Abstract
Ischemic changes are not visible on non-contrast head CT until several hours after infarction, though deep convolutional neural networks have shown promise in the detection of subtle imaging findings. This study aims to assess if dual-energy CT (DECT) acquisition can improve early infarct visibility for machine learning. The retrospective dataset consisted of 330 DECTs acquired up to 48 h prior to confirmation of a DWI positive infarct on MRI between 2016 and 2022. Infarct segmentation maps were generated from the MRI and co-registered to the CT to serve as ground truth for segmentation. A self-configuring 3D nnU-Net was trained for segmentation on (1) standard 120 kV mixed-images (2) 190 keV virtual monochromatic images and (3) 120 kV + 190 keV images as dual channel inputs. Algorithm performance was assessed with Dice scores with paired t-tests on a test set. Global aggregate Dice scores were 0.616, 0.645, and 0.665 for standard 120 kV images, 190 keV, and combined channel inputs respectively. Differences in overall Dice scores were statistically significant with highest performance for combined channel inputs (p < 0.01). Small but statistically significant differences were observed for infarcts between 6 and 12 h from last-known-well with higher performance for larger infarcts. Volumetric accuracy trended higher with combined inputs but differences were not statistically significant (p = 0.07). Supplementation of standard head CT images with dual-energy data provides earlier and more accurate segmentation of infarcts for machine learning particularly between 6 and 12 h after last-known-well.
Keywords: Dual Energy CT, Stroke, Segmentation, NnU-Net, Deep Learning, MRI
Introduction
Dual-energy Computed Tomography (DECT) is a promising technique in stroke imaging and has been shown to aid in the variety of tasks in stroke management such as distinguishing hemorrhage from contrast staining [1], visualizing infarcts, and predicting risk of hemorrhagic transformation [2]. DECT works by performing CT acquisitions at 2 different energies which can then be used to synthetically create virtual monochromatic images (VMI) [3]. Emerging literature has shown that a variety of DECT techniques can improve infarct visibility by increasing the conspicuity of cytotoxic edema and allow for earlier infarct detection with higher inter-reader agreement [2, 4–7]. Early and rapid visualization of acute infarcts on initial non-contrast head CT is of great clinical benefit because estimation of core infarct volume can affect immediate decision-making for procedures such as mechanical thrombectomy and affects long-term prognostication [8, 9]. Size of core infarct is often estimated using CT perfusion, though this requires a separate scan and software methods which may not be readily available [10] and have limitations [11].
Deep convolutional neural networks (DCNNs) are a technique in machine learning that have shown potential to identify findings beyond human detection [12]. DCNNs utilize large amounts of data to learn patterns from imaging features and this technique has demonstrated utility in tasks such as ischemic stroke segmentation on MRI [13–19]. Prior literature has relied on subjective evaluation of infarct visibility on CT using dual energy methods [4–6]. It is unclear, however, how DECT can objectively improve performance for machine learning to provide for more accurate detection and localization of acute infarcts, which has not been previously evaluated.
In this study, we compare the ability of DCNNs to segment and provide volumetric estimation of early ischemic infarcts on DECT acquisitions. We compare segmentation on (1) standard 120 kV mixed-images (2) 190 keV VMI and (3) 120 kV + 190 keV images as dual channel inputs, analyzing performance by size and time of infarct. We hypothesize that supplementation of CT images with 190 keV VMI would significantly improve accuracy of volumetric segmentation of acute ischemic infarcts and allow for earlier visualization and estimation of core infarct volumes.
Materials and Methods
This retrospective study was conducted with exemption acknowledged by the institutional review board. A Foundation of the American Society of Neuroradiology grant was used to fund this study.
Dataset
The dataset consisted of all patients who had an MRI brain examination performed at our institution between January 2016 and November 2022. Reports from the examination were screened for those that contained mention of DWI-positive acute infarct. A total of 2,510 examinations met the initial inclusion criteria. The PACS system was queried for associated CT examinations that were performed with dual energy acquisition the same day as the MRI acquisition to up to 2 days prior, totaling 956 CT examinations.
