Abstract
Background
Pregnant women have historically been excluded from participation in medication trials, in part due to the perceived risks of drug exposure to mothers and fetuses. However, little is known about pregnant women's attitudes toward risk and participation in such trials.
Aims
To address this knowledge gap and to identify factors that influence trial participation.
Materials and methods
Australian women over the age of 18, currently pregnant or within six months of delivery, were recruited to participate in an online survey (n = 623) and follow‐up interviews (n = 11). The survey investigated willingness to participate in five hypothetical drug trial scenarios of varying risk. Demographic and obstetric information, including COVID‐19 vaccination status, was also collected. The impact of these factors on trial participation was analysed using ordinal regression. Interviews were subjected to thematic framework analysis using a priori and emergent themes.
Results
Nearly half of the respondents (48%) indicated a willingness to participate in at least one of the hypothetical trials. As trial risk increased participation likelihood decreased, especially if the risk was to the fetus, regardless of benefits to the mother. COVID‐19 vaccination status and medication hesitancy were predictors of an unwillingness to participate. Three broad themes emerged from the qualitative data: risk–benefit analysis, quality of evidence, and trust.
Conclusions
Overall, participants expressed a positive attitude toward research and medication trials during pregnancy, but were concerned about fetal risk. The findings of this study may help enhance trial design and the participation of pregnant women in medication trials.
Keywords: clinical trial, fetus, mothers, pregnancy, trust
BACKGROUND
Clinical trials form a key part of the pharmaceutical regulatory approval process and are designed to provide confidence to clinicians and patients. 1 Despite substantial anatomical and physiological changes during pregnancy that affect drug pharmacokinetics, 2 pregnant women have historically been excluded from participating in clinical trials. 3
Over 90% of clinically approved drugs administered in pregnancy lack pregnancy‐specific information on safety, efficacy and pharmacokinetics. 4 In 2017, Scaffidi et al 4 reported that a mere 0.32% of global drug trials involved testing in pregnancy. Consequently, ‘off labelling’ – the process of prescribing a drug for a disease or medical condition that it is not approved to treat – is a common practice in pregnancy care. 5 This can lead to inadequate dosing, poor efficacy, or treatments with ill‐defined maternal or fetal risks. 6 , 7 , 8
There is a growing consensus among researchers and other stakeholders that it is unethical to systematically exclude pregnant women from clinical trials. 9 , 10 , 11 As Baylis 8 stated, ‘Pregnant women get sick, and sick women get pregnant.’ In fact, 40–80% of pregnant women report using at least one prescription medication, 12 while 90% report using non‐prescription medications such as over‐the‐counter pain killers and vitamin supplements. 12 , 13
In the context of research ethics and governance, pregnant women are often viewed as a ‘vulnerable’ group, defined in the Declaration of Helsinki as a population that ‘includes those who cannot give or refuse consent for themselves and those who may be vulnerable to coercion or undue influence.’ 14 , 15 Instead, pregnant women would be better classified as scientifically complex. 14 , 16 , 17 It is a common assumption that pregnant women are reluctant to participate in clinical trials; however, this is supported by limited evidence. 18 , 19 , 20 , 21 Several studies found that trial participation was conditional on risk 18 , 21 , 22 , 23 and, unsurprisingly, the potential risk of harm to the fetus. 20 , 22 , 24 , 25 , 26 In contrast, trial participation is facilitated by the rapport and communication skills of the trial recruiter and a recommendation from a medical professional. 20 , 21 , 24 , 26
The aim of this study was to assess the risk tolerance and attitudes of pregnant women in Australia with respect to medication trial participation and to explore the influence of demographic or obstetric factors on decision‐making. These data are critical in understanding how to design pregnancy medication trials that can successfully recruit participants and consequently improve the safety and efficacy of the pharmaceutical landscape in pregnancy.
MATERIALS AND METHODS
Recruitment of participants
The project was approved by the University of Western Australia Human Research Ethics Committee (2022_ET000290). Australian women over the age of 18 who were either currently pregnant or had delivered within the last six months were eligible to complete an online survey. The survey, advertised via social media groups, paid Facebook advertising, and posters displayed in general practice clinics and childcare centres, was available for eight weeks between August and September 2022. Participants provided informed consent at the commencement of the survey, and at the conclusion were invited to signify interest in participating in a semi‐structured interview. Purposive sampling was used for the interviews to ensure a diverse range of views were captured relative to the total survey cohort and the general population. Interviewees provided written informed consent. 27
Online survey
The online survey (Appendix S1), developed with community consultation, was hosted on the Qualtrics CustomerXM platform. It consisted of 38 questions and took approximately 15 min to complete. Part one (21 questions) collected participant demographics. In part two (15 questions), participants were asked to report, using a five‐point Likert scale, their willingness to participate in five hypothetical drug trials (vignettes) with increasing drug‐related risk calibrated to the Therapeutic Goods Administration's (TGA) ‘Australian categories for prescribing drugs during pregnancy’. 28 In part three, (two questions) participants were asked to identify factors that would inform their willingness to participate in clinical trial research more generally. The survey concluded with a single open‐ended question asking for any final comments. The Checklist for Reporting Results of Internet E‐Surveys (CHERRIES) 29 guidelines were followed.
