Abstract
Objective
For most patients with breast cancer undergoing breast-conserving surgery, radiotherapy reduces local recurrence and mortality. However, in basal-like breast cancer, radiotherapy is less effective due to the cells’ intrinsic radioresistance. Understanding the molecular mechanism of radioresistance in basal-like breast cancer is crucial for improving treatment and outcomes.
Methods
αB-crystallin levels were determined in different breast cancer cell lines, including basal-like breast cancer cell lines (HCC1599, MDA-MB-468, MDA-MB-231) and luminal breast cancer cell lines (MDA-MB-453, BT-474, MCF-7). To further investigate the role of αB-crystallin in breast cancer cells, we utilized short hairpin RNA lentivirus particles to knock down αB-crystallin expression in MDA-MB-231 cells and overexpressed it in MCF-7 cells via lentivirus-mediated delivery of αB-crystallin. Cell migration and invasion were assessed using transwell assays, and radiosensitivity was evaluated via colony formation assays. Flow cytometry was employed to quantify apoptotic cells and western blot was performed to explore the expression levels of invasion and migration-related proteins and apoptotic proteins. The levels of γ-H2AX and the co-localization of αB-crystallin with Caspase-3 in cells were evaluated using immunofluorescence microscopy. Immunoprecipitation followed by western blot was used to detect the interaction of αB-crystallin with Caspase-3.
Results
We have identified αB-crystallin as a pivotal regulator of migration and radiosensitivity in basal-like breast cancer. Repression of αB-crystallin expression enhanced radiation-induced DNA damage and apoptosis in basal-like breast cancer cells. Moreover, knockdown of αB-crystallin diminished its interaction with Caspase-3, resulting in an amplified activation of Caspase-3 triggered by irradiation, intensified apoptosis, and heightened radiosensitivity in basal-like breast cancer cells.
Conclusions
The overexpression of αB-crystallin not only enhances the migratory capacity of basal-like breast cancer cells but also confers radioresistance to the cells by directly interacting with Caspase-3 and inhibiting its activity. Suppression of αB-crystallin enhances radiation-induced cell death, thereby improving the radiosensitivity of basal-like breast cancer cells.
Keywords: Basal-like breast cancer, αB-crystallin, Caspase-3, apoptosis, radioresistance
Introduction
Breast cancer is the most prevalent malignant neoplasm among women globally and poses a significant threat to female health. 1 According to the molecular characteristics, breast cancer can be classified into four subtypes: luminal epithelial type A, luminal epithelial type B, Her2 (human epidermal growth factor receptor 2) overexpression type, and basal-like type. The basal-like subtype of breast cancer, comprising approximately 15% to 20% of all breast cancers, is characterized by its highly aggressive nature, frequent recurrence, absence of targeted therapeutic options, and unfavorable prognosis. 2 Breast cancer is primarily managed through surgical intervention, radiotherapy, or a combination thereof, alongside systemic chemotherapy, endocrine therapy, biological therapy, or a combination of these modalities. 3 The administration of radiotherapy is considered a crucial component in the treatment regimen for the majority of patients with breast cancer who undergo breast-conserving surgery, as it confers substantial clinical advantages including a reduction in local tumor recurrence risk and improved overall survival rates from breast cancer. 4 However, local tumor control rates remain suboptimal for basal-like breast cancer. In the case of patients with this particular subtype, inherent radioresistance of cancer cells constitutes a primary obstacle to the efficacy of radiotherapy. Therefore, elucidating the molecular mechanism underlying radioresistance in basal-like breast cancer holds significant potential for enhancing the clinical efficacy of treatment strategies for this subtype in future therapeutic interventions.
The gene test data demonstrate that αB-crystallin exhibits a significantly elevated expression level in basal-like breast cancer tissues, thereby facilitating cancer cell migration and invasion as well as promoting angiogenesis.5,6 The transplantation of tumors with αB-crystallin overexpression in nude mice has been demonstrated to induce basal-like tumor characteristics, as evidenced by previous studies. 7 The collective findings suggest that αB-crystallin serves as a reliable biomarker for basal-like breast cancer, a subtype with distinct molecular characteristics. Notably, studies have consistently reported αB-crystallin expression in 45% to 56% of basal-like breast cancer tissues.8,9 The expression sensitivity of αB-crystallin in basal-like breast cancer was found to be higher compared to CK5/6, CK14, and p63. Additionally, its specificity was observed to be higher than that of epidermal growth factor receptor and c-Kit. Therefore, αB-crystallin can be considered as a sensitive and specific marker for identifying basal-like breast cancer.10,11
αB-crystallin, a member of the mammalian small heat shock protein (sHSP) superfamily, serves as a cellular chaperone that is upregulated in response to various stimuli. This upregulation effectively mitigates the accumulation of misfolded proteins and enhances cell survival. 12 The heterotopic expression of αB-crystallin in various cell types confers protection against a diverse range of apoptotic stimuli, including tumor necrosis factor-α, chemotherapeutic agents, growth factor deficiency, and oxidative stress. This protective effect is achieved through the inhibition of Caspase-3 activation and the binding to apoptotic Bcl-2 family members (Bax, Bcl-x), thereby safeguarding mitochondria and suppressing apoptosis.13,14 Therefore, αB-crystallin is likely to enhance radiation resistance in basal-like breast cancer by suppressing apoptosis. In this study, we observed a significant upregulation of αB-crystallin expression in basal-like breast cancer cells, which was found to facilitate cell migration and confer augmented radioresistance. Conversely, the downregulation of αB-crystallin levels demonstrated an augmented sensitivity toward radiation treatment in basal-like breast cancer, thereby potentially enhancing the efficacy of clinical radiotherapy for this particular subtype.