Information related to the time the patient was last known well was mined from the clinical record. Examinations were excluded if the time the patient was last known well was documented as greater than 2 weeks prior to the CT examination or if the CT examination was performed before the clinical stroke event. If time last known well was unknown, data was still included but restricted to the training dataset. Due to limited performance of CT segmentation of small infarcts from empirical testing, MRI examinations were restricted to those with a core infarct volume more than 10 cc. Finally, examinations were excluded if there was significant degree of artifact, motion, or misregistration between CT and MRI. Of the initial 2,510 examinations, 330 met the final inclusion criteria of MRI-DECT pairs (Table 1 and 2, Figs. 1 and 2a, b). This number of studies is comparable to that used for stroke segmentation in our prior work on MRI [19], prior work on stroke segmentation on CT [20], public challenge datasets for stroke segmentation [21], and a variety of challenge datasets for which our model architecture has been trained and evaluated [22].
Table 1.
Study demographics for 330 MRI and dual energy CT pairs containing greater than 10 cc’s of infarct
| Patient Demographics | ||
|---|---|---|
| n = | 330 | |
| Age | (mean ± std) | 62.6 ± 16.3 |
| Sex | ||
| Male | 178 | |
| Female | 152 | |
| Clinical Characteristics | ||
| Last Known Well | ||
| Known | 232 | |
| Unknown/Not documented | 98 | |
| Time from Last Known Well to CT (hours) | (mean ± std) | 29.0 ± 73.4 |
| Time between CT and MRI (hours) | (mean ± std) | 17.2 ± 15.8 |
| Infarct Characteristics | ||
| Volume of Infarct (cc) | (mean ± std) | 62.6 ± 63.0 |
| Infarct Location | ||
| Supratentorial Only | 231 | |
| Infratentorial Only | 0 | |
| Supratentorial & Infratentorial | 99 | |
| Laterality | ||
| Right | 64 | |
| Left | 40 | |
| Bilateral | 226 | |
Table 2.
A comparison of demographics between the training and testing data sets
| Training Set | Test Set | |||
|---|---|---|---|---|
| Number of Cases | 263 | 67 | ||
| Number Male | 143 | 35 | ||
| Number Female | 120 | 32 | ||
| Age | (mean ± std) | 63.4 ± 16.6 | 66.1 ± 14.9 | p = 0.23 |
| Infarct volume (cc) | (mean ± std) | 64.7 ± 65.2 | 54.4 ± 54.8 | p = 0.23 |
| Time from CT to MRI (hours) | (mean ± std) | 17.3 ± 16.2 | 16.5 ± 14.3 | p = 0.72 |
| Time from last-known-well to CT (hours) | (mean ± std) | 31.0 ± 85.0 | 24.2 ± 29.8 | p = 0.52 |
Fig. 1.
Curation and exclusion criteria for studies in the dataset, identifying 330 high quality MRI- Dual Energy CT pairs with > 10 cc of infarct
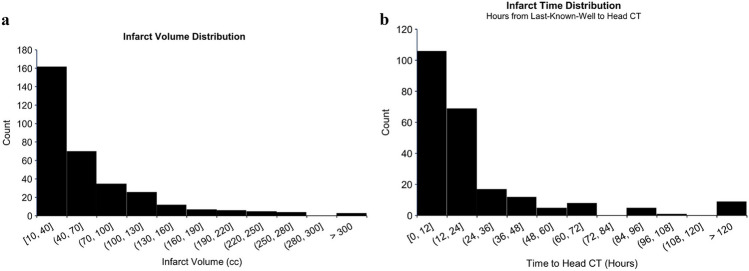
Fig. 2.
a. Distribution of infarct volumes in the dataset. Infarcts were restricted to those with a core infarct volume > 10 cc’s. b. Distribution of time from last-known-well to head CT for patients with known and documented time-last-well
MRI Imaging
MRI images were acquired on multiple scanners consisting of Siemens TrioTim 3 T, Siemens Magentom Sola 1.5 T, Siemens Avanto 1.5 T, and Siemens Aera 1.5 T. DWI images were acquired with slice thickness of 3–5 mm, repetition time of 2,600–10,700 ms, echo time of 56–107 ms, spacing between slices of 3 to 6.5 mm, and a flip angle of 90–180 degrees. Images corresponding to b = 1000 s/mm2 were retrieved from the PACS using pynetdicom [23]and subsequently anonymized using the RSNA Clinical Trials Processor [24]. Images were converted to NifTI file format using dicom2nifti [25], skull stripped using Brain Extraction Tool (BET) [26], and registered to the SRI24 anatomic atlas [27] using symmetric normalization with ANTsPy [28]. Areas of acute infarct were segmented using an nnU-Net model previously trained on 250 publicly accessible acute ischemic stroke MRI scans [21] as well as 50 manually segmented MRIs from our institution, which achieved high internal and external performance [19]. All segmentation masks were manually reviewed by a board-certified neuroradiologist (PK) and any edits were performed as needed. The segmentation masks generated from the DWI positive MRI images served as the ground truth for core infarct segmentation.
