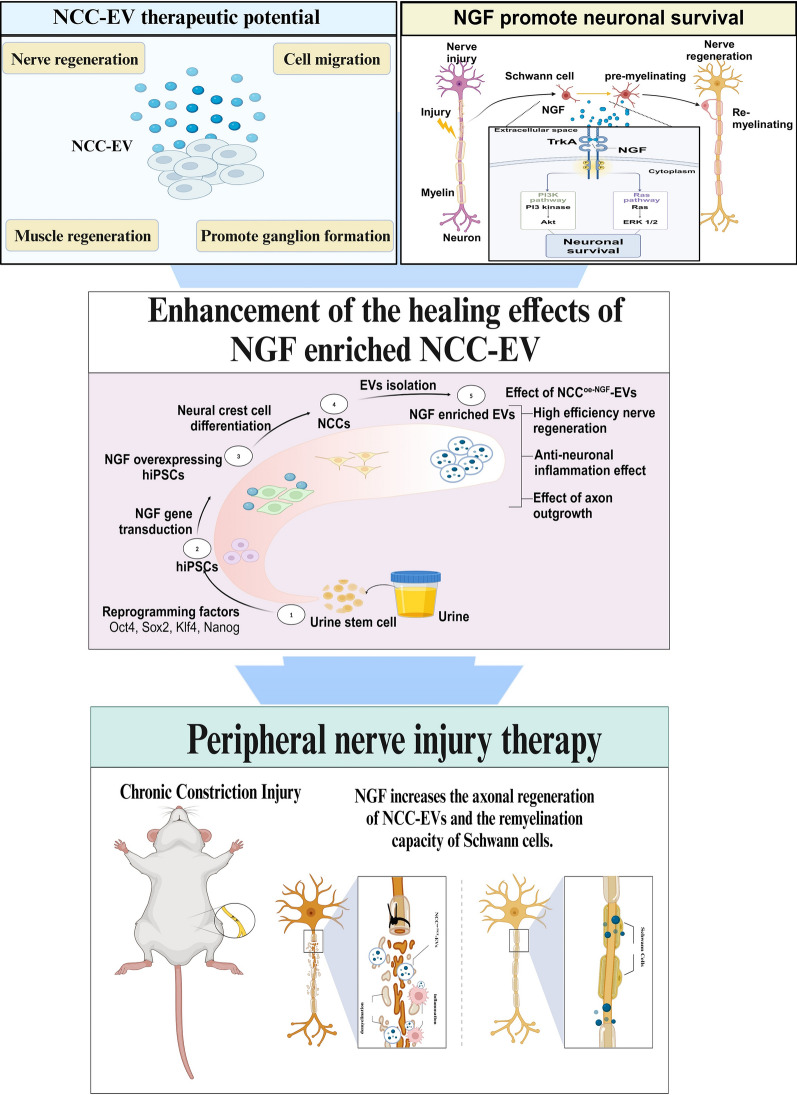
Abstract
Neurological damage caused by peripheral nerve injury can be devastating and is a common neurological disorder that, along with muscle disorders, reduces the quality of life. Neural crest cells (NCCs) are a transient cell population that occurs during embryogenesis, can differentiate into various cells upon transplantation, and has potential therapeutic effects on neurological diseases. However, there are limitations to cell therapy, such as uncontrolled differentiation and tumor formation. Extracellular vesicles (EVs), which are non-cellular potential therapeutic candidates, are nanosized membrane-bound vesicles. Studies have been reported using starch cells derived from neural cells, such as neural stem cells, because they are involved in cell-to-cell communication and carry a variety of bioactive molecules. We investigated the effects of EVs isolated from NCCs on neuronal cell death and inflammation. Additionally, we overexpressed the nerve growth factor (NGF), which is involved in neural cell growth and proliferation, in NCCs to further investigate the effects of EVs containing NGF. NCCoe-NGF-EVs showed neuroprotective and regenerative properties by modulating inflammatory pathway, promoting Schwann cell activation, and enhancing remyelination. In vitro studies on NCCoe-NGF-EVs suppressed pro-inflammatory cytokines and reduced oxidative stress-induced neuronal apoptosis through NF-κB pathway inhibition and ERK, AKT signal activation. We also evaluated the effect of EVs on neuropathy by performing in vivo study. Our results suggest that NCCoe-NGF-EV had neuroprotective effects by reducing neuronal apoptosis and promoting neuronal proliferation based on neurite outgrowth and anti-inflammation effects treated with NCCoe-NGF-EVs. In addition, NCCoe-NGF-EVs were protected muscle loss caused by peripheral nerve injury. NCCoe-NGF-EV induced regeneration of damaged nerves and inhibited cell death through anti-inflammatory effects. This study suggests the potential of NGF-enriched EVs as non-cellular therapeutic platform for peripheral neuropathies and other neuroinflammatory disorders.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40478-025-02033-9.
Keywords: Nerve growth factor, Extracellular vesicle, Neural crest cell, Urine-derived induced pluripotent stem cell, Peripheral nerve injury therapy, Neuropathy, Non-cellular therapy
Introduction
Traumatic peripheral nerve injury causes pain and loss of nerve function. Peripheral nerve injury is caused by several factors, including the aging of nerves and blood vessels, chemotherapy, diabetes, and nerve entrapment [1–5]. If the peripheral nerves or nerve roots in the spinal cord that form the peripheral nerve are compressed or injured, the muscles are also affected[6]. Currently, treatment options for peripheral nerve injury are limited and there are significant challenges in achieving complete regeneration and functional recovery. In peripheral neuropathy, the myelin sheath surrounding the axon is damaged, resulting in myelin sheath breakdown and nerve loss due to axonal cell death [7]. In a nerve injury environment, macrophages create an inflammatory microenvironment by releasing pro-inflammatory cytokines and promotes an immune response by presenting antigens to stimulate nerve repair. Macrophages activates the proliferation and division of Schwann cells. They are also involved in nerve repair through release of NGF, brain-derived growth factor (BDNF), vascular endothelial growth factor (VEGF), and transforming growth factor (TGF), all of which are involved in the regeneration and proliferation of Schwann cells during nerve injury [7–11]. NGF is involved in neuronal differentiation, binds to the TrkA receptor of the tyrosine kinase (RTK) family and p75 NTR neurons to enhance neuronal survival, proliferation, and synaptic plasticity [12]. NGF is also expected to be effective in nerve regeneration and protection against peripheral nerve injury because Schwann cells, which are essential for nerve regeneration, have a high expression of p75NTR. In nerve regeneration, p75NTR expression is increased in Schwann cells, and growth factors such as NGF and BDNF bind to p75NTR to promote Schwann cell proliferation and differentiation, thereby promoting nerve regeneration [13].
Induced pluripotent stem cells (iPSCs) can self-renew and differentiate into the three germ layers, including the nervous system. Previous studies have developed simple, cost-effective, and highly efficient reprogramming methods to generate human iPSCs from tissue-derived somatic cells, such as urine and fibroblasts, from both healthy individuals and patients [14–16]. Recently, research has been published on the generation of human iPSCs by overexpressing specific factors and exploring their differentiation mechanisms, disease modeling, and cell therapy [17, 18]. Neural crest cells (NCCs) are a population of cells that arise from the neural tube during embryogenesis and migrate to form various cell types during embryonic development. They have the potential to differentiate into mesenchymal derivatives, muscle cells, neurons, Schwann cells, and melanocytes, which have therapeutic effects such as modulation of neuroinflammation, neurogenesis, and angiogenesis in neurological diseases, corneal diseases, and other conditions, and are being used as cell therapies to treat neurological disease [19–21].
As a treatment strategy for neuronal diseases, such as Alzheimer’s disease, spinal cord injury, Parkinson’s disease, peripheral nerve injury, and brain ischemia, overexpression of nerve regeneration factors or miRNAs and proteins are being used with cell transplantation to enhance regeneration and protect damaged neurons and anti-neuroinflammatory effects [22, 23]. Stem cell-based therapies have several disadvantages such as the risk of tumorigenicity, immune response. Also, stem cells have low transport stability and a short cell lifespan, making it difficult to use them widely for treatment. To overcome this, extracellular vesicles (EVs) have recently been investigated as cell-free alternatives for cellular therapeutics [24]. EVs are non-cellular components that offer advantages over stem cell therapy in engraftment rate, tumorigenicity, and immune response [25]. They are double-membrane structures with the same components as cell membrane, ranging in size from 40 to 160 nm, and contain proteins, DNA, miRNA, and cell surface proteins. EVs can cross the blood–brain barrier and deliver proteins, miRNAs, and antibodies using recombinant virus, electroporation, and microfluidic systems, making them promising candidates for neurotherapeutic applications [26–28].
Although studies have been conducted on the differentiation of iPSCs into various neural cells and their application in treatment, there have been few reports on the application of iPSCs in disease models by differentiating them into NCCs. In this study, to investigate the potential therapeutic effects of NCC-derived EVs as a non-cellular therapeutic agent and to enhance the therapeutic efficacy of NCC-EVs in a peripheral nerve injury model. We established an NGF-overexpressing human iPSC line and differentiated them into NCCs to produce NGF-enriched EVs. By targeting inflammatory pathways, Schwann cell activation, and axonal repair, we aim to establish NGF-enriched EVs as a novel therapeutic platform for treating peripheral nerve injury and associated neuroinflammatory conditions. In addition, the administration of NCCoe-NGF-EVs after CCI to the mouse sciatic nerve to mimic a traumatic peripheral nerve environment induced nerve regrowth and remyelination, inhibited cell death, and reduced the levels of inflammatory factors. We propose that NCCoe-NGF-EVs can alleviate inflammation and promote neuroprotection and regeneration in peripheral nerve diseases and suggest that NGF-enhanced NCC-EVs in neurological diseases have potential applications in non-cellular therapies.
Materials and methods
Human urine derived stem cell isolation and iPSC generation
The experimental protocol for USCs was approved by the Institutional Review Board of the Seoul Metropolitan Government Seoul National University Boramae Medical Center (IRB No.: 30–2022-81) and Konkuk University Ethics Committee (IRB No.: 7001355-201507-BR-072) in accordance with our recent publication [29]. Briefly summarizing the protocol, 12-well (SPL Life Sciences, Republic of Korea) plate was coated with Matrigel (354,234, BD Biosciences, USA). Urine samples in container were transferred to 50 ml tubes and centrifuged at 1000 rpm for 5 min at 4 °C. The supernatants were smoothly aspirated, remaining pellets were resuspended with 25 mL of PBS in 1% P/S, and centrifuged 1000 rpm for 5 min. The supernatants were removed, leaving only 0.5 mL of the supernatants, and then resuspended in USC isolation medium. The USC isolation medium consisted of 0.5 mL Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s nutrient mixture F12 (D8437, Sigam-Aldrich, USA) containing 10% FBS (12,483,020, Gibco™, USA), 1% penicillin/streptomycin (15,140,163, Gibco™) and 0.5 mL renal epithelial growth medium (REGM, CC-3190, Lonza, Switzerland) supplemented with 10 μM of Y-27632 (HY-10071, MedChemExpress, USA). Cell suspension was seeded on culture plate. hUSCs were subcultured as single cells every 5 days using TrypLE express enzyme (12,563,029, Gibco™). The USCs were detached with TrypLE express enzyme and electroporated with 3 µg of pCXLE-hOCT3/4-shTP53, pCXLE-hSK, pCSLE-hUL and p-CXWB-EBNA by using NEON™ Transfection System 10 µl Kit (Thermo Fisher Scientific, USA). The transfected USCs were transferred to Matrigel coated 6 well and cultured in RE medium supplemented with 10 μM of Y-27632. The medium was changed every 24 h for 3 days then TeSR-E6 (05946, STEMCELL Technologies, Canada) with mycozap (VZA-2011, Lonza) was used for cell culture with 100 ng/ml of bFGF (100-18B, Peprotech, USA), and 10 μM of Y-27632 (MedChemExpress) and changed every day for a month.
Human urine derived induced pluripotent stem cell culture
Urine-derived pluripotent stem cells (UiPSCs) were cultured in TeSR-E8 medium (05990, STEMCELL Technologies) with mycozap (Lonza) and the culture medium was changed every day. When the cell confluency became about 70–80%, cells were subcultured as single cells using accutase (A6964, Sigma-Aldrich, USA) and seeded on Matrigel (BD Biosciences) coated culture dishes supplemented with 10 μM of Y-27632 (MedChemExpress).
