Abstract
The small airways comprise generations 8 to 23 of the bronchial tree, consist of airways with an internal diameter < 2 mm, and are classically difficult to assess and treat in persistent asthma. Small airways dysfunction (SAD) is integral to the asthma management paradigm because it is associated with poorer symptom control and greater levels of type 2 inflammation, and it has been proposed as a potentially treatable asthma trait. Although identification of SAD based on oscillometry has been found to be clinically useful in managing asthma, very few physicians, including specialists, use this technique as part of standard or adjunct evaluation of lung function to diagnose asthma, grade severity of airway obstruction, ascertain disease control or the risk for future exacerbations, or to make management decisions. To rectify the unrecognized value of oscillometry in the asthma community, a consortium of authors who are investigators with knowledge and experience of oscillometry wished to address the most important clinical questions raised by our colleagues who are considering using this technique, including its clinical utility. In this article, we discuss integral concepts, including applicability of oscillometry as a predictive tool for asthma exacerbations and disease control, adequacy of spirometry and oscillometry in assessing SAD, potential limitations of oscillometry, and treatment options for SAD.
Key Words: asthma, oscillometry, small airways dysfunction
Many patients with a confirmed diagnosis of asthma have uncontrolled disease despite appropriate antiinflammatory treatment.1 Among many potential reasons for this scenario is that the presence of unrecognized small airways dysfunction (SAD) confers poorer symptom control2 and greater levels of type 2 (T2) inflammation,3 and it has been proposed as a potential treatable asthma trait.4 Importantly, SAD is poorly recognized by spirometry.5 Consequently, the presence of SAD is rarely adequately investigated prior to escalating treatment to high-dose or very-high-dose inhaled corticosteroid (ICSs).6
Spirometry has been the gold standard for diagnosing and monitoring asthma. It has many strengths, including standards for manufacturers to ensure consistent measurements across instruments, for reproducibility and quality criteria during measurements, and for interpretation (including cutoff values for severity and reversibility), as well as for Global Lung Function Initiative reference values applicable to all individuals according to age, height, and sex.7,8
Spirometry requires patients to perform a forced expiratory maneuver to measure the volume of air that can be expired with maximal effort. Because it requires understanding, cooperation, and coordination, this technique is not applicable in young children and is difficult to reproducibly master in acutely unwell individuals or those with poor coordination.9 Other approaches, such as airway oscillometry, measure obstruction to airflow during regular tidal breathing, which better reflects the prevailing breathing pattern of an individual; it requires minimal cooperation and is obtained more easily and reproducibly in young children, acutely ill individuals, and those with coordination difficulties.
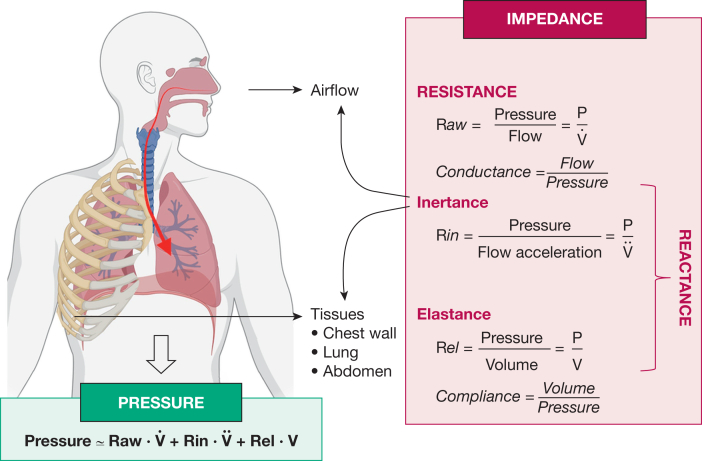
The technique superimposes sound or air waves onto normal tidal breathing and based on respiratory mechanics, provides information on resistance and reactance (Fig 1).10 Resistance largely reflects friction to airflow in the airways, whereas reactance mainly expresses the elasticity or stiffness of the respiratory system resulting primarily from the airways and lung tissues, as well as the heterogeneity of regional mechanical properties. The use of multiple wave frequencies permits better delineation of the location (peripheral vs central) of airway obstruction by exploring the impedance frequency dependency.11 An important component of peripheral airway involvement originates from the small airways, which comprise generations 8 to 23 of the bronchial tree and are defined as airways < 2 mm in internal diameter. Oscillometry is a useful test to detect SAD and, because of the portable equipment available, it can be performed in any health care setting.
Figure 1.
The first component of respiratory impedance is resistance, which is largely dependent on airway caliber. Impedance also consists of reactance, which itself comprises inertance and elastance. Inertance represents measurement of pressure losses mostly resulting from acceleration of the gas column in proximal bronchi, whereas elastance is an assessment of lung stiffness as it can be calculated as the inverse of compliance. (Reprinted, with permission, from Gochicoa-Rangel and Vargas.10)
Although identification of SAD by oscillometry has been found clinically useful in managing asthma,12 very few physicians, including specialists, use this technique as part of standard or adjunct evaluation of lung function to diagnose asthma, grade severity of airway obstruction, ascertain asthma control and the risk for future exacerbations, and make management decisions. Although first described in 1956, oscillometry is clearly not well known in the medical community. This is partly because until recently, research and the subsequent official statements13 have mainly focused on its application in patients unable to cooperate with spirometry, primarily preschool-aged children, perpetuating the perception of this technique as a substitute, only to use until or when spirometry could be performed. However, other advantages of oscillometry include greater sensitivity for detecting obstructive airway disease, bronchodilator response, airway hyperresponsiveness, and treatment responses, as well as providing insight into lung mechanics.14 Furthermore, there are now established minimal clinically important difference values for oscillometry.15,16
Ultimately, oscillometry and spirometry complement each other, giving rise to resistance and reactance as well as flow and volumes, respectively. However, the inability of spirometry to serve as the gold standard to compare with oscillometry and the absence of objective asthma diagnostic criteria in preschool-aged children further impaired confirmation of the clinical usefulness of oscillometry despite excellent psychometric properties.12 It is thus not surprising that the approach is seldom mentioned by national and international guidelines.
