Abstract
Hidradenitis suppurativa is an autoinflammatory condition resulting in painful cysts, nodules, and sinus tracts in areas of high skin on skin contact. The microenvironment of affected tissues is high in pro-inflammatory cytokines and T-helper 17 cells. Other auto-inflammatory diseases, like psoriasis, have an enhanced risk of systemic inflammation and an elevated risk of spontaneous abortion. A cohort of pregnant patients from Cerner Health Facts® was identified using a Python adaptation of a validated pregnancy identification and classification algorithm. The HS population was identified among the pregnant population and was shown to be statistically significantly associated with outcome type by Chi square. A multinomial logistic regression also indicated a statistically significant increase in the odds of a pregnant patient having a spontaneous abortion over a live birth when controlling for thyroid disease, polycystic ovarian syndrome, antiphospholipid syndrome, other inflammatory diseases, and advanced maternal age.
Introduction
Hidradenitis suppurativa (HS), is not a disease of the apocrine glands as the name suggests but is more akin to acne1. This arose due to the appearance that the disease is triggered by the obstruction of hair follicles and the subsequent dilation and rupture of the hair follicle space, or infundibulum, followed by an inflammatory response 1. Overall, HS affects females at a rate of 3:1 over males 2 and impacts 0.4% of the population 3. Those afflicted are characterized by a marked reduction in quality of life due to the extreme pain, localization to more sensitive areas, and associated depression 4.
The pathology of this disease is not entirely understood. HS lesional skin has been shown to contain increased pro-inflammatory cytokines such as Interleukin-17 (IL-17) and IL-23 5,6 as well as a downregulation of IL-37, an anti-inflammatory cytokine 7 implicated in enhancing the activity of regulatory T cells (Treg) 8. IL-17A has been shown to promote the proliferation of keratinocytes 9, which, in turn, produce IL-6 and, in the presence of IL-1, results in the differentiation of T helper 17 (Th17) cells which are characterized by the production of IL-17, IL-22, and Tumor Necrosis Factor α (TNF-α) 10. This creates a positive feedback loop and is consistent with the inflammation and pain associated with lesions. How this feedback loop gets started is unknown, however, the involvement of commensal microorganisms in the follicular infundibulum seems to be a likely candidate 11. The inflammatory microenvironment, perhaps in conjunction with uncontrolled microbial growth, eventually leads to rupture of the follicle, exacerbation of inflammation, and the formation of tunnels within the dermis 11.
Pregnancy and Inflammation
Pregnancy is considered a semi-allograft in that it contains genetic components from the same organism and from another individual of the same species. The embryo must invade the uterine lining and connect itself to the maternal blood supply. Thus, the maternal immune system must be appropriately modulated to allow for a successful pregnancy 12. As with other bodily microenvironments, Th1, Th2, Th17, cytotoxic T lymphocytes, natural killer cells, dendritic cells, Treg cells, B cells, and others not mentioned here and those yet to be fully described all play a role in defending against pathogens and in modifying the behavior of each other 13. A careful balance must be maintained.
It has been demonstrated that excess Th17 cells and associated inflammatory cytokines are present in recurrent pregnancy loss 14. IL-17 is associated with angiogenesis 15,16 and would be an expected component of early pregnancy but is also associated with embryo resorption in mice during early pregnancy when unregulated 17,16. It has also been shown that an imbalance between Treg and Th17 cells can result in recurrent pregnancy loss and preterm birth 13.
HS and Pregnancy Outcomes
The association of HS with increased IL-17 and decreased IL-37 suggests an imbalance between Th17 and Treg activity. Given this pro-inflammatory state in HS patients, it is possible that this imbalance, if systemic, could affect reproduction. However, pregnancy outcomes are not well established with respect to HS. A single center study at Henry Ford Hospital concluded that, in 202 pregnancies examined, there were no statistically significant differences in the rates of miscarriage, cesarean section, preterm birth, or stillbirth between their HS cohort and the general US population although they did not control confounders 18. A dual-center study conducted at the University of Pennsylvania and the University of California Los Angeles polled 59 female patients with HS and found a 15% rate of miscarriage 19, which is consistent with the national average 20. A retrospective analysis using the IBM Explorys platform found 1,600 females with HS over 1,862 pregnancies in which the odds ratios (OR) for miscarriage, and cesarean section were increased compared to controls when adjusting for comorbidities 21. A retrospective analysis of the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP) database found 1,021 pregnant women with HS had a greater likelihood of cesarean section when compared with non-HS pregnancies 22. Another recent retrospective study used IBM MarketScan to identify 998 pregnant women with HS and found a decreased OR for live birth and increased OR for elective termination and unspecified abortion, and cesarean section 23.