Neural crest cell differentiation and culture conditions
When UiPSCs were 70% of confluent, the cells were dissociated from culture dish using accutase (Sigma-Aldrich), centrifuged at 1000 rpm for 3 min and the supernatants were discarded. The remaining pellets were resuspended with TeSR-E6 (STEMCELL Technologies) with mycozap (Lonza). 1.98 × 106 cells were seeded at Matrigel coated 60 mm culture dish (SPL Life Sciences) with 10 μM of Y-27632 and incubated at 37 °C, 5% CO2. The next day, the culture medium was fully changed into NCC differentiation medium, composed of TeSR-E6 supplemented with N-2 (17,502,048, Gibco™), 20 μM of SB431542 (S4317, Sigma-Aldrich) and 10 μM of CHIR99021 (HY-10182, MedChemExpress). The NCC differentiation medium was daily changed for 3 days. After 3 days, the differentiated cells termed passage 0 was detached and 1.98 × 106 cells were seeded at matrigel coated 60 mm culture dish with 10 μM of Y-27632 (MedChemExpress). The culture medium was changed to NCC differentiation medium for 3 days and repeated this method until cells became passage 4.
For Neuron and Schwann cell differentiation, the neural crest cells of passage 4 were detached with accutase and centrifuged at 1000 rpm for 3 min. The supernatants were removed, and cell pellets were resuspended with culture medium. NCCs were seeded at 60 mm dish and incubated at 37 °C in a humidified atmosphere 5% CO2 for 24 h. For Neuron differentiation, the culture medium was removed and changed to Neuron differentiation medium. The contents of medium include Knockout DMEM/F-12 (12,660-012, Gibco™, USA), N-2 supplement (Gibco™, USA), 20 ng/mL of NGF (130-127-431, Miltenyi Biotec, Germany), 500 µg/mL of Dibutyryl-cAMP (1,698,950, Biogems, USA), 10 ng/mL of bFGF (Peprotech), 10 ng/mL of human CTNF (450-13, Peprotech, USA) and 1% of penicillin/streptomycin (Gibco™). For Schwann Cell differentiation, the culture medium was removed and changed to Schwann cell differentiation medium. The contents of medium contain Knockout DMEM/F-12 (Gibco™), N-2 supplement (Gibco™), 10 ng/mL of NGF (Miltenyi Biotec), 500 µg/mL of Dibutyryl-cAMP (Biogems), 10 ng/mL of human BDNF (450-02, Peprotech) and 1% of penicillin/streptomycin (Gibco™). Both culture medium was changed every other day for 14 days.
Establishment of NGF overexpressed UiPSC lines
We constructed the lentiviral plasmid vectors pCAG-MCS. Next, the constructs encoding the full-length human β-nerve growth factor (NGF) was cloned from the human Wharton’s jelly derived mesenchymal stem cells. Total RNA was extracted from using LaboPass™ Labozol reagent (CMRZ001, Cosmogenetech, Republic of Korea) according to the manufacturer’s instructions and cDNA were used to synthesize cDNA by M-MuLV Reverse Transcriptase (CMRT010, Cosmogenetech). NGF cDNA were PCR-amplified with restriction enzymes using KOD-Plus-Neo (KOD-401, Toyobo, Japan). The primers used for cloning were NGF-Xba I-F, 5`-GAA TTC TAG AAT GTC CAT GTT GTT CTA CAC-3` and NGF-BamH I-R, 5`-GAA TGG ATC CTC AGG CTC TTC TCA CA-3`. Next, the amplified NGF gene was used to insert into multiple cloning sites to obtain pCAG-NGF. Constructed vectors were confirmed by sequencing (Cosmogenetech,). LV-control or oe-NGF lentiviral particles were co-transfection of each expression plasmid with the packaging plasmids containing the gag, pol, and rev genes (provided by Dr. Min Chan Gil) into 80% confluent HEK293 T cells in 10-mm cell culture plate using PolyJet reagent (SL100688, Signagen Laboratories, USA). The medium was changed every 24 h after for 3 days. Each collected medium was concentrated to 200 µl by ultracentrifugation 25,000 rpm at 4 ℃ for 2 h (Beckman Coulter, USA). Next, feeder-free culture UiPSCs in a well of the 24-well cell culture plate were transfected with LV-Con or oe-NGF viral particles by changing the medium to TeSR-E8 medium containing concentrated viral particles and 2 μg/ml hexadimethrine bromide (H9268, Sigma-Aldrich). The medium was changed every day for Four days. 500 cells of transfected UiPSCs were plated into 6-well plate (SPL Life Sciences). After 7–10 days of culture, every cell-derived colony was manually dissected a transferred into the well of a Matrigel-coated 12-well culture plate. After expansion under feeder-free conditions, the expression levels of NGF were analyzed by RT-PCR, western blotting, and ELISA.
Reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA of the cells was extracted using LaboPass™ Labozol reagent (Cosmogenetech) according to the manufacturer’s instructions. The concentration of total RNA was measured using a NanoPhotometer (IMPLEN), and 2 µg of total RNA was used to synthesize cDNA by M-MuLV Reverse Transcriptase (Cosmogenetech). For PCR, a volume of 1 μL of cDNA template was combined with 10 pmol of forward and reverse primers, respectively, and 4 μL of rTaq Plus 5 × PCR Master Mix (ELPIS-BIOTECH, Republic of Korea). The total volume was then adjusted to 20 µL with the addition of H2O. The primer sequences are listed in the following Supplementary Table 1.
RT-PCR was conducted using the 9902 Veriti Thermal Cycler (Applied Biosystems) under the following conditions: an initial denaturing phase at 95 °C for 3 min, followed by 35 cycles of 10 s at 95 °C, 10 sat the appropriate annealing temperature, and 10 s at 72 °C, and finally, a 5 min extension phase at 72 °C. RT-PCR was performed in triplicate. The resulting products were visualized in a 1.5% agarose gel with the use of the fluorescent dye safety green. Images of the gels were captured under ultra-violet light using a Canon Powershot A520 camera (Canon, Tokyo, Japan).
For RT-qPCR, LaboPass™ Labozol reagent (Cosmo Genetech) was used to isolate total RNA from the cells, which was reverse-transcribed into complementary DNA using the LaboPass™ M-MuLV Reverse Transcriptase kit (CMRT010, Cosmo Genetech). RT-qPCR mixture was prepared using EzAmp™ qPCR 2X MasterMix (EBT-1802, ELPIS-BIOTECH, Republic of Korea) and run on a Quant Studio™3 Real-Time PCR System (Thermo Fisher Scientific). The respective methods were performed in accordance with the manufacturer’s protocol. The relative expression results were normalized based on GAPDH expression, an internal control, and the fold-change in gene expression was calculated using the comparative 2−ΔΔCt method.
EV isolation and characterization
The culture medium of NCCs was collected for 3 days. To eliminate cell debris, the harvested culture medium was centrifuged at 3000 rpm for 5 min and the supernatants were filtered through 0.2 μm Minisart filters (16534 K, Satorius) to remove remaining cell debris and apoptotic bodies. Filtered culture medium was then centrifuged at 32,000 rpm for 2 h using SW 32 Ti rotor (Optima L-90 K, Beckman Coulter, USA) at 4 °C. After ultracentrifugation, supernatants were poured out and EV samples were resuspended in 0.2 μm filtered PBS (10,010,023, Gibco™). To analyze size distributions and concentrations of EVs derived from Neural crest cells, the number of EVs was measured with NTA (Nanoparticle Tracking Analysis) using ZetaView (TWIN PMX-220, Particle Metrix) with a laser of 488 nm.
TEM
The extracellular vesicles was loaded onto a grid (FCF300-Cu, EMS). After incubation for 10 min, absorb it with 3 M paper. After washing with D.W, negative staining is performed with 1% phosphor tungstic acid. The remaining solution was absorbed with 3 M paper, soaked with D.W, and observed with a transmission electron microscope (HT7800, Hitachi).
Western blot
To isolate cell lysates, cells were lysed in RIPA buffer (CBR002, LPS solution, Korea) with protease inhibitor cocktail (87,786, Thermo Fisher Scientific) and EVs were lysed in the same. Proteins were quantified using the BCA protein assay kit (23,225, Thermo Fisher Scientific) for cells and Micro BCA protein assay kit (23,235, Thermo Fisher Scientific) for EVs. Cell lysates and EVs were prepared by adding 4X Bolt™ LDS Sample Buffer then incubating at 70 °C for 10 min. Proteins (Cells 15 μg, EVs 7 μg) were separated by 4–12% Bis–Tris Plus gels (Thermo Fisher Scientific), transferred to nitrocellulose iBlot™ 2 Transfer Stacks (Thermo Fisher Scientific) and blocked with 5% of Skim milk in TBST (10 mM Tris-HCL, 150 mM NaCl, 0.1% Tween-20) for 1 h. The membrane was incubated overnight with primary antibodies at 4 °C (Supplementary Table 2). The primary antibodies were washed three times with TBST and the membrane were incubated overnight with secondary antibodies at 4 °C and three times of washing were followed. The secondary antibodies used were Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (17,074, Cell Signaling Technology) and Horseradish peroxides (HRP)-conjugated anti-mouse IgG (7076, Cell Signaling Technology). The protein was then visualized by using Invitrogen™ iBrightTM Imagers (CL-1000, Thermo Fisher Scientific) with the procession of Clarity Western ECL substrate (170–5060, Bio-Rad Laboratories). The antibody information is listed in Supplementary Table 2.
FACS
USCs and NCCs were detached with accutase and resuspended in FACS buffer which contains PBS with 5% FBS. The cells were labeled with primary antibodies at a 1:200 ratio, reacted at 4 °C for 30 min, and centrifuged at 1000 rpm for 3 min. The primary antibodies were Anti-HNK-1/N-CAM (C6680-50 TST, Sigma aldrich), p75NTR (#8238, Cell Signaling Technology), CD34 (#130-117-775, Miltenyi Biotec), CD45 (#130-110-771, Miltenyi Biotec), CD73 (#41–0200, Invitrogen), CD90 (AF2067, R&D Systems), and CD105 (MA5-11,854, Invitrogen). The cells were washed three times with FACS buffer and reacted with the secondary antibody at a 1:500 ratio, incubated at 4 °C for 30 min. The secondary antibodies were Goat anti-mouse Alexa-Flour-488 (A-10680, Invitrogen), Goat anti-rabbit Alexa-Flour-647 (A-21245, Invitrogen), donkey anti-sheep IgG-PE (ab7009; Abcam). The cells were centrifuged at 1000 rpm for 5 min and the supernatants were removed, washed three times with FACS buffer. The fluorescence intensity of the secondary antibodies was measured by flow cytometry (CytoFLEX), Beckman Coulter, USA).
4 × 109 particles of EVs derived from NCCs were reacted for one day with Exosome-Human CD9 Isolation Reagent (10614D, Invitrogen). Using DynaMag™−2 Magnet (12321D, Invitrogen), EVs were washed twice with 0.2 μm filtered PBS then incubated with antibodies of CD63-PE (556,020, BD Pharmingen) and CD81-APC (130-119-787, Miltenyi Biotech) at 4 °C for 2 h. After incubation, antibody-labeled EVs were washed twice and measured by CytoFLEX flow cytometer (Beckman Coulter, USA).
AP staining
AP staining (Alkaline Phosphatase staining) was quantified using a Stemgent® AP Staining Kit II (Stemgent™, USA) following the manufacturer’s instructions. Briefly, UiPSCs were fixed with Fix solution at room temperature (RT) for 10 min and stained with an AP Staining Solution mixture (A, B, C, 1:1:1 ratio) at RT for 10 min in the dark. After two washes with PBS without Ca2+ and Mg2+, the images of AP-positive colonies were obtained using a light microscope evaluated microscopically (ZEISS, Axiovert 40 C).
Immunocytochemistry (ICC)
Cells were fixed with 4% paraformaldehyde (PC-2031, Biosesang) and permeabilized with PBST composed of 0.1% Triton X-100 (vol/vol, Sigma) in PBS (Gibco). Cells were then blocked with 10% normal goat serum (vol/vol, Sigma) in PBST and incubated overnight with primary antibodies in 0.1% PBST with 2% normal goat serum at 4 °C. The cells were washed three times with 0.1% PBST for 10 min and incubated overnight with the secondary antibodies in 0.1% PBST with 2% normal goat serum at 4 °C (Supplementary Table 3). The secondary antibodies used were Goat anti-mouse Alexa-Flour-488 (A-10680, Invitrogen), Goat anti-rabbit Alexa-Flour-647(A-21245, Invitrogen). The cells were then washed three times with 0.1% PBST and mounted with ProLong™ Gold Antifade Mountant with DNA stain DAPI (Invitrogen) and coverslips were added. Images were obtained using confocal microscopy (LSM 800, Carl Zeiss) was used.
