Abstract
Purpose
Due to the efficient bone targeting of mesoporous silica nanoparticles (MSNs) and polyaspartic acid (PASP), 99mTc- labeled polyaspartic acid coated mesoporous silica nanoparticles (PASP-mSiO2-DTPA-99mTc) are proposed as a potential probe for bone imaging.
Methods
Polyaspartic acid-conjugated silica nanoparticles (PASP-mSiO2) were synthesized using aqueous carbodiimide chemistry and characterized by ATR-FTR, FE-SEM, EDX, TEM, TGA and XRD. Radiolabeling of the produced nanoassembly with 99mTc was carried out via a simple DTPA chelation procedure. Aqueous dispersion of the radiolabeled nanoparticles was intravenously injected into normal mice and the bone targeting efficiency was evaluated.
Results
The PASP-mSiO2 nanoassembly was efficiently synthesized and radiolabeled with 99mTc with a high radiochemical yield (92 ± 0.5%) and sufficient in vitro stability in PBS and FBS for up to 24 h. In vivo biodistribution studies revealed a significant enhancement of radioactivity bone uptake after intravenous injection of PASP-mSiO2-DTPA-99mTc compared to radiolabeled uncoated MSNs (mSiO2-DTPA-99mTc), (13 ± 0.6% IA/gram and 5.4 ± 0.4, respectively).
Conclusion
PASP endowed MSNs with enhanced biocompatibility and highly selective bone targeting. Therefore, the proposed PASP-mSiO2-DTPA-99mTc nanoassembly has immense potential in the field of bone- imaging via single photon emitting computed tomography (SPECT).
Graphical Abstract
.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13065-025-01508-z.
Keywords: Mesoporous silica nanoparticles, Polyaspartic acid – Technetium-99m, Bone imaging
Introduction
Technetium-99m (99mTc) is currently the most widely used radiotracer for diagnostic imaging with single photon emission computed tomography (SPECT). The low-cost production and short half-life (t1/2 = 6 h) of this isotope have motivated many researchers to develop advanced SPECT bone imaging probes based on 99mTc [1]. Although, 99mTc radiopharmaceuticals are clinically applied for whole-body bone scans such as 99mTc- methylene bisphosphonate (99mTc-MDP) however, false-negative bone SPECT imaging has been reported in patients with previous bisphosphonate (BP) treatment [2]. To address this issue, recent investigations have focused on designing radiopharmaceuticals that target sites other than the bisphosphonate binding site in the bone. Nanotechnology has the potential to significantly improve the SPECT technique. 99mTc-based radiolabeling of nanoparticles has opened new avenues for the production of sensitive clinical imaging SPECT probes [3]. Mesoporous silica nanoparticles (MSNs) have been extensively explored in bone-targeted nanomedicine as therapeutic or diagnostic agents capable of exclusively reaching bone diseases [4]. MSNs are widely used for the efficient treatment of complex bone diseases such as osteoporosis [5] and bone infection [6] in addition to their use in bone tissue regeneration [7]. Functionalization of MSNs, either with bone-targeting molecules or biopolymers, has been intensively utilized to construct nanoassemblies with enhanced bone-targeting [8, 9]. Polyaspartic acid (PASP) is a water soluble polymer with a linear polyamide backbone structure that enables rapid and complete biodegradation [10]. PASP is proposed for this study due to its high affinity for hydroxyapatite making it effective as a bone targeting moiety to deliver drugs to bone [11, 12]. In vitro and in vivo studies have revealed that a short peptide sequence of the aspartic acid interacts exclusively with bone and teeth [13]. In the last decade, the aspartic acid peptide sequence has been frequently used to target small drugs to bone tissue [14]. For example, PASP has been successfully used to promote bone accumulation of small molecular weight agents, such as radiogallium-labeled bone imaging agent [15].
In this work, the attractive bone-targeting features of polyaspartc acid (PASP) and mesoporous silica nanoparticles (MSNs) have prompted us to develop a novel 99mTc -labeled nanoassembly based on PASP covalently anchored onto the surface of amino-functionalized mesoporous silica as a potential probe for bone-targeting drug delivery applications. The proposed imaging nanoprobe is featured with a safe biological fate due to the biodegradability of PASP and the biocompatibility of MSNs.
Materials and methods
Materials
Sterile Milli-Q ultrapure water with a resistivity of 18.2 MΩ·cm at 25 °C was used throughout the work. N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) 98%, N, N′-dicyclohexylcarbodiimide (DCC), diethylenetriamine-pentaacetic acid (DTPA), cetyltrimethylammonium bromide (CTAB) were products of Sigma-Aldrich Co., Germany. Maleic anhydride, tetraethyl orthosilicate (TEOS), 3-aminopropyltriethoxysilane (APTES), pyridine and acetic anhydride were products of ACROS Organics, Belgium. Aqueous ammonia (33%) was procured locally from ADWK, Egypt. Dimethylformamide (DMF) and dimethylsulfoxide (DMSO) were of analytical grade and dehydrated by fractional distillation under reduced pressure.Technetium-99m (99mTc) was eluted in the form of 99mTcO4¯ from the 99Mo/99mTc generator, at the Radioisotope Production Facility, EAEA, Egypt.
