Abstract
Objective
Aromatic amines (AAs) have been verified as a risk factor for bladder cancer (BCa). Most existing studies have focused on specific AAs types in BCa and assessed the impact of exposure to AAs on the prognosis of BCa patients; however, there is no comprehensive exploration of the mechanism of action. Therefore, this study explored the core hub genes (CHGs) involved in the interaction between major AAs and BCa through multi-database joint analysis to clarify the molecular mechanism of the AAS-induced occurrence and development of BCa and provide innovative insights in the diagnosis and treatment of AAS-induced BCa.
Methods
After the toxicity analysis of AAs, the toxicity regulatory network of AAs in BCa was constructed through network toxicology, and the targets that showed the causal relationship with BCa were screened by Mendelian randomization (MR) analysis. Comprehensive mechanism exploration, molecular docking and drug prediction analysis were conducted on CHGs defined by the protein–protein interaction (PPI) network.
Results
The seven CHGs for the five AAs with different degrees of carcinogenicity to exert toxicity to BCa regulated the occurrence and development of BCa via multiple signaling pathways. Molecular docking confirmed the potential of the activation of these pathways caused by AAs. The results of drug prediction analysis suggested that rapamycin had a potential therapeutic prospect for AAs-induced BCa.
Conclusion
This study reveals the underlying molecular mechanism by which exposure to AAs leads to the occurrence and development of BCa, providing novel preventive and therapeutic insights for populations exposed to this exposure factor.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02730-w.
Keywords: Aromatic amines, Bladder cancer, Network toxicology, Mendelian randomization, Core hub genes, Drug prediction
Introduction
According to the International Agency for Research on Cancer (IARC) classification, numerous aromatic amines (AAs) are classified as Class I carcinogens [1]. The major sink for AAs in the environment is aquatic ecosystems (84%), followed by atmosphere (16%) [2]. In addition, AAs have been used in several industries, including rubber production, paint, various dye and drug manufacturing [3]. Regardless of the limited audiences of the above industries, another widespread source of exposure is tobacco smoke [4]. In March 2012, the USA Food and Drug Administration (FDA) developed a list of 93 hazardous and potentially hazardous components (HPHCs) in tobacco products and tobacco smoke. In the subsequent expanded list, six AAs were summarized [5]. It can be seen that the application of AAs is ubiquitous in daily life. These chemicals can enter the human body through routes such as skin and digestive tract absorption, and respiratory tract inhalation, and generate metabolites associated with toxicity and DNA damage [6]. In addition, small amounts of AAs may reach specific tissues in the free form [7].
Bladder cancer (BCa) is the 10th most common cancer globally, and it ranks 6th among the common cancers in men [3, 8]. Due to the continuous development of demography and exposure omics, people's understanding towards the occurrence and development of BCa has improved [9]. Most BCa cases are associated with external risk factors, among which smoking, various occupational exposures, and environmental exposures have been confirmed to be clear risk factors for BCa [3]. In recent years, the unique information about the high risk of BCa development after long-term exposure to AAs or their derivatives makes it a concern. However, most studies focused on single- or multi-center prospective reports on the effects of AAs and their metabolites on BCa, while there is a lack of an integrative framework to comprehensively analyze how chemotoxic substances disrupt biomolecular mechanisms to induce BCa development [10–12].
Network toxicology, a new discipline that integrates bioinformatics, systems biology, and toxicology, can systematically investigate how chemotoxic substances disrupt the homeostasis of biological systems and lead to disease progression at the molecular level [13]. Mendelian randomization (MR) is a method that uses genetic variation as an instrumental variable (IV) to explore the potential causal relationship between lifetime exposure and outcomes [14]. Therefore, this study aims to explore the potential interaction mechanisms of the five major toxic compounds in AAs [3] with BCa, and also to screen the potential therapeutic compounds against core targets associated with AAs-induced BCa toxicity by combining multiple methods including network toxicology, MR randomization, molecular docking, and drug prediction. Our study not only delves into the underlying molecular mechanisms of how AAs affect the development of BCa, but also provides novel preventive and therapeutic insights for populations exposed to this exposure factor, which can address the research gap targeting the safety problem in this population. More details of the comprehensive process in this study are presented in Graphical Abstract.
Methods
Evaluation of the toxicological properties of AAs
Based on the study published by Jubber et al. [3] in August 2023, five major AAs were extracted from the risk factors for BCa occurrence, including 2-naphthylamine (2-NA), 4-Aminobiphenyl (4-ABP), 4-Chloro-ortho-toluidine (4-CloT), Benzidine (BZ), and Ortho-toluidi (OT). Besides, the ProTox 3.0 (http://tox.charite.de/protox3/) and the ADMETlab 3.0 databases (http://admetlab3.scbdd.com/server/screening/) were employed for the toxicity evaluation of these five AAs.
Acquisition of AAs targets
To be specific, the specific targets of these five AAs were obtained from the ChEMBL (http://www.ebi.ac.uk/chembl/), Comparative Toxicogenomics (CTD, http://ctdbase.org/), STITCH (http://stitch.embl.de/), PharmMapper (http://www.lilab-ecust.cn/pharmmapper/), and SwissTargetPrediction (http://www.swisstargetprediction.ch/) databases. The species was restricted to Homo sapiens. After genes normalized through the UniProt database (http://www.uniprot.org/) were merged and the duplicates were removed, the Venn diagram was plotted to visualize the results.
Mining of BCa-related targets
DrugBank (https://go.drugbank.com/), PharmGkb (https://www.pharmgkb.org/), GeneCards (https://www.genecards.org/), OMIM (https://www.OMIM.org/) databases and Therapeutic Target Database (TTD, https://db.idrblab.net/ttd/) were used for mining BCa-related targets. The search keyword was set as “bladder cancer”. The targets retrieved from each database were merged and visualized with the Venn diagram.
Construction of the toxicity regulatory network
The AAs targets and BCa-related targets were intersected to obtain the potential targets of AAs that induced BCa toxicity. The network files of these five AAs in regulating BCa were sorted out and visualized with the Cytoscape software 3.10.2 (https://www.cytoscape.org/).
