Abstract
Background
Thyroid hormones influence the function of essentially every system of the body, including the cardiovascular and metabolic system. Thyroid hormone replacement with levothyroxine (LT4) is the mainstay of pharmacological management for people with (especially clinically overt) hypothyroidism, and it is important to ensure the cardiovascular and metabolic safety of this treatment. This is especially so as in hypothyroidism, cardiometabolic risk factors and cardiovascular disease are highly prevalent conditions and will often coexist in an individual patient. Accordingly, we have reviewed the cardiometabolic consequences of hypothyroidism and intervention with thyroid hormone replacement.
Main body
Numerous observational studies and meta-analyses have described multiple potentially adverse cardiometabolic consequences of hypothyroidism, including exacerbation of cardiovascular and metabolic risk factors (especially dyslipidaemia), functional impairment of the heart and vasculature (including accelerated atherosclerosis) and increased risk of advanced cardiovascular outcomes. LT4 usually improves cardiometabolic risk factors in people with hypothyroidism and some (but not all) studies have reported improved vascular and cardiac function in LT4-treated populations. Observational data have suggested the possibility of improved cardiometabolic outcomes with LT4 treatment, particularly in younger people with hypothyroidism, although data from randomised, controlled trials are needed here. Importantly, LT4 (with or without additional triiodothyronine) appears to be safe from a cardiovascular perspective, as long as overtreatment and iatrogenic thyrotoxicosis are avoided.
Conclusions
Overall, the current evidence base supports intervention with LT4 to protect the cardiometabolic health of people with hypothyroidism who require thyroid hormone replacement, although more data on long-term clinical outcomes are needed.
Keywords: Hypothyroidism, Cardiometabolic risk factors, Cardiometabolic outcomes, Levothyroxine, Triiodothyronine, Drug safety
Introduction
As many as about 10% of all adults worldwide demonstrate features of clinically overt or subclinical hypothyroidism (half of which may be undiagnosed), with generally higher prevalence findings as age increases [1, 2]. The condition presents either as subclinical hypothyroidism (SCH), where thyrotropin (thyroid stimulating hormone, TSH) is elevated but free thyroxine (fT4) remains within the normal range, or the more severe, overt hypothyroidism (OH), where TSH is elevated and the level of fT4 is depressed [3]. As a prevalent and (usually) lifelong condition, considerable overlap will be found between populations with hypothyroidism and other common conditions [4]. Accordingly, it is important to note that the simultaneous occurrence of hypothyroidism (including that requiring intervention with LT4) and cardiometabolic risk factors or established cardiovascular disease is inevitably a common clinical finding.
We have known for decades that both the condition of hypothyroidism itself, and its principal pharmacologic treatment, levothyroxine (LT4), can exert a potentially profound impact on the function of the cardiovascular and metabolic system [5–7]. Cardiovascular disease is the leading cause of death worldwide [8, 9], so it is important, therefore, to optimise the cardiovascular and metabolic safety of intervention with LT4 in people with hypothyroidism, from the dual perspectives of minimising any adverse cardiovascular effects of thyroid dysfunction per se, while avoiding any possibility of new or exacerbation of cardiometabolic risk associated with its treatment [10, 11]. Here, we review the latest findings from the clinical literature on the impact of hypothyroidism and its treatment on cardiometabolic risk factors, and on clinical cardiovascular outcomes.
Our approach to this review
As described above, the purpose of our review is to consider the impacts of OH and SCH, and of intervention with LT4 to manage hypothyroidism, on cardiometabolic risk factors and on clinical cardiovascular outcomes. The clinical literature on hypothyroidism is vast. For conciseness and clarity, we have considered more important observational studies alongside meta-analyses and randomised, controlled trials for effects on the various cardiometabolic risk factors and cardiovascular outcomes reviewed below. We also include a brief summary of the clinical implications of the current evidence base for each risk factor, to aid the reader in navigating this complex clinical landscape.
Hypothyroidism and classical cardiometabolic risk factors
Table 1 contains a summary of studies that associated hypothyroidism with classical cardiometabolic risk factors. These are also summarised in Fig. 1.
Table 1.
Overview of studies of cardiovascular risk factors in people with hypothyroidism
| Ref | Type | Main findings |
|---|---|---|
| Hypertension | ||
| [12, 13] |
Obs Obs |
Increased diastolic and/or systolic BP or both for populations with OH or SCH vs. euthyroidism |
| [14] | Obs | Mendelian randomisation study showed that genetically predicted low T4 predicted hypertension |
| [15, 16] | Obs | High TSH or low T4 predicted hypertension |
| [17] | Obs | No significant association between SCH and hypertension or BP |
| [18–20] | MA | Meta-analyses associated SCH with increased risk of hypertension of variable magnitude |
| Dyslipidaemia | ||
| [14] | Obs | Mendelian randomisation study showed that genetically predicted low T4 predicted dyslipidaemia |
| [21] | Obs | Positive correlation between serum TSH and total-C, LDL-C and Apolipoprotein B |
| [22] | Obs | The lipid profile in SCH may be more atherogenic (increased lipid peroxidation) |
| [23] | Obs | No significant association of OH or SCH with dyslipidaemia after adjustment for other factors |
| [24–28] | MA | Meta-analyses confirmed significant associations of SCH with adverse lipid profiles |
| Impaired blood glucose control | ||
| [14] | Obs | Mendelian randomisation study showed that genetically predicted low T4 predicted dyslipidaemia |
| [29, 30] | Obs | OH or SCH was associated with insulin resistance, diminished insulin secretion and increased hepatic glucose production |
| [31, 32] | Obs | High-normal TSH levels or low-normal T4 (within reference ranges) predicted development of type 2 diabetes or prediabetes, respectively |
| [33, 34] | Obs | Increased prevalence of diabetes in people with hypothyroidism |
| [35] | Obs | Thyroid function was depressed in people with vs. without new-onset diabetes |
| [36] | Obs | More diabetes-associated hypoglycaemia in people with vs. without thyroid disease (unspecified) |
| Metabolic syndrome | ||
| [37] | Obs | No overall increase in metabolic syndrome prevalence with SCH, but some components were more common |
| [38] | Obs | Increased prevalence of metabolic syndrome in people with SCH vs. euthyroidism |
| [39] | Obs | Increased risk of hypothyroidism in people with metabolic syndrome |
| [20, 37, 38, 40] | MA | Increased prevalence of metabolic syndrome associated with SCH according to several diagnostic criteria (not criteria from China in one analysis) |
| [41] | SR | Thyroid function markedly influences all components of the metabolic syndrome |
| Obesity | ||
| [42] | Obs | About three-quarters of people with OH or SCH are overweight or obese |
| [43, 44] | Obs | Obese subjects are at increased risk of thyroid autoimmunity |
Study types: Obs observational study, MA meta-analysis. Other abbreviations: BP blood pressure, T4 thyroxine, OH overt hypothyroidism, SCH subclinical hypothyroidism, TSH thyrotropin
Fig. 1.
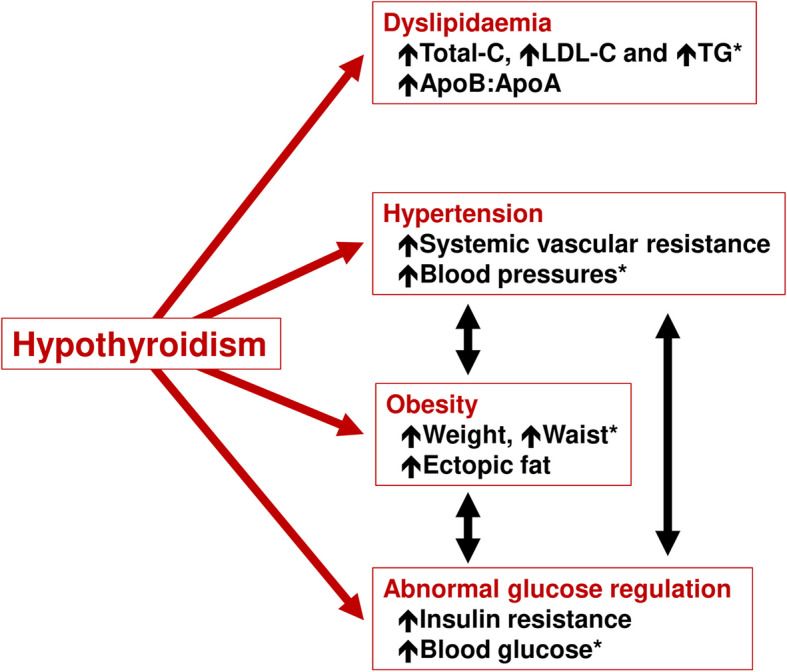
Overview of the effects of hypothyroidism on classical cardiometabolic risk factors: note that all of these are often improved by treatment with levothyroxine
*Components of the metabolic syndrome. In general, the severity of disturbances of these risk factors varies with the severity of hypothyroidism: thus, these associations maty be considered to be more established for overt hypothyroidism, although clinical studies have also described them in people with subclinical hypothyroidism (see text for further explanation and supporting references)
Blood pressure
Impact of hypothyroidism
Hypothyroidism increases systemic vascular resistance, with decreased cardiac preload and increased afterload [45]. Observational studies have demonstrated increased diastolic blood pressure (DBP), or both diastolic and systolic blood pressure (SBP), in people with hypothyroidism vs. euthyroidism [12, 13], including in a Mendelian randomisation study [14]. Conversely, people with high TSH were more likely to have hypertension in one study [15]. Measures of blood pressure (BP) have been shown to correlate significantly with markers of thyroid function, such as T4 or TSH [12, 15, 16], although variation in TSH did not explain a higher prevalence of hypertension in people with SCH [17]. Observational data have not always reported marked associations between hypothyroidism and increased BP in people with SCH [46]. Long-term data from 621 LT4-treated subjects in Brazil showed that the use of antihypertensive treatment increased over time, indicating that periodic review of BP and other cardiovascular risk factors is needed for this population [47].
