Abstract
This study aims to develop a novel antibacterial hydrogel scaffold composed of gelatin (Gel), amniotic membrane extract (AME), and aminophylline (AMP) for neural regeneration. We investigate its ability to sustain AMP release, inhibit bacterial growth, and promote neural differentiation of human Wharton’s jelly mesenchymal stem cells (hWJMSCs) via the CREB pathway, addressing unmet needs in neural tissue engineering. The composite hydrogels were synthesized and characterized using various methods and techniques, including X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), Fourier transform infrared spectroscopy (FTIR), porosity, contact angle, water uptake, thermogravimetric analysis (TGA), biodegradation, tensile strength, drug release, and antibacterial activity. Biocompatibility tests (MTT assay, AO/EB staining) confirmed > 95% viability of hWJMSCs over six days and their differentiation to the neural cells was analyzed through immunocytochemistry (ICC) staining and real-time reverse transcription–polymerase chain reaction (RT-PCR) at different time points. The results demonstrate the successful synthesis of porous hydrogels with desirable properties, including hydrophilicity, thermal stability, biodegradability, and mechanical strength. The hydrogels support the sustained release of AMP (53.18% over 336 h) and exhibit antibacterial activity against Pseudomonas aeruginosa (90.52 ± 0.26%) and Staphylococcus aureus (93.06 ± 0.34%) due to the presence of penicillin and streptomycin (P–S) antibiotics. The biocompatibility results show that the hydrogels do not have a cytotoxic effect on the viability of human WJMSCs. The neural differentiation of human WJMSCs seeded on surface hydrogels was confirmed by evaluating specific neural markers at both protein and gene levels. In conclusion, the new antibacterial gel-based hydrogel can support the release of AMP and after further evaluation, can be introduced as a new candidate for neural repair applications.
Subject terms: Biochemistry, Biotechnology, Cell biology, Stem cells
Introduction
Neurodegenerative disorders (NDs) encompass various neurological conditions distinguished by the presence of misfolded proteins and irregular cell death in particular areas of the brain and spinal cord. Such diseases stand as significant public health issues, causing mortality and disability, particularly in older individuals. While current treatments, including chemical medication, surgery, and therapy, are insufficient to halt or reverse the progression of these diseases, researchers are exploring alternative approaches like gene therapy, stem cell therapy, and neuroprotective agents to find more effective treatments1–3.
Regenerative medicine and tissue engineering are novel therapeutic methods for regenerating or replacing damaged cells, tissues, or organs. In recent years, biomaterial scaffolds combined with growth factors and different types of stem cells (e.g., embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem/stromal cells (MSCs), and brain-derived neural stem cells (NSCs) have been used as attractive candidates to cure and promote tissue regeneration in neurodegenerative diseases2,4.
Stem cell therapy using MSCs shows promise for treating neurological disorders like Parkinson’s disease (PD), Amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), and multiple sclerosis (MS)5. Wharton’s jelly mesenchymal stem cells (WJMSCs), which come from the human umbilical cord, are one of the best sources of MSCs for cell transplantation and regenerative medicine therapy. The human WJMSCs exhibit low immunogenicity, robust immunomodulatory activity, easy accessibility, no ethical or legal issues, high expression of pluripotency markers (e.g., Sox2, Nanog, and Oct4), and a high differentiation potential6,7.
Over the past decade, researchers have investigated using 2D (two-dimensional) and 3D (three-dimensional) cell cultures for neural tissue engineering applications8. Hydrogels, a three-dimensional, water-swollen, cross-linked hydrophilic polymer network, have emerged as an ideal scaffold for neural tissue regeneration and restoration. Their exceptional biodegradability, biocompatibility, porosity, bioactivity, and flexibility have paved the way for successful neural tissue regeneration, marking a significant achievement in regenerative medicine9,10. Biological hydrogel materials can provide a favorable milieu for nerve development and repair and help avoid further nerve injury11.
Gelatin (Gel) is a water-soluble, natural, protein-based polymer derived from collagen’s irreversibly heat-induced and enzymatic hydrolysis. Gel-based hydrogel has been extensively used in tissue engineering (e.g., skin, bone, and nerve), drug delivery, cell encapsulation, and the food industry12,13. Studies have shown that hybrid Gel-based hydrogels, in combination with another natural-synthetic polymer, are ideal candidates for therapies for neural disorders such as traumatic brain injury (TBI)14 and AD15.
The amniotic membrane (AM), also known as the amnion, constitutes the innermost layer of the fetal membranes and holds a variety of growth factors and cytokines. The amniotic membrane extract (AME) is obtained by hydrating freeze-dried human AM16,17. The human AM-based scaffold is a natural biological material used in various regenerative tissues, such as skin burns17, oral18, ocular19,20, and nervous systems11.
In vitro, neurons can be differentiated from MSCs through growth factors, co-culture conditions, exosomes, micro-RNA, chemical compounds21, and natural products22. Aminophylline (AMP) is a member of the methylxanthine family and an inexpensive intravenous drug used to treat chronic obstructive pulmonary disease (COPD), cardiovascular diseases, and asthma. It can block adenosine receptors and stimulate cerebrospinal fluid (CSF) secretion and the central nervous system23–25.
This study aimed to create a new biodegradable and antibacterial hydrogel scaffold by combining Gel, AME, and AMP. The properties of the hydrogel scaffolds were extensively analyzed using various methods, and we investigated their effects on cell viability and neural differentiation. Additionally, the study examined differentiated cells expressing neural markers using immunocytochemistry (ICC) and real-time RT-PCR techniques.
Recent advancements in neural tissue engineering emphasize the importance of multifunctional hydrogels that combine antibacterial properties with neurogenic potential. For instance, Kong et al. (2022) demonstrated that antibacterial scaffolds loaded with silver nanoparticles significantly enhance neural regeneration while mitigating infection risks, though their long-term cytotoxicity remains a concern26. Similarly, Huang et al. (2025) developed a GelMA-hyaluronic acid hydrogel for sustained neurotrophic factor delivery, yet their system lacked intrinsic antibacterial activity27. Notably, Manrui et al. (2025) identified aminophylline’s role in suppressing ferroptosis via the miR-128-3p/Slc7a11 axis in traumatic brain injury models, underscoring its neuroprotective potential28, However, no prior study has combined AMP with AME in a Gel-based scaffold to exploit both CREB-mediated differentiation and antibacterial efficacy a critical gap our work addresses.
This study pioneers the use of AMP in a scaffold to modulate the CREB pathway, a critical regulator of neural progenitor differentiation. While AMP’s role in blocking phosphodiesterase (PDE) is known, its integration into a hydrogel for sustained cAMP/PKA signaling in stem cells and the resultant CREB phosphorylation has not been previously demonstrated.
This study aims to address the limitations of current neural regeneration strategies9,29,30 by developing a multifunctional hydrogel scaffold that combines Gel, AME, and AMP to synergistically promote neural differentiation of human WJMSCs through the CREB pathway. Specifically, we hypothesize that the sustained release of AMP from the hydrogel will enhance neural differentiation via cAMP/PKA-mediated CREB activation, while the antibacterial properties of penicillin–streptomycin (P-S) within the scaffold. This dual-functional platform uniquely addresses the unmet need for scaffolds that simultaneously prevent infection and promote neural regeneration.
Results
MTT Assay and AO/EB staining-based cell viability analysis
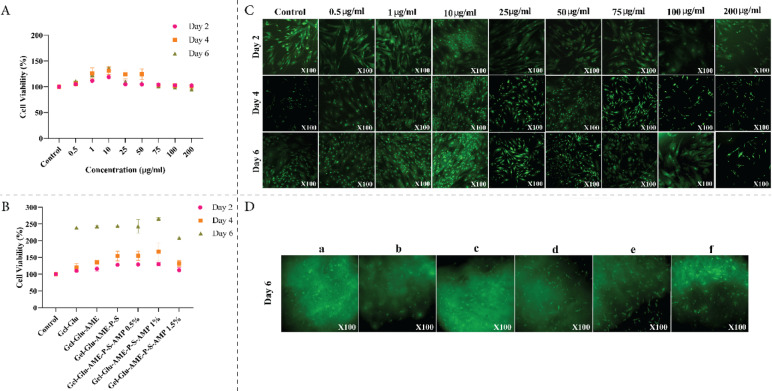
In this study, the cell proliferation and biocompatibility rate of treated human WJMSCs with various concentrations (ranging from 0.5 to 200 mg/ml) of AMP and hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP (0.5%, 1%, and 1.5% (v/v))) were assessed using an MTT assay and AO/EB staining for different incubation over 6 days. As shown in Fig. 1A, on the 2nd and 4th days, AMP at concentrations of 0.5, 1, 10, 25, and 50 μg/ml showed no cytotoxicity and promoted high proliferation of human WJMSCs compared to the control group. On the 6th day, AMP at concentrations of 0.5 to 75 μg/ml showed cell proliferation and growth, but AMP at 100 and 200 μg/ml showed decreased cell viability (no significant difference with control). The percentage of human WJMSCs viability under AMP at 10 μg/mL concentration compared to the control group on 2, 4, and 6 days is 118.84 ± 0.42, 131.18 ± 7.34 and 136.19 ± 1.20, respectively. The Acridine Orange assay was performed on day 6 to assess cell viability, morphology, and adherence to scaffolds, as this interval is critical for evaluating retained morphological integrity and viability (key indicators of cell-scaffold interaction and biocompatibility). The human WJMSCs showed significant enhancement under AMP at a concentration of 10 μg/mL (P < 0.05), which was utilized in subsequent experiments. As shown in Fig. 1B, on the 2nd, 4th and 6th days, the viability, proliferation, and biocompatibility of the human WJMSCs on hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP (0.5%, 1%, and 1.5% (v/v))) were significantly higher than the control group (P < 0.05). However, on the 6th day, the cell viability of Gel-Glu-AME-P-S-AMP1% hydrogel scaffold indicates a significant increase compared with the control group (P < 0.0001) and other concentrations (0.5 and 1.5% (v/v) of AMP (P < 0.0007). The percentage of human WJMSCs viability cultured on Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold compared to the control group at 2, 4, and 6 days is 130.30 ± 4.87, 167.24 ± 26.78 and 265.82 ± 2.24, respectively. The Gel-Glu-AME-P-S-AMP 1% composite hydrogel was selected for further experiments. A significant difference was observed between the Gel-Glu-AME-P-S-AMP 1% hydrogel on days 4 and 6 compared to the control group on days 2, 4, and 6 (P < 0.0001). The Gel-Glu-AME-P-S-AMP 1% hydrogel showed a significant difference compared to the Gel-Glu hydrogel on various days: day 2 (P < 0.0008), day 4 (P < 0.0083) and day 6 (P < 0.0001). Also, a significant difference was observed between the Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold on day 6 and the Gel-Glu hydrogel on days 2 and 4 (P < 0.0001).
