Abstract
Background
On the basis of the contribution of the gut microbiota to hypertension development, a novel strategy involving fecal microbiota transplantation (FMT) has been proposed to treat hypertension, but its efficacy has not been investigated in the clinic.
Methods
In a randomized, blinded, placebo-controlled clinical trial (2021/03–2021/12, ClinicalTrials.gov, NCT04406129), hypertensive patients were recruited from seven centers in China, and received FMT or placebo capsules orally at three visits. The patients were randomized at a 1:1 ratio in blocks of four and stratified by center by an independent statistician. The intention-to-treat principle was implemented, as all randomized participants who received at least one intervention were included. The primary outcome was the decrease in office systolic blood pressure (SBP) from baseline to the day 30 visit. Adverse events (AEs) were recorded through the 3-month follow-up to assess safety measures. Alterations in BP, the fecal microbiome, and the plasma metabolome were assessed via exploratory analyses.
Results
This study included 124 patients (mean age 43 years, 73.4% men) who received FMT (n = 63) or placebo (n = 61) capsules. The numbers of participants who experienced AEs (13 (20.6%) vs. 9 (14.8%), p = 0.39) and the primary outcome (6.28 (11.83) vs. 5.77 (10.06) mmHg, p = 0.62) were comparable between the groups. The FMT group presented a decrease in SBP after 1 week of FMT, with a between-arm difference of − 4.34 (95% CI, − 8.1 to − 0.58; p = 0.024) mmHg, but this difference did not persist even after repeated intervention. After FMT, shifts in microbial richness and structure were identified and the abundance of the phyla Firmicutes and Bacteroidetes was altered. Decreases in the abundances of Eggerthella lenta, Erysipelatoclostridium ramosum, Anaerostipes hadrus, Gemella haemolysans, and Streptococcus vestibularis and increases in the abundances of Parabacteroides merdae, Prevotella copri, Bacteroides galacturonicus, Eubacterium sp. CAG 180, Desulfovibrio piger, Megamonas hypermegale, Collinsella stercoris, Coprococcus catus, and Allisonella histaminiformans were identified and correlated with office SBP. Those species were also correlated with responding and inversely office SBP-associated metabolites including tyrosine, glutamine, aspartate, phenylalanine, methionine, serine, sarcosine, and/or asparagine.
Conclusions
Safety but unsustainable BP reduction was observed in the first trial of the effects of FMT on hypertension. Additional intervention studies on specific microbes with metabolite-targeting and BP-modulating features are needed.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-025-02118-6.
Keywords: Fecal microbiota transplantation, Hypertension, Microbiome, Metabolome
Background
Hypertension is a prevalent disease affecting approximately 1.28 billion people aged 30–79 years worldwide. Despite the diverse new pharmacological and other treatment options that have been developed, few hypertension treatments are available in the clinic. Only 23% of women and 18% of men achieve adequate blood pressure (BP) control [1], and approximately 8.5 million global deaths [2]. Indeed, novel therapeutic approaches are urgently needed to prevent comorbidities and death in hypertensive patients.
Many studies support the role of the microbiota in BP homeostasis, including indirect associations, cause‒and‒effect relationships, and even interventional relationships [3, 4]. Microbial dysbiosis occurs in both animal models and diverse cohorts in hypertensive and prehypertensive states [3, 5–7]. Under germ-free (GF) conditions, hypertension cannot be induced by angiotensin II [8]. Our previous study was the first to establish the ability of fecal microbiota transplantation (FMT) from hypertensive patients to modulate the gut microbiome and elevate BP in animals maintained under GF conditions [7]. Notably, hypertensive phenotypes can be transferred via different models: (1) FMT from stroke-prone spontaneously hypertensive rats (SHRs) into control Wistar-Kyoto (WKY) rats [9]; (2) FMT from mice with angiotensin II-induced hypertension lacking prebiotic fiber into GF mice through short-chain fatty acid (SCFA) reduction through short chain fatty acid (SCFA) reductionthrough short chain fatty acid (SCFA) reduction [10]; and (3) FMT from high-salt-induced hypertension (hSIH) models into normotensive controls, with increased corticosterone levels but reduced Bacteroides and arachidonic acid, and vice versa [11]. Specific microbes also affect with the bioactivity and/or metabolism of antihypertensive medications [12, 13]. The hypothesis of BP regulation via microbiota intervention is thereby proposed.
To date, multiple methods for gut microbiome modulation, such as prebiotics, probiotics, and antibiotics, have been explored. Meta-analysis of the effects of prebiotics [14] and probiotics [15] on hypertension treatment revealed modest changes in systolic BP of − 0.9 (95% CI − 2.5 to − 0.6) mmHg and − 2.05 (95% CI − 3.87 to − 0.24) mmHg, respectively. A case study revealed a decrease in BP in patients with resistant hypertension after the use of broad-spectrum antibiotics (vancomycin, ciprofloxacin, and rifampin) [16]. In animals with DOCA-salt-induced hypertension, high-fiber diet consumption and the administration of acetate, a SCFA produced by the gut microbiota via fiber fermentation, were found to decrease BP and protect target organs [17]. Additionally, the efficacy of probiotics such as Lactobacillus and Bifidobacterium in treating hypertension are being assessed through ongoing preclinical and clinical studies [13, 18]. However, the specific microorganisms associated with hypertension pathogenesis are currently unknown, which greatly hinders the identification of agents for hypertension treatment via the above approaches.
A strategy for modulating the entire gut microbial composition via FMT from healthy donors to recipients was developed. FMT has been officially recommended as a treatment for recurrent and resistant Clostridium difficile infection since 2013 [19] and was developed to treat intestinal and extraintestinal noncommunicable diseases such as inflammatory bowel disease (IBD), melanoma, obesity, metabolic syndrome, and diabetes [20–24]. Notably, a newly developed route for FMT allows for sustained administration with fewer adverse effects (AEs) than invasive delivery approaches [21, 25]. In the present study, we conducted a randomized, blinded, placebo-controlled trial to assess the effects and safety of oral FMT on hypertension. As exploratory analyses, alterations in the fecal microbiome and plasma metabolome in response to the intervention, as well as the correlations of these changes with alterations in BP, were assessed.
Methods
Study overview and participants
A randomized, blinded, placebo-controlled clinical trial of FMT [26] (Supplementary Materials) that included 126 patients with hypertension was conducted between 2021/03/17 and 2021/12/24 at seven centers in China, including Fuwai Hospital, Chinese Academy of Medical Science, Fuwai Yunnan Cardiovascular Hospital, Qilu Hospital of Shandong University, the Second Affiliated Hospital of Shantou University Medical College, People’s Hospital of Ji Xian District, Shanxi Bethune Hospital, and South University of Science and Technology Hospital.
Eligible participants were 18–60 years of age, provided written informed consent, and were initially diagnosed with grade one hypertension (or had not received antihypertensive medications for at least 1 month), defined as three measurements of 140 mmHg ≤ office systolic BP (SBP) < 160 mmHg and/or 90 ≤ office diastolic BP (DBP) < 100 mmHg on different days, according to the “2010 Chinese Guidelines for Prevention and Treatment of Hypertension” [27]. Patients were excluded if they used antibiotics, probiotics, or antihypertensive agents within 1 month before the study; if they had participated in other clinical trials of hypertension within the last 3 months; or if they were diagnosed with secondary hypertension. Other exclusion criteria included severe hepatic or renal diseases, stroke, coronary revascularization, myocardial infarction, NYHA class III–IV heart failure, sustained atrial fibrillation, severe valvular diseases, dilated or hypertrophic cardiomyopathy, rheumatic or congenital cardiac disease, cognitive impairment or severe neuropsychiatric comorbidities, and pregnancy and/or lactation, as assessed by investigators.
Scheduled visits occurred at baseline and on day 1, day 7, day 14, day 30, day 60, and day 90. Fecal and blood samples were collected and stored at − 80 °C in a freezer for metagenomic and metabolomic profiling. Printed case report forms (CRFs) and an electronic data capture systems (EDC) system (http://47.107.145.115/fmtEDC/) with information on demographic features, cardiovascular disease (CVD) risk factors, office BP, and other measures, including 24-h ambulatory BP monitoring (ABPM) data, electrocardiography data, routine blood and urine test results, C-reactive protein (CRP) levels, the results of liver and renal function analyses, fasting blood glucose (GLU) and lipids, brachial–ankle pulse wave velocity (baPWV) and the ankle–brachial index (ABI), randomization and capsule prescription information, 24-h dietary recall data, sample collection information, comorbid diseases, and treatment data, and AE data, were prepared.
This investigator-initiated trial, registered on ClinicalTrials.gov (NCT04406129, 28 May 2020), was approved by the Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences (approval NO. 2020–1334, 14 April 2020), and all collaborating implementation centers. Written informed consent was obtained before participant enrollment.
Randomization and blinding
Randomization allocation sequencing was performed at a 1:1 ratio in blocks of four and stratified by center by an independent statistician via SAS 9.4 software (SAS Institute, Cary, NC, USA). Staff from the Medical Research & Biometrics Center, National Center for Cardiovascular Diseases, generated the random codes, and only an independent staff member from the responsible center maintained the codes that were incorporated into the EDC system and created sealed emergency envelopes for each participant. Packaged intervention capsules are prepared in accordance with the Good Clinical Practice (GCP) guidelines. Study participants and all personnel who recruited and followed up with participants and collected and analyzed data, including investigators, patient care providers, and/or data analysts, were blinded to the treatment conditions.