Materials and methods
Cell culture and irradiation
The cell lines utilized in this investigation were acquired from the Cell Bank of Type Culture Collection at the Chinese Academy of Sciences (Shanghai, China). HCC1599 (RRID: CVCL_1256), MDA-MB-468 (RRID: CVCL_0419), and MDA-MB-231 (RRID: CVCL_0062) cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco, Australia), along with 100 U/mL penicillin and 100 μg/mL streptomycin. MDA-MB-453 (RRID: CVCL_0418), BT-474 (RRID: CVCL 0179), and MCF-7 (RRID: CVCL_0031) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) enriched with 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin. The cells were irradiated using an RS2000 X-ray device (RS-2000 Pro; Rad Source, USA) at a dose rate of 1.16 Gy/min.
Transwell assay
Cell migration and invasion were assessed using Boyden chambers with a diameter of 6.5 mm and pores measuring 8 μm (Cat. No: 353097; Corning, USA). The upper chambers were either coated or uncoated with Matrigel (Cat. No: 356234; Corning) to detect invasion or migration, respectively. Following a 6-hour serum-free starvation period, 40,000 cells per well were seeded into the upper chambers and cultured in serum-free medium. In the lower chamber, 600 μL of medium containing 10% FBS was added. Cells were cultured continuously for either 12 (migration) or 24 hours (invasion). Subsequently, nonmigrating cells and Matrigel in the upper chamber were eliminated using a cotton swab. The filters containing invaded cells were individually fixed using 4% paraformaldehyde, stained with 0.1% crystal violet solution, and visualized under a light microscope (IX73; Olympus, Japan).
Colony formation assay
The cell radiosensitivity was assessed using a colony formation assay. Varying numbers of cells were seeded into six-well plates (150, 200, 400, 1000, and 2000 per well). After a 24-hour incubation, the cells were exposed to X-ray irradiation at room temperature with doses of 0, 2, 4, 6, and 8 Gy. The colonies were allowed to grow for a duration of 2 weeks after radiation exposure. Subsequently, the colonies were rinsed twice with PBS solution and fixed using 70% ethanol before being stained with crystal violet solution (0.1%). Colonies consisting of more than 50 cells were counted. The percentage of colony formation rate for each group was calculated as follows: (number of clones divided by the initial number of seeded cells) multiplied by one hundred percent (%). The survival fraction (SF) for the control group was considered as one hundred percent (%), while the SF for each experimental group was determined by dividing its clone formation rate by that of the control group and multiplying it by one hundred percent (%).
Real-time quantitative PCR
Total RNA was extracted using Trizol reagent (Cat. No: 15596026CN; Invitrogen, USA). Subsequently, the extracted RNA was reverse-transcribed into cDNA using the PrimeScript RT Reagent Kit (Cat. No: RR037A; Takara, Japan). Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using PowerUp™ SYBR® Green Master Mix (Cat. No: A25918; Thermo Fisher Scientific, USA) on a Life Technologies system (ViiA7; Thermo Fisher Scientific, USA). The primers for αB-crystallin were designed as follows: forward primer: 5ʹ-GCACTTCTCCCCAGAGGAAC-3ʹ; reverse primer: 5ʹ-CCATTCACAGTGAGGACCCC-3ʹ. For glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the primers were as follows: forward primer: 5ʹ-GCACCGTCAAGGCTGAGAAC-3ʹ; reverse primer: 5ʹ-TGGTGAAGACGCCAGTGGA-3ʹ. GAPDH was used as an internal normalization control. The mean values of six independent replicates for each gene were calculated. Relative gene expression levels were quantified using the 2–ΔΔCt method.
Gene silencing and overexpression
Short hairpin RNA (shRNA) sequences targeting αB-crystallin were annealed and ligated into the pU6-shRNA-CMV-Puro lentiviral vector. The sequences of αB-crystallin shRNA: sense, 5′-CCGGGACTAGTGCTGAAGCTTATTATTCAAGAGATAATAA GCTTCAGCACTAGTCTTTTTG-3′; antisense, 5′-AATTCAAAAAGACTAGTGCTGAAGCTTATTATCTCTT GAATAATAAGCTTCAGCACTAGTC-3′; and target, CTAGTGCTGAAGCTTATTA. The empty vector was used as a control. Lentiviral vector pCDH-EF1-MCS-T2A-Puro carrying the cDNA of αB-crystallin was constructed for the purpose of overexpressing αB-crystallin. An empty vector was used as a negative control. The titer of all the aforementioned lentiviral vectors was 1 × 10^8 TU/mL. The viral stock solution was subsequently diluted at a ratio of 1:10 for the infection of target cells. All lentiviral vectors carried the puromycin resistance gene and were purchased from Sangon Biotech (Shanghai, China). Human breast cancer cell lines were infected with lentiviruses for 24 hours and then cultured in fresh medium. After incubation for 48 hours, media containing puromycin at a concentration of 1.5 μg/mL was added to select cells that exhibited stable expression of the desired genes.
