Abstract
Background
Alopecia areata (AA) is an autoimmune disorder and the second most common form of non-scarring hair loss, affecting approximately 1.7–2% of the global population. Although it predominantly presents before the age of 40, AA can occur at any age and is strongly associated with genetic predisposition. Environmental, hormonal, and psychological factors have been implicated in disease onset and progression. Recent evidence highlights the immunomodulatory role of vitamin D, traditionally recognized for its functions in bone metabolism and calcium homeostasis, in skin health and immune regulation, raising interest in its potential relevance to AA pathogenesis and treatment.
Summary
This review examines the epidemiology, genetic predisposition, and immunological mechanisms underlying AA, emphasizing the loss of immune privilege in hair follicles as a key pathogenic event. Additionally, it examines the role of vitamin D and its receptor (VDR) in hair follicle homeostasis, discussing the association between vitamin D deficiency, altered VDR expression, and disease severity. Emerging clinical data suggest that vitamin D supplementation and analogs, such as calcipotriol, may serve as adjunctive therapeutic strategies, with potential benefits in mitigating disease severity and reducing relapse rates.
Key Messages
(1) Vitamin D serves as a critical immunomodulator in AA, regulating follicular immune privilege and key inflammatory pathways involved in disease pathogenesis. (2) Vitamin D deficiency is frequently observed in patients with AA and has been associated with increased disease severity, prolonged duration, and a higher risk of relapse. (3) Emerging evidence supports the therapeutic potential of vitamin D analogs and supplementation in AA, positioning vitamin D as a promising adjunct or primary treatment modality.
Keywords: Hair diseases; Alopecia; Alopecia areata; Alopecia totalis; Alopecia focalis; Alopecia universalis; Non-scarring alopecia; Trichology; Vitamins; Vitamin D; Vitamin D receptor; 1,25 dihydroxyvitamin D; 25-Hydroxyvitamin D; Vitamin D deficiency; Autoimmune disorder; Calcipotriol; Paricalcitol; SALT score
Introduction
Alopecia areata (AA) represents the second most common form of non-scarring alopecia, with a prevalence ranging from 1.7% to 2% in the general population [1]. This condition predominantly manifests before the age of 40; however, it can occur across all age-groups. A significant genetic predisposition is evident, as 10–42% of affected individuals report a family history of AA, often correlating with an earlier disease onset [2]. Research has suggested associations between AA and various contributing factors, including emotional, physical, hormonal, and infectious influences [3].
Recent evidence underscores vitamin D’s role in AA pathogenesis and management. Known for regulating calcium homeostasis and bone health, it also modulates immune responses and supports skin and hair integrity. This review explores AA’s epidemiology, genetic predisposition, and immunological mechanisms, alongside vitamin D’s immunomodulatory properties and therapeutic potential.
Immunology of AA
Patients with AA experience hair growth abnormalities due to an inflammatory disorder, beginning with the breakdown of immune privilege (IP) in hair follicles. IP allows tissues to evade immune responses through NK-cell inhibition, antigen sequestration, and physical barriers. NK-cell inhibition is mediated by non-classical MHC molecules HLA-G and HLA-E, increased macrophage migration inhibitory factor (MIF), reduced NKG2D expression, decreased IFN-γ production, and upregulated killer cell immunoglobulin-like receptor inhibitors (KIR). Antigen sequestration involves suppressed MHC-I production, regulated by inhibitory cytokines and growth factors like TGF-β, α-MSH, IL-10, calcitonin-related peptide, IGF-1, somatostatin, and PD-L1. Additionally, the lack of lymphatic drainage and the extracellular matrix acts as protective barriers against immune infiltration. Studies show that disruptions in follicular IP contribute to immune tolerance loss, a key factor in AA development (Table 1), particularly in genetically predisposed individuals [4].
Table 1.
Comparisons between vitamin D immune functions and immune mechanisms in AA
| Vitamin D (1,25(OH)2D3) | AA | Immune mechanisms |
|---|---|---|
| Cytokines | ||
| Decreases IFN-γ | Increased IFN-γ | Increase MHC I–II follicle expression [4, 5] |
| Decreases TNF-α (Th1 profile) | Increased TNF-α | Increase MHC I follicle expression |
| Decreases activated cell pathways by CXCL-9 | Increased CXCL-9 chemotaxis | Lymphocyte chemotaxis [6] |
| Decreases CXCL-10 production | Increased CXCL-10 concentration | Lymphocyte chemotaxis |
| Decreases CXCL-11 production | Increased CXCL-11 production | Lymphocyte chemotaxis |
| Inhibition of IL-2 production by Th1 lymphocytes | Increased IL-2 production by T CD8 + lymphocytes | Stimulation of TNF-α and IL-1 production/Autoantigen recognition [7] |
| Decreases RNA transcription and production of IL-1 | Increased levels of IL-1 | Follicle inflammation by lymphocytes [8] |
| Inhibition of IL-17 production by Th17 lymphocytes | Increased levels of IL-17 | Induction and maintenance of autoimmune inflammation [9, 10] |
| Immune cells | ||
| Inhibition of Th1 and Th17 lymphocytes | Infiltration by Th1 and Th17 | Follicle inflammation [11] |
| Increases a tolerogenic phenotype of DC, Treg lymphocytes, and production of IL-10 | Autoreactive DC (plasmacytoid cells) | Induction of autoimmune inflammation [12] |
| Decreases production of NK cells | Increased NKG2D + cells | Hair follicle destruction |
IFN, interferon; TNF-α, tumor necrosis factor alpha; CXCL, C-X-C motif ligand; IL, interleukin.
