Abstract
Gray blight is a serious foliar disease that significantly threatens tea plant cultivation. Although dynamic histone methylation was reported in regulating plant immunity, the specific roles of this epigenetic modification in tea plant disease resistance have yet to be fully elucidated. This study demonstrates that the protein arginine methyltransferase CsPRMT5, which catalyzes the symmetric dimethylation of histone H4R3 (H4R3sme2), is involved in the tea plant response to gray blight. Transcription of CsPRMT5 and the level of histone H4R3 methylation in tea were downregulated following infection by the fungal pathogen Pseudopestalotiopsis (Ps). A negative correlation was observed between the resistance of tea plants to Ps and the expression level of CsPRMT5 across various cultivars. Downregulation of CsPRMT5 expression led to reduced H4R3sme2 levels, elevated expression of defense-related genes, and lower reactive oxygen species (ROS) production after Ps infection, thus enhancing pathogen resistance of tea. Furthermore, complementation of Atprmt5 mutant with CsPRMT5 restored the susceptibility to Ps infection in Arabidopsis. Chromatin Immunoprecipitation Sequencing (ChIP-seq)and Chromatin Immunoprecipitation quantitative PCR (ChIP-qPCR) analyses revealed that CsPRMT5 binds to defense-related genes, including CsMAPK3, and regulates their expression through H4R3sme2 modification. Collectively, the results indicate that CsPRMT5 negatively regulates the immune response to pathogens through repressing CsMAPK3 expression in tea plants.
Introduction
Tea, a nonalcoholic beverage appreciated globally, provides substantial economic, health, and cultural advantages, establishing it as a fundamental aspect of daily life for millions [1, 2]. However, tea plants encounter numerous biotic and abiotic stresses [3]. Gray blight is one of the most destructive fungal leaf diseases, inflicting severe damage on tea leaves and leading to considerable economic losses [4–6]. To date, little is known about the molecular mechanisms by which tea plants adapt to the infection of Ps, which limits the further design of molecular breeding.
Recent research has highlighted the molecular mechanisms underlying tea plant defense against gray blight disease, emphasizing the critical roles of oxidative bursts and hormones in modulating plant immune responses [6–8]. After Ps infection, the expression of essential genes related to phenylpropanoid and flavonoid production increase, including phenylalanine ammonia-lyase (PAL), while genes associated with photosynthesis decrease concurrently in tea plants [6, 9]. Additionally, the transcription of the defensive response genes is tightly regulated by numerous transcription factors, including WRKY and NAC [9–11]. This intricate reprogramming of gene expression necessitates a swift and highly coordinated response at both the transcriptional and post-transcriptional levels.
Increasing evidence suggests that epigenetic processes, including DNA methylation and histone modification, play an essential role in the regulation of transcription, significantly influencing plant immune responses. Histone methylation, a prevalent and critical epigenetic modification, occurs on both lysine and arginine residues. Emerging findings indicate that histone lysine methylation marks play essential roles in plant responses to stresses [12–14]. For instance, JMJ27 activates defense genes like PR1 and PR3, while also repressing negative regulators of defense genes by modulating H3K9 methylation [13]. Similarly, the H3K4 demethylase JMJ14 regulates immunological responses by regulating the deposition of H3K4me3 marks, which in turn affects the transcription of key defense genes, including PR1, FMO1, and SNI1 [14]. Additionally, OsJMJ705 removes H3K27me3 from defense-related genes, thereby enhancing resistance [15].
In eukaryotes, histone arginine methylation is a widespread and essential post-translational modification crucial for various biological activities [16, 17]. It is categorized into three types based on the modification form: monomethyl arginine (MMA), asymmetric dimethyl arginine (ADMA), and symmetric dimethyl arginine (SDMA) [16]. The enzymes that catalyze this modification, known as protein arginine methyltransferases (PRMTs), are classified into four groups based on substrate specificity [18]. Type I PRMTs (PRMT1, PRMT3, PRMT4/CARM, PRMT6, and PRMT8) catalyze the formation of ADMA at H3R2/H4R3 sites, activating transcription and ribosomal synthesis, with PRMT1 playing a crucial role in early mouse development. Type II (PRMT5, PRMT9), which mediates the deposition of SDMA at H3R8/H4R3 sites, represses gene expression. Type III (mainly PRMT7), which only generates MMA, and Type IV, which specifically methylates secondary amine groups on arginine residues (a process found only in yeast) [19–21]. The function of PRMTs is highly conserved across evolution, and abnormal expression is closely associated with developmental defects and cancer in mammals.
Recently, genetic studies in model plant Arabidopsis thaliana have shown that mutations in AtPRMT3, AtPRMT4a/4b, AtPRMT5, AtPRMT6, and AtPRMT10 lead to significant phenotypic abnormalities (such as delayed flowering and root development defects), confirming the universal regulatory role of arginine methylation in growth and development [22–25]. Among these, PRMT5 (AtSKB1) serves as a core regulatory factor by catalyzing the symmetric dimethylation of histone H4R3 (H4R3sme2), which inhibits transcription of target genes and is involved in plant growth and development and abiotic [25–31]. Additionally, PRMT5 can dynamically modify non-histone substrates (such as LSM4 and AGO2), thereby antagonistically regulating plant responses to both biotic and abiotic stresses [32–34].
Furthermore, recent studies have demonstrated that PRMT5 is involved in plant immunity across multiple species [25]. In Arabidopsis, AtPRMT5 functions as a negative regulator of the immune response to oomycetes and AvrRpt pathogens [35, 36]. Specifically, bacterial infection leads to a decrease in PRMT5 expression, accompanied by reduced arginine methylation of key proteins such as AGO2 and LSM4 [33–35]. Through its dual regulation of AtAGO2, PRMT5 modulates the plant’s immune response to pathogens. In contrast, the role of OsPRMT5 in rice (Oryza sativa) resistance to blast disease is distinct. Research indicates that OsPRMT5 acts as a positive regulator of immunity in rice. Upon infection with the rice blast fungus, OsPRMT5 regulates AGO2 activity by arginine methylation and interacts with miR1875 to influence the rice immune response [33]. The results suggest that PRMT5 may have varied modes of action among different species, and its role in the disease resistance mechanisms of tea plants requires further investigation.
