , 63 , 64 One trial was excluded after manuscript screening because its primary outcome was safety and tolerability, and the UPDRS was measured in the ON‐state only. 61 Rasagiline is likely efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.11.2. Summary
Five trials evaluated the use of rasagiline for reducing OFF‐time duration compared with a placebo. 59 , 60 , 62 , 63 , 64 These studies found a statistically significant, though not clinically meaningful, reduction in OFF‐time ranging from 0.5 to 0.9 h/day at doses of either 0.5 mg/day 59 , 60 or 1 mg/day. 59 , 60 , 62 , 63 , 64 Four trials examined the effects of rasagiline on improving off‐medication state motor symptoms (MDS‐UPDRS‐III or UPDRS‐III) and disability (MDS‐UPDRS‐II or UPDRS‐II) compared with placebo. 59 , 60 , 62 , 63 These four trials reported a statistically significant but not clinically meaningful improvement in motor subscores (ranging from 1.6 to 5.6 points) and OFF‐time disability subscores (ranging from 1.0 to 1.7 points). Additionally, they observed an increase in ON‐time duration without troublesome dyskinesia, ranging from 0.5 to 1.2 h, at doses of either 0.5 or 1 mg/day. One trial noted a dose‐dependent increased risk of dyskinesia. Three trials assessed quality of life using the PDQ‐39 and found a statistically significant but not a clinically meaningful improvement with rasagiline at either dose of 0.5 or 1.0 mg/day. 59 , 60 , 64
2.12. Selegiline
2.12.1. Efficacy Conclusion
Five trials met the criteria for the PICOT. 65 , 66 , 67 , 68 , 69 The conclusion on the efficacy of selegiline for treating motor fluctuations on (attempted) optimal oral levodopa therapy is that there is insufficient evidence. The main concerns were low quality of the evidence, small sample sizes leading to imprecision, and heterogeneity in the results of the studies considered.
2.12.2. Summary
Four trials evaluated the use of selegiline for reducing OFF‐time compared with a placebo. 65 , 66 , 67 , 68 Only one study reported a meaningful reduction in OFF‐time, with at least a 1‐h decrease. 68 Another study documented a statistically significant reduction in end‐of‐dose and early morning akinesia during selegiline treatment, as well as increase in the mean duration of a levodopa dose's effect, though it did not clearly meet the 1‐h threshold. 65 Two other trials showed no significant difference in OFF‐time improvement with selegiline versus placebo. 66 , 67 Three trials assessed the use of selegiline for increasing ON‐time. 66 , 67 , 68 Only one trial reported a clinically meaningful benefit regarding dyskinesia‐free ON‐time compared with placebo. 68 Another study found no significant difference in ON‐time without dyskinesia at combined weeks 10 and 12, though it did report a statistically significant increase in dyskinesia‐free ON‐time at 12 weeks. 67 A third study showed no significant difference in ON‐time improvement with selegiline versus placebo. 66 Motor impairment was evaluated in three trials with mixed results. 65 , 66 , 69 Two studies assessed disability outcomes, with one showing significant improvement with selegiline using the Columbia University Rating Scale, 65 while the other found no difference between selegiline and placebo on the UPDR‐ II and the Schwab and England Activities of Daily Living Scale. 66
2.13. Safinamide
2.13.1. Efficacy Conclusion
Four trials met the eligibility criteria for this question. 70 , 71 , 72 , 73 Safinamide is efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.13.2. Summary
Four trials evaluated the use of safinamide for improving OFF‐time and ON‐time compared with a placebo. These studies found a clinically meaningful reduction in OFF‐time (around 1 h compared with placebo) and an increase in ON‐time without troublesome dyskinesia (also around 1 h compared with placebo). 70 , 71 , 72 , 73 Motor impairment in the off‐medication state was not evaluated in any of these studies. Regarding quality of life, assessed using the PDQ‐39, results varied. Only one study reached the 5‐point threshold for clinically meaningful improvement in PDQ‐39, but the confidence interval was large. 70 One study showed an improvement of more than 5 points only at the 100 mg dose. 71 Two studies did not meet this threshold. 71
2.14. Istradefylline
2.14.1. Efficacy Conclusion
Eight trials met the eligibility criteria for this question. Istradefylline is likely efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.14.2. Summary
Eight trials evaluated the use of istradefylline at doses of 10, 20, 40, and 60 mg once daily compared with placebo. 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 One study compared istradefylline combined with sham repetitive transcranial magnetic stimulation (rTMS) with a placebo combined with rTMS. 81 The reduction in OFF‐time with istradefylline was inconsistent and of unclear clinical relevance, ranging from 12 min (in larger studies) to 114 min (in smaller studies). Similarly, the increase in ON‐time without (troublesome) dyskinesia was inconsistent and of unclear clinical relevance, ranging from 12 to 48 min. Notably, studies that assessed ON‐time with (troublesome) dyskinesia reported an increase, ranging from 6 to 60 min. 76 , 77 , 78 , 80 There was no improvement in UPDRS‐III scores in the on‐medication state or off‐medication state compared with placebo, with changes ranging from −2.5 and 1.4 points (on‐medication state) and −0.4 to 2.2 points (off‐medication state). Regarding disability, there was no improvement in on‐medication state UPDRS‐II score, and an inconsistent and clinically insignificant improvement in off‐medication state UPDRS‐II scores, ranging from 0.3 to 1.8 points. Quality of life changes were assessed in only one trial, but quantitative results were not reported. 75
2.15. Amantadine
2.15.1. Efficacy Conclusion
Three trials met the eligibility criteria for this question. 82 , 83 , 84 One trial was excluded after title and abstract screening due to a short study duration caused by high dropout rates. 85 Amantadine extended release is likely efficacious in increasing ON‐time and decreasing OFF‐time in PD patients with motor fluctuations on (attempted) optimal oral levodopa therapy. There is insufficient evidence for the use of amantadine immediate release.
2.15.2. Summary
Two trials evaluated amantadine extended release as an add‐on therapy to improve motor fluctuations compared with a placebo. 82 , 83 They found a clinically meaningful increase in ON‐time (without troublesome dyskinesia, with troublesome dyskinesia, and with dyskinesia overall). While the reduction in OFF‐time was not clinically meaningful, the difference compared with the placebo group suggested a significant treatment effect. Washout of amantadine significantly worsened levodopa‐induced dyskinesia and ON‐time with troublesome dyskinesia, without significantly increasing OFF‐time. 84 Neither trial assessed changes in off‐medication state MDS‐UPDRS‐II or UPDRS‐III scores, nor did they evaluate quality of life. 82 , 83 , 84
2.16. Terguride
2.16.1. Efficacy Conclusion
One trial met the eligibility criteria for this question. 86 The efficacy conclusion is insufficient evidence for terguride to improve motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.16.2. Summary
One trial evaluated the use of terguride for reducing OFF‐time and improving both off‐medication and on‐medication states compared with placebo. 86 Terguride did not meaningfully improve scores on the Columbia University Rating Scale (measuring motor impairment) in either on‐medication or off‐medication states, and there was no significant reduction in OFF‐time compared with placebo after 3 months. Additionally, the authors did not find an increased risk of dyskinesia.
2.17. Nicotine
2.17.1. Efficacy Conclusion
A single trial met the eligibility criteria for the question. 87 The efficacy conclusion is insufficient evidence regarding nicotine use for treatment of motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.17.2. Summary
Only one trial assessed the potential benefit of nicotine. 87 Conducted as an open‐label study with no placebo‐treated arm, patients were assigned to either transdermal nicotine therapy or no additional treatment. Regarding motor impairment in the off‐medication state, there was no significant difference in MDS‐UPDRS‐III scores between the groups. 87 Nicotine demonstrated improvement in unblinded secondary outcomes such as disability and activities of daily living (UPDRS‐II in the off‐medication state), as well as dyskinesias (UPDRS‐IV). This suggests possible benefits for patients treated with nicotine; however, one cannot exclude a placebo effect. 87 These findings would need to be confirmed in larger, double‐blind, placebo‐controlled studies. Changes in OFF‐ and ON‐times duration were not evaluated in this study.