A vignette‐based approach has been used to assess the willingness of pregnant women to participate in vaccine trials. 18 , 22 , 30 The first three scenarios presented in this study (Appendix S2) represent trials of increasing drug‐related risk to the pregnant woman and/or fetus. Scenarios four and five were both calibrated to the same level of drug‐related risk, with the risk in scenario four assigned to the mother, and the risk in scenario five to the fetus. Each participant was presented with the scenarios in the same order.
Semi‐structured interviews
Semi‐structured interviews, lasting approximately 60 min (either in person or by videoconferencing by participant preference), were conducted by a graduate (Honours) student researcher (EQ). 31 Participants were asked to again consider each of the hypothetical drug trial scenarios and to describe their decision‐making process with respect to willingness to participate and perceived risk to mother and fetus (Appendix S3). The influence of the COVID‐19 pandemic on participants' views of medical research and decision‐making was also explored. Interviews were recorded and transcribed by the interviewer. Interviewees were deidentified, given pseudonym initials, and provided an opportunity to validate their transcript.
Interview analysis occurred simultaneously with data collection. Developing themes were immediately corroborated with incoming and previous data. 32 Saturation was achieved after nine interviews. A further two interviews were conducted post‐saturation for confirmation. 27 A coding framework was developed using NVivo software (QSR International) employing the template analysis technique described by Brooks et al. 33 and the a priori themes of evidence, risk, and trust as identified by Jaffe et al. 22 The interview data were corroborated with data captured via the single open‐ended question concluding the online survey and analysed using the same coding framework. Qualitative data surrounding the COVID‐19 pandemic were coded using a separate framework (Appendix S3).
Statistical analysis
Categorical data are presented as frequency counts (percentages); descriptive analysis of categorical and demographic data was conducted in Jamovi (https://www.jamovi.org). To assess the influence of demographic and obstetric factors on risk tolerance, ordinal regression analysis was performed using SPSS statistics software (IBM). For ordinal regression analysis of scenarios one, two and three, each participant was assigned an average risk tolerance score. The score was calculated by assigning a value to each of the response options: ‘very likely’ = 1, ‘likely’ = 2, ‘not sure’ = 3, ‘unlikely’ = 4, and ‘very unlikely’ = 5. From this, the responses of each participant from scenarios one to three were summed to obtain an overall score. Based on this overall score, participants were sorted into three participation categories: ‘likely’ (score 3–7), ‘not sure’ (score 8–10), and ‘unlikely’ (score 11–15). For scenarios four and five, ordinal regression analysis was performed using the categories ‘likely’, ‘not sure’, and ‘unlikely.’ Factors were considered statistically significant if the following conditions were met: an overall model fit (P ≤ 0.05), both the Pearson and deviation goodness of fit (P ≥ 0.05), and the individual factor itself (P ≤ 0.05). The Nagelkerke pseudo R 2 parameter is also provided.
RESULTS
Survey participation and cohort demographics
There were 875 participants who attempted the online survey, and 623 completed all questions and were included in the analysis (Fig. 1). Preliminary analysis revealed that the exclusion of the 257 incomplete surveys had no substantive impact on the study results and conclusion. The majority of respondents were aged 25 to 34 years (70%), had a Bachelor's degree or higher (65%), a household income of greater than $100 000 (80%), and were not religious (75%) (Table 1). There was an almost equal split between respondents living in major cities and remote/regionally. Most respondents were born and raised in Australia (80%); 3.5% were Aboriginal or Torres Strait Islander.
Figure 1.
