Abstract
Objective
Breast cancer (BC) cells exhibit mutations over time, conferring resistance to therapeutic approaches. We attempted to ascertain the efficacy of selected hormonal therapy for advanced BC.
Methods
This is a systematic review and meta-analysis of clinical trials. We searched Medline, PubMed, Cochrane Library, Web of Science, and others. Studies that investigated the effectiveness of hormonal therapy for HR positive (HR+) advanced BC were included. The outcomes were progression-free survival (PFS), overall survival (OS), and objective response rate (ORR). A random-effect meta-analysis model was employed. The study protocol was registered with the International Prospective Register of Systematic Reviews: CRD42023431939.
Results
Twenty-one studies were included in the meta-analysis with an overall sample size of 8,482. ORR and PFS between aromatase inhibitors (AIs) and other hormonal therapies: selective oestrogen receptor degrader, selective oestrogen modulator (SERM) and androgen inhibitors showed no significant difference (OR = 1.122 [0.917–1.374], p = 0.263; OR = 0.010 [0.000–1.292], p = 0.063), respectively. Subgroup analysis showed a statistically significant difference in ORR in favour of patients who received SERM compared to AI (OR = 1.362 [1.033–1.795], p = 0.028). For OS, no significant difference was observed among anastrozole, letrozole, and exemestane recepients (OR = 1.718 [0.021–139.128], p = 0.809).
Conclusion
Given the above findings, clinical decisions could be based on factors such as the line of cancer treatment, adverse events, drug dosing, and individual drug benefits. Although newer combination therapies are being adopted, the agents explored in this review are still widely used in clinical practice for HR+ BC.
Keywords: Efficacy, Breast cancer, Advanced breast cancer, Hormonal therapy, Meta-analysis
Highlights of the Study
A non-significant improvement in progression-free survival was noted in patients who received aromatase inhibitors compared to other hormonal therapies reviewed.
Patients treated with selective oestrogen modulators and selective oestrogen degraders had a higher objective response rate than patients treated with aromatase inhibitors.
No significant difference was observed between anastrozole and other aromatase inhibitors-letrozole and exemestane.
Introduction
As the most commonly diagnosed life-threatening cancer in women, breast cancer (BC) is a matter of global concern owing to its substantial mortality and morbidity rates. In 2020, there were approximately 2.3 million incident cases of BC reported globally, resulting in 685,000 deaths [1]. The projected rise of this burden is anticipated to exceed 40% by 2040 [2]. Regardless of treatment with adjuvant chemotherapy, the 5-year survival rate for metastatic BC is below 30% [3]. A broad spectrum of BC treatment options is available; nevertheless, endocrine treatment, in both adjuvant and advanced settings, is recommended for hormone receptor-positive BCs [4, 5], which comprise around 70–80% of all BCs [6]. Although treatment choices are influenced by factors such as tumour biology, disease stage is the primary determinant [7].
Hormonal therapies recommended for post-menopausal women with HR-positive (HR+) cancers in the advanced stage include aromatase inhibitors (AIs), selective oestrogen receptor modulators (SERMs), and selective oestrogen receptor degraders (SERDs). AIs such as anastrozole block oestrogen production, SERMs such as tamoxifen bind to oestrogen receptors to prevent oestrogen binding, and SERDs like fulvestrant degrade the oestrogen receptors. These hormonal therapies can also be administered with targeted drugs such as cyclin-dependent kinase 4/6 inhibitors, mTOR inhibitors, and histone deacetylase inhibitors [8]. These targeted drugs were developed to target the molecular pathways promoting endocrine therapy resistance. It is anticipated that these targeted approaches would restore sensitivity to the endocrine therapy, rather than reversing drug resistance [8]. In the context of metastasis, the recommended treatments provide early advantages for most patients, resulting in either disease stability or reduction in the tumour size. However, BC cells continue to exist and progress. Hormonal therapy will not be effective for a significant number of patients with HR+ breast malignancies, either as a result of de novo resistance or acquired resistance mechanisms [9].
Breast malignancies exhibit mutations over time, and confer resistance to therapy, ultimately advancing the disease [10]. ESR1 mutation which is the most common mechanism of resistance causes oestrogen receptor activation that is not dependent on oestrogen, resulting in resistance to AIs [11]. However, these mutations may not confer resistance to SERD and SERM as much as aromatase inhibition [10, 12]. Even in the absence of oestrogen, which AIs seek to reduce, oestrogen receptors in ESR1 mutations can still signal for cell proliferation. In contrast to AIs, which focus on oestrogen deprivation, SERM and SERD can still attach to the mutant receptor to inhibit its activity [12, 13]. Other mechanisms for resistance to therapy include; epigenetic modifications, tumour microenvironment, drug efflux mechanism, activation of growth factor signalling and PI3K/Akt pathway [9, 14–17]. These mechanisms comprise changes in the oestrogen receptors themselves, activation of alternative pathways, and DNA modifications thus conferring resistance to different hormonal therapies based on their mechanism of action [17]. Thus, it is important to compare these therapies, especially in advanced BC (ABC).
New combinations of hormonal therapy drugs have been developed as a result of this tumour resistance, increasing the choices for hormonal therapy. Due to the approval and abundance of available agents, identifying the optimal treatment can be daunting; therefore, clinicians rely on evidence-based guidelines to aid in selecting the appropriate treatment approach. While combination strategies are being implemented, it is important to explore the baseline efficacy of hormonal therapies and inform decision-making in situations where the use of combination therapies is not possible due to cost, lack of access, and patient characteristics. In this review, we attempt to ascertain the efficacy of three classes of hormonal therapy – AIs, SERM, and SERD – in treating advanced hormone receptor-positive BC.
Methods
Study Design and Registration
This is a systematic review of clinical trials with meta-analysis, comparing the effects of hormonal therapy drugs for post-menopausal women with HR+ ABC. The reporting of this study was structured using the 2020 update of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [18]. The study protocol was submitted to the International Prospective Register of Systematic Reviews (PROSPERO) for registration; registration number-CRD42023431939.
Eligibility Criteria
Inclusion Criteria
The following served as the criteria for inclusion: (1) studies that investigated the effectiveness of hormonal therapy in post-menopausal women with ABC, (2) studies investigating this effect in HR+/unknown BCs, (3) clinical trials, irrespective of the language, study location, sample size, and test statistics, (4) peer-reviewed literature.
Exclusion Criteria
The following studies were excluded: (1) studies involving mixed populations of both early and ABC, in which it is difficult to extract data solely for ABC, (2) studies involving other types of BC other than HR+ cancers, (3) non-clinical trials, (4) and studies involving non-human subjects.
Outcome Measures
Overall survival (OS), progression-free survival (PFS), time to progression, and the objective response rate (ORR) are the primary clinical endpoints. These clinical endpoints were measured as per the standard definitions [19] across the included studies. However, studies utilizing non-standard endpoint definitions were identified in the risk of bias assessment and rated to have “some concern.” Also, data from each publication were collected as secondary data, including the article ID, setting, sample size, participant demographics, treatment protocol, and results/conclusions.
OS is characterized as the time interval between randomization and death from any cause, which is measured by the intention to treat the population [19]. PFS refers to the time from randomization to the first sign of disease progression or death from any cause. In contrast to OS, it covers incremental alterations in every phase of treatment [19].
Time to progression is the time from randomization to the first sign of disease progression. ORR evaluates the effects of a given treatment on the size and total tumour load, for a minimum time. It is the sum of the partial and complete responses [19].
Information Sources
At least, two databases are needed to ensure optimum and efficient search results, thus, a literature search was conducted using a combination of various electronic databases [20]. The databases include Medline, PubMed, Cochrane Library, CINAHL, Web of Science, Scopus, and African Journal. We searched the databases from their inception to June 2023. Additionally, the reference list of identified systematic reviews was searched and relevant studies were included.
Search Strategy Development
The literature search was conducted using a variety of search terms developed from keywords from the title and abstract of relevant papers and through consultations by the primary investigator, librarian, and information specialist. A draft PubMed search strategy was developed by the previously mentioned personnel. A second librarian who not part of this study reviewed the initial PubMed draft and modifications were made accordingly. The search terms were then adjusted to correspond with the syntax and subject headings of the remaining databases. Boolean operators (AND, OR), MeSH terms, and phrase searches were employed correctly.
Data Management
All literature search results were exported into EndNote 20 and duplicate articles were removed. In EndNote 20, we screened (title and abstract) all articles according to the eligibility criteria and articles that meet the inclusion requirements were downloaded for full-text screening. To aid the screening process, we developed screening forms that include eligibility questions based on the inclusion criteria. The flow of articles, both included and excluded, is arranged in EndNote 20 for the PRISMA flow chart generation.
