Abstract
Introduction
Atopic dermatitis (AD) is a chronic, relapsing disease that can start at any age and has a significant negative impact on quality of life, including a significant itch burden. Here we report the proportion of patients in a real-world study achieving a complete/almost complete resolution of itch, as measured by the Peak Pruritus Numeric Rating Scale (PP-NRS) and improvement in overall disease severity score (ODS), in patients aged ≥ 12 years with moderate-to-severe AD up to 3 years after commencing dupilumab treatment.
Methods
PROSE is an ongoing, prospective, observational, multicenter registry in the USA and Canada, collecting real-world data from patients aged ≥ 12 years with moderate-to-severe AD who initiated dupilumab in accordance with country-specific prescribing information. Assessments include patient-reported PP-NRS (range 0–10) and clinician-measured ODS score (range 0–4).
Results
A total of 857 patients were enrolled, of whom 42% were male and 6.4% were adolescents aged ≥ 12 to < 18 years. The mean [standard deviation (SD)] age was 40.1 (17.9) years, and the duration of AD was 17.4 (16.2) years. The subsequent mean (SD) duration of dupilumab treatment was 23.1 (13.7) months. The proportion of patients achieving complete/almost complete itch resolution (PP-NRS score of 0 or 1) improved consistently over time, from 2.7% (17/622) of patients at baseline to 56.3% (58/103) at 3 years. Additionally, by year 3, 65.1% (54/83) of patients had an ODS score of no/minimal disease (score of 0 or 1), versus 2.2% (19/852) at baseline.
Conclusions
In this real-world setting of the PROSE registry, adult and adolescent patients with moderate-to-severe AD followed up for up to 3 years after the initiation of dupilumab treatment experienced sustained and substantial improvement in pruritus and ODS, using the stringent endpoints of PP-NRS 0 or 1 and ODS 0 or 1.
Trial Registration
ClinicalTrials.gov identifier: NCT03428646.
Graphical Abstract

Keywords: Atopic dermatitis, Disease control, Dupilumab, Efficacy, Patient-reported outcomes, Real-world study, Safety
Plain Language Summary
Atopic dermatitis (AD) is a long-term condition with rashes, inflammation and intense itching that disturbs sleep and daily activities. Dupilumab is used to treat AD when topical medications are not adequate. Our aim was to find out how many patients showed no or minimal itch and AD severity when patients use dupilumab over the long term in the real world. The PROSE real-world registry collected information on 857 adults and adolescents with AD who were prescribed dupilumab by their doctors. Patients in the registry reported their itch weekly on a scale from 0 (no itch) to 10 (worst possible itch). At the study start, and up to 36 months later, their doctor graded the severity of their AD from 0 (no disease) to 4 (severe disease). We measured how many PROSE patients had no/minimal itch and AD severity (in both cases, scores of 0 or 1) for up to 3 years after they started dupilumab treatment. At the study start, 2.7% of the patients had no or minimal itch. For those patients still being observed after 36 months (about 12% of the starting sample), 56.3% of the patients had no or almost no itch; the severity of AD improved similarly. These results show that many patients with AD receiving dupilumab can experience complete or almost complete itch and/or AD relief, although other medications could also have helped these patients improve. Our results are useful for doctors treating AD.
Key Summary Points
| Patients with moderate-to-severe atopic dermatitis (AD) experience a substantial health burden dominated by pruritus which can profoundly impact sleep, daily functioning and quality of life. |
| The PROSE registry was designed to collect real-world data from patients aged ≥ 12 years with moderate-to-severe AD who initiate dupilumab in accordance with country-specific prescribing information. |
| Data from the PROSE registry study underwent an interim analysis to assess the effectiveness of using dupilumab in treating AD in adolescents and adults in a real-world setting over 3 years, as assessed using clinician-assessed measures of AD disease severity and patient-reported measures of symptoms and quality of life. |
| These data showed that the majority of patients achieved complete/almost complete resolution of patient-reported itch and clinician-reported overall disease severity at 3 years after first commencing dupilumab treatment. |
Digital Features
This article is published with digital features, including a graphical abstract to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.28554257.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing disease that can start at any age and has a significant negative impact on quality of life [1]. Patients with moderate-to-severe disease experience a substantial burden dominated by pruritus, which can profoundly impact sleep, daily functioning and quality of life [1, 2].