Mendelian randomization (MR) analysis
Next, MR analysis was performed to screen the candidate targets that might have causal relationship with BCa. First of all, data of BCa patients were downloaded from the GWAS database (https://www.ebi.ac.uk/gwas/) (registration number: GCST90041857), including 2264 BCa patients and 454,084 healthy controls. Through searching and processing in the eQTLGen Consortium (https://www.eqtlgen.org/), the blood cis-eQTL (expression quantitative trait loci) data of 2,534 genes were obtained. In this study, single nucleotide polymorphisms (SNPs) with genome-wide significance (p < 5 × 10–8) were used as the instrumental variables (IV). To ensure the validity of the selected tool, SNPs were clustered according to the linked disequilibrium (LD) criteria (window size = 10,000 kb and r2 < 0.001), so as to avoid weak instrumental bias and horizontal pleiotropy. The MR analysis was implemented using the “TwoSampleMR”, “MendelianRandomization” and “MVMR” packages in R software version 4.4.1. Three methods including inverse variance weighted (IVW), weighted median and MR-Egger were used to determine the MR estimates. The IVW analysis of Wald ratios for individual SNPs was deemed as the primary outcome. In further sensitivity analysis, the heterogeneity among SNPs was evaluated using Cochran’s Q statistics. Meanwhile, the horizontal pleiotropy was assessed by MR-Egger intercept method.
Screening of core hub targets (CHGs) and plotting of the protein–protein interaction (PPI) network
The genes with significant differences after MR analysis were uploaded to the STRING database (https://cn.string-db.org/), with the species being limited to Homo sapiens and the medium confidence > 0.4. After removing the free nodes, the PPI network diagram of CHGs was obtained.
Pathway enrichment analysis of CHGs
The pathway enrichment analysis of CHGs was performed using R software. In brief, “org.Hs.eg.db” R package were employed for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. The threshold of P-value was set at 0.05.
The relationship regulatory network between CHGs and specific pathways
The CHGs were uploaded to ClueGO plugin of Cytoscape, with the Network Specificity being selected as Medium, the P-value threshold being < 0.05, and the Cluster being set at 3. Both CluePedia and yFiles Layout Algorithms plugins were utilized to visualize the relationship regulatory network between CHGs and specific pathways.
Vina molecular docking
The molecular docking technology was used to explore whether AAs showed potential binding effects with CHGs. In particular, the 2D structures of AAs were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), which were later optimized into the 3D structures with Chem3D software (https://www.3dchem.com/). Subsequently, the crystal structures of core targets were downloaded from the RCSB Protein Data Bank (PDB, https://www.rcsb.org/). Thereafter, molecular docking was completed by AutoDock Tools 1.5.6 (http://autodock.scripps.edu/) and AutoDock Vina 1.1.2 (https://vina.scripps.edu/). In addition, PyMOL software 3.0 (https://www.pymol.org/) was also employed to visualize the key ligand-receptor interactions analyzed by the Protein–ligand Interaction Profiler (PLIP) database (http://plip-tool.biotec.tu-dresden.de/plip-web/plip/index/).
Drug prediction analysis
It is essential to identify small molecule therapeutic drugs based on CHGs. Therefore, we downloaded the DSigDBv1.0 Detailed.txt dataset from the DsigDB database (https://dsigdb.tanlab.org/), and used R software “org.Hs.eg.db” and “DOSE” packages to predict the potential therapeutic drugs that might target CHGs. The P-value threshold was set to 0.05.
Analysis of expression of CHGs in BCa
The mRNA expression profiles of bladder urothelial carcinoma (BLCA) were downloaded from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). Subsequently, the expression difference of CHGs between tumor tissues and adjacent non-tumor tissues was analyzed by the “limma” package of R software. Finally, the CHGs immunohistochemical (IHC) results were collected from The Human Protein Atlas (HPA, https://www.proteinatlas.org/) database.
Results
Molecular structures of AAs
The 2D and 3D structures of 2-NA, 4-ABP, 4-CloT, BZ and OT were searched in the PubChem database. As shown in Fig. S1, the basic structure of the five AAs was composed of a benzene ring and amino groups. In addition, both 4-CloT and OT contained one methyl group, while the chlorine group was also present in 4-CloT alone.
Toxicity distribution of AAs
To evaluate the toxicity distribution network of AAs, the toxicity radar maps were plotted based on the ProTox database. As shown in Fig. S2, the five AAs showed different degrees of ecotoxicity, carcinogenicity, neurotoxicity, and blood–brain barrier (BBB) obstruction. In addition, the carcinogenic toxicity scores and toxicity grades predicted by the ADMETlab database are presented in Table S1, proving the potential high-risk carcinogenicity of AAs.
Prediction of the targets of five AAs on BCa
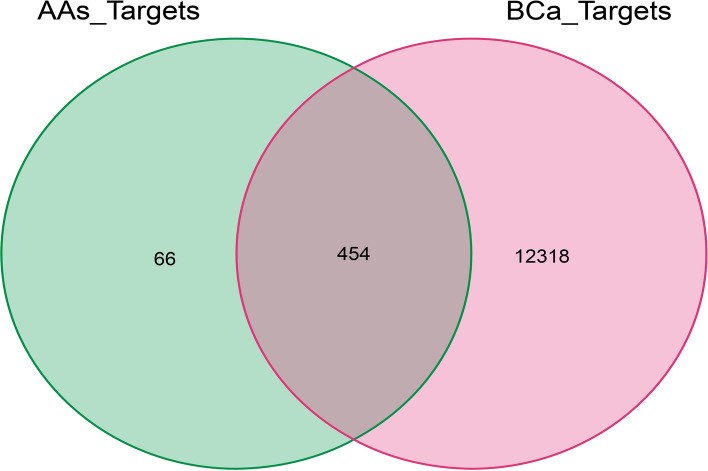
202, 235, 123, 191 and 61 potential targets were generated for 2-NA, 4-ABP, 4-CloT, BZ and OT, respectively (Fig. S3A-E). By summarizing these targets and deleting the duplicate data, a total of 520 AAs-specific targets were obtained (Fig. S3F). Additionally, altogether 12,772 BCa-related targets were identified (Fig. S3G). By intersecting the AAs-specific targets with BCa-related targets, 454 toxic targets by which AAs might induce the occurrence and development of BCa were obtained (Fig. 1).
Fig. 1.
The intersection of the AAs-related targets and BCa-related targets
The toxicity regulatory network of five AAs
A subfile was constructed based on the 454 toxicity targets and uploaded to Cytoscape for mapping the toxicity regulatory network. As observed from Fig. 2, 2-NA, 4-ABP, 4-CloT, BZ, and OT targeted 183, 203, 106, 173, and 52 BCa-related targets, respectively. Meanwhile, there were common targets between any two AAs. Noteworthily, these five AAs could simultaneously interact with 12 targets, suggesting that these toxic substances have multi-target properties and may have extensive toxic potential and biological activities.
Fig. 2.
