Abstract
Background
Non-small cell lung cancer (NSCLC) is a significant global health challenge, with 2% of cases fuelled by RET rearrangements. RET inhibitors (RETi) have revolutionized treatment for these patients, but resistance remains an important clinical challenge limiting therapy effectiveness. This study investigated the mechanisms underlying resistance to RETi.
Methods
NSCLC cells were exposed to increasing doses of RETi (pralsetinib/BLU-667 and selpercatinib/LOXO-292) to generate resistant cells. RNA-Sequencing analysis identified differentially expressed genes in resistant versus sensitive cells, followed by in vitro and in vivo functional assays to explore novel therapeutic strategies. Additionally, tumor biopsies from RET-rearranged NSCLC patients who exhibited cancer progression on RET inhibitor therapy were analyzed.
Results
RNA-sequencing analysis revealed the upregulation of the EGFR signaling pathway and hyperactivation of AP1 complex members in resistant cells compared to sensitive cells. Silencing of EGFR and AP1 complex members significantly reversed drug resistance, whereas EGFR overexpression reduced the sensitivity of parental Lc2/AD cells to RET inhibitors. Furthermore, the combination of RET and EGFR inhibitors showed synergistic antitumor activity in vitro and hindered tumor growth in mouse models with resistant cell xenografts. Notably, we observed a significant increase in EGFR expression in tumor biopsies from NSCLC patients treated with RET inhibitors who experienced disease progression, further validating the clinical relevance of our findings.
Conclusions
This study elucidates EGFR's role in mediating resistance to RET inhibitors in NSCLC patients. These findings offer insights into therapeutic adaptation and explore personalized combinations of RET and EGFR inhibitors for improved clinical outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13046-025-03392-w.
Keywords: Non-small cell lung cancer, RET, RET inhibitors, EGFR, Drug resistance, Combination targeted therapy
Introduction
Non–small cell lung cancer (NSCLC) is one of the leading causes of cancer-related deaths worldwide [1]. Among the subtypes of NSCLC, adenocarcinoma is undergoing a significant surge, representing approximately 50% of all cases [2]. Approximately 2% of adenocarcinoma NSCLCs harbor RET (REarranged during Transfection) rearrangements [3]. RET oncogene is activated primarily through chromosomal inversion or translocation, leading to the fusion of the Ret C-terminal kinase domain with the N-terminal portion of an unrelated protein [4]. The most common RET fusion partners in lung cancer are KIF5B (70–90%) and CCDC6 (10–25%) [5–7]. Fusions result in chimeric proteins with intact kinase domains, determining constitutive activation for ligand-independent dimerization and phosphorylation. Consequently, this event enhances Ret downstream signaling pathways, including mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), phosphatidylinositide 3-kinase (PI3 K), and c-Jun N-terminal kinase (JNK) [8, 9]. RET genomic alterations in NSCLC disrupt the physiological activation of proliferating and apoptotic pathways as well as differentiation and chemotaxis, ultimately leading to uncontrolled cell growth [10].
The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) recently accelerated the approval of selpercatinib/LOXO- 292 and pralsetinib/BLU- 667 for adult patients with advanced RET fusion-positive NSCLC, marking significant progress in targeted therapy. These approvals were based on promising results from the LIBRETTO- 001 (NCT03157128) and ARROW (NCT03037385) clinical trials [11–19]. Despite positive results, not all patients benefit from these treatments, and almost all of them eventually progress. While on-target resistance mutations are identified in only 10–15% of cases, the majority of resistance mechanisms seem to involve off-target pathways, such as the activation of bypass receptor tyrosine kinases [20]. Furthermore, nearly half of the patients exhibited no identifiable genomic resistance mechanisms, limiting the efficacy of new-generation RET inhibitors to a specific patient subset. Therefore, the development of novel therapeutic strategies to circumvent RET inhibitor resistance is an unmet clinical need.
Herein, we report the results of our study, in which we identified hyperactivation of EGFR signaling as a potential mechanism of resistance to RET inhibition. We demonstrated that EGFR hyperactivation, due to aberrant regulation of AP1 complex members, drives a proliferative phenotype in RET fusion-positive NSCLC cells resistant to BLU-667 and LOXO-292. Notably, our preclinical data revealed that targeting EGFR resensitized resistant cells and xenografts to RET inhibition. These findings were further supported by the observation of increased EGFR expression in patients with RET rearrangement who progressed to RET inhibitor therapy. These results provide a scientific rationale supporting the combined use of RET and EGFR inhibitors, potentially delaying the emergence of resistance and overcoming it when it arises.
Methods
Cell lines and inhibitors
The human non-small cell lung cancer cell line Lc2/AD (harboring CCDC6-RET fusion) was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in ATCC recommended media (RPMI- 1640:F12 = 1:1) supplemented with 10% fetal bovine serum (FBS) (Gibco, Waltham, MA, USA) and 1 × antibiotic/antimycotic (Gibco). The human papillary thyroid cancer cell line TPC- 1 (harboring CCDC6-RET fusion) was kindly provided by Prof. Massimo Santoro (Medicina Molecolare e Biotecnologie Mediche, Università degli Studi di Napoli Federico II, Napoli, Italy). The cells were maintained in DMEM supplemented with 10% FBS (Gibco, Waltham, MA, USA) and 1 × antibiotic/antimycotic (Gibco). All the cell lines used in this study were maintained at 37 °C in a humidified atmosphere of 5% CO2 in air. Drug-resistant cells (BluR and LoxoR) were generated after 6 months of treatment with increasing doses of BLU- 667 (Pralsetinib-SelleckChem-Houston, TX, USA; #S8716) and LOXO- 292 (Selpercatinib; SelleckChem-Houston, TX, USA; #S8781), with final concentrations reaching up to 500 nM. All the cell lines were tested for mycoplasma contamination and authenticated via short tandem repeat (STR) profiling by BMR Genomics. All experiments on these cell lines were performed between the 4 th and 16 th passages.
Clonogenic and dose‒response assays
For clonogenic assays, sensitive and drug-resistant cells (5 × 104/well) were seeded in triplicate in 6-well plates in complete media. After 24 h, the cells were treated with vehicle (DMSO), BLU-667, LOXO-292 (final concentration of 500 nM), afatinib (SelleckChem, Houston, TX, USA; #S1011; final concentration of 50 nM), osimertinib (SelleckChem, Houston, TX, USA; # S7297; final concentration of 200 nM) or erlotinib (SelleckChem, Houston, TX, USA; # S7786; final concentration of 1 µM). The media were replenished every 3 days until the control wells reached 70–80% confluency. The monolayers were then fixed and stained with 20% methanol/80% water/0.25% crystal violet for 20 min, washed with water, and dried. Then, the plates were scanned in Brother MFCL2710DW (Brother) at 300 dpi, the stained cells were solubilized with 20% acetic acid solution, and the absorbance was quantified via spectrophotometric detection at 590 nm using a plate reader (GloMax® Discover Microplate Reader, Promega).
For the dose‒response assays, parental and drug-resistant cell lines were seeded in 96-well plates, and after 24 h, the cells were treated with DMSO or increasing concentrations of BLU-667 or LOXO-292 (12 doses ranging from 0.1 nM to 10 μM, threefold dilution). Six days later, a CellTiter-Glo Luminescent Cell Viability Assay (Promega; #G8461) was performed. An equal volume of CellTiter-Glo reagent was added to the culture medium in each well. After 2 min on an orbital shaker to induce cell lysis, the absorbance was quantified by spectrophotometric detection using a plate reader (GloMax® Discover Microplate Reader (Promega).
To test the synergy between BLU-667/LOXO-292 and afatinib, cells were treated with increasing doses of each drug alone or in combination every 72 h until the vehicle-treated controls reached ∼90% confluence. The combination indices were determined using the Chou‒Talalay method based on the median-effect equation. The resulting combination index (CI) offers a quantitative definition of the additive effect (CI = 1), synergism (CI < 1), and antagonism (CI > 1) of drug combinations.
Immunoblot analysis
The cells were washed with PBS and lysed with radioimmunoprecipitation assay (RIPA) buffer (#sc-24948, Santa Cruz, USA) according to the manufacturer’s instructions. Snap-frozen tumor fragments were homogenized using a TissueLyser (Qiagen) and lysed in RIPA lysis buffer. The lysates were kept on ice for 30 min, vortexed, and then centrifuged at 13,400 rpm for 20 min at 4 °C. The protein concentrations in the supernatants were quantified using a BCA protein assay kit (Pierce™; #23,225). Total protein (20 μg) was separated on bis–tris 3–8% gradient gels (NuPAGE) and transferred to nitrocellulose membranes using the Trans-Blot® Turbo™ RTA Mini Nitrocellulose Transfer Kit (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% nonfat dry milk at room temperature for 45 min, followed by overnight incubation with primary antibodies at 4 °C. All the following antibodies were purchased from Cell Signaling Technology or Santa Cruz Biotechnology: EGFR (1:1000 - #sc- 1005); phospho-EGFR (Tyr1068- 1:1000 #2234); ERK2 (1:1000- #sc- 1647); phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204 - 1:1000- #9101); FosB (1:1000- #2251); Fra1 (1:1000- #sc- 28310); Ret (1:500- #3220); phospho-Ret (Tyr905- 1:500- #3221); Shc (1:500- #2432); phospho-Shc (Tyr239/240- #2434); and α/β Tubulin (1:1000- #2148). The nitrocellulose membranes were washed and incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibodies for 45 min at room temperature. Immunoreactive proteins were visualized by enhanced chemiluminescence using SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA). The films were imaged using Brother MFCL2710DW (Brother) at 300 dpi. Densitometric analysis was conducted using ImageJ 1.54 d software (NIH, Bethesda, MD, USA), and results are presented as fold change (FC). Intensity values were normalized to the expression signals of loading controls.
