Abstract
Purpose
The purpose of this study was to assess the impact of testosterone therapy on mood and cognitive symptoms in perimenopausal and postmenopausal women.
Methods
A retrospective cohort study undertaken in a UK specialist menopause clinic. 510 women using hormone replacement therapy (HRT) with persistent low libido, cognitive and negative mood symptoms were treated with testosterone cream or gel for 4 months. A modified version of the Greene Climacteric Scale was used to measure self-reported symptom frequency and severity at baseline and 4 months after initiating treatment.
Results
All nine cognitive and mood symptoms significantly improved across the study period. Mood improved more than cognition (47% of women reported an improvement in mood vs. 39% reported an improvement in cognition; 34% vs. 22% decrease in mean symptom scores, respectively). Regarding libido, 52% of women reported an improvement; mean symptom score decreased by 33%.
Conclusion
Transdermal testosterone therapy for 4 months was associated with significant improvements in mood and cognition. Further research including randomised clinical trials are needed to establish the long-term efficacy and safety of testosterone for the treatment of menopausal cognitive and psychological symptoms.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00737-024-01513-6.
Keywords: Menopause, Mental health, Cognition, Mood, Hormone replacement therapy, Testosterone
Highlights
The findings of this pilot study suggest that transdermal testosterone therapy has a beneficial effect on cognitive and mood-related symptoms in peri- and postmenopausal women. Mood improved more than cognition, suggesting that testosterone may have differential effects on neuropsychiatric symptoms and highlighting the need for further research to determine which women are most likely to benefit from testosterone therapy.
Mood and libido improved to a similar degree, suggesting that testosterone may have benefits beyond the treatment of Hypoactive Sexual Desire Disorder in postmenopausal women, the only indication for which testosterone is currently licensed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00737-024-01513-6.
Introduction
Perimenopause and menopause are associated with neurological symptoms resulting from fluctuating and declining sex steroid hormone levels in the brain (Brinton et al. 2015). Approximately two thirds of women report subjective cognitive impairment - ‘brain fog’ - with wide-ranging symptoms including fatigue, difficulty concentrating, poor memory, reduced verbal fluency, and reduced ability to multitask and plan (Reuben et al. 2021). Negative mood symptoms such as low mood, anxiety, irritability, loss of confidence and drive, and feeling overwhelmed, are also common (Leonhardt 2019; Maki et al. 2019; Riecher-Rössler 2020; O’Reilly et al. 2023). The risk of a major depressive episode is increased 2- to 4-fold (Bromberger et al. 2011). Suicidal thoughts are up to eight times more prevalent in perimenopausal women (Usall et al. 2009), which may contribute to a higher suicide rate in women aged 45 to 64 years in the UK compared with younger and older women (Statistics 2022; Branch 2023).
Hormone replacement therapy (HRT) – oestrogen with or without a progestogen – is the most effective treatment for menopausal symptoms including cognitive impairment and negative mood symptoms (Riecher-Rössler and Geyter 2007; Kulkarni 2018; Behrman and Crockett 2023) Some women require additional psychiatric support and/or antidepressant medication, especially those with a past history of depression, but there is no clear evidence of benefit for selective serotonin reuptake inhibitors (SSRIs) when used to treat low mood in perimenopausal women without a formal diagnosis of clinical depression (NICE 2019).
Menopausal women with cognitive impairment and negative mood symptoms may also benefit from testosterone replacement therapy. Testosterone improves libido and vulvovaginal health, and has anti-inflammatory, cardioprotective, bone-protective, and neuroprotective effects (Davis 2021). In men, testosterone deficiency is associated with fatigue, depressed mood, and cognitive impairment (Hackett et al. 2023). In women, circulating testosterone levels decline with age and are up to 50% lower in menopausal women compared with younger adult women (Davis 2023), which is likely to contribute to symptoms including brain fog and depressed mood in mid-life.
A recent meta-analysis concluded that there is insufficient randomised clinical trial (RCT) evidence to support the use of testosterone to treat any menopausal symptom other than low libido (Islam et al. 2019). Consequently, current guidelines only recommend testosterone to treat low sexual desire in menopausal women, if HRT alone is not effective (Davis et al. 2019; NICE 2019). Nevertheless, women are often prescribed testosterone in clinical practice because the available data, and physiological rationale, suggest overall benefit.
The aim of this study was to assess the effect of transdermal testosterone on cognitive and negative mood symptoms in perimenopausal and postmenopausal women with persistent symptoms despite standard HRT (oestrogen with or without progesterone). We focussed on women using transdermal testosterone because, compared with other testosterone formulations (oral, intramuscular, and subcutaneous pellets), transdermal testosterone is body-identical (biochemically identical to ‘natural’ testosterone) and better tolerated. Side effects such as acne and localised hair growth at the site of application are usually mild, easily treated and reversible; and transdermal testosterone is not associated with an increased risk of serious adverse events when used in physiologic doses (i.e., doses that approximate physiological testosterone concentrations in premenopausal women) (Davis et al. 2019; Parish et al. 2021). Oral and injectable formulations containing synthetic testosterones alien to the female body are rarely prescribed due to their inferior safety profile.