Donor screening and FMT capsule manufacturing
Seventeen rigorously selected healthy volunteer donors provided stool for all participants in accordance with the current consensus [28–30]. Prospective donor candidates sequentially underwent questionnaire interviews; psychological tests; physical examinations; and blood, stool, and urea laboratory tests to exclude the possibility of transmissible infectious diseases and potential dysbiosis-related diseases. Each fresh stool sample weighing 100–200 g was put into a cold storage box (4–8 °C) immediately after collection and delivered to the Oriental Yeekang laboratory within 1 h. Each collection from a single donor was mixed with precooled normal saline, filtered, centrifuged, lyophilized, and finally placed into 20–35 FMT capsules, which were coded, stored at − 80 °C, and delivered via dry ice. Each capsule contains collection from a single donor. At each visit, each patient receives capsules from a single donor. However, patients can receive FMT capsules from up to three different donors at the different visits to ensure the diversity and stability of transplantation. The protocol is presented in detail in the “Supplementary Material for Donor Screening and FMT Capsule Manufacturing.”
Interventions
Eligible participants were randomized to the “FMT capsule” group or the “placebo capsule” group. One course of coded capsules (20 capsules per visit, 1–3 × 1012 bacteria per FMT capsule) was given on days 1, 7, and 14 after randomization, and medication adherence and AEs were classified via the Common Terminology Criteria for Adverse Events (CTCAE) (Version 4.03) recorded. The placebo capsules contained identically appearing powder without donor stool or any active drug. The participants fasted for 4 h prior to and 2 h after the intervention. The capsules were recovered in a 37 °C water bath and taken orally under clinical supervision, with a 100% compliance rate.
Outcomes
The primary outcome was defined as the change in office SBP from baseline to the day 30 visit. Office BP measurements were performed in accordance with the Chinese Hypertension Guideline [27], after participants in seated position resting for at least 5 min and be measured two times at each arm with at least 1 min apart (and the third time if over 5 mmHg difference observed between the previous measurements) by trained clinic staffs. The arm with higher BP readings was measured in follow-up visits. Office BP levels were calculated using the average of readings. The secondary outcomes were other indicators related to BP evaluation, safety concerns, and the potential mechanisms, including (1) office SBP and DBP; (2) average SBP and DBP measured via 24-h ABPM; (3) the ABI, baPWV, GLU levels, blood lipid levels, and body mass index (BMI); (4) the number of participants that experienced AEs; and (5) fecal microbiota and plasma metabolites, as determined via metagenomic and metabolomic analysis.
Sample size
A sample size of 120 hypertensive patients (PASS Version 15) [26] yielded 80% statistical power to demonstrate a 5 mmHg difference in SBP of the “FMT capsule” group superior to the “placebo capsule” group (standard deviation of 8.6 mmHg) at a one-sided alpha level of 0.025 and a 20% rate of loss to follow-up.
DNA extraction and metagenomic sequencing
Total DNA was extracted using the cetyltrimethylammonium bromide (CTAB) method, and all the samples were sequenced on the Illumina NovaSeq 6000 platform, with 2 × 150-bp paired-end reads. The DNA quality was evaluated with a NanoDrop spectrophotometer, a Qubit 2.0 fluorometer, and agarose gel electrophoresis (AGE). Quality control for raw sequencing reads was performed with Trimmomatic (V0.36). Considering the possibility of host contamination in the samples, the clean data were aligned to the human reference genome hg19 with SOAPaligner (V2.21, parameters: − r 2−m 200 − × 400). After removing host reads, taxonomical and functional profiling were performed using MetaPhlAn (version 3.0.13) and HUMAnN3 (v3.0.0) with default parameters.
Metabolomic profiling
Untargeted metabolite screening was performed on a Q Exactive HFX Orbitrap mass spectrometer (Thermo, CA). The resulting raw spectrogram was processed using TraceFinder 3.2 (ThermoFisher). For analysis of amino acids and trimethylamine N-oxide (TMAO)-related metabolites, processed samples were injected onto a silica column (2.0 * 150 mm, Luna 5u Silica 100 A; Phenomenex, Torrance, CA) at a flow rate of 0.4 ml/min using an LC- 20 AD Shimadazu pump system and a SIL- 20 AXR autosampler interfaced with an API 6500Q-TRAP mass spectrometer (AB SCIEX, Framingham, MA). For analysis of SCFA, samples were separated on an Agilent DB-WAX capillary column (30 m × 0.25 mm ID × 0.25 µm) for gas chromatography, and an Agilent 7890 A/5975 C gas chromatography‒mass spectrometer was used for GC‒MS analysis. MSD ChemStation software was used to extract the chromatographic peak area and retention time.
Statistical analysis of clinical outcomes
All tests were performed using SAS software(version 9.4). The intention-to-treat principle was implemented, as all randomized participants that received at least one intervention were included, and per-protocol analysis was used for sensitivity analysis. For superiority analysis, primary analysis was performed with a one-sided alpha level of 0.025. Other analyses were a two-sided level of 0.05. Categorical variables are presented as counts (percentages) and were compared between arms via the likelihood ratio chi-square test or Fisher’s exact test. Continuous variables are presented as means (SDs), medians (IQRs), or confidence intervals (CIs) and were analyzed via Student’s t test or the Wilcoxon rank sum test. For the primary analysis, analysis of covariance (ANCOVA) was performed, adjusting for the center effect, baseline effect, and interaction term if significant (p < 0.05). A post hoc mixed-effects model was performed after unblinding for sensitivity analysis. Fixed effects included the treatment group, center, baseline effect, and interaction term if significant (p < 0.05). The non-nested random intercept referred to batch effects (individuals receiving capsules from the same donor). The last observation carried forward (LOCF) method was used for missing data in the primary analysis and sensitivity analysis, including the complete case (CC) and data using the multiple imputation (MI, 100 imputations) methods. Subgroup analyses based on age, sex, BMI, baseline SBP and DBP, history of diabetes or hyperlipidemia, and 10-year risk of CVD were performed. For secondary outcomes, t tests were used to compare continuous data between arms at visits and within groups. The number of AEs was compared via the likelihood ratio chi-square test or Fisher’s exact test.
Statistical analysis of metagenome and metabolome
All analyses were performed using R (version 4.3.2). The following differences in response to FMT intervention were analyzed (Fig. S1): (1) differences between the FMT and placebo groups; (2) differences within the FMT group between the baseline and follow-up timepoints; and (3) parallel intergroups at baseline. The correlations of changes in various parameters with changes in office SBP were also analyzed. Alpha diversity indices were calculated for different species. Beta diversity analysis was conducted using the Bray‒Curtis distance matrix. The Wilcoxon rank sum test and paired Wilcoxon rank sum test were used to identify differences in microbes and metabolites between and within groups, respectively. Features with a false discovery rate (FDR) adjusted p-value < 0.05 (corrected via the Benjamini–Hochberg method) were considered significantly different. Partial Spearman’s correlations (pSRCs) were evaluated, adjusting for age, sex, BMI, center, hypertension course, previous history of antihypertensive medication use, baseline office SBP and DBP, and baseline phenotype level. Correlations with a p-value < 0.05 were considered statistically significant. Office SBP-related features that changed both within the FMT group and between groups were then included in a zero-inflated beta regression (ZIBR) model to identify differences in abundance or the presence of features between groups over time. Features with an adjusted p-value < 0.05 (corrected via the Benjamini–Hochberg method), according to the group beta regression model, group logarithmic model, or group joint model, were considered significantly different. The functional enrichment of the metabolites was assessed using MetaboAnalyst. Intracenter, cross-center, and leave-one-center-out (LODO) validations of office SBP-related differentially abundant genera/species were performed for the FMT and control samples collected at D7, D14, D30, D60, and D90 using xMarkerFinder. For multiomics analyses, namely, correlation analyses among clinical phenotypes, metabolite levels, and microbial abundance, pSRC analysis was performed as previously described. The protocol and statistical analysis plan are described in detail in the Supplementary Materials.
Results
Baseline characteristics
Among 180 patients assessed for eligibility (Fig. 1), 124 participants received at least one dose of intervention and were included in the intention-to-treat analysis; there were 63 patients in the FMT group and 61 patients in the placebo group; and the patients in the two groups had comparable hypertension courses of 2.18 (2.78) and 2.03 (3.26) years, respectively. The treatment compliance rate of patients in the two groups was 100%. Baseline features, including demographics; previous antihypertensive agent use; office BP levels; ABPM measurements; atrial stiffness; previous medical history; living habits; inflammatory markers levels; GLU, lipid, and creatinine (CREA) levels; CVD risk; and dietary habits and components but not office DBP levels, were balanced across arms(Tables 1 & S1). The mean ages of the recruited participants were 41.8 (9.3) and 44.2 (9.0) years. Males accounted for 47 (74.6%) and 44 (72.1%) of the patients, and the mean office BP and 24-h BP levels were 146.1/94.2 vs. 147.6/95.9 mmHg and 137.8/90.2 vs. 139.2/90.9 mmHg, respectively.
Fig. 1.

Flow diagram of the trial of FMT for hypertension
Table 1.