This research investigates the function of CsPRMT5 in response to Ps infection in tea plants. Our results demonstrate that CsPRMT5-silenced plants upregulated several immune stress response genes, including CsWRKY40, CsNAC, and CsMAPK3, to enhance plant resistance to the gray blight pathogen. Conversely, the overexpression of CsPRMT5 increased the vulnerability of tea plants to Ps. Following Ps infection, H4R3sme2 levels decrease as CsPRMT5 dissociates from the chromatin, thereby facilitating the activation of CsMAPK3 expression. The findings demonstrate that CsPRMT5 functions as a negative regulator of the defense response in tea plants against gray blight, offering new insights into the role of histone arginine methylation in plant immunity.
Results
The histone arginine methylation dynamics in response to gray blight
Although many studies have demonstrated that histone lysine methylation contributes to plant disease resistance, the dynamic changes in arginine methylation levels after pathogen infection remain poorly understood [12]. Consequently, we evaluated the histone dimethylation levels at many arginine sites following Ps infection at 0, 24, 48, and 72 h postinoculation (hpi). Western blot analysis was performed using specific antibodies against H4R3sme2, H4R3ame2, H3R2ame2, and H3R17ame2. As shown in Fig. 1a, the methylation levels at distinct arginine sites fluctuated after varying durations of Ps inoculation. We observed a significant decrease in H3R2ame2 levels at 48 h, followed by a gradual recovery at 72 h. Conversely, the level of H3R17ame2 significantly increased at both 48 and 72 h. The methylation level at the H4R3 site remained unchanged, while the level of H4R3sme2 significantly decreased over time. These findings indicate that plant disease responses are complex processes, involving the methylation of several arginine residues on histones. Various histone methylation alterations may serve unique functions in modulating this response.
Figure 1.
CsPRMT5 is repressed by the pathogen Pseudopestalotiopsis (Ps) infection. (a) Histone methylation levels in tea plant leaves following different periods of Ps infection. Histone proteins were extracted at 0, 24, 48, and 72 hpi. Numbers below the blots indicate the quantified relative band intensities of the corresponding methylation site. (b) The expression pattern of PRMT family genes. FPKM values are represented by a heatmap. (c) Relative expression of CsPRMT5 in leaves after Ps infection. Data were denoted as means ± SD (n = 3). Statistical significance (P < 0.05) was marked with distinct letters, based on Duncan’s multiple range test. (d) Immunoblot analysis of CsPRMT5 protein levels after Ps infection. Numbers below the blots indicate the quantified relative band intensities, and the value at time 0 was set to 1.
To identify PRMT genes associated with plant defense response through histone arginine methylation regulation, we performed a genome-wide analysis of the tea PRMT family based on their similarity to AtSKB1 (Table S1). We identified a total of nine PRMT genes. Among these, eight genes were classified into Type I (PRMT1A-C, PRMT4A-B, PRMT10A-C), which catalyze the asymmetric dimethylation of histone H3 at R2 and R17, and histone H4 at R3. One gene was classified as Type II (PRMT5), which catalyzes the symmetric dimethylation of histone H4 at R3 (Fig. 1b). Interestingly, the expression pattern of PRMT5 in the transcriptomic data shows a consistent decline trend over time with the level of H4R3sme2 after pathogen infection (Fig. 1b). Subsequently, we examined the transcript and protein levels of CsPRMT5 in tea plants after Ps infection. A significant reduction in CsPRMT5 transcript and protein levels was also observed at 24 and 48 hpi (Fig. 1c and d). These findings indicate that the expression of CsPRMT5 was inhibited after Ps infection. Previous research has demonstrated that AtPRMT5 contributes to plant immunity by catalyzing the arginine methylation of AtAGO2 and LSM4 [33]. Based on these findings, we chose CsPRMT5 in response to gray blight infection of tea plant for further study.
The correlation between disease resistance and CsPRMT5 expression in different tea plant cultivars
To investigate the correlation between CsPRMT5 expression levels and disease resistance in different tea cultivars, we randomly selected the following seven cultivars for disease resistance experiments (Fig. 2a). The second leaf was selected for pathogen inoculation, and the expression of CsPRMT5 in unaffected leaves of each cultivar was quantified. Lesion areas were measured 5 days postinoculation (dpi) (Fig. 2b and d). The results showed that the varieties FY6 and PYTZ had relatively low CsPRMT5 expression levels and exhibited high disease resistance (Fig. 2b and c). Conversely, the varieties SCZ and WNZ had higher CsPRMT5 transcript levels and showed lower disease resistance (Fig. 2b and c). The lesion area and CsPRMT5 expression levels were found to be positively correlated across different tea cultivars significantly (r = 0.828, **P < 0.01) (Fig. 2e).
Figure 2.
Disease resistance of tea plants was negatively correlated with the expression of CsPRMT5. (a) Phenotypes of different tea plant varieties. Scale bars = 1 cm. Inoculate the second leaf with the pathogen (Ps). (b) Transcript and protein levels of CsPRMT5 in different tea plant varieties. Data were denoted as means ± SD (n = 3); statistical significance (P < 0.05) was marked with distinct letters, based on Duncan’s multiple range test. (c) Phenotypes of different tea plant varieties at 5 dpi. (d) Total area of lesions in different tea plant varieties after infection. (e) Correlation between disease resistance and expression level of CsPRMT5 in different varieties using Pearson’s correlation, **P < 0.01.