Autoimmunity in the Hair Follicle
The immune response in AA primarily targets melanin peptides involved in melanogenesis during the anagen phase, as well as keratinocyte-derived antigens. As a result, active disease predominantly affects pigmented hairs, sparing nonpigmented ones and leading to leucotrichia during regrowth. Inflammation within the follicle induces hallmark changes such as matrix cell vacuolization, increased hair shaft fragility causing breakage at the ostium, premature transition from anagen to telogen phases, and aberrant MHC I and II expression in the precortical region, perpetuating autoimmunity [13]. Concurrently, elevated malondialdehyde levels and oxidative stress contribute to tissue damage, accompanied by dendritic and lymphocyte infiltration [14, 15].
Vitamin D: Metabolism and Activation
Vitamin D, a steroid hormone derived from cholesterol, exists in two primary forms. Ergocalciferol (D2) is synthesized from UV-irradiated yeast sterol ergosterol and occurs naturally in sun-exposed mushrooms. Cholecalciferol (D3) is produced in the skin from 7-dehydrocholesterol or obtained from lanolin and oily fish. After synthesis, vitamin D undergoes metabolism in the liver and kidney, forming its major circulating (25(OH)D) and hormonal (1,25(OH)2D) forms. Additionally, several tissues, including the skin, immune cells, parathyroid gland, intestinal epithelium, prostate, and breast, locally produce 1,25(OH)2D, exerting paracrine and autocrine effects. The renal enzyme 1-hydroxylase, which generates 1,25(OH)2D, is regulated by parathyroid hormone, FGF23, calcium, and phosphate levels [16].
The vitamin D receptor (VDR) mediates most, but not all, of the effects of 1,25(OH)2D. Acting as a transcription factor, VDR partners with other transcriptional regulators, such as the retinoid X receptor. When bound to 1,25(OH)2D, this complex modulates gene transcription by either enhancing or suppressing cofactors’ interactions. Notably, VDR is not confined to cells involved in bone and mineral homeostasis (e.g., bone, gut, kidney) but is widely expressed across various cell types [17]. As a result, the effects of 1,25(OH)2D are extensive, influencing numerous physiological and pathological processes. Its roles include inhibiting cellular proliferation, promoting differentiation, reducing angiogenesis, stimulating insulin production, and enhancing macrophage cathelicidin production [12].
There is no consensus on the normal or adequate values of vitamin D. Different international organizations recommend different cutoff values; the Endocrine Society considers serum levels of 25(OH)D between 30 and 100 ng/mL (75–250 nmol/L) as adequate, while other organizations, such as the 14th Consensus for Vitamin D Nutritional Guidelines and the Institute of Medicine, suggest thresholds of 25 or 20 ng/mL (62 or 50 nmol/L), respectively. Due to this variability, the definition of vitamin D deficiency differs. Generally, deficiency is characterized by 25(OH)D levels below 20 ng/mL (50 nmol/L), while insufficiency is defined as levels between 21 and 29 ng/mL (52.5–72.5 nmol/L) [18, 19].
In addition to these controversies, studies indicate that 97.5% of the population can maintain adequate bone health with levels of 20 ng/mL, while 50% can do so at 16 ng/mL. Consistent with this, the Recommended Dietary Allowance established by the Institute of Medicine in 2011 suggests a daily intake of 600–800 IU for all adults [20].
Vitamin D and the Hair Follicle
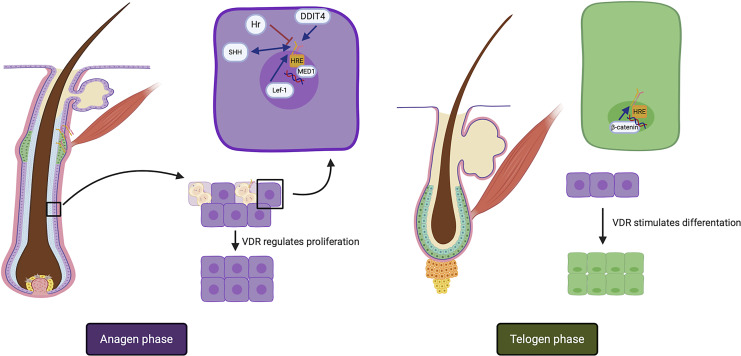
Keratinocytes within hair follicles exhibit immunoreactivity to VDR, particularly during the anagen phase, marking a crucial facet of hair biology (Fig. 1). VDR expression is discerned in key components of the pilosebaceous unit, including the external root sheath, hair follicular bulb, and sebaceous glands [21]. This expression contributes significantly to the regulation of hair cycling, particularly during post-embryonic stages [8]. Interestingly, alopecia in humans has been linked to disruptions in VDR function in several different types [22]. Research has revealed a connection between vitamin D deficiency, VDR-related disorders, and the development of non-scarring alopecia conditions, encompassing female pattern alopecia, telogen effluvium, and AA [23]. Table 2 provides a comprehensive overview of the factors associated with VDR activity and their influence on the intricate hair cycle dynamics.