EV uptake assay
EVs with concentration of 1 × 109 particles/mL derived from NCC and NCCoe-NGF were reacted with 1,1′Dioctadecyl-3,3,3′3′-Tetramethylindotricarbocyaninelodide (Invitrogen) at 37 °C for 1 h in accordance with our recent publication [30, 31]. 2 × 106 cells of BV2 cells and 1 × 106 cells of SH-SY5Y cells were seeded at a 60 mm dish and incubated 24 h in culture medium with 10% FBS. The culture medium was removed then changed to fresh medium without FBS with a treatment of 1 × 108 particles/mL of EVs and incubated at 37 °C in 5% CO2 for 6 h. Cells were dissociated with 0.25% trypsin–EDTA (Gibco) and resuspended in PBS. The fluorescence intensity was measured with flow cytometry (CytoFLEX, Beckman Coulter, USA). Also, Images were obtained using confocal microscopy (LSM 800, Carl Zeiss) was used.
Measurement of nitrite (NO2-) production
2 × 105 cells of BV2 cells were seeded in a 24-well plate and incubate 24 h. 1 × 108 particles/mL of NCC-EVs were pre-treated about 6 h with serum free DMEM-low media and 100 ng/mL LPS (lipopolysaccharide, Sigma-Aldrich) were treated with new serum free media. After 24 h, cell supernatant per group was harvested and cell debris removed through centrifuge at 3000 rpm for 1 min. The concentration of nitrite, an indirect indicator of NO production, was measured in the culture supernatant of LPS-stimulated BV2 cells using the Griess reagent. The Griess reagent consisted of a 1:1 mixture of 1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in distilled water. 100 µl of the Griess reagent and 100 µl of the cell supernatant were loaded onto a 96-well plate and incubated at RT, 10 min. The amount of nitric oxide was measured at an absorbance of 540 nm by using a Bio-RAD x-Mark™ spectrophotometer (Bio-Rad Laboratories).
Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturer`s instructions, the concentration of NGF (EHNGF, Invitrogen), TNF-α (BMS607-3, Invitrogen), IL-6 (RAB0308, Invitrogen) levels were quantified using cell culture conditioned media and EVs. Samples were taken from the conditioned media of each cell culture. The measurement was performed using a 1:1000 dilution of conditioned media according to the manufacturer’s instructions. Samples were read on a Bio-RAD x-MarkTM spectrophotometer (Bio-Rad Laboratories).
Cell viability assay
To determine anti-apoptotic effect of EVs on SH-SY5Y neuroblastoma cells, H2O2 was used to induce neurotoxicity mediated cell apoptosis. 2 × 106 cells were seeded in a 60 mm cell culture dish and incubated at 37 °C, 5% CO2 for 24 h. The culture medium was removed, EVs with a concentration of 1 × 108 particles/mL were pre-treated for 6 h with fresh serum free media with 1% P/S and H2O2 was then treated at a concentration of 700 μM. After 6 h, the medium was replaced to fresh medium with Cellrix Viability Assay Kit (B1007-500, Medifab). Cell viability was measured by quantifying an absorbance of 450 nm using a Bio-RAD x-Mark™ spectrophotometer. And we performed cell viability visualization using the LIVE/DEAD Cell Imaging Kit (R37601, Life Technology) according to the manufacturer’s instructions. In brief, the live green and dead red reagents were then mixed to make 2X stock and added to the cells at RT for 15 min and images were obtained using confocal microscopy (LSM 800, Carl Zeiss). Cells with green fluorescence signal represent live cells, while cells with red fluorescence signal represent dead cells.
Neurite outgrowth assay
Undifferentiated SH-SY5Y cells were seeded in Matrigel-coated 4-well culture slide (SPL) at densities of 1 × 105 cells/well on Day − 1. The culture medium was removed then changed to neurobasal media with or without a treatment of 1 × 108 particles/mL of EVs and incubated at 37 °C in 5% CO2 for 6 h. Following 6 h, each medium was removed, replaced with fresh neurobasal medium and incubated for 3 days at 37 °C in 5% CO2. After 3 days, cells were fixed using 4% PFA, stained for neurites using βIII-tubulin antibody and imaged using confocal microscopy (LSM 800, Carl Zeiss). The neurite outgrowths of the SH-SY5Y cells were measured using Image J.
Animal and CCI model surgical procedures
All animal experiments were performed in accordance with the guidelines of the Konkuk University Institutional Animal Care and Use Committee (IACUA: KU24170). 8-week-old Balb/C female mice (20–25 g) were purchased JA BIO (Korea). The mice were housed for 1 week before the experiment for acclimatization in a well-ventilated room under a standard 12 h light/dark cycle with a regulated ambient temperature of 20–25 ℃. Food and water were provided freely. The CCI induction model for the sciatic nerve was used to induce neuropathic pain as previously reported [32]. Mice were anesthetized at ratio of 4:1 with Alfaxan (10 mg/ml) and Rompun (23.32 mg/ml). A surgical incision was made along the femur under aseptic conditions in the region of the sciatic nerve. Two ligatures (4/0 silk sutures) were then placed around the nerve to induce a CCI. Following the closure of the surgical wound and the application of disinfectant, the animals were returned to their cages for recovery. For all groups, von-Frey test was performed every 3–4 days and the rotarod test every 7 days. After 3 days of CCI induced, each mouse was injected into the site of sciatic nerve injury region with PBS (injury group) and 1 × 109 particles suspended in PBS. The mice were divided into four groups as follows: (1) sham group, (2) PBS-treated group (only injury), (3) NCC-EV-treated group, and (4) NCCoe-NGF-EV-treated group (n = 10 mice in each group). All animals were sacrificed 14 days after CCI induction and nerves, and gastrocnemius muscles were isolated for analysis.
Von-Frey test
To confirm pain-like behavior like hypersensitivity, Paw-withdrawal thresholds (PWT) was measured using Von-Frey filaments according to the up-down method. CCI-induced mic`e were placed on a stainless mesh and acclimated for 10 min. After acclimating, von-Frey filaments (Jeung Do Bio & Plant, Korea) were stimulated in the hind paws. We chose a von-Frey filament of 1 g and reduced the filament force as the foot rose and gradually increased the filament force if there was no response. We rested for 5 min between each filament stimulus. For each mouse individual, PWT was measured and recorded two times to quantify the tensile force level of the plantar response.
Accelerating rotarod test
In this experiment, we assessed gait and motor neuron function in mice using a rotarod (Kyungdo Bio & Plant, Korea) to induce forced motor activity as previously reported [33]. We placed the mice on rotarod and subjected them to an acceleration speed. Before starting the experiment, the mice were adapted to train for 5 min at 4 rpm. After adaptation, the trials followed a program in which the speed was increased from 4 to 30 rpm for the first 3 min and then fixed at 30 rpm for 2 min. After adaptation, the trials followed a program in which the speed was increased from 4 to 30 rpm for the first 3 min and then fixed at 30 rpm for 2 min. This was done twice for each group, and between trials the mice rested for 30 min to avoid exhaustion. If the rat fell off the bar or completed turn while hanging on, it was considered to have fallen, and the time was recorded.
Wet muscle evaluation and histological analysis
The gastrocnemius is the largest muscle innervated by the sciatic nerve and begins to atrophy after sciatic nerve injury. To assess nerve regeneration under nerve injury conditions, we measured the weight and fiber surface area of the gastrocnemius muscle immediately after sacrificing mice. Gastrocnemius muscles from both legs were harvested and weighed. For subsequent muscle tissue analysis, muscle sections were fixed in 4% PFA in PBS, dehydrated and embedded in paraffin. Muscle tissue was sectioned transversely at 4–5 μm. Histological evaluation of myofibrillar nerve tissue was then performed using Hematoxylin and eosin (H&E) staining and Masson`s trichrome (MT) staining. The cross-section area and collagen volume were measured using Image J.
Immunohistochemistry
Mice were sacrificed, and the sciatic nerve (distal from the injury site) were collected and fixed using 4% PFA in PBS and immersed in 30% sucrose solutions until the tissues sunk. Afterward, tissues were embedded into a mold with optimal cutting temperature (OCT) compound (Leica, Wetzlar, Germany) with a careful orientation of the tissue. Then, it was placed over dry ice for several minutes for equilibration and subsequently stored at − 80 °C until cryosection. For cryosection, tissue specimens were mounted on a Leica CM1850 (Leica) in a specimen chuck containing OCT compound and then loaded into a specimen holder with the holder blade against the specimen. Tissue blocks were then cut into 15 μm sections and immunohistochemistry was performed to analyze neural structure and nerve regeneration. For immunohistochemistry, the sections were permeabilized with 1% SDS for 5 min at RT and blocked with PBST (0.1% Triton X-100 in PBS) containing 10% normal goat serum at RT for 1 h. Next, samples were incubated with primary antibodies in PBST containing 2% normal goat serum (Supplementary Table 3). After washing three times with PBST, samples were incubated with secondary antibodies at RT for 3 h. The secondary antibodies used Goat anti-mouse Alexa-Flour-488 (A-10680, Invitrogen), Goat anti-rabbit Alexa-Flour-647(A-21245, Invitrogen). After washing, samples were then washed three times with PBST and mounted with ProLong™ Gold Antifade Mountant with DNA stain DAPI (Invitrogen) and coverslips were added. Images were obtained using confocal microscopy (LSM 800, Carl Zeiss). Three randomly selected fields from each slide were analyzed using Image J.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software). All experiments were performed independently at least in triplicate. Data were expressed as the mean ± SD. Adjusted p-values were calculated using student`s t-test and one-way ANOVA followed by Tukey’s multiple comparison`s post‑hoc test to determine statistical significance. In all figures, the asterisk symbol indicates statistical significance as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Results
USC isolation and establishment of UiPSC, further isolation of EVs from NCC differentiated from UiPSC
Following the isolation of stem cells from midstream urine, iPSCs were generated using the Yamanaka factor. First, we isolated USCs and then performed FACS analysis to identify MSC expression markers and showed that negative markers (CD34 and CD45) were expressed at less than 1%, whereas positive markers (CD73 and CD90) were expressed at over 90%, and CD105 was expressed at over 80% (Supplementary Fig. 1A). These results confirmed that USCs possess the same characteristics as mesenchymal stem cells derived from other tissues. Next, we produced iPSCs from USCs using OSKM, as described in the methods and materials, as reported previously [34, 35]. The immunofluorescence staining of stemness markers, including Oct4, Nanog, SSEA-4, and Tra-1–60, confirmed that differentiation of the UiPSC lines was successfully established (Supplementary Fig. 1B). Then UiPSCs were cultured for 14 days and subsequently differentiated into NCCs. RT-qPCR was performed to measure the expression levels of SOX10, AP2α, P75NTR, and PAX3 mRNA at each passage during differentiation from UiPSCs to NCCs. As a result, the expression levels of SOX10, AP2α, and P75NTR gradually increased during differentiation into NCCs, while PAX3 showed a tendency to increase in passage 2 during differentiation into NCCs and then decrease again (Fig. 1A). FACS analysis indicated that the ratio of double-positive cells for p75NTR and HNK-1 in NCCs derived from UiPSCs exceeded 90%, confirming that NCCs were well differentiated from UiPSC lines (Fig. 1B). Immunofluorescence staining for positive markers of NCCs (Nestin, SOX10, and AP2α) and the negative marker (PAX6) demonstrated stable maintenance of NCC properties (Fig. 1C). Then EVs were isolated from NCCs and characterized by identifying their size, morphology using TEM which showed proper morphology of EVs and ZetaView (NTA), which revealed that the size of most particles ranged from 120 to 130 nm, and zeta-potential was under − 30 mV (Fig. 1D, E). To further evaluate the EV characteristics, we performed western blot analysis, which indicated that NCC-EVs expressed EV-positive markers, including the tetraspanin proteins CD9 and CD63, whereas negative markers, such as calnexin and GM130, were not detected in NCC-EVs (Fig. 1F). Finally, FACS analysis confirmed that the expression levels of the positive EV markers, CD63-PE and CD81-APC, were over 90% in NCC-EV (Fig. 1G). These results demonstrate that UiPSCs successfully differentiate into NCCs, and that EVs isolated from NCCs well maintain their shape and properties.
Fig. 1.
