Abstract
Background
KRAS exon 2 mutations are highly prevalent in human malignancies, making them attractive targets for detection and monitoring in cell-free DNA (cfDNA) of cancer patients. Drop-off assays designed for digital polymerase chain reaction (ddPCR drop-off) span entire mutational hotspots and detect any mutated allele within the covered region, overcoming a major limitation of mutation-specific ddPCR assays. We therefore set out to develop a novel KRAS codon 12/13 ddPCR drop-off assay for the robust, highly sensitive and specific detection of KRAS exon 2 hotspot mutations in cfDNA.
Methods
We designed, optimized and extensively validated a KRAS codon 12/13 ddPCR drop-off assay. We compared assay performance to a commercially available KRAS multiplex assay. For clinical validation, we analyzed plasma samples collected from patients with KRAS-mutated gastrointestinal malignancies.
Results
Limit of detection of the newly established ddPCR drop-off assay was 0.57 copies/µL, limit of blank was 0.13 copies/µ. The inter-assay precision (r2) was 0.9096. Our newly developed KRAS ddPCR drop-off assay accurately identified single nucleotide variants in 35/36 (97.2%) of circulating tumor DNA-positive samples from the patient validation cohort. Assay cross-validation showed that the newly established KRAS codon 12/13 ddPCR drop-off assay outperformed a commercially available KRAS multiplex ddPCR assay in terms of specificity. Moreover, the newly developed assay proved to be suitable for multiplexing with mutation-specific probes.
Conclusion
We developed and clinically validated a highly accurate ddPCR drop-off assay for KRAS exon 2 hot-spot detection in cfDNA with broad applicability for clinic and research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13000-025-01637-y.
Keywords: Liquid biopsy, Droplet digital polymerase chain reaction (ddPCR), KRAS, Drop-off assay, Cell-free DNA (cfDNA), Circulating-tumor DNA (ctDNA), Precision medicine
Background
The analysis of circulating tumor DNA (ctDNA) in the plasma of cancer patients has emerged into a promising diagnostic tool of precision oncology [1–9]. A broad range of technologies has been developed to detect and quantify tumor-derived alterations in cell-free DNA (cfDNA) with high sensitivity and specificity [10–19]. Moreover, multiple studies have highlighted the potential of ctDNA analyses for a wide range of applications, including early cancer detection, tumor molecular profiling, detection and monitoring of minimal residual disease (MRD), as well as the analysis of treatment response dynamics [4, 7, 20–30]. One of the key advantages of liquid biopsies (LBs) is their non-invasiveness, which enables longitudinal analyses with high temporal resolution, making dynamic response assessment during cancer treatment a highly attractive aspect of personalized cancer therapy [23, 31–33]. Furthermore, LBs can provide unique insights into tumor molecular heterogeneity and its evolution during cancer treatment, thereby minimizing the sampling bias of tissue biopsies [34]. Several interventional clinical trials are currently assessing the potential of serial LBs to guide cancer treatment, with focus on MRD detection and selection of adjuvant treatment based on cfDNA analysis [6, 35].
A key technical challenge for cfDNA analysis is the often low tumor fraction in cfDNA and low concentration of cfDNA [6]. Established methods used to analyse cfDNA are real-time PCR, digital PCR, and next generation sequencing [10, 12, 14, 15, 17, 19, 20, 36–41]. In a cross-comparison, digital droplet PCR (ddPCR) stands out with high specificity (100%), relatively high sensitivity (66.7–90%), low limit of detection (LoD) (0.01%), and moderate cost [11, 12, 37]. Droplet digital polymerase chain reaction accurately detects and quantifies very low levels of target DNA by counting individual mutant molecules without the need for a reference standard. This allows the detection of tumor mutations in plasma samples with low tumor fraction in low concentrations of cfDNA. Additionally, ddPCR is characterized by excellent reproducibility, with high precision and low variability across samples, making it a reliable tool for clinical diagnostic applications. ddPCR is therefore an ideal tool for the analysis of cfDNA fragments in the bloodstream of cancer patients, particularly in cases where the tumor fraction and cfDNA concentration are low.
However, when studying a hotspot region with a wide range of potential nearby mutations, the available fluorophores in a ddPCR system can become a limiting factor. As a result, most assays are only able to detect a limited number of mutations per reaction, limiting the real-life applicability for the clinical management of cancer patients [42–45]. To overcome these limitations, we designed, established, and optimized a KRAS drop-off assay detecting all genomic alterations within codon 12 (G12) and 13 (G13) of exon 2. A drop-off assay is based on the principle of detecting mismatches in DNA sequences using two fluorescent probes, both complementary to the wild-type sequence, resulting in a double positive signal. When there is a genetic variation or mutation, only one probe can bind to the target DNA, leading to a reduced or ‘dropped-off’ fluorescent signal. This indicates the presence of the genetic alteration, allowing for precise mutation detection [46, 47]. In clinical practice, such a drop-off assay, while not replacing state-of-the-art molecular diagnostics assay, could be particularly valuable as a broadly applicable molecular monitoring tool during cancer treatment. We performed both technical and clinical validation of our assay to assess its utility in detecting KRAS mutations. Technical validation involved re-analyzing samples from our already established pancreatic cancer (pancreatic ductal adenocarcinoma, PDAC) patient cohort, as KRAS mutations occur in 90–95% of all cases of PDAC [10, 18, 48]. Clinical validation was subsequently carried out using samples collected from patients with gastrointestinal malignancies undergoing adjuvant or palliative systemic treatment at our institution.
Methods
Human blood samples
All patients gave written informed consent for collection and analysis of blood samples. Local institutional review boards (IRB) in Freiburg (EK-Freiburg project number 46/18) and Zurich (BASEC-Nr. 2020-01 104) approved all relevant procedures and analyses. Venous blood samples from metastasized PDAC patients for technical validation of the KRAS drop-off assay were previously collected at the university hospital Freiburg [49]. For clinical validation numerous new plasma samples of patients with gastrointestinal malignancies were collected using commercially available cfDNA blood collection tubes (Ruwag, Bettlach, Switzerland, cat. no. 218997) at the university hospital Zurich and analyzed in its laboratories. Samples from both cohorts were collected prior to therapy and at various time points during the course of disease, as part of routine clinical follow-ups. Oncological treatment was performed as per standard of care and blinded to (mutant KRAS) cfDNA results.
CfDNA extraction
Plasma was extracted through two subsequent centrifugation steps, as previously described, and frozen at -80 °C until cfDNA extraction [49]. cfDNA was then extracted from each 2-4 ml plasma following the SEP/SBS protocol of the PME-free circulating DNA extraction kit (Analytik Jena, cat. no. 845-IR-0003050) according to manufacturer’s instructions and as previously described [49]. DNA was stored at -20 °C until cfDNA quantification with the Qubit™ 4 fluorometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA, cat. no. Q32866). DNA concentrations from up to 5 mL of plasma typically ranged from 0.1 to 20 ng/µL. To prevent damage to the ddPCR droplets, a maximum of 60 ng per well was applied. cfDNA quantification was a crucial step in the workflow to ensure the appropriate input amount for ddPCR, maintaining assay sensitivity and preventing droplet overloading. Moreover, quantifying cfDNA provided an initial quality control measure for the plasma samples, allowing the identification of samples with insufficient cfDNA yield, which may indicate poor plasma quality or improper handling.
Probes and primers
Locked nucleic acid (LNA)-based probes and corresponding primers for the KRAS G12/G13 drop-off assay were designed using Beacon Designer v.8.20 software (Premier Biosoft, Palo Alto, California, USA) and manufactured by Integrated DNA Technologies (IDT, Inc., Coralville, Iowa, USA). The placement of LNA bases was chosen to enhance specificity at the G12/G13 loci rather than relying solely on their ability to increase melting temperature (Tm). This approach ensures that the probes effectively discriminate between mutant and wild-type sequences, thereby improving assay sensitivity and reducing the likelihood of nonspecific binding. The wild type (WT) or drop-off probe, spanning the mutation hotspot, was labelled with hexachlorofluorescein (HEX), the reference probe with 6-carboxyfluorescein (FAM). Sequences of primer and probes are listed in Table 1.
Table 1.
