Abstract
Background
Angelica-based formulas can improve quality of life in patients with endometrial cancer. However, the results remain controversial, and their mechanisms of action are unclear. In this study, we systematically explored the effects and mechanism of action of Angelica sinensis (AS) in endometrial cancer (EC) using network pharmacology and molecular docking.
Methods
We systematically searched PubMed, Embase, Cochrane Library, Web of Science, Chinese Science and Technology Journals (CQVIP), China Academic Journals (CNKI), Wanfang, and Chinese Biomedical Literature database (SinoMed). Nine randomized controlled trials were enrolled in the study. In network pharmacology, ingredients of Angelica sinensis were screened, endometrial cancer related genes were then identified and the 'Herb-Ingredient-Target-Disease' network constructed. Molecular docking was finally employed for in silico simulation matching between representative Angelica sinensis ingredients and their target genes.
Results
The meta-analysis of this research provides evidence to support the efficacy of angelica-based formulas in the treatment of endometrial cancer. Network pharmacology demonstrated that EGFR, TP53, CTNNB1, CCND1, and HRAS are the core targets of endometrial cancer, and ferulic acid and caffeic acid are the major bioactive ingredients of Angelica sinensis. Molecular docking showed that ferulic acid and caffeic acid can closely bind core targets.
Conclusions
Our study is the first to systematically apply bioinformatics methods—including meta-analysis, network pharmacology, and molecular docking—to explore the pharmacological and molecular mechanisms of Angelica sinensis in endometrial cancer treatment. Results of our study provide valuable scientific insights into the underlying mechanisms of Angelica sinensis in the treatment of endometrial cancer, serving as a crucial foundation for future research in this area.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02619-8.
Keywords: Angelica sinensis, Ferulic acid, Caffeic acid, Endometrial cancer, Network pharmacology, Molecular docking, PI3K/AKT signal pathway
Introduction
Endometrial cancer (EC) is the second most common gynecological malignancy worldwide, the first in developed countries [1]. It was estimated that 66,200 new cases and 13,030 subsequent deaths occurred in 2023 in the United States[2]. Owing to the impact of factors such as population aging, obesity epidemic, and prolonged exposure to estrogen, the global incidence of endometrial cancer is projected to persistently increase in the forthcoming decades [3]. Currently, carboplatin plus paclitaxel administered intravenously every 21 days is the global standard first-line chemotherapy for advanced or recurrent EC, but it can cause more frequent and severe neutropenia, hepatic events, and myalgia [4]. Therefore, finding drugs or treatments with definite clinical and minor side effects is an urgent problem.
Traditional Chinese medicine (TCM) has been widely used in many countries and has been proven to enhance the efficacy of chemotherapy, radiotherapy, targeted therapy, and immunotherapy, while reducing their associated side effects [5]. Owing to its holistic regulatory capacity targeting multiple pathways, TCM has demonstrated significant efficacy in cancer therapy, as evidenced by numerous studies [6–11]. Angelica sinensis (AS), known as Danggui in Chinese, is the root of Angelica sinensis (Oliv.) Diels (Apiaceae) and has been used for thousands of years in traditional Chinese medicine. It is renowned for its efficacy in treating various gynecological disorders that are often challenging to manage with conventional therapies, such as menstrual irregularities, amenorrhea, and dysmenorrhea, thus also called 'Female Ginseng' [12].Current researches indicate that AS has various effects, such as anticancer, immune modulation, radioprotection, memory enhancement, hematopoietic activity, neuroprotection, and antioxidation [12–15]. However, there is a lack of credible studies concerning the mechanism of AS in treating EC. Consequently, we have decided to conduct a new study utilizing meta-analysis and network pharmacology methods.
In this study, we first conducted a meta-analysis to gather as many published articles on the treatment of EC with AS as possible. This helped us confirm the effectiveness of AS in treating EC. We then applied network pharmacology to predict the potential mechanism by which AS exerted therapeutic effects on cancer. This provided a research basis for the next steps, such as cellular and animal experiments, and also laid a foundation for further clinical use. Additionally, we hope that this study will offer new ideas for oncologists in clinical decision making.
Materials and methods
Meta-analysis
Search strategy
We systematically searched PubMed, Embase, Cochrane Library, Web of Science, Chinese Science and Technology Journals (CQVIP), China Academic Journals (CNKI), Wanfang, and Chinese Biomedical Literature database (SinoMed) to evaluate the efficacy of Chinese herbal compound containing Angelica sinensis on endometrial carcinoma. The search keywords included “endometrial carcinoma” AND (“Angelica sinensis” OR “Chinese angelica” OR “danggui” OR “TCM”) AND (“randomized” OR “randomised” OR “RCT”), and the search strategy was confirmed after multiple preliminary searches. All articles were published before April 27, 2023, and no restriction on language was applied.
Criteria for inclusion and exclusion
Studies that met the following PICOS criteria were included: (1) participants: endometrial carcinoma patients; (2) intervention: A traditional Chinese medicine compound containing angelica is used; (3) comparator: Chemotherapy; (4) outcomes: quality of life (QOL), immune function (IF), pain level (PL), recurrent rate (RR), TCM symptom score; (5) study design: RCT.
Exclusion criteria: non-RCTs, animals and cells experiment, editorials, letters, meta-analysis, expert opinions, abstracts, case reports and reviews without original data, and studies lacking control groups. In addition, studies and data according to the following situation were also excluded: Raw data cannot be extracted; the outcomes of patients were not reported; studies from uniform institutions with identical data.
Data extraction
All variables included in the study were extracted by two reviewers (ZL and CJ) independently, including the first author, year of publication, sample sizes of treatment group and control group, tumor stage, intervention, TCM duration, and clinical outcome indicators. Two reviewers discussed differences. When differences cannot be resolved, the third reviewer (QW) shall solve them.