Western blot assay
Radioimmunoprecipitation assay buffer buffer was utilized for the extraction of total proteins, and protein concentration was determined using a DC Protein Assay Kit I (Cat. No:500-0111; Bio-Rad, USA). All protein samples were subjected to denaturation at 100°C for 5 minutes. Equal quantities of protein were separated via sodium dodecyl sulfate polyacrylamide gel electrophoresis and subsequently transferred onto a PVDF (polyvinylidene fluoride) membrane. The membranes were immersed in nonfat milk (5%) to block heterogeneous antigens for 1 hour at room temperature, followed by overnight incubation with primary antibodies at 4°C. After three washes with PBST (PBS supplemented with Tween-20), the membranes were exposed to horseradish peroxidase-conjugated secondary antibody at room temperature for 1 hour. Signals were detected using an ECL (enhanced chemiluminescence) kit (Cat. No: 6883; Cell Signaling Technology, USA) and recorded utilizing a polychromatic fluorescence chemiluminescence imaging analysis system (FluorChem E; Protein Simple, USA). Relative densitometry values for the protein blots were analyzed using Image J 1.8.0 software. The details of the antibodies utilized in the western blot analysis are presented in Table 1.
Table 1.
Primary and secondary antibodies used for western blot.
| Antibody | Company | Cat. No: | Clone number | Dilution |
|---|---|---|---|---|
| αB-crystallin | Abcam | 230722 | 6D11 | 1:500 |
| N-cadherin, | Cell Signaling Technology | 13116 | D4R1H | 1:1000 |
| E-cadherin, | Cell Signaling Technology | 3195 | 24E10 | 1:1000 |
| Vimentin | Cell Signaling Technology | 5741 | D21H3 | 1:1000 |
| Snail | Cell Signaling Technology | 3879 | C15D3 | 1:1000 |
| Bax | Cell Signaling Technology | 5023 | D2E11 | 1:1000 |
| Bcl-2 | Cell Signaling Technology | 3498 | D17C4 | 1:1000 |
| Cleaved Caspase-3 | Cell Signaling Technology | 9661 | Asp175 | 1:1000 |
| β-Actin | Cell Signaling Technology | 8457 | D6A8 | 1:1000 |
| Anti-rabbit IgG | Cell Signaling Technology | 7074 | 1:2000 | |
| Anti-mouse IgG | Cell Signaling Technology | 7076 | 1:2000 |
Assessment of apoptosis and Caspase-3 activity
The cells were harvested 48 hours after exposure to a radiation dose of 4 Gy of X-rays. Subsequently, flow cytometry (Verse; BD Biosciences, USA) was employed to quantify apoptotic cells using the Annexin V-PE/7-AAD Apoptosis Detection Kit (Cat. No: 40295S; Cell Signaling Technology, USA), following the manufacturer's guidelines. Forty-eight hours after radiation exposure, the cells were harvested and lysed on ice for 5 minutes using a lysis buffer (Cat. No: 7018S; Cell Signaling Technology, USA). The lysates were then centrifuged at 10,000 × g for 10 minutes at 4°C. The resulting supernatant was transferred to a 96-well plate at a volume of 100 μL per well. Caspase-3 activity was subsequently measured using Caspase-3 activity assay kits (Cat. No: 5723S; Cell Signaling Technology, USA) in accordance with the manufacturer's instructions.
Immunofluorescence
Cells were grown on glass cover slips in a 24-well plate. After treatment, the cells were fixed with a solution containing 4% paraformaldehyde for 15 minutes. Subsequently, they were rinsed three times with PBS and permeabilized using 0.2% Triton X-100 at room temperature for 15 minutes. To prevent nonspecific binding, the cells were then blocked with a solution containing 5% bovine serum albumin for 1 hour. Following this, they were incubated overnight at a temperature of 4°C with the primary antibody specific to γ-H2AX (1:500; Cat. No: ab303656; Clone number: N1-431; Abcam), αB-crystallin (1:50; Cat. No: ab230722; Clone number: 6D11; Abcam), and Caspase-3 (1:25; Cat. No: 32351; Clone number: E87; Abcam). After washing the samples three times with PBST, the cover slips were incubated with fluorescent secondary antibodies: Anti-Rabbit IgG-Alexa Fluor 488 (1:500; Cat. No: ab150077; Abcam) or Anti-Mouse IgG-Alexa Fluor 647 (1:500; Cat. No: ab150115; Abcam) for 1 hour at room temperature in the dark. The samples underwent another round of washing thrice with PBST before being mounted using an anti-quenching reagent along with 4', 6-diamidino-2-phenylindole (Cat. No: P0131; Beyotime, China). Finally, foci representing γ-H2AX protein expression levels were quantified by examining at least six random fields under a Confocal Laser Scanning Microscope (FV1200; Olympus, Japan).
Co-Immunoprecipitation
Cells were lysed with a specialized buffer included in the Antibody Cross-linking IP/Co-IP Kit (Cat. No: P2180S; Beyotime, China) for subsequent immunoprecipitation analysis. And 50 µL of total cell extracts were incubated overnight at 4°C with anti-αB-crystallin antibodies (1:20; Cat. No: ab230722; Clone number: 6D11; Abcam), anti-Caspase-3 antibody (1:20; Cat. No: 32351; Clone number: E87; Abcam), or anti-IgG antibody (1:20; Clone number: EPR25A; Abcam). Subsequently, the antibody–protein complex was mixed with protein A/G plus-agarose beads and allowed to interact at room temperature for 2 h. The protein-coated beads were then separated and heated in a loading buffer. Finally, the precipitated protein complexes underwent western blot analysis.