Sequences of primers and probes of the KRAS G12/G13 drop-off assay
| Primer | ||
|---|---|---|
| Gene | Direction | |
| KRAS | Forward | 5‘ – CAA GAT TTA CCT CTA TTG TTG GA – 3‘ |
| Reverse | 5‘ – GTG TGA CAT GTT CTA ATA TAG TC – 3‘ | |
| Probes | ||
| Gene | Target | |
| KRAS | drop-off | 5‘ – /5HEX/CTA C + GC C + AC C + AG C + TC CA/3IABkFQ/ – 3‘ |
| Reference | 5‘ – /56-FAM/ATT AG + C TG + T AT + C GT + C AAG G/3IABkFQ/ – 3‘ | |
HEX, Hexachloro-Flouresceine; FAM, 6-Carboxyflourescein; 3IABkFQ, 3’ Iowa Black® FQ, Qeuncher from IDT
Design of the KRAS drop-off ddPCR assay
For the design of the KRAS drop-off ddPCR assay, we utilized the high affinity required for TaqMan probe hybridization, meaning that even a single altered base in the DNA sequence can prevent the probe from binding. Therefore, we designed a 17-bp long, HEX-labeled, LNA-based probe that spans the mutation hotspot within exon 2, codons 12/13 of the KRAS gene, specifically complementary to the wild-type sequence (drop-off probe, Table 1). Additionally, we designed a 19-bp long LNA-based FAM-labeled probe (Reference probe) complementary to the KRAS wild-type sequence to quantify the overall number of molecules present in the sample (Table 1). This reference probe is located within the same amplicon, 9 bp upstream of the drop-off probe, but does not overlap with the target region. Consequently, if there is no alteration in KRAS codons 12/13 of exon 2, wild-type molecules yield a double positive (HEX + FAM) signal. In the presence of a mutation-induced mismatch, the suboptimal hybridization of the drop-off probe leads to a reduced HEX signal, shifting the droplet cloud towards a solely FAM-positive population. This shift is proportional to the percentage of mutant molecules. The reference probe thus serves as an internal control, confirming that the cfDNA fragment of interest was present in the sample and that the changes observed with the drop-off probe are due to the biological process being studied (e.g., a mutation) rather than errors or inconsistencies in the experiment. Overall, we designed the probes and primers to be as short as possible to account for the fragmented nature of ctDNA, which results from nucleosomal degradation and apoptotic processes. The use of LNA bases thereby allows for the design of shorter probes while maintaining high binding specificity, making them ideal for the detection of short DNA targets.
DdPCR
For the analysis of cfDNA extracted from patient plasma samples, a standardized DNA sample volume of 10 µl per well was used. For the analysis of DNA extracted from tissue samples, a maximum of 60 ng DNA was used. Primers, probes, template DNA, fluorescein (VWR, Radnor, Pennsylvania, USA, cat. no. 0681–100 g) and nuclease-free water (Ambion by Thermo Fisher Scientific, Waltham, Massachusetts, USA, cat.no. AM9932) were added to the ddPCR Perfecta Multiplex qScript Tough Mix_5X (Quantabio, Beverly, Massachusetts, USA, cat. no. 95147-250) following manufacturer’s instructions. Final concentrations of primers and probes were 1 µM for primers and 250 nM for the drop-off and reference probes, respectively. This reaction mix was transferred into Sapphire Chips for the Naica™ crystal digital PCR system (Stilla Technologies, cat. no. C14012) following manufacturer’s instructions. The Naica™ Geode (Stilla Technologies, cat. no. H14000) was used to automatically generate droplets and perform PCR on the same instrument. PCR cycling conditions for the KRAS G12/G13 drop-off assay are shown in Supplemental Table 1. Samples were subsequently analyzed on a Naica™ Prism3 (Stilla Technologies, cat. no. H22000) using the Crystal Reader software v. 3.1.6.3.SP1 provided by Stilla Technologies. The absolute concentration of the targeted nucleic acids was then calculated using the Crystal Miner software v. 3.1.6.3 provided by Stilla Technologies.
Assay controls and ddPCR cluster formation
For each run, a minimum of one well per control was used, including negative control (WT only), positive control (mutant + WT), and no template control. The WT only control contained recombinant DNA from IDT in the form of a gBlock for the KRAS WT sequence as template (fragment length 400 to 500 bp). The positive control contained both recombinant WT gBlock DNA and mutant gBlock DNA, corresponding to the specific mutation at a molar ratio of approximately 1:1, calculated as 5000 copies of template DNA per well. For the mutant DNA, the KRAS G12D alteration was chosen, as it is one of the most frequent KRAS alterations. However, we have confirmed that our drop-off assay also works effectively with other variants, including KRAS G12V, G12R, G12A, G12C, G12S, and G13D, as positive controls. Based on the controls performed for the ddPCR experiment, the individual clusters were formed using the Crystal Miner software. In our KRAS drop-off assay, an orange cluster was included to represent the double-positive droplets, indicating co-encapsulation of both double-positive and FAM-only positive droplets within a single droplet. This co-encapsulation results in the formation of a new cluster, as seen in Fig. 1C. For the KRAS Kit from ID Solutions®, the clusters were generated according to the manufacturer’s instructions, and therefore, no orange cluster was present in this assay. The droplet clusters for the drop-off and ID Solutions® assay were each saved and used as a template for all following runs, so that each run was analyzed with the exact same droplet clusters.
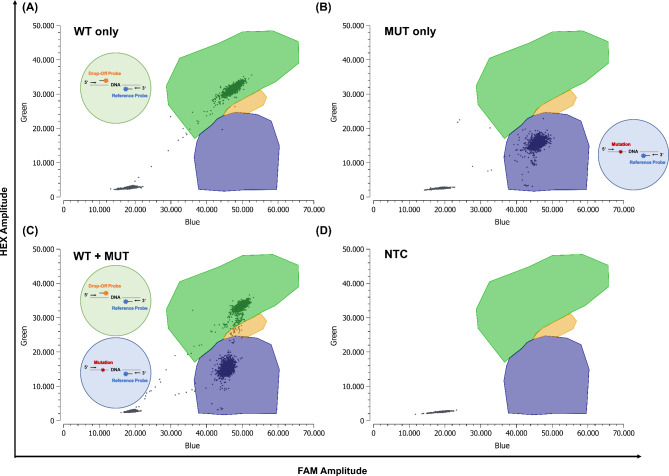
Fig. 1.
Technical validation - In-house designed KRAS ddPCR drop-off assay. Primers and locked nucleic acid (LNA)-based probes were designed, and the assay optimized individually regarding cycling conditions. drop-off assay ddPCR approach with only wild-type (WT) DNA (A), only mutant (MUT) DNA (B), both WT and MUT DNA (C) and a no template control (NTC) (D). Green: droplets positive for KRAS WT, Blue: MUT KRAS droplets, Orange: double positive droplets (WT + MUT), Gray: empty droplets. Green circle: In the case of a wild-type sequence, both probes bind, resulting in a double-positive signal. Blue circle: In the case of a mutation, only the reference probe binds to the DNA, resulting in a single FAM-positive signal. Figure 1B depicts a mutation with 100% variant allele frequency, while Fig. 1C shows a mixture of mutant and WT DNA. Based on the four scenarios (WT only, MUT only, WT + MUT, NTC), individual clusters were identified using Crystal Miner software. In our KRAS drop-off assay, an orange cluster was included to represent double-positive droplets, indicating the co-encapsulation of both double-positive and FAM-only positive droplets within a single droplet. This co-encapsulation results in the formation of a new cluster. DNA, deoxyribonucleic acid; FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein
Quantification of the target DNA concentration
The number of copies/µL of the target nucleic acids was automatically calculated by the Crystal Miner software based on predefined clusters and the number of positive droplets detected in the sample. Crystal Miner employs Poisson statistics to determine the concentration by considering both the number of positive droplets and the total number of droplets analyzed. When calculating the variant allele frequency (VAF) of a sample, it is important to account for the random co-encapsulation of mutant and WT DNA in some droplets, which can result in double-positive droplets that are often indistinguishable from WT only droplets. To avoid underestimating the mutant DNA concentration, this double-positive population must be subtracted. The mutant DNA concentration is then determined by the proportion of FAM-positive droplets among those that are either single FAM-positive or double-negative. With this approach, even in the drop-off setting, ddPCR provides absolute quantification of target DNA without the need for standard curves, whereas next-generation-sequencing (NGS) quantification relies on read counts and may be influenced by factors such as sequencing depth, amplification bias, and bioinformatic pipelines.