To rectify the unrecognized value of oscillometry in the asthma community, a consortium of authors who as investigators have contributed to the current knowledge of oscillometry wished to address the most important clinical questions raised by their colleagues who are considering using this technique, including its clinical utility. The major indications for evaluating SAD with oscillometry are: diagnosing asthma,11 assessing disease control,17 and assessing response to therapy.18 Therefore, airway oscillometry could be considered for assessment of any patient at every asthma review.
How I Do It
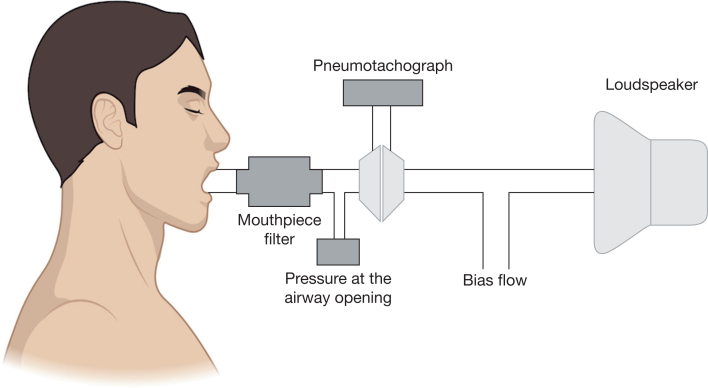
Airway oscillometry is an easy-to-perform pulmonary function test that superimposes sound or air waves onto physiologic breathing to obtain readings such as airway resistance and lung compliance. Multiple devices are available commercially. Because forced or prolonged expiratory maneuvers such as those required by spirometry and fractional exhaled nitric oxide (Feno) measurements directly affect airway geometry, it is required that oscillometry be performed first, prior to any additional lung function testing. Nose clips are a necessity, as well as asking the patient to place their hands on their cheeks, with a slight head tilt upward. Patients are then asked to breathe through a mouthpiece normally for 20 to 30 seconds, ideally with 3 repeated measurements. The individual components of an oscillometer are shown in Figure 2.10 The mean value for each parameter is then accepted if it adheres to a coefficient of variability criteria ≤ 10% for adults and ≤ 15% in children.11
Figure 2.
Oscillometry determines breathing mechanics by superimposing small external pressure signals generated from the loudspeaker onto the spontaneous breathing of the individual at the mouthpiece. Flow is measured by a heated screen pneumotachograph. (Reprinted, with permission, from Gochicoa-Rangel and Vargas.10)
Case
A 26-year-old medical student (height, 165 cm; BMI, 24 kg/m2; never used tobacco use) attended a severe asthma center for persistent cough and breathing difficulties, despite high-dose fluticasone furoate at a daily beclomethasone dipropionate equivalent dose of 2000 μg, vilanterol, umeclidinium, montelukast, and fexofenadine. In the clinic, the patient’s inhaler technique was optimal, and she was adherent to her medication assessed according to the medication possession ratio. However, the patient’s Asthma Control Questionnaire score was 4.0 (optimal value, 0; worst value, 6) and she had required 2 courses of oral corticosteroids over the past 12 months for exacerbations. Investigations were as follows: FEV1, 2.88 L 85% predicted; FEV1/FVC, 0.84; resistance at 5Hz (Rrs5), 0.42 kPa/L per second (z score, 2.1); difference in resistance between 5 and 20 Hz (Rrs5-20), 0.09 kPa/L per second (z score, 1.9); reactance at 5 Hz (Xrs5), –0.19 kPa/L per second (z score, –2.0); area under the reactance curve (AX), 1.34 kPa/L (z score, 1.96); Feno, 95 ppb; blood eosinophil count, 540 cells/μL; total IgE, 366 kU/L; aspergillus IgG, 39 mg/mL, and aspergillus IgE, 0.03 kU/L. High-resolution CT imaging reveals minor mucus plugging but no other significant abnormality.
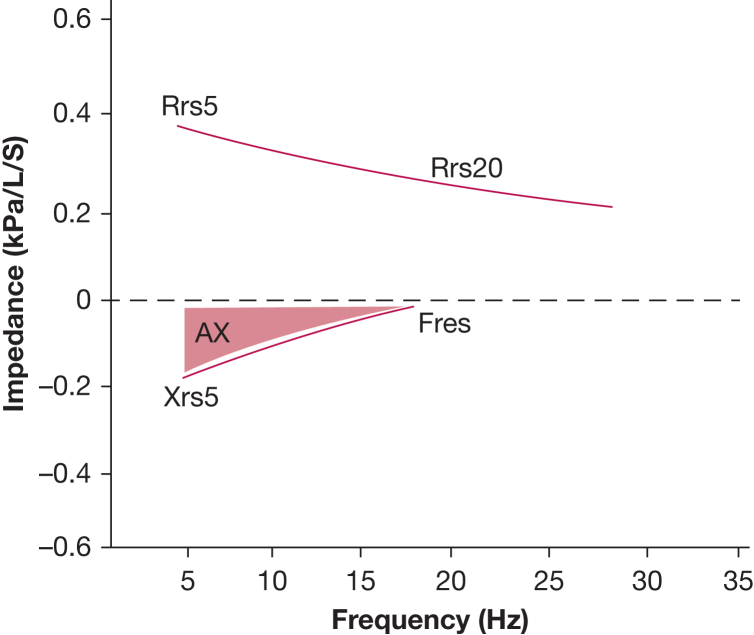
In this case, the patient presented with preserved spirometry but high T2 inflammatory biomarkers with evidence of SAD, identified by oscillometry. Due to the United Kingdom’s eligibility criteria for biologics generally requiring at least 4 exacerbations in the past 12 months, the attending physician switched the patient’s inhalers to extra-fine beclomethasone dipropionate, formoterol fumarate, and glycopyrronium bromide at the same beclomethasone dipropionate equivalent dose of ICSs.19 The patient returned 6 months later with no further exacerbations and an Asthma Control Questionnaire score of 1.4. Her oscillometry measurements were also markedly improved: Rrs5-20, 0.05 kPa/L per second (z score, 0.75); Xrs5, –0.09 kPa/L per second (z score, 0.4); and AX, 0.49 kPa/L (z score, 0.33); these between-visit changes exceeded biological variability values in adults of 0.04 kPa/L per second for Rrs5-20 and 0.39 kPa/L for AX.15 An example oscillogram is shown in Figure 3.