The toxicity target relationship network of the five AAs. Blue color stands for the five AAs, and yellow–red color represent their target genes (with the darker color and greater circle representing the more AAs connected)
CHGs screened by MR analysis and PPI network analysis
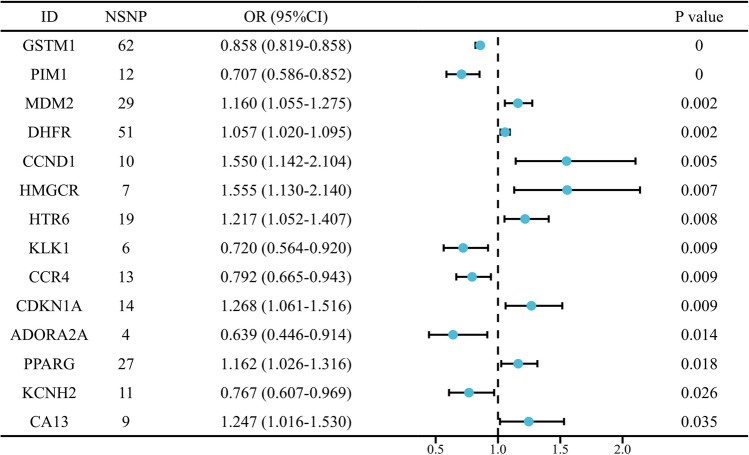
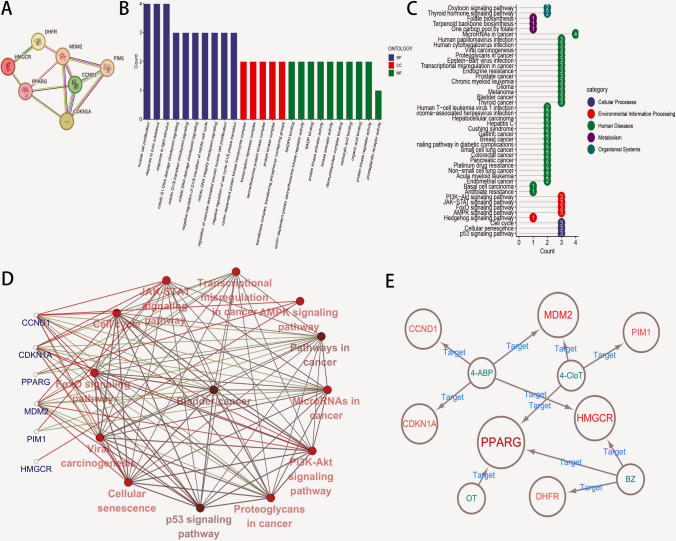
After two-sample MR analysis, the top 20 genes were obtained according to the P-value obtained by the IVW method, among which, the P values of 14 genes were of significant differences (P < 0.05) (Table S2). The detailed MR analysis (Fig. S4A) and sensitivity analysis results (Fig. S4B) for the 14 genes with significant causal relationships were displayed, and forest maps were drawn to intuitively reflect their respective risk effects on BCa (Fig. 3). After constructing the PPI network, the free nodes were eliminated to ensure that each target was interconnected. According to the results, seven closely related genes were finally selected as CHGs (including CCND1, CDKN1A, DHFR, HMGCR, MDM2, PIM1 and PPARG) (Fig. 4A).
Fig. 3.
Forest plot of the causal relationship between 14 toxicity targets and BCa (NSNPs: Number of single nucleotide polymorphisms; OR Odds ratio, 95%CI 95% Confidence interval; P < 0.05)
Fig. 4.
The PPI network and functional enrichment analysis of CHGs. A The PPI network diagram of CHGs. B The bar chart of CHGs related to BP, CC and MF modules in GO annotation (P < 0.05). C The lollipop chart of CHGs in KEGG pathway enrichment analysis (P < 0.05). D The network regulatory analysis of the toxicity mechanisms of CHGs in BCa. The blue color stands for CHGs, the red and green lines represent genes that may act on the pathway, the black line suggests the interaction between pathways, and the darker color of the red circle indicates the more significant P value (P < 0.05). E The relationship network diagram of AAs with specific CHGs
A comprehensive study of the molecular mechanisms underlying CHGs
To better understand the potential mechanisms by which exposure to AAs might lead to BCa, CHGs were subjected to GO analyses to comprehensively evaluate the biological processes (BPs), cellular components (CCs), and molecular functions (MFs). A total of 436 GO terms were obtained from GO annotation (P < 0.05; Fig. 4B, S5A). In terms of BP, CHGs were mainly involved in multiple mitosis-related cell cycle processes, which described the macroscopic regulatory processes that they realized specific toxic functions in BCa. Various protein kinase complexes, transcription- and transfer-related complexes were enriched in the CC module, indicating the specific localization of toxic functions in the cells. As for MF category, various protein kinase activity pathways were mainly involved in this process, highlighting the cytotoxic catalytic and signal transduction activities of these multi-gene product complexes at the cellular microscopic level.
In addition, a total of 44 significantly enriched pathways were generated in the KEGG module (P < 0.05; Fig. 4C, Fig. S5B). Among them, Cellular Process was mediated by cell cycle, cellular senescence, and p53 signaling pathways. PI3K-Akt, JAK-STAT, FoxO, and AMPK signaling pathways, which were strongly associated with the occurrence and development of BCa, were involved in Environmental Information Processing [15–18]. Furthermore, a few CHGs participated in the Metabolic Process and Organismal Systems. Noteworthily, numerous cancers were enriched in Human Disease. Here, the cancer type studied in this work was among them, demonstrating the strong associations with CHGs that interacted with AAs.
To better understand how CHGs regulated BCa via a specific pathway, ClueGO and CluePedia plugins of Cytoscape was utilized to draw the gene and pathway relationship diagram centered on “Bladder Cancer”. From Fig. 4D, CCND1, CDKN1A, and MDM2 might regulate BCa via the JAK-STAT, PI3K-Akt, p53, and FoxO signaling pathways. Also, BCa might be regulated by these CHGs-associated biological processes including cell cycle, cellular senescence, viral carcinogenesis and proteoglycans in cancer (P < 0.05). Therefore, the CHGs in which AAs exert toxic biological behavior on BCa can regulate BCa via various signaling pathways, thus promoting the occurrence and development of BCa.