Technical validation
The KRAS drop-off assay was optimized using recombinant mutated DNA spiked into recombinant KRAS WT DNA as template. Probe and primer sequences are listed in Table 1, and assay conditions are detailed in Supplemental Table (1) Once established and optimized with recombinant DNA, the drop-off assay was technically validated with 12 cfDNA samples from our previously established PDAC patient cohort [49]. Samples from this cohort were chosen because NGS on tumor tissue had already been peformed to assess the KRAS mutational status (KRAS G12V, D, R, C and A). In addition, the mutational VAFs of these samples were known, as they had previously been analyzed using highly sensitive and mutation-specific single-target assays in the laboratories of the Comprehensive Cancer Center Freiburg (CCCF), part of the University Hospital Freiburg [49]. Samples with known high and low concentrations of mutant KRAS in plasma were selected and re-analyzed with both the drop-off assay and the respective single-target assay for technical validation. For this, we used our previously established single-target assays and optimized them on the Naica™ crystal digital PCR system, as the initial VAF results had been obtained on the Bio-Rad QX100/200 system. The assays for KRAS G12C, D, R and V were redesigned to obtain optimal separation of individual droplet clusters on the Naica™ system. Sequences of the new primer and probes are listed in Supplemental Table (2). DdPCR was performed as described above. Cycling conditions were optimized individually for all assays to achieve best separation of the different droplet clusters. PCR conditions of the resulting single-target assays are summarized in Supplemental Table 1. Spillover compensation of the Crystal Reader program was used to analyze ddPCR results, allowing for improved clustering.
Clinical validation
For the clinical validation of our drop-off assay, 45 samples gathered from patients with gastrointestinal malignancies receiving adjuvant or palliative systemic treatment at our institution were analyzed. The KRAS mutation status of the matched tumor tissue samples was already known due to routinely performed certified molecular pathology workup (NGS analysis). Thirty-six samples harbored a KRAS mutation (KRAS G12V, G12D, G12R, G12C, G12A, G12S, or G13D), while nine samples were KRAS WT. CtDNA positivity was confirmed with respective highly sensitive and mutation-specific single-target assays [49]. If available, tissue samples from the primary tumor were also tested using the KRAS drop-off assay to confirm the KRAS mutational status (data not shown).
Calculation of limits of detection and blank
To determine the limit of blank (LoB) for the drop-off assay, 20 WT only samples were analyzed. Samples were collected from patients with gastrointestinal tumors, with previously confirmed KRAS WT status determined through routine molecular pathology examination.The concentration of the samples ranged from 0.1 ng/µl to 10 ng/µl. A maximum of 60 ng per well was used to avoid damaging the ddPCR droplets. The LoB was calculated using the formula: LoB = meanblank + 1.645 × (SDblank), as recommended in published guidelines and previously described [49, 50]. The LoD was then calculated based on the measured LoB and the standard deviation (SD) obtained from 18 replicates of a sample known to contain a low concentration of the analyte (1:2000). Mutant DNA was therefore spiked into WT only samples (gBlocks from IDT, fragment length 400–500 bp). The LoD was determined using the formula: LoD = LoB + 1.645 × (SDlow concentration sample). If a sample’s concentration was at or below the LoB, the target was considered not detected. Concentrations between the LoB and LoD indicated that the target was detected but not quantifiable with high confidence, while concentrations above the LoD (and LoB) were considered true positives, indicating reliable detection and quantification of the target nucleic acid.
Commercial KRAS drop-off kit
For comparison, patients’ plasma samples were also analyzed with the commercial KRAS drop-off Kit from ID Solutions® (ID Solutions® by Labgene Scientific SA, Châtel-St-Denis, Switzerland, cat.no IDKRAS(s)-50). This kit is a ready-to-use multiplex test designed for the detection and quantification of mutations in codons 12 and 13 of exon 2, as well as codon 61 of exon 3, in the KRAS gene using digital PCR. Manufacturer’s instructions were followed for use of the assay. Step 7 (cooling) of the ddPCR protocol was not performed as this resulted in better droplet quality. Performing the cooling step (the final step of the PCR) led to the destruction of many droplets, which appeared as rainfall in the 2D plot of the ddPCR analysis software. Additionally, the exposure time was reduced to 75 ms for FAM and 150 ms for HEX, as this significantly reduced droplet oversaturation and improved data interpretation. Assay controls were performed analogous to our in-house established KRAS drop-off assay.
Statistical analysis
All ddPCR runs were analyzed using the Crystal Reader software v. 3.1.6.3.SP1. The absolute concentration of copies/µl was then calculated with the Crystal Miner software v. 3.1.6.3. Both software programs were provided by Stilla Technologies. GraphPad Prism version 9.4.1 (GraphPad Software, Inc., La Jolla, CA) was used for all statistical analyses.
Results
KRAS drop-off assay
In our previous work, we designed and clinically validated multi-target ddPCR assays by combining two to six mutation-specific probes together with the corresponding wild-type probe [49]. Assay performance was not inferior to commercially available assays and LoD, LoB and inter-assay precision were excellent throughout [49]. However, such multi-target assays only allow the detection of a limited number of mutations per reaction, and screening capacity is restricted because the mutations must be known a priori. To overcome these limitations we established a KRAS drop-off ddPCR assay with the procedures detailed in the Methods section. WT molecules result in a double positive (HEX + FAM) signal (Fig. 1, top left). In case of a mutation-induced mismatch, the suboptimal hybridization of the drop-off probe results in a reduced HEX signal. This causes the droplet cloud to shift towards a solely FAM-positive population, proportional to the percentage of mutant molecules (Fig. 1, top right + bottom left). A complete shift to a solely FAM-positive population indicates the presence of a mutation with 100% VAF (Fig. 1, top right), whereas a partial shift suggests a mixture of wild-type and mutant DNA (Fig. 1, bottom left). Supplemental Fig. 1 shows results obtained with the commercial KRAS drop-off Kit from ID Solutions® in parallel experiments for comparison. Our KRAS drop-off assay enables the concurrent detection of several genomic alterations within exon 2 codon 12/13 of the KRAS gene in one experiment while using minimal patient sample volume (Fig. 2). The drop-off assay demonstrated equivalent performance for all tested alterations, ensuring consistent sensitivity and specificity across the covered mutations.
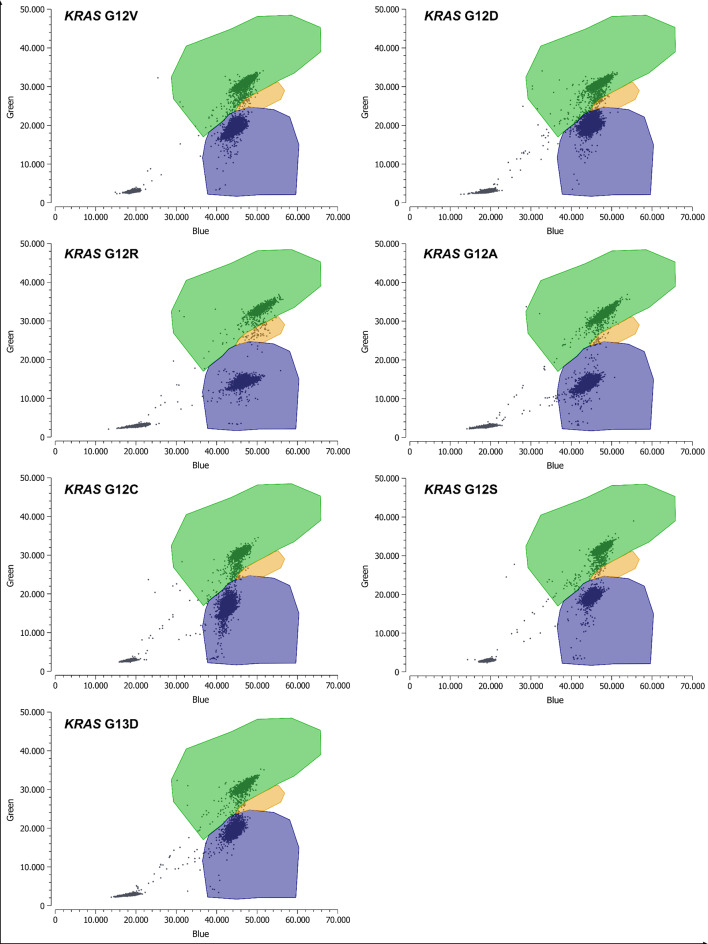
Fig. 2.