Materials and methods
Study design and population
This was a single-centre, UK-based, retrospective cohort study performed as part of a service evaluation. Women attending the Newson Health Menopause and Wellbeing Centre in Stratford-upon-Avon between 1st August 2021 and 31st July 2022 were eligible for inclusion if (1) they had been using transdermal oestrogen with or without a progestogen (oral micronised progesterone or the levonorgestrel-releasing intrauterine system) for at least three months, (2) they had persistent low libido and at least one mood or cognitive symptom, (3) they had initiated transdermal testosterone without a concurrent oestradiol dose change, (4) they had completed a menopause symptom questionnaire (MSQ) at baseline (prior to initiation of testosterone), and (5) they had been reviewed and had completed an MSQ four months after initiating testosterone therapy. MSQ forms were collected as part of routine clinical care. Informed consent was obtained from all patients for their data to be used for research and audit purposes.
Outcomes
Characteristics of the study population (demographics, testosterone formulation, and MSQ scores) were extracted from the medical records.
Symptom scores
Prior to each clinical encounter, all patients completed the MSQ, a modified version of the Greene Climacteric Scale (GCS) (Greene 1976) (Supplementary File 1). The Greene Climacteric Scale is a 21-item questionnaire that measures the number and severity of menopausal symptoms and has been validated for use in different populations (Greene 1998). Patients rate the severity of each symptom using a 4-point Likert scale, from 0 (‘not at all’) to 3 (‘extremely’).
The MSQ includes 18 of the 21 symptoms listed in the original GCS. Three items have been removed (‘excitable’, ‘parts of body feel numb’, and ‘loss of feeling in hands or feet’) and replaced with 5 new items (‘memory problems’, ‘tinnitus’, ‘pins and needles in any part of the body’, ‘urinary symptoms’, and ‘symptoms due to vaginal dryness’).
The focus of this study was to assess the impact of testosterone on neuropsychological symptoms. Consequently, only data pertaining to cognition (‘feeling tired or lacking in energy’, ‘memory problems’, and ‘difficulty in concentrating’), and mood (‘feeling tense of nervous’, ‘irritability’, ‘attacks of anxiety or panic’, ‘feeling unhappy or depressed’, ‘loss of interest in most things’, ‘crying spells’, and ‘loss of interest in sex’) was extracted from the medical records (10 items; overall score range 0–30).
Testosterone treatment
Patients were treated with one of three transdermal testosterone formulations according to patient preference; AndroFeme® 1% (10 mg/ml), 0.5 ml daily (Lawley Pharmaceuticals Ltd, Perth, Western Australia), Testim 50 mg/5 g, one tube over 10 days(Endo Ventures Ltd, Dublin, Ireland), or Testogel 40.5 mg/2.5 g, one sachet over 8 days (Besins Healthcare, Paris, France).
Data analysis
Statistical analysis was performed using R software v4.3.
All patients were included in the baseline analysis for symptom prevalence. Per symptom, only patients who scored a 1, 2 or 3 before starting testosterone were included when analysing the percentage of women who reported symptom improvement and the change in symptom severity score at 4 months. Patients who assigned a score of 0 to a symptom at baseline were excluded from the 4-month analysis for that individual symptom. For example, a patient who scored ‘fatigue’ a 1, 2, or 3 at baseline was included in the 4-month analysis for change in ‘fatigue’ symptom score; but if the same patient scored ‘tearfulness’ a 0 at baseline, she was not included in the 4-month analysis for change in ‘tearfulness’ symptom score.
Counts and percentages were used to summarise categorical and ordinal variables. The mean (SD) and the median (IQR) were used to summarise continuous normal and non-normal variables, respectively. McNemar’s test was used to compare symptom prevalence before and after treatment. A paired t-test was used to compare the mean symptom scores before and after treatment. Hypothesis testing was performed at a 5% level of significance.
Results
Baseline characteristics
510 women with persistent menopausal symptoms despite ≥ 3 months of HRT (oestrogen with or without a progestogen) initiated transdermal testosterone therapy during the study period, without a concurrent oestradiol dose increase (Supplementary file 2). The mean age was 54 years (IQR 50–58 years). 339 women (66%) were postmenopausal, 171 women (34%) were perimenopausal. 462 (91%) were using oestrogen combined with a progestogen – either micronised progesterone (n = 428) or the Mirena coil (n = 34); 48 (9%) were using oestrogen alone. 88 women (17%) were co-prescribed antidepressants during the study interval.
Most women were treated with AndroFeme (n = 443, 87%); 41 women (8%) received Testim and 26 (5%) received Testogel. The mean time from testosterone initiation to first review appointment was 121 ± 36.4 days.
