Abstract
Rhinoviruses (RV) are the major cause of the common cold and acute exacerbations of asthma and chronic obstructive pulmonary disease. Toll-like receptors (TLRs) are a conserved family of receptors that recognize and respond to a variety of pathogen-associated molecular patterns. TLR3 recognizes double-stranded RNA, an important intermediate of many viral life cycles (including RV). The importance of TLR3 in host responses to virus infection is not known. Using BEAS-2B (a human bronchial epithelial cell-line), we demonstrated that RV replication increased the expression of TLR3 mRNA and TLR3 protein on the cell surface. We observed that blocking TLR3 led to a decrease in interleukin-6, CXCL8, and CCL5 in response to poly(IC) but an increase following RV infection. Finally, we demonstrated that TLR3 mediated the antiviral response. This study demonstrates an important functional requirement for TLR3 in the host response against live virus infection and indicates that poly(IC) is not always a good model for studying the biology of live virus infection.
The Toll-like receptors (TLRs) are a recently described family of evolutionarily conserved pathogen recognition receptors, which function by recognition of and response to specific conserved motifs unique to microorganisms (pathogen-associated molecular patterns). To date, 10 human TLRs have been described, with specific pathogen-associated molecular patterns identified for 9 of them (5, 17, 19). In response to ligand binding, TLR signaling through MyD88 leads to activation of NF-κB and mitogen-activated protein kinases, resulting in inflammatory cytokine release and recruitment of cells required for host defense (reviewed in reference 1).
In relation to antiviral responses, TLR3 has been shown to recognize double-stranded RNA (dsRNA) (2), and TLR7 and TLR8 have been shown to recognize single-stranded RNA (17, 19). TLR3 is able to signal independently of MyD88, via a Toll/interleukin-1 (IL-1) receptor domain containing adapter-inducing beta interferon (IFN-β) (TRIF), leading to NF-κB activation and also IFN-regulatory factor 3 activation, leading to the production of IFN-β (49, 50). Activation of TLR3 via dsRNA has been shown to lead to increased TLR3 expression (26, 41), inflammatory cytokine release (29, 38, 41), and potent IFN-β responses (6).
While a great deal of work has investigated TLR3 and its potential role in antiviral responses, the majority of work has used synthetic poly(IC) to model virus infection (2, 6, 20, 28, 32, 38). Five studies have investigated the role of TLR3 in live virus infections. One reported that TLR3 was up-regulated by measles virus infection via IFN-α/β (44), another that virus stimulation of mast cells led to IFN-α but not inflammatory cytokine release (25), and a third that influenza infection led to proinflammatory and IFN-β production though TLR3 (16). None of these studies investigated the role of TLR3 in virus replication. A further recent publication demonstrated that TLR3 was not required for protection against disease in four different virus infections in mice and, further, did not contribute to adaptive immune responses (7). The final study demonstrated a role for TLR3 in some proinflammatory responses following respiratory syncytial virus infection but did not find evidence for a role for TLR3 in virus replication (37). There is, therefore, no published data demonstrating directly that activation of TLR3 inhibits virus replication and very little investigating its role in the context of live virus infection.
In addition, a thorough examination of the role of TLR3 in infection with a clinically important viral pathogen, in the context of its natural host cell type, has yet to be performed. We therefore wished to investigate the functional role of surface TLR3 activation in the target cell of a virus type causing important human illness.
Rhinoviruses (RVs) are the major cause of the common cold (27). They are also the major cause of acute exacerbations of asthma (3, 12, 22, 31, 47) and chronic obstructive pulmonary disease (COPD) (36, 40). Asthma affects up to one-third of children, and COPD is the fourth leading cause of death worldwide; in both diseases, acute exacerbations are the major cause of morbidity, mortality, and health care costs. The primary target of RV infection in this context is the bronchial epithelial cell, which has been shown to express TLR3 and to respond to its activation by poly(IC) (41).
Therefore, to further investigate the role of TLR3 in defense against an important human virus in a human context, we investigated whether TLR3 was up-regulated in response to RV infection of bronchial epithelial cells and whether blocking activation of TLR3 with specific antibody interfered with proinflammatory cytokine production and RV replication. We further compared live virus with poly(IC), to determine whether poly(IC) is a good model for studying the role of TLR3 in the pathogenesis of live virus infections. We report the first demonstration of an important functional role for human TLR3 in response to a clinically important human RNA virus and conclude that poly(IC) is not a good model for live virus infection in the context of examining inflammatory responses to virus infection.
MATERIALS AND METHODS
Cell and viral culture.
The human bronchial epithelial cell line BEAS-2B was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Invitrogen). RV serotypes 16, 9, and 1B were grown in HeLa cells and prepared as previously described (33). Viruses were titrated on HeLa cells to ascertain their 50% tissue culture infective dose (TCID50)/ml (23). The identities of all RVs were confirmed by titration on HeLa cells and neutralization using serotype-specific antibodies. UV inactivation was performed as previously described (21, 33) and filtered virus was produced by passing RV stocks through a 30-kDa membrane (Millipore) at 10,000 × g for 5 min.