Instruments
UV-Vis absorption measurements were recorded using a computerized recording on a Cary 300 spectrophotometer, from Agilent Technologies. ATR-FTIR was measured using a Bruker VERTEX 80 (Germany) combined with Platinum Diamond ATR, with a range of 4000–400 cm-1 with a resolution of 4 cm-1, and a refractive index of 2.4. The morphological structure was studied using field emission scanning electron microscopy (FE-SEM) using with a JEOL instrument, JXA–840 A. Energy dispersive X-ray spectroscopy (EDS) was done on an INCAx–Sight from Oxford Instruments. Transmission electron microscope (TEM) images were recorded on a JEM-2100, Jeol electron microscope. Dynamic light scattering (DLS) instrument from PSS, Santa Barbara, CA, USA, using the 632 nm line of a He-Ne laser as the incident light with an angle of 90o and Zeta potential with an external angle of 18.9o. Thermal gravimetric analysis (TGA) was performed under nitrogen using a Perkin Elmer Thermogravimetric Analyzer TGA7, USA (RT to 600 ºC) with a heating rate of 10 °C min-1. X-ray diffraction (XRD) data were collected on a PANalytical EMPYREAN diffractometer from Holland with an operating voltage of 45 kV using CuKα as a radiation source. Diffraction patterns were recorded in the angular range of 10–80 with a step width of 0.02 s. Proton Nuclear Magnetic Resonance (1H-NMR) spectra were recorded on a Bruker 400 MHz NMR using d6-chloroform as a solvent. For γ counting, a γ-Scintillation counter (Scaler Ratemeter SR7, Nuclear enterprises LTD, USA fitted with a well type NaI (TI) crystal) was utilized.
Synthesis
Synthesis of mSiO 2-NH2 Nanoparticles
Amino-functionalized mesoporous silica nanoparticles were prepared using a one-pot co-condensation method as previously described by Wada et al. [16] with slight modifications. In brief, a mixture of CTAB (0.7 g, 1.9 mmol) in Milli-Q ultrapure water (350 mL) and 3 ml of 2 M NaOH were stirred at 75 °C for 15 min. TEOS (3.5 mL, 18 mmol) and APTES (0.43 mL, 2.04 mmol) were then added and the mixture was stirred for 3 h. The white solid was isolated by filtration under reduced pressure using a glass frit and subsequently washed with water and ethyl alcohol. After drying at 60 °C, CTAB was removed by soxhlet extraction using a solution of isopropyl alcohol (100 mL) and HCl 37% (5 mL).
Synthesis of mSiO2-DTPA nanoparticles
Firstly DTPA dianhydride (DTPA-DA) was prepared as previously described [17]. For the preparation of mSiO2-DTPA, a mixture of silica (300 mg), DTPA-DA (357 mg, 1 mmol), EDC.HCl (230 mg, 1.2 mmol), NHS (115 mg, 1 mmol) and 15 mL of anhydrous DMF was heated at 50 °C in the dark under static argon for 3 h. After cooling to room temperature, the yellowish product was separated by centrifugation (8000 rpm, 10 min). The solid was washed with a mixture of methanol, DI water and DMF (10:3:0.5). Finally the solid was thoroughly washed with acetone and dried under reduced pressure. The solid was stored in the dark under argon at 4 °C.
Synthesis of polyaspartic acid (PASP)
This biocompatible water soluble polymer was prepared from maleic anhydride and aqueous ammonia through a two- step procedure [18]. FTIR (KBr): ν (cm-1) = 538, 633, 708, 846, 940, 1109, 1236, 1351, 1392, 1549, 1712 (C = O carboxylate), 2880 (CH aliphatic), 2892 (CH aliphatic), 3100–3500 broad (OH). PASP acid was prepared as previously described by our group.1H-NMR (400 MHz, TMS, δ in ppm) 4.71 (CH in α segment), 4.57 (CH in β segment), 2.7 (CH2 β segment), Fig.SI.
Synthesis of PASP-mSiO2 Nanoparticles
Initially, an aqueous solution of PASP (pH 5.2) was subjected to lyophilization at -45°C. Subsequently, a mixture of the lyophilized PASP (100 mg), EDC.HCl (30 mg), NHS (30 mg), 5 mL of anhydrous DMSO and 15 mL of PBS (pH = 4.4) was sonicated for 20 min. To this solution, 300 mg of mSiO2-NH2 was added drop wise. The mixture was then stirred (400 rpm) in the dark for 2 days. The solid was separated by centrifugation (8000 rpm, 10 min). The yellowish white solid was thoroughly washed with DI (3 × 20 mL) and methanol (3 × 20 mL) and dried under reduced pressure. The solid was stored in the dark under argon at 4 °C.
Synthesis of PASP-mSiO2-DTPA nanoparticles
DTPA dianhydride (30 mg) of the prepared mixture was added to a mixture of NHS (30 mg) and DCC (100 µL) previously dissolved in 5 mL of anhydrous DMSO. The mixture was then sonicated at 50 °C for 3 h using an ultrasonic water bath. After cooling to room temperature, 20 mg of mSiO2-PASP was added and the mixture was magnetically stirred (400 rpm) in the dark for 48 h. The nanoparticles were collected through centrifugation and washed with DI (3 × 20 mL) and ethyl alcohol (3 × 20 mL). The yellowish white solid was then dried under reduced pressure and stored under argon.
99 mTc radiolabeling
Radiolabeling of mSiO2-DTPA nanoparticles and PASP-mSiO2-DTPA nanoparticles with 99mTc was performed using SnCl2.2H2O as a reducing agent [19]. Briefly, 0.1 M HCl solution containing different amounts of SnCl2.2H2O (0.5–4 µg) was added to sealed evacuated vials each containing 1 mg of mSiO2-DTPA nanoparticles or PASP-mSiO2-DTPA nanoparticles dispersed in 1 mL of water. The radiolabeling reaction started with the addition of 0.5 mL 99mTcO4− (37 MBq). The reaction mixture was incubated at ambient temperature (25 ± 5 ºC) for different time intervals (5–30 min) to determine the optimum reaction time. The radiochemical yield was estimated using thin layer chromatographic technique (TLC) utilizing silica gel- coated aluminum foil strips as a stationary phase. Two eluents were used; the first one was acetone for the determination of the free technetium (99mTcO4−) percentage. The second eluent was a mixture of water, ethanol and ammonium hydroxide (5: 2: 1 v/v/v) for the determination of reduced hydrolyzed 99mTc and stannous colloid. All experiments were performed in triplicate and the radioactivity was estimated using a well type NaI (TI) gamma counter.