Dupilumab is a fully human monoclonal antibody that blocks the shared receptor component for interleukin (IL)−4 and IL-13, and thus inhibits the signaling of both cytokines. These cytokines are thought to be key drivers of the type 2 inflammation that characterizes AD and a number of other diseases that are often comorbid with AD, such as asthma, allergic rhinitis and food allergies [3]. Clinical trials of dupilumab in AD have demonstrated significant improvements in signs and symptoms of AD, including pruritus and quality of life [4, 5], with good efficacy and acceptable safety results reported for up to 5 years of dupilumab treatment in a clinical trial extension study and two real-world studies [6–8]. A retrospective analysis of 356 patients treated with dupilumab found a correlation between improvements in Peak Pruritus Numeric Rating Scale (PP-NRS) scores and both Dermatology Life Quality Index (DLQI) and Patient-Oriented Eczema Measure scores [9]. In addition, the authors of a pooled analysis of two phase 3 trials also reported improvements in quality of life measures in parallel with PP-NRS improvements following 2 weeks of treatment, which were maintained through week 16 [5].
PROSE is an ongoing, prospective, observational, multicenter registry in the USA and Canada, designed to collect real-world data from patients aged ≥ 12 years with moderate-to-severe AD who initiate dupilumab in accordance with country-specific prescribing information (ClinicalTrials.gov identifier: NCT03428646). A previous interim analysis of PROSE registry data over a 2-year period revealed substantial and sustained improvements in AD signs [as measured by the Eczema Area and Severity Score (EASI), symptoms (including PP-NRS), quality of life (DLQI) and a one-item clinician-assessed global severity question, the Overall Disease Severity (ODS) score] [10].
As an observational registry study, no specific protocol response definitions were mandated in PROSE. The ODS score asks the clinician to rate the answer on a 0 to 4 scale to the question, “Taking into account all important aspects characterizing the severity of AD, how would you grade your patient’s AD at this time?”. A related measure of clinician-assessed global AD severity, the Investigator’s Global Assessment (IGA), which only addresses the severity of certain lesional signs, is universally utilized in clinical trials of advanced systemic AD treatments; it is often also rated on a 0 to 4 scale.
For itch, the percentage of patients in a treatment group experiencing at least a 4-point reduction in PP-NRS is a typical response definition in clinical trials of advanced systemic AD treatments, including those for dupilumab [11, 12]. Recently, achievement of a PP-NRS score of 0/1 has started to be used as an endpoint in randomized clinical studies assessing new treatments for moderate-to-severe AD, and has been described as a stringent, high-threshold endpoint [13–16]. While this endpoint is considered to be stringent by some, no analysis has shown whether a response definition based on PP-NRS scores of 0 or 1 is clinically distinguishable from any other cutoff point. Nevertheless, the availability of data reporting this endpoint in patients treated with some advanced therapies may allow for indirect comparisons, with the caveat that a different study design and population differences must also be considered.
The present interim analysis of the PROSE study was conducted to report the proportion of patients achieving PP-NRS scores of 0/1 over time, and also the achievement of no/minimal disease (a score of 0 or 1) on the clinician-assessed ODS, in patients older than 12 years with moderate-to-severe AD, 3 years after starting dupilumab treatment.
Methods
The detailed design of the PROSE registry has been reported previously [17]. Briefly, PROSE is an ongoing, prospective, observational, multicenter registry of patients with AD treated with commercially available dupilumab, as per country-specific prescribing information, across the USA and Canada; enrollment was completed as of 2022. At the baseline visit, eligible patients aged ≥ 12 years with moderate-to-severe AD received their first administration of dupilumab. Subsequently, there were no further restrictions on the use of dupilumab or concomitant medications that were deemed necessary by the treating physician. Patients were encouraged to remain in the registry even if dupilumab treatment was discontinued, although patients were not allowed to restart dupilumab. The PROSE study received institutional review board/ethics committee approval, and all patients provided informed consent. Data were anonymized in compliance with the Health Insurance Portability and Accountability Act (HIPAA). All patients with up to 3 years of data are included in this paper, based on an interim data analysis with a database lock date of November 2022.