Statistics
Graphs were generated and statistical analyses were conducted using GraphPad Prism 8.3.0 software. The data were assessed for normality using the Shapiro-Wilk test. Normally distributed data were compared between two groups using unpaired two-tailed t-tests. For non-normal data, the Wilcoxon rank test was used. Statistical significance was defined as p ≤ 0.05. Each experiment was independently repeated at least three times, and all data are expressed as means ± standard deviation (SD).
Results
αB-crystallin facilitates cell migration in basal-like breast cancer cells
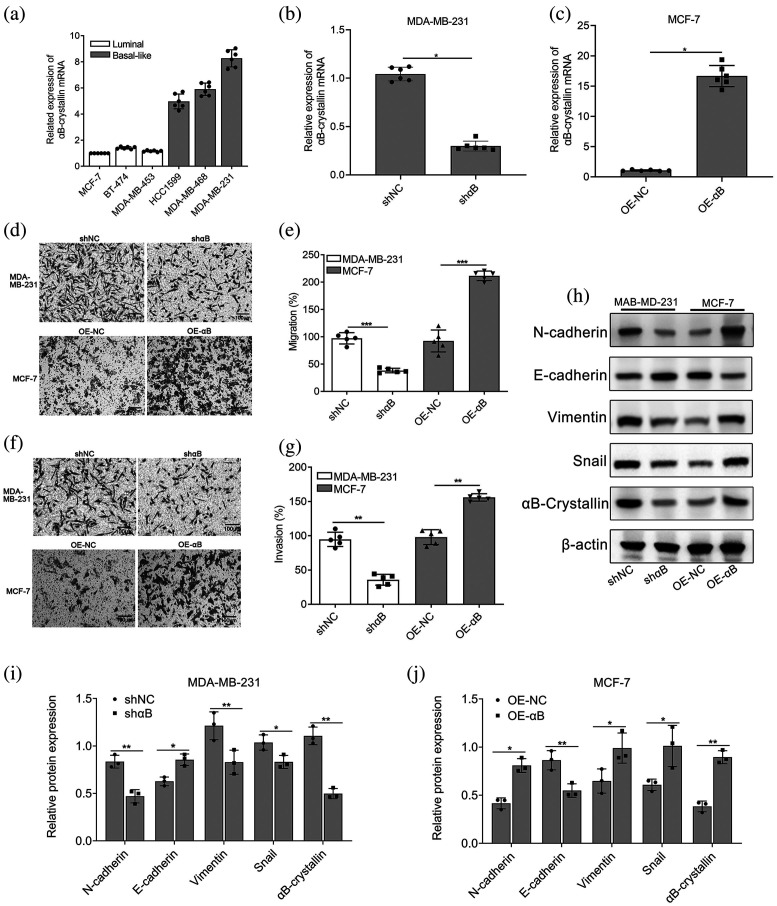
Most studies have shown that basal-like breast cancer exhibits highly aggressive biological behavior, is prone to early hematologic metastasis, and has low disease-free survival and overall survival. Therefore, αB-crystallin levels were determined in different breast cancer cell lines, including basal-like breast cancer cell lines (HCC1599, MDA-MB-468, MDA-MB-231) and luminal breast cancer cell lines (MDA-MB-453, BT-474, MCF-7). The results showed that αB-crystallin was highly expressed in basal-like cell lines compared to luminal cell lines (Figure 1(a)). The results demonstrated that the expression of αB-crystallin was significantly elevated in basal-like cell lines compared to luminal cell lines, with MDA-MB-231 cells exhibiting the highest expression and MCF-7 cells displaying the lowest levels. To further elucidate the role of αB-crystallin in breast cancer cells, we employed shRNA lentivirus particles to knock down αB-crystallin expression in MDA-MB-231 cells, while overexpressing it in MCF-7 cells through lentivirus-mediated delivery of αB-crystallin (Figure 1(b) and (c)). Transwell assays revealed that suppression of αB-crystallin in MDA-MB-231 cells significantly attenuated their invasive and migratory capacities compared to the control shRNA-treated cells (shNC). Conversely, overexpression of αB-crystallin in MCF-7 cells enhanced their migratory and invasive abilities when compared to the negative control (NC) (Figure 1(d)–(g)). Western blotting analysis confirmed the correlation between αB-crystallin and genes associated with epithelial-mesenchymal transition (EMT). The results demonstrated that, compared with the NC group, overexpression of αB-crystallin significantly upregulated the protein expression levels of mesenchymal markers N-cadherin and Vimentin, as well as the transcription factor Snail in MCF-7 cells (Figure 1(h) and (j)). Conversely, interfering with the expression of αB-crystallin in MDA-MB-231 cells markedly suppressed the expression of EMT-associated proteins compared to the shNC group (Figure 1(h) and (i)). These results strongly suggest that sufficient expression of αB-crystallin facilitates the metastasis of basal-like breast cancer cells.
Figure 1.
