Abstract
Background
Finite element analysis (FEA) for endocrown restorations (ER) on maxillary molars has rarely been investigated, despite its high incidence in gross decay and being treated endodontically. This study aimed to analyze the influence of various preparation features (margin type, pulp chamber extension, occlusal thickness) and restorative material (zirconia, lithium disilicate, nanoceramic resin) on the stress distribution pattern of maxillary first molar ER using three-dimensional FEA.
Material/Methods
Six three-dimensional finite element models (models A–F) were created for ER maxillary molars, considering preparation features – margins (flat butt, 20° bevel, shoulder), pulpal extension (2 mm/4 mm), and occlusal thickness (3 mm) – for 3 ER materials (zirconia, lithium disilicate, nanoceramic resin). The closing phase of mastication was loaded with 600 Newtons of functional load from an apple bolus. Von Mises Stress (VMS) was used to calculate tooth component and ER stress distributions. Standard criteria were used to calculate cement layer failure risk.
Results
Resin nanoceramic shoulder margins with 2- and 4-mm PE (models E and F) had higher stress concentrations on the tooth preparation surface, cervical enamel, buccal roots, and ER intaglio surface. Higher elastic modulus (ME) materials had higher stress concentrations inside ER and less stress in the cement layer, facilitating tooth bonding. Resin nanoceramic distributed stresses more uniformly on ER/tooth tissues.
Conclusions
Different margin and pulpal extension preparation features and restorative materials cause varied ER stress distribution. Shoulder margin created considerably larger stresses in dental tissue and ER than flat butt margin, regardless of material type. Increased pulpal extension reduced tooth tissue stresses but increased ER.
Keywords: Adhesives; Ceramics; Dental Bonding; Dentistry, Operative; Endodontics
Introduction
Dental caries is one of the most prevalent microbial diseases affecting both deciduous and permanent dentitions across all ages: 37% in children aged 2–8 years, 58% in adolescents aged 12–19 years, and 90% in people aged 20 years and older [1]. Fortunately, endodontic treatment of teeth affected by caries approaching the tooth pulp significantly reduces the mortality and morbidity of such teeth. Global estimates indicate that 8.2% of the world’s population has an endodontically-treated tooth (ETT), with 55.7% having at least 1 ETT [2]. Depending upon the involved tooth and the complexity of its root canal anatomy, important anatomical structures like marginal ridges and tooth amount (enamel and dentin) are further lost while gaining access to the canal apex [3]. Earlier perceptions that ETT induces water loss (non-collagen bound) resulting in a decrease in elastic modulus (ME)/hardness/resistance to traction have been largely negated, with the primary reason being loss of structural integrity of the tooth (due to caries and cavity preparation) [4]. Endodontic procedures, occlusal cavity preparations, and large cavity preparations (mesio-occlusal distal) have been shown to reduce cusp durability in healthy human teeth by 5%, 20%, and 63%, respectively [5]. There is widespread agreement that prompt restoration of ETT with direct [6] or indirect restorations [7] is necessary following endodontic treatment, governed by its location and strategic function [2]. While direct restorations using posterior restorative resins have been associated with a high risk of tooth fracture and polymerization-shrinkage-linked microleakage [6], cuspal coverage restorations (onlays, crowns) enhance fracture resistance and long-term clinical outcomes of ETT [8]. Higher short- and long-term (5 years (94.2% survival rate) and 10 years, respectively] survival rates were found for indirect restorations when compared to direct restorations (survival rate 63%) [9,10]. Depending upon the extent of caries-associated tooth structure loss, these teeth at times require placement of a post followed by core fabrication before an indirect restoration is cemented in place. Post and cores, however, require further internal tooth structure reduction (58.3% average) [11], compounded by the risk of iatrogenic root perforations [12]. Dentinal adhesives and bonding, together with the emphasis on minimally invasive dentistry, have paved the way for advanced dental ceramic techniques (sintered, pressable, milled, castable, slip cast) and materials (feldspar, leucite, lithium silicate/disilicate, zirconia, and hybrids [polymer-infiltrated ceramic network]) [13].
The original ‘monoblock restoration’ concept of Pissis (1995) [14] was further developed and termed endocrown restoration (ER) [15], with the basis of a conservative coronal restoration for ETT. The endocrown restoration is basically an onlay type restoration that is fabricated as a one-piece construct and is designed in the form of an internal retainer adhesively retained by pulp chamber internal axial walls. Endocrown restoration/s (ER/ERs) prevents tooth tissue removal and requires less chair time for fabrication [16]. It is particularly suitable for complex clinical situations (eg, wide dentinal defects, short or tortuous roots, small root canals, or limited interocclusal space) [4,11,15]. When compared to conventional crowns, it requires minimal tooth preparation for retainer installation and prevents recontamination during disobturation [11,15]. However, it is contraindicated technically when inadequate surfaces are available for bonding, shallow pulp chambers (<3 mm), and narrow cervical margins (<2 mm) [17]. Patients with group function occlusion, parafunctional habits, and steeper cusps (young permanent teeth) are also not ideal and therefore not advised [18–20]. ERs were initially constructed using glass-ceramics; however, due to the stiffness and brittleness of these materials, fractures that extend to the root are quite frequent [21]. However, in vitro and clinical trials have reported ERs have similar or even higher fracture resistance and survival rates (99% at 44.7 months) [19], when compared with post-core (prefabricated fiber post) [22] and crown restorations [23]. The higher success rates of ER are attributed to the design of the intracoronal retainer, which does not allow stress concentration at the floor of the pulp chamber, while at the same time directing the stresses to the adjacent natural tooth structure to endure physiological loading [18]. Einhorn et al [24] concluded that preparation design parameters, mechanical behavior, and resin bonding strength significantly influence the success of ERs, with various other authors adding influencing factors like marginal forms [14,25,26], abduction angle [27,28], chamber (pulp) extension depth [27,29], pulp chamber wall thickness [18,27], and occlusal thickness [25,30,31]. Depending upon the number of axial walls, the endocrown preparation primarily has 3 basic designs: class 1 (double axial walls), class 2 (single axial wall), and class 3 (no axial wall) [32], with class 3 having low clinical performance due to lower stress density [33]. The biomechanical principles of endocrown tooth preparation are similar to those of any indirect restorations covering functional and nonfunctional cusps. These include marginally inclined axial walls with an approximate angulation of a minimum of 6 degrees (average 8–10) within the pulp chamber [34], and even the pulp floor, with 1.5–3 mm cusp lessening with a definite and prominent margin, preferably a flat buttock or straight shoulder [27,30]. Margin forms have significant influences on the mechanical performance of ERs. Although the butt joint margin showed less marginal leakage than the shoulder finish line when dye penetration was used, Abo-Elmagd et al [35] did find that margin design does not alter the vertical marginal gap of ERs. Additionally, Sakrana et al found no significant effect of finish line type (shoulder/chamfer) on fracture resistance [36]. Due to the very small axial wall, shoulder margin is also advised [17,23]. Taha et al found that shoulder finish line fracture resistance was higher than butt joint fracture resistance, despite the same failure mode [25]. The authors recommended a shoulder finish line for better force distribution, less pressure on the pulpal floor, less cement thickness, and less shrinkage polymerization and restoration stress. Greater intra-pulpal extension was associated with improved mechanical characteristics, retention, load transfer, and fracture prevention and intensity in endocrowns with different compression curl patterns, according to research by Darota et al [29]. During function, restorative rotation increased and fracture resistance decreased as a result of less extension [29]. Extending into the pulp chamber 4 mm rather than 2 mm improves bonding surfaces and load-to-failure values [37], according to Hayes et al, even if the lateral force on the cervical area causes more non-restorable fractures. However, Rocca et al found that conventional crowns and endocrowns with 2–4 mm pulpal depths had similar fatigue resistance, and failures in both groups were irreparable (under CEJ) [38]. Although 2 mm is the suggested occlusal thickness for an endocrown, the effect of this thickness on fracture resistance is debatable [39]. Zhu et al suggest a thickness of 1.5 mm to avoid bulk fractures [18], but fracture load values rise with increasing occlusal thicknesses while decreasing in the presence of surface defects [40]. In agreement with Taha et al [25], endocrowns with different occlusal thicknesses (3.5 mm) were able to withstand loads greater than those seen in the clinic (850 N in molars) without having any significant differences in various occlusal thicknesses. Generally, the left-over natural tooth tissue and endocrown bulk affect stress distribution in the ER/tooth complex. The greater the endocrown thickness, the more stress added to the restoration.
