Abstract
Background
The Lateral Organ Boundaries Domain (LBD) gene family, encompassing plant-specific LOB domain proteins, plays essential roles in various aspects of plant growth and development, and has continuously diversified its functions across numerous species. However, studies on LBD genes in the Orchidaceae family remain limited. To our knowledge, this is the first systematic investigation of the LBD gene family in Cymbidium, a genus that exhibits remarkable species and trait diversity within the Orchidaceae.
Results
In this study, we identified 122 LBD genes within the genomes of four Cymbidium species, distributed on 20 chromosomes. These genes were classified into class I (109 members, including 16 in subclass Ic/d) and class II (13 members), with protein lengths ranging from 94—477 amino acids. Promoter sequences of CymLBD genes revealed various cis-elements significant for light, hormonal, biotic, and abiotic stress responses. Transcriptomic analysis revealed tissue- and stage-specific expression of CymLBD genes in Cymbidium, and RT-qPCR and yeast one-hybrid assays indicated that CsiLBD27 may regulate floral patterning by directly binding to the CsiSEP3 promoter and activating its transcription. Under ABA treatment, the genes CsiLBD13, CsiLBD19, and CsiLBD21 displayed tissue-specific expression changes, suggesting hormone-responsive regulation. In Cymbidium ensifolium, 16 CenLBD genes were differentially expressed, while in Cymbidium mannii, 9 CmaLBD gene expression exist obvious circadian rhythm. GO and KEGG enrichment of 1074 and 399 predicted target genes, respectively, indicating a diverse range of functions for LBDs.
Conclusions
Collectively, this study provides the first comprehensive insight into the evolutionary dynamics, regulatory mechanisms, and functional roles of LBD genes in Cymbidium. These findings offer a valuable genetic resource for understanding floral and vegetative development in Orchidaceae and uncover potential novel functions of LBD genes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-025-11701-y.
Keywords: Cymbidium, LBD, Flower development, Expression profile, Genetic variation
Background
Lateral Organ Boundaries (LOB) Domain (LBD) proteins, also referred to as the ASYMMETRICLEAVES2-like (AS2) gene family, comprise a set of transcription factors specific to plants, originating from charophyte algae [1–3]. Numerous studies underscore the significant role of these LBD transcription factors in the development and growth of plants. They are essential in shaping and developing various plant parts, including roots, leaves, flowers, and other lateral structures [4, 5]. The LBD gene family is divided into two main categories: classes I and II. This classification is based on the LOB domain's unique protein sequences at the N-terminus. Class I possesses a distinct C-motif (CX2CX6CX3C, where'C'signifies conserved cysteine residues and'X'represents non-conserved amino acids) which can potentially bind to the 5’-GCGGCG-3’ DNA motif, and includes a Gly–Ala–Ser (GAS) domain comprising 49 amino acids, typically starting with FX2VH and ending with DP (V/I) YG sequences. It also features a leucine-zipper-like coiled-coil motif (LX6LX3LX6L). The first discovered member of the LBD family, known as lateral organ boundaries (LOB), is involved in LBD proteins'interaction with other proteins [6–8]; In contrast, class II includes a conserved zinc finger-like motif, likely playing a role in protein dimerization [9]. The LBD proteins'C-terminal variable region is instrumental in regulating the expression of genes downstream [10, 11]. The inaugural LBD gene was identified in Arabidopsis, distinguished by a unique gene expression pattern due to an enhancer trap insertion, noticeable in a cell band at the adaxial base of all lateral organs [12]. This gene family has been identified in numerous higher plants, with varying numbers of members, such as 32 members in rice (Oryza sativa) [13], 43 in Arabidopsis (Arabidopsis thaliana) [14], 40 in melon (Cucumis melo) [15], 44 genes in maize (Zea mays) [16], 50 in grapevine (Vitis vinifera) [17], 51 in danshen (Salvia miltiorrhiza) [18], 55 in Moso bamboo (Phyllostachys edulis) [19], 58 in apple (Malus domestica) [20], 90 in wheat (Triticum aestivum) [21] and 126 in rape (Brassica napus) [22].
LBD genes exhibit a range of expression patterns, varying from temporal to spatial, and are implicated in a multitude of biological processes [12, 23]. Specifically, the AtAS2 and OsAS2 genes show selective expression on the proximal surfaces of developing floral organs, influencing their development in plants [3, 24]. Genes such as AthLBD37, AthLBD38, AthLBD39, and OsaLBD37 play a pivotal role in regulating nitrogen metabolism [25–27]. The AthLBD33 and AthLBD18 genes are capable of enhancing lateral root growth by stimulating E2 Fa expression [28]. In apple, the MdLBD13 protein is known to suppress anthocyanin production and nitrogen absorption [29]. In maize, the ZmIG1 gene is crucial for the development of female gametes and leaf axial differentiation [30]. Overexpressing the eucalyptus gene EgLBD37 leads to increased internode length and higher lignin content, while EgLBD29 overexpression results in shorter plant growth and reduced circular growth. Furthermore, overexpressing EgLBD22 enhances the phloem's fiber content [9]. Under drought conditions, StLBD2-6 and StLBD3-5 expressions are induced in potato stems [31]. The GmLBD12 gene in soybean responds to various abiotic stresses and hormonal treatments including indole acetic acid (IAA), abscisic acid (ABA), and salicylic acid (SA) [32]. In wheat, TaLBD16-4D can alter plant architecture and flowering timing, potentially through its involvement in the auxin pathway, leading to early flowering and various architectural changes [33]. Additionally, MdLBD41 significantly boosts the formation of adventitious shoots in genetically modified tobacco. [34].
The Orchidaceae family is one of most ancient and diverse families of flowering plants. Its flowers exhibit unique morphological characteristics and hold substantial research value. A key distinguishing feature of orchids is the gynostemium structure, in which the pistil and stamen are fused, a characteristic that sets them apart from other species. The study of this structure holds immense research value [35]. Among orchids, species within the Cymbidium genus are particularly representative of this diversity, with their rich natural variation providing invaluable material for studying the functions of key genes. For example, Cymbidium sinense (C. sinense) exhibit a wide range of floral morphologies, where the petals and column can undergo mutual transformation, and their number can either increase or decrease [36]. In contrast, Cymbidium ensifolium (C. ensifolium) show diverse leaf colors, including mutations that lead to petal-like modifications and variations in leaf pigmentation [37]. Cymbidium goeringii (C. goeringii) stands out for its delicate flowers, fragrance, broad foliage, and cold tolerance [38], while Cymbidium mannii (C. mannii) excels in diverse habitats, with elongated leaves, epiphytic growth, and cassulacean acid metabolism, producing vibrant, colorful flowers [39]. These phenotypic variations offer unique insights into the genetic mechanisms governing morphological development in orchids. These striking phenotypic traits provide a valuable model system for dissecting the genetic basis of morphological diversification in orchids. While modern biotechnological breeding offers promising and efficient avenues for enhancing superior traits in flowering plants, genetic engineering in orchids remains constrained by significant technical barriers. Despite substantial advances in understanding the developmental and metabolic pathways underlying floral architecture, fragrance biosynthesis, leaf variegation, and the accumulation of pharmacologically active compounds, the regulatory networks coordinating these processes remain poorly defined in many orchid species. To address this gap, we sought to investigate whether LBD plays a specific function in Cymbidium, we conducted a thorough comparative genomic analysis of LBD genes in these four Cymbidium species. Our objectives were to: (1) identify and locate LBD genes on ten chromosomes in Cymbidium; (2) categorize Cymbidium LBD genes using comparative genomics; (3) reveal tissue-specific expression patterns across four Cymbidium species; and (4) comprehensively characterize the structural features and potential regulatory networks of 122 CymLBD genes, with yeast one-hybrid assays conducted to experimentally validate the predicted interaction between a representative pair of regulatory and target genes. These results provide a systematic and comprehensive set of Cymbidium LBD genes and their putative regulatory targets, offering valuable genetic resources for uncovering novel functional roles of LBD transcription factors and for refining the molecular regulatory networks associated with key traits in Orchidaceae, thereby supporting future molecular breeding strategies.
Results
Identification and characterization of LBD genes in four Cymbidium species
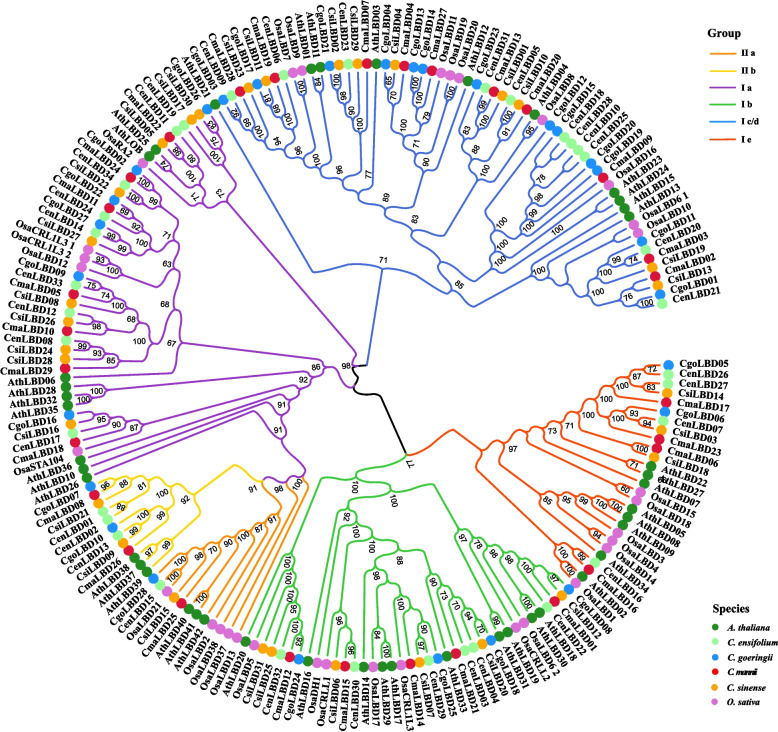
Based on the criteria outlined in the materials and methods section, we successfully identified 122 LBD genes featuring a complete LOB domain across four Cymbidium species. Specifically, there were 28 LBD genes in C. goeringii, 29 in C. mannii, 31 in C. sinense, and 34 in C. ensifolium. To determine the evolutionary divergence and functional specialization of CymLBD genes, we adopted a classification method consistent with that of Arabidopsis and rice LBD genes. Furthermore, we conducted a phylogenetic analysis of these 122 CymLBD genes alongside 32 from rice and 43 from Arabidopsis (Fig. 1 and Table S1), which enabled us to categorize CymLBD genes into two primary classes, I and II, with class I subdivided into five subgroups classes (Ia-e); and class II into two subgroups (IIa and IIb), as depicted in Fig. 1. Notably, the count of class I LBD genes in the studied species was over nine times higher than that of class II. Among these, subgroup IIa was the smallest with only 4 LBD genes, while subgroup Ic/d was the largest, comprising 43 genes (see Table S1). Interestingly, a comparative analysis with the LBD gene families previously reported in 14 other species revealed that Class I genes account for the highest proportion in all four Cymbidium species, all exceeding 88.24%, which is comparable to Prunus mume (87.80%) and Cucumis melo (87.50%). Notably, C. sinense exhibits an exceptionally high proportion of 90.32% (Table S2), suggesting that Class I CsiLBD genes may have evolved novel functions specific to C. sinense.
