ABSTRACT
Objective
Calcium homeostasis is regulated by the effects of parathyroid hormone (PTH) and vitamin D on the bones, GI tract, and kidneys. Post‐thyroidectomy hypoparathyroidism and resultant hypocalcemia are common complications associated with prolonged hospitalization and higher costs. The long‐term use of proton pump inhibitors has been associated with metabolic disturbances, including hypocalcemia. The purpose of this study is to determine the rate of hypocalcemia following thyroidectomy in patients taking proton pump inhibitors.
Materials and Methods
Patients treated with total thyroidectomy between 2012 and 2022 were identified via the TriNetX Research Network. The rate of transient (0–6 months following thyroidectomy) and permanent (6–12 months following thyroidectomy) postoperative hypocalcemia was compared between patients with and without a prescription for proton pump inhibitors.
Results
Of 33,309 patients, 21.3% (n = 7081) took proton pump inhibitors before surgery. 50.9% and 10.76% of thyroidectomy patients taking proton pump inhibitors had hypocalcemia compared to 48.3% and 7.22% of patients without proton pump inhibitors at 0–1 and 6–12 months, respectively. Patients prescribed proton pump inhibitors had a significantly increased risk of experiencing hypocalcemia at 0–1, 1–6, and 6–12 months. Patients taking proton pump inhibitors were also at increased risk of visiting the emergency department at 1 and 6 months following surgery.
Conclusions
Patients taking proton pump inhibitors may be more likely to experience short‐term and permanent hypocalcemia after thyroid surgery. The current study is the largest to date, indicating an increased risk of hypocalcemia after thyroidectomy in patients taking proton pump inhibitors.
Level of Evidence
3.
Keywords: calcium, hypocalcemia, PPIs, proton pump inhibitors, thyroidectomy
Patients taking proton pump inhibitors may be more likely to experience of short‐term and permanent hypocalcemia after thyroid surgery. The current study is the largest to date, indicating an increased risk of hypocalcemia after thyroidectomy in patients taking proton pump inhibitors.
1. Introduction
Thyroidectomies are among the most common surgeries performed. Thyroid surgery is the definitive management option for thyroid malignancies as well as benign diseases such as multinodular goiter with compression symptoms [1]. Hypocalcemia is a known complication of thyroidectomy [2]. Patients undergoing thyroidectomy are at risk for devascularization or accidental avulsion of the parathyroid glands [3, 4, 5]. According to the literature, the incidence of transient postoperative hypoparathyroidism and permanent hypoparathyroidism ranges from 0.3% to 49% and 0% to 13%, respectively [6].
A few studies have looked at predictive factors for increased risk of developing hypocalcemia following total thyroidectomy. These include age, sex, preoperative hypocalcemia levels, Graves' disease, prolonged surgery time, and preoperative low PTH levels, all of which may increase the risk for postoperative hypocalcemia after thyroidectomy [2, 7, 8]. Few studies have specifically looked at proton pump inhibitors as one of these factors.
Proton pump inhibitors (PPIs) are commonly prescribed medications. They account for 5%–10% of all prescriptions, and their use continues to increase [9, 10, 11]. PPIs are indicated for many common pathologies such as gastroesophageal reflux disease (GERD), gastric and duodenal ulcers, Barrett's esophagus, and the treatment of Helicobacter pylori infection [12]. As such, they are a mainstay in the short‐term remittance of painful symptoms and the long‐term prevention of esophageal adenocarcinoma [13]. Their mechanism of action works by decreasing gastric acid production by parietal cells [14]. These medications have a favorable side‐effect profile in the short term; however, side effects with extended use include serious metabolic disorders, including hypocalcemia [15].
A prior study has shown a significantly increased incidence of short‐term and permanent hypocalcemia after parathyroidectomy in patients taking a PPI [16]; therefore, it could be hypothesized that patients taking a PPI leading up to thyroidectomy may similarly be more susceptible to postoperative hypocalcemia. In the present study, we seek to determine the rate of short‐term and permanent hypocalcemia following thyroidectomy in a large group of patients with and without PPIs from multiple centers across the United States. To the authors' knowledge, this is the largest study to date in this group of patients.
2. Materials and Methods
2.1. Data Source
The study data were obtained from the TriNetX Research Network (Cambridge, MA) on May 1, 2023. TriNetX is a global federated health research network that provides access to a database of electronic medical records. These include procedures, diagnoses, medications, and laboratory values, all from large healthcare organizations (HCOs) [17]. The TriNetX platform utilizes statistical summaries and aggregated counts of de‐identified information. The platform does not provide personal data or protected health information to its users. Consequently, this study was exempted from Institutional Review Board (IRB) review, and the requirement for informed consent was waived (STUDY00018629).
2.2. Participants
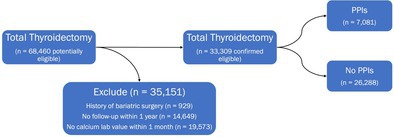
We queried the database and identified patients who underwent total thyroidectomy between 2012 and 2022. Patients were excluded if they had a history of bariatric surgery [18]. Additional measures were taken to ensure high‐quality data and that patients were not lost to follow‐up: Patients were required to have a calcium laboratory value recorded within 1 month of the date of thyroidectomy and have active records for the duration of the study period. Those who met inclusion and exclusion criteria were sorted into a cohort with a PPI prescription and those without. Cohort creation is graphically represented by Figure 1.
