Abstract
Objective
This study aimed to analyze the characteristics of adverse reactions in cancer patients treated with Pembrolizumab based on the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS) database, and to assess the characteristics and risk factors of fatality reports.
Methods
The study data was sourced from the FAERS database, collecting adverse event reports related to Pembrolizumab from 2013 to June 2024. The main analysis variables included gender, age, cancer type, country, reporter type, and adverse reaction outcomes. Descriptive statistics, univariate analysis, and multivariate Logistic regression models were used to assess the relationship between each variable and fatal outcome.
Results
A total of 46,883 adverse reactions were collected, including 5,483 reports with fatal outcomes. The number of events has been increasing since 2013, especially peaking in 2022 and 2023. The United States and Japan had the highest number of adverse reaction reports. The number of serious events reported increased significantly with age, especially in the 51–65 and 66–80 age groups. The age of patients who died was concentrated in the elderly group (≥ 65 years old), and the median treatment duration time of pembrolizumab was 17 days. Analysis showed that gender (OR = 0.75; 95%CI: 0.71–0.80, p < 0.01), age (OR = 0.89; 95%CI: 0.84–0.96, p < 0.01), and ingredients count (OR = 1.92; 95%CI: 1.84–2.01, p < 0.01) were significantly associated with the treatment duration of pembrolizumab.
Conclusion
The serious adverse reactions in cancer patients treated with Pembrolizumab are closely related to patient individual characteristics and cancer types. It is necessary to strengthen the monitoring of high-risk groups such as the elderly in clinical treatment to reduce the risk of fatal outcomes.
Keywords: Adverse reactions, Immunotherapy, Pembrolizumab, Prognostic factors, FAERS
Introduction
Pembrolizumab is a monoclonal antibody targeting the immune checkpoint programmed death receptor 1 (PD-1) [1]. In the field of cancer treatment, pembrolizumab has triggered a revolution in immunotherapy, especially in the treatment of a variety of advanced and metastatic tumors [2, 3]. Since it was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of advanced melanoma in 2014 [2, 4], the indications of pembrolizumab have gradually expanded to multiple tumor types, including non-small cell lung cancer [5], head and neck cancer [6], and hepatocellular carcinoma [7]. Its wide application further establishes the advantages of immune checkpoint inhibitors (ICIs) in modern tumor treatment.
Although pembrolizumab has significant anti-tumor activity and improves the overall survival of multiple tumors, its accompanying immune-related adverse events (irAEs) have also received increasing attention [8]. Compared with traditional chemotherapy or targeted therapy, ICIs induce a wide range of irAEs, involving multiple organ systems, including the skin, gastrointestinal tract, liver, endocrine system, lungs, and heart [8]. These irAEs are usually highly heterogeneous, ranging from self-limiting rashes or fatigue to fatal complications such as immune myocarditis, myasthenia gravis, and acute liver failure [9]. Therefore, how to timely identify, intervene, and manage these adverse reactions has become an important direction in clinical tumor treatment.
The U.S. FSA Adverse Event Reporting System (FAERS) is a global voluntary reporting database for adverse drug events [10], providing a valuable real-world data resource that can help us identify and evaluate adverse reactions of drugs in a wide range of populations. Through a large-scale retrospective analysis of the FAERS database, healthcare providers may capture the spectrum of adverse reactions associated with pembrolizumab, especially those rare or serious adverse events that may not be fully reflected in clinical trials.
Existing studies have shown that the incidence of adverse reactions to pembrolizumab varies with individual patient differences and different medication regimens [11, 12]. For example, elderly patients and patients with certain specific cancer types may be more susceptible to severe immune-related adverse reactions [13]. In addition, drug use patterns and adverse drug reaction reporting systems in different countries and regions may also affect the reporting frequency and pattern of adverse events [14]. Therefore, in-depth analysis of adverse reactions in pembrolizumab treatment combined with real-world data from different populations and regions is worthy for optimizing tumor immunotherapy strategies and improving patient safety.
The purpose of this study is to systematically review the adverse reaction reports of pembrolizumab treated cancer patients in the FAERS database, focusing on the characteristics of adverse reactions in patients who died. By evaluating the differences between patients with severe adverse reactions and those with non-serious adverse reactions in terms of baseline characteristics, cancer type, adverse reaction spectrum, and reporter type, we aim to reveal potential factors that may be associated with the severity of adverse reactions and provide real-world evidence for clinical treatment.
Methods
Data source
The data for this study came from the FAERS database. FAERS is a spontaneously reported adverse event database that is regularly updated and open to the public, and contains reports of various adverse reactions that occur after the use of drugs [15]. This study collected all adverse reaction reports related to pembrolizumab from January 2013 to June 2024. Although Pembrolizumab was approved for clinical use in 2014, adverse event reports related to the drug were included in the FAERS database starting from 2013, reflecting reports from clinical trials and investigational use before its official market approval. These reports include patient demographic characteristics, drug information, cancer type, severity of adverse reactions, event outcomes (such as death or non-death), reporter type, and the country where the event occurred.
Data filtering
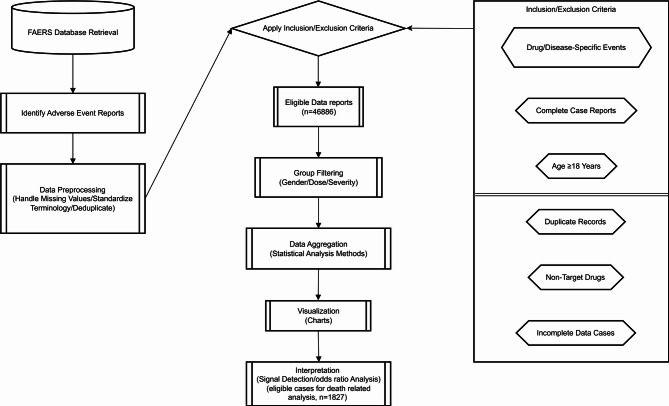
The steps of data screening are shown in Fig. 1. In order to ensure the accuracy of the data and the relevance of the research, this study screened the data according to the following criteria:
Fig. 1.
