Abstract
Cells constituting the placental barrier secrete soluble factors that may participate in controlling human immunodeficiency virus type 1 (HIV-1) transmission from the mother to the fetus. In this study, we asked whether placental soluble factors (PSF) could limit cell-cell contact inducing HIV-1 production that occurs after inoculation of HIV-1-infected peripheral blood mononuclear cells (HIV-1+ PBMCs) onto trophoblast-derived BeWo cells grown as tight and polarized barriers in a two-chamber system. The activity of recombinant chemokines and cytokines expressed by placental tissue and of factors secreted by either early or term placentae of HIV-1-negative women, was analyzed. We identified chemokines (RANTES and MIP-1β) and cytokines (tumor necrosis factor alpha and interleukin-8) that decreased and increased, respectively, viral production in trophoblast barrier cells inoculated with HIV-1+ PBMCs. Unexpectedly, factors secreted by either early or term placentae of HIV-1-negative women enhanced viral production. Nevertheless, the same PSF did not favor infection of trophoblastic barriers with cell-free HIV-1 and strongly reduced viral production in PBMCs infected with cell-free HIV-1. Moreover, PSF contained chemokines (RANTES and MIP-1β) and a cytokine, leukemia inhibitory factor, exhibiting a strong anti-HIV-1 activity in our model of cell-to-cell infection. Together these data suggested that at the maternal interface the global activity of PSF is related to the synergistic action of several soluble factors with a balance in favor of an enhancing activity on the passage of viruses across the trophoblast barrier. This could explain the presence of viral sequences in trophoblasts in all placentae of HIV-1-infected women.
In all human immunodeficiency virus type 1 (HIV-1)-infected women with no preventive antiretroviral therapy during pregnancy, trophoblasts that constitute the first layer of the placental barrier in direct contact with the maternal blood contain viral sequences (23). Nevertheless, more than 90% of the children born from these women are protected against HIV-1 during pregnancy (16, 20), indicating that HIV-1 infection and spread through the placenta are tightly controlled. To determine whether the trophoblastic barrier contributes in limiting in utero transmission of HIV-1, we have developed a model of trophoblastic barrier through which cell-free HIV-1 does not pass (15). In this model, human trophoblast-derived BeWo cells form a tight and polarized cellular barrier. Polarized cells are not productively infected by HIV-1, unless HIV-1-infected (HIV-1+) peripheral blood mononuclear cells (PBMCs) are inoculated onto the apical pole, as described for nonpolarized trophoblasts (1, 8). Even in this case, a translocation of selected maternal HIV-1 quasispecies is observed across the trophoblastic barrier (15).
Additional levels of control have to take place at the materno-fetal interface to efficiently limit the transmission of HIV through the trophoblastic barrier and spread to underlying placental cells. Hence, several mechanisms have been proposed to explain the protection of the fetus against maternal HIV during pregnancy. Maternal antibodies (28) and CD4+ T lymphocytes (11, 18) might contribute in limiting in utero transmission of HIV-1. Placental cells secrete a vast array of soluble factors, including growth factors, soluble receptors, and major histocompatibility complex (MHC) class I molecules, such as soluble HLA-G, cytokines, chemokines, and hormones, as well as factors that have not been identified yet (4, 22). These soluble factors most likely exert a concerted activity to create a suppressive environment at the materno-fetal interface, allowing the embryo's semiallogenic tissues to be tolerated by the mother's immune system (22) and the fetus to develop properly. Among these factors, chemokines and cytokines with a known potent anti-HIV-1 activity are detected (4, 6, 9, 24, 26, 27). However, cytokines, such as inflammatory cytokines, the activity of which facilitates HIV infection and replication in different cell types, are also expressed during the various phases of pregnancy.
Indirect evidence indicates that, indeed, placental cytokines and chemokines may influence HIV replication in placental cells, particularly in trophoblasts. In women in whom viral load is controlled with antiretroviral therapy, trophoblasts bear no detectable level of HIV sequences but express less inflammatory cytokine and chemokine mRNAs than trophoblasts isolated from placentae of HIV-1-negative women (24). In contrast, expression of inflammatory cytokines is elevated in trophoblasts with a high level of HIV transcripts (18). Along with these findings, interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) were found to stimulate the transcriptional activity of HIV long terminal repeats expressed transiently in isolated trophoblast cells (33) or in trophoblastic cell lines infected with HIV-1 particles pseudotyped with HIV-1 envelope glycoproteins (32), whereas alpha interferon (IFN-α) and IFN-β suppress the transcription driven from the viral promoter (33). Moreover, a correlation between in utero transmission of HIV-1 and expression of Th2 cytokines (IL-4 and IL-10) and leukemia inhibitory factor (LIF) has been reported (3, 26). However, whether these soluble factors, alone or in association, could limit infection of the first placental barrier and/or HIV-1 spread remains to be elucidated.
In this study, we asked whether cytokines and chemokines secreted by placental tissue and supernatants of placental explants containing the same cytokines and chemokines could control infection of trophoblastic barriers inoculated with HIV-1+ PBMCs.
MATERIALS AND METHODS
Reagents and antibodies.
The study used the factor recombinant human regulated upon activation normal T-cell-expressed and secreted (RANTES); macrophage inflammatory protein 1β (MIP-1β); stromal cell-derived factor-1 (SDF-1α/β); IL-16; IL-8; TNF-α; neutralizing anti-MIP-1α, -MIP-1β, and -RANTES antibodies; and control normal goat serum and human LIF soluble receptor (rhLIF-SR) (R&D, United Kingdom). Anti-CD54-phycoerythrin (PE) (ICAM-1; Becton Dickinson), isotype-matched, PE-labeled, control antibodies (Pharmingen, Beckman Coulter), anti-CD106 (VCAM-1) (Beckman Coulter) or mouse immunoglobulin G1 (IgG1) (Beckman Coulter) and anti-CD11b-PE and anti-CD11c-PE (Beckman Coulter) were used at a final concentration of 5 to 10 μg/ml. Goat anti-mouse IgG [F(ab′)2-PE] (DAKO, France) (5 μg/ml) was used as the secondary antibody.