Baseline characteristics of the study participants
| Characteristic | FMT group (N = 63) | Placebo group (N = 61) |
|---|---|---|
| Age, years | 41.8 ± 9.3 | 44.2 ± 9.0 |
| < 38 | 23 (36.5%) | 14 (23.0%) |
| 38– < 48 | 22 (34.9%) | 21 (34.4%) |
| ≥ 48 | 18 (28.6%) | 26 (42.6%) |
| Sex, No. (%) | ||
| Female | 16 (25.4%) | 17 (27.9%) |
| Male | 47 (74.6%) | 44 (72.1%) |
| BMI, kg/m2 | 26.27 ± 3.79 | 26.73 ± 3.10 |
| Years with HTN, years | 2.18 ± 2.78 | 2.03 ± 3.26 |
| Office blood pressure | ||
| Systolic, mmHg | 146.1 ± 5.13 | 147.6 ± 5.88 |
| Diastolic, mmHg | 94.2 ± 4.95 | 95.9 ± 3.41 |
| Distribution of SBP, mmHg | ||
| < 143.7 | 28 (44.4%) | 15 (24.6%) |
| 143.7– < 149 | 17 (27.0%) | 22 (36.1%) |
| ≥ 149 | 18 (28.6%) | 24 (39.3%) |
| 24 h mean blood pressure | ||
| Systolic, mmHg | 137.8 ± 7.62 | 139.2 ± 8.83 |
| Diastolic, mmHg | 90.2 ± 5.02 | 90.9 ± 5.82 |
| Inflammatory markers | ||
| CRP, mg/L | 2.04 ± 2.35 | 2.16 ± 2.89 |
| WBC count, × 10^9/L | 6.69 ± 1.89 | 6.19 ± 1.71 |
| Serum creatine, μmol/L | 79.75 ± 15.50 | 79.39 ± 15.60 |
| Fasting serum glucose, mmol/L | 5.48 ± 0.79 | 5.65 ± 1.06 |
Categorical data are compared between groups using likelihood ratio chi-square test or Fisher’s exact test. Continuous variables are compared between groups using two sample t-test
BMI, body mass index; HTN, hypertension; SBP, systolic blood pressure; CRP, c reactive protein; WBC, white blood cell
Primary outcome
The changes in office SBP from baseline to day 30 were parallel between arms, with decreases of 6.28 (11.83) mmHg in the FMT arm and 5.77 (10.06) mmHg in the placebo arm (p = 0.62, Table 2). Sensitivity analysis of the complete dataset and via MI methods for missing data revealed consistent observations, as did per-protocol analysis and post hoc analysis using the linear mixed model with random effects of donors’ effects (Table S2).
Table 2.
Primary outcome measure of office SBP
| Outcome | FMT group (N = 63) | Placebo group (N = 61) | P value |
|---|---|---|---|
| Primary outcome, office SBP, mmHg | |||
| Baseline | 146.05 ± 5.13 | 147.61 ± 5.88 | |
| Day 30 | 139.78 ± 11.04 | 141.93 ± 11.81 | |
| Change | 6.28 ± 11.83 | 5.77 ± 10.06 | 0.62 |
| Subgroup analysis, change in office SBP (baseline–Day 30), mmHg | |||
| Age, years | |||
| < 38 | 4.38 ± 10.04 | 6.20 ± 12.18 | 0.63 |
| 38– < 48 | 2.91 ± 10.81 | 6.27 ± 8.72 | 0.35 |
| ≥ 48 | 12.81 ± 13.07 | 5.14 ± 10.21 | 0.029 |
| Sex | |||
| Female | 8.34 ± 15.14 | 7.59 ± 10.94 | 0.43 |
| Male | 5.57 ± 10.58 | 5.07 ± 9.74 | 0.88 |
| BMI, kg/m2 | |||
| 18.5– < 24 | 3.47 ± 9.32 | 5.50 ± 13.24 | 0.98 |
| ≥ 24 | 7.15 ± 12.36 | 5.83 ± 9.48 | 0.43 |
| Baseline office SBP, mmHg | |||
| < 143.7 | 3.44 ± 11.01 | 7.21 ± 11.98 | 0.20 |
| 143.7– < 149 | 5.65 ± 10.06 | 4.80 ± 9.38 | 0.16 |
| ≥ 149 | 11.28 ± 13.50 | 5.76 ± 9.70 | 0.11 |
| Baseline office DBP, mmHg | |||
| < 93.5 | 6.31 ± 13.25 | 6.54 ± 10.09 | 0.72 |
| 93.5– < 97.5 | 2.37 ± 9.85 | 5.90 ± 10.73 | 0.29 |
| ≥ 97.5 | 8.72 ± 10.92 | 5.24 ± 9.76 | 0.16 |
| 10-year risk of CVD on the basis of Framingham risk scoring | |||
| < 15% | 5.09 ± 11.68 | 5.96 ± 10.58 | 0.84 |
| ≥ 15% | 8.82 ± 12.04 | 5.51 ± 9.47 | 0.30 |
The covariance analysis with adjusting center, baseline effect, and interactive term between the center and treatment group (if applicable) is used in above analysis. The baseline refers to the baseline office SBP. Mean and SD are presented. Difference and 95% CI of change in office SBP are estimated by ANCOVA in ITT dataset after missing data imputed by LOCF method
Notably, a significant change of 7.65 (95% CI 0.78–14.51) mmHg in office SBP change was observed in participants aged over 48 years old (Table 2), with a decrease in SBP of 12.81 (13.07) in the FMT group and 5.14 (10.21) in the placebo group (p = 0.029). However, no difference was observed in other subgroup analyses, including subgroup analyses based on sex, BMI, baseline SBP and DBP, history of diabetes or hyperlipidemia, or 10-year risk of CVD. Interestingly, an increasing difference between the FMT and placebo arms was observed for baseline SBP (3.44 (11.01) versus 7.21 (11.98) for baseline SBP < 143.7 mmHg, 5.65 (10.06) versus 4.80 (9.38) for baseline SBP 143.7– < 149 mmHg, and 11.28 (13.50) versus 5.76 (9.70) for baseline SBP ≥ 149 mmHg), despite not reaching statistical significance (Table 2).
Secondary outcomes
There were no significant differences between arms in secondary outcomes, i.e., BP-related indices (SBP, DBP, 24 h SBP, 24 h DBP, day SBP, day DBP, night SBP, and night DBP) assessed in office or by ABPM from baseline to follow-up timepoints beginning at day 30, i.e., the termination of intervention (Table S3). Notably, significant heterogeneity existed in office SBP on day 7, the visit after the first intervention, with an office SBP of 138.9 (9.4) mmHg in the FMT group and 143.2 (11.7) mmHg in the placebo group. A trend toward a lower level of office SBP after FMT gradually decreased between groups over time: − 4.34 (95% CI, − 8.1 to − 0.58, p = 0.024) on day 7, − 3.46 (95% CI, − 7.52 to 0.59, p = 0.09) on day 14, − 2.16 (95% CI, − 6.24 to 1.92, p = 0.30) on day 30, − 0.95 (95% CI, − 5.12 to 3.22, p = 0.65) on day 60, and − 0.92 (95% CI, − 5.16 to 3.33, p = 0.67) on day 90 (Fig. 2A).
Fig. 2.
Office SBP and microbial diversity after FMT and placebo intervention. A The line plots show changes in office SBP (upper) and differences (lower) between the FMT (red line) and control (CTL, blue line) arms over the follow-up period. The means and S.D.s are presented. The 95% confidence intervals (CIs) of the difference between two groups were calculated by normal approximation for continuous variables. Intragroup analysis was performed via two-sample t tests.B Changes in alpha diversity revealed by the number of species. Between- (upper) and within-group differences (lower) were analyzed via the Wilcoxon rank sum test (*, p < 0.05). C Bar chart revealing seven phyla of donors and hypertensive participants. D Heatmap showing changes in four differential phyla in the FMT group compared with donors over the follow-up period. The p values were obtained through the Wilcoxon rank sum test with FDR adjustment (*, adjusted p < 0.05; +, adjusted p < 0.01). FC, fold of change. E Principal coordinate analysis (PCoA) presents intragroup differences in beta diversity evaluated by the Bray‒Curtis distance. The p values were obtained through permutational multivariate analysis of variance (PERMANOVA)
To explore the potential effects of microbiota modulation on cardiometabolic health and arterial stiffness, changes in CRP, GLU and lipid levels, BMI, baPWV, and the ABI from baseline to the day 90 visit were measured. However, no significant difference was observed (Table S3).
AEs
No serious AEs or differences in AEs between arms were observed throughout the study. There were a total of 13 (20.6%) participants that experienced 15 AEs in the FMT arm assessed, including one with diarrhea, four with hyperlipidemia, five with hyperuricemia, one with elevated GLU level, three with an abnormal urinary microalbumin‒CREA ratio, and one with a hive. In the placebo group, 9 (14.8%) participants experienced a total of 13 AEs, including seven cases of hyperlipidemia, two cases of hyperuricemia, one case of elevated GLU level, one case of constipation, and two cases of an abnormal urinary microalbumin‒CREA ratio (Table 3).
Table 3.