FIGURE 1.

Cohort creation. PPIs, proton pump inhibitors.
2.3. Outcome Measures
Data were gathered regarding demographics, medical comorbidities, and pre and postoperative calcium, magnesium, vitamin D, and PTH levels. Surgical factors, including neck dissection, parathyroid autotransplantation, and vocal cord paralysis, were also collected. Indications for thyroidectomy, such as thyroid cancer, Graves' disease, and autoimmune thyroiditis, were also collected. Hypocalcemia was analyzed during three time periods following thyroidectomy: from the date of surgery through the first month, the first month through to 6 months, and from 6 months to 1 year after surgery. Hypocalcemia documented within 6 months after surgery was defined as transient or short‐term. Hypocalcemia documented after 6 months from Surgery was defined as permanent. We defined hypocalcemia as a laboratory serum calcium ≤ 8.4 mg/dL or ionized calcium ≤ 4.6 mg/dL or ≤ 1.1 mmol/L. Visits to the emergency department were also recorded from 0 to 1 month and 1 to 6 months after surgery. The diagnosis and procedure codes for the queries are listed in Supporting Information 1.
2.4. Statistical Methods
All statistical analyses were performed through the TriNetX platform. Short‐term and permanent hypocalcemia rates and emergency department visits were compared between Surgery patients prescribed a PPI and Surgery controls not prescribed a PPI. T‐tests were utilized to compare the mean pre and postoperative calcium, magnesium, vitamin D, and PTH levels between the two cohorts. To mitigate confounding factors, two additional analyses were performed. In the first analysis, groups were propensity score matched according to demographics (age, sex, race, ethnicity) and prior calcium, magnesium, and vitamin D supplementation. In the second sensitivity analysis, groups were matched for demographics, clinical/surgical factors (neck dissection, parathyroid autotransplantation, vocal cord paralysis), and indications for surgery (thyroid cancer, Graves' disease, autoimmune thyroiditis). Propensity score matching was performed utilizing a 1:1 nearest neighbor algorithm with a standard deviation caliper of 0.1. Significance was defined as p < 0.05 for all statistical tests.
3. Results
3.1. Description of Cohort
Sixty‐eight thousand four hundred and sixty patients (n = 68,460) were identified who underwent thyroidectomy between 2012 and 2022. We excluded 19,573 patients without a documented calcium level within 1 month of surgery, 14,649 who did not have active records for the duration of the study period, and 929 with a history of bariatric surgery. This left 33,309 patients eligible for analysis. Of this group, 21.3% (n = 7081) had a PPI prescription leading up to Surgery. The cohort demographics and pertinent clinical information are shown in Table 1. Fifty‐four percent of patients prescribed a PPI were diagnosed with GERD, compared to 12% of patients without a prescription (p < 0.001). Patients prescribed PPIs were older (p < 0.001) and were more likely to be male (p < 0.001). Patients in the PPI group also had a significantly increased likelihood of having a prior prescription for calcium, magnesium, and vitamin D supplementation (p < 0.001).
TABLE 1.
Sample demographics and clinical features (n = 33,309).
| Demographic/clinical feature | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| PPI use and thyroidectomy (n = 7081) | No PPI and thyroidectomy (n = 26,228) | p | PPI use and thyroidectomy (n = 7017) | No PPI and thyroidectomy (n = 7017) | p | |
| Age | 55 ± 14.7 | 48.7 ± 16.6 | < 0.001 | 54.9 ± 14.6 | 55.3 ± 14.6 | 0.07 |
| Sex | ||||||
| Male | 1873 (26.5%) | 6045 (23.0%) | < 0.001 | 1845 (26.3%) | 1758 (25.1%) | 0.09 |
| Female | 5208 (73.5%) | 20,180 (76.9%) | < 0.001 | 5172 (73.7%) | 5258 (74.9%) | 0.10 |
| Unknown | 0 (0%) | < 10 (< 0.04%) | 0.10 | 0 (0%) | 0 (0%) | — |
| Race | ||||||
| White | 4485 (63.3%) | 17,521 (66.8%) | < 0.001 | 4450 (63.4%) | 4416 (62.9%) | 0.55 |
| Black or African American | 1207 (17.0%) | 3587 (13.7%) | < 0.001 | 1191 (17.0%) | 1192 (17.0%) | 0.98 |
| Asian | 190 (2.7%) | 1011 (3.9%) | < 0.001 | 189 (2.7%) | 164 (2.3%) | 0.18 |
| Native Hawaiian or Other Pacific Islander | 22 (0.3%) | 90 (0.3%) | 0.68 | 22 (0.3%) | 13 (0.2%) | 0.13 |