CHGs can dock with multiple AAs simultaneously
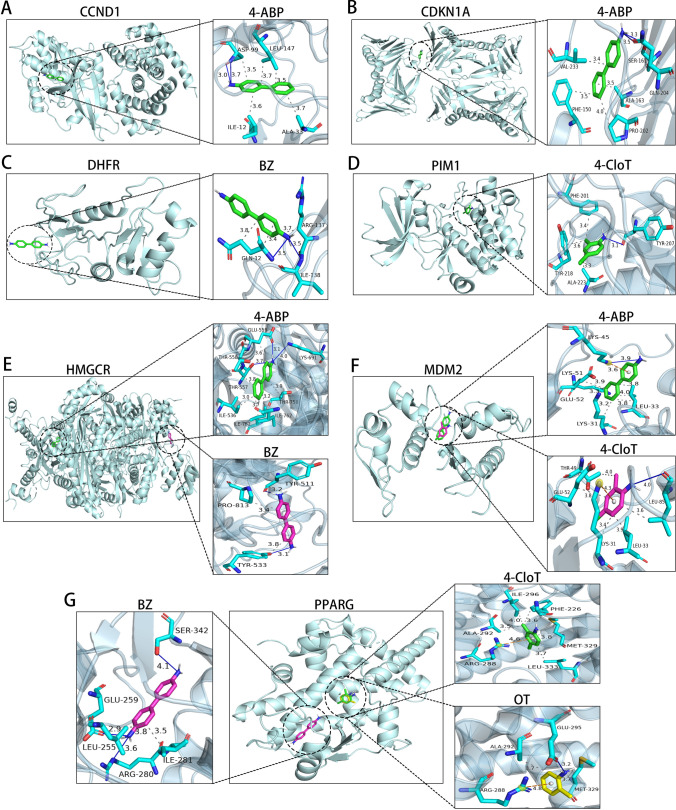
The relationship network of AAs in regulating CHGs was derived from the toxicity regulatory network of AAs to BCa-related targets. As displayed in Fig. 4E, individual AAs can target multiple CHGs simultaneously. Interestingly, 2-NA did not dock with any CHGs, but it does not mean that 2-NA showed lower biotoxicity to BCa. Subsequently, on the basis of the regulatory network of AAs on CHGs, molecular docking analysis was performed to systematically evaluate the binding efficiency between the four AAs and specific CHGs. All four AAs demonstrated successful docking with their respective targets (Fig. 5). In short, several binding modes existed for mono-ligand-receptors: (1) multi-site secondary bond formation with identical residues, (2) multi-site/multi-secondary bond engagement with the same residue, and (3) multi-residue interactions mediated by identical secondary bonds at shared binding sites (Fig. 5A–D). Furthermore, the capacity of CHGs to accommodate multiple ligands through these mechanisms highlights their intrinsic multi-toxicant binding potential (Fig. 5E–G). The binding energy of each ligand-receptor docking mode is less than − 5 kcal/mol, signifying good binding stability (Table S3).
Fig. 5.
Molecular Docking Analysis of AAs with Specific CHGs. Molecular docking of A CCND1, B CDKN1A, C DHFR, D PIM1, E HMGCR, F MDM2, and G PPARG with corresponding connected toxic ligands. Blue straight line represents hydrogen bonds, gray dashed line stands for hydrophobic interactions, and the yellow dashed line indicates π–π stacking
Drug prediction analysis
On the basis of CHGs, the potential therapeutic drugs that might target CHGs were predicted with the DsigDB database. Figure 6A presents the top ten therapeutic drugs according to P-value. Among them, 2–4-Thiazolidinedione, gamma-Tocopherol, Netoglitazone, Paraoxon-methyl and Brassinolide targeted three CHGs; pravastatin and triclosan targeted four CHGs, while lovastatin and folic acid targeted five CHGs. Most importantly, rapamycin was bound to six CHGs, indicating its superior potential therapeutic effect against AAs-induced BCa (P < 0.05). Figure 6B shows the top 30 therapeutic drugs according to P-value (P < 0.05). These drugs provide new insights into the prevention and treatment of specific BCa populations exposed to AAs.
Fig. 6.
Therapeutic Drug Predictions for CHGs. A The relationship diagram of CHGs with therapeutic drugs targeting the genes. The greater yellow circle stands for the more genes connected with the therapeutic (P < 0.05). B The bar chart showing the top 30 therapeutic drugs targeting specific CHGs ranked according to the P value (P < 0.05)
Differential expression analysis of CHGs
To quantify the differential expression of CHGs between BCa and its adjacent non-tumor tissues, the mRNA expression profiles of TCGA-BLCA cohort were downloaded for subsequent analysis. The results revealed that DHFR and HMGCR were significantly up-regulated in tumor tissues, whereas CDKN1A and PIM1 were significantly down-regulated (P < 0.05; Fig. S6), indicating that they might play a crucial role in the pathogenesis of BCa. Interestingly, the expression of CCND1, MDM2 and PPARG in tumor tissues showed an up-regulating trend, yet the differences were not statistically significant, this did not necessarily imply that they did not play a significant role in the development of BCa.
In the HPA database, the differential expression of CDKN1A, DHFR, HMGCR, and PIM1 proteins in the normal and tumor groups was consistent with their mRNA expression. Furthermore, the expression of CCND1, MDM2, and PPARG seemed to increase in the tumor group relative to the normal group, verifying that their protein expression levels might show potential specificity compared with their mRNA expression levels that showed no difference (Fig. S7). It is proved that protein abundance may be influenced by numerous factors, such as post-translational modification of protein, subcellular localization of protein, and protein–protein interactions [19, 20].
Discussion
BCa is a major global health issue, with smoking and specific occupational exposures being verified to be the most established risk factors [3]. According to statistics, about 50% of BCa cases are caused by smoking [21]. Smoke from tobacco products contains a variety of AAs, which are thought to be the main cause of BCa [4]. A study of 422,100 participants followed for 30 years showed that smoking increased the risk of BCa by about 2–3 times [22]. It is worth noting that a study from Dobaradaran et al. found that cigarette butts (CBs) can also release AAs into the environment, and the average level of AAs in fresh CBs after smoking is higher [23]. Exposure to occupational carcinogens is the second most common risk factor for BCa in industrialized countries, accounting for approximately 5.7% of newly diagnosed BCa cases [24]. Typically, AAs are one of the most important risk factors, which are primarily generated in industrial settings dealing with dyes, paints, petrochemicals, and metals [25]. At present, relevant studies have suggested that the standardized mortality rate (SMR) and standardized incidence rate (SIR) associated with BCa increased in dye workers with long-term exposure to AAs [12, 26]. At the same time, with the development of modern industry, the emission of AAs has increased significantly, among untreated or insufficiently treated industrial wastewater and tail gas are the main ways for AAs to enter the environment. The cumulative effect of their emission also poses a threat to the ecological environment and human health that cannot be ignored. A study from Chinthakindi et al. investigated 119 indoor dust samples from collected from five provinces in China and found that 10 of the 35 AAs were present in more than 80% of the samples [27]. The common AAs that affect BCa include 2-NA, 4-ABP, 4-CloT, BZ, and OT [3]. Unfortunately, information regarding the genotoxicity of these AAs is insufficient and consistent results have not been obtained. Despite the increasing number of studies concerning the associations between AAs and BCa, most studies focus on identifying the specific types of carcinogenic AAs in BCa and assessing the impact of exposure to AAs on the prognosis of BCa patients. There is a lack of a comprehensive framework for the study of how AAs disrupt the biomolecular mechanism of the organism to induce the occurrence and development of BCa.