Fig. 1.
Relative cell viability of human WJMSCs via MTT assay in the presence of (A) different concentrations of AMP (0.5, 1, 10, 25, 50, 75, 100, and 200 μg/ml) and (B) cultured on Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP (0.5%, 1%, and 1.5% (v/v)) hydrogel scaffolds after 2, 4, and 6 days of cell seeding. The data were presented as mean ± SD and two-way ANOVA was performed with Tukey’s multiple comparisons test. Fluorescence micrographs of AO/EB stained human WJMSCs in the presence of (C) different concentrations of AMP (0.5, 1, 10, 25, 50, 75, 100, and 200 μg/ml) after 2, 4, and 6 days of cell culture and (D) cultured on different hydrogel scaffolds ((a) Gel-Glu, (b) Gel-Glu-AME, (c) Gel-Glu-AME-P-S, (d) and Gel-Glu-AME-P-S-AMP 0.5%, (e) Gel-Glu-AME-P-S-AMP 1%, and (f) Gel-Glu-AME-P-S-AMP 1.5%) after 6 days of cell culture. For AO/EB staining, living cells emitted green fluorescence, while dead cells displayed red fluorescence. The results of the MTT assay and AO/EB staining show that all the hydrogel polymers are biocompatible and that there is no significant cytotoxic effect of all composite hydrogels and AMP on the human WJMSCs over 6 days. AMP at 10 μg/ml concentration and Gel-Glu-AME-P-S-AMP1% composite hydrogel were selected for further investigations.
As shown in Fig. 1C, on the 2nd, 4th, and 6th days, AO/EB showed the human WJMSCs in the presence of different concentrations of AMP (0.5, 1, 10, 25, 50, 75, 100, and 200 μg/ml) have normal morphology and apparent nucleus and green cytoplasm. Figure 1C shows that the human WJMSCs in 0.5–75 μg/ml concentrations exhibit normal morphology and density, while 100 and 200 μg/ml concentrations show a slight decrease in cell density (no significant difference with control) on the 6th day.
As shown in Fig. 1D, on the 6th day, AO/EB showed the human WJMSCs cultured on hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP (0.5%, 1%, and 1.5% (v/v))) have normal morphology and apparent nucleus and green cytoplasm. Figure 1D shows that all hydrogel scaffolds exhibit proper biocompatibility. However, the Gel-Glu-AME-P-S-AMP1% hydrogel scaffold demonstrated higher viability and density than other AMP-containing groups on the 6th day. The stained samples were examined under fluorescent microscopy, where living cells emitted green fluorescence, while dead cells displayed red fluorescence.
Analyses of synthesized hydrogel scaffolds
Analyses of FTIR and XRD
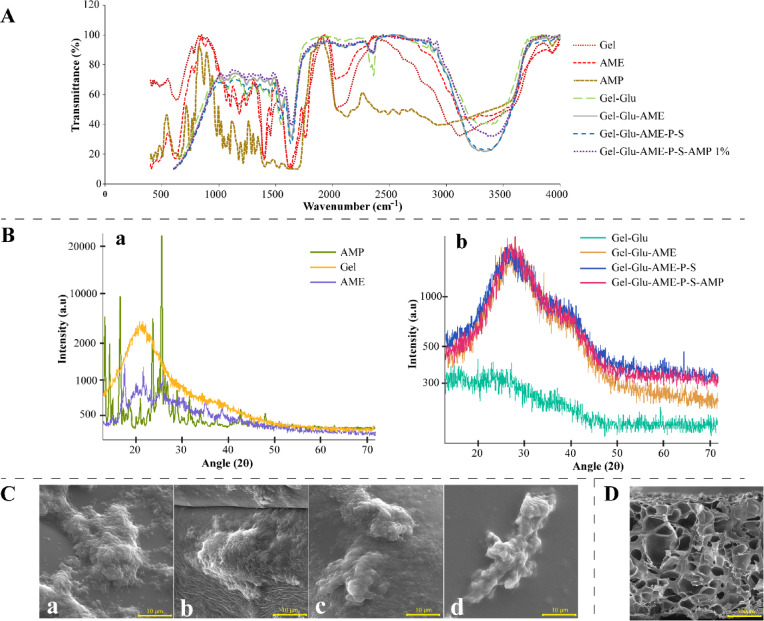
Figure 2A shows the FTIR spectra of pure (Gel, AME, AMP) and hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%). The pure Gel and AME show characteristic absorption peaks at 3120 cm−1 and 3232 cm−1 due to hydrogen bond water and amide A (N–H stretching), 1638 cm−1 and 1616 cm−1 due to amide-I (C=O stretching), respectively. The peaks at around 1400–1550 cm−1 are due to amide-II (C–N stretching, N–H bending, and symmetric and asymmetric bending vibrations of the methyl group). The peaks at around 1240–1670 cm−1 due to amide-III (peak at 1163 cm−1 and 1180 cm−1 for Gel and AME, respectively), and the peaks at around 1210–1300 cm−1 are related to C–N stretching and N–H bending in a plane with C–C stretching and C=O bending vibrations. The peak at around 1100 cm−1 corresponds to the phosphodiester group of nucleic acid, phospholipids, and glycolipids in the AME. The peaks at 640–650 cm−1 are linked apparently to planar deformation vibration of the C=O bond in amide IV31,32.
Fig. 2.
The FTIR spectra of (A) pure (Gel, AME, AMP), and hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%. The XRD spectrum (B) of (a) pure (Gel, AME, AMP), and (b) hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%. FESEM micrographs of (C) seeded human WJMSCs on (a) Gel-Glu (SEM MAG: 350 kX; SEM kV: 15 kV), (b) Gel-Glu-AME, (c) Gel-Glu-AME-P-S, and (d) Gel-Glu-AME-P-S-AMP 1% (SEM MAG: 5 kX; SEM kV: 15 kV) hydrogel scaffolds. FESEM micrographs of (D) interconnected porous structure of Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold (SEM MAG: 100X; SEM kV: 15 kV).
The FTIR spectra of pure AMP show the peaks at around 500–1500 cm−1 are related to the vibrations of the aromatic ring. The broad absorption band from the region 3000–3500 cm−1 is due to water molecules and O–H stretching vibrations, which overlap peaks of C–H and N − H. The peaks at 1403 and 1691 cm−1 are due to the C = O stretching vibration of amide and ring stretching, respectively33. The FTIR spectra of Gel-Glu displayed peaks at 3389 cm−1 and 2900 cm−1 due to O–H stretching vibrations of hydrogen bonds and C–H stretching vibrations, respectively. The 2035 cm−1 peak of Gel has been removed, and the 1546 cm−1 peak has been added, which, together with the 1450 cm−1 peak, indicates the cross-linking of Glu with Gel and the formation of aldimine (the cross-link of CHO aldehyde and the amino acid group of protein lysine). The FTIR spectra of Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1% displayed the addition of the AME, P–S, and AMP 1% to Gel-Glu due to the decreased O–H stretching at 3000–3500 cm−1. Additionally, there is an increase in alkene and amide bonds at 1000–2400 cm−1.
Figure 2B shows the XRD spectrum of pure (Gel, AME, AMP) and hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%). The XRD spectrum of pure Gel showed a broad peak located at 2θ ~ 19°, related to the triple-helical crystalline structure of Gel34. The pure AME showed six sharp peaks at 2θ values of 15°, 20°, 25°, 36°, 40° and 45° related to the triple-helical crystalline structure of collagen35. The pure AMP showed two sharp peaks at 2θ values of 12° and 23°, related to the 002 and 004 planes of the crystal structure of AMP36. Adding Glu to Gel creates bonds between the gelatin chains, which prevent their conformation into three-helical gelatin structures and show that the crystal structure has become amorphous. Additionally, with the addition of AME, P-S, and AMP 1% to Gel-Glu hydrogel, the previous peaks of Gel disappeared, and a broad peak at 2θ = 26 and a small peak at 2θ = 41 were created, which shows that the amorphous structure has been transformed apparently into a semi-crystalline structure.
FESEM analyses
Following two weeks of neural differentiation, as depicted in Fig. 2C, the FESEM micrographs show that differentiated cells have suitable attachment, growth, proliferation, spreading, and normal morphology on Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds. Also, as shown in Fig. 2D, the FESEM micrographs before the cell seeding show that the Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold exhibits interconnected porous morphology with average pore sizes of 153.92 μm.
Analyses of water contact angle, porosity, and water uptake ability
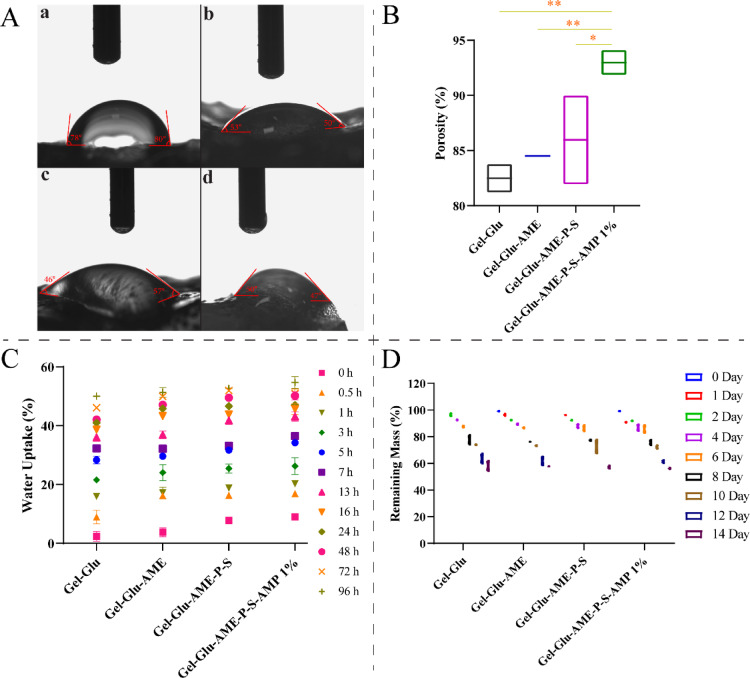
Contact angles greater and less than 90° indicate a hydrophobic and hydrophilic surface, respectively37. Figure 3A shows that the Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds have suitable hydrophilic properties with water contact angles of 78–80°, 53–50°, 46–57°, and 50–47°, respectively. Figure 3B represents that the porosity of the different interconnected porous hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%) was 82.49 ± 1.30%, 84.51 ± 0.11%, 84.97 ± 4.03%, and 92.98 ± 1.14%, respectively. The porosity results indicate that all four hydrogel scaffolds showed proper porosity. The porosity of Gel-Glu-AME-P-S-AMP 1% hydrogel significantly increased compared to other hydrogels (P < 0.01 and P < 0.05). Figure 3C displays water uptake variations in different hydrogel scaffolds over time. On the 96th hour, the average water uptake in Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds were 50.07 ± 0.76%, 51.30 ± 1.19%, 52.72 ± 0.01%, and 54.70 ± 1.43%, respectively. Also, there was no significant difference between composite hydrogels at the 96th hour.
