Abstract
Background
In this study, we aimed to compare the mid-term clinical and radiographic outcomes of highly porous and hydroxyapatite (HA)-coated porous titanium cups using the same cementless cup system in patients undergoing total hip arthroplasty (THA). We investigated the differences in radiolucent line (RLL) formation, cup migration, and clinical outcomes between the two surface processing methods.
Methods
This retrospective study included 209 hips (116 in the highly porous group and 93 in the HA porous group) with a minimum follow-up period of over 4 years. Using standardized anteroposterior pelvic radiographs, we conducted radiographic evaluations to assess cup orientation, initial gaps, and RLLs. Migration was analyzed using Einzel-Bild-Rontgen Analysis (EBRA), focusing on a subset of 28 randomly selected cups from each group as determined by power analysis. Clinical outcomes were measured using the Japanese Orthopedic Association (JOA) hip score. The primary outcome was the incidence of cup revision, and the secondary outcomes were postoperative JOA scores and the incidence of initial gaps and RLLs.
Results
No cup revisions were made in either group. Both groups had significant postoperative improvements in the JOA scores, with no differences observed during the follow-up period. At baseline, the initial gaps were observed at similar rates (highly porous vs. HA porous [51% vs. 52%]; p = 0.97). However, at the 5-year follow-up, the initial gaps were resolved in nearly all cases. In the highly porous group, RLLs were significantly more frequent during the first-year follow-up (23% vs. 2%; p < 0.0001). However, they decreased in all cases, and none of them exhibited RLL expansion. EBRA migration analysis revealed no significant differences between the groups.
Conclusion
Both groups demonstrated comparable mid-term clinical and radiographic outcomes. The highly porous group exhibited higher initial RLL incidences. However, RLL diminished over time without compromising the migration or clinical results. Both designs are reliable options for cementless THA.
Keywords: Highly porous titanium cups, Cementless cup system, Mid-term outcomes, Bone ingrowth, Hydroxyapatite-coated porous cups, Cup migration, Clinical outcomes, Three-dimensional-printed porous design
Background
Total hip arthroplasty (THA) is an excellent treatment option for patients with severe hip joint disorders in terms of cost-effectiveness and significant clinical improvement [1]. The survival rates of long-term implants have exceeded 90% over the last 10 years [2]. However, early implant failure due to aseptic loosening remains a significant concern [3], necessitating revision surgeries, which affect patient-reported outcomes negatively [4]. Aseptic loosening of the acetabular cups due to poor initial fixation and insufficient bone ingrowth is critical for long-term implant success [5]. Therefore, improving implant longevity remains a critical issue in orthopedic surgery.
Historically, the evolution of the surface design and processing in cementless THA played a critical role in the survival rates of long-term implant survival, particularly for acetabular cups [6]. In cementless cups, conventional porous coatings such as porous plasma spray (PPS) processing with or without hydroxyapatite (HA) coating have demonstrated excellent clinical outcomes and durability for over 10 years [7, 8]. Recently, the three-dimensional (3D) highly porous processing, designed to mimic the trabecular structure of cancellous bone, was developed using advanced manufacturing techniques such as 3D printing technology. These highly porous structures are hypothesized to enhance bone ingrowth and provide superior initial stability based on biomechanical and osteogenic studies using animal models [9, 10]. However, reports of an early postoperative radiolucent line (RLL) and aseptic loosening in some cases have raised concerns regarding the effectiveness of highly porous coatings [11]. Comparisons of the clinical and radiographic outcomes between conventional porous coatings and newer highly porous coatings remain unclear.
Therefore, we aimed to elucidate the impact of different surface processing techniques within the same cup system by comparing the postoperative clinical and radiographic outcomes of the conventional HA porous with the highly porous coatings in the mid-term period. We hypothesized that the highly porous cups would demonstrate clinical and radiographic outcomes equivalent to those of HA-porous cups. From this comparison, we aim to provide a clearer understanding of the benefits and potential drawbacks of highly porous surface processing in cementless cups.