Fig. 1.
Phylogenetic tree of LBD proteins from Cymbidium (Cen, Cymbidium ensifolium; Cgo, Cymbidium goeringii; Cma, Cymbidium mannii; Csi, Cymbidium sinense), Arabidopsis (Ath, Arabidopsis thaliana), and rice (Osa, Oryza sativa). The values above each branch represent the percentage of bootstrap support (> 60%) for the branch points, based on 1,000 resamplings
The clustering patterns within the phylogenetic tree revealed that Cymbidium species'LBD members generally grouped together within the same subgroup, similar to the clustering of Arabidopsis LBD genes. This pattern suggests significant divergence between monocot and dicot LBD genes throughout evolution. A high degree of protein sequence similarity within the same subgroup from Cymbidium species indicates a relatively conservative evolutionary trajectory for these genes. Key gene characteristics such as protein sequence length (in amino acids), molecular weight (MW), isoelectric point (pI), and others are detailed in Table S1. The length of the CenLBD, CsiLBD, CmaLBD, and CgoLBD proteins varied from 94 to 309, 94 to 302, 97 to 477, and 94 to 368 amino acids, respectively. Notably, there was significant variation in DNA length across different subfamilies, with the longest DNA segments in various subfamilies distributed among different species: Ic/d in CmaLBD13, Ia in CsiLBD22, IIb in CgoLBD10, Ib and Ie in CenLBD30 and CenLBD16, respectively. The average length of CgoLBD proteins was approximately 214.18 amino acids, while CenLBDs averaged around 198.00 amino acids. The average molecular weights of the CmaLBDs, CgoLBDs, CenLBDs, and CsiLBDs were 23,359.74 Da, 23,700.74 Da, 21,806.56 Da, and 22,254.57 Da, respectively. The isoelectric points ranged from 4.77 to 9.92, and the instability coefficients varied between 31.21 and 84.98. Out of the Cymbidium LBD gene family members, 11 were identified as stable proteins with stability coefficients below 40. The aliphatic index values spanned from 65.32 to 98.02, and hydrophilicity indexes ranged from −0.558 to 0.301.
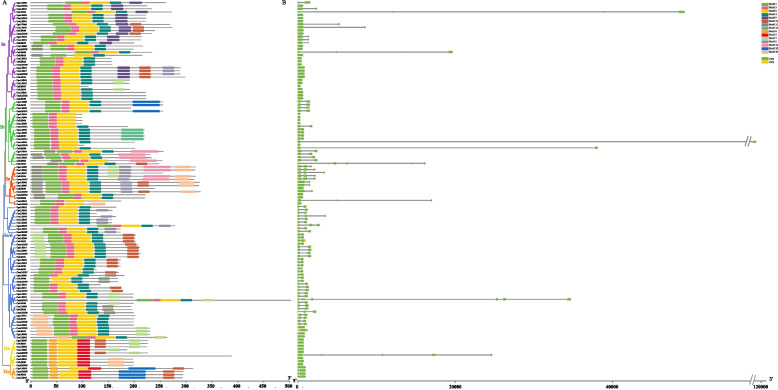
Gene structure and conserved motif analysis of CymLBD genes
In this study, we further delved into the evolutionary relationships of LBD proteins in Cymbidium by constructing phylogenetic trees for four species (see Fig. 2). In line with their classification, Class I splits into four subclasses (Ia, Ib, Ic/d, and Ie) comprising 32, 21, 43, and 13 members of the LBD gene family respectively. Class II segregates into IIa and IIb, encompassing 4 and 9 members, respectively. To decode the functional areas of CymLBDs, MEME was employed to predict conserved motifs. This analysis with MEME software revealed 15 distinct conserved motifs (labeled motif 1 to motif 15) within the four Cymbidium LBDs, illustrated in schematic diagrams (Table S3).
Fig. 2.
The phylogenetic relationship, conserved motifs, and gene structure of the CymLBD gene family in four Cymbidium species. A The conserved motifs of the CymLBD gene family; B The gene structure of the CymLBD gene family
Focusing on LBD proteins, their motif numbers ranged from three to nine in C. sinense, C. goeringii, and C. mannii, and from three to seven in C. ensifolium. These varying motif compositions might suggest diverse functionalities. Notably, Motif 1 features the CX2CX6CX3C zinc finger-like domain, while motifs 2 and 3, containing the GAS domain, were prevalent in almost all CymLBD proteins. Class I CymLBDs uniquely exhibited Motif 4, harboring the LX6LX3LX6L leucine zipper-like domain, and this motif was absent in Class II CymLBDs, aligning with the classification basis of LBDs and underscoring the reliability of this study’s CymLBD classification. Additionally, specific motifs like 5–10 were unique to certain subgroups; for instance, Motifs 5 and 8 were mainly found in Class II genes but not in Class I. Similarly, Motifs 5 and 12 appeared exclusively in Class Id; Motif 9 in Class Ia; and Motif 13 in Class Ib.
We also performed comparative analysis of intron–exon structures by aligning genomic DNA with full-length cDNA (Fig. 2). The gene structure patterns across the four Cymbidium species were similar. With 1 to 11 exons, CymLBDs fell into four categories based on exon count. Notably, 33 (27.05%) of the LBD genes had a single exon, distributed as 5 (17.86%) in C. goeringii, 8 (23.53%) in C. ensifolium, 10 (34.48%) in C. mannii, and 10 (32.26%) in C. sinense. A majority, 65 (53.28%), had 2 exons, distributed among the species. Additionally, 24 (19.67%) of the LBD genes possessed 3 exons. Significantly, the intron–exon structures corresponded with the phylogenetic clusters of CymLBDs, indicating a strong correlation between the intron–exon. configuration and the phylogenetic relationships among CymLBDs.
Analysis of cis-regulatory elements (CREs) in CymLBD promoters
In the exploration of gene regulation mechanisms, CREs play a pivotal role. For this analysis, we selected the 2000 bp region upstream of the CymLBD gene transcription start site as the putative promoter region. After extracting the putative promoter sequences of the CymLBD genes, we utilized the PlantCARE database to identify CREs (Table S4). Figure 3 showcases 18 representative cis-acting elements.
Fig. 3.
The cis-regulatory elements identified within the presumptive promoter regions of CymLBD genes. A Distribution of cis-acting elements identified in the 2000 bp up stream promoter region of CymLBD genes; B The quantity of cis-acting elements on putative promoters of CymLBD genes
Beyond the fundamental cis-acting elements, numerous motifs were identified that are involved in various regulatory processes. Light regulation is mediated by elements like CAAT-box, GT1-motif, 3-AF1 binding site, Sp1, ACE, MRE, among others. Elements like LTR cater to low-temperature response, while STRE, F-box, TC-rich repeats, and AC-II are linked to defense and stress responses. ARE elements are key in anaerobic induction. Additionally, the MYB binding site, crucial for drought induction and flavonoid biosynthesis regulation (MBS, MBSI), and various hormone regulatory elements were found: salicylic acid (TCA-element and SAER), methyl jasmonate (TGACG-motif and JERE), auxin (TGA-element and AuxRR-core), abscisic acid (ABRE and AAGAA-motif), and gibberellin (P-box, TATC-box, GARE-motif, and CARE). Elements linked to tissue-specific expression (CCGTC and CAT-box) and those important for developmental processes/cell differentiation (such as MSA-like, circadian, GCN4_motif, etc.) were also detected.
Among these, the abscisic acid responsiveness elements emerged as the most abundant, extensively present in both Class I and Class II CymLBD promoter regions, covering 110 CymLBDs. Auxin-responsive elements predominantly appeared in Class I CymLBDs. A noteworthy observation was that some subgroups, like Class Ic/d, showed similar cis-acting elements in their promoter regions, with all CymLBDs in this subgroup containing MYB binding sites. In conclusion, these findings suggest that the expression of LBD genes in Cymbidium is influenced by a diverse array of cis-acting elements, which are intricately linked to hormone regulation, growth and development, and stress responses.