Fig. 3.
Surface hydrophilicity and contact angle of (A) different hydrogel scaffolds (a) Gel-Glu, (b) Gel-Glu-AME, (c) Gel-Glu-AME-P-S, and (d) Gel-Glu-AME-P-S-AMP 1%. The porosity rate of (B) Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds. The water uptake rate of (C) the hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%.) at 96h. The remaining mass profile of (D) the hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%) was immersed in PBS at pH 7.4.
Analyses of biodegradation, TGA, tensile, and drug release
Figure 3D shows the biodegradation curves of the Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P–S, and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds after different periods in PBS at 37 °C. Following incubation for 14 days, the average percentage remaining mass of the Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1% samples was 58.14 ± 3.87%, 57.91 ± 0.13%, 57.20 ± 1.20%, and 56.31 ± 0.52%, respectively. Also, there was no significant difference between various hydrogels.
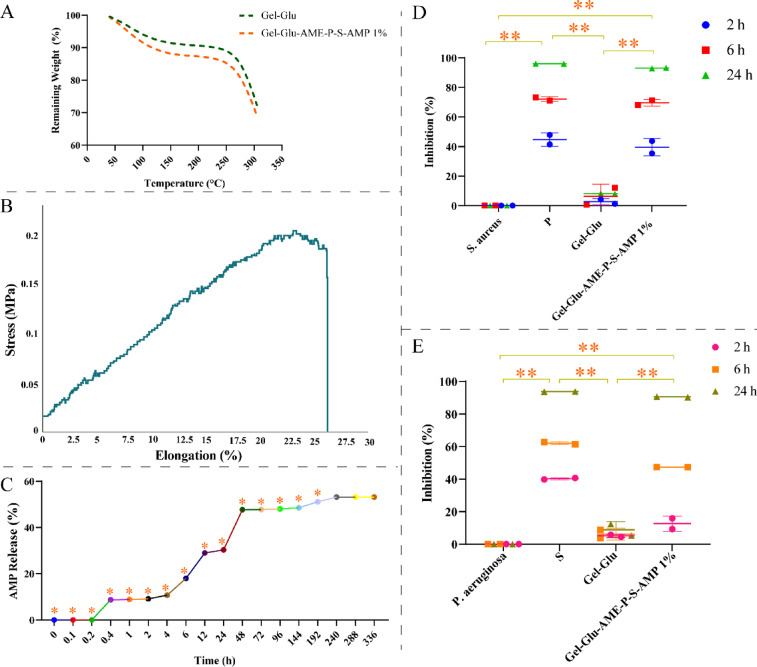
The thermal analysis of Gel-Glu and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds was performed by TGA, and the results are in Fig. 4A. The first weight loss of Gel-Glu and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds was observed at 47 °C and 43 °C, respectively, and was about 1%. The residual masses for Gel-Glu and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds at 300 °C were about 74% and 71%, respectively. The Gel-Glu-AME-P-S-AMP 1% showed the lowest remaining mass rate compared to the Gel-Glu hydrogel. Also, there was no significant difference between hydrogel scaffolds. Figure 4B illustrates the stress-elongation curve for the Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold. The elastic modulus and percentage elongation at break of Gel-Glu-AME-P-S-AMP 1% were 600 kPa and 26%, respectively. The result of the mechanical properties of Gel-Glu-AME-P-S-AMP 1% is shown in Table 1. Figure 4C shows the in vitro drug release percentage of AMP from Gel-Glu-AME-P-S-AMP 1% hydrogel. According to Fig. 4C, there was no observed AMP release until the first 20 min. However, after 20 min, the release of the AMP rate increased slightly and continued until 240 h, after which the release amount remained constant at 240, 288, and 336 h; 53.18% of AMP was released. The significant release of AMP from the Gel-Glu-AME-P-S-AMP 1% composite hydrogel was observed over time (P < 0.05).
Fig. 4.
TGA curves of (A) the Gel-Glu and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds. The stress-elongation curve of (B) Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold. In vitro releases of (C) AMP from Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold in PBS at pH = 7.4. Antibacterial assays of the Gel-Glu and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds against (D) S. aureus and (E) P. aeruginosa. The data are presented as mean ± SD (*P < 0.05, **P < 0.01).
Table 1.
Mechanical properties of Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold.
| Sample | Stress (MPa) | Modulus at break (MPa) |
Elongation at break (%) |
|---|---|---|---|
| Gel-Glu-AME-P-S-AMP 1% | 0.16 | 0.6 | 26.34 |
The sustained AMP release profile (53.18% over 14 days) contrasts sharply with rapid-release systems, ensuring prolonged CREB activation critical for late-stage neural marker expression (e.g., ENO2, TUBB3).
Antibacterial activity
The antibacterial activities of the Gel-Glu and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds were represented in terms of inhibition by MIC assay in Fig. 4D and E, at 24 h. The Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold has an apparent inhibitory influence on the growth of P. aeruginosa (90.52 ± 0.26%) and S. aureus (93.06 ± 0.34%). Also, the positive control sample (P and S antibiotics) showed 96.07 ± 0.11% and 93.80 ± 0.12% inhibition, respectively. The Gel-Glu-AME-P-S-AMP 1% displayed influential antibacterial activity against P. aeruginosa and S. aureus, confirming the robust antibacterial effect of the P–S antibiotic (P < 0.01).
The scaffold’s antibacterial efficacy against common neurosurgical pathogens (Fig. 4D–E) addresses a critical limitation of existing neural scaffolds, which often lack infection-mitigation strategies. This dual functionality positions our hydrogel as a safer option for clinical translation compared to single-purpose designs.
2D and 3D cultures (neural differentiation of human WJMSCs)
Protein expression analysis
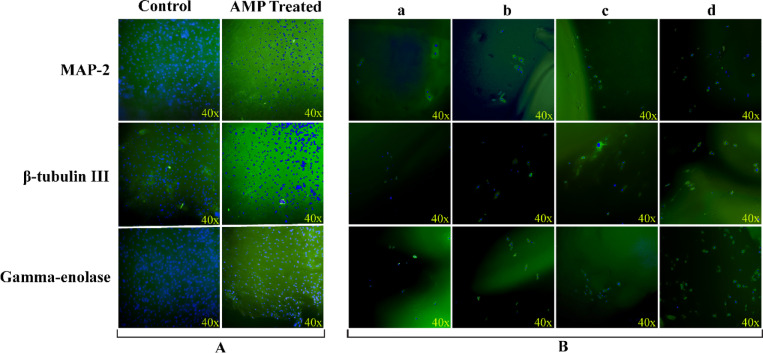
The cellular localization and protein expression of neural markers, including (Gamma-enolase, β-tubulin III, and Microtubule-Associated Protein 2 (MAP-2)) were investigated using ICC staining. On the 14th day, as shown in Fig. 5A and B, the protein expression of Gamma-enolase, β-tubulin III, and MAP-2 in the AMP-treated group was higher than in the control group. Also, the protein levels of neural markers in the Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold group were higher than other hydrogel groups lacking the AMP drug. On the 14th day, the protein expression of Gamma-enolase in Gel-Glu-AME-P-S-AMP 1% composite hydrogel was higher than other neural markers (β-tubulin III, and MAP-2).
Fig. 5.
The protein expression of neural markers (MAP-2, β-tubulin III, and Gamma-enolase) in neural-differentiated cells in the presence of (A) AMP (10 μg/ml) and (B) hydrogel scaffolds ((a) Gel-Glu, (b) Gel-Glu-AME, (c) Gel-Glu-AME-P-S, and (d) Gel-Glu-AME-P-S-AMP 1%) after 14 days of neural induction. Green and blue (DAPI) staining indicate protein and nuclei of differentiated cells, respectively.
Gene expression analysis
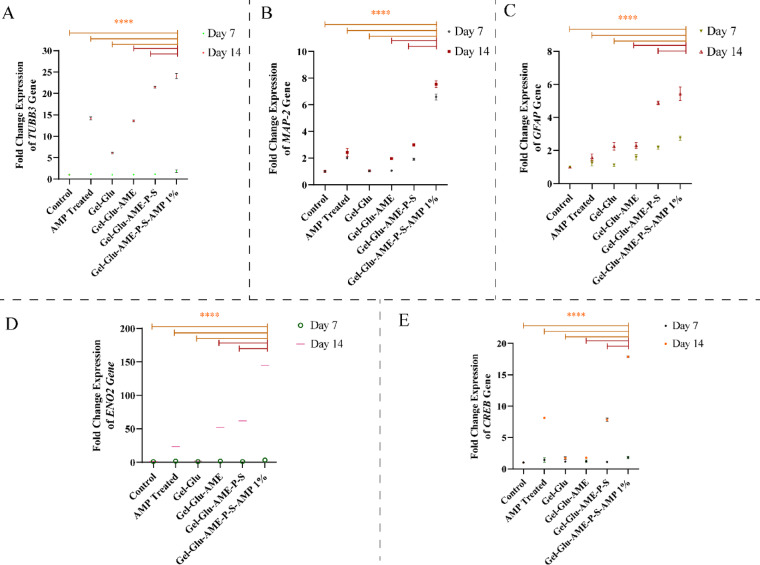
Gene expression levels of MAP-2, tubulin beta class III or TUBB3, neuron-specific enolase or ENO2, glial fibrillary acidic protein or GFAP, and cyclic adenosine monophosphate (cAMP) responsive element (CRE) binding protein (CREB) were assessed in human WJMSCs differentiated for 7 and 14 days in 2D and 3D cultures. Figure 6A, B, C, D, and E show that gene expression levels increased in the Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1% hydrogel scaffolds and AMP (10 μg/ml) groups compared to the control group on both days 7 and 14. Figure 6A, B, C, D, and E show that the fold change ratio for expression of TUBB3, MAP-2, GFAP, ENO2, and CREB in Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold for 7 days after neural differentiation was 1.80 ± 0.42, 6.60 ± 0.33, 2.76 ± 0.20, 3.20 ± 1.10, and 1.81 ± 0.27, respectively. Also, the fold change ratio for gene expression of TUBB3, MAP-2, GFAP, ENO2, and CREB in Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold for 14 days after neural differentiation was 24.08 ± 0.88, 7.55 ± 0.35, 5.43 ± 0.58, 144.65 ± 0.64, and 17.85 ± 0.21, respectively. On the 14th day, the expression levels of genes in all differentiated groups in 2D and 3D cultures were higher than on the 7th day. Figure 6A, B, C, D, and E show that the Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold group has significantly higher gene expression levels than the other hydrogel groups lacking the AMP drug (P < 0.0001). Also, the gene expression of neural markers and CREB in the AMP-treated group was higher than in the control group. Figure 6A, B, C, D, and E show that the fold change ratio for gene expression of TUBB3, MAP-2, GFAP, ENO2, and CREB in the AMP-treated group for 7 days after neural differentiation was 1.14 ± 0.06, 2.01 ± 0.06, 1.23 ± 0.24, 1.80 ± 0.28, and 1.42 ± 0.47, respectively. Additionally, the fold change ratio for gene expression of TUBB3, MAP-2, GFAP, ENO2, and CREB in the AMP-treated group at 14 days of neural deafferentation was 14.17 ± 0.47, 2.43 ± 0.44, 1.57 ± 0.31, 23.43 ± 0.31, and 8.14 ± , respectively.