This study used a combination of multiple methods to specifically evaluate the interaction patterns between the five AAs and BCa. After confirming that the five AAs shared the common toxicity-carcinogenicity, we first constructed a toxicity regulatory network. The results showed that the five AAs could simultaneously interact with 12 targets related to the biological behavior of BCa. This means that these AAs may display the broad toxicity potential. In the core part of this study, seven CHGs were obtained by combining MR analysis and PPI network analysis. By the comprehensive mechanism exploration of CHGs, the strong correlations between these CHGs and BCa were verified, and CHGs might regulate the occurrence and development of BCa via multiple signaling pathways were clarified. Further, the drug prediction analysis results suggested that rapamycin bound to six CHGs, indicating their superior therapeutic potential against AAs-induced BCa.
We have comprehensively summarized the functions of seven CHGs in BCa. Regarding cell cycle and proliferation regulation, CCND1 encodes cyclin D1, a protein that regulates the G1-to-S phase transition of the cell cycle, thereby promoting cell cycle progression. Studies have confirmed that CCND1 is highly expressed in BCa and is associated with poor prognosis [28]. Multiple studies have demonstrated that CCND1 influences the biological functions of BCa through various pathways [29, 30]. Specifically, CCND1 plays a crucial role in the G1/S transition of the cell cycle by forming a complex with CDK4/6 and acting as a regulatory subunit. Perfluorooctanoic acid (PFOA), commonly found in cosmetics and interior decoration products, has been reported to promote breast cancer progression by increasing the expression levels of cyclin D and CDK4/6 via endocrine disruption pathways [31]. CDKN1A, also known as P21, functions as a cell cycle inhibitor [32]. Recent studies have highlighted its potential role in immunotherapy and targeted therapy for BCa [33, 34]. Through the TCGA pan-cancer atlas study, it was found that 10.46% of bladder urothelial carcinomas have CDKN1A alterations, which is the highest proportion among all types of cancer. Although smoking is a known risk factor for lung cancer, only 1.44% of lung squamous cell carcinomas and 1.06% of lung adenocarcinomas show CDKN1A alterations. This indicates that despite exposure to common carcinogens, the driving mechanisms of CDKN1A in bladder cancer and lung cancer are different (truncating mutations for bladder cancer and missense mutations for lung cancer), and it can serve as a biomarker for predicting clinical outcomes [34]. Additionally, mutations in potential biomarkers such as RAB44, TERT, MUC16, HRNR, and FLG are significantly enriched in BCa cases with CDKN1A alterations [34]. 4-ABP preferentially forms specific adducts at two codons in the TP53 gene, leading to dysregulation of downstream CDKN1A [35]. BZ can downregulate p21 mRNA levels and reduce p21 protein expression, stimulating cells to transition from G1 to S and G2 phases, thereby contributing to the occurrence and development of BCa. The close relationship between these two AAs and CDKN1A aligns well with the findings of this study.
As a negative regulator of p53, MDM2 restricts cell cycle arrest and apoptosis [36]. It promotes tumor cell proliferation, migration, and invasion, demonstrating a positive correlation with the development of BCa [37]. Research indicates that individuals exposed to certain occupational hazards, such as plastic products, paints, polycyclic hydrocarbons, and chemical solvents, exhibit more aggressive forms of BCa through alterations in the MDM2-p73-P14ARF pathway [38]. Regarding MDM2-related biomarkers, most research has focused on the interaction between MDM2 and p53. For instance, circNUDT21 can promote BCa progression via the miR-16-1-3p/MDM2/p53 axis [39]. Compared with BCa, other cancers may exhibit the same or different MDM2 pathogenic mechanisms under environmental exposure. Studies have shown that in some populations in Northeast China, the MDM2-rs2279744 SNP is associated with exposure factors for lung cancer such as pesticide contact, occupational exposure to gasoline/diesel, smoking duration, and heavy cooking fumes [40]. That is, the MDM2-rs2279744 SNP under multiple cancer-inducing exposure factors can regulate MDM2 protein expression, weaken the p53 stress response pathway by directly blocking p53 transcriptional activity and mediating p53 protein degradation [41, 42], and thereby lead to lung cancer. Other studies have shown that SNP309G is a risk allele for spontaneous tumor formation. MDM2 SNP309G/G mice exposed to low-dose ionizing radiation have an increased susceptibility to lymphoma, breast cancer, glioblastoma, and histiocytic sarcoma compared with MDM2 SNP309T/T mice. However, MDM2 SNP309T/T mice exposed to ultraviolet B and Benzo(a)pyrene have a higher risk of developing skin squamous cell carcinoma than MDM2 SNP309G/G mice [43], demonstrating that the driving mechanisms of MDM2 SNP309 in different tumors under environmental exposure are not the same. However, multiple studies have shown that the association between MDM2 SNP309 polymorphism and bladder cancer risk is heterogeneous, and large-sample and well-designed studies are still needed to confirm the association between MDM2 SNP309 polymorphism and bladder cancer susceptibility [44]. Additionally, p53, TET1, and NRF2 can serve as potential prognostic biomarkers associated with MDM2 in bladder cancer [39]. PIM1, a serine-threonine kinase, facilitates early transformation, cell proliferation, and survival during tumorigenesis [45]. According to Guo et al., PIM1 plays a crucial role in the survival and drug resistance of BCa cells, making it a promising therapeutic target [45]. PIM1 also elucidates the pathogenic mechanisms of various cancers under different carcinogenic environmental exposures. Following exposure to cigarette smoke (CS), the upregulation of PIM1 gene expression in lung tissue, corresponding to reduced methylation levels, can prevent cell death induced by CS and neutrophilic airway inflammation [46]. This mechanism may contribute to the pathogenesis of chronic obstructive pulmonary disease (COPD) or COPD-associated lung cancer triggered by CS-induced neutrophilic airway inflammation. Additionally, studies have demonstrated that exposure to benzene and its metabolites, such as phenols and catechols, enhances PIM1 activity, which may promote leukemia progression by modulating c-MYB activity. Specifically, this includes the phosphorylation of c-MYB co-activator p100 by PIM1, as well as the phosphorylation of other PIM1 substrates like CDC25A [47]. Since PIM1 acts as a downstream effector of transcription factors like c-MYC, it can be predicted to jointly predict the clinical outcome of BCa [48].