| American Indian or Alaska Native | < 10 (< 0.1%) | 46 (0.2%) | 0.53 | < 10 (0.1%) | < 10 (0.1%) | 1.0 |
| Unknown | 1170 (16.5%) | 3973 (15.1%) | 0.005 | 1158 (16.5%) | 1226 (17.5%) | 0.13 |
| Ethnicity | ||||||
| Not hispanic or Latino | 4970 (70.2%) | 17,314 (66.0%) | < 0.001 | 4914 (70.0%) | 4916 (70.1%) | 0.97 |
| Hispanic or Latino | 487 (6.9%) | 2014 (7.7%) | 0.02 | 485 (6.9%) | 432 (6.2%) | 0.07 |
| Unknown | 1624 (22.9%) | 6900 (26.3%) | < 0.001 | 1618 (23.1%) | 1669 (23.8%) | 0.31 |
| Risk factors for hypocalcemia after thyroidectomy | ||||||
| Previous need for vitamin/mineral supplementation | ||||||
| Prior calcium supplementation | 1980 (28.0%) | 3887 (14.8%) | < 0.001 | 1918 (27.3%) | 1900 (27.1%) | 0.73 |
| Prior vitamin D supplementation | 1712 (24.2%) | 4047 (15.4%) | < 0.001 | 1665 (23.7%) | 1678 (24.0%) | 0.80 |
| Prior magnesium supplementation | 1099 (15.5%) | 1338 (5.1%) | < 0.001 | 1035 (14.8%) | 936 (13.3%) | 0.02 |
| Indication for thyroidectomy | ||||||
| Thyroid cancer | 3217 (45.4%) | 12,926 (49.3%) | < 0.001 | 3217 (45.4%) | 3194 (45.1%) | 0.70 |
| Graves' disease | 671 (9.5%) | 3083 (11.8%) | < 0.001 | 671 (9.5%) | 631 (8.9%) | 0.24 |
| Autoimmune thyroiditis | 736 (10.4%) | 3189 (12.2%) | < 0.001 | 736 (10.4%) | 679 (10.0%) | 0.11 |
| Surgical factors | ||||||
| Central neck dissection | 1176 (16.6%) | 5056 (19.3%) | < 0.001 | 1176 (16.6%) | 1152 (16.3%) | 0.59 |
| Lateral neck dissection | 634 (9.0%) | 2415 (9.2%) | 0.51 | 634 (9.0%) | 561 (8.0%) | 0.03 |
| Parathyroid Autotransplantation | 835 (11.8%) | 3386 (12.9%) | 0.01 | 835 (11.8%) | 776 (11.0%) | 0.12 |
| Paralysis of vocal cord | 124 (1.8%) | 336 (1.3%) | 0.003 | 124 (1.8%) | 86 (1.2%) | 0.008 |
Abbreviation: PPI, proton pump inhibitor.
3.2. Outcomes
3.2.1. Incidence of Postoperative Hypocalcemia
Patients taking PPIs who underwent thyroidectomy had significantly increased rates of hypocalcemia within 0–1 month (relative risk, 95% confidence interval, p‐value) (1.05, 1.03–1.08, < 0.001), 1–6 months (1.47, 1.37–1.57, < 0.001), and 6–12 months (1.49, 1.38–1.61, < 0.001) following surgery compared to patients without a PPI prescription. Patients taking a PPI were more likely to visit the Emergency Department at the 0–1 month (1.44, 1.31–1.59, < 0.001) and 1–6 months (1.86, 1.71–2.01, < 0.001) time points than patients not taking a PPI. The incidence of hypocalcemia in each group is shown in Table 2.
TABLE 2.
Rate of hypocalcemia and emergency department visits following thyroidectomy (before matching).
| Time point (months) | PPI use and thyroidectomy (n = 7081) | Total thyroidectomy alone (n = 26,228) | RR (95% CI) | p |
|---|---|---|---|---|
| 0–1 | 3606 (50.9%) | 12,678 (48.3%) | 1.05 (1.03–1.08) | < 0.001 |
| 1–6 | 959 (13.54%) | 2421 (9.23%) | 1.47 (1.37–1.57) | < 0.001 |
| 6–12 | 762 (10.76%) | 1894 (7.22%) | 1.49 (1.38–1.61) | < 0.001 |
| Emergency department visits | ||||
| 0–1 | 520 (7.3%) | 1338 (5.1%) | 1.44 (1.31–1.59) | < 0.001 |
| 1–6 | 804 (11.35%) | 1605 (6.12%) | 1.86 (1.71–2.01) | < 0.001 |
Abbreviations: PPI, proton pump inhibitor; RR, relative risk.
3.2.2. Biochemical Values Before and After Surgery
Table 3 compares the average serum calcium, calcidiol, and PTH levels at various time points before and after thyroidectomy between patients taking a PPI and those not taking a PPI. Prior to surgery, patients taking PPIs had, on average, lower calcium levels (mean difference, [95% confidence interval], p‐value) (−0.1, [−0.12, −0.08], < 0.001) and higher PTH levels (21, [12.9, 29.0], < 0.001) than those without a PPI prescription. In both cohorts, serum calcium, calcidiol, and PTH sharply decreased the week following surgery before gradually increasing. However, only calcidiol returned to the preoperative baseline, while both serum calcium and PTH levels fell short in both groups. PTH levels remained significantly higher among patients prescribed a PPI compared to those without a prescription at 0–1 week (3.0, [1.1, 4.9], 0.002), 1 week–1 month (6.0, [3.8, 8.2], < 0.001), 1–6 months (6.0, [2.2, 9.8], 0.002) and 6–12 months (8.0, [2.3, 13.7], 0.006). There was no significant difference in calcidiol levels between groups before (0.0, [−1.2, 1.2], 1.0) and through 12 months after Surgery (1.0 [−0.3, 2.3], 0.13).
TABLE 3.