A recent meta-analysis described significant elevations of BP in middle-aged women with SCH (odds ratio [OR] 1.64 [95%CI 1.18 to 2.27]), while there was no significant association in older women (OR 0.97 [0.80 to 1.16]) [18]. Another such analysis, of observational studies, reported only a small average difference in BP of 1.5/0.4 mmHg between populations with SCH and euthyroidism [19]. An analysis of the effects of hypothyroidism on the incidence of the metabolic syndrome (described below) also found an increase in BP in the hypothyroid group [20].
Impact of LT4
A reduction in DBP (− 2.6 mmHg [95%CI –0.4 to –4.8], p = 0.021) was observed in 60 patients with OH or SCH who were prescribed LT4 and followed for 12 weeks [48]. LT4 treatment reduced BP in an observational study in 30 women with elevated TSH vs. healthy control subjects [49]. In another study, an increase in DBP was observed in a population who underwent sudden, severe hypothyroidism due to withdrawal of LT4 treatment following total thyroidectomy, which suggests opposite influences of hypothyroidism per se and LT4 on BP [50].
A recent (2024) meta-analysis of 34 studies showed that intervention with LT4 for SCH was associated with an average reduction (95%CI) in BP of − 4.0 (–4.6 to –6.5)/ − 2.1 (–0.6 to –3.7) mmHg (both p < 0.05) [51]. An earlier analysis (2018) reported that average reductions (95%CI) in BP associated with LT4 treatment were − 2.5 (–0.3 to –4.6)/ − 0/9 (–2.3 to 0.6) mmHg (p = 0.024/p = NS) in patients enrolled in randomised trials and − 4.8 (–3.1 to –6.5)/ − 2.7 (–1.4 to –4.1) mmHg (p < 0.001 for each) [52]. Another meta-analysis, which focussed on older people with SCH, found no effect of LT4 on BP, although other cardiovascular risk factors improved (see below) [53].
Hypothyroidism and hypertension: summary of clinical implications.
The association of hypothyroidism with increased blood pressure has not been a universal finding (especially for SBP), although multiple observational and meta-analytic studies have reported such a finding. In addition, meta-analyses have suggested that intervention with LT4 reduces BP to a clinically significant extent in people with hypothyroidism. The observation in one study that effects of LT4 on BP were larger in observational studies than randomised trials suggests that the former may overestimate the contribution of reduced BP to overall cardiovascular risk management on this population.
Dyslipidaemia
Impact of hypothyroidism
Clinically significant hyperlipidaemia, with increases in ApoB-containing lipoproteins (e.g. total- and low-density lipoprotein cholesterol [LDL-C]) and triglycerides, is a well-recognised biochemical consequence of hypothyroidism, including SCH [1, 3, 54–56]. Effects on high density lipoprotein (HDL-C) are variable, although there is usually an increase in the ratio of ApoB:ApoA-containing lipoproteins. [56] Disturbances of multiple pathophysiological processes contribute to the effects of hypothyroidism on the lipid profile, including increased cholesterol production (e.g. via inhibition of hepatic HMG-CoA reductase and increased intestinal cholesterol absorption) and reduced clearance of cholesterol (e.g. via reduced density of hepatic LDL receptors), as well as other processes that contribute to an atherogenic lipid profile, including inhibition of reverse cholesterol transport [54–58]. Genetically predicted low T4 was associated significantly with dyslipidaemia [14]. Serum TSH has been shown to correlate positively with total cholesterol (total-C), LDL-cholesterol (LDL-C) and ApoB [21]. Increased lipid peroxidation in people with SCH may indicate a more atherogenic lipid profile in this population [22].
Meta-analyses found adverse lipid profiles in people with SCH, including in populations with comorbid polycystic ovary syndrome, a strongly insulin resistant state [20, 24–28]. Overall, it has been estimated that as many as 90% of people with OH have dyslipidaemia [57]. Not all studies have associated hypothyroidism with dyslipidaemia after adjustment for other factors, however [23].
Impact of LT4
Randomised, placebo-controlled trials have demonstrated modest, but clinically significant, reductions in indices of hyperlipidaemia, including total-C and LDL-C, in people with SCH after treatment with LT4 [59–63].Observational data also supported a reduction of total-C, LDL-C, HDL-C, VLDL-C and triglycerides after LT4 treatment in people with OH or SCH [21, 22, 48, 64–68]. Meta-analysis of studies conducted in people with SCH found that treatment with LT4 improved multiple aspects of the lipid profile (total-C, LDL-C, triglycerides and ApoB) [53, 69].
Hypothyroidism has also been associated with increased deposition of ectopic fat in the liver [57], which is a strong predictor of future dyslipidaemia, hyperglycaemia, metabolic-dysfunction-associated fatty liver disease (MAFLD) and increased cardiometabolic risk [70, 71]. LT4 treatment reduced liver lipid content in an observational study in euthyroid people with type 2 diabetes and MAFLD. While comparable data from populations with a diagnosis of hypothyroidism are awaited, MAFLD has been described as a state of "intrahepatic hypothyroidism" [72]. Treatment with LT4 also reduced ectopic fat in the heart in a population with SCH [73].
Hypothyroidism and dyslipidaemia: summary of clinical implications.
Clinically significant dyslipidaemia is a well-known complication of hypothyroidism, and a substantial evidence base of clinical data supports improvement of the lipid profile following thyroid hormone replacement with LT4. However, the disturbances of the lipid profile associated with SCH may be relatively mild, and the effects of LT4 on lipids may be relatively modest. In addition, dyslipidaemia is a common condition and may occur independently in parallel with hypothyroidism. Care should be taken to identify and correct an abnormal lipid profile with standard lipid modifying therapy, according to prevailing cardiovascular management guidelines, in addition to correction of thyroid hormone levels with LT4, where indicated clinically.
Blood glucose control
Impact of hypothyroidism
Insulin resistance appears to accompany hypothyroidism [29], with diminished pancreatic insulin secretion and increased hepatic glucose production, both of which are likely to predispose the patient to the development of fasting hyperglycaemia [30]. A prospective cohort study showed that higher TSH predicted a higher risk of developing type 2 diabetes, even within the reference range for this parameter [31]. Low-normal FT4 has been shown elsewhere to predict the presence of prediabetes [32]. Several other studies (reviewed elsewhere) have described epidemiological associations between the presence of diabetes and hypothyroidism [14, 33, 34]. A cross-sectional study in hospitalised patients showed that FT4, triiodothyronine (T3) and TSH were lower in people with vs. without new-onset diabetes [35]. Thyroid dysfunction in general has been shown to increase the risk of hypoglycaemia [36], particularly in the context of rare autoimmune disturbances of insulin action such as insulin autoimmune syndrome (Hirata's disease) or type B insulin resistance syndrome [74, 75].
A meta-analysis found no increase in the risk of incident diabetes in people with SCH [76], although others found that the presence of diabetes significantly predicted the presence of comorbid SCH [77] and TSH above its reference range predicted a 26% increase in the risk of diabetes [78]. Two additional meta-analyses have described greater severity of diabetes complications in people with SCH and diabetes, especially in the microvasculature [77, 79].
Impact of LT4
No randomised, controlled trials of LT4 are available from populations with type 2 diabetes and hypothyroidism. Observational data from populations with SCH [66, 67] or more severe hypothyroidism [48, 80] have suggested improvements in blood glucose and/or indices or insulin resistance; although no effect on insulin resistance was seen in another study in patients with primary hypothyroidism [64]. Another observational study suggested that LT4 had little effect on HbA1c in people without diabetes [81]. Finally, a systematic review reported a reduced rate of progression of diabetic nephropathy associated with LT4 treatment vs. placebo, although long-term data were lacking [82].
Hypothyroidism and impaired glycaemic control: summary of clinical implications.
There appear to be epidemiologic and pathophysiological connections between hypothyroidism and disturbances of blood glucose control, although these seem to be limited in extent and not seen universally in clinical studies. The meta-analytic evidence of improved renal outcome in people with diabetes who received LT4 is intriguing, and more randomised, controlled trials are urgently needed from this population. Hypothyroidism is treated with LT4, and LT4 may reduce the hypoglycaemic effect of antidiabetic drugs: therefore, patients' blood glucose levels should be frequently monitored, and if necessary, the dosage of anti-diabetic medications should be adjusted when initiating or adjusting LT4 therapy.