In vitro stability study
The stability of technetium- 99 m labeled mSiO2-DTPA nanoparticles and PASP-mSiO2-DTPA nanoparticles was investigated in PBS and fetal bovine serum albumin (FBS). The experiments were performed by incubating 1 mL of aqueous dispersions of radiolabeled nanoparticles with 3 mL of PBS or FBS for 24 h at 37ºC. At different time intervals (0.5, 1, 2, 4, 6, and 24 h) aliquots were taken out and the free technetium percentage was determined using TLC under the same conditions mentioned before.
Hydroxyapatite microspheres binding assay
To determine the degree of binding of the synthesized nanoparticles to bone, a hydroxyapatite micro sphere binding assay was conducted. Initially, HA was prepared as described in the literature [20]. Dispersion of technetium radiolabeled mSiO2-DTPA-99mTc nanoparticles and PASP-mSiO2-DTPA- 99mTc nanoparticles (3.7 MBq in 1 mL PBS) were incubated with 5 mg HA for 2 h at 37 °C. The incubated sample was then separated by centrifugation at 500 rpm for 5 min; and the radioactivity of the collected supernatant was measured. This experiment was repeated three times. The relative binding affinity of the radiolabeled nanoparticles was calculated using the following equation:
 |
Biocompatibility study
The biocompatibility of both mSiO2-DTPA nanoparticles and PASP-mSiO2-DTPA nanoparticles was assessed using an MTT assay. The cytotoxicity was assessed against human foreskin fibroblast cells (Hs27) as representative of normal human cells. The impact of concentrations (0–200 µg /mL) of nanoparticles on cell viability was determined by incubating them with the cells for 48 h at 37 °C.
Biodistribution study
Evaluation of the bone targeting efficiency of the synthesized silica nanoparticles was evaluated by studying the biodistribution profile of the 99mTc radiolabeled nanoparticles in normal male Swiss Albino mice (25–30 g). Briefly, a 150 µL aqueous dispersion of 99mTc radiolabeled mSiO2-DTPA nanoparticles or PASP-mSiO2-DTPA nanoparticles containing 100 µg nanoparticles and equivalent to 500 µCi was injected intravenously into the tail vein of each normal mouse. At various time intervals (0.5, 1, 2, 4 and 6 h post- injection, with 5 mice for each time point), the mice were anesthetized and sacrificed humanely by cervical dislocation. Different mice organs were dissected and washed twice with saline and weighed. Samples of fresh blood, bone and muscle were collected and weighed. Blood, bone and muscles were assumed to be 7, 10 and 40% of the total body weight, respectively [21]. The radioactivity of each organ was measured using a gamma counter and expressed as a percentage of the injected activity per gram organ or body fluid (% IA/gram organ or body fluid). All results are presented as the mean value ± standard deviation (SD).
Statistical analysis
Statistical analysis of the data was performed using One-way ANOVA. The differences were considered statistically significant at p < 0.05.
Results and discussion
Synthesis and characterization
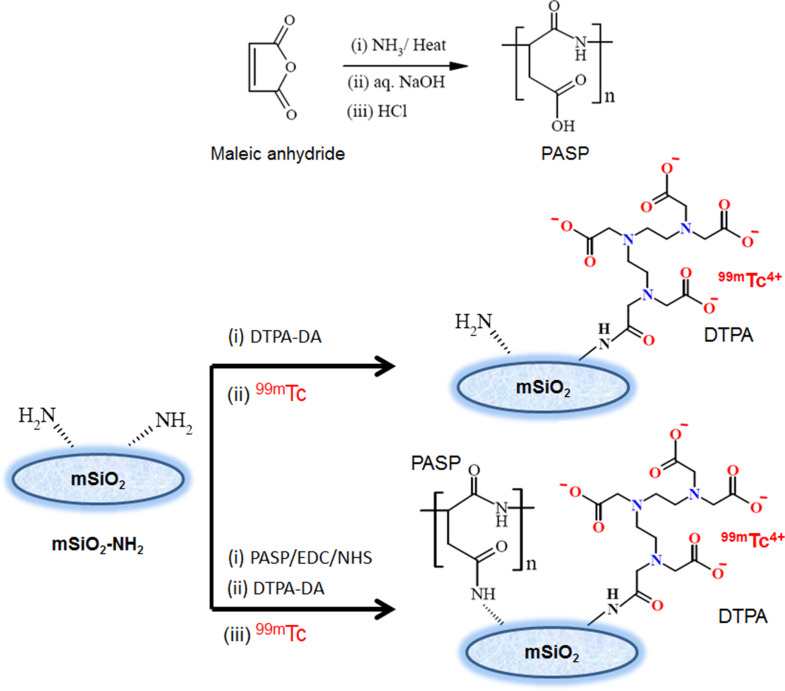
For sensitive and non-invasive SPECT bone-imaging, the moiety to be radiolabeled should interact exclusively with bone without toxic degradable products. Mesoporous silica nanoparticles (MSNs) and polyamino acids such as polyaspartic acid (PASP) show great potential in this regard. The aim of this work was to develop an effective bone-targeting probe based on the biocompatibility and bone-targeting features of both mesoporous silica nanoparticles (mSiO2-NH2) and polyaspartic acid (PASP). First, a facile one-pot method was used to prepare amino-functionalized mesoporous silica nanoparticles (mSiO2-NH2) byco-condensation of silica precursor TEOS with an amino silane coupling agent (APTS) in the presence of CTAB as a shape directing agent [16]. Compared to the classical two-step method, the co-condensation method is more facile and proved to ensure a more homogeneous distribution of amino groups on the surface of the silica nanoparticles [22]. When further covalent binding of active molecules (e.g. drugs or molecules for active targeting, etc.) to the surface of silica is intended, the homogeneous distribution of anchoring amino groups is of utmost importance. Amino-functionalized silica (mSiO2-NH2) nanoparticles were then conjugated to diethylene- triaminepentaacetic dianhydride (DTPA-DA) to produce mSiO2-DTPA nanoparticles (Fig. 1) [23]. In another probe, the biocompatible PASP was synthesized and subsequently anchored onto the amino groups present at the surface of mSiO2-NH2 nanoparticles to produce SiO2-PASP nanoparticles (Fig. 1). Subsequently, DTPA was anchored to the unreacted amino groups to produce PASP-mSiO2-DTPA nanoparticles. Both types of nanoparticles were successfully radiolabeled with Technetium-99m to produce mSiO2-DTPA-99mTc nanoparticles and PASP-mSiO2-DTPA-99mTc nanoparticles suitable for in vivo SPECT bone imaging. The carboxylic groups were activated to NHS-ester groups via simple aqueous carbodiimide chemistry in all coupling reactions.
