Abstract
Fibre optic sensors (FOSs) have developed as a transformative technology in healthcare, often offering unparalleled accuracy and sensitivity in monitoring various physiological and biochemical parameters. Their applications range from tracking vital signs to guiding minimally invasive surgeries, enabling advancements in medical diagnostics and treatment. However, the integration of FOSs into biomedical applications faces numerous challenges. This article describes some challenges for adopting FOSs for biomedical purposes, exploring technical and practical obstacles, and examining innovative solutions. Significant challenges include biocompatibility, miniaturization, addressing signal processing complexities, and meeting regulatory standards. By outlining solutions to the stated challenges, it is intended that this article provides a better understanding of FOS technologies in biomedical settings and their implementation. A broader appreciation of the technology, offered in this article, enhances patient care and improved medical outcomes.
Keywords: fibre optic sensors, biomedical applications, biocompatibility, glucose measurement, signal processing
1. Introduction
As sensor technology advances, Fibre Optic Sensors (FOSs) have gained prominence for their accuracy, high sensitivity, and resistance to electromagnetic interference. These characteristics make them particularly attractive for biomedical applications, where accurate and reliable measurements are crucial [1,2]. From monitoring physiological parameters to aiding in minimally invasive surgeries, fibre optic sensors hold the potential to revolutionize medical diagnostics and treatment [3].
The adoption of fibre optic sensors in the biomedical field requires several obstacles to be overcome. Integrating these sensors into medical devices requires overcoming challenges related to biocompatibility, miniaturization, and effective and efficient signal processing. Additionally, ensuring the robustness, real-time responsivity, and reliability of FOSs in the dynamic and often harsh environments of the human body presents another layer of complexity. Also, their use requires meeting international standards, which can also be challenging in the pathway to commercialization [4,5].
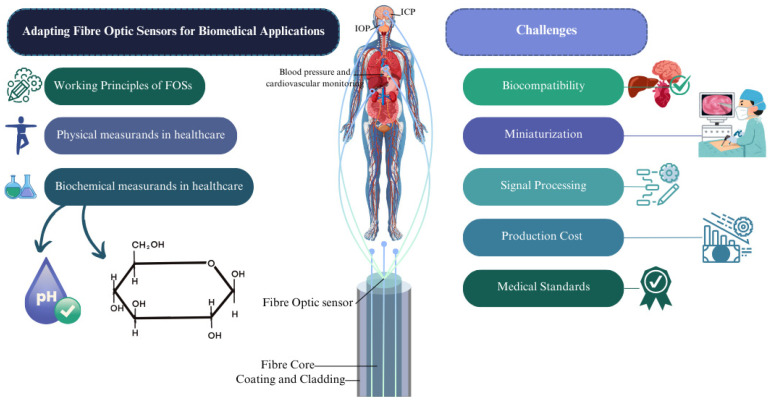
This review explores the key challenges encountered in adopting fibre optic sensors for biomedical applications. It presents the background and working principles of these sensors, along with an assessment of key physical and biochemical measurands. The review then examines the challenges associated with FOS implementation, focusing on issues such as biocompatibility, miniaturization, signal processing, manufacturing costs, various medical standards, and ethical considerations relevant to biomedical use. In the final section, the conclusion highlights potential future directions for fibre optic sensors in both the scientific community and real-world applications. Figure 1 provides an abstract overview of the review, highlighting the key challenges associated with the adoption of FOSs in real-world applications, based on different physical and biochemical measurands.
Figure 1.
An abstract of the review, showing challenges regarding adoption of FOSs in real-world applications.
1.1. Background
FOSs transmit light through optical fibres, where changes in properties such as intensity, phase, or wavelength indicate specific physiological conditions. In the biomedical field, FOSs enable the real-time monitoring of vital signs, including pressure and temperature; facilitate biochemical detection; and support minimally invasive surgical procedures [6]. Despite their advantages, several challenges hinder their widespread adoption in healthcare. Key issues include ensuring biocompatibility, scaling manufacturing to meet industry standards, and developing efficient real-time signal processing algorithms. Overcoming these barriers is critical for integrating FOS technology into routine medical diagnostics and treatment, where it holds the potential to enhance accuracy and reliability in patient care [7].
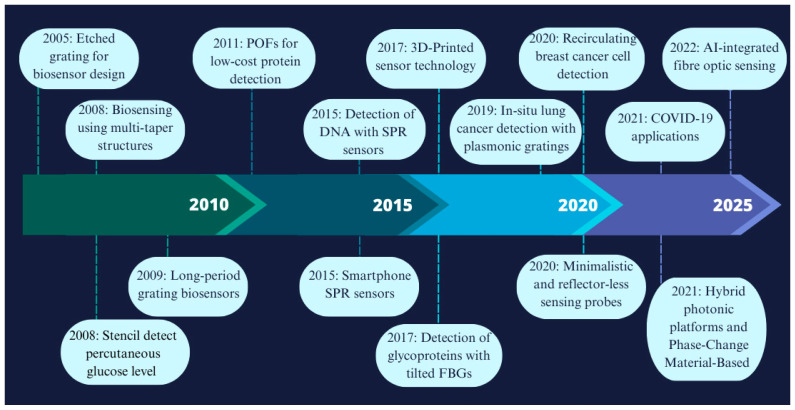
The origins of biosensing technology trace back to 1956, when Leland C. Clark Jr. (referred to as the “father of biosensors”) developed the first true biosensor for oxygen detection. A breakthrough came in 1962 when Clark introduced the first glucose biosensor, a technology that remains widely used and is continually evolving. Initially based on the detection of hydrogen peroxide, glucose biosensors have since progressed from basic laboratory demonstrations to commercialized devices, and now to advanced wearable and non-invasive systems for real-time monitoring [8]. Figure 2 illustrates some of the main achievements in fibre optic biosensor technology since 2005 and highlights the most significant milestones in the field [9].
Figure 2.
A chronology of some of the main achievements in fibre optic biosensor technology [9,10,11,12,13].
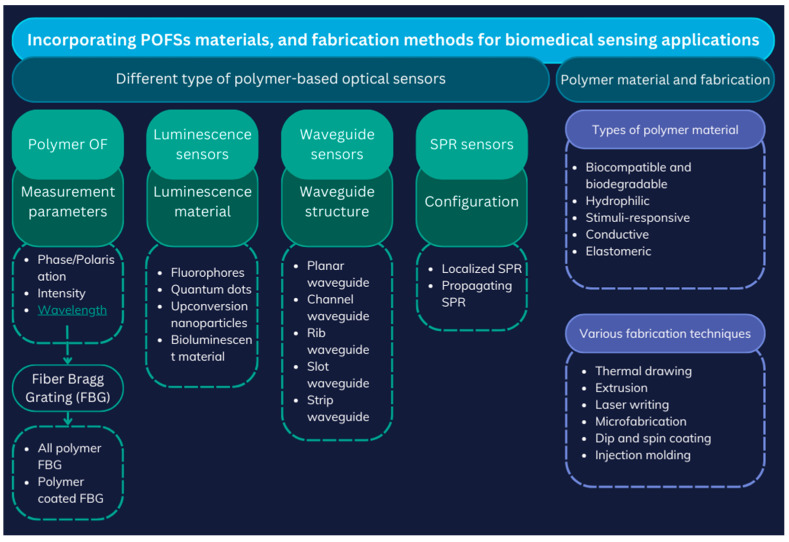
Polymer-based (or plastic) optical fibre sensors (POFSs) represent a significant advancement in FOS technology, offering flexible and adaptable sensing solutions. These sensors can operate based on various principles, including waveguide-based [14,15] mechanisms, luminescence [16], Surface Plasmon Resonance (SPR) [17], and optical fibre-based sensing, and they are suitable for both single-point and multi-point applications [18,19,20]. A particular focus is placed on polymer optical fibre sensors, due to their applications in biosensing. Polymeric materials, such as biodegradable, biocompatible, hydrophilic, stimuli-responsive, conductive, and molecularly imprinted polymers, further enhance biomedical sensing capabilities [21]. Additionally, various fabrication techniques for polymer optical fibres (POFs), including thermal drawing, extrusion, laser writing, microfabrication, and advanced coatings, are included in Figure 3 as classifications of different types of fibres and fabrication techniques [3,22,23].
Figure 3.
Different types of polymer-based FOSs with fabrication techniques; adopted idea: [22].
1.2. Working Principles of FOSs
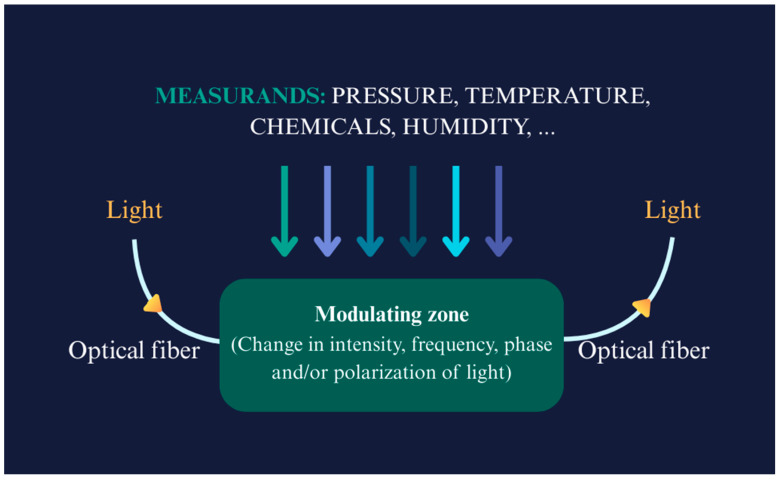
FOSs vary widely in their physical appearances and characteristics, and their operating principles are correspondingly diverse. Primary FOS classification splits into intrinsic and extrinsic sensors [18]. Intrinsic FOSs rely on light–matter interaction occurring wholly within the fibre itself, where changes in light properties (intensity, phase, polarization, or wavelength) occur due to external influences such as temperature, pressure, chemicals, humidity, or strain. On the other hand, extrinsic fibre optic sensors use the optical fibre merely to transmit light to and from an external sensing element. Fibre Bragg Gratings (FBGs) [24,25] have represented a revolution in sensing using optical fibres. Their working principle and reflection of specific light wavelengths or shifts in response to different measurands is shown in Figure 4. Interferometric sensors (of which the FBG is an example) generally measure phase changes in light caused by external perturbations [4,26,27]. The FBGs’ interaction with light as it passes through the grating planes depends on the Bragg condition, and the first-order Bragg condition can be stated as shown below:
| (1) |
where denotes the effective refractive index, while represents the grating period, and stands for the Bragg wavelength.
Several FOS and polymer-based optical fibre (POF) sensor working principles cannot be included in this article owing to page length restrictions; e.g., fluorescence-based sensors [28,29] detect variations in fluorescence emitted by certain materials subjected to external light excitation and are often used for biochemical measurements. Other types of FOSs include interferometric-based devices such as Fabry–Pérot [26,30], Surface Plasmon Resonance (SPR) [31,32], and Optical Coherence Tomography (OCT) [33,34] devices, utilized in imaging applications and often incorporating specialist fibre, e.g., Photonic Crystal Fibre (PCF) [28,35]. They all leverage the interaction between light and the external environment to provide sensitive and accurate measurements in a wide range of applications [27,36,37,38,39]. They can be categorized in various ways, including fibre type, application, and sensing mechanisms, as outlined in Table 1.
Figure 4.
Working principles of a fibre optic sensor with different measurands (showed in arrows with different colours); adopted idea: [40].
Table 1.