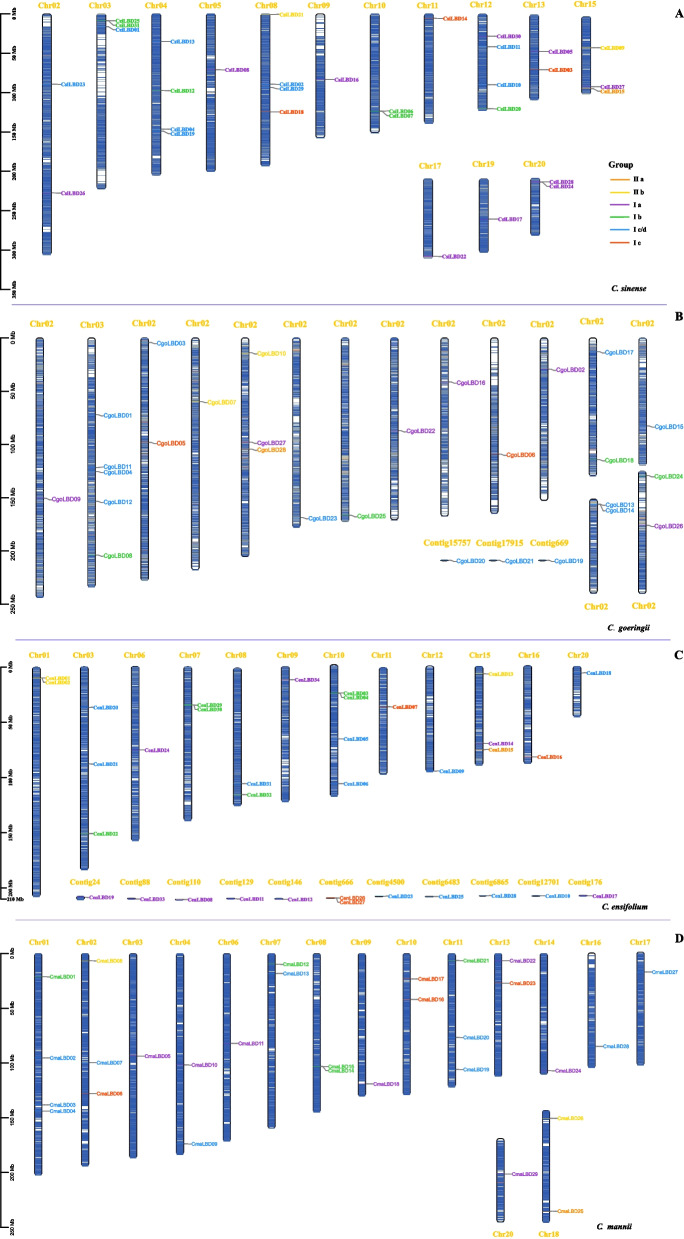
Chromosomal location, collinearity and evolution analysis of CymLBD genes
Utilizing publicly available genomic data for C. sinense, C. ensifolium, C. goeringii, and C. mannii, we were able to examine the gene locations and chromosome lengths, enabling an analysis of the distribution of genes across chromosomes. This analysis revealed that the CymLBD genes are distributed non-uniformly across the twenty chromosomes of Cymbidium, as illustrated in Fig. 4. Specifically, Chr03 (Chromosome 03) contains the highest count of LBD genes (12, representing 9.84%), followed by Chr10 with 9 (7.38%), Chr15 with 8 (6.56%), and Chr07 with 7 (5.74%). Notably, an equal number of CymLBD genes (6, equating to 4.92%) was observed on five chromosomes. A lack of a clear positive relationship between the length of a chromosome and its LBD gene count was noted, with no evident chromosomal preference in the distribution of the two CymLBD classes. MCScanX analysis revealed a singular tandem duplication event in C. sinense and C. mannii (involving CsiLBD06/07 and CmaLBD14/15), and four such events in C. ensifolium (CenLBD01/02, CenLBD03/04, CenLBD26/27, and CenLBD29/30). Additionally, 23 CymLBD genes, located on duplicated segments of the Cymbidium genome (referenced in Table S5), corresponded to 12 segmental duplication gene pairs. This suggests that the evolution of some CymLBDs may have been influenced by gene duplication, with segmental duplications being a significant evolutionary driver. Moreover, the majority of duplicated gene pairs belonged to Class I, comprising approximately 79.41% of all CymLBD genes.
Fig. 4.
Chromosomal distribution of the LBD genes in four Cymbidium species. Chromosome (Chr) names in yellow are on the up, and gene names are on the right. The scale on the left is in megabases (Mb). Gradient colors from red (high) to blue (low) indicate gene density in heat maps on Cymbidium chromosomes by setting the estimated inheritance interval to 300 kb. A C. sinense; B C. goeringii; C C. ensifolium; D C. mannii
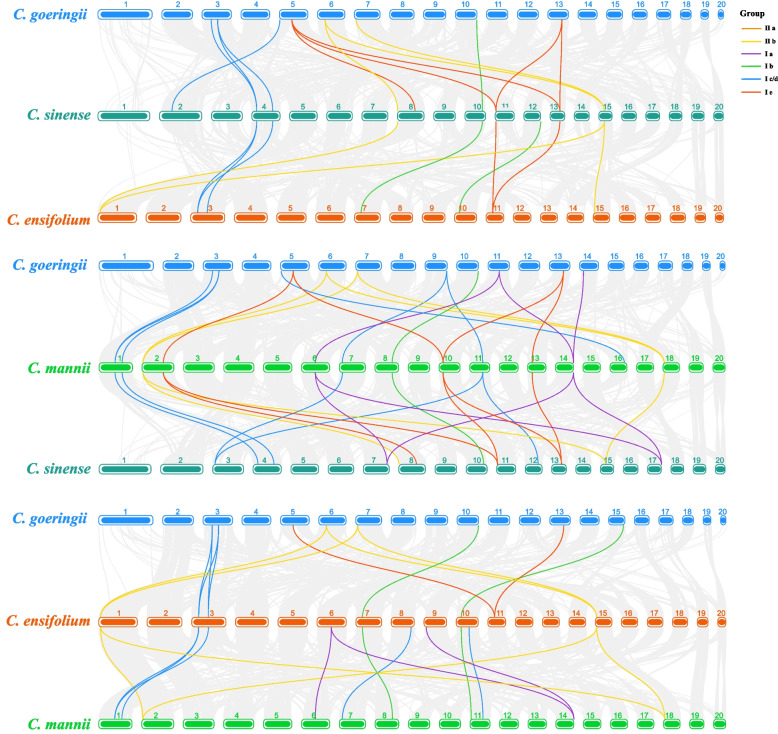
To delve into the evolutionary pressures shaping LBD gene functional divergence post-duplication, a study was conducted on the nonsynonymous to synonymous substitution ratio (Ka/Ks) for each duplicated LBD gene pair (data presented in Table S5). A total of 18 duplicated CymLBD pairs were predicted to be under positive selection (Ka/Ks < 1), indicating that the Cymbidium LBD gene family might have undergone considerable purifying selection pressure during its evolutionary course. This purifying selection likely played a crucial role in preserving the LBD genes'structural integrity over time. An intergenomic synteny analysis among various orchid species was conducted to understand the evolutionary links of CymLBD genes (as illustrated in Fig. 5). The analysis revealed the presence of 27, 26, 30, 24, 25, and 27 collinear gene pairs between C. sinense and C. ensifolium, C. sinense and C. goeringii, C. sinense and C. mannii, C. ensifolium and C. goeringii, C. ensifolium and C. mannii, and C. goeringii and C. mannii, respectively (detailed in Table S6). These findings highlight a closer evolutionary relationship between the LBD genes of C. sinense and C. mannii. Thus, the findings suggest that the LBD genes might have undergone genomic rearrangements during genome duplication events.
Fig. 5.
LBD genes synteny analysis of four Cymbidium species. Grey lines in the background indicate collinear blocks between four Cymbidium species, while color lines highlight syntenic LBD gene pairs. Species names is on the left
Analyzing CymLBD gene expression in various Cymbidium tissues
Research indicates that LBD genes are integral to the growth and development of various plant organs or tissues, including lateral roots, stems, leaves, and flowers. This study examined the expression patterns of 122 CymLBD genes in different floral tissues such as sepals, petals, lips, and gynostemiums across various flower mutants, as well as in other tissues including roots, stems, leaves, and fruits. Genes with minimal expression in all tested samples were excluded from the study. This is illustrated in Fig. 6.
Fig. 6.