Study Selection and Data Extraction
Two reviewers independently conducted the title and abstract screening. Screening conflicts were resolved in consultation with a third reviewer. Full-text screening was also carried out by the same two reviewers. The PRISMA diagram shows the flow of studies throughout the selection process, as well as the reasons behind exclusions (shown in Fig. 1). Two reviewers independently extracted data from the selected articles using an Excel spreadsheet template. Both reviewers extracted 20% of the articles independently until a good inter-rater agreement was achieved. Subsequently, one reviewer extracted the remaining 80% of the included articles. Only data that aligned with the inclusion criteria were extracted, thus, only data for post-menopausal women clearly identified in the individual studies (methods and results) were extracted.
Fig. 1.
Flow of studies as illustrated by the PRIMA flow chart.
Risk of Bias Assessment
Each publication’s quality and risk of bias were evaluated using the Cochrane risk of bias tool for randomized trials [21]. A revised version of this tool (RoB 2.0) was utilized. The Cochrane RoB 2.0 is suitable for individual, parallel-group, and cluster-randomized trials [22]. The tool requires rating the risk of bias as “high,” “low,” or “unclear” [21]. Two independent reviewers carried out the risk of bias assessment. Disagreements were resolved in consultation with a third reviewer.
Data Analysis and Assessment of Heterogeneity
We employed a random-effect meta-analysis model to evaluate the effectiveness of hormonal plus targeted therapies. Effect sizes, when required, were translated to standardized mean differences [23]. We computed the mean and standard deviation for studies that summarized age using median and interquartile ranges by employing the formula in Hozo et al. [24]. Subgroup analysis was performed based on the different classes of hormonal therapies included in the study irrespective of the line of treatment. With the level of significance set at 0.05, statistical analysis was carried out using the Comprehensive Meta-analysis version 3.
Protocol Amendment
In the initial registered protocol, we sought to investigate studies that assessed the efficacy of hormonal and/or targeted therapies for ABC. However, following the full-text screening, we had over 200 relevant articles, hence the authors decided to address each of the 3 initial research questions separately to include further inclusion criteria (based on HR/HER2 status and menopausal status) to maximize focus and clarity. Thus, we further grouped the relevant articles into those assessing the efficacy of combined hormonal and targeted therapies, hormonal therapy alone, and targeted therapy alone. The search strategy is added as a supplementary file.
Results
The database search yielded 7,149 relevant studies (CINAHL 359, Cochrane 82, Medline 1,151, PubMed 2,107, Scopus 3,276, Web of science 174). De-duplication further reduced the articles to 4,667. Following the title, abstract, and full-text screening, over 200 articles meeting our previous protocol inclusion criteria remained. Further grouping of the articles yielded 38 studies evaluating the efficacy of different hormonal therapy classes in post-menopausal women with HR+ or unknown ABC (shown in Fig. 1).
Risk of Bias and Quality Assessment
Cochrane ROB2 was used to assess the risk of bias in the included articles. A total of 15 studies were rated to have “some concerns” in the overall risk of bias while a low risk of bias was recorded for the other 23 articles in all the domains. The major sources of bias were in the domains of randomization, deviations from the intended intervention and measurement of outcomes (Table 1). Issues with randomization were majorly on blinding and heterogeneous baseline participant characteristics which might influence the findings of the included studies. Deviations from intended interventions were noted to be due to the event of adverse side effects, toxicities, disease progression, and change in clinical practice could introduce bias affecting the conclusions of the study if not properly accounted for.
Table 1.
Risk of bias assessment using the Cochrane ROB 2.0 for RCTs
| Authors | Bias due to randomization | Bias due to deviations from intended intervention | Bias due to missing outcome data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|
| Buzdar et al. [25] (2002) | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Campos et al. [26] (2009) | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Gershanovich et al. [27] (1997) | Some concern | Some concern | Low risk | Low risk | Low risk | Some concern |
| Maung and O’Shaughnessy [28] (2001) | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Osborne et al. [29] (2002) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Robertson et al. [30] (2016) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Pritchard et al. [31] (2010) | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Robertson et al. [32] (2012) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bonneterre et al. [33] (2000) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Thurlimann et al. [34] (2004) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Rose et al. [35] (2003) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Xu et al. [36] (2011) | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Bajetta et al. [37] (1994) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zilembo et al. [38] (1995) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Chia et al. [39] (2008) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Di Leo et al. [40] (2010) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Di Leo et al. [41] (2014) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Goss et al. [42] (2007) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Howell et al. [43] (2004) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Krop et al. [44] (2020) (Cohort 1) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Krop et al. [44] (2020) (Cohort 2) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lipton et al. [45] (2008) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Llombart-Cussac et al. [62] (2012) | Some concern | Some concern | Low risk | Some concern | Low risk | Some concern |
| Mauriac et al. [46] (2009) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Mehta et al. [47] (2019) | Some concern | Some concern | Low risk | Some concern | Low risk | Some concern |
| Mourisden et al. [48] (2001) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Nabholtz et al. [49] (2000) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Robertson et al. [50] (2009) | Some concern | Some concern | Low risk | Low risk | Low risk | Some concern |
| Arpino et al. [51] (2003) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Gershanovich et al. [52] (1998) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ellis et al. [53] (2015) | Some concern | Some concern | Low risk | Some concern | Low risk | Some concern |
| Ingle et al. [54] (1997) | Some concern | Some concern | Low risk | Low risk | Low risk | Some concern |
| Mourisden [55] (2007) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ohno et al. [56] (2010) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Paridaens et al. [57] (2008) | Low risk | Some concern | Low risk | Some concern | Low risk | Some concern |
| Thurlimann et al. [58] (1996) | Low risk | Some concern | Low risk | Low risk | Low risk | Some concern |
| Wang et al. [59] (2023) | Low risk | Some concern | Low risk | Some concern | Low risk | Some concern |
| Yamamoto et al. [60] (2013) | Low risk | Some concern | Low risk | Some concern | Low risk | Some concern |
| Zhang et al. [61] (2016) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Study and Participant Characteristics
Included studies were either phase II or III randomized clinical trials, two of these studies are OS reports of the primary study, hence, the true overall sample size was 14, 056. Median age ranged from 54 to 73 years. Participants were post-menopausal women with HR+/unknown ABC who received hormonal therapies (Table 2).
Table 2.