Adverse events related to the study interventions
| FMT group (N = 63) | Placebo group (N = 61) | P value | |
|---|---|---|---|
| Participants | 13 (20.6%) | 9 (14.8%) | 0.39 |
| Total adverse events | 15 (23.8%) | 13 (21.3%) | |
| Hyperlipidemia | 4 (6.3%) | 7 (11.5%) | 0.31 |
| Hyperuricemia | 5 (7.9%) | 2 (3.3%) | 0.44 |
| Elevated fasting serum glucose | 1 (1.6%) | 1 (1.6%) | 1.00 |
| Abnormal urinary mALB/Cr | 3 (4.8%) | 2 (3.3%) | 1.00 |
| Hives | 1 (1.6%) | 0 (0.0%) | 1.00 |
| Diarrhea | 1 (1.6%) | 0 (0.0%) | 1.00 |
| Constipation | 0 (0.0%) | 1 (1.6%) | 0.49 |
Categorical data are compared between the two groups using likelihood ratio chi-square test or Fisher’s exact test. Hyperlipidemia (defined as low density lipoprotein-cholesterol ≥ 3·4 mmol/L, triglyceride ≥ 1·7 mmol/L, total cholesterol ≥ 5·2 mmol/L), elevated fasting serum glucose (defined as fasting glucose > 6·1 mmol/L), hyperuricemia (defined as uric acid > 420 μmol/L), abnormal urinary mALB/Cr (microalbumin to creatinine ratio, defined as > 30 mg/gCr)
Changes in microbial diversity
To elucidate the potential mechanisms underlying the effect of microbial intervention on BP, metagenomic profiling of samples collected at all visits was performed. The bacterial richness (number of species) incrementally increased within the group from baseline to day 14 after FMT, persisted until day 30, and day 60, but recovered on day 90, and was distinct from that of the placebo group at specific visits (Fig. 2B). However, bacterial evenness revealed by the Shannon, Pielou, and Simpson indices was comparable between the groups (adjusted p > 0.05), which indicates that the microbial structure in hypertensive recipients was resistant to one course of intervention (Fig.S2). From PERMANOVA analysis, shifts from baseline in the microbial structure within the FMT group occurred after the intervention (Fig.S3), with changes detected at the day 30, 60, and 90 visits (p < 0.05). The microbial structure changed between the groups over a similar period (Fig. 2E). There were no differences in alpha or beta diversity across visits within the placebo group (Fig.S3).
Engraftment of donor microbiota after FMT
Distinctions in microbial structure between donor and FMT recipients, which were assessed via the Bray‒Curtis distance, were consistently observed throughout the study (Fig.S4); however, the microbiome composition was altered in recipients, indicating that a single course of FMT is insufficient to restore a normal microbial composition in hypertensive patients. The main differentially abundant phyla (Fig. 2C) in hypertensive patients [31], Firmicutes and Bacteroidetes, were parallel between the FMT group and donors (adjusted p > 0.05) after intervention but the changes were reversed after day 30 (Fig. 2D & S4 C). Within-group alterations in phyla after FMT also reached statistical significance at the day 30 visit, with a decreased abundance of Firmicutes and an increased abundance of Bacteroidetes; the latter phylum was negatively correlated with office and 24-h SBP but was not significantly different from that in the placebo group (Fig.S4B). Additionally, changes in the abundance of 11 (55%) of the top 20 abundant genera were observed in response to FMT, although these changes were not maintained after the intervention ceased (Fig. S5).
Changes in microbial composition and correlations with clinical phenotype
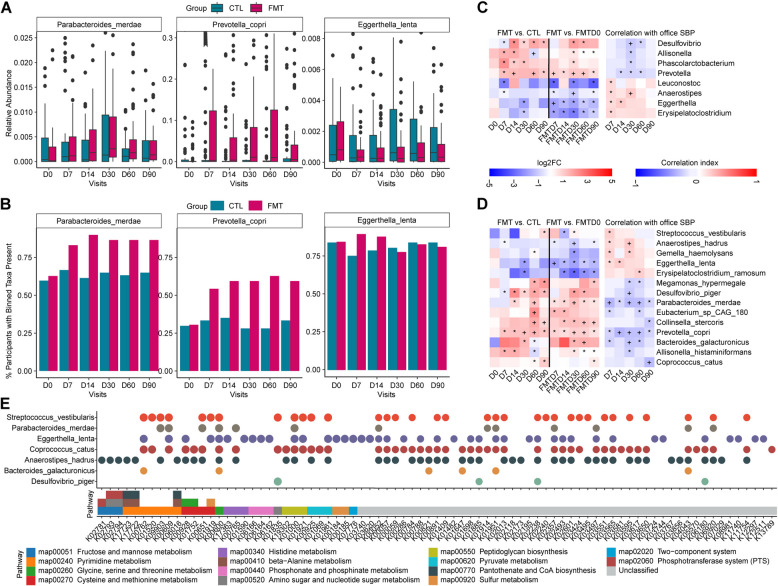
Through MetaPhlAn analysis, 218 species and 97 genera were identified across 748 samples covering 17 donors, 124 participants, and 7 visits. A total of 17 differential genera were detected between arms and within the FMT arm from baseline (Fig.S6 A, Table S5). Partial Spearman correlation analysis (Fig. 3C, Table S6) revealed that the abundances of four genera whose abundance decreased, Eggerthella, Anaerostipes, Leuconostoc, and Erysipelatoclostridium were correlated with office SBP. Among them, Erysipelatoclostridium comprising strains producing IgA proteases [32], a feature we observed in the renal microbiota of hypertensive hosts [33], presented with pathogenic features of bacterial invasion in immunocompromised individuals. Genera increased in abundance (Fig. 3C&S7 A) have been identified and reversely associated with office SBP, including Desulfovibrio, Prevotella, Allisonella, and Phascolarctobacterium, the genera also decreased in individuals with nonalcoholic fatty liver disease [34] and responds to the antidiabetic agents metformin and berberine [35].
Fig. 3.
Alterations in microbiome profile after FMT and placebo intervention. A Box plot showing the relative abundance of examples of differential species, including Parabacteroides merdae, Prevotella copri, and Eggerthella lenta, between the FMT (red) and control (blue) arms across visits on the basis of a zero-inflated beta regression (ZIBR) model, adjusted p < 0.05. B The bar plot shows the percentage of participants with species from Fig. 3A present in the FMT and control (CTL) arms across visits. C‒D Heatmap showing office SBP-related, differential genera (C, upper) and species (D, lower) that changed within the FMT arm and between the FMT and control arms (left), as well as their correlations with office SBP over follow-up visits (right), adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, and baseline office SBP and DBP, using partial Spearman correlation analysis (*, adjusted p < 0.05; +, adjusted p < 0.01). FC, fold of change. E A total of 97 office-SBP-associated KOs changed both within the FMT group and between groups from Fig. S12; the KEGG pathways and the corresponding office SBP-associated, differential species from Fig. 3D annotated from those KOs are shown
Notably, 35 species differed within the FMT group and between arms (Fig.S6B, Table S5). Among them, 14 species correlated with office SBP (Fig. 3D, Table S6). The differential genera and species that responded to FMT were identified to coexist in both the donors and FMT recipients at baseline (Fig.S8). The abundances of Parabacteroides merdae, Bacteroides galacturonicus, Eubacterium sp. CAG 180, Prevotella copri, Desulfovibrio piger, Megamonas hypermegale, Collinsella stercoris, Coprococcus catus, and Allisonella histaminiformans were increased after FMT and were negatively correlated with office SBP. The abundances of Erysipelatoclostridium ramosum, Anaerostipes hadrus, Gemella haemolysans, Eggerthella lenta, and Streptococcus vestibularis, decreased after FMT, were presented with BP-elevating features. Interestingly, the increases of Parabacteroides merdae, Prevotella copri, and the decrease of Eggerthella lenta were consistently observed in abundance and/or prevalence within the FMT group (Fig. 3D), compared with donors (Fig.S8). These findings were repeatedly confirmed between the intervention groups through ZIBR models for longitudinal change evaluation (Fig. 3A&B).
Validation of microbial changes across clinical centers
To assess the reproducibility of the differential microbes as biomarkers across study centers, intra-center, cross-center, and leave-one-center-out (LODO) prediction validations were performed using the complete set of FMT and control samples via random forest classifier models (Fig.S9, Table 4&S4&S16). The average area under the curve (AUCs) using the profile of 8 genera were 0.75, 0.60, and 0.68 at the day 7 visit; 0.71, 0.56, and 0.66 at the day 14 visit; 0.76, 0.65, and 0.71 at the day 30 visit; 0.83, 0.56, and 0.61 at the day 60 visit; and 0.68, 0.52, and 0.61 at the day 90 visit, for intra-center, cross-center, and LODO predictions, respectively. For validation using the profile of 14 species, the AUCs were 0.72, 0.57, and 0.66 on day 7; 0.70, 0.55, and 0.58 on day 14; 0.80, 0.58, and 0.64 on day 30; 0.91, 0.64, and 0.73 on day 60; and 0.78, 0.64, and 0.71 on day 90, for intra-center, cross-center, and LODO predictions, respectively. The AUCs during follow-up visits were improved after removing center 4, ranging from 0.74 to 0.93, 0.62 to 0.76, and 0.61 to 0.78 for the 14 species; and from 0.70 to 0.87, 0.55 to 0.72, and 0.64 to 0.75 for the 8 genera, for intra-center, cross-center, and LODO predictions, respectively.
Table 4.
Average AUCs for intra-center, cross-center, and leave-one-center-out prediction validations on FMT and control samples
| Validation method | Average AUCs at visits | ||||
|---|---|---|---|---|---|
| D7 | D14 | D30 | D60 | D90 | |
| Based on 14 species | |||||
| Intra-center | 0.72 | 0.70 | 0.80 | 0.91 | 0.78 |
| Cross-center | 0.57 | 0.55 | 0.58 | 0.64 | 0.64 |
| LODO | 0.66 | 0.58 | 0.64 | 0.73 | 0.71 |
| Based on 14 species excluding center 4 | |||||
| Intra-center | 0.74 | 0.76 | 0.82 | 0.93 | 0.80 |
| Cross-center | 0.64 | 0.62 | 0.67 | 0.76 | 0.74 |
| LODO | 0.70 | 0.61 | 0.70 | 0.78 | 0.75 |
Prediction validations are performed using xMarkerFinder on FMT and CTL samples at D7, D14, D30, D60, and D90 visits
AUC, area under the curve, LODO leave-one-center-out
Microbial function analysis via KEGG orthology
Although no inter-group differences were detected, the KEGG ortholog (KO) structure (Fig. S10&S11) was significantly altered within the FMT group at the day 7, 14, and 30 visits, prior to microbial structure alterations (Fig. 1E&S3). HUMAnN2 analysis revealed that 4338 KOs and 210 KOs were altered within the FMT group and between arms, and that 97 KOs (Fig. 3E&S12, Table S7), which partially overlapped with BP-related KOs identified in a previous cohort [36], were associated with office SBP. The main annotated species included Parabacteroides merdae, Desulfovibrio piger, Bacteroides galacturonicus, Coprococcus catus, Eggerthella lenta, Anaerostipes hadrus, and Streptococcus vestibularis (Fig. 3E&S13, Table S8). The following KEGG pathways (Fig. 4C, Table S9) were annotated: (1) production of metabolites, such as alanine, aspartate, glutamate, lysine, serine, cysteine, methionine, phenylalanine, tyrosine, and tryptophan; (2) persistence factors associated with genetic processing (i.e., DNA repair and recombination proteins); (3) environmental communication (i.e., ABC transporters and two-component systems); and (4) virulence factors such as peptidoglycan biosynthesis and cationic antimicrobial peptide (CAMP) resistance.