Technical validation - Screening for KRAS mutations with generic drop-off assay. Detection of KRAS G12 V/D/R/A/C/S and G13D with the KRAS drop-off assay is shown. Green: droplets positive for KRAS WT, Blue: MUT KRAS droplets, Orange: double positive droplets (WT + MUT), Gray: empty droplets. Based on the controls performed for the ddPCR experiment (WT only, MUT only, WT + MUT, NTC), the individual clusters were formed using the Crystal Miner software. In our KRAS drop-off assay, an orange cluster was included to represent the double-positive droplets, indicating co-encapsulation of both double-positive and FAM-only positive droplets within a single droplet. This co-encapsulation results in the formation of a new cluster. FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein; MUT, mutant; NTC, no template control; WT, wild-type
Multiplexing for specific mutations of interest in the drop-off setting
Since the Stilla Naica™ crystal digital PCR system offers three different fluorophores, we added a third Cy5-labeled probe to the PCR reaction mix in a second step, which specifically binds to KRAS G12C. The use of the spillover compensation in the crystal reader program enabled even stronger cluster formation, as illustrated in Fig. 3. The KRAS drop-off assay including KRAS G12C was optimized individually using recombinant DNA (Supplemental Table 1). Utilizing this third probe in the drop-off setting enables simultaneous screening for KRAS alterations at exon 2 codon 12/13 of the KRAS gene and specific identification of KRAS G12C, a clinically relevant and therapeutically actionable mutation in many solid malignancies [51]. Furthermore, our assays demonstrate the capability to multiplex for additional specific mutations of interest, such as TP53 variants (Supplemental Fig. 2), increasing the assay’s utility in broader contexts. Detecting TP53 or other single nucleotide variants (SNVs) can increase the sensitivity of the assay by capturing a wider array of mutational events that may be relevant for disease progression or treatment resistance.
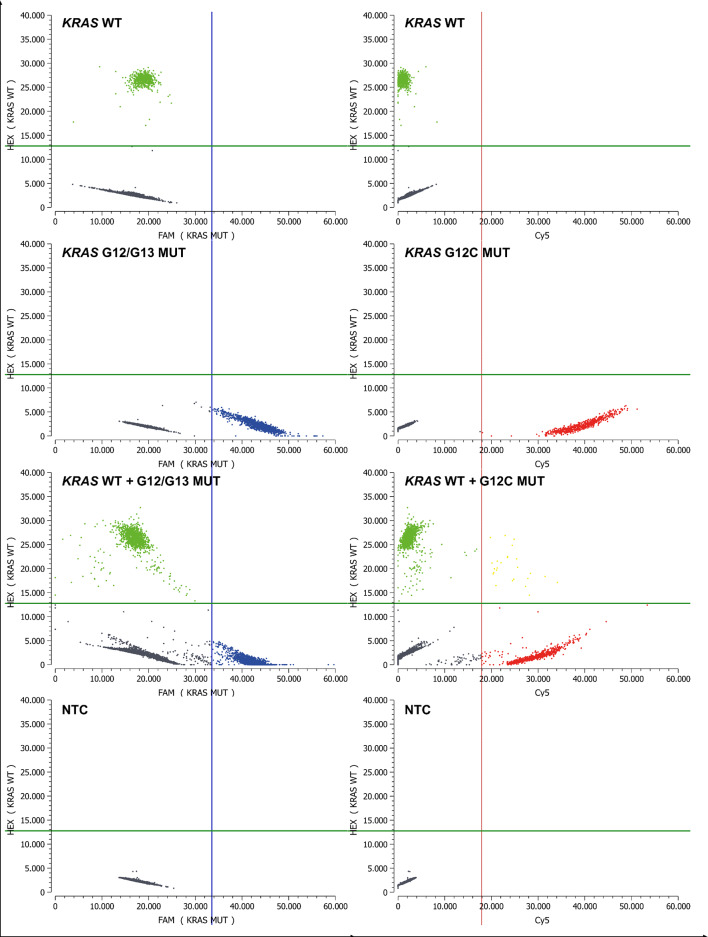
Fig. 3.
Technical validation - drop-off ddPCR setting with specific detection of KRAS G12C. Simultaneous screening for genomic KRAS alterations at exon 2 codon 12/13 of the KRAS gene and specific detection of KRAS G12C. Green: droplets positive for KRAS WT, Blue: MUT KRAS G12/G13 droplets, Gray: empty droplets, Red: MUT KRAS G12C positive droplets, Yellow: double positive droplets (WT and KRAS G12C mutant). Based on the controls performed for the ddPCR experiment (WT only, MUT only, WT + MUT, NTC), the individual thresholds for the fluorescence channels were determined using the Crystal Miner software. Green lines: Threshold between HEX negative and positive droplets, blue lines: Threshold between FAM negative and positive droplets, red lines: Threshold between Cy5 negative and positive droplets. Cy5, cyanin-5; FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein; MUT, mutant; NTC, no template control; WT, wild-type
drop-off assay technical validation
To assess assay sensitivity (LoD), we evaluated replicates of a sample known to have a low analyte concentration and computed the LoD following the procedure outlined in Materials and Methods. The minimum calculated LoD for the KRAS drop-off assay was determined to be 0.57 copies/µL. The LoB was examined analyzing 20 KRAS WT samples, resulting in a LoB of 0.13 copies/µL. Above this threshold, a sample was considered true positive. Overall, there was no correlation between the number of WT copies/µL and the false-positive rate (Supplemental Fig. 3A), and dilution linearity of the KRAS drop-off assay was excellent throughout (Supplemental Fig. 3B). Once established and optimized with recombinant DNA, the drop-off assay was technically validated with cfDNA samples from our already established PDAC patient cohort, with known mutational VAFs of those samples.
For this, the single-target assays were optimized for the Naica™ Crystal Digital PCR system (Supplemental Fig. 4). After optimization, the KRAS single-target assays were used to evaluate the interassay accuracy between the generic single-target and the KRAS drop-off assay, a key component of the technical validation (Fig. 4). For this purpose, ctDNA-positive patient samples were analyzed concurrently, and VAFs were plotted against each other in Fig. 4B, revealing an outstanding correlation (r2 = 0.9096) between the single-target and the drop-off assay. Interassay precision for the commercial KRAS kit from ID Solutions® versus single-target assays and versus generic drop-off assay was assessed for comparison (Fig. 4). In 12/12 samples (100%) tested, the KRAS drop-off assay correctly identified the KRAS mutation, as confirmed by tissue NGS mutational analysis and respective single-target assays (Fig. 4). Overall, as little as 1–5 ng of input DNA is enough to reliably detect alterations with ddPCR, and we aimed to use a minimum of 10 ng of total DNA per ddPCR reaction (equivalent to approximately 3,000 genome equivalents for human DNA).