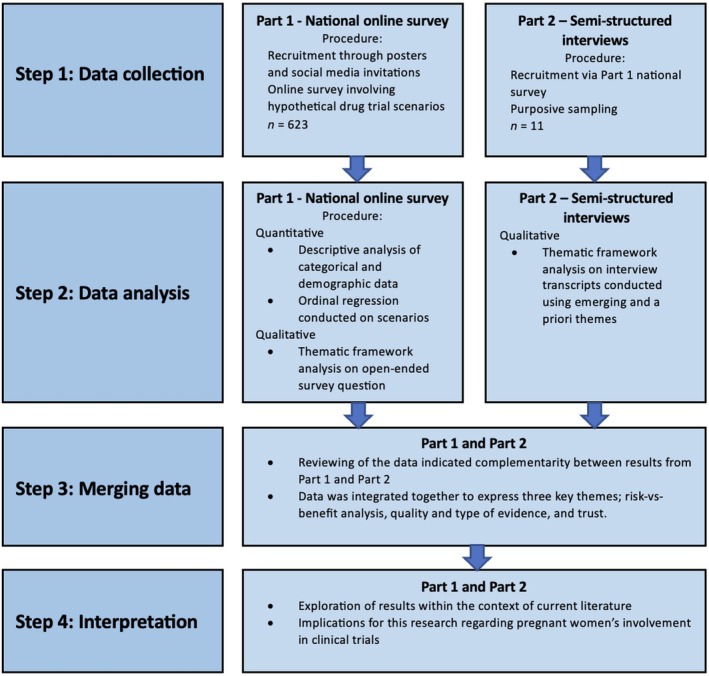
Study design.
Table 1.
Demographics of the online survey respondents (n = 623). Categories based on the Australian Bureau of Statistics population census
| Demographic | Number of women (%) |
|---|---|
| Age | |
| 18–24 | 46 (7.4) |
| 25–29 | 180 (28.9) |
| 30–34 | 254 (40.7) |
| 35–39 | 123 (19.7) |
| 40+ | 20 (3.2) |
| Highest completed level of education | |
| Did not complete high school | 20 (3.2) |
| Completed Year 12 | 57 (9.2) |
| Certificate (TAFE or University level) | 92 (14.8) |
| Diploma | 48 (7.7) |
| Bachelor degree | 217 (34.8) |
| Postgraduate qualification | 189 (30.3) |
| Occupation area | |
| Health care and social assistance | 251 (40.3) |
| Education and training | 97 (15.6) |
| Wholesale and retail trade | 33 (5.3) |
| Professional, scientific and technical services | 31 (4.9) |
| Administrative and support services | 28 (4.5) |
| Financial and insurance services | 23 (3.7) |
| Public administration and safety | 18 (2.9) |
| Agriculture, forestry and fishing | 17 (2.7) |
| Construction | 16 (2.6) |
| Arts and recreation services | 16 (2.6) |
| Homemaker/stay at home mum | 14 (2.3) |
| Mining | 10 (1.6) |
| Accommodation and food services | 10 (1.6) |
| Electricity, gas, water and waste services | 9 (1.5) |
| Other a | 50 (8.0) |
| Household income | |
| $0–$50,000 | 20 (3.2) |
| $50,000–$100,000 | 108 (17.3) |
| $100,000–$150,000 | 188 (30.2) |
| $150,000–$200,000 | 177 (28.4) |
| $200,000 + | 130 (20.8) |
| Aboriginal and/or Torres Strait Islander | |
| Yes | 22 (3.5) |
| Background | |
| Born and raised in Australia | 503 (80.7) |
| Born in Australia and raised by immigrant parents | 37 (5.9) |
| Born overseas and arrived in Australia as a child | 42 (6.7) |
| Born overseas and arrived in Australia as an adult | 41 (6.6) |
| Residential location | |
| Major city | 312 (50.1) |
| Regional | 285 (45.7) |
| Remote | 26 (4.2) |
| Religion | |
| None | 469 (75.3) |
| Catholic | 66 (10.6) |
| Anglican | 23 (3.7) |
| Christian (not a major faction) | 47 (7.5) |
| Other | 18 (2.9) |
| Long‐term health conditions | |
| Yes | 325 (52.2) |
| Pregnancy status at survey | |
| First trimester | 49 (7.9) |
| Second trimester | 107 (17.2) |
| Third trimester | 110 (17.7) |
| Post‐birth; within 6 months of delivery | 357 (57.3) |
| COVID ‐ 19 vaccination status | |
| No | 38 (6.1) |
| Yes, before pregnancy | 216 (34.7) |
| Yes, while pregnant | 357 (57.3) |
| Yes, after delivery | 12 (1.9) |
Other includes manufacturing, transport, postal and warehousing, information media and telecommunications, rental, hiring and real estate services, animal services, beauty services, defence force, disability pension, unemployed, funeral director, and legal services.