Methodological quality assessment
According to the RCT quality assessment criteria developed by the Cochrane Collaboration Assessment Tool, we assessed the methodological quality in seven dimensions to clarify the bias situation of the study and the reliability of the results, including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases.
Statistics analysis
Cochrane Review Manager software (RevMan, version 5.4) was utilized for statistical analysis. For dichotomous outcomes, results were expressed as a risk ratio (RR) with 95% confidence intervals (CI). The continuous variables were assessed for treatment effects using mean differences (MD) since the studies included in this paper all used the same scales. The meaning of HR values was determined according to different outcome indicators. P < 0.05 was considered statistically significant. Heterogeneity was assessed using the Q test and I2 statistics. A chi-squared P < 0.1 or an I2 statistic > 50% was regarded as heterogeneity. A fixed-effects model was performed on the data to estimate the combined effect sizes.
Network pharmacology research
Identification of ingredients and targets of AS
Notably, compared to the conventional method of obtaining the active ingredients of traditional Chinese medicine from databases such as TCMSP, this study directly obtained the chemical components of AS from literature, with higher reliability. The Swiss target prediction (STP, http://www.swisstargetprediction.ch/, Retrieval date: 8 May, 2023) was used for target fishing. STP can predict potential targets for biologically active small molecules based on a combination of 2D and 3D structural similarities [16]. And according to a recent study, among the current nine mainstream target fishing tools, STP tends to produce the most reliable predictions while enriching more targets [17].
Obtaining targets of EC based on microarray data analysis
Microarray data analysis is an emerging technology in the twenty-first century [18]. Its main advantage is the ability to detect the expression of tens of thousands of genes simultaneously and comprehensively, and to identify genes that may be affected by diseases in a short period of time through gene chips [19, 20]. In this study, we screened differential genes of EC based on a high-throughput sequencing dataset (GSE115810) stored in the GEO database (https://www.ncbi.nlm.nih.gov/geo/, accessed on 8 May 2022) [21]. Specifically, we selected 3 control samples and 17 endometrial cancer samples as the reference group and the test group, respectively, for difference analysis. The experimental procedures were as follows: data were downloaded from the GEO database through the GEO query package, probes corresponding to multiple molecules were removed, and when probes corresponding to the same molecule were encountered, only the probe with the largest signal value was retained, and then the data were normalized again through the normalize Between Arrays function of the limma package, and then the samples were viewed through box plots for the normalized situation, followed by a difference analysis of the two groups using the limma package [22, 23]. The results of the differential gene analysis were then visualized using the ComplexHeatmap package [24].
Screening AS-related targets against EC
In order to obtain more comprehensive targets related to EC, we obtained EC-related targets from GeneCards (https://www.genecards.org/, accessed on 9 May 2023) as a complement to differential genes. We set a relevance score > 10 as the threshold for obtaining more accurate targets. Then, they were merged with the differential genes obtained based on GEO, and intersected with the targets of AS to obtain AS-related targets against EC.
The intersection targets were imported into the STRING platform (https://string-db.org/, Retrieval date: 15 May, 2023) to construct a PPI network. Cytoscape 3.10.0 software for visualization, the network topology parameters were analyzed, and the core targets of AS in EC treatment with degree ≥ 50 were selected.
Herb-ingredient-target-disease network construction
In this study, we constructed Herb-Ingredient-Target-Disease (HITD) network of the screened core targets by Cytoscape 3.10.0 and calculated the degree value of the ingredient, and the top 10 degree values were selected as potential active ingredients.
Functional and pathway enrichment analysis of core targets
To elucidate the mechanism of AS in the treatment of EC, we import the core targets into the DAVID database (https://david.ncifcrf.gov/, Retrieval date: 16 May, 2023). Then we conducted comprehensive enrichment analyses, including Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). The GO analysis encompassed categories such as biological process (BP), cellular component (CC), and molecular function (MF).
Molecular docking
Two core ingredients from the primary bioactive component of AS were selected and docked with five proteins from the core targets. We got the 3D structure of the core targets in the PDB database (https://www.rcsb.org/, Date retrieved: 9 July 2023), while obtaining the 2D or 3D structure of the molecular ligand from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/, Date retrieved: 9 July 2023). They were then uploaded to CB-Dock2 (https://cadd.labshare.cn/cb-dock2/) for molecular docking. CB-Dock2, a molecular docking site that can automatically preprocesses receptors and ligands, outperformed most popular blind docking tools [25].