Fig. 6.
Relative gene expression of (A) TUBB3, (B) MAP-2, (C) GFAP, (D) ENO2, and (E) CREB in neural differentiated cells in the presence of AMP (10 μg/ml) and hydrogel scaffolds (Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%) at 7 and 14 days. The data are shown as mean ± SD (****P < 0.0001).
On the 14th day, the gene expression of ENO2 in the Gel-Glu-AME-P-S-AMP 1% composite hydrogel and AMP-treated group was higher than other neural markers (TUBB3, MAP-2, and GFAP).
Discussion
In this study, we evaluated the differentiation of human WJMSCs into neurons and astrocytes using an antibacterial and biocompatible hydrogel scaffold containing AMP drugs as inducers.
The characterization results indicate the successful synthesis of antibacterial hydrogels containing Gel, AME, and AMP with appropriate properties. Hydrogels’ physical and biochemical properties play critical roles in changing cellular processes, such as survival rate, growth, adhesion, and differentiation of neural cells38.
Gel and human AM have emerged as promising tools in neuronal tissue engineering due to their biocompatibility, biodegradability, low antigenicity, and biomechanical properties8,13,39. Our findings on cell viability showed that all the hydrogel polymers are biocompatible and that there is no significant cytotoxic effect of all composite hydrogels and AMP on the human WJMSCs over six days in vitro. Studies related to Gel-based hydrogel have reported similar results on cellular viability and proliferation of other cells, such as human adipose stem cells (ASCs)40, human bone marrow-derived mesenchymal stem cells (BM-MSCs)15, human MSCs41, human umbilical cord mesenchymal stem cells (UC-MSCs)42, and human iPSCs43,44. Souza et al. similarly reported that a hydrogel film containing a human amniotic membrane modified with methacryloyl domains (AMMA) used in neural tissue repair could induce adhesion, proliferation, and differentiation of NSCs, indicating that the amniotic membrane used in the hydrogel lacked cytotoxicity45. KIM et al. demonstrated similar results for AMP at a concentration of 10 μg/ml by MTT assay. This concentration exerts anti-apoptotic effects against cigarette smoke extract-induced MRC-5 cell death46. Also, according to the FESEM images (Fig. 2C), all hydrogel scaffolds investigated in this study (because of their biocompatible properties) are suitable for cell attachment and growth.
In this study, we used FTIR spectroscopy to determine the formation and presence of new chemical bonds and functional groups in the produced hydrogel scaffolds. The FTIR peaks of Gel and AME are similar due to their protein structures, with intensity differences based on the number of functional groups. Gel absorption bands are in the amide bond region, while AME’s peaks are in the collagen extracellular matrix index31,32. AMP consists of two theophylline molecules and ethylenediamine. The presence of ethylenediamine improves its solubility in water47. The AMP structure consists of peaks related to the aromatic structure, C-H, N–H, C=O, C-N, and hydrogen bonds of water molecules with carbonyl and amine groups33,48. The FTIR spectrum results of Gel, and AME are similar to previous studies and confirm the structure of Gel, and AME31,32. Furthermore, the FTIR peaks of the AMP structure are the same as that of the theophylline structure33,48. Kadkhodaie-Elyaderani et al. found vibrational bands in the FTIR spectra of S at 3420 cm−1, 1650 cm−1, and 1505 cm−1 due to the phenolic O–H group, the aromatic C=C double bond group, and the amino group, respectively.49. Wu’s study reported P peaks at 2550 cm−1 due to SH stretching vibrations50. The FTIR analysis revealed that Gel, Glu, AME, P–S, and AMP exist in a composite hydrogel structure. XRD analysis supports these findings, and the results of XRD are consistent with previous studies34,35,49,51 and confirm the structure of Gel, AME, and P-S. Moreover, the XRD pattern of the AMP structure is the same as that of the theophylline structure36.
The optimal pore size and porosity of more than 90% of scaffolds are crucial in determining their properties, including cell proliferation, migration, infiltration, mechanical strength, and diffusion rate of nutrients and waste removal41,52,53. Our FESEM and porosity findings showed that the composite hydrogel containing AMP has an interconnected porous structure and high porosity, similar to other Gel composite hydrogels41,52. The composite hydrogel containing AMP provides suitable conditions for neural cell growth, proliferation, and adhesion, consistent with previous studies that suggest neural cell growth requires a pore size within 10 to 100 μm and 90% porosity54–56. The current results from FESEM and porosity analysis are associated with the cell viability percentage of the hydrogels. These findings suggest the potential of the composite hydrogel containing AMP for applications in neural engineering.
The scaffold surface’s hydrophilic nature is desirable for promoting cell attachment, interactions, and proliferation in tissue engineering37,53. The composite hydrogel contact angle result indicates that it belongs to the hydrophilic group with a high surface wettability. Furthermore, these findings support the present results of the MTT and FESEM analyses. Ghasemi-Mobarakeh et al. also reported that the Gel composite scaffold has hydrophilicity properties and is suitable for nerve regeneration57.
The swelling rate of scaffolds is essential in cell-to-scaffold interactions, biodegradation, and bioactivity in tissue regeneration. The biodegradation and swelling rate of biomaterials depends on several factors such as polymer type, density of polymer molecules, crosslinker molecules, biomaterial chemical, and mechanical specifications, and enzyme existence at the injury zone54,58. In this study, the composite hydrogel containing AMP showed the highest degradation and water uptake rate. In contrast, the Gel-Glu hydrogel showed the lowest degradation rate and water uptake ability compared to other hydrogels. Also, adding AME, P-S, and AMP to the Gel hydrogel slightly increased its degradation and water uptake ability rate compared to the Gel hydrogel (control). The rate of biodegradation results supports the result of Madaninasab et al. indicating that the Gel composite hydrogel containing 2 wt.% carboxyl graphene has a biodegradation rate of about 40% after 14 days, making it suitable for neural tissue repair59. Also, Chen et al. similarly reported that a Gel-based composite hydrogel has about 17% degradation rate in PBS up to 72 h, and is appropriate for TBI60. The different hydrogel scaffolds show no significant difference in water uptake and biodegradation rates.
The hydrogel scaffolds have suitable water uptake rates due to their high porosity, and the presence of Gel, AME, P-S, and AMP is known as hydrophilic polymers. Hydrogels should degrade slowly and swell minimally for effective central nervous system (CNS) regeneration. A higher percentage of swelling leads to scaffolds’ weight loss and structural deformation54. The water uptake data support the results of Sahin et al., indicating that the poly (2-hydroxyethyl methacrylate) hydrogel conduit containing methacrylate gelatin (GelMA)-methacrylate hyaluronic acid (HaMA) IPN has a water content of 50% and is appropriate for peripheral nerve regeneration61.
The mechanical properties (tensile strength) of hydrogel scaffolds significantly impact the use of biomaterials as medical devices in surgery and tissue engineering53. According to Fig. 4B, the composite hydrogel has a tensile modulus of 600 kPa. Borschel et al. reported that the acellular nerve tissue has a tensile modulus of 576 kPa62. Additionally, Sahin et al. have successfully synthesized a Gel composite hydrogel with a tensile modulus of 570.9 kPa for peripheral nerve regeneration. Sahin et al. reported that this hydrogel has demonstrated its suitability for cell migration, attachment, and application in nerve tissue, instilling confidence in its potential for nerve tissue application61.
Furthermore, the 92.98% porosity and 600 kPa tensile modulus mirror native neural tissue mechanics, reducing stress-shielding and promoting axonal alignment critical for functional recovery.
Thermo-responsive hydrogels are a promising option for delivering drugs to injured nerve tissues63. According to the TGA results, the composite hydrogel containing AMP demonstrated thermal stability within the body temperature range for medical applications. This result is consistent with biodegradation and water uptake rate results. The remaining mass result of Gel-based hydrogel is similar to the Bagher et al. survey. This result suggests olfactory ecto-mesenchymal stem cells with thermal stability (about 70% remaining mass at 300 °C) can be effectively differentiated into motor neuron-like cells on chitosan-aniline pentamer/gelatin/agarose hydrogels64.
Hydrogels can provide a stable and controlled release of drugs, allowing for sustained delivery over an extended period. The porosity, pore sizes, and swelling ratio of hydrogels and drug solubility can control the drug release rate from polymer-based hydrogels65.
The significant and sustained release of AMP from the composite hydrogel containing AMP is probably related to the water uptake rates of the hydrogel, as AMP was blended sufficiently within the hydrogel. The sustainability of the AMP for at least 48 h introduces this hydrogel as appropriate for drug delivery systems in neural tissue engineering. This result is similar to the study by Ooi et al. on releasing theophylline drugs from gel composite hydrogel66. The present result of AMP release is related to the swelling ratio of hydrogels.
Antibacterial scaffolds are essential for nerve tissue engineering, as they prevent bacterial infection and promote recovery by preventing inflammation26. After neurosurgery, the average risk of intracranial infection due to common bacterial agents (e.g., S. aureus and P. aeruginosa) is 6.1%67. The result of the antibacterial effect of the composite hydrogel containing P-S antibiotic is consistent with M. Tamer et al.'s research findings. The resulting study68 showed the hydrogel Polyvinyl alcohol (PVA)/Kaolin composite sponges loaded with P-S antibiotic have antibacterial effects vs. Gram-negative and Gram-positive bacteria, including Shigella sp, P. aeruginosa, Proteus vulgaris (P. vulgaris), S. aureus, and Streptococcus pyogenes (S. pyogenes).
The integration of antibacterial activity (93% inhibition of S. aureus and P. aeruginosa) with CREB-mediated neural differentiation (17.85-fold CREB upregulation) represents a significant advancement in neural scaffold design. Current strategies, such as silver nanoparticle-loaded hydrogels (Kong et al.) or growth factor-eluting scaffolds (Li et al.), often prioritize one functionality at the expense of the other. For example, while silver nanoparticles excel in infection control, their cytotoxicity limits long-term neural integration. Conversely, growth factor-based scaffolds lack intrinsic antimicrobial properties, necessitating adjunct antibiotics that disrupt stem cell microenvironments (Sahin et al.).
Our Gel-AME-AMP scaffold circumvents these trade-offs by synergizing P-S antibiotics with AMP’s neurogenic effects, offering a clinically viable solution to simultaneously mitigate infection risks and enhance regeneration (a dual capability absent in prior literature).