Figure 3.
Oscillogram depicting resistance and reactance curves. The difference in resistance between 5 Hz (Rrs5) and 20 Hz (Rrs20) corresponds to peripheral airway resistance (Rrs5-20). The resonant frequency (Fres) is the frequency at which reactance equals zero. The area under the curve between reactance at 5 Hz (Xrs5) and Fres corresponds to the reactance area (AX).
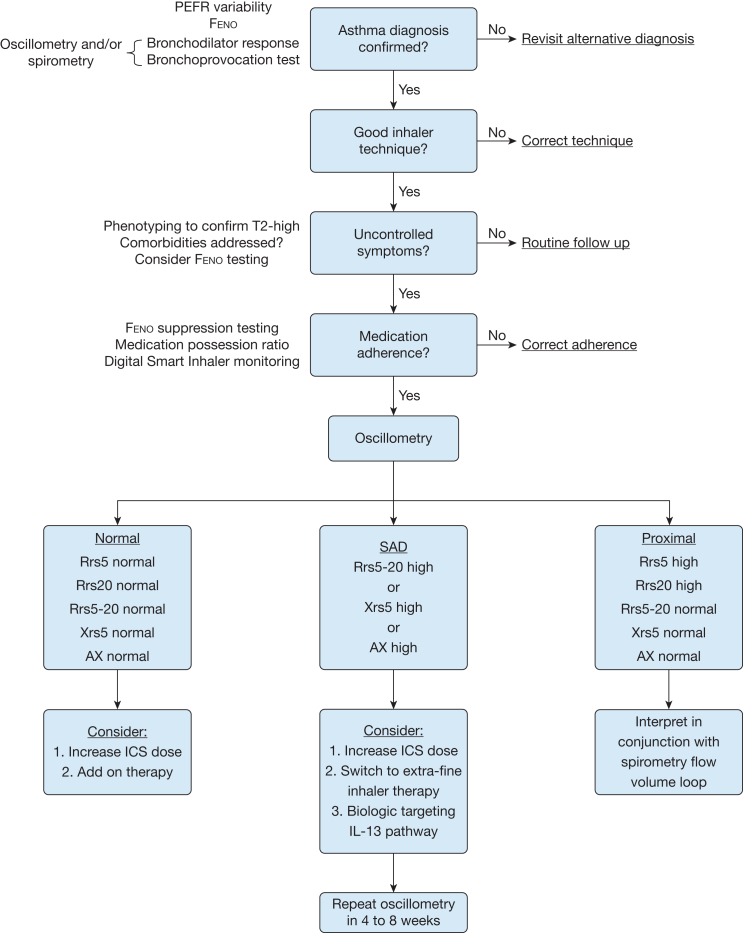
After confirming asthma diagnosis and good inhaler technique, if a patient attends clinic with uncontrolled symptoms despite objective evidence of medication adherence, we recommend further assessment with airway oscillometry. This could be offered in conjunction with Feno and spirometry as point-of-care investigations.
Normal impedance would be characterized by all oscillometry parameters being within normal range. Further phenotyping with Feno, blood eosinophil counts, and total IgE may be helpful. In the absence of elevated T2 inflammation markers, uncontrolled symptoms might be related to pathophysiology-driven T2 inflammatory low and steroid-insensitive disease. With a T2 high signature, increasing the ICS dose may provide adequate suppression of airway inflammation.
SAD is reflected by high Rrs5, Xrs5, and AX values; as resistance at 20 Hz remains within normal limits, the Rrs5-20 is also elevated. Options to treat SAD include an increase in ICS dose or switching to extra-fine therapy, which has been shown to improve symptoms and airway inflammation.20,21 There is some evidence that suppressing IL4/13-mediated inflammation is effective at improving SAD.18,22,23 If oscillometry identifies proximal airway disease, we would recommend interpreting this result in the context of the spirometric flow volume loop because it may detect intrathoracic or extrathoracic upper airway obstruction (Fig 4).
Figure 4.
Proposed clinical algorithm to guide interpretation of airway oscillometry. AX = area under the reactance curve; Feno = fractional exhaled nitric oxide; ICS = inhaled corticosteroid; PEFR = peak expiratory flow rate; Rrs5 = resistance at 5 Hz; Rrs5-20 = difference in resistance between 5 and 20 Hz; Rrs20 = resistance at 20 Hz; SAD = small airways dysfunction; T2 = type 2 high; Xrs5 = reactance at 5 Hz.