ERs survival and success rates are also influenced by the surface area of the tooth available for bonding [15], which is why the clinical performance of ER for a premolar tooth has been reported to be inferior than for molars or between anteriors and posteriors [41]. Recommendation of ER as a prosthetic treatment includes for ET incisors [21], premolars [42], and molars with gross tissue losses. ER are not the first treatment of choice when it comes to restoring anterior teeth, due to their limited surface area available for bonding and higher non-axial forces on the tooth. The first choice is usually a post/core crown, either prefabricated or custom-made, depending upon the amount of remaining tooth structure. ERs are fabricated using various methodologies, including digital workflows using restorative materials like feldspathic ceramics [43], glass-infiltrated ceramics (eg, leucite, lithium disilicate) [14,23], resin composites and nanoceramics [18,44], zirconia-reinforced lithium silicate [19,45], and polymer-infiltrated ceramic network (PICN) [46]. These materials directly influence the outcome of ER since each individual material can respond differently to similar masticatory force [31]. Chairside CAD/CAM technology is commonly used, providing macro-mechanical and micro-mechanical retention. The restorative effect of endocrowns is closely related to and highly influenced by the CAD/CAM materials [47]. Leucite-, lithium disilicate-, and zirconia-reinforced silicate ceramics are preferable due to their esthetics, translucency, biocompatibility, anti-corrosion, anti-aging, and anti-abrasion qualities, and mechanical advantages [45]. Ceramic materials are brittle; therefore, porcelain fractures frequently cause failure [14,45]. Resin nanofillers provide the material with a modulus of elasticity (ME) (18.0 GPa) like dentin [31], thereby producing less stress on teeth. Resin-based restorations have better flexural strength and less crack propagation than CAD/CAM ceramics [45,48]. Resin-based composites are also easy to cut, easy to repair in the mouth, and gentle on grinding burs [16,20]. Materials exhibiting a high ME are documented to induce lower stress by effectively conveying functional forces, whereas those with a low ME generate more stress, leading to detrimental loading [23,34,45]. Depending upon the material, the thickness of the ER is determined. ER made of zirconia with a 2-mm thickness has similar fracture resistance as that of lithium disilicate ER that is 4.5 mm thick [30].
Finite element analysis (FEA) is a commonly used biomechanical research technique to evaluate stress distribution on materials or oral tissues, comparing stress distribution on various restorative materials, and predicting clinical performance [49]. FEA is cost-effective, efficient, and continuously improves accuracy, avoiding expensive and time-consuming in vitro and in vivo experiments [50,51]. It can distinguish stress concentration regions that might undergo failure and can be calculated using finite element method (FEM) simulations. Digital model simulations and non-invasive investigations can validate the deployment of newly manufactured materials, minimizing time and expense compared to in vitro testing [52]. Photoelastic strain gauge methods are used in simulation methods. Most of the recent studies using FEA and newer composite and ceramic ER materials with different preparation designs have been performed on the mandibular molar [49–53], while there is only 1 study that investigated the role of filling material in relation to lithium disilicate restorative materials in ER for the maxillary molar [27]. There are vast anatomical and functional variations between maxillary and mandibular molars, including greater faciolingual dimensions, a smaller number of cusps, different functional cusps, a smaller distolingual cusp and distobuccal root, and more roots for maxillary molars. The inclination of maxillary molars is more centered around their roots. Even dental decay tends to be more pronounced in mandibular molars due to early eruption and food collection. Therefore, the stresses observed under FEA for maxillary molars are expected to be different than those of mandibular molars. This FEA study was therefore aimed to analyze the stress distribution in a virtually modelled ET maxillary first molar provided with different endocrown preparation features (margin, intrapulmonary extension, occlusal thickness). The objective was to determine which type of margin (flat buttock, shoulder, bevel) and intrapulmonary extension (2 and 4 mm) at the constant occlusal thickness (3 mm) would produce stresses on restoration, tooth structures, and cementing surface. The study also sought to predict which ER material (ceramic or resin) has a higher risk of debonding failures, which will allow clinicians to choose a better bonding cement in practice. The null hypothesis for this study WAs that various margins, materials, and extents of pulp will not alter the stress distribution in restoration, dental remnants, and cement layers.
Material and Methods
Ethics
This study was approved by the Dental Ethics Committee of the College of Dentistry at Jazan University (Saudi Arabia) (CODJU) during the academic session of 2024.
Study Design
The study utilized an experimental comparative approach with predetermined definite independent preparation features: endocrown margins (flat butt, 20-degree bevel, shoulder), pulpal extension (2 and 4 millimeters), ER materials (in-ceram zirconia, IPS e.max CAD, Grandio blocs), occlusal thickness (3 mm), dependent stress distributions (von Mises stresses), and failure risk (cohesive and adhesive) variables.
Sample Size
Based on the number of independent variables (6 designs), the total number of subgroups that would be formed were first outlined. In each subgroup within a group, the number of samples that would represent the independent variables was then derived using online sample size determination software (Nquery, v7.0; Informer Technologies, USA). The power analysis was then performed using 80% power assumption, 0.28 effect size, and 0.05 type 1 rate of error, showing that 18 test samples (6 tests for each restoration type) were needed.
Preparation Features and Designs
Three preparation features that significantly influence the stress distribution of ER, including the margin type (flat butt, 20-degree bevel, shoulder) [14,24,25,54], the pulpal extension (2 and 4 mm) [20,29,38,53], and the occlusal thickness (constant 3 mm) [25–27], were first outlined on a computer using routine computer windows (Windows 10 Pro, Microsoft Corporation) and software (MS PowerPoint, version 20H2, OS build 19042, 1466).
Generating Geometric Endocrown Model and Grouping
To create a stereolithography file, a three-dimensional scan of an intact maxillary first molar was made from microcomputed tomographic imaging (microCT, Quantum GX; PerkinElmer) and its related software (SolidWorks, Dassault System). Using a medical interactive image control system, the acquired data related to the scanned tooth was first divided into different tooth components (enamel, dentin, alveolar bone, both compact and cancellous, ER) by varying pixel densities and a three-point cloud. Non-Uniform Rational B-Splines (NURBS), being mathematical representatives of three-dimensional geometry, then were used to reconstruct the various geometrical shapes of the tooth and surrounding structures. The relevant shapes are derived from a mesh or a point cloud and are very precise and accurate. After that, CAD software (SolidWorks 2014, Dassault Systèmes) was used to rebuild the model in 3 dimensions. Gutta percha was utilized to replace pulp tissue and then covered with a resin (flowable, Dentsply Sirona). The ER’s intaglio surface was adjusted so that both ER and bonding cement could have a consistent shape/number of contacting faces, which in turn minimizes processing interference [27]. The final model of the digital maxillary first molar was then replicated 6 times to represent the 6 model designs for the study. Preparation features of the ER were then made using various commands (cut plane, axis integration, mesh refinement). Each sectioned model had preparation features as outlined in Figure 1A. From the original ET maxillary first molar model, 6 ER models with different preparation features were thus created (Figure 1B). Based on the differences in preparation features, the 6 ER models were: ER model A (flat butt margin, 2 mm pulpal extension, 3 mm occlusal thickness of endocrown); ER model B (flat butt margin, 4 mm pulpal extension, 3 mm occlusal thickness); ER model C (20 degree bevel margin, 2 mm pulpal extension, 3 mm occlusal thickness); ER model D (20 degree bevel margin, 4 mm pulpal extension, 3 mm occlusal thickness); ER model E (shoulder margin, 2 mm pulpal extension, 3 mm occlusal thickness); and ER model F (shoulder margin, 4 mm pulpal extension, 3 mm occlusal thickness) (Figure 1B). Other constant preparation features included an internal pulpal axial wall with a taper of 8 degrees. The negative duplication of the ER, which would fit into the preparation, was then formed with the corresponding portion being sliced by the software tool. A cement layer of 120 μm thickness was also modelled between the restoration surface and the ER preparation surface. The 6 ER models were then duplicated, followed by 3 different restorations: zirconia-based (In-Ceram Zirconia, Vita Zahnfabrik, H. Rauter GmbH & Co. KG Postfach, Bad Säckingen); lithium disilicate-based IPS e.max CAD (Ivoclar Vivadent AG, Liechtenstein); and nanoceramic resin-based Grandio blocs (VOCO GmbH Cuxhaven, Germany).
Figure 1.
(A) Line diagram showing preparation features (occlusal thickness, margins, pulpal extension) for endocrown that formed the basis of their designs. (B) Finite element models of endodontically treated maxillary first molar restored with endocrown using various preparation features. Model A (flat butt margin, 2 mm pulpal extension, 3 mm occlusal thickness; Model B (flat butt margin, 4 mm pulpal extension, 3 mm occlusal thickness); Model C (20 degree bevel margin, 2 mm pulpal extension, 3 mm occlusal thickness); Model D (20 degree bevel margin, 4 mm pulpal extension, 3 mm occlusal thickness); Model E (shoulder margin, 2 mm pulpal extension, 3 mm occlusal thickness); Model F (shoulder margin, 4 mm pulpal extension, 3 mm occlusal thickness). M – margin; PCE – pulp chamber extension; OT – occlusal thickness; mm – millimeters, ° – degrees. Figure created using MS PowerPoint, version 20H2 (OS build 19042,1466), Windows 11 Pro, Microsoft Corporation).