The human WJMSCs can differentiate into different types of neurons and enhance neurogenesis and neuroprotection by secreting various growth factors, cytokines, and chemokines6,7. Several studies have shown that WJMSCs can improve neuronal function in patients with hypoxic-ischemic encephalopathy69 and storke70, as well as in an animal model of spinal cord injury (SCI)71, TBI72, and AD73.
This study investigated the expression of neural differentiation markers, including MAP-2, TUBB3, ENO2, and GFAP, in 2D and 3D cultures. MAP2, as a late neuronal marker, promotes dendrite elongation, stabilizes microtubules in dendrites, modulates synaptic plasticity, and regulates neuronal cytoskeleton organization74,75. TUBB3, as an early neuronal marker, is a pivotal gene for neural differentiation in cells that have a vital role in neuronal growth, maintenance of the axonal and dendritic structure, and guidance of growing axons during neural development76. By addressing both infection (90.52% P. aeruginosa inhibition) and regeneration (24.08-fold TUBB3 expression), this platform could significantly improve outcomes in high-risk procedures like peripheral nerve grafting or deep brain stimulation.
ENO2 is a neuroectoderm marker highly expressed in neural cells during the development and formation of nerve cells44,77. GFAP (neurogenesis marker) is an intermediate filament protein in astrocytes, playing significant roles in cell signaling and cytomechanics44,78.
This study’s findings showed that the expression of specific neural differentiation markers and CREB as pathway markers in 3D culture using a composite hydrogel containing AMP increases compared to the 2D culture and other hydrogel scaffolds, which lack AMP at 14 days. This result is similar to the previous reports, in which the neural differentiation of WJMSCs increased in 3D scaffolds compared to the 2D system7,79. Additionally, previous reports have successfully utilized Gel composites to induce neural differentiation of various stem cells, as revealed through the upregulation of MAP-2, β-tubulin III, ENO2, GFAP, and Olig2 (oligodendrocyte marker)44,80–82.
The acquired data conclusively demonstrate that AMP drugs with a hydrogel scaffold facilitate the differentiation of WJMSCs into neurons and astrocytes. Xu et al. found that AMP makes protection against ferroptotic neuronal death in mice with controlled cortical impact, offering potential treatment for TBI patients through the microRNA(miR)-128-3p/Slc7a11 axis83.
CREB is a transcription factor activated through phosphorylation at Serine 133 by the cAMP-dependent protein kinase A (PKA) pathway. CREB plays a crucial role in cellular processes such as cell growth, differentiation, and the survival of embryonic and adult neural progenitors and hematopoietic cells30,84. Compared to the control, the CREB expression in the composite hydrogel containing AMP and AMP-treated (2D) increased significantly. Based on the CREB expression and AMP mechanism, we conclude that AMP inhibition of phosphodiesterase (PDE) increases intracellular cAMP concentrations, leading to neural differentiation by the activation of cAMP-dependent protein kinase A (PKA) and phosphorylation of CREB. Kim et al. demonstrated that hippocampal HiB5 cells treated with dibutyryl-cyclic AMP (dbcAMP) differentiate into neuronal cells in vitro through activating the PKA signaling pathway and phosphorylation of CREB85. Also, Tio et al. utilized retinoic acid (RA), dbcAMP, and 3-isobutyl-1-methylxanthine (IBMX) to prompt neural differentiation of human UC-MSCs. After treatment, results show an increase in the expression of GFAP, NSE, tyrosine hydroxylase (TH), Nurr-1, and neurofilament genes (NF-L, NF-M, NF-H) compared to undifferentiated human UC-MSCs. Two drugs (dbcAMP and IBMX) induce neuronal differentiation through increased cAMP levels and activation of the PKA86.
Mohammed et al. investigated the effect of theophylline on neural stem cell differentiation in vitro. They reported that theophylline had no cytotoxic effect on neural stem cells at doses used in this research and no effect on neural stem cell differentiation87. Paladino et al. reported that WJMSCs treated with Forskolin, an activator of the adenylyl cyclase enzyme that increases cAMP levels, could be differentiated into dopaminergic neurons by increasing the expression of MAP-2 and NF-M proteins. These findings suggest that WJMSCs could be a promising approach for treating NDs88.
According to the present results, the composite hydrogel containing Gel, AME, P-S, and AMP, with an interconnected porous structure, biocompatible, biodegradable, antibacterial, hydrophilic, and drug-release properties, was appropriate for neural differentiation.
Our findings align with Cruz et al. (2023), who reported enhanced neuronal marker expression (TUBB3, MAP-2) in GelMA hydrogels80, but diverge in demonstrating AMP’s dual role as a neurogenic and antibacterial agent as a novelty over prior single-functional systems. The observed CREB upregulation (17.85-fold) corroborates Manrui et al. (2025), who linked AMP to neuroprotection via cAMP/PKA signaling28, yet our work uniquely ties this mechanism to 3D scaffold-mediated human WJMSCs differentiation. Furthermore, the 93% inhibition of S. aureus surpasses Tamer et al. (2021) PVA/Kaolin sponges (85% inhibition)68, likely due to synergistic P-S/AMP effects.
Unlike previous studies focusing on single aspects of neural repair (e.g., biocompatibility or growth factor delivery), our scaffold uniquely combines antibacterial protection with sustained neurogenic induction. For instance, while AME-based scaffolds or Gel hydrogels have shown regenerative potential, none have incorporated AMP for neural differentiation or P-S for infection control.
The observed CREB upregulation not only validates AMP’s role as a neurogenic inducer but also unveils a scaffold-driven synergy between biochemical and biomechanical cues. Unlike 2D cultures, the hydrogel’s 3D architecture amplifies mechanotransduction signals (e.g., integrin activation, cytoskeletal remodeling) that converge with AMP-induced cAMP/PKA signaling to hyperactivate CREB. This dual stimulation mechanism (previously unreported in neural scaffolds) suggests our platform could be adapted to target other CREB-dependent processes, such as synaptic plasticity in neurodegenerative diseases. For instance, in Alzheimer’s disease models, CREB activation rescues memory deficits by enhancing neurotrophin expression; our scaffold’s sustained AMP release might offer a localized, minimally invasive strategy to modulate such pathways.
Study limitations and future perspective
While promising, key translational challenges remain. First, in vivo validation is needed to assess scaffold degradation rates and immune responses, as murine models often underestimate human inflammatory reactions to xenogeneic AME. Second, AMP’s narrow therapeutic window (cytotoxicity at > 100 µg/ml) necessitates dose optimization for chronic use. Third, the scalability of freeze-drying protocols must be addressed for mass production. Future work should explore (1) large-animal neural defect models, (2) combinatorial delivery of AMP with neurotrophic factors (e.g., BDNF), and (3) 3D bioprinting to customize scaffold geometry for patient-specific lesions.
Conclusion
This study successfully developed a multifunctional hydrogel scaffold composed of Gel, AME, and AMP that integrates antibacterial efficacy with neurogenic potential. The scaffold’s interconnected porous structure (153.92 ± 12.5 μm pore size, 92.98% porosity) and hydrophilic surface (50–47° contact angle) provided an optimal microenvironment for hWJSC adhesion and proliferation, as evidenced by > 95% viability over six days (Figs. 1B, 3A). Its sustained AMP release (53.18% over 336 h) and antibacterial activity (93.06% inhibition of S. aureus) addressed critical limitations of existing neural scaffolds, which often lack dual functionality (Figs. 4C–E).
The scaffold’s 3D architecture and AMP delivery synergistically enhanced neural differentiation, driving significant upregulation of TUBB3 (24.08-fold), CREB (17.85-fold), and ENO2 (144.65-fold) at 14 days,outcomes surpassing 2D cultures (Figs. 5, 6). These results align with the Discussion’s emphasis on CREB pathway activation as a mechanistic bridge between scaffold design (e.g., porosity, sustained release) and biological performance.
This study establishes a proof-of-concept for multifunctional neural scaffolds but underscores the need for translational validation. Future work will (1) evaluate the hydrogel’s performance in rodent spinal cord injury models to assess functional recovery and immune response, (2) optimize AMP release kinetics using computational modeling to balance efficacy and safety, and (3) explore 3D bioprinting for patient-specific lesion customization. Success in these areas could position Gel-AME-AMP hydrogels as a first-line option for neural repair, reducing the $150 billion annual burden of neurodegenerative disease complications in aging populations.
Materials and methods
Cell culture of human WJMSCs
The human WJMSCs (Royan Stem Cell Technology Co, Iran) were cultured using liquid high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM-h) with 10% (v/v) fetal bovine serum (FBS), 10,000 units/ml of penicillin, and 10,000 μg/ml of streptomycin (P-S) (BioIdea, Tehran, Iran). The 75 cm2 tissue culture flasks of cells were maintained at a precise 37 °C with 5% CO2 in an incubator for cell growth and viability. The human WJMSCs at passages 3–4 were used to evaluate in vitro cell viability and neural differentiation.
Synthesis and sterilization of hydrogel scaffolds
Gel type A (derived from porcine skin at a concentration of 150 mg/ml; Sigma-Aldrich, USA) was dissolved in distilled water under magnetic stirring at 50 °C until completely dissolved. Subsequently, we added the AME (10 mg/ml, Lifecell, Tehran, Iran), 1% P-S antibiotics (v/v), and varying concentrations (0.5%, 1%, and 1.5% v/v) of the AMP drug (Xochem, Germany) to the Gel solution. The mixture was stirred until a homogeneous solution was obtained, then we transferred it to a microplate and incubated it at 4 °C, − 20 °C, and − 80 °C for 4, 12, and 24 h, respectively. The resulting hydrogel scaffolds containing Gel, Gel-AME, Gel-AME-P-S, and Gel-AME-P-S-AMP (at 0.5%, 1%, and 1.5% volume/volume) were lyophilized for 24 h to create 3D porous structures using a freeze dryer (Christ, Germany). Eventually, the lyophilized hydrogels were soaked in a 0.25% (v/v) glutaraldehyde solution (Glu, Sigma-Aldrich, USA) in distilled water and cross-linked for 6 h at room temperature. Thereon, the cross-linked hydrogel scaffolds containing Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP (at 0.5%, 1%, and 1.5% v/v) were rinsed six times with deionized water to remove excess Glu solution. Hydrogel scaffolds were sterilized for 2 h with P-S and washed (several times) with sterile phosphate-buffered saline (PBS; Merck, Germany). Before cell seeding, the hydrogel scaffolds were immersed in DMEM with 1% (v/v) P-S and incubated for 24 h at 37 °C.