Lung cancer spheroid assay
The Lc-2/ad, BluR, and LoxoR cell lines were seeded in quadruplicate into Ultra-Low attachment 96 plates (#CLS3474, Corning) at a density of 5 × 103 cells per well in 100 µL of 10% RPMI- 1640:F12. After 48 h, images were captured (T0) using an InvitrogenTM EVOSTM FL imaging system (20X magnification). The spheroids were treated with the indicated drugs for 72 h. The area of the spheroids was quantified using ImageJ 1.53 software (NIH, Bethesda, MD, USA) and normalized to the T0 area. Spheroids transfected with siCTRL and siEGFR were established 48 h after siRNA transfection. They were then treated, and the areas were measured as described above.
siRNA transfection
Small interfering RNAs (siRNAs) targeting EGFR (#226,558,293; #226,558,296; #226,558,299 hs.Ri. EGFR), FOSL1 (#229,852,154; #229,852,157; #229,852,160; hs.Ri. FOSL1), and FOSB (#233,819,949; #233,819,952; #233,819,955; hs.Ri. FOSB) were purchased from Integrated DNA Technology. The cells were transfected with 50 nM siRNA via Lipofectamine RNAiMAX® transfection reagent (Thermo Fisher Scientific) following the manufacturer’s instructions. Forty-eight hours post-transfection, the cells were seeded in medium supplemented with 10% FBS in ultralow attachment 96-well plates (5 × 103/well for spheroid assays), 60-mm plates (for immunoblot analysis), or 6-well plates for the clonogenic assay or cell count (5 × 104/well) and treated with the indicated drug concentrations.
Plasmid overexpression
Human pLenti-C-mGFP control (PS100071 V) and mGFP-tagged-EGFR (RC217384L4 V) lenti-ORF particles were purchased from OriGene. In Lc2/ad parental cells, lenti-ORF particles (10^7 TU/mL) were transfected with 8 µg/mL polybrene (Sigma‒Aldrich) to generate stably transduced cell lines. After 48 h of incubation, the transduced cells were selected with 1 µg/mL puromycin, and the cell culture media was changed every 72 h.
Cell cycle analysis by propidium iodide staining
Lc-2/ad, BluR, and LoxoR cells were seeded in 100-mm plates. The cells were incubated in serum-free media for 24 h and then treated with media containing 10% FBS + the indicated drugs for 24 h. Next, the cells were washed with PBS and fixed in 99% methanol for 3 h at − 20 °C. The cells were then incubated with FxCycle™ PI/RNase Staining Solution (Invitrogen™) following the manufacturer’s instructions. Fluorescence-activated cell sorting (FACS) analysis was performed usingvia a FACSVerse™ (BD Biosciences).
Immunofluorescence assay
2 × 104 Lc-2/ad, BluR, and LoxoR were seeded in sterile chamber slides (Nunc Lab-Tek) for 24 h and treated with BLU-667 and LOXO-292 for 24 h. The cells were fixed in paraformaldehyde and 4% in 1X PBS for 30 min. Fixed cells were washed with 1X PBS and then incubated in blocking buffer (0.5% BSA in 1X PBS) for 45 min, followed by an overnight incubation with an anti-EGFR rabbit polyclonal antibody (1:200; #sc- 1005) at 4 °C. The chamber slides were washed with 1X PBS and incubated with a donkey anti-rabbit IgG (H + L) cross-reaction secondary antibody, Cy™2 (1:200; Jackson ImmunoResearch; #711–225 - 152), for 1 h. Fluorescent mounting medium with DAPI was used as the mounting solution (Abcam; #104,139). Images were collected using a fully automated inverted microscope.
ChIP q‒PCR analysis
BluR cells were cultured in 100-mm dishes and incubated with serum-free RPMI- 1640:F12 medium containing 1% formaldehyde for 10 min at room temperature. Crosslinking was quenched using glycine. The plates were subsequently washed three times with ice-cold 1X PBS three times, and the cells were scraped off the plate with ice-cold PBS containing Halt™ Protease Inhibitor Cocktail (Thermo Scientific™- Catalog: # 87,786). The cells were subsequently centrifuged at 720 × g for 10 min at 4 °C. The pellets were subsequently resuspended in 1.5 mL of sonication buffer (1% SDS, 10 mmol/L EDTA, 50 mmol/L Tris–HCl, pH 8.0–8.1) supplemented with protease inhibitors. Chromatin was sonicated via a Bioruptor. Sonicated chromatin was adjusted to a final concentration of 200 mM NaCl and incubated at 65 °C overnight. The following day, the chromatin was incubated with RNase A for 30 min at 37 °C, followed by incubation with proteinase K for 1 h at 42 °C. DNA was purified via a QIAquick PCR Purification Kit (Cat 28,106). DNA shearing was checked, with an average fragment size of 200–750 bp. To conduct chromatin immunoprecipitation (ChIP), the sonicated chromatin was eluted with ChIP dilution buffer (0.01% SDS buffer, 1.1% Triton X- 100, 1.2 mmol/L EDTA, 167 mmol/L NaCl, 16.7 mmol/L Tris–HCl, pH 8.0–8.1) with protease inhibitors and precleared with Gammabind G Sepharose beads (GE Healthcare, Cat no. 17088501) previously washed three times with ChIP-dilution buffer and blocked in 0.5% BSA at 4 °C for 1 h. The precleared chromatin was incubated at 4 °C overnight with primary antibody. The following day, Gammabind G Sepharose beads were added to the antibody pulldowns for 2 h at 4 °C. Next, the beads were washed once in Buffer I (0.1% SDS, 1% Triton X- 100, 2 mmol/L EDTA, 20 mmol/L Tris–HCl, 150 mmol/L NaCl, and protease inhibitors, pH 8.0–8.1), once in Buffer II (0.1% SDS, 1% Triton X- 100, 2 mmol/L EDTA, 20 mmol/L Tris–HCl, 500 mmol/L NaCl, and protease inhibitors), once in Buffer III (0.25 M LiCl, 1% NP40, 1% Na-deoxycholate, 1 mmol/L EDTA, 10 mmol/L Tris–HCl, pH 8.0–8.1) and twice in TE buffer, pH 8.0–8.1. The complexes were eluted from the beads in elution buffer (0.1 M NaHCO3, 1% SDS) at 65 °C for 10 min twice in a thermomixer (Chromatin Immunoprecipitation (ChIP) Assay Kit- Catalog #17–295). Eluates from the antibody pulldowns and sonicated chromatin (as input controls) were adjusted to 200 mmol/L NaCl and incubated at 65 °C overnight. The following day, the DNA was purified using a QIAquick PCR Purification Kit (QIAGEN; Catalog #28,104). The ChIP products and input DNA were analyzed in triplicate by quantitative PCR (qPCR) using iTaq Universal SYBR Green Supermix and a CFX Connect Real-Time PCR Detection System (Bio-Rad). Actinβ was used as a housekeeping gene for data normalization, and relative gene expression was measured using the 2−ΔΔ Ct method. qPCR oligo pairs for the EGFR promoter based on Cistrome BD data were analyzed in the UCSC genome browser: 5’- ATGACTCTGCTTGGTGCAGAA- 3’ (Fwd); 5’- TCACACTTGAGTCTTGGCCCC- 3’ (Rev); 5’- ACCCATGGCTGGTTGCAATA- 3’ (Fwd); 5’- AGAGCCAGCGTCGGATAATG- 3’ (Rev); 5’- CCTGGCCAAAGGGTCATCAT- 3’ (Fwd); and 5’- CTGTCTCACCCAGTCCACAC- 3’ (Rev).
Oncomine tumor mutation load assay
Each sample was tested using The Oncomine Tumor Mutational Load Assay (Thermo Fisher Scientific) on the Ion Torrent S5 GS NGS platform (Thermo Fisher Scientific) according to the manufacturer’s instructions. This panel was able to evaluate single-nucleotide variants, copy number variations, and insertions/deletions in 409 cancer-related genes (a complete gene list is available at https://www.thermofisher.com/order/catalog/product/A3790 9#/A37909). Briefly, 10 ng of DNA from each sample was manually amplified, according to the manufacturer’s instructions. The libraries were then diluted to 30 ng and pooled for sequencing analysis. The pool was automatically loaded onto the Ion 540 chip according to the manufacturer’s instructions. Sequencing data were analyzed using Ion Reporter Suite software (v.5.0.2.1; Thermo Fisher Scientific) using a customized workflow. Data analysis was performed as follows: variants with ≥ 5 × allele coverage and a quality score ≥ 20 within an amplicon coverage of at least 500 × alleles were called. Finally, the frequency of each mutant allele was recorded.
RNA-Sequencing and gene set enrichment analysis
Parental and drug-resistant cells were seeded in triplicate in 100-mm dishes and treated with DMSO, BLU-667, or LOXO-292. After 24 h, the cells were harvested, and RNA was purified using the RNeasy Plus Kit (QIAGEN; #74,134). A Qubit fluorimeter was used to determine the sample concentration. Genewiz (Germany) conducted library preparation, RNA-sequencing analysis, and reading quality control. Gene set enrichment analysis (GSEA) was performed on normalized data using GSEA_4.3.2. Connectivity map (https://clue.io/about), DepMap (https://depmap.org/portal/interactive/), and Integrated Motif Activity Response Analysis (ISMARA; https://ismara.unibas.ch/mara/) were conducted using online tools.