CsPRMT5 is a negative regulator of gray blight resistance
To further investigate the role of CsPRMT5 in Ps resistance in tea plants, we transiently suppressed its expression in tea leaves using a gene-specific antisense oligonucleotide (AsODN) approach, as previously described. The new shoots of tea plants were treated by asODN and sense oligonucleotide (sODN) (Fig. 3a). RT-qPCR and immunoblot assays revealed a significant decrease in both CsPRMT5 transcript and protein levels in AsODN-treated tea plants compared to the control group treated with sODN. This reduction was accompanied by a notable decrease in H4R3sme2 levels (Fig. 3b and c). Additionally, we observed that the lesion area in the CsPRMT5-silenced plants was smaller than the control after 5 dpi (Fig. 3d). Pathogenesis-related (PR) genes, which play a key role in the immune response of tea plants, were upregulated early during Ps infection in both AsODN- and sODN-treated plants. However, PR gene expression was significantly higher in CsPRMT5-silenced plants than in the control (Fig. 3e).
Figure 3.
The pathogen resistance phenotypes of CsPRMT5-silenced tea plants. (a) The schematic diagram of AsODN gene silencing. (b) The relative expression levels of CsPRMT5. (c) Immunoblot analysis of levels of CsPRMT5 and H4R3sme2 in AsODN and sODN plants. (d) Disease symptoms after Ps infection in CsPRMT5-silenced tea plants and control. Scale bars = 0.1 mm. (e) The relative expression levels of PR genes. (f) Diaminobenzidine (DAB) staining, (g) H2O2 content, (h) CAT activity, and (i) POD activity of tea plant leaves 48 h after Ps inoculation.
Subsequently, we overexpressed CsPRMT5 driven by the 35S promoter in tea leaves via a transient expression method. RT-qPCR analysis demonstrated a significant upregulation of CsPRMT5 expression. Compared to the control plants that expressed the empty vector (EV), CsPRMT5-overexpressing tea plants had increased lesion areas and reduced resistance to Ps, corroborating the previously described findings (Fig. S1). Reactive oxygen species (ROS) accumulation and scavenging are common defense responses of plants against pathogen infection. We measured the activity of key ROS scavenging enzymes (Fig. 3f and i). Consistent with the disease resistance phenotypes, compared to sODN plants, H2O2 levels were lower in CsPRMT5-silenced plants, and the activities of POD and SOD enzymes were higher. In contrast, ROS accumulation was significantly higher in CsPRMT5-overexpressing leaves compared to EV (Fig. S1). These findings indicate that CsPRMT5 acts as a negative regulator of gray blight resistance in tea plants.
The overexpression of CsPRMT5 reduced disease resistance in transgenic Arabidopsis
To investigate the function of CsPRMT5 on disease resistance in other plants, we overexpressed CsPRMT5 in the Arabidopsis skb1 mutant (35S::CsPRMT5/skb1-1, hereafter as Com2) and challenged wild-type (WT), skb1-1 mutant, and Com2 plants with Ps strain. The skb1-1 mutant plants showed significantly enhanced resistance to Ps infection, while the Com2 plants were more susceptible (Fig. 4b). Disease severity was categorized into four levels based on the percentage of the green area lost in half leaves. Statistical analysis indicated that Com2 and WT plants exhibited a greater proportion of Level 4 severity and a diminished proportion of Level 1 severity, whereas the skb1-1 mutant had the inverse trend, with an increased proportion of Level 1 severity and a reduced proportion of Level 4 severity (Fig. 4c). Consistent with the phenotypic results, AtPR1 was significantly induced in Com2 plants (Fig. 4d). Moreover, we observed stronger H2O2 accumulation and lower SOD and POD activity in Com2 leaves, whereas H2O2 levels were almost unchanged in skb1 (Fig. 4e and g). Our results show that CsPRMT5 negatively regulates plant resistance to gray blight.
Figure 4.
Heterologous expression of CsPRMT5 reduced disease resistance in transgenic Arabidopsis. (a) CsPRMT5 and H4R3sme2 levels of WT, skb1-1, Com2 Arabidopsis plants. (b) Phenotypes of 5-week-old Arabidopsis WT, skb1-1, and Com2 plants with disease symptoms after Ps infection. The CK treatment (mock) was inoculated with water. Photographs were taken at 5 dpi. (c) Representative leaves with fungal infection symptoms of WT, skb1-1, and Com2 plants and the statistics of different grades of disease symptoms after Ps infection. (d) Relative expression levels of AtPR1, (e) H2O2 content, (f) POD activity, and (g) SOD activity. Scale bars = 1 cm.
Pathogen-responsive genes are upregulated in CsPRMT5-silenced plants
To clarify the molecular processes by which CsPRMT5 regulates tea plant immunity, we performed transcriptome sequencing to identify pathogen-associated genes governed by CsPRMT5. In CsPRMT5-silenced plants, a total of 748 upregulated genes and 217 downregulated genes were identified, with a log₂ (fold-change) ≥1.0 serving as the cut-off under normal growth conditions (Fig. 5a). Since CsPRMT5-mediated H4R3sme2 is generally associated with gene repression, the transcription of its targeted genes is anticipated to be stimulated in CsPRMT5-silenced plants. Therefore, we concentrated on the upregulated genes (Table S2). Among these genes, 48 were categorized into ‘response to biotic stimulus’ in Gene Ontology (GO) analysis (Fig. 5b; Table S4). This group includes 14 genes that were previously reported to be associated with host defense and disease resistance, such as WRKY, receptor-like kinases, and ankyrin repeat proteins; the transcriptomic data used for the heatmap are sourced from Tan et al. (Fig. 5c; Table S3) [6].
Figure 5.
CsPRMT5 regulates the expression of immune-related genes. (a) Numbers of DEGs in CsPRMT5-silenced plants compared to sODN plants. Tea plant samples with efficient CsPRMT5 silencing were subjected to transcriptome analysis through RNA sequencing. (b) GO enrichment analysis of upregulated DEGs in CsPRMT5-silenced plants relative to sODN plants. (c) A heatmap showing normalized expression levels of pathogen-related genes in tea leaves following different periods of Ps infection. TPM (log2) of genes as z-scores. (d) The relative expression of potential CsPRMT5 target genes.