Metabolic syndrome
Impact of hypothyroidism
The metabolic syndrome comprises a constellation of five cardiometabolic risk factors (hypertension, hyperglycaemia, hypertriglyceridaemia, low HDL-C, and abdominal obesity) that are associated closely and pathophysiological with insulin resistance [83]. There is no doubt that the presence of the metabolic syndrome confers increased risk of cardiometabolic events and mortality, although most or all of this excess risk appears to be due to that conferred by the five risk factors contained within it [84].
Meta-analytic [20, 37, 40, 41] data and observational data from China [38] have confirmed a significantly increased risk of various diagnostic definitions of the metabolic syndrome associated with hypothyroidism vs. euthyroidism, although one of these studies found no significant increase in the criteria derived in China [20]. Increases in the risk of elevated obesity, blood pressure, and triglycerides, and of low HDL-C, drove the increased risk of metabolic syndrome in these analyses, consistent with the studies reviewed above. Conversely, people with the metabolic syndrome appear to be at increased risk of developing hypothyroidism [39].
Impact of LT4
LT4 has been shown to improve the various risk factors associated with the metabolic syndrome, as described elsewhere in this review and in uncontrolled studies in people who meet the diagnostic criteria for the metabolic syndrome [41, 85]. A study that involved withdrawal of LT4 treatment from subjects with thyroid cancer rendered athyreotic with radioactive iodine found that the resulting deterioration of cardiometabolic risk factors was more severe in patients with more metabolic syndrome components at baseline [86].
Hypothyroidism and metabolic syndrome: summary of clinical implications.
Few studies have been performed in populations with hypothyroidism and metabolic syndrome, especially involving the administration of LT4. The adverse effect of the metabolic syndrome on cardiometabolic outcomes can be explained largely by the effects of the individual risk factors. Accordingly, the evidence for exacerbation of metabolic syndrome components by hypothyroidism, and the available evidence for improved cardiometabolic risk factors with LT4, may be seen in the general context of improvements of these individual risk factors as described elsewhere in this review.
Obesity
Impact of hypothyroidism
Obesity and excess adiposity are other well-described clinical consequences of hypothyroidism [1, 3]. A retrospective study from Spain showed that 76% of a population with hypothyroidism were overweight or obese, compared with 59% of people with hyperthyroidism [42]. Overweight/obesity is seen both in terms of increased body mass index (BMI) and waist circumference. While the TSH level correlates positively with BMI, the relationship between thyroid status and obesity is complex. Morbid obesity per se increases TSH, and bariatric surgery leads to a reduction in TSH without a change in the level of FT4 [87]. Increased adiposity may alter the set point of thyroid homeostasis, favouring a higher level of TSH for a given level of FT4, perhaps associated with increased secretion of leptin [87]. The observation that obese subjects have higher risk of a diagnosis of hypothyroidism [43] may reflect similar issues of confounding. However, an observational study found that obesity increased the risk of OH and thyroid autoimmunity, suggesting that other mechanisms may be at play [44].
Impact of LT4
In an observational study, treatment of people with OH with LT4 for 12 weeks resulted in improvements in weight, body mass index, waist circumference, and subcutaneous (but not visceral) fat [48]. However, a meta-analysis did not report a significant reduction in BMI in people with hypothyroidism receiving LT4 during treatment periods of up to one year [88]. Much of the weight gain in hypothyroidism often consists of excess salt and water [89], and people taking LT4 for hypothyroidism should not expect to lose substantial amounts of weight. For example, a retrospective evaluation of 95 patients starting LT4 reported a mean reduction in BMI of − 0.1 kg/m2, from a baseline mean value of 29.3 kg/m2, which indicates an overweight-to-obese population, on average [90]. Increased hunger during LT4 treatment has been implicated as one possible reason for limited weight loss during thyroid hormone replacement, according to one recent study [91].
Accordingly, thyroid hormone replacement with LT4 is not a rational strategy for achieving weight loss and changes in weight in this setting are unlikely to represent a mechanism for reducing cardiometabolic risk [92]. Overweight or obesity associated with excess fat, especially ectopic fat deposited in the liver, heart, pancreas and muscle, is a major driver of diabetes and adverse cardiometabolic outcomes and should be addressed in addition to the management of thyroid function [93].
Hypothyroidism and obesity: summary of clinical implications.
Hypothyroidism is associated with overweight/obesity, although this relationship is complex. Weight loss on LT4 is usually modest. Administration of LT4 should never be seen as a strategy for achieving weight loss.
Hypothyroidism and vascular and cardiac function
Table 2 and Fig. 2 summarise the results of individual studies that reported the impact of hypothyroidism on the function of the heart and/or vasculature. The effects of LT4 on these parameters are summarised in Table 3 and Fig. 2.
Table 2.
Overview of studies of effects of hypothyroidism on both vascular and cardiac structure as well as function and adverse cardiovascular outcomes
| Ref | Type | Main findings |
|---|---|---|
| Impaired vascular or cardiac function | ||
| [94] | Obs | A "quasi-experimental study" significantly associated SCH with impaired endothelial dysfunction |
| [95] | MA | A meta-analysis of 10 studies found decreased mean endothelial function in a population with SCH vs. euthyroid population |
| [96] | MA | A meta-analysis reported increased arterial stiffness (increased pulse wave velocity and diminished nitrate-induced vasodilatation) associated with SCH vs. a euthyroid population |
| [97] | Obs | Poor prognosis in patients with SCH, heart failure, TSH > 7 mIU/L and low triiodothyronine levels |
| [98] | Obs | Impaired diastolic cardiac function for people with SCH vs. control subjects |
| [99] | Obs | Impaired systolic and diastolic function observed in "mild SCH" (4.2 < TSH < 10.0 mIU/L) vs. controls |
| [100] | Obs | A magnetic resonance imaging technique revealed diffuse myocardial injuries in the hearts of people with SCH |
| Atherosclerosis | ||
| [94] | Obs | No impact of SCH on cIMT |
| [94–96, 101] | MA | Meta-analyses reported increased mean cIMT in populations with SCH vs. euthyroid subjects |
| [102] | MA | Larger increase in cIMT in a population with SCH where TSH was > 10 mIU/L |
Study types: Obs observational study, MA meta-analysis. Other abbreviations: cIMT carotid intima-media thickness, SCH subclinical hypothyroidism, TSH thyrotropin
Fig. 2.

Overview of the effects of LT4 on cardiac structure and function in clinical studies
aSee text and Fig. 1. bThese potential benefits have not been seen in all studies (see text). The order of progression of cardiovascular disease shown here is for illustrative purposes and does not always occur in this order (e.g. the onset of heart failure may not be preceded by an adverse cardiovascular event). AI: atherogenic index; CAC: coronary artery calcification; cIMT: carotid intima-media thickness; CRI: coronary risk index; CV: cardiovascular; FMD: flow-mediated vasodilatation; LT4: levothyroxine; PWV: pulse wave velocity; RCT: randomised, controlled trial. See text for further explanation and supporting references
Table 3.