αB-crystallin facilitates cell migration in basal-like breast cancer cells. (a) Relative αB-crystallin expression levels in basal-like breast cancer cell lines HCC1599, MDA-MB-468, MDA-MB-231, and in luminal breast cancer cell lines MDA-MB-453, BT-474, MCF-7. (b) and (c) The expression levels of αB-crystallin in MDA-MB-231 and MCF-7 cells transfected with αB-crystallin knockdown or overexpression lentiviral vectors were quantified using qRT-PCR, with GAPDH serving as the internal loading control. Data are represented as means ± SD (n = 6; Wilcoxon rank test; *p < 0.05). (d)–(g): Migration (d) and (e) and invasion (f) and (g) activities of MDA-MB-231, MCF-7, and control cells were analyzed using a Transwell assay. Representative images (100×, Scale bar: 100 μm) and summarized quantitative results are presented separately. Data are represented as means ± SD (n = 5; unpaired two-tailed Student's t-test; *p < 0.05, **p < 0.01, ***p < 0.001). (h)–(j) Western blot analysis of EMT markers (E-cadherin, N-cadherin, Vimentin, and Snail). β-Actin was utilized as the internal reference for normalization. The histogram presents the results of the quantitative analysis of the protein bands. Data are represented as means ± SD (n = 3; unpaired two-tailed Student's t-test; *p < 0.05, **p < 0.01). EMT: epithelial-mesenchymal transition; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; OE-αB: cells transfected with αB-crystallin overexpression vectors; qRT-PCR: quantitative real-time polymerase chain reaction; shαB: cells transfected with αB-crystallin-specific shRNA; shNC and OE-NC: cells transfected with corresponding empty vectors.
αB-crystallin enhances radioresistance in basal-like breast cancer cells
The results demonstrated an increased number of γ-H2AX foci in αB-crystallin-silenced MDA-MB-231 cells compared to the control group following exposure to 4 Gy of X-rays (Figure 2(a) and (b)). Conversely, overexpression of αB-crystallin led to a reduction in γ-H2AX foci in irradiated MCF-7 cells (Figure 2(c) and (d)). The radiosensitization impact of αB-crystallin silencing in breast cancer cells was confirmed through a clonogenic assay. Results demonstrated that the colony survival fraction of MDA-MB-231 cells transfected with αB-crystallin-shRNA significantly decreased after X-ray irradiation compared to shNC cells (Figure 2(e)). Conversely, overexpression of αB-crystallin significantly enhanced the survival fraction of MCF-7 cells irradiated by X-ray (Figure 2(f)). These findings suggest that downregulation of αB-crystallin enhances the radiosensitivity of basal-like breast cancer cells to X-ray irradiation.
Figure 2.
αB-crystallin enhances radioresistance in basal-like breast cancer cells. (a) and (c) Representative images of γ-H2AX foci formation were acquired in MDA-MB-231 + shαB, MCF-7 + OE-αB, and control cells at 2-hour post 4 Gy of X-ray irradiation. Red fluorescence indicates γ-H2AX foci. These images were captured using a 60× objective lens and subsequently magnified locally to facilitate detailed observation. Scale bar: 10 μm. (b) and (d) The number of foci in each group was normalized to those in shNC or OE-NC cells from the 0 Gy group for quantification of γ-H2AX foci formation. (e) and (f) Colony formation and survival curve analysis were conducted on MDA-MB-231 + shαB, MCF-7 + OE-αB, and control cells following exposure to varying doses of X-ray irradiation (0, 2, 4, 6, and 8 Gy). Data are represented as means ± SD (n = 5; unpaired two-tailed Student's t-test; *p < 0.05, **p < 0.01). OE-αB: cells transfected with αB-crystallin overexpression vectors; shαB: cells transfected with αB-crystallin-specific shRNA; shNC and OE-NC: cells transfected with corresponding empty vectors.
Downregulation of αB-crystallin enhances radiation-induced apoptosis in basal-like breast cancer cells
Flow cytometry assay revealed that inhibition of αB-crystallin resulted in enhanced radiation-induced apoptosis in αB-crystallin-silenced MDA-MB-231 cells compared to control cells (Figure 3(a) and (b)). Conversely, overexpression of αB-crystallin significantly attenuated radiation-induced apoptosis in MCF-7 cells (Figure 3(c) and (d)). Similarly, in the radiation-treated MDA-MB-231 cells, compared with the control group, the knockdown of αB-crystallin resulted in increased levels of Bax and cleaved Caspase-3, as well as decreased expression of Bcl-2 (Figure 3(e) and (g)). Alternatively, overexpression of αB-crystallin in irradiated MCF-7 cells led to decreased levels of Bax and Cleaved Caspase-3, along with an increase in Bcl-2 expression (Figure 3(f) and (h)).
Figure 3.
Downregulation of αB-crystallin enhances radiation-induced apoptosis in basal-like breast cancer cells. (a)–(d) The apoptosis rate was estimated using flow cytometry in MDA-MB-231 + shαB, MCF-7 + OE-αB, and control cells at 48 h after exposure to 4 Gy of X-rays. Data are represented as means ± SD (n = 6; unpaired two-tailed Student's t-test; **p < 0.01). (e)–(h) Western blot analysis was performed to evaluate the expression levels of proteins associated with apoptosis at 48 hours after 4 Gy of X-ray irradiation. The histogram presents the results of the quantitative analysis of the protein bands. Data are represented as means ± SD (n = 3; unpaired two-tailed Student's t-test; *p < 0.05, **p < 0.01). OE-αB: cells transfected with αB-crystallin overexpression vectors; shαB: cells transfected with αB-crystallin-specific shRNA; shNC and OE-NC: cells transfected with corresponding empty vectors.