Study characteristics of included studies
| Study ID | Study design | Setting | Sample size | Cancer type | Age (median) |
|---|---|---|---|---|---|
| Buzdar et al. [25] (2002) | Phase III randomized active-control trial | Multicentre, International | I1: 542 | HR+ | – |
| I2: 550 | |||||
| Campos et al. [26] (2009) | Randomized parallel-group trial | 39 sites in Europe and the USA | I1: 64 | HR+/unknown | I1: 61 (43–88) |
| I2: 64 | I2: 64.5 (42–84) | ||||
| Gershanovich et al. [27] (1997) | Randomized phase III trial | Multicentre | I1: 157 | HR+/unknown | I1: 60.9 (38.0–85.0) |
| I2: 157 | I2: 62.2 (35.0–82.0) | ||||
| I3: 149 | I3: 59.6 (31.0–90.0) | ||||
| Maung and O’Shaughnessy [28] (2001) | Phase II randomized trial | – | I1: 61 | HR+/unknown | I1: 62 (37–85) |
| I2: 59 | I2: 63 (46–87) | ||||
| Osborne et al. [29] (2002) | Phase III randomized double-dummy trial | North America | I1: 206 | HR+ | – |
| I2: 194 | |||||
| Robertson et al. [30] (2016) | Phase III randomized double-dummy trial | Multicentre, International | I1: 230 | HR+ | I1: 64·0 (38–87) |
| I2: 232 | I2: 62·0 (36–90) | ||||
| Pritchard et al. [31] (2010) | Phase II randomized trial | Multicentre, International | I1: 47 | HR+ | I1: 63 (42–88) |
| I2: 51 | I2: 69 (38–85) | ||||
| I3: 46 | I3: 67 (49–85) | ||||
| Robertson et al. [32] (2012) | Phase II randomized trial | Multicentre | I1: 102 | HR+ | I1: 66 (40–89) |
| I2: 103 | I2: 68 (48–87) | ||||
| Bonneterre et al. [33] (2000) | Randomized double-blind study | Multicentre, International | I1: 340 | HR+/unknown | I1: 67 (34–91) |
| I2: 328 | I2: 66 (41–92) | ||||
| Thurlimann et al. [34] (2004) | Randomized double-blind study | Multicentre, Swiss | I1: 31 | HR+/unknown | 68 (47–85) |
| I2: 29 | |||||
| Rose et al. [35] (2003) | Randomized phase IIIb/IV trial | Multicentre, International | I1: 356 | HR+/unknown | 63 (27–92) |
| I2: 357 | |||||
| Xu et al. [36] (2011) | Phase III randomized double-dummy trial | Multicentre, China | I1: 121 | HR+ | 54.1 |
| I2: 113 | |||||
| Bajetta et al. [37] (1994) | Phase II randomized trial | Milan | I1: 72 | HR+/unknown | I1: 59 (46–75) |
| I2: 71 | I2: 60 (31–71) | ||||
| Zilembo et al. [38] (1995) | Phase II randomized trial | Milan | I1: 24 | HR+/unknown | I1: 63 (49–75) |
| I2: 28 | I2: 62 (49–72) | ||||
| Chia et al. [39] (2008) | Phase III RCT | Multicentre | I1: 351 | HR+ | I1: 63 (38–88) |
| I2: 342 | I2: 63 (32–91) | ||||
| Di Leo et al. [40] (2010) | Phase III randomized trial | Multicentre, International | I1: 361 | ER+ | 61 |
| I2: 374 | |||||
| Di Leo et al. [41] (2014) | Phase III randomized trial | Multicentre, International | I1: 361 | ER+ | 61 |
| I2: 374 | |||||
| Goss et al. [42] (2007) | Phase III randomized trial | Multicentre, International | I1: 434 | ER/PgR + | I1: 65 |
| I2: 431 | I2: 63 | ||||
| Howell et al. [43] (2004) | Double-blind randomized trial | Multicentre, International | I1: 313 | ER/PgR + or unknown | I1: 67 (43–93) |
| I2: 274 | I2: 66 (43–92) | ||||
| Krop et al. [44] (2020) (Cohort 1) | Phase II randomized trial | Not stated | I: 63 | HR+, HER2 normal | I1: 59 (34–85) |
| C: 64 | I2: 65 (34–89) | ||||
| Krop et al. [44] (2020) (Cohort 2) | Phase II randomized trial | Not stated | I: 60 | HR+, HER2 normal | I: 58 (35–83) |
| C: 60 | C: 61 (34–89) | ||||
| Lipton et al. [45] (2008) | Phase III double-dummy trial | Multicentre, International | I1: 453 | HR+ or unknown | I1: 65 (31–96) |
| I2: 454 | I2: 64 (31–93) | ||||
| Llombart-Cussac et al. [62] (2012) | Phase II randomized trial | Multicentre, Spain | I1: 47 | HR+ | I1: 67.9 (45–94) |
| I2: 50 | I2: 72.6 (46–85) | ||||
| Mauriac et al. [46] (2009) | Phase III randomized trial | Multicentre | I1: 351 | HR+ | I1: 63 (39–87) |
| I2: 342 | I2: 63 (34.5–88) | ||||
| Mehta et al. [47] (2019) | Open-label randomized trial | Multicentre | I: 349 | HR+ | I: 65 (36–91) |
| C: 345 | C: 65 (27–92) | ||||
| Mourisden et al. [48] (2001) | Phase III randomized trial | Multicentre, International | I1: 453 | HR+ or unknown | I1: 65 (31–96) |
| I2: 454 | I2: 64 (31–93) | ||||
| Nabholtz et al. [49] (2000) | Double-blind randomized trial | Multicentre, USA and Canada | I1: 171 | HR+ or unknown | I1: 68 (30–88) |
| I2: 182 | I2: 67 (40–92) | ||||
| Robertson et al. [50] (2009) | Phase II randomized trial | Multicentre, International | I1: 89 | ER/PgR + or unknown | I1: 66 (40–89) |
| I2: 93 | I2: 68 (68–87) | ||||
| Arpino et al. [51] (2003) | Double-blind randomized trial | Multicentre | I1: 108 | HR + | I1: 59.1 (30–90) |
| I2: 111 | I2: 59.9 (30–90) | ||||
| Gershanovich et al. [52] (1998) | Open-label comparative trial | Multicentre, International | I1: 185 | HR+ or unknown | I1: 64 |
| I2: 192 | I2: 66 | ||||
| I3: 178 | I3: 65 | ||||
| Ellis et al. [53] (2015) | Phase II randomized trial | Multicentre, International | I1: 102 | HR+ | <65 or ≥65 years |
| I2: 103 | |||||
| Ingle et al. [54] (1997) | Phase II randomized trial | I1: 46 | ER/PgR + and unknown | I1: 65 (40–81) | |
| I2: 45 | I2: 66 (49–85) | ||||
| Mourisden [55] (2007) | Phase III double-blind randomized trial | I1: 453 | HR+ or unknown | I1: 65 (31–96) | |
| I2: 454 | I2: 64 (31–93) | ||||
| Ohno et al. [56] (2010) | Phase II randomized trial | Multicentre, Japan | I1: 45 | ER+ | I1: 61 (50–77) |
| I2: 51 | I2: 62 (43–86) | ||||
| I3: 47 | I3: 61 (45–83) | ||||
| Paridaens et al. [57] (2008) | Phase III randomized trial | Multicentre | I1: 182 | ER/PgR + | I1: 63 (37–86) |
| I2: 189 | I2: 62 (37–87) | ||||
| Thurlimann et al. [58] (1996) | Phase III randomized trial | Multicentre | I1: 107 | HR+ or unknown | I1: 65 (40–83) |
| I2: 105 | I1: 65 (39–87) | ||||
| Wang et al. [59] (2023) | Phase II randomized open-label trial | Multicentre | I1 = 75 | ER+/HER2- | I1: 62 (45–80) |
| I2 = 64 | I2: 63 (46–76) | ||||
| Yamamoto et al. [60] (2013) | Phase III randomized trial | Not indicated | I1: 46 | HR+/HER2- | I1: 63 (51–87) |
| I2: 45 | I2: 62 (49–87) | ||||
| Zhang et al. [61] (2016) | Phase III double-blind randomized trial | Multicentre, China | I1: 109 | ER+ | I1: 55 (26–80) |
| I2: 110 | I2: 55 (31–76) |
HR, hormone receptor; HER2, human epidermal growth factor receptor 2; ER, oestrogen receptor; I1, intervention 1; I2, intervention 2; C, control group; PgR, progesterone receptor.
Summary of Treatment and Outcomes
Patients comprised both those who had not received any prior therapy/or had just initiated an endocrine therapy ≤14 days before randomization in the advanced setting (20 studies), and patients who had received any systemic therapy in the advanced setting (18 studies; out of which 6 only received chemotherapy and patients were endocrine-naive). The classes of hormonal therapy included in these studies were primarily AIs, selective oestrogen modulators/degraders, and androgen inhibitors (1 study). Overall, ORR ranged from 2.2 to 46%, median PFS ranged from 3.6 to 16.6 months, and OS ranged from 19.9 to 54.1 months. The summary of the study treatment protocols and outcomes is presented in Table 3.
Table 3.