Overview of studies of effects of levothyroxine on vascular and cardiac structure and function,a and clinical cardiovascular outcomes
| Ref | Type | Main findings |
|---|---|---|
| Vascular structure and function | ||
| [62] | RCT | Increased FT4 was the strongest multivariate predictor of improved endothelial function in a RCT of LT4 vs. placebo in a population with SCH |
| [65] | Obs | Improved endothelial function during LT4 treatment in a population of women with overt hypothyroidism |
| [48] | Obs | Improved indices of cardiovascular risk during treatment of patients with “clinical hypothyroidism” and TSH > 10 mIU/L with LT4 (atherogenic index, coronary risk index, Framingham risk score) |
| [94] | Obs | Administration of LT4 for 2 months to people with SCH resulted in a significant improvement in endothelial function, but with no effect on cIMT |
| [96] | Obs | Improved cIMT and reduced cardiac epicardial fat during treatment of people with SCH with LT4 vs. a control group or vs. baseline |
| [49, 68] | Obs | cIMT was reduced during treatment with LT4 in subjects with elevated TSH or a diagnosis of SCH |
| [73] | Obs | Administration of LT4 to a population with SCH reduced cIMT and epicardial fat |
| [101, 103] | MA | cIMT in patients with SCH who received LT4, especially for > 6 months |
| Cardiac function | ||
| [104] | RCT | LT4 increased early diastolic velocity and early-to-late diastolic velocity and reversed increases in IVRT seen in SCH |
| [105] | RCT | LT4 reversed SCH-associated increases in pre-ejection/ejection time ratio and IVRT, peak transmitral flow velocity in late diastole (peak A) and cyclic variation index |
| [106] | RCT | LT4 improved IVRT, LVEF and CO in patients with chronic CHF |
| [107, 108] | RCT | LT4 increased LVEF, the shifted to right the slope of the end-systolic stress relation, reduced systemic vascular resistance and in increased CO in subjects with idiopathic dilated cardiomyopathy |
| [109] | RCT | No effect of LT4 on systolic or diastolic function in patients with mild SCH |
| [99] | Obs | LT4 increased LVEF and E/e' ratio and reduced myocardial performance index and lower global longitudinal strain in patients with new-onset mild SCH |
| [110] | Obs | 12 months of LT4 improved multiple aspects of left and right ventricular structure dynamics in a population with SCH |
| [111] | Obs | 12 months of LT4 improved PCr to ATP ratio in the hearts of people with SCH (improved myocardial bioenergetics) |
| [112, 113] | Obs | LT4 treatment was associated with increased risk of acute HF decompensation in people with HFpEF who were already receiving LT4 (the reason for treatment was unknown) |
| [114] | MA | LT4 improved (CO), left ventricular ejection fraction (LVEF), and the ratio of peak E velocity/peak A velocity in people with SCH |
| Clinical cardiovascular outcomes | ||
| [115, 116] | Obs | The risk of CVD or mortality was increased in untreated, but not LT4-treated patients with SCH from a database in Scandinavia; both under treatment and over treatment increased the risk of these outcomes |
| [48] | Obs | LT4 reduced Framingham CHD risk score in people with “clinical hypothyroidism” |
| [117] | Obs | > 1 year of LT4 reversed the increased risk of new CHD in patients with Hashimoto's thyroiditis |
| [118, 119] | Obs | LT4 did not affect cardiovascular outcomes significantly in populations with SCH ± CVD |
| [120] | Obs | LT4 reduced risk of mortality or CHD in people with SCH aged < 65 y, but not in older patients |
| [121] | MA | LT4 reduced risk of all-cause or cardiovascular mortality or CHD in people with SCH aged < 65 y, but not in older patients |
aSee Table 2 for effects of overt hypothyroidism or SCH on cardiovascular structure and function. Study types: RCT randomised controlled trial, Obs observational study, MA meta-analysis. Other abbreviations: CHD coronary heart disease, cIMT carotid intima-media thickness, CVD cardiovascular disease, CO cardiac output, HF heart failure, HFpEF HF with preserved ejection fraction, IVRT isovolumetric relaxation time, LVEF left ventricular ejection fraction; SCH: subclinical hypothyroidism
Impact of hypothyroidism
Impaired endothelium-derived vascular relaxation is an early event in the development of clinical cardiovascular disease and is associated strongly with an increased future risk of atherosclerosis and adverse cardiovascular outcomes. The presence of SCH was associated with reduced endothelial function, measured using flow-mediated vasodilatation, in a "quasi-experimental" study [94] and in a meta-analysis of 10 studies [95]. The same analysis reported a significant increase in mean carotid intima-media thickness (cIMT) in the SCH vs. euthyroid groups [95] that suggests strongly that SCH was associated with an increase in the overall burden of atherosclerosis. Other meta-analysis reported similar associations between endothelial function, cIMT progression or increased coronary artery calcification (another marker of atherosclerosis) [96, 101], especially where TSH was > 10 mIU/L [102]. One of these analyses also associated SCH with increased pulse wave velocity, a marker of increased arterial stiffness [96].
In the heart, hypothyroidism has been associated particularly with diastolic myocardial dysfunction and exacerbation of pre-existing heart failure [97, 98, 122]. Impairment of systolic cardiac function has also been described in populations with hypothyroidism and impairment of myocardial performance appears to correlate with the level of TSH [99, 100].
Impact of LT4
Increased FT4 was the strongest predictor of improved endothelial function in a randomised, placebo-controlled trial in people with SCH [62]. Another observational study reported improved endothelial function after treatment of women with primary hypothyroidism with LT4 [65]. Treatment of people with OH with LT4 for 12 weeks resulted in improvements in several parameters relating to vascular function, such as atherogenic index and coronary risk index [48]. A meta-analysis demonstrated significant benefit for LT4 on cIMT whether LT4 was compared with a control group (placebo or no treatment) or before and after administration in the same patients [96]. Additional meta-analytic [101, 103] and observational [49, 68, 73] data have also documented reduced cIMT during treatment with LT4. Importantly, an observational study [96] and a meta-analysis [103] study showed that larger effects on cIMT were observed with longer durations of LT4 treatment (> 6 months), which would be consistent with a long-term improvement in cardiovascular risk. Not all studies reported reduced cIMT on LT4 treatment, however, including in a randomised, placebo-controlled trial [123].
Randomised trials have described improvements in regional systolic or diastolic cardiac performance with LT4 vs. control or placebo in populations with SCH [104, 105], or cardiac insufficiency without thyroid dysfunction [106–108]although not all trials have demonstrated such a clinical benefit [109]. These trials were generally small, however, and trials in more substantial populations are needed. Observational studies of relatively long duration (up to 1 year) [99, 110, 111] and a meta-analysis [114], have also demonstrated reversal of deficits in cardiac performance associated with hypothyroidism when patients treated with LT4 were compared with control groups.
A database study where LT4 treatment was associated with increased risk of acute cardiac decompensation in people with heart failure with preserved ejection fraction and normal TSH levels provides a note of caution in this area [112]. However, interpretation of this study is severely hampered by a lack of information on thyroid status and the reason for active treatment in the LT4 group [113].
Hypothyroidism and vascular/cardiac structure and function: summary of clinical implications.
Hypothyroidism-associated structural and functional abnormalities in the heart and vasculature are well described. These adverse changes are not unexpected, given the adverse impact of hypothyroidism on cardiovascular risk factors, described above. Indeed, it is well known that endothelial dysfunction and subclinical atherosclerosis (among other adverse changes) provide a clear path from individual cardiovascular risk factors to adverse clinical cardiovascular outcomes, which are described below. While signs of reversal of vascular and cardiac abnormalities during LT4 treatment are promising, more and larger randomised trials will be needed to realise this potential.
Clinical cardiovascular outcomes
Impact of hypothyroidism (Table 2)
A cohort of 3,021 people with SCH in South Korea was followed for cardiovascular outcomes for 12 years [124]. Compared with euthyroid control subjects, those with SCH and TSH in the highest quartile (> 6.7 mIU/L) were at increased risk of all-cause death (hazard ratio [HR] 2.12 [95%CI 1.27 to 3.56]) and cardiovascular events (HR 1.92 [95%CI 1.21 to 3.04). Higher risks were seen in subjects aged < 65 years with high CV risk at baseline (HRs (3.50 [95%CI 1.50 to 8.16] and 3.37 [95%CI 1.46 to 9.57], respectively). Other observational studies have reported higher rates of coronary heart disease or cardiovascular events in people with SCH vs. euthyroid status [33, 115, 116, 125, 126], including in populations with pre-existing coronary disease [127] or heart failure [128]. A large meta-analysis (35 studies) found a significant association between SCH and adverse cardiovascular outcomes in younger patients (< 65 years) and those with elevated cardiovascular risk [129]. Additional meta-analyses reported similar findings, with larger risks of coronary heart disease and associated mortality in people with SCH and TSH > 10 mIU/L [130, 131]. Higher FT4 and lower TSH, consistent with hyperthyroidism, were associated with increased risk of cardiovascular mortality in a large observational study [132], which may emphasise the importance of not over titrating LT4, thereby inducing a state of iatrogenic hyperthyroidism.
An observational study associated SCH with cerebrovascular disease [133], although a meta-analysis did not confirm this [134]. Finally, a Mendelian randomisation study significantly associated genetically predicted levels of FT4 with increased risk of coronary heart disease and stroke [14].
Impact of LT4
A large database study from Scandinavia reported that both under- and over-treatment with LT4 were associated with increased risk of coronary heart disease, suggesting that appropriate TSH-guided management of thyroid homeostasis is more appropriate than prescribing LT4 per se [115, 116]. Long-term treatment of patients with autoimmune Hashimoto's thyroiditis with LT4 (> 1 year, vs. no treatment) reduced the risk of new coronary heart disease to the level seen in subjects without this condition [117]. In another study, treatment of 60 people with OH with LT4 for 12 weeks reduced the mean Framingham risk score, consistent with a reduction in the 10-year risk of adverse coronary outcomes [48]. Other observational studies did not report a significant effect of LT4 on cardiovascular outcomes in overall populations with SCH [118] or SCH with comorbid cardiovascular disease [119]. A subgroup analysis of one of these studies [118] and another study based on UK primary care data [120] reported a reduced risk of all-cause mortality and ischaemic heart disease events, respectively, in subjects with SCH aged < 65 years, with no significant effect in older subjects in either study. A large meta-analysis of studies in populations with SCH (7 studies, N = 21,055) reported that LT4 reduced all-cause and cardiovascular mortality only in patients aged < 65 years, with no significant effect in older patients or in the overall population [121].
The tendency for LT4 to be associated with reduced risk of adverse cardiovascular or mortality outcomes in younger subjects is intriguing. TSH levels tend to increase naturally with age without a corresponding increase in FT4, which has prompted speculation that SCH, in particular, may be over diagnosed in the elderly population [87]. Accordingly, it is possible that older populations with a diagnosis of hypothyroidism (especially SCH) will be enriched with subjects whose diagnosis of hypothyroidism may be open to question.
Hypothyroidism and clinical cardiovascular outcomes: summary of clinical implications.
We still await demonstration of improved clinical cardiovascular outcomes associated with thyroid hormone replacement with LT4 in a randomised, controlled trial. Real world evidence suggests such a benefit in younger people with SCH. Conversely, TSH-guided treatment of hypothyroidism with LT4 appears safe from a cardiovascular perspective, with no evidence of an increased risk of adverse cardiovascular outcomes.