CT Imaging
DECT images were acquired on Siemens Somatom Force and Philips IQon – Spectral CT with dual energy acquisition at 80 and 150 kV. Images were reconstructed to form mixed 120 kV images, reflecting standard 120 kVp single-energy CT images, as well as 190 keV VMI images. VMI images reflect synthetic construction to generate images at a hypothetical energy level that simulate the results of acquisition with a monoenergetic x-ray beam at that energy. We used 190 keV images as they are part of the standard DECT protocol at our institution as 190 keV images optimize visualization of intracranial hemorrhage [29], which has added utility in stroke imaging, and has been reported as improving visualization of edema [30]. Since most VMI images were of variable resolution, typically with a 1 mm slice thickness, the VMI images were down-sampled to match the thick-resolution standard CT images using ANTsPy with symmetric normalization [28]. Both the VMI and standard CT images were identically skull stripped using BET [26] and registered to the SRI24 anatomic atlas [27] using symmetric normalization with ANTsPy [28] to match the imaging plane, orientation, and resolution of the MRI images.
Network
80% of the dataset consisting of the 330 MRI-DECT pairs was used for training and validation with the remaining 20% used as the hold-out test set (Table 2). In order to perform detailed subgroup analysis on infarct timing, the 20% hold-out test-set was selected randomly from the subset of patients for whom last-known-well was known and documented.
We used nnU-Net [22], which is a state-of-the-art deep learning segmentation model that performs automated self-configuration from image preprocessing to model development with elements of preprocessing, architecture design, training, and post-processing automatically configured and optimized by the algorithm. nnU-Net has demonstrated high performance for medical imaging segmentation tasks on 23 public datasets [22] and high performance for stroke segmentation on MRI from our prior work [19]. For our model development, nnU-Net training was performed using the 3D full resolution configuration, using the DWI-positive MRI segmentation mask as the ground truth. nnU-Net automatically optimizes the training parameters, requiring no further manual configuration. Training was performed on (1) 120 kV images alone (2) 190 keV images alone and (3) 120 kV and 190 keV in combination used as dual channel inputs in the nnU-Net model. Training was restricted to 200 epochs per fold, with 5 folds for cross-validation, totaling 1,000 epochs. While 1,000 epochs per fold is standard for nnU-Net, training was restricted to 200 epochs per fold as we found that models tended to converge earlier and still performed well as in prior work [19]. Inference was subsequently performed using the hold-out test set. Training and testing was performed on eight NVIDIA A6000 48 GB graphics cards.
Statistical Analysis
The primary performance metric was Dice score which was evaluated with Python. Dice score is a measure of similarity between two sets and indicates the level of overlap between segmentations ranging from 0 to 1, with 1 indicating perfect overlap. This was done case by case, reporting average and standard deviation, as well as on a global level of all aggregate cases, due to the varying size of infarcts between cases. Dice scores were assessed on the hold-out set and compared between CT acquisitions using paired t-test analysis. Surface Dice similarity coefficients were also calculated at tolerance thresholds of 1 mm, 5 mm, and 10 mm according to prior published segmentation work [31] using the Surface Distance Python library [32] with similar statistical analysis. Surface Dice similarity coefficients refer to a measure of overlap of two surfaces as opposed to volumetric overlap as with standard Dice [31]. Secondary analysis was performed to assess false positive, false negative rates, and the mean absolute error in predicting core infarct volume. Subgroup analysis was performed by size of infarct as well as the timing of the infarct, as determined by the last-known-well documentation in the clinical notes.
Results
Segmentation Performance
Global aggregate Dice scores measured 0.616, 0.645, 0.665 on the hold out-test set for segmentation on standard 120 kV images, 190 VMI, and combined channel inputs respectively with mean ± standard deviation of 0.500 ± 0.279, 0.531 ± 0.273, and 0.544 ± 0.271 for infarcts of all size and times (Figs. 3, 4, 5 and 6). On paired t-test analysis, differences were statistically significant for standard images versus combined inputs (p < 0.01) though not statistically significant for standard versus VMI (p = 0.05), or VMI versus combined images (p = 0.08).