This study seeks to understand the impact of HS on pregnancy outcomes while controlling for confounding variables. The covariates chosen are conditions that tend to be comorbid with HS, like inflammatory bowel disease 4,24 or polycystic ovarian syndrome (PCOS) 25, or may confound negative pregnancy outcomes due to associations with Th17/Treg imbalance like antiphospholipid syndrome (APS) 26, thyroid disease 27,28, systemic lupus erythematosus 29,30, rheumatoid arthritis 31,32, and psoriasis 33.
Methods
A pregnancy datamart was constructed from Cerner Health Facts® (HF) using pypreg 34, a Python package developed by the author that adapts a validated algorithm 35 to accept both ICD9 and ICD10 diagnosis and procedure codes. The resulting datamart was cleaned for analysis by including only those patients indicated to be female without also being recorded as male.
Patients receiving a diagnosis of HS or PCOS at any point in their record in HF were considered to be positive for those conditions to account for delays in diagnosis as well as limitations with retrospective data. Thyroid disease, APS, and other inflammatory diseases (ID) were considered longitudinally with respect to each pregnancy. Presence during or prior to a pregnancy was recorded as positive for the condition for that pregnancy. Advanced maternal age was derived from the patient’s age at the time of the outcome and dichotomized as ≤35 and <35. The timespan of a pregnancy was estimated using the admitted date of the outcome encounter and the beginning of the pregnancy start window as determined by the Moll algorithm 35 as not all pregnancies in the datamart were able to have the date of the last menstrual periods determined. The independent variables described above are defined in Table 1.
Table 1.
Description of independent variables used to study pregnancy outcomes.
Univariate statistics were conducted to assess the association of HS with pregnancy outcome type. A multinomial logistic regression was also performed to control for covariates as described in Table 1. The multinomial regression was calculated using the nnet 36 R package. All statistics were performed in R version 4.3.1 37. P-values less than or equal to 0.05 were considered significant.
Results
Of the seven pregnancy outcome types available in the datamart (live birth, stillbirth, delivery of unknown type, ectopic, trophoblastic, therapeutic abortion, and spontaneous abortion), the “delivery of unknown type” outcome was excluded from analysis. This was due to containing misclassifications and representing a lack of specificity in the source data rather than being a true pregnancy outcome type. This left a total population of 1,572,279 pregnancies with 3,475(0.22%) associated with an HS patient.
A Chi square test of independence indicated significance (X2=73.359, df=5, p<0.001) in the relationship between HS and pregnancy outcome. However, a negligible effect size was also indicated (Cramer’s V=0.007). A residual plot indicated a negative contribution for live birth to the Chi square statistic and positive contributions for each other negative outcome type (Figure 1).
Figure 1.

Residual plot showing the strength of association with HS and pregnancy outcome. Larger and darker circles indicate a stronger contribution to the Chi square statistic, red is a negative association, and blue is a positive association.
A multinomial logistic regression was run using the pregnancy outcome as the dependent variable and the variables listed in Table 1 as independent variables. The model statistics for each pregnancy outcome (ectopic, spontaneous abortion, therapeutic abortion, stillbirth, and trophoblastic) are shown in Tables 2-6 respectively as compared against live birth.
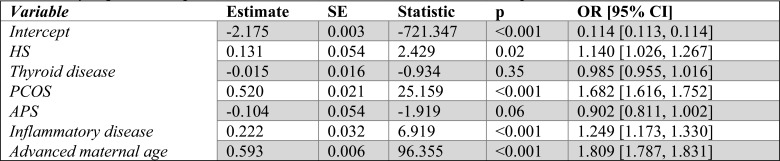
Table 2.
Multinomial logistic regression statistics when comparing Ectopic to Live birth. ORs are recovered by exponentiating the coefficient estimates which are in terms of log odds.