Recently introduced FOSs based on fibre type and their applications, with assessment on their sensing mechanisms.
| Fibre Type | Application | Sensitivity | Sensing Mechanism | Ref. |
|---|---|---|---|---|
| SMF | Pressure | 263.15 pm/kPa | FPI | [41] |
| OF | Pressure (IOP) | Low baseline drift (<2.8 mmHg) over >4.5 years | FPI with OCT | [34] |
| MMF | Pressure | 2.49 nm/kPa | Interference-based sensing | [42] |
| OF | Pressure/temperature | 55.468 nm/MPa (pressure), 0.01859 nm/°C (temperature) | FPI with MEMs | [43] |
| U-shaped MMF | Biosensing | 1251.44 nm/RIU | LSPR | [44] |
| PC fibre | Biosensing | 12,000 nm/RIU and 16,000 nm/RIU |
SPR | [45] |
| D-shaped OF | Biosensing | 5161 nm/RIU | SPR | [46] |
| D-shaped OF | Biosensing | 4122 nm/RIU | LMR | [47] |
| D-shaped PC fibre | Biosensing | 21,700 nm/RIU | SPR | [48] |
| D-shaped PC fibre | Biosensing | 20,000 nm/RIU | SPR | [49] |
| Plastic OF | Cholesterol detection | 140 mg/dL to 250 nm/dL | - | [50] |
| SMF | Temperature | 210.25 KHz/°C | Vernier effect | [51] |
| Fibre tip integrated ZnO-nanowire-nanograting | Temperature | 0.066 nW/°C | Bragg reflection | [52] |
| MMF with spherical end | Pressure/temperature | 0.139 mV/kPa (pressure), 0.87 mV/°C (temperature) | RI modulation using MEMS-based silicon | [53] |
| SMF with a Hollow Silica Tube (HST) | Pressure | 396 pm/kPa | FPI | [54] |
| SMF with FBG | Pressure | 1.466 pm/kPa | FBG array | [55] |
| Ultra-miniature fibre optic sensor | Pressure (IPP) | (r ≥ 0.7, p < 0.001) | Diaphragm-based FO integrated with a proportional–integral–derivative (PID) | [56] |
| Distributed OF | Pressure | 65.920 μϵ/kPa | Axial strain change detection with a sensitizing structure | [57] |
Also, fibre optic sensors (FOSs) can be classified, based on their measurands, into physical and biochemical types. Physical FOSs detect pressure, temperature, and strain by analysing optical signal changes. Biochemical FOSs identify specific analytes, such as glucose or pH, e.g., using functionalized coatings. This classification highlights their versatility in biomedical and industrial applications. This review focuses on their applications in biomedical sensing.
1.3. Physical Measurands in Healthcare
FOSs are well suited for providing measurements of various physical measurands and are becoming increasingly accepted for patient monitoring and diagnostics. These measurands include temperature, pressure, strain, flow, liquid level, displacement, vibration, rotation, radiation, and biochemical markers. For example, body temperature measurements are vital for tracking temperature fluctuations during surgeries, post-operative care, and critical care settings, ensuring patient stability and the early detection of infections [58,59]. Pressure sensors are extensively used to monitor several medical pressure parameters, including blood pressure, Intracranial Pressure (ICP) [60,61], and Intraocular Pressure (IOP) [62,63], which are critical for managing conditions including hypertension, Traumatic Brain Injuries (TBIs) [64], and glaucoma [63]. Strain sensors are employed to monitor respiration by measuring chest wall movements, offering valuable data for respiratory therapy [4] and sleep studies, and managing conditions including asthma or chronic obstructive pulmonary disease (COPD) [5,65]. By providing real-time data, fibre optic sensors offer enhanced clinical decision making and improved patient outcomes, and contribute to the advancement of personalized medicine [66,67].
Biomechanical measures encompass the physical parameters of the human body, focusing on physical structure and movement, and accurate measurement is crucial for the successful monitoring of various parameters. FOSs can measure strain and deformation in tissues and organs, providing critical data for orthopaedic and rehabilitation applications [1,3]. For instance, it is possible to monitor the stress and strain on bones and joints during physical activities, aiding in the assessment and treatment of musculoskeletal disorders. Additionally, they have been used for posture monitoring and ulcer formation detection in patients who are required to use a wheelchair [68]. Furthermore, these sensors are employed in developing prosthetics and wearable devices, providing real-time feedback on mechanical performance and interaction with the body. By measuring these biomechanical parameters, FOSs support the diagnosis, treatment, and rehabilitation of various conditions and advance the field of biomechanics in healthcare [5,69,70,71,72,73].
1.4. Biochemical Measurands in Healthcare
All biochemical measurands could be considered vital parameters in healthcare, providing essential information about patients’ whole physiological and metabolic states. FOSs are increasingly used to measure these biochemical markers with high sensitivity and specificity. One significant application of FOSs is in continuous glucose monitoring (CGM), particularly for diabetes management. These sensors can measure glucose levels in blood or interstitial fluid, providing real-time data that aid in maintaining optimal glycaemic control [74,75,76,77]. pH monitoring is used in assessing metabolic conditions and the body’s acid–base balance, which is vital in some critical care settings and surgical procedures [78,79,80,81]. Additionally, fibre optic sensors are used for blood component detection [75], to detect specific proteins [82,83], enzymes [76,84,85], and hormones [35,86,87], aiding in the diagnosis and monitoring of various diseases, such as cancer [88,89,90] and hormonal imbalances [36,91]. These sensors can be functionalized to detect biomarkers at the molecular level, enabling early disease detection and the tracking of treatment efficacy. By measuring these biochemical parameters, fibre optic sensors provide data that present real time monitoring opportunities, enhancing diagnostic accuracy and treatment monitoring, which are directly related to biochemical measurands [36,92,93].
FOSs are adept at detecting various substances in both gas and liquid phases. In the gas phase, FOSs can detect a wide range of gases, including oxygen, carbon dioxide, and Volatile Organic Compounds (VOCs) [94,95,96,97] with high sensitivity, useful in respiratory monitoring [65,98] and detecting gases in environmental health studies. In the liquid phase, FOSs are widely used for measuring biochemical substances, such as glucose, electrolytes, and pH levels in bodily fluids like blood, urine, and saliva [91,99,100,101]. This capability is essential for continuous glucose monitoring in diabetic patients and assessing kidney function or urinary protein [100,102,103]. Accurate, real-time measurements can be achieved using different sensing mechanisms, based on different responsive materials. These sensors also provide access to a vast range of use cases, as described in Table 2 [28,104,105]. Table 2 provides an overview of recently developed FOSs designed for pH measurement and glucose detection, as well as the detection of certain cancer biomarkers and hormones, highlighting their fibre type and detection range with the responsive material.
Table 2.
Overview of recent FOSs for pH, glucose, cancer biomarker, and hormone measurement/detection.
| Sensing Application | Responsive Material with Fibre Type | Detection Range | Sensitivity | LOD (Limit of Detection) | Ref. |
|---|---|---|---|---|---|
| pH | PANi with TFBG | 2–12 | minimum of 30 pm/pH maximum of 82 pm/pH | - | [106] |
| PAAm hydrogel with SPR | 8–10 | 13 nm/pH at | - | [107] | |
| gold nanoparticle-functionalized fibre optic probes with FPI | 2–12 | 1.95 nm/pH | - | [80] | |
| Hydrogel + polymer microarrays with miniature optical fibre | 5.5–8 | mean precision of 0.10 pH units | - | [79] | |
| glucose | GO/GOD with LPFG | 0–8 mM | ∼0.24 nm/mM | - | [108] |
| GOD with multimode microfibre | 0.0–166.67 mM | 1.74 nm/(mg/mL) | - | [109] | |
| 3-APBA with LDOF | 0–50 mM | 2.6 μWmM−1 | [74] | ||
| GO with LPFG | 0∼1 wt% | 6.229 dB/wt% | [110] | ||
| GO/GOD with PCF | 10 g/L to 70 g/L | - | - | [111] | |
| SPR with Microsphere optical fibre | 0–200 mg/dL | 0.1688 nm/(mg/dL) | 4 mg/dL | [112] | |
| Gold nanoparticles (AuNPs) and LSPR with TOF | 1.328–1.393 (5–45 wt%) | For bare TOF: 1265%/RIU For AuNP-decorated TOF: 2032%/RIU |
- | [113] | |
| GO/GOD with PS-LPFG inscribed on high-birefringence fibre (HBF) | 5–25 mM | ∼20.8 pm/mM | - | [114] | |
| Gold-coated plasmonic layer with PCF | Not specified | 2500 nm/RIU (wavelength), 152 RIU−1 (amplitude) | - | [115] | |
| SPR with enzymatic reaction | 0–400 mg/d | 3.10 pm/(mg/dL) | - | [76] | |
| gold nanoparticle-functionalized fibre optic probes with FPI | 1 μM–1 M | 3.25 nm/mM | - | [80] | |
| cancer biomarkers | Tapered fibre optic interferometer cascaded with FBG for HER2 protein | - | - | 2 ng/mL | [90] |
| FOSs catheter embedded for CD44 protein | - | - | 4.68 aM | [116] | |
| Ti3C2-supported gold nanorod hybrid nanointerfaces with microfibre, integrated with hybrid nanointerfaces | - | - | 13.8 zM (buffer), 0.19 aM (30% serum) | [117] | |
| hormones | Oestrogen receptor on gold-coated polystyrene with Spoon-shaped SPR | - | - | 0.1 pM | [91] |
| Thin gold layer with 7-core fibre + SMF for Insulin | - | - | 10−8 g/mL | [118] | |
| Oestrogen receptor with gold-coated tilted fibre Bragg grating (TFBG) | - | - | 1.5 × 10−3 ng/mL | [119] | |
| Anti-cortisol antibody on AuPd-coated with SPR on plastic optical fibre (POF) for cortisol | 0.005–10 ng/mL | - | 1 pg/mL | [120] | |
| Anti-cortisol antibody on gold-coated D-shaped SPR for cortisol | 0.01–100 ng/mL | 0.65 ± 0.02 nm/log(ng/mL) | 1.46 ng/mL | [121] | |
| Gold nanoparticles with FOS microfluidic channel for thyroglobulin (Tg) | - | - | 93.11 fg/mL | [122] |
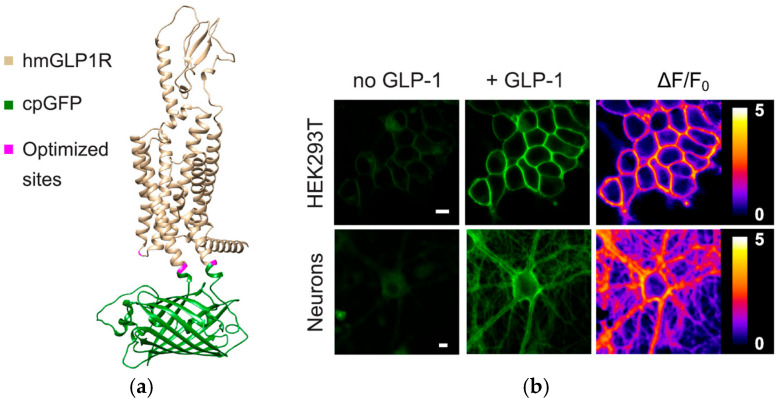
FOSs have also demonstrated potential in monitoring glucagon-like peptide-1 (GLP-1), a critical biomarker in glucose metabolism and insulin regulation. The ability to continuously monitor GLP-1 levels offers significant advantages in managing diabetes and other metabolic disorders. A genetically encoded sensor (GLPLight1) has been developed by engineering a circularly permuted green fluorescent protein into the human GLP-1 receptor (GLP1R), as shown in Figure 5. This sensor accurately detects receptor conformational activation in response to pharmacological ligands, as indicated by its fluorescence signal [123,124].
Figure 5.
The development and optical properties of GLPLight1 were examined using structural modelling and fluorescence imaging. (a) The structural model, generated using Alphafold [125], depicts the human glucagon-like peptide-1 receptor (GLP1R) in gold, the circularly permuted green fluorescent protein (cpGFP) in green, and mutagenesis target residues in magenta. (b) Fluorescence imaging in HEK293T cells and primary cortical neurons demonstrated an increase in fluorescence intensity. Pixel-wise ΔF/F0 images further confirmed these fluorescence changes, supporting the sensor’s effectiveness in detecting GLP-1 interactions. Image source: [124].