Meshing and Convergence (Figure 2A, 2B)
Figure 2.
Components for finite element analysis (A) cement layer (B) components associated with endocrown restoration analysis (C) Food modelling on the occlusal surface and the load. Figure created using MS PowerPoint, version 20H2 (OS build 19042,1466), Windows 11 Pro, Microsoft Corporation).
The final geometric designs of each model included the monolithic endocrowns of ERs, the resin cement (120 μm), flowable composite, gutta percha, dentine, enamel, periodontal ligament, and compact and cancellous alveolar bone (Figure 2A, 2B). The geometries of the 6 different models were then transferred in STEP format to finite elemental analysis software (ANSYS version 10; ANSYS, Inc., Canonsburg, PA, USA). In the software, the geometric designs were further divided into various meshes composed of tetrahedral elements and nodes. A continuous geometric space is discretized into mathematically specified, reliably formed parts by means of a mesh. Because of this discretization, computers can simulate the physical consequences by numerically solving the governing equations. All 3 of these factors – convergence, precision, and simulation speed – are affected by the mesh quality. An essential part of finite element analysis (FEA) is convergence, which is the point at which the computed solution stabilizes and does not vary much, while the numerical model parameters, like mesh density or iteration steps, are fine-tuned. This test was then conducted with a 10% mesh control, growth rate 1.2–1.5, and edge length ≤1/5 of the smallest structure circumference, which determined the number of nodes/elements needed to generate models. The specific number of nodes/elements used for various components during FEA in this study is presented in Table 1. The composition of restorative materials and their mechanical properties determined in previous studies and used during FEA are presented in Table 2 [55–63]. The assumption was made that all structures were homogeneously distributed, were isotropic, and had linear elastic properties.
Table 1.
Number of elements and nodes employed for designing various models of endocrowns with different specifications (margin, pulp chamber extension, occlusal thickness).
| Structures | Model designs | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| Specifications | M – Flat Butt PCE – 2 mm OT – 3 mm |
M – Flat Butt PCE – 4 mm OT – 3 mm |
M – 20° bevel PCE – 2 mm OT – 3 mm |
M – 20° bevel PCE – 4 mm OT – 3 mm |
M – Shoulder PCE – 2 mm OT – 3 mm |
M – Shoulder PCE – 4 mm OT – 3 mm |
|
| Dentine | Elements | 92762 | 82197 | 92267 | 83384 | 93909 | 84370 |
| Nodes | 20209 | 18182 | 20209 | 18393 | 20421 | 18472 | |
| Enamel | Elements | 8675 | 8340 | 2257 | 7604 | 5304 | 5250 |
| Nodes | 2558 | 2450 | 7634 | 2295 | 1953 | 1939 | |
| Periodontal ligament | Elements | 14297 | 14297 | 14297 | 14297 | 14297 | 14297 |
| Nodes | 4869 | 4869 | 4869 | 4869 | 4869 | 4869 | |
| Cortical bone | Elements | 80172 | 80172 | 80172 | 80172 | 80172 | 80172 |
| Nodes | 18393 | 18393 | 18393 | 18393 | 18393 | 18393 | |
| Cancellous bone | Elements | 28385 | 28385 | 28385 | 28385 | 28385 | 28385 |
| Nodes | 6382 | 6382 | 6382 | 6382 | 6382 | 6382 | |
| Resin cement | Elements | 25884 | 15715 | 25884 | 15715 | 25884 | 15715 |
| Nodes | 5797 | 3626 | 5797 | 3626 | 5797 | 3626 | |
| Endocrown | Elements | 18072 | 21390 | 19290 | 23033 | 19915 | 23491 |
| Nodes | 4317 | 5048 | 4608 | 5464 | 4865 | 5639 | |
| Gutta percha | Elements | 16684 | 16684 | 16684 | 16684 | 16684 | 16684 |
| Nodes | 4578 | 4578 | 4578 | 4578 | 4578 | 4578 | |
| Total | Elements | 284931 | 267180 | 279236 | 269274 | 284550 | 268364 |
| Nodes | 67103 | 63528 | 72470 | 64000 | 67258 | 63898 | |
M – margin type; PCE – pulp chamber extension; OT – occlusal thickness; CAD – computer assisted/aided diagnosis.
Model descriptions: model A = flat butt margin +2 mm PCE +3 mm OT; model B = flat butt margin +4 mm PCE + 3 mm OT; model C = 20° bevel margin +2 mm PCE +3 mm OT; model D = 20° bevel +4 mm PCE +3 mm OT; model E = shoulder margin +2 mm PCE +3 mm OT; model F = shoulder margin +4 mm PCE +3 mm OT.
Table 2.
Composition of different restorative materials tested and their respective properties used for finite element analysis.
| Material | Elastic modulus (GPA) | Poison’s ratio |
|---|---|---|
| Tooth enamel [55] | 84.10 | 0.33 |
| Dentin [55] | 18.60 | 0.31 |
| Cortical bone [56] | 13.7 | 0.03 |
| Bone alveolar (cancellous bone) [56] | 1.37 | 0.30 |
| Gutta percha [57] | 0.69 | 0.45 |
| Periodontal ligament (0.15 to 0.21mm wide) [57] | 0.07 | 0.45 |
| Core buildup resin [58] | 7.17 | 0.23 |
| Luting cement: aNx3 Kerr (120 micron thick) [59] | 7.4 | 0.35 |
| Restorative material 1: bin-Ceram Zirconia [60,61] | 210 | 0.26 |
| Restorative material 2: cIPS e.max CAD [62] | 100.00 | 0.20 |
| Restorative material 3: dGrandio blocs [54,63] | 18 | 0.26 |
| Apple [56] | 0.01 | 0.3 |
FEA – finite element analysis; GPA – giga pascals; mm – millimeter
Resin cement – Base [Barium aluminoborosilicate glass (30–60%), Ytterbium fluoride (10–<30%), Ethoxylated bisphenol-A dimethacrylate (<15%), Urethane dimethacrylate (<10%), Triethylene glycol dimethacrylate (<10%), Hydroxyethylmethacrylate (<10%), Fumed silica(<5%), Bisphenol-A diglycidyl methacrylate (<5%), Ethyldimethylaminobenzoate (<0.5%)]; catalyst [Barium aluminoborosilicate glass (30–60%), Ytterbium fluoride (10–<30%), Triethylene glycol dimethacrylate (<10%), Ethoxylated bisphenol-A dimethacrylate (<10%), Urethane dimethacrylate (<10%), Fumed silica (<5%), Bisphenol-A diglycidyl methacrylate (<5%), Hydroxyethylmethacrylate (<5%), Peppermint oil (<0.5%)]; Indirect bond strengths [zirconia – 23.7Mpa, porcelain – 30.6 Mpa, composite – 35.9 Mpa]; shear bond strength [33.8 Mpa to dentine]; tensile strength [51.9 Mpa], Linear thermal expansion coefficient (0.0165).
In-ceram zirconia – composition zirconium dioxide (ZrO2) (91–94%), yttrium oxide (Y2O3) (4–6%), hafnium oxide (HfO2) (<3%), aluminum oxide (Al2O3) and silicon dioxide (SiO2) (<1%).
IPS e.max CAD - composition: SiO2 (57.0–80.0), Lithium oxide (Li2O) (11–19%), Potassium oxide (K2O) (<3%), phosphorous pentoxide (P2O5), ZrO2 (<11%), zinc oxide (ZnO) (<8%), (Al2O3) (<8%), MgO (<8%), pigments (<5%)
GrandioSO (Resin-based restoratives – Nanoceramic Hybrid CAD/CAM Material) – composition: 86% Nanohybrid Filler, 14% UDMA+ DMA (no firing or sintering required, available in low translucency for anterior (shades A1, A2, A3, A3.5, B1, C2, BL) and high translucency posteriors (A1, A2, A3, A3.5).
Application of Simulated Masticatory Load (Figure 2C)
The simulated load was decided to be 600 Newtons in accordance with the methodology explained in previous related studies [14,27,30,54]. The functional analysis was performed by considering the occlusal contact between a bolus of food and the ER occlusal surface with opposing occlusal contact during the regular chewing cycle while closing the mandible (Figure 2C (a, b)). The simulated solid food was the pulp of a regular apple with predetermined settings (ME10 Mpa, Poisson’s ratio 0.3) (Table 2). The occlusal contact elements between the occlusal surface and bolus were kept as sliding type, mimicking the grinding movements of the masticatory cycle. The border condition was set by fixing a zero nodal displacement while being applied to the alveolar bone tissue base. Previous simulations of masticatory loads at different points on the occlusal surface does not represent the ideal masticatory situation where the food bolus is present. Therefore, applying masticatory load through a bolus mimics the actual clinical situation than merely choosing subjective points.