Cell viability study
The cytotoxicity of the different groups of synthesized hydrogel scaffolds and various concentrations (0.5, 1, 10, 25, 50, 75, 100, and 200 μg/ml) of AMP dissolved in distilled water was evaluated utilizing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and AO/EB staining over 6 days after treatment. The human WJMSCs were cultured in flasks as monolayers to reach 70–80% confluence and trypsinized in an exponentially growing phase with 1 ml of 0.25% (v/v) trypsin–EDTA (BioIdea, Tehran, Iran). We carefully counted every cell using a hemocytometer. Next, 104 suspension cells were seeded on the surface of different hydrogel scaffolds and incubated for 2, 4, and 6 days. In another experiment, 104 suspension cells were seeded in 96-well plates and then incubated overnight. The human WJMSCs were treated with various concentrations of AMP and incubated for 2, 4, and 6 days. In the control groups, the untreated cells were grown only in the complete medium for 2, 4, and 6 days.
For the MTT assay, after 2, 4, and 6 days of different exposure times, the human WJMSCs were incubated with 100 μl of MTT (Sigma-Aldrich, USA) mixture at a concentration of 0.5 mg/ml in DMEM for 4 h at 37 °C and 95% humidity. After incubation, the medium was removed from each well, and 100 µl of Dimethyl sulfoxide (DMSO; Merck, Germany) was added to dissolve the resultant formazan crystals. We utilized the ELISA plate reader (Bio-Tek Instruments, Winooski, VT, USA) to determine the absorbance of dissolved formazan at a wavelength of 570 nm. The following equation estimated the cell viability percentage89:
Live/dead human WJMSCs were evaluated by dual AO/EB (Merck, Germany) fluorescent staining. For this assay, the supernatant was removed from each well and washed with PBS. Then, the cells were mixed with 100 µl of the dual AO/EB fluorescent staining solution (100 µg/ml of AO and 100 µg/ml of EB in PBS) for five minutes in the dark at room temperature. The dual AO/EB staining solution was removed and the human WJMSCs were rinsed with PBS. Finally, the fluorescence images of human WJMSCs were observed using fluorescent microscopy (Nikon, Tokyo, Japan).
Characterization of synthesized hydrogel scaffolds
FTIR and XRD analyses
Different synthesized hydrogel scaffolds were analyzed using FTIR spectroscopy (AVATAR, Thermo, USA) to determine their chemical combinations and functional groups at room temperature in the 400–4000 cm−1 wavelength range (4 cm⁻1 resolution, 32 scans). The XRD patterns that determined the crystal structures of the dried hydrogel scaffolds were recorded in a diffractometer with PAN analytical, X’pert PRO 2000 at 40 kV, 30 mA, Cu Kα radiation (λ = 1.5405 Å) with a scan rate of 2θ = 1°/min (10–80° range) (PW1730, PHILIPS, Poland).
FESEM
The FESEM microscopy was performed to study the morphology and structure of the hydrogel scaffold, cell adhesion, and neural-differentiated cells onto scaffolds. For FESEM observations, after 14 days of neural differentiation, the supernatant of the cell-scaffold samples was removed and rinsed with PBS. Then, the cell-scaffold samples were fixed for 60 min in 2.5% Glu dissolved in PBS at pH = 7.4. For dehydration, the cell-scaffold samples were rinsed with PBS and immersed in graded ethanol of 60–100% (twice) for 10 min (each time) and dried (room temperature). Finally, the cell-scaffold specimens were gold-coated and imaged by FESEM (TESCAN, VEGA3, Czech Republic) to study the cell adhesion and morphology of the differentiated cells. Images were acquired at 5–15 kV after gold sputtering.
Water contact angle measurement and water uptake ability
The surface wettability of the hydrogel scaffolds was assessed by measuring contact angles using the liquid drop shape (4 µl distilled water) method and a digital camera supplied with a contact angle goniometer (CAG-20 SE, JIKAN, Iran) at room temperature. For water uptake measurements, freeze-dried hydrogel scaffolds were weighed and absorbed in 2 ml of PBS (pH = 7.4) at 37 °C for 0, 0.5, 1, 3, 5, 7, 13, 16, 24, 48, 72, and 96 h until being saturated. After each time point, the hydrogel specimens were removed from PBS, dried on filter paper, and weighed again. The water uptake ability percentage of hydrogel scaffold samples (Wt) was determined (below equation)41.
Porosity measurement and in vitro biodegradation rate
Freeze-dried hydrogel scaffolds (Ws) and bottles filled with absolute ethanol (Merck, Germany) were weighed (W1). Freeze-dried hydrogel scaffolds were immersed in a bottle of absolute ethanol and maintained for 10 min. The weight of the bottle filled with absolute ethanol after immersing the freeze-dried hydrogels was measured as W2. After removing the absolute ethanol-impregnated hydrogel scaffolds, the weight of the bottle filled with absolute ethanol was recorded as W3. We calculated the porosity of the hydrogel scaffolds using Archimedes’ Principle90.
where ρe is the density of the ethanol.
The in vitro degradation of dried-hydrogel scaffolds was examined by absorbing them in 2 ml of PBS (pH = 7.4) at 37 °C for 0, 1, 2, 4, 6, 8, 10, 12, and 14 days. After immersion, the hydrogel scaffolds were carefully removed from the PBS mixture, dried at 50 °C for five hours, and weighed again. The remaining mass percent of hydrogels was calculated according to the following Eq. 91:
The weight of the hydrogel before and after immersion at each time point is represented by Mi and Mf, respectively.
Mechanical testing (tensile strength) and TGA
We tested the mechanical properties of the Gel-Glu-AME-P-S-AMP 1% hydrogel scaffold with a length of 35 mm, width of 0.8 mm, and thickness of 0.5 mm using a Universal Testing Machine (Uni-axial, STM-20, SANTAM, Iran) at a crosshead speed of 10 mm/min and room temperature. We conducted the TGA analysis of hydrogel scaffolds (Gel-Glu and Gel-Glu-AME-P-S-AMP 1%) using the TGA STA6000 instrument (PerkinElmer Ltd., Buckinghamshire, UK) from 40 to 300 °C. Also, we maintained the temperature ramp (10 °C/min) and tested the hydrogel scaffolds in a flowing nitrogen atmosphere (N2) at a flow rate of 20 ml min−1.
Study of drug release (in vitro)
The release of AMP from hydrogel scaffolds (Gel-Glu-AME-P-S and Gel-Glu-AME-P-S-AMP 1%) was estimated utilizing a nanodrop spectrophotometer set (ND-1000, Wilmington, Delaware, USA) at 271 nm. The hydrogel scaffolds were submerged in PBS at physiological conditions (37 °C and pH = 7.4) for 14 days. We removed 2 μl of the AMP release medium and replaced it with 2 μl of new PBS at regular intervals. The various concentrations of the standard AMP solution in distilled water were used to make the calibration curve equation for determining the release of AMP in the hydrogel. Finally, the amount of AMP release percentage was calculated using Eq. 92.
Antibacterial activity
The antibacterial activity of the hydrogel scaffolds (Gel-Glu and Gel-Glu-AME-P-S-AMP 1%) was evaluated against Pseudomonas aeruginosa (P. aeruginosa, PTCC 1074; Gram-negative bacteria) and Staphylococcus aureus (S. aureus, ATCC 33591; Gram-positive bacteria) (Iranian Biological Resource Center, Tehran, Iran) using a minimum inhibitory concentration (MIC) assay. We cultured the bacteria in a 15 ml falcon tube holding 2 ml of sterile liquid Mueller–Hinton (M-H) broth at 37 ºC for 24 h. For MIC assay, 5 μl of bacterial suspension with a concentration of 0.5 McFarland standard (1.5 × 108 Colony Forming Unit, or CFU) was added to a 96-well microtiter plate containing 100 μl of M–H broth. Then, the bacterial suspension was treated with hydrogel scaffolds. Negative, blank, and positive controls were prepared using bacterial suspension, M-H medium, and P/S antibiotics. The plate was then incubated for 2, 6, and 24 h at 37 ºC. Optical density (OD) at 600 nm was quantified using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The inhibition percentage is calculated using the following formula68:
Neural differentiation of human WJMSCs in 2D and 3D cultures
The human WJMSCs (3–4 passages) were cultured on six different groups, including ((Gel-Glu, Gel-Glu-AME, Gel-Glu-AME-P-S, and Gel-Glu-AME-P-S-AMP 1%) hydrogels, treated with an optimum concentration (10 μg/mL) of AMP, and control). To induce neural differentiation, the samples were treated using DMEM-h supplemented with 2.5 mM forskolin, 2.5 Μm retinoic acid, 50 μg/ml indomethacin (Sigma Aldrich, USA), and varying FBS concentrations at 5% CO2 and 37 °C. We used three different media with varying FBS concentrations over 14 days to induce neural differentiation. The FBS concentration was 10% for the first 1–4 days, 5% for 5–10 days, and 2% for 11–14 days. About 50% of the neural differentiation medium was renewed every 48 h. Immunocytochemistry and real-time RT-PCR analyzed the expression levels of protein and genes in the differentiated cells.
Immunocytochemistry
After 14 days of differentiation, the cells were washed with PBS (pH = 7.4) and fixed with 4% paraformaldehyde (Sigma-Aldrich, USA)/PBS for 20 min at 4 °C and 5 min at room temperature. The cells were washed with cold PBS and treated with Triton-X100 (Sigma-Aldrich, Oakville, Canada; 0.4% (v/v); 10 min at room temperature) to increase their permeability. After rinsing with PBS, the cells were blocked thoroughly with a mixture of goat serum (Sigma-Aldrich, Oakville, Canada)/PBS-Tween 20 (5% (v/v)) for 45 min at room temperature. The primary antibodies of Gamma-enolase (1:100 dilution), β-tubulin III (1:50 dilution), and MAP-2; 1:300 dilution) (all from Santa Cruz Biotechnology, Inc., Dallas, Texas, United States) in BSA (Sigma-Aldrich, Oakville, Canada)/PBS, 0.2% (v/v) was added to the cells and incubated overnight at 4 °C. The next day, the cells were washed with PBS-Tween 20 (0.1% (v/v); 5 min) and rinsed with BSA/PBS (1% v/v; 30 min at room temperature). We added secondary antibodies of FITC-conjugated goat anti-mouse IgG (in BSA/PBS 0.2% (v/v); Invitrogen, USA) for 3 h at room temperature. Finally, after washing with PBS, DAPI (4′,6-diamidino-2-phenylindole) (Sigma-Aldrich, St Louis, MO, USA) staining solution (10% (v/v)) to stain the nucleus of differentiated cells was added, and the neural differentiated cells were recorded under a fluorescent microscope (Olympus, Japan).