Quantitative analysis of survey data
Participation willingness
Overall, 48% of respondents reported a willingness (‘likely’ or ‘very likely’) to participate in at least one of the first three hypothetical drug trial scenarios. However, willingness to participate decreased markedly as drug‐related risk increased, with 39%, 24%, and 12% of respondents ‘very likely’ or ‘likely’ to participate in scenarios one (TGA category A), 2 (category B) and 3 (category C), respectively (Fig. 2a). Ordinal regression analysis identified only three demographic factors to be significantly predictive of modified risk appetite across the three scenarios: routine vaccination status (model fit 0.03), COVID‐19 vaccination status (model fit <0.001) and medication hesitancy (model fit <0.001), with women who are unvaccinated or hesitant about taking medication predicted to be the least willing to participate (Appendix S4).
Figure 2.
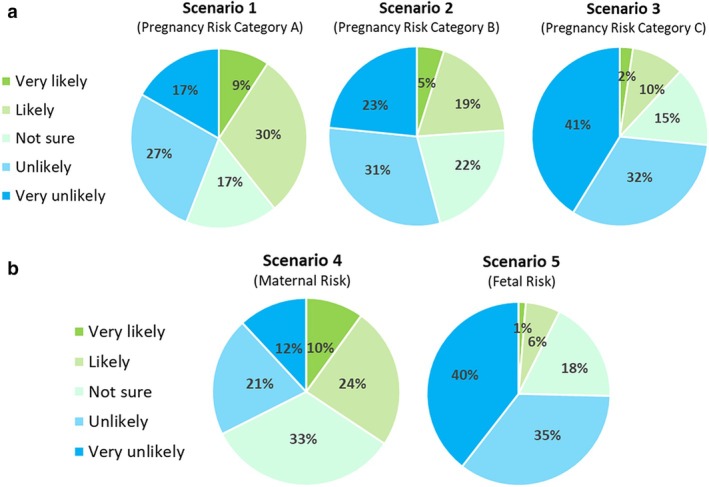
(a) Participant willingness to participate in hypothetical drug trial scenarios one, two, and three, representing scenarios of increasing risk. (b) Participant willingness to participate in hypothetical drug trial scenarios four and five, where risk was either focused on the mother (4) or fetus (5). Willingness is indicated in green, while unwillingness is in blue.
Maternal versus fetal risk
Respondents were far more likely (34% vs 7%) to be willing to participate in scenario four (TGA Category D: maternal risk, fetal benefit) than scenario five (Category D: fetal risk, maternal benefit) (Fig. 2b). The ordinal regression model predicted women unvaccinated against COVID‐19 to be three times (3.04; 95% CI, 1.603–5.782; Wald χ2(1) = 11.57, P = 0.001) more unlikely to participate in hypothetical drug trial scenario four than vaccinated women. There were no statistically significant predictors for participation in scenario five.
Factors informing risk tolerance
Ninety‐seven percent of respondents reported fetal harm as ‘very important’ in their decision‐making process (Fig. 3), while only 39% of participants reported concerns for their own safety as ‘very important’. The desire to help other pregnant women was ‘very important’ to 23% of respondents. Eighty‐two percent of respondents reported direct evidence of trial drug safety in human pregnancy to be ‘very important’. Evidence of safety in incidental pregnancies (women who became pregnant during a trial) was considered ‘very important’, but safety evidence from non‐pregnant human trials and animal studies was less so. Respondents placed considerable trust in a recommendation from an obstetrician, doctor, or midwife, with 56% ranking this as ‘very important’ (Fig. 3). In contrast, nearly half of all respondents reported recommendations from family/friends or the government to be ‘not at all important’.
Figure 3.
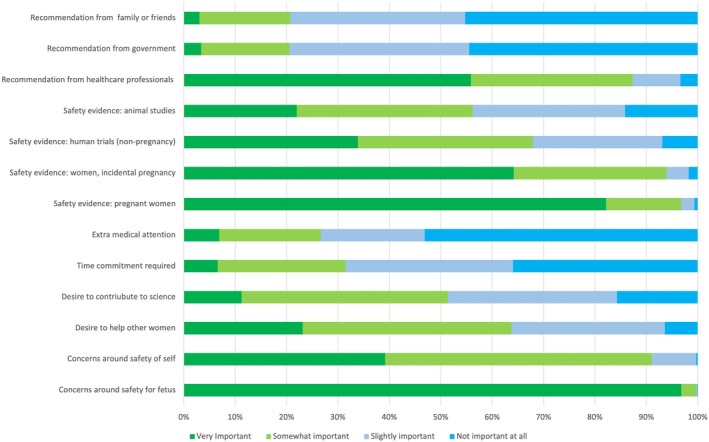
Participant ranking of the importance of 13 factors affecting trial participation. High importance is indicated in green, while low importance is in blue.