Results
Meta-analysis
Characteristics of included studies
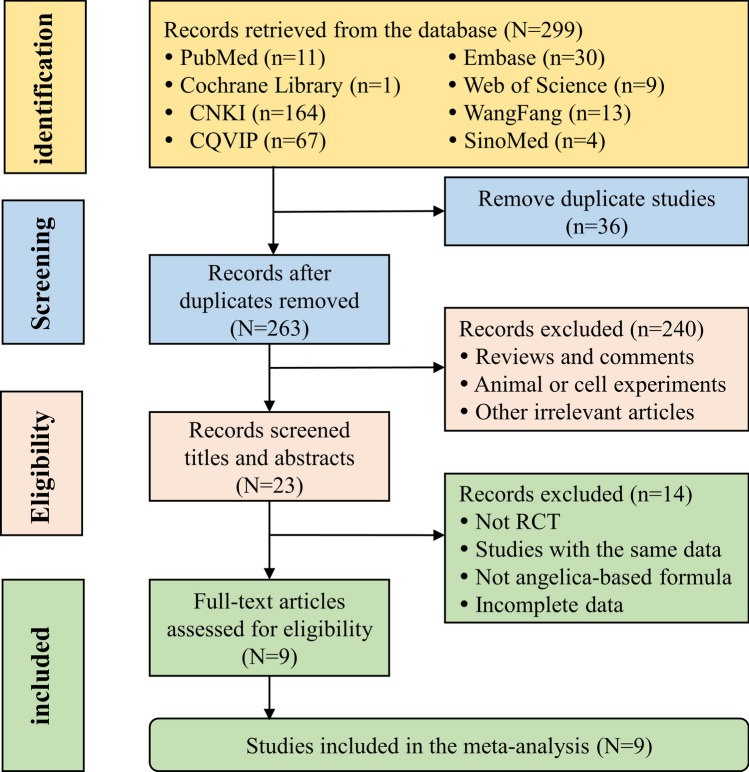
As shown in Fig. 1, a total of 299 literatures were retrieved. After further rigorous screening, nine studies containing 889 samples were finally included in this meta-analysis. The basic characteristics of all included articles were shown in Table 1. These studies were published between 2011 and 2022. There were 444 patients with endometrial cancer treated with chemotherapy or herbal compound without angelica. Correspondingly, 445 patients added herbal compound containing angelica to their original treatment.
Fig. 1.
Flow diagram for selecting eligible studies
Table 1.
Characteristics of the included studies
| Study | Sample size (C/T) | Mean age (years) (C/T) | Intervention (C) | Intervention (T) | Outcomes |
|---|---|---|---|---|---|
| Sun (2011) | 30/30 | 55.13/56.73 | Chemotherapy | plus formulas containing angelica | ①② |
| Zhang (2014) | 48/48 | 45.3 ± 7.9/45.2 ± 8.1 | Chemotherapy | plus formulas containing angelica | ①③⑧ |
| Wan (2015) | 33/33 | 56.5 ± 6.3/55.8 ± 5.4 | Chemotherapy | plus formulas containing angelica | ①② |
| Wang (2015) | 39/39 | 61.2 ± 9.1/62.9 ± 9.5 | Chemotherapy | plus formulas containing angelica | ①③⑧ |
| Nie(2016) | 150/150 | 43.8 ± 3.7/44.4 ± 3.1 | Chemotherapy | plus formulas containing angelica | ①③⑧ |
| Xu (2017) | 50/50 | 49.7 ± 4.8/48.9 ± 4.7 | Chemotherapy | plus formulas containing angelica | ①⑧ |
| Liu (2017) | 30/30 | 54.2 ± 6.9/55.4 ± 7.2 | JDYQF | plus formulas containing angelica | ②④⑤⑥⑦ |
| Wang (2019) | 49/49 | 53.2 ± 5.4/ 54.1 ± 5.8 | JDYQF | plus formulas containing angelica | ④⑤⑥⑦ |
| Ding (2022) | 15/16 | 57.23 ± 5.65/57.17 ± 5.62 | JDYQF | plus formulas containing angelica | ④⑤⑥⑦ |
T: treatment group; C: control group; JDYQF: Jie Du Yi Qi Fang; quality of life; TCM symptom score; pain level; IgG (g/L); IgA (g/L); CD4+(g/L); NK cell (g/L); recurrent rate
Risk of bias analysis outcomes
The bias diagram of a total of 9 included literatures is shown in Fig. 2.
Fig. 2.
Risk assessment of the included studies. A Respectively; B Summary
Efficacy of angelica-based formulas on endometrial cancer
QOL, PL, RR and TCM symptom score were used to assess the clinical efficacy of angelica-based formulas in endometrial cancer.
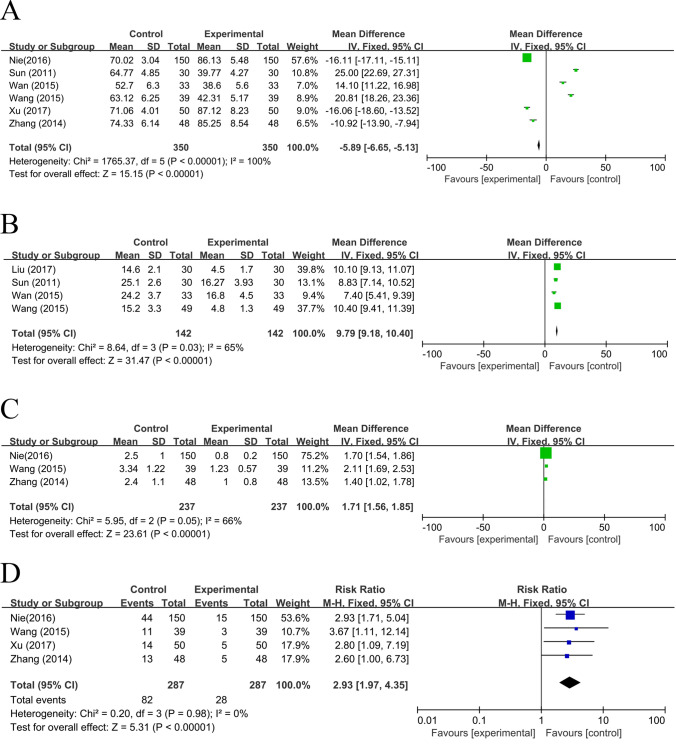
Six studies reported QOL, and a total of 700 samples were included in this meta-analysis. As shown in Fig. 3A, angelica-based formulas can effectively improve the QOL of patients (MD = − 5.89, 95%CI[ − 6.65, − 5.13], I2 = 100%, P < 0.05).
Fig. 3.