Fig. 1.
Schematic representation for preparation of PASP, mSiO2-DTPA nanoparticles, and PASP-mSiO2-DTPA nanoparticles
The prepared nanoparticles were characterized by FTIR, TEM, FE-SEM, EDX, XRD, and TGA. The FTIR spectra of mSiO2-NH2 nanoparticles and PASP-mSiO2-NH2 nanoparticles matched well with the data previously reported for mSiO2-NH2 [22] and PASP [18]. The broad peak at 3000–3600 cm− 1 assigned to the Si-OH group of silica nanoparticles or adsorbed water molecules [24a]. The most characteristic region in the FTIR spectra (1200 cm− 1 -2000 cm− 1) of mSiO2-NH2 nanoparticles and PASP-mSiO2-NH2 nanoparticles is shown in Fig. 2a. PASP-coated silica showed two bands at 1714 cm− 1 and 1780 cm− 1 corresponding to stretching vibrations of the carboxylic carbonyl group and amide carbonyl group, respectively [24a]. This indicates that some carboxylic groups of PASP linked to the amino groups at the surface of mSiO2-NH2 forming an amide linkage, while other groups remain unbound to silica. Vibration peaks in the range of 400 cm− 1 − 1400 cm− 1 are characteristic for symmetric stretching and bending of the Si-O-Si bond. The bending mode of the amino groups is observed at 1646 cm− 1. The C-H stretching modes of the methylene groups of the aminopropyl chain and PASP chains give rise to signals in the 2848–2920 cm− 1 range.
Fig. 2.
ATR-FTIR spectra, a, UV-Visible spectra, b and Thermal gravimetric analysis (TGA), c of mSiO2 nanoparticles and PASP-mSiO2 nanoparticles
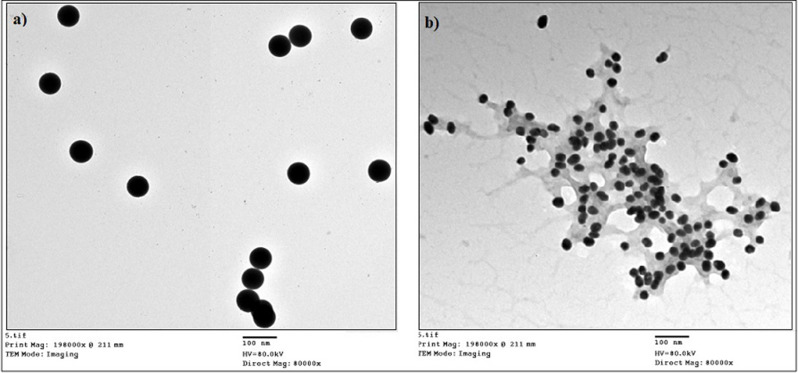
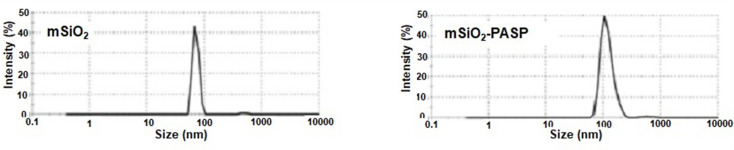
UV-Vis spectra showed that all prepared nanoparticles are inactive in the visible region of spectra (400–700 nm). However, the absorption profiles in the UV region (200–400 nm) (Fig. 2b). Silica is optically inactive in this region, while conjugation to DTPA or PASP resulted in an increase in absorbance in the UV region. This observation gives rise to believe in particular changes of the chemical bonding schemes at the surface of silica nanoparticles. Thermal gravimetric analysis (TGA) of PASP, mSiO2 nanoparticles and PASP-mSiO2 nanoparticles was carried out under a nitrogen atmosphere to avoid any expected thermal degradation (Fig. 2c). In contrast to the high thermal stability of mSiO2 nanoparticles, pure PASP undergoes a rapid thermal degradation after 200 °C. Considering these results and comparing the thermograph of mSiO2 nanoparticles and PASP-mSiO2 nanoparticles, it can be concluded that the PASP layer represents about 10% of the weight of PASP-mSiO2 nanoparticles. X- ray diffraction patterns of mSiO2 and PASP-mSiO2 are shown in Fig. SII. MSiO2 showed some degree of crystallinity in the range of 2θ = 10–30 [23]. Interestingly, PASP coat showed a higher degree of crystallinity as indicated by sharp peaks from 2θ = 20–30 [18]. TEM images (Fig. 3a and b) indicate that both mSiO2-NH2 and mSiO2-PASP nanoparticles are monodispersed and have a spherical morphology. Coating with PASP did not change either size (~ 58 ± 6 nm) or shape of mSiO2-NH2 (size was ascertained by measuring 50 particles in each material). Coating with PASP resulted in the formation of a polymer corona around mSiO2-NH2. The formation of a corona structure is well known when inorganic nanoparticles are coated with a hydrophilic polymer [24b]. FE-SEM images of both mSiO2 nanoparticles, PASP-mSiO2 nanoparticles showed monodispersed spherical nanoparticles of a smooth surface (Fig. 4a and b). The mean diameters of mSiO2 nanoparticles, PASP-mSiO2 nanoparticles were found to be around 80 and 95 nm, respectively. Energy dispersive X-ray (EDX) analysis of mSiO2 nanoparticles and PASP-mSiO2 nanoparticles (Fig. 4c and d; Table 1) showed an increase in the percent content of carbon and oxygen while there was a decrease in