Pre and postoperative biochemical values (before matching).
| Biochemical values | PPI use and thyroidectomy | No PPI and thyroidectomy | Mean difference (95% CI) | p |
|---|---|---|---|---|
| Presurgery (within 6 months prior) | ||||
| Serum calcium (mg/dL) a | 9.4 ± 0.6 (n = 5377) | 9.5 ± 0.6 (n = 17,094) | −0.1 (−0.12 to −0.08) | < 0.001 |
| Calcidiol (ng/mL) | 30 ± 15 (n = 842) | 30 ± 15 (n = 2805) | 0.0 (−1.2 to 1.2) | 1.0 |
| PTH (pg/mL) | 90 ± 174 (n = 1420) | 69 ± 119 (n = 4430) | 21 (13.0 to 29.0) | < 0.001 |
| Postsurgery (0–1 week) | ||||
| Serum calcium (mg/dL) a | 8.7 ± 0.7 (n = 6321) | 8.7 ± 0.7 (n = 23,342) | 0.0 (−0.02 to 0.02) | 1.0 |
| Calcidiol (ng/mL) | 26 ± 14 (n = 214) | 26 ± 14 (n = 760) | 0.0 (−2.13 to 2.13) | 1.0 |
| PTH (pg/mL) | 35 ± 66 (n = 3572) | 32 ± 46 (n = 13,317) | 3.0 (1.1 to 4.9) | 0.002 |
| Postsurgery (1 week–1 month) | ||||
| Serum calcium (mg/dL) a | 9.1 ± 0.8 (n = 3492) | 9.2 ± 0.7 (n = 12,002) | −0.1 (−0.13 to −0.07) | < 0.001 |
| Calcidiol (ng/mL) | 29 ± 14 (n = 450) | 30 ± 15 (n = 1635) | −1.0 (−2.5 to 0.5) | 0.20 |
| PTH (pg/mL) | 38 ± 58 (n = 1554) | 32 ± 33 (n = 5866) | 6.0 (3.8 to 8.2) | < 0.001 |
| Postsurgery (1–6 months) | ||||
| Serum calcium (mg/dL) a | 9.1 ± ± 0.7 (n = 3840) | 9.1 ± 0.6 (n = 12,308) | 0.0 (−0.02 to 0.02) | 1.0 |
| Calcidiol (ng/mL) | 32 ± 15 (n = 834) | 31 ± 14 (n = 2894) | 1.0 (−0.1 to 2.1) | 0.07 |
| PTH (pg/mL) | 47 ± 65 (n = 1372) | 41 ± 64 (n = 5006) | 6.0 (2.2 to 9.8) | 0.002 |
| Postsurgery (6–12 months) | ||||
| Serum calcium (mg/dL) a | 9.1 ± 0.7 (n = 3237) | 9.1 ± 0.6 (n = 9377) | 0.0 (−0.03to 0.03) | 1.0 |
| Calcidiol (ng/mL) | 33 ± 15 (n = 670) | 32 ± 15 (n = 2178) | 1.0 (−0.3 to 2.3) | 0.13 |
| PTH (pg/mL) | 50 ± 60 (n = 732) | 42 ± 71 (n = 2285) | 8.0 (2.3 to 13.7) | 0.006 |
Note: Reference ranges: calcium (8.5–10.5 mg/dL), calcidiol (30–60 ng/mL), PTH (15–65 pg/mL).
Abbreviations: CI, confidence interval; PPI, proton pump inhibitor; PTH, parathyroid hormone.
There are multiple laboratory codes indicating serum calcium and ionized calcium; however, these values represent the most commonly documented code for each.
Subgroup analysis 1 week after surgery revealed 15.6% of PPI users and 16.2% of nonusers had at least one instance of PTH measured below the reference range (≤ 15 pg/mL) in the same time period. Average calcium level was significantly lower in the PPI group at 8.6 ± 0.9 mg/dL compared to those without PPIs at 8.8 ± 0.9 mg/dL (−0.2, [−0.3, −0.1], p < 0.001). Among patients with low PTH ≤ 15 pg/mL, average calcium level at 1 week–1 month was lower in the PPI group (9.1 ± 1.2 mg/dL) compared to the control (9.2 ± 0.9 mg/dL). However, this difference was not significant (−0.1, [−0.23, 0.03], 0.2). At 1–6 months and 6–12 months, calcium levels were the same in both cohorts at 8.9 ± 0.9 mg/dL (0.0, [−0.1, 0.1], 1.0) and 8.8 ± 0.8 mg/dL (0.0, [−0.2, 0.2], 1.0), respectively.
3.2.3. Propensity Score Matching and Sensitivity Analysis
After propensity score matching for age, sex, race, ethnicity, and documented prior prescription for calcium, magnesium, and vitamin D supplementation, patients taking PPIs continued to have significantly higher rates of hypocalcemia at 0–1, 1–6, and 6–12 months after thyroidectomy compared to patients without a PPI prescription (Table 4). This held true in a sensitivity analysis controlling for demographics, indication for thyroidectomy (thyroid cancer, Graves' disease, and autoimmune thyroiditis), and surgical factors (route of dissection, parathyroid autotransplantation, and vocal cord paralysis) (Table 5).
TABLE 4.