Symptom prevalence
Symptom prevalence at baseline, and the number (%) of patients whose symptom scores improved after initiating testosterone therapy are presented in Tables 1 and 2, and Fig. 1.
Table 1.
The number (%) of patients who reported symptoms at baseline, and the number (%) of patients who reported an improvement (a reduction in symptom score from ‘3’ to ‘2’,‘1’ or ‘0’; ‘2’ to ‘1’ or ‘0’; or ‘1’ to ‘0’) after 4 months of testosterone therapy
| Symptom | Symptom prevalence at baseline (n = 510) | % of patients who reported symptom improvement at 4 months | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Cognitive symptoms | |||||
| Feeling tired or lacking in energy | 470 | 92% | 201 | 43% | < 0.001 |
| Memory problems | 462 | 91% | 157 | 34% | < 0.001 |
| Difficulty in concentrating | 427 | 84% | 174 | 41% | < 0.001 |
| Mood symptoms | |||||
| Feeling tense or nervous | 415 | 81% | 156 | 38% | < 0.001 |
| Irritability | 392 | 77% | 147 | 38% | < 0.001 |
| Feeling unhappy or depressed | 327 | 64% | 142 | 43% | < 0.001 |
| Loss of interest in most things | 325 | 64% | 183 | 56% | < 0.001 |
| Attacks of anxiety of panic | 290 | 59% | 144 | 50% | < 0.001 |
| Crying spells | 212 | 42% | 117 | 55% | < 0.001 |
| Loss of interest in sex | 510 | 100% | 265 | 52% | < 0.001 |
Table 2.
The distribution of symptom scores (‘0’ - ‘not at all’, ‘1’ - ‘a little’, ‘2’ - ‘quite a bit’, and ‘3’ - ‘extremely’) at baseline and 4 months after initiating testosterone therapy, and the change in prevalence of moderate-severe symptoms (score ‘2’ + ‘3’) across the study period (the absolute decrease and the % reduction in number of women reporting moderate or severe symptoms)
| Score | Pre testosterone | Post testosterone | % decrease |
% improvement | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 2 + 3 | 2 + 3 | |
| Cognitive symptoms | ||||||||||
| Feeling tired or lacking in energy |
40 (7.84%) |
209 (40.98%) |
186 (36.47%) |
75 (14.71%) |
67 (13.14%) |
259 (50.78%) |
140 (27.45% |
44 (8.63%) |
15.1% | 29.5% |
| Memory problems |
48 (9.41%) |
271 (53.14%) |
161 (31.57%) |
30 (5.88%) |
96 (18.82%) |
271 (53.14%) |
124 (24.31%) |
19 (3.73%) |
9.4% | 25.1% |
| Difficulty in concentrating |
83 (16.27%) |
269 (52.75%) |
131 (25.69%) |
27 (5.29%) |
132 (25.88%) |
280 (54.90%) |
86 (16.86%) |
12 (2.35%) |
11.8% | 38.0% |
| Mood symptoms | ||||||||||
| Feeling tense or nervous |
95 (18.63%) |
286 (56.08%) |
113 (22.16%) |
16 (3.14%) |
138 (27.06%) |
280 (54.90%) |
83 (16.27%) |
9 (1.76%) |
7.3% | 28.7% |
| Irritability |
118 (23.14%) |
274 (53.73%) |
103 (20.20%) |
15 (2.94%) |
147 (28.82%) |
287 (56.27%) |
70 (13.73%) |
6 (1.18%) |
8.2% | 35.6% |
| Feeling unhappy or depressed |
183 (35.88%) |
247 (48.43%) |
68 (13.33%) |
12 (2.35%) |
239 (46.86%) |
215 (42.16%) |
52 (10.20%) |
4 (0.78%) |
4.7% | 30.0% |
| Loss of interest in most things |
185 (36.27%) |
216 (42.35%) |
98 (19.22%) |
11 (2.16%) |
270 (52.94%) |
182 (35.69%) |
52 (10.20%) |
6 (1.18%) |
10.0% | 46.8% |
| Attacks of anxiety or panic |
220 (43.14%) |
206 (40.39%) |
71 (13.92%) |
13 (2.55%) |
263 (51.57%) |
197 (38.63%) |
45 (8.82%) |
5 (0.98%) |
6.7% | 40.5% |
| Crying spells |
298 (58.43%) |
169 (33.14%) |
39 (7.65%) |
4 (0.78%) |
355 (69.61%) |
124 (24.31%) |
26 (5.10%) |
5 (0.98%) |
2.4% | 27.9% |
| Loss of interest in sex |
0 (0%) |
159 (31.18%) |
187 (36.67%) |
164 (32.16%) |
81 (15.58%) |
224 (43.92%) |
148 (29.02%) |
57 (11.18%) |
28.6% | 41.6% |
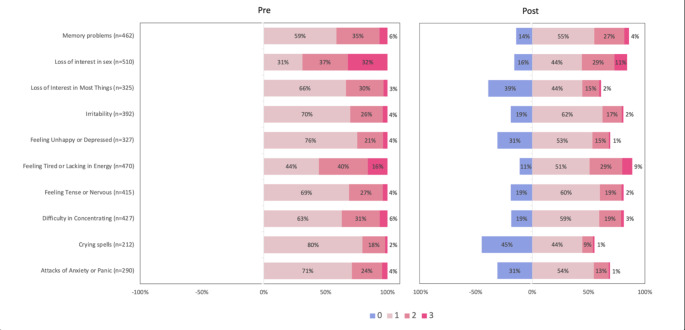
Fig. 1.