Forest plot displayed the efficacy of angelica-based formulas on endometrial cancer. A Quality of Life (QOL): Pooled analysis of 6 studies showed significant improvement in QOL with angelica-based formulas (MD = − 5.89, 95% CI [ − 6.65, − 5.13], I2 = 100%, P < 0.05); B TCM Symptom Score: Meta-analysis of 4 studies indicated significant reduction in symptom scores (MD = 9.79, 95% CI [9.18, 10.40], I2 = 65%, P < 0.05), suggesting effective symptom management; C Pain Level (PL): Analysis of 3 studies demonstrated significant pain reduction (MD = 1.71, 95% CI [1.56, 1.85], I2 = 66%, P < 0.05); D Recurrent Rate (RR): Pooled data from 4 studies showed significantly lower recurrence risk (RR = 2.93, 95% CI [1.97, 4.35], I2 = 0%, P < 0.05)
Four studies reported TCM symptom score, and a total of 284 samples were included in this meta-analysis. As shown in Fig. 3B, angelica-based formulas can effectively decrease the TCM symptom score of patients, improving patients' TCM symptoms (MD = 9.79, 95%CI[9.18,10.40], I2 = 65%,P < 0.05).
Three studies reported PL, and a total of 474 samples were included in this meta-analysis. As shown in Fig. 3C, angelica-based formulas can effectively decrease the PL of patients, relieving the patient's pain. (MD = 1.71, 95%CI[1.56,1.85], I2 = 66%, P < 0.05).
Four studies reported RR, and a total of 574 samples were included in this meta-analysis. As shown in Fig. 3D, angelica-based formulas can effectively decrease the RR of patients, reducing tumor recurrence (RR = 2.93, 95%CI[1.97,4.35],I2 = 0%, P < 0.05).
Effect of AS-based formulas on immune function in patients with endometrial cancer
IgG, IgA, NK cell and CD4+T cell were used to assess the immune function of the patient. As shown in Fig. 4, three studies (Ding (2022), Liu (2017), Wang (2019)) reported on immune-related indicators. As shown in Fig. 4A and B, the IgG (MD = 2.17, 95%CI[1.80,2.54], I2 = 51%, P < 0.05) and IgA (MD = 0.39, 95%CI[0.33, 0.45], I2 = 30%, P < 0.05) levels were differentially reduced in the test group compared to the control group. In contrast, the NK cell (MD = − 1.84, 95%CI[ − 2.20, − 1.47], I2 = 0%, P < 0.05) and CD4+T cell (MD = − 4.61, 95%CI[ − 5.34, − 3.88], I2 = 32%, P < 0.05) of the test group showed different degrees of improvement, as shown in Fig. 4C and D.
Fig. 4.
Forest plot displayed the effect of angelica-based formulas on immune function in patient. A IgG levels (MD = 2.17, 95% CI [1.80–2.54]); B IgA levels (MD = 0.39, 95% CI [0.33–0.45]); C NK cell activity (MD = − 1.84%, 95% CI [ − 2.20 to − 1.47]); D CD4 + T cell counts (MD = − 4.61, 95% CI [ − 5.34 to − 3.88])
Network pharmacology research
The ingredients and targets of AS
We obtained 165 chemical components of Angelica sinensis from the literature [26]. A total of 1032 targets were obtained by predicting the targets of these molecules and eliminating some compounds without targets.
Obtaining targets of EC
The total number of genes after microarray data analysis based on GSE115810 is 12,548, among which, there are 210 genes that satisfy the threshold of |log2(FC)|> 1 & p value < 0.05. The normalized signal intensity of the sample was shown in Fig. 5A. The medians of almost all samples are basically at one level, indicating a good degree of normalization between samples. The results of the differential gene analysis were shown in Fig. 5B and Fig. 5C. These differentially expressed genes were further merged with EC targets obtained from GeneCards, totaling 605 targets.
Fig. 5.
Microarray data analysis based on GEO. A Normalized signal intensity of the sample. B Volcanic map of gene differences. Red represents high expression in the experimental group and blue represents high expression in the control group. C Heat map of gene differences. Red represents high expression in the experimental group and blue represents high expression in the control group. Color shades represent the strength of expression
Establishment of PPI network and core target screening
We established the protein–protein interaction (PPI) network on the STRING platform, incorporating 106 intersecting targets. For the screening of AS-related targets against EC, we identified 29 core targets based on DC greater than 50 (with a median DC value of 36) (Fig. 6C).
Fig. 6.