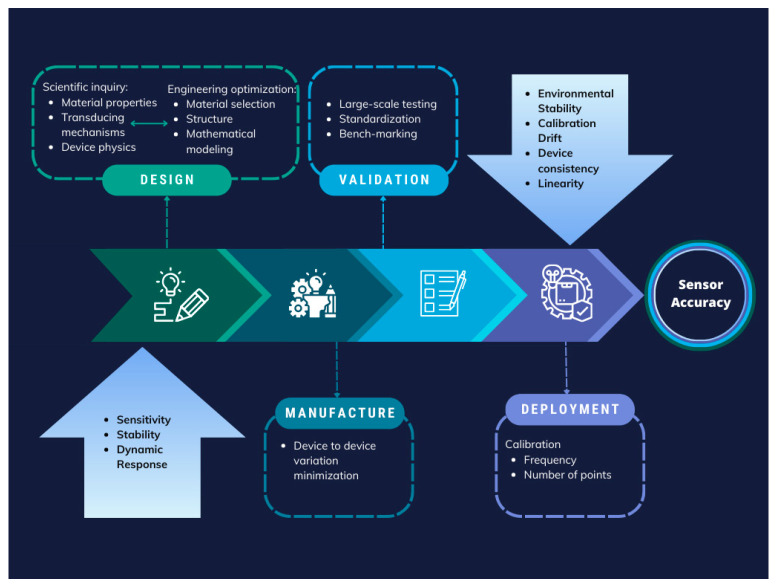
Ensuring accurate parameter values is paramount in sensor technology, particularly in the case of fibre optic sensors for biomedical applications. A comprehensive approach to achieving high accuracy must span the entire lifecycle of sensor development and deployment, as shown in the example step graph of Figure 6. This entails fundamental research into materials during the design phase to comprehend material properties, transduction mechanisms, and device physics, ultimately leading to optimized materials and device structures. Iteration between scientific inquiry and engineering optimization enhances sensor accuracy, considering factors such as the 3S’s (size, speed, and sensitivity).
Figure 6.
Step graph of an approach to sensor accuracy assurance during the design, manufacturing, validation, and deployment of sensor technology; factors to consider are highlighted in blue in the first and the last steps. Adopted with permission from [126]. Copyright 2025 American Chemical Society.
Fabricated device accuracy and consistency are pivotal in moving towards manufacturability and deployment. Additionally, large-scale validation employing standardized procedures and benchmarking against gold-standard measurements are indispensable for obtaining reliable calibration curves [126].
2. Challenges for FOSs in Biomedical Applications
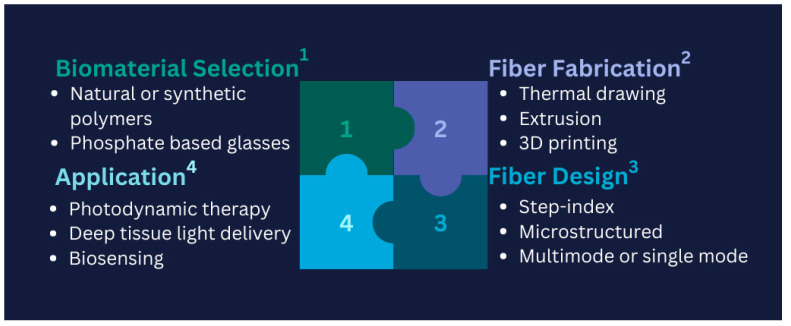
Developing biodegradable and biocompatible optical fibres for biomedical applications presents several key challenges. Firstly, material selection is critical, as the fibres must degrade safely in the body without releasing toxic byproducts. Natural or synthetic polymers, such as polylactic acid (PLA) or phosphate-based glasses, must be carefully evaluated for their mechanical properties and degradation rates to match specific biomedical requirements. Secondly, fabrication techniques such as thermal drawing, extrusion, or 3D printing must be optimized to ensure that the fibres maintain structural integrity while also being scalable for clinical applications. Thirdly, fibre design plays a significant role; features like microstructure or step-index profiles must balance light-guiding efficiency with mechanical flexibility and controlled biodegradability. Finally, application-specific considerations must be addressed, such as ensuring effective light delivery in photodynamic therapy or accurate biosensing under dynamic physiological conditions. PLA-based fibres have shown promise due to their adjustable degradation rates and biocompatibility. However, challenges such as mechanical fragility and inconsistent degradation in physiological environments necessitate further research. Similarly, phosphate-based glass fibres are attractive for their complete dissolution in biological fluids, but optimizing their mechanical properties without compromising their optical performance remains a significant challenge. These challenges highlight the need for continued advancements in material science and fibre design to achieve reliable, biodegradable, and biocompatible optical fibres for diverse biomedical applications [127]. Figure 7 illustrates a schematic representation of the four key challenges encountered in developing biodegradable and biocompatible optical fibres.
Figure 7.
Schematic puzzle of the four significant challenges in developing biodegradable and biocompatible optical fibres; adopted idea: [127].
2.1. Biocompatibility
Ensuring non-toxicity for any material to be used in fabricating sensors is one of the primary challenges to achieving biocompatibility. The selection of materials needs to ensure that the FOSs are mechanically safe, non-toxic, and do not provoke an immune response when implanted in or used on the human body. This requires extensive testing to ensure that the materials do not cause inflammation, allergic reactions, or any other adverse effects [69,128]. This is generally conducted by regulating bodies, such as the USA’s Food and Drug Administration (FDA) (Section 2.5 of this article).
FOSs often require coatings to enhance biocompatibility. These coatings must be able to withstand the harsh biological environment without degrading. Ensuring the long-term stability and functionality of these coatings is crucial for the sensors’ reliable performance and regulatory approval. Furthermore, sensors often must endure various sterilization methods such as autoclaving, gamma irradiation, or chemical sterilization. A significant challenge remains to design sensors that maintain their performance characteristics post-sterilization, as these processes can sometimes compromise sensor integrity and functionality [24,129]. Table 3 presents an overview of biomaterials used in fabricating optical fibres, highlighting their base materials, advantages, and disadvantages, based on the literature.
Table 3.
| Material Type | Material Example | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Natural | Proteins: silk Polysaccharides: alginate, cellulose, agarose, chitosan, gelatine |
biocompatibility and biodegradability |
limited design flexibility, restricted availability and quantity, batch-to-batch variability, low mechanical strength, and potential immunogenicity | [131,132,133,134,135] |
| Synthetic | Hydrogels: Polyethylene Glycol (PEG), Pluronic (Poloxamer) Citrate-based elastomers: poly (Octamethylene Citrate) (POC), poly (Octamethylene Maleate Citrate) (POMC), Polymer-Based: Polyvinyl Chloride (PVC), SU-8 (Negative Photoresist Polymer), Poly (L-Lactic Acid) (PLLA), Poly (D, L-Lactic Acid) (PDLLA), Poly (L-Lactic-Co-Glycolic Acid) (PLGA), Poly (D,L-Lactic-Co-Glycolic Acid) (PDLGA), Poly-ε-Caprolactone (PCL) Inorganic materials: Calcium–Phosphate Glass (PGs) Silicon-Based Materials: Silicon, Polydimethylsiloxane (PDMS) |
adaptable and flexible structure, tunable biodegradability, and customizable physical, mechanical, and chemical characteristics | biocompatibility should be verified and the rigidness and brittleness of glass should be confirmed |
[22,127,136,137,138,139] |
| Hybrid Biomaterials (Natural and Synthetic) | Chitosan and Polystyrene Membranes/PAA Silk Fibroin Film, Agarose hydrogel (AG) with gold nanoparticles (AuNPs) | biocompatibility, mechanical strength and tunable properties for PAA, controlled permeability, chemical resistance | limited flexibility, surface modification required for some, degradation issues, Processing complexity, for AuNPs, agglomeration of AuNPs, and limited long-term stability | [133,134,140,141,142] |
2.2. Miniaturization, Durability, and Longevity
Reducing the size of fibre optic sensors without sacrificing sensitivity or accuracy is a significant ongoing challenge. Miniaturized sensors must still be capable of delivering accurate and reliable measurements [143]. Also, small-sized sensors need to be seamlessly integrated into medical devices and systems, often requiring custom design solutions [103]. This integration must ensure that the sensors do not interfere with the overall device performance and that they are easy to incorporate into existing medical infrastructure [92]. Scaling up miniaturized FOSs while maintaining quality and reliability remains a major challenge, especially for long-term biomedical applications [144,145].
Nanostructured materials have emerged as a solution to many of these challenges. The unique optical and biological properties of nanomaterials, such as size-dependent signal amplification, plasmon resonance, and enhanced charge-transfer abilities, improve the sensitivity, specificity, limit of detection, response time, and signal-to-noise ratio of fibre optic biosensors. The integration of biocompatible nanomaterials with selective bioreceptors has established a strategy for advancing FOS performance. Recent literature explores different dimensions of nanomaterials (0D, 1D, 2D, and 3D structures), although a detailed discussion is beyond the scope of this review [146,147].
Notable technologies and advancements include LSPR (Localized Surface Plasmon Resonance) fibre optic biosensing platforms [145]; THz-metasurface-mediated nano-biosensors [148]; hybrid nano-structured SPR biosensors, developed for breast and cervical cancer detection [149]; and using nanoparticles for biosensing a variety of viruses, such as SARS-CoV-2 [150]. These approaches significantly contribute to miniaturization efforts and functional enhancements. However, challenges like material stability, reproducibility, large-scale manufacturability, and environmental sustainability remain key barriers to clinical translation. Some of the innovations, such as green synthesis methods, continuous flow nanomaterial production, and advanced surface engineering techniques, are actively addressing these issues and paving the way toward the next generation of reliable, scalable, and sustainable diagnostic tools [151].
Also, based on the usage of a sensor, it could be advantageous if the sensor can be delivered inside a standard medical catheter, as this often overcomes the problem of mechanical robustness and durability [152,153,154].
2.3. Signal Processing, Data Integration, and Interoperability
Biological environments are often inherently noisy, e.g., external electromagnetic interference from scanning equipment (MRI and CT) can interfere with the signals detected by fibre optic sensors. If it is impossible to make the sensor immune to these sources of interference, developing advanced algorithms and signal processing techniques to filter out this noise becomes crucial for accurate measurements. The signals from FOSs often require sophisticated interpretation, especially when monitoring dynamic biological processes. This necessitates the development of advanced computational models and machine learning algorithms to analyse and interpret the data accurately [155,156,157].
Many types of medical applications require real-time data processing and feedback. Ensuring that the sensor systems can handle the computational load and provide timely, accurate information is a significant and ongoing technical challenge. FOSs must be compatible with healthcare IT systems and electronic health records (EHRs). Ensuring seamless integration and data interoperability is therefore essential for effective use in clinical settings [158,159].
Developing standardized data protocols to ensure that data from fibre optic sensors can be easily shared and interpreted across different platforms and systems is crucial. This includes ensuring sound data security and maintaining patient privacy. Finally, at this stage, providing user-friendly interfaces that allow healthcare professionals to interact with and interpret data from fibre optic sensors is important for their adoption. This involves developing intuitive software and visualization tools [92,160,161].
2.4. Production Cost and Manufacturing
High production costs can hinder the widespread adoption of fibre optic sensors. Developing cost-effective manufacturing processes without compromising quality and performance is crucial for making the sensors affordable [42,93]. Additionally, it may be necessary to accommodate further scaling up of production while maintaining consistency and reliability. The latter requires advanced manufacturing techniques and stringent quality control measures to ensure each sensor meets the required standards. Finally, achieving economies of scale to achieve lower costs involves improving manufacturing processes and increasing market demand and production volumes. This can also be challenging in the early stages of technology adoption [162,163].
2.5. Medical Standards and Regulatory Approval
Medical and/or biomedical FOSs must meet stringent regulatory standards set by organizations such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency) [164,165,166,167,168,169]. This involves extensive testing to demonstrate safety, efficacy, and reliability.
Ensuring compliance with international standards for medical devices is critical. This includes adhering to ANSI (American National Standards Institute)/AAMI (Association for the Advancement of Medical Instrumentation) or ISO standards, such as AAMI/ISO 10993, ISO 13485, and AAMI TIR42: Technical Information Report (TIR), not a formal standard, but rather guidance for evaluating biocompatibility in alignment with ISO 10993, which helps manufacturers interpret biocompatibility requirements for regulatory compliance [164,170,171].
Also, regulatory frameworks governing medical devices within the European Union include the EU Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR) [167,168,170]. Obtaining regulatory approval can be lengthy and complex, requiring a long lead time and many resources. This can delay the introduction of new fibre optic sensor technologies to the market and their wider application. Table 4 outlines the regulatory standards governing using fibre optic sensors in medical applications.
Table 4.