Measurements
The VMS for the ER and the dental structures were calculated from the outcomes of uniaxial tensile tests. The VMS is utilized to predict the yielding of materials subjected to complicated loads. When the distortion energy of 2 stress levels is the same, then the VMS is also the same. The normal stress that was perpendicular to the trajectory of insertion (X axis) provided the measures for the resin cement layer. Failure risks were designated as either cohesive or adhesive. The cohesive failure risk for the resin cement was calculated by using the formula peak stress value divided by the material tensile strength. The adhesive failure risk was calculated by using the mathematical formula of stress peak values divided by the adhesive bond strength of the material to the dentin [14,31,56,64].
Results
The VMS distribution colorimetric graph outcomes were attained for each material in relation to the endocrown preparation (intaglio surface) and buccal roots (Figure 3), endocrown and resin cement intaglio surfaces (Figure 4), overall tooth/ER structures (Figure 5), and cumulative effect of preparation features and restorative materials on remaining coronal tooth structures (Figure 6). The VMS (Mpa) observed on various tooth components (enamel, dentin, root) and ER for various preparation features and restorative materials are presented numerically in Table 3.
Figure 3.
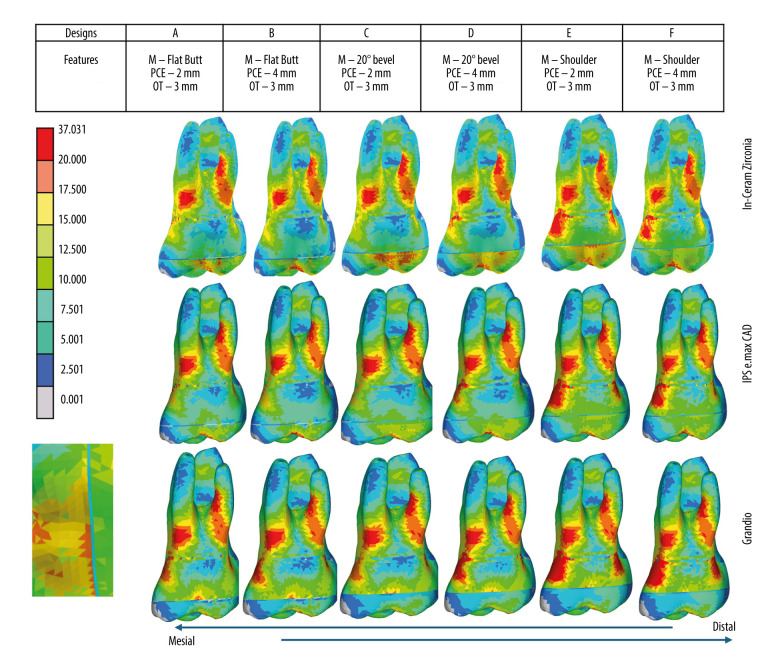
Stress distribution (Mpa) of endocrown preparation (intaglio surface) and buccal roots under occlusal loading in different models based on preparation features (margin, pulp chamber extension, occlusal thickness) and restorative materials used [Zirconia (In-Ceram Zirconia, Lithium disilicate (IPS e.max CAD), Resin Nanoceramic (Grandio blocs)]. Colorimetric Graphs Obtained from FEA Compiled and Created Using MS PowerPoint, Version 20H2 (OS Build 19042,1466), Windows 11 Pro, Microsoft Corporation).
Figure 4.
Stress distribution (Mpa) of (a) endocrown intaglio surface (b) cement intaglio surface under occlusal loading in different models based on preparation features (margin, pulp chamber extension, occlusal thickness) and restorative materials used [Zirconia (In-Ceram Zirconia, Lithium disilicate (IPS e.max CAD), Resin Nanoceramic (Grandio blocs)]. Colorimetric Graphs Obtained from FEA Compiled and Created Using MS PowerPoint, Version 20H2 (OS Build 19042,1466), Windows 11 Pro, Microsoft Corporation).
Figure 5.
Stress distribution (Mpa) on overall structures (endodontic restoration and buccal roots) under occlusal loading in different models based on preparation features (margin, pulp chamber extension, occlusal thickness) and restorative materials used [Zirconia (In-Ceram Zirconia, Lithium disilicate (IPS e.max CAD), Resin Nanoceramic (Grandio blocs)]. Colorimetric Graphs Obtained from FEA Compiled and Created Using MS PowerPoint, Version 20H2 (OS Build 19042,1466), Windows 11 Pro, Microsoft Corporation).
Figure 6.
Cumulative effect of various preparation features and (margin, pulp chamber extension, occlusal thickness) and restorative materials [Zirconia (In-Ceram Zirconia, Lithium disilicate (IPS e.max CAD), Resin Nanoceramic (Grandio blocs)] of an endocrown restoration on developing the stress concentration areas on the remaining tooth structure. Colorimetric Graphs Obtained from FEA Compiled and Created Using MS PowerPoint, Version 20H2 (OS Build 19042,1466), Windows 11 Pro, Microsoft Corporation).
Table 3.
Distribution of groups according to material type and preparation characteristics (margins, occlusal thickness, and pulpal extension) of the total von Mises stresses (Mpa) measured in tooth components and endocrown restoration.
| Model | Design features | Material | Enamel | Dentin | Root | Endocrown |
|---|---|---|---|---|---|---|
| A | M – Flat Butt PCE – 2 mm OT – 3 mm |
In-Ceram Zirconia | 17.59 | 13.86 | 24.93 | 23.40 |
| IPS e.max CAD | 17.54 | 13.56 | 24.95 | 20.96 | ||
| Grandio blocs | 22.96 | 10.62 | 25.18 | 19.80 | ||
| B | M – Flat Butt PCE – 4 mm OT – 3 mm |
In-Ceram Zirconia | 13.81 | 5.75 | 24.16 | 30.30 |
| IPS e.max CAD | 14.37 | 4.80 | 24.40 | 23.03 | ||
| Grandio blocs | 21.96 | 8.75 | 25.46 | 20.31 | ||
| C | M – 20° bevel PCE – 2 mm OT – 3 mm |
In-Ceram Zirconia | 17.67 | 12.69 | 23.41 | 22.75 |
| IPS e.max CAD | 17.06 | 12.74 | 23.93 | 19.76 | ||
| Grandio blocs | 25.14 | 9.27 | 24.04 | 18.57 | ||
| D | M – 20° bevel PCE – 4 mm OT – 3 mm |
In-Ceram Zirconia | 14.04 | 4.80 | 24.40 | 29.61 |
| IPS e.max CAD | 15.07 | 5.01 | 24.05 | 21.99 | ||
| Grandio blocs | 23.28 | 9.04 | 25.13 | 19.97 | ||
| E | M – Shoulder PCE – 2 mm OT – 3 mm |
In-Ceram Zirconia | 19.91 | 13.97 | 24.30 | 28.86 |
| IPS e.max CAD | 20.18 | 10.12 | 24.34 | 26.87 | ||
| Grandio blocs | 27.99 | 9.42 | 24.72 | 19.18 | ||
| F | M – Shoulder PCE – 4 mm OT – 3 mm |
In-Ceram Zirconia | 18.22 | 5.82 | 23.14 | 32.04 |
| IPS e.max CAD | 18.71 | 6.00 | 23.41 | 23.07 | ||
| Grandio blocs | 27.34 | 8.99 | 24.62 | 20.55 |
M – margin type; PCE – pulp chamber extension; OT – occlusal thickness; CAD – computer assisted/aided diagnosis.
Model descriptions: model A = flat butt margin +2 mm PCE +3 mm OT; model B = flat butt margin +4 mm PCE +3 mm OT; model C = 20° bevel margin +2 mm PCE +3 mm OT; model D = 20° bevel +4 mm PCE +3 mm OT; model E = shoulder margin +2 mm PCE +3 mm OT; model F = shoulder margin +4 mm PCE +3 mm OT.