The shift in the distribution of symptom severity scores across the 4-month study period. Scores of ‘3’ (‘extremely’) are shaded dark pink, scores of ‘2’ (‘quite a bit’) are shaded mid-pink, scores of ‘1’ (‘a little’) are shaded light pink, and scores of ‘0’ (‘not at all’) are shaded blue. A ‘left shift’ (pink to blue) indicates symptom improvement. For each symptom, only patients reporting symptoms at baseline (score 1, 2 or 3) are included
Cognitive symptoms were more frequently reported than mood symptoms at baseline (mean prevalence 89%,range 84–92%, vs. 64.5%,range 42–81%, respectively; Table 1). ‘Feeling tired or lacking in energy’ and ‘memory problems’ were the most prevalent cognitive symptoms (92% and 91%, respectively); ‘feeling tense or nervous’ and ‘irritability’ were the two most frequently reported mood symptoms (81% and 77% of women, respectively). ‘Crying spells’ were the least prevalent symptom (42% of women). 100% of women reported reduced libido, as this was an inclusion criterion.
When stratified by symptom severity (Table 2), loss of libido was the most bothersome symptom at baseline (68.83% of women scored ‘loss of interest in sex’ a ‘2’ - ‘quite a bit’, or ‘3’ - ‘extremely’). Cognitive symptoms were more bothersome than mood symptoms; 39.87% (range 30.98–51.18%) of women rated their cognitive symptoms moderate or severe (a score of ‘2’ or ‘3’), compared with 18.40% (range 8.43–25.30%) of women with mood symptoms. Fatigue and memory problems were most likely to be assigned a score of ‘2’ or ‘3’ (51.18% and 37.45% of women, respectively). ‘Tense/nervous’ and ‘irritability’ were the most bothersome mood symptoms (assigned a score of ‘2’ or ‘3’ by 25.30% and 23.14% of women, respectively). ‘Crying spells’ were the least bothersome symptom (8.43% of women).
All 10 symptoms significantly improved four months after initiating testosterone therapy (Table 1). 39% (range 34–43%) of women with cognitive symptoms reported an improvement vs. 47% (range 38–56%) of women with mood symptoms. The three symptoms most likely to improve were ‘loss of interest in most things’ (56% of women reported an improvement, p < 0.001), ‘crying spells’ (55% of women, p < 0.001), and ‘loss of interest in sex’ (52% of women, p < 0.001). ‘Memory problems’ were the least likely to improve (34% of women reported an improvement, p < 0.001).
When stratified by symptom severity (Table 2; Fig. 1), the number of women with moderate or severe symptoms (rated ‘2’ or ‘3’) decreased by approximately one third (35%) across the study period. The prevalence of moderate-to-severe cognitive symptoms decreased by 31% vs. moderate-to-severe mood symptoms decreased by 37% vs. moderate-to-severe ‘loss of interest in sex’ decreased by 42%. The greatest improvements were observed in women with ‘loss of interest in most things’ (the number of women with moderate to severe symptoms decreased by 47%), ‘loss of interest in sex’ (42% reduction), ‘attacks of anxiety and panic’ (41% reduction), and ‘crying spells’ (41% reduction).
Between 41.8% and 66.7% of women reported no change in individual symptom scores during the study period, mainly because the majority of women without a particular symptom at baseline didn’t develop that symptom during the study interval (Supplementary files 3 and 4). For example, 298 women did not experience ‘crying spells’ at baseline, and 260 women did not report ‘crying spells’ at follow up (only 38 women developed new-onset ‘crying spells’).
Individual symptom scores increased in 6.3 to 18.0% of women (a new symptom or increase in symptom severity) over the 4-month study interval (Supplementary file 3).
Symptom severity scores
Mean symptom scores at baseline and four months after initiating testosterone therapy, and the change in the mean symptom scores, are presented in Table 3; Fig. 2.
Table 3.