Fig. 4.
Changes in metabolic features after FMT and placebo intervention. A Bar plot showing the relative abundance of metabolites, including asparagine, methionine, phenylalanine, sarcosine, and tyrosine, identified through the ZIBR model with FDR adjusted p-value < 0.05, on the basis of the eight office SBP-associated, differentially abundant metabolites from targeted metabolomic profiling within the FMT group and between arms from Fig. S15. B Venn diagrams present differentially abundant metabolites within the FMT group and between the group after intervention at the day 30 visit (left, 693 metabolites) and the day 90 visit (right, 1165 metabolites) from untargeted metabolomic profiling analysis. C Bar plot showing that the KEGG pathways annotated from 97 office SBP-associated KOs changed both within the FMT group and between the groups (Fig. S12). Between-group differences and within-group differences were analyzed via the Wilcoxon rank sum test and paired Wilcoxon rank sum test, respectively, with the p value adjusted by the FDR. D Heatmaps showing 31 metabolites changed within the FMT group and/or between groups (medium) from targeted metabolomic profiling analysis for amino acids, TMAO pathway-related metabolites, and SCFA; and their correlations with the 14 office-SBP-related differential species after interventions at the day 30 visit (left); and their correlations with office SBP over the study period (right), adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, and baseline office SBP and DBP, using partial Spearman correlation analysis. Between-group differences and within-group differences were analyzed via the Wilcoxon rank sum test and paired Wilcoxon rank sum test, respectively, with FDR adjustment (*, p < 0.05; +, p < 0.01). FC, fold of change
Alterations in plasma metabolites after the FMT intervention
To explore the effects of FMT on the metabolic profiles, untargeted metabolomic analysis via LC‒MS was performed on 60 randomly selected participants (30 in each group based on a block of 4) at baseline and at the day 30 and day 90 visits, with 4696 metabolites identified (Fig.S1B). The metabolic structure determined via PCoA could barely discriminate between the FMT and placebo groups at each visit, indicating stable or recovered circulating metabolites 1 month after FMT (Fig. S14). However, the levels of 33 metabolites, such as derivatives of acetate, butyric acid, glutamic acid, phenylalanine, lysine, carnitine, kynurenic acid, indoles, and steroids annotated to lipid and amino acid metabolism, were altered within the FMT group and between groups (Fig. 4B, Tables S10–S12).
Then, targeted mass spectrometry of KO-annotated, untargeted profiling, and previously reported hypertension-related metabolic pathways of amino acids, TMAO and SCFA [13], was performed on samples from all participants at baseline, and at the day 30 and day 90 visits (Fig.S15, Table S13). Among them, glutamine, aspartate, asparagine, tyrosine, phenylalanine, methionine, serine, and sarcosine were altered both within the FMT group and between arms, corresponding to microbial KOs alterations and/or responding metabolites from untargeted analysis. The level of these eight metabolites increased after FMT prescription and were inversely correlated with office SBP (Fig. 4D, Table S14). Additionally, changes in the levels of asparagine, phenylalanine, methionine, serine, and sarcosine were repeatedly confirmed via ZIBR models (Fig. 4A).
In contrast, the levels of TMAO pathway-related metabolites and SCFA, metabolites that are thought to be associated with hypertension, were not different between the FMT group and the placebo group (Figs .4D&S15), although changes within the FMT group were observed. SCFA such as acetic acid, propionic acid, and butyric acid were decreased within the FMT group and positively associated with office SBP. TMAO precursors such as choline, betaine, and carnitine were increased within the FMT group and negatively correlated with office SBP at the day 30 visit.
Correlations among the levels of all targeted metabolites, the abundance of differentially abundant microbes, and office SBP level were then explored (Figs .4D&S15, Table S15). The abundances of opportunistic pathogens, including Eggerthella lenta, Erysipelatoclostridium ramosum, Anaerostipes hadrus, Streptococcus vestibularis, and Gemella haemolysans, which were negatively associated with the levels of the aforementioned differentially abundant amino acids and/or TMAO precursors but positively correlated with the level of butyric acid, decreased after FMT. The opposite change was observed for the levels of those metabolites, and the abundance of species such as Bacteroides galacturonicus, Prevotella copri, Desulfovibrio piger, Parabacteroides merdae, and Allisonella histaminiformans increased after FMT.
Discussion
This is the first randomized, blinded, multicenter, clinical trial of FMT for primary hypertension treatment (Fig. 5). A short-term decrease in BP amelioration was detected, with a significant between-arm difference of − 4.34 (95% CI, − 8.1 to − 0.58) mmHg within the first week of FMT intervention, but this difference did not persist after intervention termination or until the day 30 visit. Subgroup analysis revealed that those aged over 48 years significantly benefited from FMT even at the 1-month follow-up, with an intergroup difference of 7.65 (95% CI, 0.78–14.51) mmHg. The oral capsules used in this study were prepared with freeze-dried microbiota, which were found to be equally effective as fresh microbiota in rCDI treatment [37]. The use of frozen materials and capsulized FMT techniques allows for accessibility, which is restricted by comprehensive and strict donor selection processes, the avoidance of infectious agents, and the need to follow guidelines and expert consensus when preparing materials for FMT [28–30] (Supplementary Materials), as well as safety and compliance, particularly among patients with chronic disorders requiring repeated intervention, as it does not require endoscopy. In line with our observations, a recent retrospective study of 73 Chinese hypertensive patients receiving invasive microbiota transplantation reported a reduction of 5.09 (15.51) mmHg in the office SBP (p = 0.009) during the first hospital stay of a median of 8 (5–10) days; however, this reduction could not be maintained until the 1-month follow-up, with a changed SBP of 2.23 (17.54) mmHg (p = 0.28) [38]. Four clinical trials of FMT for obesity and/or metabolic syndrome also yielded data on BP changes [23, 39–41]. These changes were out of statistical significance after isolated FMT intervention via capsules or invasive tubes [39–41]. However, the use of oral FMT capsules in combination with high-fermentable or low-fermentable fiber supplementation decreased office SBP level over 12 weeks [23]. Interestingly, high-fiber diet modulates gut microbiota function and high-fermented diet intervene microbiota diversity and host inflammation [42]. Strategies such as fiber (prebiotics), and fermented products (postbiotics) such as acetate, presented with promising results in hypertension intervention from animal models [17]. An insight to restoration of microbial functional network rather than merely the compositional changes is important.
Fig. 5.
Structured graphical abstract of the study. This is the first multicenter randomized, blinded, placebo-controlled clinical trial to explore the safety and efficacy of FMT in treating hypertension. FMT via oral capsules is safe for hypertension treatment, with self-limiting or mild AEs observed, and is comparable with the placebo. However, FMT has only a 1-week BP-attenuating effect that is eliminated with intervention termination and does not support our hypothesis for the primary outcome of office SBP change at the day- 30 follow-up. Alterations and interactions of 8 genera, 14 species, 97 KOs, and 8 amino acids in response to FMT and with BP-affecting features were identified via multiomics analysis of metagenomic, untargeted and targeted metabolomic data
In this study, we identified 8 genera and 14 species responding to FMT interventions. Upon replication analysis, the differential, office SBP-related microbes successfully discriminated the FMT group from the control group across centers, except for center 4 from Shandong Province. Possible explanations include the following: (1) a relatively high salt diet, which was previously reported to contribute to hypertension and to affect the gut microbiota [11, 43] (Shandong is one of the largest salt-producing and salt-consuming provinces [44, 45] in China); and (2) the proximity of center 4 to the ocean. Opportunistic pathogens such as Eggerthella lenta, which is capable of causing extraintestinal infections such as bacteremia, abscesses, or endocarditis, decreased after FMT, and showed BP-elevating features during visits. In contrast, among the species whose abundance was increased after FMT, Parabacteroides merdae maintained inverse correlations with office SBP over the study period. Recent studies have revealed the cardiovascular-protecting effect of Parabacteroides merdae, which attenuates atherosclerotic plaque in animals and resists cardiovascular damage via the degradation of branched chain amino acids such as leucine, isoleucine, and valine [46]. Additionally, we observed that the abundance of Prevotella copri, a species with pathogenic potency in rheumatoid arthritis [47, 48], was inversely correlated with BP over time. Interestingly, Prevotella copri has been identified as a positive indicator of postprandial glucose and cardiometabolic metabolism [49]; is more common in non-Western populations that consume high-fiber and low-fat diets [50]; and is the dominant taxon in the heathy microbiota of malnourished young individuals fed formula [51]. Strain-specific preferences for diverse metabolic patterns have been reported for Prevotella copri (i.e., carbohydrate metabolism, BCAA production via protein metabolism, and succinate production) [50, 52], which may explain the controversial impacts of Prevotella copri on diseases, deserving stepwise explorations.