Endocrown Tooth Preparation and Buccal Roots (Table 3, Figures 3, 4)
Zirconia and lithium disilicate restorations did not present any stress concentration in any of the preparation features on the endocrown preparations’ intaglio surface. The stress concentration areas for zirconia were mainly in the root areas, with distobuccal roots showing a higher stress concentration area than mesio buccal roots. For both zirconia and lithium disilicate, the shoulder margin preparation, irrespective of the pulpal extension, showed stress concentration areas shifting to the coronal tooth structure (Figures 3, 4). The influence of pulpal extension on stress distribution of both ceramic restorations was observed when pulpal extension was 4 mm, with stress concentration areas being shifted to the coronal tooth structure. The 2 mm pulpal extension did not produce any stress on the crown. For resin nanoceramic, however, more stress concentration areas were observed on the preparation feature (margin), especially on the axial walls of the shoulder margin (Figure 3). For resin nanoceramic, all preparation features showed a higher influence on stress distribution, with a lesser influence of pulp chamber extension. In addition to higher stress concentrations at the margins, the resin nanoceramic ER showed higher VMS on enamel (21.96–27.99) and root (24.04–25.46) than zirconia (enamel 13.81–19.91, root 23.14–24.93) and lithium disilicate (enamel 14.37–20.18), root (23.41–24.95) across various models of ERs (Table 3). The resin nanoceramic, however, showed less stress on dentin than zirconia and lithium disilicate ERs for models A, C, and E, while showing higher stress on dentin in models B, D, and E. This indicates that resin nanoceramic was affected more by the intrapulpal extension of 4 mm since models B, D, and E had 4 mm extension and were less influenced by margin type. Independent of preparation features, the resin nanoceramic showed stress concentration in the coronal aspect for all models, with shoulder margin showing the highest stress concentrations in coronal tooth structure. The colorometric graph also shows that resin nanoceramic produced stresses on the dentinoenamel junction on the intaglio surface of the endocrown preparations for all models (Figure 3).
Overall Tooth (Crown and Root)/ER Structures (Table 3, Figure 4)
All restorative materials, irrespective of the margin features, produced stresses on the root of the maxillary first molar, whereas differences existed for stress distribution on the occlusal surface and margins at the crown. The distobuccal root of the maxillary first molar was observed to have stresses that extended along the length of the root, more concentrated where the root meets the crown. The range of VMS (Mpa) for all preparation features and materials was 23.14–25.46 Mpa, with the least stress for resin ceramic when preparation features were shoulder margin, 4 mm pulpal extension, and 3 mm occlusal thickness (model F), and the highest being for resin ceramic (model B – flat butt, 4 mm pulp chamber extension, and 3 mm occlusal thickness). In the coronal portion of the crown, the stresses were observed on the mesiobuccal surfaces, with resin ceramic showing more stresses than ceramic ERs. Flat butt and 20-degree bevel margins with either 2 or 4 mm of pulpal extension showed more coronal stress than the shoulder margin ER. However, with zirconia ceramic, more stresses were observed on the occlusal and buccal surface of the restoration near the cavosurface margins of the restoration (Figure 4).
Endocrown Restoration/Resin Cement Intaglio Surfaces (Figure 5)
The intaglio ER surface and the cement layer provide a comprehensive picture of the stresses generated within ER and the risk of cement failure due to such stresses. The resin ceramic ER shows no stresses being generated within the material for all preparation features across all models (Figure 5). However, the VMS stresses generated show that increased pulpal extension was associated with higher stresses within ER when margins and occlusal thickness were constant. Lithium disilicate-based ER shows less stress concentration than zirconia across all models, irrespective of preparation features. For both though, the stresses are concentrated on the axial surfaces and the axiopulpal line angle with 4 mm pulpal extension showing more stresses than 2 mm pulpal extension. Another difference in stress distribution between zirconia and lithium disilicate is that in zirconia ERs, the stresses are spread to the margin area towards the external surface of the restoration. Zirconia ERs show the highest VMS generated, with model E being the highest (32.04 Mpa) (Table 3). An increase in pulpal extension from 2 to 4 mm was associated with higher stress generated within ER for both ceramics, except model F for lithium disilicate, where 4 mm extension showed less stress on ER than 2 mm pulpal extension. The intaglio surface of the cement shows that the stresses generated were within the limits of 7.5 Mpa without any undue VMS being generated within the cement layer.
Cumulative Effect of Preparation Features and Restorative Materials on Remaining Coronal Tooth Structures
Regardless of the preparation features, the stresses at the cervical area of the crown near the cementoenamel junction after ER placement were observed in all models for all 3 restorative materials (Figure 6). For all restorative materials, the 2 mm pulpal extension was observed to be more strongly associated with cervical crown stress than the 4 mm pulpal extension. Less stress on remaining natural teeth in the cervical crown region was observed with flat butt margins, independent of material type. There was a reduction in cervical crown stress for shoulder margins if the pulpal extension was extended from 2 to 4 mms in ceramic ER, while no stress reduction was observed for nanoceramic resin ER.
Cement Failure Risk (Table 4)
Table 4.
Standard stress peak levels (Mpa) and their relative risk of cohesive and adhesive failures in the resin cement layer.
| Model | Design features | Material | Cement NX3 stress peak (MPA) | Failure risk | |
|---|---|---|---|---|---|
| Cohesive | Adhesive | ||||
| A | M – Flat Butt PCE – 2 mm OT – 3 mm |
Zirconia | 1.778 | 0.0114 | 0.0176 |
| Lithium disilicate | 4.658 | 0.0119 | 0.0183 | ||
| Nanoceramic resin | 0.868 | 0.0164 | 0.0253 | ||
| B | M – Flat Butt PCE – 4 mm OT – 3 mm |
Zirconia | 1.222 | 0.0167 | 0.0256 |
| Lithium disilicate | 3.506 | 0.0209 | 0.0322 | ||
| Nanoceramic resin | 0.596 | 0.0235 | 0.0361 | ||
| C | M – 20° bevel PCE – 2 mm OT – 3 mm |
Zirconia | 1.675 | 0.0246 | 0.0378 |
| Lithium disilicate | 3.757 | 0.0310 | 0.0477 | ||
| Nanoceramic resin | 0.856 | 0.0322 | 0.0495 | ||
| D | M – 20° bevel PCE – 4 mm OT – 3 mm |
Zirconia | 1.089 | 0.0323 | 0.0497 |
| Lithium disilicate | 4.344 | 0.0342 | 0.0526 | ||
| Nanoceramic resin | 0.62 | 0.0344 | 0.0528 | ||
| E | M – Shoulder PCE – 2 mm OT – 3 mm |
Zirconia | 1.786 | 0.0644 | 0.0989 |
| Lithium disilicate | 3.344 | 0.0675 | 0.1037 | ||
| Nanoceramic resin | 1.613 | 0.0723 | 0.1111 | ||
| F | M – Shoulder PCE – 4 mm OT – 3 mm |
Zirconia | 1.278 | 0.0731 | 0.1123 |
| Lithium disilicate | 3.797 | 0.0836 | 0.1285 | ||
| Nanoceramic resin | 1.681 | 0.0897 | 0.1378 | ||
M – margin type; PCE – pulp chamber extension; OT – occlusal thickness; CAD – computer-assisted/aided diagnosis.
Model descriptions: model A = flat butt margin +2 mm PCE +3 mm OT; model B = flat butt margin +4 mm PCE +3 mm OT; model C = 20° bevel margin +2 mm PCE +3 mm OT; model D = 20° bevel +4 mm PCE +3 mm OT; model E = shoulder margin +2 mm PCE +3 mm OT; model F = shoulder margin +4 mm PCE +3 mm OT.
Statistical analysis: cohesive failure risk = stress peak value/tensile strength; adhesive failure risk = stress peak value/adhesive bond strength of the concerned material to dentin.
The cement failure risk was assessed for both cohesive and adhesive types of cement failures (Table 4). All the simulated models with various preparation features and different restorative materials showed a higher adhesive failure risk (0.0176–0.1378) when compared to the cohesive type of failure (0.0114–0.0897). The same was observed when individual material types were considered for failure risk type. The lowest risk of cement failure for all restorative materials was for model A (flat butt margin, 2 mm pulpal extension, 3 mm occlusal thickness), while the highest failure risk was for model F (shoulder margin, 4 mm pulpal extension, 3 mm occlusal thickness).
Discussion
This study estimated the stress dissemination of endodontically-treated maxillary first molars with different common preparation features of margin, pulpal extension, and 3 different restorative materials with varied modulus of elasticity. The clinical effectiveness and longevity of ERs are determined by the mechanical qualities of the restoration material as well as existing design factors. Based on the findings and interpretations of the study results, the null hypothesis is rejected since data show that preparation features like margin type and pulpal extension and type of restorative material used significantly affect the biomechanical stress distribution in the function of maxillary molar ERs. Compared to earlier studies [18,27,28,31,56] where functional load application has been centered on a single loading point (vertical, horizontal, oblique) at 1 time interval based on the tripod concept of occlusal contacting (which views a minimum 3 points of contact on each individual cusp of a tooth), this study replicated the functional load of occlusal contacts that closely resembles mastication by applying the food bolus (apple) to the simulated ER surface, thereby distributing the occlusal load evenly rather than at 1 point, as used in recent studies [54,64,65]. This replication of functional loading is based on the fact that functional interactions during occlusion are more often at the regional than point level and that the texture of the food chewed influences the motor activity of chewing in the oral cavity [64,66]. Stress distributions generated during FEA tests are highly dependent upon the functional loads; therefore, close mimicking of the actual conditions is necessary for clinical application [64].