Consortium Response to the State of Evidence
Despite evidence-based asthma guidelines, a considerable number of adults and children with asthma still experience poor disease control associated with an increased risk of asthma exacerbations, pulmonary function loss, and increased health care utilization.24 Poor control in the presence of normal spirometry should call for the investigation of possible undetected SAD as well as other comorbidities and lifestyle factors, including nonadherence. Spirometry measurement of FEV1 provides a good assessment of larger airways but is less sensitive to SAD even with the use of mid-expiratory flow as forced expiratory flow at 25% to 75% of FVC (FEF25-75) and FEV1/FVC ratio.9 Indeed, the peripheral airways are the major (and sometimes only) sites of airway obstruction as evidenced by a reported overall clinic-based prevalence of SAD affecting at least 50% of adults and children with persistent asthma.25,26
Utility of Oscillometry as a Predictive Tool
Oscillometry has been shown to be a practical in-office device that has been validated by several established measures of peripheral airway function.27 It is effort independent, measures impedance, including resistance and reactance, and has been shown to be effective in identifying asthma in young children28 and adults29 and uncontrolled asthma.25,30 It is also effective at predicting loss of asthma control,31 risk of exacerbations,32 and future loss of lung function in adolescents.33
Using oscillometry, heterogeneity in Rrs5-20, low-frequency Xrs5, or AX between 5 Hz and the resonant frequency can also be used to assess the small airways.34 Xrs5 and AX are thought to reflect peripheral lung compliance, which is reduced in patients with SAD; these parameters also reflect mechanical heterogeneity that may involve the central airways. In adult patients with moderate to severe persistent asthma who were not taking biologics, the presence of abnormal values of either Rrs5-20 ≥ 0.10 kPa/L per second or AX ≥ 1.0 kPa/L were associated with worse disease control as measured by using the Asthma Control Questionnaire score.35 It has previously been shown that Rrs5-20 is a direct measure of anatomical narrowing of the small airways by computational modeling.36 Although certain parameters such as Rrs5 or resistance at 20 Hz are used more often in this article, it is worth noting that different frequencies are also utilized depending on the age of the patient (eg, resistance at 7 Hz or 8 Hz to account for breathing interference in young patients) or type of device.
In a large cohort of adults with and without asthma, the Assessment of Small Airways Involvement in Asthma (ATLANTIS) studies reported that SAD determined by oscillometry was present in a large percentage of patients with asthma across the Global Initiative for Asthma treatment spectrum; a higher prevalence of, and more severe, SAD was observed in individuals with more severe asthma.2,24,25 The results showed a significant association between SAD established by oscillometry and clinically important outcomes, including symptom control and exacerbation frequency. Indeed in a 12-month longitudinal follow-up study, a composite ordinal score of abnormal resistance (Rrs5-R20) and reactance (AX and Xrs5) independently predicted future asthma control and exacerbations in a multivariate analysis, whereas FEV1 was not retained as a significant predictor in the model following adjustment for the ordinal oscillometry score.32
The consortium concluded that the use of oscillometry in clinical practice could increase the health providers’ ability to determine the presence of SAD in their patients and those at higher risk for asthma exacerbation. Their recommendation was that SAD ascertained by oscillometry should be added to the list of risk factors of poor asthma outcomes. One study also found that measuring SAD by oscillometry in patients with asthma aged between 17 and 85 years, with an FEV1 < 80%, resulted in better identification of poor asthma control and exacerbations than either factor alone.17 These findings strongly suggest that evaluation for oscillometry SAD should be considered in any future asthma guideline program.
Spirometry Does Not Adequately Assess SAD
Despite its convenience, FEF25-75 is not an optimal way of measuring small airways function due to its volume dependence, inherent variability, and lack of specificity.9 Moreover, this parameter is an assessment of moderate to larger airways. In a study of patients with asthma with preserved spirometry (mean FEF25-75 91% and mean FEV1/FVC 0.79),5 oscillometry identified patients with worse symptoms and greater levels of T2 inflammation than FEF25-75. In that study, a cutoff value of FEF25-75 < 65% predicted correctly identified only 2.5% of patients with SAD, whereas an oscillometry threshold of Rrs5-20 > 0.07 kPa/L per second identified 46.6% (nearly 19-fold more). Indeed, no relationship between FEF25-75 percent predicted and peripheral airway inflammation by trans-bronchial biopsy has been documented.37
It is well known that spirometry does not exhibit good correlation with asthma symptoms, possibly due to its poor sensitivity for detecting SAD, which presents with symptoms perceived by patients. In 1 pediatric study, 41% of children with asthma had evidence of SAD despite good symptom control.38 Using receiver-operating characteristic analysis, the addition of FEF25-75 < 65% to Xrs5 did not significantly increase sensitivity to detect SAD. In this study, Xrs5 was superior to FEF25-75 at detecting SAD in children aged 4 to 18 years.39
Although FEV1/FVC is useful for evaluating airway obstruction, it is less sensitive than oscillometry for identifying SAD.5 In patients in whom FEV1 or FEV1/FVC was greater than or equal to the lower limit of normal, there was complete consensus among the consortium that oscillometry would identify additional patients with SAD than possible with spirometry alone. This is exemplified by a study reporting worse symptom control and more frequent asthma exacerbations in those with combined impairment of spirometry and oscillometry compared with abnormal spirometry alone.35
Potential Limitations of Oscillometry
What are the limitations of oscillometry? Perhaps the most important limitation is the lack of Global Lung Function Initiative reference equations as established for spirometry.40 Of note, a common concern is the perception that reference values may be ethnicity specific. However, we reported a > 80% agreement between reference equations developed in Hispanic participants compared with White children for Rrs5, AX, and Xrs5.24 Furthermore, we found minimal variation in impulse oscillometry reference values for Rrs5 and Xrs5 according to ethnicity in Asian, European, and North and South American children and adults.41 Values in the latter study were comparable to commercially available, age-appropriate regression equations based on reference values determined primarily in White children and adults, suggesting potential application across populations. In this regard, we were able to replicate these conclusions in a separate cohort.42
Although there can be significant differences in reference values in various populations of normal children compared with those developed in a specific population,43 we validated equations from different populations with asthma by determining which equation best identified uncontrolled asthma.44 This offers a practical approach to establish which equations best fit the provider’s asthma population and again found no significant differences between Hispanic and White populations.