The expressional pattern of LBD genes in four Cymbidium species. A, B C. sinense. Floral bud0, dormant lateral buds; floral bud1, 1 − 5 mm floral bud; floral bud2, 6 − 10 mm floral bud; floral bud3, 11 − 15 mm floral bud; floral bud4, 16 − 20 mm floral bud; floral bud5, blooming flower. LaPV, labellum-like perianth variety; MPV, multi-perianth variety; NLV, null-lip variety; GPV, gynostemium-like perianth variety; The numbers 1 to 4 correspond to individual floral organs, specifically the sepal, petal, labellum, and gynostemium, respectively. C Expression profiles across various tissues and organs of C. ensifolium. D Expression profiles in different leaf variants of C. ensifolium. E Tissue-specific expression profiles in C. goeringii. F Tissue-specific expression analysis in C. mannii. G Circadian expression analysis in C. mannii. Leaf tip segments (10 cm) were collected at 4-h intervals over a 24-h period, beginning at 08:00 (ZT0, lights on) and ending at 08:00 the following day (ZT24), with sampling at ZT0, ZT4, ZT8, ZT12, ZT16, ZT20, and ZT24. Lights were turned off at 20:00 (ZT12) to initiate the dark phase. The purple frame highlights tissues focusing on LBD gene tissue specificity and developmental roles, the blue frame emphasizes petal morphology and flower shape, the green frame addresses variations in leaf color, and the orange frame focuses on circadian rhythm-related aspects
Given the rich and distinctive floral morphological variations observed in C. sinense, we first investigated the organ-specific expression profiles of CsiLBD genes in roots, stems, leaves, flowers, and fruits. A more detailed analysis was then conducted in floral tissues, considering different floral organs (sepals, petals, lips, and gynostemiums), floral developmental stages (including dormant lateral buds, 1–5 mm, 6–8 mm, 11–15 mm, 16–20 mm floral buds, and blooming flowers), and a range of floral variants (standard flowers, gynostemium-like perianth, multi-perianth, labellum-like perianth, and null-lip types). The CsiLBD genes were categorized into three clusters based on their expression patterns (as shown in Fig. 6A). Group 1 genes showed expression in ovules at the gynostemium and in 6–15 mm floral buds. Group 2 genes were predominantly found in stems, leaves, flowers, and fruits. Interestingly, tissue-specific expressions of CsiLBD genes were noted in various floral tissues among different flower varieties (Fig. 6B). For instance, CsiLBD03 and CsiLBD27 exhibited higher expression in the gynostemium, highlighting their potential role in its formation. To explore the potential functions of LBD genes in C. sinense, we utilized transcriptomic data to assess the expression changes of the CsiLBD gene family under abscisic acid (ABA) treatment. Our findings revealed that CsiLBD12 exhibited over a two-fold increase in expression in leaves without treatment, whereas CsiLBD28 demonstrated a similar increase in flowers. Upon ABA treatment, CsiLBD21 showed more than a nine-fold expression increase in flowers, while CsiLBD13 and CsiLBD19 exhibited increases of over 25-fold and 17-fold in leaves, respectively. These results suggest that CsiLBD genes may play a role in the regulation of floral and leaf development in C. sinense through ABA-responsive pathways (Supplementary Fig. 1).
Similarly, the expression of CenLBD genes was examined in various organs (roots, pseudobulbs, leaves, and bracts), with flowers being further dissected into tissues (sepals, petals, lips, and gynostemiums), development stages (1–5 mm, 6–8 mm, 11–15 mm, 16–20 mm floral buds, and blooming flowers), and leaf variegation types (standard, wild leaf, yellow-green leaf, yellow leaf, white leaf, green leaf, and perianth-like leaf). A heat map categorized CenLBD genes into three groups based on their expression (Fig. 6C). Group 1 genes were primarily expressed in roots and leaves, whereas group 2 genes were more prevalent in petals and lips. Group 3 genes were predominantly expressed in 1–15 mm floral buds, with some in bracts. Notably, most genes showed high expression in white and perianth-like leaves, with CenLBD05 being specifically prominent in green leaves and CenLBD24 showing lower expression in yellow and yellow-green leaves (Fig. 6D).
In C. goeringii, the expression of CgoLBD genes was analyzed in roots, stems, flower buds, flowers, and fruits. These genes were divided into four groups based on their expression patterns (Fig. 6E), with the first group showing high expression in roots. Groups 2 and 3 genes were expressed in fruits and flowers, respectively, while group 4 genes were highly expressed in flower buds. In C. mannii, species valued for its medicinal properties and known for exhibiting diurnal rhythmicity in metabolite accumulation, the expression patterns of CmaLBD gene were analyzed across various organs (young leaves, mature leaves, flower buds, mature flowers, and fruits) and during rhythmic oscillations (ZT0 h, ZT4 h, ZT8 h, ZT12 h, ZT16 h, ZT20 h, and ZT24 h). The genes were categorized into five groups based on their expression in these organs (Fig. 6F). While most genes showed high expression at ZT0 h, CmaLBD13 and CmaLBD16 were more expressed at ZT4 h, CmaLBD13 and CmaLBD26 at ZT8 h, CmaLBD20 at ZT12 h, CmaLBD16 at ZT16 h, and CmaLBD02 and CmaLBD11 at ZT18 h, with CmaLBD02 and CmaLBD25 exhibiting elevated levels at ZT24 h.
Evaluating CsiLBD gene expression through quantitative Real-Time PCR (RT-qPCR) Analysis
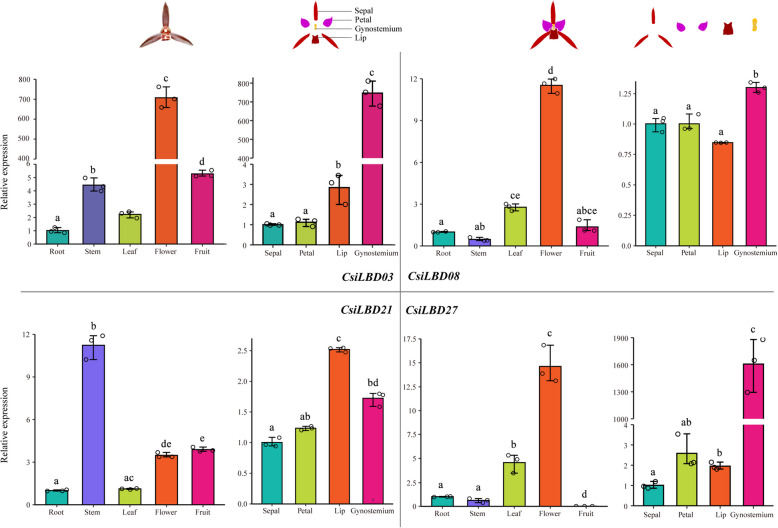
The transcriptome data suggesting the potential regulatory roles of CsiLBDs in floral patterning was intriguing. To validate the accuracy of this transcriptome data and the results of functional clustering analysis, four selected CsiLBD genes underwent RT-qPCR experiments. The outcomes of these experiments were in strong agreement with the transcriptome sequencing data, as depicted in Fig. 7. These four CsiLBD genes displayed varied expression patterns across different tissues. For instance, three genes from class I (CsiLBD03, CsiLBD08, and CsiLBD27) showed elevated expression in floral tissues, indicating their likely significant role in the development of flowers. Notably, CsiLBD03 and CsiLBD27 manifested pronounced expression in the gynostemium, hinting at their potential involvement in the formation and growth of this structure. Additionally, a notable expression of CsiLBD21 was detected in the lip. The RT-qPCR findings corroborated the transcriptome sequencing results, further highlighting the role of CsiLBD21, which was also markedly expressed in the stem, suggesting its possible extended functions in this area as well. The specific primers used are detailed in Table S7.
Fig. 7.
RT-qPCR analysis of four genes (CsiLBD03, CsiLBD08, CsiLBD21, and CsiLBD27) at different tissues of C. sinense. Significant differences analyzed between the tissues by Student's t-test, using R (means with different lowercase letters show a significant difference, significance level 0.05)
Uncovering and characterizing target genes of CymLBD in Cymbidium
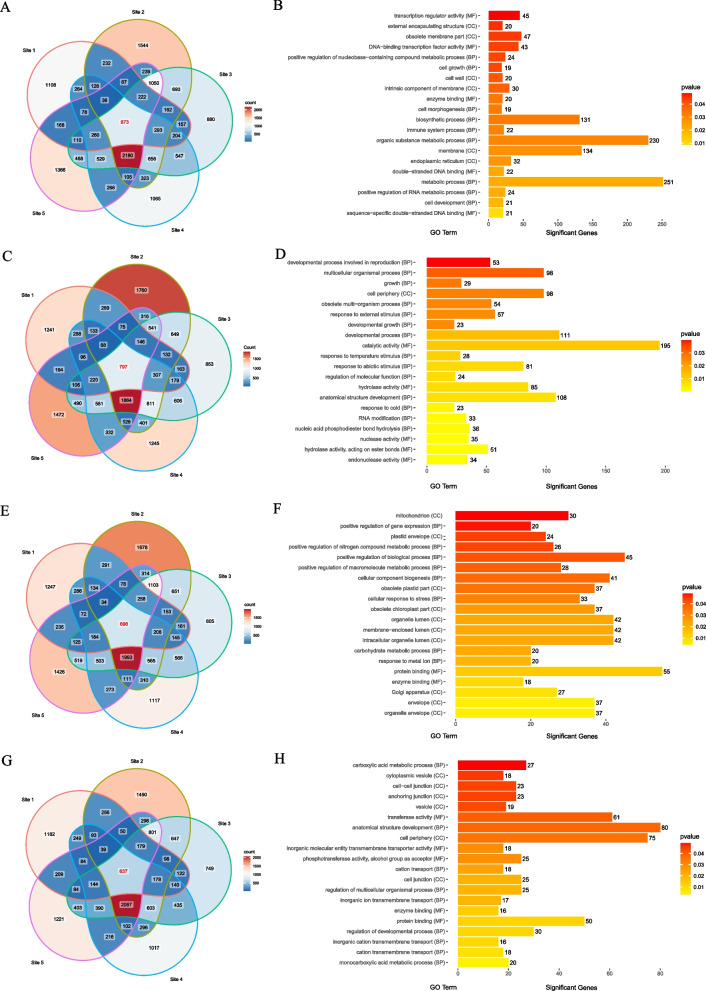
To investigate potential downstream genes influenced by LBD genes in Cymbidium and to ascertain their roles, we analyzed the 2000 bp upstream promoter sequences of genes from four Cymbidium species using the JASPAR database. This was done to identify consensus LBD motifs. We discovered a total of 873, 797, 698, and 637 target genes in C. ensifolium, C. sinense, C. goeringii, and C. mannii respectively, as presented in Fig. 8A, C, E, and G. An analysis was conducted to understand the biological roles of these target genes, involving both GO enrichment and KEGG pathway analysis. According to our findings, a total of 342, 317, 215, and 200 targets were assigned GO terms for C. ensifolium, C. sinense, C. mannii, and C. goeringii respectively (refer to Table S8). The GO analysis revealed diverse protein functions among the target genes, including metabolic processes (251 in C. ensifolium), catalytic activities (195 in C. sinense), development of anatomical structures (80 in C. mannii), and protein bindings (55 in C. goeringii). The most enriched top 20 GO terms for these target genes are depicted in Fig. 8B, D, H, and F. While GO analysis provides insights into enriched gene categories, KEGG analysis offers a deeper understanding of biological pathways. KEGG pathway enrichment analysis indicated that 159, 129, 69, and 42 genes significantly align with KEGG database pathways in C. ensifolium, C. sinense, C. goeringii, and C. mannii, respectively (as listed in Table S9). These target genes are mainly involved in pathways like metabolism (85 in C. ensifolium), signaling and cellular processes (69 in C. sinense), carbohydrate metabolism (43 in C. goeringii), and amino acid metabolism (23 in C. mannii) with statistical significance (p < 0.05). These findings suggest that CymLBDs could influence various pathways by regulating a range of target genes. To validate the reliability of the predicted targets, we focused on CsiSEP3 (Mol016808), a key floral development gene predicted to contain an LBD binding motif in its promoter. we integrated transcriptome data and RT-qPCR analysis, as shown in Supplementary Fig. 2, yeast cells co-transformed with the CsiLBD27 activation construct and the CsiSEP3 promoter reporter construct exhibited strong growth on selection medium and developed blue coloration on X-Gal-containing medium. This result indicates that CsiLBD27 can activate the promoter of CsiSEP3, providing preliminary experimental validation for the predicted LBD regulatory network and support the functional relevance of the candidate gene set identified in this study.