Fig. 1.
Functions of VDR in the hair cycle and factors associated with VDR. In the anagen phase, VDR increases keratinocyte proliferation in the outer root sheath in the first stages and suppresses it in late stages. DDIT4, SHH/HH and the Lef-1/HH pathway, and MED1 in the vitamin D response element interact with VDR in this phase. In the catagen phase, VDR suppresses proliferation and stimulates keratinocyte differentiation. The Wnt/β catenin pathway plays a role in VDR stimulation in this phase. VDR, vitamin D receptor; SHH, sonic hedgehog protein; DDIT4, DNA damage-inducible transcript 4; HRE, hormone response element.
Table 2.
Main signaling pathways and proteins associated with VDR in the hair cycle
| Signaling pathway/protein | Effect in VDR | Functions in hair cycle |
|---|---|---|
| DDIT4 | Synthesis stimulation | Induction in epithelial differentiation |
| Hair follicle growth in anaphase phase by mTOR blockage [21] | ||
| Hr protein | Synthesis and functional inhibition | Elicits tonic inhibition of hair follicle cycle by Hr-VDR-RXRα complex [22] |
| SHH/HH signaling pathway | Co-dependency | Mesenchymal-epithelial interactions [8] |
| MED1 | VDR interacting protein complex member | Hair differentiation during first hair follicle cycle in the VDR/β-catenin pathway |
| Onset of the anagen phase | ||
| Promotion of terminal keratinocyte differentiation | ||
| Suppression of interfollicular epidermal differentiation and proliferation [24] | ||
| β-catenin/Wnt signaling pathway | Inhibition in keratinocytes and stimulation in hair follicle | Increase in follicular differentiation [21] |
| Lef 1/Wnt signaling pathway | N-terminal ligand | Epidermal keratinocyte proliferation |
| Stimulation of stem cells to form a follicular unit [23] |
DDIT4, DNA damage-inducible transcript 4; Hr, hairless; SHH, sonic hedgehog; HH, hedgehog; MED, transcription co-activator mediator; Lef 1, lymphoid enhancer-binding factor 1.
Vitamin D and AA
Immune Functions of Vitamin D in AA
The levels of 25(OH)D exhibit significant fluctuations throughout the year, with peak concentrations occurring during summer and lower levels observed in winter. This cyclical pattern is influenced by latitude, which determines the intensity of sunlight that reaches the exposed skin surfaces. Notably, patients afflicted with chronic AA tend to experience a higher incidence of relapses during the winter season, while experiencing fewer relapses during the summer months [25, 26]. Recent findings by Zhao et al. [27] have revealed that individuals with AA and other forms of alopecia consistently exhibit lower serum vitamin D levels when compared to age- and sex-matched control groups. Interestingly, the disparity in serum vitamin D levels between AA patients and controls was not statistically significant during the autumn season. This observation may be attributed to the seasonal decrease in vitamin D serum levels observed in the control group, which is consistent with the relatively lower serum levels observed in AA patients.
The immunomodulatory role of vitamin D has been established for decades. 1,25(OH)2D modulates T helper-1 (Th1) inflammatory activity, a key factor in AA pathogenesis [11]. Additionally, Th17 cells, CTLA-4, and the JAK-STAT pathway are involved in AA, with recent studies linking these mechanisms to vitamin D’s immune-regulating effects. By influencing JAK-STAT signaling, vitamin D reduces pro-inflammatory cytokine production, including interferons and interleukins, exerting an anti-inflammatory effect. Studies in C3H/Hej mice have demonstrated that JAK inhibitors suppress Th1 and IFN-γ pathways, reinforcing their therapeutic relevance in AA [6]. Chronic AA patches often show increased Th17 lymphocytes, correlating with disease persistence and poor treatment response. Elevated IL-17 and IL-22 levels are also associated with AA severity, although the exact role of Th17 in AA’s clinical presentation remains under investigation [9].
Research indicates that Th17 lymphocytes contribute to hair follicle bulb damage and the autoimmune response in AA. Activation of the VDR by 1,25(OH)2D3 can mitigate this process by reducing Th17 cell generation and inhibiting IL-17 production through suppression of NFAT, RUNx1, and RORγt [10]. This further highlights the connection between vitamin D levels and the immunology of chronic AA.
CTLA-4, an immunosuppressive protein regulating self-reactive T lymphocytes, contributes to the inflammatory processes in AA. Notably, ipilimumab, an anti-CTLA4 therapy for metastatic melanoma, has been linked to AA development. Certain genetic variations, such as the CT60 polymorphism in the CTLA4 gene, correlate with a lower AA risk. Vitamin D enhances CTLA-4 production and its ligand signaling in antigen-presenting cells, leading to reduced activation of new T lymphocytes, especially in the Th17 environment [28]. Figure 2 illustrates key pathways linking vitamin D to AA immunology.
Fig. 2.