The mean symptom score at baseline and four months after initiating transdermal testosterone therapy, and the change in mean symptom score.
| Symptom | n | Mean symptom score at baseline Mean (SD) |
Mean symptom score at 4 months Mean (SD) |
Change in symptom score | p |
|---|---|---|---|---|---|
| Cognitive symptoms | |||||
| Feeling tired or lacking in energy | 470 | 1.71 (0.72) | 1.36 (0.80) | − 0.36 | < 0.001 |
| Memory problems | 462 | 1.48 (0.62) | 1.21 (0.73) | − 0.28 | < 0.001 |
| Difficulty in concentrating | 427 | 1.43 (0.61) | 1.06 (0.69) | − 0.37 | < 0.001 |
| Mood symptoms | |||||
| Feeling tense of nervous | 415 | 1.35 (0.55) | 1.04 (0.68) | − 0.31 | < 0.001 |
| Irritability | 392 | 1.34 (0.55) | 1.01 (0.65) | − 0.33 | < 0.001 |
| Feeling unhappy or depressed | 327 | 1.28 (0.53) | 0.86 (0.70) | − 0.42 | < 0.001 |
| Loss of interest in most things | 325 | 1.37 (0.55) | 0.79 (0.75) | − 0.58 | < 0.001 |
| Attacks of anxiety of panic | 290 | 1.33 (0.56) | 0.85 (0.69) | − 0.49 | < 0.001 |
| Crying spells | 212 | 1.22 (0.46) | 0.67 (0.70) | − 0.55 | < 0.001 |
| Loss of interest in sex | 510 | 2.01 (0.80) | 1.35 (0.88) | − 0.66 | < 0.001 |
Fig. 2.
The percentage change (improvement) in mean symptom score from baseline (prior to testosterone treatment) to four months after initiating testosterone therapy. Cognitive symptoms are highlighted in blue, mood symptoms in green, and libido in purple
At baseline, ‘loss of interest in sex’, ‘feeling tired or lacking in energy’, and ‘memory problems’ achieved the highest symptom scores, and ‘crying spells’ achieved the lowest score (Table 3). All 10 symptom scores significantly improved across the four-month study period (Fig. 2). The mean cognitive symptom score decreased by 22% (range 18 to 26%) vs. the mean mood-related symptom score decreased by by 34% (range 23–45%). Libido decreased by 33%.
Discussion
Summary of findings
Cognitive and negative mood symptoms were highly prevalent in this real-world cohort of 510 perimenopausal and postmenopausal women, despite use of oestrogen with or without a progestogen for at least three months. Transdermal testosterone therapy for four months was associated with significant improvements in all mood and cognitive symptoms (reductions in both symptom frequency and severity).
Overall, mood improved more than cognition: 47% of women reported an improvement in mood vs. 39% of women reported an improvement in cognition; 37% of women with moderate-to-severe mood symptoms reported an improvement vs. 31% of women with moderate-to-severe cognitive symptoms; mean symptom scores decreased by 34% vs. 22% respectively. The improvement in mood was comparable to the improvement in libido: 47% of women reported an improvement in mood vs. 52% reported an improvement in libido; the mean mood symptom score decreased by 34% vs. the libido symptom score decreased by 33%. The finding that mood and libido improved to a similar degree is not surprising given that libido is a mood – an affective state, or a ‘pervasive and sustained feeling tone that is experienced internally’ (Sekhon and Gupta 2024). Further, libido and mood are closely interlinked; women are more likely to feel ‘in the mood’ for sex if they are energised and their mood is positive, whereas fatigue and negative mood symptoms (stress, irritability, low mood, anxiety) have a deleterious effect on sexual desire (Arcos-Romero and Calvillo 2023).
Testosterone is currently only recommended for menopausal women with low sexual desire, if HRT alone is not effective (off-license use) (NICE 2015; Davis et al. 2019). Our results suggest that testosterone therapy also benefits mood and cognition and may be a useful adjunct to oestrogen and progestogen therapy for peri- and postmenopausal women with neuro-psychiatric symptoms. Further, testosterone therapy may obviate the need for higher oestrogen doses in women with persistent menopausal symptoms after initiating HRT - an ‘oestrogen-sparing effect’ – reducing the risk of oestrogenic side effects such as bleeding and mastalgia, and facilitating customisation of HRT dose and regimen. Accordingly, patients using standard HRT with ongoing mood and cognitive symptoms can consider a trial of testosterone therapy (off-license use) provided they understand that the available evidence is limited, and have been supported to make an informed treatment choice.
Research in context
Our results align with those of previous studies that have demonstrated improvements in mood and cognition in women treated with testosterone therapy (Sherwin and Gelfand 1985; Sherwin 1988a, b; Dias et al. 2006; Shah et al. 2006; Davison et al. 2011; Glaser et al. 2011; Davis et al. 2014).