Characterization of UiPSC-derived NCCs and NCC-EVs. A RT-qPCR was used to measure the expression of NCC related genes (SOX10, AP2α, p75NTR, PAX3). The relative gene expression levels were analyzed in triplicate and normalized to endogenous GAPDH. Data are shown as mean ± SD (n = 3). B FACS analysis to measure double positive expression of NCC markers. C The image shows the expression of NCC positive markers (Nestin, SOX10, AP2α) and negative marker (PAX6) using a fluorescence microscopy. Scale bar = 20 μm. D The particle size of NCC-EVs was measured with ZetaView and the morphology was observed with transmission electron microscopy. Scale bar = 100 nm. E The Zeta potential of NCC-EVs was measured with ZetaView. F The expression level of EVs markers in the whole cell lysates and EVs from NCC was confirmed by western blotting. The EVs positive markers are CD9, CD63, and negative markers are GM130 (Golgi marker), Calnexin (ER marker) and β-actin. G The expression of CD81 and CD63 in each group of EVs was detected by FACS using Exosome-Human CD9 Dynabeads. For multiple comparisons of groups, Statistical significance was determined using one-way ANOVA was performed followed by post hoc Tukey’s multiple comparison. **P < 0.01, ***P < 0.001, ****P < 0.0001
NCC-EVs promote neuronal differentiation and anti-inflammatory effects in vitro
We investigated whether NCC-EVs are effective in promoting neural cell differentiation and alleviating inflammatory responses. SH-SY5Y were used to identify the effect of NCC-EVs on neuronal differentiation. SH-SY5Y cells were treated with NCC-EVs and cultured for 3 days, after which they were immunostained with Tuj1. This staining confirmed that neurite outgrowth in EV-treated cells compared to that in untreated cells longer neurites (Fig. 2A–B). Furthermore, Western blotting was performed to determine the expression levels of ERK, AKT, Tuj1, and NF-H, which are associated with neural cell differentiation and neurite outgrowth. The results showed that the expression of P-ERK and P-AKT was increased in the NCC-EVs group compared to that in the control group (Fig. 2C). Next, to confirm the anti-inflammatory effects of EVs on LPS-induced inflammation in BV2 cells, we measured nitrite production by treating BV2 cells with various concentrations of LPS for 24 h and confirmed that nitrite was produced from BV2 cells in an LPS concentration-dependent manner (Fig. 2D). NCC- EVs were then pre-treated at 37 °C for 6 h, and LPS was used to induce inflammation for 24 h. Nitrite production and pro-inflammatory gene expression were reduced in the EVs-treated group (Fig. 2E). Moreover, the mRNA expression of pro-inflammatory cytokines genes (TNF-α, IL-6) was significantly reduced in EV treatment group (Fig. 2F–G). These results confirmed that NCC-EVs support neural cell differentiation by promoting neurite outgrowth in SH-SY5Y cells and attenuate the inflammatory environment in BV2 cells.
Fig. 2.
NCC-EVs promote neurite outgrowth and attenuate neuronal inflammation. A–B Fluorescence microscopy images of Tuj1 immuno-stained SH-SY5Y cells which were treated with NCC-EVs and incubated for 3 days Scale bar = 20 μm and average neurite length each group. Data are shown as mean ± SD (n = 3). C The western blot image shows the expression level of P-ERK, T-ERK, P -AKT, T-AKT, Tuj1, NF-H, and β-actin in SH-SH5y cells treated with EVs from each cell lines and incubated for 3 days. D The graph shows the relative Nitrite concentration in supernatants of BV2 cells exposed to LPS at various concentrations (100, 500, 1000 ng/ml) for 24 h. Data are shown as mean ± SD (n = 3). E The result shows comparative nitrite concentration of LPS-induced BV2 cells after 6 h of pre-treatment with NCC-EVs. Data are shown as mean ± SD (n = 3). F–G RT-qPCR was used to measure the expression of pro-inflammatory gene (TNF-α, IL-6). The relative gene expression levels were analyzed in triplicate and normalized to endogenous GAPDH. Data are shown as mean ± SD (n = 3). Statistical significance was determined using Student`s t-test. For multiple comparisons of groups, Statistical significance was determined using one-way ANOVA was performed followed by post hoc Tukey’s multiple comparison. ***P < 0.001, ****P < 0.0001
Establishment of NGF overexpressing UiPSCs
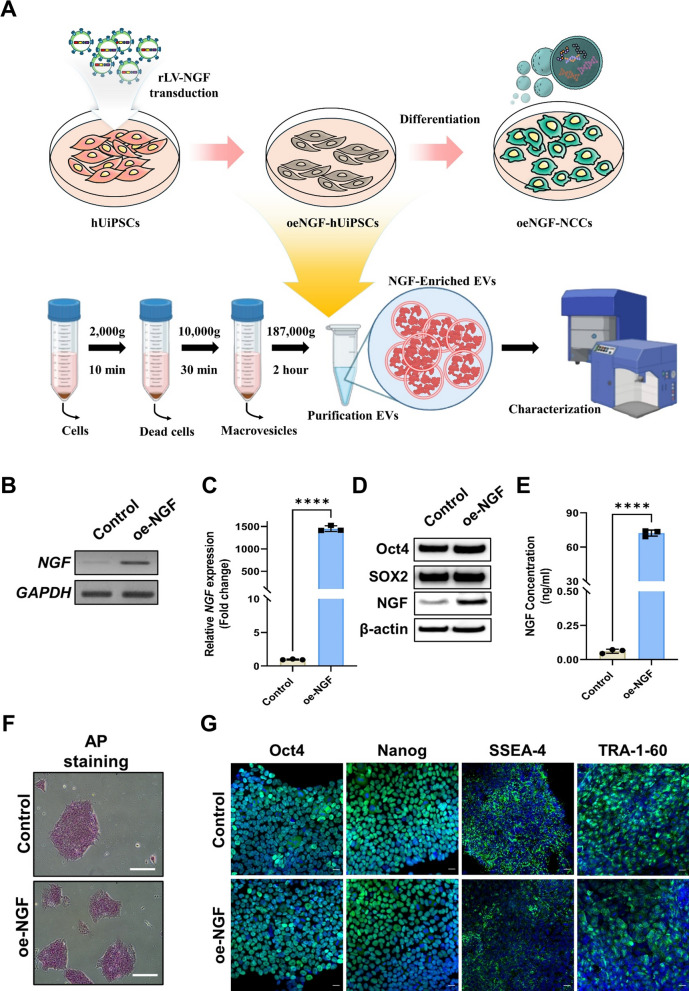
To investigate whether NGF could further enhance the effect of NCC-EVs, we generated NGF-overexpressing UiPSC cell lines by transducing NGF into UiPSCs via lentivirus and differentiated them into NCCs for 14 days to isolate EVs (Fig. 3A). UiPSCs were transfected with LV-Con or oe-NGF lentiviral particles. Single cell-derived LV-Con or oe-NGF UiPSC colonies were manually selected and expanded. The gene expression level of NGF in the constructed UiPSC lines was measured using RT-PCR, and was found to be overexpressed in the oe-NGF group (Fig. 3B, C). Furthermore, to confirm the expression of NGF at the protein level, western blotting and ELISA were performed, revealing significant overexpression in the oe-NGF group, and the protein expression levels of Oct4 and SOX2 in each cell line were similar to those in the Control group (Fig. 3D, E). AP and immunofluorescence staining of stemness markers, including Oct4, Nanog, SSEA-4, and Tra-1–60, confirmed that differentiation of the LV-Con and oe-NGF UiPSC lines was not affected by the recombinant lentivirus (Fig. 3F, G). These results confirmed that cell lines with increased NGF expression were successfully established without affecting the undifferentiated state of NGF-overexpressing UiPSCs.
Fig. 3.
Establishment of a human urine-derived induced pluripotent stem cell (hUiPSC) line overexpressed NGF. A Schematic design for the establishment of UiPSCs and NGF-overexpressing cell lines and subsequent isolation of EVs from NCCoe-NGF. B The levels of NGF and GAPDH expression in each cell lines were determined using RT-PCR analysis. C RT-qPCR was used to measure the expression of NGF. The relative gene expression levels were analyzed in triplicate and normalized to endogenous GAPDH. Data are shown as mean ± SD (n = 3). D Western blot analysis was used to measure the expression levels of Oct4, SOX2, NGF and β-actin in each UiPSC lines. E The levels of NGF protein were measured using ELISA in the conditioned media derived from each UiPSC cell lines. Data are shown as mean ± SD (n = 3). F Representative images showing the AP activity in the each UiPSC cell lines. Scale bar = 20 μm. G Fluorescence microscopy images of the expression of Oct4, Nanog, SSEA-4 and TRA-1–60 in the individual UiPSC cell lines. Scale bar = 20 μm. Statistical significance was determined using Student`s t-test. ****P < 0.0001
Characterization of NCCs differentiated from NGF-overexpressing UiPSCs
We investigated whether NCCs differentiated from NGF-overexpressing UiPSCs express NGF and maintain the characteristics of NCCs. The results of NGF expression levels confirmed that NCCoe-NGF are at significantly higher levels than NCC-Con (Fig. 4A, C). In addition, western blot and ELISA were performed to measure NGF protein expression levels, and it was found that NCCoe-NGF expressed NGF at significantly higher levels than NCC-Con (Fig. 4B, D). In all experimental groups, the percentage of cells that were positive for both p75NTR and HNK-1 exceeded 90%, confirming that the overexpression of NGF did not affect the differentiation rate of UiPSCs into NCCs (Fig. 4E). Immunofluorescence staining for positive markers of NCCs, including Nestin, SOX10, and AP2α, as well as the negative marker PAX6, demonstrated the properties of the NCCs were maintained stably, irrespective of the presence or absence of NGF overexpression (Fig. 4F–G). To confirm the multipotency of NCCs, we further differentiated them into neuronal and Schwann cells and observed their specific protein expression by immunostaining. These cells differentiated terminally in the presence or absence of NGF overexpression (Supplementary Fig. 2A–B). These findings indicate the successful construction of NGF-overexpressing NCCs from UiPSCs, while preserving cell-specific properties and differentiation rates. The following experiments were performed using these two cell lines: control and oe-NGF.
Fig. 4.
Characterization of NCCs generated from NGF overexpressed UiPSC lines. A The levels of NGF and GAPDH expression in each NCC lines were determined using RT-PCR analysis. B Western blot analysis was used to measure the expression levels of NGF and β-actin in each NCC lines. C RT-qPCR was used to measure the expression of NGF. The relative gene expression levels were analyzed in triplicate and normalized to endogenous GAPDH. Data are shown as mean ± SD (n = 3). D The levels of NGF protein were measured using ELISA in the conditioned media derived from each NCC lines. Data are shown as mean ± SD (n = 3). E FACS analysis to measure double positive expression of NCC surface markers (HNK-1, p75NTR). F–G Microscopic images show the expression of Nestin, Sox10, PAX6, and AP2α in each NCC lines using a fluorescence microscopy. Scale bar = 20 μm. Statistical significance was determined using Student`s t-test. ****P < 0.0001
Isolation and characterization of EVs from NGF-overexpressed NCCs
We investigated whether NGF overexpression altered the properties of EVs. We evaluated the morphology, size, and zeta potential of NCCoe-NGF-EVs using TEM and Zetaview, which revealed that the size of most particles ranged from 120 to 130 nm, and the zeta potential was under − 30 mV. Our findings confirmed that NCC-derived EVs differentiated from NGF-overexpressing cell lines did not significantly alter the size or zeta potential of EVs (Fig. 5A–C). Furthermore, the NGF protein ELISA data showed that NCC-derived EVs derived from NGF-expressing UiPSCs were loaded with NGF (Fig. 5D). To further evaluate the effect of NGF on EV characteristics, western blot analysis was performed. This analysis indicated that NCCoe-NGF-EVs expressed EV-positive markers, including the tetraspanin proteins CD9 and CD63. In contrast, negative markers, such as calnexin and GM130, were not detected in NCCoe-NGF-EVs (Fig. 5E). Finally, FACS analysis demonstrated that the expression levels of the positive EV markers, CD63-PE and CD81-APC, exceeded 95% (Fig. 5F). These findings provide evidence that NGF proteins are loaded into NCC-derived EVs while maintaining the properties of these EVs.
Fig. 5.