αB-crystallin interacts with Caspase-3 to attenuate its enzymatic activity
Recent studies have revealed an intriguing interaction between αB-crystallin and Caspase-3, a key enzyme involved in programmed cell death or apoptosis. It has been demonstrated that the binding of αB-crystallin to Caspase-3 inhibits the enzymatic activity of the latter, thereby preventing excessive apoptosis. This interaction highlights the importance of αB-crystallin as a protective factor against apoptotic processes. To determine whether αB-crystallin binds to Caspase-3 in breast cancer cells, we immunoprecipitated αB-crystallin from MDA-MB-231 and MCF-7 cells. As shown in Figure 4(a) and (b), αB-crystallin was found to co-immunoprecipitate with Caspase-3. To evaluate the effect of X-ray irradiation on the interaction between αB-crystallin and Caspase-3, an immunofluorescence assay was conducted. The results demonstrated a significant reduction in the interaction between αB-crystallin and Caspase-3 in MDA-MB-231 cells following αB-crystallin knockdown, whereas an enhanced interaction was observed in MCF-7 cells overexpressing αB-crystallin (Figure 4(c) and (d)). Similarly, the activities of Caspase-3 were evaluated, demonstrating that knockdown of αB-crystallin enhanced Caspase-3 activity in MDA-MB-231 cells, whereas overexpression of αB-crystallin decreased Caspase-3 activity in MCF-7 cells following 4 Gy X-ray irradiation (Figure 4(e) and (f)). These findings indicate that αB-crystallin binds to Caspase-3, thereby inhibiting its enzymatic activity and consequently modulating Caspase-3-mediated apoptosis.
Figure 4.
αB-crystallin interacts with Caspase-3 to attenuate its enzymatic activity. (a) and (b) Immunoprecipitation followed by western blot was used to detect the interaction of αB-crystallin with Caspase-3 in MDA-MB-231 and MCF-7 cells. (c) and (d) The co-localization of αB-crystallin (red) and Caspase-3 (green) in cells was detected using an immunofluorescence assay. Nuclei were stained with DAPI (blue). These images were acquired using a 60× objective lens and subsequently magnified locally to facilitate detailed observation. Scale bar: 10 μm. (e) and (f) The activities of Caspase-3 were assessed using Caspase-3 activity kits in MDA-MB-231 + shαB, MCF-7 + OE-αB, and control cells at 48 hours post-exposure to 4 Gy of X-ray irradiation. Data are represented as means ± SD (n = 5; unpaired two-tailed Student's t-test; *p < 0.05, **p < 0.01). DAPI: 4', 6-diamidino-2-phenylindole; OE-αB: cells transfected with αB-crystallin overexpression vectors; shαB: cells transfected with αB-crystallin-specific shRNA; shNC and OE-NC: cells transfected with corresponding empty vectors.
Discussion
Breast cancer is one of the most prevalent types of cancer affecting women worldwide. Among its subtypes, basal-like breast cancer is known for its aggressive nature and poor prognosis. Understanding the molecular mechanisms underlying tumor progression and metastasis in this subtype is essential for developing effective therapeutic strategies. Recent studies have highlighted the involvement of αB-crystallin, a member of the small heat shock protein family, in facilitating cell migration and invasion in cancer cells. 15 We investigated the expression levels of αB-crystallin across common breast cancer cell lines and discovered that it was most prominently expressed in the basal-like breast cancer cell line MDA-MB-231, while exhibiting minimal expression in the luminal breast cancer cell line MCF-7. Compared with MCF-7 cells, MDA-MB-231 cells exhibited significantly stronger migration and invasion capabilities. However, the suppression of αB-crystallin markedly attenuated the migratory and invasive abilities of MDA-MB-231 cells, suggesting that excessive expression of αB-crystallin plays a critical role in promoting the migration and invasion of breast cancer cells. Mechanistically, αB-crystallin may play a role in epithelial-mesenchymal transition (EMT), a process that is closely associated with tumor metastasis. αB-crystallin induces EMT-associated transcription factors such as Snail and Slug through activation of signaling pathways like transforming growth factor beta/Smad or phosphoinositide 3-kinase/Akt/mechanistic target of rapamycin cascades.16–18 These transcription factors repress E-cadherin expression while upregulating mesenchymal markers like N-cadherin and vimentin, thereby enhancing invasive properties of basal-like breast cancer cells. The present study also revealed that knockdown of αB-crystallin significantly modulated the expression of proteins associated with EMT and effectively inhibited the migratory capacity of basal-like breast cells. Therefore, it is clinically viable to inhibit the expression of αB-crystallin to reduce metastasis and recurrence, thereby enhancing the therapeutic efficacy for breast cancer.