Influence of Preparation Features
Three different margin types for ER were analyzed, with shoulder preparation being the most conservative of all since it preserves marginal tooth tissue by creating a short axial wall, which tends to improve load distribution at the margins by countering the shear stresses through the walls [24]. The shoulder margin thus incorporates a circumferential ferrule around the preparation, the use of which has been advocated by many authors [18,26,28]. Our results showed that for both ceramic ERs with high MOE, the shoulder margin preparation produced more stresses on the crown, which were independent of the pulpal extension. The shoulder margin also produced stress concentration areas on the margins and axial walls in nanoceramic ER, with higher stress being observed in coronal tooth structure and less stress in the roots (with 4 mm pulpal extension). Another significant finding for shoulder margin preparation in this study was that there was a reduction in cervical crown stress for shoulder margins if the pulpal extension was extended from 2 to 4 mms in ceramic ER, while no stress reduction was observed for nanoceramic resin ER. In terms of cement risk failure, shoulder margin was associated with the highest failure risk (model F). Because of this stress distribution, the endocrown edge, enamel, and the area surrounding the impacted restoration are more likely to experience fractures and leaking. These findings may not agree with the results obtained in previous studies [17,23,25,36] that advocated use of the shoulder finish line for better force distribution. Our findings on stress distribution are, however, in accordance with Zheng et al [64], who observed stress concentration areas in the axial walls of the ER, cervical enamel region, and margin interface. Previous studies suggest short axial walls with a shoulder finish line can counteract shear stresses and improve load distribution [24], but this increases the risk of fractures at the endocrown margin or enamel [64]. Moreover, the shoulder margin preparations for ERs are clinically more challenging and highly technique-sensitive than flat butt or bevel margins. On the contrary, both flat and bevel margins showed a more homogenous stress distribution on different tooth structures and ER for ceramic ER in this study, which agrees with previous studies [17,22,23,64,65]. The features of flat butt margin for both ceramics being homogenous stress distribution on the endocrown tooth preparation areas (intaglio preparation), less occlusal and cavosurface margin stress on ER, less cervical stress on remaining natural tooth structure, and lowest cement failure risk for all materials (model A). However, the flat but was associated with more stress generation in the intaglio surface (axial wall and pulp extension line angles) of zirconia restoration when the pulpal extension exceeded 4 mm. This indicates that the stresses may be better distributed across the tooth structure and that a stable surface would be better able to endure them [19,26,45]. The 20-degree bevel angle has been observed to ameliorate the stress concentrations [67]. Concurrently, such margin design disperses compressive and shear forces effectively, thereby lessening the likelihood of subsequent ER fractures [64]. Our study considered keeping the occlusal thickness uniform for all models since variations could confound the stress patterns on those obtained and influenced by other preparation features. However, Tribst et al [31] found that as the ER becomes thin, the stress concentrations on it become higher. Increased thickness of ER also increases its fracture resistance [25] while invariably increasing the ER and tissue stress [51]. No consensus exists on pulp chamber expansion depth for endocrowns currently. Kuijper et al found that an extension depth of 0, 2, or 4 mm in the pulp chamber had no effect on the load-to-fracture ratio of glass-ceramic ERs with consistent remaining dental tissue following severe thermomechanical aging [68]. In their study, Dartora et al [29] utilized 3 distinct CAD/CAM molar ERs with pulp chamber extension depths of 1, 3, and 5 mm. They found that mechanical performance under axial load improved as the extension depth increased. Although a 5-mm extension depth improves masticatory force transmission to the root, increases adhesive retention surface area, and deepens the pulp chamber extension for an endocrown, it can harm the pulp chamber floor of mandibular molars [51]. The risk of catastrophic fracture under a load directed at 45° to the tooth axis has been reported to increase with increasing pulp depths (2, 3, and 4 mm) [37]. Therefore, it is recommended that the ER pulp extension not be deeper. Ideally, ETT have been observed to undergo fracture loads of 720–1326 N, depending upon the tooth involved, the direction and type of forces, the amount of remaining natural tooth structure, and the type of restoration used [22,25].
Influence of Material Type
This study examined how restorative material affected the stress distributions of ETT that had undergone ER. The results show that stress maxima in the restorations themselves are proportionate to the ME of the restorative material, independent of the restoration margin types. Zirconia with the highest ME (210 GPa) among the investigated restorative materials had the highest stresses on the restoration, while resin nanoceramic had the lowest. Although these do not exceed the zirconia fracture strength, they could be detrimental when situated at endocrowns in lower and edge areas, leading to failure. The undersurface or intaglio surface of the zirconia restoration was particular highly stressed, which is in accordance with previous studies [29,53,54,64]. Separation of the restoration can also be initiated in highly stressed locations, which is particularly problematic when they are positioned on the bonding surface, which is over the cement layer and natural tooth structure [38]. When the ME of the prosthesis and substrate differ significantly, it can lead to stress intensity at the interface, cement layer degradation, and an increase in fatigue mechanisms inside the layer [39]. The surface energy of restorative resins generally is affected by clinical procedures [69]. Consistent with research on entire crowns [14], the resinous nanoceramic material (Grandio bulk) had a lower stress variation between dentin and endocrown and released more energy than dentin alone [70].
Cement Failure Risk
The thickness layer of the cement in the study was 120 μm, which has been previously used [14,24], and has been a matter of debate since bonding failures in this layer are strongly correlated with the durability of adhesive restorations [64]. As the material’s rigidity increases, the stress stops extending to the cement layer, which was also supported by the findings of our study for maxillary molar endocrowns. The cement failure risk results (Table 4) show that the risk of debonding can be decreased by choosing a material with a higher ME. These results agree with previous studies that investigated the influence of ME of restorative material on the adhesive and cohesive bond strengths of luting agents [18,27,31,54,56,64,65]. The cement area is very small after proper cementation in clinical practice; therefore, there are few noticeable differences among the restorative materials, despite their significance. Careful cementation is always required because, as exhibited in the colored graphs, materials with a low ME permit visible entry of stress to the cement. The cement layer, including the endocrown’s mechanical performance and longevity, needs additional research. Individual mechanical properties of the resin cement were observed to have a direct effect on failure risk (both adhesive and cohesive). The cement we used has a tensile strength of 51.9 MPa, which is much higher than some of the cements used in other studies [31,45]. The restoration margins are also more prone to infiltration and leakage due to the centrifugal contraction that occurs during cement polymerization shrinkage. Cavities can emerge when biofilm accumulates in the space between a restoration and a tooth. Furthermore, the cement layer thickness affects the polymerization shrinkage effect and structural flaws and can be influenced by the endocrown’s internal adaptation.
Study Strength and Limitations
Our literature search found 3 FEA studies on maxillary first molar, but none investigated the preparation parameters and restorative materials that we studied. Our study also analyzed stress distributions on ER while varying the margin types and pulpal extension for ceramic and resin nanoceramic restorative materials. The limitations of the study were that other choices of CADCAM restorative materials were not included, like leucite-reinforced ceramics, and that the occlusal thickness of the ER was not investigated. There are different chewing patterns and occlusion types (class 2 and class 3 malocclusion) involved in mastication, which also need to be investigated. Subsequently, the non-axial loading that occurs during parafunctional habits of the teeth was not investigated. Additional in vitro tests on fracture resistance are necessary to confirm the findings of this FEA study, considering the stress dispersal pattern of an endocrown with varying pulp chamber extension depths and occlusal thicknesses according to the materials’ Young’s modulus and Poisson’s ratio.
Conclusions
Within the scope and limitations of this FEA study on maxillary first molars for ERs, it may be concluded that no single factor for preparation or restorative material can be exclusively used to guide a clinician in restoring ETT with ER. However, among the margin types, the flat butt and 20-degree bevel do not stress the endocrown preparation area, while shoulder margin is associated with higher stresses, especially with resin nanoceramics. The shoulder margin is also associated with inducing coronal stresses for all materials. Considering 2–4 mm of pulpal extension, the 4 mm extension increases coronal stresses on enamel for both lithium disilicate and resin nanoceramic, with less influence on zirconia. Less stresses exist on ER if the low ME material is used, while increasing the stress on tooth tissue and cement. All restorative materials, irrespective of preparation design, generate stress on the remaining natural tooth structure, with resin nanoceramic generating the most. The adhesive failure risk for ER is greater than the cohesive failure risk, irrespective of preparation features or material used.