Screening of core targets. A The intersection of AS and EC targets. B The PPI network of core targets. C Screening process of core targets. Targets with DC greater than 50 were selected
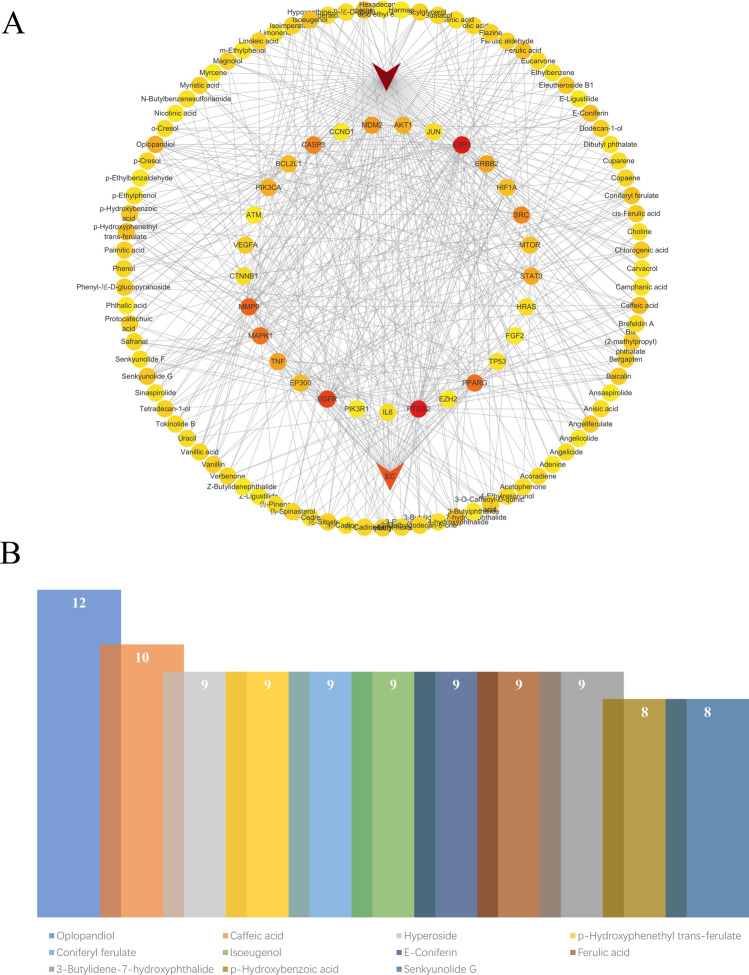
Herb-ingredient-target-disease network construction
The H-I-T-D network was shown in Fig. 7A. The results of the network topology analysis of the H-I-T-D network are presented in Fig. 7B. The results showed that oplopandiol, caffeic acid, hyperoside, ferulic acid, which are the main active ingredients in AS, played key roles in the treatment of EC.
Fig. 7.
Network construction. A The H-I-T-D network. B The degree values of important nodes in network
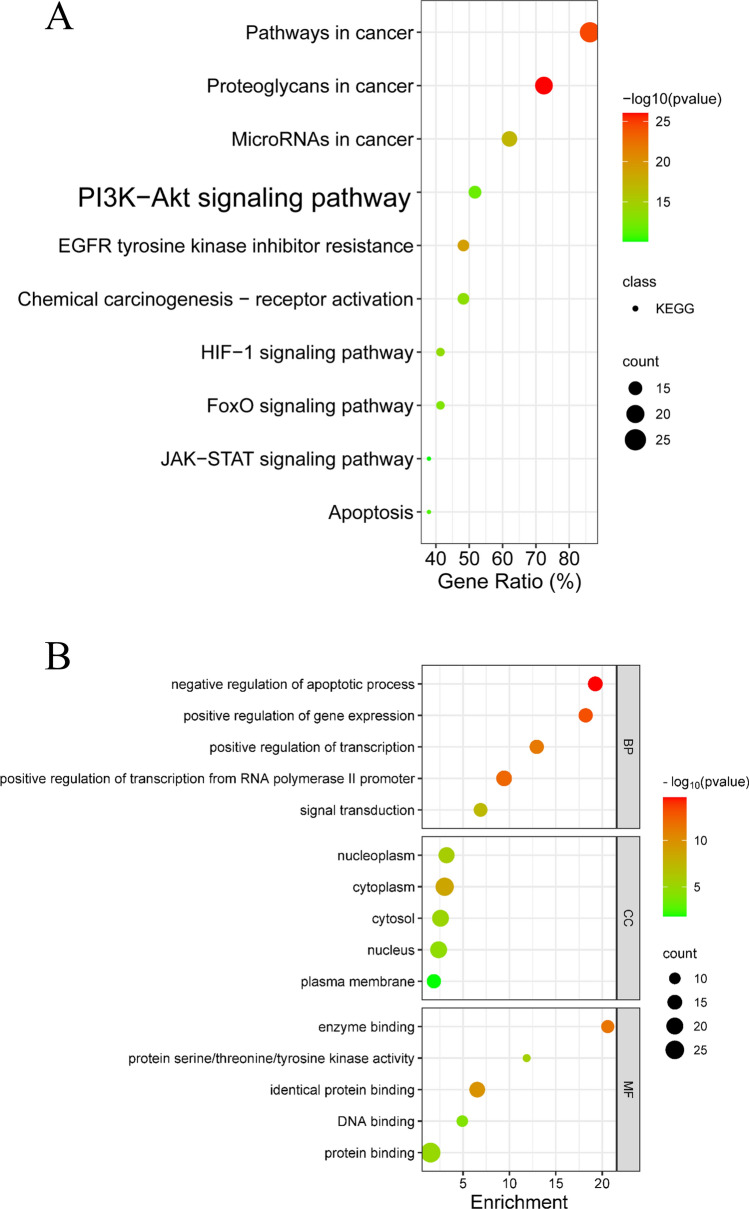
Functional and pathway enrichment analysis of core targets
By importing 29 core targets into the DAVID database, we performed KEGG and GO analyses, resulting in 343 biological process (BP) entries such as negative regulation of apoptotic process and positive regulation of gene expression, 35 molecular function (MF) entries such as nucleus, cytoplasm, and cytosol, as well as 65 cellular component (CC) entries such as enzyme binding, protein serine/threonine/tyrosine kinase activity, and identical protein binding. In addition, we obtained a total of 143 KEGG pathways, including the PI3K/AKT signaling pathway. After removing items that are evidently unrelated to this study, the remaining entries were sorted based on their count values, and the top-ranked items were visualized (Fig. 8).
Fig. 8.
Functional and pathway enrichment analysis of core targets. A The top10 KEGG pathways. B The top5 entries of BP, CC and MF
Molecular docking
As shown in Table 2, the binding energy of quercetin, kaempferol with EGFR, TP53, CTNNB1, CCND1 and HRAS was all less than − 5.0 kcal·mol-1, showing good binding force. The binding of EGFR and TP53 to ferulic acid and caffeic acid is shown in Fig. 9.