2.18. Zonisamide
2.18.1. Efficacy Conclusion
Two trials met the eligibility criteria for this question. 88 , 89 Zonisamide is likely efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.18.2. Summary
Two trials, conducted by the same group of researchers, evaluated the use of zonisamide at doses of 25, 50, and 100 mg once daily compared with a placebo. 88 , 89 The dropout rate was considerable, around 20%, and asymmetric in one trial. 88 Compared with placebo, OFF‐time reduction ranged from ~40 to 85 min, showing a dose‐dependent gradient. On‐medication UPDRS‐III improvement ranged between 1.5 and 3.8 points compared with the placebo. Inconsistencies were noted between the two trials. The efficacy of zonisamide in improving disability was assessed in one study. 89 Zonisamide 50 mg improved the off‐medication state UPDRS‐II by approximately 1 point, which was statistically but not clinically significant. Changes in ON‐time and quality of life were not assessed in these trials.
2.19. Perampanel
2.19.1. Efficacy Conclusion
Two trials met the eligibility criteria for this question. 90 , 91 The efficacy conclusion is insufficient evidence regarding perampanel use for treatment of motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.19.2. Summary
Two trials evaluated the use of perampanel for reducing OFF‐time compared with a placebo but failed to demonstrate its superiority. 90 , 91 Only one study assessed its benefits for ON‐time and disability. 90 In these areas, perampanel also failed to show significant improvement compared with the placebo. Additionally, motor impairment in the off‐medication state and HR‐QoL were not evaluated in any of these studies.
2.20. Helicobacter pylori Eradication
2.20.1. Efficacy Conclusion
One trial met the eligibility criteria for this question. 92 The efficacy conclusion is insufficient evidence regarding the eradication of Helicobacter pylori (HP) for the treatment of motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.20.2. Summary
One trial involving 34 patients evaluated the use of HP eradication (omeprazole, amoxicillin, clarithromycin) for motor fluctuations, comparing it with an antioxidant treatment with allopurinol in a double‐blind fashion. 92 Eligible patients had to have positive serology and positive stool testing for HP infection, confirmed by upper gastrointestinal endoscopy. Levodopa dosages remained unchanged during the study. Patients who underwent HP eradication showed significant improvement in motor impairment, with a prolonged on‐medication state measured by UPDRS‐III after 3 months, suggesting that HP infection may affect levodopa absorption. The prevalence of dyskinesias was not reported in this study.
2.21. Coenzyme Q10
2.21.1. Efficacy Conclusion
One trial met the eligibility criteria for this question. 93 Coenzyme Q10 is not efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.21.2. Summary
One trial evaluated the use of Coenzyme Q10 for reducing OFF‐time compared with placebo. 93 There were no differences between the Coenzyme Q10 and the placebo in terms of OFF‐time duration, ON‐time duration, motor impairment during off‐medication states, or disability scores.
2.22. Exenatide
2.22.1. Efficacy Conclusion
Two trials met the eligibility criteria for this question. 94 , 95 The efficacy conclusion is insufficient evidence for exenatide to improve motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.22.2. Summary
In two small trials—one a double‐blind trial 95 and the other an open‐label trial with blinded video assessment of the MDS‐UPDRS‐III 94 —the potential neuroprotective effects of exenatide were evaluated in patients experiencing wearing off. Both trials showed a difference in favor of exenatide on the MDS‐UPDRS‐III in the off‐medication state, with improvements of approximately 4 points, the threshold for the MCID. However, in the double‐blind trial, no differences were observed between exenatide and placebo on other outcomes, including the MDS‐UPDRS‐II in the on‐medication state, the PDQ‐39, or OFF‐time, and ON‐time durations. 95 Due to the small sample sizes, the potential effect hovering around the MCID, and the positive results being limited to a single measure, exenatide is classified as having insufficient evidence.
2.23. Subthalamic Nucleus Deep Brain Stimulation
2.23.1. Efficacy Conclusion
High‐quality evidence indicates that subthalamic nucleus (STN) deep brain stimulation (DBS) is efficacious to improve motor impairment in patients with PD who experience motor fluctuations on oral levodopa therapy. Additionally, STN DBS is also efficacious in improving disability, as measured by activities of daily living in the off‐medication state, and in improving HR‐QoL.
2.23.2. Summary
Eight RCTs evaluated the efficacy of STN DBS in treating motor fluctuations in patients with PD, comparing it to BMT or delayed stimulation. 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 Seven studies reported improvement in motor impairment, as measured by the UPDRS‐III in the off‐medication state, with an average improvement of 18.6 points in the STN DBS group. 96 , 97 , 98 , 99 , 100 , 101 , 102 One sham (subtherapeutic stimulation) controlled trial showed a difference in the increase in ON‐time without troublesome dyskinesia of 3.0 h (P < 0.0001). 102 Three studies reported the effect of DBS on number of hours spent in the on‐medication state, with an average difference between the STN DBS group and control of 2.6 h. 96 , 97 , 99 , 101 , 102 Four studies assessed the effect of STN DBS on disability, as measured by activities of daily living in the off‐medication state, showing moderate evidence for improvement ranging from 4.5 to 8.8 points on the UPDRS‐II. 96 , 97 , 98 , 99 Four studies evaluated the effect of STN DBS on quality of life, 96 , 98 , 99 , 102 indicating that STN DBS improves quality of life, with improvement ranging between 6.5 and 10.0 points on the PDQ‐39 summary index. Considering the surgical nature of the intervention, the concerns for blinding was raised for patient‐reported outcomes on the studies that did not use a sham control; thus the evidence for STN DBS on HR‐QoL measures and activities of daily living was downgraded to moderate. 102
2.24. Internal Globus Pallidus DBS
2.24.1. Efficacy Conclusion
Five studies met the eligibility criteria for this question, 100 , 104 , 105 , 106 , 107 of which two also had longer follow‐up periods published. 108 , 109 Bilateral internal globus pallidus (GPi) DBS is likely efficacious to treat motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.24.2. Summary
Two trials compared bilateral GPi and STN DBS versus best BMT. 104 , 106 Neither study separated the results of GPi DBS from STN DBS. In one study, 106 only 4 of 178 patients received GPi DBS, with the remainder receiving STN DBS; therefore, this study is not discussed further in the GPi DBS section of the review. The other study, from the United States Veterans Affairs Cooperative Studies Program (CSP) 468 study group, compared 121 DBS participants (61 GPi DBS and 60 STN DBS) with 134 participants receiving BMT. 104 Although patients were blinded to the surgical target (GPi vs. STN) and UPDRS‐III score ratings were done by neurologists blinded to treatment (DBS vs. BMT), the study nevertheless has a high risk of bias because patients were aware of surgical/stimulation (vs. BMT) status. Participants receiving DBS (of either target) showed a significant improvement in ON‐time without troublesome dyskinesia of 4.6 h compared with BMT, which showed no improvement. 104 A number of other metrics also improved with DBS compared with BMT, including off‐medication state motor impairment (UPDRS‐III score was 10.6 points better with DBS than with BMT), quality of life (PDQ‐39 score was 12 points better with DBS), and disability (UPDRS‐II was 4.6 points better with DBS). 104 The second phase of this Veterans Affairs study compared GPi (n = 152) versus STN (n = 147) targets 105 and found similar improvements in motor function after either bilateral GPi or bilateral STN DBS. In the GPi group, the primary outcome (change from baseline to 24 months in off‐medication, on‐stimulation UPDRS‐III scores) improved by a mean of 11.8 points (95% CI 9.5–14.1 points) versus 10.7 points (95% CI 8.5–12.9 points) in the STN group. There was a trend for greater improvements with GPi versus STN DBS in various secondary outcomes, including ON‐time without troublesome dyskinesia and PDQ‐39. 105 Improvements remained stable and comparable between targets at 36 months’ follow‐up, 108 although Mattis Dementia Rating Scale (MDRS) scores declined faster in the STN group (note, however, that the STN group was already slightly worse than GPi patients on some baseline neurocognitive tests). 108