Analysis of gene expression
The expression of neural critical genes (MAP-2, TUBB3, ENO2, and GFAP) as well as CREB as a transcription factor was analyzed using real-time RT-PCR at the 7th and 14th days of differentiation. The materials were from Tehran Cavosh Clon, Tehran, Iran. Ribonucleic acid (RNA) was first extracted from differentiated cells using the TRIzol reagent (RiboEX, geneAll). The RNA concentration was calculated by reading absorbance in a nanodrop. We synthesized the complementary DNA (cDNA) using the cDNA Synthesis Kit (Parstous, Iran). Real-time RT-PCR was performed for 40 cycles using gene-specific primers listed in Table 2 and Maxima RealQ Plus 2 × Master Mix Green (High Rox) (Ampliqon, Denmark) with an Applied Biosystems System (ABS, Waltham, MA, USA). The ΔΔCt formula was used to analyze the expression level of target genes and detect beta-actin (β-actin) as a housekeeping gene.
Table 2.
Primer sequences used for real-time RT-PCR.
| Gene | Primers F (top), R (bottom) | Product size (bp) |
|---|---|---|
| β-actin |
F: TTCTACAATGAGCTGCGTGTGG R: GTGTTGAAGGTCTCAAACATGAT |
119 |
| MAP-2 |
F: AGTTCCAGCAGCGTGATG R: CATTCTCTCTTCAGCCTTCTC |
97 |
| ENO2 |
F: GGAGAACAGTGAAGCCTTGG R: GGTCAAATGGGTCCTCAATG |
238 |
| GFAP |
F: GCAGACCTTCTCCAACCT G R: ACTCCTTAATGACCTCTCCATC |
127 |
| TUBB3 |
F: GATCGGAGCCAAGTTCTG R: GTCCATCGTCCCAGGTTC |
177 |
| CREB |
F: CCACTGTAACGGTGCCAACT R: GCTGCATTGGTCATGGTTAATGT |
217 |
Significance value bold.
Statistical study
Data are presented as mean ± standard deviation (SD) from six independent replicates (n = 6). For comparisons across multiple groups (e.g., hydrogel compositions, AMP concentrations), two-way ANOVA with Tukey’s post-hoc test was applied to account for time-dependent variables (e.g., days 2, 4, 6). One-way ANOVA with Tukey’s post-hoc was used for single-factor comparisons (e.g., porosity, and AMP release). For gene expression fold changes (ΔΔCt method), two-way ANOVA with Tukey’s post-hoc test and Sidak’s correction compared differentiated groups to controls. Two-way ANOVA with Tukey’s post-hoc test was used for antibacterial activity, water uptake, and biodegradation tests. Statistical significance was set at p < 0.05. All analyses were performed using GraphPad Prism 8.0.2 (USA), following guidelines for biomedical data analysis93–96.
Acknowledgements
We would like to thank the guidance and support from Islamic Azad University, Tehran Medical Branch. Also, some illustrations featured in the graphical abstract are obtained from Biorender.com under an individual subscription.
Author contributions
A.S. S.J. Investigation, Data curation, Data analysis, Software, Writing-original draft. H.A. Methodology (Synthesis of hydrogel), Data validation, Review & Editing. Z.J. Methodology, Formal Analysis, Data validation, Review & Editing. S.S. Methodology, Review & Editing. A.S. Supervision, Project administration, Conceptualization, Review & Editing, Methodology, Data validation.
Data availability
Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This project was evaluated by the Research Ethics Committees of the Islamic Azad Tehran Medical Sciences University - Pharmacy and Pharmaceutical Branches Faculty and has Approval ID: IR.IAU.PS.REC.1402.301.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siafaka, P. I. et al. Protein and gene delivery systems for neurodegenerative disorders: where do we stand today?. Pharmaceutics14, 2425 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei, M., Yang, Z., Li, S. & Le, W. Nanotherapeutic and stem cell therapeutic strategies in neurodegenerative diseases: A promising therapeutic approach. Int. J. Nanomed.18, 611–626 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabouni, N. et al. Role of curcumin and its nanoformulations in the treatment of neurological diseases through the effects on stem cells. J. Drug Target31, 243–260 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Pina, S. et al. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials12, 1824 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shariati, A. et al. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: a promising frontier. Eur. J. Cell Biol.99, 151097 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Lija, S. & Eswari, S. Wharton jelly mesenchymal stem cells: A potential candidate for neuronal trans-differentiation. (2023).
- 7.Hosseini, S. M. et al. Differentiation of Wharton’s jelly mesenchymal stem cells into neurons in alginate scaffold. Neural Regen. Res.10, 1312–1316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, Y.-Y. et al. Gelatin-based hydrogel for three-dimensional neuron culture application. ACS Omega8, 45288–45300 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Politrón-Zepeda, G. A., Fletes-Vargas, G. & Rodríguez-Rodríguez, R. Injectable hydrogels for nervous tissue repair—a brief review. Gels10, 190 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma, X. et al. Design and fabrication of polymeric hydrogel carrier for nerve repair. Polymers14, 1549 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, Z.-Y. et al. Fresh human amniotic membrane effectively promotes the repair of injured common peroneal nerve. Neural Regen. Res.14, 2199–2208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Seedi, H. R. et al. Gelatin nanofibers: Recent insights in synthesis, bio-medical applications and limitations. Heliyon9, e16228 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, S. et al. Three-dimensional bioprinting sodium alginate/gelatin scaffold combined with neural stem cells and oligodendrocytes markedly promoting nerve regeneration after spinal cord injury. Regen. Biomater.9, rbac038 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajkumar, M. et al. Gelatin/polyethylene glycol-loaded magnesium hydroxide nanocomposite to attenuate acetylcholinesterase, neurotoxicity, and activation of GPR55 protein in rat models of Alzheimer’s disease. J. Chem. Neuroanat.133, 102337 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Li, J. et al. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int. J. Biol. Macromol.187, 200–213 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Hao, X. et al. Amniotic membrane extract-enriched hydrogel augments the therapeutic effect of menstrual blood-derived stromal cells in a rat model of intrauterine adhesion. Biomater. Adv.142, 213165 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Farzan, R. et al. Effects of amniotic membrane extract and deferoxamine on angiogenesis in wound healing: an in vivo model. J. Wound Care27, S26–S32 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Kothari, C. R. et al. Use of amnion as a graft material in vestibuloplasty: a clinical study. Br. J. Oral Maxillofac. Surg.50, 545–549 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Ghiasi, M., Hashemi, M., Salimi, A., Tavallaie, M. & Aghamollaei, H. Isolation, culture, optimization and validation of human corneal stromal keratocytes from discarded corneal tissue. J. Appl. Biotechnol. Rep.10, 910–917 (2023). [Google Scholar]
- 20.Asl, N. S. et al. Amniotic membrane extract eye drop promotes limbal stem cell proliferation and corneal epithelium healing. Cell J. (Yakhteh)20, 459 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiménez-Acosta, M. A., Hernández, L. J. R., Cristerna, M. L. P., Tapia-Ramírez, J. & Meraz-Ríos, M. A. Neuronal differentiation protocols of mesenchymal stem cells. Adv. Biosci. Biotechnol.13, 15–71 (2022). [Google Scholar]
- 22.An, J. et al. Modulatory Effects of natural products on neuronal differentiation. Neuropsychiatry8, 1593–1611 (2018). [Google Scholar]
- 23.Abdelaziz, T. S. A., Abdou, K., Mahmoud, N. Y. & Ibrahim, I. M. Trans-nasal sphenopalatine ganglion block versus intravenous aminophylline injection for treating postdural puncture headache after cesarean section under spinal anesthesia. J. Cell. Mol. Anesth.8, 265–273.
- 24.Klimovic, S. et al. Aminophylline induces two types of arrhythmic events in human pluripotent stem cell–derived cardiomyocytes. Front. Pharmacol.12, 789730 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aghabiklooei, A. The efficacy of aminophylline on raising consciousness in benzodiazepines-intoxicated patients. Asia Pacific J. Med. Toxicol.7, 17–19 (2018). [Google Scholar]
- 26.Kong, Y., Wang, D., Wei, Q. & Yang, Y. Nerve decellularized matrix composite scaffold with high antibacterial activity for nerve regeneration. Front. Bioeng. Biotechnol.9, 840421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, L. et al. Advancements in GelMA bioactive hydrogels: Strategies for infection control and bone tissue regeneration. Theranostics15, 460 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manrui, L. et al. Aminophylline targets miR-128-3p/Slc7a11 axis to attenuate neuronal ferroptosis after traumatic brain injury. Cell. Mol. Life Sci.82, 1–23 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, J. et al. Near infrared photothermal conversion materials: mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B9, 7909–7926 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Landeira, B. S. et al. Activity-independent effects of CREB on neuronal survival and differentiation during mouse cerebral cortex development. Cereb. Cortex28, 538–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majidnia, E., Ahmadian, M., Salehi, H. & Amirpour, N. Development of an electrospun poly (ε-caprolactone)/collagen-based human amniotic membrane powder scaffold for culturing retinal pigment epithelial cells. Sci. Rep.12, 6469 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahmani, S., Olad, A. & Rahmani, Z. Preparation of self-healable nanocomposite hydrogel based on Gum Arabic/gelatin and graphene oxide: Study of drug delivery behavior. Polym. Bull.80, 4117–4138 (2023). [Google Scholar]
- 33.Lin, H.-L., Hsu, P.-C. & Lin, S.-Y. Theophylline–citric acid co-crystals easily induced by DSC–FTIR microspectroscopy or different storage conditions. Asian J. Pharm. Sci.8, 19–27 (2013). [Google Scholar]
- 34.Venezia, V. et al. Adding humic acids to gelatin hydrogels: A way to tune gelation. Biomacromol23, 443–453 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramasamy, P. et al. Bio-fabrication of human amniotic membrane Zinc oxide nanoparticles and the wet/dry HAM dressing membrane for wound healing. Front. Bioeng. Biotechnol.9, 695710 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bobrovs, R., Seton, L. & Dempster, N. The reluctant polymorph: investigation into the effect of self-association on the solvent mediated phase transformation and nucleation of theophylline. CrystEngComm17, 5237–5251 (2015). [Google Scholar]
- 37.Dave, K. & Gomes, V. G. Interactions at scaffold interfaces: Effect of surface chemistry, structural attributes and bioaffinity. Mater. Sci. Eng., C105, 110078 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Zhu, N. et al. Multistructured hydrogel promotes nerve regeneration. Mater. Today Adv.21, 100465 (2024). [Google Scholar]