Microbial function after FMT was also analyzed in this study. Alterations in KOs occur prior to alterations in microbial structure, and KOs were shared among the above differential microbes. In addition to features related to within-host adaptation and virulence, we observed that altered microbial KOs were mainly involved amino acid-related pathways. After metabolomic profiling analysis, the levels of glutamine, aspartate, phenylalanine, tyrosine, serine, methionine, sarcosine, and asparagine were confirmed to be increased within the FMT group and within groups, and these metabolites were found to interact with differential species after FMT. Community-based cohorts indicated an inverse association among glutamine levels and cardiometabolic factors such as BP and cardiovascular mortality risk [53, 54]. Recent in vivo and clinical studies have revealed the ability of glutamine to lower BP and protect against left ventricular hypertrophy [53, 55]. Moreover, glutamine interferes with the gut microbiota via multiple mechanisms [56–58], i.e., (1) reducing the Firmicutes-to-Bacteroidetes ratio; (2) increasing the abundance of the Prevotella genus; (3) increasing immunoglobulin A levels, promoting intestinal bacterial aggregation; and (4) limiting bacterial translocation in patients and animal models. Similarly, we observed associations of the levels of metabolites such as aspartate and tyrosine with clinical phenotypes and species. A Mendelian randomization study suggested a genetically predicted inverse association between aspartate levels and BP [59]. Tyrosine, a nonessential aromatic amino acid derived from phenylalanine, is the precursor of neurotransmitters such as catecholamines and dopamine, linking gut–brain communication, and reduces BP in SHRs in which norepinephrine is released in the brain [60]. Microbe‒metabolite interactions and their functions in BP modulation are therefore indicated and deserve further exploration.
Interestingly, this study revealed that SCFA levels decreased within the FMT arm and were positively associated with office SBP. These observations align with a recent cross-sectional study of 241 Hong Kong Chinese individuals that reported elevated total SCFA, propionic acid, and acetic acid levels in the blood of hypertensive individuals [61]. Notably, there is a conflicting phenomenon regarding the direction of associations between fecal SCFA and BP levels [62, 63], and the oral administration of SCFA decreases BP levels in animal models [10, 17]. In addition, although the protective role of SCFA has also been observed in patients cohorts and animal models of other cardiometabolic diseases, including type 2 diabetes, metabolic syndrome, and obesity. In clinical trials, FMT combined with supplementation of fibers, which can ferment SCFA [23], or probiotics alone [64] barely improved clinical parameters of evaluating glucose tolerance and insulin resistance. Possible explorations and validation of the asymmetry between blood SCFA levels and BP are needed, and may involve studies on SCFA-sensing receptors, bidirectional inflammation-related effects, and endothelial functions.
Although the changes in the abundance of the primary differentially abundant phyla in our recipients, Firmicutes and Bacteroidetes, were in parallel with those in donors after FMT, these changes were reversed after intervention termination and at the day 30 follow-up visit. One-course FMT intervention in our study did not influence the evenness or significant similarity of microbial structure between donors and recipients, although it changed the microbial composition and function in the FMT recipients. Additionally, the differential taxa between and within arms after intervention were present in both donors and recipients at baseline, which aligns with a perspective for preadaptation, enabling engraftment with similar microbial taxa [65, 66]. This indicated the resistance of the recipient microbiome, corresponding to the short-term decrease in BP observed. Meta-analyses of clinical trials on BP regulation via prebiotics or probiotics [14, 15, 67] have found that factors, such as the intervention duration (≥ 8 weeks or ≥ 4 weeks), multiple species, consumption dose (≥ 1011 or ≥ 2 × 1010 colony-forming units per day), age (< 60 years), and diabetes mellitus comorbidity, affect decreases in BP. Moreover, the issue of samples from mixed donors’ batch or a single donor for recipients remains to be explored [65, 66]. Indeed, a clinical trial (ClinicalTrials.NCT05608447) involving repeated FMT interventions used in this study is currently being conducted to explore the medium-term effects of FMT on primary hypertension. Further studies on anchoring donors using high-throughput sequencing and machine learning approaches may lead to the development of matching interventions via microbiota transfer.
Limitations
This study has several limitations. This multicenter study included only Chinese patients from a limited geographical region, which may limit the generalizability of the study observations to individuals of diverse races or genetic backgrounds. The sample size from each center was limited to allow validation across multiple centers. Data on the metabolomic profiles of plasma at days 7, 14, and 60 are lacking because of ethical considerations and the invasiveness of blood sample collection, constraining more profound analysis of alterations in the levels of metabolites over time and their potential interactions with microbe species, particularly in the period during which the BP decreased early after FMT intervention. The follow-up period was 3 months, and information on the long-term safety of FMT is limited. In addition, a one-course FMT intervention was administered at three visits over 2 weeks; these visits were designed to occur early in the study, with limited available references on oral FMT capsule treatment, which may explain in part the resilience of the host microbiota in hypertensive patients. Additionally, the reproducibility of FMT capsules can be influenced by the donors and laboratories used, despite the use of general guidelines or expert consensus, which may affect the clinical ability of FMT to treat diseases and deserve caution. Finally, this study used MetaPhlAn3 and HUMAnN3 for microbial taxa and function analysis. For future research endeavors that aim to perform a more in-depth analysis of specific microbial genomes, the metagenome-assembled genomes are recommended into the analytical framework.
Conclusion
In this first randomized clinical study of FMT in patients with hypertension, one course of treatment via oral FMT capsules presented similar safety concerns as the placebo did within 3 months of follow-up and resulted in a short-term BP reduction after intervention initiation; however, this decrease was gradually reversed over time. Multiomics analysis revealed alterations in the microbiome composition, functional KOs, and changes in specific amino acid levels in response to FMT. Further studies involving more intense modulation of the gut microbiota in patients with hypertension and explorations of the antihypertensive mechanisms of specific microbes with metabolite-targeting abilities are recommended.
Supplementary Information
Supplementary Material 1: Figure S1. The Metagenomic and Metabolomic Analysis Flowcharts. Figure S2. Microbial Diversity Within and Between Groups. Figure S3. Microbial Diversity Within Groups. Figure S4. Microbial Composition of the Donors and FMT Group. Figure S5. Microbial Composition of Genera Between Donors and the FMT Group. Figure S6. Microbiome Profile Within and Between the Groups and Clinical–Phenotype Association. Figure S7. Examples of office SBP-associated Differential Genera and Species Within and Between Groups. Figure S8. Changes in Microbial Abundance Between Donors and the FMT Group. Figure S9. AUC matrix using 14 species (left panel) and 8 genera (right panel) at five follow-up timepoints. Figure S10. Diversity of KEGG Orthology Between Groups. Figure S11. Diversity of KEGG Orthology within Groups. Figure S12. Differential KOs Within and Between Arms Associated with Clinical Office SBP. Figure S13. Correlations Between Office SBP-Related and Differential Species and KOs. Figure S14. Untargeted Metabolomic Analysis. Figure S15. Targeted Metabolomic Analysis. Figure S16. Clinical Phenotype Associations with Differentially Abundant Species. Figure S17. Clinical Phenotype Associations with Differentially Abundant Metabolites.
Supplementary Material 2: Table S1. Extended Baseline Characteristics of the Study Participants. Table S2. Sensitivity and Subgroup Analysis of the Primary Endpoint. Table S3. Secondary Outcome Measures Compared Between Groups. Table S4. Average AUCs based on 8 Genera for Intra-center, Cross-center, and Leave-One-Center-Out Prediction Validations on FMT and Control Samples.
Supplementary Material 3: Table S5. Detailed information on the differential microorganisms within the FMT group and between the FMT and control groups. Table S6. Partial Spearman's correlation between the altered gut microbial signatures (phylum, genera, species) and office SBP over follow-up visits adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, baseline office SBP and DBP. Table S7. Detailed information on significantly altered KEGG Orthology (KO, HUMAnN analysis) within the FMT group and between the FMT and control groups. Table S8. Species annotation to 14 species of 97 office-SBP-related, altered KOs within and between groups. Table S9. Table S10. Detailed information on differential plasma metabolites from untargeted metabolomic profiling analysis. KEGG pathway annotation of 97 office-SBP-related, altered KOs within and between groups. Table S11. Functional enrichment of differential plasma metabolites from untargeted metabolomic profiling analysis via MetaboAnalyst. Table S12. Partial Spearman's correlation between the altered untargeted metabolites and office SBP over follow-up visits adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, baseline office SBP and DBP. Table S13. Detailed information on differential plasma metabolites from targeted metabolomic profiling analysis (amino acids, TMAO-related metabolites, SCFAs). Table S14. Partial Spearman's correlation between the altered targeted metabolites and office SBP over follow-up visits adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, baseline office SBP and DBP. Table S15. Partial Spearman's correlation between the altered microbial signatures and metabolites over follow-up visits adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, baseline office SBP and DBP. Table S16. Number of included samples for Random Forest classifier. Table S17. The quality control metrics of gut metagenomics profling analysis.
Acknowledgements
We thank Wei Li, Yang Wang, Yi Sun, and Qianqian Wei from the Medical Research & Biometrics Center, National Center for Cardiovascular Diseases, Fuwai Hospital Chinese Academy of Medical Sciences for statistical analysis consultation, EDC system and centralized randomization system construction, and data management. We appreciate all personnel involved in the study from the investigation centers and collaborating parties, including but not limited to Xiurong Guo, Enhua Cao, Hailei Wu, Shutong Wang, Lu Cheng, Xuefang Yan, Zhen Chen, Dan Qi, Changyi Zhang, Weiqiang Wu, Zhichao Chen, Yonghong Yuan, Ruishan Zhang, Xian Qiu, Yun Ti, Yajing Zhao, Wen Zhang, Youqing Liang, Yunjian Li, Qian Wang, and all study participants, for their contribution.