Rate of hypocalcemia following thyroidectomy—propensity score matching (matching for demographics and prior vitamin supplementation).
| Time point (months) | PPI use and thyroidectomy (n = 7081, 7017 after matching) | Total thyroidectomy alone (n = 26,228, 7017 after matching) | RR (95% CI) | p |
|---|---|---|---|---|
| 0–1 | 3573 (50.92%) | 3206 (45.69%) | 1.11 (1.08–1.15) | < 0.001 |
| 1–6 | 941 (13.41%) | 678 (9.66%) | 1.39 (1.27–1.52) | < 0.001 |
| 6–12 | 752 (10.72%) | 546 (7.78%) | 1.38 (1.24–1.53) | < 0.001 |
Abbreviations: PPI, proton pump inhibitor; RR, relative risk.
TABLE 5.
Rate of hypocalcemia following thyroidectomy—sensitivity analysis (matching for demographics, clinical diagnoses/indication, and surgical factors).
| Time point (months) | PPI use and thyroidectomy (n = 7081, 7081 after matching) | Total thyroidectomy alone (n = 26,228, 7081 after matching) | RR (95% CI) | p |
|---|---|---|---|---|
| 0–1 | 3606 (50.93%) | 3279 (46.31%) | 1.10 (1.06–1.14) | < 0.001 |
| 1–6 | 959 (13.54%) | 679 (9.59%) | 1.41 (1.29–1.55) | < 0.001 |
| 6–12 | 762 (10.76%) | 533 (7.53%) | 1.43 (1.29–1.59) | < 0.001 |
Abbreviations: PPI, proton pump inhibitor; RR, relative risk.
4. Discussion
Symptomatic hypocalcemia is a substantial source of postoperative morbidity for individuals undergoing thyroid surgery. Hypocalcemia has been described to occur in up to 60% of patients. While this is typically transient, hypocalcemia may be permanent in up to 7% of patients [2]. Many factors influence postoperative hypocalcemia in the setting of thyroidectomy [19]. Intraoperative ligation of or damage to the inferior thyroid artery may compromise the blood supply to parathyroid glands. The parathyroid glands may also suffer unintended trauma or necessary resection [4, 5, 20].
Numerous studies have examined preoperative risk factors for hypocalcemia after thyroid Surgery. These include low preoperative serum calcium, 25‐hydroxyvitamin D, and PTH, as well as advanced age, female sex, and preoperative diagnosis [2, 7, 8]. Surgical factors and the degree of damage to parathyroid glands are understandably the greatest risk factors [4, 5, 20, 21]. Patients are also now routinely tested for postoperative PTH and serum calcium, as low levels can accurately predict early and long‐term need for calcium supplementation [8, 22, 23, 24]. Oral calcium, vitamin D, and, occasionally, magnesium supplementation are usually sufficient to mitigate transient and permanent hypocalcemia [2, 25].
In the present study, the average PTH levels of the PPI group were significantly higher than those of patients not on a PPI both preoperatively and throughout all time points postoperatively. Subgroup analysis of patients with very low postoperative PTH revealed a small but significant negative difference in calcium levels in the PPI group 1 week after surgery. Average calcium levels increased in both groups at 1 month and gradually decreased throughout the following 6 and 12 month time points without significant differences between groups. This is consistent with what is known of PTH as a risk factor for post‐thyroidectomy hypocalcemia and suggests PTH may play a role in the short‐term risk of transient hypocalcemia in patients taking PPIs. The risk of permanent hypocalcemia can be better explained by PPI‐induced hypochlorhydria and decreased gastrointestinal calcium absorption. This could elicit a secondary hyperparathyroidism augmented by increased gastrin due to high gastric pH. Chronic hypergastrinemia has been shown to have an independent, hypertrophic effect on the parathyroid glands [26, 27]. This suggests that the increased risk of permanent postoperative hypocalcemia among PPI users is not due to decreased PTH but is more likely due to persistent hypochlorhydria and malabsorption of calcium. For this reason, oral calcium citrate supplementation may be preferred over the more commonly prescribed calcium carbonate as there is evidence this form is better absorbed at higher pH [28].
Of note, many studies document the benefits of using a postoperative PTH level to predict the risk of hypocalcemia [22, 29, 30, 31]. While the existing literature varies on the use of postoperative PTH and there is no universally agreed upon threshold, a decrease in postoperative PTH generally predicts hypocalcemia [32]. Our finding of relatively increased PTH in patients taking PPIs suggests that postoperative PTH measurements might be misleadingly high. Further studies comparing postoperative PTH levels between surgery patients with and without PPIs may yield interesting results.
PPIs are commonly prescribed and, in the United States, can also be obtained over‐the‐counter [9, 10, 33]. As patients' PPI use increases and persists for long periods, so do concerns about adverse effects. These include an increased risk of chronic kidney disease, pneumonia, dementia, Clostridioides difficile colitis, and malabsorption of important vitamins and minerals such as calcium, magnesium, and vitamin B12 [15]. PPI‐induced hypochlorhydria directly decreases calcium bioavailability, as it depends on low gastric pH. Similarly, PPIs can induce hypomagnesemia, impairing PTH release and further decreasing calcium levels [34, 35]. In the present study, we found patients taking PPIs were 1.47 and 1.49 times more likely to experience short‐term and permanent hypocalcemia after thyroidectomy, respectively.