Qualitative thematic analysis
Twenty‐six individuals were invited from a pool of 112 volunteers for semi‐structured interview. Three First Nations women were invited, but none were available for interview. Demographic information of the final 11 interviewees can be found in Appendix S5. Where a pseudonym is not attributed to a quotation, the data were extracted from the online survey. Three clear themes emerged from the thematic analysis of the qualitative data: (1) risk–benefit analysis; (2) quality and type of evidence; and (3) trust in healthcare professionals.
Theme 1: Risk–benefit analysis
During the decision‐making process, participants routinely weighed up the benefits and risks to themselves and their baby from the perspective of both the health condition outlined in the vignettes and the safety of the trial drug.
I would rather suffer so my baby is kept safe.
Participants commonly expressed a desire to prevent or avoid fetal malformations, especially when considering participation in trial scenarios four and five. Rarely, participants identified or acknowledged the indirect benefits trial participation may have for their baby through improved maternal health.
It has got minor effects on fetal growth, and even though they are resolved after birth, I would still be hesitant to try it. – ZR in response to scenario three about a drug for gestational diabetes
Participants shared concerns about being the first in a pregnancy trial of a new drug; the lack of long‐term safety evidence or research; the inability of the fetus to provide consent; the lack of direct benefit from participation; and the emotional distress or ‘mum guilt’ which could result if trial participation caused harm to the fetus. This manifested as an unwillingness to discuss trial participation with friends/family in case of judgement. While often hesitant to participate themselves, there was considerable support among participants for the inclusion of pregnant women in medication trials more generally.
I guess for all of the scenarios, potential benefits are you know, adding to the wealth of knowledge out there for other people to make decisions too. Some people have to be the ones that started it. – JJ
Theme 2: Quality and type of evidence
Participants shared frustration with the lack of available safety data for drugs prescribed for use in pregnancy.
The significant delay and seeming lack of desire/urgency in completing pregnancy drug trials even after the drugs are available to the general public is a significant issue.
The quality and type of evidence participants said would be useful during decision‐making (enablers) included: safety and efficacy data from previous pregnancy trials; online access to existing drug safety data; information about the health problem being treated, even if they couldn't fully understand it; and less commonly safety and efficacy data from animal studies.
I have a level of trust in science where I'm like, well, they're not going to necessarily study it on something that has absolutely no correlation to what it might act like in a human. – AH in relation to animal studies
Evidence obtained from small studies of pregnant women, non‐pregnant women, and animals was also queried for translatability.
Just that it is not widely tested, so it could have unforeseen side effects in the future and that the trial that it has been tested in is very small, so it may not have covered all the sorts of risks involved. – MK
Theme 3: Trust
All participants said they would discuss trial participation with their partner, reflecting a view that decisions made that might affect the fetus should be made jointly. Friends and family had less influence unless they had medical knowledge. However, as risk increased across the scenarios, the consensus was that the final decision would ultimately rest with woman.
Certainly, my partner would definitely be included in the decision‐making process because it is his baby too. – JJ
All participants said they would consult a medical professional about trial participation. Rapport with that medical professional, specifically their degree of understanding of their personal medical history and values, was an essential contributor to trust; length of association was not as important. Participants with pre‐existing medical conditions said they would consult their general practitioner, specialist, and/or their primary pregnancy care provider. It was critical this adviser was independent of the trial.
So, I guess just in general depending on the trial, but not even really depending on the trial, I would speak to whatever doctors are looking after me or my child, like anyone that I'm already in contact with medically in that sense. – AH
Trust in information provided by government websites was mixed, with some participants saying they would consider these sources during decision‐making, while others were concerned about bias. The need to avoid another ‘thalidomide disaster’ was mentioned frequently across the interviews and end‐of‐survey open‐text comments.
It's good to get the insight from government recommendation, but with a lot of things I'd like to get more independent confirmation as well. – AS
DISCUSSION
Despite long‐term advocacy for the inclusion of pregnant women in clinical trials, and clear evidence that their exclusion is discriminatory and an impediment to the development of new and safe therapeutics in pregnancy, pregnant women are still actively excluded. 4 , 6 , 7 One common assumption is that pregnant women are reluctant to participate and hard to recruit to trials. Only 20% of women from a Canadian obstetrics and gynaecology clinic, for example, said they would participate in a hypothetical medication trial. 25
If perceived risk is a key determinant of trial participation, it follows that recruitment success will be dependent on the level of risk and how it is presented. Our study was, therefore, designed to assess the impact of risk (defined by the TGA categories for prescribing drugs during pregnancy) on Australian pregnant women's willingness to participate in a medication trial. We found that almost half of our respondents would participate in at least one trial, with likelihood of participation decreasing with increasing risk. Three key themes regarding trial participation decision‐making emerged: pregnant women perform a risk–benefit analysis, appraise the quality and type of evidence supporting the trial, and trust in healthcare professionals. These themes are similar to those reported in the Jaffe et al 22 Zika virus vaccination study.