Fig. 3.
Sample segmentation prediction on 120 kV and 190 keV images with similar high performance on an established infarct. (a) Standard CT at 120 kV demonstrating an established right middle cerebral artery territory infarct with (b) accurate infarct localization. Similar performance is demonstrated on 190 keV images (c-d). Ground truth DWI images (e) and segmentation (f)
Fig. 4.
Sample segmentation of a subtle right cerebral hemisphere infarct. (a, b) Segmentation on standard CT at 120 kV demonstrates patchy areas of segmented infarct in the right frontal temporal, and occipital lobes. Segmentation on 190 keV images (c-d) more accurately localizes the infarct seen on ground truth DWI images (e–f)
Fig. 5.
Example of a subtle infarct detected only on 190 keV images. (a) Mixed 120 kV images demonstrating no obvious abnormality. The algorithm did not segment any infarct on these images. (b) 190 keV images showing a very subtle area of hypoattenuation in the left occipital lobe which is segmented by the model (c). (d) Ground truth MRI showing acute infarct in this region
Fig. 6.
Example of a segmentation failure case. (a) Mixed 120 kV images demonstrate a chronic area of infarct in the right occipital lobe. The algorithm did not segment any infarct on these images. On 190 keV images (b), the model segments a portion of the parenchyma adjacent to the chronic area of infarct (c). Ground truth MRI (d) shows the infarct is in the left frontal lobe and was incorrectly segmented by the model
Similar trends were observed with surface Dice similarity coefficients (Fig. 7). At a tolerance of 1 mm, scores measured 0.27 ± 0.17, 0.28 ± 0.16, and 0.29 ± 0.16 for standard, VMI, and combined images respectively with statistical significance between standard and combined images (p = 0.01). With a threshold of 10 mm, scores reached 0.72 ± 0.33, 0.76 ± 0.32, and 0.76 ± 0.31, though these differences were not statistically significant (p = 0.06 for standard versus combined).
Fig. 7.

A comparison of surface Dice similarity coefficients at varying tolerance thresholds for segmentation performed on standard CT images, 190 keV virtual monochromatic images, and combined channel inputs. Differences were statistically significant between standard and combined channel inputs at tolerance thresholds of 1 mm and 5 mm (p < 0.05). Error bars indicate standard error of the mean
Combined channel inputs significantly reduced the false negative rate over standard images (0.428 ± 0.305 versus 0.489 ± 0.308; p < 0.01) but demonstrated no statistically significant difference in false positive rate (0.002 ± 0.003 versus 0.002 ± 0.003; p = 0.28).
Absolute error in predicting the core infarct volume trended lower with 190 VMI and combined channel inputs but differences were not statistically significant (mean absolute error of 22.2 ± 26.1 cc, 20.4 ± 25.5 cc, and 18.8 ± 22.7 cc for standard, VMI, and combined inputs respectively; p = 0.07 for standard vs combined) (Fig. 8).
Fig. 8.
(a) A comparison of predicted infarct volume versus true infarct volume for segmentation performed on standard CT images as well as standard CT images combined with 190 virtual monochromatic images. (b) Differences in infarct segmentation reflecting predicted minus true infarct volumes
By Time
Segmentation performance significantly improved after 6 h from last known well with Dice scores of 0.211 ± 0.238 before 6 h and 0.649 ± 0.181 after 6 h for combined channel inputs (p < 0.001). Segmentation performance continued to trend higher beyond 6 h (Dice of 0.589 ± 0.210 for segmentation for 6–12 h versus 0.681 ± 0.158 for > 12 h, p = 0.04) and plateaued around 12 h (Figs. 9 and 10). Differences between standard images and combined channel inputs were statistically significant for infarcts between 6–12 h (p = 0.04) and 24–48 h (p = 0.03) but not at other times (Fig. 10).
Fig. 9.

Dice scores for infarct segmentation on standard 120 kV and combined 120 kV + 190 keV virtual monochromatic images by time of infarct. Segmentation performance generally improved with greater time from last-known-well and with combined inputs
Fig. 10.