Methods
In this retrospective cohort study, we analyzed 163 consecutive patients (214 hips) who underwent primary THA with G7 acetabular cup system (Zimmer Biomet, Warsaw, IN, USA) at the Institute of Science, Tokyo Hospital, between March 2014 and January 2017. Our institutional review board approved this study. We excluded patients with < 4 years of follow-up or those with insufficient imaging evaluations. Based on the exclusion criteria, 14 patients were excluded; 13 (14 hips) were due to insufficient follow-up, and 1 patient (2 hips) was due to insufficient imaging. We enrolled 149 patients (198 hips). Patients were divided into two groups based on the G7 cup surface processing: G7 OsseoTi highly porous titanium cup (highly porous group; 109 hips) and G7 PPS BoneMaster cup coated HA (HA porous group; 89 hips). The G7 OsseoTi surface employed CT-based 3D printing to mimic the human cancellous bone structure. This surface was manufactured using a proprietary layer-by-layer printing process to achieve a mean pore size of 475 microns and 70% porosity. The G7 PPS BoneMaster surface was produced using PPS titanium coating with a thin layer of hydroxyapatite. The cup selection gradually shifted from HA porous cups to highly porous cups as the latter became available. In all cases, the type of stem used in this series was Taperloc Complete Microplasty Stem (Zimmer Biomet, Warsaw, IN, USA), except for one case in which a changeable neck stem with M/L Taper and Kinectiv Stem (Zimmer Biomet, Warsaw, IN, USA) were used due to anteversion adjustment. Four high-volume expert arthroplasty surgeons performed all surgeries. The acetabulum was under-reamed by 1 mm, and all the cementless cups were inserted using the press-fit technique in both groups. Adjunctive one or two screw fixation was performed at the surgeon’s discretion. A highly cross-linked polyethylene liner and ceramic head were used in all cases. The target cup angles were set at 40° in radiographic inclination and 15°–20° in radiographic anteversion. Demographic data, including age, sex, body mass index (BMI), and underlying hip pathology, were collected from medical records. Surgical details such as approach, cup size, the number of screws, and cup revision surgery used were documented. Clinical outcomes were assessed using the Japanese Orthopedic Association (JOA) hip score (maximum 100 points); a high JOA score was indicative of better hip function [12]. Data was collected preoperatively and postoperatively at 1 year, 2–3 years (recorded as 2-year postoperative data), and 4–6 years (recorded as 5-year postoperative data).
Radiographic analysis
Radiographic evaluations were conducted at baseline (within 1 week postoperatively) and at regular postoperative intervals: 1 year, 2–3 years (recorded as 2-year postoperative data), and 4–6 years (recorded as 5-year postoperative data). All anteroposterior (AP) pelvic radiographs were obtained in a standardized manner (patient standing in an upright position and full weight-bearing) [13]. AP pelvic radiographs were analyzed to measure the cup inclination and anteversion angle and the presence of the initial gap and RLLs. The cup inclination angle was determined by measuring the angle between the inter-teardrop line and the major oblique axis of the cup. The cup anteversion angle was measured on AP pelvic radiographs using the method described by Lewinnek et al. [14]. The locations of the initial gap and RLL were classified using the acetabular zones proposed by DeLee and Charnley. The initial gap was defined as a gap of ≥ 0.5 mm between the cup and the acetabular host bone that could be detected on the postoperative radiographs within 1 week postoperatively. RLL was defined as radiolucency ≥ 0.5 mm without an initial gap [15]. Reliability assessments were conducted to detect the initial gap and RLL. Two independent orthopedic surgeons (TT and YT) not involved in the THA surgery tested inter and intra-observer agreements using a subset of 20 randomly selected hips, with measurements repeated at least 4 weeks apart. The inter-observer agreement rates for detecting the initial gaps and RLLs were 93% and 97%, respectively, and the intra-observer agreement rates were 99% and 99%, respectively. Using the McNemar test, no significant differences were observed in detection rates between inter- and intra-observer agreements for initial gaps (p = 0.32, p = 1.0, respectively) or RLLs (p = 0.56, p = 0.32, respectively), indicating equivalent detection reliability.