Two other studies conducted double‐blind comparisons between bilateral GPi versus bilateral STN DBS. 100 , 107 , 109 In one study, 65 participants received GPi DBS and 63 received STN DBS with a follow‐up duration of 1 year. 107 The primary outcome was a scale assessing functional status and disability. There was no significant difference in the primary outcome between the GPi‐DBS and STN‐DBS groups, although the improvement was numerically smaller with GPi DBS (3.0 points vs. 7.7 points with STN DBS). 107 Likewise, there was a trend for lesser improvements in UPDRS‐II and quality of life scores with GPi versus STN DBS. A significantly lesser improvement was found for off‐medication UPDRS‐III score with GPi DBS compared with STN DBS (by 11.4 points vs. 20.3 points, P = 0.03). 107 At 3 years follow‐up, improvement in off‐medication UPDRS‐III scores was again less with GPi DBS versus STN DBS. 109 There were no differences in cognitive, mood, or behavioral outcomes. 109 An earlier pilot study involving 20 patients (10 GPi, 10 STN) who completed 12‐month follow‐up concluded that both procedures improved motor features of PD, including off‐medication UPDRS‐II and UPDRS‐III. 100
2.25. Pallidotomy
2.25.1. Efficacy Conclusion
Four trials met the eligibility criteria for this question. 110 , 111 , 112 , 113 , 114 Unilateral pallidotomy is likely efficacious in treating motor fluctuations (ie, off‐medication motor impairment) in PD patients on (attempted) optimal oral levodopa therapy, compared with medical therapy. 110 , 111 One trial demonstrated that unilateral pallidotomy was inferior to STN DBS in addressing PD motor fluctuations, 112 , 113 while another small trial suggested that unilateral pallidotomy was equally effective when compared with unilateral subthalamotomy. 114
2.25.2. Summary
The quality of evidence from trials comparing unilateral pallidotomy to medical therapy trials is limited by the lack of subject blinding (no studies included a sham control arm) and small sample sizes. Two trials evaluated the efficacy of unilateral pallidotomy in treating off‐medication motor impairment compared with medical therapy 110 , 111 and found a statistically significant and clinically meaningful improvement of UPDRS‐III scores (with assessors blinded to treatment assignment in both studies) by ~15 points in the pallidotomy group at 6‐month follow‐up, with no significant score change in the medical therapy group. 110 , 111 Both trials also reported improvement in disability as measured by UPDRS‐II and the Schwab and England Activities of Daily Living Scale. Potential benefits in OFF‐time, ON‐time, and quality of life were only assessed by one trial, 110 leading to insufficient evidence to conclude on these outcomes. In comparison with STN DBS, one trial showed that bilateral STN DBS was more effective than unilateral pallidotomy in improving off‐medication state motor impairment at 6 months (UPDRS‐III 19 vs. 7 points improvement) and at 12 months (24 vs. 12 points improvement), indicating longer‐term superiority. 112 , 113 Meanwhile, in one very small trial (n = 10), both unilateral pallidotomy and unilateral subthalamotomy improved off‐medication state motor impairment and Schwab and England Activities of Daily Living Scale, with no significant differences between the groups.
2.26. Subthalamotomy
2.26.1. Efficacy Conclusion
Two trials met the eligibility criteria for this question. 114 , 115 There is insufficient evidence to support the use of unilateral or bilateral subthalamotomy for treating motor fluctuations in PD. Severe hemiballismus has been reported as a major adverse event in 20%–50% of patients randomized to undergo unilateral or bilateral subthalamotomy.
2.26.2. Summary
Two trials met the eligibility criteria for this question but had very small sample sizes. 114 , 115 One study compared unilateral subthalamotomy with unilateral pallidotomy (n = 10), while another compared bilateral subthalamotomy, unilateral subthalamotomy with contralateral STN DBS, and bilateral STN DBS (n = 16). No studies compared subthalamotomy to BMT. All procedures led to significant improvements in off‐medication state motor impairments and functional scales, but without significant differences between groups.
2.27. Bilateral Zona Incerta DBS
2.27.1. Efficacy Conclusion
One study met the eligibility criteria for this question. 116 The efficacy conclusion is insufficient evidence regarding the use of bilateral zona incerta DBS for the treatment of motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.27.2. Summary
One trial meeting the review eligibility criteria compared bilateral zona incerta DBS with BMT in PD. 116 Only 19 patients completed the trial and evaluations at 6 months (9 DBS, 10 BMT). Motor impairment, as assessed with off‐medication UPDRS‐III, was improved by 41% compared with baseline in the zona incerta DBS group, with a strong effect on tremor, whereas the score was unchanged in the BMT group. Quality of life (PDQ‐39 summary index) improved from baseline in both groups, but without significant differences between groups. As with most DBS studies, this trial has a high risk of bias due to blinding issues, with only the outcome assessors (for the UPDRS‐III) blinded to treatment allocation, but not the patients.
2.28. Glial Cell‐Derived Neurotrophic Factor
2.28.1. Efficacy Conclusion
One trial met the eligibility criteria for the question. 117 , 118 The efficacy conclusion is insufficient evidence regarding glial cell‐derived neurotrophic factor (GDNF) use for treatment of motor fluctuations in PD patients on (attempted) optimal oral levodopa therapy.
2.28.2. Summary
Motor impairment in the off‐medication state was evaluated in one trial with GDNF. 117 , 118 This trial compared bilateral intraputamenal infusions of GDNF (120 mg per putamen) with a placebo but failed to show its superiority in improving UPDRS‐III scores in the off‐medication state at a 40‐week follow‐up. 117 The open‐label extension study also did not demonstrate significant improvement in motor scores with GDNF. 118
Both studies assessed benefits for disability, finding no significant difference between the GDNF and placebo groups, or between the GDNF/GDNF group and placebo/GDNF groups in the open‐label extension study. 117 , 118 Similarly, GDNF did not show improvement in quality‐of‐life outcomes (PDQ‐39 and EQ‐5D), which were evaluated as supplementary endpoints. 117 , 118 Regarding dyskinesias, the UPDRS‐IV score remained essentially unchanged between baseline and weeks 40 and 80 in both groups, with no significant treatment differences between GDNF and placebo or between GDNF/GDNF and placebo/GDNF. 118 Changes in OFF‐ and ON‐times were not evaluated in these studies. The trial detailed total OFF‐time, good quality ON‐time, and ON‐time with troublesome dyskinesias, finding no significant differences between the GDNF and placebo groups. 117 The open‐label extension study also failed to show significant improvement of PD diary‐based outcomes. 118
3. Discussion
In this new systematic review, levodopa extended release, pramipexole immediate release and extended release, ropinirole immediate release, rotigotine, opicapone, safinamide, and bilateral STN DBS were assessed as efficacious, and continuous intestinal levodopa infusion, continuous subcutaneous levodopa, continuous subcutaneous apomorphine, ropinirole prolonged release, ropinirole patch, continuous subcutaneous apomorphine, entacapone, rasagiline, istradefylline, amantadine controlled release, zonisamide, bilateral GPi DBS, and pallidotomy were assessed as likely efficacious for the treatment of motor fluctuations in people with PD who are already being treated with levodopa. In the current review, the dopamine agonists pergolide, bromocriptine, and cabergoline, and the catechol‐O‐methyltransferase (COMT) inhibitor tolcapone are no longer mentioned, unlike in the previous review. This is either due to insufficient scientific evidence or because they are no longer available for the treatment of PD. Meanwhile, more alternatives are available. As a result of the new methodology, the efficacy conclusions for continuous intestinal levodopa infusion, prolonged release ropinirole, entacapone, rasagiline, zonisamide, bilateral GPi DBS, and pallidotomy have been revised from efficacious to likely efficacious in this review, compared with the previous one. This is due to, for example, the fact that the new method required a longer follow‐up period, concerns with precision, and blinding. The duration of follow‐up of previously included studies was also the reason for changing some classifications from efficacious to insufficient evidence (eg, intermittent apomorphine injections).
Since 2002, the MDS EBM Committee has reviewed the scientific literature on PD treatments. These reviews used a systematic evaluation method to assess the quality of included studies. Conclusions on the effectiveness of interventions were based on evidence level and clinical relevance. Initially, there was room for nuanced conclusions based on expert opinion due to the relative lack of high‐quality RCTs for some widely used treatments, like levodopa. The first RCT comparing levodopa to a placebo for early PD was published in 2004, despite its long‐term clinical use. 119 Nowadays, the quality of clinical studies is better, and there are many more treatments available for PD. Therefore, it was decided to adapt the methods for the reviews to a modified GRADE methodology. The literature search, as well as the risk of bias assessment and the conclusions, were standardized. Another important modification is that the duration of follow‐up of the included studies is at least 3 months.