DHFR is an enzyme that plays a critical role in folate metabolism by converting dihydrofolate into tetrahydrofolate [49]. Chen et al. demonstrated that prolonged exposure to CS extract (CSE) significantly reduces DHFR transcription in urothelial cells, mediated by free radicals such as hydrogen peroxide [50]. This downregulation of DHFR may lead to widespread reductions in the circulation of methyl donors for one-carbon metabolites, including folic acid and methionine, resulting in DNA methylation defects [51]. Such abnormal methylation states can induce genomic instability and DNA fragmentation, thereby promoting carcinogenesis [52]. Given that folate metabolism is commonly disrupted in cancer, other enzymes involved in this pathway, such as SHMT2, MTHFD2, and MTHFD1L, may also play significant roles. These genes could potentially synergize with DHFR to influence the clinical prognosis of BCa [53, 54]. HMGCR is essential for mevalonate synthesis and has been linked to environmentally induced cancers [55]. Studies have shown that high exposure to perfluoroalkyl substances (PFAS) in drinking water regulates the HMGCR gene via cancer-related miRNAs [53]. As a target of lipid-lowering drugs, changes in HMGCR expression affect serum lipid metabolite levels [54], indirectly influencing tumors associated with lipid metabolism. Although specific studies on HMGCR’s role in BCa under environmental exposures are lacking, higher HMGCR expression correlates with BCa occurrence and progression. Liu et al. identified HMGCR as an inhibitor of ferroptosis, inferring that it played a negative role during the development of BCa [56]. Additionally, FXR reduces the malignant metastatic ability of BCa cells by downregulating MMP2, SREBP2, and HMGCR through the AMPK signaling pathway, suggesting these genes may serve as prognostic markers for BCa associated with HMGCR [57]. PPARG, a ligand-activated transcription factor, is downregulated in the basal/squamous subtype of tumors, indicating that loss of PPARG signaling may contribute to BCa formation [58]. Environmental exposure influences PPARG agonists, which upregulate arsenite-induced PPARG in urothelial cells, reducing the expression of proteins related to the basal subtype of BCa and attenuating its invasiveness [59]. In summary, the precise relationship between CHGs and BCa suggests that AA-induced effects on BCa are comprehensive and complex. By intervening in the aberrant expression of multiple genes, AAs can alter various cellular biological behaviors, leading to BCa development.
The molecular docking results of this study also confirmed this view. The binding of each AA to CHGs showed high binding stability, besides, different AAs enhanced the affinity with CHGs through the multi-site and multi-secondary bond binding mode. Therefore, AAs might exert their potential biotoxic and carcinogenic effects by regulating several tumor-related signaling pathways. These results provide important molecular mechanism support for the AAs-induced occurrence of BCa, and also reflect the potential therapeutic benefits of CHGs as the therapeutic targets. Through further drug prediction analysis, we focused our results on rapamycin, which bound to six CHGs. The mammalian rapamycin target protein mTOR pathway is critical for regulating cell function, and the abnormal activation of this pathway can promote tumor progression. The high mTOR activity is detected in approximately 70% of urothelial cancers, suggesting that this pathway plays a key role in the occurrence and development of BCa [60]. Rapamycin is a mTOR inhibitor, which regulates cell proliferation, survival and metabolism by suppressing the mTOR pathway. The Bacillus Calmette-Guérin (BCG) vaccine has been utilized in the treatment of BCa for several decades. Despite its initial success, approximately 25–45% of patients do not respond to BCG therapy, and around 40% experience recurrence [61]. In contrast to BCG’s broad-spectrum immune activation, mTOR inhibitors facilitate personalized therapy by targeting specific molecular characteristics. Moreover, unlike immune checkpoint inhibitors (ICIs), which can induce immune-related adverse reactions, the side effects associated with mTOR inhibitors are generally manageable through dose adjustment and are less likely to lead to secondary resistance. Additionally, previous studies have indicated that combining mTOR inhibitors with chemotherapy or autophagy modulators can enhance therapeutic efficacy. As discovered in the study using a clinically relevant animal model conducted by Andrade D et al., the integration of rapamycin into BCG combined infusion chemotherapy exhibited the best effect [62]. In human studies, rapamycin has also been verified to induce the BCG-specific γδ T cells to enhance its therapeutic effect on BCa, demonstrating the long-term safety and tolerance [63]. Certainly, according to the findings of Ferro et al. [64], the independent predictors of BCG treatment response encompass multifocality, lymphovascular invasion, and the occurrence of repeat transurethral resection of the bladder (re-TURB). Furthermore, these factors have prompted us to investigate their potential role in predicting the therapeutic efficacy of rapamycin when combined with BCG. Collectively, based on targeting six AAs-related CHGs, rapamycin has potential specific antitumor efficacy against AAs-induced BCa compared with other treatment modalities.
This study, for the first time, integrates network toxicology and MR analysis methods to elaborate the interactions between five AAs and BCa, and successfully constructs the comprehensive framework of AAs affecting the occurrence and development of BCa. Certainly, there are certain limitations in the study. First of all, all the target data in this study were obtained from online databases. Although these data were screened and sorted out at various stages, there were still some unavoidable data biases. Secondly, to further enhance its clinical and scientific impact, a more extensive analysis could be conducted by integrating previously reported bladder cancer risk factors with AAs. This approach may offer a more comprehensive perspective on the underlying mechanisms. At the same time, experimental verification for the specific characterizations of the seven CHGs in BCa cells and tissues is still lacking. Therefore, further in vivo and in vitro studies are still warranted to clarify the mechanisms of action between these CHGs and AAs-induced BCa, and relevant prospective experiments should be performed to evaluate and predict the therapeutic effects and biosafety of the therapeutic drugs including rapamycin for AAs-induced BCa patients.
Conclusion
This study revealed the potential molecular mechanisms of BCa occurrence and development caused by AAs exposure and identified seven CHGs involved in multiple signaling pathways (Fig. S8). Comprehensive enrichment analysis and molecular docking results confirmed the possible role of CHGs in AAs-induced BCa. Drug prediction revealed rapamycin as a promising therapeutic drug. These findings provide new prevention and treatment insights for people exposed to AAs and lay the foundation for future research and clinical applications.
Supplementary Information
Acknowledgements
Not applicable.
Author contributions
Conceptualization: Haoyu Xu, Junwu Li, Wei Tang; methodology: Haoyu Xu, Junwu Li, Wei Tang; data curation: Haoyu Xu, Junwu Li; formal analysis: Haoyu Xu, Junwu Li; visualization: Senlin Peng; projection administration: Wei Tang; funding acquisition: Wei Tang; writing—original draft: Haoyu Xu, Junwu Li; writing—review and editing: Senlin Peng, Yuanyuan Bai, Wei Tang.
Funding
The study was funded by the Chongqing Key Projects for Technological Innovation and Application Development, China (cstc2019jscx-gksbx0007).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haoyu Xu and Junwu Li contributed equally to this work.