Immune functions of vitamin D in the pathophysiology of AA. 1,25(OH)2D3 induces dendritic cells (DC) into a tolerogenic state and increases the CTLA-4 receptor in T cells. In Th1 lymphocytes, VDR stimulation by 1,25(OH)D inhibits IL-1 RNA translation, IL-2 production, and the JAK-STAT pathway. Cytokine production of fibroblasts, histiocytes, and endothelial cells are reduced by 1,25(OH)2D3, with decreased levels of IFN-γ, a crucial cytokine in AA, which induces MHC I and II expression in the hair follicle and initiation of the inflammatory process. 1,25(OH)2D3 reduces NK production and Th17 functions, the latter by inhibition of nuclear transcription factors RUNx1, RORγι, and NFAT. In contrast, 1,25(OH)2D3 stimulates Treg production and functions. CTLA-4, cytotoxic T-lymphocyte antigen 4; JAK, Janus kinase receptor; STAT, signal transducer and activator of transcription protein; CXCL, C-X-C motif ligand; IFNg, interferon gamma; HRE, hormone response element; MHC, major histocompatibility complex; RUNX1, Runt-related transcription factor 1; NFAT, activated T-cell nuclear factor; RORγι, retinoic-acid-receptor-related orphan nuclear receptor gamma; VDR, vitamin D receptor.
Serum Levels of Vitamin D in AA
A retrospective study conducted by Tran et al. [29] assessed vitamin D levels in patients with AA and compared them with those in individuals diagnosed with other forms of alopecia and non-alopecia controls. The study revealed that while patients with AA or other forms of alopecia did not exhibit lower levels of 25(OH)D compared to the controls, AA patients displayed a statistically significant higher proportion of individuals with vitamin D deficiency and an overall lower average level of 25(OH)D. These findings suggest that vitamin D may play a more prominent role in the pathogenesis of AA given its autoimmune nature [29].
In a study conducted by Lim et al. [30] in Philadelphia, which focused on children and adolescents, it was observed that non-white ethnicity, higher Fitzpatrick skin type, and non-summer season were significantly associated with an increased likelihood of vitamin D insufficiency. Interestingly, even in areas with a higher monthly UV index, suboptimal vitamin D levels persist [30]. Notably, AA patients exhibited a higher prevalence of vitamin D deficiency than healthy individuals, a trend consistent across various populations, including those with a high prevalence of vitamin D deficiency (Table 3) [31].
Table 3.
Studies of vitamin D serum levels in AA patients
| Author | Study group | Vitamin D determination | Vitamin D deficiency/insufficiency parameters | Vitamin D serum levels | Prevalence vitamin D deficiency/insufficiency | Association with AA | |||
|---|---|---|---|---|---|---|---|---|---|
| AA | C | AA | C | AA | C | ||||
| Zhao et al. [27] (2020) | 443 | 2,079 | ELISA | N/R | 46.8 nmol/L (quartile: 45.2, 48.2 nmol/L) | 52.7 nmol/L (quartile: 51.6, 53.7 nmol/L) | N/R | N/R | Yes |
| Rehman et al. [32] (2019) | 135 | 135 | N/R | I <30 ng/mL | 22.8±10.03 ng/mL | 27.3±10.5 ng/mL | I 34.8% | I 34.1% | Yes |
| D <20 ng/mL | D 37.8% | D 23.7% | |||||||
| Lee et al. [5] (2018) | 1,255 | 783 | N/A | D <20 ng/mL | N/R | N/R | D 75.5% | N/R | Yes |
| Tsai and Huang [33] (2018) | 456 | N/R | N/A | N/R | N/R | N/R | N/R | N/R | Yes |
| Daroach et al. [24] (2018) | 30 | 30 | N/R | I <30 ng/mL | 7.6±4.5 ng/mL | 15.8±11.5 ng/mL | D + I | D + I 73.3% | Yes |
| D <20 ng/mL | 96.7% | ||||||||
| Gade et al. [34] (2018) | 45 | 45 | ELISA | N/R | 17.9±5.8 ng/mL | 30.6±6.2 ng/mL | N/R | N/R | Yes |
| Darwish et al. [35] (2017) | 30 | 20 | ELISA | N/R | 7.5±6.2 ng/mL | 31.7±12.3 ng/mL | N/R | N/R | Yes |
| Bhat et al. [36] (2017) | 50 | 35 | Chemiluminescence | D <30 ng/mL | 16.6 (5.9) ng/mL | 25.49 (1.02) ng/mL | 42% | 28.6% | Yes |
| Erpolat et al. [37] (2017) | 41 | 32 | High-resolution liquid chromatography | I <30 ng/mL | 8.1 ng/mL (5.0–38.6 ng/mL) | 9.8 ng/mL (3.6–38.5 ng/mL) | I 3.1% | I 9.8% | No |
| D <20 ng/mL | D 93.8% | D 85.3% | |||||||
| Ghafoor et al. [38] (2017) | 30 | 30 | ELISA | I <30 ng/mL | 13.5 ng/mL | 22.5 ng/dL | N/R | N/R | Yes |
| D <20 ng/mL | |||||||||
| Bakry et al. [39] (2016) | 60 | 60 | ELISA | I <50–75 nmol/L | 44.04±15.61 nmol/mL (21.6–80.5 nmol/mL) | 66.1±17.4 nmol/mL (29.8–95.5 nmol/mL) | D + I | D + I | Yes |
| D <50 nmol/L | 83.3% | 23.3% | |||||||
| Fawzi et al. [22] (2016) | 20 | 20 AGA | ELISA and biopsy for VDR determination | N/A | N/A | N/A | N/A | N/A | Yes |
| 20 C | |||||||||