Intervention and outcomes in the included studies
| Study ID | Prior therapies in the advanced setting | Agent/dose | Dose modifications | Treatment duration/follow-up | Outcomes |
|---|---|---|---|---|---|
| Buzdar et al. [25] (2002) | None | I1: 40 mg/day droloxifene + placebo tamoxifen | NI | Mean treatment duration | ORR |
| I2: 20 mg/day tamoxifen + placebo droloxifene | I1: 196 (8–920) days | I1: 27.1% | |||
| I2: 218 (6–969) days | I2: 31.7% | ||||
| p = 0.14 | |||||
| Campos et al. [26] (2009) | Chemotherapy and hormonal therapy | I1: exemestane 25 mg/day I2: anastrozole 1 mg/day | NI | I1: 17 weeks | ORR (95% CI) |
| I1: 10.9 (4.5–21.3) % | |||||
| I2: 15.6 (7.8–26.9) % | |||||
| Median TTP | |||||
| I1: 3.7 (0.7–34.3) months | |||||
| I2: 18.5 weeks | I2: 4.2 (0.9–0.2) months | ||||
| Median OS | |||||
| I1: 30.5 (1.1–41.3) | |||||
| I2: 33.3 (2.6–44.7) | |||||
| Gershanovich [27] (1997) | ≤14 days tamoxifen | I1: 1 toremifene 60 mg tablet daily | No dose modifications were allowed | Minimum treatment duration was 2 months | ORR |
| I1: 20.4% | |||||
| I2: 28.7% | |||||
| I3: 20.8% | |||||
| Median TTP | |||||
| I1: 4.9 (3.8–7.3) months | |||||
| I2: 6.1 (4.5–8.0) months | |||||
| I3: 5.0 (3.7–6.2) months | |||||
| I2: 2 toremifene 60 mg tablets twice a day | Median follow-up 20.5 months | I3:I1-HR, 1.015 (0.79–1.31), p = 0.905 | |||
| I3:I2-HR, 1.124 (0.87–1.46), p = 0.374 | |||||
| Median OS | |||||
| I1: 25.4 (20.8–31.0) months | |||||
| I3: tamoxifen 40 mg tablet daily | I2: 23.8 (20.9–29.7) months | ||||
| I3: 23.4 (18.4–34.2) months | |||||
| I3:I1-HR, 0.96 (0.72–1.28), p = 0.802 | |||||
| I3:I2-HR, 1.02 (0.76–1.36), p = 0.854 | |||||
| Maung and O’Shaughnessy, [28] (2001) | 1 prior chemotherapy | I1: oral exemestane 25 mg/day | – | – | ORR |
| I2: tamoxifen 20 mg/day | I1: 40.9% | ||||
| I2: 13.6% | |||||
| Osborne [29] (2002) | 1 prior endocrine therapy except for fulvestrant and AIs | I1: fulvestrant 250 mg every 28 days + matching placebo | – | Median follow-up of 16.8 months | Median TTP (95.14% CI) |
| I1: 5.4 months | |||||
| I2: 3.4 months | |||||
| HR, 0.92 (0.74–1.14) | |||||
| p = 0.43 | |||||
| I2: oral anastrozole 1 mg once daily + matching placebo | ORR (95.14% CI) | ||||
| I1: 17.5% | |||||
| I2: 17.5% | |||||
| OR, 1.01 (0.59–1.73) | |||||
| p = 0.96 | |||||
| Robertson [30] (2016) | 1 prior chemotherapy | I1: IM fulvestrant | No fulvestrant dose reductions were permitted | Median Rx duration | Median PFS |
| I1: 16.6 (3.83–20.99) months | |||||
| 500 mg on days 0, 14, 28, and every 28 days | I2: 13.8 (11.99–16.59) months | ||||
| Thereafter + anastrozole placebo | HR, 0.80 (0.64–1.00) | ||||
| I2: oral anastrozole 1 mg | I1: 14.7 (0.9–37.7) months | ORR | |||
| I1: 46% | |||||
| 500 mg on days 0, 14, 28, and every 28 days | I1: 14.7 (0.9–37.7) months | I2: 45% | |||
| Thereafter + anastrozole placebo | OR 1.07 (0.72–1.61), p = 0.7290 | ||||
| Pritchard et al. [31] (2010) | Endocrine therapy | I1: fulvestrant 250 mg (AD) | – | – | Median TTP |
| I1: 3.1 months | |||||
| I2: fulvestrant 250 mg (LD) | I2: 6.1 months | ||||
| I3: 6.0 months | |||||
| I3: fulvestrant 250 mg (HD) | ORR | ||||
| I1: 8.5 (2.4–20.4) % | |||||
| I2: 5.9 (1.2–16.2) % | |||||
| I3: 15.2 (6.3–28.9) % | |||||
| Robertson et al. [32] (2012) | None | I1: IM fulvestrant 500 mg (500 mg/month plus 500 mg on day 14 of month 1) | – | Median follow-up for TTP I1: 18.8 months | Median TTP |
| I1: 23.4 months | |||||
| I2: anastrozole 1 mg/day orally | I2: 12.9 months | I2: 13.1 months | |||
| HR, 0.64 (0.46–0.90), p = 0.01 | |||||
| Bonneterre et al. [33] (2000) | None | I1: anastrozole 1 mg once daily + tamoxifen placebo | None | Median follow-up: 19 months | ORR |
| I1: 32.9% | |||||
| I2: 32.6% | |||||
| I2: tamoxifen: 20 mg once daily + anastrozole placebo | p = 0.787 | ||||
| Median TTP (95% CI) | |||||
| I1: 8.2 months | |||||
| I2: 8.3 months | |||||
| HR, 0.99 (0.86-N/R) | |||||
| p = 0.941 | |||||
| Thurlimann et al. [34] (2004) | None | Anastrozole: 1 mg once daily + tamoxifen placebo | None | Median follow-up | Median TTP |
| Tamoxifen: 20 mg once daily + anastrozole placebo | 66.3 (3.9–81.6) months | I1: 11.3 (3.2–16.8) months | |||
| I2: 8.3 (4.6–16.6) months | |||||
| Rose et al. [35] (2003) | Endocrine and 1 prior chemotherapy | I1: letrozole 2.5 mg | – | Median duration of treatment | Median TTP |
| I1: 5.7 (5.1–6.0) months | |||||
| I2: 5.7 (4.6–6.1) months | |||||
| I1: 5.9 months | p = 0.92 | ||||
| ORR (90% CI) | |||||
| I1: 19.1 (15.7–22.9) % | |||||
| I2: anastrozole 1 mg | I2: 5.6 months | I2: 12.3 (9.6–15.6) % | |||
| OR, 1.70, p = 0.013 | |||||
| Median OS | |||||
| I1: 22.0 (19.6–24.6) | |||||
| I2: 20.3 (18.0–23.1) | |||||
| HR, 0.95, p = 0.624 | |||||
| Xu [36] (2011) | Endocrine | I1: IM fulvestrant 250 mg every 4 weeks with matching daily anastrozole placebo | – | Mean duration of treatment | Median TTP |
| I1: 110 days | |||||
| I2: 159 days | |||||
| HR, 1.314 (0.948–1.822) | |||||
| p = 0.101 | |||||
| I2: anastrozole 1 mg daily | I2: 174.3 days | ORR | |||
| I1: 10% | |||||
| with matching placebo to fulvestrant monthly | I2: 14% | ||||
| OR, 0.631 (0.244–1.635) | |||||
| p = 0.343 | |||||
| Bajetta [37] (1994) | Endocrinee and chemotherapy | I1: IM formestane 250 mg every 2 weeks | – | – | Median TTP |
| I1: 8 (8–46) months | |||||
| I2: 9 (2–35) months | |||||
| Median OS | |||||
| I1: 30 (1–46) months | |||||
| I2: IM formestane 500 mg every 2 weeks | I2: 22 (2–47) months | ||||
| ORR | |||||
| I1: 28% | |||||
| I2: 46% | |||||
| p = 0.026 | |||||
| Zilembo et al. [38] (1995) | None | I1: IM formestane 250 mg every 2 weeks | – | Median treatment duration | ORR |
| I2: IM formestane 500 mg every 2 weeks | I1: 7 months | I1: 33 (14–53) % | |||
| I2: 9 months | I2: 46 (28–64) % | ||||
| Chia et al. [39] (2008) | None | I1: IM fulvestrant 500 mg loading dose on day 0, 250 mg on day 14 and 28 then 250 mg every 28 days + matching placebo | None | Median follow-up: 13 months | ORR (95% CI) |
| I1: 7.4% | |||||
| I2: 6.7% | |||||
| I2: exemestane 25 mg once daily + matching placebo | OR, 1.12 (0.578–2.186) | ||||
| p = 0.736 | |||||
| Median TTP (95% CI) | |||||
| I1: 3.7 months | |||||
| I2: 3.7 months | |||||
| HR, 0.96 (0.819–1.133) p = 0.65 | |||||
| Di Leo et al. [40] (2010) | None | I1: IM fulvestrant 500 mg on days 0, 14, and 28 then every 28 days (±3 days) thereafter | None | Median duration | Median PFS (95% CI) |
| I1: 6.5 months | |||||
| I2: 5.5 months | |||||
| I2: IM fulvestrant 250 mg + one placebo injection on days 0 and 14 then every 28 days | I1: 174 days (10–1,441) | HR, 0.80 (0.68–0.94) p = 0.006 | |||
| ORR (95% CI) | |||||
| I1: 9.1% | |||||
| I1: 145 days (7–1,387) | I2: 10.2% | ||||
| OR, 0.94 (0.57–1.55) | |||||
| p = 0.795 | |||||
| Di Leo et al. [41] (2014) | None | I1: IM fulvestrant 500 mg on days 0, 14, and 28 then every 28 days (±3 days) thereafter | None | Median duration | Median OS (95% CI) |
| I1: 174 days (10–1,441) | I1: 26.4 months | ||||
| I2: IM fulvestrant 250 mg + one placebo injection on days 0 and 14 then every 28 days | I1: 145 days (7–1,387) | I2: 22.3 months | |||