The expression of pathogen-responsive genes, including CsMAPK3, CsPP2C, and CsDSP1, was significantly induced following pathogen infection [37, 38], and their transcript levels were higher in CsPRMT5-silenced plants compared with sODN-treated plants, which is consistent with the RNA-seq analysis (Fig. 5d). These results suggest that CsPRMT5 negatively modulates the transcription of pathogen-induced genes.
CsPRMT5 modulates resistance to gray blight by manipulating CsMAPK3 expression
In Arabidopsis, SKB1 associates with the FLC promoter and catalyzes H4R3sme2, leading to transcriptional suppression [27, 32]. To investigate whether the elevated expression of defense genes was due to decreased H4R3sme2 levels, we first conducted a whole-genome ChIP-seq analysis using anti-H4R3sme2 antibodies (Table S4). We found that there were methylation modifications in the chromosome where pathogen-responsive genes, including CsMAPK3, CsPP2C, and CsMYB73, were located (Fig. 6a). We verified the binding of CsPRMT5 and H4R3sme2 in the promoters of CsMAPK3 using ChIP-qPCR/ChIP-PCR (Chromatin Immunoprecipitation PCR). Consistently, CsPRMT5 binding was significantly enriched near or at the promoter regions (Fig. 6b and Fig. S2). Upon pathogen infection, the degree of chromatin binding of CsPRMT5 and the methylation level of MAPK3 both decreased, while the transcription level of CsMAPK3 increased (Fig. 6b and c). In addition, tea leaves with overexpressed CsMAPK3 showed smaller lesion areas than that of the EV leaves, while CsMAPK3-silencing led to larger lesion areas (Fig. 6d and e).
Figure 6.
The binding of CsPRMT5 to the chromatin of resistance genes and the levels of H4R3sme2 decreased by Ps infection in tea plants. (a) ChIP-seq analysis of CsPRMT5 association with chromatin containing defense-related genes (The experiment used anti-H4R3sme2 antibodies). (b) CsMAPK3 gene structure. Chromatin extracted from tea leaves was treated with Ps for 24 h. (c) The expression of CsMAPK3 in leaves infected relative to the double-distilled water control (CK). Template RNA isolated from tea leaves was treated with Ps for 24, 48, or 72 h. (d) Disease symptoms following Ps infection in CsMAPK3-overexpressing and control. Scale bars = 2 mm. (e) Disease symptoms following Ps infection in CsMAPK3-silenced tea plants (CsMAPK3-TRV) and control (TRV) plants.
Building on these findings, we propose a hypothetical model outlining the role of CsPRMT5 in the tea plant defense response (Fig. 7). In the pathogen infection, CsPRMT5 binds to chromatin to inhibit the expression of disease-resistance genes through arginine methylation to maintain normal plant growth. In contrast, when tea plants are infected by fungi, the expression of CsPRMT5 decreases. Consequently, less CsPRMT5 associates with the chromatin and H4R3sme2 level declines, thereby releasing the transcriptional suppression of pathogen-responsive genes and increasing the activity of ROS scavenging enzymes, which plays a positive role in the disease resistance of tea plants.
Figure 7.
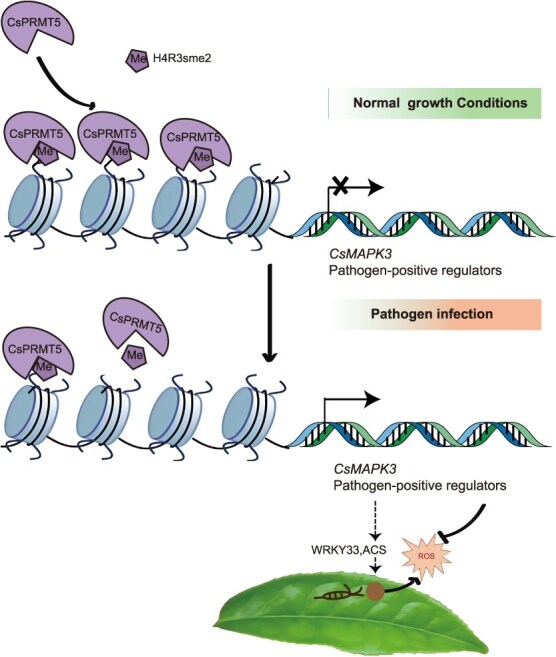
A model of CsPRMT5 regulation of CsMAPK3 expression represses resistance to gray blight in tea plant. CsPRMT5 binds to defense-related genes, including CsMAPK3, and regulates their expression through H4R3sme2 modification. Under normal growth conditions, CsPRMT5 associates with CsMAPK3 to mediate its H4R3sme2, inhibiting the expression of CsMAPK3. After infection by the gray blight pathogen (Ps), the expression of CsPRMT5 is suppressed, leading to less CsPRMT5 association with chromatin and a decline in H4R3sme2 levels, thereby releasing the transcriptional suppression of pathogen-induced CsMAPK3 and enhancing plant resistance.
Discussion
In this study, we propose a mechanism by which CsPRMT5-mediated histone H4R3 dimethylation negatively regulates resistance to gray blight in tea plants (Fig. 7). As an important mechanism of epigenetic regulation, histone modifications (such as acetylation and lysine methylation) have been widely reported implicating in a variety of biological processes [39], while the specific role of histone arginine methylation in plant immunity remains unclear. In this study, we showed that CsPRMT5 regulates plant disease defense epigenetically through the H4R3sme2 in tea plants. The levels of H4R3sme2 are closely associated with the external environment. In CsPRMT5-silenced tea plants and skb1-1 mutant, genes involved in plant immune responses were upregulated and the absence of H4R3sme2 enhanced plant resistance to Ps infection (Fig. 5). Our findings strongly suggest that CsPRMT5 contributes to the defense against gray blight through the modulation of resistance-related genes in plants.
