Abstract
In Venus’s mesosphere, the observation/model discrepancy of molecular oxygen, O2, abundance has been a long-standing puzzle. Chlorine atoms have been proposed as a catalyst to oxidize carbon monoxide through the formation of chloroformyl radicals (ClCO), removing O2 and ultimately generating CO2. However, relevant kinetic studies of this catalytic cycle are scarce and highly uncertain. In this work, we report the spectrum of the ClCO radical between 210–520 nm using a multipass UV–Vis spectrometer coupled to a pulsed-laser photolysis flow reactor at 236–294 K temperature and 50–491 Torr pressure ranges. High-level ab initio calculations were performed to simulate the observed spectrum and to investigate the electronic structure. In addition, we observed the formation of molecular chlorine, Cl2, and phosgene, Cl2CO, suggesting that both the terminal chlorine and the central carbon in the ClCO radical are reactive towards chlorine atoms. Most importantly, the reported spectrum will enable future measurements of essential kinetic parameters related to ClCO radicals, which are important in regulating the O2 abundance in Venus’s mesosphere.
Subject terms: Optical spectroscopy, Atmospheric chemistry
In Venus’s mesosphere, chlorine atoms have been proposed as a catalyst to oxidize carbon monoxide, remove O2, and generate CO2, but relevant kinetic studies of this catalytic cycle are scare and uncertain. Here, the authors report the UV-Vis spectra of chloroformyl radicals under relevant conditions, enabling future measurements of essential kinetic parameters related to the ClCO radicals.
Introduction
Chorine (Cl) chemistry plays an important role in a wide range of industrial applications, including water treatment1, drug discovery2, development of photovoltaic materials3,4 organic synthesis5, and air pollution6. These processes involve the formation of Cl-containing intermediates. A well-known example is the catalytic destruction of the ozone layer by chlorine atoms in Earth’s stratosphere, a phenomenon described in the work that led to the award of the 1995 Nobel Prize7.
Beyond Earth, chlorine chemistry has been proposed to play a pivotal role in regulating the concentration of molecular oxygen in Venus’s mesosphere8. The upper atmosphere of Venus (60−90 km, 300–150 K)9 is composed primarily of CO210,11, which is photolyzed (CO2 + hν → CO + O) by the ultraviolet (UV) radiation from the Sun. On the night side, O atoms recombine, emitting light from excited state O2 molecules (O + O + M → O2* + M, O2* (A1Δg) → O2 (X3∑g−) + hv)12. However, previous telescope observations have shown that oxygen is essentially absent ([O2]/[CO2] < 3 × 10−7)13, a result that cannot be resolved by current photochemical models, which overestimate oxygen concentrations, [O2], by more than a factor of ten near the upper cloud layer14,15.
Direct oxidation of CO by O(3P) or O2 proceeds by high reaction barriers and is too slow to impact [O2]. As shown in Fig. 1, the chlorine-initiated catalytic cycle of CO oxidation through the formation of chloroformyl radicals (ClCO)16 has been proposed to consume O2 and generate peroxychloroformyl radicals (ClC(O)OO). The catalytic cycle is completed by reaction of ClC(O)OO with either Cl atoms, O atoms, SO or SO2 (X in Fig. 1) to generate CO2 and release the Cl atom back. To accurately estimate the effects of Cl atoms on CO oxidation, it is essential to have well-constrained kinetic parameters related to ClCO, paramount of which is the ClCO equilibrium constant (KClCO). To the best of our knowledge, studies of ClCO are scarce with only one study examining KClCO17, determined by monitoring the disappearance of Cl atoms at various CO concentrations, with an uncertainty of ± a factor of three (2 standard deviations). This uncertainty leads to several orders of magnitude difference in the modeled O2 levels (109–1013 cm−3) throughout the Venus upper atmosphere15.
Fig. 1. The catalytic cycle of the Cl-initiated oxidation of CO in the Venus mesosphere.
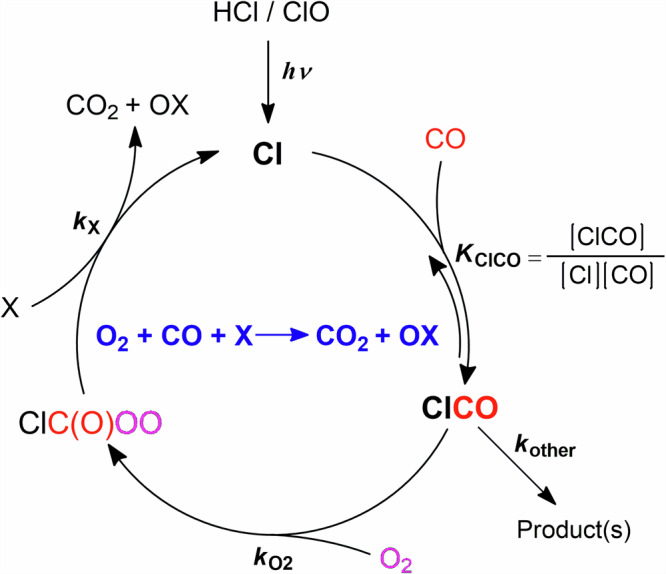
X denotes the species proposed to be oxidized by the ClC(O)OO radicals in Venus’s photochemical models (e.g., Cl, O, SO and SO2).
Direct spectroscopic and kinetic measurements of the ClCO radical would provide a unique pathway to accurately measure KClCO. Existing spectroscopic studies of the ClCO radical include an electron spin resonance measurement18, and mid-infrared (IR) measurements both in an Ar-matrix19 and in the gas phase20. Despite theoretical studies that have predicted ClCO to have moderate absorption cross section in the UV–Vis range21, direct measurements are still absent from the literature. Leveraging stronger cross sections in the UV–Vis range would allow direct detection of the ClCO radical with excellent signal-to-noise ratio to determine KClCO with reduced uncertainty.
In this work, we have recorded the UV–Vis absorption spectrum of gas-phase ClCO using a multipass UV–Vis spectrometer coupled to a pulsed-laser photolysis flow reactor22. High-level ab initio calculations were conducted to elucidate the electronic structure and to simulate the recorded spectrum. Finally, chemical insights into the catalytic CO oxidation and the implications in the Venus mesosphere are discussed.