A comparison of infarct segmentation performance on standard 120 kV, 190 keV virtual monochromatic images, and combined inputs by time since last-known-well. Low performance was observed on all models within 6 h of infarct. Segmentation performance improved after 6 h with significantly improved performance of 190 keV and combined channel inputs between 6 and 12 h. * indicates p < 0.05 on paired t-test analysis
By Volume of Infarct
Segmentation performance significantly improved with volume of the infarct and continued to improve with increasing size up to approximately 60 cc’s (Dice of 0.752 ± 0.037 for infarcts between 60–80 cc versus 0.715 ± 0.133 for infarcts > 80 cc’s for combined inputs; p = 0.52) (Figs. 11 and 12). Comparing standard images with combined inputs, differences were only significant for the largest infarcts > 80 cc (Dice of 0.634 ± 0.204 for standard images versus 0.716 ± 0.133 for combined inputs; p < 0.01) (Fig. 12).
Fig. 11.

Dice scores for infarct segmentation on standard 120 kV and combined 120 kV + 190 keV virtual monochromatic images by volume of infarct. Segmentation performance generally improved with larger infarcts and with combined inputs
Fig. 12.

A comparison of infarct segmentation performance on standard 120 kV, 190 keV virtual monochromatic images, and combined inputs by volume of infarct. Segmentation performance improved with larger infarcts. * indicates p < 0.05 on paired t-test analysis
Discussion
Our results illustrate that supplementation of standard CT images with dual energy 190 VMI images improves infarct visibility for deep learning algorithms, and this is particularly true for infarcts between 6–12 h, providing for earlier and more accurate segmentation.
Our work reaffirms the results previously suggested from qualitative analysis that dual energy acquisition offers better visibility of early ischemic infarcts [2, 4–7]. Proposed methods include altering contrast between gray and white matter and improving image quality by reducing artifacts such as in the posterior fossa [2, 4, 5]. These can improve the contrast between cytotoxic edema from infarcts and surrounding normal brain parenchyma, consequently improving infarct segmentation. In our work, this led to a significant reduction in false negative rate for the deep learning algorithms, but no significant difference in false positive rate.
Infarcts are often not visible on non-contrast head CT until several hours after infarction and our results demonstrate a similar phenomenon for deep learning segmentation. We found low Dice scores for all algorithms within the first 6 h of infarction due to the lack of perceptible CT changes. We did however find that supplementation with VMI images provides a statistically significant albeit small improvement in performance between 6 and 12 h after infarction, and this may be where subtle changes related to cytotoxic edema are better perceived with DECT.
The inter-evaluator Dice score for infarct segmentation of human experts on DWI sequences has been reported as 0.76 ± 0.14 [16]. Representative work using deep learning for segmentation of infarcts on MRI sequences range from Dice scores of 0.67 to 0.85 [13–19]. In our work, we observed Dice scores of 0.68 ± 0.16 for infarcts of all sizes 12 h after infarction with Dice scores upwards of 0.73 ± 0.11 for large volume infarcts > 70 cc. For older and larger infarcts, this performance is comparable to MRI. These results show great promise in allowing for more accurate volumetric segmentation of infarct volumes from non-contrast DECT. This may offer rapid estimation of infarct volumes particularly within 6–24 h where mechanical thrombectomy may still be a consideration, though would not include information regarding the ischemic penumbra which would require perfusion imaging. For early ischemic changes on CT, our observed Dice are also similar to the inter-reader agreement of neuroradiologists and prior machine learning segmentation of early ischemia on standard CT images, demonstrating Dice scores of 0.25–0.56 [20].
Our results are not without limitation. In this study, we utilized 190 keV VMI images to supplement standard CT images, which we utilize for stroke imaging. While this is the practice at our institution, it is not routine standard of care, and would require additional post-processing for institutions that acquire dual energy CT but perform alternate post-processing. The benefit of 190 keV VMI images is that it also allows distinction of iodine staining from hemorrhage which is an important clinical question in stroke imaging [29, 30]. We show here that supplementation of 190 keV images statistically improves visibility of infarcts for machine learning algorithms though this small difference may not necessarily imply a clinical or human-perceptible difference. The lack of benefit from 0 to 6 h also limits clinical applicability as this is the timeframe where thrombolytics can be considered. Furthermore, our dataset was restricted to infarcts with larger than 10 cc of core infarct, which limits utility for small infarcts.