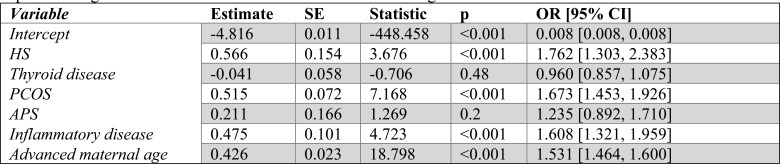
Table 6.
Multinomial logistic regression statistics when comparing Trophoblastic to Live birth. ORs are recovered by exponentiating the coefficient estimates which are in terms of log odds.
Ectopic
When considering ectopic pregnancies, all covariates except APS were statistically significant. The odds of a pregnancy ending as ectopic rather than live birth were increased for patients identified with HS by 73.2% (46.1%105.4%) or PCOS by 87.5% (73.9%-102.1%) and a patient with a concurrent or previous diagnosis of ID by 41.1% (25.4%-58.9%) or advanced maternal age by 40.2% (36.7%-43.8%). Patients with a concurrent or previous diagnosis of thyroid disease had increased odds of live birth over ectopic pregnancy by 6.8% (0.2%-13.9%). The reported p-value for the APS term is the result of rounding, the 95% confidence interval confirms non-significance. (Table 2).
Spontaneous abortion
When considering spontaneous abortion pregnancies, HS, PCOS, ID, and advanced maternal age were all statistically significant. The odds of a pregnancy ending in spontaneous abortion rather than live birth were increased for patients identified with HS by 14% (2.6%-26.7%) or PCOS by 68.2% (61.6%-75.2%) and a patient with a concurrent or previous diagnosis of ID by 24.9% (17.3%-33%) or advanced maternal age by 80.9% (78.7%-83.1%) (Table 3).
Table 3.
Multinomial logistic regression statistics when comparing Spontaneous abortion to Live birth. ORs are recovered by exponentiating the coefficient estimates which are in terms of log odds.
Therapeutic abortion
When considering therapeutic abortion pregnancies, HS, thyroid disease, APS, ID, and advanced maternal age were all statistically significant. The odds of a pregnancy ending with a therapeutic abortion rather than live birth were increased for patients identified with HS by 52.9% (27.6%-83.3%) and a patient with a concurrent or previous diagnosis of ID by 22.7% (8.1%-39.3%) and 47.8% (44.2%-51.5%) for patients of advanced maternal age. However, the odds were reduced for patients with a concurrent or previous diagnosis of thyroid disease by 40% (30.2%-50.5%) and APS by 31.4% (4%-66.2%) (Table 4).
Table 4.
Multinomial logistic regression statistics when comparing Therapeutic abortion to Live birth. ORs are recovered by exponentiating the coefficient estimates which are in terms of log odds.
Stillbirth
When considering stillbirth pregnancies, HS, PCOS, ID, and advanced maternal age were all statistically significant. The odds of a pregnancy ending with a stillbirth rather than live birth were increased for patients identified with HS by 76.2% (30.3%-138.3%) or PCOS by 67.3% (45.3%-92.6%) and a patient with a concurrent or previous diagnosis of ID by 60.8% (32.1%-95.9%) or advanced maternal age by 53.1% (46.4%-60%) (Table 5).
Table 5.
Multinomial logistic regression statistics when comparing Stillbirth to Live birth. ORs are recovered by exponentiating the coefficient estimates which are in terms of log odds.
Trophoblastic
When considering trophoblastic pregnancies, thyroid disease, PCOS, ID, and advanced maternal age were all statistically significant. The odds of a pregnancy ending as trophoblastic rather than a live birth were increased for patients identified with PCOS by 106.4% (79.9%-136.9%) and a patient with a concurrent or previous diagnosis of thyroid disease by 31.7% (18.4%-46.4%), ID by 45.2% (16.2%-81.3%), or advanced maternal age by 79% (70.9%87.6%) (Table 6).
Discussion and Conclusions
In this study, pregnancy outcomes were examined to learn how they were impacted by HS. A Chi square test of independence demonstrated significance, thus the null hypothesis that HS and pregnancy outcome are not associated was rejected. However, the negligible effect size observed indicates that this observation could be spurious due to being overpowered or rare.