Placental soluble factors (PSF) were 24-h culture supernatants of placental explants prepared as previously described (24) in accordance with French ethics guidelines. Briefly, isolated villi (3 g in 20 ml) from early or term placentae were incubated for 24 h in RPMI medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 1% penicillin-streptomycin, and 1% sodium bicarbonate (provided by GIBCO, BRL). Supernatants were harvested, submitted to centrifugation, aliquoted, and frozen at −80°C until use. Samples were used only once. Placentae of nine HIV-1-negative women were available for this study.
Cells.
The human choriocarcinoma BeWo cell line (ATCC CCL98) was maintained in 5% CO2-95% air at 37°C in medium (Dulbecco's modified Eagle medium [25 mM glucose], 20% heat-inactivated FCS, 20 mM glutamine, 50 IU penicillin/ml, and 50 μg/ml streptomycin [GIBCO BRL, Gaithersburg, MD]). PBMCs were obtained after Ficoll-Paque centrifugation of buffy coats (Etablissement Français du Sang, Rungis, France). PBMCs from seven different HIV-1- and hepatitis C virus-negative volunteers were used. PBMCs were activated with 100 pg/ml phytohemagglutinin (PHA) and cultivated in RPMI 1640, 10% FCS, 20 mM glutamine, antibiotics, and 5 U IL-2/ml.
Virus and HIV-infected PBMCs.
Two second passage stocks of HIV-1 A204 with a 50% tissue culture infective doses (TCID50) of 104 to 3.2 × 104/ml, respectively, were used. A204 is a clinical isolate containing X4 and R5 viruses, originally isolated from a mother whose child had been infected in utero (29). HIV-1+ PBMCs were obtained by inoculating 100 TCID50 of HIV-1 A204 onto 106 PHA-IL-2-activated PBMCs and were used at the peak of viral production (at day 7 postinfection), as determined by measuring reverse transcriptase activity in the supernatant of HIV-infected cells just before each experiment.
Infection of trophoblastic barriers.
Tight and polarized trophoblastic barriers were grown in a two-chamber system, as previously described (15). Under these conditions of culture, BeWo cells form tight junctions and express both CXCR4 and CCR5 (15). To reconstitute the placental soluble environment, barriers were incubated overnight in medium (RPMI, 10% FCS, 20 mM glutamine, antibiotics) containing or not containing (control) PSF (in a proportion of 1:1 by volume or as indicated) or increasing doses of human recombinant cytokine or chemokine. In neutralization experiments, trophoblastic barriers were incubated in the presence of medium containing PSF, first incubated for 30 min at 37°C in the presence of either control antibodies, neutralizing antibodies, or rh-LIFsR. Infections were performed in triplicates, in the presence or absence of PSF (± neutralizing reagents). HIV-1+ PBMCs (2 × 105 or 2 × 106 cells) or cell-free HIV-1 (100 TCID50) was inoculated onto the apical pole of trophoblastic barriers for 3 h at 37°C. Then, cell-free HIV or HIV-infected cells were removed. For this purpose, the apical surface of BeWo cell monolayers was carefully washed five times, using 4× 500 μl of prewarmed medium (RPMI 1640 medium plus 10% FCS, 20 mM glutamine, and antibiotics) and 1× 500 μl of prewarmed (37°C) trypsin-EDTA. Concurrently and separately, the basolateral surface of each trophoblastic barrier was washed by incubating inserts into four consecutive wells containing 1.5 ml of medium (RPMI 1640 medium plus 10% FCS, 20 mM glutamine, and antibiotics) and in one well containing 1.5 ml of prewarmed trypsin-EDTA. After these five consecutive washes of apical and of basolateral poles, barriers were cultured in new wells containing indicator cells (1 × 106 to 1.5 × 106 PBMCs) plated into the lower chamber. The amount of virus produced by BeWo cells was amplified by these indicator cells and quantified by measuring HIV-1 p24 antigen in the supernatant using a commercial kit (Coulter, France). The barriers' integrity was determined before each experiment by measuring the transepithelial resistance for tightness, as previously described (15), and at the end of each experiment, by quantifying the passage of a dye, Blue Evans, across trophoblastic layers for permeability. Blue Evans was added (or not) into the apical supernatant, and cellular barriers were incubated for 4 to 6 h at 37°C. The absence (or presence) of the dye in the basolateral supernatant was measured by optical density at λ = 650 nm. Unless trophoblastic barriers were damaged (in which case, data were excluded), data are given as a mean of 3 determinations ± standard error of the mean (SEM). Each experiment was performed two to seven times.
Flow cytometry analysis of surface molecule expression.
BeWo cells grown as tight and polarized monolayers (2 × 105 cells per filter) and incubated overnight in the presence or the absence of PSF were washed using PBS containing 5% fetal bovine serum (FBS). Cells were then mechanically detached from polycarbonate membranes and incubated in the presence of human Igs (Tegeline; Institut Français du Sang, France) for 30 min at 4°C to block nonspecific binding. After one washing step, cells were stained either with directly labeled antibodies or with unlabeled first antibody, added at the final concentration of 0.5 or 10 μg/ml, and a fluorescent secondary antibody, goat anti-mouse IgG (F(ab[prime])2-PE. Isotype control monoclonal antibodies were used at the same concentration as the primary antibody. After two washing steps, cells were analyzed by flow cytometry. Ten thousand events were analyzed for each sample.