Consistent with the findings of Palmer et al, 25 our respondents had a positive attitude toward medical research and saw the importance of medication trials during pregnancy. A direct benefit to the participating mother‐fetus dyad was a clear enabler to participation. 20 , 21 , 34 Willingness to participate was particularly high when the direct benefit was a reduced risk of fetal malformation (scenario 4). In contrast, our respondents rarely recognised the indirect benefits of trial participation to the fetus. A clear explanation of the direct and indirect benefits of trial participation could therefore aide recruitment.
It was common in our study for risks of trial participation to be more obvious to participants and for them to be given more weight in the decision‐making process. The key finding, that fetal risk was the primary influencing factor, supports previous work. 20 , 22 , 24 , 25 , 26 Most women were willing to take on greater levels of maternal risk to prevent adverse fetal health outcomes. 25 , 26 However, risk‐averse respondents often failed to identify the risks of not participating in a trial (not having access to the new drug). 35 , 36 ‘Mum guilt’ may prevent women from active intervention, with some of our respondents reporting they would prefer to do nothing or to explore non‐medicinal interventions. Yet, the vast majority of our participants reporting taking medications or supplements in pregnancy. 4 , 37 Studies to ascertain pregnant women's knowledge about the safety of the drugs they take would be worthwhile. 11
Nearly all of our respondents (96%) would engage their partner in trial participation decision‐making, a greater commitment to shared decision‐making than previously reported, 21 , 38 but felt that ultimately the decisions was theirs to make. 21 , 38 Brinchmann, Forde and Nørtvedt (2002) found parents would prefer not to have the final say in life‐or‐death situations given their lack of medical knowledge and emotional burden. 39 Confirming other studies, friends, and family were not particularly influential, except when they had medical knowledge. 21 , 38 Consulting with a medical professional regarding trial participation was important to our respondents, but only if rapport had already been developed. 20 , 21 , 38 This highlighted the importance of their healthcare provider having an understanding of the woman's medical history and personal values, plus some independence from the trial and the trial researchers.
Similar to Kenyon et al, 24 our respondents acknowledged the importance of contributing to science and helping other pregnant women, but did not rate this as a strong influencing factor for trial participation. Promoting societal benefit is, therefore, unlikely to significantly increase recruitment. The frequent mention of the thalidomide tragedy as a barrier to participation, despite this occurring over 60 years ago, 40 emphasises the importance of taking time to explain the research process and trial safeguards at recruitment.
COVID‐19 vaccination status and attitudes toward recommended medication use during pregnancy were the only statistically significant predictors of trial participation in our study. This suggests that enriching for specific participant demographics is unlikely to improve recruitment. Unvaccinated women were less likely to be willing to participate in a hypothetical drug trial, which is unsurprising given correlations between COVID‐19 vaccine hesitancy and a lack of trust in medical research. 41 , 42
Study strengths include the large sample size broadly reflective of Australian population demographics, 43 its risk‐focused vignette approach, and the mixed method study design. Assessing the impact of the COVID‐19 pandemic on attitudes toward medical research and health advice allows useful comparisons with pre‐pandemic studies. The opt‐in survey approach, and English language restrictions, mean the findings may not be entirely reflective of the Australian population, or applicable outside of Australia. The relatively high proportion of healthcare workers, and the lack of First Nations representation in the interviews, were also limitations.
In conclusion, this study found that pregnant Australian women value medical research and feel positive toward medication trials in pregnancy. A significant proportion of pregnant women are open to trial participation, provided they are given the appropriate resources by a trusted individual to undertake a meaningful risk–benefit analysis before decision‐making. The findings of this study can assist researchers in optimising the process of recruiting and consenting women into medication trials in pregnancy.
FUNDING
No direct funding was received for this study.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
Supporting information
Appendix S1. Participant information and consent.
Appendix S2. Hypothetical drug trial scenarios.
Appendix S3. Semi‐structured interview guiding question.
Appendix S4. Logistic regresion data.
Appendix S5. Demographic details of the interviewees.