Results and discussion
The UV–Vis absorption spectrum of the ClCO radical
To investigate the ClCO spectrum between 270–510 nm, oxalyl chloride, (ClCO)2, was photolyzed at 193 nm, producing two Cl atoms and two CO molecules with a yield of unity at 236 K and a total pressure of 50 Torr23,24. The available energy after the decomposition of (ClCO)2 (~247 kJ mol−1) is distributed as 13% in the Cl atoms and 87% in the CO molecules25, leading to highly excited CO molecules. These hot CO molecules will be stabilized either through collision with the buffer gas or by IR emission26. Based on the reported forward and backward rate coefficients of the Cl + CO reaction (k+ = 3.2 × 10−33 cm6 s−1 and k− = 1.5 × 10−15 cm3 s−1)17, we expected a prompt formation of the ClCO absorption signal peaking around 100 µs at 50 Torr of CO. A high (ClCO)2 concentration (~1016 cm−3) was used to ensure a strong signal. However, a large fraction of the 193 nm photons were absorbed by (ClCO)2, resulting in an estimated transmittance of 18%, based on the absolute cross section of (ClCO)2 (σ(ClCO)2(193 nm)27 = 3.8 × 10−18 cm2) and the length of the flow reactor, excluding the purge region (L = 45 cm). This absorption resulted in inhomogeneous radical formation down the length of the flow reactor.
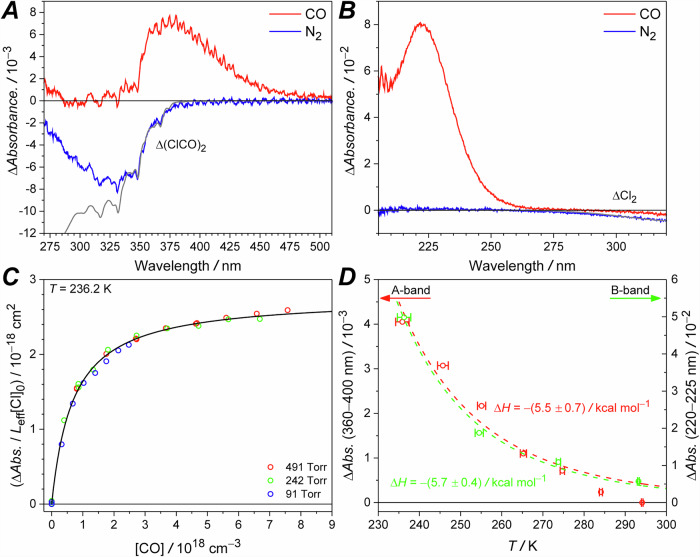
Figure 2A shows the recorded spectra of the (ClCO)2 photolysis system in pure N2 and CO at a total pressure of 50 Torr and at 236 K. The negative signal below 380 nm was attributed to the photodepletion of (ClCO)2, Δ(ClCO)2. The introduction of CO produced a new spectral feature, characterized by two series of vibrational progressions in the 360–475 nm range. A significant deviation was observed from the Δ(ClCO)2 near 275 nm. The origin of this deviation is unclear. Although direct Cl2 formation has been proposed from the photolysis of (ClCO)2, the Cl2 quantum yield for 193 nm photolysis is likely smaller than 5%24,28,29. Moreover, Cl2 has a maximum absolute cross section near 330 nm30,31, which is far from 275 nm. Any influence from the direct photolysis products of (ClCO)2 were eliminated by subtracting the recorded spectrum in the presence of CO from that in the presence of N2. Additionally, the overall spectral profile is supported by the agreement between the experimental results and theoretical simulations (see Fig. 3).
Fig. 2. The recorded spectra of the ClCO radical and the observed signal intensities as a function of CO concentration and temperature.
A Representative spectra of CO or N2 as the buffer gas at a delay time 72 μs, 50 Torr and 236 K for an average of 12266 laser shots using 193 nm photolysis of (ClCO)2. The gray line shows the contribution due to depletion of (ClCO)2. B Representative spectra of pure CO or N2 as the buffer gas at a delay time 145 μs, 50 Torr, and 236 K for an average of 6144 laser shots using 351 nm photolysis of Cl2. The gray line shows the contribution due to depletion of Cl2. C The normalized signal intensities in the 220–225 nm window as a function of [CO] at a delay time 145 μs, 236 K, and different total pressures. The [Cl2] at 491 Torr, 242 Torr, and 91 Torr are 7.5 × 1015 cm−3, 6.9 × 1015 cm−3, and 6.2 × 1015 cm−3, respectively. The black line shows the fit to all data points. D The recorded signal intensities near 380 nm (red) and near 223 nm (green) as a function of temperature. The dashed lines show the fitting of the van’t Hoff equation to all the data points in each dataset, yielding the estimated enthalpy change. The quoted error ranges are one standard deviation from the fit only.
Fig. 3. The UV–Vis spectrum of the ClCO radical.
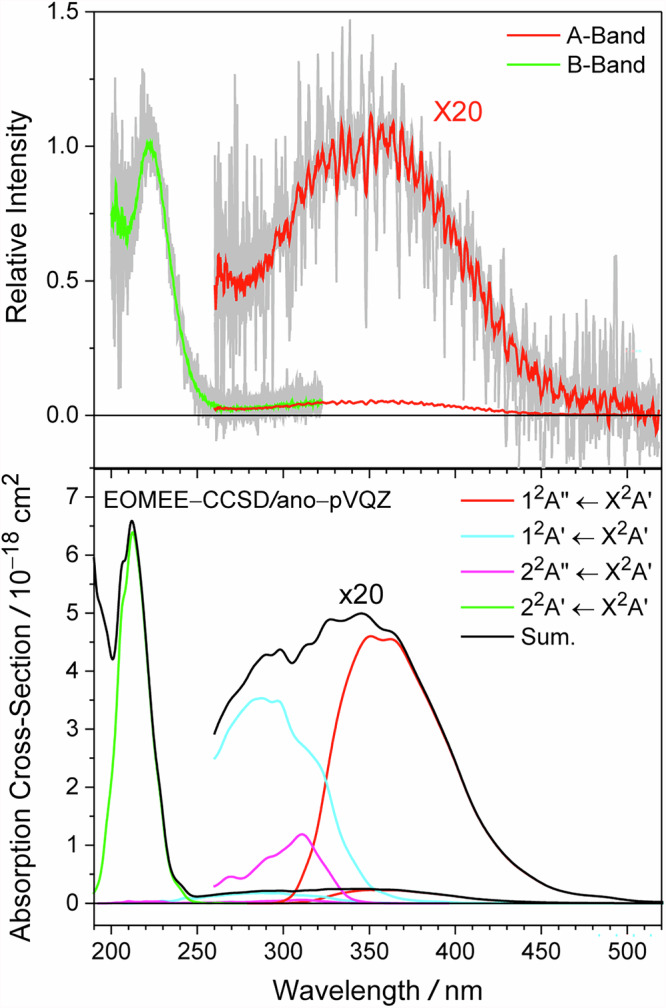
The upper panel shows the recorded A-band (red) and B-band (green) spectra of the ClCO radical at a total pressure of 50 Torr and at 236 K for an average of 12266 and 6144 laser shots, respectively. The lower panel shows the simulated spectrum (black) and the contributions from four low-lying excited states (red, cyan, magenta, and green). The simulated spectrum was constructed using the nuclear ensemble approach by sampling the ground state geometry based on a Wigner distribution with 5000 structures and calculating the vertical excitation energies and oscillator strength for each geometric structure at the EOMEE-CCSD/ano-pVQZ level of theory.