Flow chart of identifying eligible reports
Inclusion criteria: All adverse event reports that identified pembrolizumab as “Primary Suspect Drug” (PSD) were screened, and the outcome of the adverse reaction (death or non-death) must be clearly stated in the report. This study specifically focuses on reports where the patient experienced a fatal outcome, and the death was considered possibly related to pembrolizumab treatment. Not all patients in the study had fatal outcomes.
Exclusion criteria: Reports lacking key information (such as age, gender, event outcomes, etc.) and reports with duplicate records were excluded. All included reports were strictly deduplicated to ensure that each patient’s report was unique.
Adverse reaction classification
Adverse events were coded and classified according to the Preferred Terms (PT) of the International Medical Dictionary for Respiratory Diseases (MedDRA), and further classified into the corresponding System Organ Class (SOC) [15]. Serious adverse events (SAEs) and non-serious adverse events (NSAEs) were focused on, and death reports were analyzed according to event outcomes. Case priority events refer to adverse reactions that are classified as requiring expedited reporting due to their severity and potential impact on patient safety, as defined by the FAERS database [15].
Statistical analysis
Data processing and statistical analysis were performed using R 4.4.1 and python 3.12 software. The classification of adverse reaction types used the MedDRA terminology set, and data were extracted using the FAERS dashboard [16]. Baseline characteristics of all patients and adverse events included in the study were analyzed. Continuous variables were described using mean ± SD or median (interquartile range, IQR), and categorical variables were expressed as frequency. The differences between groups in categorical variables were analyzed using the Chi-square test or Fisher’s exact test, and independent sample t-test or Mann-Whitney U test were used for continuous variables. Logistic regression models were used to evaluate the associations between gender, age, cancer type, and reporting country with adverse reaction outcomes (SAEs or NSAEs). The results were expressed as odds ratios (OR) and their 95% confidence intervals (CI). The Proportional Reporting Ratio (PRR), Reporting Odds Ratio (ROR), and Relative Reporting Ratio (RRR) are key statistical measures used in pharmacovigilance to detect signals of adverse drug reactions (ADRs) [17]. PRR compares the frequency of an adverse event for a specific drug to other drugs, highlighting potential safety concerns when the ratio is greater than 1. ROR assesses the odds of an adverse event being reported for a drug compared to all other drugs, suggesting a possible association if the value is significantly greater than 1. RRR quantifies the relative incidence of adverse events for a specific drug, providing insights into its relative safety profile. To analyze the risk factors of death, subgroups were set according to treatment duration time for analysis to further explore the risk factors that may affect the duration time of pembrolizumab. All analyses were considered statistically significant at p ≤ 0.05.
Results
Overall trends of pembrolizumab related adverse events
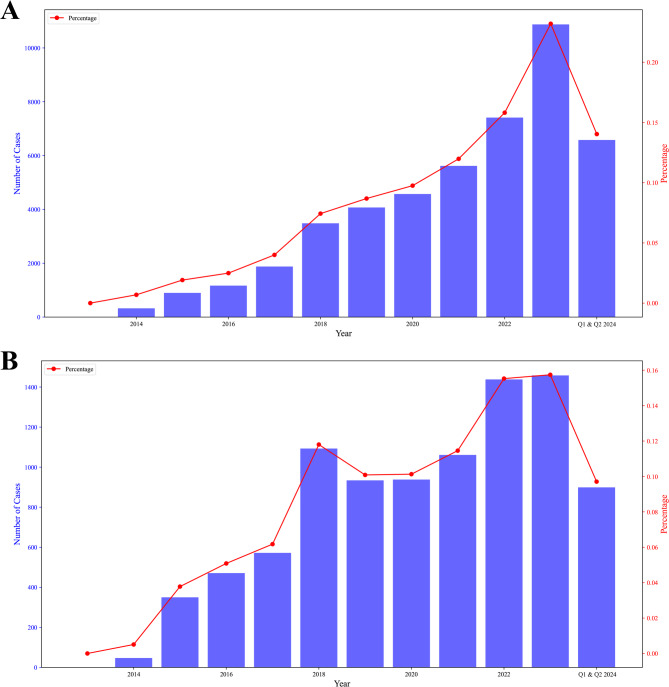
This study collected a total of 46,883 adverse event reports related to pembrolizumab, including 43,131 serious adverse events (SAEs), accounting for approximately 91.98%, and 3,752 non-serious adverse events (NSAEs), accounting for approximately 8.02%. From 2013 to 2023, the number of adverse event reports (Fig. 2A) and the number of deaths (Fig. 2B) of pembrolizumab showed an upward trend, especially in 2022 and 2023, when the reports reached a peak.
Fig. 2.