Migration analysis with einzel-bild-rontgen analysis
Cup migration was analyzed using Einzel-Bild-Rontgen Analysis (EBRA) software (German: Einzel–Bild–Röntgen–Analyze), a validated method for assessing implant migration without markers [13]. A power analysis was conducted before the migration analysis to ensure statistical robustness. Based on the preliminary measurement, a standard deviation of 1.3 mm, and a clinically meaningful difference of 1.0 mm [13, 16], a sample size of 28 hips per group was required to achieve 80% power, with a significance level of 0.05. For migration analysis, 28 cups were randomly selected from both groups to assess potential differences in migration between the two groups. Migration was assessed as the total displacement vector calculated with the Pythagorean Theorem, and a clinically significant threshold of total migration was defined as ≥ 2 mm within the first 2 years [16].
Statistical analysis
Statistical analyses were performed using the STATA 16 software (StataCorp LP, College Station, TX, USA). All continuous data are presented as means ± standard deviations (SD). We performed the Shapiro–Wilk test to assess the normality of the data. For continuous parameters with a normal distribution, we used the unpaired t-test for statistical comparisons. For non-normally distributed data, we applied the non-parametric Mann–Whitney U test. Chi-square analyses were used to assess the associations between discrete variables (the presence of initial gaps and RLLs). The significance level was set at p < 0.05 for all tests.
Results
The highly porous and HA porous groups had comparable demographic and clinical characteristics except for the follow-up period and head diameter (Table 1). The mean age, BMI, cup diameter, number of fixation screws, cup alignment, and surgery time were similar between the two groups. Both groups showed balanced distributions of sex, THA diagnosis, operation site, and surgical approach. The slight difference in the follow-up periods was due to the timing of availability of each cup. The head diameter was slightly smaller in the HA porous group.
Table 1.
Patients’ demographic and clinical characteristics
| Patient characteristics | Highly porous group (n = 109) |
HA porous group (n = 89) |
p-value |
|---|---|---|---|
| Age (years)*† | 63.0 ± 10.8 | 62.4 ± 13.0 | 0.63 |
| Sex, female/male‡ | 89/20 | 76/13 | 0.48 |
| Body mass index (kg/m2)*† | 24.2 ± 3.8 | 23.3 ± 3.5 | 0.18 |
| Diagnosis‡ | |||
|
Primary hip osteoarthritis, n (%) Secondary osteoarthritis due to dysplasia, n (%) Osteonecrosis of the femoral head, n (%) |
9 (8) 95 (87) 5 (5) |
4 (4) 76 (86) 9 (10) |
0.46 |
| Operation side, R/L‡ | 56/53 | 44/45 | 0.79 |
| Follow-up period (years)** † | 5.7 ± 0.8 (3.7–6.9) | 6.2 ± 0.8 (3.8–6.9) | < 0.001 |
| Surgical approach‡ | |||
|
Anterolateral, n (%) Posterolateral, n (%) |
11 (10) 98 (90) |
16 (18) 73 (82) |
0.11 |
| Cup diameter (mm)*† | 50.3 ± 2.9 | 50.2 ± 2.5 | 0.83 |
| Head diameter (mm)*† | 32.7 ± 1.8 | 31.9 ± 2.1 | 0.005 |
| Number of fixation screws*† | 0.03 ± 0.2 | 0.01 ± 0.1 | 0.88 |
| Cup inclination (degrees)*†† | 37.9 ± 4.6 | 38.4 ± 4.0 | 0.13 |
| Cup anteversion (degrees)*† | 18.5 ± 5.3 | 17.3 ± 5.3 | 0.15 |
| Surgery time (min)*† | 88.1 ± 25.9 | 82.0 ± 17.7 | 0.16 |
*Values are presented as the mean ± SD
**Values are presented as the mean ± SD (range)
† Mann-Whitney test
††Unpaired t-test
‡ Pearson’s chi-squared test
Statistically significant p-values are presented in bold
During the observation period, no cup revision occurred in either group. Both groups demonstrated significant improvements in the JOA scores postoperatively, with no significant differences observed at any follow-up interval (Table 2). The preoperative scores were comparable, and the postoperative scores exceeded 90 points in both groups, reflecting excellent clinical outcomes.
Table 2.