| Narang et al. [40] (2016) | 22 | N/A | N/R | D <20 ng/mL | N/R | N/A | D 91% | N/A | N/A |
| Thompson et al. [41] (2016) | 133 | 55,929 | N/R | N/R | 29 ng/mL | N/R | N/R | N/R | No |
| Mahamid et al. [42] (2014) | 23 | 20 | ELISA | I <30 ng/mL | 11.3±10.1 ng/mL | 21.5±13.6 ng/mL | D + I | D + I | Yes |
| D <20 ng/mL | 69.5% | 25% | |||||||
| Çerman et al. [7] (2014) | 86 | 44 vitiligo 58 C | Liquid chromatography and random mass spectrometry | D <20 ng/mL | 11.8±6.2 ng/mL | Vitiligo 16.1±7.9 ng/mL | D 90.7% | Vitiligo 70.5% | Yes |
| C 23.6±9.03 ng/mL | C 32.8% | ||||||||
| D’Ovidio et al. [43] (2013) | 156 | 148 | Chemiluminescence | D <20 ng/mL | N/R | N/R | 42.4% | 29.5% | Yes |
| Nassiri y cols [44] (2013) | 28 | 44 | Chemiluminescence | I <30 ng/mL | N/R | N/R | I 50% | I 45.4% | No |
| D <10 ng/mL | D 31.1% | D 52.3% | |||||||
| Yilmaz et al. [11] (2012) | 42 | 42 | ELISA | I <50 nmol/L | 33.4±17.7 nmol/L | 51.2±21.1 nmol/L | I 85% | N/R | Yes |
C, controls; AA, alopecia areata; D, deficiency; I, insufficiency; N/A, no application; N/R, not registered; AGA, androgenetic alopecia.
Some researchers have proposed a connection between vitamin D deficiency and clinical manifestations of AA [45]. In a study by Daroach et al. [24], serum vitamin D levels in AA patients were inversely correlated with disease severity and duration. To address this, they administered cholecalciferol at a dosage of 60,000 IU once a week for 12 weeks to patients with low serum vitamin D levels. Remarkably, approximately 27 percent of supplemented patients experienced complete hair repopulation, although no significant differences in the Severity of Alopecia Tool (SALT) score were observed. Supplementation did not achieve a level exceeding 30 ng/mL, which is a threshold associated with immunomodulatory effects [24]. Furthermore, Rehman et al. [32] conducted a study that demonstrated a link between vitamin D deficiency and AA severity, as assessed by the SALT score. They found that patients with multiple patches on the scalp and body, along with nail involvement, exhibited lower serum vitamin D levels than patients with a single patch and those without nail involvement [32].
VDR plays a crucial role in regulating the hair cycle, and its dysfunction has been linked to AA. Fawzi et al. [22] evaluated VDR levels in AA patients using serum analysis, biopsies, immunohistochemistry, and enzyme-linked immunosorbent assay (ELISA). To establish comparative benchmarks, the study included androgenetic alopecia patients and a control group. Results showed significantly lower VDR levels in both serum and tissue of AA and androgenetic alopecia patients compared to controls. Interestingly, no significant differences were found between the two alopecia groups. Additionally, VDR tissue levels negatively correlated with AA severity and inflammation, highlighting its potential role in AA pathogenesis [24, 39].
Some studies have failed to establish a consistent link between lower vitamin D serum levels and AA in patients than in healthy individuals. For instance, Nassiri et al. [44] conducted an analysis involving 28 Iranian patients with AA and 44 control subjects, but they did not find a significant relationship between AA and vitamin D. The authors postulated that the high prevalence of vitamin D deficiency observed in the control group could account for these results, which are primarily attributable to religious dress traditions. Additionally, the control group had a median age higher than the AA group, which might have contributed to lower vitamin D serum levels associated with aging and a relatively small sample of AA patients [44]. Similarly, a recent cross-sectional study conducted by Lizarondo et al. [46] in the Philippines compared the serum levels of 25-hydroxyvitamin D in 29 AA patients and 29 healthy controls. This study indicated a tendency toward an increased likelihood of vitamin D deficiency in AA patients, although this difference was not statistically significant [46].
In contrast, Thompson et al. [41] investigated whether vitamin D deficiency served as a risk factor for AA in a cohort derived from the Nurses’ Health Study (NHS), initiated in 1976 and comprising 121,700 nurses aged 30–55 years. This cohort was regularly surveyed for lifestyle and medical history. The authors integrated various factors affecting serum vitamin D levels, including ethnicity, UVB radiation exposure, body mass index, supplementation, and diet, into a predictive model. Their findings did not identify vitamin D as a risk factor for AA [41].