| HR = 0.81 (0.69–0.96) | |||||
| p = 0.02 | |||||
| Goss et al. [42] (2007) | None | I1: atamestane 5 100 mg tabs (3 before or after breakfast, 2 before or after dinner) daily + toremifene one 60 mg tab in the morning daily | None | – | ORR (95% CI) |
| I1: 30 (26–35) % | |||||
| I2: 36 (31–40) % | |||||
| OR, 1.27 (0.95–1.69) | |||||
| p = 0.10 | |||||
| I2: letrozole 2.5 mg tab in the morning + 5 placebo tabs daily (3 before or after breakfast, 2 before, or after dinner) | OS (95% CI) | ||||
| I1: 3.01 years | |||||
| I2: 2.79 years | |||||
| HR, 0.99 (0.92–1.06) | |||||
| p = 0.70 | |||||
| Howell et al. [43] (2004) | None | I1: fulvestrant 250 mg once monthly (i.e., every 28±3 days) + placebo 20 mg daily | None | Median Rx. duration | ORR |
| I1: 8.3 (0.9–26.5) months | I1: 31.6% | ||||
| I2: 9.3 (0.9–25.1) months | I2: 33.9% | ||||
| I2: tamoxifen 20 mg daily orally + placebo to match fulvestrant | Median follow-up: 31.1 months | Median OS (95% CI) | |||
| I1: 36.9 months | |||||
| I2: 38.7 months | |||||
| HR, 1.29 (1.01–1.64), p = 0.04 | |||||
| Krop et al. [44] (2020) (Cohort 1) | None | I1: enzalutamide 160 mg daily + exemestane 50 mg daily | None | I1: 40.9 weeks | ORR (95% CI) |
| I: 31% (17–48) | |||||
| Control group: 19% (9–34) | |||||
| p = 0.2216 | |||||
| I2: exemestane 25 mg + placebo daily | Control group: 25.7 weeks | Median PFS (95% CI) | |||
| I: 11.8 months (7.3–15.9) | |||||
| Control group: 5.8 months (3.5–10.9) | |||||
| HR, 0.82 (0.5–1.26) p = 0.3631 | |||||
| Krop et al. [44] (2020) (Cohort 2) | One endocrine therapy and one chemotherapy | I1: enzalutamide 160 mg daily + exemestane 50 mg daily | None | Median Rx. duration | ORR (95% CI) |
| I: 10% (3–23) | |||||
| Control group: 5% (1–16) | |||||
| p = 0.3968 | |||||
| I2: exemestane 25 mg + placebo daily | I: 10.2 weeks | Median PFS (95% CI) | |||
| I: 3.6 months (1.9–5.5) | |||||
| I: 10.2 weeks | Control group: 3.9 months (2.6–5.4) | ||||
| HR, 1.02 (0.66–1.59) p = 0.9212 | |||||
| Lipton et al. [45] (2008) | One chemotherapy and endocrine therapy | I1: letrozole 2.5 mg once daily | None | Median duration of study | ORR (95% CI) |
| I1: 30% (26–35) | |||||
| I2: tamoxifen 20 mg once daily | 18 months | I2: 20% (17–24) | |||
| OR, 1.71 (1.26–2.31) p = 0.0006 | |||||
| Llombart-Cussac et al. [62] (2012) | Single line of chemotherapy (4 weeks before randomization) | I1: exemestane 25 mg daily orally | None | Median follow-up of 9.1 months (0.07–79.96) | ORR (95% CI) |
| I1: 36.2% (18.5–45.9) | |||||
| I2: 46% (32.2–59.8) | |||||
| I2: anastrozole 1 mg daily orally | Median OS (95% CI) | ||||
| I1: 19.9 months (15.32–24.46) | |||||
| I2: 48.3 months (18.3–78.3) | |||||
| HR, 1.33 (0.78–2.25), p = 0.296 | |||||
| Mauriac et al. [46] (2009) | None | I1: IM fulvestrant 500 mg on day 0, 250 mg on days 14 and 28 then 250 mg every 28±3 days thereafter | None | – | ORR (95% CI) |
| I1: 15.1% | |||||
| I2: exemestane 25 mg once daily orally | I2: 16% | ||||
| OR, 1.28 (0.47–3.09) p = 0.33 | |||||
| Mehta et al. [47] (2019) | None | I: fulvestrant 500 mg on day 1, 250 mg on days 14 and 28 then 250 mg every 28 days thereafter + anastrozole standard dose | None | Median follow-up of 7 years | Median OS (95% CI) |
| I: 49.8 months | |||||
| Control group: 42 months | |||||
| Control group: anastrozole: standard dose | HR, 0.82 (0.69–0.98) | ||||
| p = 0.03 | |||||
| Median PFS (95% CI) | |||||
| I: 15 months | |||||
| Control group: 13.5 months | |||||
| HR, 0.81 (0.69–0.94) p = 0.007 | |||||
| Mourisden et al. [48] (2001) | One chemotherapy and endocrine therapy | I1: letrozole 2.5 mg once daily | None | Median duration of study | ORR (95% CI) |
| I1: 30% (26–35) | |||||
| I2: 20% (17–24) | |||||
| I2: tamoxifen 20 mg once daily | 18 months | OR, 1.71 (1.26–2.31) p = 0.0006 | |||
| Median TTP | |||||
| I1: 9.4 months | |||||
| I2: 6.0 months | |||||
| HR, 0.70 (0.60–0.82) p = 0.0001 | |||||
| Nabholtz et al. [49] (2000) | None | I1: anastrozole 1 mg once daily | None | Median follow-up of 17.7 months | ORR |
| I2: tamoxifen 20 mg once daily | I1: 21.1% | ||||
| I2: 17% | |||||
| Robertson et al. [50] (2009) | None | I1: fulvestrant 500 mg on days 0, 14±3, 28±3 then every 28±3 days | None | I1: 9.2 months (1–20.5) | ORR (95% CI) |
| I2: 6.1 months (0–19.8) | I1: 36% | ||||
| I2: anastrozole 1 mg once daily | Median follow-up: 8 months and 5.9 months | I2: 35.5% | |||
| OR, 1.02 (0.56–1.87) p = 0.947 | |||||
| Arpino et al. [51] (2003) | None | I1: idoxifene one 20 mg + one 40 mg tablet daily/first 21 days (loading dose) then one 40 mg tab daily | None | – | ORR |
| I1: 13% | |||||
| I2: 9% | |||||
| p = 0.39 | |||||
| I2: tamoxifen one 20 mg + one placebo daily for first 21 days then one 20 mg daily throughout | Median TTP | ||||
| I1: 166 days (140–230) | |||||
| I2: 140 days (110–185) | |||||
| p = 0.32 | |||||
| Gershanovich et al. [52] (1998) | None | I1: letrozole 2.5 mg once a day | None | Median treatment duration of about 5 months | ORR (95% CI) |
| I1: 19.5% (13.8–25.2) | |||||
| I2: 16.7% (11.4–21.9) | |||||
| I3: 12.4% (7.5–17.2) | |||||
| Median TTP (95% CI) | |||||
| I1: 3.4 months | |||||
| I2: 3.3 months | |||||
| I3: 3.2 months | |||||
| I2: letrozole 0.5 mg once a day | Median follow-up duration: 20 months | RR, 0.76 (0.57–0.9) p = 0.008 | |||
| Median OS (95% CI) | |||||
| I1: 28 months | |||||
| I3: aminoglutethimide 250 mg twice daily + corticosteroid support | I2: 21 months | ||||
| I3: 20 months | |||||
| RR, 0.69 (0.56–0.92) p = 0.021 | |||||
| Ellis et al. [53] (2015) | None | I1: fulvestrant 500 mg on days 0, 14, and 28 then every 28 days subsequently | None | Follow-up of about 6 months | Median OS (95% CI) |
| I1: 54.1 months | |||||
| I2: anastrozole: 1 mg daily | I2: 48.4 months | ||||
| H, 0.70 (0.50–0.98) p = 0.04 | |||||
| Ingle et al. [54] (1997) | Chemotherapy | I1: letrozole 0.5 mg per day | None | Minimum follow-up of 6 months | ORR (95% CI) |
| I2: letrozole 2.5 mg per day | I1: 20% (11–34) | ||||
| I2: 22% (13–36) | |||||
| Mourisden [55] (2007) | Chemotherapy | I1: letrozole 2.5 mg once daily orally | None | Median follow-up was 32 months | Median OS |
| I1: 34 months | |||||
| I2: tamoxifen 20 mg once daily orally | I2: 30 months | ||||
| p = 0.53 | |||||
| Ohno et al. [56] (2010) | Endocrine therapy | I1: fulvestrant (approved dose): 250 mg days 0 and 28 then every 28 days thereafter + 2 placebo injections on day 14 | None | Median duration of treatment | ORR (95% CI) |
| I1: 197 days | I1: 11.1% (3.7–24.1) | ||||
| I2: fulvestrant (loading dose): 500 mg at day 0, 250 mg at days 14 and 28 then every 28 days thereafter | I2: 225 days | I2: 17.6% (8.4–30.9) | |||
| I3: 213 days | I3: 10.6% (3.5–23.1) | ||||
| Followed up for at least 24 weeks | Median TTP | ||||
| I3: fulvestrant (high dose): 500 mg on days 0, 14, and 28 then every 28 days thereafter | I1: 6.0 months | ||||
| I2: 7.5 months | |||||
| I3: 6.0 months | |||||
| Paridaens et al. [57] (2008) | Radiotherapy and chemotherapy | I1: exemestane 25 mg once daily orally | None | I1: 11.5 months (10.18–13.54) | ORR (95% CI) |
| I1: 46% | |||||