Regulatory standards for fibre optic sensors in medical applications [164,165,166,167,168,169,170,171,172].
| Regulatory Body | Standard/Guideline | Scope and Relevance to FOSs |
|---|---|---|
| FDA (USA) | FDA Medical Device Approval Process | Safety, efficacy, and reliability assessments ensure FOSs meet regulatory requirements before market approval. |
| NCAs (EU) | Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) | Regulation of general medical devices in the EU governs their safety and performance. |
| ISO | ISO 13485/ISO 10993 | Quality management system for medical devices/Biocompatibility evaluation of medical devices. |
| AAMI/ANSI | AAMI TIR42 | Guidance on biocompatibility evaluation, which supports compliance with ISO 10993 for medical FOSs. |
2.6. Ethical Considerations
When developing and deploying sensor technologies for biomedical applications, there is a range of ethical challenges that researchers and clinicians need to consider. These include ensuring user autonomy by preventing unnecessary dependence on the technology or burdening participants, and promoting beneficence by balancing societal benefit against individual risk while protecting participant privacy and dignity. Transparency is essential, as participants should be informed that they may not receive direct clinical benefit and that the research is for broader knowledge generation. Issues of justice particularly regarding equitable access, data usage, and potential commercialization must be addressed. Informed consent processes should be clear, dynamic, and include explicit examples of data collection. Researchers must communicate that participation does not equate to guaranteed well-being and foster trust through respectful engagement. Oversight mechanisms and conflict-of-interest disclosures are vital, as is ensuring that researchers understand both the technical and social impacts of these technologies, including compliance with local privacy laws [173].
FOSs offer advantages for biomedical applications, including high sensitivity, immunity to electromagnetic interference, compact size, biocompatibility, and the ability for real-time, minimally invasive monitoring. Their versatility allows for the detection of a wide range of physical, chemical, and biochemical measurands with excellent spatial resolution [174]. However, despite these strengths, FOSs also face notable disadvantages. Manufacturing complexity, especially for miniaturized and nanostructured designs, remains a major challenge. Long-term stability in harsh biological environments, signal degradation over time, the need for specialized interrogation systems, and high production costs can limit large-scale clinical deployment [3]. Moreover, achieving consistent reproducibility and meeting regulatory standards adds additional barriers to translation from laboratory prototypes to real-world medical devices [175,176]. Table 5 summarizes the key challenges involved in developing FOSs for use in biomedical applications.
Table 5.
Challenges in developing FOSs for biomedical applications.
| Challenge | Key Considerations | Refs. |
|---|---|---|
| Biocompatibility | - Ensuring non-toxicity, immune safety, and mechanical stability of materials - Coatings for biocompatibility |
[127,128] |
| Miniaturization and Nanomaterials | - Maintaining accuracy in miniaturized sensors - Integration into medical devices - Advancements in nanomaterials for improved sensitivity - Long-term sensor reliability |
[145,146] |
| Signal Processing and Data Integration | Fast and accurate data processing - Ensuring seamless system integration with medical devices - Real-time data transmission and processing |
[158,159] |
| Production Cost and Manufacturing | - Balancing cost-effectiveness with high quality - Ensuring scalability in production |
[93,161] |
| Medical Standards and Regulatory Approval | - Adhering to FDA and other regulatory body requirements - Ensuring sensor stability after sterilization - Ensuring human safety testing and approval |
[164,165,167,170] |
| Ethical Considerations | - Ensuring patient privacy, safety, and secure data transmission - Obtaining informed consent and preventing misuse of health data |
[173] |
3. Future Perspectives
Looking ahead, the integration of advanced materials, green nanotechnology, and hybrid photonic platforms is expected to address many of the current limitations of FOSs. The convergence of fibre optic sensing with machine learning [177], AI technologies [178,179], IoT (Internet of Things) technologies [180], robotics [181], tactile sensing [182], and wearable devices [183] opens new possibilities for personalized and predictive healthcare. Future research will likely focus on improving sensor robustness, achieving scalable manufacturing, enhancing environmental sustainability, and ensuring ethical and regulatory compliance for clinical applications. Ultimately, fibre optic biosensors are poised to become a critical part of next-generation diagnostics, offering real-time insights and improving outcomes across a wide range of medical fields [176,184].
4. Conclusions
FOSs have been transformative in healthcare, offering high accuracy and versatility in monitoring physiological and biochemical parameters, including temperature, pressure, strain, and biochemical markers, enhancing diagnostics and patient outcomes. However, the widespread adoption of FOS technology in healthcare faces several critical challenges, including biocompatibility, miniaturization, durability, and robust signal processing. Addressing these challenges requires interdisciplinary collaboration across material science, engineering, and medical practice to develop reliable, scalable, and clinically viable sensor systems.
Innovative advancements in biocompatible materials, fabrication techniques, and signal processing algorithms continue to push the boundaries of what FOSs can achieve in medicine. Standardization and regulatory approval remain key hurdles that must be overcome to facilitate their transition from research laboratories to commercial medical devices. Throughout the process of addressing all challenges, ethical considerations must also be contemplated. Future research efforts should focus on enhancing sensor integration within existing medical systems, improving long-term reliability and stability, and developing AI-driven analytical methods for accurate data interpretation for personalized and predictive healthcare.
In overcoming these challenges, fibre optic sensors have the potential to revolutionize biomedical sensing, paving the way for more precise diagnostics, personalized treatment plans, and improved patient outcomes. As the field advances, the synergy between optical sensor technology and emerging biomedical innovations will shape the future of healthcare, making real-time, minimally invasive monitoring an integral part of medical practice.
Acknowledgments
The authors express sincere gratitude to colleagues and collaborators who provided valuable insights and support throughout this research. A postgraduate research bursary from the Atlantic Technological University supported this research.
Abbreviations
The following abbreviations are used in this manuscript:
| FOSs | Fibre Optic Sensors (FOSs) |
| POSs | Polymer-based optical sensors |
| OF | Optical Fibre |
| SPR | Surface Plasmon Resonance |
| POFs | Polymer Optical Fibres |
| FBGs | Fibre Bragg Gratings |
| OCT | Optical Coherence Tomography |
| IOP | Intraocular Pressure |
| PCF | Photonic Crystal Fibre |
| SMF | Single-mode Fibre |
| FPI | Fabry–Pérot Interferometer |
| MMF | Multi-mode Fibre |
| MEMs | Micro-Electromechanical Systems |
| LSPR | Localized Surface Plasmon Resonance |
| LMR | Lossy Mode Resonance |
| HST | Hollow Silica Tube |
| PID | Proportional Integral Derivative |
| PANi | Polyaniline |
| TFBG | Tilted Fibre Bragg Grating |
| PAAm | Polyacrylamide |
| GO | Graphene Oxide |
| GOD | Glucose Oxidase |
| LPFG | Long-period Fibre Grating |
| TOFI | Tapered Optical Fibre Interferometer |
| 3-APBA | 3-Aminophenylboronic Acid |
| LDOF | Lossy Dielectric Optical Fibre |
| HBF | High-birefringence fibre |
| PLA | Polylactic acid |
| FDA | Food and Drug Administration |
| PEG | Polyethylene Glycol |
| POC | Poly (Octamethylene Citrate) |
| POMC | Poly (Octamethylene Maleate Citrate) |
| PVC | Polyvinyl Chloride |
| SU-8 | Negative Photoresist Polymer |
| PLLA | Poly (L-Lactic Acid) |
| PDLLA | Poly (D, L-Lactic Acid) |
| PLGA | Poly (L-Lactic-Co-Glycolic Acid) |
| PDLGA | Poly (D, L-Lactic-Co-Glycolic Acid) |
| PCL | Poly (ε-Caprolactone) |
| PGs | Phosphate Glass |
| PDMS | Polydimethylsiloxane |
| PAA | Polyacrylic Acid |
| AG | Agarose Hydrogel |
| AuNPs | Gold Nanoparticles |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| EHRs | Electronic Health Records |
| NCAs | National Competent Authorities |
| ANSI | American National Standards Institute |
| AAMI | Association for the Advancement of Medical Instrumentation |
| TIR | Technical Information Report |
| MDR | Medical Devices Regulation |
| IVDR | In Vitro Diagnostic Regulation |
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors Elfed Lewis and Sanober Farheen Memon are supported by the Higher Education Authority (HEA), the Department of Further and Higher Education, Research, Innovation and Science (DFHERIS) and the Shared Island Fund for the North-South Research Programme under the OXI-SMART Project.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Abdhul Rahuman M.A., Kahatapitiya N.S., Amarakoon V.N., Wijenayake U., Silva B.N., Jeon M., Kim J., Ravichandran N.K., Wijesinghe R.E. Recent Technological Progress of Fiber-Optical Sensors for Bio-Mechatronics Applications. Technologies. 2023;11:157. doi: 10.3390/technologies11060157. [DOI] [Google Scholar]
- 2.Roriz P., Frazão O., Lobo-Ribeiro A.B., Santos J.L., Simões J.A. Review of fiber-optic pressure sensors for biomedical and biomechanical applications. J. Biomed. Opt. 2013;18:050903. doi: 10.1117/1.JBO.18.5.050903. [DOI] [PubMed] [Google Scholar]
- 3.Ngiejungbwen L.A., Hamdaoui H., Chen M.Y. Polymer optical fiber and fiber Bragg grating sensors for biomedical engineering Applications: A comprehensive review. Opt. Laser Technol. 2024;170:110187. doi: 10.1016/j.optlastec.2023.110187. [DOI] [Google Scholar]
- 4.Bartnik K., Koba M., Śmietana M. Advancements in optical fiber sensors for in vivo applications—A review of sensors tested on living organisms. Measurement. 2024;224:113818. doi: 10.1016/j.measurement.2023.113818. [DOI] [Google Scholar]
- 5.Padha B., Yadav I., Dutta S., Arya S. Recent Developments in Wearable NEMS/MEMS-Based Smart Infrared Sensors for Healthcare Applications. ACS Appl. Electron. Mater. 2023;5:5386–5411. doi: 10.1021/acsaelm.3c00860. [DOI] [Google Scholar]