Vitamin D in the Treatment of AA
Recent developments have seen the evaluation of vitamin D analogs, synthetic counterparts of 1,25(OH)2D3, in the treatment of AA. In 2021, Papadimitriou et al. [47] reported on 3 cases of AA, including alopecia totalis, universalis, and focalis, treated with vitamin D analogs (specifically oral calcitriol, procalcitol, and high doses of cholecalciferol) in three young girls aged 1 to 5 years. Notably, none of these patients had concurrent autoimmune skin disease. Various dosing regimens of oral cholecalciferol were administered, followed by oral calcitriol and its analog, paricalcitol. Two of the patients achieved remission at 6 months, and 1 at 3 months, with only 1 patient experiencing recurrence, which subsequently improved upon increasing the dose of calcitriol [47].
In 2012, Kim et al. [48] documented a 7-year-old boy with a single AA patch who responded successfully to topical calcipotriol. After 3 months of treatment at 50 μg/mL, partial and complete regrowth was observed at 6 weeks and 3 months, respectively, with no relapses [48]. Çerman et al. [49] later reported a 75% improvement in moderate-to-severe AA using calcipotriol cream in 48 patients. Similarly, Narang et al. [40] found that 59.1% of 22 patients with patchy AA (<40% scalp involvement) showed hair regrowth with calcipotriol 0.005% lotion, though 18.2% experienced recurrence.
Corticosteroids are central to AA management, yet recent research suggests calcipotriol may offer comparable benefits. Molinelli et al. [50] compared topical calcipotriol 0.005% with clobetasol propionate 0.05% over 24 months, finding faster regrowth and fewer relapses with calcipotriol, without significant efficacy differences.
Alam et al. [51] tested calcipotriol 0.005% ointment combined with mometasone 0.1% cream versus mometasone alone in single patchy AA cases (<10% scalp involvement). The combination therapy improved treatment efficacy (SALT score reduction of 4.24 vs. 3.39 points), though 3 patients developed mild side effects, including erythema and dermatitis [51].
El Taieb et al. [52] evaluated topical calcipotriol, NB-UVB phototherapy, and their combination in 60 AA patients. Both treatments were effective; nonetheless, combining them did not enhance results. Interestingly, calcipotriol increased serum vitamin D levels in some patients. Emerging treatments include intralesional vitamin D3. A 2022 randomized study by Rashad et al. [53] examined its efficacy in 60 focal AA patients. The vitamin D3 group showed significant clinical improvement with no relapses, while mild pain and spot bleeding were the only reported side effects [53].
Despite promising findings, these studies have limitations, including inconsistent disease activity measures and treatment adherence. Most trials involved mild-to-moderate patchy AA. While calcipotriol shows potential as an alternative to steroids and phototherapy, more randomized trials are necessary to refine its role in AA treatment and assess oral supplementation.
Conclusions
AA is a multifactorial inflammatory disorder shaped by autoimmune, environmental, and genetic factors. Increasing evidence highlights the pivotal role of vitamin D in AA pathogenesis and treatment, primarily through its immunomodulatory effects and regulation of follicular homeostasis via VDR interactions. Given these functions, routine vitamin D screening may be warranted, particularly in patients with severe or recurrent disease. While the optimal serum threshold for immune modulation remains undefined, maintaining levels between 20 and 30 ng/mL (50–75 nmol/L) appears reasonable. Vitamin D supplementation may serve as a valuable adjunctive therapy, particularly in deficient individuals, with oral cholecalciferol showing promise.
The strong association between vitamin D deficiency and AA underscores the need for proactive evaluation of vitamin D status in affected patients. Although early studies suggest that vitamin D analogs such as calcipotriol may enhance clinical outcomes and reduce relapse rates, current evidence remains inconclusive. Further large-scale, randomized controlled trials are essential to refine treatment protocols, optimize dosing strategies, and identify patient subgroups most likely to benefit. With continued research, vitamin D could evolve from a supportive measure to a cornerstone therapy in AA management.
Conflict of Interest Statement
This letter has not been published and is not being considered for publication elsewhere, in whole or in part, in any language. All authors have read and approved the submission of the letter in its current form. All authors declare no conflict of interest.
Funding Sources
Rafael Zubiran was supported by the Intramural Research Program of the NHLBI at the National Institutes of Health. No other funding was received from any source during the preparation of this manuscript.
Author Contributions
Erick Alejandro Jiménez-Herrera, Bianca Eunice López-Zenteno, and Ricardo Parra-Guerra wrote the original draft in support with Carlos Barrera-Ochoa and Rafael Zubirán. Eduardo Corona-Rodarte and Luis Enrique Cano-Aguilar ensured scientific accuracy of the paper. Daniel Asz-Sigall was responsible for the design and critical revision of the manuscript. All authors approve the final version of the manuscript for submission.
Funding Statement
Rafael Zubiran was supported by the Intramural Research Program of the NHLBI at the National Institutes of Health. No other funding was received from any source during the preparation of this manuscript.