| I2: 6.57 months (5.78–10.91) | I2: 31% | ||||
| OR, 1.85 (1.21–2.82) p = 0.005 | |||||
| Median follow-up | Median PFS (95% CI) | ||||
| I2: tamoxifen 20 mg once daily orally | I1: 9.9 months (8.7–11.8) | ||||
| 29 months (20–53) | I2: 5.8 months (5.3–8.1) | ||||
| Median OS (95% CI) | |||||
| I1: 37.2 months (29.2–45.5) | |||||
| I2: 43.3 months (32.8–51.6) | |||||
| Thurlimann et al. [58] (1996) | None | I1: tamoxifen 20 mg/day p.o. | None | Minimum of 2 months treatment duration | ORR (95% CI) |
| I1: 27% (21–35) | |||||
| I2: 20% (13–29) | |||||
| I2: fadrozole 1 mg p.o twice a day | Follow-up of 3 years | OR, 0.56 (0.28–1.11) p = 0.26 | |||
| Wang et al. [59] (2023) | None | I1: IM fulvestrant 500 mg on days 0, 14, and 28 then every 28 thereafter ±3 days | None | 156 weeks treatment duration | Median PFS (95% CI) |
| I1: 8.5 months | |||||
| I2: 5.6 months | |||||
| I2: exemestane: 25 mg/day orally | HR, 0.62 (0.42–0.91) | ||||
| p = 0.0014 | |||||
| ORR (95% CI) | |||||
| I1: 19.5% (10.63–28.33) | |||||
| I2: 6% (0.3–11.64) p = 0.017 | |||||
| Yamamoto et al. [60] (2013) | Non-steroidal AI | I1: toremifene 120 mg daily | None | Median follow-up period | ORR (95% CI) |
| I1: 11.6% (5.1–24.5) | |||||
| I2: 2.2% (1.2–16.7) | |||||
| p = 0.069 | |||||
| I1: 69 weeks (13–144) | Median PFS (95% CI) | ||||
| I1: 7.3 months | |||||
| I2: exemestane 25 mg daily | I1: 69 weeks (13–144) | I2: 3.7 months | |||
| HR, 0.61 (0.38–0.99) p = 0.045 | |||||
| I1: 69 weeks (13–144) | Median OS (95% CI) | ||||
| I1: 32.3 months | |||||
| I2: 21.9 months | |||||
| HR, 0.60 (0.26–1.39) p = 0.22 | |||||
| Zhang et al. [61] (2016) | Endocrine therapy | I1: IM fulvestrant 500 mg (two 5 mL) on days 0, 14, and 28 then every 28 days thereafter | None | Treatment exposure | Median PFS (95% CI) |
| I1: 8.0 months (5.5–10.9) | |||||
| I1: 6.5 months | I2: 4.0 months (2.9–5.7) | ||||
| I2: IM fulvestrant 250 mg (one 5 mL + one placebo injection) on days 1 and 28 then every 28 days thereafter + 2 placebo injection on day 14 | HR, 0.75 (0.54–1.03) p = 0.078 | ||||
| I2: 3.8 months | ORR | ||||
| I1: 14.4% | |||||
| I2: 9.1% |
I1, intervention 1; I2, intervention 2; OR, odds ratio; HR, hazard ratio; NI, no information; IM, intramuscular.
Meta-Analysis of Included Studies
Results of the subgroup analysis of the ORR between AIs and other hormonal therapies (SERD, selective oestrogen modulator, and androgen inhibitors) showed a non-significant difference in ORR in patients who received AI compared to other hormonal therapies (OR = 1.122 [0.917–1.374], p = 0.263). The odds of obtaining ORR in patients receiving AI are 1.1 compared to those receiving SERM, SERD, or androgen inhibitors; however, this was not significant. Substantial heterogeneity in study characteristics was observed between studies (I2 = 1.795) (shown in Fig. 2).
Fig. 2.
Forest plot displaying analysis of the efficacy of AI versus other (SERM, SERD, and androgen inhibitor) in terms of ORR.
Given the heterogeneity observed, subgroup analysis comparing individual drug classes was performed. Subgroup analysis comparing the ORR between patients who received AI and those who received SERD showed a non-significant difference in ORR (OR = 0.975 [0.798–1.191], p = 0.806). The odds of obtaining ORR in patients receiving AI are approximately 1 compared to those receiving SERD, indicating no difference in odds. No substantial heterogeneity in study characteristics was observed between studies (I2 = 0.396) (shown in Fig. 3). For patients receiving SERM compared to AI, a statistically significant difference in ORR in favour of patients who received SERM (OR = 1.362 [1.033–1.795], p = 0.028). The odds of obtaining ORR in patients receiving SERM are 1.4 compared to those receiving AI. However, substantial heterogeneity in study characteristics was equally observed between studies (I2 = 1.795) (shown in Fig. 4).
Fig. 3.
Forest plot displaying sub-group analysis of the efficacy of AI versus SERD only in terms of ORR.
Fig. 4.
Forest plot displaying sub-group analysis of the efficacy of AI versus SERM only in terms of ORR.
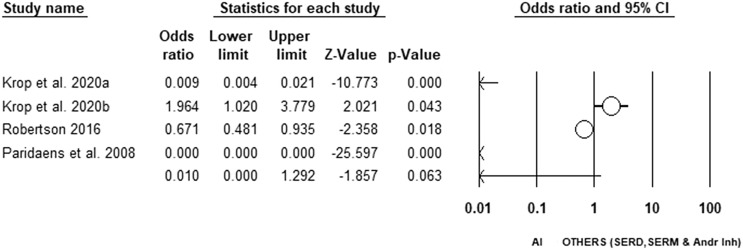
In terms of PFS, we observed a non-statistically significant difference in PFS for patients receiving AI compared to SERM, SERD, and androgen inhibitors. The odds of obtaining PFS in patients receiving AI are lower compared to those receiving SERM, SERD or androgen inhibitors; however, it was not statistically significant (OR = 0.010 [0.000–1.292], p = 0.063). Substantial heterogeneity in study characteristics was observed (I2 = 99.547) (shown in Fig. 5).
Fig. 5.
Forest plot displaying analysis of the efficacy of AI versus other (SERM, SERD, and androgen inhibitor) in terms of PFS.
For OS, a non-statistically significant difference was observed between anastrozole and other AIs (letrozole and exemestane) (OR = 1.718 [0.021–139.128], p = 0.809). The odds of obtaining OS in patients receiving letrozole and exemestane are 1.7 compared to those receiving anastrozole; however, the difference was not statistically significant. No substantial heterogeneity in study characteristics was observed between studies (I2 = 99.380) (shown in Fig. 6).
Fig. 6.
Forest plot displaying analysis of the efficacy of anastrozole versus other (exemestane and letrozole) in terms of OS.
Discussion
Once the decision is made on whether endocrine therapy or chemotherapy is the most optimal initial treatment, the next step is to establish if single therapy or combination therapy will produce the most positive outcomes for the patient [63]. Multiple studies have sought to investigate the effectiveness of different classes of hormonal therapy [47, 59, 60]. In this review, we evaluate these studies and present a subgroup analysis of hormonal classes and agents.
Oestrogen receptor deprivation in post-menopausal women, achieved through the reduction of oestrogen synthesis with AIs or by directly targeting the receptors using selective oestrogen receptor modulators (SERM) or SERDs, has emerged as a crucial strategy for treating hormone receptor-positive (HR+) BC. In this study, patients who received AI had a 1.1 odds of obtaining objective responses (complete or partial) than those who received other hormonal therapies. However, this lacked statistical significance. Similarly, when comparing AI to other hormonal therapies for PFS, we found that patients who received AI had a non-statistically significant improvement in PFS. The observed differences in PFS and ORR did not reach statistical significance. This could suggest either a true equivalence between therapies or insufficient power to detect small but clinically relevant differences [64]. For ABCs, AIs have supplanted SERMs such as tamoxifen due to their partial agonist activity [10, 65]. Regarding survival, AIs have demonstrated superiority over tamoxifen [66]. However, this was not observed in our study. ABC often develops resistance to endocrine therapies, including ESR1 mutations which are the most common. These mutations can lead to oestrogen receptor activation independent of oestrogen levels, thus reducing AI effectiveness more than SERM or SERD.