Comparison of the JOA score between the highly porous and HA porous groups
| JOA score | Highly porous group | HA porous group | p-value |
|---|---|---|---|
| Preoperative | 51.6 ± 13.3 | 50.5 ± 13.7 | 0.38 |
| Follow-up, 1 year | 93.5 ± 5.7 | 92.6 ± 6.8 | 0.62 |
| 2 years | 93.7 ± 5.3 | 93.5 ± 6.3 | 0.89 |
| 5 years | 94.2 ± 6.3 | 92.3 ± 7.1 | 0.07 |
All values are presented as the mean ± SD
The variables were evaluated using the Mann–Whitney U test
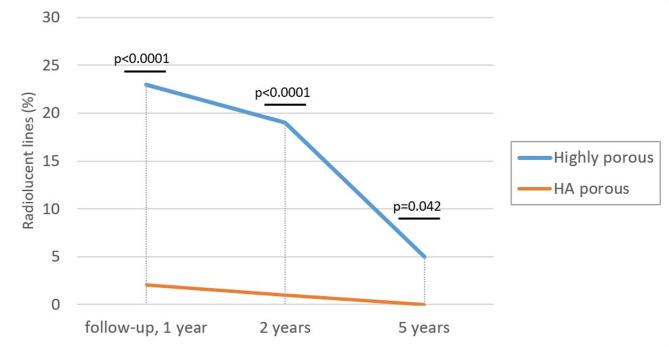
The initial gaps were observed at comparable rates in both groups, and no significant differences were observed during the entire follow-up period (Table 3). At baseline, initial gaps were present in > 50% of the cases in both groups; however, at the 5-year follow-up, the incidence decreased to approximately 1% in both groups. At all postoperative intervals, RLLs were significantly more frequent in the highly porous group than in the HA group (Fig. 1). However, the incidence of RLLs decreased over time in both groups. Furthermore, in all cases with RLLs, there was no progressive widening of the RLLs or expansion of the affected zones over time (Table 4). Migration analysis using EBRA revealed no significant differences in the total migration between both groups at any follow-up period (Table 5). In addition, both groups exhibited stable migration patterns, with no cases exceeding the clinical threshold of 2 mm within the first 2 years.
Table 3.
Comparison of the initial gap between the highly porous and HA porous groups
| Parameter | DeLee zone | Highly porous group (109 hips) | HA porous group (89 hips) |
p-value |
|---|---|---|---|---|
| Initial gap (hips) | One or more zones | 56 (51%) | 46 (52%) | 0.97 |
| 1 zone | 18 (17%) | 8 (9%) | 0.12 | |
| 2 zone | 43 (39%) | 39 (44%) | 0.54 | |
| 3 zone | 4 (4%) | 5 (6%) | 0.51 | |
| Follow-up, 1 year | One or more zones | 13 (12%) | 8 (9%) | 0.50 |
| 1 zone | 6 (6%) | 3 (3%) | 0.47 | |
| 2 zone | 7 (6%) | 4 (4%) | 0.56 | |
| 3 zone | 0 (0%) | 1 (1%) | 0.27 | |
| 2 years | One or more zones | 4 (4%) | 2 (2%) | 0.56 |
| 1 zone | 2 (2%) | 1 (1%) | 0.68 | |
| 2 zone | 2 (2%) | 0 (0%) | 0.20 | |
| 3 zone | 0 (0%) | 1 (1%) | 0.27 | |
| 5 years | One or more zones | 1 (1%) | 1 (1%) | 0.89 |
| 1 zone | 1 (1%) | 0 (0%) | 0.37 | |
| 2 zone | 0 (0%) | 0 (0%) | 1.0 | |
| 3 zone | 0 (0%) | 1 (1%) | 0.27 |
The variables were evaluated using Pearson’s chi-squared test
Fig. 1.
Incidence of radiolucent lines in highly porous and HA porous groups at 1 year, 2 years, and 5 years postoperatively
Table 4.
Comparison of the radiolucent lines between the highly porous and HA porous groups
| Radiolucent lines | ||||
|---|---|---|---|---|
| Follow-up | DeLee zone | Highly porous group | HA porous group | p-value |
| 1 year | One or more zones | 25 (23%) | 2 (2%) | < 0.0001 |
| 1 zone | 10 (9%) | 1 (1%) | 0.014 | |
| 2 zone | 4 (4%) | 0 (0%) | 0.068 | |
| 3 zone | 20 (18%) | 1 (1%) | < 0.0001 | |
| 2 years | One or more zones | 21 (19%) | 1 (1%) | < 0.0001 |
| 1 zone | 9 (8%) | 1 (1%) | 0.023 | |
| 2 zone | 4 (4%) | 0 (0%) | 0.068 | |
| 3 zone | 17 (16%) | 0 (0%) | < 0.0001 | |
| 5 years | One or more zones | 5 (5%) | 0 (0%) | 0.042 |
| 1 zone | 3 (3%) | 0 (0%) | 0.12 | |
| 2 zone | 0 (0%) | 0 (0%) | 1.0 | |
| 3 zone | 2 (2%) | 0 (0%) | 0.2 | |
The variables were evaluated using Pearson’s chi-squared test
Statistically significant p-values are presented in bold text
Table 5.