Characterization of NCCoe-NGF-derived extracellular vesicles. A The particle size of NCCoe-NGF-EVs were measured by ZetaView. B The Zeta potential of EVs NCCoe-NGF were measured by ZetaView. C Transmission electron microscopy images showing the extracellular vesicles morphology of NCCoe-NGF-EVs. Scale bar = 100 nm. D The levels of NGF protein were measured using ELISA in EVs from each NCCs. Data are shown as mean ± SD (n = 3). E The expression level of EVs markers in the whole cell lysates and EVs from NCCoe-NGF was confirmed by western blotting. The EVs positive markers are CD9, CD63, and negative markers are GM130 (Golgi marker), Calnexin (ER marker) and β-actin. F The expression of CD81 and CD63 in NCCoe-NGF-EVs was detected by FACS using Exosome-Human CD9 Dynabeads. Statistical significance was determined using Student`s t-test. ****P < 0.0001
NCCoe-NGF-EVs promote neuronal differentiation and attenuate nerve cell apoptosis by H2O2-induced oxidative stress in SH-SY5Y cell
We investigated whether NGF in NCCoe-NGF-EVs could enhance the neurite outgrowth effect of NCC-EVs. Initially, we observed that EVs could be internalized by SH-SY5Y cells. Both types of EVs were labeled with DiR and incubated with SH-SY5y cells at 37 °C for 6 h. The presence of red fluorescence within the cells confirmed successful internalization of EVs (Fig. 6A). Furthermore, FACS analysis showed that under the same conditions, NCCoe-NGF-EVs (66.8%) internalized a higher amount of EVs compared to NCC-EVs (41.1%) (Fig. 6B). SH-SY5Y cells were then treated with EVs and cultured for 3 days, western blotting was performed to determine the expression levels of ERK, AKT, Tuj1, and NF-H, which are associated with neuronal differentiation. The results showed that the expression of P-ERK and P-AKT was increased in the NCCoe-NGF-EVs group compared to that in the NCC-EV group, but no obvious difference was observed in the expression of the neuronal markers Tuj1 and NF-H (Fig. 6C). Then each group of cells were immunostained with Tuj1, confirming that neurites grew in EV-treated cells compared to that in untreated cells. Notably, cells cultured with NCCoe-NGF-EVs exhibited significantly longer neurites (Fig. 6D–E). Next, investigating neuroprotection effect during H2O2-induced oxidative stress in SH-SY5Y cells, cells were pre-treated with NCC-EVs and NCCoe-NGF-EVs for 6 h then treated with 700 μM of H2O2 in fresh serum free medium for 6 h. Using CellRix, cell viability in the NCCoe-NGF-EVs groups was greater than that in the oxidative stress group, revealing that NCCoe-NGF-EVs can enhance neuroprotective effects (Supplementary Fig. 3A). In addition, we captured images of the live/dead assay after 6 h of pretreatment with EVs and 6 h of H2O2 treatment, which showed more cell death (red) in the H2O2-induced oxidative stress group, whereas the NCC-EV and NCCoe-NGF-EV groups showed weak dead signals and more live signals (green), indicating that EVs can protect cells from death during oxidative stress (Supplementary Fig. 3C–D). As previously reported [36, 37], using western blotting, we investigated whether NCCoe-NGF-EVs inhibit cell apoptosis by regulating the expression of ERK and AKT signaling during oxidative stress-induced apoptosis. Neuronal cell death was inhibited in the EV-treated groups, with increased expression of P-AKT and P-ERK and decreased expression of the apoptosis-related protein Bax. We also observed increased expression of the synaptic plasticity protein, nNOS (Supplementary Fig. 3B). In summary, NCCoe-NGF-EVs not only promoted the differentiation and proliferation of neuronal cells by enhancing the expression of Tuj1 and NF-H, proteins related to neuronal differentiation, and by activating the P-ERK and P-AKT pathways, which are related to proliferation, thereby extending neurites, but also played a role in preventing cell death induced by oxidative stress.
Fig. 6.
NCCoe-NGF-EVs promote neurite outgrowth in SH-SY5Y nerve cells. A Confocal microscopy images of SH-SY5Y nerve cells were acquired after treatment of DiR-labelled EVs from NCC and NCCoe-NGF lines for 6 h. Scale bar = 20 μm. B FACS analysis of SH-SY5Y cells treated DiR-labelled EVs from each cell lines for 6 h shows the EV uptake rate to SH-SY5Y cells. C The western blot image shows the expression level of P-ERK, T-ERK, P -AKT, T-AKT, Tuj1, NF-H, and β-actin in SH-SH5y cells treated with EVs from each cell lines and incubated for 3 days. D–E Fluorescence microscopy images of Tuj1 immuno-stained SH-SY5Y cells which were treated with EVs from each cell lines and incubated for 3 days Scale bar = 20 μm and average neurite length each group. Data are shown as mean ± SD (n = 3). For multiple comparisons of groups, Statistical significance was determined using one-way ANOVA was performed followed by post hoc Tukey’s multiple comparison. *P < 0.05, ***P < 0.001, ****P < 0.0001
Effect of NCCoe-NGF-EVs on LPS-induced neuronal inflammation
We further investigated the anti-inflammatory effects of EVs on LPS-induced inflammation in BV2 cells in vitro. Initially, to assess the internalization capacity of EVs, DiR labelled EVs were incubated with BV2 cells at 37 °C for 6 h, demonstrating successful uptake of EVs into the cytoplasm (Fig. 7A). Subsequently, using FACS analysis, we identified the cellular uptake rate by measuring the fluorescence intensity of DiR-labelled EVs incubated with cells at 37 °C for 6 h. We observed a higher cellular uptake capacity of NCCoe-NGF-EVs (87.6%) compared with that of NCC-EVs (70.5%) (Fig. 7B). Following this, EVs were pre-treated at 37 °C for 6 h, and LPS was used to induce inflammation for 24 h. Nitrite production and pro-inflammatory gene expression were similarly decreased in both EV groups (Fig. 7C). Moreover, the mRNA expression of pro-inflammatory cytokines was significantly reduced in both EV treatment groups. Specifically, the expression level of TNF-α, COX-2, and IL-1β in NCCoe-NGF-EVs was lower than those in the NCC-EVs group, while the expression level of IL-6 was higher, but there was no significant difference between the two groups (Supplementary Fig. 4). Furthermore, we observed a significant decrease in the protein levels of pro-inflammatory cytokine in both EV treatment groups (Fig. 7D). Additionally, we analyzed the phosphorylation levels of JNK and NF-κB, which are related to the synthesis of pro-inflammatory cytokines, suggesting that EV treatment can decrease inflammation-related signaling pathways, especially in the NCCoe-NGF-EVs treatment group (Fig. 7E). Finally, we evaluated the effect of EVs on LPS-induced inflammation by immunostaining for Iba-1, which revealed reduced expression levels of Iba-1 in LPS activated BV2 cells treated with EVs (Fig. 7F–G). Taken together, these results show that NCC-derived EVs have anti-inflammatory effects that prevent LPS-induced inflammation, with no significant differences in the presence or absence of NGF.
Fig. 7.
Inhibitory effect of NCCoe-NGF-EVs on LPS-induced neuronal inflammation. A Confocal microscopy images of BV2 cells were acquired after treatment of DiR-labelled EVs from NCC and NCCoe-NGF lines for 6 h. Scale bar = 20 μm. B FACS analysis of BV2 cells treated DiR-labelled EVs from each cell lines for 6 h shows the EV uptake rate to BV2 cells. C Graph shows comparative expression level of Nitrite contained in the supernatant of LPS-induced BV2 cells after 6 h of pre-treatment with EVs derived from NCC and NCCoe-NGF lines. Data are shown as mean ± SD (n = 3). D ELISA assays were used to determine the protein levels of TNF-α (left panel) and IL-6 (right panel) in the cell supernatants of BV2 cells stimulated with LPS with or without each EV pre-treatment. Data are shown as mean ± SD (n = 3). E Western blot image shows the expression level of P-JNK, P-NF-kB, T-NF-kB, and β-actin in BV2 cells stimulated with LPS with or without each EV pre-treatment. F–G Fluorescence microscopy images confirming the expression levels of F-actin and Iba-1 in LPS-induced BV2 cells and a graph showing the relative immunoreactivity for Iba-1 expression in BV2 cells (relative to control). Data are shown as mean ± SD (n = 3). Scale bar = 20 μm. For multiple comparisons of groups, Statistical significance was determined using one-way ANOVA was performed followed by post hoc Tukey’s multiple comparison. *P < 0.05, **P < 0.01, ****P < 0.0001
NCCoe-NGF-EVs recovery CCI-induced neuropathy pain and reduces loss of function in the gastrocnemius muscle
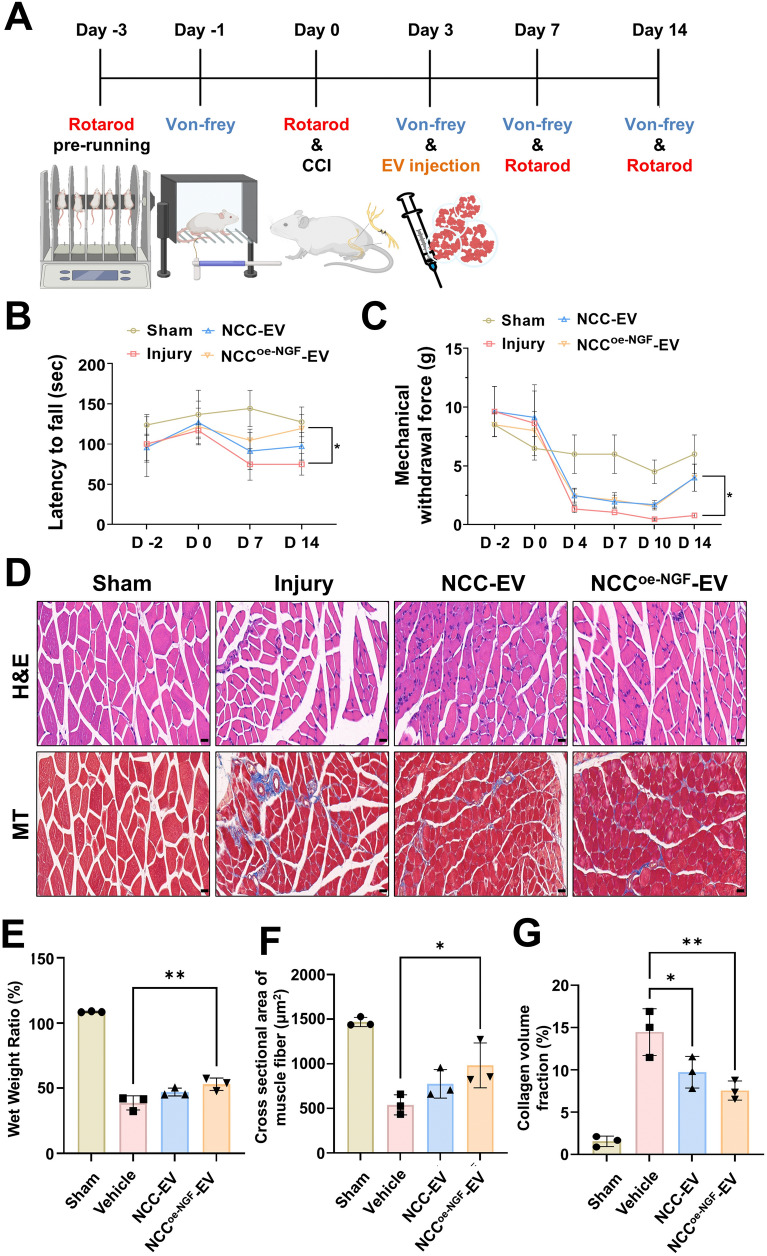
We established the mice model of CCI to investigate the therapeutic effects of NCCoe-NGF-EV on functional recovery, neuronal regeneration, and inflammatory modulation. We ligated the right sciatic nerve of mice twice to induce chronic compression for 2 weeks, and 3 days after surgery, we injected NCC-EVs and NCCoe-NGF-EVs into the muscles near the injury site. Rotarod and Von-Frey tests were conducted to confirm behavioral changes upon injury (Fig. 8A). We investigated the degree of recovery of motor function following nerve injury using the rotarod test, and the degree of recovery of pain sensitivity following nerve injury using the Von-Frey filament to quantify pain-like behavior like hypersensitivity. The results showed that the injured group exhibited reduced motor function and consistently lower pain sensitivity. This result confirms that sciatic nerve ligation induces nerve damage. We tested the recovery of motor function and pain sensitivity using EVs. The results showed that the NCC-EV and NCCoe-NGF-EV treated groups had a reduced loss of motor function compared with that of the injury group. We also observed a significant recovery of motor function in the NCCoe-NGF-EV group compared to the injury group and recovery of motor function in the NCC-EV group, but the difference was not significant. When we examined the recovery of pain sensitivity by nerve injury, we found a significant recovery from day 7 in both the NCC-EV and NCCoe-NGF-EV groups compared with that of the injury group (Fig. 8B–C). Next, we harvested the gastrocnemius muscle 2 weeks after induction of the CCI model to compare changes in muscle weight and structure [38, 39]. Gastrocnemius muscles were stained with H&E and Masson’s trichrome to analyze the structural changes. Microscopic images showed that the injury group had more collagen and fibers, and the EV group had less collagen and fibers. There was also a reduction in muscle fibers in the injury group compared with that of the EV group (Fig. 8D). Comparing the amount of muscle loss by measuring the wet weight of gastrocnemius muscles on both sides of the mice, we observed nerve ligation-induced muscle loss and found that the percentage of muscle loss in the NCCoe-NGF-EV group was significantly increased compared with that in the injury group (Fig. 8E). Furthermore, a comparison of the cross-sectional area of muscle fibers stained with H&E showed that the area of muscle fibers decreased in the injury group and increased in the NCCoe-NGF-EV group, indicating that NCCoe-NGF-EVs have a protective effect against nerve injury-induced muscle fiber loss (Fig. 8F). Lastly, by measuring the collagen volume fraction in the gastrocnemius muscle stained with Masson’s trichrome, the data confirmed that CCI can induce accumulation of collagen, as seen in the injury group, and NCCoe-NGF-EVs can significantly reduce collagen accumulation and protect the fibrosis of the gastrocnemius muscle induced by CCI (Fig. 8G). In summary, NCC -EV can attenuated functional and morphological changes (such as muscle loss, collagen deposition, and fibrosis) in the gastrocnemius muscle after nerve injury and improved exercise performance and pain, and NGF can enhance therapeutic effects.