In the subgroup analysis for each of the therapies, the ORR for patients treated with SERM was significantly higher than that of patients treated with AI, but no significant difference in ORR was observed between AI and SERD. Decision-making to use any of these therapies for ABC may be influenced by factors such as the physician’s assessments of the drug’s benefits, line of treatment, adverse reactions and risks, as well as patient preferences. For post-menopausal women, AIs are the recommended first-line endocrine therapy, either alone or in combination with targeted agents such as cyclin-dependent kinase 4/6 inhibitors [5]. For second-line treatment, it is recommended to administer fulvestrant, a SERD, at a dose of 500 mg using a loading schedule [5]. However, in this meta-analysis, previous hormonal therapy use and the number of lines of treatment were not considered. Furthermore, variations in patient demographics, tumour biology, treatment duration, dose modifications, and prior treatments could dilute the observed treatment effect. In the study, patients had varying treatment histories, some having received previous systemic therapy while others were endocrine-naïve. This variation could affect treatment responses and introduce confounding effects which were not considered in the meta-analysis.
In terms of OS, there was no statistically significant difference between anastrozole and the other AIs (letrozole and exemestane). This may indicate that the three AIs are comparable options for the treatment of ABC. There is a lack of clinical evidence to support the superiority of one AI over the other [10]. However, when taken at the recommended levels, letrozole is said to be a more powerful AI than anastrozole, leading to a higher level of inhibition of aromatase. Geisler et al. [67] found that anastrozole achieved an average aromatase inhibition rate of 97.1%. Conversely, letrozole effectively suppressed aromatase activity by more than 99.1% when administered at typical doses [67]. Thus, drug dosing may contribute to the efficacy of one AI over the other.
Several limitations have been observed in this study. Firstly, the study was exclusive to ABC and excluded studies with mixed stages. Performing a sensitivity analysis for stage-specific outcomes may be more beneficial. Secondly, the high heterogeneity (I2) observed in the meta-analysis of some of the outcomes may affect the interpretability of the results. The analysis with high heterogeneity included a few studies (3 and 4). When a meta-analysis has few studies I2 should be interpreted cautiously [68]. Thirdly, subgroup analysis based on patient-specific outcomes or factors such as previous lines of treatment and prior therapies for ABC was not carried out and may be required. Lastly, the methodological quality of the included studies may be questionable given the rating of “some concern” with the risk of bias tool.
Conclusion
Patients receiving SERM obtained a significantly higher ORR than those who received AI. There was no significant difference in ORR between patients receiving AI and SERD, thus, clinical decisions could be based on factors such as the line of Ca treatment, tumour biology, adverse events, and individual drug benefits. Furthermore, in terms of OS, the three AIs are comparable options for ABC. Although the uptake of newer combination therapies to deal with drug resistance is being adopted and may impact the relevance of this study, these agents explored in this review are still widely used in clinical practice.
Future RCTs and reviews should consider exploring several factors that could impact drug metabolism and moderate the effect of these hormonal therapies such as prior treatment history, and comorbidities. RCTs should explore the efficacy of these therapies in subgroups such as biomarker-defined subgroups (e.g., ESR1-mutant vs. wild-type tumours), lines of treatment in the advanced setting, etc., for better comparisons. The long-term outcomes and OS in patients receiving these hormonal therapies were only explored in a few studies. Future RCTs may consider exploring that. For methodological quality, future RCTs should consider controlling for deviations in intended treatments and where inevitable could use intention to treat analysis. Clear information on the randomization process and study blinding should be provided. The process of matching could be used to control for heterogeneous participant characteristics with adequate subgroup analysis.
Statement of Ethics
The study protocol was submitted to the International Prospective Register of Systematic Reviews (PROSPERO) for registration; registration number CRD42023431939.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Vitalis C. Okwor: conceptualization, methodology, visualization, project administration, resources, supervision, and writing – review and editing. Juliet C. Okwor: conceptualization, methodology, visualization, project administration, resources, supervision, and writing – review and editing. Maryjane K. Ukwuoma: conceptualization, methodology, data curation, visualization, writing – initial draft, and writing – review and editing. Sara B. Mitha: data collection, resources, and editing. Martins C. Nweke: conceptualization, methodology, data curation, analysis, visualization, writing – review and editing, and supervision. All authors approved of the version to be published.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Supplementary Material.
Supplementary Material.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer. 2021;124(1):13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol. 2018;29(8):1634–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol. 2016;34(25):3069–103. [DOI] [PubMed] [Google Scholar]
- 6. Jager A, de Vries EGE, der Houven van Oordt CWM, Neven P, Venema CM, Glaudemans AWJM, et al. A phase 1b study evaluating the effect of elacestrant treatment on estrogen receptor availability and estradiol binding to the estrogen receptor in metastatic breast cancer lesions using 18F-FES PET/CT imaging. Breast Cancer Res. 2020;22(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lux MP, Maass N, Schütz F, Schwidde I, Fasching PA, Fehm T, et al. Breast cancer interpretation of new and known data. Geburtsh Frauenheilk. 2013;73(6):584–98. [Google Scholar]
- 8. Bian L, Xu FR, Jiang ZF. Endocrine therapy combined with targeted therapy in hormone receptor-positive metastatic breast cancer. Chin Med J. 2020;133(19):2338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reinert T, de Paula B, Shafaee MN, Souza PH, Ellis MJ, Bines J. Endocrine therapy for ER-positive/HER2-negative metastatic breast cancer. Chin Clin Oncol. 2018;7(3):25. [DOI] [PubMed] [Google Scholar]
- 11. Grinshpun A, Chen V, Sandusky ZM, Fanning SW, Jeselsohn R. ESR1 activating mutations: from structure to clinical application. Biochim Biophys Acta Rev Cancer. 2023;1878(1):188830. [DOI] [PubMed] [Google Scholar]
- 12. Spoerke J, Gendreau S, Walter K, Qiu J, Wilson TR, Savage H, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7:11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herzog SK, Fuqua SAW. ESR1 mutations and therapeutic resistance in metastatic breast cancer: progress and remaining challenges. Br J Cancer. 2022;126(2):174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozyurt R, Ozpolat B. Molecular mechanisms of anti-estrogen therapy resistance and novel targeted therapies. Cancers. 2022;14(21):5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tokunaga E, Hisamatsu Y, Tanaka K, Yamashita N, Saeki H, Oki E, et al. Molecular mechanisms regulating the hormone sensitivity of breast cancer. Cancer Sci. 2014;105(11):1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rasha F, Sharma M, Pruitt K. Mechanisms of endocrine therapy resistance in breast cancer. Mol Cell Endocrinol. 2021;532(532):111322. [DOI] [PubMed] [Google Scholar]
- 17. Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37(4):496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delgado A, Guddati AK. Clinical endpoints in oncology: a primer. Am J Cancer Res. 2021;11(4):1121–31. [PMC free article] [PubMed] [Google Scholar]
- 20. Ewald H, Klerings I, Wagner G, Heise TL, Stratil JM, Lhachimi SK, et al. Searching two or more databases decreased the risk of missing relevant studies: a metaresearch study. J Clin Epidemiol. 2022;149:154–64. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savović J, Weeks L, Sterne JA, Turner L, Altman DG, Moher D, et al. Evaluation of the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borenstein M. Effect sizes for continuous data. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. Russell Sage Foundation; 2009. p. 221–35. [Google Scholar]
- 24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buzdar A, Hayes D, El-Khoudary A, Yan S, Lønning P, Lichinitser M, et al. Phase III randomized trial of droloxifene and tamoxifen as first-line endocrine treatment of ER/PgR-positive advanced breast cancer. Breast Cancer Res Treat. 2002;73(2):161–75. [DOI] [PubMed] [Google Scholar]