Adhesion assay.
PHA-IL-2-activated noninfected (HIV-1 negative) and infected (HIV-1+) PBMCs (2 × 106 cells/ml) from the same donor were stained with 0.5 μg/ml (10 mM) of Cell Tracker-Green 5-chloro-methylfluorescein diacetate (CMFDA) (Molecular Probes, Eugene, Oregon), as recommended by the manufacturer. HIV-1-negative and HIV-1+ PBMCs were kept unstained, as a control for background fluorescence. Cells were then inoculated onto BeWo cell layers that had been incubated or not overnight in the presence of supernatant of placental explants. After 3 h of contact, nonadherent cells were removed by washing BeWo monolayers with 0.5 ml of PBS plus 5% FBS. Cells were detached mechanically by pipetting them up and down and analyzed by flow cytometry using a flow cytometer (XL2 EPICS Coulter). PBMCs and BeWo cells were separated by size. Nonadherent PBMCs were much smaller and fluorescent and thus were easily distinguishable from BeWo cells and from PBMCs adhering to BeWo cells. Twenty thousand events were analyzed for each sample.
Statistical analysis.
Statistical significance was determined using a nonparametric Mann-Whitney test. P values of <0.05 were regarded as significant.
RESULTS
Productive infection of BeWo cells grown as a tight and polarized trophoblastic barrier can be increased or decreased by cytokines and chemokines expressed by the placenta.
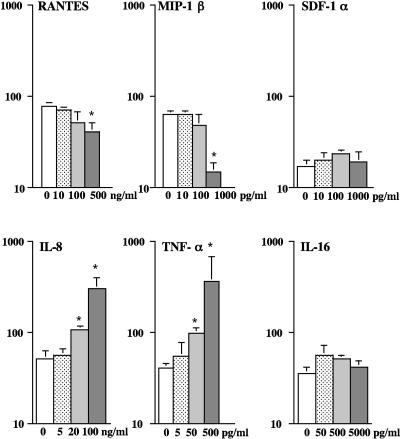
We first tested the activity of recombinant cytokines and chemokines in terms of the capacity of trophoblastic barriers to be infected with cell-associated HIV-1. All were expressed by placental tissue (4, 6, 9, 24) and exhibited either a known activity against HIV-1 (RANTES, MIP-1β, SDF-1, and IL-16) (2, 5, 21) or an activity facilitating HIV-1 replication (IL-8 and TNF-α) (17, 18, 20). Doses were chosen to correspond to the amounts of these cytokines and chemokines in PSF (9, 24) or for SDF-1, reported to be active on HIV-1 entry (21). The results showed that trophoblastic barriers incubated in the presence of RANTES or of MIP-1β, before and during inoculation of HIV-1+ PBMCs, produced significantly (P < 0.05) less infectious viruses than untreated barriers (Fig. 1, upper panels). SDF-1 or IL-16 had no detectable activity (Fig. 1, upper and lower panels), whereas IL-8 or TNF-α significantly increased (P < 0.05) the amount of virus produced, in a dose-dependent manner (Fig. 1, lower panels). Altogether, these data show that, individually, certain cytokines and chemokines expressed and secreted by placental tissue can reduce or increase HIV-1 production in trophoblastic barriers.
FIG. 1.
Cytokines and chemokines expressed by placental tissue can reduce or increase viral production in trophoblastic barriers inoculated with HIV-1-infected PBMCs. BeWo cells grown as tight and polarized trophoblastic layers were incubated overnight in the presence or absence (control) of medium containing increasing doses of recombinant human RANTES, MIP-1β, SDF-1-α, IL-8, TNF-α, or IL-16. BeWo cells were then inoculated for 3 h with HIV-1-infected PBMCs in medium containing or not each of the chemokines or cytokines. After extensive washes, trophoblastic barriers were cultured for 5 days in new wells containing indicator cells. Data represent the amount of HIV-1 p24 antigen (log) detected in the supernatant of indicator cells at day 7 postinfection, are given as the mean ± SEM of duplicates or triplicates, and are representative of two independent experiments. *, P < 0.05, Mann-Whitney test.
PSF enhanced the production of HIV-1 in trophoblastic barriers inoculated with HIV-1-infected PBMCs.
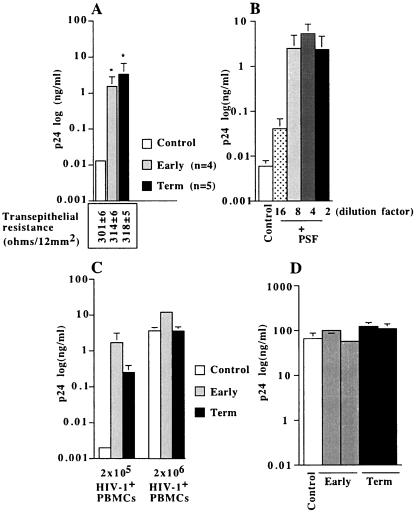
We then determined whether PSF that contain all these cytokines and chemokines (9, 24) were active on the productive infection of trophoblastic barriers. The results showed that PSF, from either early (n = 4) or term placentae (n = 5) of HIV-1-negative women, increased the amount of infectious viruses produced by trophoblastic barriers, compared to that in medium without PSF (Fig. 2A). This activity was dose dependent (Fig. 2B) and detected under a nonsaturating condition of infection (2 × 105 HIV-1-infected PBMCs) (Fig. 2C). As a control, we verified that HIV-1-infected PBMCs incubated in the presence of PSF for 3 h, (thus for a length of time corresponding to the time of inoculation onto trophoblastic barriers in the presence of PSF) did not produce more virus than untreated HIV-1-infected PBMCs (Fig. 2D). PSF neither altered the transepithelial resistance of trophoblastic barriers (Fig. 2A) nor allowed the passage of Blue Evans (not shown) across barriers, demonstrating that PSF did not alter their integrity. Altogether, these data showed that PSF increased viral production in trophoblastic barriers inoculated with HIV-1-infected PBMCs.