Radiotherapy serves as a primary modality for the conservative treatment of many cancers; however, the intrinsic radioresistance of cancer cells significantly diminishes its therapeutic efficacy. Basal-like breast cancer is characterized by its highly aggressive nature and resistance to conventional therapies, including radiotherapy. Radiotherapy functions by inducing DNA damage in tumor cells, thereby instigating their demise. However, αB-crystallin facilitates efficient repair of damaged DNA by interacting with other proteins involved in DNA repair pathways. This ultimately enables the survival and sustained proliferation of tumor cells despite exposure to radiation.19,20 We observed a significant reduction in radioresistance when αB-crystallin expression was downregulated in basal-like breast cancer cells. Conversely, the overexpression of αB-crystallin in highly radiosensitive luminal breast cancer cells led to a marked enhancement in radioresistance. However, radioresistant cells frequently evade apoptosis via multiple mechanisms. They might upregulate anti-apoptotic proteins such as Bcl-2 or downregulate pro-apoptotic factors like Bax, thereby preventing the activation of caspases, which are key enzymes in the execution of apoptosis. αB-crystallin exhibits an anti-apoptotic effect on basal-like breast cancer cells following irradiation, thereby suppressing programmed cell death initiated by severe cellular stress or damage caused by radiotherapy.21,22 This study demonstrated that the downregulation of αB-crystallin augmented DNA damage and apoptosis in basal-like breast cancer cells induced by radiation. Further research aimed at developing targeted strategies or inhibitors against αB-crystallin could potentially enhance the efficacy of radiotherapy for basal-like breast cancer.
By inhibiting apoptosis signaling pathways, αB-crystallin prevents the programmed cell death of irradiated tumor cells and promotes their survival instead. Elucidating the mechanisms by which αB-crystallin enhances radioresistance in basal-like breast cancer provides critical insights into potential therapeutic targets, thereby facilitating the optimization of treatment strategies for patients with this aggressive subtype. αB-crystallin has been identified as a pivotal regulator of Caspase-3 enzymatic activity, which is crucial for programmed cell death or apoptosis. By interacting with Caspase-3, αB-crystallin effectively functions as a potent inhibitor, thereby attenuating its enzymatic activity. 23 The interaction between αB-crystallin and Caspase-3 functions as a protective mechanism against excessive cellular apoptosis, thereby contributing to the maintenance of cellular homeostasis and prevention of unnecessary cell loss. 24 Additionally, the present research demonstrated that the interaction between αB-crystallin and Caspase-3 not only attenuates its enzymatic activity but also modulates its substrate specificity, thereby implying a potential influence of αB-crystallin on downstream signaling pathways regulated by activated Caspase-3. However, the precise molecular mechanism underlying the downstream effects of the interaction between αB-crystallin and Caspase-3 has not been fully elucidated. Ongoing research is being conducted to address this knowledge gap. It is hypothesized that specific regions or domains within both proteins play a crucial role in their binding affinity and subsequent modulation of Caspase-3 activity. 25 Comprehending these structural intricacies has the potential to pave the way for therapeutic strategies targeting this interaction in diverse pathologies characterized by aberrant apoptosis. However, αB-crystallin is expressed not only in cancer cells but also in other normal cells, such as lens cells. Therefore, specifically inhibiting αB-crystallin in tumor cells while preserving its function in normal cells will be a significant challenge and an important research direction for our future studies.
Conclusion
In summary, our findings indicate that αB-crystallin enhances the radioresistance of basal-like breast cancer cells through its interaction with Caspase-3, thereby inhibiting Caspase-3-mediated apoptosis. This interaction between αB-crystallin and Caspase-3 offers valuable insights into the intricate regulatory network governing radiation-induced apoptosis. Further investigation into the complex interplay between these two proteins promises significant advancements in our understanding of apoptotic pathways and may lead to the identification of novel therapeutic strategies for basal-like breast cancer.
Supplemental Material
Supplemental material, sj-docx-1-sci-10.1177_00368504251343177 for αB-crystallin enhances radioresistance in basal-like breast cancer cells by suppressing caspase-3 activation by Yan Yuan, Xubaihe Zhang, Ying Xu, Yangyang Ge and Anqing Wu in Science Progress
Acknowledgements
The authors thank all the staff of Key Laboratory of Radiation Damage and Treatment of Jiangsu Provincial Universities and Colleges for expert technical assistance and administrative support. We sincerely thank Professor Dong Yu for providing experimental site.
Footnotes
ORCID iDs: Yan Yuan https://orcid.org/0009-0002-1592-2975
Ying Xu https://orcid.org/0000-0001-7504-2588
Anqing Wu https://orcid.org/0000-0002-9009-640X
Author’ contributions: YY was involved in writing—original draft, visualization, investigation, formal analysis, and data curation; XZ and YX in visualization, investigation, formal analysis, and data curation; YG in visualization, methodology, and investigation; and AW in supervision, project administration, funding acquisition, conceptualization, and writing—review and editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Suzhou Fundamental Research Project, National Natural Science Foundation of China, Basic Research Program of Nantong Science and Technology Bureau (grant number SJC2023001, 12205215, JC12022103).