the percent content of silicon after anchoring with PASP. As shown in Fig. 5, DLS analysis revealed that conjugation of PASP increased the hydrodynamic size of silica nanoparticles from 95 ± 7 nm (PDI 0.412) to 120 ± 9 nm (PDI 0.521). This increase can be attributed to the outermost layer of PASP and the enhanced water absorbability of PASP-mSiO2 nanoparticles [24a]. The zeta potentials of mSiO2 NPs and PASP-mSiO2 NPs in their aqueous dispersions were + 18.22 and + 10.11, respectively. The positively charged surface of amino modified silica is due to the amino groups on the surface of the NPs. The surface positive charge was decreased after substitution of some of the surface amino groups with anionic polyaspartate.
Fig. 3.
TEM images of mSiO2 nanoparticles, a, and PASP-mSiO2 nanoparticles, b
Fig. 4.
FE-SEM images of mSiO2 nanoparticles, a, and PASP-mSiO2 nanoparticles, b, EDX mapping, d of mSiO2 nanoparticles, c, and PASP-mSiO2 nanoparticles
Table 1.
Data of EDX analysis of mSiO2-NH2 nanoparticles and PASP-mSiO2 nanoparticles
| Element | % C | % N | % O | % Si |
|---|---|---|---|---|
| mSiO2-NH2 | 15 | 10.92 | 51.29 | 14.16 |
| PASP-mSiO2 | 20.48 | 11.81 | 53.12 | 12.59 |
Fig. 5.
DLS images of mSiO2 nanoparticles, a, and mSiO2- PASP nanoparticles
X-ray diffraction patterns of mSiO2 nanoparticles and PASP-mSiO2 nanoparticles are shown in Fig. SI. In addition to the peaks characteristic of silica nanoparticles, the anchoring of PASP resulted in the appearance of new peaks at higher 2theta values.
99 mTc radiolabeling and in vitro Stability study
The radiolabeling of mSiO2 nanoparticles and PASP-mSiO2 nanoparticles with 99mTc was conducted using a DTPA chelation procedure (Fig. 1). MSiO2-DTPA nanoparticles and PASP-mSiO2-DTPA nanoparticles were radiolabeled with 99mTc in high yield as indicated by TLC analysis. This analysis technique is a preferable method for radiochemical yield estimation because it is simple, fast and reliable [23]. According to the TLC conditions applied in this study, the radiolabeled nanoparticles remain at the baseline with the colloid (Rf = 0) when using acetone as an eluent while the free technetium (99mTcO4−) moves with the solvent front (Rf =0.9-1). When using the previously mentioned mixture as an eluent, the colloid remains at the baseline while silica nanoparticles and the free technetium move with the eluent (Rf =0.6–0.7 and Rf = 0.9-1, respectively). The maximum radiolabeling yield achieved for mSiO2-DTPA nanoparticles and PASP-mSiO2-DTPA nanoparticles was (97 ± 0.5% and 92 ± 0.5%, respectively) using 1 µg of SnCl2.2H2O. The radiochemical yield was determined after different time intervals and the results revealed that the optimum reaction time for obtaining maximum yield was 15 min. High in vitro stability (ranging from 96 to 98% stable radiolabeled nanoparticles) was observed for both types of radiolabeled silica nanoparticles in PBS and FBS up to 24 h. This convenient stability has encouraged further biodistribution studies.
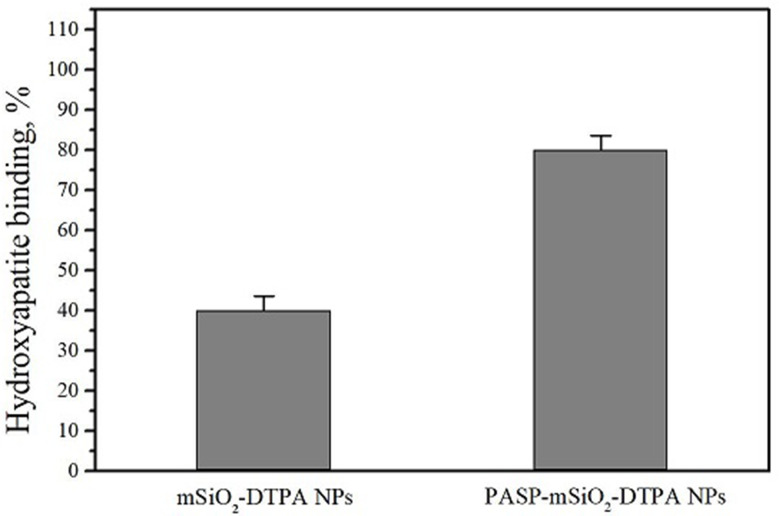
Hydroxyapatite binding affinity
The human body’s bone are rich in hydroxyapatite (HA) so enhancement of nanoparticles bone targeting can be achieved by increasing HA binding affinity. In this study, in vitro evaluation of the effect of poly aspartic acid conjugation to silica nanoparticles on bone affinity was performed by determining the relative binding of the radiolabeled nanoparticles to HA microsphere. The results shown in Fig. 6 revealed higher HA affinity percent of PASP-mSiO2-DTPA nanoparticles compared to mSiO2-DTPA nanoparticles (80 ± 3% and 40 ± 3.5%, respectively) confirming the potential role of poly aspartic acid conjugation in enhancing the silica nanoparticles HA affinity and accumulation in the bone [11].