References
- 1.International Agency for Research on Cancer. Agents classified by the IARC monographs, vol 1–132; 2022. https://monographs.iarc.who.int/list-of-classifications. Accessed 05 July 2022.
- 2.Urbaniak M, Chinthakindi S, Martinez A, et al. Occurrence of primary aromatic amines and nicotine in sediments collected from the United States. Sci Total Environ. 2022;851(Pt 1): 158102. 10.1016/j.scitotenv.2022.158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jubber I, Ong S, Bukavina L, et al. Epidemiology of bladder cancer in 2023: a systematic review of risk factors. Eur Urol. 2023;84(2):176–90. 10.1016/j.eururo.2023.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Guo J, Villalta PW, Weight CJ, et al. Targeted and untargeted detection of dna adducts of aromatic amine carcinogens in human bladder by ultra-performance liquid chromatography-high-resolution mass spectrometry. Chem Res Toxicol. 2018;31(12):1382–97. 10.1021/acs.chemrestox.8b00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng T, Reilly SM, Feng C, et al. Harmful and potentially harmful constituents in the filler and smoke of tobacco-containing tobacco products. ACS Omega. 2022;7(29):25537–54. 10.1021/acsomega.2c02646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem Res Toxicol. 2011;24(8):1169–214. 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherer G, Riedel K, Pluym N, et al. Assessment of the exposure to aromatic amines in users of various tobacco/nicotine products. ACS Omega. 2022;7(45):41775–82. 10.1021/acsomega.2c06130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Compérat E, Amin MB, Cathomas R, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet. 2022;400(10364):1712–21. 10.1016/S0140-6736(22)01188-6. [DOI] [PubMed] [Google Scholar]
- 10.Stojanovic J, Milovanovic S, Pastorino R, et al. Occupational exposures and genetic susceptibility to urinary tract cancers: a systematic review and meta-analysis. Eur J Cancer Prev. 2018;27(5):468–76. 10.1097/CEJ.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 11.Rushton L, Hutchings SJ, Fortunato L, et al. Occupational cancer burden in Great Britain. Br J Cancer. 2012;107(Suppl 1):S3-7. 10.1038/bjc.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano M, Shinagawa T, Eitaki Y, et al. Risk of bladder cancer in male Japanese workers exposed to ortho-toluidine and other aromatic amines. Int Arch Occup Environ Health. 2021;94(6):1427–39. 10.1007/s00420-021-01658-2. [DOI] [PubMed] [Google Scholar]
- 13.He N, Zhang J, Liu M, et al. Elucidating the mechanism of plasticizers inducing breast cancer through network toxicology and molecular docking analysis. Ecotoxicol Environ Saf. 2024;284: 116866. 10.1016/j.ecoenv.2024.116866. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson E, Glymour MM, Holmes MV, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2:6. 10.1038/s43586-021-00092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi M, Liu J, Mei C, et al. TEAD4 functions as a prognostic biomarker and triggers EMT via PI3K/AKT pathway in bladder cancer. J Exp Clin Cancer Res. 2022;41(1):175. 10.1186/s13046-022-02377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog RW, Suzuki M. Adenoviral gene therapy for bladder cancer. Cell. 2023;186(5):893. 10.1016/j.cell.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Ao X, Ding W, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17(1):104. 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin L, Yuan F, Dai G, et al. Blockage of O-linked GlcNAcylation induces AMPK-dependent autophagy in bladder cancer cells. Cell Mol Biol Lett. 2020;25:17. 10.1186/s11658-020-00208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee S, Cakil ZV, Gallant K, et al. Light-activatable ubiquitin for studying linkage-specific ubiquitin chain formation kinetics. Adv Sci (Weinh). 2025;12(6): e2406570. 10.1002/advs.202406570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Li A, Zhang Z, et al. Posttranslational modifications: regulation of nitrogen utilization and signaling. Plant Cell Physiol. 2021;62(4):543–52. 10.1093/pcp/pcab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306(7):737–45. 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob L, Freyn M, Kalder M, et al. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: a retrospective study of 422,010 patients followed for up to 30 years. Oncotarget. 2018;9(25):17420–9. 10.18632/oncotarget.24724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobaradaran S, Mutke X, Schmidt TC, et al. Aromatic amines contents of cigarette butts: fresh and aged cigarette butts vs unsmoked cigarette. Chemosphere. 2022;301: 134735. 10.1016/j.chemosphere.2022.134735. [DOI] [PubMed] [Google Scholar]
- 24.Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118(8):1130–41. 10.1038/s41416-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alouini S. Risk factors associated with urothelial bladder cancer. Int J Environ Res Public Health. 2024;21(7):954. 10.3390/ijerph21070954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciocan C, Godono A, Franco N, et al. Mortality from bladder cancer in dyestuff workers exposed to aromatic amines: a 73-year follow-up. Med Lav. 2022;113(2): e2022017. 10.23749/mdl.v113i2.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinthakindi S, Zhu Q, Liao C, et al. Profiles of primary aromatic amines, nicotine, and cotinine in indoor dust and associated human exposure in China. Sci Total Environ. 2022;806(Pt 4): 151395. 10.1016/j.scitotenv.2021.151395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Gu P, Xie R, et al. Heterogeneous nuclear ribonucleoprotein K is associated with poor prognosis and regulates proliferation and apoptosis in bladder cancer. J Cell Mol Med. 2017;21(7):1266–79. 10.1111/jcmm.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Chen X, Xie R, et al. DANCR promotes metastasis and proliferation in bladder cancer cells by enhancing IL-11-STAT3 signaling and CCND1 expression. Mol Ther. 2019;27(2):326–41. 10.1016/j.ymthe.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying Y, Li J, Xie H, et al. CCND1, NOP14 and DNMT3B are involved in miR-502-5p-mediated inhibition of cell migration and proliferation in bladder cancer. Cell Prolif. 2020;53(2): e12751. 10.1111/cpr.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierozan P, Jerneren F, Karlsson O. Perfluorooctanoic acid (PFOA) exposure promotes proliferation, migration and invasion potential in human breast epithelial cells. Arch Toxicol. 2018;92(5):1729–39. 10.1007/s00204-018-2181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst). 2016;42:63–71. 10.1016/j.dnarep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Ding Y, Liu Y, et al. Identification of mutated peptides in bladder cancer from exomic sequencing data reveals negative correlation between mutation-specific immunoreactivity and inflammation. Front Immunol. 2020;11: 576603. 10.3389/fimmu.2020.576603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnoff TE, El-Deiry WS. CDKN1A/p21(WAF1), RB1, ARID1A, FLG, and HRNR mutation patterns provide insights into urinary tract environmental exposure carcinogenesis and potential treatment strategies. Am J Cancer Res. 2021;11(11):5452–71. [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Z, Hu W, Rom WN, et al. 4-aminobiphenyl is a major etiological agent of human bladder cancer: evidence from its DNA binding spectrum in human p53 gene. Carcinogenesis. 2002;23(10):1721–7. 10.1093/carcin/23.10.1721. [DOI] [PubMed] [Google Scholar]