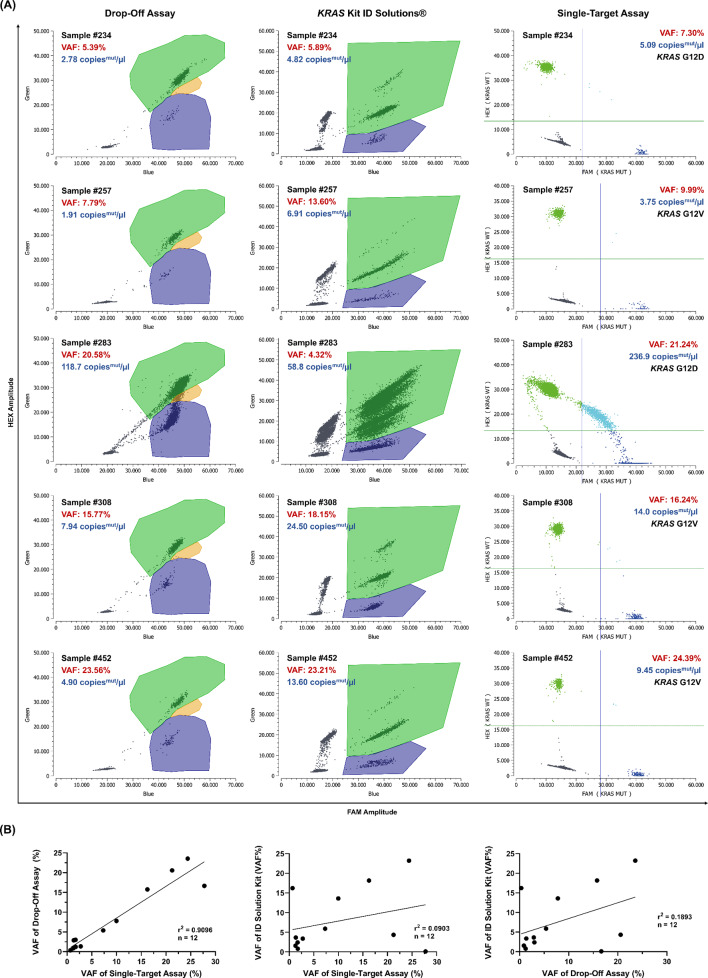
Fig. 4.
Technical validation - Inter-assay precision of the KRAS ddPCR drop-off assay.cfDNA samples from pancreatic cancer patients were analyzed with different assays as indicated. (A) drop-off assay (left column) vs. corresponding single-target assays (right column) vs. the commercial KRAS kit from ID Solutions® (middle column). The commercial kit shows a distinct subclone population in the HEX channel, visible as a second population within the double-positive (WT + MUT) droplet cloud region (green), likely caused by off-target amplification from a homologous region on chromosome 6. Analysis of sample 283 shows a more accurate estimation of VAF and KRAS wild type allele frequency by the drop-off assay. Green: droplets positive for KRAS WT, Blue: MUT KRAS droplets, Orange: double positive droplets (WT + MUT), Gray: empty droplets. Green lines: Threshold between HEX negative and positive droplets, blue lines: Threshold between FAM negative and positive droplets. (B) Variant allele frequencies (VAFs) were plotted against each other. cfDNA, cell-free DNA; FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein; MUT, mutant; WT, wild-type
During cfDNA analysis using the KRAS Kit from ID Solutions®, a subclone was observed in the HEX channel, which increased proportionally with the amount of input DNA (Fig. 4, middle part). This phenomenon likely arises from off-target amplification due to the high sequence homology (95% identity over 118 of 124 base pairs) between the KRAS amplicon and a region on human chromosome 6 (GRCh38.p14). When the amplicon is too short, partial binding of primers and probes to chromosome 6 can occur, leading to the formation of subclones, which manifest as distinct sub-populations in the 2D droplet plot, particularly in the double-positive (HEX + FAM) droplet cloud region. This issue is more pronounced in samples with higher cfDNA input, potentially resulting in inaccurately low allele frequency estimates. In our study, this limitation was evident when comparing allele frequencies obtained using the commercial KRAS drop-off kit with those from the single-target assays and the in-house designed drop-off assay, where correlation values were notably lower (r² = 0.0903 and r² = 0.1893, respectively; Fig. 4B).
Screening patient samples for the presence of KRAS mutations (Clinial Validation)
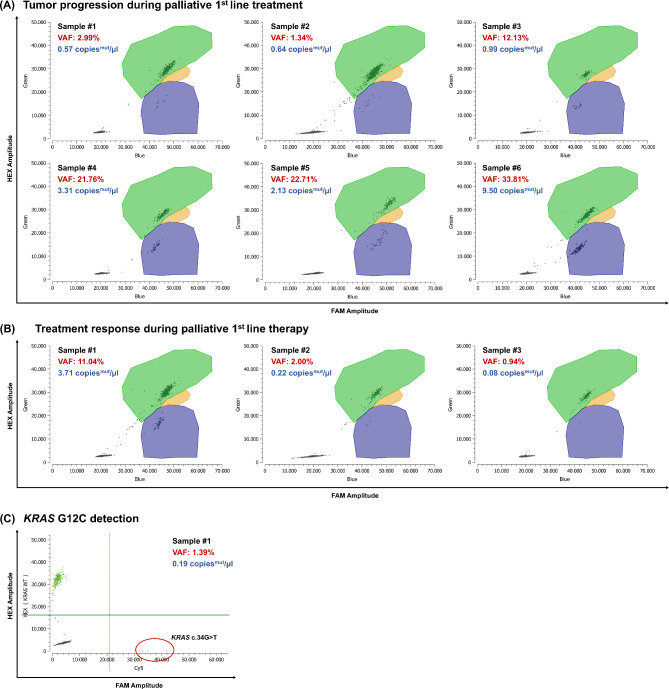
For further clinical validation of the KRAS drop-off assay, we extracted cfDNA from plasma samples collected from patients with gastrointestinal cancers undergoing adjuvant or palliative systemic treatment at our institution. The KRAS drop-off assay correctly identified the KRAS SNV in 35 out of 36 ctDNA-positive patient samples tested, resulting in a diagnostic sensitivity of 97.22%. KRAS mutational status was known through routinely performed NGS analysis, and ctDNA positivity was confirmed with respective single-target assays. Additionally, the assay detected all nine KRAS WT samples correctly as KRAS WT, yielding a diagnostic specificity of 100%. These results indicate that the assay demonstrates a high level of accuracy in distinguishing KRAS mutations from KRAS WT samples, making it a reliable tool for clinical diagnostic purposes. Notably, ctDNA kinetics reflected individual patients’ disease course, as illustrated in two index cases (Fig. 5).
Fig. 5.
Clinical validation - Clinical response prediction by mutant KRAS cfDNA kinetics. cfDNA samples from three different pancreatic cancer patients during palliative systemic treatment were analyzed with the KRAS ddPCR drop-off assay. Sample 1 is the baseline sample taken before starting systemic treatment. (A) Tumor progression during palliative first-line treatment. (B) Treatment response indicated by decreasing mutant copies/µl. (C) Example of specific KRAS G12C detection in cfDNA. Green: droplets positive for KRAS WT, Blue: MUT KRAS droplets, Orange: double positive droplets (WT + MUT), Gray: empty droplets. Green lines: Threshold between HEX negative and positive droplets, red lines: Threshold between Cy5 negative and positive droplets. cfDNA, cell-free DNA; Cy5, cyanin-5; FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein; MUT, mutant; WT, wild-type
Taken together, a total of 45 patient samples were analyzed with the drop-off assay and the respective single-target assays for positive and negative percent agreement validation. The VAFs of the analyzed samples ranged from 0.0 to 33.81%, and interassay precision was excellent throughout (r² = 0.9835, Supplemental Fig. 3C). Our assay achieved an overall percent agreement of 97.78% (Supplemental Table 3).
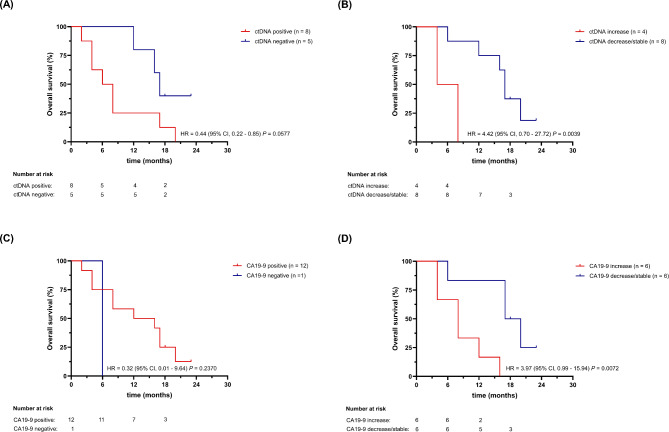
Patient samples with detectable ctDNA showed decreased overall survival (OS) compared to patient samples without measurable ctDNA ((hazard ratio (HR) = 3.1 (P = 0.0602)) as illustrated in Fig. 6 top left. A longer OS (HR = 4.4 (P = 0.0039)) was observed with measurable decrease or stable amount of detected ctDNA over time, compared to patient samples with increasing ctDNA load, as shown in Fig. 6. Similarly, Fig. 6 shows the OS of the same patients depending on the presence of the tumor marker CA19-9 (HR = 0.032, P = 0.237). Patients with stable to decreasing dynamics of CA 19 − 9 had longer OS, as compared with patients with increasing tumormarker (HR = 3.97, P = 0.0072). These findings highlight the potential value of monitoring multiple biomarkers, such as ctDNA and CA19-9, to gain a more comprehensive understanding of treatment response and prognosis. In summary, we developed and preliminary clinically validated a KRAS hot spot ddPCR assay tailored for cfDNA analysis in cancer patients with wide-ranging applicability in clinical and translational research.