Another potential problem is a lack of uniform measurement across oscillometer devices. Indeed, it has been repeatedly shown that reference values can vary significantly in different devices in the same pediatric population,42,45 suggesting a great need for harmonization of devices. Recently, it was suggested that oscillometry ratios might reduce issues related to comparative bias when interpreting absolute values from different devices.46 An example of this was shown in a cohort of patients with asthma who underwent airway oscillometry using 2 commercially available devices.47 In this study, there were interdevice differences in resistance and reactance measurements in the same patients. In this regard, assessing peripheral airway resistance as a percentage of total airway resistance as Rrs5-20/Rrs5 might perhaps obviate this bias. The results of the original study46 were inconclusive, and larger prospective studies assessing this ratio are therefore indicated.
Most of the consortium agreed with the utilization of reference equations validated for their specific oscillometry device, whereas one-half of the group used equations already developed for a similar population. In sum, the data presented strongly suggest that, despite some work in progress issues, identification of SAD with oscillometry is currently ready to be included as part of clinical practice to establish asthma severity as well as identify disease control status and risk of exacerbations.
Technology Readiness, Physician Interpretability, and Device Standardization
Airway oscillometry technology has seen significant advances, with devices now capable of providing high-quality measurements that are easy to perform and reproducible. The readiness of this technology for widespread clinical use depends on factors such as the availability of reliable, user-friendly equipment, the speed and accuracy of data collection, and its integration within existing electronic health record systems. Modern oscillometry devices are portable, require minimal patient cooperation, and produce results quickly, making them suitable for both clinical and research settings. However, limitations remain, including cost of the device.
One of the challenges with adopting airway oscillometry is ensuring that physicians can easily interpret the results. For effective use in clinical practice, clear, standardized interpretation guidelines or algorithms are essential. Training programs for health care providers can also help improve their understanding of oscillometry. Furthermore, physicians using oscillometry should be aware of artifacts from breathing interference, particularly when interpreting low-frequency resistance in children aged < 8 years.11
To ensure consistent and reliable results across different settings, device standardization is crucial. Standardization efforts should include agreed-upon methodologies for device calibration, test protocols, and interpretation of results.11 Collaborative efforts between manufacturers and physicians can help create uniform guidelines for device operation, performance, and result interpretation. Oscillometry ratios such as the Rrs5-20/Rrs5 have been proposed to obviate the risk of between-device bias, and they now need to be validated in other populations and settings.46
Treatment Options in Patients With Confirmed SAD
SAD seems to be a treatable trait that has successfully been managed with extra-fine ICSs.48 The identification of SAD could guide therapeutic decisions. For example, in the presence of SAD, several options are available. First, it is always good clinical practice to ascertain correct inhaler technique and adherence. Second, one might consider using an inhaled corticosteroid with a smaller particle size that better reaches the peripheral airways.49 However, it is worth emphasizing here that no head-to-head comparisons of the same drug in 2 different formulations has yet been performed to show that targeting SAD improves clinical outcomes compared with a nontargeted approach.
The peripheral airways have a high density of both beta2-adrenergic agonists and corticosteroid receptors.41 Aerosols with a mass median aerodynamic diameter of 0.8 to 3 μm are thought to be appropriate for reaching the peripheral airways,50 with extra-fine ICSs having a mass median aerodynamic diameter < 2 μm.49 Although there have been studies showing the clinical benefit of extra-fine ICSs,51 and others showing greater improvement of SAD with extra-fine ICSs,41 few studies have shown improvements in both clinical benefits and SAD.48 Third, stepping up therapy as ICSs or the addition of another agent may be considered to prevent further morbidity.5
From the ATLANTIS study, in which the prevalence of SAD increased with increasing Global Initiative for Asthma-defined treatment steps (ie, reflecting asthma severity), it is worthwhile considering stepping up extra-fine ICS therapy.25 In this regard, a randomized controlled trial of patients with more severe asthma and preserved spirometry (FEV1 ≥ 80%) showed that budesonide/formoterol as finer particle ICSs conferred improvements in both peripheral airway resistance (Rrs5-20) and reactance (AX) as well as asthma control test scores compared with fluticasone propionate/salmeterol.48
Due to lack of supportive evidence, most of us agreed that step-up ICS therapy would not be indicated in the immediate setting if a patient was entirely asymptomatic but exhibited evidence of SAD. Switching to an extra-fine formulation of the same dose would be theoretically more appropriate here, although the consortium admits that trial evidence is lacking in this area. The exception in our recommendation perhaps would be if a patient had previously presented with a frequent exacerbation history or evidence of significant airway inflammation as a markedly raised Feno. Evaluation of adherence and inhaler technique was suggested prior to systematically switching to extra-fine ICSs to reduce the likelihood of airway remodeling in children with asthma.