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Heng C. Tooth decay is the most prevalent disease. Fed Pract. 2016;33(10):31–33. [PMC free article] [PubMed] [Google Scholar]
- 2.León-López M, Cabanillas-Balsera D, Martín-González J, et al. Prevalence of root canal treatment worldwide: A systematic review and meta-analysis. Int Endod J. 2022;55(11):1105–27. doi: 10.1111/iej.13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alghamdi NS, Alamoudi RA, Baba SM, et al. A scanning electron microscopy study comparing 3 obturation techniques to seal dentin to root canal bioceramic sealer in 30 freshly extracted mandibular second premolars. Med Sci Monit. 2023;29:e940599. doi: 10.12659/MSM.940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CYKJ, Lin YS, Chang YH. Fracture resistance and failure modes of CEREC endocrowns and conventional post and core-supported CEREC crowns. J Dent Sci. 2009;4(3):110–17. [Google Scholar]
- 5.Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod. 1989;15(11):512–16. doi: 10.1016/S0099-2399(89)80191-8. [DOI] [PubMed] [Google Scholar]
- 6.Suksaphar W, Banomyong D, Jirathanyanatt T, Ngoenwiwatkul Y. Survival rates from fracture of endodontically treated premolars restored with full-coverage crowns or direct resin composite restorations: A retrospective study. J Endod. 2018;44:233–38. doi: 10.1016/j.joen.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Tang W, Wu Y, Smales RJ. Identifying and reducing risks for potential fractures in endodontically treated teeth. J Endod. 2010;36:609–17. doi: 10.1016/j.joen.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Singh M, Singh S, Kumar L, et al. Evaluation of retentive strength of 50 endodontically-treated single-rooted mandibular second premolars restored with cast post cores using 5 common luting (cement) agents. Med Sci Monit. 2024;30:e944110. doi: 10.12659/MSM.944110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shu X, Mai QQ, Blatz M, et al. Direct and indirect restorations for endodontically treated teeth: A systematic review and meta-analysis, IAAD 2017 Consensus Conference Paper. J Adhes Dent. 2018;20(3):183–94. doi: 10.3290/j.jad.a40762. [DOI] [PubMed] [Google Scholar]
- 10.Sailer I, Makarov NA, Thoma DS, Zwahlen M, Pjetursson BE. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs) Dent Mater. 2015;31:603–23. doi: 10.1016/j.dental.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Pissis P. Fabrication of a metal-free ceramic restoration utilizing the monobloc technique. Pract Period Aesthet Dent. 1995;7(5):83–94. [PubMed] [Google Scholar]
- 12.Meshni AA, Al Moaleem MM, Mattoo KA, et al. Radiographic evaluation of post-core restorations fabricated by dental students at Jazan University. J Contemp Dent Pract. 2018;19(1):66–72. doi: 10.5005/jp-journals-10024-2213. [DOI] [PubMed] [Google Scholar]
- 13.Sayed ME, Lunkad H, Mohana EJ, et al. The impact of repeated steam sterilization cycles on the efficacy of chairside adjustment kits for polishing monolithic multi-layered zirconia dental restoration material. Med Sci Monit. 2024;30:e944502. doi: 10.12659/MSM.944502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Zheng Z, Wu M, et al. Influence of restorative material and cement on the stress distribution of endocrowns: 3D finite element analysis. BMC Oral Health. 2021;21:495. doi: 10.1186/s12903-021-01865-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bindl A, Richter B, Mörmann WH. Survival of ceramic computer-aided design/manufacturing crowns bonded to preparations with reduced macroretention geometry. J Prosthet Dent. 2006;95(1):81. [PubMed] [Google Scholar]
- 16.Rocca GT, Sedlakova P, Saratti CM, et al. Fatigue behavior of resin-modified monolithic CAD-CAM RNC crowns and endocrowns. Dent Mater. 2016;32(12):e338–50. doi: 10.1016/j.dental.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Fages M, Bennasar B. The endocrown: A different type of all-ceramic reconstruction for molars. J Can Dent Assoc. 2013;79:d140. [PubMed] [Google Scholar]
- 18.Zhu J, Rong Q, Wang X, et al. Influence of remaining tooth structure and restorative material type on stress distribution in endodontically treated maxillary premolars: A finite element analysis. J Prosthet Dent. 2017;117(5):646–55. doi: 10.1016/j.prosdent.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Sedrez-Porto JA, Rosa WL, da Silva AF, et al. Endocrown restorations: A systematic review and meta-analysis. J Dent. 2016;52:8–14. doi: 10.1016/j.jdent.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Rocca GT, Krejci I. Crown and post-free adhesive restorations for endodontically treated posterior teeth: From direct composite to endocrowns. Eur J Esthet Dent. 2013;8(2):156–79. [PubMed] [Google Scholar]
- 21.Zarone F, Sorrentino R, Apicella D, et al. Evaluation of the biomechanical behavior of maxillary central incisors restored by means of endocrowns compared to a natural tooth: A 3D static linear finite elements analysis. Dent Mater. 2006;22(11):1035–44. doi: 10.1016/j.dental.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 22.Guo J, Wang Z, Li X, et al. A comparison of the fracture resistances of endodontically treated mandibular premolars restored with endocrowns and glass fiber post-core retained conventional crowns. J Adv Prosthodont. 2016;8:489–93. doi: 10.4047/jap.2016.8.6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattoo K, Etoude HS, Hothan HM. Failure of an all ceramic posterior fixed partial denture as a consequence of poor clinical application of diagnostic data. JAMDSR. 2023;11(3):44–48. [Google Scholar]
- 24.Einhorn M, DuVall N, Wajdowicz M, et al. Preparation ferrule design effect on endocrown failure resistance. J Prosthodont. 2019;28:237–42. doi: 10.1111/jopr.12671. [DOI] [PubMed] [Google Scholar]
- 25.Taha D, Spintzyk S, Schille C, et al. Fracture resistance and failure modes of polymer infiltrated ceramic endocrown restorations with variations in margin design and occlusal thickness. J Prosthodont Res. 2018;62:293–97. doi: 10.1016/j.jpor.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Ruan W, He J, et al. Clinical efficacy of different marginal forms of endocrowns: Study protocol for a randomized controlled trial. Trials. 2019;20:454. doi: 10.1186/s13063-019-3530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tribst JPM, Lo Giudice R, Dos Santos AFC, et al. Lithium disilicate ceramic endocrown biomechanical response according to different pulp chamber extension angles and filling materials. Materials. 2021;14:1307. doi: 10.3390/ma14051307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, Wang D, Rong Q, et al. Effect of central retainer shape and abduction angle during preparation of teeth on dentin and cement layer stress distributions in endocrown-restored mandibular molars. Dent Mater J. 2020;39:464–70. doi: 10.4012/dmj.2019-050. [DOI] [PubMed] [Google Scholar]
- 29.Dartora NR, de Conto Ferreira MB, Moris ICM, et al. Effect of intracoronal depth of teeth restored with endocrowns on fracture resistance: In vitro and 3-dimensional finite element analysis. J Endod. 2018;44:1179–85. doi: 10.1016/j.joen.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Haralur SB, Alamrey AA, Alshehri SA, et al. Effect of different preparation designs and all ceramic materials on fracture strength of molar endocrowns. J Appl Biomater Funct Mater. 2020;18:2280800020947329. doi: 10.1177/2280800020947329. [DOI] [PubMed] [Google Scholar]
- 31.Tribst JPM, Dal Piva AMO, Madruga CFL, et al. Endocrown restorations: Influence of dental remnant and restorative material on stress distribution. Dent Mater. 2018;34:1466–73. doi: 10.1016/j.dental.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Belleflamme MM, Geerts SO, Louwette MM, et al. No post-no core approach to restore severely damaged posterior teeth: An up to 10-year retrospective study of documented endocrown cases. J Dent. 2017;63:1–7. doi: 10.1016/j.jdent.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Gulec L, Ulusoy N. Effect of endocrown restorations with different CAD/CAM materials: 3D finite element and weibull analyses. Biomed Res Int. 2017;2017:5638683. doi: 10.1155/2017/5638683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magne P, Carvalho AO, Bruzi G, et al. Influence of no-ferrule and no-post buildup design on the fatigue resistance of endodontically treated molars restored with resin nanoceramic CAD/CAM crowns. Oper Dent. 2014;39(6):595–602. doi: 10.2341/13-004-L. [DOI] [PubMed] [Google Scholar]
- 35.Abo-Elmagd A, Abdel-Aziz M. Influence of marginal preparation design on microleakage and marginal gap of endocrown cemented with adhesive resin cement. Dent J. 2015;61(5481):5489. [Google Scholar]