Fig. 8.
NCCoe-NGF-EVs reduces CCI-induced loss of function in the gastrocnemius muscle. A Schematic diagram of establishment of CCI model and schedule of rotarod and von-Frey to examine behavioral improvement according to EVs injection. B–C Graph of the latency to fall for each group using the Rotarod for 2 weeks. And the Von-Frey method was used to determine the mechanical force required to measure a 50% withdrawal response in each group. Data are shown as mean ± SD (n = 10 per group) D Image of mouse gastrocnemius muscle sectioned and stained with H&E and MT staining to show muscle cross-sectional area and collagen accumulation. E Wet weight ratios were measured to evaluate functional recovery of the gastrocnemius muscle. Data are shown as mean ± SD (n = 3 per group). F Graph shows cross sectional area of muscle fiber stained with H&E. Data are shown as mean ± SD (n = 3 per group). Scale bar = 20 μm G Collagen accumulation and muscle fibrosis were confirmed by measuring collagen volume fraction in gastrocnemius muscle stained with Masson’s trichrome. Data are shown as mean ± SD (n = 3 per group). For multiple comparisons of groups, a one-way ANOVA was performed followed by post hoc Tukey’s multiple comparison. *P < 0.05, **P < 0.01
NCCoe-NGF-EVs restores neurological function by promoting remyelinating in the CCI model
We investigated the remyelination process to assess the neuronal regenerative effects of NCCoe-NGF-EVs in the CCI model. To determine whether EVs increase the expression of growth factors involved in nerve regeneration following nerve injury, we first measured the gene expression levels of BNDF and NT-3. Both BNDF and NT-3 were significantly higher in the NCCoe-NGF-EV group than in the injury group, and NGF protein expression was also upregulated in the tissue (Fig. 9A–B). To further explore the changes in factors related to nerve repair and remyelination at the protein level, we performed western blotting analyses. Notably, NCCoe-NGF-EVs significantly increased the expression of the neuroplasticity marker nNOS and induced neuronal repair by enhancing the phosphorylation of EKR and AKT. This, in turn, led to an increase in the expression of axonal markers GAP43 and NF-H (Fig. 9B–E). Additionally, we examined the expression of p75NTR, a Schwann cell marker involved in myelination, and observed a significant increase in its expression following NCCoe-NGF-EV treatment. To assess the extent of nerve repair, we stained axons and myelin. Sciatic nerve tissues, which were ligated for 2 weeks, were harvested, transversely sectioned, and immunostained with NF-H (axon marker) and MBP (myelin marker) to determine whether EVs induce remyelination after nerve injury. In the sham group, the myelin sheath wrapped around the axon, a characteristic of normal myelinated axon structure. In contrast, the injury group exhibited a decreased percentage of myelinated axons, along with a reduction in MBP expression. In addition, the percentage of myelinated axons were higher in the NCC-EV group than in the injury group, although the difference was not statistically significant. Importantly, the NCCoe-NGF-EV group exhibited a significant decrease and increase in the percentage of myelinated axons compared to the sham and injury groups, respectively (Fig. 9F–G). These findings suggest that NCCoe-NGF-EVs promote remyelination following nerve injury.
Fig. 9.
NCCoe-NGF-EVs repairs neurological function by remyelinating the damaged sciatic nerve. A RT-qPCR was used to measure the expression of growth factor gene (BDNF and NT-3). The relative gene expression levels were analyzed in triplicate and normalized to endogenous GAPDH. Data are shown as mean ± SD (n = 3 per group). B The protein levels of NF-H, nNOS, p75NTR, NGF, GAP-43, T-ERK, P-ERK, T-AKT, P-AKT, and β-actin in each group were determined using western blotting. C–E Relative signal intensity of NF-H, nNOS, and p75NTR normalized by β-actin. Data are shown as mean ± SD (n = 3 per group). F Representative images of the immunohistochemistry showing the NF-H and MBP in sciatic nerve after induction of CCI and EVs administration using confocal microscopy. Scale bar = 10 μm. G Graph showing the MBP expression to NF-H expression ratio of myelinated axons. Data are shown as mean ± SD (n = 3 per group). For multiple comparisons of groups, a one-way ANOVA was performed followed by post hoc Tukey’s multiple comparison. *P < 0.05, **P < 0.01, ****P < 0.0001
NCCoe-NGF-EVs alleviate neuropathy by modulating neuronal cell death and inflammatory responses
Finally, we determined whether NCCoe-NGF-EVs could alleviate the neuropathological changes induced by chronic nerve constriction injury. Neuronal death, which is triggered by ligation-induced nerve injury, was assessed via cell death marker, cleaved caspase-3, and NF-H staining. We observed a significant increase in neuronal death in the injury group compared to that in the sham group. To determine whether neuronal death was inhibited by EVs, we assessed cell death across different groups and found that cell death was highly induced in the injury group, while both the NCC-EV and NCCoe-NGF-EV groups demonstrated significant neuroprotective effects, as evidenced by a reduction in cell death compared to the injury group (Fig. 10A–B). To further investigate the underlying mechanisms, we performed western blot analysis to determine the protein expression of inflammatory factors. The data revealed increased TNF-α, Iba-1, COX-2, P-NF-κB, and T-NF-κB protein expression in all groups. The data revealed increased TNF-α, P-NF-κB, Iba-1, and COX-2 expression in the injury group. However, protein expression of these inflammatory factors was significantly reduced in the NCCoe-NGF-EV group (Fig. 10C). Additionally, mRNA expression levels of the inflammatory factors TNF-α, IL-6, and COX-2 increased in the injury group and significantly decreased in both the NCC-EV and NCCoe-NGF-EV groups. Notably, the gene expression levels of TNF-α and IL-6 were significantly lower in the NCCoe-NGF-EV group than that in the NCC-EV group (Fig. 10D). Collectively, these findings suggest that NCCoe-NGF-EVs alleviate CCI-induced neuropathy by reducing the expression of proinflammatory cytokines and preventing neuronal cell death though downregulation of apoptosis-related proteins (Fig. 10E).
Fig. 10.
NCCoe-NGF-EVs modulate neuronal cell death and inflammation to alleviate neuropathy. A Representative immunohistochemistry images showing NF-H and cleaved caspase-3 in sciatic nerve after CCI induction and EV administration using confocal microscopy. Scale bar = 10 μm. B The result shows the relative immunoreactivity for cleaved caspase-3 expression in sciatic nerve (relative to sham). Data are shown as mean ± SD (n = 3 per group). C The protein levels of TNF-α, Iba-1, COX-2, T-NFκB, P- NFκB, and β-actin in each group were determined using western blotting. D Relative mRNA expression levels of mTNF-α, mCOX-2, and IL-6 were determined using RT-qPCR. Data are shown as mean ± SD (n = 3 per group). E Representative image summarizing the nerve regeneration effects of NCCoe-NGF-EVs in the CCI model, promoting axon regrowth, remyelination, and anti-inflammation and anti-apoptosis. For multiple comparisons of groups, a one-way ANOVA was performed followed by post hoc tukey’s multiple comparison. *P < 0.05, **P < 0.01. ***P < 0.001. ****P < 0.0001
Discussion
Stem cell therapies, such as iPSCs and MSCs, show promise for nerve regeneration in neurological diseases resulting from traumatic pain-related peripheral nerve injury [40, 41]. iPSCs can be differentiated into various nerve cell types, particularly NCCs, which have multipotent capabilities that promote neurite outgrowth and nerve regeneration [42–46]. While direct cell transplantation is a potential therapeutic strategy for nerve injury, it is associated with some limitations, including immune rejection and abnormal differentiation. To overcome these limitations, EVs have emerged as a non-cellular therapy. EVs modulate immune responses and avoid the risk of tumorigenesis owing to their stability, ability to cross the blood–brain barrier, and immune modulation properties, EVs serve as promising candidates for the treatment of neurological diseases [27, 47–52]. In addition, advancements in bioreactor-based mass production, high-efficiency separation techniques, and targeted delivery optimization strategies enable the large-scale production, purification, and storage of EVs for clinical applications [53–56]. Several in vivo models of peripheral nerve injury are used to evaluate peripheral nerve recovery, each with its own advantages and disadvantages. When selecting a peripheral nerve injury model, it is important to consider the desired outcomes and the specific environment of injury [57]. The CCI model, which involves sciatic nerve compression, induces an inflammatory response around the nerve, leading to neuronal cell death and demyelination. This injury also affects the gastrocnemius muscle, which is connected to the sciatic nerve, resulting in muscle atrophy, collagen accumulation, and fibrosis. As inflammation progresses, pain-like behaviors such as increased hypersensitivity in the hindfoot, are exacerbated, promoting sensitivity to even minor stimuli[58]. Various treatment strategies for peripheral nerve injury are being investigated for short- and long-term effects, focusing on nerve regeneration and functional recovery [59–61]. While these studies have not confirmed long term efficacy and safety outcomes, short-term treatment effects have been verified. Consequently, in our study, we overexpressed NGF, which has been previously reported to repair peripheral nerve injury through autophagy and Schwann cell activation [62], in UiPSC-NCCs. We loaded NGF into EVs (NCCoe-NGF-EVs) and used the CCI model to evaluate the in vivo efficacy of NCCoe-NGF-EVs. NCCoe-NGF-EVs exhibited nerve repair, anti-inflammatory, and anti-apoptotic effects.
In this study, we used an endogenous loading strategy that leverages natural exosome biogenesis pathways to establish a cell line capable of producing NGF-incorporated NCC-EVs. These vesicles effectively alleviate inflammation and cell death after neuronal injury. Specifically, we hypothesized that overexpression of NGF in NCCs promote the formation of NGF-containing late endosomes, which are subsequently packaged into EVs as multivesicular bodies (MVBs) mature and fuse with the plasma membrane. To create the cell line, we generated iPSCs from human urine, overexpressed NGF using a lentiviral vector, and differentiated the iPSCs into NCCs over a 14-day period. We found that even with NGF overexpression, the UiPSCs successfully differentiated into NCCs, maintaining both their stem cell properties and characteristic features of NCCs in the differentiated state. EVs are small biologically active particles, arranging in size from 40 to 160 nm, with a typical zeta potential of approximately − 10 to − 50 mV. The positive markers for EVs include tetraspanin proteins, including CD9, CD63, and CD81, while negative markers include GM130 and calnexin [63]. We confirmed that the EVs isolated from NGF-expressing NCCs were approximately 120 nm, a zeta potential of less than − 30 mV, and expressed characteristic the CD markers. To investigate NGF incorporation into EVs, ELISA analysis confirmed successful NGF incorporation, showing a 190-fold increase in NGF concentration in NCCoe-NGF-EVs (9.7 ng/ml) compared to NCC-EVs (0.05 ng/ml). Furthermore, the physicochemical properties of NCCoe-NGF-EVs, including size (~ 120 nm) and zeta potential (< − 30 mV), were consistent with those of NCC-EVs. These results indicated that NGF is efficiently incorporated into EVs via the MVB pathway, while preserving the essential properties of EVs.
Considering the potential clinical application of EV therapy, it is crucial to evaluate the target tissue delivery efficiency and in vivo distribution of EVs more accurately [64]. Several studies have used techniques such as nano-luciferase, radioisotope labeling, and IVIS imaging to determine the tissue-specific distribution and in vivo dynamics of EVs [65, 66]. Although biodistribution and kinetics were not examined in this study, we confirmed the uptake of EVs into neuronal and immune cells in vitro using DiR-labeled EVs, suggesting that EVs have the potential to be incorporated into neural tissues when administered in vivo. We found that DiR-labelled EVs were incorporated into SH-SY5Y cells and BV2 cells, with NCCoe-NGF-EVs demonstrating more efficient intracellular delivery compared to NCC-EVs under the same experimental conditions.