FIG. 2.
Enhancing activity of soluble factors secreted by placental tissue obtained from early or term placentae of HIV-1-negative women on viral production in trophoblastic barriers inoculated with HIV-1-infected PBMCs. BeWo cells grown as tight and polarized monolayers were incubated overnight in culture medium containing either nothing (Control, white histograms) or soluble factors secreted by placental explants (PSF) from early (gray histograms) or term (black histograms) placentae (dilution, 1:3 by volume or as indicated [B]). BeWo cells were then inoculated for 3 h with 2 × 105 or 2 × 106 HIV-1-infected-PBMCs, in the presence or the absence of PSF. After removal of HIV-1-infected PBMCs by five extensive washes, trophoblastic barriers were cultured in new wells containing fresh indicator cells. In parallel, HIV-1-infected PBMCs were incubated or not (control) for a duration of 3 h with PSF (n = 2 from early placenta and n = 2 from late placenta), and the amount of virus produced by the cells was determined 5 days later. (A) PSF secreted by early (n = 4) or term (n = 5) placentae increased HIV-1 production. The transepithelial resistance of trophoblastic barriers incubated overnight in the presence or absence of PSF from early or term placentae is indicated under each corresponding bar. (B) Dose-dependent activity of PSF secreted by an early placenta. (C) Enhancing activity of PSF under nonsaturating (2 × 105 HIV-1+ PBMCs) and saturating (2 × 106 HIV-1+ PBMCs) conditions of infection. Data represent the amount of HIV-1 p24 antigen (log) produced by BeWo cells and amplified in indicator cells detected in the supernatant of indicator cells at day 7 postinfection, are given as the mean ± SEM of duplicates or triplicates, and are representative of two to seven independent experiments. (D) PSF did not increase the amount of virus produced by HIV-1+ PBMCs. Data represent the amount of HIV-1 p24 antigen (log) produced by HIV-1+ PBMCs at day 5 postincubation and are given as the mean ± SEM of triplicates. *, P < 0.05, Mann-Whitney test.
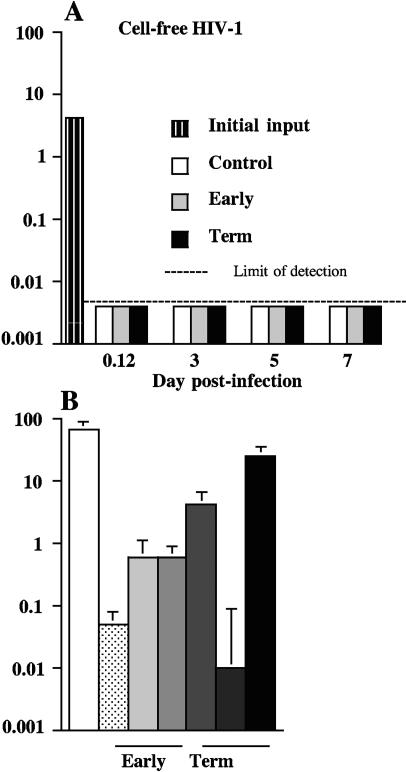
PSF do not change the resistance of trophoblastic barriers to cell-free HIV infection and attenuate the susceptibility of PBMCs to infection with cell-free HIV-1.
It is now well established that infection of primary trophoblasts or of trophoblast-derived cell lines with cell-free HIV-1 is poorly or not at all productive (7, 15, 32). Our results showing that PSF increased viral production in these cells inoculated with HIV-1-infected PBMCs suggested that PSF could also alter the resistance of BeWo cells to cell-free virus. To test this hypothesis, trophoblastic monolayers were incubated in the presence of PSF and then inoculated with cell-free HIV-1 A204 at a dose of 100 TCID50 (5 to 10 ng of p24) for 3 h. The amounts of virus that had crossed barriers during these 3 h (Fig. 3A, 0.12 h postinfection) or that were produced in BeWo cells at different times postinfection, after removal of the inoculum, extensive washes, and culture in new wells containing fresh indicator cells, were measured. In parallel, PHA-IL-2-activated PBMCs were incubated overnight in the presence or the absence of PSF and then inoculated with cell-free HIV-1 (100 TCID50), as a control for infection. The results showed that PSF did not facilitate the passage of cell-free HIV-1 across tight and polarized BeWo cells (Fig. 3A), confirming that trophoblast BeWo cells were not productively infected with cell-free HIV-1 and that no viral particle crossed barriers whether PSF were added or not to cell cultures. Thus, PSF did not alter the integrity of monolayers. Moreover, no virus was produced in BeWo cells inoculated with cell-free virus, whether PSF were incubated or not with PSF (Fig. 3A). In PHA-IL-2 activated PBMCs, HIV-1 204 replicated (Fig. 3B). In these cells, all PSF from early or term placentae (n = 3 each) but one induced a 2- to 3-log decrease in the amount of virus produced (Fig. 3B). Altogether, these data show that in cells that are resistant to cell-free HIV-1 infection, namely in polarized trophoblast-derived BeWo cells, PSF did not release the restriction. The data further show that during acute infection of cells permissive for HIV-1 replication, PSF exhibit an anti-HIV-1 activity, as previously described (14, 26, 30).
FIG. 3.