To control other covariates and remove the multiple comparisons problem, a multinomial logistic regression was employed. When considering the effect of HS on pregnancy outcomes, while holding all other covariates constant, HS raised the odds ratio of having a spontaneous abortion by 14% (2.6%-26.7%), an ectopic pregnancy by 73.2% (46.1%-105.4%), a therapeutic abortion by 52.9% (27.6%-83.3%), and a stillbirth by 76.2% (30.3%-138.3%) as compared against live birth. With respect to the increase in spontaneous abortion odds, this is consistent with expectations where a pro-inflammatory state could result in a deleterious microenvironment for the developing embryo. Similarly, the increased odds in therapeutic abortion are consistent with expectations as this outcome type is associated with a variety of motivating issues including depressive disorders 38,39, with which HS is also associated 4. However, the precise underlying cause behind these therapeutic abortions is not known. The finding of an increase in the odds ratio for stillbirth and ectopic pregnancies over live births appear to be novel as previous literature has reported no difference in the HS population 18,23. This finding could be due to a larger sample size and accounting for covariates, although it may be possible that the stillbirths have an association with preterm birth and thus may also be susceptible to imbalances in the Th17 microenvironment 13.
This study has several limitations. Retrospective data can contain inaccuracies and may not provide a full picture of a patients’ diagnoses. It is possible that some independent variables may have been incorrectly labeled due to the lack of codes present in HF. While HF is a large secondary database, it only contains information from Cerner EHR facilities that elected to contribute data to HF. Patient care for these conditions could be managed elsewhere and not recorded during the pregnancy. Many spontaneous abortions occur early in pregnancy outside of a medical facility and thus are never recorded. The pregnancy datamart, while consistent with national figures, has not been formally validated against chart review. The model treats each pregnancy as an independent event and could suffer from missing or incorrectly represented terms. HS flare state and severity could not be assessed as notes data is not available in HF nor were a sufficient number of HS patients with C-reactive protein lab results present in the data. Other conditions that could be confounded with HS and pregnancy outcome like BMI and smoking status were not considered. BMI is not always a reliable measure in HF due to missingness, longitudinal inconsistency, incorrect units, or being recorded in imperial units. Smoking status similarly has recording inconsistencies. This study also has several strengths. This study has the largest population of pregnant HS patients investigated to my knowledge, makes use of multinomial logistic regression to attempt to control for covariates without multiple testing, draws from a large secondary database with contributions from across the US, and extensive efforts were made to clean the source database before building the pregnancy datamart.
Many people struggle with their reproductive goals. Having a greater understanding of how a person’s existing diagnoses can impact these goals is important in empowering them to make informed decisions. This study indicates that HS increases the odds of negative pregnancy outcomes over live birth. As a result, it is imperative that HS patients seeking to become pregnant receive appropriate care to align with their goals. Addressing the Th17/Treg imbalance could lead to better outcomes in patients with HS and other conditions associated with a Th17/Treg imbalance. This is generally achieved with systemic biologic treatment, but guidelines for IL-17 antagonists recommend against their use during pregnancy 40. Although, there is some evidence reporting no adverse effects of secukinumab exposure during pregnancy and no difference in the rate of spontaneous abortion from the general population in psoriasis patients 41,42. More study is needed to determine safety and if the observed non-difference in negative outcomes from the general population is an improvement for HS patients and others with a Th17/Treg imbalance condition.
Figures & Tables
References
- 1.Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! an appeal to (finally) abandon a misnomer. International Journal of Dermatology. 2005 July;44:535–540. doi: 10.1111/j.1365-4632.2004.02536.x. [DOI] [PubMed] [Google Scholar]
- 2.Jemec GBE. Hidradenitis suppurativa. New England Journal of Medicine. 2012 January;366:158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 3.Jfri A, Nassim D, O’Brien E, Gulliver W, Nikolakis G, Zouboulis CC. Prevalence of hidradenitis suppurativa. JAMA Dermatology. 2021 August;157:924. doi: 10.1001/jamadermatol.2021.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zouboulis VA, Zouboulis KC, Zouboulis CC. Hidradenitis suppurativa and comorbid disorder biomarkers, druggable genes, new drugs and drug repurposing—a molecular meta-analysis. Pharmaceutics. 2021 December;14:44. doi: 10.3390/pharmaceutics14010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Frontiers in Immunology. 2018. p. 9. [DOI] [PMC free article] [PubMed]
- 6.Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. Journal of the American Academy of Dermatology. 2011 October;65:790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Shanmugam VK, Jones D, McNish S, Bendall ML, Crandall KA. Transcriptome patterns in hidradenitis suppurativa: support for the role of antimicrobial peptides and interferon pathways in disease pathogenesis. Clinical and Experimental Dermatology. 2019 April;44:882–892. doi: 10.1111/ced.13959. [DOI] [PubMed] [Google Scholar]
- 8.Wang DW, Dong N, Wu Y, Zhu XM, Wang CT, Yao YM. Interleukin-37 Enhances the Suppressive Activity of Naturally Occurring CD4+CD25+ Regulatory T Cells. Scientific Reports. 2016 December. p. 6. [DOI] [PMC free article] [PubMed]
- 9.Ma WY, Jia K, Zhang Y. IL-17 promotes keratinocyte proliferation via the downregulation of C/EBPα. Experimental and Therapeutic Medicine. 2015 December;11:631–636. doi: 10.3892/etm.2015.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunological Reviews. 2008 June;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frew JW. Comprehensive guide to hidradenitis suppurativa. 2022.