One author suggested that there is already ample evidence portraying SAD in those without symptoms as an independent risk factor for exacerbations and poor asthma control.32 Therefore, it is thought that patients should be started on low-dose, extra-fine ICS therapy or have their treatment escalated by 1 step even in the absence of symptoms or abnormal spirometry. The rationale here is that preventing exacerbations and future lung function decline outweighs the risks of adverse events.52
The consortium unanimously agreed that stepping up extra-fine ICS therapy would require an evaluation of risk vs benefit. Factors in favor of escalating therapy include the presence of symptoms and more severe SAD.
Lastly, there is some evidence that the presence of SAD may influence the choice of biologic used. A recent case matching indirect comparison of the anti-IL-5 receptor alpha benralizumab vs the anti-IL-4 receptor alpha dupilumab has shown the latter to be more effective at improving SAD measured by Rrs5-20 and AX in those selected a priori for SAD.18 The mechanism for this would be due, at least in part, to the effect of IL13 inhibition on bronchial smooth muscle tone and possibly mucus plugging. However, we are not aware of any studies using SAD to inform biologic choice.
Thus, the need exists for randomized clinical trials comparing extra-fine ICSs vs non-extra-fine ICSs in improving SAD and asthma clinical status in children and adults with persistent asthma. This would be important not only clinically but would also show the central role of SAD in determining the clinical state of asthma control and exacerbations.
Priorities for Future Research
Although oscillometry exhibits good properties, some research gaps remain. First, a head-to-head comparison of current standard of care based on spirometry in terms of asthma diagnosis and management vs an SAD-directed approach using oscillometry would be desired, ideally powered on asthma control. Second, there is a significant need for harmonization between different oscillometry devices; until then, one should systematically use device-specific reference equations. Any future exacerbation risk prediction score integrating oscillometry will have to account for this interdevice heterogeneity or possibly use parameters reported as a ratio to obviate the between-device bias. Third, similar to cutoff points being applied to diagnose bronchodilator response for oscillometry based on 95% CIs in the healthy population, we need to confirm oscillometry thresholds for airway hyperresponsiveness. Oscillometry has the advantage of being more sensitive than spirometry, and therefore less constrictor agonist agent would be required to diagnose asthma using airway hyperresponsiveness per se.
Conclusions
The evidence suggests a clear advantage for including airway oscillometry into clinical practice for more accurate assessment, prediction of those at risk of deterioration, and management decision guidance. Clinicians would benefit from greater knowledge in terms of advantages and limitations to prescribe and interpret oscillometry. Further studies are ongoing to investigate the additive value of oscillometry to conventional spirometry and Feno. If a patient is not clinically responding to therapy despite normal spirometry, this is a strong indication for oscillometry. We recommend that oscillometry be considered for use in specialized care and community practice.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: R. C. reports institutional grants from Chiesi; advisory boards for AstraZeneca; personal fees (talks) from AstraZeneca, Chiesi, and Thorasys; support for attending meetings from AstraZeneca, Chiesi, NIOX, and Sanofi-Regeneron; and honoraria (drafting educational material) from AstraZeneca and Vitalograph. L. G.-R. reports personal fees for conferences from GlaxoSmithKline, Chiesi, and Thorasys; and support for attending European Respiratory Society Congress from Chiesi. E. A. G. has received institutional grants from Gilead, Circassia, Chiesi, Propeller Health, Helicon Health, Adherium Ltd, and AstraZeneca; and personal fees from Circassia and Sanofi. F. M. D. has received unrestricted research funds from AstraZeneca, Covis Pharma, GlaxoSmithKline, Merck Canada, Novartis, Teva, Trudell Medical, GlaxoSmithKline, and MEDTEQ in partnership with Thorasys Inc; honoraria for consultancy work from AstraZeneca, Covis Pharma, Sanofi, Teva, and Thorasys Inc; and honoraria as an invited speaker from Covis Pharma, Jean-Coutu Pharmacy, and Brunet Pharmacy. None declared (M. C., P. C., S. P. G.).
References
- 1.Bateman E.D., Boushey H.A., Bousquet J., et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 2.Cottini M., Licini A., Lombardi C., Berti A. Prevalence and features of IOS-defined small airway disease across asthma severities. Respir Med. 2021;176 doi: 10.1016/j.rmed.2020.106243. [DOI] [PubMed] [Google Scholar]
- 3.Kuo C.R., Jabbal S., Lipworth B. Is small airways dysfunction related to asthma control and type 2 inflammation? Ann Allergy Asthma Immunol. 2018;121(5):631–632. doi: 10.1016/j.anai.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Cottini M., Lombardi C., Comberiati P., et al. Oscillometry defined small airways dysfunction as a treatable trait in asthma. Ann Allergy Asthma Immunol. 2025;134(2):151–158. doi: 10.1016/j.anai.2024.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Cottini M., Bondi B., Bagnasco D., et al. Impulse oscillometry defined small airway dysfunction in asthmatic patients with normal spirometry: prevalence, clinical associations, and impact on asthma control. Respir Med. 2023;218 doi: 10.1016/j.rmed.2023.107391. [DOI] [PubMed] [Google Scholar]