Fig. 8.
Venn diagram of consensus motif in different LBD DNA binding site (Site 1, MA1673.1; Site 2, MA2021.1, Site 3, MA2022.1, Site 4, MA2398.1, and Site 5, MA2418.1) of Cymbidium. Top 20 enriched Gene Ontology (GO) terms for candidate CymLBD target genes. MF, molecular function; CC, cellular component; BP, biological process. A, B C. ensifolium. C, D C. sinense. E, F C. goeringii. G, H C. mannii
Moreover, the target genes were found to possess over 100 different protein domains, including Phosphate acyltransferases, UDP-glycosyltransferases, MADS-box, cytochrome P450 family, and zinc finger domains. This diversity indicates that CymLBDs may regulate a broad spectrum of target genes, impacting the growth and development of Cymbidium (detailed in Table S10).
Discussion
We focus on Cymbidium, one of the most naturally diverse and scientifically valuable genera within orchids [40]. As a plant-specific transcription factor family, LBD genes have been identified in about 35 plant species and play key roles in regulating plant growth and development [41]. However, research on the LBD gene family in Cymbidium has been lacking. Thus, a comprehensive classification of LBD genes in Cymbidium is essential to understand their roles more clearly. From the genomes of four Cymbidium species, excluding sequences lacking complete LOB domain structures, we identified 122 protein sequences that form the CymLBD gene family. The phylogenetic analysis, encompassing the LBD gene family from four Cymbidium species along with Arabidopsis and rice, revealed that CymLBD genes are categorized into two subgroups (Class I and Class II), with Class I (109 genes, 89.34%) and Class II (13 genes, 10.66%). Despite minor structural variances between these subclades, significant differences were noted in the conserved protein motifs. This distribution is similar to that observed in Arabidopsis [3], where Class I members outnumber those in Class II. The phylogenetic relationships established in this study align with those reported in prior research [13, 42]. Our motif analysis of CymLBD (as illustrated in Fig. 2) demonstrates that all Class I members, with the exception of a few in C. ensifolium (CenLBD03, CenLBD04, CenLBD06, CenLBD22), C. sinense (CsiLBD17, CsiLBD20, CsiLBD25), C. mannii (CmaLBD15, CmaLBD16), and C. goeringii (CgoLBD18), possess the intact LX6LX3LX6L motifs. This finding underscores the validity of the Class I and Class II classification in our study. Furthermore, other motifs like 9 and 10 were predominantly found in the highly variable C-terminal domain of CymLBD proteins, with members of the same subclass usually sharing similar motif patterns, in line with findings from earlier studies [43].
Analysis of the gene exon/intron structure revealed that a vast majority of the CymLBDs (97.54%, or 119 out of 122) possess no more than two introns. This pattern mirrors the exon/intron struc.
0.ure of most LBD genes in other plant species, indicating a degree of conservation in gene structure throughout evolution. However, notable structural variances were observed in the Class I c/d members of the gene family, with the number of introns in these CymLBD genes ranging from none to ten. This variation suggests that Class I c/d family members may have experienced gene segment splicing or insertion events during their evolutionary history [44]. In addition, the number of introns in a gene affects the speed of the expression process and the mRNA editing process, as a result, genes with less number of introns can be induced faster [45].
In the realm of gene expression regulation, the interaction between transcription factors (TFs) and Cis-regulatory elements (CREs) in gene promoter regions upstream is crucial for the transmission of biological signals [46]. Our study identified several regulatory motifs in the predicted promoter regions of CymLBD genes, relating to hormonal control, growth, development, and responses to biotic and abiotic stresses. Predominantly, ABA responsiveness elements emerged as the most abundant cis-acting elements, found in most CymLBDs (110 out of 123). ABA plays numerous vital roles in plant growth and development, such as influencing tillering, flowering, seed development, and maturation [47]. In Arabidopsis, for example, AthLBD14's expression is downregulated by ABA throughout the various stages of lateral root development [48]. Following treatment with abscisic acid (ABA), genes CsiLBD13, CsiLBD19, and CsiLBD21 demonstrated elevated expression in either leaves or flowers, indicating their potential regulatory roles in the growth and developmental processes of these tissues.
Tandem duplication and segmental duplication are acknowledged as the primary mechanisms driving gene family expansion throughout the evolution of species. Furthermore, it has been suggested that segmental duplications play a more critical role in gene family enlargement compared to tandem duplications [49]. In the genome of C. ensifolium, our research identified four pairs of tandem duplicated CenLBD genes and two pairs of segmental duplicated CenLBD genes (as detailed in Table S8). The nonsynonymous to synonymous substitution ratio (Ka/Ks) for these duplicated gene pairs was found to be higher in comparison to C. mannii (1, 4), C. goeringii (0, 4), and C. sinense (1, 2). This might shed light on why the CenLBD gene family in C. ensifolium has more members than the LBD gene families in the other three Cymbidium species.
Synteny analysis across different species is instrumental in shedding light on their evolutionary links and relationships. In our research, we carried out a synteny analysis of LBD genes among the four Cymbidium species, as shown in Fig. 5. Out of 159 pairs of collinear genes, 19 appeared with a frequency exceeding five times, including five genes each in C. mannii, C. goeringii, and C. sinense, but only four in C. ensifolium. These findings imply that these 19 genes likely share core functions across the Cymbidium species [50].
Regarding tissue-specific expression patterns, our study noted that certain CymLBD gene members displayed specific expression across all tissues. Analyzing these patterns suggested that select CymLBD genes might play roles in unique physiological processes. For instance, genes like CenLBD01, CsiLBD21, and CgoLBD07 were predominantly expressed in roots, similar to their orthologs AthLBD37 and AthLBD38 in A. thaliana [26, 51], which implies their potential involvement in shaping the root morphology of Cymbidium. Other genes such as CsiLBD03, CsiLBD08, CsiLBD12, CsiLBD14, CsiLBD19, and CsiLBD27 were notably active in various floral tissues. This mirrors the impact of OsIG1, a LBD-like gene in rice, which influences the expression of EG1, OsMADS6, and OsMADS1, leading to double ovules and abnormal development in floral organs [52, 53]. To delve deeper into the functions of CymLBDs and their potential downstream target genes, GO annotation and enrichment analysis were conducted. A total of 1074 target genes were identified, predominantly enriched in biological processes (193), molecular functions (59), and cellular components (45), as detailed in Table S8. One notable target gene, CsiAG (Mol017710), emerged as a key regulator of gynostemium development in C. sinense [54, 55]. This finding, corroborated by RT-qPCR and AlphaFold3 predict results (Supplementary Fig. 3), suggests that CsiLBD may influence the development of the gynostemium through the C. sinense AGAMOUS (CsiAG) gene. This finding is consistent with previous studies reporting that the development of the orchid gynostemium is co-regulated by AG and SEP genes [56]. Together, these results provide deeper insights into the regulatory roles of CymLBDs in floral organ development.
Although this study confirmed the direct regulatory role of CsiLBD27 on CsiSEP3 through yeast one-hybrid assays, floral development, particularly the formation of floral morphology, is governed by a complex regulatory network that may also involve other transcription factors or epigenetic modifications. In addition, no SNP mutations were identified in the promoter region that could account for the observed expression differences, suggesting that upstream transcriptional regulation or factors affecting RNA stability may also contribute to the regulation of CsiSEP3 expression. Future studies are needed to further investigate the upstream regulatory network and potential cooperative regulatory mechanisms involved.
These findings underscore the significant potential application value of the candidate genes in orchid molecular breeding, warranting further comprehensive experimental validation and breeding applications. For instance, biological and abiotic stress treatments, such as hormones and light, could be applied to orchid species to further investigate the expression changes of CymLBD. Additionally, CRISPR-Cas9-mediated knockout or overexpression of the core candidate CymLBD genes should be employed to observe phenotypic changes. Moreover, a number of predicted upstream and downstream regulatory genes, along with interacting proteins, could be further validated through techniques such as chromatin immunoprecipitation sequencing, yeast one-hybrid assays, dual-luciferase reporter systems, and bimolecular fluorescence complementation. The candidate genes identified in this study warrant further investigation, as they have the potential to aid molecular breeding for traits such as stress resistance, floral morphology, and leaf color variation, ultimately contributing to the advancement of orchid breeding.