Baseline was defined as the day that the patient received the initial dose (i.e. loading dose) of commercially available dupilumab. ODS was assessed by the clinician at each clinic visit. In answer to the question “Taking into account all important aspects characterizing the severity of AD, how would you grade your patient’s AD at this time?”, the clinician would rate the patient as either 0 (no disease), 1 (minimal disease), 2 (mild disease), 3 (moderate disease) or 4 (severe disease). During the prior week, patients reported pruritus using the PP-NRS (score ranging from 0 to 10, with 0 being no itch and 10 being worst possible itch). Clinic visits were scheduled per the clinician’s standard of care; for analytic purposes, clinic visits were fit into visit windows, such that the post-baseline visits were month 3 (± 1 month), month 6 (± 2 months) and every 6 months (± 2 months) thereafter. PP-NRS was assessed outside of clinic visits weekly and captured via diaries or call center interactions. Patients who discontinued dupilumab (permanently or temporarily) were still eligible and encouraged to continue in the study. Patients who left the study before the 60-month visit were asked to complete an early termination visit. Adverse events (AEs) were recorded for the duration of the study and were coded using the Medical Dictionary for Regulatory Activities version 22.0.
Results
The sociodemographic, treatment history, disease characteristics and disease burden of the initial 315 patients included in the PROSE registry have been reported previously [14], as has a detailed analysis of the disease burden and safety data for the 632 patients analyzed at 2 years during a prior interim analysis [18]. By the cutoff date for the present interim analysis, 857 patients had been enrolled in PROSE, with 189 (22.1%) having withdrawn from the study at the time of the data cutoff. The most common reasons for discontinuing from the study were withdrawal of consent (76 patients, 8.9%) and loss to follow-up (30 patients, 3.5%). In this study 42% of patients were male and 6.4% were adolescents (aged ≥ 12 to < 18 years). The mean [standard deviation (SD)] age of patients included was 40.1 years (17.9), with a mean (SD) AD treatment duration of 17.4 (16.3) years.
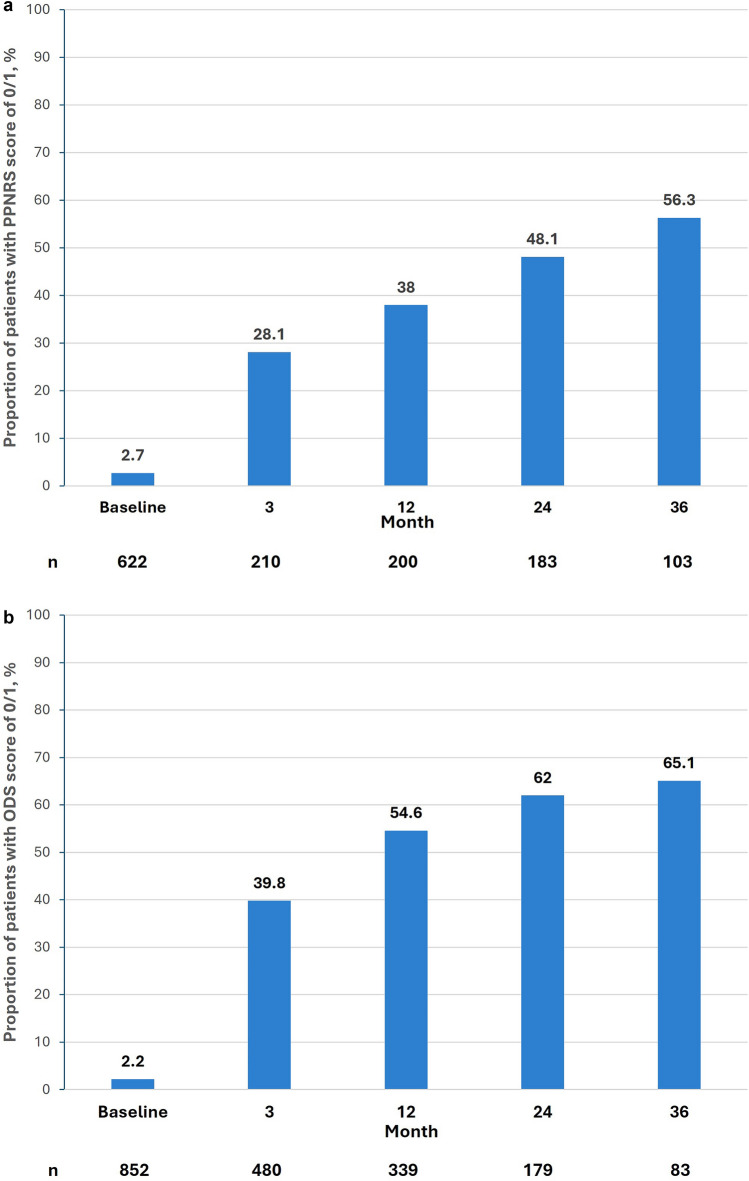
At the time of this analysis, the mean (SD) duration of dupilumab treatment was 23.1 (13.7) months, with 103 patients completing 3 years of treatment. Itch, as measured by the PP-NRS, improved consistently from a baseline mean (SD) value of 7.1 (2.26) to a mean (SD) score of 1.9 (2.35) at 3 years. In addition, the proportion of patients achieving a complete/near complete resolution score of 0 or 1 for PP-NRS improved consistently over time from 2.7% (17/622) at baseline to 56.3% (58/103) at 3 years in this patient group (Fig. 1a).