RCT data regarding the impact of testosterone on negative mood symptoms is limited. In 1985, a double-blind RCT (n = 43) found that surgically menopausal women randomised to testosterone alone, or testosterone combined with oestrogen, had more energy, improved wellbeing, and greater improvements in somatic and psychological symptom scores compared with women treated with oestrogen alone or a placebo (Sherwin and Gelfand 1985). In 2006, a 24-week, double-blind RCT assessed the effect of adding methyltestosterone with or without HRT to venlafaxine in 72 women with postmenopausal depression. A trend towards increased remission rates was observed in each treatment group relative to venlafaxine plus placebo, but only women in the venlafaxine plus methyltestosterone group were significantly more likely to remit. Unfortunately, the study lacked power because the dropout rate was high (a third of the women dropped out, mainly due to venlafaxine-related side effects), and the authors were unable to draw definitive conclusions (Dias et al. 2006).
Similarly, there is a paucity of RCT data regarding the impact of testosterone on menopausal cognitive symptoms. In 1988, a small RCT demonstrated a decrease in cognitive symptom scores following bilateral oophorectomy that was prevented in women treated with oestrogen, testosterone, or a combination of both hormones (Sherwin 1988b). The improvement in symptom scores was similar across all three treatment groups and concurrent with changes in circulating hormone levels. However, the sample size was small (n = 50), women were treated with synthetic hormones administered by monthly depot injection, and testosterone levels exceeded the upper limit of the normal reference range during the treatment phase. Thus, the improvement in cognitive function in women treated with intramuscular testosterone benzoate may have been due to a pharmacologic effect, rather than a physiologic effect of testosterone. More recent RCTs (combined n = 177) have demonstrated a beneficial effect of transdermal testosterone on learning and memory both in women using and not using concurrent oestrogen (Shah et al. 2006; Davison et al. 2011; Davis et al. 2014).
Because RCT data is limited, systematic reviews and meta-analyses have mainly pooled data from trials in which the primary outcome was the effect of testosterone on sexual function, and concluded that testosterone does not improve cognition or mood in menopausal women (Islam et al. 2019; Stuursma et al. 2022). However, the studies included have generally been small (cognitive symptoms: 3 RCTs, n = 213 women including 102 women treated with testosterone; depressive symptoms: 4 RCTs, n = 636 women including 313 women treated with testosterone) and vary considerably in design (Islam et al. 2019). Women have received different doses and formulations of testosterone (creams, patches, oral, or intramuscular), over periods ranging from 12 to 26 weeks, in studies that have used different measurement scales to assess similar endpoints, with mixed results. ‘Absence of evidence is not evidence of absence’ (Feres and Feres 2023), and well-designed, adequately powered, RCTs are urgently needed to assess the effect of testosterone therapy on menopausal cognitive and negative mood symptoms.
Strengths and limitation
The present study has several strengths. The impact of testosterone on mood and cognition was assessed in 510 women, which is more than the number of women treated with testosterone in all the RCTs combined (n = 415) (Islam et al. 2019). All women receiving combined HRT prior to and concurrent with testosterone therapy used either micronised progesterone (n = 428) or the Mirena coil (n = 34). None received systemic, synthetic progestins, which can exert androgenic effects that might have confounded the results. Testosterone was initiated three or more months after starting HRT or an HRT dose adjustment. Clinical response to HRT is variable but most women respond within three months at which time the dose and/or regimen are adjusted if there are ongoing symptoms (BMS 2020). Consequently, it is likely that the benefit observed during the study period resulted from the addition of testosterone to the HRT regimen, rather than a delayed or latent effect of oestradiol. The use of a validated symptom questionnaire that measured changes in both depressive and anxiety-related mood symptoms enabled us to collect more comprehensive data than previous studies that have mainly focused on a limited number of depressive symptoms. Questionnaires were completed in real-time, at baseline and at the time of the review consultation, reducing the risk of recall bias.
This study is also subject to several limitations. First, this was a retrospective cohort study with no control arm. The clinical improvement may therefore represent a placebo effect or result from confounding factors not adjusted for in our analysis. For example, we did not include data regarding the effect of testosterone on other menopausal symptoms such as night sweats and insomnia, that can also negatively impact mood and cognition. Additionally, antidepressant use was recorded at baseline but not at 4 months, and it is possible that some women initiated antidepressant therapy or received other treatment for psychological symptoms during the study interval. Second, we did not assess adherence to treatment. No studies have measured adherence to transdermal testosterone therapy (cream and gel) in women. Transdermal testosterone is usually well tolerated (Davis et al. 2019; Parish et al. 2021) and, in our clinical experience, few women discontinue treatment due to side effects. However, other factors such as inconvenience, forgetfulness, and lack of perceived benefit (possibly due to poor transdermal absorption - discussed further below), may result in suboptimal adherence and limit the potential benefits associated with testosterone therapy. Third, there is significant interindividual variation in transdermal drug absorption (Farahmand and Maibach 2009; Singh and Morris 2011), and some women need higher doses to achieve physiological levels. For the purpose of this study we did not collect data regarding serum testosterone levels before and during treatment, and therefore we were unable to confirm whether physiological levels were achieved in all women. Given that all women received a standard testosterone dose, it is unlikely that therapeutic levels were achieved across the whole cohort and further research is needed to assess clinical response following dose optimisation. Fourth, the study duration was short. Mood and cognitive symptoms fluctuate in perimenopause and early menopause, and longer studies are needed to assess efficacy in women treated with testosterone for more than four months. Moreover, it can take up to 6 months to see a clinical response to testosterone therapy (Panay 2022), assuming levels are in the physiological range. Benefit is therefore likely to have been underestimated because outcomes were assessed after only four months. Finally, women attending a specialist menopause clinic may be more likely to experience difficult symptoms and/or have issues with absorption compared with women managed in primary care. As such, symptom frequency at baseline and the prevalence of ‘poor absorbers’ (women with subtherapeutic testosterone levels and persistent symptoms) may be higher than that of women in the general population, which decreases the generalisability of our results.