Furthermore, we assessed Dice segmentation scores on a per-scan basis. Dice scores are a standard for machine learning segmentation, though have several limitations in the context of stroke imaging. In this study, segmentation scores reflect total infarct volume, which does not differentiate between a single large infarct or multiple small infarcts. An algorithm may demonstrate high Dice scores by segmenting large areas of infarcts, while missing numerous small infarcts. This is particularly relevant for our dataset where all infratentorial infarcts were accompanied by supratentorial infarcts, which limits ability to assess for performance on posterior fossa infarcts, which have reduced visibility on CT [4]. Furthermore, Dice scores can be artificially elevated for larger infarcts due to the mathematical calculation of the number of correctly segmented voxels, which can account for elevated Dice scores for larger infarcts rather than a true reflection of improved visibility. Future work would need to assess segmentation in specific locations to ensure adequate performance on all vascular territories and infarct shapes and sizes. Finally, our methodology does not account for interval infarction that may occur in the 48 h between DECT and MRI acquisition.
Conclusions
Our results demonstrate the benefit for DECT for earlier and more accurate segmentation of acute ischemic infarcts for deep learning algorithms with reduction in false negative rates particularly between 6 and 12 h from time-last-well. These results provide a framework for estimation of core infarct volumes from initial non-contrast DECT.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Peter Kamel, Mazhar Khalid, Rachel Steger, Uttam Bodanapally, and Dheeraj Gandhi. The technical aspects of the study were performed by Peter Kamel, Adway Kanhere, Pranav Kulkarni, and Vishwa Parekh. The first draft of the manuscript was written by Peter Kamel and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Funding for this project was provided by a Foundation of the American Society of Neuroradiology Grant.
Data Availability
The data that support the findings of this study are available from the corresponding author, PK, upon reasonable request. The data are not publicly available due to privacy of patient information.
Declarations
Ethics Approval
An IRB exemption was granted to conduct this project by the University of Maryland Institutional Review Board.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Hedent S, Hokamp NG, Laukamp KR, et al. Differentiation of Hemorrhage from Iodine Using Spectral Detector CT: A Phantom Study. AJNR Am J Neuroradiol. 2018;39(12):2205-2210. 10.3174/ajnr.A5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangesius S, Janjic T, Steiger R, et al. Dual-energy computed tomography in acute ischemic stroke: state-of-the-art. Eur Radiol. 2021;31(6):4138-4147. 10.1007/s00330-020-07543-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaddam DS, Dattwyler M, Fleiter TR, Bodanapally UK. Principles and Applications of Dual Energy Computed Tomography in Neuroradiology. Semin Ultrasound CT MR. 2021;42(5):418-433. 10.1053/j.sult.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 4.Hixson HR, Leiva-Salinas C, Sumer S, Patrie J, Xin W, Wintermark M. Utilizing dual energy CT to improve CT diagnosis of posterior fossa ischemia. J Neuroradiol. 2016;43(5):346-352. 10.1016/j.neurad.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 5.Mohammed MF, Marais O, Min A, et al. Unenhanced Dual-Energy Computed Tomography: Visualization of Brain Edema. Invest Radiol. 2018;53(2):63-69. 10.1097/RLI.0000000000000413 [DOI] [PubMed] [Google Scholar]
- 6.van Ommen F, Dankbaar JW, Zhu G, et al. Virtual monochromatic dual-energy CT reconstructions improve detection of cerebral infarct in patients with suspicion of stroke. Neuroradiology. 2021;63(1):41-49. 10.1007/s00234-020-02492-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taguchi K, Itoh T, Fuld MK, Fournie E, Lee O, Noguchi K. “X-Map 2.0” for Edema Signal Enhancement for Acute Ischemic Stroke Using Non–Contrast-Enhanced Dual-Energy Computed Tomography. Invest Radiol. 2018;53(7):432–439. 10.1097/RLI.0000000000000461 [DOI] [PubMed]
- 8.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. New England Journal of Medicine. 2018;378(1):11-21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378(8):708-718. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ted. W. Post, ed. Mechanical thrombectomy for acute ischemic stroke. In: UpToDate. ; 2023.