Overall trends of pembrolizumab related adverse events in recent years. A number of adverse events by years (2013–2023 and 2024 Q1-Q2); B number of died cases by years (2013–2023 and 2024 Q1-Q2)
Patient characteristics
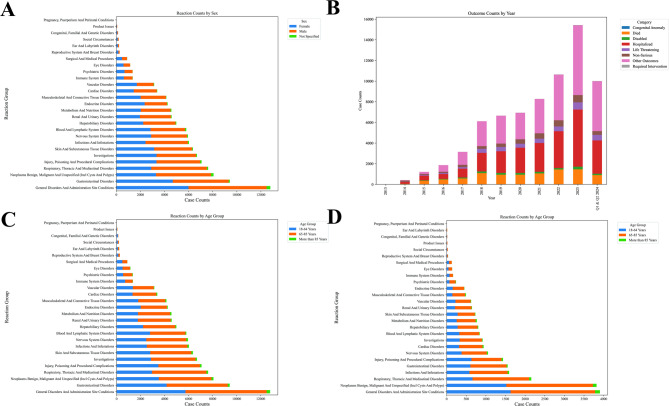
In terms of gender (Fig. 3A), the cumulative number of adverse event reports in male patients was higher than that in female patients (57452 for male vs. 51422 for female). Although the overall number of adverse event reports in male patients was higher, certain adverse reactions, such as Social Circumstances, Endocrine Disorders, and Reproductive System and Breast Disorders, were more commonly reported in females. For outcomes, high-risk outcomes (hospitalizations, life-threatening events) demonstrated exponential growth (43% annual increase) from 2013 to 2023, peaking at 5,535 and 700 cases respectively (Fig. 3B). Anomalous 2023 surges (e.g., 6,754 “Other Outcomes”) suggests systemic reporting inconsistencies or external interventions requiring validation of data integrity and classification criteria (Fig. 3B). In terms of age, the incidence of serious adverse events in elderly patients (≥ 65 years old) was significantly higher than that in young patients, and the mortality rate in elderly patients was higher (Fig. 3C and D). In terms of adverse reaction types, the most common adverse reactions related to pembrolizumab included: progression of malignant tumors (6052, 12.91%), diarrhea (2412, 5.14%), death (2241, 4.78%), fatigue (2113, 4.51%), in addition to other adverse reactions such as loss of appetite, fever and rash (Fig. 3). In terms of geographical distribution, adverse event reports from Japan and the United States accounted for the largest proportion, with 12,616 cases reported in Japan and 11,045 cases reported in the United States, accounting for a total of 50.50% of global reports.
Fig. 3.
General features of adverse events in patients treated with pembrolizumab. A number of cases in different reaction groups by sex; B number of various outcomes in different years (2013–2023 and 2024 Q1-Q2); C number of cases in different reaction groups by age; D number of died cases in different reaction groups by age
Analysis of risk factors related to serious adverse reactions
Logistic regression analysis showed that gender, age, and reporter type were significantly associated with the severity of adverse events. Specifically, the odds ratio of serious adverse events in female patients was 0.76 (95% CI: 0.73–0.79; P < 0.01), indicating that the risk of serious adverse events in female patients was significantly lower than that in male patients. The risk of elderly patients increased with age, with the odds ratio of serious adverse events increasing by 1.01 (95% CI: 1.007–1.011; P < 0.01) for every 1-year increase in patient age, indicating that age increase was statistically significantly associated with increased severity of adverse events. In addition, the severity of case priority events was significantly higher than that of other types, with an odds ratio as high as 2.47 (95% CI: 2.32–2.64; P < 0.01). At the same time, we also found that the odds ratios of regimen ingredient count of less than 2 (OR = 0.92; 95%CI:0.901–0.934; P < 0.001), and reporter type of medical staff (OR = 1.17; 95%CI:1.083–1.271; P < 0.001) showed statistical significance, suggesting that these factors may also be a risk factor for serious adverse events.
Baseline characteristics of deceased patients
After screening, 1827 death reports were finally included in the analysis (Table 1). Among the 1827 patients who died, 39.1% (715 cases) were female, 58.0% (1060 cases) were male, and 2.8% (52 cases) were of unknown gender. The most commonly used treatment regimen was single- agent pembrolizumab treatment, accounting for about 35.3% (645 cases). Among other treatment regimens, pembrolizumab combined with lenvatinib accounted for 20.9% (381 cases), and pembrolizumab combined with carboplatin and pemetrexed accounted for 7.5% (137 cases). Among the patients who died, non-small cell lung cancer (23.9%) and cervical cancer (32.4%) were the most common types of cancer. Melanoma patients accounted for 8.5%, and renal cancer patients accounted for 10.1%. 88.1% of the patient reports of deaths were classified as “expedited” treatment, and 93.9% of adverse events were reported by medical professionals. The mean age of patients who died was 66.3 years, with a standard deviation of 11.3 years. The mean age of female patients was 64.0 years, and the mean age of male patients was 67.7 years. The mean time to adverse events in patients who died was 67.8 days, with a standard deviation of 137.7 days, indicating that the time to adverse events was highly variable. In the distribution of the number of treatment components, 35.2% of patients received single-drug therapy, while the proportion of multidrug combination therapy was higher (60% of patients received combination therapy with more than two drugs).
Table 1.