We also investigated the ClCO spectrum in the 210–320 nm range by photolyzing molecular chlorine, Cl2, at 351 nm. Figure 2B shows the recorded spectra of the Cl2 photolysis system in pure N2 and CO at a total pressure of 50 Torr and at 236 K. The negative absorption near 310 nm was attributed to the photodepletion of Cl2, ΔCl2, and was used to estimate the initial Cl atom concentration, [Cl]0, for further analyses. The photolysis laser transmittance exceed 95% due to the small absorption cross section of Cl2 near 351 m (σCl2(351 nm) = 1.9 × 10−19 cm2)30,31, despite the high [Cl2] = 5.8 × 1015 cm−3. Therefore, we expected homogeneous radical formation along the flow tube22.
As shown in Fig. 3, we assigned the absorption band with a vibrational progression near 380 nm and the structureless band peaking around 223 nm to the A-band and the B-band of the ClCO radical, respectively. In Fig. 2C, the B-band intensities, recorded at a delay time of 145 μs and 236 K, exhibit a rapid rise at low [CO] and reach a maximum at high [CO]. Since the observed signal intensities were proportional to the [Cl]0, we normalized the signal intensities to [Cl]0 = (11.7 ± 1.7) × 1013 cm−3, (10.0 ± 1.4) × 1013 cm−3 and (8.9 ± 1.3) × 1013 cm−3 at 498 Torr, 241 Torr and 91 Torr, respectively. At high [CO], the slow increase suggests that the ClCO → Cl + CO reaction is occurring as a result of the ClCO equilibrium.
By assuming rapid equilibrium, we can ignore the loss of Cl atoms. We derived formula (1) from the definition of the ClCO equilibrium constant and the mass balance relation, [Cl]0 = [Cl] + [ClCO], to analyze the observed CO dependence.
| 1 |
The fitting in Fig. 2C yielded a KClCO(236 K) = (1.5 ± 0.2) × 10−18 cm3, which is 1.5 times smaller than the literature value17, but still within the uncertainty (1.2 × 10−18 cm3 < KClCO(236 K) < 8.8 × 10−18 cm3). This analysis of the CO dependence provides a method to determine KClCO from relative intensity measurements, thus avoiding errors in absolute concentrations or rate coefficients propagates into the uncertainty of KClCO.
Figure 2D shows the signal intensities of the A-band and B-band at 236–294 K. Fitting the data to the van’t Hoff equation yielded an enthalpy change of ΔH = −(5.5 ± 0.7) kcal mol−1, which is 25% smaller than the literature. The experimental17 and theoretical32 enthalpy changes of the Cl + CO → ClCO reaction are ΔH°(0 K) = −(6.9 ± 0.7) kcal mol−1 and −5.9 kcal mol−1, respectively. Both data sets show near identical temperature dependences, implying that the origins of both absorption bands are the same.
Figure 3 shows the ClCO spectrum obtained by subtracting the absorption spectra recorded with CO from those recorded with N2. The A-band spectrum has a clear vibrational progression, and its overall shape closely matches the simulated spectrum. At wavelengths longer than 340 nm, the same vibronic progression was observed using 248 nm photolysis of both (ClCO)2 and thionyl chloride (Cl2SO) as Cl atom precursors (Fig. S1). We note that interference from unknown species affected the spectra of the Cl2SO photolysis system (Fig. S3), and so we only use this data as a qualitative comparison for the spectral signatures. The vibronic progression contains a strong and a weak series, with average spacings of 409 and 408 cm−1 (Fig. S2), respectively. A consistent difference of 215 cm−1 between these two series between 340 and 440 nm suggests the presence of a vibrational mode with a frequency of 624 cm−1.
The B-band absorption cross section was determined based on the observed CO dependence, while the A-band absorption cross section was derived by normalizing the absorption signal in the 270–300 nm window to the B-band profile. We determined the absolute cross section of σClCO(223 nm) to be ~3 × 10−18 cm2. This value should be considered as a lower bound since complete scavenging of the Cl atoms is hard to quantify without kinetic analysis. Although the estimated absolute cross section is roughly two times smaller than theoretical predictions, the ratio of the predicted cross sections of the peaks of the B band and the A band, σ(212 nm)/σ(345 nm) = 6.6 × 10−18 cm2/2.5 × 10−19 cm2 = 26, is consistent with the observed relative intensity of the peaks (~20) as shown in Fig. 3. For the uncertainty reported in this section, see Supplementary Information “Error Analysis”.
The electronic structure of the ClCO radical
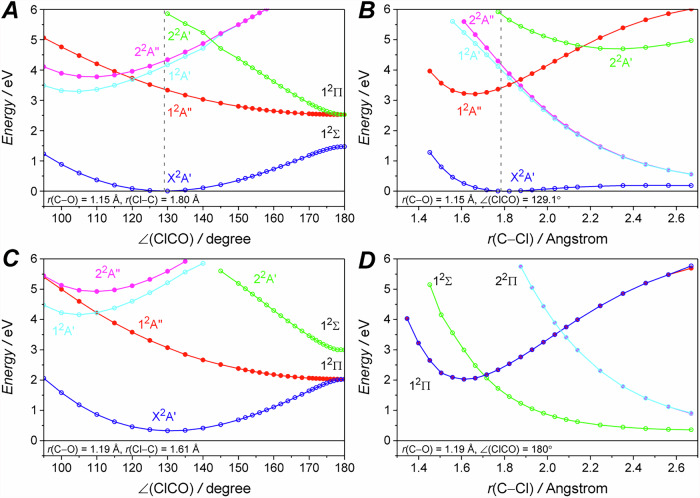
Table 1 summarizes the ClCO calculation results. The geometry optimization yielded a ground state structure with a C−O bond length of 1.15 Å, a C−Cl bond length of 1.80 Å, and a bond angle of 129.1 degrees, consistent with previous calculations20,21,33. The harmonic vibrational frequencies are 367 cm−1 for the bending mode, 606 cm−1 for the CCl stretching mode, and 1963 cm−1 for the CO stretching mode, which agree with previous IR measurements19,20. Four low-lying excited states were identified, with their orbital transition detailed in Table S1 and Table S2.