Activity of soluble factors secreted by placental tissue obtained from early or term placentae of HIV-1-negative women on viral production in trophoblastic barriers and PHA-IL-2-activated PBMCs inoculated with cell-free HIV-1. (A) BeWo cells grown as tight and polarized monolayers or (B) PHA-IL-2-activated-PBMCs were incubated overnight in culture medium containing, as indicated, either nothing (Control; white histograms) or soluble factors secreted by placental explants (PSF) from early (dots and gray histograms; n = 3) or term (dark gray to black histograms; n = 3) placentae (dilution, 1:3 by volume). Cells were then inoculated for 3 h with cell-free HIV-1 (100 TCID50/106 cells), freshly diluted into medium containing or not PSF. After removal of cell-free HIV-1 by five extensive washes, trophoblastic barriers were cultured in new wells containing fresh indicator cells. Data represent the amount of HIV-1 p24 antigen (log) produced by BeWo cells at various times postinoculation and by HIV-1+ PBMCs at day 7 postinfection. Data are given as the mean ± SEM of triplicates and are representative of three independent experiments.
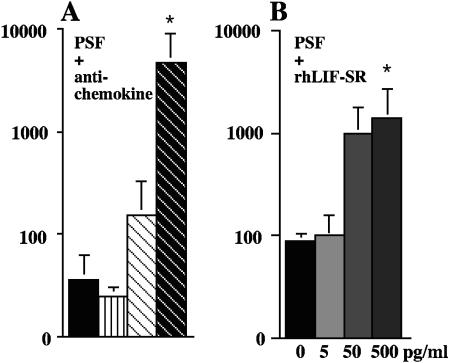
PSF contain factors decreasing the production of HIV-1 in trophoblastic barriers inoculated with HIV-1-infected PBMCs.
We then asked whether in PSF, cytokines and chemokines reported to have an anti-HIV-1 activity and present in detectable amounts were active in our model of cell-to-cell infection. Thus, PSF were incubated with neutralizing reagents specific for RANTES, MIP-1α, MIP-1β, or LIF, a cytokine the expression of which correlates with the protection against in utero transmission of HIV-1 (26). The results showed that neutralization of RANTES and to some extent of MIP-1β, but not of MIP-1α, in PSF resulted in an enhancement in viral production, as compared to viral production in barriers incubated with PSF mixed with control antibodies (Fig. 4A). Similarly, addition of increasing doses of rhLIF-SR into PSF progressively enhanced viral production (Fig. 4B). These data demonstrated that PSF contained factors that actively reduce cell-to-cell contact-inducing HIV-1 production in trophoblast-derived cells.
FIG. 4.
Soluble factors secreted by placental tissue obtained from early or term placentae of HIV-1-negative women contain factors exhibiting an anti-HIV-1 activity on viral production in trophoblastic barriers inoculated with HIV-1-infected PBMCs. BeWo cells grown as tight and polarized monolayers were incubated overnight in culture medium containing soluble factors secreted by placental explants (PSF) from term placentae (dilution, 1:4 by volume) mixed with (A) either normal goat serum (goat Igs, 55 μg/ml), neutralizing anti-MIP-1α (αMIP-1α; 25 μg/ml), anti-MIP-1β (αMIP-1β; 25 μg/ml), or anti-RANTES (αRANTES; 15 μg/ml). (B) increasing doses of rhLIF-SR. Barriers were then inoculated for 3 h with either 2 × 105 or 2 × 106 HIV-1-infected PBMCs, freshly diluted into media containing or not PSF mixed or not (control) with neutralizing reagents. After removal of HIV-1-infected PBMCs by five extensive washes, trophoblastic barriers were cultured in new wells containing fresh indicator cells. Data represent the amount of HIV-1 p24 antigen (log) produced by BeWo cells and amplified in indicator cells detected in the supernatant of indicator cells at day 7 postinfection, are given as the mean ± SEM of duplicates or triplicates, and are representative of two independent experiments. *, P < 0.05, Mann-Whitney test.
PSF increases neither adhesion molecule expression nor binding of HIV-1-infected PBMCs to BeWo cells.
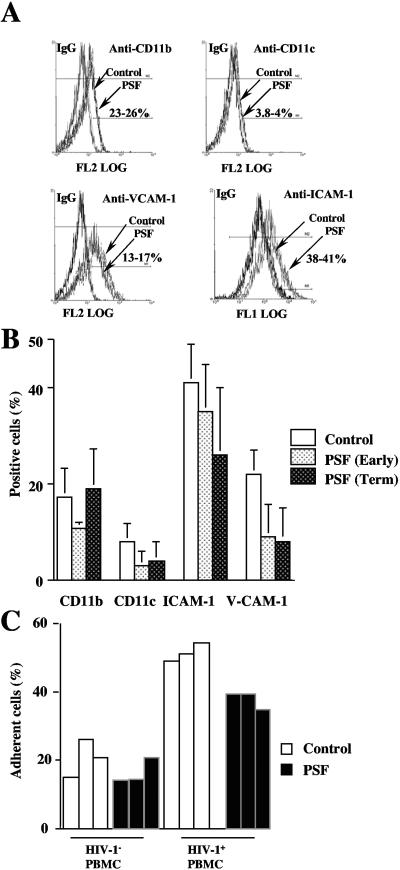
The present results showing that PSF increased the amount of infectious virus produced by BeWo cells inoculated with cell-associated HIV-1 suggested that PSF could facilitate contacts between HIV-1-infected PBMCs and BeWo cells and consequently cell-to-cell transmission of HIV-1. From HIV-1-infected PBMCs to another trophoblast cell line, JAR, the transmission of virus and binding of HIV-1-infected PBMCs to these cells involve LFA-1-ICAM-1 (CD11a-CD18) but neither LFA-1-ICAM-2, LFA-1-ICAM-3, nor VCAM-1-VLA-4 interaction (1). To determine whether PSF could facilitate contacts between HIV-1-infected PBMCs and BeWo cells, we therefore analyzed the activity of PSF on cell surface expression of adhesion molecules ICAM-1 and V-CAM-1 and of two other control cell surface antigens, CD11b and CD11c. The capacity of HIV-1-infected PBMCs to bind to BeWo cells was also determined. Flow cytometry analysis showed that expression of CD11b was similar whether or not BeWo cells were incubated with PSF, whereas cell surface expression of CD11c was almost undetectable (Fig. 5 A and B). Expression of ICAM-1 and V-CAM-1 was not increased but slightly decreased, especially in BeWo cells treated with PSF expressed by term placenta (Fig. 5A and B).