- 12.Druckmann R, Druckmann MA. Progesterone and the immunology of pregnancy. The Journal of Steroid Biochemistry and Molecular Biology. 2005 December;97:389–396. doi: 10.1016/j.jsbmb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Jafarpour R, Pashangzadeh S, Mehdizadeh S, Bayatipoor H, Shojaei Z, Motallebnezhad M. Functional significance of lymphocytes in pregnancy and lymphocyte immunotherapy in infertility: a comprehensive review and update. International Immunopharmacology. 2020 October;87:106776. doi: 10.1016/j.intimp.2020.106776. [DOI] [PubMed] [Google Scholar]
- 14.Fu B, Tian Z, Wei H. Th17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol Immunol. 2014 July;11:564–570. doi: 10.1038/cmi.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Numasaki M. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2002 October;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 16.Osborne LM, Brar A, Klein SL. The role of Th17 cells in the pathophysiology of pregnancy and perinatal mood and anxiety disorders. Brain, Behavior, and Immunity. 2019 February;76:7–16. doi: 10.1016/j.bbi.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Addio F, Riella LV, Mfarrej BG, Chabtini L, Adams LT, Yeung M, et al. The Link between the PDL1 Costimulatory Pathway and Th17 in Fetomaternal Tolerance. The Journal of Immunology. 2011 November;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyons AB, Peacock A, McKenzie SA, Jacobsen G, Naik HB, Shi VY, et al. Retrospective cohort study of pregnancy outcomes in hidradenitis suppurativa. British Journal of Dermatology. 2020 June;183:945–947. doi: 10.1111/bjd.19155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adelekun AA, Villa NM, Hsiao JL, Micheletti RG. Pregnancy in hidradenitis suppurativa—patient perspectives and practice gaps. JAMA Dermatology. 2021 February;157:227. doi: 10.1001/jamadermatol.2020.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ACOG. ACOG practice bulletin no. 200: early pregnancy loss. Obstetrics & Gynecology. 2018 November;132:e197–e207. doi: 10.1097/AOG.0000000000002899. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick L, Hsiao J, Tannenbaum R, Strunk A, Garg A. Adverse pregnancy and maternal outcomes in women with hidradenitis suppurativa. Journal of the American Academy of Dermatology. 2021 June. [DOI] [PubMed]
- 22.Althagafi H, Czuzoj-Shulman N, Abenhaim HA. Pregnancy outcomes in patients with hidradenitis suppurativa. American Journal of Obstetrics and Gynecology. 2021 February;224:S314–S315. [Google Scholar]
- 23.Sakya SM, Hallan DR, Maczuga SA, Kirby JS. Outcomes of pregnancy and childbirth in women with hidradenitis suppurativa. Journal of the American Academy of Dermatology. 2022 January;86:61–67. doi: 10.1016/j.jaad.2021.05.059. [DOI] [PubMed] [Google Scholar]
- 24.Melnik BC, John SM, Chen W, Plewig G. T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa/acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. British Journal of Dermatology. 2018 June. [DOI] [PubMed]
- 25.Phan K, Charlton O, Smith SD. Hidradenitis suppurativa and polycystic ovarian syndrome: systematic review and meta-analysis. Australasian Journal of Dermatology. 2019 July. p. 61. [DOI] [PubMed]
- 26.Yan H, Li B, Su R, Gao C, Li X, Wang C. Preliminary Study on the Imbalance Between Th17 and Regulatory T Cells in Antiphospholipid Syndrome. Frontiers in Immunology. 2022 May. p. 13. [DOI] [PMC free article] [PubMed]