Table 1.
The Geometries, Harmonic Frequencies, and Vertical Transition Energies of the ClCO Radical Calculated at the CCSD/ano-pVQZ level of theory
| Geometry Optimized | X2A′ | 12Π | |||
|---|---|---|---|---|---|
| Geometry | r(C–O)/Å | 1.153 | 1.192 | ||
| r(C–Cl)/Å | 1.796 | 1.611 | |||
| ∠(ClCO)/degree | 129.07 | 180 | |||
| Frequency/cm−1 | ClCO bending | 367.1 | 411.4 | ||
| CCl stretching | 605.7 | 733.7 | |||
| CO stretching | 1962.5 | 2013.6 | |||
| Vertical Transition Energya/eV | 12A′ | 4.34 (1.62 × 10−3)b | 12Π | 0.0 | |
|
22A′ 12A″ |
5.91 (4.43 × 10−2) 3.45 (1.67 × 10−3) |
12∑ | 1.05 | ||
| 22A″ | 4.55 (2.21 × 10−4) | 22Π | 6.91 | ||
aCalculated at EOMEE-CCSD/ano-pVQZ level.
bOscillator strength is shown in parentheses.
With an oscillator strength on the order of 10−2 and a vertical transition energy (5.91 eV, 210 nm) slightly larger than the observed B-band position, the 22A′ ← X2A′ transition is the most likely candidate for the stronger absorption band near 223 nm. On the other hand, the transitions to the 12A′, 12A″, and 22A″ states have oscillator strengths on the order of 10−3 and vertical excited energies that lie between the peaks of the A-band and B-band, potentially contributing to the weaker absorption band near 360 nm.
The presence of vibrational progression on the red side of the A-band suggests the existence of a stable excited state. To gain further insights, we generated four representative slices of the potential energy surface (PES) of the ClCO radical along either the bending angle (∠ClCO) or the C–Cl bond length (r(C–Cl)), using MR-EOM-CC calculations with an “11 electron in 8 orbital CASSCF” reference averaged over 5 states.
Starting from the bent X2A′ state geometry, Fig. 4B shows that the 12A″ state and the 22A′ state have stable energy minima near r(C–Cl) = 1.65 Å and 2.4 Å, respectively. Figure 4A shows that both states have energy minima at linear geometry and become degenerate to form the 12Π state, while the X2A′ state becomes the 12Σ state. At linear geometry, Fig. 4D shows that a symmetry-allowed conical intersection34 near r(C–Cl) = 1.7 Å between the bonded 12Π state and the repulsive 12Σ state. This conical intersection blurs the correlation between the X2A′, 12A″ and 22A′ states and the 12Π and 12Σ states, as the 12A″ state can become degenerate with either the X2A′ state (Fig. 4C) or the 22A′ state (Fig. 4A) depending on the r(C–Cl). As a result, the stabilities of the excited states are determined by their C–Cl bond length at the minimum energy geometry.
Fig. 4. The slices of the potential energy surface of the ClCO radical.
A/C and B/D shows the potential energy curve along the bending angle (∠ClCO) and C–Cl bond length (r(C–Cl)) with the rest degrees of freedom fixed at the X2A′ geometry and the 12Π geometry for (A/B) and (C/D), respectively.
We conclude that the 22A′ state is repulsive and the 12A″ state primarily contributes to the observed vibrational progressions. Indeed, the simulated spectrum also shows that the red side of the A-band is dominated by the 12A″ ← X2A′ transition in Fig. 3. The appearance of the two series of vibrational progressions could be explained by the dependence of the excited state lifetime on the vibrational angular momentum, as observed in HCO and FCO (see Supplementary Information “Comparison with the HCO and FCO”).
The molecular orbital diagram and reactivity of ClCO
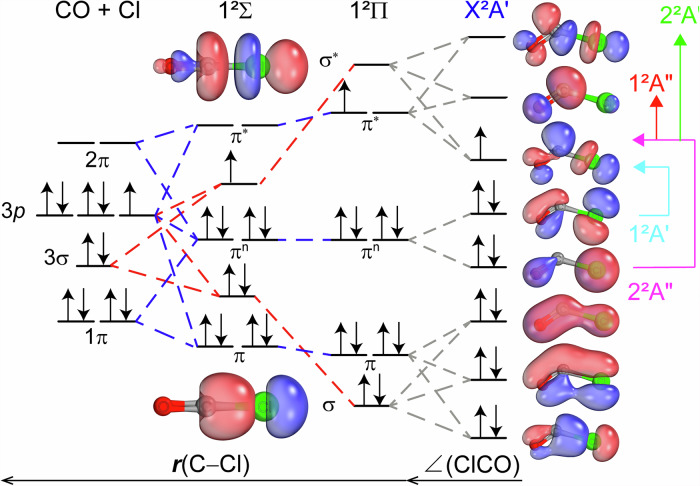
The molecular orbital (MO) diagram (Fig. 5) summarizes the quantum calculations performed in this study. First, we considered the 3p orbitals of the Cl atom and the 1π, 3σ, and 2π orbitals of the CO molecule. At the linear geometry, the 3p orbital of the Cl atom along the molecular axis interacts with the 3σ orbital of the CO molecule. This interaction generates a pair of bonding (σ) and anti-bonding (σ*) orbitals, characterized along the C–Cl bond. Additionally, the out-of-axis orbitals from the Cl atom and the CO molecule contribute to the formation of bonding (π), non-bonding (πn), and anti-bonding (π*) orbitals.
Fig. 5. Molecular orbital correlation diagram showing the valence electronic structure of the ClCO radical.
The 3p orbitals of the Cl atom and the 1π, 3σ, and 2π orbitals of the CO molecule were considered. The red dashed and blue dashed lines connect orbitals with σ and π characteristics along the C–Cl bond, respectively. The gray dashed lines connect orbitals at linear and bend geometries. The vertical and horizontal arrows indicate the alpha and beta electron transitions from the X2A′ ground state, respectively.
We note that the energy gap between the σ/σ* pair increases as the C–Cl bond length decreases. Eventually, the energy of the σ* orbital can surpass that of the π* orbital as r(Cl–C) continues to decrease. When the σ* orbital energy is lower than the π* orbital energy, the unpaired electron prefers to occupy the σ* orbital, resulting in the repulsive 12Σ state. Conversely, occupation of the π* orbital results in the bound 12Π state as shown in Fig. 4D. The bending motion induces the mixing of the σ* and π* orbitals, lowering the energy of the singly occupied molecular orbital (SOMO) while increasing its anti-bonding character along the C–Cl bond. As a result, the stronger anti-bonding character of the SOMO relative to the πn orbitals makes the 12A′ state and the 22A″ state repulsive.