Baseline characteristics of died patients related to adverse events of pembrolizumab by sex
| Factors | Groups | Male (N = 1060) | Female (N = 715) | Unknown (N = 52) | Total (N = 1827) |
|---|---|---|---|---|---|
| Top 9 Regimen | Pembrolizumab | 391 (36.9%) | 208 (29.1%) | 46 (88.5%) | 645 (35.3%) |
| Other | 225 (21.2%) | 121 (16.9%) | 4 (7.7%) | 350 (19.2%) | |
| Lenvatinib; Pembrolizumab | 138 (13%) | 243 (34%) | 0 (0%) | 381 (20.9%) | |
| Carboplatin; Pembrolizumab; Pemetrexed | 107 (10.1%) | 30 (4.2%) | 0 (0%) | 137 (7.5%) | |
| Carboplatin; Pembrolizumab; Taxane | 50 (4.7%) | 32 (4.5%) | 0 (0%) | 82 (4.5%) | |
| Axitinib; Pembrolizumab | 26 (2.5%) | 7 (1%) | 0 (0%) | 33 (1.8%) | |
| Pembrolizumab; Pemetrexed | 16 (1.5%) | 11 (1.5%) | 1 (1.9%) | 28 (1.5%) | |
| Cisplatin; Pembrolizumab; Pemetrexed | 15 (1.4%) | 7 (1%) | 0 (0%) | 22 (1.2%) | |
| Pembrolizumab; Taxane | 92 (8.7%) | 56 (7.8%) | 1 (1.9%) | 149 (8.2%) | |
| Ingredient counts | 1 | 391 (36.9%) | 207 (29%) | 46 (88.5%) | 644 (35.2%) |
| 2 | 258 (24.3%) | 301 (42.1%) | 1 (1.9%) | 560 (30.7%) | |
| 3 | 262 (24.7%) | 122 (17.1%) | 0 (0%) | 384 (21%) | |
| 4 | 53 (5%) | 47 (6.6%) | 3 (5.8%) | 103 (5.6%) | |
| 5 | 37 (3.5%) | 24 (3.4%) | 2 (3.8%) | 63 (3.4%) | |
| 6 | 11 (1%) | 5 (0.7%) | 0 (0%) | 16 (0.9%) | |
| 7 | 4 (0.4%) | 1 (0.1%) | 0 (0%) | 5 (0.3%) | |
| 8 | 23 (2.2%) | 3 (0.4%) | 0 (0%) | 26 (1.4%) | |
| 9 | 21 (2%) | 5 (0.7%) | 0 (0%) | 26 (1.4%) | |
| Cancer related diagnosis | Cervix Cancer | 364 (34.3%) | 223 (31.2%) | 5 (9.6%) | 592 (32.4%) |
| Cholangiocarcinoma | 0 (0%) | 1 (0.1%) | 0 (0%) | 1 (0.1%) | |
| Colorectal Cancer | 22 (2.1%) | 10 (1.4%) | 1 (1.9%) | 33 (1.8%) | |
| Endometrial Cancer | 0 (0%) | 145 (20.3%) | 0 (0%) | 145 (7.9%) | |
| Head And Neck Cancer | 54 (5.1%) | 9 (1.3%) | 0 (0%) | 63 (3.4%) | |
| Malignancy | 52 (4.9%) | 24 (3.4%) | 44 (84.6%) | 120 (6.6%) | |
| Melanoma | 89 (8.4%) | 66 (9.2%) | 0 (0%) | 155 (8.5%) | |
| Non-Small Cell Lung Cancer | 329 (31%) | 105 (14.7%) | 2 (3.8%) | 436 (23.9%) | |
| Renal Cancer | 140 (13.2%) | 45 (6.3%) | 0 (0%) | 185 (10.1%) | |
| Uterine Cancer | 10 (0.9%) | 87 (12.2%) | 0 (0%) | 97 (5.3%) | |
| Case priority | Non-Expedited | 120 (11.3%) | 114 (15.9%) | 0 (0%) | 234 (12.8%) |
| Expedited | 934 (88.1%) | 595 (83.2%) | 52 (100%) | 1581 (86.5%) | |
| Direct | 6 (0.6%) | 6 (0.8%) | 0 (0%) | 12 (0.7%) | |
| Reporter type | Consumer | 66 (6.2%) | 42 (5.9%) | 2 (3.8%) | 110 (6%) |
| Healthcare Professional | 993 (93.7%) | 672 (94%) | 50 (96.2%) | 1715 (93.9%) | |
| Not Specified | 1 (0.1%) | 1 (0.1%) | 0 (0%) | 2 (0.1%) | |
| Age group | < median age | 494 (46.6%) | 415 (58%) | 20 (38.5%) | 929 (50.8%) |
| ≥median age | 566 (53.4%) | 300 (42%) | 32 (61.5%) | 898 (49.2%) | |
| Age | Mean ± Standard Deviation | 67.7 ± 10.6 | 64.0 ± 12.1 | 69.4 ± 9.3 | 66.3 ± 11.3 |
| Treatment duration (days) | Mean ± Standard Deviation | 67.8 ± 134.8 | 60.8 ± 133.7 | 163.4 ± 203.2 | 67.8 ± 137.7 |
Note: Other in Top 9 Regimen includes a variety of combination therapies that are less commonly used but still reported. Ingredient counts indicate the number of drugs in the treatment regimen (1 = single-drug, up to 9 = nine-drug combinations)
Signal mining of adverse events in deceased patients
We analyzed the common pembrolizumab-related adverse events in patients who died and evaluated the signal strength of the top 50 adverse events based on indicators such as the proportional reporting rate (PRR), relative risk ratio (RRR), and reporting odds ratio (ROR) (Table 2). These adverse events included immune-mediated hyperthyroidism (PRR = 621.98, RRR = 156.24, ROR = 622.32, n = 3), immune-mediated hypothyroidism (PRR = 552.87, RRR = 151.51, ROR = 556.10, n = 32), tumor pseudoprogression (PRR = 345.54, RRR = 130.20, ROR = 346.49, n = 15), and immune-mediated pneumonitis (PRR = 310.99, RRR = 124.99, ROR = 311.84, n = 15). Other adverse events with high frequency of reporting include immune-mediated renal disorder (PRR = 233.24, RRR = 110.29, ROR = 233.62, n = 9), immune-mediated hepatic disorder (PRR = 225.62, RRR = 108.56, ROR = 227.14, n = 37). Some adverse events have high signal strength but low number of reports. For example, immune-mediated pancreatitis and immune-mediated encephalitis have strong signals (ROR 88.90 and 168.85, respectively), but low number of reported events, suggesting that these events are relatively rare adverse reactions.