Our results regarding the risk of hypocalcemia after thyroidectomy stand in contrast to a retrospective study of 371 patients by Gerges et al. They found no significant difference in the incidence and duration of symptomatic hypocalcemia after total or completion thyroidectomy between patients who were and were not taking PPIs [36]. Their study analyzed patients from a single center and utilized symptomatic hypocalcemia, duration of symptoms, and emergency department visits as outcome measures. The present study collected data from over 50 HCOs using the TriNetX research network, with a qualifying sample size of 33,309 patients. The large sample size increases the power of this study and makes the results more generalizable. In addition, we used solely laboratory values to define hypocalcemia, which are more sensitive than diagnosis codes. This increased our confidence that patients truly had active, biochemical hypocalcemia.
Concerning visits to the emergency department following surgery, historically, 21.9% of ED visits within 30 days of thyroidectomy have been found to be due to hypocalcemia [37]. Young et al. studied patients with recent parathyroid or thyroid surgery and found those taking a PPI to be 1.8 times more likely to be evaluated in the emergency department [38]. We found similar results. In our study, patients on a PPI were 1.86 times more likely to visit the emergency department following surgery. This is a relevant outcome for future quality improvement studies and is a significant measure of morbidity.
Greater than 20% of patients in this sample had a prescription for a PPI; this is a clinically significant group. Patients who continue PPIs may benefit from early or preoperative calcium and vitamin D supplementation in anticipation of the increased risk. Magnesium should be screened, monitored, and replenished as necessary [16, 23, 39, 40].
The current work represents the largest study to date analyzing the effect of PPI use on hypocalcemia following thyroidectomy. The greatest strengths of our study include our large sample size of patients and our use of laboratory‐defined hypocalcemia to make the study generalizable, highly powered, and internally valid. There are still limitations to be acknowledged.
Limitations of this study include its retrospective design, limited granularity of the data, and the potential for variability in reporting. As a retrospective study, it is impossible to determine patient adherence to either PPIs or postoperative prescriptions. Furthermore, it is not guaranteed that all patients obtained laboratory testing at the specified time points, beyond the first month. This opens the possibility of undercounting patients with hypocalcemia. To ameliorate this issue, we specified that all patients must keep active records with their respective HCOs for the duration of the study period with at least one visit from 6 to 12 months after thyroidectomy. This increases our confidence that patients without documented laboratory values either indeed did not have hypocalcemia or at the very least were asymptomatic. Given this is a retrospective study, it not only would have been limiting to guarantee patients obtain lab values at regular intervals, but it could also inappropriately bias the study and inflate the reported risk of hypocalcemia. Emergency department visits also represent a broad outcome measure, and we can neither deduce their presentation nor their diagnosis on discharge. There may also be variability in calcium documentation and surgical factors between providers due to including records from many HCOs. This may affect reporting and makes it impossible to determine whether operating surgeons had identified or preserved healthy parathyroid tissue, which could influence hypocalcemia rates.
5. Conclusions
Hypocalcemia following thyroidectomy is often temporary and resolves in most patients. We found that patients taking PPIs may be at increased risk of transient as well as permanent hypocalcemia. Surgeons and patients should be aware of this risk and may consider discontinuing PPIs on a case‐by‐case basis in anticipation of Surgery. Further study is needed to conclusively determine whether holding PPIs reduces postoperative risk of presenting to the ED and returns the risk of hypocalcemia down to baseline.
Disclosure
The authors have nothing to report.
Supporting information
Supporting Information 1 Diagnosis and procedure codes.
Acknowledgments
The authors thank Caia Hypatia for their assistance with manuscript preparation and submission.
Funding: This work was supported by the National Center for Advancing Translational Sciences (UL1 TR002014). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This study was presented at the Triological Society Combined Sections Meeting; January 25, 2024; West Palm Beach, FL, USA and the Pennsylvania Academy of Otolaryngology—Head and Neck Surgery Annual Meeting; June 14, 2024; Hershey PA, USA.