In previous updates, the reviews from before were used as a basis, and these were then supplemented with new studies. The current review is entirely new: from a completely new literature search to the efficacy conclusions. Because it was expected that renewing the reviews would require considerable work, and to assess the feasibility of the revised method of the MDS EBM review, we first started with the PICOT motor fluctuations of the treatment of PD motor symptoms and not immediately with all subareas of the treatment of motor symptoms review. The other parts of the motor symptoms review will follow shortly. The RCTs on High Intensity Focused Ultrasound, potentially eligible for the review, were published after our search. We anicipate that Focused Ultrasound may be adressed in future updates of the review.
With regard to the choice of an intervention, in addition to effectiveness, the adverse effect profile is also relevant. For an adequate assessment of adverse effects, more types of research and sources of information are important than just RCTs. Therefore, we mention possible, particular, severe side effects, but we refer to the SmPC for medications and available (national) databases for adverse effect profiles.
There are many options available for treating motor fluctuations. In clinical practice, the most common approach is to start by adjusting the dosing schedule of levodopa, which may involve taking more frequent doses throughout the day or shortening the interval between doses. Additionally, promoting the predictability of levodopa absorption is important; this can be achieved by taking it on an empty stomach and ensuring normal gastrointestinal transit time through the treatment of constipation. However, not all of these practical adjustments have been investigated in randomized trials. If this does not provide sufficient improvement, other interventions may be necessary, with the obvious choice being to start with oral or transdermal medication options and, if these are not beneficial enough, switch to a more invasive pump treatment or neurosurgical procedure.
The challenge lies in determining which medication to initiate first from the wide array of options available for someone who has developed motor fluctuations. There are few comparative studies with long‐term follow‐up to support a particular choice. One study relevant to this situation is the second PD MED RCT. 120 In this pragmatic RCT, a total of 500 patients with PD, with a mean age of 73.0 years (SD 8.2 years), who developed motor fluctuations and did not have dementia, were randomly assigned to open‐label treatment with a dopamine agonist, a monoamine oxidase type B (MAO‐B) inhibitor, or a COMT inhibitor. Over a median of 4.5 years of follow‐up, participants in the dopamine agonist group had a mean PDQ‐39 mobility score that was 2.4 points (95% CI −1.3 to 6.0 points) better than that of the combined MAO‐B and COMT groups; however, this difference was not statistically significant. Participants in the MAO‐B group had mean PDQ‐39 mobility scores that were 4.2 points (95% CI 0.4–7.9 points; P = 0.03) better than those in the COMT group. This study highlights the clinical difficulties clinicians face when weighing the evidence and recommending a particular treatment to a given patient. In addition to the varying degree of certainty of the efficacy of an intervention, its safety and individual tolerability must be taken into account, together with each patient's preference.
It may not always be necessary to exhaustively try all available oral options before switching to one of the more invasive treatments. The results of the EARLYSTIM trial showed that STN DBS was superior to medical therapy in patients with PD and early motor complications. 96 It would be helpful in making a choice between the various options if more studies became available that compare treatments head‐to‐head. For example, comparisons between DBS and infusion therapies, as well as between medications like safinamide and rasagiline, would provide valuable insights. Also, the fact that different outcome measures have been used in different studies and treatments also makes it more difficult to combine the results of the studies in a meta‐analysis.
The current review is not the same as a guideline for the treatment of PD and does not prioritize therapeutic choices, but rather reflects the available evidence. For example, although STN DBS has a higher efficacy designation than some of the oral dopamine agonists, this does not imply that STN DBS should be used prior to these medications in treating motor fluctuations. Neither can it be concluded that oral treatments that have an efficacious designation are more efficacious, in terms of their magnitude of effect, compared with GPi DBS or infusion therapies with a likely efficacious designation. Similarly, in clinical practice, consideration must be given to the patient profile and disease stage.
When drafting a guideline, in is important to consider not only the effectiveness of an intervention but also factors such as its availability and costs, as well as any alternatives within a specific country or region. Additionally, a patient's social environment, preferences, lifestyle, and cultural aspects should also be taken into account. Our review could serve as a valuable resource for developing guidelines for the treatment of PD.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
R.M.A.d.B.: 1A, 1B, 1C, 2A, 2B.
R.K.: 1A, 1C, 2B.
B.E.K.S.S.: 1A, 1C, 2B.
M.P.: 1A, 1C, 2B.
S.‐Y.L.: 1A, 1C, 2B.
T.A.M.: 1A, 1C, 2B.
S.P.L.: 1A, 1C, 2B.
M.C.: 1A, 1C, 2B.
C.A.: 1A, 1C, 2B.
A.H.T.: 1A, 1C, 2B.
V.B.: 1A, 1C, 2B.
J.M.D.: 1A, 1C, 2B.
B.H.: 1A, 1C, 2B.
C.‐H.L.: 1A, 1C, 2B.
L.A.K.: 1A, 1C, 2B.
I.L.: 1C, 2B.
R.S.: 1A, 1C, 2B.
K.S.: 1A, 1C, 2B.
J.C.: 1A, 2B.
C.S.: 1A, 2B.
S.H.F.: 1A, 2B.
M.A.S.: 1A, 1C, 2B.
Financial Disclosures
R.M.A.d.B. received research grants from Medtronic, Bial, ZonMw, AMC Foundation, ROMO Foundation, and Stichting ParkinsonFonds, all paid to the institution. R.K. has received honoraria for lecturing and consulting from AbbVie, Bial, Britannia, Merz, Neuroderm, Novartis, Stada, Supernus, UCB, and Zambon. B.E.K.S.S. has no disclosures to report. M.P. received speaking honoraria (lectures) from Candoro Ethics Austria. S.‐Y.L. has received stipends from the International Parkinson and Movement Disorder Society (MDS) as Chair of the Asian‐Oceanian Section, and Science Advances as Associate Editor (Neuroscience). He reports consultancies from The Michael J. Fox Foundation for Parkinson's Research, the Aligning Science Across Parkinson's‐Global Parkinson's Genetics Program (ASAP‐GP2), and Neurotorium Editorial Board; honoraria for lecturing from the MDS, Lundbeck, Eisai, and Medtronic; and research grants from the Malaysian Ministry of Education Fundamental Research Grant Scheme and The Michael J. Fox Foundation for Parkinson's Research. T.A.M. has received honoraria for participating in Consulting and Advisory Boards for AbbVie, Ipsen, International Parkinson and Movement Disorder Society, CHDI Foundation/Management, Roche, and Medtronic; has received research grants from EU Joint Programme – Neurodegenerative Disease Research, uOBMRI, Ontario Research Fund, CIHR, The Michael J. Fox Foundation for Parkinson's Research, Parkinson Canada, Parkinson Research Consortium, and Brain Canada; and has received salary from UOMA. S.P.L. consulted for ELEA laboratories and received honoraria from IPMDS; has also received grants from the Agencia De Promocion Científica Y Técnica and honoraria for manuscript writing from IPMDS; and owns stock options from Teleneurosolutions LLC. M.C. has received honoraria and financial support to attend neurology meetings from Bial, Zambon, AbbVie, Stada, Medtronic, Boston Scientific, Ipsen, Allergan, Merz, Italofarmaco, and Rovi; and for the previous 12 months has received fees from an observational study conducted by AbbVie. C.A. has no disclosures to report. A.H.T. has received speaker's honoraria from Eisai and Orion Pharma, a research grant from The Michael J. Fox Foundation for Parkinson's Research, the Global Parkinson's Genetic Program, and a stipend from Elsevier as Associate Editor of Parkinsonism and Related Disorders. V.B. received research grants from Allergan, Ipsen, and Paladin Labs and has served on an advisory board for Biogen. J.M.D. reports research grants from ZonMw (Netherlands governmental fund for health research); Medtronic for a randomized trial comparing deep brain stimulation and continuous intrajejunal levodopa in advanced Parkinson's disease; Stichting Parkinson Nederland (charitable foundation); Amsterdam Neuroscience (academic research institute); and Medtronic, all paid to the institution. B.H. reports honoraria from Novartis AG, AbbVie, and Bial, and grants from the Austrian Science Fund (FWF), outside the submitted work. C.‐H.L. has no disclosures to report. L.A.K. has received support from AbbVie for scientific conference attendance and speaker honoraria from Zambon. I.L. undertakes research supported by the National Institutes of Health (NIH) grants: U01NS112010, U01NS100610, R01AG063911, R61NS141119‐01; U01NS112010; P30AG062429‐06; and R25NS10893; The Michael J. Fox Foundation for Parkinson's Research, Parkinson's Foundation, Lewy Body Association, CurePSP, Roche, AbbVie, Lundbeck, EIP‐Pharma, Alterity, Novartis, and UCB. She is a member of the Scientific Advisory Board for the Rossy PSP Program at the University of Toronto, Aprinoia, and Amydis; and she receives her salary from the University of California San Diego and as Chief Editor of Frontiers in Neurology. R.S. has no disclosures to report. K.S. reports honoraria from the International Parkinson and Movement Disorders Society, grants from the Austrian Science Fund (FWF), The Michael J. Fox Foundation for Parkinson's Research, and the International Parkinson and Movement Disorder Society, as well as personal fees from Teva, UCB, Lundbeck, AOP Orphan Pharmaceuticals AG, AbbVie, Roche, and Grünenthal, outside the submitted work. J.C. has no specific funding that was received for this work, and declares that there are no additional disclosures to report. C.S. is an employee of CHDI Management, Inc. (advisors for CHDI Foundation), and has received consultancy honorarium from Pfizer, Kyowa Kirin, vTv Therapeutics, GW Pharmaceuticals, Neuraly, Neuroderm, Neuroxpharm, Inflictis, Biocodex, Thelonious Mind, Novartis, Biogen Green Valley Pharmaceuticals, and Pinteeon Pharmaceuticals. S.H.F. receives clinic support from the Edmond J. Safra Foundation for Parkinson Research; Parkinson Foundation, and the Toronto Western and General Foundation; research funding from The Michael J. Fox Foundation for Parkinson's Research, NIH (Dystonia Coalition); Parkinson Canada; Weston Foundation; honoraria from the International Parkinson and Movement Disorder Society; consultancy/speaker fees from AbbVie; and royalties from Oxford University Press. M.A.S. has received grants from Parkinson's UK, the Michael J. Fox Foundation for Parkinson's Research, and the Medical Research Council (MRC); and honoraria for lecturing and consulting from Bial and Medtronic.