FIG. 5.
Soluble factors secreted by placental tissue obtained from early or term placentae of HIV-1-negative women modify neither cell surface expression of ICAM-1 nor binding of HIV-1-infected PBMCs to polarized BeWo cells. BeWo cells grown as a tight and polarized monolayer were incubated overnight in the presence (gray and black histograms) or the absence (Control; white histogram) of media containing soluble factors secreted by placental explants from early and term placentae. (A and B) Cells were detached mechanically from their polycarbonate support; immunostained using antibodies specific for either CD11b, CD11c (control), ICAM-1, or V-CAM-1; and analyzed by flow cytometry. In panel A, data represent fluorescence profiles of 10,000 cells; numbers represent the percentage of specific labeling obtained in four independent experiments. (B) Data obtained by flow cytometry and illustrated in panel A. (C) CMFDA-labeled noninfected (HIV-1− PBMC) and HIV-1-infected PBMCs (HIV-1+ PBMC) were inoculated for 3 h onto the apical pole of BeWo monolayers. After three washing steps, cells were analyzed by flow cytometry. Data represent the number of CMFDA-labeled PBMCs binding to BeWo cells that were distinguishable from unbound CMFDA-labeled PBMCs by size. Data are representative of three independent experiments.
Binding analysis showed that the capacity of noninfected PBMCs to bind to polarized BeWo cells was similar whether trophoblasts were incubated in the presence or the absence of PSF (Fig. 5C). As expected, the percentage of adherent cells was increased when HIV-1-infected PBMCs were inoculated onto polarized BeWo cells. Remarkably, adhesion of HIV-1-infected cells to BeWo cells was not increased and even slightly decreased when BeWo cells were incubated in the presence of PSF (Fig. 5C). Together, these results indicated that PSF increased neither the expression of adhesion molecules nor the number of HIV-1-infected PBMCs bound to BeWo cells.
DISCUSSION
In this study, we have shown that cell-to-cell contact-induced HIV-1 production in BeWo cells grown as a tight and polarized cellular monolayer is enhanced in the presence of human recombinant TNF-α or IL-8. Interestingly, the active doses of these inflammatory cytokines correspond to amounts of IL-8 and of TNF-α detected in placentae of HIV-1-infected women (18), in particular in women suffering coinfections, such as infection with Plasmodium falciparum (31), and for whom the risk of mother-to-child HIV-1 infection in utero is increased (25).
Similarly, we found that PSF from normal pregnancy that do contain both factors enhancing and factors reducing HIV-1 infection (18, 24) increase HIV-1 production in trophoblast-derived BeWo cells inoculated with HIV-1-infected PBMCs. Nevertheless, PSF do not modify the low or lack of permissiveness of these cells to cell-free HIV-1 infection. These data demonstrate that even in the presence of factors increasing HIV-1 production, signals induced by cell-to-cell contact are absolutely required for trophoblast-derived BeWo cells to be productively infected. This is consistent with previous studies indicating that (i) the initial transmission of virus from HIV-1-infected cells to trophoblasts requires appropriate signaling through adhesion molecule LFA-1/ICAM-1 (1, 32) and (ii) the rate of HIV-1 replication could depend on additional signals provided by cytokines present in the environment (18, 32).
We show here that the presence of RANTES, MIP-1β, and LIF in the environment restricts the amount of virus produced by trophoblast-derived cells even in the presence of factors activating HIV-1 production. Both the initial transmission of virus and the first azidothymidine (AZT)-sensitive phase of HIV-1 replication, but not the second round of HIV-1 RNA synthesis, which is not sensitive to AZT or to neutralizing antibody (11, 13), could be limited by these chemokines and by LIF. In agreement with this hypothesis, it is now well established that binding of chemokines on CCR5 prevents HIV-1 entry through CCR5 (5), whereas LIF inhibits HIV-1 replication at a step after entry and before retrotranscription (26). Nevertheless, the present results indicate that in the presence of a mix of factors enhancing and inhibiting HIV-1, factors enhancing HIV are more potent and overcome the activity of factors with an anti-HIV-1 activity on cell-to-cell contact-induced HIV-1 production in BeWo cells. Moreover, the present results showing that PSF increases neither ICAM-1 expression nor adherence of HIV-1-infected PBMCs to BeWo cells indicate that this increase in viral production is not related to an increase in the number of infected cells interacting with BeWo cells and eventually fusing with trophoblasts. Rather, we suspect that this increase in viral production might reflect an increase in the later phase of HIV-1 replication which is not sensitive to AZT (11, 13). Because the active doses of recombinant TNF-α and IL-8 were about 10 times higher than the doses of the same cytokines in the PSF we used (9), it is likely that the enhancing activity of PSF results from the synergistic activity of several factors, which may include those of IL-8 and TNF-α.