Abbreviations
- FMT
Fecal microbiota transplantation
- BP
Blood pressure
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- AE
Adverse event
- GF
Germ-free
- SHRs
Spontaneously hypertensive rats
- WKY
Wistar-Kyoto;
- SCFA
Short-chain fatty acid
- hSIH
High-salt-induced hypertension
- IBD
Inflammatory bowel disease
- CRF
Case report form
- EDC
Electronic data capture
- CVD
Cardiovascular disease
- Ang
Angiotensin
- CI
Confidence interval
- BMI
Body mass index
- ABI
Ankle–brachial index
- LAACI
Large artery atherosclerotic cerebral infarction
- TIA
Transient ischemic attack
- PCI
Percutaneous transluminal coronary intervention
- CABG
Coronary artery bypass grafting
- NYHA
New York Heart Association
- ABPM
24-Hour ambulatory blood pressure monitoring
- ECG
Electronic cardiogram
- PWV
Pulse wave velocity
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- K
Potassium
- Na
Sodium
- Cl
Chloride
- BUN
Urea nitrogen
- CREA
Creatinine
- UA
Uric acid
- TC
Total cholesterol
- TG
Triglyceride
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- GLU
Fasting blood glucose
- GCP
Good Clinical Practice
- ID
Identification
- SD
Standard deviation
- IQR
Interquartile range
- LOCF
Last-observation-carried-forward
- CC
Complete case
- MI
Multiple imputation
- CTCAE
Common Terminology Criteria for Adverse Events
- STEP
Strategy of Blood Pressure Intervention in Elderly Hypertensive Patients
- AUC
Area under the curve
- LODO
Leave-one-center-out
- ENA
European Nucleotide Archive
- EI
Electron bombardment ionization
- CV
Coefficient of variation
- PERMANOVA
Permutational multivariate analysis of variance
- FDR
False discovery rate
- pSRC
Partial Spearman’s correlations
- ZIBR
Zero-inflated beta regression
- KO
KEGG orthology
- CAMP
Cationic antimicrobial peptide
- TMAO
Trimethylamine n-oxide
- BCAA
Branched-chain amino acids
- CDI
Clostridium difficile infection
Authors’ contributions
LYF contributes to literature search, study design and implementation, data collection, analysis, interpretation, and paper writing. JRC, QZ, YYZ and YW contribute to study methodology, data analysis, interpretation, and paper drafting. JR, YRC, JFY, LW, ZHG, PLB, BPZ, XYL, WJW, ZZC, QNG contribute to study implementation, data collection, and paper drafting. LMZ contributes to the data collection and interpretation, and paper reviewing critically for important intellectual content. JC contributes to study design and supervision, funding acquisition, data interpretation, and paper reviewing critically for important intellectual content. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. LYF and JC have directly accessed and verified the underlying data reported in the manuscript.
Funding
This work was supported by National Science and Technology Major Project for the Prevention and Treatment of Cancer, Cardiovascular and Cerebrovascular Diseases, Respiratory Diseases, and Metabolic Diseases (Grant No.2024ZD0526804, 2024ZD0526800); Beijing Research Ward Excellence Program, BRWEP (Grant No. BRWEP2024W012060100, BRWEP2024W012060105); National Natural Science Foundation of China (Grant No. 82300564, 82330013, 81630014, 81825002); Beijing Municipal Science & Technology Commission (Grant No. Z231100004623009, Z241100009024041); Beijing Natural Science Foundation (Grant No. L248105); Beijing Hospitals Authority Clinical medicine Development of special funding support (Grant No. ZLRK202511); CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M- 1–007); Key project of Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (Grant No. CI2021 A00920); Beijing Outstanding Young Scientist Program (Grant No. BJJWZYJH01201910023029); and Beijing Municipal Science & Technology Commission (Grant No.Z231100004623009).
Data availability
Sequence data that support the findings of this study have been deposited in the European Nucleotide Archive with the primary accession code PRJEB72385(Project: PRJEB72385, https://www.ebi.ac.uk/ena/browser/view/PRJEB72385) and the private link https://figshare.com/s/3d6633ce046aa475fb74. Editors and reviewers can reach the data via search the project ID or use the link provided on above database.
Declarations
Ethics approval and consent to participate
This investigator-initiated trial, registered on ClinicalTrials.gov (NCT04406129, 28 May 2020), was approved by the Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences (approval NO. 2020–1334, 14 April 2020). Written informed consent was obtained before participant enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luyun Fan, Junru Chen, Qi Zhang, Jie Ren, Youren Chen, Jinfeng Yang, and Lu Wang are co-first authors.
Contributor Information
Lemin Zheng, Email: zhengl@bjmu.edu.cn.
Jun Cai, Email: caijun7879@126.com.
References
- 1.Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–80. [DOI] [PMC free article] [PubMed]
- 2.Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18(11):785–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muralitharan RR, Jama HA, Xie L, Peh A, Snelson M, Marques FZ. Microbial peer pressure: the role of the gut microbiota in hypertension and its complications. Hypertension. 2020;76(6):1674–87. [DOI] [PubMed] [Google Scholar]
- 4.Finlay BB, Humans C, Microbiome t. Are noncommunicable diseases communicable? Science. 2020;367(6475):250–1. [DOI] [PubMed]
- 5.Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR Jr, et al. Gut microbiota composition and blood pressure. Hypertension. 2019;73(5):998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5(9):e003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H, et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. 2020;141(17):1393–403. [DOI] [PubMed] [Google Scholar]
- 11.Yan X, Jin J, Su X, Yin X, Gao J, Wang X, et al. Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ Res. 2020;126(7):839–53. [DOI] [PubMed] [Google Scholar]
- 12.Yang T, Mei X, Tackie-Yarboi E, Akere MT, Kyoung J, Mell B, et al. Identification of a gut commensal that compromises the blood pressure-lowering effect of ester angiotensin-converting enzyme inhibitors. Hypertension. 2022;79(8):1591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Donnell JA, Zheng T, Meric G, Marques FZ. The gut microbiome and hypertension. Nat Rev Nephrol. 2023;19(3):153–67. [DOI] [PubMed] [Google Scholar]
- 14.Evans CE, Greenwood DC, Threapleton DE, Cleghorn CL, Nykjaer C, Woodhead CE, et al. Effects of dietary fibre type on blood pressure: a systematic review and meta-analysis of randomized controlled trials of healthy individuals. J Hypertens. 2015;33(5):897–911. [DOI] [PubMed] [Google Scholar]
- 15.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64(4):897–903. [DOI] [PubMed] [Google Scholar]
- 16.Galla S, Chakraborty S, Cheng X, Yeo J, Mell B, Zhang H, et al. Disparate effects of antibiotics on hypertension. Physiol Genomics. 2018;50(10):837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964–77. [DOI] [PubMed] [Google Scholar]
- 18.Mähler A, Wilck N, Rauch G, Dechend R, Müller DN. Effect of a probiotic on blood pressure in grade 1 hypertension (HYPRO): protocol of a randomized controlled study. Trials. 2020;21(1):1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98. [DOI] [PubMed] [Google Scholar]
- 20.Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321(2):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haifer C, Paramsothy S, Kaakoush NO, Saikal A, Ghaly S, Yang T, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2022;7(2):141–51. [DOI] [PubMed] [Google Scholar]
- 22.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mocanu V, Zhang Z, Deehan EC, Kao DH, Hotte N, Karmali S, et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med. 2021;27(7):1272–9. [DOI] [PubMed] [Google Scholar]
- 24.Ng SC, Xu Z, Mak JWY, Yang K, Liu Q, Zuo T, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. 2022;71(4):716–23. [DOI] [PubMed] [Google Scholar]
- 25.Allegretti JR, Mullish BH, Kelly C, Fischer M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet. 2019;394(10196):420–31. [DOI] [PubMed] [Google Scholar]
- 26.Fan L, Ren J, Chen Y, Wang Y, Guo Z, Bu P, et al. Effect of fecal microbiota transplantation on primary hypertension and the underlying mechanism of gut microbiome restoration: protocol of a randomized, blinded, placebo-controlled study. Trials. 2022;23(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(7):579–615. [PubMed] [Google Scholar]
- 28.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haifer C, Kelly CR, Paramsothy S, Andresen D, Papanicolas LE, McKew GL, et al. Australian consensus statements for the regulation, production and use of faecal microbiota transplantation in clinical practice. Gut. 2020;69(5):801–10. [DOI] [PubMed] [Google Scholar]
- 30.Ng SC, Kamm MA, Yeoh YK, Chan PKS, Zuo T, Tang W, et al. Scientific frontiers in faecal microbiota transplantation: joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut. 2020;69(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N, et al. The gut microbiome in hypertension: recent advances and future perspectives. Circ Res. 2021;128(7):934–50. [DOI] [PubMed] [Google Scholar]
- 32.Milosavljevic MN, Kostic M, Milovanovic J, Zaric RZ, Stojadinovic M, Jankovic SM, et al. Antimicrobial treatment of Erysipelatoclostridium ramosum invasive infections: a systematic review. Rev Inst Med Trop Sao Paulo. 2021;63: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu XY, Li J, Zhang Y, Fan L, Xia Y, Wu Y, et al. Kidney microbiota dysbiosis contributes to the development of hypertension. Gut Microbes. 2022;14(1):2143220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17(5):279–97. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmu J, Salosensaari A, Havulinna AS, Cheng S, Inouye M, Jain M, et al. Association between the gut microbiota and blood pressure in a population cohort of 6953 individuals. J Am Heart Assoc. 2020;9(15):e016641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318(20):1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong HJ, Zeng HL, Cai YL, Zhuang YP, Liou YL, Wu Q, et al. Washed microbiota transplantation lowers blood pressure in patients with hypertension. Front Cell Infect Microbiol. 2021;11:679624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913-6.e7. [DOI] [PubMed] [Google Scholar]