T3 treatment and cardiovascular risk factors
The sections of this review that have described the effects of thyroid hormone replacement on cardiometabolic risk factors and outcomes have focused on evaluations of LT4, as LT4 monotherapy is the guideline-driven standard of care for the management of hypothyroidism at this time [92, 135, 136]. The use of T3 within the personalised management of patients with OH appears to be common in real-life clinical practice [136], despite a lack of support for this approach in current guidelines for the management of hypothyroidism [135, 136].
LT4 acts as a prohormone for T3, which is the physiologically active thyroid hormone [137]. Observations of persistent hypothyroid-like symptoms in a substantial minority of people with hypothyroidism and well controlled TSH on LT4 monotherapy have stimulated interest in the therapeutic use of LT4 and T3 in combination, which could potentially better reflect the physiological situation. Clinical trials in this area in the early part of the current century showed little or no benefit for the LT4 + T3 combination vs. LT4 monotherapy [136]. However, subsequent discussion of design limitations of these trials has prompted a new generation of trials in subjects more likely to benefit compared with the relatively unselected hypothyroid populations recruited by the earlier trials, e.g. due to low T3 or the presence of polymorphisms in deiodinase enzymes that might suggest reduced availability of T3 at the level of target tissues for thyroid hormones [136]. A brief account of the effects of T3 on cardiometabolic risk factors and outcomes, focussing on more recent studies, will be included here for completeness.
A recent (2022) randomised trial found that T3 monotherapy improved quality of life measured using the validated, disease-specific ThyPro instrument, in women with residual hypothyroid-like symptoms on LT4 monotherapy or LT4 + T3, although there was no difference between groups for BP (average BP was not elevated in this population at baseline) [138]. Another randomised trial (2016) reported no difference between the effects of LT4 + T3 and T3 alone on BP or lipids [139].
With regard to cardiovascular function, a recent (2022) meta-analysis that analysed the effects of T3 treatment in subjects with low T3 reported an increase in cardiac index in adults undergoing cardiac surgery (high quality evidence), but no benefit in adults with heart failure or after myocardial infarction (low quality evidence) [140]. An acute increase in endothelial function was seen in an experimental study in human subjects [141]. Improved diastolic cardiac function was observed in a population without cardiovascular risk factors [142], and improved cardiac function has been seen in T3-treated patients with heart failure [143–145].
Currently, therefore, evidence for an additional effect of T3 on cardiometabolic parameters in people with hypothyroidism already treated with LT4 is scarce. This is perhaps unsurprising, as it is known already that intervention with LT4 improves risk factors such as dyslipidaemia and hypertension, and recent trials in this area have focussed more on the possibility of improving residual symptoms such as fatigue with additional T3. The suggestions of improved cardiac function with T3, described above, are intriguing. Further research is needed in populations with low T3 syndrome (a state of low T3 sometimes associated with cardiac conditions or surgery), which is associated with a poor clinical prognosis [146].
Importantly, adding T3 to LT4 therapy appears to be safe from a cardiometabolic perspective in people with hypothyroidism: there seems to be little evidence for an increased risk of the adverse cardiovascular sequelae of iatrogenic thyrotoxicosis, as long as routine measure to avoid over treatment with thyroid hormones are respected [147]. More recent evaluations of LT4 + T3 combination therapy have tended to use a lower ratio of LT4:T3 (about 14:1 or higher), compared with earlier studies (as high as about 1:5), which may mimic the euthyroid state more accurately and therefore maintain tolerability [147].
Summary and conclusions
The studies summarised above show clearly that hypothyroidism (especially OH but also SCH), leads to adverse changes in cardiometabolic risk factors, such as dyslipidaemia, hypertension, obesity and hyperglycaemia. Effects on these risk factors may interact to exacerbate the situation further: for example, obesity worsens insulin resistance, especially in the setting of ectopic fat deposition in the liver, heart and muscle, which in turn tend to exacerbate sub-optimal blood glucose control, hypertension and hypertriglyceridaemia, among other effects (Fig. 1). Hypothyroidism is also associated with an increased likelihood of increased systemic vascular resistance, impaired diastolic dysfunction, decreased systolic function, and reduced preload. Damage to cardiovascular tissues caused by these adverse changes accumulates over time and leads to atherosclerosis, endothelial dysfunction, left ventricular hypertrophy and − ultimately − morbid cardiovascular events and death (Fig. 2). In addition, decreases in systolic and diastolic function in people with hypothyroidism contribute to impaired quality of life during exercise and even rest, contributing significantly to impaired quality of life and adding to the burden of disease [148].
The clinical evidence base suggests that TSH-optimised thyroid hormone replacement with LT4 may improve some cardiometabolic disease risk factors, particularly diastolic hypertension and dyslipidaemia. Real world evidence suggests that these benefits feed through to reduced manifestations of vascular and cardiac dysfunction, with suggestions of a reduced risk of adverse clinical cardiometabolic outcomes in younger people in SCH (Fig. 1). Randomised studies are needed to confirm this potential benefit of LT4 (and to provide further confirmation of the apparent good cardiovascular safety of this treatment) and to clarify the metabolic mechanisms by which alterations in thyroid homeostasis may influence the level of cardiometabolic risk in people with hypothyroidism.
Acknowledgements
A medical writer (Dr Mike Gwilt, GT Communications) provided editorial assistance funded by Merck Healthcare KGaA, Darmstadt, Germany.
Abbreviations
- DBP
Diastolic blood pressure
- BP
Blood pressure(s)
- SBP
Systolic blood pressure
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- VLDL-C
Very low-density lipoprotein cholesterol
- MAFLD
Metabolic-dysfunction-associated fatty liver disease
- HMG-CoA
3-Hydroxy-3-methylglutaryl coenzyme A
- ApoB
Apolipoprotein B
- BP
Blood pressure
- SCH
Subclinical hypothyroidism
- OH
Overt hypothyroidism
- OR
Odds ratio
- LT4
Levothyroxine
- T4
Thyroxine
- FT4
Free thyroxine
- T3
Triiodothyronine
- TSH
Thyrotropin (thyroid stimulating hormone)
- BMI
Body mass index
- cIMT
Carotid intima-media thickness
Authors' contributions
G.J.K. led the development of the article text. G.J.K., Y.L. and L.P. contributed to and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Merck Healthcare KGaA, Darmstadt, Germany, funded editorial assistance (see below) and the article processing charge; no other funding applied.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
GJK’s institution, The Johannes Gutenberg University (JGU) Medical Center, received research-associated funding from Merck Healthcare KGaA, Darmstadt, Germany. LP received fees for seminars/congresses from Merck and Sandoz. YL declared no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chiovato L, Magri F, Carlé A. Hypothyroidism in context: where we’ve been and where we’re going. Adv Ther. 2019;36(Suppl 2):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301–16. [DOI] [PubMed] [Google Scholar]
- 3.Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: a review. JAMA. 2019;322:153–60. [DOI] [PubMed] [Google Scholar]
- 4.McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study. Thyroid. 2011;21:837–43. [DOI] [PubMed] [Google Scholar]
- 5.Razvi S, Jabbar A, Pingitore A, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. 2018;71(16):1781–96. [DOI] [PubMed] [Google Scholar]
- 6.Zúñiga D, Balasubramanian S, Mehmood KT, Al-Baldawi S, Zúñiga SG. Hypothyroidism and cardiovascular disease: a review. Cureus. 2024;16(1): e52512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh JP, Bremner AP, Bulsara MK, et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med. 2005;165:2467–72. [DOI] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80:2361–71. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Cardiovascular Diseases. Available at https://www.who.int/health-topics/cardiovascular-disease. Accessed Sept 2024.