Table 2.
The binding energy of compound and core targets (kcal/mol)
| Target | Target | Compound | Affinity |
|---|---|---|---|
| (PDB ID) | (kcal/mol) | ||
| EGFR | 6LUD | Ferulic acid | − 5.8 |
| Caffeic Acid | − 5.9 | ||
| Paclitaxel | − 9.2 | ||
| Carboplatin | − 4.8 | ||
| Carboplatin Paclitaxel | − 9.2 | ||
| TP53 | 4RG2 | Ferulic acid | − 7.2 |
| Caffeic Acid | − 6.9 | ||
| Paclitaxel | − 7.2 | ||
| Carboplatin | − 5.6 | ||
| Carboplatin Paclitaxel | − 7.9 | ||
| CTNNB1 | 7AFW | Ferulic acid | − 5.2 |
| Caffeic Acid | − 5.4 | ||
| Paclitaxel | − 7.4 | ||
| Carboplatin | − 4.9 | ||
| Carboplatin Paclitaxel | − 7.4 | ||
| CCND1 | 2W96 | Ferulic acid | − 6.4 |
| Caffeic Acid | − 6.2 | ||
| Paclitaxel | − 8.4 | ||
| Carboplatin | − 5.1 | ||
| Carboplatin Paclitaxel | − 8.6 | ||
| HRAS | 4XRV | Ferulic acid | − 6.5 |
| Caffeic Acid | − 6.7 | ||
| Paclitaxel | − 8.4 | ||
| Carboplatin | − 6.0 | ||
| Carboplatin Paclitaxel | − 8.4 |
Fig. 9.
Ingredient-target molecular docking. A Ferulic acid-EGFR. B Caffeic acid-EGFR. C Ferulic acid-TP53. D Caffeic acid-TP53. (E)Heat map of molecular docking results
Discussion
Endometrial cancer is the 6th most common cancer in women worldwide. Because of the impact of factors such as population aging, obesity epidemic, and prolonged exposure to estrogen, the global incidence of endometrial cancer is still persistently increasing. Chemotherapy is one of the common treatments used in the treatment of endometrial cancer. Currently, carboplatin plus paclitaxel administered intravenously every 21 days is the global standard first-line chemotherapy for advanced or recurrent EC, but it can cause several side effects. However, with the extensive application of carboplatin, the incidence of hematological toxicities, such as neutropenia, is increasingly on the rise. Furthermore, it is worth noting that animal toxicological studies have confirmed the potential of carboplatin to induce kidney injury. Therefore, there is an urgent demand in current research to identify a low-toxic or non-toxic alternative therapeutic agent. More and more studies have shown the therapeutic effect of Angelica sinensis on endometrial cancer, but the exact mechanism remains unclear. To explore the efficacy and mechanism of AS in the treatment of EC and its effective targets, we used a method of integrating meta-analysis and network pharmacology analysis.
We ultimately included 9 studies, employing QOL, IF, PL, RR, and Traditional Chinese Medicine syndrome score to assess the clinical efficacy of angelica-based formulas in endometrial cancer. The present meta-analysis revealed that angelica-based formulas can effectively improve patients' QOL and TCM symptoms, while significantly decrease patients' PL, RR and TCM symptom score. Additionally, angelica-based formulas would differentially decrease IgG and IgA levels, but NK cells and CD4 + T cells had different degrees of improvement, which suggests that angelica-based formulas have an improvement effect on immune function.
We also performed network pharmacology to explore the specific effects of bioactive components on EC. The H-I-D-T network revealed that the primary bioactive components of Angelica sinensis are oplopandiol, caffeic acid, hyperoside, ferulic acid, etc. Among them, ferulic acid and caffeic acid have been confirmed in numerous studies to exhibit anti-cancer effects [27–30]. A recent study demonstrated that caffeic acid suppresses the self-renewal and migratory capacity of cancer stem cells (CSCs) both in vitro and in vivo by inhibiting PI3K/AKT signaling, a pathway associated with tumorigenesis, therapy resistance, and stem-like properties, thereby effectively eliminating CSC characteristics in cancer [31]. Besides, caffeic acid and its derivatives can inhibit P-glycoprotein (P-gp) to reduce drug efflux and reverse resistance, while also suppressing proteasome activity to enhance cisplatin efficacy. Ferulic acid is a phenolic compound composed of free radicals, exhibiting various effects such as anticancer, anti-inflammatory, antidiabetic, anti-aging, and neuroprotective properties [27, 28, 32]. A study found that ferulic acid inhibits the transport function of P-gp in drug-resistant tumor cells, reduces tumor cell resistance to paclitaxel, and upregulates apoptosis signals in paclitaxel-induced resistant cells, confirming its chemosensitizing effect [33].
The PPI network revealed that tumor protein p53 (TP53), catenin beta 1 (CTNNB1), G1/S-specific cyclin-D1 (CCND1), GTPase HRas (HRAS), and epidermal growth factor receptor (EGFR) were the five most relevant targets. TP53 is an important tumor-suppressor gene that encodes the tumor-suppressor protein p53, which plays a critical role in normal cell growth and tumor prevention [34]. EGFR is a ubiquitously expressed transmembrane receptor tyrosine kinase[35]. Several cancer types including EC express elevated levels of EGFR relative to normal tissues[36]. EGFR can form a dimer with the ligand epidermal growth factor (EGF), leading to receptor autophosphorylation, triggering downstream cell signaling pathways such as PI3K/AKT and JAK/STAT [37]. KEGG enrichment analysis showed that AS treatment of EC involves multiple signaling pathways, with the PI3K/AKT and JAK/STAT pathways being significantly enriched. Relevant studies also indicate that the PI3K/AKT signaling pathway plays a crucial role in regulating cell survival, proliferation, and growth in endometrial cancer [38–40]. Additionally, the JAK/STAT3 signaling pathway, plays a role in virtually every aspect of immune regulation, from recognizing tumor cells to mediating tumor-induced immune evasion [41].