Comparison of postoperative total migration between the highly porous and HA porous groups
| Total migration (mm) | |||
|---|---|---|---|
| Follow-up | Highly porous group | HA porous group | p-value |
| 1 year* | 1.12 0.51 0.51 |
1.20 0.66 0.66 |
0.63 |
| 2 years* | 1.60 0.85 0.85 |
1.65 0.79 0.79 |
0.82 |
| 5 years** | 1.94 ± 1.3 | 2.10 ± 1.2 | 0.53 |
All values are presented as the mean ± SD
* The p-value was evaluated using the unpaired t-test
** The p-value was evaluated using the Mann–Whitney U test
Discussion
In this study, we highlight the clinical and radiological outcomes of cementless THA performed with highly porous cups, compared with conventional HA-coated porous cups in the same cup system. Our findings indicate that the highly porous cup provided satisfactory clinical outcomes despite a higher incidence of RLLs in the early postoperative period. These RLLs gradually disappeared after 5 years postoperatively, indicating successful long-term osseointegration.
Tamaki et al. reported the short-term outcomes of the G7 OsseoTi highly porous cup compared with the Trident HA-coated porous cup (Stryker, Mahwah, NJ, USA) [15]. Notably, both groups exhibited excellent clinical outcomes; however, RLLs were observed exclusively in the highly porous OsseoTi cup group at rates of up to 22.8% at 24 months postoperatively, without any cases requiring revision surgery. This finding is consistent with our study, which further extended the follow-up period and confirmed that these RLLs tend to disappear after 5 years postoperatively, indicating stable osseointegration. By focusing on the same G7 cup system, this study provides important information regarding the long-term reliability of the highly porous processing of G7 OsseoTi.
The mechanisms underlying our findings are likely multifactorial. For example, Carli et al. evaluated the Tritanium primary cup (Stryker) and compared it to the Trident HA-coated porous cup over a 5-year follow-up period [17]. They observed a progressive increase in RLLs only with the Tritanium cup, which was designed to be highly porous, requiring cup revision due to aseptic loosening in 1.8% of cases. Histological examination revealed fibrous fixation with reduced osseointegration in the retrieved Tritanium cups. The highly porous design of the Tritanium cup featured excessive porosity (72.5%) and larger mean pore diameter (546 μm) compared with other highly porous cups. This design employed a deposition process in which pure titanium was applied to a scaffold, creating a porous surface [17]. This technique achieved a high degree of porosity; however, it introduced structural randomness and variability in pore size and distribution. These factors may contribute to a lower elastic modulus, which induces suboptimal osseointegration or micromotion, finally leading to aseptic loosening. In contrast, the OsseoTi cup used in this study demonstrated a highly controlled and uniform porous surface. The design incorporated 70% porosity and a mean pore diameter of 475 μm through an advanced 3D printing technique called Electron Beam Melting [18]. These factors in the OsseoTi cup may contribute to stable osseointegration beyond the initial RLL phase. These findings indicate the critical role of the production process for porous surfaces and structural design, including optimized porosity and pore diameter, in achieving effective bone ingrowth. Our results suggest that both surface designs are viable for primary THA. However, we believe that whenever new surface technologies are introduced, such as highly porous coatings designed to mimic human cancellous bone and promote early osseointegration, their presumed clinical advantages must be critically assessed through long-term clinical follow-up to confirm whether they are truly achieved.