Conclusion
In this pilot study, use of transdermal body-identical testosterone for four months was associated with significant improvements in cognition and mood in a real-world cohort of perimenopausal and postmenopausal women attending a UK-based specialist menopause clinic. This supports the notion that testosterone has neuropsychological benefits beyond libido, and signals the need for further research including well-designed, sufficiently powered clinical trials, to establish the long-term efficacy and safety of testosterone for the treatment of menopausal mood and cognitive symptoms Assessment of clinical outcomes in surgically menopausal women and women with premature ovarian insufficiency is also needed, to establish the efficacy and safety of testosterone therapy in different patient populations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementarymaterials.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arcos-Romero AI, Calvillo C (2023) Sexual health and Psychological Well-Being of women: a systematic review. Healthcare 11:3025. 10.3390/healthcare11233025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman S, Crockett C (2023) Severe mental illness and the perimenopause. BJPsych Bull 1–7. 10.1192/bjb.2023.89 [DOI] [PMC free article] [PubMed]
- BMS (2020) BMS – Tools for Clinicians. In: Guide for Clinicians. https://thebms.org.uk/wp-content/uploads/2022/12/04-BMS-TfC-HRT-Guide-NOV2022-A.pdf. Accessed 18 Aug 2024
- Branch HSI (2023) Investigation report: Care delivery within community mental health teams. https://www.hssib.org.uk/patient-safety-investigations/care-delivery-within-community-mental-health-teams/investigation-report/#42-impact-of-menopause-on-mental-health. Accessed 5 Feb 2024
- Brinton RD, Yao J, Yin F et al (2015) Perimenopause as a neurological transition state. Nat Rev Endocrinol 11:393–405. 10.1038/nrendo.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Chang Y-F et al (2011) Major depression during and after the menopausal transition: study of women’s Health across the Nation (SWAN). Psychol Med 41:1879–1888. 10.1017/s003329171100016x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR (2021) Use of Testosterone in Postmenopausal Women. Endocrinol Metab Clin North Am 50:113–124. 10.1016/j.ecl.2020.11.002 [DOI] [PubMed] [Google Scholar]
- Davis SR (2023) Testosterone for women: certainties and uncertainties. Climacteric 26:21–24. 10.1080/13697137.2022.2146492 [DOI] [PubMed] [Google Scholar]
- Davis SR, Jane F, Robinson PJ et al (2014) Transdermal testosterone improves verbal learning and memory in postmenopausal women not on oestrogen therapy. Clin Endocrinol 81:621–628. 10.1111/cen.12459 [DOI] [PubMed] [Google Scholar]
- Davis SR, Baber R, Panay N et al (2019) Global Consensus position Statement on the Use of Testosterone Therapy for women. J Clin Endocrinol Metab 104:4660–4666. 10.1210/jc.2019-01603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison SL, Bell RJ, Gavrilescu M et al (2011) Testosterone improves verbal learning and memory in postmenopausal women: results from a pilot study. Maturitas 70:307–311. 10.1016/j.maturitas.2011.08.006 [DOI] [PubMed] [Google Scholar]
- Dias RS, Kerr-Corrêa F, Moreno RA, et al (2006) Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause 13:202–211. 10.1097/01.gme.0000198491.34371.9c [DOI] [PubMed]
- Farahmand S, Maibach HI (2009) Transdermal drug pharmacokinetics in man: interindividual variability and partial prediction. Int J Pharm 367:1–15. 10.1016/j.ijpharm.2008.11.020 [DOI] [PubMed] [Google Scholar]
- Feres M, Feres MFN (2023) Absence of evidence is not evidence of absence. J Appl Oral Sci 31:ed001. 10.1590/1678-7757-2023-ed001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, York AE, Dimitrakakis C (2011) Beneficial effects of testosterone therapy in women measured by the validated menopause rating scale (MRS). Maturitas 68:355–361. 10.1016/j.maturitas.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Greene JG (1976) A factor analytic study of climacteric symptoms. J Psychosom Res 20:425–430. 10.1016/0022-3999(76)90005-2 [DOI] [PubMed] [Google Scholar]
- Greene JG (1998) Constructing a standard climacteric scale. Maturitas 29:25–31. 10.1016/s0378-5122(98)00025-5 [DOI] [PubMed] [Google Scholar]