- 6.Yu L., Kim B.J., Meng E. Chronically implanted pressure sensors: Challenges and state of the field. Sensors. 2014;14:20620–20644. doi: 10.3390/s141120620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Presti D.L., Massaroni C., Leitao C.S.J., Domingues M.D.F., Sypabekova M., Barrera D., Floris I., Massari L., Oddo C.M., Sales S., et al. Fiber Bragg Gratings for Medical Applications and Future Challenges: A Review. IEEE Access. 2020;8:156863–156888. doi: 10.1109/ACCESS.2020.3019138. [DOI] [Google Scholar]
- 8.Yoo E.H., Lee S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors. 2010;10:4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tosi D., Sypabekova M., Bekmurzayeva A., Molardi C., Dukenbayev K. Optical Fiber Biosensors: Device Platforms, Biorecognition, Applications. Academic Press; Cambridge, MA, USA: 2021. [Google Scholar]
- 10.Venketeswaran A., Lalam N., Wuenschell J., Ohodnicki P.R., Badar M., Chen K.P., Lu P., Duan Y., Chorpening B., Buric M. Recent Advances in Machine Learning for Fiber Optic Sensor Applications. Adv. Intell. Syst. 2021;4:2100067. doi: 10.1002/aisy.202100067. [DOI] [Google Scholar]
- 11.Xu Y., Wu X., Guo X., Kong B., Zhang M., Qian X., Mi S., Sun W. The Boom in 3D-Printed Sensor Technology. Sensors. 2017;17:1166. doi: 10.3390/s17051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadha U., Bhardwaj P., Agarwal R., Rawat P., Agarwal R., Gupta I., Panjwani M., Singh S., Ahuja C., Selvaraj S.K., et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022;109:21–51. doi: 10.1016/j.jiec.2022.02.010. [DOI] [Google Scholar]
- 13.Ayyanar N., Sreekanth K.V., Raja G.T., Rajan M.S.M. Photonic Crystal Fiber-Based Reconfigurable Biosensor Using Phase Change Material. IEEE Trans. Nanobioscience. 2021;20:338–344. doi: 10.1109/TNB.2021.3065006. [DOI] [PubMed] [Google Scholar]
- 14.Wang J., Dong J. Optical waveguides and integrated optical devices for medical diagnosis, health monitoring and light therapies. Sensors. 2020;20:3981. doi: 10.3390/s20143981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng C., Yang C., Zhao H., Liang L., Zheng C., Chen C., Qin L., Tang H. Optical Waveguide Refractive Index Sensor for Biochemical Sensing. Appl. Sci. 2023;13:3829. doi: 10.3390/app13063829. [DOI] [Google Scholar]
- 16.Leiner M.J.P. Luminescence chemical sensors for biomedical applications: Scope and limitations. Anal. Chim. Acta. 1991;255:209–222. doi: 10.1016/0003-2670(91)80049-Y. [DOI] [Google Scholar]
- 17.Englebienne P., Van Hoonacker A., Verhas M. Surface plasmon resonance: Principles, methods and applications in biomedical sciences. Spectroscopy. 2003;17:255–273. doi: 10.1155/2003/372913. [DOI] [Google Scholar]
- 18.Grattan L.S., Meggitt B.T. Optical Fiber Sensor Technology: Fundamentals. Springer; New York, NY, USA: 2010. [(accessed on 1 March 2025)]. Available online: https://books.google.com/books?id=hbp1cgAACAAJ. [Google Scholar]
- 19.Pirzada M., Altintas Z. Recent progress in optical sensors for biomedical diagnostics. Micromachines. 2020;11:356. doi: 10.3390/mi11040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong L., Zhong H., Wan S., Yu J. Single-point curved fiber optic pulse sensor for physiological signal prediction based on the genetic algorithm-support vector regression model. Opt. Fiber Technol. 2024;82:103583. doi: 10.1016/j.yofte.2023.103583. [DOI] [Google Scholar]
- 21.Pendão C., Silva I. Optical Fiber Sensors and Sensing Networks: Overview of the Main Principles and Applications. Sensors. 2022;22:7554. doi: 10.3390/s22197554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagar M.A., Janner D. Polymer-Based Optical Guided-Wave Biomedical Sensing: From Principles to Applications. Photonics. 2024;11:972. doi: 10.3390/photonics11100972. [DOI] [Google Scholar]
- 23.Soge A.O., Dairo O.F., Sanyaolu M.E., Kareem S.O. Recent developments in polymer optical fiber strain sensors: A short review. J. Opt. 2021;50:299–313. doi: 10.1007/s12596-021-00699-7. [DOI] [Google Scholar]
- 24.Zhang Z., Zhang C., Zuo S. A Novel Bioinspired Whisker Sensor for Gastrointestinal Endoscopy. IEEE/ASME Trans. Mechatronics. 2023;29:636–646. doi: 10.1109/TMECH.2023.3289955. [DOI] [Google Scholar]
- 25.Theodosiou A. Recent Advances in Fiber Bragg Grating Sensing. Sensors. 2024;24:532. doi: 10.3390/s24020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu C., Zheng H., Ma L., Yao Z., Liu B., Huang J., Rao Y. Advances in Fiber-Optic Extrinsic Fabry-Perot Interferometric Physical and Mechanical Sensors: A Review. IEEE Sens. J. 2023;23:6406–6426. doi: 10.1109/JSEN.2023.3244820. [DOI] [Google Scholar]
- 27.Elsherif M., Salih A.E., Muñoz M.G., Alam F., AlQattan B., Antonysamy D.S., Zaki M.F., Yetisen A.K., Park S., Wilkinson T.D., et al. Optical Fiber Sensors: Working Principle, Applications, and Limitations. Adv. Photonics Res. 2022;3:2100371. doi: 10.1002/adpr.202100371. [DOI] [Google Scholar]
- 28.Moeglen Paget B., Vinod Ram K., Zhang S., Perumal J., Vedraine S., Humbert G., Olivo M., Dinish U.S. A review on photonic crystal fiber based fluorescence sensing for chemical and biomedical applications. Sens. Actuators B Chem. 2024;400:134828. doi: 10.1016/j.snb.2023.134828. [DOI] [Google Scholar]
- 29.Azmi A.N., Wan Ismail W.Z., Abu Hassan H., Halim M.M., Zainal N., Muskens O.L., Wan Ahmad Kamil W.M. Review of Open Cavity Random Lasers as Laser-Based Sensors. ACS Sensors. 2022;7:914–928. doi: 10.1021/acssensors.1c02749. [DOI] [PubMed] [Google Scholar]
- 30.Fatima Domingues M., Direito I., Sousa C., Radwan A., Antunes P., Andre P., Helguero L., Alberto N. Optical Fibre FPI End-Tip based Sensor for Protein Aggregation Detection; Proceedings of the 2022 IEEE International Conference on E-health Networking, Application & Services (HealthCom); Genoa, Italy. 17–19 October 2022; pp. 129–134. [DOI] [Google Scholar]
- 31.Wang F., Li X., Wang S., Cao Y., Zhang L., Zhao Y., Dong X., Zheng M., Liu H., Lu W., et al. 3D fiber-probe surface plasmon resonance microsensor towards small volume sensing. Sens. Actuators B Chem. 2023;384:133647. doi: 10.1016/j.snb.2023.133647. [DOI] [Google Scholar]
- 32.Mi H., Wang Y., Jin P., Lei L. Design of a ultrahigh-sensitivity SPR-based optical fiber pressure sensor. Optik. 2013;124:5248–5250. doi: 10.1016/j.ijleo.2013.03.097. [DOI] [Google Scholar]
- 33.Samimi K., Contreras Guzman E., Wu M., Carlson L., Feltovich H., Hall T.J., Myers K.M., Oyen M.L., Skala M.C. Optical coherence tomography of human fetal membrane sub-layers during loading. Biomed. Opt. Express. 2023;14:2969. doi: 10.1364/BOE.489691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui P.C., Shtyrkova K., Zhou C., Chen X., Chodosh J., Dohlman C.H., Paschalis E.I. Implantable self-aligning fiber-optic optomechanical devices for in vivo intraocular pressure-sensing in artificial cornea. J. Biophotonics. 2020;13:e202000031. doi: 10.1002/jbio.202000031. [DOI] [PubMed] [Google Scholar]
- 35.Nithish A.N., Patel S.K., Ayyanar N., Surve J., Rajaram S., Deepa S.N., Nguyen T.K., Al-Zahrani F.A. Terahertz Women Reproductive Hormones Sensor Using Photonic Crystal Fiber with Behavior Prediction Using Machine Learning. IEEE Access. 2023;11:75424–75433. doi: 10.1109/ACCESS.2023.3277955. [DOI] [Google Scholar]
- 36.Gupta B.D., Pathak A., Shrivastav A.M. Optical Biomedical Diagnostics Using Lab-on-Fiber Technology: A Review. Photonics. 2022;9:86. doi: 10.3390/photonics9020086. [DOI] [Google Scholar]
- 37.Uniyal A., Srivastava G., Pal A., Taya S., Muduli A. Recent Advances in Optical Biosensors for Sensing Applications: A Review. Plasmonics. 2023;18:735–750. doi: 10.1007/s11468-023-01803-2. [DOI] [Google Scholar]
- 38.Katrenova Z., Alisherov S., Abdol T., Molardi C. Status and future development of distributed optical fiber sensors for biomedical applications. Sens. Bio-Sensing Res. 2024;43:100616. doi: 10.1016/j.sbsr.2023.100616. [DOI] [Google Scholar]
- 39.Butt M.A., Kazanskiy N.L., Khonina S.N., Voronkov G.S., Grakhova E.P., Kutluyarov R.V. A Review on Photonic Sensing Technologies: Status and Outlook. Biosensors. 2023;13:568. doi: 10.3390/bios13050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenato L. Fiber-optic sensors for geo-hydrological applications: Basic concepts and applications. Rend. Online Soc. Geol. Ital. 2014;30:51–54. doi: 10.3301/ROL.2014.11. [DOI] [Google Scholar]
- 41.Zhang S., Lei Q., Hu J., Zhao Y., Gao H., Shen J., Li C. An optical fiber pressure sensor with ultra-thin epoxy film and high sensitivity characteristics based on blowing bubble method. IEEE Photonics J. 2021;13:6800510. doi: 10.1109/JPHOT.2021.3055872. [DOI] [Google Scholar]
- 42.Jauregui-Vazquez D., Gutierrez-Rivera M.E., Garcia-Mina D.F., Sierra-Hernandez J.M., Gallegos-Arellano E., Estudillo-Ayala J.M., Hernandez-Garcia J.C., Rojas-Laguna R. Low-pressure and liquid level fiber-optic sensor based on polymeric Fabry–Perot cavity. Opt. Quantum Electron. 2021;53:237. doi: 10.1007/s11082-021-02871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S., Wang J., Li W., Liu Y., Li J., Jia P. A MEMS-Based High-Fineness Fiber-Optic Fabry–Perot Pressure Sensor for High-Temperature Application. Micromachines. 2022;13:763. doi: 10.3390/mi13050763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An C.L.I., Hen Z.L.I., Huanglu S.L.I., Hang Y.A.Z., Aoping B., Un S., Uehao Y.Y.U., En H.A.R., Iang S.H.J. LSPR optical fiber biosensor based on a 3D composite structure of gold nanoparticles and multilayer graphene films. Opt. Express. 2020;28:6071–6083. doi: 10.1364/OE.385128. [DOI] [PubMed] [Google Scholar]
- 45.Selvendran S., Susheel A., Tarun P.V., Muthu K.E., Raja A.S. A novel surface plasmon based photonic crystal fiber sensor. Opt. Quantum Electron. 2020;52:290. doi: 10.1007/s11082-020-02403-8. [DOI] [Google Scholar]
- 46.Melo A.A., Santiago M.F., Silva T.B., Moreira C.S., Cruz R.M. Investigation of a D-shaped optical fiber sensor with graphene overlay. IFAC-PapersOnLine. 2018;51:309–314. doi: 10.1016/j.ifacol.2018.11.623. [DOI] [Google Scholar]
- 47.Tien C., Lin H., Su S. High Sensitivity Refractive Index Sensor by D-Shaped Fibers and Titanium Dioxide Nanofilm. Adv. Condens. Matter Phys. 2018;2018:1–6. doi: 10.1155/2018/2303740. [DOI] [Google Scholar]
- 48.Wu T., Shao Y., Wang Y., Cao S., Cao W., Zhang F., Liao C., He J., Huang Y., Hou M., et al. Surface plasmon resonance biosensor based on gold-coated side-polished hexagonal structure photonic crystal fiber. Opt. Express. 2017;25:227–231. doi: 10.1364/OE.25.020313. [DOI] [PubMed] [Google Scholar]
- 49.Haque E., Hossain A., Ahmed F., Namihira Y. Surface Plasmon Resonance Sensor Based on Modified D -Shaped Photonic Crystal Fiber for Wider Range of Refractive Index Detection. IEEE Sens. J. 2018;18:8287–8293. doi: 10.1109/JSEN.2018.2865514. [DOI] [Google Scholar]
- 50.Yunianto M., Permata A.N., Eka D., Ariningrum D., Wahyuningsih S., Marzuki A. Design of a Fiber Optic Biosensor for Cholesterol Detection in Human Blood Design of a Fiber Optic Biosensor for Cholesterol Detection in Human Blood. IOP Conf. Ser. Mater. Sci. Eng. 2017;176:012014. doi: 10.1088/1757-899X/176/1/012014. [DOI] [Google Scholar]
- 51.Cheng Y., Wang Y., Song Z., Lei J. High-sensitivity optical fiber temperature sensor based on a dual-loop optoelectronic oscillator with the Vernier effect. Opt. Express. 2020;28:35264–35271. doi: 10.1364/OE.410922. [DOI] [PubMed] [Google Scholar]