Xenograft studies
All animal procedures were performed in accordance with the institutional guidelines of the University of Naples Animal Care Committee and the Declaration of Helsinki. BluR cells (7 × 106 cells per mouse) were suspended in PBS and Matrigel (Corning #356,234) at a 1:1 ratio and injected subcutaneously into the right flank of 6-week-old female Balb/c (nu +/nu +) mice (Charles River). Approximately 4 weeks after tumor cell injection, mice bearing tumors measuring ≥ 200 mm3 were randomized (five per group) to treatment with 1) vehicle (5% DMSO, 40% PEG300, 5% Tween, and 50% ddH₂O, by oral gavage); 2) BLU- 667 (10 mg/kg b.i.d., by oral gavage); 3) afatinib (20 mg/kg, q.d. by oral gavage); and 4) BLU-667 plus afatinib, for 15 days. Animal weights and tumor diameters (measured with calipers) were measured twice weekly, and the tumor volume in mm3 was calculated using the following formula: volume = width2 × length/2. At the end of the treatment, the tumors were harvested and snap-frozen in liquid nitrogen before total protein extraction and immunoblot analysis.
Patient cohort and immunohistochemistry (IHC)
Patient biopsies for immunohistochemistry analysis were obtained from Gustave Roussy (Paris), IRCCS (Rome) and IRCCS (Milan). Pre-treatment biopsies were collected within six months before starting selpercatinib. Post-treatment biopsies were obtained at primary or metastatic sites after that progression disease (PD) was assessed. For paired biopsies (pre- and post- selpercatinib), two 4-μm thick sections from each tissue block were cut at the microtome: one section was attached to a normal slide and stained with hematoxylin/eosin for adequacy evaluation, and one section was attached to positively charged slides for subsequent IHC. Soon after the sections were cut, the slides were placed in an oven at 48 °C for 1 h and then stored at room temperature until the next day.
IHC staining was performed using an automated staining system (BenchMark XT; Ventana Medical Systems, Inc., Arizona, USA) using the UltraView Universal DAB Detection Kit. The standard IHC protocol was used, and pre-diluted anti-EGFR (DAKO EGFR pharmDx kit for Gustave Roussy samples and polyclonal anti-epidermal growth factor receptor/EGFR/ErbB1 (Quartet-ref. 2-EP040 - 10 – biocyc GmbH & Co. KG) antibodies were used for IRCCS (Rome and Milan) samples at 37 °C for 32 min. Briefly, after deparaffinization and rehydration, the paraffin-embedded tissue sections were blocked with 3% hydrogen peroxide for 4 min at room temperature. Antigen retrieval was performed on the selected samples using CC1 solution (Ventana Medical Systems, Inc., Arizona, USA) containing EDTA (pH 8.0) at 95 °C for 64 min. The tissue sections were then incubated with UltraView Universal HRP Multimer and developed with 3,3′-diaminobenzidine (DAB) for 4 min. Counterstaining was performed with hematoxylin for 8 min and a bluing reagent for 4 min.
EGFR expression was assessed by an expert pathologist, who assigned values ranging from 0 (no staining or membranous staining in < 10% of tumor cells) to 1/2/3 (incomplete, weak/moderate, and strong and complete staining in > 10% of tumor cells) using the H score (0–300).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software, version 10.2 (San Diego, California, USA). Data were expressed as mean ± standard deviation (SD). For comparisons between two groups, Student’s t-test was used, while two-way analysis of variance (ANOVA) was applied for comparisons among three or more groups. For RNA-seq data comparisons, Wilcoxon test was utilized. Correlations were analyzed using Pearson’s correlation analysis. A p-value of less than 0.05 was considered statistically significant.
Results
Generation and characterization of CCDC6-RET NSCLC cell lines resistant to BLU-667 and LOXO-292
To investigate the signaling pathways that could sustain resistance to RET inhibition, we generated NSCLC cells resistant to the drugs BLU-667/pralsetinib and LOXO-292/selpercatinib, herein defined as BluR and LoxoR cells, respectively. Briefly, Lc2/AD NSCLC cells (harboring the CCDC6-RET fusion) were chronically exposed to increasing doses of BLU-667 or LOXO-292 (Fig. 1a). After 6 months, resistant cells grew in the presence of 500 nM of either compound, whereas in parental cells the treatment completely abrogated both monolayer growth and spheroid formation (Fig. 1b, Supplementary Fig. 1a, left and right panels). Moreover, dose‒response proliferation assays confirmed that the BluR and LoxoR cells were less responsive to each drug than the Lc2/AD parental cells (Fig. 1c, left and right panels; Suppl. Fig. 1b‒c). To further support these findings, cell cycle analysis demonstrated that, in response to RET inhibitors (RETi), Lc-2/AD-sensitive cells showed an increased percentage of cells in the G1 phase and a decreased percentage of cells in the G2/M phase. In contrast, BluR and LoxoR cells showed a defective G1 phase increase, underlying the acquisition of resistance to RETi (Supplementary Fig. 1d-e).
Fig. 1.
CCDC6-RET NSCLC cells resistant to BLU-667 and LOXO-292. a Schematic representation illustrating the generation of BLU-667-resistant (BluR) and LOXO-292-resistant (LoxoR) Lc2/AD cells. b Left, images of crystal violet-stained monolayers of Lc2/AD, BluR, and LoxoR cells seeded in 6-well plates and treated with vehicle, BLU-667 (500 nM) or LOXO-292 (500 nM). Right, bar graph showing quantification by spectrophotometric detection of the integrated intensity values as % of vehicle-treated controls in Lc2/AD (black), BluR (red), and LoxoR (blue) cells (ns p > 0.05, ****p < 0.0001; 2way ANOVA Bonferroni’s multiple comparisons). c Twelve-point dose–response curves of parental Lc2/AD (black line) and BluR (red line), and Lc2/AD (black line) and LoxoR (blu line) cells treated with BLU-667 (left) or LOXO-292 (right). After 6 days of treatment, cells were lysed and quantified with ATP cell titer Glo. Each data point represents the percentage of cell viability relative to vehicle-treated controls. d Immunoblots of Lc2/AD parental and BluR cells treated with BLU-667 (500 nM). After treatment for 4 h, whole-cell lysates were prepared and subjected to immunoblot analyses with the indicated antibodies. Numbers above blots indicate FC variations compared to vehicle-treated Lc2/AD cells. Images are representatives from three independent experiments. e Heatmap showing unsupervised hierarchical clustering of differentially expressed genes (FDR < 0.01) in BluR versus Lc2/AD parental cells upon BLU-667 treatment for 24 h. Up- and down-regulated genes are represented in red and green, respectively. f Gene set enrichment analysis (GSEA) of significantly de-regulated pathways in BluR cells versus Lc2/AD cells shown with Normalized Enriched Score (NES) and FDR q-value. For all panels, data are expressed as mean ± standard deviation (SD) of three separate experiments, indicated by error bars
To assess whether the insensitivity of resistant cells to RET inhibitors resulted from newly acquired mutations in RET oncogene or in other known oncogenic drivers in NSCLC (such as MET or KRAS, already known as mechanisms of resistance to RETi [21]), we conducted a mutational screening for 409 cancer-related gene abnormalities. Interestingly, molecular alterations in LC2/AD (i.e., KMT2D, COL1 A1, and PARP1) were mirrored in BluR cells (Suppl. Fig. 2), ruling out the possibility that new mutations in RET oncogene or other driver genes in BluR cells could be responsible for drug resistance.
Hence, we hypothesized that the acquisition of resistance to BLU-667 and LOXO-292 could be sustained by the activation of alternative signaling pathways, as previously reported for other tyrosine kinase inhibitors [22–29]. Immunoblotting suggested that RETi continued to impair Ret activation in BluR and LoxoR cells, as indicated by the effects on p-Ret and p-SHC (Fig. 1d, Supplementary Fig. 3a). However, upon chronic treatment with RETi, BluR and LoxoR cells showed sustained phospho-ERK (an effector of mitogen-activated protein kinase (MAPK)) (Fig. 1d, Supplementary Fig. 3a), despite the presence of BLU-667 and LOXO-292.
Thus, to investigate the transcriptional reprogramming sustaining resistance to RET inhibition, RNA-Sequencing of BluR, LoxoR, and parental Lc-2/AD cells (± BLU-667 or ± LOXO-292 for 24 h) was performed. Heatmaps revealed the peculiar transcriptional signatures of BluR and LoxoR cells compared with the Lc2/AD cells (Fig. 1e, Supplementary Fig. 3b). Gene set enrichment analysis (GSEA; [30]) of the differentially regulated genes in the BluR and LoxoR cells revealed hyperexpression of pro-proliferative signaling pathways, such as E2F-responsive and G2-M checkpoint genes, which was consistent with the enhanced survival capabilities of the resistant cells compared with the sensitive parental cells (Fig. 1f, Supplementary Fig. 3c).