This MO diagram indicates that the SOMO correlates with the 2π orbital of the CO molecule, which has π anti-bonding character along the CO triple bond. Therefore, the CO bond strength in the ClCO radical is weaker than that of the CO molecule, as suggested by a smaller CO vibrational frequency20. The shape of the SOMO shows nearly equal character from the 3p orbital of the Cl atom and the 2π orbital of the CO molecule, as the EPR measurement18 suggests a value of 0.42 for the unpaired electron density in the chlorine 3p orbital of the ClCO radical. This distribution of the unpaired electron implies that both the terminal chlorine and the central carbon will act as reaction centers in the ClCO radical.
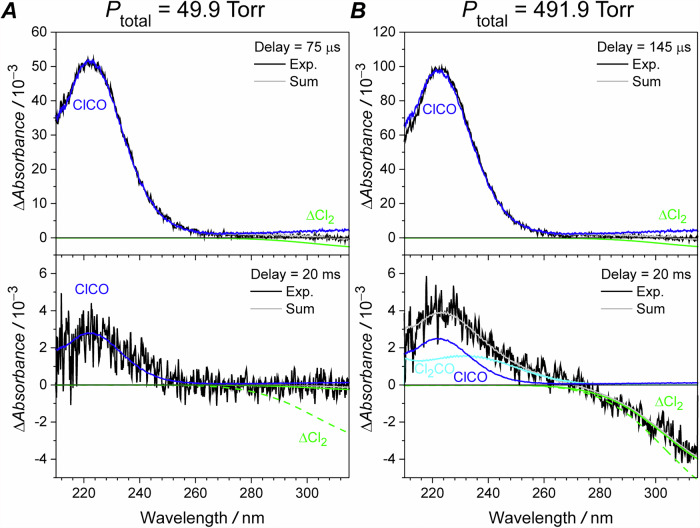
As shown in Fig. 6, we have observed the reformation of Cl2 at 49.9 Torr and at a delay time of 20 ms. Moreover, the reformation of Cl2 is inhibited at 491.9 Torr while Cl2CO is observed. We are unable to fit the increase in absorbance at 260 nm with the ClCO and Cl2 spectra alone, however inclusion of the Cl2CO spectrum allowed us to resolve this discrepancy. The formation of both Cl2 and Cl2CO in our system indicates that both the terminal chlorine and central carbon are potential reactive centers in ClCO, as suggested by the MO diagram.
Fig. 6. The recorded spectra from the photolysis of Cl2 molecules at 351 nm in the presence of CO.
Experimental conditions at 236 K are Ptotal = 49.9 Torr, P(CO) = 49.9 Torr, P(N2) = 0 Torr, P(Cl2) = 83.7 mTorr for (A) and are Ptotal = 491.9 Torr, P(CO) = 45.6 Torr, P(N2) = 446.3 Torr, P(Cl2) = 181.6 mTorr for (B). The recorded spectra were deconvolved into the contributions of ClCO formation (blue, see the B band profile in upper panel of Fig. 3) and Cl2 depletion31 (green), except for the one recorded at a 20 ms delay time and 491.9 Torr, where phosgene54 (cyan) was considered. The gray lines show the fitting results. The dashed green lines in both lower panels present the fitted ΔCl2 from the corresponding upper panels.
Photolyzing Cl2 in the presence of CO at room temperature and one atmosphere pressure is a well- known recipe for synthesizing Cl2CO, although studies on the reaction mechanism are sparse35,36. In our system, the Cl + ClCO reaction is the most plausible candidate for explaining the observed pressure effect on the formation of Cl2 and Cl2CO, as the Cl + ClCO → Cl2CO association reaction could be enhanced at higher pressures, while the chlorine extraction is pressure independent. In addition, the ClCO self-reaction is another potential candidate for the formation of Cl2 and Cl2CO. A weaker pressure effect may be expected if it is an elementary reaction not involving multiple steps or formation of energetically hot intermediates.
The chemical insights and potential implications
The catalytic role of Cl atom could be understood by the balance between energy gain and entropy cost in the formation of ClCO. The Cl–CO bond energy, D0(Cl–CO) = 6.37 kcal mol−1 (Table S3), is comparable to the entropy cost for bringing a water molecule to the reactants under typical atmospheric conditions37. In Earth’s atmosphere, the energy gain from the formation of two hydrogen bonds (~7 kcal mol−1)38 is required to compensate the entropy cost for catalyzing the self-reaction of HO239 and the reactions of Criegee intermediates with water vapor40,41. If the Cl-CO bond was too strong, a third reactant would be required to extract the Cl atom from the central carbon, preventing the release of a reactive Cl atom. For example, the F-initiated CO oxidation is not considered catalytic due to the stronger F–CO bond energy, D0(F–CO) = 22.3 kcal mol−142,43. On the other hand, if the Cl–CO bond was too weak, the concentration of ClCO would be too low to consume O2 with a sufficiently fast rate. We posit that the Cl–CO bond energy is perhaps in a “Goldilocks zone”, giving the highest catalytic efficiency analogous to the concept of the Sabatier principle.
Furthermore, this first direct observation of the ClCO radical in the UV–Vis region provides a new way to determine important parameters related to ClCO with reduced uncertainty at 236 K (e.g., KClCO and the rate coefficient for the ClCO + O2 reaction). The ClCO absorption signals near 223 nm over 236–294 K were strong, showing that the KClCO could be determined from the observed CO dependence using Cl2 as the chlorine precursor over 150–300 K for accurately modeling the catalytic effect of the Cl-initiated CO oxidation in Venus’s mesosphere. A better estimation of the O2 profile as a function of altitude is paramount, as it will be directly compared to measurements taken by the upcoming DAVINCI descent probe, scheduled for launch in the early 2030s44,45.
Conclusions
In this work, the UV–Vis spectrum of the ClCO radical was recorded at 236-294 K and 50-491 Torr using a multipass absorption spectrometer coupled to a temperature-controlled flow reactor. A weak (A-band) and a strong (B-band) absorption band were observed, with peaks near 360 nm and 223 nm, respectively. The A-band shows two series of vibronic progressions, both with an average spacing of 409 cm−1 and a shift of 215 cm−1. Conversely, the B-band is structureless. The CO dependence of the B-band signal intensities yielded an absorption cross section of σClCO(223 nm) near 3 × 10−18 cm2 and ClCO equilibrium constant of KClCO(236 K) = (1.45 ± 0.17) × 10−18 cm3.