Histone methylation plays a fundamental role in regulating various developmental processes and is also involved in silencing repetitive sequences to maintain genome stability [17]. Pathogen-induced histone methylation modifications can activate host cell signaling cascades, activating or inhibiting the expression of defense genes, thereby enhancing or suppressing plant resistance responses. The most extensively studied modifications are the methylation/demethylation of histone lysine residues, with modifications at the H3K4, H3K27, and H3K36 sites upregulating SA/JA signaling pathway genes or R genes, positively regulating plant resistance to Pst DC3000 and Xoo [40]. In contrast, the regulatory mechanisms of histone arginine methylation in plant immunity remain to be further explored. This study finds that histone arginine methylation exhibits dynamic changes during pathogen infection. The repressive mark H4R3sme2 mediated by PRMT5 significantly decreases upon pathogen infection, which is consistent with previous reports in potatoes [41].
It is noteworthy that PRMT5, as a multifunctional regulatory factor, regulates flowering time by inhibiting FLOWERING LOCUS C (FLC) gene expression through H4R3sme2 and is also involved in salt stress response [32]. During salt stress or ABA treatment, PRMT5/SKB1 dissociates from chromatin, leading to a decrease in H4R3sme2 levels, which activates FLC and stress-responsive genes and enhances pre-mRNA splicing efficiency. Vernalization or photoperiod treatment dynamically alters the H4R3sme2 levels on the FLC chromatin to regulate the flowering process. Additionally, PRMT5/SKB1-mediated H4R3sme2 deposition in the promoter region of the Ib subgroup bHLH transcription factors alleviates gene repression under iron deficiency conditions by reducing the modification levels, enhancing Arabidopsis iron uptake ability [42]. Together, these findings suggest that PRMT5 participates in plant development and environmental adaptation by dynamically regulating H4R3sme2.
This study further reveals the novel function of CsPRMT5 in plant immunity. Transcriptome analysis showed that differentially expressed genes in CsPRMT5-silenced tea plants are significantly enriched in the plant–pathogen interaction pathway, which is highly consistent with the transcriptome of the Arabidopsis skb1-1 mutant (Fig. S3; Table S6). Key immune regulators MAPK3, PP2C, DSP1, WRKY40, and POD are significantly upregulated in the silenced lines, and both CsPRMT5-silenced tea plants and Arabidopsis prmt5 mutants exhibit enhanced disease resistance. More importantly, this study provides the first evidence that CsPRMT5 regulates plant resistance through the modulation of ROS balance. In the CsPRMT5-silenced plants, the expression of POD genes is significantly upregulated, resulting in a decrease in H2O2 and O2− levels, which effectively mitigates oxidative damage caused by pathogen infection. In contrast, the CsPRMT5-overexpressing plants accumulate excessive ROS, leading to increased tissue damage and reduced resistance. These findings not only confirm that CsPRMT5 is involved in regulating gray blight disease resistance in tea plants but also reveal its role in precisely regulating defense responses and oxidative damage by coordinating ROS dynamic balance.
PRMT5 participates in gene expression regulation, RNA processing, pre-mRNA splicing, and protein homeostasis maintenance through the extensive methylation of histone and nonhistone substrates. Previous studies have revealed that PRMT5 regulates plant immunity by methylating nonhistone proteins such as AGO2 and LSM4. In Arabidopsis, PRMT5 regulates the selective splicing function of the splicing body component LSM4 through arginine methylation. Bacterial infection reduces LSM4 methylation levels, enhancing plant resistance, a mechanism related to reduced intron retention of immune-related genes. Moreover, PRMT5-mediated AGO2 methylation has been shown to finely regulate pathogen defense responses in both rice and Arabidopsis [33, 35]. This study elucidates that PRMT5 participates in tea plant immune regulation through H4R3sme2 modification. Notably, under pathogen infection conditions, the reduced binding ability of CsPRMT5 to chromatin leads to a decrease in H4R3sme2 levels, which subsequently activates the transcription of immune-regulatory genes such as MAPK3. However, the molecular sensing mechanism by which CsPRMT5 perceives pathogen signals and dynamically regulates H4R3 methylation remains to be further elucidated.
Previous studies have confirmed that PRMT5 (SKB1) is crucial for plant growth and development. Compared to WT plants, the Arabidopsis skb1 mutant exhibits traits such as late flowering, darker leaves, and slower growth rates. In tea plants, PRMT5 shows different expression patterns in various tissues, with significantly higher expression in young leaves (Fig. S4). However, the specific functions of PRMT5 in different developmental stages of tea plants (such as germination and flowering) remain largely unexplored and warrant further investigation. Studies on the Arabidopsis skb1-1 mutant show that the loss of PRMT5 leads to growth inhibition but enhances disease resistance, revealing its pivotal role in coordinating resource allocation. PRMT5 binds to chromatin and deposits the repressive marker H4R3sme2, directly suppressing the expression of the flowering regulator gene FLC. Pathogen infection triggers a decrease in PRMT5 protein levels, which in turn reduces the methylation modification of the spliceosome component LSM4, enhancing resistance by optimizing the alternative splicing of immune-related genes. This bidirectional dynamic modification suggests that PRMT5 may function as a ‘molecular switch’ to balance growth and immune metabolic conflicts through epigenetic reprogramming.
This study reveals a new mechanism by which CsPRMT5 negatively regulates H4R3sme2 levels to suppress disease resistance in tea plants, providing an epigenetic target for crop disease resistance breeding. Future research could focus on: elucidating how CsPRMT5 collaborates with DNA methylation/histone acetylation to coregulate the defense gene network; clarifying the functional division and cross-regulation between CsPRMT5 and other PRMT members in tea plants (such as CsPRMT3, CsPRMT10); exploring the conservation and specificity of PRMT5-mediated disease resistance pathways in economic crops like Solanum lycopersicum and Zea mays, and assessing their potential for broad-spectrum disease resistance.