This study has some limitations. First, this was a single-center retrospective analysis conducted in an academic hospital, raising the potential for selection bias. The G7 PPS BoneMaster cup (HA porous group) had a slightly longer mean follow-up period due to earlier market availability; however, other demographic data were largely comparable between both groups. Second, we could not evaluate the effects of screw fixation as 98% of all patients in our study did not use screws. However, we thought that it had minimal influence on the outcomes. Third, the femoral components were not included in the analysis. Variations in stem design and alignment might influence the stress distribution of the acetabular cup. However, 99% of the cases in this study utilized the same cementless stem (Taperloc complete microplasty stem; Zimmer Biomet). No periprosthetic fractures were observed around stems during the observation period. Fourth, the timing of radiographic follow-up was not strictly standardized because of the retrospective design of the study. Radiographic data were collected for 2–3 years postoperatively but were grouped as 2-year evaluations, which might have influenced our results. Compared with the findings of Tamaki et al. [15], our study demonstrated a more pronounced reduction in RLLs in the highly porous group between 1 and 2 years postoperatively. We believe that in the highly porous group, RLLs begin to decrease between 2 and 3 years postoperatively as osseointegration progresses. Therefore, further studies are needed to determine the exact timing of RLL reduction. Fifth, although the observed cup migration values at 5 years, 1.94 mm in the highly porous group and 2.10 mm in the conventional porous group, might appear relatively large, we believe they indicate stable cup fixation. Wyss et al. reported that a gradual decrease in cup migration over time was a critical indicator of successful results using EBRA-based analyses [19]. In our study, both groups demonstrated a consistent reduction in migration over time, suggesting stable cup fixation. Moreover, although EBRA utilizes a comparability algorithm to exclude radiographs with substantially different pelvic tilts or rotations, subtle changes in pelvic tilt over mid-term follow-up might still influence measurement accuracy. Finally, our data was based on a mid-term follow-up. As follow-ups beyond 10 years have not been conducted, further studies with longer follow-up periods are necessary to elucidate the impact of highly porous surfaces.
Conclusion
The highly porous surface processing in cementless cups was associated with a higher incidence of early RLL formation compared with conventional HA porous coating. However, this does not adversely affect mid-term clinical outcomes or implant stability. Both surface-processing techniques can provide reliable fixation and excellent clinical outcomes.
Acknowledgements
This study was supported by JSPS KAKENHI (grant number JP-23K15694). We thank Yasushi Takenouchi, MD, for his assistance with radiography.
Abbreviations
- HA
Hydroxyapatite
- THA
Total hip arthroplasty
- RLL
Radiolucent line
- EBRA
Einzel-Bild-Rontgen Analysis
- JOA
Japanese Orthopedic Association
- 3D
Three-dimensional
- BMI
Body mass index
- AP
Anteroposterior
- PPS
Porous plasma spray
Author contributions
TT, HK, and JT contributed substantially to the conception of the study. TO contributed to the statistical analysis of the results. KM, MH, RT, RS supervised the study. All authors contributed to the manuscript revision and approved the final version of the manuscript for publication.
Funding
None.
Data availability
The data sets used in this study are available from the corresponding author upon request.
Declarations
Ethical approval and consent to participate
All procedures were conducted in accordance with the principles of the Declaration of Helsinki. The Ethics Committee of the Institute of Science Tokyo, Tokyo, Japan, approved this study. Since participant data was anonymized and because of the voluntary nature of the study, we opted out of the need for informed consent for this study. Written consent to participate was not required for this study design.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–19. 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Bayliss LE, Culliford D, Monk AP, Glyn-Jones S, Prieto-Alhambra D, Judge A, et al. Effect of patient age at intervention on the risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet. 2017;389:1424–30. 10.1016/S0140-6736(17)30059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt M, Hooper G, Frampton C, Rothwell A. Survival outcomes of cemented stems compared with uncemented stems in primary total hip replacement. World J Orthop. 2014;5:591–6. 10.5312/wjo.v5.i5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada S, Hamai S, Shiomoto K, Hara D, Fujii M, Ikemura S, et al. Patient-reported outcomes after primary or revision total hip arthroplasty: A propensity score-matched Asian cohort study. PLoS ONE. 2021;16:e0252112. 10.1371/journal.pone.0252112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugano N, Nishii T, Nakata K, Masuhara K, Takaoka K. Polyethylene sockets and alumina ceramic heads in cemented total hip arthroplasty. A ten-year study. J Bone Joint Surg Br. 1995;77:548–56. 10.1302/0301-620X.77B4.7615596. [PubMed] [Google Scholar]