References
- 1. Pratt H, King L, Messenger A, Christiano AM, Sundberg JP. Alopecia areata. Nat Prim Rev. 2017;3:17011–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miteva M, Villasante A. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiménez-Herrera EA, Rios-Garza Z, Peralta-Pedrero ML, Jurado-Santa Cruz F, Morales-Sánchez MA. Prognostic factors in Mexican patients with patchy and other types of Alopecia Areata. Skin Appendage Disord. 2020;6(5):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018;179(5):1033–48. [DOI] [PubMed] [Google Scholar]
- 5. Lee S, Kim BJ, Lee CH, Lee WS. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(7):1214–21. [DOI] [PubMed] [Google Scholar]
- 6. Anzai A, Wang EHC, Lee EY, Aoki V, Christiano AM. Pathomechanisms of immune-mediated alopecia. Int Immunol. 2019;31(7):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170(6):1299–304. [DOI] [PubMed] [Google Scholar]
- 8. Saini K, Mysore V. Role of vitamin D in hair loss: a short review. J Cosmet Dermatol. 2021;20(11):3407–14. [DOI] [PubMed] [Google Scholar]
- 9. Loh SH, Moon HN, Lew BL, Sim WY. Role of T helper 17 cells and T regulatory cells in alopecia areata: comparison of lesion and serum cytokine between controls and patients. J Eur Acad Dermatol Venereol. 2018;32(6):1028–33. [DOI] [PubMed] [Google Scholar]
- 10. Fawaz L, Mrad MF, Kazan JM, Sayegh S, Akika R, Khoury SJ. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. 2016;166-167:59–71. [DOI] [PubMed] [Google Scholar]
- 11. Yilmaz N, Serarslan G, Gokce C. Vitamin D concentrations are decreased in patients with alopecia areata. Vitam Miner. 2012;1:105–9. [Google Scholar]
- 12. Zhang S, Miller DD, Li W. Non-musculoskeletal benefits of vitamin D beyond the musculoskeletal system. Int J Mol Sci. 2021;22(4):2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertolini M, Pretzlaff M, Sulk M, Bähr M, Gherardini J, Uchida Y, et al. Vasoactive intestinal peptide, whose receptor-mediated signalling may be defective in alopecia areata, provides protection from hair follicle immune privilege collapse. Br J Dermatol. 2016;175(3):531–41. [DOI] [PubMed] [Google Scholar]
- 14. Abdel Fattah NSA, Ebrahim A, El Okda ES. Lipid peroxidation/antioxidant activity in patients with alopecia areata. J Eur Acad Dermatol Venereol. 2011;25(4):403–8. [DOI] [PubMed] [Google Scholar]
- 15. Mijailović B, Mladenović T, Hrnjak M, Karadaglić D, Nikolić B. Kontaktna termometrija lezija alopecije areate [Contact thermometry of lesions in alopecia areata]. Vojnosanit Pregl. 1997;54(1):31–3. [PubMed] [Google Scholar]
- 16. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(12):3908 [published correction appears in J Clin Endocrinol Metab. 2024 Sep 16;109(10):e1991. [DOI] [PubMed] [Google Scholar]
- 17. Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess. 2009;183:1–420. [PMC free article] [PubMed] [Google Scholar]
- 18. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 19. Henry HL, Bouillon R, Norman AW, Gallagher JC, Lips P, Heaney RP, et al. 14th Vitamin D Workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2010;121(1–2):4–6. [DOI] [PubMed] [Google Scholar]
- 20. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2015;148:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fawzi MM, Mahmoud SB, Ahmed SF, Shaker OG. Assessment of vitamin D receptors in alopecia areata and androgenetic alopecia. J Cosmet Dermatol. 2016;15(4):318–23. [DOI] [PubMed] [Google Scholar]
- 23. Amor KT, Rashid RM, Mirmirani P. Does D matter? The role of vitamin D in hair disorders and hair follicle cycling. Dermatol Online J. 2010;16(2):3. [PubMed] [Google Scholar]
- 24. Daroach M, Narang T, Saikia UN, Sachdeva N, Sendhil Kumaran M. Correlation of vitamin D and vitamin D receptor expression in patients with alopecia areata: a clinical paradigm. Int J Dermatol. 2018;57(2):217–22. [DOI] [PubMed] [Google Scholar]
- 25. Putterman E, Castelo-Soccio L. Seasonal patterns in alopecia areata, totalis, and universalis. J Am Acad Dermatol. 2018;79(5):974–5. [DOI] [PubMed] [Google Scholar]
- 26. Wilson-Barnes SL, Hunt JEA, Williams EL, Allison SJ, Wild JJ, Wainwright J, et al. Seasonal variation in vitamin D status, bone health and athletic performance in competitive university student athletes: a longitudinal study. J Nutr Sci. 2020;9:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao J, Sheng Y, Dai C, Qi S, Hu R, Rui W, et al. Serum 25 hydroxyvitamin D levels in alopecia areata, female pattern hair loss, and male androgenetic alopecia in a Chinese population. J Cosmet Dermatol. 2020;19(11):3115–21. [DOI] [PubMed] [Google Scholar]
- 28. Megiorni F, Mora B, Maxia C, Gerardi M, Pizzuti A, Rossi A. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in Alopecia Areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305(7):665–70. [DOI] [PubMed] [Google Scholar]