Materials and methods
Plant materials and growth conditions
This study used the ‘Shuchazao’ cultivar as the primary experimental material, which was cultivated in the Experimental Tea Garden of Anhui Agricultural University (latitude 31.86°N, longitude 117.27°E). To test disease resistance of different cultivars, seven tea plant cultivars, including ‘Longjing 43’ (LJ43), ‘Shuchazao’ (SCZ), ‘Wuniuzao’ (WNZ), ‘Fuyun 6’ (FY6), ‘Longjingchangye’ (LJCY), ‘Zhenong 113’ (ZN113), and ‘Pingyangtezao’ (PYTZ). Arabidopsis thaliana (Columbia-0, Col-0), mutant skb1-1 (salk_065814), and the complementary line (35S::CsPRMT5 skb1-1) previously obtained by the lab were used to study the resistance against Ps.
Pathogen inoculation
The Ps strain used is named EC-4, and its pathogenicity was reported by Wang et al [5]. The strain of Ps a was cultured on potato dextrose agar (PDA) medium at 28°C for 5 days, and then the spores were collected by centrifugation at 6000 × g for 10 min. The spore concentration was adjusted to 106/ml with sterile water for subsequent inoculation tests. A sterile needle was used to create wounds on each leaf, and 50 μl of the spore suspension was inoculated onto the tea leaves. The control group plants were inoculated with the same amount of sterile distilled water. After inoculation, the leaves were covered with a thin film to maintain high humidity and promote fungal growth. The film was removed 24 h later, and regular cultivation continued. Arabidopsis was grown in nutrient soil at 22°C, with 75% humidity and a 16 h/8 h (light/darkness) photoperiod. A 15 μl spore suspension of the same concentration was applied to 5-week-old leaves of transgenic Arabidopsis, and with 80% humidity. The disease progression was observed in the later stages, and the lesion area was quantified using ImageJ software.
Transient overexpression and gene silencing in tea leaves
Transient gene expression and silencing were conducted following a previously established protocol [8]. The complete cDNAs of the target genes were incorporated into the expression vector pCAMBIA1305, which contains the GFP tag, to produce the donor plasmids [43]. The primers are included in Table S5. The donor plasmids were integrated into the Agrobacterium tumefaciens GV3101 (pSoup-p19). Leaf samples were collected 48 hpi for further gene expression profiling and evaluation of disease resistance.
AsODNs directed against CsPRMT5 were formulated utilizing Soligo software [43], with sODNs employed as controls (Table S1). Tender shoots were selected for the AsODN experiment and submerged in 100 μM AsODN/sODN; after 48 h, the leaves were collected [8]; the VIGS method was utilized to mute CsMAPK3. The 54- to 327-bp fragments from the 5′ regions of CsMAPK3 were employed as silencing pieces.
Immunoblot analysis
Western blot examination of total proteins was conducted with anti-CsPRMT5 polyclonal antibodies (Wuhan Aibo Tech; projected molecular weight, 72 kDa). Histone-enriched proteins were obtained following the methodology outlined by Houben et al. [44], and immunoreacted samples, as detailed by Connolly et al., were utilized to assess CsPRMT5 levels [45]. Immunoblot analysis was conducted using antibodies against Anti-Histone H4/Anti-H4R3sme2 (Shanghai Aibo Antibody; anticipated molecular mass, 10/15 kDa). ACTIN served as the loading control (Shanghai Bioengineering; anticipated molecular weight, 42 kDa). The original images of the immunoblot analysis have been added to Table S7.
Histochemical staining of plants and assessment of reactive oxygen species
The leaves infected with the pathogen were positioned in 3-methylene-diaminobenzidine (DAB) solution (1 mg/ml, pH 3.8) and incubated at 37°C for 8 h. The enzyme activity of SOD and POD in the leaves were measured using kits (Beijing Solabao Technology Co., Beijing, China) [46].
ChIP-qPCR assay
We conducted ChIP analysis as previously described with minor modifications [47]. Samples infected by Ps for 24 h were used for the ChIP-qPCR assay. The samples were placed in a 50-ml centrifuge tube, and 1% formaldehyde was added for cross-linking under vacuum conditions for 30 min. Then, 0.125 mol/l glycine was added to terminate the cross-linking. The plant material was pulverized in liquid nitrogen, and the nuclei were extracted. The chromatin was then fragmented using sonication. DNA fragments bound to proteins were immunoprecipitated with anti-H4R3sme2 (Shanghai Aibo Antibody, 1:5000 dilution). The immunoprecipitated DNA fragments were extracted and purified for ChIP-qPCR assays. The percentage input was calculated by 1% × 2 (Ct)1%Input Sample-(Ct)IP Sample. The dilution factor (IDF) was 100, and the Input DNA was 1%.
RNA-seq analysis
Total RNA was extracted using the RNA Pure Plant Kit (Tiangen). Subsequently, the quality and concentration of the extract were assessed using agarose gel electrophoresis and the NanoDrop 2000 spectrophotometer (Thermo). The library was then constructed and sequenced using the Illumina sequencing platform. After filtering out low-quality reads, all remaining high-quality clean sequencing reads were aligned to the tea plant reference genome (http://tpdb.shengxin.ren/). AsODN- and sODN-treated samples from one bud and one leaf were extracted and sent to Novogene Technology Co., Ltd. for sequencing. Each sample included three biological replicates. Differentially expressed genes (DEGs) in response to AsODN treatment were defined using a threshold of fold change ≥1.00 and P ≤ 0.05. The data of gray blight are sourced from the public database of Anhui Agricultural University (http://tpia.teaplant.org/index.html).
Statistical analysis
All studies were performed with at least three distinct biological replicates. All measurements were performed in duplicate. A one-way analysis of variance (ANOVA) was utilized to evaluate statistically significant changes between the control and experimental plants, employing Duncan’s test (P < 0.05) executed through SPSS.