EGFR hyperactivation promotes resistance to RET inhibitors
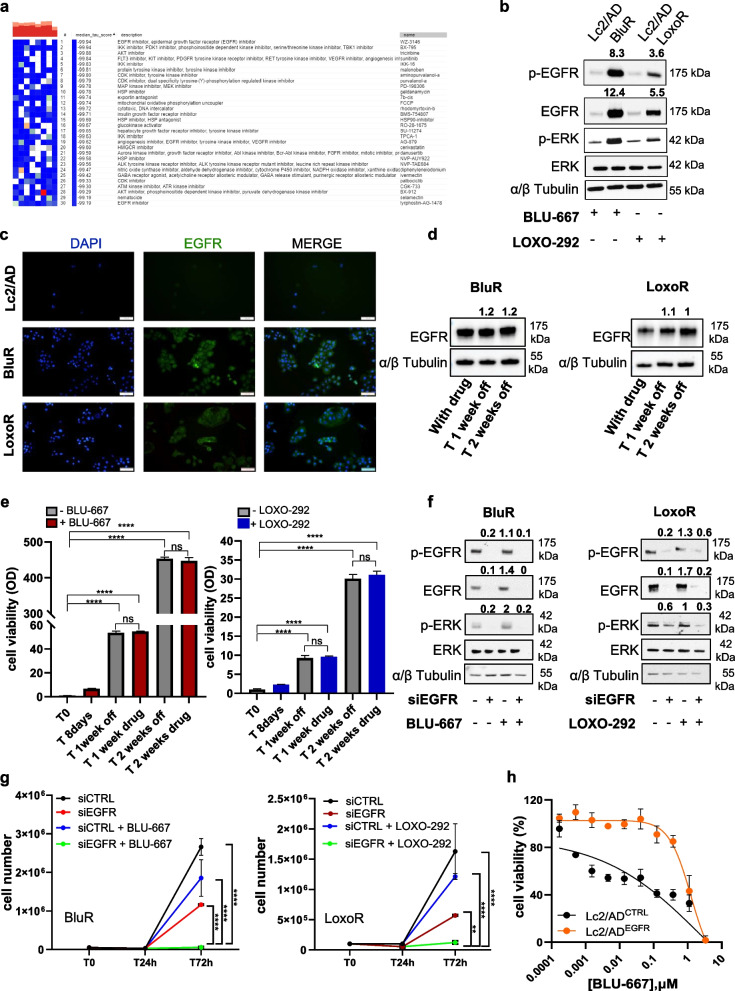
We sought to identify potential deregulated pathways that might promote resistance to RET inhibitors. Leveraging RNA-Sequencing data, we conducted connectivity map analysis to determine which pathway inhibition could most effectively reverse the resistant phenotype. The top 150 upregulated genes in RETi-resistant cells were selected according to the protocol, which generated a list of drugs capable of eliciting a gene expression pattern opposite to the observed signature [30]. Interestingly, EGFR pathway inhibitors emerged as the top candidates for reversing the transcriptional signature associated with resistance to RET inhibitors, with the most negative median score correlating with the best drug (Fig. 2a). This result prompted us to investigate the effects of EGFR inhibition in resistant models.
Fig. 2.
EGFR expression and activation regulates sensitivity to RET inhibitors. a. Connectivity map analysis to identify drugs that could potentially reverse the expression of resistance-associated genes. b Immunoblots of Lc2/AD parental, BluR and LoxoR cells treated with BLU-667 (500 nM) or LOXO-292 (500 nM). After treatment for 24 h, whole-cell lysates were prepared and subjected to immunoblot analyses with the indicated antibodies. Numbers above blots indicate FC variations compared to their counterpart Lc2/AD cells, treated with BLU-667 or LOXO-292. Images are representatives from three independent experiments. c Immunofluorescence staining of EGFR (Cy2) in Lc2/AD, BluR, and LoxoR cells. Nuclei were counterstained in blue (DAPI) (magnification 20 ×). d Immunoblots of BluR (left) and LoxoR (right) cells treated with BLU-667 (500 nM) or LOXO-292 (500 nM) for 8 days and then washed out for 1 and 2 weeks. Whole-cell lysates were prepared and subjected to immunoblot analyses with the indicated antibodies. Numbers above blots indicate FC variations compared to cells under treatment for 8 days. Images are representatives from three independent experiments. e Bar graph showing quantification by spectrophotometric detection of the integrated intensity values as % of T0, in BluR (left) and LoxoR (right). Cells were treated with the drugs for 1 week and then allowed to grow in media containing the respective drugs (BLU-667 in red and LOXO- 292 in blu) or without drugs (gray). (**p < 0.01; ****p < 0.0001; 2way ANOVA Bonferroni’s multiple comparisons). f Immunoblots of BluR (left) and LoxoR (right) cells knocked down for scramble or EGFR siRNAs and treated or not with BLU-667 and LOXO-292, respectively. After treatment for 72 h, whole-cell lysates were prepared and subjected to immunoblot analyses with the indicated antibodies. Numbers above blots indicate FC variations compared to cells transfected with scramble siRNAs. Images are representatives from three independent experiments. (**p < 0.01; ****p < 0.0001; 2way ANOVA Bonferroni's multiple comparison). g Line chart indicating cell number of BluR (left panel) and LoxoR (right panel) cells treated with scramble small interfering RNAs (siCTRL) or siRNAs targeting EGFR (siEGFR) in the presence or not of BLU-667 and LOXO-292, respectively. h Twelve-point dose–response curves of Lc2/AD over-expressing GFP control vector (pLenti-C-mGFP-P2 A-Puro; Lc2/ADCTRL, black line) or EGFR over-expressing cells (Lc2/ADEGFR, orange line) treated with BLU-667. After 6 days of treatment, cells were lysed and quantified with ATP cell titer Glo. Each data point represents the percentage of cell viability relative to vehicle-treated controls. For all panels, each point represents the mean ± SD from three independent experiments
Hence, we first evaluated EGFR expression in cell lines resistant to both RET inhibitors using immunoblot and immunofluorescence assays. Notably, we observed an increase in EGFR expression and activation, as well as an increase in p-ERK levels, in BluR and LoxoR cells (Fig. 2b-c). Hence, we hypothesized that EGFR hyperactivation could trigger compensatory mechanisms to RET inhibition, in turn activating MAPK signaling.
Then, we investigated whether the transcriptional reprogramming sustaining resistance was maintained after the withdrawal of the drugs. Interestingly, we found that BluR and LoxoR cells, following the removal of BLU-667 and LOXO-292 for 2 weeks, respectively, exhibited sustained EGFR levels (Fig. 2d, left and right panels) and growth profiles comparable to those of cells continuously exposed to the drugs (Fig. 2e, left and right panels).
Hence, to elucidate the causal role of EGFR in conferring resistance to RET inhibitors, we explored the effects of EGFR silencing on BluR and LoxoR cell growth. We observed that the combination of EGFR silencing and RET inhibition led to decrease in ERK activation (Fig. 2f) and a drastic and significant reduction in cell viability and spheroid formation (Fig. 2g, Suppl. Fig. 4a). Notably, EGFR knockdown alone did not elicit any effects on Lc-2/AD parental cells (Suppl. Figure 4b-c). Conversely, we stably overexpressed EGFR in Lc- 2/AD cells (Lc- 2/ADEGFR) (Suppl. Figure 4d). Compared with the Lc- 2/AD control (Lc- 2/ADCTRL), Lc- 2/ADEGFR exhibited reduced sensitivity to BLU-667 and LOXO-292 associated with ERK activation (Fig. 2h, Suppl. Figure 4e-f).
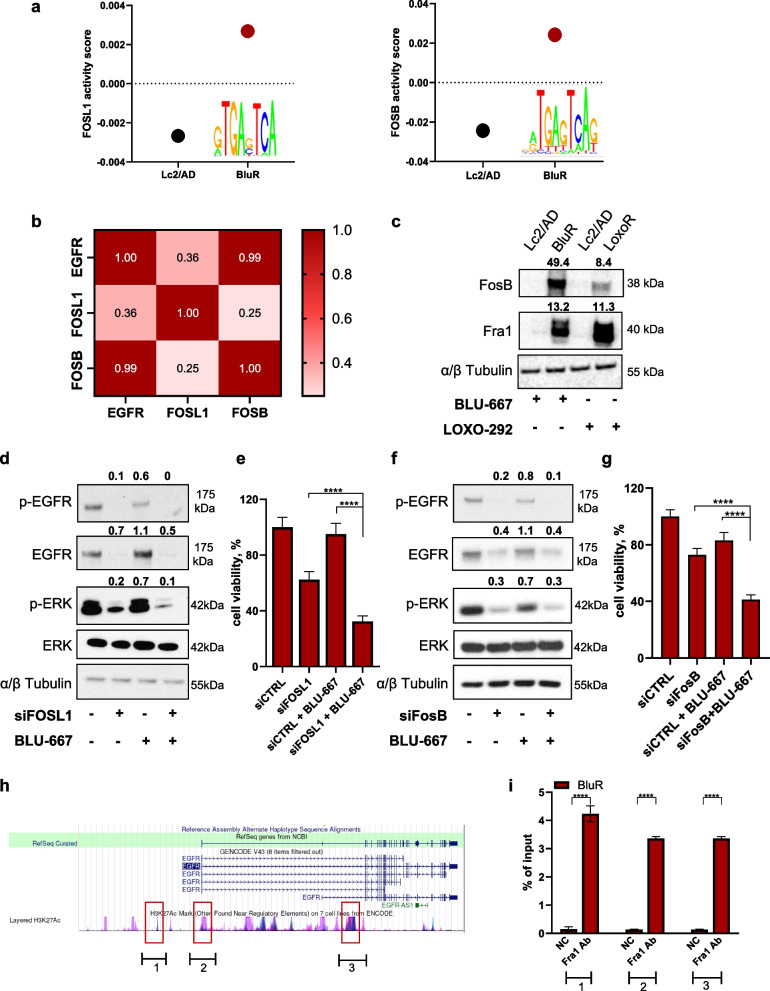
Fig. 4.