ACKNOWLEDGEMENTS
We would like to thank all our study participants, without whom this research would not have been possible. We would also like to thank Associate Professor Zoe Bradfield and Dr Erin Kelty, who were the examiners of EQ's Honour's thesis, for providing critique and advice on the project. This study was presented in part at the Science on the Swan Conference, May 2023, Perth Convention & Exhibition Centre, Perth, Western Australia. Open access publishing facilitated by The University of Western Australia, as part of the Wiley ‐ The University of Western Australia agreement via the Council of Australian University Librarians.
REFERENCES
- 1. National Institutes of Health . What Are Clinical Trials and Studies? Vol. 2022. Bethesda, MD: National Institutes of Health, 2022. [Google Scholar]
- 2. Pariente G, Leibson T, Carls A et al. Pregnancy‐associated changes in pharmacokinetics: A systematic review. PLoS Med 2016; 13: e1002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sewell CA, Sheehan SM, Gill MS et al. Scientific, ethical, and legal considerations for the inclusion of pregnant people in clinical trials. Am J Obstet Gynecol 2022; 227: 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scaffidi J, Mol BW, Keelan JA. The pregnant women as a drug orphan: A global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG 2017; 124: 132–140. [DOI] [PubMed] [Google Scholar]
- 5. Herring C, McManus A, Weeks A. Off‐label prescribing during pregnancy in the UK: An analysis of 18,000 prescriptions in Liverpool Women's hospital. Int J Pharm Pract 2010; 18: 226–229. [DOI] [PubMed] [Google Scholar]
- 6. Merkatz RB. Inclusion of women in clinical trials: A historical overview of scientific, ethical, and legal issues. J Obstet Gynecol Neonatal Nurs 1998; 27: 78–84. [DOI] [PubMed] [Google Scholar]
- 7. Goldkind SF, Sahin L, Gallauresi B. Enrolling pregnant women in research‐‐lessons from the H1N1 influenza pandemic. N Engl J Med 2010; 362: 2241–2243. [DOI] [PubMed] [Google Scholar]
- 8. Baylis F. Pregnant women deserve better. Nature 2010; 465: 689–690. [DOI] [PubMed] [Google Scholar]
- 9. Caritis SN, Venkataramanan R. Obstetrical, fetal, and lactation pharmacology‐a crisis that can no longer be ignored. Am J Obstet Gynecol 2021; 225: 10–20. [DOI] [PubMed] [Google Scholar]
- 10. Kaye DK. The moral imperative to approve pregnant women's participation in randomized clinical trials for pregnancy and newborn complications. Philos Ethics Humanit Med 2019; 14: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Concept Foundation . R&D Blueprint for Accelerating Innovation for Mothers. Geneva: Concept Foundation, 2021. [Google Scholar]
- 12. Lee E, Maneno MK, Smith L et al. National patterns of medication use during pregnancy. Pharmacoepidemiol Drug Saf 2006; 15: 537–545. [DOI] [PubMed] [Google Scholar]
- 13. Lyerly AD, Faden RR. Mothers matter: Ethics and research during pregnancy. Virtual Mentor 2013; 15: 775–778. [DOI] [PubMed] [Google Scholar]
- 14. Wild V. How are pregnant women vulnerable research participants? Int J Feminist Approaches Bioethics 2012; 5: 82–104. [Google Scholar]
- 15. World Medical Association Inc . Declaration of Helsinki 2008, 9th edn. Ferney‐Voltaire: World Medical Association Inc, 2008; 5. [Google Scholar]
- 16. van der Zande ISE, van der Graaf R, Oudijk MA, van Delden JJM. Vulnerability of pregnant women in clinical research. J Med Ethics 2017; 43: 657–663. [DOI] [PubMed] [Google Scholar]
- 17. Blehar MC, Spong C, Grady C et al. Enrolling pregnant women: Issues in clinical research. Womens Health Issues 2013; 23: e39–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldfarb IT, Jaffe E, James K, Lyerly AD. Pregnant women's attitudes toward Zika virus vaccine trial participation. Vaccine 2018; 36: 6711–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodger MA, Makropoulos D, Walker M et al. Participation of pregnant women in clinical trials: Will they participate and why? Am J Perinatol 2003; 20: 69–76. [DOI] [PubMed] [Google Scholar]
- 20. Monteiro TM, Katz L, Bento SF et al. Reasons given by pregnant women for participating in a clinical trial aimed at preventing premature delivery: A qualitative analysis. BMC Pregnancy Childbirth 2019; 19: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smyth RM, Jacoby A, Elbourne D. Deciding to join a perinatal randomised controlled trial: Experiences and views of pregnant women enroled in the magpie trial. Midwifery 2012; 28: E478–E485. [DOI] [PubMed] [Google Scholar]