Conclusion
The identification and classification of LBD transcription factor families is essential in the functional studies of LBD genes in orchid. However, there are few reports in this area. To complement this aspect of research, we identified and classified the CymLBD families for the first time. Our research marks a pioneering effort to systematically and comprehensively identify 122 LBD genes within the genomes of four Cymbidium species, categorizing them into six distinct subfamilies and locating them across 20 chromosomes. Analysis of CymLBD gene promoters uncovered a range of cis-elements crucial for responses to light, hormones, and both biotic and abiotic stresses. Synteny analysis highlighted 159 sets of collinear gene pairs, underscoring the evolutionary significance of duplication in CymLBDs. Integrating transcriptome profiling, RT-qPCR validation, and gene functional annotation, our results highlight the widespread involvement of CymLBD genes in vegetative and floral development. In particular, yeast one-hybrid assays confirmed that CsiLBD27 directly binds to the promoter region of CsiSEP3, suggesting a novel regulatory pathway potentially involved in floral morphogenesis. Taken together, this study provides important insights into the evolutionary history and functional diversity of LBD genes in Cymbidium, offering a valuable resource for further investigations into their roles within complex regulatory networks governing orchid development.
Materials and methods
Plant materials, growth conditions, and treatment
In this research, representative orchid plants (C. sinensis ‘Qihei’) were housed in a growth chamber at the Institute of Environmental Horticulture of the Guangdong Academy of Agricultural Sciences (Guangzhou, China). The chamber was set to maintain an artificial climate condition with 80% relative humidity and a temperature regime of 25 °C during a 16-h day and 18 °C during an 8-h night. We collected the roots, stems, leaves, flowers, and fruits from adult plants aged two years, and subsequently preserved them at −80℃ following rapid freezing in liquid nitrogen. In addition, we treated two-year-old C. sinensis'Qihei'plants with 100 μM abscisic acid (ABA) through weekly spray applications. This treatment was continued for a duration of one month. Subsequently, both leaves and flowers were harvested from these plants. To preserve their biological integrity, these samples were rapidly frozen using liquid nitrogen and then stored at a temperature of −80℃.
Identification and analysis of LBD protein sequences from four Cymbidium species
For our study, the LBD gene sequences from A. thaliana and O. sativa were retrieved from the TAIR (The Arabidopsis Information Resource) and the Ensembl Plants databases. We then employed the BLAST search tool (with an E-value threshold of 1e-5 and a minimum identity of 60%) to locate genes in the genomes of the four Cymbidium species that are homologous to these sequences [57]. Subsequently, the Pfam database (version 35.0) [http://pfam.xfam.org/] was utilized to identify conserved structural domains (specifically DUF260, PF03195) within the candidate protein sequences. Any sequences lacking or possessing incomplete conserved domains were excluded manually. Moreover, the identified conserved domains underwent additional validation through the SMART (Simple Modular Architecture Research Tool) database [http://smart.embl-heidelberg.de/]. Ultimately, this process yielded 122 LBD protein sequences from the Cymbidium species, each characterized by conserved structural domains. These sequences were systematically named according to their chromosomal location.
Conducting alignments and phylogenetic analysis of Cymbidium LBD genes
Conducting Alignments and Phylogenetic Analysis of Cymbidium LBD Genes [58]. We aligned LBD protein sequences from A. thaliana, O. sativa, and the four Cymbidium species (C. sinense, C. ensifolium, C. goeringii, and C. mannii) using the MAFFT alignment tool [59]. Subsequently, a maximum likelihood phylogenetic tree (ML tree) was constructed based on these alignments, employing 10,00 bootstrap replications in the IQtree software (version 2.2) [60]. The phylogenetic tree's results were then visualized using Evolview (version 3) [61]. Additionally, the conserved domains within the LBD protein sequences were meticulously compared and edited with the aid of Geneious Prime (version 2021.1.1) (https://www.geneious.com) [62].
Analysis of gene structure, motifs, and cis-regulatory elements in Cymbidium LBD genes
The intron–exon structures of the LBD genes in Cymbidium were determined using GFF annotation files from the respective Cymbidium genomes. The MEME online tool (available at http://meme-suite.org/) [63] was utilized for the protein sequence analysis to identify conserved motifs in the CymLBD proteins. The analysis was conducted with the following parameter settings: repetition mode set to"any,"a maximum of 15 motifs, and a preferred motif width ranging from 6 to 100 amino acids. The TBtools software (Version 1.0987) was employed to isolate the 2000-bp region upstream of the transcription start site of each Cymbidium LBD gene [64]. Following this, the PlantCare website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was used for predicting cis-acting elements within the presumptive promoter regions of these LBD genes [65]. Visualization of these findings was also conducted using TBtools (Version 1.0987) [64].
Mapping chromosomal locations and analyzing gene duplication in Cymbidium
All Cymbidium LBD genes were anchored to their corresponding chromosomes using TBtools (Version 1.0987) [64], based on the physical location data from the Cymbidium genome database. To examine the synteny between orthologous LBD genes in Cymbidium and rice, gene duplication events were scrutinized using the Multicollinearity Scanning Toolkit (MCScanX), with the application of default settings [66], The synteny and duplication findings were visualized using TBtools (Version 1.0987) [64].
In the assessment of Ka/Ks ratios, seventeen pairs of homologous genes were pinpointed through BLASTn, adhering to two specific criteria: (1) a sequence similarity above 75%, and (2) an alignment region exceeding 75% of the length of the longer sequence among the pair [67]. The KaKs_Calculator2.0 tool was then employed to calculate the synonymous (Ks) and nonsynonymous (Ka) substitution rates, as well as the Ka/Ks ratio for these homologous gene pairs [68].
Analysis of Cymbidium LBDs using transcriptome data from RNA-Seq
For this study, transcriptome datasets of four chosen orchid species (C. sinense, C. ensifolium, C. goeringii, and C. mannii) [54, 69–71] were acquired from the NCBI Sequence Read Archive (SRA) databas (available at https://www.ncbi.nlm.nih.gov/sra). The acquired SRA files were transformed into fastq format utilizing the SRA Toolkit's fastq-dump function. We then employed Trimmomatic software to cleanse the raw reads according to standard quality criteria. To identify erroneous sequences, the sequencing reads were aligned to the genomes using the RSEM (RNA-Seq by Expectation Maximization) software [72]. Transcripts exhibiting either FPKM (Fragments Per Kilobase of transcript per Million mapped reads) or TPM (Transcripts Per Million) values below 1 were excluded from further analysis. For the visualization of the data, heatmaps were created using TBtools along with the pheatmap package in R (Version 2.2.2) [73].
Discovering and characterizing target genes of CymLBD
In our research, we aimed to identify genes potentially regulated by LBD. This was achieved by analyzing the 2000 bp sequence upstream of the transcription start site, which is considered the putative promoter region. The LBD DNA binding site consensus motifs (MA1673.1, MA2021.1, MA2022.1, MA2398.1, and MA2418.1) were sourced from the JASPAR CORE database (available at https://jaspar.genereg.net/) [74]. To pinpoint LBD-binding motifs within the Cymbidium promoter region, we employed the Motif FIMO tool from the MEME suite, setting a threshold of p < 1 × e-4 for selecting the final LBD target gene candidates. Furthermore, we utilized AlphaFold3 [75] to predict the potential regulatory effects of representative proteins on the DNA sequences within the promoter regions of potential target genes. Additionally, the identified candidate genes were then functionally annotated using the Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) databases for comprehensive understanding.
Yeast one‑hybrid assay
The full-length coding sequence (CDS) of CsiLBD27 was cloned into the pB42 AD vector to generate the fusion construct pB42 AD-CsiLBD27. Promoter regions of CsiSEP3 (Mol016808) involved in floral patterning in C. sinense were amplified from genomic DNA and inserted into the pLacZi vector. Different combinations of pLacZi constructs containing different promoter fragments, along with either pB42 AD-CsiLBD27 or the empty pB42 AD vector, were co-transformed into Saccharomyces cerevisiae strain EGY48 using the Yeastmaker™ Yeast Transformation System 2 (Clontech, USA). The co-transformation of empty pB42 AD and pLacZi vectors served as a negative control. Transformed yeast colonies were first cultured on SD/-Trp/-Ura medium at 30 °C for 3 days and subsequently transferred to SD/-Trp/-Ura/Gal/Raf medium supplemented with X-Gal for an additional 3 days at 30 °C to evaluate transcriptional activation.
Evaluating LBD gene expression via quantitative Real-Time PCR (RT-qPCR)
For a detailed examination of LBD gene expression patterns, we utilized RT-qPCR. The extraction of total RNA from various tissues of C. sinense was performed using the FastPure Plant Total RNA Isolation Kit (produced by Vazyme Biotech Co., Ltd., Nanjing, China). The synthesis of first-strand DNA was carried out using the TransScript® All-in-One First-Strand cDNA Synthesis SuperMix suitable for qPCR (provided by TransGen Biotech, Beijing, China). We designed primers for both candidate and internal reference genes for RT-qPCR using Primer Premier 5 software, and their specificity was verified through a primer blast on the NCBI platform. The RT-qPCR assays were conducted using the Hieff® qPCR SYBR Green Master Mix (Low Rox Plus) from Yeasen, Shanghai, China. As a reference for normalization, we selected the actin gene from C. sinense (Mol022529). The relative expression levels of the target genes were determined using the 2−ΔΔCT method [76]. and the expression data presented here represent the average of three biological replicates.