- 52.Cao H., Li D., Zhou K., Chen Y. Demonstration of a ZnO-Nanowire-Based Nanograting Temperature Sensor. Photonic Sens. 2023;13:1–7. doi: 10.1007/s13320-022-0663-8. [DOI] [Google Scholar]
- 53.Zhou N., Jia P., Liu J., Ren Q., An G., Liang T., Xiong J. MEMS-based reflective intensity-modulated fiber-optic sensor for pressure measurements. Sensors. 2020;20:2233. doi: 10.3390/s20082233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., Zheng Y., Liang D., Zhang Y., Guo J., Lian S., Yu Y., Du C., Ruan S. Fiber-Tip Fabry-Perot Cavity Pressure Sensor With UV-Curable Polymer Film Based on Suspension Curing Method. IEEE Sens. J. 2022;22:6651–6660. doi: 10.1109/JSEN.2022.3153993. [DOI] [Google Scholar]
- 55.Qureshi K.K. Detection of Plantar Pressure Using an Optical Technique; Proceedings of the 2021 7th International Conference on Engineering, Applied Sciences and Technology (ICEAST); Pattaya, Thailand. 1–3 April 2021; pp. 77–80. [DOI] [Google Scholar]
- 56.Yoshida T., Tsuruoka N., Haga Y., Kinoshita H., Lee S.S., Matsunaga T. Automatic irrigation system with a fiber-optic pressure sensor regulating intrapelvic pressure for flexible ureteroscopy. Sci. Rep. 2023;13:22853. doi: 10.1038/s41598-023-47373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han J., Chen M., Wen J., Yang T., Dong Y. High sensitivity fiber optic esophageal pressure sensor based on OFDR. J. Phys. Conf. Ser. 2023;2581:012007. doi: 10.1088/1742-6596/2581/1/012007. [DOI] [Google Scholar]
- 58.Shang C., Fu B., Tuo J., Guo X., Li Z., Wang Z., Xu L., Guo J. Soft Biomimetic Fiber-Optic Tactile Sensors Capable of Discriminating Temperature and Pressure. ACS Appl. Mater. Interfaces. 2023;15:53264–53272. doi: 10.1021/acsami.3c12712. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H., Cong B., Zhang F., Qi Y., Hu T. Simultaneous measurement of refractive index and temperature by Mach–Zehnder cascaded with FBG sensor based on multi-core microfiber. Opt. Commun. 2021;493:126985. doi: 10.1016/j.optcom.2021.126985. [DOI] [Google Scholar]
- 60.Narayan V., Mohammed N., Savardekar A.R., Patra D.P., Notarianni C., Nanda A. Noninvasive Intracranial Pressure Monitoring for Severe Traumatic Brain Injury in Children: A Concise Update on Current Methods. World Neurosurg. 2018;114:293–300. doi: 10.1016/j.wneu.2018.02.159. [DOI] [PubMed] [Google Scholar]
- 61.He C., Teng C., Xiong Z., Lin X., Li H., Li X. Intracranial pressure monitoring in neurosurgery: The present situation and prospects. Chin. Neurosurg. J. 2023;9:14. doi: 10.1186/s41016-023-00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mimura M., Akagi T., Kohmoto R., Fujita Y., Sato Y., Ikeda T. Measurement of vitreous humor pressure in vivo using an optic fiber pressure sensor. Sci. Rep. 2023;13:18233. doi: 10.1038/s41598-023-45616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raveendran R., Prabakaran L., Senthil R., Yesudhason B.V., Dharmalingam S., Sathyaraj W.V., Atchudan R. Current Innovations in Intraocular Pressure Monitoring Biosensors for Diagnosis and Treatment of Glaucoma—Novel Strategies and Future Perspectives. Biosensors. 2023;13:663. doi: 10.3390/bios13060663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ordookhanian C., Nagappan M., Elias D., Kaloostian P.E. Traumatic Brain Injury: Pathobiology, Advanced Diagnostics and Acute Management. BoD–Books on Demand; Norderstedt, Germany: 2018. Management of Intracranial Pressure in Traumatic Brain Injury. [DOI] [Google Scholar]
- 65.Zhao C., Liu D., Xu G., Zhou J., Zhang X., Liao C., Wang Y. Recent advances in fiber optic sensors for respiratory monitoring. Opt. Fiber Technol. 2022;72:103000. doi: 10.1016/j.yofte.2022.103000. [DOI] [Google Scholar]
- 66.Tosi D., Poeggel S., Iordachita I., Schena E. Fiber Optic Sensors for Biomedical Applications. Elsevier Inc.; Amsterdam, The Netherlands: 2018. [DOI] [Google Scholar]
- 67.Poeggel S., Duraibabu D., Kalli K., Leen G., Dooly G., Lewis E., Kelly J., Munroe M. Recent improvement of medical optical fibre pressure and temperature sensors. Biosensors. 2015;5:432–449. doi: 10.3390/bios5030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.González-Cely A.X., Diaz C.A.R., Callejas-Cuervo M., Bastos-Filho T. Optical fiber sensors for posture monitoring, ulcer detection and control in a wheelchair: A state-of-the-art. Disabil. Rehabil. Assist. Technol. 2023;19:1773–1790. doi: 10.1080/17483107.2023.2234411. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X., Wang C., Zheng T., Wu H., Wu Q., Wang Y. Wearable Optical Fiber Sensors in Medical Monitoring Applications: A Review. Sensors. 2023;23:6671. doi: 10.3390/s23156671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Najafzadeh A., Gunawardena D.S., Liu Z., Tran T., Tam H.Y., Fu J., Chen B.K. Application of fibre bragg grating sensors in strain monitoring and fracture recovery of human femur bone. Bioengineering. 2020;7:98. doi: 10.3390/bioengineering7030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Tommasi F., Romano C., Lo Presti D., Massaroni C., Carassiti M., Schena E. FBG-Based Soft System for Assisted Epidural Anesthesia: Design Optimization and Clinical Assessment. Biosensors. 2022;12:645. doi: 10.3390/bios12080645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X., Zhou H., Chen M., He Y., Zhang Z., Gan J., Yang Z. Highly Sensitive Strain Sensor Based on Microfiber Coupler for Wearable Photonics Healthcare. Adv. Intell. Syst. 2023;5:2200344. doi: 10.1002/aisy.202200344. [DOI] [Google Scholar]
- 73.Zhao J., Zhang S., Sun Y., Zhou N., Yu H., Zhang H., Jia D. Wearable Optical Sensing in the Medical Internet of Things (MIoT) for Pervasive Medicine: Opportunities and Challenges. ACS Photonics. 2022;9:2579–2599. doi: 10.1021/acsphotonics.2c00898. [DOI] [Google Scholar]
- 74.Elsherif M., Hassan M.U., Yetisen A.K., Butt H. Hydrogel optical fibers for continuous glucose monitoring. Biosens. Bioelectron. 2019;137:25–32. doi: 10.1016/j.bios.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Qu W., Chen Y., Ma C., Peng D., Bai X., Zhao J., Liu S., Luo L. Application of Optical Fiber Sensing Technology and Coating Technology in Blood Component Detection and Monitoring. Coatings. 2024;14:173. doi: 10.3390/coatings14020173. [DOI] [Google Scholar]
- 76.Zhang J., Mai X., Hong X., Chen Y., Li X. Optical fiber SPR biosensor with a solid-phase enzymatic reaction device for glucose detection. Sens. Actuators B Chem. 2022;366:131984. doi: 10.1016/j.snb.2022.131984. [DOI] [Google Scholar]
- 77.Li Y., Luo S., Gui Y., Wang X., Tian Z., Yu H. Difunctional Hydrogel Optical Fiber Fluorescence Sensor for Continuous and Simultaneous Monitoring of Glucose and pH. Biosensors. 2023;13:287. doi: 10.3390/bios13020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Werner J., Belz M., Klein K., Sun T., Grattan K.T.V. Fiber optic sensor designs and luminescence-based methods for the detection of oxygen and pH measurement. Measurement. 2021;178:109323. doi: 10.1016/j.measurement.2021.109323. [DOI] [Google Scholar]
- 79.Gong J., Tanner M.G., Venkateswaran S., Stone J.M., Zhang Y., Bradley M. Analytica Chimica Acta A hydrogel-based optical fibre fluorescent pH sensor for observing lung tumor tissue acidity. Anal. Chim. Acta. 2020;1134:136–143. doi: 10.1016/j.aca.2020.07.063. [DOI] [PubMed] [Google Scholar]
- 80.Khan R.R., Watekar A.V., Kang S. Fiber-Optic Biosensor to Detect pH and Glucose. IEEE Sens. J. 2018;18:1528–1538. doi: 10.1109/JSEN.2017.2786279. [DOI] [Google Scholar]
- 81.Steinegger A., Wolfbeis O.S., Borisov S.M. Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020;120:12357–12489. doi: 10.1021/acs.chemrev.0c00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paltusheva Z.U., Ashikbayeva Z., Tosi D. Highly Sensitive Zinc Oxide Fiber-Optic Biosensor for the Detection of CD44 Protein. Biosensors. 2022;12:1015. doi: 10.3390/bios12111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song H., Wang Q., Zhao W. A novel SPR sensor sensitivity-enhancing method for immunoassay by inserting MoS2 nanosheets between metal film and fiber. Opt. Lasers Eng. 2020;132:106135. doi: 10.1016/j.optlaseng.2020.106135. [DOI] [Google Scholar]
- 84.Cai J., Liu Y., Shu X. Long-Period Fiber Grating Sensors for Chemical and Biomedical Applications. Sensors. 2023;23:542. doi: 10.3390/s23010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen X., Xiao L., Li X., Yi D., Zhang J., Yuan H., Ning Z., Hong X., Chen Y. Tapered Fiber Bioprobe Based on U-Shaped Fiber Transmission for Immunoassay. Biosensors. 2023;13:940. doi: 10.3390/bios13100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vijayalakshmi D., Ayyanar N., Manimegalai C.T., Alzahrani F.A. Photonic crystal fiber—Based biosensor for detection of women reproductive hormones. Opt. Quantum Electron. 2023;55:442. doi: 10.1007/s11082-023-04672-5. [DOI] [Google Scholar]
- 87.Villegas-cantoran D.S., Gómez C.L., Gómez-pavón L.C., Zaca-morán P., Castillo-lópez D.N., Luis-ramos A., Muñoz-pacheco J.M. Quantification of hCG Hormone Using Tapered Optical Fiber Decorated with Gold Nanoparticles. Sensors. 2023;23:8538. doi: 10.3390/s23208538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohammed N.A., Khedr O.E., El-Rabaie E.S.M., Khalaf A.A.M. Early detection of brain cancers biomedical sensor with low losses and high sensitivity in the terahertz regime based on photonic crystal fiber technology. Opt. Quantum Electron. 2023;55:230. doi: 10.1007/s11082-022-04515-9. [DOI] [Google Scholar]
- 89.Perspectives F. Overview of optical biosensors for early cancer detection: Fundamentals, applications and future perspectives. Biology. 2023;12:232. doi: 10.3390/biology12020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun D., Ran Y. Label-Free Detection of Cancer Biomarkers Using an In-Line Taper Fiber-Optic Interferometer and a Fiber Bragg Grating. Sensors. 2017;17:2559. doi: 10.3390/s17112559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arcadio F., Seggio M., Pitruzzella R., Zeni L., Bossi A.M., Cennamo N. An Efficient Bio-Receptor Layer Combined with a Plasmonic Plastic Optical Fiber Probe for Cortisol Detection in Saliva. Biosensors. 2024;14:351. doi: 10.3390/bios14070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naresh V., Lee N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors. 2021;21:1109. doi: 10.3390/s21041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leitão C., Pereira S.O., Marques C., Cennamo N., Zeni L., Shaimerdenova M., Ayupova T., Tosi D. Cost-Effective Fiber Optic Solutions for Biosensing. Biosensors. 2022;12:575. doi: 10.3390/bios12080575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prasanth A., Meher S.R., Alex Z.C. Metal oxide thin films coated evanescent wave based fiber optic VOC sensor. Sens. Actuators A Phys. 2022;338:113459. doi: 10.1016/j.sna.2022.113459. [DOI] [Google Scholar]
- 95.Pathak A.K., Viphavakit C. A review on all-optical fiber-based VOC sensors: Heading towards the development of promising technology. Sens. Actuators A Phys. 2022;338:113455. doi: 10.1016/j.sna.2022.113455. [DOI] [Google Scholar]