- 40.Leong KSW, Jayasinghe TN, Wilson BC, Derraik JGB, Albert BB, Chiavaroli V, et al. Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA Netw Open. 2020;3(12):e2030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartstra AV, Schüppel V, Imangaliyev S, Schrantee A, Prodan A, Collard D, et al. Infusion of donor feces affects the gut-brain axis in humans with metabolic syndrome. Mol Metab. 2020;42:101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature. 2017;551(7682):585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li LM, Rao KQ, Kong LZ, Yao CH, Xiang HD, Zhai FY, et al. A description on the Chinese national nutrition and health survey in 2002. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(7):478–84. [PubMed] [Google Scholar]
- 45.Wang Q. Review and prospective on salt archaeology in Shandong. Huaxia archaeology. 2012;4:59–69. [Google Scholar]
- 46.Qiao S, Liu C, Sun L, Wang T, Dai H, Wang K, et al. Gut Parabacteroides merdae protects against cardiovascular damage by enhancing branched-chain amino acid catabolism. Nat Metab. 2022;4(10):1271–86. [DOI] [PubMed] [Google Scholar]
- 47.Stoll ML. Genetics, Prevotella, and the pathogenesis of rheumatoid arthritis. The Lancet Rheumatology. 2020;2(7):e375–6. [DOI] [PubMed] [Google Scholar]
- 48.Nii T, Maeda Y, Motooka D, Naito M, Matsumoto Y, Ogawa T, et al. Genomic repertoires linked with pathogenic potency of arthritogenic Prevotella copri isolated from the gut of patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82(5):621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021;27(2):321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prasoodanan PKV, Sharma AK, Mahajan S, Dhakan DB, Maji A, Scaria J, et al. Western and non-western gut microbiomes reveal new roles of Prevotella in carbohydrate metabolism and mouth-gut axis. NPJ Biofilms Microbiomes. 2021;7(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raman AS, Gehrig JL, Venkatesh S, Chang HW, Hibberd MC, Subramanian S, et al. A sparse covarying unit that describes healthy and impaired human gut microbiota development. Science. 2019;365(6449):eaau4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Filippis F, Pasolli E, Tett A, Tarallo S, Naccarati A, De Angelis M, et al. Distinct genetic and functional traits of human intestinal Prevotella copri strains are associated with different habitual diets. Cell Host Microbe. 2019;25(3):444–53. [DOI] [PubMed] [Google Scholar]
- 53.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng Y, Hu FB, Ruiz-Canela M, Clish CB, Dennis C, Salas-Salvado J, et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvención con DIeta MEDiterránea (PREDIMED) trial. J Am Heart Assoc. 2016;5(9):e003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L, Xu L, Li J, Wang H, Sun J, Yu Z, et al. The association of dietary glutamine supplementation with the development of high salt-induced hypertension in rats. Front Nutr. 2022;9:1011739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Souza AZ, Zambom AZ, Abboud KY, Reis SK, Tannihão F, Guadagnini D, et al. Oral supplementation with L-glutamine alters gut microbiota of obese and overweight adults: a pilot study. Nutrition. 2015;31(6):884–9. [DOI] [PubMed] [Google Scholar]
- 57.Ren W, Wang K, Yin J, Chen S, Liu G, Tan B, et al. Glutamine-induced secretion of intestinal secretory immunoglobulin A: a mechanistic perspective. Front Immunol. 2016;7:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perna S, Alalwan TA, Alaali Z, Alnashaba T, Gasparri C, Infantino V, et al. The role of glutamine in the complex interaction between gut microbiota and health: a narrative review. Int J Mol Sci. 2019;20(20):5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao JV, Kwok MK, Schooling CM. Effect of glutamate and aspartate on ischemic heart disease, blood pressure, and diabetes: a Mendelian randomization study. Am J Clin Nutr. 2019;109(4):1197–206. [DOI] [PubMed] [Google Scholar]
- 60.Sved AF, Fernstrom JD, Wurtman RJ. Tyrosine administration reduces blood pressure and enhances brain norepinephrine release in spontaneously hypertensive rats. Proc Natl Acad Sci U S A. 1979;76(7):3511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Virwani PD, Qian G, Hsu MSS, Pijarnvanit T, Cheung CN, Chow YH, et al. Sex differences in association between gut microbiome and essential hypertension based on ambulatory blood pressure monitoring. Hypertension. 2023;80(6):1331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verhaar BJH, Collard D, Prodan A, Levels JHM, Zwinderman AH, Bäckhed F, et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur Heart J. 2020;41(44):4259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tilves C, Yeh HC, Maruthur N, Juraschek SP, Miller E, White K, et al. Increases in circulating and fecal butyrate are associated with reduced blood pressure and hypertension: results from the SPIRIT trial. J Am Heart Assoc. 2022;11(13):e024763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11(1):5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porcari S, Benech N, Valles-Colomer M, Segata N, Gasbarrini A, Cammarota G, et al. Key determinants of success in fecal microbiota transplantation: from microbiome to clinic. Cell Host Microbe. 2023;31(5):712–33. [DOI] [PubMed] [Google Scholar]
- 66.He R, Li P, Wang J, Cui B, Zhang F, Zhao F. The interplay of gut microbiota between donors and recipients determines the efficacy of fecal microbiota transplantation. Gut Microbes. 2022;14(1):2100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chi C, Li C, Wu D, Buys N, Wang W, Fan H, et al. Effects of probiotics on patients with hypertension: a systematic review and meta-analysis. Curr Hypertens Rep. 2020;22(5):34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Figure S1. The Metagenomic and Metabolomic Analysis Flowcharts. Figure S2. Microbial Diversity Within and Between Groups. Figure S3. Microbial Diversity Within Groups. Figure S4. Microbial Composition of the Donors and FMT Group. Figure S5. Microbial Composition of Genera Between Donors and the FMT Group. Figure S6. Microbiome Profile Within and Between the Groups and Clinical–Phenotype Association. Figure S7. Examples of office SBP-associated Differential Genera and Species Within and Between Groups. Figure S8. Changes in Microbial Abundance Between Donors and the FMT Group. Figure S9. AUC matrix using 14 species (left panel) and 8 genera (right panel) at five follow-up timepoints. Figure S10. Diversity of KEGG Orthology Between Groups. Figure S11. Diversity of KEGG Orthology within Groups. Figure S12. Differential KOs Within and Between Arms Associated with Clinical Office SBP. Figure S13. Correlations Between Office SBP-Related and Differential Species and KOs. Figure S14. Untargeted Metabolomic Analysis. Figure S15. Targeted Metabolomic Analysis. Figure S16. Clinical Phenotype Associations with Differentially Abundant Species. Figure S17. Clinical Phenotype Associations with Differentially Abundant Metabolites.
Supplementary Material 2: Table S1. Extended Baseline Characteristics of the Study Participants. Table S2. Sensitivity and Subgroup Analysis of the Primary Endpoint. Table S3. Secondary Outcome Measures Compared Between Groups. Table S4. Average AUCs based on 8 Genera for Intra-center, Cross-center, and Leave-One-Center-Out Prediction Validations on FMT and Control Samples.
Supplementary Material 3: Table S5. Detailed information on the differential microorganisms within the FMT group and between the FMT and control groups. Table S6. Partial Spearman's correlation between the altered gut microbial signatures (phylum, genera, species) and office SBP over follow-up visits adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, baseline office SBP and DBP. Table S7. Detailed information on significantly altered KEGG Orthology (KO, HUMAnN analysis) within the FMT group and between the FMT and control groups. Table S8. Species annotation to 14 species of 97 office-SBP-related, altered KOs within and between groups. Table S9. Table S10. Detailed information on differential plasma metabolites from untargeted metabolomic profiling analysis. KEGG pathway annotation of 97 office-SBP-related, altered KOs within and between groups. Table S11. Functional enrichment of differential plasma metabolites from untargeted metabolomic profiling analysis via MetaboAnalyst. Table S12. Partial Spearman's correlation between the altered untargeted metabolites and office SBP over follow-up visits adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, baseline office SBP and DBP. Table S13. Detailed information on differential plasma metabolites from targeted metabolomic profiling analysis (amino acids, TMAO-related metabolites, SCFAs). Table S14. Partial Spearman's correlation between the altered targeted metabolites and office SBP over follow-up visits adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, baseline office SBP and DBP. Table S15. Partial Spearman's correlation between the altered microbial signatures and metabolites over follow-up visits adjusted for age, sex, BMI, center, hypertension disease course, previous history of antihypertensive medication, baseline office SBP and DBP. Table S16. Number of included samples for Random Forest classifier. Table S17. The quality control metrics of gut metagenomics profling analysis.
Data Availability Statement
Sequence data that support the findings of this study have been deposited in the European Nucleotide Archive with the primary accession code PRJEB72385(Project: PRJEB72385, https://www.ebi.ac.uk/ena/browser/view/PRJEB72385) and the private link https://figshare.com/s/3d6633ce046aa475fb74. Editors and reviewers can reach the data via search the project ID or use the link provided on above database.