Fig. 1.
Assessment of PP-NRS and ODS over 36 months following initiation of dupilumab treatment. Data are presented for all patients with data at each time point. a PP-NRS score of 0/1 (complete/near-complete itch resolution) over time, b ODS of 0/1 over time. ODS Overall Disease Severity, PP-NRS Peak Pruritus Numeric Rating Scale
At baseline, most patients (88.9%) had an ODS score of moderate (55.9%) or severe (33.0%), with the remaining 11.1% of patients having mild disease (8.3%), minimal disease (1.6%). No disease (0.6%) or missing (0.6%). By year 3, 65.1% (54/83) of patients had an ODS score of no or minimal disease (score of 0 or 1) (Fig. 1b).
In the 3-year cohort, AE rates were similar to those at 2 years and were reported at a rate of 27.7 AEs per 100 patient-years; serious AEs were reported at a rate of 1.6 serious AEs per 100 patient-years.
Discussion
Real-world evidence from registry studies like PROSE offers valuable insights into the disease burden of AD and supports the effectiveness of treatments in more realistic clinical settings—compared to strictly controlled clinical trials—for patients with a wider range of disease burden. A recent meta-analysis of 22 real-world studies with durations of up to 52 weeks of dupilumab-treated patients with AD demonstrated sustained and clinically meaningful effectiveness, supporting its long-term use [19].
The protocol for the PROSE registry did not mandate specific response definitions. In clinical trials for dupilumab, the main clinician-assessed primary and co-primary endpoints were the achievement of clear or almost clear skin, as assessed by the IGA, and a ≥ 75% improvement from baseline to endpoint in EASI score (also known as the EASI-75). In the present analysis, we focused on AD severity over time based on the proportion of patients whose ODS, as assessed by the clinician, was 0 or 1 (no or minimal disease).
This update from the PROSE registry aimed to facilitate a deeper understanding of the real-world disease burden in terms of the resolution of chronic itch in patients with moderate-to-severe AD, up to 3 years after starting dupilumab. The proportion of patients achieving complete/near-complete resolution of itch increased over time, with a sustained pattern of improvement during the first 3 years of the PROSE registry, a time frame not generally captured in clinical trial research. For itch, we measured response using a PP-NRS score of 0 or 1, as has recently been used by authors examining other treatment modalities for AD [13, 15, 20].
This research adds to recent clinical studies which utilize a stringent measure for PP-NRS (complete/near-complete itch resolution), in contrast to the more commonly utilized clinically meaningful 3-point and 4-point improvement in PP-NRS used in most AD clinical studies, including those studying dupilumab [11, 12]. Itch resolution using the PP-NRS of 0/1 is a stringent, high-threshold and relevant endpoint that may offer an appropriate tool to evaluate the effectiveness of patient care in the real-world as well as in the clinic [21] and be a useful way to show a high-threshold response to treatment in moderate-to-severe AD [16].
The patients included in this analysis had a high disease burden at baseline, with baseline itch and ODS scores indicative of moderate-to-severe AD [22]. This analysis of the PROSE registry of dupilumab-treated patients with AD demonstrates that with effective treatment, most patients can achieve almost complete control of itch.