AP1 complex members regulate EGFR expression in cells resistant to RET inhibitors. a Graph showing activity score of transcriptional factor FOSL1 (left) and FOSB (right) in Lc2/AD and BluR cells based on RNA-Sequencing data using Integrated System for Motif Activity Response Analysis (ISMARA). b Schematic representation of Pearson correlation for expression levels of EGFR, FOSL1 and FOSB genes, based on our RNA-Sequencing data. c Immunoblots of Lc2/AD and BluR cells treated with BLU-667 (500 nM) and Lc2/AD and LoxoR cells treated with LOXO-292 (500 nM) for 24 h. Whole-cell lysates were prepared and subjected to immunoblot analyses with the indicated antibodies. Numbers above blots indicate FC variations compared to their counterpart Lc2/AD cells, treated with BLU-667 or LOXO-292. Images are representatives from three independent experiments. d, f Immunoblots of BluR cells knocked down for scramble (siCTRL) or FOSL1 (siFOSL1; d) or FOSB (siFOSB; f) siRNAs and treated or not with BLU-667. After treatment for 72 h, whole-cell lysates were prepared and subjected to immunoblot analyses with the indicated antibodies. Numbers above blots indicate FC variations compared with cells transfected with scramble siRNAs. Images are representatives from three independent experiments. e, g Bar graph showing cell viability of BluR cells transfected with siRNAs against FOSL1 (e) and FOSB (g) and treated with BLU-667 (500 nM). Data are plotted as a percentage of vehicle-treated cells transfected with siRNAs scramble (siCTRL) (****p < 0.0001; 2way ANOVA). h Schematic representation of the genomic locus encompassing EGFR gene. In the top part the exon/intron structure. In the bottom part, ENCODE tracks for H3K27Ac mapping across promoter and introns of EGFR are represented as coverage curves (peaks). i ChIP-qPCR with a Fra1 antibody or normal rabbit IgG (control), at the EGFR regulator regions. Enrichment values are expressed as percent (%) of input. (****p < 0.0001; Student's t-test). For all panels, each point represents the mean ± SD from three independent experiments
Furthermore, since Lc2/AD represents the only available model harboring RET rearrangement for NSCLC, we employed TPC1 cells, a papillary thyroid cancer (PTC) cell line carrying CCDC6-RET fusion. RET fusions occur in 10–20% of patients with PTC, qualifying these patients for treatment with RET inhibitors. Thus, we aimed to evaluate whether EGFR signaling plays a similar role in conferring resistance to RET inhibitors in this model. Surprisingly, we observed a lower sensitivity to BLU-667 and LOXO-292 in TPC1 cells (Suppl. Figure 5a) compared with Lc2/AD cells. Interestingly, we observed early increases in EGFR and p-ERK levels upon RETi treatment (Suppl. Figure 5b, left and right panels). Notably, concurrent silencing of EGFR and treatment of cells with either BLU-667 or LOXO-292 significantly impaired TPC1 viability, unlike RET inhibition or EGFR knockdown (Suppl. Figure 5c, left and right panels).
Concurrent pharmacologic inhibition of RET and EGFR restores BLU-667 and LOXO-292 sensitivity in vitro and in vivo
Based on these results, we tested the effects of combining EGFR inhibitors with either BLU-667 or LOXO-292 on RETi-resistant cells. EGFR is pharmacologically inhibited by afatinib, a clinically approved irreversible EGFR inhibitor. Interestingly, afatinib led to a significant reduction in cell viability when combined with BLU- 667 or LOXO- 292 in BluR and LoxoR cells (Fig. 3a-b). Single-agent treatments did not affect proliferation or ERK activation, but concurrent treatment with BLU-667/LOXO-292 and afatinib resulted in robust blockade of MAPK signaling and viability (Fig. 3a-c). These findings were further validated using the first-generation EGFR inhibitor erlotinib and the third-generation EGFR inhibitor osimertinib, which significantly resensitized BluR and LoxoR cells to RET inhibitors (Supplementary Fig. 6a-f). Furthermore, to verify whether the combinations of BLU-667/afatinib and LOXO-292/afatinib had synergistic or additive effects, we assessed drug interactions via the Chou‒Talalay method [31]. Combination studies using increasing concentrations of BLU-667 or LOXO-292 and afatinib demonstrated striking synergy between these agents in inhibiting the growth of BluR and LoxoR cells (combination index, CI < 1 indicates synergy; BluR CI = 0.16; LoxoR CI = 0.10; Fig. 3d).
Fig. 3.
The combination of RET and EGFR inhibitors impairs tumor growth in vitro and in vivo. a Images of crystal violet-stained monolayers of BluR and LoxoR cells seeded in 6-well plates and treated with vehicle, BLU-667 (500 nM) or LOXO-292 (500 nM) (RET i), afatinib (50 nM), and the combination (RET i + afatinib). b Bar graph showing quantification by spectrophotometric detection of the integrated intensity values as % of vehicle-treated controls in BluR (red) and LoxoR (blue) cells. (***p < 0.001; ****p < 0.0001; 2way ANOVA Bonferroni’s multiple comparisons). c Immunoblots of BluR (left) and LoxoR (right) cells treated with BLU-667 or LOXO-292 ± afatinib (left and right panels, respectively). Whole-cell lysates were prepared and subjected to immunoblot analysis with the indicated antibodies. Numbers above blots indicate FC variations compared to vehicle-treated cells. Images are representative of three independent experiments. d Viability assay to test the synergy between BLU-667 (left) or LOXO-292 (right) and afatinib in BluR and LoxoR cells, respectively. Cells were treated with increasing concentrations of each drug alone or with both drugs every 72 h until the vehicle-treated controls reached ~ 90% confluency. Cell monolayers were then lysed with ATP cell titer Glo reagent and the number of viable cells was determined based on the quantification of ATP, indicating metabolically active cells. Combination indices were determined using the Chou-Talalay test. Numbers inside each box indicate the ratio of viable cells to untreated cells from three independent experiments. e Line chart indicating the number of Lc2/AD cells treated with vehicle, BLU-667 (500 nM), LOXO-292 (500 nM), afatinib (50 nM), and their combinations for 30 days. (**p < 0.01, ***p < 0.001; 2way ANOVA Bonferroni’s multiple comparisons).Representative images of cells treated with BLU-667 ± afatinib and LOXO-292 ± afatinib for 30 days. f Immunoblots of Lc2/AD cells treated with BLU-667, LOXO-292, afatinib and the combination for 25 days. Whole-cell lysates were prepared and subjected to immunoblot analysis with the indicated antibodies. Numbers above blots indicate FC variations compared to vehicle-treated cells. Images are representative of three independent experiments. g BluR xenografts established in Balb/c mice. Once tumors reached ≥ 200 mm3, the mice were randomized to treatment with vehicle, BLU-667 (10 mg/kg b.i.d.), afatinib (20 mg/kg q.d.), or both drugs. The BluR tumor growth was monitored for 3 months. Each data point represents the mean tumor volume in cm3 ± SEM (n =5 mice per arm). i Whole-cell lysates were prepared from proteins extracted from BluR xenografts after four days of treatment and subjected to immunoblot analyses with the indicated antibodies. Numbers above blots indicate FC variations compared to vehicle-treated tumors. Images are representative of three independent experiments. For all panels, each point represents the mean ± SD from three independent experiments
Similar to the NSCLC BluR and LoxoR models, concomitant treatment with RET inhibitors and afatinib significantly decreased TPC1 cell viability (Suppl. Figure 7a, left and right panels). Impairment of cell viability was associated with a reduction in p-ERK (Suppl. Figure 7b, left and right panels). Finally, we also evaluated whether BLU-667/LOXO-292 had a synergistic effect with afatinib via the Chou‒Talalay method and we observed notable synergism between RET and EGFR inhibitors (BLU-667/afatinib CI = 0.40; LOXO-292/afatinib CI = 0.1, Suppl. Figure 7c, left and right panels), indicating a crucial role of EGFR in TPC1 sensitivity to RETi.
Next, we investigated whether combined RET/EGFR inhibition could delay the development of drug resistance. After 25 days of treatment with the single agents BLU-667 or LOXO-292, cells began to proliferate again, whereas no regrowth was observed under combinatory treatment with afatinib (Fig. 3e) This regrowth following RET inhibitors treatment may be driven by EGFR activation, which is suppressed by the combination therapy (Fig. 3f). Moreover, upon removal of inhibitors, cells began to proliferate and recolonize the wells with higher regrowth in cells treated with BLU-667 or LOXO-292 alone compared to the growth rates observed in cells that received combination therapy (Suppl. Fig. 8a).
Finally, we tested the safety and efficacy of combining afatinib with BLU-667 in vivo. BluR xenografts in Balb/c nude mice revealed that treatment with BLU-667 or afatinib alone had no effects on tumor growth, whereas the combination significantly affected tumor growth (Fig. 3g). Importantly, the combined treatment did not negatively affect the body weights of the mice (Suppl. Fig. 8b). Western blot analysis of tumors treated with afatinib plus BLU-667 revealed downregulation of p-ERK levels (Fig. 3h), which was consistent with the in vitro findings.