Table 2.
AEs signal detection in patients who treated with pembrolizumab
| Adverse event | PRR | RRR | ROR | Event counts |
|---|---|---|---|---|
| immune-mediated hyperthyroidism | 621.976 | 156.244 | 622.315 | 3 |
| immune-mediated hypothyroidism | 552.868 | 151.509 | 556.103 | 32 |
| tumour pseudoprogression | 345.542 | 130.203 | 346.486 | 15 |
| immune-mediated adrenal insufficiency | 345.542 | 130.203 | 346.171 | 10 |
| immune-mediated pneumonitis | 310.988 | 124.995 | 311.837 | 15 |
| immune-mediated thyroiditis | 248.79 | 113.632 | 249.061 | 6 |
| immune-mediated renal disorder | 233.241 | 110.29 | 233.622 | 9 |
| immune-mediated hepatic disorder | 225.619 | 108.564 | 227.143 | 37 |
| immune-mediated dermatitis | 190.048 | 99.634 | 190.428 | 11 |
| immune-mediated cholangitis | 186.593 | 98.68 | 186.898 | 9 |
| immune-mediated encephalitis | 168.452 | 93.387 | 168.849 | 13 |
| krebs lungen-6 increased | 165.86 | 92.589 | 165.98 | 4 |
| immune-mediated myocarditis | 160.087 | 90.77 | 161.874 | 61 |
| adrenocorticotropic hormone deficiency | 142.536 | 84.873 | 142.82 | 11 |
| immune-mediated nephritis | 138.217 | 83.33 | 138.367 | 6 |
| tumour hyperprogression | 130.942 | 80.642 | 131.227 | 12 |
| immune-mediated adverse reaction | 117.671 | 75.428 | 118.119 | 21 |
| hypothalamo-pituitary disorder | 103.663 | 69.442 | 103.775 | 6 |
| immune-mediated enterocolitis | 99.516 | 67.565 | 100.385 | 48 |
| immune-mediated myasthenia gravis | 98.726 | 67.202 | 98.905 | 10 |
| immune-mediated pancreatitis | 88.854 | 62.498 | 88.902 | 3 |
| vitiligo | 77.747 | 56.816 | 77.873 | 9 |
| limbic encephalitis | 77.747 | 56.816 | 77.831 | 6 |
| autoimmune myositis | 75.391 | 55.553 | 75.445 | 4 |
| immune-mediated lung disease | 74.424 | 55.029 | 74.801 | 28 |
| eastern cooperative oncology group performance status worsened | 72.214 | 53.817 | 72.619 | 31 |
| immune-mediated hepatitis | 70.735 | 52.995 | 71.105 | 29 |
| blood corticotrophin decreased | 69.108 | 52.081 | 69.171 | 5 |
| immune-mediated hypophysitis | 69.108 | 52.081 | 69.146 | 3 |
| paraneoplastic neurological syndrome | 69.108 | 52.081 | 69.146 | 3 |
| immune-mediated myositis | 57.476 | 45.218 | 57.766 | 28 |
| cortisol decreased | 56.543 | 44.641 | 56.574 | 3 |
| myasthenia gravis | 54.853 | 43.586 | 55.438 | 59 |
| autoimmune colitis | 54.559 | 43.401 | 54.608 | 5 |
| autoimmune myocarditis | 54.559 | 43.401 | 54.608 | 5 |
| fulminant type 1 diabetes mellitus | 54.085 | 43.102 | 54.143 | 6 |
| cholangitis sclerosing | 52.487 | 42.086 | 52.676 | 20 |
| pericarditis malignant | 48.782 | 39.681 | 48.817 | 4 |
| urogenital fistula | 47.844 | 39.061 | 47.87 | 3 |
| encephalitis autoimmune | 40.454 | 34.012 | 40.511 | 8 |
| thrombophlebitis migrans | 39.491 | 33.332 | 39.547 | 8 |
| malignant neoplasm progression | 24.405 | 21.94 | 39.038 | 2112 |
| tumour associated fever | 38.873 | 32.893 | 38.915 | 6 |
| iga nephropathy | 36.587 | 31.249 | 36.606 | 3 |
| hypopituitarism | 33.62 | 29.069 | 33.692 | 12 |
| myasthenia gravis crisis | 33.172 | 28.735 | 33.196 | 4 |
| myasthenic syndrome | 31.55 | 27.515 | 31.589 | 7 |
| trousseau’s syndrome | 31.099 | 27.173 | 31.132 | 6 |
| myositis | 29.885 | 26.246 | 30.14 | 48 |
| pericardial effusion malignant | 29.618 | 26.041 | 29.634 | 3 |
Abbreviations: PRR: Proportional Reporting Ratio; RRR: Relative Reporting Ratio; ROR: Reporting Odds Ratio
Analysis of treatment duration distributions in died patients
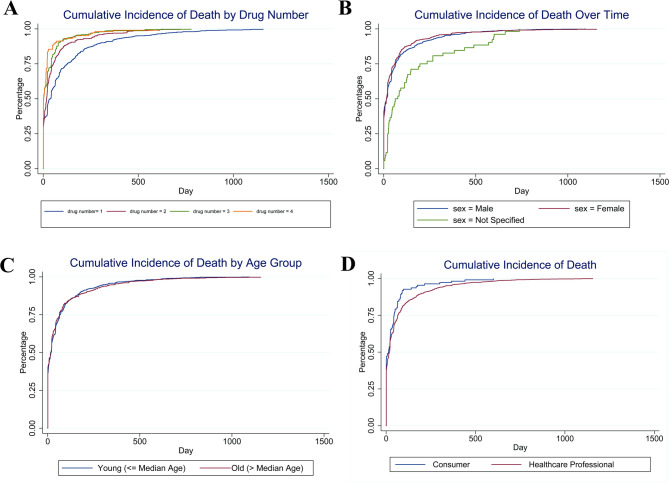
We divided the deceased patients into two groups according to the median duration time. The number of drug components (Fig. 4A), gender (Fig. 4B), age (Fig. 4C), and category of reporting personnel (Fig. 4D) were then analyzed. The results showed that various combination treatment regimens such as “carboplatin + pembrolizumab + pemetrexed” and “carboplatin + pembrolizumab + paclitaxel” showed a significant effect on the treatment duration. Compared with pembrolizumab monotherapy, these combination regimens were significantly associated with a shorter treatment duration (e.g., “carboplatin + pembrolizumab + paclitaxel” OR = 0.26, p < 0.01), suggesting that combination therapy may be associated with shorter treatment duration. As the number of drug components increased, the treatment time was also significantly reduced. Compared with a single component, a treatment regimen containing three components was significantly associated with a shorter duration time (OR = 0.40, p < 0.01). At the same time, patients who required expedited treatment showed a shorter pembrolizumab treatment time (OR = 0.53, p < 0.01), suggesting that expedited cases may be associated with earlier occurrence of adverse events (Table 3).
Fig. 4.
Treatment time of pembrolizumab in died patients. A Treatment time of pembrolizumab by drug number; B Treatment time of pembrolizumab by sex; C Treatment time of pembrolizumab by median age; D Treatment time of pembrolizumab by reporter
Table 3.
Analysis of factors related to the treatment time of pembrolizumab in died patients
| Factors | < median duration (N = 907) | ≥median duration (N = 920) | |
|---|---|---|---|
| Top 9 Regimen | Pembrolizumab | 241 (26.6%) | 404 (43.9%) |
| Other | 234 (25.8%) | 116 (12.6%) | |
| Lenvatinib; Pembrolizumab | 185 (20.4%) | 196 (21.3%) | |
| Carboplatin; Pembrolizumab; Pemetrexed | 66 (7.3%) | 71 (7.7%) | |
| Carboplatin; Pembrolizumab; Taxane | 57 (6.3%) | 25 (2.7%) | |
| Axitinib; Pembrolizumab | 14 (1.5%) | 19 (2.1%) | |
| Pembrolizumab; Pemetrexed | 7 (0.8%) | 21 (2.3%) | |
| Cisplatin; Pembrolizumab; Pemetrexed | 17 (1.9%) | 5 (0.5%) | |
| Pembrolizumab; Taxane | 86 (9.5%) | 63 (6.8%) | |
| Ingredient counts | 1 | 241 (26.6%) | 403 (43.8%) |
| 2 | 270 (29.8%) | 290 (31.5%) | |
| 3 | 230 (25.4%) | 154 (16.7%) | |
| 4 | 74 (8.2%) | 29 (3.2%) | |
| 5 | 42 (4.6%) | 21 (2.3%) | |
| Sex | Male | 537 (59.2%) | 523 (56.8%) |
| Female | 364 (40.1%) | 351 (38.2%) | |
| Case priority | Non-Expedited | 101 (11.1%) | 133 (14.5%) |
| Expedited | 804 (88.6%) | 777 (84.5%) | |
| Age group | < median age | 466 (51.4%) | 463 (50.3%) |
| ≥median age | 441 (48.6%) | 457 (49.7%) | |
| Cancer related diagnosis | Cervix Cancer | 327 (36.1%) | 265 (28.8%) |
| Cholangiocarcinoma | 15 (1.7%) | 18 (2%) | |
| Colorectal Cancer | 70 (7.7%) | 75 (8.2%) | |
| Endometrial Cancer | 33 (3.6%) | 30 (3.3%) | |
| Head And Neck Cancer | 37 (4.1%) | 83 (9%) | |
| Malignancy | 24 (2.6%) | 131 (14.2%) | |
| Melanoma | 254 (28%) | 182 (19.8%) | |
| Non-Small Cell Lung Cancer | 87 (9.6%) | 98 (10.7%) | |
| Renal Cancer | 60 (6.6%) | 37 (4%) | |
| Reporter type | Consumer | 57 (6.3%) | 53 (5.8%) |
| Healthcare Professional | 848 (93.5%) | 867 (94.2%) |
Note: Other in Top 9 Regimen includes a variety of combination therapies that are less commonly used but still reported. Ingredient counts represent the number of drugs in the regimen (1 = single-drug, up to 5 = five-drug combinations). The range of Ingredient counts differs from Table 1 as Table 3 focuses on treatment duration subgroups, where regimens with > 5 components were rare
Discussion
This study, through a retrospective analysis of pembrolizumab-related adverse events in the FAERS database, revealed the diversity and severity of adverse reactions caused by this drug in tumor treatment, especially in elderly patients and male patients, where the incidence of serious adverse reactions (such as death) was significantly higher. Consistent with previous literatures [1, 7, 12], as an immune checkpoint inhibitor, irAEs caused by pembrolizumab involve multiple organ systems, including the gastrointestinal tract, skin, and endocrine system, suggesting that it needs to be closely monitored in clinical applications.
This study found that male patients reported a higher incidence of specific serious adverse events than female patients, especially in the death outcome group. Differences in the reactivity and physiological status of male patients may explain this phenomenon [18]. In addition, elderly patients (≥ 65 years old) are more susceptible to serious adverse reactions, especially in the presence of other chronic diseases or immunosuppression [19]. This result is consistent with the findings of existing studies [18, 19] that elderly patients have a higher incidence of adverse reactions after treatment with ICIs.
The above differences may be linked to immunosenescence and sex-related immune differences [20–22]. Immunosenescence, the gradual deterioration of the immune system with aging, is characterized by reduced T-cell function, chronic low-grade inflammation, and impaired immune homeostasis [23]. These changes could lead to an exaggerated immune response or increased susceptibility to irAEs when exposed to immune checkpoint inhibitors like pembrolizumab [24]. Previous studies have suggested that older patients may have a diminished ability to regulate immune activation, making them more prone to severe toxicities [25]. Sex differences in immune response have also been well-documented. Male patients typically exhibit lower baseline immune activation compared to females, which may partially explain why immune-related toxicities differ between sexes [26]. Additionally, combination therapies involving pembrolizumab and other agents may further modulate immune adverse events. Chemotherapy, for example, has been shown to increase tumor antigen release, thereby enhancing immune activation but also potentially triggering systemic inflammation [27]. Similarly, some tyrosine kinase inhibitors (TKIs) or anti-angiogenic agents used in combination with pembrolizumab could alter the tumor immune microenvironment [28, 29]. These interactions may explain why certain treatment regimens are associated with an increased risk of severe adverse events. Therefore, tailoring immunotherapy based on age, sex, and drug combinations may ultimately help improve outcomes while minimizing toxicities.
In this study, the most common adverse reactions associated with pembrolizumab included diarrhea, and fatigue, which were mainly caused by overactivation of the immune system. These results are highly consistent with irAEs reported in previous literatures [8, 12]. In particular, the death events suggests that pembrolizumab may cause severe immune system reactions in patients with advanced cancer. Based on this, clinicians should be particularly pay attention to the immune response of elderly patients when using this drug, and strengthen monitoring and management. The study found that the United States and Japan are the countries with the most reports of adverse events of pembrolizumab, which may be related to the high frequency of use of pembrolizumab in these two countries and their acceptance of the adverse event reporting system. Most reports were submitted by medical professionals, reflecting those adverse reactions are more adequately monitored in clinical practice. However, the awareness of spontaneous reporting of adverse reactions by patients and the public still needs to be further improved to ensure more comprehensive monitoring.
Although the FAERS database provided a lot of data support for this study, the limitations of its spontaneous reporting system cannot be ignored. The voluntary and incomplete nature of the reports may lead to an underestimation of the incidence of adverse reactions. In addition, the lack of background information of the patients (such as concomitant medication, specific treatment regimen, etc.) limited the in-depth analysis of the effects of certain variables in this study. Therefore, future studies should combine more detailed patient data and further evaluate the safety of pembrolizumab through clinical trials.
This study summarizes a safety reference for the use of pembrolizumab in the treatment of cancer patients. During the diagnosis and treatment process, attention should be paid to the treatment response of elderly patients, and the monitoring of irAEs should be strengthened. At the same time, the geographical distribution differences of adverse reactions and the characteristics of reports mainly submitted by medical professionals suggest that the monitoring of adverse reactions of pembrolizumab in clinical practice is relatively sufficient, but the awareness of spontaneous reporting by patients and the public still needs to be further improved.
Acknowledgements
The authors would like to express their gratitude to the U.S. Food and Drug Administration for providing access to the Adverse Event Reporting System database, which was instrumental in conducting this study. We also acknowledge the contributions of all healthcare professionals and patients who have reported adverse events, enabling the collection of valuable data for research purposes.
Abbreviations
- FAERS
U.S. Food and Drug Administration’s Adverse Event Reporting System
- FDA
U.S. Food and Drug Administration
- ICIs
Immune Checkpoint Inhibitors
- irAEs
Immune-Related Adverse Events
- PD-1
Programmed Death Receptor 1
- KL-6
Krebs Lungen-6
- SAEs
Serious Adverse Events
- NSAEs
Non-Serious Adverse Events
- OR
Odds Ratio
- CI
Confidence Interval
- PRR
Proportional Reporting Ratio
- ROR
Reporting Odds Ratio
- RRR
Relative Reporting Ratio
- ADR
Adverse Drug Reaction
- PSD
Primary Suspect Drug
- SD
Standard Deviation
- IQR
Interquartile Range
- R
Statistical software version 4.4.1
- MedDRA
Medical Dictionary for Regulatory Activities
- PT
Preferred Terms
- SOC
System Organ Class
Author contributions
HX and DC contributed to the study concept and design, data analysis, and drafting of the manuscript. HX, YH, and HH was involved in data collection and interpretation. NZ and DC performed the statistical analysis. All authors participated in the critical revision of the manuscript for content. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data used in this study were obtained from publicly available database (https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis). The relevant data are available from the corresponding authors upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As this research was based on publicly available data, no ethical approval was required. Informed consent was not applicable as the study was retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huilin Xu, Email: xhlcdd@163.com.
Dedong Cao, Email: caodedong123@whu.edu.cn.