Supporting information
Data S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Goetz C, Koller W, Poewe W. Management of Parkinson's disease: an evidence‐based review. Mov Disord 2002;17(Suppl. 4):S1–S–166. 10.1002/mds.5555 [DOI] [PubMed] [Google Scholar]
- 2. Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence‐based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord 2018;33(8):1248–1266. 10.1002/mds.27372 [DOI] [PubMed] [Google Scholar]
- 3. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64(4):383–394. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 4. Katzenschlager R, de Bie RMA, Costa J, Sampaio C. MDS Evidence Based Medicine Committee: Revision of the methodological process for Systematic Reviews: Adoption of the Modified GRADE system; 2021. https://www.movementdisorders.org/MDS-Files1/PDFs/EBM-Papers/EBMCommitteeMethodologyUpdateSept2021.pdf.
- 5. Lefebvre C, Glanville J, Briscoe S, et al. Searching for and selecting studies. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons Ltd; 2019:67–107. 10.1002/9781119536604.ch4. [DOI] [Google Scholar]
- 6. Glanville J, Foxlee R, Wisniewski S, Noel‐Storr A, Edwards M, Dooley G. Translating the Cochrane EMBASE RCT filter from the Ovid interface to Embase.com: a case study. Health Info Libr J 2019;36(3):264–277. 10.1111/hir.12269 [DOI] [PubMed] [Google Scholar]
- 7. Cochrane Central Register of Controlled Trials (CENTRAL); https://www.cochranelibrary.com/central/about-central.
- 8. Lobbestael G. DedupEndNote (Version 1.0.0); 2023.
- 9. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hauser RA, Gordon MF, Mizuno Y, et al. Minimal clinically important difference in Parkinson's disease as assessed in pivotal trials of pramipexole extended release. Parkinsons Dis 2014;2014:467131. 10.1155/2014/467131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horváth K, Aschermann Z, Ács P, et al. Minimal clinically important difference on the motor examination part of MDS‐UPDRS. Parkinsonism Relat Disord 2015;21(12):1421–1426. 10.1016/j.parkreldis.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 12. Horvath K, Aschermann Z, Kovacs M, et al. Minimal clinically important differences for the experiences of daily living parts of movement disorder society – sponsored unified Parkinson's disease rating scale. Mov Disord 2017;32(5):789–793. 10.1002/mds.26960 [DOI] [PubMed] [Google Scholar]
- 13. Horváth K, Aschermann Z, Kovács M, et al. Changes in quality of life in Parkinson's disease: how large must they be to be relevant? Neuroepidemiology 2017;48(1–2):1–8. 10.1159/000455863 [DOI] [PubMed] [Google Scholar]
- 14. Wolters EC, Horstink M, Roos RAC, Jansen ENH. Clinical efficacy of Sinemet CR 50/200 versus Sinemet 25/100 in patients with fluctuating Parkinson's disease: an open, and a double‐blind, double‐dummy, multicenter treatment evaluation. Clin Neurol Neurosurg 1992;94(3):205–211. [DOI] [PubMed] [Google Scholar]
- 15. Wolters EC, Tesselaar HJ. International (NL‐UK) double‐blind study of Sinemet CR and standard Sinemet (25/100) in 170 patients with fluctuating Parkinson's disease. J Neurol 1996;243(3):235–240. [DOI] [PubMed] [Google Scholar]
- 16. Hutton JT, Morris JL, Roman GC, Imke SC, Elias JW. Treatment of chronic Parkinson's disease with controlled‐release carbidopa/levodopa. Arch Neurol 1988;45(8):861–864. [DOI] [PubMed] [Google Scholar]
- 17. Hauser RA, Hsu A, Kell S, et al. Extended‐release carbidopa‐levodopa (IPX066) compared with immediate‐release carbidopa‐levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double‐blind trial. Lancet Neurol 2013;12(4):346–356. [DOI] [PubMed] [Google Scholar]
- 18. Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa‐carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double‐blind, double‐dummy study. Lancet Neurol 2014;13(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung SJ, Calopa M, Ceravolo MG, et al. Effects of levodopa‐carbidopa intestinal gel compared with optimized medical treatment on nonmotor symptoms in advanced Parkinson's disease: INSIGHTS study. Parkinsons Dis 2022;2022:1216975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soileau MJ, Aldred J, Budur K, et al. Safety and efficacy of continuous subcutaneous foslevodopa‐foscarbidopa in patients with advanced Parkinson's disease: a randomised, double‐blind, active‐controlled, phase 3 trial. Lancet Neurol 2022;21(12):1099–1109. 10.1016/S1474-4422(22)00400-8 [DOI] [PubMed] [Google Scholar]
- 21. Mizuno Y, Yanagisawa N, Kuno S, et al. Randomized, double‐blind study of pramipexole with placebo and bromocriptine in advanced Parkinson's disease. Mov Disord 2003;18(10):1149–1156. [DOI] [PubMed] [Google Scholar]
- 22. Wong KS, Lu CS, Shan DE, Yang CC, Tsoi TH, Mok V. Efficacy, safety, and tolerability of pramipexole in untreated and levodopa‐treated patients with Parkinson's disease. J Neurol Sci 2003;216(1):81–87. [DOI] [PubMed] [Google Scholar]
- 23. Moller JC, Oertel WH, Koster J, Pezzoli G, Provinciali L. Long‐term efficacy and safety of pramipexole in advanced Parkinson's disease: results from a European multicenter trial. Mov Disord 2005;20(5):602–610. [DOI] [PubMed] [Google Scholar]
- 24. Poewe WH, Rascol O, Quinn N, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: a double‐blind, double‐dummy, randomised controlled trial. Lancet Neurol 2007;6(6):513–520. [DOI] [PubMed] [Google Scholar]
- 25. Schapira AH, Barone P, Hauser RA, et al. Extended‐release pramipexole in advanced Parkinson disease: a randomized controlled trial. Neurology 2011;77(8):767–774. [DOI] [PubMed] [Google Scholar]
- 26. Mizuno Y, Yamamoto M, Kuno S, et al. Efficacy and safety of extended‐ versus immediate‐release pramipexole in Japanese patients with advanced and L‐dopa‐undertreated Parkinson disease: a double‐blind, randomized trial. Clin Neuropharmacol 2012;35(4):174–181. [DOI] [PubMed] [Google Scholar]
- 27. Rascol O, Lees AJ, Senard JM, Pirtosek Z, Montastruc JL, Fuell D. Ropinirole in the treatment of levodopa‐induced motor fluctuations in patients with Parkinson's disease. Clin Neuropharmacol 1996;19(3):234–245. [DOI] [PubMed] [Google Scholar]
- 28. Lieberman A, Olanow CW, Sethi K, et al. A multicenter trial of ropinirole as adjunct treatment for Parkinson's disease. Ropinirole Study Group. Neurology 1998;51(4):1057–1062. [DOI] [PubMed] [Google Scholar]
- 29. Mizuno Y, Nomoto M, Hasegawa K, et al. Rotigotine vs ropinirole in advanced stage Parkinson's disease: a double‐blind study. Parkinsonism Relat Disord 2014;20(12):1388–1393. [DOI] [PubMed] [Google Scholar]
- 30. Brunt ER, Brooks DJ, Korczyn AD, Montastruc JL, Stocchi F. A six‐month multicentre, double‐blind, bromocriptine‐controlled study of the safety and efficacy of ropinirole in the treatment of patients with Parkinson's disease not optimally controlled by L‐dopa. J Neural Transm 2002;109(4):489–502. [DOI] [PubMed] [Google Scholar]