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data availability statement: The datasets utilized and/or examined in this study can be obtained from the corresponding author upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. 2021/02/05. [DOI] [PubMed] [Google Scholar]
- 2.Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res 2015; 5: 2929–2943. 2015/12/23. [PMC free article] [PubMed] [Google Scholar]
- 3.Kunkler IH, Williams LJ, Jack WJL, et al. Breast-conserving surgery with or without irradiation in early breast cancer. N Engl J Med 2023; 388: 585–594. 2023/02/16. [DOI] [PubMed] [Google Scholar]
- 4.Lago BM, Bello SDS, Gondim GRM, et al. Breast cancer after thoracic radiotherapy in young patients: what does the radiologist need to know? Radiol Bras 2023; 56: 145–149. 2023/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Schootbrugge C, Bussink J, Span PN, et al. AlphaB-crystallin stimulates VEGF secretion and tumor cell migration and correlates with enhanced distant metastasis in head and neck squamous cell carcinoma. BMC Cancer 2013; 13: 128. 2013/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu A, Zhang L, Luo N, et al. Limb-bud and heart (LBH) inhibits cellular migration, invasion and epithelial-mesenchymal transition in nasopharyngeal carcinoma via downregulating alphaB-crystallin expression. Cell Signal 2021; 85: 110045. 2021/05/18. [DOI] [PubMed] [Google Scholar]
- 7.Moyano JV, Evans JR, Chen F, et al. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest 2006; 116: 261–270. 2006/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosman JD, Yehiely F, Evans JR, et al. Regulation of alphaB-crystallin gene expression by the transcription factor Ets1 in breast cancer. Breast Cancer Res Treat 2010; 119: 63–70. 2009/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Schootbrugge C, van Asten F, Nagtegaal ID, et al. AlphaB-crystallin expression is correlated with phospho-ERK1/2 expression in human breast cancer. Int J Biol Markers 2013; 28: e365–e370. 2013/06/01. [DOI] [PubMed] [Google Scholar]
- 10.Koletsa T, Stavridi F, Bobos M, et al. AlphaB-crystallin is a marker of aggressive breast cancer behavior but does not independently predict for patient outcome: a combined analysis of two randomized studies. BMC Clin Pathol 2014; 14: 28. 2014/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang JY, Ni YB, Chan SK, et al. Androgen receptor expression shows distinctive significance in ER positive and negative breast cancers. Ann Surg Oncol 2014; 21: 2218–2228. 2014/03/19. [DOI] [PubMed] [Google Scholar]
- 12.Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), alphaB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol 2012; 44: 1622–1631. 2012/04/24. [DOI] [PubMed] [Google Scholar]
- 13.Mao YW, Liu JP, Xiang H, et al. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ 2004; 11: 512–526. 2004/01/31. [DOI] [PubMed] [Google Scholar]
- 14.Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem 2001; 276: 16059–16063. 2001/03/29. [DOI] [PubMed] [Google Scholar]
- 15.Rashidieh B, Bain AL, Tria SM, et al. Alpha-B-crystallin overexpression is sufficient to promote tumorigenesis and metastasis in mice. Exp Hematol Oncol 2023; 12: 4. 2023/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa K, Sreekumar PG, Spee C, et al. alphaB-crystallin regulates subretinal fibrosis by modulation of epithelial-mesenchymal transition. Am J Pathol 2016; 186: 859–873. 2016/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu A, Zhang L, Chen J, et al. Limb-bud and heart (LBH) mediates proliferation, fibroblast-to-myofibroblast transition and EMT-like processes in cardiac fibroblasts. Mol Cell Biochem 2021; 476: 2685–2701. 2021/03/06. [DOI] [PubMed] [Google Scholar]
- 18.Ren Z, Dong Z, Xie P, et al. PNU282987 inhibits amyloid-beta aggregation by upregulating astrocytic endogenous alphaB-crystallin and HSP-70 via regulation of the alpha7AChR, PI3K/Akt/HSF-1 signaling axis. Mol Med Rep 2020; 22: 201–208. 2020/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu R, Aplin AE. alphaB-crystallin is mutant B-RAF regulated and contributes to cyclin D1 turnover in melanocytic cells. Pigment Cell Melanoma Res 2010; 23: 201–209. 2010/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita A, Taniwaki T, Kaikoi Y, et al. Protective role of the endoplasmic reticulum protein mitsugumin23 against ultraviolet C-induced cell death. FEBS Lett 2013; 587: 1299–1303. 2013/04/02. [DOI] [PubMed] [Google Scholar]
- 21.Mahalingam S, Karmakar S, Santhoshkumar P, et al. Effect of structural changes induced by deletion of (54)FLRAPSWF(61) sequence in alphaB-crystallin on chaperone function and anti-apoptotic activity. Int J Mol Sci 2021; 22: 10771. 2021/10/14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caporossi D, Parisi A, Fantini C, et al. AlphaB-crystallin and breast cancer: role and possible therapeutic strategies. Cell Stress Chaperones 2021; 26: 19–28. 2020/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahomi RB, Wang B, Raghavan CT, et al. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem 2013; 288: 13022–13035. 2013/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attia SA, Truong AT, Phan A, et al. alphaB-crystallin peptide fused with elastin-like polypeptide: intracellular activity in retinal pigment epithelial cells challenged with oxidative stress. Antioxidants (Basel) 2023; 12: 1817. 2023/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahsan SM, Bakthisaran R, Tangirala R, et al. Nucleosomal association and altered interactome underlie the mechanism of cataract caused by the R54C mutation of alphaA-crystallin. Biochim Biophys Acta Gen Subj 2021; 1865: 129846. 2021/01/15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sci-10.1177_00368504251343177 for αB-crystallin enhances radioresistance in basal-like breast cancer cells by suppressing caspase-3 activation by Yan Yuan, Xubaihe Zhang, Ying Xu, Yangyang Ge and Anqing Wu in Science Progress