- 29. Tran PT, Chen A, Yi L, Goh C. Vitamin D levels in alopecia areata and other alopecias: a retrospective case–control study at a single institution. Int J Trichol. 2022;14(5):175–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim RK, Castelo-Soccio L, Putterman E, Qureshi AA, Cho E. Predictors of vitamin D insufficiency in children and adolescents with alopecia areata. Cureus. 2022;14(3):e22934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharifi A, Vahedi H, Honarvar MR, Alipoor B, Nikniaz Z, Rafiei H, et al. Vitamin D increases CTLA-4 gene expression in patients with mild to moderate ulcerative colitis. Middle East J Dig Dis. 2019;11(4):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rehman F, Dogra N, Wani MA. Serum vitamin D levels and alopecia areata- A hospital based case-control study from north-India. Int J Trichology. 2019;11(2):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsai TY, Huang YC. Vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;78(1):207–9. [DOI] [PubMed] [Google Scholar]
- 34. Gade VKV, Mony A, Munisamy M, Chandrashekar L, Rajappa M. An investigation of vitamin D status in alopecia areata. Clin Exp Med. 2018;18(4):577–84. [DOI] [PubMed] [Google Scholar]
- 35. Darwish NMM, Marzok HF, Gaballah MAM, Abdellatif HE. Serum level of vitamin D in patients with alopecia areata. Egypt J Basic Appl Sci. 2017;4(1):9–14. [Google Scholar]
- 36. Bhat YJ, Latif I, Malik R, Hassan I, Sheikh G, Lone KS, et al. Vitamin D level in alopecia areata. Indian J Dermatol. 2017;62(4):407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erpolat S, Sarifakioglu E, Ayyildiz A. 25-hydroxyvitamin D status in patients with alopecia areata. Postepy Dermatol Alergol. 2017;34(3):248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghafoor R, Anwar MI. Vitamin D deficiency in alopecia areata. J Coll Physicians Surg Pak. 2017;27(4):200–2. [PubMed] [Google Scholar]
- 39. Bakry OA, El Farargy SM, El Shafiee MK, Soliman A. Serum Vitamin D in patients with alopecia areata. Indian Dermatol Online J. 2016;7(5):371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Narang T, Daroach M, Kumaran MS. Efficacy and safety of topical calcipotriol in management of alopecia areata: a pilot study. Dermatol Ther. 2017;30(3):e12464. [DOI] [PubMed] [Google Scholar]
- 41. Thompson JM, Li T, Park MK, Qureshi AA, Cho E. Estimated serum vitamin D status, vitamin D intake, and risk of incident alopecia areata among US women. Arch Dermatol Res. 2016;308(9):671–6. [DOI] [PubMed] [Google Scholar]
- 42. Mahamid M, Abu-Elhija O, Samamra M, Mahamid A, Nseir W. Association between vitamin D levels and alopecia areata. Isr Med Assoc J. 2014;16(6):367–70. [PubMed] [Google Scholar]
- 43. d’Ovidio R, Vessio M, d’Ovidio FD. Reduced level of 25-hydroxyvitamin D in chronic/relapsing Alopecia Areata. Dermatoendocrinol. 2013;5(2):271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nassiri S, Saffarian Z, Younespour S. Association of vitamin D level with alopecia areata. Iran J Dermatol. 2013;16(1):1–5. [Google Scholar]
- 45. Narang T, Daroach M, Sendhil M. Efficacy and safety of topical calcipotriol in management of alopecia areata: a pilot study. Dermatol Ther. 2016;30(3). [DOI] [PubMed] [Google Scholar]
- 46. Lizarondo FPJ, Gervasio MKR, Chamberlin CVS, Gnilo CMS, Silva CY. Determination of serum 25-hydroxyvitamin D levels in patients with alopecia areata and their comparison with levels in healthy controls: a cross-sectional study. JAAD Int. 2021;5:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Papadimitriou DT, Bothou C, Dermitzaki E, Alexopoulos A, Mastorakos G. Treatment of alopecia totalis/universalis/focalis with vitamin D and analogs: three case reports and a literature review. World J Clin Pediatr. 2021;10(6):192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim DH, Lee JW, Kim IS, Choi SY, Lim YY, Kim HM, et al. Successful treatment of alopecia areata with topical calcipotriol. Ann Dermatol. 2012;24(3):341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Çerman AA, Solak SS, Altunay İ, Küçükünal NA. Topical calcipotriol therapy for mild-to-moderate alopecia areata: a retrospective study. J Drugs Dermatol. 2015;14(6):616–20. [PubMed] [Google Scholar]
- 50. Molinelli E, Campanati A, Brisigotti V, Sapigni C, Paolinelli M, Offidani A. Efficacy and safety of topical calcipotriol 0.005% versus topical clobetasol 0.05% in the management of alopecia areata: an intrasubject pilot study. Dermatol Ther. 2020;10(3):515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alam M, Amin SS, Adil M, Arif T, Zahra FT, Varshney I. Comparative study of efficacy of topical mometasone with calcipotriol versus mometasone alone in the treatment of alopecia areata. Int J Trichology. 2019;11(3):123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. El Taieb MA, Hegazy EM, Ibrahim HM, Osman AB, Abualhamd M. Topical calcipotriol vs narrowband ultraviolet B in treatment of alopecia areata: a randomized-controlled trial. Arch Dermatol Res. 2019;311(8):629–36. [DOI] [PubMed] [Google Scholar]
- 53. Rashad AF, Elgamal E, Fouda I. Intralesional vitamin D3 in treatment of alopecia areata: a randomized controlled clinical trial. J Cosmet Dermatol. 2022;21(10):4617–22. [DOI] [PubMed] [Google Scholar]