- 31. Im JH, Ha JH, Cho IS, Lee MC. Ropinirole as an adjunct to levodopa in the treatment of Parkinson's disease: a 16‐week bromocriptine controlled study. J Neurol 2003;250(1):90–96. [DOI] [PubMed] [Google Scholar]
- 32. Watts RL, Lyons KE, Pahwa R, et al. Onset of dyskinesia with adjunct ropinirole prolonged‐release or additional levodopa in early Parkinson's disease. Mov Disord 2010;25(7):858–866. [DOI] [PubMed] [Google Scholar]
- 33. Barone P, Lamb J, Ellis A, Clarke Z. Sumanirole versus placebo or ropinirole for the adjunctive treatment of patients with advanced Parkinson's disease. Mov Disord 2007;22(4):483–489. [DOI] [PubMed] [Google Scholar]
- 34. Mizuno Y, Abe T, Hasegawa K, et al. Ropinirole is effective on motor function when used as an adjunct to levodopa in Parkinson's disease: STRONG study. Mov Disord 2007;22(13):1860–1865. [DOI] [PubMed] [Google Scholar]
- 35. Pahwa R, Stacy MA, Factor SA, et al. Ropinirole 24‐hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology 2007;68(14):1108–1115. [DOI] [PubMed] [Google Scholar]
- 36. Zesiewicz TA, Chriscoe S, Jimenez T, Upward J, Davy M, VanMeter S. A randomized, fixed‐dose, dose‐response study of ropinirole prolonged release in advanced Parkinson's disease. Neurodegener Dis Manag 2017;7(1):61–72. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Z, Wang J, Zhang X, et al. The efficacy and safety of ropinirole prolonged release tablets as adjunctive therapy in Chinese subjects with advanced Parkinson's disease: a multicenter, double‐blind, randomized, placebo‐controlled study. Parkinsonism Relat Disord 2013;19(11):1022–1026. [DOI] [PubMed] [Google Scholar]
- 38. Reichmann H, Cooper J, Rolfe K, Martinez‐Martin P. Sleep duration and “on” time during different periods of the day and night in patients with advanced Parkinson's disease receiving adjunctive ropinirole prolonged release. Parkinsons Dis 2011;2011:354760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hattori N, Mochizuki H, Hasegawa K, et al. Ropinirole patch versus placebo, ropinirole extended‐release tablet in advanced Parkinson's disease. Mov Disord 2020;35(9):1565–1573. 10.1002/mds.28071 [DOI] [PubMed] [Google Scholar]
- 40. Stocchi F, Giorgi L, Hunter B, Schapira AH. PREPARED: comparison of prolonged and immediate release ropinirole in advanced Parkinson's disease. Mov Disord 2011;26(7):1259–1265. [DOI] [PubMed] [Google Scholar]
- 41. LeWitt PA, Lyons KE, Pahwa R. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER study. Neurology 2007;68(16):1262–1267. [DOI] [PubMed] [Google Scholar]
- 42. Nomoto M, Mizuno Y, Kondo T, et al. Transdermal rotigotine in advanced Parkinson's disease: a randomized, double‐blind, placebo‐controlled trial. J Neurol 2014;261(10):1887–1893. [DOI] [PubMed] [Google Scholar]
- 43. Nicholas AP, Borgohain R, Chana P, et al. A randomized study of rotigotine dose response on “off” time in advanced Parkinson's disease. J Parkinsons Dis 2014;4(3):361–373. [DOI] [PubMed] [Google Scholar]
- 44. Zhang Z‐X, Liu C‐F, Tao E‐X, et al. Rotigotine transdermal patch in Chinese patients with advanced Parkinson's disease: a randomized, double‐blind, placebo‐controlled pivotal study. Parkinsonism Relat Disord 2017;44:6–12. [DOI] [PubMed] [Google Scholar]
- 45. Hattori N, Nomoto M, 6500‐004 Study Group . Sustained efficacy of apomorphine in Japanese patients with advanced Parkinson's disease. Parkinsonism Relat Disord 2014;20(8):819–823. [DOI] [PubMed] [Google Scholar]
- 46. Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson's disease with persistent motor fluctuations (TOLEDO): a multicentre, double‐blind, randomised, placebo‐controlled trial. Lancet Neurol 2018;17(9):749–759. 10.1016/S1474-4422(18)30239-4 [DOI] [PubMed] [Google Scholar]
- 47. Olanow CW, Factor SA, Espay AJ, et al. Apomorphine sublingual film for off episodes in Parkinson's disease: a randomised, double‐blind, placebo‐controlled phase 3 study. Lancet Neurol 2020;19(2):135–144. 10.1016/S1474-4422(19)30396-5 [DOI] [PubMed] [Google Scholar]
- 48. Brooks DJ, Sagar H. Entacapone is beneficial in both fluctuating and non‐fluctuating patients with Parkinson's disease: a randomised, placebo controlled, double blind, six month study. J Neurol Neurosurg Psychiatry 2003;74(8):1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fenelon G, Gimenez‐Roldan S, Montastruc JL, et al. Efficacy and tolerability of entacapone in patients with Parkinson's disease treated with levodopa plus a dopamine agonist and experiencing wearing‐off motor fluctuations. A randomized, double‐blind, multicentre study. J Neural Transm 2003;110(3):239–251. [DOI] [PubMed] [Google Scholar]
- 50. Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M. Efficacy and safety of entacapone in Parkinson's disease patients with suboptimal levodopa response: a 6‐month randomized placebo controlled double blind study in Germany and Austria (Celomen study). Acta Neurol Scand 2002;105(4):245–255. [DOI] [PubMed] [Google Scholar]
- 51. Reichmann H, Boas J, MacMahon D, Myllyla V, Hakala A, Reinikainen K. Efficacy of combining levodopa with entacapone on quality of life and activities of daily living in patients experiencing wearing‐off type fluctuations. Acta Neurol Scand 2005;111(1):21–28. [DOI] [PubMed] [Google Scholar]
- 52. Rinne UK, Larsen JP, Siden A, Worm‐Petersen J. Entacapone enhances the response to levodopa in Parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology 1998;51(5):1309–1314. [DOI] [PubMed] [Google Scholar]
- 53. Entacapone improves motor fluctuations in levodopa‐treated Parkinson's disease patients. Parkinson Study Group. Ann Neurol 1997;42(5):747–755. [DOI] [PubMed] [Google Scholar]
- 54. Deuschl G, Vaitkus A, Fox GC, Roscher T, Schremmer D, Gordin A. Efficacy and tolerability of entacapone versus cabergoline in parkinsonian patients suffering from wearing‐off. Mov Disord 2007;22(11):1550–1555. [DOI] [PubMed] [Google Scholar]
- 55. Lew MF, Somogyi M, McCague K, Welsh M. Immediate versus delayed switch from levodopa/carbidopa to levodopa/carbidopa/entacapone: effects on motor function and quality of life in patients with Parkinson's disease with end‐of‐dose wearing off. Int J Neurosci 2011;121(11):605–613. [DOI] [PubMed] [Google Scholar]
- 56. Ferreira JJ, Lees A, Rocha J‐F, Poewe W, Rascol O, Soares‐da‐Silva P. Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end‐of‐dose motor fluctuations: a randomised, double‐blind, controlled trial. Lancet Neurol 2016;15(2):154–165. 10.1016/S1474-4422(15)00336-1 [DOI] [PubMed] [Google Scholar]
- 57. Lees AJ, Ferreira J, Rascol O, et al. Opicapone as adjunct to levodopa therapy in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol 2017;74(2):197–206. 10.1001/jamaneurol.2016.4703 [DOI] [PubMed] [Google Scholar]