- 10.Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab. 2010;95:1734–40. [DOI] [PubMed] [Google Scholar]
- 11.Iervasi G, Molinaro S, Landi P, et al. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167:1526–32. [DOI] [PubMed] [Google Scholar]
- 12.Saito I, Ito K, Saruta T. Hypothyroidism as a cause of hypertension. Hypertension. 1983;5:112–5. [DOI] [PubMed] [Google Scholar]
- 13.Piantanida E, Gallo D, Veronesi G, et al. Masked hypertension in newly diagnosed hypothyroidism: a pilot study. J Endocrinol Invest. 2016;39:1131–8. [DOI] [PubMed] [Google Scholar]
- 14.Wang JJ, Zhuang ZH, Yu CQ, et al. Assessment of causal direction between thyroid function and cardiometabolic health: a Mendelian randomization study. J Geriatr Cardiol. 2022;19:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saltiki K, Voidonikola P, Stamatelopoulos K, Mantzou E, Papamichael C, Alevizaki M. Association of thyroid function with arterial pressure in normotensive and hypertensive euthyroid individuals: A cross-sectional study. Thyroid Res. 2008;1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal A, Figenschau Y, Jorde R. Blood pressure in relation to serum thyrotropin: The Tromsø study. J Hum Hypertens. 2006;20:932–6. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Jiang F, Shan Z, et al. A cross-sectional survey of relationship between serum TSH level and blood pressure. J Hum Hypertens. 2010;24:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Prasitlumkum N, Randhawa S, Banerjee D. Association between subclinical hypothyroidism and incident hypertension in women: a systematic review and meta-analysis. J Clin Med. 2021;10: 3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Y, Xie H, Zeng Y, Zhao X, Tian Z, Zhang S. Association between subclinical hypothyroidism and blood pressure–a meta-analysis of observational studies. Endocr Pract. 2014;20:150–8. [DOI] [PubMed] [Google Scholar]
- 20.Ding X, Zhao Y, Zhu CY, et al. The association between subclinical hypothyroidism and metabolic syndrome: an update meta-analysis of observational studies. Endocr J. 2021;68:1043–56. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira P de F, Reuters VS, Ferreira MM, et al. Lipid profile in different degrees of hypothyroidism and effects of levothyroxine replacement in mild thyroid failure. Transl Res. 2008;151:224–31. [DOI] [PubMed]
- 22.Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khazan M, Amouzegar A, Gharibzadeh S, Mehran L, Tohidi M, Azizi F. Prevalence of hypothyroidism in patients with dyslipidemia: Tehran Thyroid Study (TTS). Horm Metab Res. 2014;46:980–4. [DOI] [PubMed] [Google Scholar]
- 24.Pergialiotis V, Konstantopoulos P, Prodromidou A, Florou V, Papantoniou N, Perrea DN. Management of endocrine disease: The impact of subclinical hypothyroidism on anthropometric characteristics, lipid, glucose and hormonal profile of PCOS patients: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R159–66. [DOI] [PubMed] [Google Scholar]
- 25.Liu XL, He S, Zhang SF, et al. Alteration of lipid profile in subclinical hypothyroidism: a meta-analysis. Med Sci Monit. 2014;20:1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treister-Goltzman Y, Yarza S, Peleg R. Lipid profile in mild subclinical hypothyroidism: systematic review and meta-analysis. Minerva Endocrinol (Torino). 2021;46:428–40. [DOI] [PubMed] [Google Scholar]
- 27.Xing Y, Chen J, Liu J, Ma H. The impact of subclinical hypothyroidism on patients with polycystic ovary syndrome: a meta-analysis. Horm Metab Res. 2021;53:382–90. [DOI] [PubMed] [Google Scholar]
- 28.de Medeiros SF, de Medeiros MAS, Ormond CM, Barbosa JS, Yamamoto MMW. Subclinical hypothyroidism impact on the characteristics of patients with polycystic ovary syndrome. a meta-analysis of observational studies. Gynecol Obstet Invest. 2018;83:105–15. [DOI] [PubMed] [Google Scholar]
- 29.Maratou E, Hadjidakis DJ, Kollias A, et al. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol. 2009;160:785–90. [DOI] [PubMed] [Google Scholar]
- 30.Cicatiello AG, Di Girolamo D, Dentice M. Metabolic effects of the intracellular regulation of thyroid hormone: old players, new concepts. Front Endocrinol (Lausanne). 2018;9:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaker L, Ligthart S, Korevaar TI, et al. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC Med. 2016;14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SW, Jeon JH, Moon JS, et al. Low-normal free thyroxine levels in euthyroid male are associated with prediabetes. Diabetes Metab J. 2019;43:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandt F, Thvilum M, Almind D, et al. Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register-based study. PLoS ONE. 2013;8: e66711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vemula SL, Aramadaka S, Mannam R, et al. The impact of hypothyroidism on diabetes mellitus and its complications: A Comprehensive Review. Cureus. 2023;15: e40447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Yi M, Deng X, Li W, Chen Y, Zhang X. Evaluation of the thyroid characteristics and correlated factors in hospitalized patients with newly diagnosed type 2 diabetes. Diabetes Metab Syndr Obes. 2022;15:873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal S, Makuch S, Dróżdż M, et al. The impact of hypoglycemia on patients with diabetes mellitus: a cross-sectional analysis. J Clin Med. 2022;11: 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eftekharzadeh A, Khamseh ME, Farshchi A, Malek M. The Association Between Subclinical Hypothyroidism and metabolic syndrome as defined by the ATP III Criteria. Metab Syndr Relat Disord. 2016;14:137–44. [DOI] [PubMed] [Google Scholar]
- 38.He J, Lai Y, Yang J, et al. The relationship between thyroid function and metabolic syndrome and its components: a cross-sectional study in a Chinese Population. Front Endocrinol (Lausanne). 2021;12: 661160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang CH, Yeh YC, Caffrey JL, Shih SR, Chuang LM, Tu YK. Metabolic syndrome is associated with an increased incidence of subclinical hypothyroidism - A Cohort Study. Sci Rep. 2017;7:6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Lv X, Yue F, Wei D, Liu W, Zhang T. Subclinical hypothyroidism and the risk of metabolic syndrome: A meta-analysis of observational studies. Endocr Res. 2016;41:158–65. [DOI] [PubMed] [Google Scholar]
- 41.Iwen KA, Schröder E, Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. 2013;2:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ríos-Prego M, Anibarro L, Sánchez-Sobrino P. Relationship between thyroid dysfunction and body weight: a not so evident paradigm. Int J Gen Med. 2019;12:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Lin H, Li Q, et al. Association between different obesity phenotypes and hypothyroidism: a study based on a longitudinal health management cohort. Endocrine. 2021;72:688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahdavi M, Amouzegar A, Mehran L, Madreseh E, Tohidi M, Azizi F. Investigating the prevalence of primary thyroid dysfunction in obese and overweight individuals: Tehran thyroid study. BMC Endocr Disord. 2021;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jabbar A, Razvi S. Thyroid disease and vascular risk. Clin Med (Lond). 2014;14(Suppl 6):s29-32. [DOI] [PubMed] [Google Scholar]
- 46.Cai P, Peng Y, Chen Y, Wang Y, Wang X. Blood pressure characteristics of subclinical hypothyroidism: an observation study combined with office blood pressure and 24-h ambulatory blood pressure. J Hypertens. 2021;39:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ettleson MD, Penna GCE, Wan W, Benseñor IM, Laiteerapong N, Bianco AC. TSH trajectories during levothyroxine treatment in the Brazilian longitudinal study of adult health (ELSA-Brasil) cohort. J Clin Endocrinol Metab. 2024 (advance publication online, 10.1210/clinem/dgae294). [DOI] [PMC free article] [PubMed]
- 48.Maiti R, Mohanty RR, Mishra A, Dey A, Verma N. Levothyroxine therapy and predictors of cardiovascular risk in clinical hypothyroidism: a prospective cohort study. Cureus. 2022;14: e30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gluvic Z, Sudar E, Tica J, et al. Effects of levothyroxine replacement therapy on parameters of metabolic syndrome and atherosclerosis in hypothyroid patients: a prospective pilot study. Int J Endocrinol. 2015;2015: 147070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fommei E, Iervasi G. The role of thyroid hormone in blood pressure homeostasis: evidence from short-term hypothyroidism in humans. J Clin Endocrinol Metab. 2002;87:1996–2000. [DOI] [PubMed] [Google Scholar]
- 51.Darouei B, Amani-Beni R, Abhari AP, Fakhrolmobasheri M, Shafie D, Heidarpour M. Systematic review and meta-analysis of levothyroxine effect on blood pressure in patients with subclinical hypothyroidism. Curr Probl Cardiol. 2024;49: 102204. [DOI] [PubMed] [Google Scholar]
- 52.He W, Li S, Zhang JA, Zhang J, Mu K, Li XM. Effect of levothyroxine on blood pressure in patients with subclinical hypothyroidism: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2018;9:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao C, Wang Y, Xiao L, Li L. Effect of levothyroxine on older patients with subclinical hypothyroidism: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022;13: 913749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su X, Peng H, Chen X, Wu X, Xijie W, Wang B. Hyperlipidemia and hypothyroidism. Clin Chim Acta. 2022;27:61–70. [DOI] [PubMed] [Google Scholar]
- 55.Kahaly GJ. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid. 2000;10:665–79. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Peng D. Update on dyslipidemia in hypothyroidism: the mechanism of dyslipidemia in hypothyroidism. Endocr Connect. 2022;11: e210002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mavromati M, Jornayvaz FR. Hypothyroidism-associated dyslipidemia: potential molecular mechanisms leading to NAFLD. Int J Mol Sci. 2021;22: 12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J. 2011;5:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu P, Liu R, Chen X, et al. Can levothyroxine treatment reduce urinary albumin excretion rate in patients with early type 2 diabetic nephropathy and subclinical hypothyroidism? A randomized double-blind and placebo-controlled study. Curr Med Res Opin. 2015;31:2233–40. [DOI] [PubMed] [Google Scholar]
- 60.Monzani F, Caraccio N, Kozàkowà M, et al. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo- controlled study. J Clin Endocrinol Metab. 2004;89:2099–106. [DOI] [PubMed] [Google Scholar]