AP1 complex members modulate resistance to selective RET inhibitors regulating EGFR expression
We aimed to uncover the biological mechanisms underlying EGFR overexpression in BluR and LoxoR cells, which are ultimately responsible for resistance to RETi. We interrogated our RNA-Sequencing data to determine putative transcription factors regulating EGFR expression in resistant cells by using the Integrated System for Motif Activity Response Analysis (ISMARA) [32]. By cross-referencing the top activated transcription factors obtained by ISMARA with known EGFR regulators, we identified Fra1 (encoded by FOSL1) and FosB (encoded by FOSB), both well-known members of the AP1 complex, as the most activated factors in resistant cells (Fig. 4a). Notably, interrogating the available omics datasets in DepMap portal, we found that FOSL1 and FOSB expression positively correlated with EGFR expression in different cancer types (Suppl. Figure 9a-b; Pearson coefficients of 0.281 and 0.597, respectively), including lung cancer (Suppl. Figure 9c-d; Pearson coefficients of 0.647 and 0.290, respectively). The positive correlation between FOSL1/FOSB and EGFR was further confirmed by our RNA-Sequencing data (Fig. 4b). Thus, we verified the expression of these two transcription factors by western blot analysis, which revealed higher expression of Fra1 and FosB in BluR and LoxoR cells than in Lc2/AD cells (Fig. 4c). We subsequently knocked down FOSL1 and FOSB in BluR and LoxoR cells to verify whether they could recapitulate the effects of EGFR silencing. Both siFOSL1 and siFOSB reduced EGFR expression and activation (Fig. 4d, 4f), further confirming their roles in regulating EGFR. Moreover, the combination of either siFOSL1 or siFOSB with BLU- 667 or LOXO-292 significantly decreased BluR and LoxoR viability (Fig. 4e, 4 g; Suppl. Figure 10a-b). Conversely, similar to the events occurring upon EGFR knockdown, in sensitive Lc2/AD cells, siFOSL1 and siFOSB did not elicit any additive effects on BLU-667 or LOXO-292 treatment, considering both cell count and spheroid growth analysis (Suppl. Figure 10c-f).
Finally, to determine the regulatory role of AP1 complex members in EGFR expression, we performed ChIP‒qPCR analysis to investigate whether direct binding of Fra1 or FosB occurs in EGFR regulatory regions. For this purpose, we first interrogated the Cistrome Data Browser to verify the putative binding regions of AP1 complex members on the EGFR promoter or enhancer regions. Based on the available Chip-Seq datasets, we selected eight candidate regions common to different databases as potentially bound by Fra1 (representative image in Fig. 4h; no data were available for FosB). Interestingly, three of the eight selected regions were amplified by qPCR following Fra1-selective chromatin immunoprecipitation (Fig. 4i). Conversely, chromatin immunoprecipitation using a specific anti-FosB antibody did not yield similar results. Hence, our analysis confirmed that Fra1, but not FosB, binds to EGFR regulatory regions and modulates its expression.
EGFR levels modulation in NSCLC patients treated with RET inhibitors: clinical insights
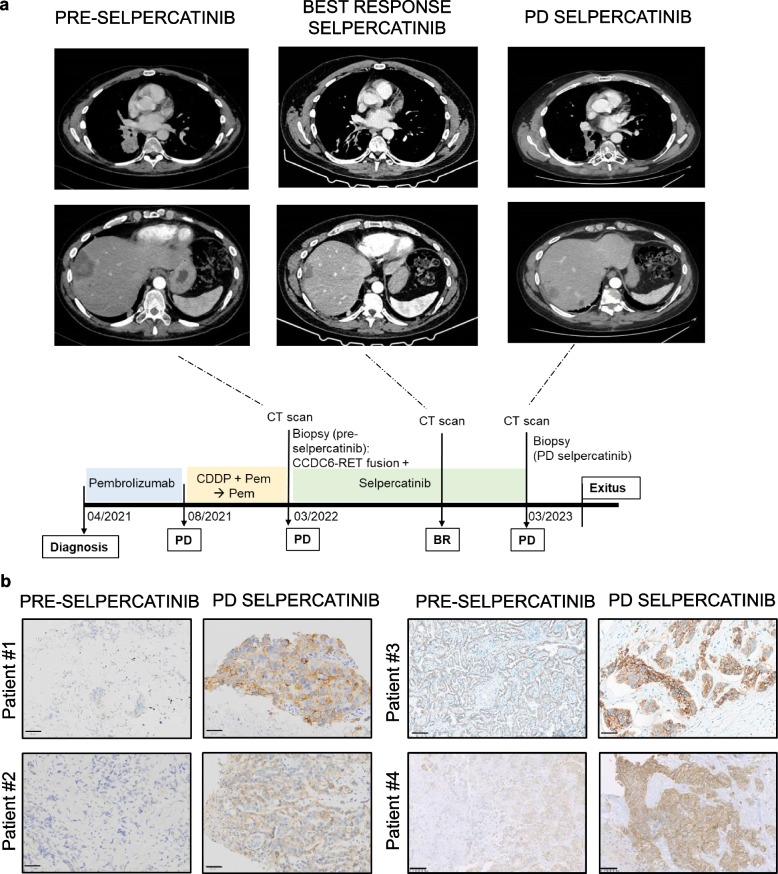
Our preclinical data revealed that EGFR overexpression is a key event that underpins resistance to RET inhibition. Therefore, we examined the clinical relevance of EGFR as a resistance mechanism in a cohort of patients treated with RETi. For six patients diagnosed with RET-rearranged NSCLC treated with RET inhibitors, we found available matched biopsies, including a RETi-baseline biopsy (before the start of the RETi, no more than six months before strating treatment) and a biopsy collected at the time of progression from RETi (RETi-PD). For all patients, we examined EGFR protein levels by immunohistochemistry (IHC) at RETi-baseline and after progression biopsies. In four out of six patients, the findings align with our hypothesis.
In particular, Patient #1 was treated with selpercatinib after progressing to platinum + pemetrexed + bevacizumab as first-line treatment. After 6 months of RETi, the patient experienced pleural, adrenal, and bone progression. Similarly, patient #2, following progression after chemoimmunotherapy in combination with platinum + pemetrexed + pembrolizumab, started second-line treatment with selpercatinib and continued treatment for 6 months until the treatment was stopped for progression in the liver and peritoneum sites. After progressing on vinorelbine, Patient #3 was characterized as RET-rearranged and enrolled for selpercatinib treatment, which was administrated for eleven months. All three patients had KIF5B-RET fusion.
At the time of diagnosis, patient #4 (with available representative computerized axial tomography; Fig. 5a) exhibited high (80%) PDL- 1 expression, but RET fusion status was not known (other molecular alterations investigated: wild-type EGFR, BRAF, and KRAS; no rearrangements in ALK and ROS1; tested negative for MET and NTRK alterations). After treatment with anti-PD- 1 pembrolizumab as the first-line therapy, the patient progressed after an initial clinical response. A biopsy of the liver metastasis indicated the presence of a CCDC6-RET fusion; therefore, the patient was administered the RET inhibitor selpercatinib as a subsequent intervention. Despite an initial partial response, after one year of treatment, the patient experienced disease progression with the appearance of new lung, pelvic, and liver secondary lesions (Fig. 5a). Biopsy of the new metastatic lesion in the pelvis was performed for comprehensive analysis.
Fig. 5.
Immunohistochemistry analysis reveals increased EGFR expression in disease-progression tumor biopsies. a Contrast-enhanced computed-tomography images of the thorax (upper) and abdomen (bottom) were acquired at baseline (on the left), showing a mass in the inferior lobe of the right lung, and a liver metastasis with central low attenuation (compatible with necrosis) and enhanced margins. These target lesions are shown at best response (central, best response) and at progression after selpercatinib treatment (right), where new liver lesions appeared (bottom panel showing the new lesion). CDDP = Cisplatin, cis-diamminedichloroplatinum; Pem = pemetrexed; PD = progression disease; BR = best response b Representative images of tissue sections subjected to IHC for EGFR. For patient #1, representative images of lung (pre-selpercatinib) and adrenal (Progression disease, PD selpercatinib) sections; for patient #2, representative images of brain metastasis (pre-selpercatinib) and liver (PD selpercatinib); for patient #3, representative images of liver (pre-selpercatinib and PD selpercatinib); for patient #4, representative images of liver (pre-selpercatinib) and pelvis (PD selpercatinib); Scale bar 50 µm
Interestingly, we discovered that, compared with pre-selpercatinib biopsies, post-progression treatment tissues presented discernible increases in EGFR protein levels in four patients (three cases switching from absent/weak staining in pre-treatment samples to moderate/strong staining in post-treatment tissues and one case from moderate to strong; Fig. 5b). Hence, these clinical findings mirror the results of our preclinical models of RET resistance.
Discussion
Over the past decade, molecular targeted therapies have become increasingly pivotal in the therapeutic landscape of oncogene-addicted cancers. Notably, pralsetinib and selpercatinib have demonstrated unprecedented efficacy in the treatment of patients with lung or thyroid tumors, driven by RET gene rearrangements. However, despite the remarkable responses in many cases, the emergence of resistance mechanisms poses significant challenges to the clinical efficacy of these therapies.
Clinical evidence has highlighted the reactivation of the MAPK pathway in selpercatinib-resistant tumors, driven by secondary RET mutations or alterations in other driver genes, such as MET amplification and RAS or BRAF mutations [21]. However, a well-documented occurrence in solid cancers is signaling rebound prompted by transcriptional reprogramming rather than gene alterations [24–26, 33, 34]. Hu et al. demonstrated in preclinical models that MAPK reactivation induced by treatment with BLU-667 led to the upregulation of Aurora A/B kinases, which resulted in tumor regression when inhibited in combination with RET inhibition [35].