- 26. Campos SM, Guastalla JP, Subar M, Abreu P, Winer EP, Cameron DA. A comparative study of exemestane versus anastrozole in patients with postmenopausal breast cancer with visceral metastases. Clin Breast Cancer. 2009;9(1):39–44. [DOI] [PubMed] [Google Scholar]
- 27. Gershanovich M, Garin A, Baltina D, Kurvet A, Kangas L, Ellmén J. A phase III comparison of two toremifene doses to tamoxifen in postmenopausal women with advanced breast cancer. Eastern European Study Group. Breast Cancer Res Treat. 1997;45(3):251–62. [DOI] [PubMed] [Google Scholar]
- 28. Maung K, O’Shaughnessy J. Randomized Phase II Trial Comparing Exemestane to Tamoxifen for First-Line Hormonal Therapy of Postmenopausal Patients with Metastatic Breast Cancer. Clin Breast Cancer. 2001;2(2):110–112. [Google Scholar]
- 29. Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–95. [DOI] [PubMed] [Google Scholar]
- 30. Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997–3005. [DOI] [PubMed] [Google Scholar]
- 31. Pritchard KI, Rolski J, Papai Z, Mauriac L, Cardoso F, Chang J, et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2). Breast Cancer Res Treat. 2010;123(2):453–61. [DOI] [PubMed] [Google Scholar]
- 32. Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized 'FIRST' study. Breast Cancer Res Treat. 2012;136(2):503–11. [DOI] [PubMed] [Google Scholar]
- 33. Bonneterre J, Thürlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18(22):3748–57. [DOI] [PubMed] [Google Scholar]
- 34. Thürlimann B, Hess D, Köberle D, Senn I, Ballabeni P, Pagani O, et al. Anastrozole (‘Arimidex') versus tamoxifen as first-line therapy in postmenopausal women with advanced breast cancer: results of the double-blind cross-over SAKK trial 21/95--a sub-study of the TARGET (Tamoxifen or “Arimidex” Randomized Group Efficacy and Tolerability) trial. Breast Cancer Res Treat. 2004;85(3):247–54. [DOI] [PubMed] [Google Scholar]
- 35. Rose C, Vtoraya O, Pluzanska A, Davidson N, Gershanovich M, Thomas R, et al. An open randomised trial of second-line endocrine therapy in advanced breast cancer. comparison of the aromatase inhibitors letrozole and anastrozole. Eur J Cancer. 2003;39(16):2318–27. [DOI] [PubMed] [Google Scholar]
- 36. Xu B, Jiang Z, Shao Z, Wang J, Feng J, Song S, et al. Fulvestrant 250 mg versus anastrozole for Chinese patients with advanced breast cancer: results of a multicentre, double-blind, randomised phase III trial. Cancer Chemother Pharmacol. 2011;67(1):223–30. [DOI] [PubMed] [Google Scholar]
- 37. Bajetta E, Zilembo N, Buzzoni R, Noberasco C, Di Leo A, Bartoli C, et al. Endocrinological and clinical evaluation of two doses of formestane in advanced breast cancer. Br J Cancer. 1994;70(1):145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zilembo N, Bajetta E, Noberasco C, Buzzoni R, Vicario G, Bono A, et al. Formestane: an effective first-line endocrine treatment for advanced breast cancer. J Cancer Res Clin Oncol. 1995;121(6):378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26(10):1664–70. [DOI] [PubMed] [Google Scholar]
- 40. Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–600. [DOI] [PubMed] [Google Scholar]
- 41. Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goss P, Bondarenko IN, Manikhas GN, Pendergrass KB, Miller WH Jr, Langecker P, et al. Phase III, double-blind, controlled trial of atamestane plus toremifene compared with letrozole in postmenopausal women with advanced receptor-positive breast cancer. J Clin Oncol. 2007;25(31):4961–6. [DOI] [PubMed] [Google Scholar]
- 43. Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22(9):1605–13. [DOI] [PubMed] [Google Scholar]
- 44. Krop I, Abramson V, Colleoni M, Traina T, Holmes F, Garcia-Estevez L, et al. A Randomized Placebo Controlled Phase II Trial Evaluating Exemestane with or without Enzalutamide in Patients with Hormone Receptor-Positive Breast Cancer. Clin Cancer Res. 2020;26(23):6149–57. [DOI] [PubMed] [Google Scholar]
- 45. Lipton A, Leitzel K, Chaudri-Ross HA, Evans DB, Ali SM, Demers L, et al. Serum TIMP-1 and response to the aromatase inhibitor letrozole versus tamoxifen in metastatic breast cancer. J Clin Oncol. 2008;26(16):2653–8. [DOI] [PubMed] [Google Scholar]
- 46. Mauriac L, Romieu G, Bines J. Activity of fulvestrant versus exemestane in advanced breast cancer patients with or without visceral metastases: data from the EFECT trial. Breast Cancer Res Treat. 2009;117(1):69–75. [DOI] [PubMed] [Google Scholar]
- 47. Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Overall survival with fulvestrant plus anastrozole in metastatic breast cancer. N Engl J Med. 2019;380(13):1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mouridsen H, Gershanovich M, Sun Y, Pérez-Carrión R, Boni C, Monnier A, Apffelstaedt J, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19(10):2596–606. [DOI] [PubMed] [Google Scholar]
- 49. Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18(22):3758–67. [DOI] [PubMed] [Google Scholar]
- 50. Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27(27):4530–5. [DOI] [PubMed] [Google Scholar]
- 51. Arpino G, Nair Krishnan M, Doval Dinesh C, Bardou VJ, Clark GM, Elledge RM. Idoxifene versus tamoxifen: a randomized comparison in postmenopausal patients with metastatic breast cancer. Ann Oncol. 2003;14(2):233–41. [DOI] [PubMed] [Google Scholar]
- 52. Gershanovich M, Chaudri HA, Campos D, Lurie H, Bonaventura A, Jeffrey M, et al. Letrozole, a new oral aromatase inhibitor: randomised trial comparing 2.5 mg daily, 0.5 mg daily and aminoglutethimide in postmenopausal women with advanced breast cancer. Letrozole International Trial Group (AR/BC3). Ann Oncol. 1998;9(6):639–45. [DOI] [PubMed] [Google Scholar]
- 53. Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiówka M, Hewson N, et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II FIRST Study. J Clin Oncol. 2015;33(32):3781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ingle JN, Johnson PA, Suman VJ, Gerstner JB, Mailliard JA, Camoriano JK, et al. A randomized phase II trial of two dosage levels of letrozole as third-line hormonal therapy for women with metastatic breast carcinoma. Cancer. 1997;80(2):218–24. [DOI] [PubMed] [Google Scholar]
- 55. Mouridsen HT. Letrozole in advanced breast cancer: the PO25 trial. Breast Cancer Res Treat. 2007;105(Suppl 1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ohno S, Rai Y, Iwata H, Yamamoto N, Yoshida M, Iwase H, et al. Three dose regimens of fulvestrant in postmenopausal Japanese women with advanced breast cancer: results from a double-blind, phase II comparative study (FINDER1). Ann Oncol. 2010;21(12):2342–7. [DOI] [PubMed] [Google Scholar]
- 57. Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(30):4883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thürlimann B, Beretta K, Bacchi M, Castiglione-Gertsch M, Goldhirsch A, Jungi WF, et al. First-line fadrozole HCI (CGS 16949A) versus tamoxifen in postmenopausal women with advanced breast cancer. Prospective randomised trial of the Swiss Group for Clinical Cancer Research SAKK 20/88. Ann Oncol. 1996;7(5):471–9. [DOI] [PubMed] [Google Scholar]
- 59. Wang J, Cai L, Song Y, Sun T, Tong Z, Teng Y, et al. Clinical efficacy of fulvestrant versus exemestane as first-line therapies for Chinese postmenopausal oestrogen-receptor positive /human epidermal growth factor receptor 2 -advanced breast cancer (FRIEND study). Eur J Cancer. 2023;184:73–82. [DOI] [PubMed] [Google Scholar]
- 60. Yamamoto Y, Ishikawa T, Hozumi Y, Ikeda M, Iwata H, Yamashita H, et al. Randomized controlled trial of toremifene 120 mg compared with exemestane 25 mg after prior treatment with a non-steroidal aromatase inhibitor in postmenopausal women with hormone receptor-positive metastatic breast cancer. BMC Cancer. 2013;13:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Q, Shao Z, Shen K, Li L, Feng J, Tong Z, et al. Fulvestrant 500 mg vs 250 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer: a randomized, double-blind registrational trial in China. Oncotarget. 2016;7(35):57301–57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Llombart-Cussac A, Ruiz A, Antón A, Barnadas A, Antolín S, Alés-Martínez JE, et al. Exemestane versus anastrozole as front-line endocrine therapy in postmenopausal patients with hormone receptor-positive, advanced breast cancer: final results from the Spanish Breast Cancer Group 2001-03 phase 2 randomized trial. Cancer. 2012;118(1):241–7. [DOI] [PubMed] [Google Scholar]
- 63. Manohar PM, Davidson NE. Updates in endocrine therapy for metastatic breast cancer. Cancer Biol Med. 2021;19(2):202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tsang R, Colley L, Lynd LD. Inadequate statistical power to detect clinically significant differences in adverse event rates in randomized controlled trials. J Clin Epidemiol. 2009;62(6):609–16. [DOI] [PubMed] [Google Scholar]
- 65. Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract. 2007;61(12):2051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98(18):1285–91. [DOI] [PubMed] [Google Scholar]
- 67. Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20(3):751–7. [DOI] [PubMed] [Google Scholar]
- 68. von Hippel PT. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol. 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.