- 22. Jaffe E, Lyerly AD, Goldfarb IT. Pregnant women's perceptions of risks and benefits when considering participation in vaccine trials. Vaccine 2020; 38: 6922–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Osarfo J, Adjei RO, Magnussen P, Tagbor HK. Participation of Ghanaian pregnant women in an antimalarial drug trial: Willingness, experiences and perceptions. Trans R Soc Trop Med Hyg 2021; 115: 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kenyon S, Dixon‐Woods M, Jackson CJ et al. Participating in a trial in a critical situation: a qualitative study in pregnancy. Qual Saf Health Care 2006; 15: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer S, Pudwell J, Smith GN, Reid RL. Optimizing participation of pregnant women in clinical trials: Factors influencing decisions about participation in medication and vaccine trials. J Obstet Gynaecol Can 2016; 38: 945–954. [DOI] [PubMed] [Google Scholar]
- 26. Mohanna K, Tunna K. Withholding consent to participate in clinical trials: Decisions of pregnant women. Br J Obstet Gynaecol 1999; 106: 892–897. [DOI] [PubMed] [Google Scholar]
- 27. Saunders B, Sim J, Kingstone T et al. Saturation in qualitative research: Exploring its conceptualization and operationalization. Qual Quant 2018; 52: 1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Therapeutic Goods Administration . Australian Categorisation System for Prescribing Medicines in Pregnancy, Vol. 2022. Woden, ACT: The Department of Health, 2022. [Google Scholar]
- 29. Eysenbach G. Improving the quality of web surveys: The checklist for reporting results of internet E‐surveys (CHERRIES). J Med Internet Res 2004; 6: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans SC, Roberts MC, Keeley JW et al. Vignette methodologies for studying clinicians' decision‐making: Validity, utility, and application in ICD‐11 field studies. Int J clin. Health Psychol 2015; 15: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kallio H, Pietilä AM, Johnson M, Kangasniemi M. Systematic methodological review: Developing a framework for a qualitative semi‐structured interview guide. J Adv Nurs 2016; 72: 2954–2965. [DOI] [PubMed] [Google Scholar]
- 32. Busetto L, Wick W, Gumbinger C. How to use and assess qualitative research methods. Neurol Res Pract 2020; 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brooks J, McCluskey S, Turley E, King N. The utility of template analysis in qualitative psychology research. Qual Res Psychol 2015; 12: 202–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McQuaid F, Jones C, Stevens Z et al. Factors influencing women's attitudes towards antenatal vaccines, group B streptococcus and clinical trial participation in pregnancy: An online survey. BMJ Open 2016; 6: e010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lyerly AD, Little MO, Faden R. The second wave: Toward responsible inclusion of pregnant women in research. Int J Fem Approaches Bioeth 2008; 1: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyerly AD, Mitchell LM, Armstrong EM et al. Risk and the pregnant body. Hastings Cent Rep 2009; 39: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Therapeutic Goods Administration . Prescribing Medicines in Pregnancy Database. Woden, ACT: Department of Health, 2022. [Google Scholar]
- 38. Houghton G, Kingdon C, Dower M et al. What women think about consent to research at the time of an obstetric emergency: A qualitative study of the views of a cohort of World Maternal Antifibrinolytic Trial participants. BJOG 2018; 125: 1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brinchmann BS, Førde R, Nortvedt P. What matters to the parents? A qualitative study of parents' experiences with life‐and‐death decisions concerning their premature infants. Nurs Ethics 2002; 9: 388–404. [DOI] [PubMed] [Google Scholar]
- 40. Kim JH, Scialli AR. Thalidomide: The tragedy of birth defects and the effective treatment of disease. Toxicol Sci 2011; 122: 1–6. [DOI] [PubMed] [Google Scholar]
- 41. Palamenghi L, Barello S, Boccia S, Graffigna G. Mistrust in biomedical research and vaccine hesitancy: The forefront challenge in the battle against COVID‐19 in Italy. Eur J Epidemiol 2020; 35: 785–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kleitman S, Fullerton DJ, Zhang LM et al. To comply or not comply? A latent profile analysis of behaviours and attitudes during the COVID‐19 pandemic. PLoS One 2021; 16: e0255268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Australian Bureau of Statistics . Snapshot of Australia Canberra. Belconnen, ACT: Australian Bureau of Statistics, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Participant information and consent.
Appendix S2. Hypothetical drug trial scenarios.
Appendix S3. Semi‐structured interview guiding question.
Appendix S4. Logistic regresion data.
Appendix S5. Demographic details of the interviewees.