- 6.Global Initative for Asthma, Main Report. 2024. Global Initative for Asthma website. Accessed June 1, 2024. https://ginasthma.org/2024-report/
- 7.Graham B.L., Steenbruggen I., Miller M.R., et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quanjer P.H., Stanojevic S., Cole T.J., et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the Global Lung Function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen J.E., Sun X.G., Wasserman K. Discriminating measures and normal values for expiratory obstruction. Chest. 2006;129(2):369–377. doi: 10.1378/chest.129.2.369. [DOI] [PubMed] [Google Scholar]
- 10.Gochicoa-Rangel L., Vargas M.H. How best to choose an oscillometer and reference equations for your patients with asthma. Ann Allergy Asthma Immunol. 2025;134(2):159–164. doi: 10.1016/j.anai.2024.11.009. [DOI] [PubMed] [Google Scholar]
- 11.King G.G., Bates J., Berger K.I., et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55(2) doi: 10.1183/13993003.00753-2019. [DOI] [PubMed] [Google Scholar]
- 12.Ducharme F.M., Chan R. Oscillometry in the diagnosis, assessment, and monitoring of asthma in children and adults: review article. Ann Allergy Asthma Immunol. 2025;134(2):135–143. doi: 10.1016/j.anai.2024.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Beydon N., Davis S.D., Lombardi E., et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175(12):1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 14.Kaminsky D.A., Simpson S.J., Berger K.I., et al. Clinical significance and applications of oscillometry. Eur Respir Rev. 2022;31(163) doi: 10.1183/16000617.0208-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan R., Misirovs R., Lipworth B. Repeatability of impulse oscillometry in patients with severe asthma. Eur Respir J. 2022;59(1) doi: 10.1183/13993003.01679-2021. [DOI] [PubMed] [Google Scholar]
- 16.Abdo M., Kirsten A.M., von Mutius E., et al. Minimal clinically important difference for impulse oscillometry in adults with asthma. Eur Respir J. 2023;61(5) doi: 10.1183/13993003.01793-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan R., Lipworth B.J. Determinants of asthma control and exacerbations in moderate to severe asthma. J Allergy Clin Immunol Pract. 2022;10(10):2758–2760.e1. doi: 10.1016/j.jaip.2022.06.042. [DOI] [PubMed] [Google Scholar]
- 18.Chan R., Stewart K., Kuo C.R., Lipworth B. Evaluation of dupilumab and benralizumab on peripheral airway resistance and reactance. Allergy. 2024;79(10):2862–2864. doi: 10.1111/all.16214. [DOI] [PubMed] [Google Scholar]
- 19.Chan R, RuiWen Kuo C, Lipworth B. Pragmatic clinical perspective on biologics for severe refractory type 2 asthma. J Allergy Clin Immunol Pract. 2020;8(10):3363–3370. doi: 10.1016/j.jaip.2020.06.048. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M., Niimi A., Ueda T., et al. Effect of inhaled corticosteroids on small airways in asthma: investigation using impulse oscillometry. Pulm Pharmacol Ther. 2009;22(4):326–332. doi: 10.1016/j.pupt.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Hozawa S., Terada M., Hozawa M. Comparison of the effects of budesonide/formoterol maintenance and reliever therapy with fluticasone/salmeterol fixed-dose treatment on airway inflammation and small airway impairment in patients who need to step-up from inhaled corticosteroid monotherapy. Pulm Pharmacol Ther. 2014;27(2):190–196. doi: 10.1016/j.pupt.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Chan R., Lipworth B. Real-life effects of dupilumab on airway oscillometry in severe uncontrolled asthma. Ann Allergy Asthma Immunol. 2023;131(5):664–666. doi: 10.1016/j.anai.2023.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Svenningsen S., Kjarsgaard M., Haider E., et al. Effects of dupilumab on mucus plugging and ventilation defects in patients with moderate-to-severe asthma: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2023;208(9):995–997. doi: 10.1164/rccm.202306-1102LE. [DOI] [PubMed] [Google Scholar]
- 24.Galant S.P., Fregeau W., Pabelonio N., Morphew T., Tirakitsoontorn P. Standardized IOS reference values define peripheral airway impairment-associated uncontrolled asthma risk across ethnicity in children. J Allergy Clin Immunol Pract. 2020;8(8):2698–2706. doi: 10.1016/j.jaip.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 25.Postma D.S., Brightling C., Baldi S., et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019;7(5):402–416. doi: 10.1016/S2213-2600(19)30049-9. [DOI] [PubMed] [Google Scholar]
- 26.Nuttall A.G., Beardsmore C.S., Gaillard E.A. Ventilation heterogeneity in children with severe asthma. Eur J Pediatr. 2021;180(11):3399–3404. doi: 10.1007/s00431-021-04101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppenheimer B.W., Goldring R.M., Berger K.I. Distal airway function assessed by oscillometry at varying respiratory rate: comparison with dynamic compliance. COPD. 2009;6(3):162–170. doi: 10.1080/15412550902918410. [DOI] [PubMed] [Google Scholar]
- 28.Song T.W., Kim K.W., Kim E.S., Park J.W., Sohn M.H., Kim K.E. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol. 2008;19(8):763–768. doi: 10.1111/j.1399-3038.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 29.Mandilwar S., Thorve S.M., Gupta V., Prabhudesai P. Role of impulse oscillometry in diagnosis and follow-up in bronchial asthma. Lung India. 2023;40(1):24–32. doi: 10.4103/lungindia.lungindia_251_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y., Aledia A.S., Tatavoosian A.V., Vijayalakshmi S., Galant S.P., George S.C. Relating small airways to asthma control by using impulse oscillometry in children. J Allergy Clin Immunol. 2012;129(3):671–678. doi: 10.1016/j.jaci.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y., Aledia A.S., Galant S.P., George S.C. Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. J Allergy Clin Immunol. 2013;131(3):718–723. doi: 10.1016/j.jaci.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Kraft M., Richardson M., Hallmark B., et al. The role of small airway dysfunction in asthma control and exacerbations:a longitudinal, observational analysis using data from the ATLANTIS study. Lancet Respir Med. 2022;10(7):661–668. doi: 10.1016/S2213-2600(21)00536-1. [DOI] [PubMed] [Google Scholar]