When peripheral nerve damage occurs, Wallerian degeneration is triggered by the breakdown of the myelin sheath surrounding axons, leading to demyelination and subsequent nerve cell death, impairing nerve function. Several studies have evaluated therapies for peripheral nerve injury from two perspectives. First, nerve regeneration effects are evaluated by examining remyelination, myelin debris removal, Schwann cell activation, and growth factor expression. The extent of remyelination in injured nerve is assessed through immunostaining for myelin sheet markers such as MBP, MAG, and MPZ, as well as axonal markers such as NF-200 and NF-H. Additionally, Schwann cell-related proteins, such as S100β and p75NTR, are examined to assess Schwann cell involvement in repair processes [62, 67–70]. In this study, we used both in vitro and in vivo models to investigate the neuroprotective, neuroregenerative and anti-inflammatory effects of NCCoe-NGF-EVs. As previously reported, NCCoe-NGF-EV treatment of SH-SY5Y cells induced axonal growth by enhancing the expression of axonal markers (Tuj-1 and NF-H) through ERK and AKT signaling pathway activation via endocytosis [71]. We also investigated the anti-apoptotic effects of NCCoe-NGF-EV in response to H₂O₂-induced oxidative stress and found that these vesicles effectively inhibited oxidative stress-induced neuronal cell death by increasing the expression of neuroplasticity marker nNOS and decreasing the expression of apoptotic protein BAX, as well as reducing the percentage of dead cells, as determined by live/dead cell staining using confocal microscopy. Our findings suggest that NCCoe-NGF-EVs induce neuronal regeneration by inhibiting neuronal cell death, promoting neurite outgrowth and increasing nNOS expression through the ERK and AKT signaling pathways. Consistent with the in vitro results, NCCoe-NGF-EVs also induced axon regeneration in injured nerves by increasing the expression of NF-H and GAP-43 through activation of ERK/AKT signaling. Additionally, NCCoe-NGF-EVs upregulated the expression of the neuroplasticity protein nNOS. To further confirm remyelination, MBP, and NF-H, immunostaining was performed on the sciatic nerve, revealing a significant increase in the number of myelinated axons in the NCCoe-NGF-EV-treated group compared to the injury group. This effect was associated with an increase in p75NTR protein expression, which is involved in Schwann cell activation. Our results provide strong evidence that NCCoe-NGF-EVs can effectively repair peripheral nerve injury and demonstrate that the strategy of incorporating NGF into EVs is a promising approach for nerve regeneration.
The second objective was to evaluate the suppression of the inflammatory environment and the inhibition of cell apoptosis at the site of nerve injury. Upon peripheral nerve injury, two types of macrophages are activated. M1 macrophages release inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, which contribute to the inflammatory response. In contrast, M2 macrophages promote nerve cell proliferation by releasing growth factors, including NGF, BDNF, NT-3, and vascular endothelial growth factor. M2 macrophages also activate Schwann cells to remove myelin debris and induce remyelination, facilitating nerve regeneration [7, 8, 10, 72]. In the early stages of nerve injury, M1 macrophages release inflammatory cytokines at the injury site, which contribute to cell death. Consequently, many studies utilize in vitro models to assess the neuroprotective and anti-inflammatory effects of potential nerve injury treatments [73–75]. Our results showed that in a BV2 cells in vitro model of inflammation induced by LPS treatment, NCCoe-NGF-EVs significantly reduced nitrite production and effectively downregulated the expression of proinflammatory cytokine genes (TNF-α, IL-6, IL-1β, COX-2) as well as the protein levels of P-NFκB and Iba-1. However, the effects of NCCoe-NGF-EVs in alleviating neuroinflammation and suppressing cell death were less pronounced compared to NCC-EVs. Furthermore, in an in vivo CCI model, we observed that NCCoe-NGF-EVs exhibited anti-apoptotic and anti-inflammatory effects. Additionally, NCCoe-NGF-EVs suppressed neuronal apoptosis by suppressing the expression of NK-κB, BAX, and cleaved caspase-3. These anti-inflammatory and anti-apoptotic effects also contributed to the inhibition of nerve injury-induced muscle atrophy and collagen accumulation, ultimately restoring motor function and pain sensitivity.
In summary, our results demonstrate the potential of NCCoe-NGF-EVs to promote regeneration and attenuate inflammation in peripheral nerve injury, highlighting their potential as an EV-based therapeutic strategy. While this study assessed the therapeutic effects of NCCoe-NGF-EVs through specific gene and protein analyses, the detailed composition of the EV cargo and the precise mechanisms of action remain unclear. Future studies should utilize proteomics and transcriptomics approaches to investigate the contributions of specific EV components to their neuroregenerative and anti-inflammatory effects.
Conclusion
In conclusion, we successfully differentiated NCCs from NGF-overexpressing UiPSCs to produce NGF-enriched NCC-derived EVs, which hold potential for neuroregenerative and protective applications in peripheral nerve injury. NCCoe-NGF-EVs activated ERK and AKT signaling pathways, promoting neurogenesis, neuronal proliferation, and protection against oxidative stress-induced cell death. Additionally, NCCoe-NGF-EVs demonstrated neuroprotective and regenerative effects through nerve regeneration, growth factor upregulation, apoptosis inhibition, anti-inflammatory activity, and protection against muscle atrophy in the CCI model. Thus, NCCoe-NGF-EVs enhanced NGF expression in the nerve population, leading to improved sciatic nerve repair. This highlights the improved efficacy of NCCoe-NGF-EVs in peripheral nerve injury. Further research is needed to investigate the specific therapeutic mechanisms of NCCoe-NGF-EVs, focusing on long-term efficacy, in vivo distribution, and EV cargo mechanism analysis. Despite these limitations, our study provides evidence supporting the potential of NCCoe-NGF-EVs as a novel non-cellular therapeutic strategy for the treatment of peripheral nerve injury.
Supplementary Information
Acknowledgements
We would like to thank Editage for English language editing and Biorender.com for their support with the drawings.
Abbreviations
- USC
Urine-derived stem cell
- iPSC
Induced-pluripotent stem cell
- TEM
Transmission electron microscope
- NTA
Nano particle tracking analysis
- EV
Extracellular vesicle
- NCC-EV
Neural crest cell derived-extracellular vesicle
- NCCoe-NGF-EV
NGF overexpressed neural crest cell derived-extracellular vesicle
- NGF
Nerve growth factor
- CCI
Chronic constriction injury
- NCC
Nerve crest cell
- MBP
Myelin basic protein
- GAP-43
Growth associated protein 43
- NF-H
Neurofilament heavy chain
Author contributions
SGC, HCY, and YJK designed experiments; HCY, YJK, and JKS performed experiments and contributed to data analysis; SBJ, YJK, NHL, and KWS conducted partial experiments; JHL, SWK and MCC provided resources; SGC conceptualized and funded; HCY, JKS, and SGC were involved in writing and editing of manuscripts; All authors read and approved the final manuscript.
Funding
This study was supported by a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korean government (Ministry of Science and ICT and Ministry of Health & Welfare; grant numbers: 24A0203L1 and 25B0101L1) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT; grant number: NRF-2022R1A2C2092291).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The experimental protocol for USCs was approved the Institutional Review Board of the Seoul Metropolitan Government Seoul National University Boramae Medical Center (IRB No.: 30-2022-81) and the Konkuk University Ethics Committee (IRB No.: 7001355-201507-BR-072). The in vivo experiments were performed according to the guidelines of the Konkuk University Institutional Animal Care and Use Committee (IACUA, KU24170).
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: The terms ‘IL-6’ and ‘IL-1β’ were placed incorrectly in the sentence under the Results section of this article and it has been updated correctly.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hancheol Yeo, Yoo Jung Kim, and Jaekwon Seok have contributed equally to this work.
Change history
8/27/2025
The original online version of this article was revised: The terms ‘IL-6’ and ‘IL-1β’ were placed incorrectly in the sentence under the Results section of this article and it has been updated correctly.
Change history
9/8/2025
A Correction to this paper has been published: 10.1186/s40478-025-02108-7
References
- 1.Li J, Guan R, Pan L (2023) Mechanism of Schwann cells in diabetic peripheral neuropathy: a review. Medicine (Baltimore) 102:e32653. 10.1097/MD.0000000000032653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silver S, Ledford CC, Vogel KJ, Arnold JJ (2021) Peripheral nerve entrapment and injury in the upper extremity. Am Fam Physician 103:275–285 [PubMed] [Google Scholar]
- 3.Lim TK, Shi XQ, Johnson JM, Rone MB, Antel JP, David S et al (2015) Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia, contributing to the genesis of neuropathic pain. J Neurosci 35:3346–3359. 10.1523/JNEUROSCI.4040-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng FY, Xiao WH, Bennett GJ (2011) The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 176:447–454. 10.1016/j.neuroscience.2010.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuffler DP, Foy C (2020) Restoration of neurological function following peripheral nerve trauma. Int J Mol Sci. 10.3390/ijms21051808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shea JE, Garlick JW, Salama ME, Mendenhall SD, Moran LA, Agarwal JP (2014) Side-to-side nerve bridges reduce muscle atrophy after peripheral nerve injury in a rodent model. J Surg Res 187:350–358. 10.1016/j.jss.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 7.Gu D, Xia Y, Ding Z, Qian J, Gu X, Bai H et al (2024) Inflammation in the peripheral nervous system after injury. Biomedicines. 10.3390/biomedicines12061256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Msheik Z, El Massry M, Rovini A, Billet F, Desmouliere A (2022) The macrophage: a key player in the pathophysiology of peripheral neuropathies. J Neuroinflamm 19:97. 10.1186/s12974-022-02454-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dervan A, Franchi A, Almeida-Gonzalez FR, Dowling JK, Kwakyi OB, McCoy CE et al (2021) Biomaterial and therapeutic approaches for the manipulation of macrophage phenotype in peripheral and central nerve repair. Pharmaceutics. 10.3390/pharmaceutics13122161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu P, Peng J, Han GH, Ding X, Wei S, Gao G et al (2019) Role of macrophages in peripheral nerve injury and repair. Neural Regen Res 14:1335–1342. 10.4103/1673-5374.253510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou N, Gu T, Xu Y, Liu Y, Peng L (2023) Challenges and progress of neurodrug: bioactivities, production and delivery strategies of nerve growth factor protein. J Biol Eng 17:75. 10.1186/s13036-023-00392-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martorana F, Gaglio D, Bianco MR, Aprea F, Virtuoso A, Bonanomi M et al (2018) Differentiation by nerve growth factor (NGF) involves mechanisms of crosstalk between energy homeostasis and mitochondrial remodeling. Cell Death Dis 9:391. 10.1038/s41419-018-0429-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei C, Guo Y, Ci Z, Li M, Zhang Y, Zhou Y (2024) Advances of Schwann cells in peripheral nerve regeneration: from mechanism to cell therapy. Biomed Pharmacother 175:116645. 10.1016/j.biopha.2024.116645 [DOI] [PubMed] [Google Scholar]
- 14.Huang P, Li Y, Nasser MI, Guo H, Huang H, Zhao M et al (2020) Urine-derived induced pluripotent stem cells in cardiovascular disease. Cardiol Res Pract 2020:3563519. 10.1155/2020/3563519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi T, Cheung M (2021) Urine-derived induced pluripotent/neural stem cells for modeling neurological diseases. Cell Biosci 11:85. 10.1186/s13578-021-00594-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Son N, Son YS, Jung CR, Son MY (2023) Generation of a human fibroblast-derived induced pluripotent stem cell line KRIBBi009-A from a patient with breast cancer. Stem Cell Res 68:103060. 10.1016/j.scr.2023.103060 [DOI] [PubMed] [Google Scholar]