Fig. 6.
Clinical validation - Overall survival (OS) analyses for gastrointestinal cancer patients undergoing systemic treatment. Top left: Kaplan-Meier estimate of OS for gastrointestinal cancer patients stratified by KRAS mutant cfDNA positivity (ctDNA positive) during systemic treatment. Top right: Kaplan-Meier estimate of OS for gastrointestinal cancer patients stratified by KRAS mutant cfDNA changes during systemic treatment: ctDNA decrease/stable versus increase. Bottom left: Kaplan-Meier estimate of OS for gastrointestinal cancer patients stratified by CA 19 − 9 positivity during systemic treatment. Bottom right: Kaplan-Meier estimate of OS for gastrointestinal cancer patients stratified by CA 19 − 9 changes during systemic treatment: CA 19 − 9 decrease/stable versus increase
Discussion
We report the successful development and preliminary clinical validation of a novel KRAS exon 2 drop-off ddPCR assay with improved specificity compared to commercially available assays. A key innovation of our assay is its ability to multiplex for additional specific mutations through the incorporation of a third mutation-specific probe, which increases sensitivity and allows for more personalized and precise therapy monitoring. Similar to commercially available drop-off assays, our assay was able to detect and quantify all KRAS mutations in exon 2 codon 12/13 from plasma cfDNA, including previously unknown mutations [42–45]. Moreover, in comparison to a standard single-target ddPCR assay, our KRAS drop-off assay demonstrated to retain an analytical sensitivity on par with a classical ddPCR assay, while simultaneously covering a broad spectrum of molecular alterations, such as SNVs, deletions, and insertions, in a single reaction [11, 12, 37]. Overall, our KRAS drop-off assay demonstrated exceptional sensitivity, specificity, and precision without showing any cross-reactivity. However, a drop-off assay is designed to detect only specific mutations in the target region and cannot distinguish between individual KRAS G12/G13 subtypes. Additionally, it may miss mutations that occur outside the probe-binding region, making it unsuitable for broad-spectrum mutation detection. To partially overcome this limitation, we show that our assay allows for multiplexing, therefore enabling the detection of additional specific mutations of interest, such as KRAS G12C or TP53, through the addition of a third fluorophore. In theory, the more fluorescence channels available, the more specific mutations can be incorporated into the drop-off assay setting, further enhancing both the detection capacity and sensitivity of the assay. From a clinical perspective, being able to screen for multiple mutations in one reaction is an ideal tool for the analysis of cfDNA fragments in the bloodstream of cancer patients, particularly in cases where the tumor fraction and cfDNA concentration are low. The specific detection of KRAS G12C aligns with current clinical relevance, as targeted therapies are presently available only for this mutation. Therefore, the ability to detect all KRAS G12/G13 mutations in a single assay while specifically identifying KRAS G12C provides an efficient approach for mutation screening in a clinically meaningful manner.
Designing a ddPCR assay for the mutation hotspot region of the KRAS gene presents a significant challenge due to the amplicon’s overlap with a sequence on human chromosome 6 (GRCh38.p14). Out of 124 base pairs, 118 (95%) are identical, which can cause partial binding of the primer and probes to chromosome 6 if the amplicon is too short. This may lead to the formation of subclones in the 2D plot of the ddPCR analysis, which can often be observed when analysing patient cfDNA samples with commercially available kits, a limitation that becomes more relevant in samples with a higher input of cfDNA, probably contributing to inaccurately low allele frequencies. In our work, this was also reflected in the comparison of allele frequencies with the KRAS Kit from ID Solutions®, as the correlation with the single-target assays (r2 = 0.0903) and the in-house designed drop-off assay (r2 = 0.1893) was considerably lower. However, since the primer locations of the ID Solution® Kit remain undisclosed, this hypothesis cannot be verified. To circumvent this cross-reactivity, we positioned the reverse primer outside of this region.
We successfully validated our assay system using plasma samples collected from patients with gastrointestinal cancers receiving adjuvant or palliative systemic treatment at our institution. Results of the drop-off assay matched those obtained with the respective single-target assays in terms of VAF detection. Furthermore, the KRAS drop-off assay correctly identified SNVs in 35/36 (97.22%) of ctDNA positive samples, underscoring its potential for clinical application. In our study cohort, despite limited sample size, detection of increasing ctDNA in patient samples resulted in shorter OS (HR = 4.4 (P = 0.0039)), underlining the prognostic relevance of the assay. While our assay demonstrates high analytical performance, further steps are required to establish its suitability for clinical decision-making and potential use in therapeutic guidance. To meet regulatory requirements, the assay needs to undergo formal validation in accordance with established clinical and laboratory standards, such as SN EN ISO 15,189. Additionally, accreditation within a certified clinical laboratory setting is necessary to ensure compliance with these standards. Future work will focus on fulfilling these requirements, including expanding validation in a larger cohort and demonstrating clinical utility in real-world settings.
Over the last decade, LB has emerged as a powerful tool for monitoring tumor molecular dynamics and early molecular tumor response during cancer treatment. By employing highly sensitive and specific methods like ddPCR, low-frequency mutations can be detected with high accuracy, which is crucial for tailoring personalized multimodality treatments and maximize efficacy while limiting toxicity [23, 31–33]. In contrast, NGS provides broader mutational coverage, detecting multiple mutations across entire genes, though its sensitivity for rare variants is generally lower (around 1–5%) unless deep sequencing is performed. Thus, ddPCR is ideal for highly sensitive, targeted mutation detection, while NGS excels in comprehensive mutational profiling. Compared to tissue NGS, ddPCR results can be obtained within two days, enabling real-time molecular monitoring of cancer patients in a time- and cost-efficient manner without requiring extensive bioinformatics expertise [11, 52, 53]. Analyzing a single sample costs approximately 20 Swiss francs per well, which includes DNA extraction, primers and probes, as well as all other consumables and maintenance. Since KRAS mutations are highly prevalent in human malignancies, optimizing cfDNA KRAS drop-off assays has the potential to further advance the field of molecular diagnostics and precision oncology. To our best knowledge, the assay presented in this work is the only assay capable to specifically detect the KRAS G12C mutation, even in a drop-off setting [47]. The prevalence of the KRAS G12C mutation varies among cancer types, accounting for approximately 10–15% of NSCLC cases, 3–4% of CRC cases, and a smaller proportion of other solid tumors [54–58]. The recent development of selective small-molecule inhibitors such as sotorasib (AMG 510), adagrasib (MRTX849) olomorasib and selumetinib, targeting KRAS G12C, have transformed the treatment landscape for cancer patients harboring this alteration [59–61]. Therefore, it is of relevance to identify this KRAS mutation in a minimially invasive manner. However, we do not cover more rarely occurring KRAS exon 3 and exon 4 mutations, which is a limitation of our assay system. Additionally, while the assay presented in this study is effective for detecting SNVs, it faces challenges in identifying certain mutations, particularly structural variations such as large insertions or deletions, due to the fragmented nature of ctDNA.