- 39.Chen, L. et al. Gelatin nanofiber-reinforced decellularized amniotic membrane promotes axon regeneration and functional recovery in the surgical treatment of peripheral nerve injury. Biomaterials300, 122207 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Wang, C.-Y. et al. Polymeric gelatin scaffolds affect mesenchymal stem cell differentiation and its diverse applications in tissue engineering. Int. J. Mol. Sci.21, 8632 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azizipour, E. et al. A novel hydrogel scaffold contained bioactive glass nanowhisker (BGnW) for osteogenic differentiation of human mesenchymal stem cells (hMSCs) in vitro. Int. J. Biol. Macromol.174, 562–572 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Yao, M. et al. Dual-enzymatically cross-linked gelatin hydrogel enhances neural differentiation of human umbilical cord mesenchymal stem cells and functional recovery in experimental murine spinal cord injury. J. Mater. Chem. B9, 440–452 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Distler, T. et al. Neuronal differentiation from induced pluripotent stem cell-derived neurospheres by the application of oxidized alginate-gelatin-laminin hydrogels. Biomedicines9, 261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.KarbalaeiMahdi, A. et al. Neural differentiation of human induced pluripotent stem cells on polycaprolactone/gelatin bi-electrospun nanofibers. Mater. Sci. Eng., C78, 1195–1202 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Sousa, J. P. et al. Amniotic membrane-derived multichannel hydrogels for neural tissue repair. Adv. Healthcare Mater.13, 2400522 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim, Y. J. et al. Protective effect of aminophylline against cigarette smoke extract-induced apoptosis in human lung fibroblasts (MRC-5 Cells). Basic Clin. Pharmacol. Toxicol.109, 17–22 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Kosutova, P., Mikolka, P., Mokra, D. & Calkovska, A. Anti-inflammatory activity of non-selective PDE inhibitor aminophylline on the lung tissue and respiratory parameters in animal model of ARDS. J. Inflamm.20, 10 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunasekaran, S., Sankari, G. & Ponnusamy, S. Vibrational spectral investigation on xanthine and its derivatives—theophylline, caffeine and theobromine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.61, 117–127 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Kadkhodaie-Elyaderani, A. et al. Medicated scaffolds prepared with hydroxyapatite/streptomycin nanoparticles encapsulated into polylactide microfibers. Int. J. Mol. Sci.23, 1282 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, Q., Cao, C., Yan, F. & Sheng, Z. Synthesis of chiral penicillamine-coated gold nanoparticles and effect on PC12 cells for the treatment of Alzheimer’s disease. J. Cluster Sci.31, 1071–1075 (2020). [Google Scholar]
- 51.Kumar, M., Sodhi, K. K., Singh, P., Agrawal, P. K. & Singh, D. K. Synthesis and characterization of antibiotic-metal complexes [FeCl3 (L1) 2H2O and Ni (NO3) 2 (L2) 2H2O] and enhanced antibacterial activity. Environ. Nanotechnol., Monit. Manag.11, 100209 (2019). [Google Scholar]
- 52.Goodarzi, H., Jadidi, K., Pourmotabed, S., Sharifi, E. & Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol.126, 620–632 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Suamte, L., Tirkey, A., Barman, J. & Babu, P. J. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf.1, 100011 (2023). [Google Scholar]
- 54.Hasanzadeh, E. et al. Injectable hydrogels in central nervous system: Unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater. Today Bio20, 100614 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jurga, M. et al. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomater.32, 3423–3434 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Yuan, N. et al. Neural stem cell transplantation in a double-layer collagen membrane with unequal pore sizes for spinal cord injury repair. Neural Regen. Res.9, 1014–1019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghasemi-Mobarakeh, L., Prabhakaran, M. P., Morshed, M., Nasr-Esfahani, M.-H. & Ramakrishna, S. Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials29, 4532–4539 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Fekrazad, R., Tondnevis, F. & Abolhasani, M. M. In-vitro evaluation of novel polycaprolactone/chitosan/carbon nano tube scaffold for tissue regeneration. J. Biomed. Phys. Eng.12, 395 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madaninasab, P., Mohammadi, M. & Labbaf, S. Electroconductive gelatin/alginate/graphene hydrogel based scaffold for neural tissue repair. Macromol. Mater. Eng.310, 2400229 (2025). [Google Scholar]
- 60.Chen, T. et al. Loading neural stem cells on hydrogel scaffold improves cell retention rate and promotes functional recovery in traumatic brain injury. Mater. Today Bio19, 100606 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahin, D. A., Son, C. D. & Hasirci, V. Fabrication and characterization of pHEMA hydrogel conduit containing GelMA-HaMA IPN for peripheral nerve regeneration. Explor. BioMat-X1, 34–57 (2024). [Google Scholar]
- 62.Borschel, G. H., Kia, K. F., Kuzon, W. M. Jr. & Dennis, R. G. Mechanical properties of acellular peripheral nerve. J. Surg. Res.114, 133–139 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Gao, Y., Zhang, T.-L., Zhang, H.-J., Gao, J. & Yang, P.-F. A promising application of injectable hydrogels in nerve repair and regeneration for ischemic stroke. Int. J. Nanomed.19, 327–345 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bagher, Z. et al. Conductive hydrogel based on chitosan-aniline pentamer/gelatin/agarose significantly promoted motor neuron-like cells differentiation of human olfactory ecto-mesenchymal stem cells. Mater. Sci. Eng., C101, 243–253 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Lee, C.-S. & Hwang, H. S. Starch-based hydrogels as a drug delivery system in biomedical applications. Gels9, 951 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ooi, S. Y., Ahmad, I. & Amin, M. C. I. M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crops Prod.93, 227–234 (2016). [Google Scholar]
- 67.Velnar, T., Kocivnik, N. & Bosnjak, R. Clinical infections in neurosurgical oncology: An overview. World J. Clin. Cases11, 3418 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamer, T. M. et al. Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin–streptomycin for wound dressing applications. Sci. Rep.11, 3428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kabataş, S. et al. Wharton’s jelly-derived mesenchymal stem cell transplantation in a patient with hypoxic-ischemic encephalopathy: a pilot study. Cell Transplant.27, 1425–1433 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milczarek, O. et al. Comparative analysis of the results of stroke treatment with multiple administrations of Wharton’s Jelly mesenchymal stem cells-derived HE-ATMP and standard conservative treatment: Case series study. Cell Transplant.32, 09636897231195145 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krupa, P. et al. The effect of human mesenchymal stem cells derived from Wharton’s jelly in spinal cord injury treatment is dose-dependent and can be facilitated by repeated application. Int. J. Mol. Sci.19, 1503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng, T. et al. Wharton’s jelly transplantation improves neurologic function in a rat model of traumatic brain injury. Cell. Mol. Neurobiol.35, 641–649 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hour, F. Q. et al. Magnetic targeted delivery of the SPIONs-labeled mesenchymal stem cells derived from human Wharton’s jelly in Alzheimer’s rat models. J. Control. Release321, 430–441 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Harada, A., Teng, J., Takei, Y., Oguchi, K. & Hirokawa, N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol.158, 541–549 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soltani, M. H. et al. Microtubule-associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. Am. J. Pathol.166, 1841–1850 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tischfield, M. A. et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell140, 74–87 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie, J., Du, R., Li, Q. & Li, L. The relationship between neuron-specific enolase, high sensitivity C reactive protein, and diabetic peripheral neuropathy in Chinese patients with type 2 diabetes: A prospective nested case–control analysis. Int. J. Diabetes Dev. Countries44, 190–199 (2024). [Google Scholar]
- 78.Mamber, C. et al. GFAPδ expression in glia of the developmental and adolescent mouse brain. PLoS ONE7, e52659 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salmanvandi, M., Haramshahi, S. M. A., Mansouri, E. & Alizadeh, A. The effect of rosmarinic acid on neural differentiation of wartons jelly-derived mesenchymal stem cells in two-dimensional and three-dimensional cultures using chitosan-based hydrogel. Basic Clin. Neurosci.14, 117 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cruz, E. M. et al. A gelatin methacrylate-based hydrogel as a potential bioink for 3D bioprinting and neuronal differentiation. Pharmaceutics15, 627 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin, C. A. et al. Adipose tissue derived stromal cells in a gelatin-based 3D matrix with exclusive ascorbic acid signalling emerged as a novel neural tissue engineering construct: an innovative prototype for soft tissue. Regen. Biomater.9, rbac031 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asghari, N., Irani, S., Pezeshki-Moddaress, M., Zandi, M. & Mohamadali, M. Neuronal differentiation of mesenchymal stem cells by polyvinyl alcohol/Gelatin/crocin and beta-carotene. Mol. Biol. Rep.49, 2999–3006 (2022). [DOI] [PubMed] [Google Scholar]
- 83.Xu, Y. et al. Aminophylline targets miR-128–3p/Slc7a11 axis to attenuate neuronal ferroptosis after traumatic brain injury. Cell. Mol. Life Sci.91, 1637 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang, H., Xu, J., Lazarovici, P., Quirion, R. & Zheng, W. cAMP response element-binding protein (CREB): A possible signaling molecule link in the pathophysiology of schizophrenia. Front. Mol. Neurosci.11, 255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim, G., Choe, Y., Park, J., Cho, S. & Kim, K. Activation of protein kinase A induces neuronal differentiation of HiB5 hippocampal progenitor cells. Mol. Brain Res.109, 134–145 (2002). [DOI] [PubMed] [Google Scholar]
- 86.Tio, M., Tan, K. H., Lee, W., Wang, T. T. & Udolph, G. Roles of db-cAMP, IBMX and RA in aspects of neural differentiation of cord blood derived mesenchymal-like stem cells. PLoS ONE5, e9398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohammed, B. G., Abd, A. A. & Mustafa, N. Molecular study of theophylline effect on the neural stem cells differentiation in vitro.
- 88.Paldino, E. et al. Induction of dopaminergic neurons from human Wharton’s jelly mesenchymal stem cell by forskolin. J. Cell. Physiol.229, 232–244 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Shahrousvand, M., Sadeghi, G. M. M., Shahrousvand, E., Ghollasi, M. & Salimi, A. Superficial physicochemical properties of polyurethane biomaterials as osteogenic regulators in human mesenchymal stem cells fates. Colloids Surf., B156, 292–304 (2017). [DOI] [PubMed] [Google Scholar]
- 90.Norouz, F., Halabian, R., Salimi, A. & Ghollasi, M. A new nanocomposite scaffold based on polyurethane and clay nanoplates for osteogenic differentiation of human mesenchymal stem cells in vitro. Mater. Sci. Eng., C103, 109857 (2019). [DOI] [PubMed] [Google Scholar]
- 91.Shahrousvand, M., Ghollasi, M., Zarchi, A. A. K. & Salimi, A. Osteogenic differentiation of hMSCs on semi-interpenetrating polymer networks of polyurethane/poly (2-hydroxyethyl methacrylate)/cellulose nanowhisker scaffolds. Int. J. Biol. Macromol.138, 262–271 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Ghahremani-Nasab, M. et al. Synergistic effect of chitosan-alginate composite hydrogel enriched with ascorbic acid and alpha-tocopherol under hypoxic conditions on the behavior of mesenchymal stem cells for wound healing. Stem Cell Res. Ther.14, 326 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Motulsky, H. J. & Brown, R. E. Detecting outliers when fitting data with nonlinear regression–a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics7, 1–20 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKight, P. E. & Najab, J. Kruskal‐wallis test. The corsini encyclopedia of psychology, 1–1 (2010).
- 95.Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods39, 175–191 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci.14, 365–376 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request.