- 17.Choe MS, Yeo HC, Kim JS, Lee J, Lee HJ, Kim HR et al (2024) Simple modeling of familial Alzheimer’s disease using human pluripotent stem cell-derived cerebral organoid technology. Stem Cell Res Ther 15:118. 10.1186/s13287-024-03732-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi W, Choe MS, Kim SM, Kim SJ, Lee J, Lee Y et al (2024) RFX4 is an intrinsic factor for neuronal differentiation through induction of proneural genes POU3F2 and NEUROD1. Cell Mol Life Sci 81:99. 10.1007/s00018-024-05129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan A, Toh YC (2019) Human pluripotent stem cell-derived neural crest cells for tissue regeneration and disease modeling. Front Mol Neurosci 12:39. 10.3389/fnmol.2019.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Xu X, Tong Y, Zhou X, Du J, Choi IY et al (2021) Therapeutic effects of peripherally administrated neural crest stem cells on pain and spinal cord changes after sciatic nerve transection. Stem Cell Res Ther 12:180. 10.1186/s13287-021-02200-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schizas N, Konig N, Andersson B, Vasylovska S, Hoeber J, Kozlova EN et al (2018) Neural crest stem cells protect spinal cord neurons from excitotoxic damage and inhibit glial activation by secretion of brain-derived neurotrophic factor. Cell Tissue Res 372:493–505. 10.1007/s00441-018-2808-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang D, Gong F, Ge X, Lv C, Huang C, Feng S et al (2020) Neuron-derived exosomes-transmitted miR-124–3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnol 18:105. 10.1186/s12951-020-00665-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turovskaya MV, Gaidin SG, Vedunova MV, Babaev AA, Turovsky EA (2020) BDNF overexpression enhances the preconditioning effect of brief episodes of hypoxia, promoting survival of GABAergic neurons. Neurosci Bull 36:733–760. 10.1007/s12264-020-00480-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 23:812–823. 10.1038/mt.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foo JB, Looi QH, Chong PP, Hassan NH, Yeo GEC, Ng CY et al (2021) Comparing the therapeutic potential of stem cells and their secretory products in regenerative medicine. Stem Cells Int 2021:2616807. 10.1155/2021/2616807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav K, Vijayalakshmi R, Kumar Sahu K, Sure P, Chahal K, Yadav R et al (2024) Exosome-based macromolecular neurotherapeutic drug delivery approaches in overcoming the blood-brain barrier for treating brain disorders. Eur J Pharm Biopharm. 10.1016/j.ejpb.2024.114298 [DOI] [PubMed] [Google Scholar]
- 27.Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH et al (2024) Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther 9:27. 10.1038/s41392-024-01735-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Abreu RC, Ramos CV, Becher C, Lino M, Jesus C, da Costa Martins PA et al (2021) Exogenous loading of miRNAs into small extracellular vesicles. J Extracell Vesicles 10:e12111. 10.1002/jev2.12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K, Abdal Dayem A, Gil M, Yang GM, Lee SB, Kwon OH et al (2020) 3,2′-dihydroxyflavone improves the proliferation and survival of human pluripotent stem cells and their differentiation into hematopoietic progenitor cells. J Clin Med. 10.3390/jcm9030669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song K, Dayem AA, Lee S, Choi Y, Lim KM, Kim S et al (2022) Superior therapeutic activity of TGF-beta-induced extracellular vesicles against interstitial cystitis. J Control Release 348:924–937. 10.1016/j.jconrel.2022.06.045 [DOI] [PubMed] [Google Scholar]
- 31.Kim D, Lim KM, Cho JM, Park HJ, Hwang S, Dayem AA et al (2023) Ductal delivery of extracellular vesicles promote the recovery from salivary gland inflammation. J Control Release 357:235–248. 10.1016/j.jconrel.2023.03.055 [DOI] [PubMed] [Google Scholar]
- 32.Austin PJ, Wu A, Moalem-Taylor G (2012) Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp. 10.3791/3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han D, Kim HJ, Choi HY, Kim B, Yang G, Han J et al (2015) 3,2′-dihydroxyflavone-treated pluripotent stem cells show enhanced proliferation, pluripotency marker expression, and neuroprotective properties. Cell Transpl 24:1511–1532. 10.3727/096368914X683511 [DOI] [PubMed] [Google Scholar]
- 34.Kim K, Gil M, Dayem AA, Choi S, Kang GH, Yang GM et al (2020) Improved isolation and culture of urine-derived stem cells (USCs) and enhanced production of immune cells from the USC-derived induced pluripotent stem cells. J Clin Med. 10.3390/jcm9030827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M, Park J, Kim S, Han DW, Shin B, Scholer HR et al (2023) Generation of induced pluripotent stem cells from lymphoblastoid cell lines by electroporation of episomal vectors. Int J Stem Cells 16:36–43. 10.15283/ijsc22177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JH, Choi W, Lee JH, Jeon SJ, Choi YH, Kim BW et al (2009) Astaxanthin inhibits H2O2-mediated apoptotic cell death in mouse neural progenitor cells via modulation of P38 and MEK signaling pathways. J Microbiol Biotechnol 19:1355–1363. 10.4014/jmb.0906.06003 [DOI] [PubMed] [Google Scholar]
- 37.Ismail N, Ismail M, Imam MU, Azmi NH, Fathy SF, Foo JB et al (2014) Mechanistic basis for protection of differentiated SH-SY5Y cells by oryzanol-rich fraction against hydrogen peroxide-induced neurotoxicity. BMC Complement Altern Med 14:467. 10.1186/1472-6882-14-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medeiros P, de Freitas RL, Boccella S, Iannotta M, Belardo C, Mazzitelli M et al (2020) Characterization of the sensory, affective, cognitive, biochemical, and neuronal alterations in a modified chronic constriction injury model of neuropathic pain in mice. J Neurosci Res 98:338–352. 10.1002/jnr.24501 [DOI] [PubMed] [Google Scholar]
- 39.Wang B, Zhang G, Yang M, Liu N, Li YX, Ma H et al (2018) Neuroprotective effect of anethole against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Neurochem Res 43:2404–2422. 10.1007/s11064-018-2668-7 [DOI] [PubMed] [Google Scholar]
- 40.Mietto BS, Mostacada K, Martinez AM (2015) Neurotrauma and inflammation: CNS and PNS responses. Mediat Inflamm 2015:251204. 10.1155/2015/251204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavorato A, Aruta G, De Marco R, Zeppa P, Titolo P, Colonna MR et al (2023) Traumatic peripheral nerve injuries: a classification proposal. J Orthop Traumatol 24:20. 10.1186/s10195-023-00695-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kajikawa K, Imaizumi K, Shinozaki M, Shibata S, Shindo T, Kitagawa T et al (2020) Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol Brain 13:120. 10.1186/s13041-020-00662-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cefalo MG, Carai A, Miele E, Po A, Ferretti E, Mastronuzzi A et al (2016) Human iPSC for therapeutic approaches to the nervous system: present and future applications. Stem Cells Int 2016:4869071. 10.1155/2016/4869071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A et al (2012) Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE 7:e52787. 10.1371/journal.pone.0052787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones I, Novikova LN, Wiberg M, Carlsson L, Novikov LN (2021) Human embryonic stem cell-derived neural crest cells promote sprouting and motor recovery following spinal cord injury in adult rats. Cell Transpl 30:963689720988245. 10.1177/0963689720988245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones I, Novikova LN, Novikov LN, Renardy M, Ullrich A, Wiberg M et al (2018) Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury. J Tissue Eng Regen Med 12:e2099–e2109. 10.1002/term.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Si C, Gao J, Ma X (2024) Engineered exosomes in emerging cell-free therapy. Front Oncol 14:1382398. 10.3389/fonc.2024.1382398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi H, Wang M, Sun Y, Yang D, Xu W, Qian H (2021) Exosomes: emerging cell-free based therapeutics in dermatologic diseases. Front Cell Dev Biol 9:736022. 10.3389/fcell.2021.736022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Namini MS, Daneshimehr F, Beheshtizadeh N, Mansouri V, Ai J, Jahromi HK et al (2023) Cell-free therapy based on extracellular vesicles: a promising therapeutic strategy for peripheral nerve injury. Stem Cell Res Ther 14:254. 10.1186/s13287-023-03467-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL (2020) Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci. 10.3390/ijms21124407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y, Ye Y, Su X, He J, Bai W, He X (2017) MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci 11:55. 10.3389/fncel.2017.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Qin S, Wen Y, Zhao W, Huang Y, Liu J (2022) Overcoming the blood-brain barrier: exosomes as theranostic nanocarriers for precision neuroimaging. J Control Release 349:902–916. 10.1016/j.jconrel.2022.08.002 [DOI] [PubMed] [Google Scholar]
- 53.Ferreira D, Moreira JN, Rodrigues LR (2022) New advances in exosome-based targeted drug delivery systems. Crit Rev Oncol Hematol 172:103628. 10.1016/j.critrevonc.2022.103628 [DOI] [PubMed] [Google Scholar]
- 54.Lim KM, Han JH, Lee Y, Park J, Dayem AA, Myung SH et al (2022) Rapid production method with increased yield of high-purity extracellular vesicles obtained using extended mitochondrial targeting domain peptide. J Extracell Vesicles 11:e12274. 10.1002/jev2.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Min Lim K, Kim S, Yeom J, Choi Y, Lee Y, An J et al (2023) Advanced 3D dynamic culture system with transforming growth factor-beta3 enhances production of potent extracellular vesicles with modified protein cargoes via upregulation of TGF-beta signaling. J Adv Res 47:57–74. 10.1016/j.jare.2022.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rezaie J, Feghhi M, Etemadi T (2022) A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal 20:145. 10.1186/s12964-022-00959-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li A, Pereira C, Hill EE, Vukcevich O, Wang A (2022) In vitro, in vivo and ex vivo models for peripheral nerve injury and regeneration. Curr Neuropharmacol 20:344–361. 10.2174/1570159X19666210407155543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SH, Wu CC, Lin SC, Tseng WL, Huang TC, Yadav A et al (2021) Investigation of neuropathology after nerve release in chronic constriction injury of rat sciatic nerve. Int J Mol Sci. 10.3390/ijms22094746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Wu G, Li W, Zhou X, Li W, Xu X et al (2023) Plasma exosomes improve peripheral neuropathy via miR-20b-3p/Stat3 in type I diabetic rats. J Nanobiotechnol 21:447. 10.1186/s12951-023-02222-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Yi D, Hong Q, Cao J, Geng X, Liu J et al (2024) Platelet-rich plasma-derived exosomes boost mesenchymal stem cells to promote peripheral nerve regeneration. J Control Release 367:265–282. 10.1016/j.jconrel.2024.01.043 [DOI] [PubMed] [Google Scholar]
- 61.Zhao J, Ding Y, He R, Huang K, Liu L, Jiang C et al (2020) Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell Res Ther 11:360. 10.1186/s13287-020-01872-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li R, Li D, Wu C, Ye L, Wu Y, Yuan Y et al (2020) Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 10:1649–1677. 10.7150/thno.40919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dissanayake K, Midekessa G, Lattekivi F, Fazeli A (2021) Measurement of the size and concentration and zeta potential of extracellular vesicles using nanoparticle tracking analyzer. Methods Mol Biol 2273:207–218. 10.1007/978-1-0716-1246-0_15 [DOI] [PubMed] [Google Scholar]
- 64.Kang M, Jordan V, Blenkiron C, Chamley LW (2021) Biodistribution of extracellular vesicles following administration into animals: a systematic review. J Extracell Vesicles 10:e12085. 10.1002/jev2.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faruqu FN, Wang JT, Xu L, McNickle L, Chong EM, Walters A et al (2019) Membrane radiolabelling of exosomes for comparative biodistribution analysis in immunocompetent and immunodeficient mice—a novel and universal approach. Theranostics 9:1666–1682. 10.7150/thno.27891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo W, Dai Y, Chen Z, Yue X, Andrade-Powell KC, Chang J (2020) Spatial and temporal tracking of cardiac exosomes in mouse using a nano-luciferase-CD63 fusion protein. Commun Biol 3:114. 10.1038/s42003-020-0830-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Zhang Y, Chen L, Rao Z, Sun Y (2020) Ulinastatin promotes regeneration of peripheral nerves after sciatic nerve injury by targeting let-7 microRNAs and enhancing NGF expression. Drug Des Devel Ther 14:2695–2705. 10.2147/DDDT.S255158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwasaki Y, Shiojima T, Kinoshita M (1997) S100 beta prevents the death of motor neurons in newborn rats after sciatic nerve section. J Neurol Sci 151:7–12. 10.1016/s0022-510x(97)00073-7 [DOI] [PubMed] [Google Scholar]
- 69.Sun X, Ni S, Zhou Q, Zou D (2024) Exogenous NT-3 promotes phenotype switch of resident macrophages and improves sciatic nerve injury through AMPK/NF-kappaB signaling pathway. Neurochem Res 49:2600–2614. 10.1007/s11064-024-04198-6 [DOI] [PubMed] [Google Scholar]
- 70.Li L, Li Y, Fan Z, Wang X, Li Z, Wen J et al (2019) Ascorbic acid facilitates neural regeneration after sciatic nerve crush injury. Front Cell Neurosci 13:108. 10.3389/fncel.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dayem AA, Kim B, Gurunathan S, Choi HY, Yang G, Saha SK et al (2014) Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathways. Biotechnol J 9:934–943. 10.1002/biot.201300555 [DOI] [PubMed] [Google Scholar]
- 72.Rios R, Jablonka-Shariff A, Broberg C, Snyder-Warwick AK (2021) Macrophage roles in peripheral nervous system injury and pathology: Allies in neuromuscular junction recovery. Mol Cell Neurosci 111:103590. 10.1016/j.mcn.2021.103590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nam HY, Nam JH, Yoon G, Lee JY, Nam Y, Kang HJ et al (2018) Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J Neuroinflamm 15:271. 10.1186/s12974-018-1308-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jia Y, Zhang D, Yin H, Li H, Du J, Bao H (2021) Ganoderic acid A attenuates LPS-induced neuroinflammation in BV2 microglia by activating farnesoid X receptor. Neurochem Res 46:1725–1736. 10.1007/s11064-021-03303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luan Z, Liu J, Li M, Wang Y, Wang Y (2024) Exosomes derived from umbilical cord-mesenchymal stem cells inhibit the NF-kappaB/MAPK signaling pathway and reduce the inflammatory response to promote recovery from spinal cord injury. J Orthop Surg Res 19:184. 10.1186/s13018-024-04651-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.