References
- 1. Patel K. N., Yip L., Lubitz C. C., et al., “The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults,” Annals of Surgery 271, no. 3 (2020): e21–e93, 10.1097/SLA.0000000000003580. [DOI] [PubMed] [Google Scholar]
- 2. Qin Y., Sun W., Wang Z., et al., “A Meta‐Analysis of Risk Factors for Transient and Permanent Hypocalcemia After Total Thyroidectomy,” Frontiers in Oncology 10 (2021): 614089, https://www.frontiersin.org/articles/10.3389/fonc.2020.614089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosato L., Avenia N., Bernante P., et al., “Complications of Thyroid Surgery: Analysis of a Multicentric Study on 14,934 Patients Operated on in Italy Over 5 Years,” World Journal of Surgery 28, no. 3 (2004): 271–276, 10.1007/s00268-003-6903-1. [DOI] [PubMed] [Google Scholar]
- 4. Lin Y. S., Hsueh C., Wu H. Y., Yu M. C., and Chao T. C., “Incidental Parathyroidectomy During Thyroidectomy Increases the Risk of Postoperative Hypocalcemia,” Laryngoscope 127, no. 9 (2017): 2194–2200, 10.1002/lary.26448. [DOI] [PubMed] [Google Scholar]
- 5. Sitges‐Serra A., Gallego‐Otaegui L., Suárez S., Lorente‐Poch L., Munné A., and Sancho J. J., “Inadvertent Parathyroidectomy During Total Thyroidectomy and Central Neck Dissection for Papillary Thyroid Carcinoma,” Surgery 161, no. 3 (2017): 712–719, 10.1016/j.surg.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 6. Lee Y. S., Nam K. H., Chung W. Y., Chang H. S., and Park C. S., “Postoperative Complications of Thyroid Cancer in a Single Center Experience,” Journal of Korean Medical Science 25, no. 4 (2010): 541–545, 10.3346/jkms.2010.25.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Z., Zhao Q., Du J., et al., “Risk Factors for Postoperative Hypocalcaemia After Thyroidectomy: A Systematic Review and Meta‐Analysis,” Journal of International Medical Research 49, no. 3. 0300060521996911 (2021), 10.1177/0300060521996911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Păduraru D. N., Ion D., Carsote M., Andronic O., and Bolocan A., “Post‐Thyroidectomy Hypocalcemia ‐ Risk Factors and Management,” Chirurgia (Bucur) 114, no. 5 (2019): 564–570, 10.21614/chirurgia.114.5.564. [DOI] [PubMed] [Google Scholar]
- 9. Johansen M. E., Huerta T. R., and Richardson C. R., “National Use of Proton Pump Inhibitors From 2007 to 2011,” JAMA Internal Medicine 174, no. 11 (2014): 1856–1858, 10.1001/jamainternmed.2014.2900. [DOI] [PubMed] [Google Scholar]
- 10. Rotman S. R. and Bishop T. F., “Proton Pump Inhibitor Use in the U.S. Ambulatory Setting, 2002–2009,” PLoS One 8, no. 2 (2013): e56060, 10.1371/journal.pone.0056060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mishuk A. U., Chen L., Gaillard P., Westrick S., Hansen R. A., and Qian J., “National Trends in Prescription Proton Pump Inhibitor Use and Expenditure in the United States in 2002‐2017,” Journal of the American Pharmacists Association 61, no. 1 (2021): 87–94, 10.1016/j.japh.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 12. Tosetti C. and Nanni I., “Use of Proton Pump Inhibitors in General Practice,” World Journal of Gastrointestinal Pharmacology and Therapeutics 8, no. 3 (2017): 180, 10.4292/wjgpt.v8.i3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scarpignato C. and Wang D. H., “Cancer Prevention in Patients With GERD and Barrett's Esophagus—Medicine,” Foregut 1, no. 1 (2021): 50–62, 10.1177/2634516121993676. [DOI] [Google Scholar]
- 14. Welage L. S., “Pharmacologic Properties of Proton Pump Inhibitors,” Pharmacotherapy 23, no. 10 pt. 2 (2003): 74S–80S, 10.1592/phco.23.13.74S.31929. [DOI] [PubMed] [Google Scholar]
- 15. Eusebi L. H., Rabitti S., Artesiani M. L., et al., “Proton Pump Inhibitors: Risks of Long‐Term Use,” Journal of Gastroenterology and Hepatology 32, no. 7 (2017): 1295–1302, 10.1111/jgh.13737. [DOI] [PubMed] [Google Scholar]
- 16. Lorenz F. J. and Goldenberg D., “Hypocalcemia After Parathyroidectomy in Patients Taking Proton Pump Inhibitors,” American Journal of Otolaryngology 44, no. 2 (2023): 103761, 10.1016/j.amjoto.2022.103761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Topaloglu U. and Palchuk M. B., “Using a Federated Network of Real‐World Data to Optimize Clinical Trials Operations,” JCO Clinical Cancer Informatics 2 (2018): 1–10, 10.1200/CCI.17.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lorenz F. J., Tucker J., Milarachi E. N., Hearn M., King T. S., and Goldenberg D., “Hypocalcemia After Thyroidectomy in Patients With Prior Bariatric Surgery: A Propensity Score Matched Analysis From a National Administrative Database,” Thyroid 32, no. 11 (2022): 1382–1391, 10.1089/thy.2022.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cahill R. A., Harty R., Cotter S., and Watson R. G. K., “Parathormone Response to Thyroid Surgery,” American Journal of Surgery 191, no. 4 (2006): 453–459, 10.1016/j.amjsurg.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 20. Thomusch O., Machens A., Sekulla C., Ukkat J., Brauckhoff M., and Dralle H., “The Impact of Surgical Technique on Postoperative Hypoparathyroidism in Bilateral Thyroid Surgery: A Multivariate Analysis of 5846 Consecutive Patients,” Surgery 133, no. 2 (2003): 180–185, 10.1067/msy.2003.61. [DOI] [PubMed] [Google Scholar]