- 33.Knihtila H., Kotaniemi-Syrjanen A., Makela M.J., Bondestam J., Pelkonen A.S., Malmberg L.P. Preschool oscillometry and lung function at adolescence in asthmatic children. Pediatr Pulmonol. 2015;50(12):1205–1213. doi: 10.1002/ppul.23188. [DOI] [PubMed] [Google Scholar]
- 34.Cottini M., Licini A., Lombardi C., Berti A. Clinical characterization and predictors of IOS-defined small-airway dysfunction in asthma. J Allergy Clin Immunol Pract. 2020;8(3):997–1004.e2. doi: 10.1016/j.jaip.2019.10.040. [DOI] [PubMed] [Google Scholar]
- 35.Chan R., Lipworth B. Interactions between spirometry and oscillometry in patients with moderate to severe asthma. Eur Respir J. 2022;60(4) doi: 10.1183/13993003.00543-2022. [DOI] [PubMed] [Google Scholar]
- 36.Foy B.H., Soares M., Bordas R., et al. Lung computational models and the role of the small airways in asthma. Am J Respir Crit Care Med. 2019;200(8):982–991. doi: 10.1164/rccm.201812-2322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland E.R., Martin R.J., Bowler R.P., Zhang Y., Rex M.D., Kraft M. Physiologic correlates of distal lung inflammation in asthma. J Allergy Clin Immunol. 2004;113(6):1046–1050. doi: 10.1016/j.jaci.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Yun H.J., Eom S.Y., Hahn Y.S. Assessing asthma control by impulse oscillometry and fractional expiratory nitric oxide in children with normal spirometry. J Allergy Clin Immunol Pract. 2023;11(9):2822–2829.e1. doi: 10.1016/j.jaip.2023.04.039. [DOI] [PubMed] [Google Scholar]
- 39.Tirakitsoontorn P., Crookes M., Fregeau W., et al. Recognition of the peripheral airway impairment phenotype in children with well-controlled asthma. Ann Allergy Asthma Immunol. 2018;121(6):692–698. doi: 10.1016/j.anai.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Culver B.H., Graham B.L., Coates A.L., et al. Recommendations for a standardized pulmonary function report. An official American Thoracic Society Technical statement. Am J Respir Crit Care Med. 2017;196(11):1463–1472. doi: 10.1164/rccm.201710-1981ST. [DOI] [PubMed] [Google Scholar]
- 41.Galant S.P., Komarow H.D., Shin H.W., Siddiqui S., Lipworth B.J. The case for impulse oscillometry in the management of asthma in children and adults. Ann Allergy Asthma Immunol. 2017;118(6):664–671. doi: 10.1016/j.anai.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ducharme F.M., Smyrnova A., Lawson C.C., Miles L.M. Reference values for respiratory sinusoidal oscillometry in children aged 3 to 17 years. Pediatr Pulmonol. 2022;57(9):2092–2102. doi: 10.1002/ppul.25984. [DOI] [PubMed] [Google Scholar]
- 43.Gochicoa-Rangel L., Del Rio-Hidalgo R., Hernandez-Ruiz J., et al. Validating reference equations for impulse oscillometry in healthy Mexican children. Resp Care. 2017;62(9):1156–1165. doi: 10.4187/respcare.05247. [DOI] [PubMed] [Google Scholar]
- 44.Morphew T., Tirakitsoontorn P., Galant S.P. External validation can provide the clinician with IOS equations that best predict the risk of uncontrolled asthma in their population. Pediatr Pulmonol. 2023;58(1):130–139. doi: 10.1002/ppul.26170. [DOI] [PubMed] [Google Scholar]
- 45.Ducharme F.M., Jroundi I., Jean G., Lavoie Boutin G., Lawson C., Vinet B. Interdevice agreement in respiratory resistance values by oscillometry in asthmatic children. ERJ Open Res. 2019;5(1):00138–2018. doi: 10.1183/23120541.00138-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan R., Lipworth B. Identifying poorer asthma control using oscillometry ratios. J Allergy Clin Immunology In Pract. 2024;12(2):506–508.e1. doi: 10.1016/j.jaip.2023.10.048. [DOI] [PubMed] [Google Scholar]
- 47.Kuo C.R., Jabbal S., Lipworth B. I say IOS you say AOS: comparative bias in respiratory impedance measurements. Lung. 2019;197(4):473–481. doi: 10.1007/s00408-019-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hozawa S., Terada M., Hozawa M. Comparison of budesonide/formoterol Turbuhaler with fluticasone/salmeterol Diskus for treatment effects on small airway impairment and airway inflammation in patients with asthma. Pulm Pharmacol Ther. 2011;24(5):571–576. doi: 10.1016/j.pupt.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Galant S.P., Morphew T. Adding oscillometry to spirometry in guidelines better identifies uncontrolled asthma, future exacerbations, and potential targeted therapy. Ann Allergy Asthma Immunol. 2024;132(1):21–29. doi: 10.1016/j.anai.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Leach C., Colice G.L., Luskin A. Particle size of inhaled corticosteroids: does it matter? J Allergy Clin Immunol. 2009;124(suppl 6):S88–S93. doi: 10.1016/j.jaci.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 51.Sonnappa S., McQueen B., Postma D.S., et al. Extrafine versus fine inhaled corticosteroids in relation to asthma control: a systematic review and meta-analysis of observational real-life studies. J Allergy Clin Immunol Pract. 2018;6(3):907–915.e7. doi: 10.1016/j.jaip.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 52.Soremekun S., Heaney L.G., Skinner D., et al. Asthma exacerbations are associated with a decline in lung function:a longitudinal population-based study. Thorax. 2023;78(7):643–652. doi: 10.1136/thorax-2021-217032. [DOI] [PMC free article] [PubMed] [Google Scholar]