Conclusion
We established and clinically validated a novel universally adaptable KRAS drop-off ddPCR assay for the identification and quantification of mutant KRAS in plasma cfDNA, demonstrating improved specificity while retaining high sensitivity. The resulting assay system is reliable, cost-effective, and easy to implement in any research and diagnostic facility.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Supplemental Fig. 1. KRAS drop-off Kit from ID Solutions® ID Solutions® KRAS drop-off assay ddPCR approach with only wild-type (WT) DNA (top left), human genomic DNA (top right), both WT and MUT DNA (bottom left) and a no template control (NTC) (bottom right). Green: droplets positive for KRAS WT, Blue: MUT KRAS droplets, Orange: double positive droplets (WT + MUT), Gray: empty droplets. FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein
Supplementary Material 2: Supplemental Fig. 2. Multiplexing for specific mutations of interest. Simultaneous screening for genomic KRAS alterations at exon 2 codon 12/13 of the KRAS gene and specific detection of TP53 E285K. Green: droplets positive for KRAS WT, Blue: MUT KRAS G12/G13 droplets, Gray: empty droplets, Red: MUT TP53 E285K positive droplets, Yellow: double positive droplets (KRAS WT and TP53 E285K mutant). Purple: double positive droplets (KRAS MUT and TP53 E285K mutant). Cy5, cyanin-5; FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein; MUT, mutant; WT, wild-type
Supplementary Material 3: Supplemental Fig. 3. KRAS drop-off assay Validation (A) KRAS WT samples were analyzed, and the number of WT copies/µl was plotted against the false-positive rate (MUT copies/µl). (B) Dilution linearity of the KRAS drop-off assay. Calculated versus measured variant allele frequencies (VAF) are plotted as indicated. (C) VAFs of cfDNA samples analyzed with the drop-off assay and the respective single-target assay were plotted against each other. cfDNA, cell-free DNA; MUT, mutant; VAF, variant allele frequency; WT, wild-type
Supplementary Material 4: Supplemental Fig. 4. Generic KRAS single-target assays optimization To achieve optimal separation of individual droplet clusters previously designed single-target KRAS ddPCR assays were optimized on the Naica™ Crystal Digital PCR system. Spillover compensation of the Crystal Miner program was used for improved clustering. Green: droplets positive for KRAS WT, Blue: MUT KRAS droplets, Light blue: double positive droplets (WT + MUT), Gray: empty droplets. Green lines: Threshold between HEX negative and positive droplets, blue lines: Threshold between FAM negative and positive droplets. FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein; MUT, mutant; WT, wild-type
Acknowledgements
S.H. was recipient of a KRAK Physician Scientist Fellowship provided by the Science Foundation for Oncology (SFO) and later of a «Filling the Gap» 2024-2025 – Siegenthaler Fellowship.
Abbreviations
- CCCF
Comprehensive Cancer Center Freiburg
- cfDNA
Cell-free DNA
- ctDNA
Circulating-tumor DNA (ctDNA)
- ddPCR
Droplet digital polymerase chain reaction
- DNA
Deoxyribonucleic acid
- FAM
6-carboxyfluorescein
- HEX
Hexachlorofluorescein
- HR
Hazard ratio
- IDT
Integrated DNA Technologies
- IRB
Institutional review board
- KRAS
Kirsten rat sarcoma virus
- LB
Liquid biopsy
- LNA
Locked nucleic acid
- LOB
Limit of blank
- LOD
Limit of detection
- MRD
Minimal residual disease
- MUT
Mutant
- NGS
Next-generation sequencing
- OS
Overall survival
- PDAC
Pancreatic ductal adenocarcinoma
- SD
Standard deviation
- SNV
Single nucleotide variant
- VAF
Variant allele frequency
- WT
Wild type
Author contributions
The work reported in the paper has been performed by the authors. B.A-D.N., M.G., D.A, C.P., T.B., M.Z., A.W., S.H. and R.F.: investigation; D.A., S.H. and R.F.: manuscript writing; S.H. and R.F.: supervision; all authors: manuscript review.
Funding
This work was supported by an Innovationpool research grant from the University Hospital Zurich (INOV00122).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the local institutional review boards (IRB) in Freiburg (EK-Freiburg project number 46/18) and Zurich (BASEC-Nr. 2020-01 104). All patients gave written informed consent for collection and analysis of blood samples. No identifiable images or data are included in this manuscript. All study procedures were conducted in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Saskia Hussung and Ralph Fritsch contributed equally to this work and share last authorship.
References
- 1.Diaz LA Jr., Bardelli A. Liquid biopsies: genotyping Circulating tumor DNA. J Clin Oncology: Official J Am Soc Clin Oncol. 2014;32:579–86. 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alix-Panabières C, Pantel K. Clinical applications of Circulating tumor cells and Circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479–91. 10.1158/2159-8290.Cd-15-1483. [DOI] [PubMed] [Google Scholar]
- 3.Sausen M, et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun. 2015;6:7686–7686. 10.1038/ncomms8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan JCM, et al. Liquid biopsies come of age: towards implementation of Circulating tumour DNA. Nat Rev Cancer. 2017;17:223–38. 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CW, Berchem G, Kim YJ, El-Khoury V. Cell-free DNA and next-generation sequencing in the service of personalized medicine for lung cancer. Oncotarget. 2016;7:71013–35. 10.18632/oncotarget.11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020;1:276–90. 10.1038/s43018-020-0043-5. [DOI] [PubMed] [Google Scholar]
- 7.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Reviews Clin Oncol. 2013;10:472–84. 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 8.Kato S, Janku F. Cell-free DNA as a novel marker in cancer therapy. Biomark Med. 2015;9:703–12. 10.2217/bmm.15.38. [DOI] [PubMed] [Google Scholar]
- 9.Aguirre AJ, et al. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov. 2018;8:1096–111. 10.1158/2159-8290.Cd-18-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witkiewicz AK, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744–6744. 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hindson BJ, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–10. 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hindson CM, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10:1003–5. 10.1038/nmeth.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janku F, et al. Multiplex KRASG12/G13 mutation testing of unamplified cell-free DNA from the plasma of patients with advanced cancers using droplet digital polymerase chain reaction. Annals Oncology: Official J Eur Soc Med Oncol. 2017;28:642–50. 10.1093/annonc/mdw670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy SR, et al. Detecting ultralow-frequency mutations by duplex sequencing. Nat Protoc. 2014;9:2586–606. 10.1038/nprot.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malapelle U, et al. Development of a gene panel for next-generation sequencing of clinically relevant mutations in cell-free DNA from cancer patients. Br J Cancer. 2017;116:802–10. 10.1038/bjc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman AM, et al. An ultrasensitive method for quantitating Circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–54. 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman AM, et al. Integrated digital error suppression for improved detection of Circulating tumor DNA. Nat Biotechnol. 2016;34:547–55. 10.1038/nbt.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waddell N, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zviran A, et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat Med. 2020;26:1114–24. 10.1038/s41591-020-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JD et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. J Science, 359, 926–30 (2018). 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed]
- 21.Cohen JD et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. J Proceedings of the National Academy of Sciences 114, 10202–10207 (2017). 10.1073/pnas.1704961114. [DOI] [PMC free article] [PubMed]
- 22.Del Re M, et al. Early changes in plasma DNA levels of mutant KRAS as a sensitive marker of response to chemotherapy in pancreatic cancer. Sci Rep. 2017;7:7931. 10.1038/s41598-017-08297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diehl F, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tie J, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Volume 8. Science Translational Medicine; 2016. 10.1126/scitranslmed.aaf6219. [DOI] [PMC free article] [PubMed]
- 25.Watanabe F, et al. Longitudinal monitoring of KRAS-mutated Circulating tumor DNA enables the prediction of prognosis and therapeutic responses in patients with pancreatic cancer. PLoS ONE. 2019;14:e0227366–0227366. 10.1371/journal.pone.0227366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misale S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–6. 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu B, et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctdna in resected NSCLC. Nat Commun. 2021;12:6770. 10.1038/s41467-021-27022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corcoran RB, Chabner BA. Application of Cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379:1754–65. 10.1056/NEJMra1706174. [DOI] [PubMed] [Google Scholar]