Four low-lying excited states were identified at the EOM-CCSD/ano-pVQZ level of theory. Representative slices of the ClCO PES were generated using the MR-EOM-CC method on a CASSCF (11,8)-5SA reference. The ClCO spectrum was also generated purely from theoretical calculations, and its overall profile agreed with the experimental measurements. These calculations showed that the 12A′, 22A′, and 22A″ excited states are repulsive. Moreover, the observed vibrational progression on the red side of the A-band is dominated by the 12A″ ← X2A′ transition, while the blue side is dominated by the transitions to the 12A′ and 22A″ states. For the B-band, we assigned it to the 22A′ ← X2A′ transition. A molecular orbital diagram was constructed to summarize the chemical insights obtained from the ab initio calculations. The shape of the SOMO suggests that both the terminal chlorine and the central carbon can act as reactive centers, which is supported by the observations of Cl2 and Cl2CO as the end products.
Most importantly, the reported ClCO spectrum suggests that direct monitoring of the formation and decay of ClCO radicals is feasible, which opens the door to measuring relevant kinetic parameters, such as KClCO and the reaction rate coefficient of the ClCO + O2 reaction, across a wide range of temperatures and pressures with reduced uncertainty. These data can help elucidate the catalytic role of Cl atoms in the Venus atmosphere and prepare atmospheric models for upcoming Venus’s observation missions.
Methods
UV–Vis absorption measurements
For the experimental setup, a free-space broadband light source (LDLS, Energetiq EQ−99) was collimated using a parabolic mirror (Thorlabs, MPD149–F01, RFL = 101.6 mm, 90° OAP) and directed into a White cell for 10 passes, which gave an effective absorption length of Leff ≈ 450 cm. After exiting the multipass system, the light was focused into a spectrograph, installed with either a 600 grooves/mm or a 300 grooves/mm grating (Princeton Instruments, SpectraPro HRS–300). A half-high mirror within the spectrograph guided the bottom portion of the light to a photomultiplier tube (Hamamatsu R928) and the upper portion to an intensified CCD camera (Instruments PI-MAX4, 1024 × 256). A long-pass filter (Semrock LP02-257RU-30 × 40 or Rocky Mountain Instrument, Ravg < 1% at 200–280 nm, R > 98% at 351 nm) was placed at the exit of the White cell to isolate the probe beam from the photolysis excimer laser (Coherent Complex 205 F, ArF, KrF, or XeF). The experimental performance was evaluated previously22.
The chlorine atom precursors, (ClCO)2 (Sigma-Aldrich > 99%, ampule seal) and Cl2SO (Sigma- Aldrich > 99%), were delivered by a small stream of nitrogen flow in a bubbler, immersed into a temperature-controlled water bath (Fisherbrand, Isotemp 4100) at 292 K. The emerging flow was mixed with the CO (Airgas > 99.99%) and N2 flows and was guided into a temperature-regulated double-jacket flow reactor. The gas mixtures were pre-cooled through a Graham condenser (Chemglass, CG-1830-30) with a cone shape exit, reducing the temperature inhomogeneity (236–298 K, with a margin of ±1.6 K) compared to our previous setup. To remove potential impurities, the CO stream was flowed through a potassium hydroxide (Baker Analzyed > 86.7%) trap immersed into a methanol ice slush. The Cl2 (10% in He, Airgas) was used without purification. The pressures in the flow reactor (50–491 Torr) and precursor bubbler were continuously monitored using diaphragm gauges (MKS, 127AA series). The total pressure in the reactor was controlled by a throttle valve (MKS type 153).
Theoretical methods
The CFOUR program suite46 was utilized for performing various coupled cluster calculations, including single and double (CCSD) and higher order (CCSD(T) and CCSDT) computations. We selected the ano-pVXZ basis sets47, as atomic natural orbital (ANO) basis sets have demonstrated exceptional effectiveness in calculating harmonic frequencies. The frozen-core approximation was applied since these basis sets were not designed for handling all-electron correlation. For ground state geometries, we optimized using CCSD with unrestricted Hartree-Fock for open-shell molecules and restricted Hartree-Fock for closed-shell molecules. The properties of excited states were calculated using the EOMEE-CCSD method.
While the EOM-CCSD method accurately treats dynamic correlation in the excited state, it failed in describing the behavior of adiabatic states near the conical intersection and during the bond-breaking process. Therefore, to qualitatively depict the PES and account for the complex interactions of the ClCO radical—particularly due to the Renner-Teller effect and pseudo-Jahn-Teller effect—we employed the multireference equation of motion coupled-cluster (MR-EOM-CC) method. This approach used a complete active space self-consistent field five states averaged (CASSCF(11, 8)−5SA) reference within the ORCA program suite48. For the visualization of orbitals, we used the IBOView package49.
Spectral simulations
For the spectral simulations we used the ground state geometry and normal modes calculated at the CCSD/ano-pVQZ level of theory using the CFOUR program suite. A nuclear ensemble, consisting of 5000 geometries, was created using a Wigner distribution based on normal mode displacements with the Newton-X (version 2.4 B06) program50,51. The large nuclear ensemble used minimizes the numerical integration error due to the statistical sampling in the final spectrum52. For each geometry vertical excitation energies and oscillator strengths for the ten lowest energy doublet excited states were calculated at the EOMEE-CCSD/ano-pVQZ level of theory with Gaussian 16 (Rev. C.02)53 interfaced to Newton-X. The individual vertical transitions were convoluted with a 0.1 eV full width at half-maximum normalized Gaussian function, as recommended by Farahani et al.52.
We tested different basis sets for the EOMEE-CCSD calculations and found that in all cases the final spectra were almost identical to the one calculated at the EOMEE-CCSD/ano-pVQZ level of theory. We also performed spectral simulations at three different temperatures, 0 K, 236 K, and 298 K. As can be seen in Figs. S4 and S5, the differences between the spectra at the different temperatures are negligible.
Supplementary information
Acknowledgements
The experimental research herein was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under contract with the National Aeronautics and Space Administration (NASA). Financial support was provided by the NASA Solar System Workings program. W.C. thanks for the fellowship support from the Josephine de Karman Fellowship Trust. R.S. thanks the Doctoral Programme in Chemistry and Molecular Sciences (CHEMS-DP) at the University of Helsinki for support. B.N.F. thanks for the fellowship support from the Carlsberg Foundation grant number CF22-0754. Computational resources for R.S. and B.N.F. were provided by the Finnish IT Center for Science (CSC). B.N.F. and R.S. acknowledge Research Council of Finland Center of Excellence VILMA grant number 346369.