- 11.Vagal A, Wintermark M, Nael K, et al. Automated CT perfusion imaging for acute ischemic stroke: Pearls and pitfalls for real-world use. Neurology. 2019;93(20):888-898. 10.1212/WNL.0000000000008481 [DOI] [PubMed] [Google Scholar]
- 12.Kamel PI, Yi PH, Sair HI, Lin CT. Prediction of coronary artery calcium and cardiovascular risk on chest radiographs using deep learning. Radiol Cardiothorac Imaging. 2021;3(3). 10.1148/ryct.2021200486 [DOI] [PMC free article] [PubMed]
- 13.Olivier A, Moal O, Moal B, et al. Active learning strategy and hybrid training for infarct segmentation on diffusion MRI with a U-shaped network. Journal of Medical Imaging. 2019;6(04):1. 10.1117/1.JMI.6.4.044001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong KK, Cummock JS, Li G, et al. Automatic Segmentation in Acute Ischemic Stroke: Prognostic Significance of Topological Stroke Volumes on Stroke Outcome. Stroke. 2022;53(9):2896-2905. 10.1161/STROKEAHA.121.037982 [DOI] [PubMed] [Google Scholar]
- 15.Cui L, Fan Z, Yang Y, et al. Deep Learning in Ischemic Stroke Imaging Analysis: A Comprehensive Review. Biomed Res Int. 2022;2022:1-15. 10.1155/2022/2456550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CF, Hsu J, Xu X, et al. Deep learning-based detection and segmentation of diffusion abnormalities in acute ischemic stroke. Communications Medicine. 2021;1(1):61. 10.1038/s43856-021-00062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Bentley P, Rueckert D. Fully automatic acute ischemic lesion segmentation in DWI using convolutional neural networks. Neuroimage Clin. 2017;15:633-643. 10.1016/j.nicl.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo I, Lee A, Jung SC, et al. Fully Automatic Segmentation of Acute Ischemic Lesions on Diffusion-Weighted Imaging Using Convolutional Neural Networks: Comparison with Conventional Algorithms. Korean J Radiol. 2019;20(8):1275-1284. 10.3348/kjr.2018.0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamel P, Kanhere A, Kulkarni P, et al. Optimizing Acute Stroke Segmentation on MRI Using Deep Learning: Self-Configuring Neural Networks Provide High Performance Using Only DWI Sequences. Journal of Imaging Informatics in Medicine. Published online August 13, 2024. 10.1007/s10278-024-00994-2 [DOI] [PMC free article] [PubMed]
- 20.Ostmeier S, Axelrod B, Verhaaren BFJ, et al. Non-inferiority of deep learning ischemic stroke segmentation on non-contrast CT within 16-hours compared to expert neuroradiologists. Sci Rep. 2023;13(1):16153. 10.1038/s41598-023-42961-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez Petzsche MR, de la Rosa E, Hanning U, et al. ISLES 2022: A multi-center magnetic resonance imaging stroke lesion segmentation dataset. Sci Data. 2022;9(1):762. 10.1038/s41597-022-01875-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isensee F, Jaeger PF, Kohl SAA, Petersen J, Maier-Hein KH. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods. 2021;18(2):203-211. 10.1038/s41592-020-01008-z [DOI] [PubMed] [Google Scholar]
- 23.pynetdicom. 2022. Accessed December 21, 2022. https://pydicom.github.io/pynetdicom/stable/#
- 24.RSNA MIRC Clinical Trials Processor. 2022. Accessed December 21, 2022. https://mircwiki.rsna.org/index.php?title=MIRC_CTP
- 25.dicom2nifti. Accessed July 2, 2023. https://github.com/icometrix/dicom2nifti
- 26.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143-155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohlfing T, Zahr NM, Sullivan E V, Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Hum Brain Mapp. 2010;31(5):798-819. 10.1002/hbm.20906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ANTsPy. Accessed December 26, 2022. https://antspyx.readthedocs.io/en/latest/index.html
- 29.Bodanapally UK, Archer-Arroyo KL, Dreizin D, et al. Dual-Energy Computed Tomography Imaging of Head: Virtual High-Energy Monochromatic (190 keV) Images Are More Reliable Than Standard 120 kV Images for Detecting Traumatic Intracranial Hemorrhages. J Neurotrauma. 2019;36(8):1375-1381. 10.1089/neu.2018.5985 [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman WD, Pergakis M, Ahmad G, et al. Iodine-based dual-energy CT predicts early neurological decline from cerebral edema after large hemispheric infarction. Res Sq. Published online November 10, 2023. 10.21203/rs.3.rs-3508427/v1
- 31.Nikolov S, Blackwell S, Zverovitch A, et al. Clinically Applicable Segmentation of Head and Neck Anatomy for Radiotherapy: Deep Learning Algorithm Development and Validation Study. J Med Internet Res. 2021;23(7):e26151. 10.2196/26151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surface Distance Metrics. Accessed August 29, 2024. https://github.com/google-deepmind/surface-distance
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, PK, upon reasonable request. The data are not publicly available due to privacy of patient information.