Herein, we present evidence suggesting that transcriptional reprogramming, leading to EGFR overexpression and reactivation, rather than point mutations or copy number variations, may influence the response to RET inhibition. Our findings support the efficacy of combined targeting of RET and EGFR in CCDC6-RET NSCLC and thyroid cancer cells, successfully preventing tumor cell growth under RET-resistant conditions. In our NSCLC-resistant cell lines and xenograft models, targeting EGFR, in addition to RET inhibition, resensitized cells to anti-RET treatment. This effect was particularly pronounced in RET-rearranged papillary thyroid cancer cells, where early EGFR activation reduced sensitivity to RET inhibitors, and targeting EGFR with afatinib significantly enhanced the response. Notably, upfront combination therapy with RET and EGFR inhibitors prevents compensatory EGFR activation, which otherwise contributes to tumor regrowth and resistance to RET inhibitors. This highlights the therapeutic potential of dual inhibition in delaying or overcoming adaptive resistance mechanisms. Mechanistically, we found that in NSCLC cells, the transcription factor Fra1 regulates EGFR expression by directly binding to its genomic sequence, offering insights into the underlying mechanisms of EGFR overexpression in RET-resistant models. Fra1, encoded by FOSL1, is known to operate as a heterodimeric complex with other members of the activator protein- 1 (AP- 1) complex, such as FosB. High expression of Fra1 is closely associated with tumor cell proliferation, apoptosis, and migratory events, exerting oncogenic effects, and is correlated with advanced tumor stage and poor survival in non-small cell lung cancer patients [36]. Its overexpression has been described as a consequence of MAPK hyperactivation [37]. Although EGFR targeting did not modulate Fra1 expression (data not shown), gene knockdown of the AP1 complex members Fra1 and FosB modulated EGFR expression, demonstrating direct binding of Fra1 with EGFR regulatory regions. To the best of our knowledge, our findings are the first to shed light on the mechanisms underlying EGFR hyperactivation in RET-resistant models and propose strategies to overcome these events through a clinically feasible combination approach.
Notably, the preliminary clinical evidence supports our findings. Specifically, four out of six patients treated with selpercatinib exhibited increased EGFR expression upon disease progression, as evidenced by immunohistochemistry of the metastatic sites. Notably, although our preclinical findings were obtained in CCDC6-RET cell lines, we observed the same mechanism in patients carrying CCDC6-RET and the most common KIF5B-RET rearrangements. Furthermore, although small in number, the analysis of our cohort revealed that EGFR upregulation may represent a common resistance mechanism. These findings have substantial implications, suggesting the feasibility of using immunohistochemistry to assess EGFR expression levels to guide anti-EGFR therapy selection in patients with progressive disease.
In conclusion, our study underscores the importance of the combined targeting of RET and EGFR to overcome resistance mechanisms in RET-rearranged cancers. By elucidating the complex mechanisms underlying therapy adaptation, we provide valuable insights that could inform the development of novel therapeutic approaches aimed at preventing and overcoming the resistance mechanisms that currently limit the efficacy of RET inhibitors.
Supplementary Information
Acknowledgements
The authors particularly thank patients and their relatives for allowing researchers to perform investigational analyses of tumor samples.
Abbreviations
- NSCLC
Non-small cell lung cancer
- RET
REarranged during Transfection
- EGFR
Epidermal growth factor receptor
- AP1
Activator protein 1
- KIF5B
Kinesin family member 5B
- CCDC6
Coiled-Coil Domain-Containing Protein 6
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular signal-regulated kinase
- PI3K
Phosphatidylinositide 3-kinase
- JNK
C-Jun N-terminal kinase
- FDA
Food and Drug Administration
- EMA
European Medicines Agency
- ATCC
American Type Culture Collection
- FBS
Fetal bovine serum
- DMEM
Dulbecco's Modified Eagle Medium
- CI
Combination index
- PBS
Phosphate-buffered saline
- RIPA
Radioimmunoprecipitation assay
- BCA
Bicinchoninic acid assay
- FosB
FBJ murine osteosarcoma viral oncogene homolog B
- Fra1
Fos-related antigen 1
- Shc
Src homology 2 domain containing
- FOSL1
FOS-like 1
- ORF
Open Reading Frame
- GFP
Green Fluorescent Protein
- FACS
Fluorescence-activated cell sorting
- PI
Propidium Iodide
- RNase
Ribonuclease
- BSA
Bovine Serum Albumin
- ChIP
Chromatin Immunoprecipitation
- qPCR
Quantitative Polymerase Chain Reaction
- EDTA
Ethylenediaminetetraacetic acid
- HCl
Hydrochloric acid
- mM
MilliMolar
- SDS
Sodium Dodecyl Sulfate
- Fwd
Forward
- Rev
Reverse
- NGS
Next-generation sequencing
- GSEA
Gene set enrichment analysis
- ISMARA
Integrated Motif Activity Response Analysis
- DMSO
Dimethyl Sulfoxide
- PEG
Polyethylene Glycol
- BID
Bis in die
- OS
Oral administration
- PD
Progression disease
- IHC
Immunohistochemistry
- DAB
Diaminobenzidine
- MET
Mesenchymal Epithelial Transition factor
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- KMT2D
Lysine Methyltransferase 2D
- COL1 A1
Collagen Type I Alpha 1
- PARP1
Poly (ADP-Ribose) Polymerase 1
- PTC
Papillary thyroid cancer
- BRAF
B-Raf Proto-Oncogene, Serine/Threonine Kinase
- ALK
Anaplastic Lymphoma Kinase
- ROS1
ROS Proto-Oncogene 1, Receptor Tyrosine Kinase
- NTRK
Neurotrophic Tyrosine Receptor Kinase
- PD- 1
Programmed Cell Death Protein 1
Authors’ contributions
DE conceived of the study design, carried out the experiments, analyzed the data and performed the statistical analysis, wrote the manuscript. CMA, SB, carried out immunoblot and phenotypic assays and edited the manuscript. FN, AS, FP, RF contributed to the data curation and edited of the manuscript. UM, GT provided resources for oncomine tumor mutation load and immunohistochemistry assays. DB, EB, BB collected and provided human samples and provided resources for immunohistochemistry. AI, MRG performed immunohistochemistry assays. AM, MA collected and provided human samples and edited the manuscript. LF, RB: conceived of the study design, supervised the experiments, edited the manuscript and provided funding acquisition. All authors read and approved the final manuscript.
Funding
This work was supported by AIRC IG 21339 grant (RB), PRIN_PNRR- 2022-R-BIANCO–MUR 000015 (RB), PNC00001 (RB) MFAG 21505 - 2018 grant (LF), MFAG 27826 - 2022 (AS). EB was supported by AIRC IG20583, Institutional funds of the Università Cattolica del Sacro Cuore (UCSC‐Project D1), and funds from the Ministero della Salute (Ricerca Corrente 2025). DE was supported by AIRC fellowship for Italy 2021–26795.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Federico II University Ethics Committee (ref n 139/22). Participants gave informed consent to participate in the study before taking part. The animal study was performed in accordance with institutional guidelines of the University of Naples Animal Care Committee and in accordance with the Declaration of Helsinki.
Consent for publication
Not Applicable.
Competing interests
Formisano declares the following competing interests: Consultant and advisory board for Seagen, Amgen, BMS, MSD, Jansen, and Pierre Fabre Pharma. Bianco declares the following competing interests: consultant and advisory board for BMS, MSD, Pfizer, AstraZeneca, Lilly and Novartis. Servetto reports honoraria from Eli Lilly, MSD, and Janssen and travel support from Bristol-Myers Squibb and AstraZeneca. Giuseppe Lo Russo declares advisory boards, consultancies, travel accommodations, speaker fees, writing fees, pi role in profit trials: MSD, Regeneron, Roche, Lilly, BMS, Amgen, AstraZeneca, Johnson And Johnson, Merck, Novartis, Pierre Fabre, Bayer, Beigene, Pfizer, Takeda, GSK, Daiichi,Sanofi, Gilead. Besse reports sponsored research at Gustave Roussy Cancer Center from AbbVie, Amgen, AstraZeneca, Biogen, Blueprint Medicines, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Ignyta, IPSEN, Merck KGaA, Merck Sharp & Dohme, Nektar, Onxeo, Pfizer, Pharma Mar, Sanofi, Spectrum Pharmaceuticals, Takeda, and Tiziana Pharma and investigators or coinvestigators of trials for Nerviano, GlaxoSmithKline, Pfizer, Roche-Genentech, Eli Lilly, OSE Pharma, Merck Sharp & Dohme, Celgene, Stemcentrx, Ignyta, AbbVie, Loxo Oncology, AstraZeneca, and Blueprint Medicines. Aldea reports receiving expenses and research funding from Sandoz and research funding from Amgen and AstraZeneca. Marinello declares travel/accommodation from Daiichi Sankyo and not financial support from MSD. The remaining authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Luigi Formisano, Email: luigi.formisano1@unina.it.