It remains possible that in HIV-1-infected women, an increase, even weak, in the secretion by placental cells of several factors exhibiting an anti-HIV-1 activity that may be induced upon exposure of placental cells to HIV-1 present in the maternal blood could confer to PSF the capacity to reduce infection of trophoblasts. Although speculative, this hypothesis is consistent with several studies showing that in HIV-1-infected women with no therapy, placental expression of several factors, including those of LIF, is increased and correlates with protection of the fetus (3, 26). Furthermore, the present data showing that PBMCs incubated overnight with PSF and then inoculated with HIV-1 produced 100 to 1,000 times less virus than untreated PBMCs further support a role of PSF in controlling spreading of HIV-1 through the placenta, after passage across the trophoblastic barrier. We addressed the question of whether this anti-HIV-1 activity was related to IFN-like activity in PSF since previous studies demonstrated that placental cells secreted this antiviral cytokine (19, 33). Thus, the level of secretion and the mRNA content of several forms of IFN in both placental tissue and PSF were evaluated. The results of this parallel work aimed at defining ex vivo cytokine profile within the placental environment (article in preparation) clearly indicate that no IFN-α or -γ is detected in the samples of PSF we used.
In summary, this study highlights the importance of cellular activation induced after cell-to-cell contact as a potent mechanism of HIV-1 dissemination and shows that cytokines and chemokines can regulate the amount of virus produced thereupon. The global activity of PSF from HIV-1-negative women, which includes those of several chemokines and cytokines interfering with HIV-1 infection, increased this viral production. We do not exclude that in PSF derived from HIV-1-infected women, an opposite activity might be observed. Whether under conditions of infection and/or therapy an inverse balance might be observed remains to be determined.
Acknowledgments
This work was funded by grants from ANRS and SIDACTION to E.M. and F.B.S. M.D. and A.F. were supported by a postdoctoral fellowship from SIDACTION, and G.D. was supported by a fellowship (SeCyT-UNCPBA, Argentina) followed by a fellowship from ANRS.
We thank the staff of Hôpital Antoine Béclère in Clamart for providing placentae.
REFERENCES
- 1.Arias, R. A., L. D. Munoz, and M. A. Munoz-Fernandez. 2003. Transmission of HIV-1 infection between trophoblast placental cells and T-cells takes place via an LFA-1-mediated cell to cell contact. Virology 307:266-277. [DOI] [PubMed] [Google Scholar]
- 2.Baier, M., A. Werner, N. Bannert, K. Metzner, and R. Kurth. 1995. HIV suppression by interleukin-16. Nature 378:563. [DOI] [PubMed] [Google Scholar]
- 3.Behbahani, H., E. Popek, P. Garcia, J. Andersson, A. L. Spetz, A. Landay, Z. Flener, and B. K. Patterson. 2000. Up-regulation of CCR5 expression in the placenta is associated with human immunodeficiency virus-1 vertical transmission. Am. J. Pathol. 157:1811-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaouat, G., S. Zourbas, S. Ostojic, G. Lappree-Delage, S. Dubanchet, N. Ledee, and J. Martal. 2002. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. J. Reprod. Immunol. 53:241-256. [DOI] [PubMed] [Google Scholar]
- 5.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 6.Coulomb-L'Hermine, A., D. Emilie, I. Durand-Gasselin, P. Galanaud, and G. Chaouat. 2000. SDF-1 production by placental cells: a potential mechanism of inhibition of mother-to-fetus HIV transmission. AIDS Res. Hum. Retrovir. 16:1097-1098. [DOI] [PubMed] [Google Scholar]
- 7.Dolcini, G., M. Derrien, G. Chaouat, F. Barre-Sinoussi, and E. Menu. 2003. Cell-free HIV type 1 infection is restricted in the human trophoblast choriocarcinoma BeWo cell line, even with expression of CD4, CXCR4 and CCR5. AIDS Res. Hum. Retrovir. 19:857-864. [DOI] [PubMed] [Google Scholar]
- 8.Douglas, G. C., G. N. Fry, T. Thirkill, E. Holmes, H. Hakim, M. Jennings, and B. F. King. 1991. Cell-mediated infection of human placental trophoblast with HIV in vitro. AIDS Res. Hum. Retrovir. 7:735-740. [DOI] [PubMed] [Google Scholar]
- 9.Faye, A., S. Pornprasert, G. Dolcini, P. Ave, J. Taïeb, J.-L. Taupin, M. Derrien, M. Huerre, F. Barré-Sinoussi, G. Chaouat, and E. Menu. 2005. Evaluation of the placental environment with a new in vitro model of histocultures of early and term placentae: determination of cytokine and chemokine expression profiles. Placenta. 26:262-267. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Gupta, P., R. Balachandran, M. Ho, A. Enrico, and C. Rinaldo. 1989. Cell-to-cell transmission of human immunodeficiency virus type 1 in the presence of azidothymidine and neutralizing antibody. J. Virol. 63:2361-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Kok, T., P. Li, and C. Burrell. 1993. Cell-to-cell transmission of human immunodeficiency virus infection induces two distinct phases of viral RNA expression under separate regulatory control. J. Gen. Virol. 74:33-38. [DOI] [PubMed] [Google Scholar]
- 14.Kondapi, A. K., M. A. Hafiz, and T. Sivaram. 2002. Anti-HIV activity of a glycoprotein from first trimester placental tissue. Antivir. Res. 54:47-57. [DOI] [PubMed] [Google Scholar]