- 96.Buszewski B., Grzywinski D., Ligor T., Stacewicz T., Bielecki Z., Wojtas J. Detection of Volatile Organic Compounds as Biomarkers in Breath Analysis by Different Analytical Techniques. Bioanalysis. 2013;5:2287–2306. doi: 10.4155/bio.13.183. [DOI] [PubMed] [Google Scholar]
- 97.Grantzioti E., Pissadakis S., Konstantaki M. Optical Fiber Volatile Organic Compound Vapor Sensor With ppb Detectivity for Breath Biomonitoring. IEEE Sens. J. 2025;25:8224–8229. doi: 10.1109/JSEN.2025.3527765. [DOI] [Google Scholar]
- 98.Rohan R., Venkadeshwaran K., Ranjan P. Recent advancements of fiber Bragg grating sensors in biomedical application: A review. J. Opt. 2023;53:282–293. doi: 10.1007/s12596-023-01134-9. [DOI] [Google Scholar]
- 99.Tentor F., Grønholt Schrøder B., Nielsen S., Schertiger L., Stærk K., Emil Andersen T., Bagi P., Feldskov Nielsen L. Development of an ex-vivo porcine lower urinary tract model to evaluate the performance of urinary catheters. Sci. Rep. 2022;12:17818. doi: 10.1038/s41598-022-21122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo T., Liu F., Liang X., Qiu X., Huang Y., Xie C., Xu P., Mao W., Guan B.O., Albert J. Highly sensitive detection of urinary protein variations using tilted fiber grating sensors with plasmonic nanocoatings. Biosens. Bioelectron. 2016;78:221–228. doi: 10.1016/j.bios.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 101.Xiong H., Zhang X., Sun J., Xue Y., Yu W., Mou S., Hsia K.J., Wan H., Wang P. Recent advances in biosensors detecting biomarkers from exhaled breath and saliva for respiratory disease diagnosis. Biosens. Bioelectron. 2025;267:116820. doi: 10.1016/j.bios.2024.116820. [DOI] [PubMed] [Google Scholar]
- 102.Mulyanti B., Nugroho H.S., Wulandari C., Rahmawati Y., Hasanah L., Hamidah I., Pawinanto R.E., Majlis B.Y. SPR-Based Sensor for the Early Detection or Monitoring of Kidney Problems. Int. J. Biomater. 2022;2022:9135172. doi: 10.1155/2022/9135172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vogt B. Catheter-Free Urodynamics Testing: Current Insights and Clinical Potential. Res. Rep. Urol. 2024;16:1–17. doi: 10.2147/RRU.S387757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y., Hu Y., Liu Q., Lou K., Wang S., Zhang N., Jiang N., Yetisen A.K. Multiplexed optical fiber sensors for dynamic brain monitoring. Matter. 2022;5:3947–3976. doi: 10.1016/j.matt.2022.07.024. [DOI] [Google Scholar]
- 105.Zhou B., Fan K., Li T., Luan G., Kong L. A biocompatible hydrogel-coated fiber-optic probe for monitoring pH dynamics in mammalian brains in vivo. Sens. Actuators B Chem. 2023;380:133334. doi: 10.1016/j.snb.2023.133334. [DOI] [Google Scholar]
- 106.Aldaba A.L., González-Vila Á., Debliquy M., Lopez-Amo M., Caucheteur C., Lahem D. Chemical Polyaniline-coated tilted fiber Bragg gratings for pH sensing. Sens. Actuators B Chem. 2018;254:1087–1093. doi: 10.1016/j.snb.2017.07.167. [DOI] [Google Scholar]
- 107.Zhao Y., Lei M., Liu S., Zhao Q. Chemical Smart hydrogel-based optical fiber SPR sensor for pH measurements. Sens. Actuators B Chem. 2018;261:226–232. doi: 10.1016/j.snb.2018.01.120. [DOI] [Google Scholar]
- 108.Jiang B., Zhou K., Wang C., Sun Q., Yin G., Tai Z., Wilson K., Zhao J., Zhang L. Chemical Label-free glucose biosensor based on enzymatic graphene oxide-functionalized tilted fiber grating. Sens. Actuators B Chem. 2018;254:1033–1039. doi: 10.1016/j.snb.2017.07.109. [DOI] [Google Scholar]
- 109.Li Y., Ma H., Gan L., Liu Q., Yan Z., Liu D., Sun Q. Chemical Immobilized optical fiber microprobe for selective and high sensitive glucose detection. Sens. Actuators B Chem. 2018;255:3004–3010. doi: 10.1016/j.snb.2017.09.123. [DOI] [Google Scholar]
- 110.Wu C.W. S-shaped long period fiber grating glucose concentration biosensor based on immobilized glucose oxidase. Optik. 2020;203:163960. doi: 10.1016/j.ijleo.2019.163960. [DOI] [Google Scholar]
- 111.Li J., Zhang W., Tong Z., Liu J. Fiber optic sensor modified by graphene oxide–glucose oxidase for glucose detection. Opt. Commun. 2021;492:126983. doi: 10.1016/j.optcom.2021.126983. [DOI] [Google Scholar]
- 112.Li X., Gong P., Zhang Y., Zhou X. Label-Free Micro Probe Optical Fiber Biosensor for Selective and Highly Sensitive Glucose Detection. IEEE Trans. Instrum. Meas. 2022;71:7008608. doi: 10.1109/TIM.2022.3219464. [DOI] [Google Scholar]
- 113.Ujah E., Lai M., Slaughter G. Ultrasensitive tapered optical fiber refractive index glucose sensor. Sci. Rep. 2023;13:4495. doi: 10.1038/s41598-023-31127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee S.-L., Kim J., Choi S., Han J., Lee Y.W. Optical glucose detection using birefringent long-period fiber grating functionalized with graphene oxide. Opt. Eng. 2021;60:87102. doi: 10.1117/1.OE.60.8.087102. [DOI] [Google Scholar]
- 115.Rahaman M.E., Jibon R.H., Ahsan M.S., Ahmed F., Sohn I.B. Glucose Level Measurement Using Photonic Crystal Fiber–based Plasmonic Sensor. Plasmonics. 2022;17:1–11. doi: 10.1007/s11468-021-01497-4. [DOI] [Google Scholar]
- 116.Bekmurzayeva A., Ashikbayeva Z., Assylbekova N., Myrkhiyeva Z., Dauletova A., Ayupova T., Shaimerdenova M., Tosi D. Ultra-wide, attomolar-level limit detection of CD44 biomarker with a silanized optical fiber biosensor. Biosens. Bioelectron. 2022;208:114217. doi: 10.1016/j.bios.2022.114217. [DOI] [PubMed] [Google Scholar]
- 117.Li H., Huang T., Yuan H., Lu L., Cao Z., Zhang L., Yang Y., Yu B., Wang H. Combined Ultrasensitive Detection of Renal Cancer Proteins and Cells Using an Optical Microfiber Functionalized with Ti3C2 MXene and Gold Nanorod-Nanosensitized Interfaces. Anal. Chem. 2023;95:5142–5150. doi: 10.1021/acs.analchem.3c00281. [DOI] [PubMed] [Google Scholar]
- 118.Zhu T., Chah K., Chiavaioli F., Villatoro J., Caucheteur C. Gold-coated optical fiber supermode interferometer for insulin bio-sensing. Opt. Laser Technol. 2024;168:109878. doi: 10.1016/j.optlastec.2023.109878. [DOI] [Google Scholar]
- 119.Liu L., Zhang X., Zhu Q., Li K., Lu Y., Zhou X., Guo T. Ultrasensitive detection of endocrine disruptors via super fi ne plasmonic spectral combs. Light Sci. Appl. 2021;10:181. doi: 10.1038/s41377-021-00618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leitão C., Leal-Junior A., Almeida A.R., Pereira S.O., Costa F.M., Pinto J.L., Marques C. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol. Rep. 2021;29:e00587. doi: 10.1016/j.btre.2021.e00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oyez M.É.L., Acão M.A.F., Hristophe C., Aucheteur C., Egatto M.A.E.V.S., Lorinda F.M., Osta C., Eitão C.Á.L., Ereira S.Ó.O.P., Antos N.U.N.O.F.S., et al. Label-free plasmonic immunosensor for cortisol detection in a D-shaped optical fiber. Biomed. Opt. Express. 2022;13:3259–3274. doi: 10.1364/BOE.456253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim H.M., Jeong D.H., Lee H.Y., Park J.H., Lee S.K. Design and validation of fiber optic localized surface plasmon resonance sensor for thyroglobulin immunoassay with high sensitivity and rapid detection. Sci. Rep. 2021;11:15985. doi: 10.1038/s41598-021-95375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang Z., Liu L., Zhang J., Conde K., Phansalkar J., Li Z., Yao L., Xu Z., Wang W., Zhou J., et al. Glucose-sensing glucagon-like peptide-1 receptor neurons in the dorsomedial hypothalamus regulate glucose metabolism. Sci. Adv. 2022;8:eabn5345. doi: 10.1126/sciadv.abn5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duffet L., Williams E.T., Gresch A., Chen S., Bhat M.A., Benke D., Hartrampf N., Patriarchi T. Optical tools for visualizing and controlling human GLP-1 receptor activation with high spatiotemporal resolution. eLife. 2023;12:1–20. doi: 10.7554/eLife.86628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mirdita M., Schütze K., Moriwaki Y., Heo L., Ovchinnikov S., Steinegger M. ColabFold: Making protein folding accessible to all. Nat. Methods. 2022;19:679–682. doi: 10.1038/s41592-022-01488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luo Y., Abidian M.R., Ahn J.H., Akinwande D., Andrews A.M., Antonietti M., Bao Z., Berggren M., Berkey C.A., Bettinger C.J., et al. Technology Roadmap for Flexible Sensors. ACS Nano. 2023;17:5211–5295. doi: 10.1021/acsnano.2c12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gierej A., Geernaert T., Van Vlierberghe S., Dubruel P., Thienpont H., Berghmans F. Challenges in the fabrication of biodegradable and implantable optical fibers for biomedical applications. Materials. 2021;14:1972. doi: 10.3390/ma14081972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang P., Kim J.W., Gehlbach P., Iordachita I., Kobilarov M. Autonomous Needle Navigation in Retinal Microsurgery: Evaluation in ex vivo Porcine Eyes; Proceedings of the 2023 IEEE International Conference on Robotics and Automation (ICRA); London, UK. 29 May–2 June 2023; pp. 4661–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Y., Jing Z., Li R., Zhang Y., Liu Q., Li A., Zhang C., Peng W. Miniature fiber-optic tip pressure sensor assembled by hydroxide catalysis bonding technology. Opt. Express. 2020;28:948. doi: 10.1364/OE.380589. [DOI] [PubMed] [Google Scholar]
- 130.Liu Z., Zhang Z.F., Tam H., Tao X. Multifunctional Smart Optical Fibers: Materials, Fabrication, and Sensing Applications. Photonics. 2019;6:48. doi: 10.3390/photonics6020048. [DOI] [Google Scholar]
- 131.Schyrr B., Boder-pasche S., Ischer R., Smajda R., Voirin G. Fiber-optic protease sensor based on the degradation of thin gelatin films. Sens. Bio-Sens. Res. 2015;3:65–73. doi: 10.1016/j.sbsr.2014.12.004. [DOI] [Google Scholar]
- 132.Mcdonald S.R., Tao S. An optical fiber chlorogenic acid sensor using a Chitosan membrane coated bent optical fiber probe. Anal. Chim. Acta. 2024;1288:342142. doi: 10.1016/j.aca.2023.342142. [DOI] [PubMed] [Google Scholar]
- 133.Marpu S.B., Benton E.N. Shining Light on Chitosan: A Review on the Usage of Chitosan for Photonics and Nanomaterials Research. Int. J. Mol. Sci. 2018;19:1795. doi: 10.3390/ijms19061795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suna F.A.N., Yi Z., Xiangyu H., Lihong G., Huili S. Silk materials for medical, electronic and optical applications. Sci. China Technol. Sci. 2019;62:903–918. [Google Scholar]
- 135.Fujiwara E., Oku H., Cordeiro C.M.B. De Recent developments in agar—Based optical devices. MRS Commun. 2024;14:237–247. doi: 10.1557/s43579-024-00558-z. [DOI] [Google Scholar]
- 136.Arefnia F., Zibaii M.I., Layeghi A., Rostami S. Citrate polymer optical fiber for measuring refractive index based on LSPR sensor. Sci. Rep. 2024;14:18637. doi: 10.1038/s41598-024-69083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen Z., Lee J. Biocompatibility of SU-8 and Its Biomedical Device Applications. Micromachines. 2021;12:794. doi: 10.3390/mi12070794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Manvi P.K., Beckers M., Mohr B., Seide G., Gries T., Bunge C. Chapter 3. Polymer Fiber-Based Biocomposites for Medical Sensing Applications. Elsevier Inc.; Amsterdam, The Netherlands: 2019. [DOI] [Google Scholar]