Author contributions
W.C. performed all the experiments and analyzed the data. W.C. and G.H.J. worked on the MR-EOM-CC calculations. W.C. and K.T.P. recorded the ClCO B-band spectrum. R.S. and B.N.F. worked on the simulated spectrum. The corresponding author, F.A.F.W., makes the original idea and conceptualization. The draft was prepared by W.C. with further editing by W.C., R.S., B.N.F., M.P.S.A., C.J.P., F.A.F.W. All the laboratory resources were supported by F.A.F.W. and C.J.P. Computing resources for the HEAT and MR- EOM-CC calculations as well as the access of Caltech DATA repository were provided by M.O.
Peer review
Peer review information
Communications Chemistry thanks Arnaud Mahieux and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer review reports are available.
Data availability
Supporting data about error analysis, analysis of the A-band vibronic structure, details of theoretical calculations are summarized in the supplementary information. Raw data of Figures shown in this work is available on the Caltech DATA repository at 10.22002/s58nj-d4j78.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-025-01520-3.
References
- 1.Guo, K., Wu, Z., Chen, C. & Fang, J. UV/Chlorine process: an efficient advanced oxidation process with multiple radicals and functions in water treatment. Acc. Chem. Res.55, 286–297 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Chiodi, D. & Ishihara, Y. “Magic chloro”: profound effects of the chlorine atom in drug discovery. J. Med. Chem.66, 5305–5331 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Yao, H., Wang, J., Xu, Y., Zhang, S. & Hou, J. Recent progress in chlorinated organic photovoltaic materials. Acc. Chem. Res.53, 822–832 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Zhu, G. et al. Rechargeable Na/Cl2 and Li/Cl2 batteries. Nature596, 525–530 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Trost, B. M. & Fleming, I. Comprehensive Organic Synthesis: Selectivity, Strategy, and Efficiency in Modern Organic Chemistry. (Pergamon Press, 1991). [Google Scholar]
- 6.Wang, X. et al. The role of chlorine in global tropospheric chemistry. Atmos. Chem. Phys.19, 3981–4003 (2019). [Google Scholar]
- 7.Molina, M. J. & Rowland, F. S. Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. Nature249, 810–812 (1974). [Google Scholar]
- 8.Bierson, C. J. & Zhang, X. Chemical cycling in the Venusian atmosphere: a full photochemical model from the surface to 110 km. JGR Planets125, e2019JE006159 (2020). [Google Scholar]
- 9.O’Rourke, J. G. et al. Venus, the Planet: introduction to the evolution of Earth’s sister planet. Space Sci. Rev.219, 10 (2023). [Google Scholar]
- 10.Marov, M. Y. A. et al. Venera 8: measurements of temperature, pressure and wind velocity on the illuminated side of Venus 1. J. Atmos. Sci.30, 1210–1214 (1973). [Google Scholar]
- 11.Marov, M. Y. A., Lystsev, V. E., Lebedev, V. N., Lukashevich, N. L. & Shari, V. P. The structure and microphysical properties of the Venus clouds: Venera 9, 10, and 11 data. Icarus44, 608–639 (1980). [Google Scholar]
- 12.García Muñoz, A., Mills, F. P., Slanger, T. G., Piccioni, G. & Drossart, P. Visible and near-infrared nightglow of molecular oxygen in the atmosphere of Venus. J. Geophys. Res.114, E12002 (2009). [Google Scholar]
- 13.Trauger, J. T. & Lunine, J. I. Spectroscopy of molecular oxygen in the atmospheres of Venus and Mars. Icarus55, 272–281 (1983). [Google Scholar]
- 14.Krasnopolsky, V. A. Chemical composition of Venus atmosphere and clouds: some unsolved problems. Planet. Space Sci.54, 1352–1359 (2006). [Google Scholar]
- 15.Mills, F. P. & Allen, M. A review of selected issues concerning the chemistry in Venus’ middle atmosphere. Planet. Space Sci.55, 1729–1740 (2007). [Google Scholar]
- 16.DeMore, W. B. & Yung, Y. L. Catalytic processes in the atmospheres of Earth and Venus. Science217, 1209–1213 (1982). [DOI] [PubMed] [Google Scholar]
- 17.Nicovich, J. M., Kreutter, K. D. & Wine, P. H. Kinetics and thermochemistry of ClCO formation from the Cl+CO association reaction. J. Chem. Phys.92, 3539–3544 (1990). [Google Scholar]
- 18.Adrian, F. J., Cochran, E. L. & Bowers, V. A. Electron spin resonance spectra of ClCO and ClOO. J. Chem. Phys.56, 6251–6255 (1972). [Google Scholar]
- 19.Schnöckel, H., Eberlein, R. A. & Plitt, H. S. Infrared spectra of matrix isolated ClCO and ab initio calculation. J. Chem. Phys.97, 4–7 (1992). [Google Scholar]
- 20.Chen, S.-H., Chu, L.-K., Chen, Y.-J., Chen, I.-C. & Lee, Y.-P. Detection of ClCO with time-resolved Fourier-transform infrared absorption spectroscopy. Chem. Phys. Lett.333, 365–370 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Krossner, T. H., Zülicke, L., Staikova, M. & Peyerimhoff, S. D. Ab initio investigation of the electronic spectrum of the chloroformyl radical ClCO. Chem. Phys. Lett.241, 511–515 (1995). [Google Scholar]
- 22.Winiberg, F. A. F. et al. A white cell based broadband transient UV-vis absorption spectroscopy with pulsed laser photolysis reactors for chemical kinetics under variable temperatures and pressures. Rev. Sci. Instrum.94, 114103 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Baklanov, A. V. & Krasnoperov, L. N. Oxalyl chloride a clean source of chlorine atoms for kinetic studies. J. Phys. Chem. A105, 97–103 (2001). [Google Scholar]
- 24.Ghosh, B., Papanastasiou, D. K. & Burkholder, J. B. Oxalyl chloride, ClC(O)C(O)Cl: UV/vis spectrum and Cl atom photolysis quantum yields at 193, 248, and 351 nm. J. Chem. Phys.137, 164315 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Hemmi, N. & Suits, A. G. Photodissociation of oxalyl chloride at 193 nm probed via synchrotron radiation. J. Phys. Chem. A101, 6633–6637 (1997). [Google Scholar]