- 15.Lagaye, S., M. Derrien, E. Menu, C. Coïto, E. Tresoldi, P. Mauclère, G. Scarlatti, G. Chaouat, F. Barré-Sinoussi, M. Bomsel, and European Network for the Study of In Utero Transmission of HIV-1. 2001. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblast barrier by both transcytosis and infection. J. Virol. 75:4780-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lallemant, M., G. Jourdain, S. Le Coeur, S. Kim, S. Koetsawang, A. M. Comeau, W. Phoolcharoen, M. Essex, K. McIntosh, V. Vithayasai et al. 2000. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 343:982-991. [DOI] [PubMed] [Google Scholar]
- 17.Lane, B. R., K. Lore, P. J. Bock, J. Andersson, M. J. Coffey, R. M. Strieter, and D. M. Markovitz. 2001. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J. Virol. 75:8195-8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, B.-N., N. Ordonez, E. J. Popek, J.-G. Lu, A. Helfgott, N. Eriksen, H. Hammill, C. Kozinetz, M. Doyle, M. Kline, C. Langston, W. T. Shearer, and J. M. Reuben. 1997. Inflammatory cytokine expression is correlated with the level of human immunodeficiency virus (HIV) transcripts in HIV-infected placental trophoblastic cells. J. Virol. 71:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, B. N., H. Hammill, E. J. Popek, S. Cron, C. Kozinetz, M. Paul, W. T. Shearer, and J. M. Reuben. 2001. Production of interferons and beta-chemokines by placental trophoblasts of HIV-1-infected women. Infect. Dis. Obstet. Gynecol. 9:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroy, V., J. M. Karon, A. Alioum, E. R. Ekpini, N. Meda, A. E. Greenberg, P. Msellati, M. Hudgens, F. Dabis, S. Z. Wiktor et al. 2002. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS 16:631-641. [DOI] [PubMed] [Google Scholar]
- 21.Maréchal, V., F. Arenzana-Seisdedos, J.-M. Heard, and O. Schwartz. 1999. Opposite effects of SDF-1 on human immunodeficiency virus type 1 replication. J. Virol. 73:3608-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellor, A. L., and D. H. Munn. 2001. Extinguishing maternal immune responses during pregnancy: implications for immunosuppression. Semin. Immunol. 13:213-218. [DOI] [PubMed] [Google Scholar]
- 23.Menu, E., F. X. Mbopi-Keou, S. Lagaye, S. Pissard, P. Mauclere, G. Scarlatti, J. Martin, M. Goossens, G. Chaouat, F. Barre-Sinoussi et al.. 1999. Selection of maternal human immunodeficiency virus type 1 variants in human placenta. J. Infect. Dis. 179:44-51. [DOI] [PubMed] [Google Scholar]
- 24.Moussa, M., P. Roques, N. Fievet, E. Menu, J. G. Maldonado-Estrada, J. Brunerie, R. Frydman, X. Fritel, F. Herve, and G. Chaouat. 2001. Placental cytokine and chemokine production in HIV-1-infected women: trophoblast cells show a different pattern compared to cells from HIV-negative women. Clin. Exp. Immunol. 125:455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwapasa, V., S. J. Rogerson, M. E. Molyneux, E. T. Abrams, D. D. Kamwendo, V. M. Lema, E. Tadesse, E. Chaluluka, P. E. Wilson, and S. R. Meshnick. 2004. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS 18:1051-1059. [DOI] [PubMed] [Google Scholar]
- 26.Patterson, B. K., H. Behbahani, W. J. Kabat, Y. Sullivan, M. R. O'Gorman, A. Landay, Z. Flener, N. Khan, R. Yogev, and J. Andersson. 2001. Leukemia inhibitory factor inhibits HIV-1 replication and is upregulated in placentae from nontransmitting women. J. Clin. Investig. 107:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson, B. K., A. Tjernlund, and J. Andersson. 2002. Endogenous inhibitors of HIV: potent anti-HIV activity of leukemia inhibitory factor. Curr. Mol. Med. 2:713-722. [DOI] [PubMed] [Google Scholar]
- 28.Rossi, P., V. Moschese, P. A. Broliden, C. Fundaro, I. Quinti, A. Plebani, C. Giaquinto, P. A. Tovo, K. Ljunggren, and J. Rosen. 1989. Presence of maternal antibodies to human immunodeficiency virus 1 envelope glycoprotein gp120 epitopes correlates with the uninfected status of children born to seropositive mothers. Proc. Natl. Acad. Sci. USA 86:8055-8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarlatti, G., V. Hodara, P. Rossi, L. Muggiasca, A. Bucceri, J. Albert, and E. M. Fenyö. 1993. Transmission of human immunodeficiency virus type 1 (HIV-1) from mother to child correlates with viral phenotype. Virology 197:624-629. [DOI] [PubMed] [Google Scholar]
- 30.Sharma, U. K., J. Trujillo, H. F. Song, F. P. Saitta, O. B. Laeyendecker, R. Castillo, S. Arango-Jaramillo, G. Sridharan, M. Dettenhofer, K. Blakemore, X. F. Yu, and D. H. Schwart. 1998. A novel factor produced by placental cells with activity against HIV-1. J. Immunol. 161:6406-6412. [PubMed] [Google Scholar]
- 31.Suguitan, A. L., Jr., R. G. Leke, G. Fouda, A. Zhou, L. Thuita, S. Metenou, J. Fogako, R. Megnekou, and D. W. Taylor. 2003. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis. 188:1074-1082. [DOI] [PubMed] [Google Scholar]
- 32.Vidricaire, G., M. R. Tardif, and M. J. Tremblay. 2003. The low viral production in trophoblastic cells is due to a high endocytic internalization of the human immunodefiency virus type 1 and can be overcome by the pro-inflammatory cytokines TNF-alpha and IL-1. J. Biol. Chem. 278:15832-15841. [DOI] [PubMed] [Google Scholar]
- 33.Zachar, V., T. Fink, U. Koppelhus, and P. Ebbesen. 2002. Role of placental cytokines in transcriptional modulation of HIV type 1 in the isolated villous trophoblast. AIDS Res. Hum. Retrovir. 18:839-847. [DOI] [PubMed] [Google Scholar]