- 36.Zhuang C, Miao Z, Wu Y, et al. Double-edged swords as cancer therapeutics: novel, orally active, small molecules simultaneously inhibit p53-MDM2 interaction and the NF-κB pathway. J Med Chem. 2014;57(3):567–77. 10.1021/jm401800k. [DOI] [PubMed] [Google Scholar]
- 37.Wu D, Niu X, Tao J, et al. MicroRNA-379-5p plays a tumor-suppressive role in human bladder cancer growth and metastasis by directly targeting MDM2. Oncol Rep. 2017;37(6):3502–8. 10.3892/or.2017.5607. [DOI] [PubMed] [Google Scholar]
- 38.Schlott T, Quentin T, Korabiowska M, et al. Alteration of the MDM2-p73-P14ARF pathway related to tumour progression during urinary bladder carcinogenesis. Int J Mol Med. 2004;14(5):825–36. [PubMed] [Google Scholar]
- 39.Chen L, Li W, Li Z, et al. circNUDT21 promotes bladder cancer progression by modulating the miR-16-1-3p/MDM2/p53 axis. Mol Ther Nucleic Acids. 2021;26:625–36. 10.1016/j.omtn.2021.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Jin L, Cui J, et al. Mouse double minute-2 homolog (MDM2)-rs2279744 polymorphism associated with lung cancer risk in a Northeastern Chinese population. Thorac Cancer. 2015;6(1):91–6. 10.1111/1759-7714.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bond GL, Hu W, Bond EE, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119(5):591–602. 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Arva NC, Gopen TR, Talbott KE, et al. A chromatin-associated and transcriptionally inactive p53-Mdm2 complex occurs in mdm2 SNP309 homozygous cells. J Biol Chem. 2005;280(29):26776–87. 10.1074/jbc.M505203200. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz GJ, Li Y, Post SM, et al. Contrasting effects of an Mdm2 functional polymorphism on tumor phenotypes. Oncogene. 2018;37(3):332–40. 10.1038/onc.2017.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie L, Sun Y, Chen T, et al. Association between MDM2 SNP309 T>G polymorphism and the risk of bladder cancer: new data in a Chinese population and an updated meta-analysis. Onco Targets Ther. 2015;8:3679–90. 10.2147/OTT.S95353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo S, Mao X, Chen J, et al. Overexpression of Pim-1 in bladder cancer. J Exp Clin Cancer Res. 2010;29(1):161. 10.1186/1756-9966-29-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Vries M, Heijink IH, Gras R, et al. Pim1 kinase protects airway epithelial cells from cigarette smoke-induced damage and airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2014;307(3):L240-251. 10.1152/ajplung.00156.2013. [DOI] [PubMed] [Google Scholar]
- 47.Wan J, Winn LM. The effects of benzene and the metabolites phenol and catechol on c-Myb and Pim-1 signaling in HD3 cells. Toxicol Appl Pharmacol. 2004;201(2):194–201. 10.1016/j.taap.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Foulks JM, Carpenter KJ, Luo B, et al. A small-molecule inhibitor of PIM kinases as a potential treatment for urothelial carcinomas. Neoplasia. 2014;16(5):403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eich ML, Rodriguez Pena M, Chandrashekar DS, et al. Expression and role of methylenetetrahydrofolate dehydrogenase 1 like (MTHFD1L) in bladder cancer. Transl Oncol. 2019;12(11):1416–24. 10.1016/j.tranon.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen LM, Nergard JC, Ni L, et al. Long-term exposure to cigarette smoke extract induces hypomethylation at the RUNX3 and IGF2-H19 loci in immortalized human urothelial cells. PLoS ONE. 2013;8(5): e65513. 10.1371/journal.pone.0065513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z, Zhang Q, Zhang H, et al. Colorectal cancer microbiome programs DNA methylation of host cells by affecting methyl donor metabolism. Genome Med. 2024;16(1):77. 10.1186/s13073-024-01344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmoud AM, Ali MM. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients. 2019;11(3):608. 10.3390/nu11030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pikman Y, Puissant A, Alexe G, et al. Targeting MTHFD2 in acute myeloid leukemia. J Exp Med. 2016;213(7):1285–306. 10.1084/jem.20151574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nilsson R, Jain M, Madhusudhan N, et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128. 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12(7):497–503. 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Ma H, Meng L, et al. Construction and external validation of a ferroptosis-related gene signature of predictive value for the overall survival in bladder cancer. Front Mol Biosci. 2021;8: 675651. 10.3389/fmolb.2021.675651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai CR, Tsai YL, Tsai WC, et al. Farnesoid X receptor overexpression decreases the migration, invasion and angiogenesis of human bladder cancers via AMPK activation and cholesterol biosynthesis inhibition. Cancers (Basel). 2022;14(18):4398. 10.3390/cancers14184398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tate T, Xiang T, Wobker SE, et al. Pparg signaling controls bladder cancer subtype and immune exclusion. Nat Commun. 2021;12(1):6160. 10.1038/s41467-021-26421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehus AA, Bergum N, Knutson P, et al. Activation of PPARγ and inhibition of cell proliferation reduces key proteins associated with the basal subtype of bladder cancer in As3+-transformed UROtsa cells. PLoS ONE. 2020;15(8): e0237976. 10.1371/journal.pone.0237976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huan J, Grivas P, Birch J, et al. Emerging roles for mammalian target of rapamycin (mTOR) complexes in bladder cancer progression and therapy. Cancers (Basel). 2022;14(6):1555. 10.3390/cancers14061555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crispen PL, Kusmartsev S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother. 2020;69(1):3–14. 10.1007/s00262-019-02443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrade DL, Alalizadeh M, Salustiano A, et al. Bladder cancer immunomodulatory effects of intravesical nitazoxanide, rapamycin, thalidomide and bacillus Calmette-Guérin (BCG). World J Urol. 2023;41(9):2375–80. 10.1007/s00345-023-04526-5. [DOI] [PubMed] [Google Scholar]
- 63.Ji N, Mukherjee N, Reyes RM, et al. Rapamycin enhances BCG-specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study. J Immunother Cancer. 2021;9(3): e001941. 10.1136/jitc-2020-001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferro M, Barone B, Crocetto F, et al. Predictive clinico-pathological factors to identify BCG, unresponsive patients, after re-resection for T1 high grade non-muscle invasive bladder cancer. Urol Oncol. 2022;40(11):490.e13-490.e20. 10.1016/j.urolonc.2022.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.