- 27.Torimoto K, Okada Y, Nakayamada S, Kubo S, Kurozumi A, Narisawa M, et al. Comprehensive immunophenotypic analysis reveals the pathological involvement of Th17 cells in Graves’ disease. Scientific Reports. 2022 October. p. 12. [DOI] [PMC free article] [PubMed]
- 28.Phan K, Huo YR, Charlton O, Smith SD. Hidradenitis Suppurativa and Thyroid Disease: Systematic Review and Meta-Analysis. Journal of Cutaneous Medicine and Surgery. 2019 September;24:23–27. doi: 10.1177/1203475419874411. [DOI] [PubMed] [Google Scholar]
- 29.Shan J, Jin H, Xu Y. T Cell Metabolism: A New Perspective on Th17/Treg Cell Imbalance in Systemic Lupus Erythematosus. Frontiers in Immunology. 2020 May. p. 11. [DOI] [PMC free article] [PubMed]
- 30.Dao KH, Bermas BL. Systemic Lupus Erythematosus Management in Pregnancy. International Journal of Women’s Health. 2022 February;Volume 14:199–211. doi: 10.2147/IJWH.S282604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P, Qian FY, Zhang MF, Xu AL, Wang X, Jiang BP, et al. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. Journal of Leukocyte Biology. 2019 September;106:1233–1240. doi: 10.1002/JLB.4RU0619-197R. [DOI] [PubMed] [Google Scholar]
- 32.Wallenius M, Salvesen KÅ, Daltveit AK, Skomsvoll JF. Miscarriage and Stillbirth in Women with Rheumatoid Arthritis. The Journal of Rheumatology. 2015 July;42:1570–1572. doi: 10.3899/jrheum.141553. [DOI] [PubMed] [Google Scholar]
- 33.Rahmati S, Moameri H, Mohammadi NM, Norouzi M, Ghalekhani N, Beigzadeh A, et al. Impact of maternal psoriasis on adverse maternal and neonatal outcomes: a systematic review and meta-analysis. BMC Pregnancy and Childbirth. 2023 September. p. 23. [DOI] [PMC free article] [PubMed]
- 34.Walsh D. pypreg: an open-source toolkit for pregnancy research. 2024. Available from: https://github.com/dpwh24/pypreg.
- 35.Moll K, Wong HL, Fingar K, Hobbi S, Sheng M, Burrell T, et al. Task order HHSF22301001T: pregnancy outcomes validation final report. Tech. rep. U.S. Food and Drug Administration. 2020.
- 36.Venables WN, Ripley BD. 4th ed. New York: Springer; 2002. Modern applied statistics with S. [Google Scholar]
- 37.R Core Team. Vienna: 2022. R: a language and environment for statistical computing. [Google Scholar]
- 38.Mota NP, Burnett M, Sareen J. Associations between abortion, mental disorders, and suicidal behaviour in a nationally representative sample. The Canadian Journal of Psychiatry. 2010 April;55:239–247. doi: 10.1177/070674371005500407. [DOI] [PubMed] [Google Scholar]
- 39.di Giacomo E, Pessina R, Santorelli M, Rucco D, Placenti V, Aliberti F, et al. Therapeutic termination of pregnancy and women’s mental health: determinants and consequences. World Journal of Psychiatry. 2021 November;11:937–953. doi: 10.5498/wjp.v11.i11.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owczarek W, Walecka I, Lesiak A, Czajkowski R, Reich A, Zerda I, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Advances in Dermatology and Allergology. 2020;37:821–830. doi: 10.5114/ada.2020.102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallo L, Ruggiero A, Balato A, Megna M, Fabbrocini G. Secukinumab during pregnancy: a case report and review of literature. Journal of Dermatological Treatment. 2020 April;33:585–586. doi: 10.1080/09546634.2020.1750551. [DOI] [PubMed] [Google Scholar]
- 42.Kimball AB, Guenther L, Kalia S, de Jong EMGJ, Lafferty KP, Chen DY, et al. Pregnancy Outcomes in Women With Moderate-to-Severe Psoriasis From the Psoriasis Longitudinal Assessment and Registry (PSOLAR) JAMA Dermatology. 2021 March;157:301. doi: 10.1001/jamadermatol.2020.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]