Supplementary Information
Authors’ contributions
Conceptualization, G.Z. and F.Y.; methodology, Y.W.; software, Z.L and C.L.; validation, W.Z., and J.L.; formal analysis, Q.X.; investigation, J.J. and Y.W; resources, G.Z.; data curation, Z.L and Y.W.; writing original draft preparation, Z.L and Y.W.; writing review and editing, F.Y.; visualization, J.G.; supervision, Y.W.; project administration, G.Z.; funding acquisition, G.Z. and F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China (2023YFD2300904), the Guangdong Basic and Applied Basic Research Foundation (2024A1515013187, 2025A1515012658), the 2022 Provincial Rural Revitalization Strategy Special Fund Seed Industry Revitalization Project (2022-NBA-00–015), Seed industry revitalization project of the special fund for the rural revitalization strategy of Guangdong Province (2024-NPY-00–035), Innovation Team of Modern Agriculture Industry Technology System in Guangdong Province (2024CXTD12), Guangdong Academy of Agricultural Sciences Project (CYZX202406, R2020PY-JX018, R2023PY-JG023 and XTXM202201, XT202212).
Data availability
The raw RNA sequencing datasets pertinent to this study have been deposited in the National Center for Biotechnology Information (NCBI) repository and are retrievable under the Sequence Read Archive (SRA) accession numbers: PRJNA304321, PRJNA749652, PRJNA771426, and PRJNA783745. Comprehensive details of all additional datasets generated and analyzed during the course of this research are encompassed within the main body of this published article, as well as in the accompanying supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yonglu Wei and Zengyu Lin contributed equally to this work.
Contributor Information
Genfa Zhu, Email: zhugenfa@gdaas.cn.
Fengxi Yang, Email: yangfengxi@gdaas.cn.
References
- 1.Coudert Y, Dievart A, Droc G, Gantet P. ASL/LBD phylogeny suggests that genetic mechanisms of root initiation downstream of auxin are distinct in lycophytes and euphyllophytes. Mol Biol Evol. 2013;30(3):569–72. 10.1093/molbev/mss250. [DOI] [PubMed] [Google Scholar]
- 2.Chanderbali AS, He F, Soltis PS, Soltis DE. Out of the water: origin and diversification of the LBD gene family. Mol Biol Evol. 2015;32(8):1996–2000. 10.1093/molbev/msv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwakawa H, Ueno Y, Semiarti E, Onouchi H, Kojima S, Tsukaya H, Hasebe M, Soma T, Ikezaki M, Machida C. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol. 2002;43(5):467–78. 10.1093/pcp/pcf077. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Li Z, Ma B, Hou Q, Wan X. Phylogeny and functions of LOB domain proteins in plants. Int J Mol Sci. 2020;21(7):2278. 10.3390/ijms21072278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K, Wei Y, Wang Y, Tan B, Chen S, Li H: Genome-Wide Identification of LBD Genes in Foxtail Millet (Setaria italica) and Functional Characterization of SiLBD21. In Int J Mol Sci. 2023;24. [DOI] [PMC free article] [PubMed]
- 6.Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240(4860):1759–64. 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 7.Majer C, Hochholdinger F. Defining the boundaries: structure and function of LOB domain proteins. Trends Plant Sci. 2011;16(1):47–52. 10.1016/j.tplants.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Husbands A, Bell EM, Shuai B, Smith HM, Springer PS. LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res. 2007;35(19):6663–71. 10.1093/nar/gkm775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Q, Shao F, Macmillan C, Wilson IW, Van der Merwe K, Hussey SG, Myburg AA, Dong X, Qiu D. Genomewide analysis of the lateral organ boundaries domain gene family in Eucalyptus grandis reveals members that differentially impact secondary growth. Plant Biotechnol J. 2018;16(1):124–36. 10.1111/pbi.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005;43(1):47–56. 10.1111/j.1365-313X.2005.02434.x. [DOI] [PubMed] [Google Scholar]
- 11.Majer C, Xu C, Berendzen KW, Hochholdinger F. Molecular interactions of ROOTLESS CONCERNING CROWN AND SEMINAL ROOTS, a LOB domain protein regulating shoot-borne root initiation in maize (Zea mays L.). Philos Trans R Soc Lond B Biol Sci. 2012;367(1595):1542–51. 10.1098/rstb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuai B, Reynaga-Pena CG, Springer PS. The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol. 2002;129(2):747–61. 10.1104/pp.010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao D, Chen P, Chen Z, Zhang L, Wang Y, Xu L. Genome-wide analysis of the LBD family in rice: gene functions, structure and evolution. Comput Biol Med. 2023;153:106452. 10.1016/j.compbiomed.2022.106452. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura Y, Iwakawa H, Machida Y, Machida C. Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. Plant J. 2009;58(3):525–37. 10.1111/j.1365-313X.2009.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tufekci ED. Genome-wide identification and analysis of Lateral Organ Boundaries Domain (LBD) transcription factor gene family in melon (Cucumis melo L.). Peer J. 2023;11:e16020. 10.7717/peerj.16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y-M, Zhang S-Z, Zheng C-C. Genomewide analysis of LATERAL ORGAN BOUNDARIES Domain gene family in Zea mays. J Genet. 2014;93:79–91. [DOI] [PubMed] [Google Scholar]
- 17.Grimplet J, Pimentel D, Agudelo-Romero P, Martinez-Zapater JM, Fortes AM. The LATERAL ORGAN BOUNDARIES Domain gene family in grapevine: genome-wide characterization and expression analyses during developmental processes and stress responses. Sci Rep. 2017;7(1):15968. 10.1038/s41598-017-16240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu XY, Liang XY, Li X, Shen PX, Cao XY, Chen C, Song SH, Wang DH, Wang ZZ, Zhang Y: Genome-wide characterisation and expression profiling of the LBD family in Salvia miltiorrhiza reveals the function of LBD50 in jasmonate signaling and phenolic biosynthesis. Ind Crop Prod. 2020;144:112006. j.indcrop.2019.112006.
- 19.Huang B, Huang Z, Ma R, Ramakrishnan M, Chen J, Zhang Z, Yrjälä K. Genome-wide identification and expression analysis of LBD transcription factor genes in Moso bamboo (Phyllostachys edulis). BMC Plant Biol. 2021;21(1):1–22. 10.1186/s12870-021-03078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Zhang S, Su L, Liu X, Hao Y. A genome-wide analysis of the LBD (LATERAL ORGAN BOUNDARIES domain) gene family in Malus domestica with a functional characterization of MdLBD11. PLoS ONE. 2013;8(2):e57044. 10.1371/journal.pone.0057044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Zhang R, Cheng Y, Lei P, Song W, Zheng W, Nie X. Genome-wide identification, evolution, and expression analysis of LBD transcription factor family in bread wheat (Triticum aestivum L.). Front Plant Sci. 2021;12:721253. 10.3389/fpls.2021.721253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie T, Zeng L, Chen X, Rong H, Wu J, Batley J, Jiang J, Wang Y. Genome-wide analysis of the lateral organ boundaries domain gene family in Brassica napus. Genes (Basel). 2020;11(3):280. 10.3390/genes11030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivanesan I, Nayeem S, Venkidasamy B, Kuppuraj SP, Rn C, Samynathan R. Genetic and epigenetic modes of the regulation of somatic embryogenesis: A review. Biol Futura. 2022;73(3):259–77. 10.1007/s42977-022-00126-3. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Wang F, Guo J, Zhang XS. Rice OsAS2 Gene, a Member of LOB Domain Family, Functions in the Regulation of Shoot Differentiation and Leaf Development. J Plant Biol. 2009;52(5):374–81. 10.1007/s12374-009-9048-4. [Google Scholar]
- 25.Scheible WRD, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of arabidopsis in response to nitrogen. Plant Physiol. 2004;136(1):2483–99. 10.1104/pp.104.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin G, Tohge T, Matsuda F, Saito K. Scheible W-Rd: Members of the LBD Family of Transcription Factors Repress Anthocyanin Synthesis and Affect Additional Nitrogen Responses in Arabidopsis. Plant Cell. 2009;21(11):3567–84. 10.1105/tpc.109.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albinsky D, Kusano M, Higuchi M, Hayashi N, Kobayashi M, Fukushima A, Mori M, Ichikawa T, Matsui K, Kuroda H, et al. Metabolomic Screening Applied to Rice FOX Arabidopsis Lines Leads to the Identification of a Gene-Changing Nitrogen Metabolism. Mol Plant. 2010;3(1):125–42. 10.1093/mp/ssp069. [DOI] [PubMed] [Google Scholar]
- 28.Berckmans B, Vassileva V, Schmid SPC, Maes S, Parizot B, Naramoto S, Magyar Z, Kamei CLA, Koncz C, Bögre L, et al. Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by Lateral Organ Boundary Proteins. Plant Cell. 2011;23(10):3671–83. 10.1105/tpc.111.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H-H, Liu X, An J-P, Hao Y-J, Wang X-F, You C-X. Cloning and elucidation of the functional role of apple MdLBD13 in anthocyanin biosynthesis and nitrate assimilation. Plant Cell Tiss Organ Cult. 2017;130(1):47–59. 10.1007/s11240-017-1203-x. [Google Scholar]