The findings in this study are limited by the real-world design of the PROSE registry, including the lack of a comparator or placebo group and no a priori statistical hypothesis. The PROSE registry worldwide generalizability may be restricted as only patients from North America were enrolled. However recent results from the multinational GLOBOSTAD study are extremely comparable [23]. A small number of patients were clinician-rated as having mild or even no overall disease severity at baseline; presumably, this reflects either ebbing of the disease subsequent to the decision to prescribe dupilumab (and prior to the administration of the loading dose) or therapeutic benefit from other treatment modalities. Although all patients had to start dupilumab treatment at baseline to be included in the registry, there were no constraints on their subsequent dosing or usage of dupilumab, or other concomitant treatments for AD. Therefore, improvements in this patient sample cannot be fully attributed to the use of dupilumab treatment. This is an interim analysis of an ongoing study, only a minority of patients had observations at month 36, which limits interpretation of the results; further publications at the end of the study will fully reflect data from all patients that remained in the study at 3 years.
Conclusions
In this real-world setting of the PROSE registry, adult and adolescent patients with moderate-to-severe AD up to 3 years after the initiation of dupilumab treatment experienced sustained improvement in signs and symptoms, with the majority having near-complete/complete itch resolution and no/minimal disease activity. These results support and add to the previous findings from both the PROSE registry and the dupilumab clinical trial program in AD, showing that dupilumab is suitable for the long-term treatment of patients with moderate-to-severe AD.
Acknowledgments
Medical Writing/Editorial Assistance
Medical writing/editorial assistance was provided by Jaqui Hodgkinson, DPhil, MBA of Excerpta Medica, and funded by Sanofi and Regeneron Pharmaceuticals Inc., in accordance with current Good Publication Practice guidelines. There was no financial compensation outside of salary.
Author Contributions
All authors (Neal Bhatia , Charles W. Lynde, Luz Fonacier, Liyang Shao, Andrew Korotzer, Kwinten Bosman) contributed to the concept, reviewed all copies and approved the final document.
Funding
Sponsorship for this PROSE Registry and the Rapid Service Fee were funded by Sanofi and Regeneron Pharmaceuticals Inc. ClinicalTrials.gov identifier: NCT03428646. The funders participated in the conception and design of the study, analysis and interpretation of the data and drafting and critical revision of the report, and gave approval to submit.
Data Availability
The datasets generated in the current study are in the publication itself, and additional data are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Neal Bhatia reports advisor or consultant funding from AbbVie, Advanced Derm Solutions, Almirall, Arcutis, Beiersdorf, Biofrontera, BMS, BI, Ferndale, Galderma, Incyte, ISDIN, J&J, La Roche-Posay, LEO, Lilly, Novartis, Ortho, Pfizer, Regeneron, Sanofi, Sun Pharma and Verrica. Charles W Lynde is an advisor, consultant and speaker for AbbVie, Altius Pharmaceuticals, Amgen, Aralez Bio, Arcutis Antiobix, Bausch Health, Bayer, BMS, BI, Cipher Pharmaceuticals, Dermavant, Fresenius Kabi, Galderma, GSK, Innovaderm, Intega, Janssen, Kyowa Kirin, La Roche-Posay, LEO Pharma, Lilly, L’Oreal, Medexus, Merck, Pediapharm, Pfizer, Procter & Gamble, Regeneron Pharmaceuticals Inc., Roche, Sanofi, Sentrex Health Solutions, Skin, Teva, Tribute Pharmaceuticals, UCB, Valeant and Viatris. Luz Fonacier reports research and educational grants (made to NYU Langone Hospital–Long Island) from AstraZeneca, Pfizer and Regeneron Pharmaceuticals Inc.; is an advisory board/consultant for AbbVie, LEO Pharma, Lilly, Pfizer and Regeneron Pharmaceuticals Inc.; was the past President (2020–2021) of American College of Allergy, Asthma & Immunology; is the Chair (2021) of American Board of Allergy and Immunology (ABAI); was the past President (2008–2010) of Long Island Allergy Society; was the past President of International Association of Filipino Allergists and Immunologists. Liyang Shao and Andrew Korotzer are employees and shareholders of Regeneron Pharmaceuticals Inc. Kwinten Bosman is an employee and a shareholder.
Ethical approval
The study received approval from the Institutional Review Board/Ethics Committee. All patients provided informed consent, and patient data were anonymized in compliance with the HIPAA.
Footnotes
The original online version of this article was revised: Order of author names and revised version of graphical abstract updated.