Roberto Bianco, Email: robianco@unina.it.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. Available from: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–237. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0923753419317107. [DOI] [PubMed] [Google Scholar]
- 3.Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–50. Available from: https://www.nature.com/articles/nature13385. [DOI] [PMC free article] [PubMed]
- 4.Servetto A, Esposito D, Ferrara R, Signorelli D, Belli S, Napolitano F, et al. RET rearrangements in non-small cell lung cancer: Evolving treatment landscape and future challenges. Biochim Biophys Acta - Rev Cancer. 2022;1877:188810. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304419X22001354. [DOI] [PubMed] [Google Scholar]
- 5.Ju YS, Lee W-C, Shin JY, Lee S, Bleazard T, Won JK, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–45. Available from: http://genome.cshlp.org/lookup/doi/10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarfaty M, Moore A, Neiman V, Dudnik E, Ilouze M, Gottfried M, et al. RET Fusion Lung Carcinoma: Response to Therapy and Clinical Features in a Case Series of 14 Patients. Clin Lung Cancer. 2017;18:e223–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1525730416302285. [DOI] [PubMed] [Google Scholar]
- 7.Aldea M, Marinello A, Duruisseaux M, Zrafi W, Conci N, Massa G, et al. RET-MAP: An International Multicenter Study on Clinicobiologic Features and Treatment Response in Patients With Lung Cancer Harboring a RET Fusion. J Thorac Oncol. 2023;18:576–86. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556086422019943. [DOI] [PubMed] [Google Scholar]
- 8.Li AY, McCusker MG, Russo A, Scilla KA, Gittens A, Arensmeyer K, et al. RET fusions in solid tumors. Cancer Treat Rev. 2019;81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S030573721930129X. [DOI] [PubMed]
- 9.Kawai K, Takahashi M. Intracellular RET signaling pathways activated by GDNF. Cell Tissue Res. 2020;382:113–23. Available from: https://link.springer.com/10.1007/s00441-020-03262-1. [DOI] [PubMed] [Google Scholar]
- 10.Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14:173–86. Available from: https://www.nature.com/articles/nrc3680. [DOI] [PubMed] [Google Scholar]
- 11.Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, Owen D, et al. Targeting RET in Patients With RET -Rearranged Lung Cancers: Results From the Global. Multicenter RET Registry J Clin Oncol. 2017;35:1403–10. Available from: https://ascopubs.org/doi/10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbiah V, Gainor JF, Oxnard GR, Tan DSW, Owen DH, Cho BC, et al. Intracranial Efficacy of Selpercatinib in RET Fusion-Positive Non-Small Cell Lung Cancers on the LIBRETTO-001 Trial. Clin Cancer Res. 2021;27:4160–7. Available from: https://aacrjournals.org/clincancerres/article/27/15/4160/671589/Intracranial-Efficacy-of-Selpercatinib-in-RET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2020;383:813–24. Available from: http://www.nejm.org/doi/10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subbiah V, Cassier PA, Siena S, Garralda E, Paz-Ares L, Garrido P, et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion–positive solid tumors from the phase 1/2 ARROW trial. Nat Med. 2022;28:1640–5. Available from: https://www.nature.com/articles/s41591-022-01931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drilon A, Subbiah V, Gautschi O, Tomasini P, de Braud F, Solomon BJ, et al. Selpercatinib in Patients With RET Fusion-Positive Non–Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J Clin Oncol. 2023;41:385–94. Available from: https://ascopubs.org/doi/10.1200/JCO.22.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gainor JF, Curigliano G, Kim D-W, Lee DH, Besse B, Baik CS, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22:959–69. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204521002473. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Solomon B, Loong HH, Park K, Pérol M, Arriola E, et al. First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion-Positive NSCLC. N Engl J Med. 2023;389:1839–50. Available from: 10.1056/NEJMoa2309457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griesinger F, Curigliano G, Thomas M, Subbiah V, Baik CS, Tan DSW, et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. 2022;33:1168–78. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0923753422038662. [DOI] [PubMed] [Google Scholar]
- 19.Loong HHF, Goto K, Solomon BJ, Park K, Pérol M, Arriola E, et al. LBA4 Randomized phase III study of first-line selpercatinib versus chemotherapy and pembrolizumab in RET fusion-positive NSCLC. Ann Oncol. 2023;34:S1303. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0923753423042035. [Google Scholar]
- 20.Cooper AJ, Drilon AE, Rotow JK, Liu SV, Gautschi O, Smith KER, et al. First results from the RETgistry: A global consortium for the study of resistance to RET inhibition in RET -altered solid tumors. J Clin Oncol. 2023;41:9065–9065. Available from: https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.9065. [Google Scholar]
- 21.Rosen EY, Won HH, Zheng Y, Cocco E, Selcuklu D, Gong Y, et al. The evolution of RET inhibitor resistance in RET-driven lung and thyroid cancers. Nat Commun. 2022;13:1450. Available from: https://www.nature.com/articles/s41467-022-28848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocco E, Schram AM, Kulick A, Misale S, Won HH, Yaeger R, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med. 2019;25:1422–7. Available from: https://www.nature.com/articles/s41591-019-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misale S, Bozic I, Tong J, Peraza-Penton A, Lallo A, Baldi F, et al. Vertical suppression of the EGFR pathway prevents onset of resistance in colorectal cancers. Nat Commun. 2015;6:8305. Available from: https://www.nature.com/articles/ncomms9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Formisano L, Lu Y, Servetto A, Hanker AB, Jansen VM, Bauer JA, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10:1373. Available from: https://www.nature.com/articles/s41467-019-09068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Servetto A, Kollipara R, Formisano L, Lin C-C, Lee K-M, Sudhan DR, et al. Nuclear FGFR1 Regulates Gene Transcription and Promotes Antiestrogen Resistance in ER+ Breast Cancer. Clin Cancer Res. 2021;27:4379–96. Available from: https://aacrjournals.org/clincancerres/article/27/15/4379/671550/Nuclear-FGFR1-Regulates-Gene-Transcription-and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belli S, Esposito D, Allotta A, Servetto A, Ciciola P, Pesapane A, et al. Pak1 pathway hyper-activation mediates resistance to endocrine therapy and CDK4/6 inhibitors in ER+ breast cancer. npj Breast Cancer. 2023;9:48. Available from: https://www.nature.com/articles/s41523-023-00556-9. [DOI] [PMC free article] [PubMed]
- 27.Guerrero-Zotano Á, Belli S, Zielinski C, Gil-Gil M, Fernandez-Serra A, Ruiz-Borrego M, et al. CCNE1 and PLK1 Mediate Resistance to Palbociclib in HR+/HER2− Metastatic Breast Cancer. Clin Cancer Res. 2023;29:1557–68. Available from: https://aacrjournals.org/clincancerres/article/29/8/1557/725076/CCNE1-and-PLK1-Mediate-Resistance-to-Palbociclib. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belli S, Pesapane A, Servetto A, Esposito D, Napolitano F, Ascione CM, et al. Combined blockade of mTOR and p21-activated kinases pathways prevents tumour growth in KRAS-mutated colorectal cancer. Br J Cancer. 2023;129:1071–82. Available from: https://www.nature.com/articles/s41416-023-02390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cataldo ML, De Placido P, Esposito D, Formisano L, Arpino G, Giuliano M, et al. The effect of the alpha-specific PI3K inhibitor alpelisib combined with anti-HER2 therapy in HER2+/PIK3CA mutant breast cancer. Front Oncol. 2023;13. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2023.1108242/full. [DOI] [PMC free article] [PubMed]
- 30.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–50. Available from: https://pnas.org/doi/full/10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou TC. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010;70:440–6. Available from: https://aacrjournals.org/cancerres/article/70/2/440/559704/Drug-Combination-Studies-and-Their-Synergy. [DOI] [PubMed] [Google Scholar]
- 32.Balwierz PJ, Pachkov M, Arnold P, Gruber AJ, Zavolan M, van Nimwegen E. ISMARA: automated modeling of genomic signals as a democracy of regulatory motifs. Genome Res. 2014;24:869–84. Available from: http://genome.cshlp.org/lookup/doi/10.1101/gr.169508.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaeger R, Yao Z, Hyman DM, Hechtman JF, Vakiani E, Zhao H, et al. Mechanisms of Acquired Resistance to BRAF V600E Inhibition in Colon Cancers Converge on RAF Dimerization and Are Sensitive to Its Inhibition. Cancer Res. 2017;77:6513–23. Available from: https://aacrjournals.org/cancerres/article/77/23/6513/623982/Mechanisms-of-Acquired-Resistance-to-BRAF-V600E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-Mediated Reactivation of MAPK Signaling Contributes to Insensitivity of BRAF -Mutant Colorectal Cancers to RAF Inhibition with Vemurafenib. Cancer Discov. 2012;2:227–35. Available from: https://aacrjournals.org/cancerdiscovery/article/2/3/227/37286/EGFR-Mediated-Reactivation-of-MAPK-Signaling. [DOI] [PMC free article] [PubMed]
- 35.Hu X, Liu X, Khatri U, Wu J. The heterogeneous transition state of resistance to RET kinase inhibitors converges on ERK1/2-driven Aurora A/B kinases. Drug Resist Updat. 2023;68. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1368764623000419. [DOI] [PMC free article] [PubMed]
- 36.Ma K, Chang D, Gong M, Ding F, Luo A, Tian F, et al. Expression and Significance of FRA-1 in Non-Small-Cell Lung Cancer. Cancer Invest. 2009;27:353–9. Available from: http://www.tandfonline.com/doi/full/10.1080/07357900802254008. [DOI] [PubMed] [Google Scholar]
- 37.Casalino L, Talotta F, Cimmino A, Verde P. The Fra-1/AP-1 Oncoprotein: From the “Undruggable” Transcription Factor to Therapeutic Targeting. Cancers Basel. 2022;14:1480. Available from: https://www.mdpi.com/2072-6694/14/6/1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.