- 58. Takeda A, Takahashi R, Tsuboi Y, et al. Randomized, controlled study of opicapone in Japanese Parkinson's patients with motor fluctuations. Mov Disord 2021;36(2):415–423. 10.1002/mds.28322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hattori N, Takeda A, Takeda S, et al. Efficacy and safety of adjunctive rasagiline in Japanese Parkinson's disease patients with wearing‐off phenomena: a phase 2/3, randomized, double‐blind, placebo‐controlled, multicenter study. Parkinsonism Relat Disord 2018;53:21–27. 10.1016/j.parkreldis.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 60. Parkinson Study Group . A randomized placebo‐controlled trial of rasagiline in levodopa‐treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol 2005;62(2):241–248. [DOI] [PubMed] [Google Scholar]
- 61. Rabey JM, Sagi I, Huberman M, et al. Rasagiline mesylate, a new MAO‐B inhibitor for the treatment of Parkinson's disease: a double‐blind study as adjunctive therapy to levodopa. Clin Neuropharmacol 2000;23(6):324–330. [DOI] [PubMed] [Google Scholar]
- 62. Rascol O, Brooks DJ, Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double‐blind, parallel‐group trial. Lancet 2005;365(9463):947–954. [DOI] [PubMed] [Google Scholar]
- 63. Zhang L, Zhang Z, Chen Y, et al. Efficacy and safety of rasagiline as an adjunct to levodopa treatment in Chinese patients with Parkinson's disease: a randomized, double‐blind, parallel‐controlled, multi‐centre trial. Int J Neuropsychopharmacol 2013;16(7):1529–1537. [DOI] [PubMed] [Google Scholar]
- 64. Zhang Z, Shao M, Chen S, et al. Adjunct rasagiline to treat Parkinson's disease with motor fluctuations: a randomized, double‐blind study in China. Transl Neurodegener 2018;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heinonen EH, Rinne UK, Tuominen J. Selegiline in the treatment of daily fluctuations in disability of parkinsonian patients with long‐term levodopa treatment. Acta Neurol Scand Suppl 1989;126:113–118. [DOI] [PubMed] [Google Scholar]
- 66. Hubble JP, Koller WC, Waters C. Effects of selegiline dosing on motor fluctuations in Parkinson's disease. Clin Neuropharmacol 1993;16(1):83–87. [DOI] [PubMed] [Google Scholar]
- 67. Ondo WG, Sethi KD, Kricorian G. Selegiline orally disintegrating tablets in patients with Parkinson disease and “wearing off” symptoms. Clin Neuropharmacol 2007;30(5):295–300. [DOI] [PubMed] [Google Scholar]
- 68. Waters CH, Sethi KD, Hauser RA, Molho E, Bertoni JM. Zydis selegiline reduces off time in Parkinson's disease patients with motor fluctuations: a 3‐month, randomized, placebo‐controlled study. Mov Disord 2004;19(4):426–432. [DOI] [PubMed] [Google Scholar]
- 69. Teychenne PF, Parker S. Double‐blind, crossover placebo controlled trial of selegiline in Parkinson's disease—an interim analysis. Acta Neurol Scand Suppl 1989;126:119–125. [DOI] [PubMed] [Google Scholar]
- 70. Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add‐on to levodopa in Parkinson's disease with motor fluctuations. Mov Disord 2014;29(2):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hattori N, Tsuboi Y, Yamamoto A, Sasagawa Y, Nomoto M. Efficacy and safety of safinamide as an add‐on therapy to L‐DOPA for patients with Parkinson's disease: a randomized, double‐blind, placebo‐controlled, phase II/III study. Parkinsonism Relat Disord 2020;75:17–23. 10.1016/j.parkreldis.2020.04.012 [DOI] [PubMed] [Google Scholar]
- 72. Schapira AHV, Fox SH, Hauser RA, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol 2017;74(2):216–224. [DOI] [PubMed] [Google Scholar]
- 73. Wei Q, Tan Y, Xu P, et al. The XINDI study: a randomized phase III clinical trial evaluating the efficacy and safety of safinamide as add‐on therapy to levodopa in Chinese patients with Parkinson's disease with motor fluctuations. CNS Drugs 2022;36:1217–1227. 10.1007/s40263-022-00958-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hauser RA, Hubble JP, Truong DD. Randomized trial of the adenosine A2A receptor antagonist istradefylline in advanced PD. Neurology 2003;61(3):297–303. 10.1212/01.WNL.0000081227.84197.0B [DOI] [PubMed] [Google Scholar]
- 75. Hauser RA, Shulman LM, Trugman JM, et al. Study of istradefylline in patients with Parkinson's disease on levodopa with motor fluctuations. Mov Disord 2008;23(15):2177–2185. 10.1002/mds.22095 [DOI] [PubMed] [Google Scholar]
- 76. Stacy M, Silver D, Mendis T, et al. A 12‐week, placebo‐controlled study (6002‐US‐006) of istradefylline in Parkinson disease. Neurology 2008;70(23):2233–2240. 10.1212/01.wnl.0000313834.22171.17 [DOI] [PubMed] [Google Scholar]
- 77. LeWitt PA, Guttman M, Tetrud JW, et al. Adenosine A2A receptor antagonist istradefylline (KW‐6002) reduces off time in Parkinson's disease: a double‐blind, randomized, multicenter clinical trial (6002‐US‐005). Ann Neurol 2008;63(3):295–302. 10.1002/ana.21315 [DOI] [PubMed] [Google Scholar]
- 78. Mizuno Y, Hasegawa K, Kondo T, et al. Clinical efficacy of istradefylline (KW‐6002) in Parkinson's disease: a randomized, controlled study. Mov Disord 2010;25(10):1437–1443. 10.1002/mds.23107 [DOI] [PubMed] [Google Scholar]
- 79. Pourcher E, Fernandez HH, Stacy M, Mori A, Ballerini R, Chaikin P. Istradefylline for Parkinson's disease patients experiencing motor fluctuations: results of the KW‐6002‐US‐018 study. Parkinsonism Relat Disord 2012;18(2):178–184. 10.1016/j.parkreldis.2011.09.023 [DOI] [PubMed] [Google Scholar]
- 80. Mizuno Y, Kondo T. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson's disease. Mov Disord 2013;28(8):1138–1141. 10.1002/mds.25418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li ZJ, Wu Q, Yi CJ. Clinical efficacy of istradefylline versus rTMS on Parkinsons disease in a randomized clinical trial. Curr Med Res Opin 2015;31(11):2055–2058. 10.1185/03007995.2015.1086994 [DOI] [PubMed] [Google Scholar]
- 82. Oertel W, Eggert K, Pahwa R, et al. Randomized, placebo‐controlled trial of ADS‐5102 (amantadine) extended‐release capsules for levodopa‐induced dyskinesia in Parkinson's disease (EASE LID 3). Mov Disord 2017;32(12):1701–1709. 10.1002/mds.27131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pahwa R, Tanner CM, Hauser RA, et al. ADS‐5102 (amantadine) extended‐release capsules for levodopa‐induced dyskinesia in Parkinson disease (EASE LID study) a randomized clinical trial. JAMA Neurol 2017;74(8):941–949. 10.1001/jamaneurol.2017.0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ory‐Magne F, Corvol JC, Azulay JP, et al. Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology 2014;82(4):300–307. [DOI] [PubMed] [Google Scholar]
- 85. Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry 2004;75(1):141–143. [PMC free article] [PubMed] [Google Scholar]
- 86. Pacchetti C, Martignoni E, Bruggi P, et al. Terguride in fluctuating parkinsonian patients: a double‐blind study versus placebo. Mov Disord 1993;8(4):463–465. [DOI] [PubMed] [Google Scholar]
- 87. Villafane G, Thiriez C, Audureau E, et al. High‐dose transdermal nicotine in Parkinson's disease patients: a randomized, open‐label, blinded‐endpoint evaluation phase 2 study. Eur J Neurol 2018;25(1):120–127. 10.1111/ene.13474 [DOI] [PubMed] [Google Scholar]