Previous studies have confirmed that ferulic acid and caffeic acid can inhibit the proliferation of various cancer cells by suppressing the PI3K/AKT signaling pathway[29, 42, 43]. Besides, studies have shown that caffeic acid and ferulic acid can inhibit tumor cell growth and stimulate anti-tumor immunity by suppressing the JAK/STAT pathway[43, 44]. Interestingly, a study found that ferulic acid can effectively inhibit aberrant cell proliferation by upregulating the expression of TP53 while downregulating the expression of cyclin-dependent kinases CDK2, CDK4, and CDK6 [43], but in our H-I-D-T network showed that ferulic acid did not act directly on TP53, so the mechanism by which ferulic acid increases the expression of TP53 still remains to be further investigated.
Our study has several limitations. First, the inclusion of small RCTs conducted in China may introduce some biases, though we expect future high-quality RCTs in international journals to strengthen the theoretical foundation for Angelica sinensis compounds’ clinical application. Additionally, since our meta-analysis primarily involved Chinese studies, the findings may lack generalizability to other populations due to geographic bias—a reflection of TCM’s regional prevalence. Thus, multinational studies are needed to validate AS’s efficacy across diverse ethnic and geographic cohorts. The observed heterogeneity (I2 > 50%) may stem from variations in Angelica sinensis preparations and dosing regimens across the included studies, potentially influencing the consistency of therapeutic outcomes. Another issue is the lack of standardized dosing, as the included studies used varying AS formulations and dosages, potentially affecting reproducibility; future trials should adopt fixed concentrations for consistency. Furthermore, outcomes like TRR, TCM Syndrome Score, KPS, and QOL were analyzed in fewer than ten studies, precluding publication bias assessment. Most notably, the absence of experimental validation for ferulic and caffeic acid’s efficacy in endometrial cancer treatment significantly limits clinical applicability, highlighting the need for future experimental studies to explore their therapeutic potential.
Conclusions
This is the first application of bioinformatics methods, such as network pharmacology, and molecular docking, to systematically explore the pharmacological and molecular mechanism of action of AS in EC treatment. These bioinformatics and computational analyses demonstrated that ferulic acid and caffeic acid may be the main active compounds of AS conferring therapeutic effects against EC. Moreover, AS can treat EC by inhibiting cell proliferation and promoting cell apoptosis via PI3K-AKT pathway.
Supplementary Information
Acknowledgements
We acknowledge GEO databases for providing their platforms and contributors for uploading their meaningful datasets.
Abbreviations
- BP
Biological processes
- CC
Cellular component
- CI
Confidence intervals
- CNKI
China Academic Journals
- CPTAC
Clinical Proteomic Tumor Analysis Consortium
- CR
Complete response
- ETCM
Encyclopedia of Traditional Chinese Medicine
- GO
Gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MD
Mean difference
- MF
Molecular function
- EC
Endometrial cancer
- AS
Angelica sinensis
- PD
Progressive disease
- PI
Proportional Integral
- PPI
Protein–protein interaction
- HITD
Herb Ingredient Target Disease
- PR
Partial response
- QOL
Quality of life
- RR
Risk ratio
- TCM
Traditional Chinese medicine
- TCMSP
Traditional Chinese Medicine Database and Analysis Platform
Author contributions
Z.L. and C.J. are the co-first author and performed the study. Q.W. is the corresponding authors. Conceptualization, Z.L. and C.J.; methodology, Z.L.; writing—original draft preparation, Z.L., C.J. and Y.Z.; writing—review and editing, Q.W.; visualization, Z.L., C.J.; supervision, Q.W.; project administration, Q.W.; funding acquisition, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Luzhou Science and Technology Department Applied Basic Research program (No:2022-WYC-196), and the Sichuan Province Science and Technology Department of foreign (border) high-end talent introduction project (No: 2023ZHYZ0009).
Data availability
The dataset GSE115810 used in this study can be found in the GEO database (https://www.ncbi.nlm.nih.gov/geo/). Additional materials are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenyi Li and Congchao Jia have contributed equally to this work.