- 61.Zhao M, Liu L, Wang F, et al. A Worthy Finding: Decrease in total cholesterol and low-density lipoprotein cholesterol in treated mild subclinical hypothyroidism. Thyroid. 2016;26:1019–29. [DOI] [PubMed] [Google Scholar]
- 62.Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:1715–23. [DOI] [PubMed] [Google Scholar]
- 63.Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002;87:1533–8. [DOI] [PubMed] [Google Scholar]
- 64.Ostadrahimi A, Manzari T, Gohari-Lasaki S, et al. Effects of levothyroxine replacement therapy on insulin resistance in patients with untreated primary hypothyroidism. BMC Res Notes. 2023;16:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deyneli O, Akpınar IN, Meriçliler OS, Gözü H, Yıldız ME, Akalın NS. Effects of levothyroxine treatment on insulin sensitivity, endothelial function and risk factors of atherosclerosis in hypothyroid women. Ann Endocrinol (Paris). 2014;75:220–6. [DOI] [PubMed] [Google Scholar]
- 66.Velija-Asimi Z, Karamehic J. The effects of treatment of subclinical hypothyroidism on metabolic control and hyperinsulinemia. Med Arh. 2007;61:20–1. [PubMed] [Google Scholar]
- 67.Kowalska I, Borawski J, Nikołajuk A, et al. Insulin sensitivity, plasma adiponectin and sICAM-1 concentrations in patients with subclinical hypothyroidism: response to levothyroxine therapy. Endocrine. 2011;40:95–101. [DOI] [PubMed] [Google Scholar]
- 68.Ersoy I, Banu KK, Bagci O, et al. Effects of levothyroxine treatment on cardiovascular risk profile and carotid intima media thickness in patients with subclinical hypothyroidism. Acta Endocrinologica-Bucharest. 2012;8:433–42. [Google Scholar]
- 69.Kotwal A, Cortes T, Genere N, et al. Treatment of thyroid dysfunction and serum lipids: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105:dgaa672. [DOI] [PubMed] [Google Scholar]
- 70.Kim KS, Hong S, Han K, Park CY. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study. BMJ. 2024;384: e076388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sangro P, de la Torre AM, Sangro B, D’Avola D. Metabolic dysfunction-associated fatty liver disease (MAFLD): an update of the recent advances in pharmacological treatment. J Physiol Biochem. 2023;79:869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuchay MS, Isaacs S, Misra A. Intrahepatic hypothyroidism in MASLD: Role of liver-specific thyromimetics including resmetirom. Diabetes Metab Syndr. 2024;18: 103034. [DOI] [PubMed] [Google Scholar]
- 73.Yazıcı D, Özben B, Toprak A, et al. Effects of restoration of the euthyroid state on epicardial adipose tissue and carotid intima media thickness in subclinical hypothyroid patients. Endocrine. 2015;48:909–15. [DOI] [PubMed] [Google Scholar]
- 74.Cappellani D, Macchia E, Falorni A, Marchetti P. Insulin autoimmune syndrome (Hirata Disease): a comprehensive review fifty years after its first description. Diabetes Metab Syndr Obes. 2020;13:963–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Censi S, Mian C, Betterle C. Insulin autoimmune syndrome: from diagnosis to clinical management. Ann Transl Med. 2018;6:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alwan H, Villoz F, Feller M, et al. Subclinical thyroid dysfunction and incident diabetes: a systematic review and an individual participant data analysis of prospective cohort studies. Eur J Endocrinol. 2022;187:S35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han C, He X, Xia X, et al. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PLoS ONE. 2015;10: e0135233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roa Dueñas OH, Van der Burgh AC, Ittermann T, et al. Thyroid function and the risk of prediabetes and type 2 diabetes. J Clin Endocrinol Metab. 2022;107:1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu J, Yue S, Geng J, et al. Relationship between diabetic retinopathy and subclinical hypothyroidism: a meta-analysis. Sci Rep. 2015;5: 12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stanicka S, Vondra K, Pelikanova T, Vlcek P, Hill M, Zamrazil V. Insulin sensitivity and counter-regulatory hormones in hypothyroidism and during thyroid hormone replacement therapy. Clin Chem Lab Med. 2005;43:715–20. [DOI] [PubMed] [Google Scholar]
- 81.Roth J, Müller N, Kuniss N, Wolf G, Müller UA, et al. Association between glycaemic control and the intake of thiazide diuretics, beta blockers and levothyroxine in people without diabetes. Exp Clin Endocrinol Diabetes. 2019;129:443–8. [DOI] [PubMed] [Google Scholar]
- 82.Manshahia PK, Nahar S, Kanda S, et al. Systematic review to gauge the effect of levothyroxine substitution on progression of diabetic nephropathy in patients with hypothyroidism and type 2 diabetes Mellitus. Cureus. 2023;15: e44729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fahed G, Aoun L, Bou Zerdan M, et al. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int J Mol Sci. 2022;23: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guembe MJ, Fernandez-Lazaro CI, Sayon-Orea C, Toledo E, Moreno-Iribas C; RIVANA Study Investigators. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovasc Diabetol. 2020;19:195. [DOI] [PMC free article] [PubMed]
- 85.Garcia BF, da Veiga GL, Alves BCA, Gehrke FS, Fonseca FLA. Metabolic effects caused by sodium levothyroxine in subclinical hypothyroidism patients with low HDL. Obes Med. 2019;13:26–8. [Google Scholar]
- 86.An JH, Song KH, Kim DL, Kim SK. Effects of thyroid hormone withdrawal on metabolic and cardiovascular parameters during radioactive iodine therapy in differentiated thyroid cancer. J Int Med Res. 2017;45:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Razvi S, Bhana S, Mrabeti S. Challenges in interpreting thyroid stimulating hormone results in the diagnosis of thyroid dysfunction. J Thyroid Res. 2019;2019:4106816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.American Thyroid Association. Thyroid and weight. Available at https://www.thyroid.org/thyroid-and-weight/. Accessed Sept 2024.
- 90.Lee SY, Braverman LE, Pearce EN. Changes in body weight after treatment of primary hypothyroidism with levothyroxine. Endocr Pract. 2014;20:1122–8. [DOI] [PubMed] [Google Scholar]
- 91.Medici BR, Nygaard B, la Cour JL, et al. Effects of levothyroxine substitution therapy on hunger and food intake in individuals with hypothyroidism. Endocr Connect. 2023;12: e230314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sattar N, Neeland IJ, McGuire DK. Obesity and cardiovascular disease: a new dawn. Circulation. 2024;149:1621–3. [DOI] [PubMed] [Google Scholar]
- 94.Niknam N, Khalili N, Khosravi E, Nourbakhsh M. Endothelial dysfunction in patients with subclinical hypothyroidism and the effects of treatment with levothyroxine. Adv Biomed Res. 2016;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gong N, Gao C, Chen X, Fang Y, Tian L. Endothelial function in patients with subclinical hypothyroidism: a meta-analysis. Horm Metab Res. 2019;51:691–702. [DOI] [PubMed] [Google Scholar]
- 96.Yao K, Zhao T, Zeng L, et al. Non-invasive markers of cardiovascular risk in patients with subclinical hypothyroidism: a systematic review and meta-analysis of 27 case control studies. Sci Rep. 2018;8:4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kannan L, Shaw PA, Morley MP, et al. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart Fail. 2018;11: e005266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen X, Zhang N, Cai Y, Shi J. Evaluation of left ventricular diastolic function using tissue Doppler echocardiography and conventional doppler echocardiography in patients with subclinical hypothyroidism aged <60 years: a meta-analysis. J Cardiol. 2013;61:8–15. [DOI] [PubMed] [Google Scholar]
- 99.Nakova VV, Krstevska B, Kostovska ES, Vaskova O, Ismail LG. The effect of levothyroxine treatment on left ventricular function in subclinical hypothyroidism. Arch Endocrinol Metab. 2018;62:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao Z, Gao X, Liu M, et al. Diffuse myocardial injuries are present in subclinical hypothyroidism: a clinical study using myocardial T1-mapping quantification. Sci Rep. 2018;8:4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao T, Chen B, Zhou Y, et al. Effect of levothyroxine on the progression of carotid intima-media thickness in subclinical hypothyroidism patients: a meta-analysis. BMJ Open. 2017;7: e016053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis. 2013;227:18–25. [DOI] [PubMed] [Google Scholar]
- 103.Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of Thyroxin Treatment on Carotid Intima-Media Thickness (CIMT) Reduction in Patients with Subclinical Hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Franzoni F, Galetta F, Fallahi P, et al. Effect of L-thyroxine treatment on left ventricular function in subclinical hypothyroidism. Biomed Pharmacother. 2006;60:431–6. [DOI] [PubMed] [Google Scholar]
- 105.Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab. 2001;86:1110–5. [DOI] [PubMed] [Google Scholar]
- 106.Lu X, Huang J, Zhang X, et al. Effects of thyroxine on cardiac function and lymphocyte beta-adrenoceptors in patients with chronic congestive heart failure. Chin Med J (Engl). 2003;116:1697–700. [PubMed] [Google Scholar]
- 107.Moruzzi P, Doria E, Agostoni PG. Medium-term effectiveness of L-thyroxine treatment in idiopathic dilated cardiomyopathy. Am J Med. 1996;101:461–7. [DOI] [PubMed] [Google Scholar]
- 108.Moruzzi P, Doria E, Agostoni PG, et al. Usefulness of L-thyroxine to improve cardiac and exercise performance in idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;73:374–8. [DOI] [PubMed] [Google Scholar]
- 109.Gencer, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020 (advance publication online, 10.1016/j.amjmed.2020.01.018). [DOI] [PubMed]
- 110.Ilic S, Tadic M, Ivanovic B, Caparevic Z, Trbojevic B, Celic V. Left and right ventricular structure and function in subclinical hypothyroidism: the effects of one-year levothyroxine treatment. Med Sci Monit. 2013;19:960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Madathil A, Hollingsworth KG, Blamire AM, et al. Levothyroxine improves abnormal cardiac bioenergetics in subclinical hypothyroidism: a cardiac magnetic resonance spectroscopic study. J Clin Endocrinol Metab. 2015;100:E607–10. [DOI] [PubMed] [Google Scholar]
- 112.Haloot J, Mahmoud M, Kabbani M, et al. Cardiovascular outcomes of levothyroxine supplementation in patients with heart failure with preserved ejection fraction. J Cardiac Fail. 2023;29:657. [Google Scholar]
- 113.Gavrila A, American Thyroid Association. Clinical thyroidology for the public. Hypothyroidism. Levothyroxine treatment increases mortality in patients with heart failure. Available at https://www.thyroid.org/patient-thyroid-information/ct-for-patients/may-2019/vol-12-issue-5-p-9-10/. Accessed Sept 2024.