- 6.Slaven SE, Purcell RL, Mack AW, Bedard NA, Warth LC, Callaghan JJ. Results of porous-coated anatomic total hip arthroplasty without cement at 25–30 years of age: A concise follow-up of a previous report. J Bone Joint Surg Am. 2017;99:1647–53. 10.2106/JBJS.16.01420. [DOI] [PubMed] [Google Scholar]
- 7.Crawford DA, Berend KR, Adams JB, Lombardi AV. Survival of second-generation porous plasma-sprayed acetabular components after a minimum 15-year follow-up. J Surg Orthop Adv. 2019;28:31–4. [PubMed] [Google Scholar]
- 8.Lazarinis S, Mäkelä KT, Eskelinen A, Havelin L, Hallan G, Overgaard S, et al. Does hydroxyapatite coating of uncemented cups improve long-term survival? An analysis of 28,605 primary total hip arthroplasty procedures from the nordic arthroplasty register association (Nara). Osteoarthr Cartil. 2017;25:1980–7. 10.1016/j.joca.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Small SR, Berend ME, Howard LA, Rogge RD, Buckley CA, Ritter MA. High initial stability in porous titanium acetabular cups: A Biomechanical study. J Arthroplasty. 2013;28:510–6. 10.1016/j.arth.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Gu Y, Sun Y, Shujaat S, Braem A, Politis C, Jacobs R. 3D-printed porous Ti6Al4V scaffolds for long bone repair in animal models: A systematic review. J Orthop Surg Res. 2022;17:68. 10.1186/s13018-022-02960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long WJ, Nayyar S, Chen KK, Novikov D, Davidovitch RI, Vigdorchik JM. Early aseptic loosening of the tritanium primary acetabular component with screw fixation. Arthroplast Today. 2018;4:169–74. 10.1016/j.artd.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuribayashi M, Takahashi KA, Fujioka M, Ueshima K, Inoue S, Kubo T. Reliability and validity of the Japanese orthopaedic association hip score. J Orthop Sci. 2010;15:452–8. 10.1007/s00776-010-1490-0. [DOI] [PubMed] [Google Scholar]
- 13.Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: A method to measure migration of acetabular components. J Biomech. 1995;28:1225–36. 10.1016/0021-9290(94)00177-6. [DOI] [PubMed] [Google Scholar]
- 14.Park YS, Shin WC, Lee SM, Kwak SH, Bae JY, Suh KT. The best method for evaluating anteversion of the acetabular component after total hip arthroplasty on plain radiographs. J Orthop Surg Res. 2018;13:66. 10.1186/s13018-018-0767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamaki Y, Goto T, Takasago T, Wada K, Hamada D, Sairyo K. Clinical and radiological outcomes of total hip arthroplasty using a highly porous titanium cup or a conventional hydroxyapatite-coated titanium cup: A retrospective study in Japanese patients. J Orthop Sci. 2022;27:163–8. 10.1016/j.jos.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Dammerer D, Blum P, Putzer D, Tscholl A, Liebensteiner MC, Thaler M. Good mid-term results with the Trident peripheral self-locking cup: A clinical evaluation and migration measurement with EBRA. Arch Orthop Trauma Surg. 2021;141:327–32. 10.1007/s00402-020-03639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carli AV, Warth LC, de Mesy Bentley KL, Nestor BJ. Short to midterm follow-up of the tritanium primary acetabular component: A cause for concern. J Arthroplasty. 2017;32:463–9. 10.1016/j.arth.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Tsikandylakis G, Mortensen KRL, Gromov K, Troelsen A, Malchau H, Mohaddes M. The use of porous titanium coating and the largest possible head do not affect early cup fixation: A 2-year report from a randomized controlled trial. JB JS Open Access [Internet]. 2020;5:e2000107. 10.2106/JBJS.OA.20.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyss T, Kägi P, Mayrhofer P, Nötzli H, Pfluger D, Knahr K. Five-year results of the uncemented RM pressfit cup clinical evaluation and migration measurements by EBRA. J Arthroplasty. 2013;28:1291–6. 10.1016/j.arth.2012.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used in this study are available from the corresponding author upon request.