Fig. 6.
Hydroxyapatite binding affinity of mSiO2 nanoparticles, a, and mSiO2- PASP nanoparticles
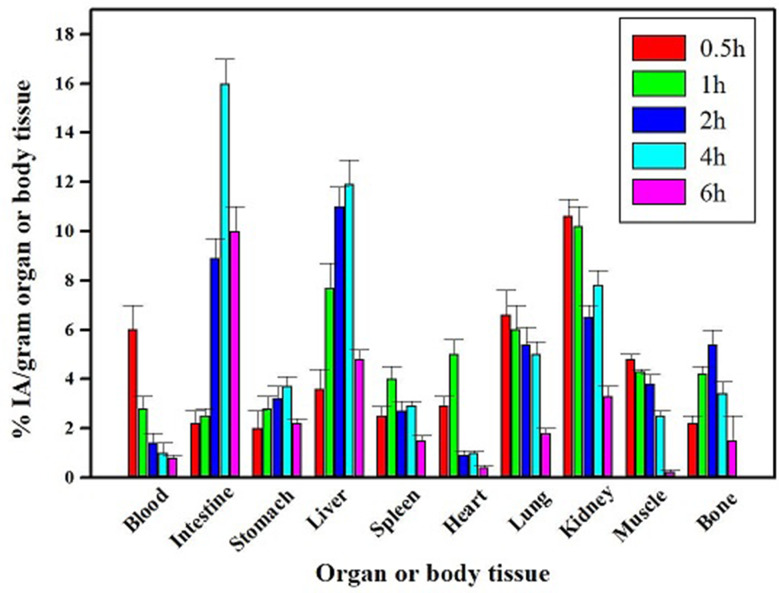
Biocompatibility and biodistribution studies
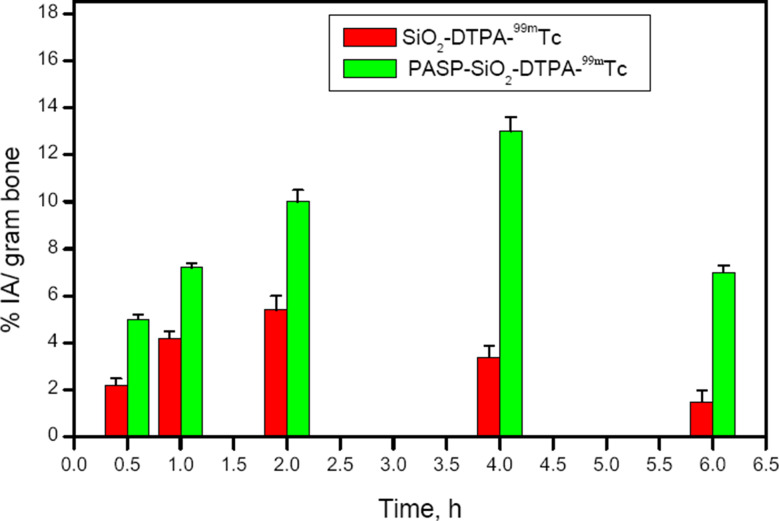
The biocompatibility of SiO2-DTPA nanoparticles and PASP-SiO2-DTPA nanoparticles was studied on a human fibroblast cell line. The MTT assay results revealed that incubation of the cells with the prepared nanoparticles at a concentration of up to 200 µg/ mL for 48 h induced undetectable cell toxicity (cell viability about 96 ± 0.5%). The biocompatibility of the prepared nanoparticles and the high in vitro stability of the radiolabeled nanoparticles encouraged performing a comparative biodistribution study. Determination of the radioactivity percentage in different organs of the experimental normal mice post intravenous injection of the 99mTc labeled nanoparticles clarifies different data as shown in Figs. 7 and 8. A higher radioactivity level was detected in the kidney after injection of PASP-SiO2-DTPA-99mTc nanoparticles compared to SiO2-DTPA-99mTc nanoparticles (26 ± 0.5% IA/gram at 2 h post injection, 10.2 ± 0.8% IA/gram 1 h post injection, respectively). This may be attributed to the enhancement of hydrophilic properties of the silica nanoparticles via anchoring with polyaspartic acid which matches with the reported renal excretion of different radiolabeled oligo-aspartic acid [15]. 99mTc-MDP, the clinically used bone imaging radiopharmaceutical also undergoes urinary excretion [25]. On the other hand, the results revealed the higher radioactivity level of SiO2-DTPA-99mTc nanoparticles in the liver and intestine (11.9 ± 1 and 16 ± 1% IA/gram 4 h post injection, respectively) compared to that of PASP-SiO2-DTPA-99mTc nanoparticles (6.2 ± 0.8% and 12.3 ± 0.7% IA/gram 4 h post injection, respectively). This indicates the hepatobiliary excretion of (SiO2-DTPA-99mTc) nanoparticles which is consistent with the behavior of the previously studied bone targeting silica nanoparticles [26]. As shown in Fig. 9 SiO2-DTPA-99mTc nanoparticles showed the maximum bone uptake (C max equal to 5.4 ± 0.4% IA/gram) at t max = 2 h post injection. A higher radioactivity accumulation level in the bone was detected after intravenous injection of PASP-SiO2-DTPA-99mTc nanoparticles as shown in Fig. 8, C max equal to 13 ± 0.6% IA/gram at t max= 4 h post injection. Figure 7 revealed the significant enhancement of radioactivity bone uptake (p < 0.05) after injection of PASP-SiO2-DTPA-99mTc at different time points attributed to the high affinity of poly aspartic acid for hydroxyapatite of the bone [2, 27]. These results are consistent with the high bone accumulation level of 67Ga-DOTA-(Asp) 8 (12.56 ± 3.09% IA/gram at 1 h post injection) which has been investigated previously as a PET bone imaging probe [15]. Interestingly, the bone uptake level of the injected synthesized SiO2-DTPA-99mTc and PASP-SiO2-DTPA-99mTc nanoparticles is higher than the previously reported bone uptake level of the injected 99mTc-MDP (2.66% ID/g) [28]. These results encourage further studies of these designed 99mTc silica nanoparticles as an alternative to traditional bisphosphonate radiopharmaceuticals. The in vivo stability of SiO2-DTPA-99mTc and PASP-SiO2-DTPA-99mTc nanoparticles was confirmed with the low radioactivity level detected in the thyroid gland (1.5 ± 0.3% and 2 ± 0.4% IA/gram, respectively) and stomach (3.2 ± 0.5% IA/gram and 3.5 ± 0.4% IA/gram, respectively) related to the free 99mTc radioisotope due to the instability of the synthesized radiopharmaceutical [30]. Additionally, the radioactivity detected in the bone also reveals the in vivo stability of the radiolabeled silica nanoparticles because 99mTc radionuclide does not accumulate in the bone in the free form [29].
Fig. 7.
Biodistribution studies results of SiO2-DTPA-99mTc after IV injection in mice, (n = 5/time point); IA for injected activity
Fig. 8.
Biodistribution studies results of PASP-SiO2-DTPA-99mTc after IV injection in mice, (n = 5/time point); IA for injected activity
Fig. 9.
Bone uptake of SiO2-DTPA-99mTc and PASP-SiO2-DTPA-99mTc, (n = 5/time point), *statistically significant difference (p < 0.05); IA for injected activity
Conclusions