- Hackett G, Kirby M, Rees RW et al (2023) The British Society for Sexual Medicine Guidelines on Male Adult Testosterone Deficiency, with statements for practice. World J Men’s Heal 41:508–537. 10.5534/wjmh.221027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam RM, Bell RJ, Green S et al (2019) Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol 7:754–766. 10.1016/s2213-8587(19)30189-5 [DOI] [PubMed] [Google Scholar]
- Kulkarni J (2018) Perimenopausal depression - an under-recognised entity. Aust Prescr 41:183–185. 10.18773/austprescr.2018.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt M (2019) Low mood and depressive symptoms during perimenopause – should General practitioners prescribe hormone replacement therapy or antidepressants as the first-line treatment? Post Reprod Heal 25:124–130. 10.1177/2053369119847867 [DOI] [PubMed] [Google Scholar]
- Maki PM, Kornstein SG, Joffe H et al (2019) Guidelines for the evaluation and treatment of Perimenopausal Depression: Summary and recommendations. J Women’s Heal 28:117–134. 10.1089/jwh.2018.27099.mensocrec [DOI] [PubMed] [Google Scholar]
- NICE (2015) Overview | Menopause: diagnosis and management | Guidance | NICE. https://www.nice.org.uk/guidance/ng23. Accessed 20 Aug 2024
- NICE (2019) Menopause: diagnosis and management. In: Menopause: diagnosis and management. www.nice.org.uk/guidance/ng23. Accessed 20 Aug 2024
- O’Reilly K, McDermid F, McInnes S, Peters K (2023) I was just a shell: Mental health concerns for women in perimenopause and menopause. Int J Ment Heal Nurs. 10.1111/inm.13271 [DOI] [PubMed] [Google Scholar]
- Panay N (2022) British Menopause Society Tool for clinicians: Testosterone replacement in menopause. Post Reprod Heal 28:158–160. 10.1177/20533691221104266 [DOI] [PubMed] [Google Scholar]
- Parish SJ, Simon JA, Davis SR et al (2021) International Society for the Study of Women’s sexual Health Clinical Practice Guideline for the use of systemic testosterone for hypoactive sexual Desire Disorder in Women. J Sex Med 18:849–867. 10.1016/j.jsxm.2020.10.009 [DOI] [PubMed] [Google Scholar]
- Reuben R, Karkaby L, McNamee C et al (2021) Menopause and cognitive complaints: are ovarian hormones linked with subjective cognitive decline? Climacteric 24:321–332. 10.1080/13697137.2021.1892627 [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A (2020) Mental Health and Illness of women. Ment Heal Illn Worldw 147–173. 10.1007/978-981-10-2369-9_9
- Riecher-Rössler A, de Geyter C (2007) The forthcoming role of treatment with oestrogens in mental health. Swiss Méd Wkly 137:565–572. 10.4414/smw.2007.11925 [DOI] [PubMed] [Google Scholar]
- Sekhon G (2024) Mood Disorder. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 32644337
- Shah S, Bell RJ, Savage G et al (2006) Testosterone aromatization and cognition in women: a randomized, placebo-controlled trial. Menopause 13:600–608. 10.1097/01.gme.0000227333.50867.4e [DOI] [PubMed] [Google Scholar]
- Sherwin BB (1988a) Affective changes with estrogen and androgen replacement therapy in surgically menopausal women. J Affect Disorders 14:177–187. 10.1016/0165-0327(88)90061-4 [DOI] [PubMed] [Google Scholar]
- Sherwin BB (1988b) Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 13:345–357. 10.1016/0306-4530(88)90060-1 [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM (1985) Differential symptom response to parenteral estrogen and/or androgen administration in the surgical menopause. Am J Obstet Gynecol 151:153–160. 10.1016/0002-9378(85)90001-8 [DOI] [PubMed] [Google Scholar]
- Singh I, Morris AP (2011) Performance of transdermal therapeutic systems: effects of biological factors. Int J Pharm Investig 1:4–9. 10.4103/2230-973x.76721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics O for N (2022) Suicides in England and Wales: 2021 registrations. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/suicidesintheunitedkingdom/2021registrations. Accessed 5 Feb 2024
- Stuursma A, Lanjouw L, Idema DL et al (2022) Surgical menopause and bilateral oophorectomy: effect of estrogen-progesterone and testosterone replacement therapy on Psychological Well-being and sexual functioning; a systematic literature review. J Sex Med 19:1778–1789. 10.1016/j.jsxm.2022.08.191 [DOI] [PubMed] [Google Scholar]
- Usall J, Pinto-Meza A, Fernández A et al (2009) Suicide ideation across reproductive life cycle of women results from a European epidemiological study. J Affect Disord 116:144–147. 10.1016/j.jad.2008.12.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementarymaterials.