Abstract
The Japan Society of Gynecologic Oncology (JSGO) guideline for the treatment of uterine body neoplasm are revised from the 2018 guideline. This guideline aimed to provide standardized care for uterine body neoplasm, indicate appropriate current treatment methods for uterine body neoplasm, minimize variances in treatment methods among institutions, improve disease prognosis and treatment safety, reduce the economic and psychosomatic burden on patients by promoting the performance of appropriate treatment, and enhance mutual understanding between patients and healthcare professionals. The guidelines were prepared through the consensus of the JSGO guideline committee, based on a careful review of evidence from the literature searches and the medical health insurance system and actual clinical practice situations in Japan. The main features of the 2023 revision are as follows: 1) The Guidelines Formulation Committee members were asked to understand Minds' medical guideline development method in advance. 2) The clinical question (CQ) was changed to Patient, Intervention, Comparison, Outcome format as much as possible. 3) Introduced the "body of evidence," which summarizes the results of research reports collected for the CQs by outcome and study design, and the strength of evidence for each body of evidence was rated from levels A to D. 4) Introduction of systematic reviews in some CQs. 5) The strength of evidence, the balance of benefits and harms, value and hope for patients, and clinical applicability were considered while drafting recommendations. Herein, we present the English version of the JSGO guidelines 2023 for the treatment of uterine body neoplasm.
Keywords: Clinical Practice Guideline, Endometrial Cancer, Treatment, Gestational Trophoblastic Disease, Sarcoma
Synopsis
The Japanese guidelines for the treatment of uterine body neoplasm 2023 aim to provide a standard of care for endometrial cancer, improve disease prognosis and treatment safety, reduce the economic and physical burden on patients, and promote mutual understanding between patients and health care professionals.
INTRODUCTION
More than 17,000 women are diagnosed with endometrial cancer annually, and approximately 2,600 women in Japan die from the disease [1]. The first edition of the Guidelines for the Treatment of Uterine Body Neoplasm was published in 2006, with subsequent revisions in 2009 [2], 2013 [3], and 2018 [4], and the fifth edition was published in 2023. The method of preparation and description style have been revised, including other guidelines published by the Japanese Society of Gynecologic Oncology (JSGO). The general changes in the guideline include: transition from a textbook-style review to a Patient, Intervention, Comparison, Outcome-style clinical question (CQ) format; more uniform method of literature collection; inclusion of a diverse committee that includes the general public, patients, and professionals; evaluation of the total body of evidence, focusing on outcomes and the certainty of the evidence, and the adoption of recommendations that consider patient values and the social context in which guidelines are used.
The 2023 edition of the guidelines for treatment of uterine body neoplasm is prepared in accordance with the “Minds Medical Practice Guideline Formulation Manual 2020 ver. 3.0.” This manual was published by Medical Information Distribution Service (Minds), an Evidence-Based Medicine promotion project that aims to improve the quality of medical care by supporting decision-making by patients and medical professionals through the dissemination of high-quality medical guidelines.
Given that surgery is commonly chosen as the initial treatment for several histological types of uterine body neoplasms, including carcinosarcoma, and for stage III and part of stage IV, as well as the early stage, the CQs on initial treatment, which were divided into several chapters in the previous edition, are summarized in Chapter 2. CQs on unresectable, advanced cancer and recurrent cancers are now summarized in Chapter 4. The new version includes 9 chapters with the following contents and 8 algorithms.
Chapter 1: Overview of the guidelines
Chapter 2: Initial treatment for endometrial cancer
Chapter 3: Postoperative adjuvant treatment for endometrial cancer
Chapter 4: Treatment of unresectable advanced/recurrent cancer
Chapter 5: Post-treatment surveillance
Chapter 6: Fertility-sparing treatment
Chapter 7: Treatment for uterine sarcoma
Chapter 8: Treatment for trophoblastic disease
Chapter 9: Document collections, including literature search terms
A consensus for each recommendation was achieved through voting by the members of the committee and external evaluation board.
CHAPTER 1: OVERVIEW OF THE GUIDELINES
1. How to use the guidelines
This guideline provides a criterion for selecting an effective therapeutic approach for uterine body neoplasms in Japan and outlines the rationale supporting this approach based on current knowledge. However, it does not exclude other treatment methods that are not described in this document. The guidelines aim to:
1) Establish appropriate treatment methods for endometrial cancer, sarcoma, and trophoblastic disease
2) Reduce disparities in treatment approaches among institutions
3) Improve treatment safety and patient prognosis
4) Reduce physical, psychological, and economic burdens on patients by providing appropriate treatment
5) Foster mutual understanding between patients and health care professionals
2. Intended audience
These guidelines are intended for healthcare professionals, such as doctors, nurses, and pharmacists involved in the care of patients with endometrial cancer, sarcoma, and trophoblastic diseases. Furthermore, these guidelines aim to enhance the understanding of the general public, including patients and their families, regarding the treatment approaches for atypical endometrial hyperplasia (AEH), endometrial cancer, sarcoma, and trophoblastic disease.
3. Diseases addressed by this guideline
This guideline addresses AEH, endometrial carcinomas, carcinosarcomas and sarcomas, and trophoblastic diseases and their recurrences.
4. Notes on using this guideline
1) Each item comprises of a CQ, recommendation, purpose, and commentary. References for the content are listed at the end of each CQ, and search formulas for collecting literature are available on the JSGO website (https://jsgo.or.jp/guideline/index.html).
2) For the CQs where 75% consensus was not achieved, the discussion and rationale behind the final recommendation were presented as "Discussion points at voting."
3) Because of the potential challenges associated with the administration of certain antineoplastic drugs under the Japanese medical care insurance system, the guidelines emphasize adherence to the recommendations of the Committee on Clinical Practice Guidelines for the Use of Anticancer Agents of the Japan Society of Clinical Oncology (JSCO).
4) Given the limited treatment options available for uterine cancer, it is not easy to make a comparative evaluation of the medical and economic aspects. Therefore, the 2023 edition basically does not evaluate the economic burden on individual patients or the healthcare system.
5. Committee members
The Guidelines Formulation Committee and Evaluation Committee included radiologists, medical oncologists, pathologists, and palliative care physicians in addition to gynecologic oncologists. Additionally, the Formulation Committee included nurses, pharmacists, patients, and the general public, who participated as external committee members from the initial stage of CQs setting.
6. Literature retrieval
This revision requested the Japan Medical Library Association (JMLA) to prepare literature search terms for a systematic database searches. The specific literature retrieval method is as follows:
1) The Formulation Committee selected an article using keywords related to the CQ, and then the JMLA prepared relevant search terms and conducted a comprehensive literature search. If the initial search yielded a large number of articles, keywords were changed or added after review by the Formulation Committee and the JMLA. The Formulation Committee examined the retrieved articles and shortlisted 20 key articles.
2) Articles in PubMed, the Japan Medical Abstract Society, and the Cochrane Library were searched for articles published between January 2017 and December 2021. Articles published before 2016 that were cited in previous guideline editions and are needed for recommendations were used as references. Articles published after January 2022 were examined separately, and some were used as references.
7. Level of evidence and grade of recommendations
1) We referred to the Minds Medical Practice Guideline Formulation Manual 2020 ver. 3.0 to determine the evidence levels for the recommendation grades [5]. The outcomes were set for each CQ. The body of evidence was assessed by considering the study designs, bias risk, and indirectness of the studies selected for each outcome (Table S1).
2) Levels of evidence in these guidelines are determined based on the factors prescribing a body of evidence that differ from the previous “levels of evidence,” which were based on study design, such as “randomized phase III.” Level of evidence might be “B (moderate),” not “A (strong),” depending on the quality of the clinical trial although evidence arises from a randomized phase III trial.
3) The “strength of recommendation” (Table S2) was evaluated by the Guideline Formulation Committee by considering the level of evidence, the balance of benefits and harms, patient values and preferences, and reimbursement status for the relevant treatment method in Japan.
4) For CQ06, CQ11, and CQ33, the Guideline Formulation Committee members conducted a systematic review for each CQ outcome. For CQs where quantitative evaluation (meta-analysis) was not possible, qualitative systematic reviews were conducted to evaluate the logic and certainty of the context.
5) The recommendation text, strength of the recommendations, and level of evidence were discussed and finalized at the seventh meeting of the Subcommittee for the Revision of Guidelines for the Treatment of Uterine Body Neoplasms. The consensus rate on these decisions was determined by voting.
6) For recommendations that failed to achieve 75% consensus, the content of the recommendation was reviewed, the “strength of recommendation” and “level of evidence” were modified, or the content of the recommendation was modified and voted again.
7) Opinions and points of discussion for the re-voted recommendations or those with a lower consensus threshold are described in re-voted CQs as “Discussion points at voting.”
8) The recommendations in CQ02-04, CQ06-08, CQ13-17, CQ23-29, CQ32, and CQ33, and those that achieved initial consensus within the Formulation Committee, were subjected to a final vote by the external committee. Therefore, the consensus for these CQs is the sum of the votes by both core and external committee members.
8. Procedure for guideline creation
The Guidelines Formulation Committee and Evaluation Committee were independently established within the Committee for Treatment Guidelines for Cervical Cancer established by the Guidelines Committee of the JSGO to create these guidelines. The Chair of the Guidelines Committee is concurrently the chair of the Committee for Treatment Guidelines for Cervical Cancer and the chair of the Guidelines Formulation Committee. After 8 meetings of the Guidelines Formulation Committee, a consensus meeting, and a period of public comments, guidelines were revised between June 2021 and April 2023.
9. Tips for activation of use and disclosure of information
1) Algorithms were developed to enhance the practical utility to the audience.
2) These guidelines are published as a pamphlet and available on the JSGO, JSCO, and Minds websites to facilitate widespread use.
3) The results of systematic reviews and meta-analyses in CQ15 [6] and CQ29 [7] have been published in internationally cited journals.
10. Responsibility for treatment
The JSGO assumes responsibility for the content of the guidelines. However, the final decision on using the guideline recommendations for individual treatment is at the discretion of the user. Responsibility for treatment outcomes should be attributed to the person directly in charge of the treatment.
11. Monitoring and revision
1) A monitoring team has been established within the Guideline Development Committee.
2) The monitoring Team will continue to collect and accumulate newly reported evidence after the completion of these guidelines.
3) The monitoring Team will collect relevant information on cases of clinical inconvenience in the use of this guideline.
4) The next revision is scheduled for 2028.
5) If it is deemed necessary to change the strength or content of a recommendation or to establish a new CQ during the interim period, the monitoring team and guideline committee chair, and vice-chair will form a new drafting committee to prepare a revised draft or a new draft recommendation.
6) The revised or newly recommended draft will be published on the website of the JSGO after approval by the Board of Directors of the Society following a consensus meeting and review of public comments.
7) Some CQs will be measured as process indicators or actual status indicators, and the implementation rate will be analyzed to evaluate the guideline recommendations using the tumor registry database of the Japanese Society of Obstetrics and Gynecology and through joint research with the National Cancer Center.
12. Funding
The development of these guidelines was solely funded by the JSGO. No external funding or support was received.
13. Conflicts of interest
The Board of the Society Conflict of Interest Committee confirmed the status of conflicts of interest disclosures of Guidelines Formulation Committee and Evaluation Committee members and their affiliates for 2020 and 2021. A total of 26 and 48 members of the Guideline Formulation Committee, respectively, and 19 and 27 members of the Evaluation Committee, respectively, reported conflicts of interest in 2020 and 2021. No member was judged to have a conflicts of interest that exceeded the socially acceptable range.
14. Summary of recommendations
Each chapter comprises the CQs, recommendations, background, objectives, explanations, and references. This article summarizes the guidelines in a question-and-answer format. Recommendations from each chapter are listed in their respective chapter titles.
15. Algorithms
The guidelines contain the following 8 algorithms and one panel:
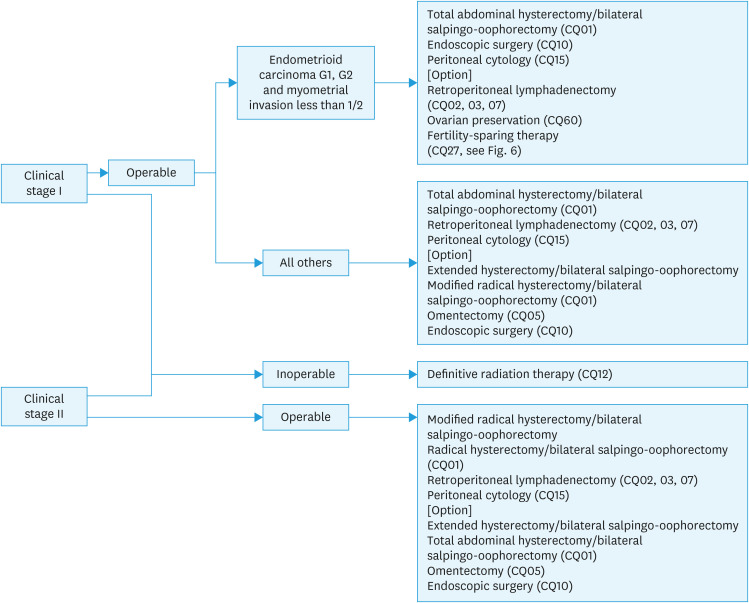
1) Initial treatment for endometrial cancer considered to be stage I or II preoperatively (Fig. 1).
2) Initial treatment for the patients diagnosed with endometrial cancer after hysterectomy and for cases with an intermediate or high risk of postoperative recurrence despite a presumed low risk preoperatively (Fig. 2).
3) Initial treatment for endometrial cancer considered to be stage III or IV preoperatively (Fig. 3).
4) Postoperative adjuvant treatment for endometrial cancer (Fig. 4).
5) Treatment of recurrent endometrial cancer (Fig. 5).
6) Fertility-sparing therapy for AEH and endometrioid carcinoma (corresponding to G1) (Fig. 6).
7) Treatment for uterine sarcoma (Fig. 7).
8) Treatment for choriocarcinoma (Fig. 8).
9) Classification of postoperative risk of recurrence of endometrial cancer (Fig. 9).
Fig. 1. Initial treatment for endometrial cancer considered to be stage I or II preoperatively.
CQ, clinical question.
Fig. 2. Initial treatment for the patients diagnosed with endometrial cancer after hysterectomy and for cases with an intermediate or high risk of postoperative recurrence despite a presumed low risk preoperatively.
CQ, clinical question.
*In the 2022 National Comprehensive Cancer Network guidelines, observation is possible for patients with a tumor of <2 cm, lymph-vascular space invasion negative, and a presumed low recurrence risk (CQ16).
Fig. 3. Initial treatment for endometrial cancer considered to be stage III or IV preoperatively.
CQ, clinical question.
*If the general condition is not worse, this refers to all patients in stage III and patients who can undergo hysterectomy and cytoreductive surgery in stage IV (CQ11).
Fig. 4. Postoperative adjuvant treatment for endometrial cancer.
CQ, clinical question.
Fig. 5. Treatment of recurrent endometrial cancer.
CQ, clinical question.
*Resection should also be considered for cases with a few small lung metastases (CQ20).
Fig. 6. Fertility-sparing therapy for AEH and endometrioid carcinoma (corresponding to G1).
AEH, atypical endometrial hyperplasia; CT, computed tomography; CQ, clinical question; MRI, magnetic resonance imaging.
Fig. 7. Treatment for uterine sarcoma.
CQ, clinical question; HGESS, high-grade endometrial stromal sarcoma; LGESS, low-grade endometrial stromal sarcoma; LMS, leiomyosarcoma; UUS, undifferentiated uterine sarcoma.
Fig. 8. Treatment for choriocarcinoma.
AcD, actinomycin-D; CQ, clinical question; FIGO, International Federation of Gynecology and Obstetrics; GTN, gestational trophoblastic neoplasia; hCG, human chorionic gonadotropin; MTX, methotrexate.
Fig. 9. Classification of postoperative risk of recurrence of endometrial cancer.
LVSI, lymph-vascular space invasion.
*Adnexa, uterine serosa, vagina, cardinal ligament, lymph node, bladder, rectum, intraperitoneal and distant metastasis.
CHAPTER 2: INITIAL TREATMENT FOR ENDOMETRIAL CANCER
CQ01: Is total hysterectomy recommended as initial surgical therapy?
Recommendations
-
1. Total hysterectomy (extrafascial) or extended simple hysterectomy is recommended for patients presumed to have no vaginal or parametrial involvement.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
-
2. Modified radical hysterectomy or radical hysterectomy for complete resection is suggested for patients with presumed vaginal or parametrial involvement.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
CQ02: Is omission of pelvic lymphadenectomy recommended for patients preoperatively estimated to be at low risk of recurrence?
Recommendation
-
1. The omission of pelvic lymphadenectomy is suggested in patients with endometrial carcinoma G1 or G2 that is presumed to be stage IA preoperatively.
Strength of recommendation: 2 (↑) level of evidence: C; consensus: 91%.
CQ03: Is pelvic and para-aortic lymphadenectomy recommended for patients who are preoperatively estimated to be at an intermediate or high-risk of recurrence?
Recommendations
-
1. Pelvic lymphadenectomy is recommended.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
-
2. In addition to pelvic lymphadenectomy, para-aortic lymphadenectomy down to the renal vein is suggested.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 82%.
Discussion points at voting: The consensus for recommendation 2 was 76%. Although there was a concern that para-aortic lymph node dissection could be easily omitted, there was also an opinion that the standard treatment group in the JCOG1412 trial was pelvic lymph node dissection, and the therapeutic significance of para-aortic lymph node dissection remains unclear.
CQ04: Is omission of pelvic lymph node dissection recommended when sentinel nodes are negative?
Recommendation
-
1. In medical institutes that are skilled in the technique of sentinel node biopsy and have the cooperation of pathologists for intraoperative diagnosis, the omission of pelvic lymph node dissection is suggested as a clinical trial.
Strength of recommendation; 2 (↑); level of evidence: B; consensus: 91%.
CQ05: Is omentectomy recommended for patients without gross evidence of omental metastases?
Recommendation
-
1. Omentectomy is suggested as a staging surgical technique if the patient is considered to have a special histological subtypes or endometrial carcinoma G3, or an extrauterine lesion is found intraoperatively.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 81%.
Discussion points at voting: Initially, the recommendation included the words "deep muscle invasion" and "positive peritoneal cytology," which received 38% consensus. There was no mention of "positive peritoneal cytology," and the recommendation did not match some of the explanatory content.
After correction, the consensus was 81%.
CQ06: Is ovarian preservation recommended for patients with preoperative stage I or II disease without gross ovarian metastases?
Systematic review
Recommendations
-
1. In principle, ovarian preservation is not recommended for initial treatment.
Strength of recommendation:1 (↓↓); level of evidence: A; consensus: 86%.
-
2. In young patients with endometrial carcinoma G1 and preoperatively estimated stage IA, if there is a strong desire for ovarian preservation, ovarian preservation is suggested after fully explaining the risks.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 95%.
Discussion points at voting: Recommendation 2 was initially “Stage I, G1, young patients with shallow myometrial invasion are suggested for ovarian preservation after fully explaining the risks associated with ovarian preservation. Strength of recommendation 2 (↑) evidence level C” with a consensus rate of 71%. The committee members asked about the principles of salpingo-oophorectomy and whether to propose ovarian preservation even for patients who did not wish to undergo ovarian preservation. The consensus was 95%.
CQ07: Is omission of pelvic lymphadenectomy recommended for surgery on elderly patients?
Recommendation
-
1. In elderly patients, perform an elderly functional assessment first. The omission of lymph node dissection is not suggested in operable patients who are presumed to be at intermediate or high risk of recurrence.
Strength of recommendation: 2 (↓); level of evidence: C; consensus: 91%.
CQ08: Is it recommended to wait for weight loss before surgery in a severely obese patient?
Recommendations
-
1. In patients with advanced-stage disease, special histologies, or endometrial carcinoma G3, wait for surgery for weight loss is not recommended.
Strength of recommendation: 1 (↓↓); level of evidence: C; consensus: 100%.
-
2. In patients with endometrial carcinoma G1 or equivalent and preoperative stage IA, wait for surgery for weight loss is suggested under the principle that the procedure should be performed at a facility with multidisciplinary cooperation.
Strength of recommendation: 2 (↑); level of evidence: D; consensus: 91%.
CQ09: Are magnetic resonance imaging (MRI), computed tomography (CT), or positron emission tomography/CT (PET/CT) recommended for treatment-decision making?
Recommendations
-
1. MRI is recommended to evaluate local extensions such as myometrial invasion and cervical stromal invasion.
Strength of recommendation: 1 (↑↑); level of evidence: A; consensus: 100%.
-
2. Evaluation of lymph node metastasis and distant metastasis by CT and MRI is recommended.
Strength of recommendation: 1 (↑↑); level of evidence: A; consensus: 100%.
-
3. Evaluation by PET/CT is suggested when it is difficult to evaluate lymph node and distant metastasis by CT or MRI.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 94%.
CQ10: Is endoscopic (laparoscopic/robotic) surgery recommended for initial treatment?
Recommendations
-
1. Endoscopic surgery is recommended for patients presumed to be in stage I.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 94%.
-
2. Endoscopic surgery is suggested for patients presumed to be in stage II.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 88%.
-
3. Endoscopic surgery is not suggested in patients with advanced-stage disease.
Strength of recommendation: 2 (↓); level of evidence: C; consensus: 88%.
CQ11: Is surgical treatment recommended for patients considered preoperatively stage IV?
Recommendation
-
1. Surgical intervention is suggested if total hysterectomy plus maximal tumor reduction is feasible.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 88%.
CQ12: Is definitive radiation therapy recommended as the initial therapy for patients with resectable tumors, but surgical resection is not indicated owing to medical reasons?
Recommendation
-
1. Definitive radiation therapy is suggested.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 94%.
CHAPTER 3: POSTOPERATIVE ADJUVANT TREATMENT FOR ENDOMETRIAL CANCER
CQ13: Is postoperative chemotherapy recommended for patients who have undergone complete surgical resection during initial surgery?
Recommendations
-
1. Chemotherapy with adriamycin (doxorubicin hydrochloride) and cisplatin (AP regimen) is recommended for high-risk patients.
Strength of recommendation: 1 (↑↑); level of evidence: A; consensus: 86%.
-
2. Chemotherapy with paclitaxel and carboplatin (TC regimen) is suggested for high-risk patients.
Strength of recommendation: 2 (↑); level of evidence: B; consensus: 82%.
-
3. Regimens for high-risk patients are also suggested for intermediate-risk patients
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 95%.
-
4. Postoperative chemotherapy is not recommended for patients at low risk of recurrence.
Strength of recommendation: 1 (↓↓); level of evidence: C; consensus: 95%.
-
5. For postoperative chemotherapy for carcinosarcoma, a 2-agent combination including ifosfamide, platinum, and paclitaxel is suggested.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 91%.
-
6. Progesterone therapy is not recommended as a postoperative adjuvant therapy.
Strength of recommendation: 1 (↓↓); level of evidence: B; consensus: 95%.
Discussion points at voting: Although the consensus rate for both recommendations was more than 75%, the AP regimen is used in only 10% of clinical settings, whereas the TC regimen is more commonly adopted. This disparity prompted discussion on whether the recommendations for AP and TC should be the same.
CQ14: Is postoperative radiation therapy recommended for patients who have undergone complete surgical resection during initial surgery?
Recommendation
-
1. Postoperative radiation therapy is suggested as an option to reduce pelvic recurrence.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 95%.
CQ15: Is postoperative adjuvant therapy recommended in patients with a low risk of recurrence and positive peritoneal cytology?
Systematic review
Recommendation
-
1. Adjuvant therapy is not suggested.
Strength of recommendation: 2 (↓); level of evidence: C; consensus: 86%.
Discussion points at voting: Although the consensus among the core members of the Formulation Committee was 94%, the consensus among the external committee members was 60%, making this the only CQ with a notable discrepancy between the Formulation Committee members and the general public. This disparity may reflect patient anxiety about not receiving adjuvant chemotherapy. Although a recommendation was made after a systematic review, evidence supporting the recommendation was deemed insufficient, resulting in “level of evidence C.”
CQ16: Is additional treatment recommended for patients diagnosed with endometrial cancer after hysterectomy?
Recommendations
-
1. Additional treatment, including staging surgery is recommended if the patient is suspected to recurrent intermediate- or high-risk cases.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 91%.
-
2. If the patient can be presumed to be in the low-risk cases for recurrence, additional treatment is not recommended and careful follow-up is suggested.
Strength of recommendation: 2 (↓); level of evidence: C; consensus: 100%.
CQ17: Is additional treatment recommended for patients initially presumed to have low-risk for recurrence but later found to have intermediate- or high-risk for recurrence after surgery?
Recommendations
-
1. Additional treatment is recommended after metastasis examination by imaging.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 86%.
-
2. Staging surgery is suggested to determine the exact surgical stage.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 95%.
CHAPTER 4: TREATMENT OF UNRESECTABLE ADVANCED/RECURRENT CANCER
CQ18: Is preoperative treatment recommended for advanced cancer that is expected to be difficult to resect or have residual disease?
Recommendation
-
1. Preoperative chemotherapy for patients with advanced-stage disease with invasion to the surrounding organs presenting challenges in surgical resection or those with distant metastases and the possibility of residual disease is suggested.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 94%.
CQ19: Is radiation therapy recommended for vaginal stump recurrence?
Recommendations
-
1. Radiotherapy is recommended.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
-
2. Surgery is suggested instead of radiotherapy.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 88%.
Discussion points at voting: The consensus for recommendation 2 was 88%, but there was a suggestion that the option of drug therapy (including hormonal therapy) should be added for vaginal stump recurrence.
CQ20: Is surgical treatment recommended in patients with recurrent sites other than the vaginal stump?
Recommendation
-
1. Surgical treatment is suggested if complete resection of the recurrent site is feasible.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
CQ21: Is chemotherapy recommended for advanced or recurrent cancers that are unresectable or have residual disease?
Recommendations
-
1. TC or AP regimen is recommended for advanced disease.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 94%.
2. For recurrent disease
-
A. TC regimen is recommended for patients who have not received prior platinum-containing chemotherapy.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
-
B. Lenvatinib plus pembrolizumab is recommended for patients who have received prior platinum-containing chemotherapy.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 81%.
-
C. Monotherapy with pembrolizumab suggested for patients with a history of platinum-containing chemotherapy and microsatellite instability (MSI)-high, deficient mismatch repair, or tumor mutational burden-high.
Strength of recommendation: 2 (↑); level of evidence: B; consensus: 88%.
-
3. Regimens, including ifosfamide, platinum, and paclitaxel is suggested for chemotherapy in patients with advanced or recurrent carcinosarcoma.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
-
4. Progestin therapy is suggested for patients with endometrioid carcinoma G1 or estrogen receptor/progesterone receptor positive.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 94%.
Discussion points at voting: The consensus rate was 35% on “strength of recommendation 2 (↑)” for recommendation 2B. Many committee members recommended “strength of recommendation 1 (↑↑)” for the lenvatinib plus pembrolizumab regimen considering the availability of evidence, increased clinical use, and guideline endorsements from the United States and Europe. A consensus rate of 81% was reached for “strength of recommendation 1 (↑↑)” after re-voting, though some suggested adjusting the recommendation based on MSI test results. Initially, recommendation 2-dimensional (2D) suggested AP regimen, TC regimen, monotherapy, and best supportive care for patients with a history of platinum therapy and for those where LP therapy is not appropriate. (strength of recommendation 2 (↑), level of evidence C). Recommendation 2D was deleted because it only describes obvious cases, and the definition of cases in which the LP regimen is not appropriate is ambiguous. However, some have expressed concerns that the significance of platinum-free therapy in endometrial cancer is inconclusive and that the removal of platinum rechallenge from the recommendation may cause confusion in the field.
CQ22: Is radiation therapy recommended for patients with advanced or recurrent cancers that are unresectable or have residual disease?
Recommendation
-
1. Radiotherapy is suggested for advanced or recurrent cancer with unresectable or residual disease for the purpose of local control or palliation.
Strength of recommendation: 2(↑); level of evidence: C; consensus: 100%.
CQ23: Is oncogene panel testing using next-generation sequencing, etc. recommended for patients with recurrent cancer?
Recommendation
-
1. A cancer gene panel test is suggested for patients with recurrent cancer who have completed or are about to complete standard treatment.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 95%.
CHAPTER 5: POST-TREATMENT SURVEILLANCE
CQ24: Is periodic surveillance recommended after definitive treatment?
Recommendations
-
1. The suggested intervals of surveillance are every 3–6 months for 1–3 years, and every 6–12 months for 4–5 years.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 86%.
-
2. Careful interviews to confirm the symptoms and pelvic examinations are recommended to diagnose pelvic recurrence.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 91%.
-
3. Vaginal stump cytology, serum tumor markers, ultrasonography, and imaging such as chest radiography and CT are suggested as appropriate, considering the risk of recurrence in each case.
Strength of recommendation: 2(↑); level of evidence C; consensus: 100%.
-
4. CT, MRI, and PET/CT evaluations are recommended to search for the lesion when recurrence is suspected.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
CQ25: Is hormone replacement therapy (HRT) recommended after treatment for endometrial cancer?
Recommendation
-
1. HRT is suggested after fully explaining the benefits and risks.
Strength of recommendation: 2 (↑); level of evidence: B; consensus: 95%.
CQ26: What factors should be considered when providing lifestyle guidance after endometrial cancer treatment?
Recommendations
-
1. Risk assessment of lifestyle-related diseases is recommended.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 95%.
-
2. Lifestyle modification guidance for patients with lifestyle-related diseases is suggested.
Strength of recommendation 2 (↑), level of evidence: B; consensus 95%.
-
3. Lower gastrointestinal endoscopic surveillance is recommended for patients with endometrial cancer diagnosed with Lynch syndrome.
Strength of recommendation: 1 (↑↑); level of evidence; B; consensus: 100%.
-
4. Genetic counseling is suggested for blood relatives of patients with endometrial cancer diagnosed with Lynch syndrome.
Strength of recommendation: 2 (↑); level of evidence: B; consensus: 100%.
CHAPTER 6: FERTILITY-SPARING THERAPY
CQ27: Is fertility-sparing therapy recommended for young patients with AEH or endometrial cancer?
Recommendation
-
1. Progesterone therapy is suggested for patients with AEH or endometrioid adenocarcinoma G1 limited to the endometrium.
Strength of recommendation: 2 (↑↑); level of evidence: C; consensus: 86%.
Discussion points at voting: The consensus rate was 71% for the initial recommendation "progesterone therapy is recommended for patients with AEH or G1 confined to the endometrium, strength of recommendation 1 (↑↑), level of evidence C". The committee members expressed concerns regarding the strength and suggested incorporating the need for confirmatory full curettage. The committee re-voted to include full curettage in the recommendation and lower the strength of recommendation to 2 (↑), resulting in a consensus rate of 86%.
CQ28: What are suitable follow-up periods and examinations after fertility preservation therapy?
Recommendation
-
Endometrial histology and transvaginal ultrasonography are suggested every 3–6 months.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 95%.
CQ29: Is conservative treatment recommended for patients with residual lesions or intrauterine recurrence after fertility preservation therapy?
Systematic review
Recommendations
-
1. Conservative treatment is not recommended for patients with residual lesions.
Strength of recommendation: 1 (↓↓); level of evidence: B; consensus: 86%.
-
2. Repeat progesterone therapy is suggested under close supervision in patients with intrauterine recurrence and a strong desire for fertility preservation.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
Discussion points at voting: Recommendation 1 initially stated that conservative therapy is not recommended (strength of recommendation 1 [↓↓] evidence level B), with a consensus rate of 50%. Committee members noted inconsistencies between the recommendation and the commentary, which included positive statements for conservative therapy. Additionally, the recommendation is too strong, and differentiating between lesion remnants and intrauterine recurrence was necessary. Based on these discussions, the CQ was revised to "Is conservative treatment recommended for patients with residual lesions or intrauterine recurrence after fertility preservation therapy?" and the consensus rate was 86%.
CQ30: Is assisted reproductive technology (ART) recommended for patients after fertility preservation therapy?
Recommendation
-
1. ART is suggested for conception.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
CHAPTER 7: TREATMENT OF UTERINE SARCOMA
CQ31: What surgical techniques are recommended for uterine sarcoma?
Recommendations
-
1. Total abdominal hysterectomy and bilateral salpingo-oophorectomy are recommended.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 88%.
-
2. Tumor reduction surgery with the aim of complete extraction is suggested.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
-
3. Lymph node dissection is not suggested for leiomyosarcoma and low-grade endometrial stromal sarcoma (ESS) without findings of lymph node metastasis.
Strength of recommendation: 2 (↓); level of evidence: B; consensus: 75%.
CQ32: Is additional surgery recommended for patients diagnosed with uterine sarcoma postoperatively?
Recommendation
-
1. Additional surgeries to complete the total hysterectomy and bilateral salpingo-oophorectomy are suggested.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
Discussion points at voting: Initially, recommendation 2 stated that “ovarian preservation for premenopausal early-stage leiomyosarcoma” (strength of recommendation 2 [↑], level of evidence C). However, there were concerns regarding whether “premenopausal” is appropriate and whether ovarian preservation should be considered for patients after enucleation, a procedure typically performed for benign disease. The deletion of recommendation 2 was initially supported by 69% consensus, with some discussion on modifying its wording. Ultimately, the committee reached a 100% consensus to delete the recommendation.
CQ33: What postoperative adjuvant therapy is recommended for patients with uterine sarcomas who have undergone complete surgical removal during the initial surgery?
Recommendations
-
1. Postoperative adjuvant therapy is not suggested for stage I leiomyosarcoma.
Strength of recommendation: 2 (↓); level of evidence: B; consensus: 95%.
-
2. Chemotherapy is suggested for postoperative adjuvant treatment of leiomyosarcoma, undifferentiated uterine sarcoma, and high-grade ESS.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 86%.
-
3. Hormone therapy is suggested when postoperative adjuvant therapy is indicated for low-grade ESS.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 95%.
CQ34: Is chemotherapy recommended for unresectable advanced or recurrent cases of uterine sarcoma?
Recommendations
-
1. Chemotherapy is suggested based on the systemic condition of patients.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 94%.
-
2. Doxorubicin monotherapy is recommended as chemotherapy.
Strength of recommendation: 1 (↑↑); level of evidence: A; consensus: 94%.
-
3. Pazopanib, trabectedin, and eribulin are suggested in the second-line and subsequent treatments.
Strength of recommendation: 2 (↑); level of evidence: B; consensus: 94%.
-
4. Hormone therapy is suggested for low-grade ESS.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
CHAPTER 8: TREATMENT OF TROPHOBLASTIC DISEASE
CQ35: What chemotherapy is recommended for an invasive mole, clinically invasive mole, or post-molar persistent human chorionic gonadotropin (hCG)?
Recommendation
-
1. Monotherapy with methotrexate or actinomycin D is recommended.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
CQ36: What chemotherapy is recommended for choriocarcinoma?
Recommendation
-
1. Multidrug regimen, including methotrexate, actinomycin D, and etoposide is recommended.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
CQ37: Is treatment other than chemotherapy recommended for choriocarcinoma?
Recommendations
-
1. Surgical treatment for uterine lesions refractory to chemotherapy or metastatic lesions is suggested with careful consideration of the indications.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 81%.
-
2. Surgical treatment is suggested for uterine lesions with difficulty in bleeding control or brain metastases with urgent bleeding and symptoms of cerebral hypertension.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
-
3. Radiotherapy is suggested for brain metastases after careful consideration of the indications.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 81%.
CQ38: What treatments are recommended for patients with placental site trophoblastic tumor or epithelioid trophoblastic tumor?
Recommendations
-
1. Total hysterectomy is suggested for patients with lesions confined to the uterus.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 100%.
-
2. Combination of surgical treatment including total hysterectomy and chemotherapy including platinum-based agents are suggested for patients with extrauterine lesions.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 94%.
CQ39: What is the recommended treatment for patients with persistently low-positive hCG?
Recommendations
-
1. Detection of the lesion and confirmation of real hCG are recommended.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
-
2. Treatment as a trophoblastic tumor is recommended if the lesion is confirmed or if the hCG tends to increase.
Strength of recommendation: 1 (↑↑); level of evidence: B; consensus: 100%.
-
3. If low levels of real hCG do not disappear after follow-up without lesions, chemotherapy and evaluation of efficacy are suggested.
Strength of recommendation: 2 (↑); level of evidence: C; consensus: 86%.
Discussion points at voting: Initially, Recommendation 2: "If the lesion is confirmed or if the hCG tends to increase, treatment is recommended based on the clinical diagnosis as a trophoblastic disease. Strength of recommendation 1 (↑↑), level of evidence B," Recommendation 3: "If low levels of real hCG do not disappear, it is suggested that chemotherapy be given to determine whether it is effective. Strength of recommendation 2 (↑), level of evidence C." The agreement rates were 69% and 19%, respectively. The consensus for recommendation 2 was 100% as a result of discussion with some modifications. Regarding recommendation 3, there were opinions that chemotherapy for all patients with persistent low-levels of hCG might be overtreatment and that easy follow-up should be avoided. After discussion, the recommendation was changed to the current recommendation, and the consensus rate was 86%.
ACKNOWLEDGEMENTS
The authors thank the Japan Society of Obstetrics and Gynecology, Japan Association of Obstetricians and Gynecologists, Japan Society of Gynecologic and Obstetric Endoscopy and Minimally Invasive Therapy, Japanese Gynecologic Oncology Group, Japanese Society for Radiation Oncology, and Japanese Society of Pathology for their comments and contributions throughout the project. Furthermore, the authors acknowledge the clerical and technical assistance provided by Ms. Toshie Yasuda of the JSGO and Ms. Yuriko Adachi of Kanehara Co., Ltd. The authors would like to thank Editage for language editing.
Participating contributors list: Members of the Guidelines Formulation Committee (alphabetical order)
Kazunari Fujino, Satoe Fujiwara, Keiko Furusawa, Masayuki Futagami, Kenichi Harano, Yuji Ikeda, Kazuhiko Ino, Haruko Iwase, Hiroaki Kajiyama, Yosuke Konno, Kenzo Kudo, Sakura Kudo, Shiho Kuji, Hiroko Machida, Kouji Matsumoto, Junji Matsuoka, Takashi Mitamura, Mayuyo Mori, Taisuke Mori, Toshinari Muramatsu, Isao Murakami, Akitoshi Nakashima, Satoshi Nagamata, Takashi Nagai, Takayuki Nagasawa, Keiichiro Nakamura, Tadaaki Nishikawa, Hidetaka Nomura, Hiroyuki Nomura, Kaoru Niimi, Yoshitake Oishi, Takashi Ota, Shinya Sato, Manabu Seino, Shogo Shigeta, Sirou Suzuki, Munetaka Takekuma, Masataka Takenaka, Yasuhisa Terao, Yoshito Terai, Takafumi Toita, Hideki Tokunaga, Naotake Tsuda, Kiyomi Toyma, Hirokazu Usui, Miho Watanabe, Hidemichi Watari, Hideaki Yahata, Ken Yamaguchi, Wataru Yamagami, Eiko Yamamoto, Hiroshi Yoshida.