- 29.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–48. 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 30.Akhoundova D, et al. ROS1 genomic rearrangements are rare actionable drivers in microsatellite stable colorectal cancer. Int J Cancer. 2022;151:2161–71. 10.1002/ijc.34257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussung S, et al. Longitudinal analysis of cell-free mutated KRAS and CA 19–9 predicts survival following curative resection of pancreatic cancer. BMC Cancer. 2021;21:49. 10.1186/s12885-020-07736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braune J et al. Circulating tumor DNA allows early treatment monitoring in BRAF- and NRAS-Mutant malignant melanoma. 20–31 (2020). 10.1200/po.19.00174 [DOI] [PubMed]
- 33.Moding EJ, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer. 2020;1:176–83. 10.1038/s43018-019-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jamal-Hanjani M, et al. Tracking the evolution of Non–Small-Cell lung cancer. N Engl J Med. 2017;376:2109–21. 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 35.Faulkner LG, Howells LM, Pepper C, Shaw JA, Thomas AL. The utility of ctdna in detecting minimal residual disease following curative surgery in colorectal cancer: a systematic review and meta-analysis. Br J Cancer. 2023;128:297–309. 10.1038/s41416-022-02017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller L, Belloum Y, Wikman H, Pantel K. Clinical relevance of blood-based ctdna analysis: mutation detection and beyond. Br J Cancer. 2021;124:345–58. 10.1038/s41416-020-01047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker M, Digital. PCR hits its Stride. Nat Methods. 2012;9:541–4. 10.1038/nmeth.2027. [Google Scholar]
- 38.Bettegowda C, et al. Detection of Circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra224. 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frampton GM, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–31. 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zill OA, et al. Cell-Free DNA Next-Generation sequencing in pancreatobiliary carcinomas. Cancer Discov. 2015;5:1040–8. 10.1158/2159-8290.CD-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolter M, Felsberg J, Malzkorn B, Kaulich K, Reifenberger G. Droplet digital PCR-based analyses for robust, rapid, and sensitive molecular diagnostics of gliomas. Acta Neuropathol Commun. 2022;10:42. 10.1186/s40478-022-01335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taly V, et al. Multiplex Picodroplet digital PCR to detect KRAS mutations in Circulating DNA from the plasma of colorectal cancer patients. Clin Chem. 2013;59:1722–31. 10.1373/clinchem.2013.206359. [DOI] [PubMed] [Google Scholar]
- 43.Pender A, et al. Efficient genotyping of KRAS mutant Non-Small cell lung cancer using a multiplexed droplet digital PCR approach. PLoS ONE. 2015;10:e0139074. 10.1371/journal.pone.0139074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bidshahri R, et al. Quantitative detection and resolution of BRAF V600 status in colorectal cancer using droplet digital PCR and a novel Wild-Type negative assay. J Mol Diagn. 2016;18:190–204. 10.1016/j.jmoldx.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Holm M, et al. Detection of KRAS mutations in liquid biopsies from metastatic colorectal cancer patients using droplet digital PCR, idylla, and next generation sequencing. PLoS ONE. 2020;15:e0239819. 10.1371/journal.pone.0239819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rausch C, et al. Double drop-off droplet digital PCR: A novel, versatile tool for mutation screening and residual disease monitoring in acute myeloid leukemia using cellular or Cell-Free DNA. J Mol Diagnostics: JMD. 2021;23:975–85. 10.1016/j.jmoldx.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Yu Q, et al. Development of multiplex drop-off digital PCR assays for hotspot mutation detection of KRAS, NRAS, BRAF, and PIK3CA in the plasma of colorectal cancer patients. J Mol Diagnostics: JMD. 2023;25:388–402. 10.1016/j.jmoldx.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185–e203113. 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hussung S, et al. Development and clinical validation of discriminatory multitarget digital droplet PCR assays for the detection of hot spot KRAS and NRAS mutations in Cell-Free DNA. J Mol Diagn. 2020;22:943–56. 10.1016/j.jmoldx.2020.04.206. [DOI] [PubMed] [Google Scholar]
- 50.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(Suppl 1):S49–52. [PMC free article] [PubMed] [Google Scholar]
- 51.Hong DS, et al. KRAS(G12C) Inhibition with Sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–17. 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saw SPL, et al. Brief report: droplet digital polymerase chain reaction versus plasma Next-Generation sequencing in detecting clearance of plasma EGFR mutations and carcinoembryonic antigen levels as a surrogate measure. JTO Clin Res Rep. 2023;4:100599. 10.1016/j.jtocrr.2023.100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia J, et al. Cross-platform comparison for the detection of RAS mutations in CfDNA (ddPCR Biorad detection assay, beaming assay, and NGS strategy). Oncotarget. 2018;9:21122–31. 10.18632/oncotarget.24950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finn SP, et al. Prognostic impact of KRAS G12C mutation in patients with NSCLC: results from the European thoracic oncology platform lungscape project. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2021;16:990–1002. 10.1016/j.jtho.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Arbour KC, et al. Treatment outcomes and clinical characteristics of patients with KRAS-G12C-Mutant Non-Small cell lung cancer. Clin cancer Research: Official J Am Association Cancer Res. 2021;27:2209–15. 10.1158/1078-0432.Ccr-20-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biernacka A, et al. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet. 2016;209:195–8. 10.1016/j.cancergen.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu G, Pei L, Xia H, Tang Q, Bi F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer. 2021;20:143. 10.1186/s12943-021-01441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS (G12C) somatic mutations across race, sex, and cancer type. N Engl J Med. 2021;384:185–7. 10.1056/NEJMc2030638. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, et al. D-1553 (Garsorasib), a potent and selective inhibitor of KRAS(G12C) in patients with NSCLC: phase 1 study results. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2023;18:940–51. 10.1016/j.jtho.2023.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Skoulidis F, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–81. 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Briere DM, et al. The KRAS(G12C) inhibitor MRTX849 reconditions the tumor immune microenvironment and sensitizes tumors to checkpoint inhibitor therapy. Mol Cancer Ther. 2021;20:975–85. 10.1158/1535-7163.Mct-20-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplemental Fig. 1. KRAS drop-off Kit from ID Solutions® ID Solutions® KRAS drop-off assay ddPCR approach with only wild-type (WT) DNA (top left), human genomic DNA (top right), both WT and MUT DNA (bottom left) and a no template control (NTC) (bottom right). Green: droplets positive for KRAS WT, Blue: MUT KRAS droplets, Orange: double positive droplets (WT + MUT), Gray: empty droplets. FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein
Supplementary Material 2: Supplemental Fig. 2. Multiplexing for specific mutations of interest. Simultaneous screening for genomic KRAS alterations at exon 2 codon 12/13 of the KRAS gene and specific detection of TP53 E285K. Green: droplets positive for KRAS WT, Blue: MUT KRAS G12/G13 droplets, Gray: empty droplets, Red: MUT TP53 E285K positive droplets, Yellow: double positive droplets (KRAS WT and TP53 E285K mutant). Purple: double positive droplets (KRAS MUT and TP53 E285K mutant). Cy5, cyanin-5; FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein; MUT, mutant; WT, wild-type
Supplementary Material 3: Supplemental Fig. 3. KRAS drop-off assay Validation (A) KRAS WT samples were analyzed, and the number of WT copies/µl was plotted against the false-positive rate (MUT copies/µl). (B) Dilution linearity of the KRAS drop-off assay. Calculated versus measured variant allele frequencies (VAF) are plotted as indicated. (C) VAFs of cfDNA samples analyzed with the drop-off assay and the respective single-target assay were plotted against each other. cfDNA, cell-free DNA; MUT, mutant; VAF, variant allele frequency; WT, wild-type
Supplementary Material 4: Supplemental Fig. 4. Generic KRAS single-target assays optimization To achieve optimal separation of individual droplet clusters previously designed single-target KRAS ddPCR assays were optimized on the Naica™ Crystal Digital PCR system. Spillover compensation of the Crystal Miner program was used for improved clustering. Green: droplets positive for KRAS WT, Blue: MUT KRAS droplets, Light blue: double positive droplets (WT + MUT), Gray: empty droplets. Green lines: Threshold between HEX negative and positive droplets, blue lines: Threshold between FAM negative and positive droplets. FAM, 6-carboxyfluorescein; HEX, hexachlorofluorescein; MUT, mutant; WT, wild-type
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.