Change history
7/21/2025
A Correction to this paper has been published: 10.1007/s13555-025-01478-z
References
- 1.Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2015;15(1):35–50. [DOI] [PubMed] [Google Scholar]
- 3.Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Ralpha antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387(10013):40–52. [DOI] [PubMed] [Google Scholar]
- 5.Cork MJ, Eckert L, Simpson EL, et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J Dermatol Treat. 2020;31(6):606–14. [DOI] [PubMed] [Google Scholar]
- 6.Beck LA, Bissonnette R, Deleuran M, et al. Dupilumab in adults with moderate to severe atopic dermatitis: a 5-year open-label extension study. JAMA Dermatol. 2024;160(8):805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boesjes CM, Kamphuis E, de Graaf M, et al. Long-term effectiveness and reasons for discontinuation of dupilumab in patients with atopic dermatitis. JAMA Dermatol. 2024;160(10):1044–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barei F, Calzari P, Valtellini L, et al. Five-year real-world drug survival of dupilumab in severe atopic dermatitis and associate predictors. J Dermatol Treat. 2024;35(1):2404718. [DOI] [PubMed] [Google Scholar]
- 9.Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals (Basel). 2022;15(7):883. [DOI] [PMC free article] [PubMed]
- 10.Simpson EL, Silverberg JI, Worm M, et al. Dupilumab treatment improves signs, symptoms, quality of life, and work productivity in patients with atopic hand and foot dermatitis: Results from a phase 3, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2024;90(6):1190-9. [DOI] [PubMed]
- 11.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48. [DOI] [PubMed] [Google Scholar]
- 12.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–303. [DOI] [PubMed] [Google Scholar]
- 13.Reich K, Silverberg JI, Papp KA, et al. Abrocitinib efficacy and safety in patients with moderate-to-severe atopic dermatitis: Results from phase 3 studies, including the long-term extension JADE EXTEND study. J Eur Acad Dermatol Venereol. 2023;37(10):2056–66. [DOI] [PubMed] [Google Scholar]
- 14.Reich K, Thyssen JP, Blauvelt A, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273–82. [DOI] [PubMed] [Google Scholar]
- 15.Yosipovitch G, Gooderham MJ, Ständer S, et al. Interpreting the relationship among itch, sleep, and work productivity in patients with moderate-to-severe atopic dermatitis: a post hoc analysis of JADE MONO-2. Am J Clin Dermatol. 2024;25(1):127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ständer S, Bhatia N, Gooderham MJ, et al. High threshold efficacy responses in moderate-to-severe atopic dermatitis are associated with additional quality of life benefits: pooled analyses of abrocitinib monotherapy studies in adults and adolescents. J Eur Acad Dermatol Venereol. 2022;36(8):1308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagel J, Nguyen TQ, Lima H, et al. Baseline demographics and severity and burden of atopic dermatitis in adult patients initiating dupilumab treatment in a real-world registry (PROSE). Dermatol Ther (Heidelb). 2022;12(6):1417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson EL, Lockshin B, Lee LW, Chen Z, Daoud M, Korotzer A. Real-world effectiveness of dupilumab in adult and adolescent patients with atopic dermatitis: 2-year interim data from the PROSE registry. Dermatol Ther (Heidelb). 2024;14(1):261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;84(1):139–47. [DOI] [PubMed] [Google Scholar]
- 20.Reich K, Lio PA, Bissonnette R, et al. Magnitude and time course of response to abrocitinib for moderate-to-severe atopic dermatitis. J Allergy Clin Immunol Pract. 2022;10(12):3228-37.e2. [DOI] [PubMed] [Google Scholar]
- 21.Penton H, Jayade S, Selveindran S, et al. Assessing response in atopic dermatitis: a systematic review of the psychometric performance of measures used in HTAs and clinical trials. Dermatol Ther (Heidelb). 2023;13(11):2549–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopra R, Vakharia PP, Sacotte R, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017;177(5):1316–21. [DOI] [PubMed] [Google Scholar]
- 23.Calzavara-Pinton P, Chu CY, Lapeere H, et al. One-year insights into the GLOBOSTAD multinational prospective observational study of patients receiving dupilumab for atopic dermatitis. Adv Ther. 2024;42:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated in the current study are in the publication itself, and additional data are available from the corresponding author on reasonable request.