References
- 1.Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, Bennouna JJJCO. Safety and antitumor activity of the anti–programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. 2018, 36(1):61–7. [DOI] [PubMed]
- 2.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu W-J, Gangadhar TCJTL. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. 2014, 384(9948):1109–17. [DOI] [PubMed]
- 3.Tan S, Zhang CW. Gao GFJSt, therapy t: seeing is believing: anti-PD-1/PD-L1 monoclonal antibodies in action for checkpoint Blockade tumor immunotherapy. 2016, 1(1):1–4. [DOI] [PMC free article] [PubMed]
- 4.Patel SP, Othus M, Chen Y, Wright GP Jr, Yost KJ, Hyngstrom JR, Hu-Lieskovan S, Lao CD, Fecher LA. Truong T-GJNEJoM: Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. 2023, 388(9):813–23. [DOI] [PMC free article] [PubMed]
- 5.Chen R, Manochakian R, James L, Azzouqa A-G, Shi H, Zhang Y, Zhao Y, Zhou K. Lou YJJoh, oncology: emerging therapeutic agents for advanced non-small cell lung cancer. 2020, 13:1–23. [DOI] [PMC free article] [PubMed]
- 6.Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, Le Q-T, Lee NY, Leidner R, Lewis RL, et al. The society for immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara SJJCO. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. 2020, 38(3):193–202. [DOI] [PubMed]
- 8.Casagrande S, Sopetto GB, Bertalot G, Bortolotti R, Racanelli V, Caffo O, Giometto B, Berti A, Veccia AJC. Immune-Related adverse events due to Cancer immunotherapy: immune mechanisms and clinical manifestations. 2024, 16(7):1440. [DOI] [PMC free article] [PubMed]
- 9.Albarrán-Artahona V, Laguna J-C, Gorría T, Torres-Jiménez J, Pascal M, Mezquita LJD. Immune-related uncommon adverse events in patients with cancer treated with immunotherapy. 2022, 12(9):2091. [DOI] [PMC free article] [PubMed]
- 10.Guo M, Shu Y, Chen G, Li J, Li FJSR. A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. 2022, 12(1):20601. [DOI] [PMC free article] [PubMed]
- 11.Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, Shen C, Duma N, Aguilera JV. Chintakuntlawar AJJo: Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. 2019, 5(7):1008–19. [DOI] [PMC free article] [PubMed]
- 12.Eun Y, Kim IY, Sun J-M, Lee J, Cha H-S, Koh E-M, Kim H, Lee. JJSr: Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. 2019, 9(1):14039. [DOI] [PMC free article] [PubMed]
- 13.Baldini C, Romano PM, Voisin A-L, Danlos F-X, Champiat S, Laghouati S, Kfoury M, Vincent H, Postel-Vinay S, Varga AJEJC. Impact of aging on immune-related adverse events generated by anti–programmed death (ligand) PD-(L) 1 therapies. 2020, 129:71–9. [DOI] [PubMed]
- 14.Yawson AA, Abekah-Nkrumah G, Okai GA, Ofori CGJTADS. Awareness, knowledge, and attitude toward adverse drug reaction (ADR) reporting among healthcare professionals in Ghana. 2022, 13:20420986221116468. [DOI] [PMC free article] [PubMed]
- 15.Hoffman KB, Dimbil M, Erdman CB, Tatonetti NP. Overstreet BMJDs: the Weber effect and the united States food and drug administration’s adverse event reporting system (FAERS): analysis of sixty-two drugs approved from 2006 to 2010. 2014, 37:283–94. [DOI] [PMC free article] [PubMed]
- 16.Kumar AJHP. The newly available FAERS public dashboard: implications for health care professionals. In., vol. 54: SAGE Publications Sage CA: Los Angeles, CA; 2019: 75–77. [DOI] [PMC free article] [PubMed]
- 17.Cutroneo PM, Sartori D, Tuccori M, Crisafulli S, Battini V, Carnovale C, Rafaniello C, Capuano A, Poluzzi E, Moretti UJFDS et al. Conducting and interpreting disproportionality analyses derived from spontaneous reporting systems. 2024, 3:1323057.
- 18.Irelli A, Sirufo MM, D’Ugo C, Ginaldi L, De Martinis MJB. Sex and gender influences on cancer immunotherapy response. 2020, 8(7):232. [DOI] [PMC free article] [PubMed]
- 19.Nardone V, Giannicola R, Giannarelli D, Saladino RE, Azzarello D, Romeo C, Bianco G, Rizzo MR, Di Meo I, Nesci AJL. Distinctive role of the systemic inflammatory profile in non-small-cell lung cancer younger and elderly patients treated with a PD-1 immune checkpoint Blockade: A real-world retrospective multi-institutional analysis. 2021, 11(11):1235. [DOI] [PMC free article] [PubMed]
- 20.Calabro A, Accardi G, Aiello A, Caruso C, Candore G. Sex and gender affect immune aging. Front Aging. 2023;4:1272118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubbels Bupp MR, Potluri T, Fink AL, Klein SL. The confluence of sex hormones and aging on immunity. Front Immunol. 2018;9:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metcalf CJE, Roth O, Graham AL. Why leveraging sex differences in immune trade-offs May illuminate the evolution of senescence. Funct Ecol. 2020;34(1):129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Dong C, Han Y, Gu Z, Sun C. Immunosenescence, aging and successful aging. Front Immunol. 2022;13:942796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Q, Wu L, Han L, Zheng X, Tong R, Li L, Bai L, Bian Y. Immune-related adverse events of immune checkpoint inhibitors: a review. Front Immunol. 2023;14:1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Li C, Zhang W, Wang Y, Qian P, Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu W, Qian X, Wang S, Gao L, Xu J, Yan J. Sex - a potential factor affecting immune checkpoint inhibitor therapy for cancers. Front Immunol. 2022;13:1024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, Martinez-Perez A, Rodrigo JP, Garcia-Pedrero JM, Gonzalez S. Chemo-Immunotherapy: A new trend in Cancer treatment. Cancers (Basel) 2023, 15(11). [DOI] [PMC free article] [PubMed]
- 28.Ciciola P, Cascetta P, Bianco C, Formisano L, Bianco R. Combining immune checkpoint inhibitors with Anti-Angiogenic agents. J Clin Med 2020, 9(3). [DOI] [PMC free article] [PubMed]
- 29.Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron 2015, 2(1). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study were obtained from publicly available database (https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis). The relevant data are available from the corresponding authors upon reasonable request.