- 26.Wu, C.-Y., Lee, Y.-P., Ogilvie, J. F. & Wang, N. S. Photolysis of oxalyl chloride (ClCO) 2 at 248 nm: emission of CO(v ‘ < 3, J ‘ < 51) detected with time-resolved Fourier Transform Spectroscopy. J. Phys. Chem. A107, 2389–2393 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Baklanov, A. V. & Krasnoperov, L. N. UV absorption spectrum and rate constant for self-reaction of silyl radicals. J. Phys. Chem. A105, 4917–4922 (2001). [Google Scholar]
- 28.Stuhr, M. et al. UV photolysis of oxalyl chloride: ClCO radical decomposition and direct Cl2${\rm Cl}_2 {\rm }$ formation pathways. Int J. Chem. Kinet.56, 482–498 (2024). [Google Scholar]
- 29.Huang, T.-K. et al. Cl2 elimination in 248 nm photolysis of (COCl)2 probed with cavity ring-down absorption spectroscopy. J. Phys. Chem. A121, 2888–2895 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Maric, D., Burrows, J. P., Meller, R. & Moortgat, G. K. A study of the UV—visible absorption spectrum of molecular chlorine. J. Photochem. Photobiol. A Chem.70, 205–214 (1993). [Google Scholar]
- 31.Burkholder, J. B. et al. JPL publication 19-5. Chemical kinetics and photochemical data for use in atmospheric. Stud. JPL Publ.19–5, 2–7 (2020). [Google Scholar]
- 32.Dixon, D. A., Peterson, K. A. & Francisco, J. S. The molecular structures and energetics of Cl2 CO, ClCO, Br 2CO, and BrCO. J. Phys. Chem. A104, 6227–6232 (2000). [Google Scholar]
- 33.Francisco, J. S. & Goldstein, A. N. The structure and dissociation energetics of low-lying states of the chloroformyl radical, ClCO. Chem. Phys.128, 367–372 (1988). [Google Scholar]
- 34.Bersuker, I. B. Pseudo-Jahn–Teller Effect—a two-state paradigm in formation, deformation, and transformation of molecular systems and solids. Chem. Rev.113, 1351–1390 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Ohta, T. Mechanism of chlorine catalytic oxidation of CO in the gas phase. Bull. Chem. Soc. Jpn. 56, 869–872 (1983).
- 36.Burns, W. G. & Dainton, F. S. The determination of the equilibrium and rate constants of the chain propagation and termination reactions in the photochemical formation of phosgene. Trans. Faraday Soc.48, 39 (1952). [Google Scholar]
- 37.Chao, W., Yin, C., Takahashi, K. & Lin, J. J.-M. Hydrogen-bonding mediated reactions of criegee intermediates in the gas phase: competition between bimolecular and termolecular reactions and the catalytic role of water. J. Phys. Chem. A123, 8336–8348 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Rocher-Casterline, B. E., Ch’ng, L. C., Mollner, A. K. & Reisler, H. Communication: determination of the bond dissociation energy (D0) of the water dimer, (H2O)2, by velocity map imaging. J. Chem. Phys. 134, 211101 (2011). [DOI] [PubMed]
- 39.Christensen, L. E. et al. Kinetics of HO2 + HO2 → H2 O2 + O2: implications for stratospheric H2 O2:implications for stratospheric H2 O2. Geophys. Res. Lett.29, 13-1–13-4 (2002). [Google Scholar]
- 40.Chao, W., Hsieh, J.-T., Chang, C.-H. & Lin, J. J.-M. Direct kinetic measurement of the reaction of the simplest Criegee intermediate with water vapor. Science347, 751–754 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Lin, L.-C., Chao, W., Chang, C.-H., Takahashi, K. & Lin, J. J.-M. Temperature dependence of the reaction of anti-CH3 CHOO with water vapor. Phys. Chem. Chem. Phys.18, 28189–28197 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Wallington, T. J., Ellermann, T., Nielsen, O. J. & Sehested, J. Atmospheric chemistry of FCOx radicals: UV spectra and self-reaction kinetics of FCO and FC(O)O2 and kinetics of some reactions of FCOx with O2, O3, and NO at 296 K. J. Phys. Chem.98, 2346–2356 (1994). [Google Scholar]
- 43.Francisco, J. S., Goldstein, A. N. & Williams, I. H. Dissociation dynamics of FCO and HCO radicals. J. Chem. Phys.89, 3044–3049 (1988). [Google Scholar]
- 44.Garvin, J. B. et al. Revealing the mysteries of Venus: the DAVINCI Mission. Planet. Sci. J.3, 117 (2022). [Google Scholar]
- 45.Widemann, T. et al. Venus evolution through time: key science questions, selected mission concepts and future investigations. Space Sci. Rev.219, 56 (2023). [Google Scholar]
- 46.Matthews, D. A. et al. Coupled-cluster techniques for computational chemistry: the CFOUR program package. J. Chem. Phys.152, 214108 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Neese, F. & Valeev, E. F. Revisiting the atomic natural orbital approach for basis sets: robust systematic basis sets for explicitly correlated and conventional correlated ab initio methods? J. Chem. Theory Comput.7, 33–43 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Neese, F. Software update: the ORCA program system—version 5.0. WIREs Comput. Mol. Sci.12, e1606 (2022).
- 49.Knizia, G. & Klein, J. E. M. N. Electron flow in reaction mechanisms-revealed from first principles. Angew. Chem. Int. Ed.54, 5518–5522 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Barbatti, M. et al. Newton-X platform: new software developments for surface hopping and nuclear ensembles. J. Chem. Theory Comput.18, 6851–6865 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crespo-Otero, R. & Barbatti, M. Spectrum simulation and decomposition with nuclear ensemble: formal derivation and application to benzene, furan and 2-phenylfuran. Theor. Chem. Acc.131, 1237 (2012). [Google Scholar]
- 52.Farahani, S., Frandsen, B. N., Kjaergaard, H. G. & Lane, J. R. Simulated electronic absorption spectra of sulfur-containing molecules present in Earth’s atmosphere. J. Phys. Chem. A123, 6605–6617 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Frisch, M. J. et al. Gaussian 16 Rev. C.02. (2019).
- 54.Meller, R., D. Boglu, & G. K. Moortgat. UV spectra of several halogenated carbonyl compounds and FTIR studies on the degradation of CF3COC1. HCFC 123 and HFC 134a. (STEP-HALOCSIDEJAFEAS Workshop, Dublin, 1991).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data about error analysis, analysis of the A-band vibronic structure, details of theoretical calculations are summarized in the supplementary information. Raw data of Figures shown in this work is available on the Caltech DATA repository at 10.22002/s58nj-d4j78.