Supplementary Material
Acknowledgements
We thank Chaoling Wei (State Key Laboratory of Tea Plant Biology and Utilization) for kindly providing Ps strain. This research was supported by the National Natural Science Foundation of China (32072624, 32402622, 32402623), the National Key R&D Program of China (2021YFD1601101), the Postdoctoral Fellowship Program of CPSF under Grant Number GZC20240006 and China Postdoctoral Science Foundation (2024 M750020), the Open Fund of State Key Laboratory of Tea Plant Biology and Utilization, SKLTOF20220115.
Contributor Information
Huanyun Peng, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Yan Wang, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Biying Zhu, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Yuanrong Wang, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Mengxue Han, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Shupei Zhang, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Tianyuan Yang, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Fei Wang, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Zhaoliang Zhang, National Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, Anhui Agricultural University, West 130 Changjiang Road, Hefei, Anhui 230036, China.
Author contributions
H.P. and Y.W. made equal contributions to this work. Z.Z. and F.W. formulated the study and devised the experiments. The experiments were conducted by H.P., Y.W., B.Z., and M.H. H.P. and Z.Z. authored the manuscript. All writers evaluated and sanctioned the final manuscript.
Data availability
The data presented in this study are available on request from the corresponding author. The raw transcriptome data have been deposited in the National Center for Biotechnology Information bioproject database under the accession number PRJNA1223124 for the RNA-seq (CsPRMT5 suppression in tea plants) and PRJNA1223117 for the ChIP-seq data, respectively.
Conflict of interest statement
None declared.
Supplementary data
Supplementary data is available at Horticulture Research online.
References
- 1. Farag MA, Elmetwally F, Elghanam R. et al. Metabolomics in tea products; a compile of applications for enhancing agricultural traits and quality control analysis of Camellia sinensis. Food Chem. 2023;404:134628 [DOI] [PubMed] [Google Scholar]
- 2. Xia EH, Li FD, Tong W. et al. Tea plant information archive: a comprehensive genomics and bioinformatics platform for tea plant. Plant Biotechnol J. 2019;17:1938–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi NN, Du YX, Ruan HC. et al. First report of Colletotrichum fructicola causing anthracnose on Camellia sinensis in Guangdong Province, China. Plant Dis. 2018;102:241 [Google Scholar]
- 4. Zheng S, Chen R, Wang Z. et al. High-quality genome assembly of Pseudopestalotiopsis theae, the pathogenic fungus causing tea gray blight. Plant Dis. 2021;105:3723–6 [DOI] [PubMed] [Google Scholar]
- 5. Wang S, Mi X, Wu Z. et al. Characterization and pathogenicity of Pestalotiopsis-like species associated with gray blight disease on Camellia sinensis in Anhui Province, China. Plant Dis. 2019;103:2786–97 [DOI] [PubMed] [Google Scholar]
- 6. Tan R, Jiao L, Huang D. et al. Comparative transcript profiling of resistant and susceptible tea plants in response to gray blight disease. Agronomy. 2024;14:565 [Google Scholar]
- 7. Hu Y, Zhang M, Lu M. et al. Salicylic acid carboxyl glucosyltransferase UGT87E7 regulates disease resistance in Camellia sinensis. Plant Physiol. 2022;188:1507–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu M, Zhao Y, Feng Y. et al. 2,4-dihydroxybenzoic acid, a novel SA derivative, controls plant immunity via UGT95B17-mediated glucosylation: a case study in Camellia sinensis. Adv Sci (Weinh). 2024;11:e2307051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang S, Liu L, Mi X. et al. Multi-omics analysis to visualize the dynamic roles of defense genes in the response of tea plants to gray blight. Plant J. 2021;106:862–75 [DOI] [PubMed] [Google Scholar]
- 10. Kang H, Fan T, Wu J. et al. Histone modification and chromatin remodeling in plant response to pathogens. Front Plant Sci. 2022;13:986940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, Guo Q, Qin LJ. et al. A Cys2His2 zinc finger transcription factor BpSZA1 positively modulates salt stress in Betula platyphylla. Front Plant Sci. 2022;13:823547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou Y, Wang L, Wang L. et al. JMJ704 positively regulates rice defense response against Xanthomonas oryzae pv. Oryzae infection via reducing H3K4me2/3 associated with negative disease resistance regulators. BMC Plant Biol. 2015;15:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutta A, Choudhary P, Caruana J. et al. JMJ27, an Arabidopsis H3K9 histone demethylase, modulates defense against Pseudomonas syringae and flowering time. Plant J. 2017;91:1015–28 [DOI] [PubMed] [Google Scholar]
- 14. Li D, Liu R, Singh D. et al. JMJ14 encoded H3K4 demethylase modulates immune responses by regulating defence gene expression and pipecolic acid levels. New Phytol. 2020;225:2108–21 [DOI] [PubMed] [Google Scholar]
- 15. Li T, Chen X, Zhong X. et al. Jumonji C domain protein JMJ705-mediated removal of histone H3 lysine 27 trimethylation is involved in defense-related gene activation in rice. Plant Cell. 2013;25:4725–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blanc RS, Richard S. Arginine methylation: the coming of age. Mol Cell. 2017;65:8–24 [DOI] [PubMed] [Google Scholar]
- 17. Liu C, Lu F, Cui X. et al. Histone methylation in higher plants. Annu Rev Plant Biol. 2010;61:395–420 [DOI] [PubMed] [Google Scholar]
- 18. Bachand F. Protein arginine methyltransferases: from unicellular eukaryotes to humans. Eukaryot Cell. 2007;6:889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee WC, Lin WL, Matsui T. et al. Protein arginine methyltransferase 8: tetrameric structure and protein substrate specificity. Biochemistry. 2015;54:7514–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ayad AA-H, Krystal D, Rong H. Non-histone arginine methylation by protein arginine methyltransferases. Current Protein and Peptide Science. 2020;21:699–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scaglione A, Patzig J, Liang J. et al. PRMT5-mediated regulation of developmental myelination. Nat Commun. 2018;9:2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scebba F, De Bastiani M, Bernacchia G. et al. PRMT11: a new Arabidopsis MBD7 protein partner with arginine methyltransferase activity. Plant J. 2007;52:210–22 [DOI] [PubMed] [Google Scholar]