- 21. Shuchleib‐Cung A., Garcia‐Gordillo J. A., Ferreira‐Hermosillo A., and Mercado M., “Risk Factors for Hypocalcemia After Total Thyroidectomy,” Cirugia y Cirujanos 90, no. 6 (2022): 765–769, 10.24875/ciru.21000579. [DOI] [PubMed] [Google Scholar]
- 22. Metere A., Biancucci A., Natili A., Intini G., and Graves C. E., “PTH After Thyroidectomy as a Predictor of Post‐Operative Hypocalcemia,” Diagnostics (Basel) 11, no. 9 (2021): 1733, 10.3390/diagnostics11091733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grodski S. and Serpell J., “Evidence for the Role of Perioperative PTH Measurement After Total Thyroidectomy as a Predictor of Hypocalcemia,” World Journal of Surgery 32, no. 7 (2008): 1367–1373, 10.1007/s00268-008-9545-5. [DOI] [PubMed] [Google Scholar]
- 24. Noureldine S. I., Genther D. J., Lopez M., Agrawal N., and Tufano R. P., “Early Predictors of Hypocalcemia After Total Thyroidectomy: An Analysis of 304 Patients Using a Short‐Stay Monitoring Protocol,” JAMA Otolaryngology. Head & Neck Surgery 140, no. 11 (2014): 1006–1013, 10.1001/jamaoto.2014.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xing T., Hu Y., Wang B., and Zhu J., “Role of Oral Calcium Supplementation Alone or With Vitamin D in Preventing Post‐Thyroidectomy Hypocalcaemia,” Medicine (Baltimore) 98, no. 8 (2019): e14455, 10.1097/MD.0000000000014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Y. X., “Chronic PPI Therapy and Calcium Metabolism,” Current Gastroenterology Reports 14, no. 6 (2012): 473–479, 10.1007/s11894-012-0290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kopic S. and Geibel J. P., “Gastric Acid, Calcium Absorption, and Their Impact on Bone Health,” Physiological Reviews 93, no. 1 (2013): 189–268, 10.1152/physrev.00015.2012. [DOI] [PubMed] [Google Scholar]
- 28. Recker R. R., “Calcium Absorption and Achlorhydria,” New England Journal of Medicine 313, no. 2 (1985): 70–73. [DOI] [PubMed] [Google Scholar]
- 29. Lecerf P., Orry D., Perrodeau E., et al., “Parathyroid Hormone Decline 4 Hours After Total Thyroidectomy Accurately Predicts Hypocalcemia,” Surgery 152, no. 5 (2012): 863–868, 10.1016/j.surg.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 30. McLeod I. K., Arciero C., Noordzij J. P., et al., “The Use of Rapid Parathyroid Hormone Assay in Predicting Postoperative Hypocalcemia After Total or Completion Thyroidectomy,” Thyroid 16, no. 3 (2006): 259–265, 10.1089/thy.2006.16.259. [DOI] [PubMed] [Google Scholar]
- 31. AlQahtani A., Parsyan A., Payne R., and Tabah R., “Parathyroid Hormone Levels 1 Hour After Thyroidectomy: An Early Predictor of Postoperative Hypocalcemia,” Canadian Journal of Surgery 57, no. 4 (2014): 237–240, 10.1503/cjs.008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathur A., Nagarajan N., Kahan S., Schneider E. B., and Zeiger M. A., “Association of Parathyroid Hormone Level With Postthyroidectomy Hypocalcemia: A Systematic Review,” JAMA Surgery 153, no. 1 (2018): 69–76, 10.1001/jamasurg.2017.3398. [DOI] [PubMed] [Google Scholar]
- 33. Inadomi J. and Fendrick A., “PPI Use in the OTC Era: Who to Treat, With What, and for How Long?,” Clinical Gastroenterology and Hepatology 3, no. 3 (2005): 208–215, 10.1016/S1542-3565(04)00717-7. [DOI] [PubMed] [Google Scholar]
- 34. Gommers L. M. M., Hoenderop J. G. J., and de Baaij J. H. F., “Mechanisms of Proton Pump Inhibitor‐Induced Hypomagnesemia,” Acta Physiologica 235, no. 4 (2022): e13846, 10.1111/apha.13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gragossian A., Bashir K., Bhutta B. S., and Friede R., “Hypomagnesemia,” in StatPearls [Internet] (StatPearls Publishing, 2025), [Updated 2023 Nov 30], https://www.ncbi.nlm.nih.gov/books/NBK500003/. [PubMed] [Google Scholar]
- 36. Gerges D., Grohmann N., Trieu V., Brundage W., and Sajisevi M., “Effect of PPIs on Symptomatic Hypocalcemia After Thyroidectomy: A Retrospective Cohort Study,” Laryngoscope Investigative Otolaryngology 6, no. 1 (2021): 150–154, 10.1002/lio2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taye A., Inabnet W. B., Pan S., et al., “Post‐Thyroidectomy Emergency Room Visits and Readmissions: Assessment From the Collaborative Endocrine Surgery Quality Improvement Program (CESQIP),” American Journal of Surgery 220, no. 4 (2020): 813–820, 10.1016/j.amjsurg.2020.02.036. [DOI] [PubMed] [Google Scholar]
- 38. Young W. G., Succar E., Hsu L., Talpos G., and Ghanem T. A., “Causes of Emergency Department Visits Following Thyroid and Parathyroid Surgery,” JAMA Otolaryngology. Head & Neck Surgery 139, no. 11 (2013): 1175, 10.1001/jamaoto.2013.4505. [DOI] [PubMed] [Google Scholar]
- 39. Luo H., Yang H., Zhao W., et al., “Hypomagnesemia Predicts Postoperative Biochemical Hypocalcemia After Thyroidectomy,” BMC Surgery 17, no. 1 (2017): 62, 10.1186/s12893-017-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Randall R. E., Rossmeisl E. C., and Bleifer K. H., “Magnesium Depletion in Man,” Annals of Internal Medicine 50, no. 2 (1959): 257–287, 10.7326/0003-4819-50-2-257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1 Diagnosis and procedure codes.