In this work, polyaspartic acid coated mesoporous silica (PASP-mSiO2) nanoparticles were synthesized by anchoring polyaspartic acid onto amino-functionalized silica nanoparticles via simple aqueous carbodiimide chemistry. The prepared nanoparticles exhibited monodispersibility, high physiological stability and biocompatibility. DTPA was covalently attached to the amino groups on the surface of the prepared nanoparticles allowing for effective labeling with the radiotracer, Technetium-99m. A biodistribution study revealed the highly selective accumulation of PASP-mSiO2 nanoparticles in bone, compared to uncoated silica nanoparticles. Due to their non-invasive and high bone-targeting features, the prepared 99mTc-labeled PASP-mSiO2 nanoparticles have high potential for safe and sensitive SPECT imaging of bone. Further studies are needed to evaluate the prepared nanoparticles as probes for targeted radionuclide palliation of metastatic bone pain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
N.B. contributed to the conceptualization of the work, synthesis process, radiolabeling and biodistribution studies. Contributed to the writing of the whole manuscript. M.S. carried out the radiolabeling reactions and contributed to the biodistribution study experiments. W.D. contributed to the conceptualization of the work, synthesis process and the writing of the manuscript. All authors have given approval to the final version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data is provided within the manuscript and supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Statement of animal rights
All procedures performed in studies involving animals were in accordance with the ethical standards of the Research Ethics Committee of the National Center for Radiation Research and Technology (REC-NCRRT), serial number F/28A/24”.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crișan G, Moldovean-Cioroianu NS, Timaru DG, Andrieș G, Căinap C, et al. Radiopharmaceuticals for PET and SPECT imaging: A literature review over the last decade. Int J Mol Sci. 2022;30:5023–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uehara T, Kiyota S, Ishii D, Ogawa K, Akizawa H, et al. Mononuclear Tc-99m-chelate-conjugated oligo-aspartic acid as a new bone imaging agent. J Nucl Med. 2006;47:520.16513622 [Google Scholar]
- 3.Mushtaq S, Bibi A, Park JE, Jeon J. Recent progress in technetium-99m-labeled nanoparticles for molecular imaging and cancer therapy. Nanomater. 2021;11:3022–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gisbert-Garzarán M, Manzano M, Vallet-Regí M. Mesoporous silica nanoparticles for the treatment of complex bone diseases: bone cancer, bone infection and osteoporosis. Pharm. 2020;12:83–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguilera-Correa JJ, Gisbert-Garzarán M, Mediero A. Antibiotic delivery from bone-targeted mesoporous silica nanoparticles for the treatment of osteomyelitis caused by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2022;154:608–25. [DOI] [PubMed] [Google Scholar]
- 6.Nie B, Huo S, Qu X, Guo J, Liu X, et al. Bone infection site targeting nanoparticle-antibiotics delivery vehicle to enhance treatment efficacy of orthopedic implant related infection. Bioact Mater. 2022;12:134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Y, Zhang P, Sun Y, Kang Q, Xu J, et al. Regeneration of large bone defects using mesoporous silica coated magnetic nanoparticles during distraction osteogenesis. Nanomed Nanotechnol Biol Med. 2019;21:102040–55. [DOI] [PubMed] [Google Scholar]
- 8.Xu c, Xiao L, Cao Y, Sun w. Mesoporous silica rods with cone shaped pores modulate inflammation and deliver BMP-2 for bone regeneration. Nano Res. 2020;13:2323–31. [Google Scholar]
- 9.Pasqua L, Napoli IE, Santo M, Greco M, Catizzone E. Mesoporous silica-based hybrid materials for bone-specific drug delivery. Nanoscale Adv. 2019;1:3269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adelnia H, Tran HDN, Little PJ, Blakey I, Ta HT. Poly (aspartic acid) in biomedical applications: from polymerization, modification, properties, degradation, and biocompatibility to applications. CS Biomater Sci Eng. 2021;7:2083–105. [DOI] [PubMed] [Google Scholar]