Members of the Guidelines Evaluation Committee (alphabetical order)
Daisuke Aoki, Kaoru Abiko, Mitsuya Ishikawa, Yutaka Ueda, Kimio Ushijima, Kouta Umemura, Yasuhiko Ebina, Katsutoshi Oda, Masanori Ono, Takashi Onda, Tetsuko Kato, Yuuko Kaneyasu, Hiroaki Kobayashi, Yuuko Sasajima, Mikiko Sato, Ayumi Shikama, Nobuyuki Susumu, Kiyoshi Takamatsu, Kazuhiro Takehara, Hiroshi Tanabe, Michihiro Tanigawa, Fumio Nagashima, Kaei Nasu, Hirotaka Nishi, Yoshiko Nishigaya, Kiyoshi Hasegawa, Kosei Hasegawa, Junzo Hamanishi, Akira Hirasawa, Takuma Fujii, Hiroyuki Fujiwara, Hisashi Masuyama, Takeshi Motohara, Hidekazu Yamada, Mayu Yunokawa, Yoshio Yoshida, Tomoko Morita, Miwa Seshita.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: N.S., B.T., T.H., A.M., T.K., T.T., H.K., H.Y., Y.M., O.A., M.M., K.Y.
- Data curation: M.T., N.S., B.T., T.H., W.H., T.M., T.Y.1, T.Y.2, K.H.
- Formal analysis: M.T., W.H., T.M., T.Y.1, T.Y.2, K.H., A.M., T.K., H.K., H.Y., Y.M., K.Y.
- Funding acquisition: T.T., K.Y.
- Investigation: M.T., W.H., T.M., T.Y.1, T.Y.2, K.H., O.A., M.M., K.Y.
- Supervision: N.S., B.T., T.H., O.A., M.M., K.Y.
- Validation: M.T., N.S., B.T., A.M., T.K., T.T., H.K., H.Y., Y.M., O.A., M.M., K.Y.
- Writing - original draft: M.T., K.Y.
- Writing - review & editing: M.T., N.S., B.T., T.H., W.H., T.M., T.Y.1, T.Y.2, K.H., A.M., T.K., T.T., H.K., H.Y., Y.M., O.A., M.M., K.Y.
T.Y.1, Yasuhisa Terao; T.Y.2, Yoshito Terai.
SUPPLEMENTARY MATERIALS
Certainty ratings for outcomes to determine grades of recommendation
Strength of recommendation
References
- 1.Cancer Information Service, National Cancer Center. Uterine body [Internet] Tokyo: Cancer Information Service, National Cancer Center; 2024. [cited 2024 Jul 1]. Available from: https://ganjoho.jp/reg_stat/statistics/stat/cancer/18_corpus_uteri.html#anchor1. [Google Scholar]
- 2.Nagase S, Katabuchi H, Hiura M, Sakuragi N, Aoki Y, Kigawa J, et al. Evidence-based guidelines for treatment of uterine body neoplasm in Japan: Japan Society of Gynecologic Oncology (JSGO) 2009 edition. Int J Clin Oncol. 2010;15:531–542. doi: 10.1007/s10147-010-0138-6. [DOI] [PubMed] [Google Scholar]
- 3.Ebina Y, Katabuchi H, Mikami M, Nagase S, Yaegashi N, Udagawa Y, et al. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int J Clin Oncol. 2016;21:419–434. doi: 10.1007/s10147-016-0981-1. [DOI] [PubMed] [Google Scholar]
- 4.Yamagami W, Mikami M, Nagase S, Tabata T, Kobayashi Y, Kaneuchi M, et al. Japan Society of Gynecologic Oncology 2018 guidelines for treatment of uterine body neoplasms. J Gynecol Oncol. 2020;31:e18. doi: 10.3802/jgo.2020.31.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minds Manual Developing Committee. Minds medical practice guideline formulation manual 2020 ver. 3.0 [Internet] Tokyo: Council for Quality Health Care; 2021. [cited 2021 Dec 20]. Available from: https://minds.jcqhc.or.jp/docs/methods/cpg-development/minds-manual/pdf/all_manual_.pdf. [Google Scholar]
- 6.Shigeta S, Konno Y, Terai Y, Morisada T, Tokunaga H, Baba T, et al. A qualitative systematic review of the significance of adjuvant therapy in patients with low-risk endometrial cancer presenting positive peritoneal cytology: a relevant study to the guideline update for endometrial cancer by the Japan society of gynecologic oncology guideline committee. Jpn J Clin Oncol. 2024;54:217–220. doi: 10.1093/jjco/hyad140. [DOI] [PubMed] [Google Scholar]
- 7.Murakami I, Machida H, Morisada T, Terao Y, Tabata T, Mikami M, et al. Effects of a fertility-sparing re-treatment for recurrent atypical endometrial hyperplasia and endometrial cancer: a systematic literature review. J Gynecol Oncol. 2023;34:e49. doi: 10.3802/jgo.2023.34.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Certainty ratings for outcomes to determine grades of recommendation
Strength of recommendation