- 30.Evans MMS. The indeterminate gametophyte1 Gene of Maize Encodes a LOB Domain Protein Required for Embryo Sac and Leaf Development. Plant Cell. 2007;19(1):46–62. 10.1105/tpc.106.047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Cao M, Chen X, Ye M, Zhao P, Nan Y, Li W, Zhang C, Kong L, Kong N, et al. Genome-Wide Analysis of the Lateral Organ Boundaries Domain (LBD) Gene Family in Solanum tuberosum. Int J Mol Sci. 2019;20(21):5360. 10.3390/ijms20215360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng S, Shi J, Hu Y, Li D, Guo L, Zhao Z, Lee GS, Qiao Y. Genome-Wide Analysis of Soybean Lateral Organ Boundaries Domain Gene Family Reveals the Role in Phytophthora Root and Stem Rot. Front Plant Sci. 2022;13:865165. 10.3835/plantgenome2016.07.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Han X, Fu X, Sun X, Chen H, Wei X, Cui S, Liu Y, Guo W, Li X et al: Overexpression of TaLBD16–4D alters plant architecture and heading date in transgenic wheat. Front Plant Sci. 2022, 13. 10.3389/fpls.2022.911993. [DOI] [PMC free article] [PubMed]
- 34.Dong H, Zheng Q, Zhou Y, Zhou Y, Bao Z, Lan Q, Li X. MdWOX4-2 modulated MdLBD41 functioning in adventitious shoot of apple (Malus domestica). Plant Physiol Biochem. 2022;186:11–8. 10.1016/j.plaphy.2022.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Pabón-Mora N, González F. The gynostemium: More than the sum of its parts with emerging floral complexities. Curr Opin Plant Biol. 2024;81:102609. 10.1016/j.pbi.2024.102609. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Guo Y, Li J, Lu C, Wei Y, Gao J, Xie Q, Jin J, Zhu G: Genome-wide association analysis identified molecular markers and candidate genes for flower traits in Chinese orchid (Cymbidium sinense). Hortic Res-England 2023:uhad206. 10.1093/hr/uhad206. [DOI] [PMC free article] [PubMed]
- 37.Cao H, Li H, Chen X, Zhang Y, Lu L, Li S, Tao X, Zhu W, Wang J, Ma L. Insight into the molecular mechanisms of leaf coloration in Cymbidium ensifolium. Front Genet. 2022;13:923082. 10.3389/fgene.2022.923082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ning HJ, Gui FF, Tian EW, Yang LY. The novel developed microsatellite markers revealed potential hybridization among Cymbidium species and the interspecies sub-division of C. goeringii and C. ensifolium. BMC Plant Biol. 2023;23(1):492. 10.1186/s12870-023-04499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Chen F, Zhang GQ, Zhang YQ, Niu S, Xiong JS, Lin Z, Cheng ZM, Liu ZJ. Origin and mechanism of crassulacean acid metabolism in orchids as implied by comparative transcriptomics and genomics of the carbon fixation pathway. Plant J. 2016;86(2):175–85. 10.1111/tpj.13159. [DOI] [PubMed] [Google Scholar]
- 40.Balilashaki K, Vahedi M, Ho TT, Niu SC, Cardoso JC, Zotz G, Khodamzadeh AA. Biochemical, cellular and molecular aspects of Cymbidium orchids: an ecological and economic overview. Acta Physiol Plant. 2022;44(2):24. 10.1007/s11738-022-03359-w. [Google Scholar]
- 41.Rong M, Gao S-X, Wen D, Xu Y-H, Wei J-H: The LOB domain protein, a novel transcription factor with multiple functions: A review. Plant Physiol Biochem. 2024:108922. 10.1016/j.plaphy.2024.108922. [DOI] [PubMed]
- 42.Jia R, Li C, Wang Y, Qin X, Meng L, Sun X: Genome-Wide Analysis of LBD Transcription Factor Genes in Dendrobium catenatum. Int J Mol Sci. 2022, 23(4). 10.3390/ijms23042089. [DOI] [PMC free article] [PubMed]
- 43.Yang Y, Yu X, Wu P. Comparison and evolution analysis of two rice subspecies LATERAL ORGAN BOUNDARIES domain gene family and their evolutionary characterization from Arabidopsis. Mol Phylogenet Evol. 2006;39(1):248–62. 10.1016/j.ympev.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Staiger D, Brown JW. Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell. 2013;25(10):3640–56. 10.1105/tpc.113.113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arab M, NajafiZarrini H, Nematzadeh G, Heidari P, Hashemipetroudi SH, Kuhlmann M. Comprehensive Analysis of Calcium Sensor Families, CBL and CIPK, in Aeluropus littoralis and Their Expression Profile in Response to Salinity. Genes. 2023;14(3):753. 10.3390/genes14030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Priest HD, Filichkin SA, Mockler TC. Cis-regulatory elements in plant cell signaling. Curr Opin Plant Biol. 2009;12(5):643–9. 10.1016/j.pbi.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Kishor PBK, Tiozon RN, Fernie AR, Sreenivasulu N. Abscisic acid and its role in the modulation of plant growth, development, and yield stability. Trends Plant Sci. 2022. 10.1016/j.tplants.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Jeon BW, Kim J. Role of LBD14 during ABA-mediated control of root system architecture in Arabidopsis. Plant Signal Behav. 2018;13(8):e1507405. 10.1080/15592324.2018.1507405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore RC, Purugganan MD. The early stages of duplicate gene evolution. Proc Natl Acad Sci USA. 2003;100(26):15682–7. 10.1073/pnas.2535513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang H, Bowers JE, Wang X, Ming R, Alam M, Paterson AH. Synteny and collinearity in plant genomes. Science. 2008;320(5875):486–8. 10.1126/science.1153917. [DOI] [PubMed] [Google Scholar]
- 51.Logacheva M, Kasianov A, Penin A, Klepikova A, Gerasimov E. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. 2016;88(6):1058–70. 10.1111/tpj.13312. [DOI] [PubMed] [Google Scholar]
- 52.Mohapatra PK, Sahu BB: Genetic Analyses of Floral Development on Rice Panicle. In: Panicle Architecture of Rice and its Relationship with Grain Filling. Cham: Springer; 2022: 97–106. 10.1007/978-3-030-67897-5_6.
- 53.Xu C, Luo F, Hochholdinger F: LOB Domain Proteins: Beyond Lateral Organ Boundaries. Trends Plant Sci. 2015, 21. 10.1016/j.tplants.2015.10.010. [DOI] [PubMed]
- 54.Yang FX, Gao J, Wei YL, Ren R, Zhang GQ, Lu CQ, Jin JP, Ai Y, Wang YQ, Chen LJ, et al. The genome of Cymbidium sinense revealed the evolution of orchid traits. Plant Biotechnol J. 2021. 10.1111/pbi.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su S, Shao X, Zhu C, Xu J, Tang Y, Luo D, Huang X: An AGAMOUS-like factor is associated with the origin of two domesticated varieties in Cymbidium sinense (Orchidaceae). Hortic Res. 2018, 5. 10.1038/s41438-018-0052-z. [DOI] [PMC free article] [PubMed]
- 56.Li Y, Zhang B, Yu H. Molecular genetic insights into orchid reproductive development. J Exp Bot. 2022;73(7):1841–52. 10.1093/jxb/erac016. [DOI] [PubMed] [Google Scholar]
- 57.Mount DW. Using the basic local alignment search tool (BLAST). Cold Spring Harb Protoc. 2007;2007(7):pdb. top17. 10.1101/pdb.top17. [DOI] [PubMed] [Google Scholar]
- 58.Members C-N. Partners: database resources of the national genomics data center, China National Center for Bioinformation in 2023. Nucleic Acids Res. 2022;51(D1):D18–28. 10.1093/nar/gkac1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura T, Yamada KD, Tomii K, Katoh K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics. 2018;34(14):2490–2. 10.1093/bioinformatics/bty121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37(5):1530–4. 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subramanian B, Gao S, Lercher MJ, Hu S, Chen WH. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47(W1):W270–5. 10.1093/nar/gkz357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9. 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202-208. 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202. 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7. 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu Z, Cavalcanti A, Chen F-C, Bouman P, Li W-H. Extent of gene duplication in the genomes of drosophila, nematode, and yeast. Mol Biol Evol. 2002;19(3):256–62. 10.1093/oxfordjournals.molbev.a004079. [DOI] [PubMed] [Google Scholar]
- 68.Wang DP, Wan HL, Zhang S, Yu J. γ-MYN: a new algorithm for estimating Ka and Ks with consideration of variable substitution rates. Biol Direct. 2009;4(1):20. 10.1186/1745-6150-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ai Y, Li Z, Sun WH, Chen J, Zhang DY, Ma L, Zhang QH, Chen MK, Zheng QD, Liu JF et al: The Cymbidium genome reveals the evolution of unique morphological traits Hortic Re. 2021, 8(1). 10.1038/s41438-021-00709-6. [DOI] [PMC free article] [PubMed]
- 70.Chung O, Kim J, Bolser D, Kim H-M, Jun JH, Choi J-P, Jang H-D, Cho YS, Bhak J, Kwak M. A chromosome-scale genome assembly and annotation of the spring orchid (Cymbidium goeringii). Mol Ecol Resour. 2022;22(3):1168–77. 10.1111/1755-0998.13537. [DOI] [PubMed] [Google Scholar]
- 71.Fan W, He ZS, Zhe M, Feng JQ, Zhang L, Huang Y, Liu F, Huang JL, Ya JD, Zhang SB, et al. High-quality Cymbidium mannii genome and multifaceted regulation of crassulacean acid metabolism in epiphytes. Plant Commun. 2023;4(5):100564. 10.1016/j.xplc.2023.100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:1–16. 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Team RC: R: A language and environment for statistical computing. R Foundation for Statistical Computing 2022.
- 74.Khan A, Fornes O, Stigliani A, Gheorghe M, Castro-Mondragon JA, Van Der Lee R, Bessy A, Cheneby J, Kulkarni SR, Tan G. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2018;46(D1):D260–6. 10.1093/nar/gkx1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, Ronneberger O, Willmore L, Ballard AJ, Bambrick J: Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024:1–3. 10.1038/s41586-024-07487-w. [DOI] [PMC free article] [PubMed]
- 76.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw RNA sequencing datasets pertinent to this study have been deposited in the National Center for Biotechnology Information (NCBI) repository and are retrievable under the Sequence Read Archive (SRA) accession numbers: PRJNA304321, PRJNA749652, PRJNA771426, and PRJNA783745. Comprehensive details of all additional datasets generated and analyzed during the course of this research are encompassed within the main body of this published article, as well as in the accompanying supplementary information files.