- 36.Sakrana A, Al-Zordk W, elameen A. Impact of marginal preparation design on the fracture resistance of endo-crown all-ceramic. IOSR-JDMS. 2019;18:11–17. [Google Scholar]
- 37.Hayes A, Duvall N, Wajdowicz M, Roberts H. Effect of endocrown pulp chamber extension depth on molar fracture resistance. Oper Dent. 2017;42(3):327–34. doi: 10.2341/16-097-L. [DOI] [PubMed] [Google Scholar]
- 38.Rocca GT, Daher R, Saratti CM, et al. Restoration of severely damaged endodontically treated premolars: The influence of the endo-core length on marginal integrity and fatigue resistance of lithium disilicate CAD-CAM ceramic endocrowns. J Dent. 2018;68:41–50. doi: 10.1016/j.jdent.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Thomas RM, Kelly A, Tagiyeva N, Kanagasingam S. Comparing endocrown restorations on permanent molars and premolars: A systematic review and meta-analysis. Br Dent J. 2020 doi: 10.1038/s41415-020-2279-y. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Sayed ME, Jain S, Ageeli AA, et al. Influence of chairside simulated adjustment (finishing and polishing) protocol and chlorhexidine mouthwash immersion on color stability and translucency of 2 and 3 preshaded multilayered monolithic zirconia. Med Sci Monit. 2024;30:e943404. doi: 10.12659/MSM.943404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roggendorf MJ, Kunzi B, Ebert J, et al. Seven-year clinical performance of CEREC-2 all-ceramic CADCAM restorations placed within deeply destroyed teeth. Clin Oral Investig. 2012;16:1413–24. doi: 10.1007/s00784-011-0642-8. [DOI] [PubMed] [Google Scholar]
- 42.Lin CL, Chang YH, Pai CA. Evaluation of failure risks in ceramic restorations for endodontically treated premolar with mod preparation. Dent Mater. 2011;27(5):431–38. doi: 10.1016/j.dental.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Aktas G, Yerlikaya H, Akca K. Mechanical failure of endocrowns manufactured with different ceramic materials: An in vitro biomechanical study. J Prosthodont. 2018;27(4):340–46. doi: 10.1111/jopr.12499. [DOI] [PubMed] [Google Scholar]
- 44.El-Damanhoury HM, Haj-Ali RN, Platt JA. Fracture resistance and microleakage of endocrowns utilizing three CAD-CAM blocks. Oper Dent. 2015;40:201–10. doi: 10.2341/13-143-L. [DOI] [PubMed] [Google Scholar]
- 45.Sedrez-Porto JA, Munchow EA, Valente LL, et al. New material perspective for endocrown restorations: effects on mechanical performance and fracture behavior. Braz Oral Res. 2019;33:e12. doi: 10.1590/1807-3107bor-2019.vol33.0012. [DOI] [PubMed] [Google Scholar]
- 46.Adawi H, Reddy KN, Mattoo K, et al. Effects of artificial aging of direct resin nano-hybrid composite on mean bond strength values for veneer ceramic samples. Med Sci Monit. 2024;30:e945243. doi: 10.12659/MSM.945243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghajghouj O, Tasar-Faruk S. Evaluation of fracture resistance and microleakage of endocrowns with different intracoronal depths and restorative materials luted with various resin cements. Materials (Basel) 2019;12(16):2528. doi: 10.3390/ma12162528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colombo M, Poggio C, Lasagna A, et al. Vickers microhardness of new restorative CAD/CAM dental materials: Evaluation and comparison after exposure to acidic drink. Materials (Basel) 2019;12(8):1246. doi: 10.3390/ma12081246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ural Ç, Çağlayan E. A 3 dimensional finite element and in vitro analysis of endocrown restorations fabricated with different preparation designs and various restorative materials. J Prosthet Dent. 2021;126(4):586–e1. doi: 10.1016/j.prosdent.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y, Fokkinga WA, Zhang Q, et al. Biomechanical properties of different endocrown designs on endodontically treated teeth. J Mech Behav Biomed Mater. 2023;140:105691. doi: 10.1016/j.jmbbm.2023.105691. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Lai H, Meng Q, et al. The synergetic effect of pulp chamber extension depth and occlusal thickness on stress distribution of molar endocrowns: A 3-dimensional finite element analysis. J Mater Sci Mater Med. 2022;33(7):56. doi: 10.1007/s10856-022-06677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aboel-Fadl AK, El-Desoky MA. Influence of endocrown pulpal extension on stress distribution in endodontically treated maxillary premolars a three-dimensional finite element analysis. Egyptian Dental Journal. 2017;63(4):3895–905. [Google Scholar]
- 53.Dartora G, Pereira GK, de Carvalho RV, et al. Comparison of endocrowns made of lithium disilicate glass-ceramic or polymer-infiltrated ceramic networks and direct composite resin restorations: fatigue performance and stress distribution. J Mech Behav Biomed Mater. 2019;100:103401. doi: 10.1016/j.jmbbm.2019.103401. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Z, He Y, Ruan W, et al. Biomechanical behavior of endocrown restorations with different CAD-CAM materials: A 3D finite element and in vitro analysis. J Prosthet Dent. 2021;125(6):890–99. doi: 10.1016/j.prosdent.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Ma L, Guess PC, Zhang Y. Load-bearing properties of minimal-invasive monolithic lithium disilicate and zirconia occlusal onlays: Finite element and theoretical analyses. Dent Mater. 2013;29:742–51. doi: 10.1016/j.dental.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tribst JP, Dal Piva AM, Lo Giudice R, et al. The influence of custom-milled framework design for an implant-supported full-arch fixed dental prosthesis: 3D-FEA study. Int J Environ Res Public Health. 2020;17:4040. doi: 10.3390/ijerph17114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rees JS, Jacobsen PH. Elastic modulus of the periodontal ligament. Biomaterials. 1997;18:995–99. doi: 10.1016/s0142-9612(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 58.Hassan AA, Sindi AS, Atout AM, et al. Assessment of microhardness of bulk-fill class II resin composite restorations performed by preclinical students: An in vitro study. European Journal of General Dentistry. 2024;13(02):158–64. [Google Scholar]
- 59.Nx3 Kerr. Available from: https://www.kerrdental.com/en-eu/dental-restoration-products/nx3-nexus-third-generation-permanent-dental-cements-and-liners.
- 60.Archangelo KC, Guilardi LF, Campanelli D, et al. Fatigue failure load and finite element analysis of multilayer ceramic restorations. Dent Mater. 2019;35(1):64–73. doi: 10.1016/j.dental.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 61.In ceram zirconia. Available from: https://www.yumpu.com/en/document/read/8159339/vita-in-ceramr-yz-for-inlabr-vident.
- 62.IPS e.max CAD. Available from: https://www.ivoclar.com/en_li/products/digital-processes/ips-e.max-cad.
- 63.Grandio blocs. Available from: https://www.dt-shop.com/index.php?id=22&L=1&artnr=0148&aw=116&pg=12&geoipredirect=1.
- 64.Zheng Z, Sun J, Jiang L, et al. Influence of margin design and restorative material on the stress distribution of endocrowns: A 3D finite element analysis. BMC Oral Health. 2022;22(1):30. doi: 10.1186/s12903-022-02063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dal Piva AMO, Tribst JPM, Borges ALS, et al. CAD-FEA modeling and analysis of different full crown monolithic restorations. Dent Mater. 2018;34(9):1342–50. doi: 10.1016/j.dental.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 66.Sterenborg B, Kalaykova SI, Loomans BAC, Huysmans M. Impact of tooth wear on masticatory performance. J Dent. 2018;76:98–101. doi: 10.1016/j.jdent.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Xiao W, Chen C, Yang T, Zhu Z. Influence of different marginal forms on endodontically treated posterior teeth restored with lithium disilicate glass-ceramic onlays: Two-year follow-up. Int J Prosthodont. 2020;33(1):22–28. doi: 10.11607/ijp.6356. [DOI] [PubMed] [Google Scholar]
- 68.de Kuijper M, Cune MS, Tromp Y, Gresnigt MMM. Cyclic loading and load to failure of lithium disilicate endocrowns: Influence of the restoration extension in the pulp chamber and the enamel outline. J Mech Behav Biomed Mater. 2020;105:103670. doi: 10.1016/j.jmbbm.2020.103670. [DOI] [PubMed] [Google Scholar]
- 69.Gupta S, Sayed ME, Gupta B, et al. Comparison of composite resin (Duo-Shade) shade guide with vita ceramic shades before and after chemical and autoclave sterilization. Med Sci Monit. 2023;29:e940949. doi: 10.12659/MSM.940949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sen N, Us YO. Mechanical and optical properties of monolithic CAD-CAM restorative materials. J Prosthet Dent. 2018;119:593–99. doi: 10.1016/j.prosdent.2017.06.012. [DOI] [PubMed] [Google Scholar]