- 88. Murata M, Hasegawa K, Kanazawa I, et al. Zonisamide improves motor function in Parkinson disease: a randomized, double‐blind study. Neurology 2007;68(1):45–50. 10.1212/01.wnl.0000250236.75053.16 [DOI] [PubMed] [Google Scholar]
- 89. Murata M, Hasegawa K, Kanazawa I, et al. Zonisamide improves wearing‐off in Parkinson's disease: a randomized, double‐blind study. Mov Disord 2015;30(10):1343–1350. 10.1002/mds.26286 [DOI] [PubMed] [Google Scholar]
- 90. Lees A, Fahn S, Eggert KM, et al. Perampanel, an AMPA antagonist, found to have no benefit in reducing “off” time in Parkinson's disease. Mov Disord 2012;27(2):284–288. [DOI] [PubMed] [Google Scholar]
- 91. Eggert K, Squillacote D, Barone P, et al. Safety and efficacy of perampanel in advanced Parkinson's disease: a randomized, placebo‐controlled study. Mov Disord 2010;25(7):896–905. [DOI] [PubMed] [Google Scholar]
- 92. Pierantozzi M, Pietroiusti A, Brusa L, et al. Helicobacter pylori eradication and l‐dopa absorption in patients with PD and motor fluctuations. Neurology 2006;66(12):1824–1829. [DOI] [PubMed] [Google Scholar]
- 93. Yoritaka A, Kawajiri S, Yamamoto Y, et al. Randomized, double‐blind, placebo‐controlled pilot trial of reduced coenzyme Q10 for Parkinson's disease. Parkinsonism Relat Disord 2015;21(8):911–916. [DOI] [PubMed] [Google Scholar]
- 94. Aviles‐Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest 2013;123(6):2730–2736. 10.1172/JCI68295.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double‐blind, placebo‐controlled trial. Lancet 2017;390(10103):1664–1675. 10.1016/S0140-6736(17)31585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schuepbach WMM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med 2013;368(7):610–622. 10.1056/NEJMoa1205158 [DOI] [PubMed] [Google Scholar]
- 97. Li D, Zhang C, Gault J, et al. Remotely programmed deep brain stimulation of the bilateral subthalamic nucleus for the treatment of primary Parkinson disease: a randomized controlled trial investigating the safety and efficacy of a novel deep brain stimulation system. Stereotact Funct Neurosurg 2017;95(3):174–182. [DOI] [PubMed] [Google Scholar]
- 98. Schüpbach WMM, Maltête D, Houeto JL, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology 2007;68(4):267–271. 10.1212/01.wnl.0000250253.03919.fb [DOI] [PubMed] [Google Scholar]
- 99. Deuschl G, Schade‐Brittinger C, Krack P, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006;355(9):896–908. [DOI] [PubMed] [Google Scholar]
- 100. Anderson VC, Burchiel KJ, Hogarth P, Fabre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 2005;62:554–560. [DOI] [PubMed] [Google Scholar]
- 101. Fonoff ET, Azevedo A, Dos Angelos JS, et al. Simultaneous bilateral stereotactic procedure for deep brain stimulation implants: a significant step for reducing operation time. J Neurosurg 2016;125(1):85–89. [DOI] [PubMed] [Google Scholar]
- 102. Vitek JL, Jain R, Chen L, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current‐controlled device in Parkinson's disease (INTREPID): a multicentre, double‐blind, randomised, sham‐controlled study. Lancet Neurol 2020;19:491–501. 10.1016/S1474-4422(20)30108-3 [DOI] [PubMed] [Google Scholar]
- 103. Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant‐current device in Parkinson's disease: an open‐label randomised controlled trial. Lancet Neurol 2012;11(2):140–149. 10.1016/S1474-4422(11)70308-8 [DOI] [PubMed] [Google Scholar]
- 104. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep‐brain stimulation for Parkinson's disease. N Engl J Med 2010;362(22):2077–2091. [DOI] [PubMed] [Google Scholar]
- 106. Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open‐label trial. Lancet Neurol 2010;9(6):581–591. 10.1016/S1474-4422(10)70093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Odekerken VJJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 2013;12(1):37–44. 10.1016/S1474-4422(12)70264-8 [DOI] [PubMed] [Google Scholar]
- 108. Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty‐six‐month outcomes. Neurology 2012;79(1):55–65. 10.1212/WNL.0b013e31825dcdc1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Odekerken V, Boel J, Schmand B, et al. GPi vs STN deep brain stimulation for Parkinson disease: three‐year follow‐up. Neurology 2016;87(7):745–746. 10.1212/WNL.0000000000003027 [DOI] [PubMed] [Google Scholar]
- 110. de Bie RM, de Haan RJ, Nijssen PC, et al. Unilateral pallidotomy in Parkinson's disease: a randomised, single‐blind, multicentre trial. Lancet 1999;354(9191):1665–1669. [DOI] [PubMed] [Google Scholar]
- 111. Vitek JL, Bakay RA, Freeman A, et al. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol 2003;53(5):558–569. [DOI] [PubMed] [Google Scholar]
- 112. Esselink RA, de Bie RM, de Haan RJ, et al. Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in PD: a randomized trial. Neurology 2004;62(2):201–207. [DOI] [PubMed] [Google Scholar]
- 113. Esselink RA, de Bie RM, de Haan RJ, et al. Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in Parkinson's disease: one year follow‐up of a randomised observer‐blind multi centre trial. Acta Neurochir 2006;148(12):1247–1255. discussion 1255 [DOI] [PubMed] [Google Scholar]
- 114. Coban A, Hanagasi HA, Karamursel S, Barlas O. Comparison of unilateral pallidotomy and subthalamotomy findings in advanced idiopathic Parkinson's disease. Br J Neurosurg 2009;23(1):23–29. [DOI] [PubMed] [Google Scholar]
- 115. Merello M, Tenca E, Pérez Lloret S, et al. Prospective randomized 1‐year follow‐up comparison of bilateral subthalamotomy versus bilateral subthalamic stimulation and the combination of both in Parkinson's disease patients: a pilot study. Br J Neurosurg 2008;22(3):415–422. 10.1080/02688690801971667 [DOI] [PubMed] [Google Scholar]
- 116. Blomstedt P, Stenmark Persson R, Hariz G‐M, et al. Deep brain stimulation in the caudal zona incerta versus best medical treatment in patients with Parkinson's disease: a randomised blinded evaluation. J Neurol Neurosurg Psychiatry 2018;89(7):710–716. 10.1136/jnnp-2017-317219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Whone A, Luz M, Boca M, et al. Randomized trial of intermittent intraputamenal glial cell line‐derived neurotrophic factor in Parkinson's disease. Brain 2019;142(3):512–525. 10.1093/brain/awz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Whone AL, Boca M, Luz M, et al. Extended treatment with glial cell line‐derived neurotrophic factor in Parkinson's disease. J Parkinsons Dis 2019;9(2):301–313. 10.3233/JPD-191576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351(24):2498–2508. 10.1056/NEJMoa033447 [DOI] [PubMed] [Google Scholar]
- 120. Gray R, Patel S, Ives N, et al. Long‐term effectiveness of adjuvant treatment with catechol‐O‐methyltransferase or monoamine oxidase B inhibitors compared with dopamine agonists among patients with Parkinson disease uncontrolled by levodopa therapy: the PD MED randomized clinical trial. JAMA Neurol 2022;79(2):131–140. 10.1001/jamaneurol.2021.4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