- 139.Han S., Shin G. Biodegradable Optical Fiber in a Soft Optoelectronic Device for Wireless Optogenetic Applications. Coatings. 2020;10:1153. doi: 10.3390/coatings10121153. [DOI] [Google Scholar]
- 140.Raghunandhan R., Chen L.H., Long H.Y., Leam L.L., So P.L., Ning X., Chan C.C. Chemical Chitosan/PAA based fiber-optic interferometric sensor for heavy metal ions detection. Sens. Actuators B Chem. 2016;233:31–38. doi: 10.1016/j.snb.2016.04.020. [DOI] [Google Scholar]
- 141.Liang D., Yu W., Pang X., Huang P., Lin Y., Xin W., Zhang R., Tao H. Silk Fibroin—Based Wearable All—Fiber Multifunctional Sensor for Smart Clothing. Adv. Fiber Mater. 2022;4:873–884. doi: 10.1007/s42765-022-00150-x. [DOI] [Google Scholar]
- 142.Rabizah S., Hasbullah M., Haziq M., Akashah N., Abdul R., Scully P.J., Gardner P. Enhancing fibre optic sensor signals via gold nanoparticle-decorated agarose hydrogels. Opt. Mater. 2023;143:114247. doi: 10.1016/j.optmat.2023.114247. [DOI] [Google Scholar]
- 143.Tarar A.A., Mohammad U., Srivastava S.K. Wearable skin sensors and their challenges: A review of transdermal, optical, and mechanical sensors. Biosensors. 2020;10:56. doi: 10.3390/bios10060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jing J., An X., Luo Y., Chen L., Chu Z., Li K.H. A Compact Optical Pressure Sensor Based on a III-Nitride Photonic Chip with Nanosphere-Embedded PDMS. ACS Appl. Electron. Mater. 2021;3:1982–1987. doi: 10.1021/acsaelm.1c00130. [DOI] [Google Scholar]
- 145.Zhang H., Zhou X., Li X., Gong P., Zhang Y., Zhao Y. Recent Advancements of LSPR Fiber-Optic Biosensing: Combination Methods, Structure, and Prospects. Biosensors. 2023;13:405. doi: 10.3390/bios13030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li M., Singh R., Wang Y., Marques C., Zhang B., Kumar S. Advances in Novel Nanomaterial-Based Optical Fiber Biosensors—A Review. Biosensors. 2022;12:843. doi: 10.3390/bios12100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sreejith S., Ajayan J., Radhika J.M., Uma Reddy N.V., Manikandan M. Recent advances in nano biosensors: An overview. Measurement. 2024;236:115073. doi: 10.1016/j.measurement.2024.115073. [DOI] [Google Scholar]
- 148.Rahman B.M.A., Viphavakit C., Chitaree R., Ghosh S., Pathak A.K., Verma S., Sakda N. Optical Fiber, Nanomaterial, and THz-Metasurface-Mediated Nano-Biosensors: A Review. Biosensors. 2022;12:42. doi: 10.3390/bios12010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seena R., Paul S., Sudheer V.R. Hybrid Nano-Structured SPR Biosensors: A Novel Approach to Breast and Cervical Cancer Detection. Plasmonics. 2025 doi: 10.1007/s11468-025-02857-0. [DOI] [Google Scholar]
- 150.Medrano-Lopez J.A., Villalpando I., Salazar M.I., Torres-Torres C. Hierarchical Nanobiosensors at the End of the SARS-CoV-2 Pandemic. Biosensors. 2024;14:108. doi: 10.3390/bios14020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thanjavur N., Bugude L., Kim Y.J. Integration of Functional Materials in Photonic and Optoelectronic Technologies for Advanced Medical Diagnostics. Biosensors. 2025;15:38. doi: 10.3390/bios15010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Meena K.V., Ravi Sankar A. Biomedical Catheters with Integrated Miniature Piezoresistive Pressure Sensors: A Review. IEEE Sens. J. 2021;21:10241–10290. doi: 10.1109/JSEN.2021.3057222. [DOI] [Google Scholar]
- 153.Friedemann M., Barz S., Voigt S., Barz T., Melloh M., Müller A., Mehner J. In-Vivo Animal Trial of a Fiber-Optic Pressure Sensor Probe with Distributed Sensing Points for the Diagnosis of Lumbar Spinal Stenosis; Proceedings of the 9th World Congress on Electrical Engineering and Computer Systems and Science; London, UK. 3–5 August 2023; pp. 1–9. [DOI] [Google Scholar]
- 154.Gan L., Wang J., Xie L., Zhou Y. A High Precision Triaxial Force Sensor Based on Fiber Bragg Gratings for Catheter Ablation. IEEE Trans. Instrum. Meas. 2023;73:7001511. doi: 10.1109/TIM.2023.3342245. [DOI] [Google Scholar]
- 155.Sadek I., Biswas J., Abdulrazak B. Ballistocardiogram signal processing: A review. Health Inf. Sci. Syst. 2019;7:10. doi: 10.1007/s13755-019-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cibula E., Pevec S., Lenardic B., Pinet E., Donlagic D. Miniature all-glass robust pressure sensor. Opt. Express. 2009;17:5098. doi: 10.1364/OE.17.005098. [DOI] [PubMed] [Google Scholar]
- 157.Zhao Z., Chen J., Yang J., Jiang Q. Photonic sensor with radio frequency power detection for body pressure monitoring. Optoelectron. Lett. 2023;19:752–755. doi: 10.1007/s11801-023-3110-1. [DOI] [Google Scholar]
- 158.Yi L., Hou B., Liu X. Optical Integration in Wearable, Implantable and Swallowable Healthcare Devices. ACS Nano. 2023;17:19491–19501. doi: 10.1021/acsnano.3c04284. [DOI] [PubMed] [Google Scholar]
- 159.Fisher C., Harty J., Yee A., Li C.L., Komolibus K., Grygoryev K., Lu H., Burke R., Wilson B.C., Andersson-Engels S. Perspective on the integration of optical sensing into orthopedic surgical devices. J. Biomed. Opt. 2022;27:010601. doi: 10.1117/1.JBO.27.1.010601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li L., Li Y., Yang L., Fang F., Yan Z., Sun Q. Continuous and Accurate Blood Pressure Monitoring Based on Wearable Optical Fiber Wristband. IEEE Sens. J. 2021;21:3049–3057. doi: 10.1109/JSEN.2020.3027919. [DOI] [Google Scholar]
- 161.Ochoa M., Algorri J.F., Roldan-Varona P., Rodriguez-Cobo L., Lopez-Higuera J.M. Recent advances in biomedical photonic sensors: A focus on optical-fibre-based sensing. Sensors. 2021;21:6469. doi: 10.3390/s21196469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xavier M.S., Tawk C.D., Zolfagharian A., Pinskier J., Howard D., Young T., Lai J., Harrison S.M., Yong Y.K., Bodaghi M., et al. Soft Pneumatic Actuators: A Review of Design, Fabrication, Modeling, Sensing, Control and Applications. IEEE Access. 2022;10:59442–59485. doi: 10.1109/ACCESS.2022.3179589. [DOI] [Google Scholar]
- 163.Shen F., Ai M., Li Z., Lu X., Pang Y., Liu Z. Pressure measurement methods in microchannels: Advances and applications. Microfluid. Nanofluidics. 2021;25:39. doi: 10.1007/s10404-021-02435-w. [DOI] [Google Scholar]
- 164.Bills E. Risk management for IEC 60601-1 third edition. [(accessed on 1 March 2025)];Biomed. Instrum. Technol. 2006 40:390–392. doi: 10.2345/i0899-8205-40-5-390.1. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17078374. [DOI] [PubMed] [Google Scholar]
- 165.Tettey F., Parupelli S.K., Desai S. A Review of Biomedical Devices: Classification, Regulatory Guidelines, Human Factors, Software as a Medical Device, and Cybersecurity. Biomed. Mater. Devices. 2024;2:316–341. doi: 10.1007/s44174-023-00113-9. [DOI] [Google Scholar]
- 166.Wirges M., Funke A., Serno P., Knop K., Kleinebudde P. Development and in-line validation of a Process Analytical Technology to facilitate the scale up of coating processes. J. Pharm. Biomed. Anal. 2013;78–79:57–64. doi: 10.1016/j.jpba.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 167.The European Parliament and the Council of the European Union Regulation (EU) 2017/746 of the European parliament and of the council on in vitro diagnostic medical devices. Off. J. Eur. Union. 2017;5:117–176. [Google Scholar]
- 168.Cruz Rivera S., Torlinska B., Marston E., Denniston A.K., Oliver K., Hoare S., Calvert M.J. Advancing UK Regulatory Science Strategy in the Context of Global Regulation: A Stakeholder Survey. Ther. Innov. Regul. Sci. 2021;55:646–655. doi: 10.1007/s43441-021-00263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Phillips J.A. Polio: Another Cause for Global Concern? Workplace Health Saf. 2015;63:92. doi: 10.1177/2165079915576935. [DOI] [PubMed] [Google Scholar]
- 170.Becker S.H. Approved American National Standards. SMPTE J. 1991;100:852–855. doi: 10.5594/J02445. [DOI] [Google Scholar]
- 171.Alden A.E. Approved American National Standards. SMPTE J. 1981;90:415–464. doi: 10.5594/J01468. [DOI] [Google Scholar]
- 172.Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes. International Organization for Standardization; Geneva, Switzerland: 2016. [(accessed on 1 March 2025)]. Available online: https://www.iso.org/standard/59752.html. [Google Scholar]
- 173.Ulrich C.M., Demiris G., Kennedy R., Rothwell E. The ethics of sensor technology use in clinical research. Nurs. Outlook. 2020;68:720–726. doi: 10.1016/j.outlook.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 174.Bujugundla R.S., Pradhan H.S. Emerging technologies for Fiber-Optic Based Sensors in Biomedical Domain: A Review and Recent Developments. IEEE Trans. Instrum. Meas. 2024;73:7010332. doi: 10.1109/TIM.2024.3481569. [DOI] [Google Scholar]
- 175.Leal-Junior A., Silva J., Macedo L., Marchesi A., Morau S., Valentino J., Valentim F., Costa M. The Role of Optical Fiber Sensors in the New Generation of Healthcare Devices: A Review. Sens. Diagn. 2024;3:1135–1158. doi: 10.1039/D4SD00032C. [DOI] [Google Scholar]
- 176.Liang L., Xie F., Jin L., Yang B., Sun L., Guan B. Optical Microfiber Biomedical Sensors: Classification, Applications, and Future Perspectives. Adv. Sens. Res. 2025:2400185. doi: 10.1002/adsr.202400185. [DOI] [Google Scholar]
- 177.Liu H., Song X., Wang X., Wang S., Yao N., Li X., Fang W., Tong L., Zhang L. Optical Microfibers for Sensing Proximity and Contact in Human-Machine Interfaces. ACS Appl. Mater. Interfaces. 2022;14:14447–14454. doi: 10.1021/acsami.1c23716. [DOI] [PubMed] [Google Scholar]
- 178.Ueno A., Hu J., An S. AI for optical metasurface. npj Nanophotonics. 2024;1:36. doi: 10.1038/s44310-024-00037-2. [DOI] [Google Scholar]
- 179.González-León K., Delgado-Macuil R.J., Vertti-Cervantes B., Muñoz-Aguirre S., Castillo-Mixcóatl J., García-Juárez M., Montes-Narvaez O., Ramírez-Sánchez E., Beltrán-Pérez G. Application of support vector machine technique to optical fiber biosensors for neuroprotector (IL-10) detection in serum samples of murine model. Opt. Laser Technol. 2025;186:112629. doi: 10.1016/j.optlastec.2025.112629. [DOI] [Google Scholar]
- 180.Zha B., Wang Z., Ma L., Chen J., Wang H., Li X., Kumar S., Min R. Intelligent Wearable Photonic Sensing System for Remote Healthcare Monitoring Using Stretchable Elastomer Optical Fiber. IEEE Internet Things J. 2024;11:17317–17329. doi: 10.1109/JIOT.2024.3356574. [DOI] [Google Scholar]
- 181.Zhang L., Tong L. A bioinspired flexible optical sensor for force and orientation sensing. Opto-Electronic Adv. 2023;6:230051. doi: 10.29026/oea.2023.230051. [DOI] [Google Scholar]
- 182.Lyu C., Li P., Zhang J., Du Y. Fiber Optic Sensors in Tactile Sensing: A Review. IEEE Trans. Instrum. Meas. 2025;74:7001816. doi: 10.1109/TIM.2025.3527487. [DOI] [Google Scholar]
- 183.Jha R., Mishra P., Kumar S. Advancements in optical fiber-based wearable sensors for smart health monitoring. Biosens. Bioelectron. 2024;254:116232. doi: 10.1016/j.bios.2024.116232. [DOI] [PubMed] [Google Scholar]
- 184.Amjad A., Xian X. Optical sensors for transdermal biomarker detection: A review. Biosens. Bioelectron. 2025;267:116844. doi: 10.1016/j.bios.2024.116844. [DOI] [PubMed] [Google Scholar]