- 23. Yan D, Zhang Y, Niu L. et al. Identification and characterization of two closely related histone H4 arginine 3 methyltransferases in Arabidopsis thaliana. Biochem J. 2007;408:113–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niu L, Zhang Y, Pei Y. et al. Redundant requirement for a pair of PROTEIN ARGININE METHYLTRANSFERASE4 homologs for the proper regulation of Arabidopsis flowering time. Plant Physiol. 2008;148:490–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pei Y, Niu L, Lu F. et al. Mutations in the type II protein arginine methyltransferase AtPRMT5 result in pleiotropic developmental defects in Arabidopsis. Plant Physiol. 2007;144:1913–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmitz RJ, Sung S, Amasino RM. Histone arginine methylation is required for vernalization-induced epigenetic silencing of FLC in winter-annual Arabidopsis thaliana. Proc Natl Acad Sci. 2008;105:411–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Zhang Y, Ma Q. et al. SKB1-mediated symmetric dimethylation of histone H4R3 controls flowering time in Arabidopsis. EMBO J. 2007;26:1934–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hong S, Song HR, Lutz K. et al. Type II protein arginine methyltransferase 5 (PRMT5) is required for circadian period determination in Arabidopsis thaliana. Proc Natl Acad Sci. 2010;107:21211–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez SE, Petrillo E, Beckwith EJ. et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature. 2010;468:112–6 [DOI] [PubMed] [Google Scholar]
- 30. Li Q, Zhao Y, Yue M. et al. The protein arginine methylase 5 (PRMT5/SKB1) gene is required for the maintenance of root stem cells in response to DNA damage. Journal of Genetics and Genomics. 2016;43:187–97 [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Ma X, Han HN. et al. AtPRMT5 regulates shoot regeneration through mediating histone H4R3 dimethylation on KRPs and pre-mRNA splicing of RKP in Arabidopsis. Mol Plant. 2016;9:1634–46 [DOI] [PubMed] [Google Scholar]
- 32. Zhang Z, Zhang S, Zhang Y. et al. Arabidopsis floral initiator SKB1 confers high salt tolerance by regulating transcription and pre-mRNA splicing through altering histone H4R3 and small nuclear ribonucleoprotein LSM4 methylation. Plant Cell. 2011;23:396–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheng C, Li X, Xia S. et al. An OsPRMT5-OsAGO2/miR1875-OsHXK1 module regulates rice immunity to blast disease. J Integr Plant Biol. 2023;65:1077–95 [DOI] [PubMed] [Google Scholar]
- 34. Agrofoglio YC, Iglesias MJ, Perez-Santángelo S. et al. Arginine methylation of SM-LIKE PROTEIN 4 antagonistically affects alternative splicing during Arabidopsis stress responses. Plant Cell. 2024;36:2219–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu P, Zhao H, Zhu P. et al. Dual regulation of Arabidopsis AGO2 by arginine methylation. Nat Commun. 2019;10:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang S, Balgi A, Pan Y. et al. Identification of methylosome components as negative regulators of plant immunity using chemical genetics. Mol Plant. 2016;9:1620–33 [DOI] [PubMed] [Google Scholar]
- 37. Schweighofer A, Kazanaviciute V, Scheikl E. et al. The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell. 2007;19:2213–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaugler P, Schneider R, Liu G. et al. Arabidopsis PFA-DSP-type phosphohydrolases target specific inositol pyrophosphate messengers. Biochemistry. 2022;61:1213–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le H, Simmons CH, Zhong X. Functions and mechanisms of histone modifications in plants. Annu Rev Plant Biol. 2025;76:9.1–9.28 [DOI] [PubMed] [Google Scholar]
- 40. Lee S, Fu F, Xu S. et al. Global regulation of plant immunity by histone lysine methyl transferases. Plant Cell. 2016;28:1640–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drozda A, Kurpisz B, Arasimowicz-Jelonek M. et al. Nitric oxide implication in potato immunity to Phytophthora infestans via modifications of histone H3/H4 methylation patterns on defense genes. Int J Mol Sci. 2022;23:4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fan H, Zhang Z, Wang N. et al. SKB1/PRMT5-mediated histone H4R3 dimethylation of Ib subgroup bHLH genes negatively regulates iron homeostasis in Arabidopsis thaliana. Plant J. 2014;77:209–21 [DOI] [PubMed] [Google Scholar]
- 43. Jiao T, Huang Y, Wu YL. et al. Functional diversity of subgroup 5 R2R3-MYBs promoting proanthocyanidin biosynthesis and their key residues and motifs in tea plant. Hortic Res. 2023;10:uhad135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Houben A, Demidov D, Caperta AD. et al. Phosphorylation of histone H3 in plants--a dynamic affair. Biochim Biophys Acta. 2007;1769:308–15 [DOI] [PubMed] [Google Scholar]
- 45. Connolly EL, Fett JP, Guerinot ML. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell. 2002;14:1347–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu L, Chen H, Zhu J. et al. miR319a targeting of CsTCP10 plays an important role in defense against gray blight disease in tea plant (Camellia sinensis). Tree Physiol. 2022;42:1450–62 [DOI] [PubMed] [Google Scholar]
- 47. Li W, Lin YC, Li Q. et al. A robust chromatin immunoprecipitation protocol for studying transcription factor-DNA interactions and histone modifications in wood-forming tissue. Nat Protoc. 2014;9:2180–93 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The raw transcriptome data have been deposited in the National Center for Biotechnology Information bioproject database under the accession number PRJNA1223124 for the RNA-seq (CsPRMT5 suppression in tea plants) and PRJNA1223117 for the ChIP-seq data, respectively.