References
- 1.Barretina-Ginesta MP, et al. SEOM-GEICO clinical guidelines on endometrial cancer (2021). Clin Transl Oncol. 2022;24(4):625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, et al. Global, Regional, and National Burden of Endometrial Cancer, 1990–2017: results from the global burden of disease study, 2017. Front Oncol. 2019;9:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller DS, et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol. 2020;38(33):3841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, et al. Positive role of Chinese herbal medicine in cancer immune regulation. Am J Chin Med. 2020;48(7):1577–92. [DOI] [PubMed] [Google Scholar]
- 6.Gao S, et al. Unveiling polysaccharides of Houttuynia cordata Thunb.: extraction, purification, structure, bioactivities, and structure-activity relationships. Phytomedicine. 2025;138:156436. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, et al. Unveiling potent bioactive compounds and anti-angiogenic pathways in Gekko swinhonis Guenther for gastric cancer therapy. J Ethnopharmacol. 2025;340:119156. [DOI] [PubMed] [Google Scholar]
- 8.Gao S, et al. Polysaccharides from Lonicera japonica Thunb.: extraction, purification, structural features and biological activities-a review. Int J Biol Macromol. 2024;281:136472. [DOI] [PubMed] [Google Scholar]
- 9.Sang Y, et al. Network pharmacology, molecular docking and biological verification to explore the potential anti-prostate cancer mechanisms of Tripterygium wilfordii Hook. F J Ethnopharmacol. 2025;338(Pt 2):119071. [DOI] [PubMed] [Google Scholar]
- 10.Shao G, et al. Therapeutic potential of traditional Chinese medicine in the prevention and treatment of digestive inflammatory cancer transformation: Portulaca oleracea L. as a promising drug. J Ethnopharmacol. 2024;327:117999. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y, et al. Pathogenesis and treatment of colitis-associated colorectal cancer: insights from traditional Chinese medicine. J Ethnopharmacol. 2025;338(Pt 2): 119096. [DOI] [PubMed] [Google Scholar]
- 12.Chen X-P, et al. Phytochemical and pharmacological studies on radix Angelica sinensis. Chin J Nat Med. 2014;11(6):577–87. [DOI] [PubMed] [Google Scholar]
- 13.Cheng YL, et al. Acetone extract of Angelica sinensis inhibits proliferation of human cancer cells via inducing cell cycle arrest and apoptosis. Life Sci. 2004;75(13):1579–94. [DOI] [PubMed] [Google Scholar]
- 14.Wei WL, et al. Angelica sinensis in China-a review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J Ethnopharmacol. 2016;190:116–41. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, et al. Structural characterization of an alkali-soluble polysaccharide from Angelica sinensis and its antitumor activity in vivo. Chem Biodivers. 2021;18(6): e2100089. [DOI] [PubMed] [Google Scholar]
- 16.Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucl Acids Res. 2019;47(W1):W357-w364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji KY, et al. Comprehensive assessment of nine target prediction web services: which should we choose for target fishing? Brief Bioinform. 2023. 10.1093/bib/bbad014. [DOI] [PubMed] [Google Scholar]
- 18.Hamamoto R. Application of artificial intelligence for medical research. Biomolecules. 2021. 10.3390/biom11010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agapito G, Arbitrio M. Microarray data analysis protocol. Methods Mol Biol. 2022;2401:263–71. [DOI] [PubMed] [Google Scholar]
- 20.Allison DB, et al. Microarray data analysis: from disarray to consolidation and consensus. Nat Rev Genet. 2006;7(1):55–65. [DOI] [PubMed] [Google Scholar]
- 21.Barrett T, et al. NCBI GEO: archive for functional genomics data sets–update. Nucl Acids Res. 2013;41:D991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis S, Meltzer PS. GEOquery: a bridge between the gene expression omnibus (GEO) and BioConductor. Bioinformatics. 2007;23(14):1846–7. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucl Acids Res. 2015;43(7): e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–9. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, et al. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucl Acids Res. 2022;50(W1):W159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma JP, et al. Phytochemical progress made in investigations of Angelica sinensis (Oliv.) Diels. Chin J Nat Med. 2015;13(4):241–9. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary A, et al. Ferulic acid: a promising therapeutic phytochemical and recent patents advances. Recent Pat Inflamm Allergy Drug Discov. 2019;13(2):115–23. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, et al. Ferulic acid-mediated modulation of apoptotic signaling pathways in cancer. Adv Protein Chem Struct Biol. 2021;125:215–57. [DOI] [PubMed] [Google Scholar]
- 29.Mirzaei S, et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: new hope in the fight against cancer. Pharmacol Res. 2021;171: 105759. [DOI] [PubMed] [Google Scholar]
- 30.Tseng JC, et al. Caffeic acid phenethyl ester suppresses EGFR/FAK/Akt signaling, migration, and tumor growth of prostate cancer cells. Phytomedicine. 2023;116: 154860. [DOI] [PubMed] [Google Scholar]
- 31.Park SR, et al. A novel therapeutic approach for colorectal cancer stem cells: blocking the PI3K/Akt signaling axis with caffeic acid. Front Cell Dev Biol. 2020;8: 585987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, et al. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021. 10.1016/j.lfs.2021.119921. [DOI] [PubMed] [Google Scholar]
- 33.Muthusamy G, et al. Ferulic acid reverses ABCB1-mediated paclitaxel resistance in MDR cell lines. Eur J Pharmacol. 2016;786:194–203. [DOI] [PubMed] [Google Scholar]
- 34.Hu J, et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol. 2021;14(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter G. The EGF receptor: a nexus for trafficking and signaling. BioEssays. 2000;22(8):697–707. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9-15. [DOI] [PubMed] [Google Scholar]
- 37.Dutta PR, Maity A. Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Cancer Lett. 2007;254(2):165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Y, et al. NLRC5 promotes cell migration and invasion by activating the PI3K/AKT signaling pathway in endometrial cancer. J Int Med Res. 2020;48(5):300060520925352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roncolato F, et al. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst Rev. 2019;10(10):CD012160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi X, et al. Research progress on the PI3K/AKT signaling pathway in gynecological cancer (Review). Mol Med Rep. 2019;19(6):4529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owen KL, Brockwell NK, Parker BS. JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers (Basel). 2019. 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li D, et al. Ferulic acid: a review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021;284: 119921. [DOI] [PubMed] [Google Scholar]
- 43.Bao X, et al. Molecular mechanism of ferulic acid and its derivatives in tumor progression. Pharmacol Rep. 2023;75(4):891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin Y, et al. Caffeic acid hinders the proliferation and migration through inhibition of IL-6 mediated JAK-STAT-3 signaling axis in human prostate cancer. Oncol Res. 2024;32(12):1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset GSE115810 used in this study can be found in the GEO database (https://www.ncbi.nlm.nih.gov/geo/). Additional materials are available from the corresponding author upon reasonable request.