- 11.Kasugai S. Selective drug delivery system to bone: small peptide (Asp)6 conjugation. J Bone Min Res. 2000;15:936–43. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Miller S, Sima M, Kopecková P, Kopecek J. Synthesis and evaluation of water-soluble polymeric bone-targeted drug delivery systems. Bioconjug Chem. 2003;14:853–9. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang L, Pan J, Zhang Y, Guo L. Synthesis of second- and third-generation asp oligopeptide conjugated dendrimers for bone-targeting drug delivery. Synth Commun. 2009;39:4039–52. [Google Scholar]
- 14.Jiang T, Yu X, Carbone EJ, Nelson C, Kan HM, et al. Poly aspartic acid peptide-linked PLGA based nanoscale particles: potential for bone-targeting drug delivery applications. Int J Pharm. 2014;475:547–57. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa K, Ishizaki A, Takai K, Kitamura Y, Kiwada T, et al. Development of novel radiogallium-labeled bone imaging agents using oligoaspartic acid peptides as carriers. PLoS ONE. 2013;8:e84335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada A, Tamaru S, Ikeda M, Hamachi I. MCM- enzyme-supramolecular hydrogel hybrid as a fluorescence sensing material for polyanions of biological significance. J Am Chem Soc. 2009;131:5321–30. [DOI] [PubMed] [Google Scholar]
- 17.Ardestani MS, Arabzadeh AJ, Heidari Z. Novel and facile methods for the synthesis of DTPA-mono-amide: a new completely revised strategy in radiopharmaceutical chemistry. J Radioanal Nucl Chem. 2010;283:447–55. [Google Scholar]
- 18.Niţà LE, Chiriac AP, Popescu CM, Neamţu I, Alecu L. Possibilities for Poly (aspartic acid) Preparation as biodegradable compound. J Optoelectron Adv Mater. 2006;8:663–6. [Google Scholar]
- 19.Amin AM, Abou Zid K, Bayoumi NA, Abd EL-hamid M. Organic synthesis and biological evaluation of novel ‘‘3 + 1’’ mixed ligands of technetium-99m Gabapentin as receptor imaging agents. J Radioanal Nucl Chem. 2010;283:55–62. [Google Scholar]
- 20.Zhang CM, Yang J, Quan ZW, Yang PP, Li CX, et al. Hydroxyapatite nano and microcrystals with multiform morphologies: controllable synthesis and luminescence properties. Cryst Growth. 2009;9:2725–33. [Google Scholar]
- 21.Bayoumi NA, Emam AN. 99mTc radiolabeling of polyethylenimine capped carbon Dots for tumor targeting: synthesis, characterization and biodistribution. Int J Rad Biol. 2021;97:977–85. [DOI] [PubMed] [Google Scholar]
- 22.Estevão BM, Miletto I, Hioka N, Marchese L, Gianotti E. Mesoporous silica nanoparticles functionalized with amino groups for biomedical applications. Chem Open. 2021;10:1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros ALB, Ferraz KSO, Dantas TCS. Synthesis, characterization, and biodistribution studies of 99mTc-labeled SBA-16 mesoporous silica nanoparticles. Mater Sci Eng C. 2015;56:181–8. [DOI] [PubMed] [Google Scholar]
- 24.Hakeem A, Zahid F, Zhan G, Yi P, Yang H, Darwish W, Abdoon A, Shata M, Elmansy M, et al. editors. Vincristine-loaded polymeric corona around gold nanorods for combination (chemo-photothermal) therapy of oral squamous carcinoma. React. Funct. Polym. 2020; 151: 104575.
- 25.Truluck CA. Nuclear medicine technology: inflammation and infection imaging. J Radiological Nurs. 2007;26:77–85. [Google Scholar]
- 26.Ren H, Chen S, Jin Y, Zhang C, Yang X, et al. Traceable and bone-targeted nanoassembly based on defectrelated luminescent mesoporous silica for enhanced osteogenic differentiation. J Mater Chem B. 2017;8:1585–93. [DOI] [PubMed] [Google Scholar]
- 27.Yokogawa K, Miya K, Sekido T, Higashi Y, Nomura M. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142:1228–33. [DOI] [PubMed] [Google Scholar]
- 28.Mandiwana V, Kalombo L, Grobler A, Zeevaart JR. 99mTc-MDP as an imaging tool to evaluate the in vivo biodistribution of solid lipid nanoparticles. Appl Radiat Isot. 2018;141:51–6. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz Z, Shani J, Soskolne WA, Touma H, Amir D. Uptake and biodistribution of technetium-99m-MD32P during rat tibial bone repair. J Nucl Med. 1993;34(1):104–8. [PubMed] [Google Scholar]
- 30.Mushtaq S, Bibi A, Park JE, Jeon J. Recent progress in Technetium-99m-Labeled nanoparticles for molecular imaging and Cancer therapy nanomaterials 2021; 11: 3022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript and supplementary information files.