- 114.Wang X, Wang H, Li Q, et al. Effect of levothyroxine supplementation on the cardiac morphology and function in patients with subclinical hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107:2674–83. [DOI] [PubMed] [Google Scholar]
- 115.Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Duration of over- and under-treatment of hypothyroidism is associated with increased cardiovascular risk. Eur J Endocrinol. 2019;180:407–16. [DOI] [PubMed] [Google Scholar]
- 116.Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Duration of hyperthyroidism and lack of sufficient treatment are associated with increased cardiovascular risk. Thyroid. 2019;29:332–40. [DOI] [PubMed] [Google Scholar]
- 117.Chen WH, Chen YK, Lin CL, Yeh JH, Kao CH. Hashimoto’s thyroiditis, risk of coronary heart disease, and L-thyroxine treatment: a nationwide cohort study. J Clin Endocrinol Metab. 2015;100:109–14. [DOI] [PubMed] [Google Scholar]
- 118.Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10: e0129793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andersen MN, Olsen AS, Madsen JC, et al. Long-term outcome in levothyroxine treated patients with subclinical hypothyroidism and concomitant heart disease. J Clin Endocrinol Metab. 2016;101:4170–7. [DOI] [PubMed] [Google Scholar]
- 120.Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811–7. [DOI] [PubMed] [Google Scholar]
- 121.Peng CC, Huang HK, Wu BB, Chang RH, Tu YK, Munir KM. Association of thyroid hormone therapy with mortality in subclinical hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2021;106:292–303. [DOI] [PubMed] [Google Scholar]
- 122.Paschou SA, Bletsa E, Stampouloglou PK, et al. Thyroid disorders and cardiovascular manifestations: an update. Endocrine. 2022;75:672–83. [DOI] [PubMed] [Google Scholar]
- 123.Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988–97. [DOI] [PubMed] [Google Scholar]
- 124.Moon S, Kong SH, Choi HS, et al. Relation of subclinical hypothyroidism is associated with cardiovascular events and all-cause mortality in adults with high cardiovascular risk. Am J Cardiol. 2018;122:571–7. [DOI] [PubMed] [Google Scholar]
- 125.Fang H, Zhao R, Cui S, Wan W. Sex differences in major cardiovascular outcomes and fractures in patients with subclinical thyroid dysfunction: a systematic review and meta-analysis. Aging (Albany NY). 2022 (advance publication online, 2022;14:10.18632/aging.204352). [DOI] [PMC free article] [PubMed]
- 126.Kim TH, Choi HS, Bae JC, et al. Subclinical hypothyroidism in addition to common risk scores for prediction of cardiovascular disease: a 10-year community-based cohort study. Eur J Endocrinol. 2014;171:649–57. [DOI] [PubMed] [Google Scholar]
- 127.Lee Y, Lim YH, Shin JH, Park J, Shin J. Impact of subclinical hypothyroidism on clinical outcomes following percutaneous coronary intervention. Int J Cardiol. 2018;253:155–60. [DOI] [PubMed] [Google Scholar]
- 128.Sato Y, Yoshihisa A, Kimishima Y, et al. Subclinical hypothyroidism is associated with adverse prognosis in heart failure patients. Can J Cardiol. 2018;34:80–7. [DOI] [PubMed] [Google Scholar]
- 129.Moon S, Kim MJ, Yu JM, Yoo HJ, Park YJ. Subclinical hypothyroidism and the risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Thyroid. 2018;28:1101–10. [DOI] [PubMed] [Google Scholar]
- 130.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ning Y, Cheng YJ, Liu LJ, et al. What is the association of hypothyroidism with risks of cardiovascular events and mortality? A meta-analysis of 55 cohort studies involving 1,898,314 participants. BMC Med. 2017;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bano A, Chaker L, Mattace-Raso FUS, et al. Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: The Rotterdam Study. Circ Res. 2017;121:1392–400. [DOI] [PubMed] [Google Scholar]
- 133.Zhang X, Xie Y, Ding C, et al. Subclinical hypothyroidism and risk of cerebral small vessel disease: A hospital-based observational study. Clin Endocrinol (Oxf). 2017;87:581–6. [DOI] [PubMed] [Google Scholar]
- 134.Chaker L, Baumgartner C, Ikram MA, et al. Subclinical thyroid dysfunction and the risk of stroke: a systematic review and meta-analysis. Eur J Epidemiol. 2014;29:791–800. [DOI] [PubMed] [Google Scholar]
- 135.Razvi S. Cardiac Safety and Efficacy of Combination Levothyroxine and L-Triiodothyronine in Patients with Athyreotic Hypothyroidism: Reassuring Initial Data but More Is Needed. Thyroid. 2023;33:1393–4. [DOI] [PubMed] [Google Scholar]
- 136.Gottwald-Hostalek U, Kahaly GJ. Triiodothyronine alongside levothyroxine in the management of hypothyroidism? Curr Med Res Opin. 2021;37:2099–106. [DOI] [PubMed] [Google Scholar]
- 137.Shahid MA, Ashraf MA, Sharma S. Physiology, thyroid hormone. StatPearls. Available at https://www.ncbi.nlm.nih.gov/books/NBK500006/. Accessed Nov 2024. [PubMed]
- 138.Bjerkreim BA, Hammerstad SS, Gulseth HL, et al. Effect of liothyronine treatment on quality of life in female hypothyroid patients with residual symptoms on levothyroxine therapy: a randomized crossover study. Front Endocrinol (Lausanne). 2022;13: 816566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaminski J, Miasaki FY, Paz-Filho G, Graf H, Carvalho GA. Treatment of hypothyroidism with levothyroxine plus liothyronine: a randomized, double-blind, crossover study. Arch Endocrinol Metab. 2016;60:562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tharmapoopathy M, Thavarajah A, Kenny RPW, et al. Efficacy and safety of triiodothyronine treatment in cardiac surgery or cardiovascular diseases: a systematic review and meta-analysis of randomized controlled trials. Thyroid. 2022;32:879–96. [DOI] [PubMed] [Google Scholar]
- 141.Napoli R, Guardasole V, Angelini V, et al. Acute effects of triiodothyronine on endothelial function in human subjects. J Clin Endocrinol Metab. 2007;92:250–4. [DOI] [PubMed] [Google Scholar]
- 142.Biondi B, Pucci M, Pontieri G, Formisano P, Esposito R. Preliminary results of a double-blind randomized controlled trial evaluating the cardiometabolic effects of levothyroxine and liothyronine compared to levothyroxine with placebo in athyreotic low-risk thyroid cancer patients. Thyroid. 2023;33:1402–13. [DOI] [PubMed] [Google Scholar]
- 143.Pingitore A, Iervasi G. Triiodothyronine (T3) effects on cardiovascular system in patients with heart failure. Recent Pat Cardiovasc Drug Discov. 2008;3:19–27. [DOI] [PubMed] [Google Scholar]
- 144.Hamilton MA, Stevenson LW, Fonarow GC, et al. Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. Am J Cardiol. 1998;81:443–7. [DOI] [PubMed] [Google Scholar]
- 145.Holmager P, Schmidt U, Mark P, et al. Long-term L-Triiodothyronine (T3) treatment in stable systolic heart failure patients: a randomised, double-blind, cross-over, placebo-controlled intervention study. Clin Endocrinol (Oxf). 2015;83:931–7. [DOI] [PubMed] [Google Scholar]
- 146.Zhao Y, Wang W, Zhang K, Tang YD. Association between low T3 syndrome and poor prognosis in adult patients with acute myocarditis. Front Endocrinol (Lausanne). 2021;12: 571765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gottwald-Hostalek U, Tayrouz Y. A review of the safety of triiodothyronine in combination with levothyroxine for the management of hypothyroidism. Curr Med Res Opin. 2024;40:2109–16. [DOI] [PubMed] [Google Scholar]
- 148.von Hafe M, Neves JS, Vale C, Borges-Canha M, Leite-Moreira A. The impact of thyroid hormone dysfunction on ischemic heart disease. Endocr Connect. 2019;8:R76-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