Infection of cells with RV.
BEAS-2B cells were cultured in 12-well tissue culture plates (Nalge Nunc) for 24 h before being placed into 2% FCS-RPMI medium for a further 24 h. Cells were infected with RV for 1 h with shaking at room temperature before the virus was removed and replaced with 1 ml of infection medium. Alternatively, cells were stimulated with 0.1 μg/ml poly(IC) (Sigma Aldrich) for 1 h before this was removed and replaced with 1 ml of infection medium containing 0.1 μg/ml poly(IC) (Sigma Aldrich). In some experiments, cells were pretreated for 1 h prior to infection with 20 μg/ml of an anti-TLR3 antibody (clone TLR3.7; eBioscience Inc.) or a mouse immunoglobulin G1 (IgG1) isotype control antibody (eBioscience Inc.). The antibody was replaced after the 1-h infection with RV or stimulation with poly(IC) at the same concentration. Cells for flow cytometry, supernatants, or RNA lysates were harvested at the times indicated. Supernatants and lysates were stored at −80°C until required.
Flow cytometric analysis of TLR3 surface protein expression.
A total of 1 × 105 cells were stained with 1 mg/ml phycoerythrin (PE)-labeled anti-TLR3 antibody (clone TLR3.7; eBioscience Inc.) or PE-labeled mouse IgG1 isotype control (BD Biosciences) for 45 min. The cells were fixed with 1% (wt/vol) paraformaldehyde and analyzed on a BD LSR Cytometer measuring 10,000 cells. The staining by the PE-labeled mouse IgG1 isotype control was indistinguishable from nonstained cells and was therefore not presented in the results. Rather, data were expressed as mean fluorescent intensity (MFI) of anti-TLR3-stained cells minus the MFI of isotype control-stained cells.
RNA extraction and reverse transcription.
RNA was extracted from cells using the RNeasy method following the manufacturer's instructions, including the optional DNase I digestion of contaminating DNA (QIAGEN). cDNA was synthesized using Omniscript RT and components as directed by the manufacturer (QIAGEN).
TaqMan real-time PCR.
Primers were purchased from Invitrogen, and probes were from QIAGEN. TaqMan analysis of TLR3 mRNA was normalized with respect to 18S rRNA. For TLR3 analysis, each reaction contained 900 nM TLR3TM.1 (AAA TTA AAG AGT TTT CTC CAG GGT GTT), 300 nM TLR3TM.2 (ATT CCG AAT GCT TGT GTT TGC), 250 nM 5′-FAM-TAMRA-labeled probe (TTT GGC CTC TTT CTG AAC AAT GTC CAG C), and 3 μl of cDNA in 1× Quantitect Probe PCR Master Mix (QIAGEN). For 18S, each reaction contained 300 nM 18STM.1 (CGC CGC TAG AGG TGA AAT TCT), 300 nM 18STM.2 (CAT TCT TGG CAA ATG CTT TCG), 175 nM 5′-FAM-TAMRA-labeled probe (5′-ACC GGC GCA AGA CGG ACC AGA), and 3 μl of cDNA diluted 1/100 in 1× Quantitect Probe PCR Master Mix (QIAGEN). The reactions were analyzed using an ABI 7000 Automated TaqMan (Applied Biosystems). The amplification cycle consisted of 50°C for 2 min, 94°C for 10 min, and 40 cycles of 94°C for 15 s and 60°C for 15 s.
Enzyme-linked immunosorbent assay to evaluate IL-6, CXCL8, and CCL5 release.
IL-6, CXCL8, and CCL5 were quantified by enzyme-linked immunosorbent assay using commercially available paired antibodies and standards, following the manufacturer's instructions (R&D Systems). The sensitivity of each assay was 7 pg/ml.
Virus titration assay.
Experimental supernatants were serially diluted in Eagles minimal essential medium containing 4% FCS (Invitrogen) and titrated on HeLa cells to determine the TCID50/ml of the RV in the supernatants (23). Each dilution was assayed in eight replicates.
Statistical analysis.
Data are presented as means ± standard errors of the means. All data were analyzed using one-way analysis of variance and Bonferroni's multiple comparison post hoc test. Data were accepted as significantly different when P was <0.05.
RESULTS
RV infection leads to an increase in TLR3 mRNA and surface protein expression.
Initial experiments were undertaken to assess the hypothesis that RV infection would increase antiviral immunity by augmenting TLR3 expression. To address this, the human bronchial cell line BEAS-2B was infected for 1 h with RV-16 at a multiplicity of infection (MOI) of 1. At various time points, cells were harvested for TLR3 mRNA analysis or for flow cytometric analysis of TLR3 surface protein.
At 6 h after the initial infection, TLR3 mRNA expression was increased in comparison to uninfected cells, although the increase was not statistically significant (Fig. 1A). This continued to a peak in expression at 24 h postinfection compared to uninfected cells (P < 0.001), with a 40-fold increase following RV infection. The virally induced mRNA expression fell at 48 h postinfection but was still significantly higher than uninfected cells (P < 0.05) with 11 times more mRNA.
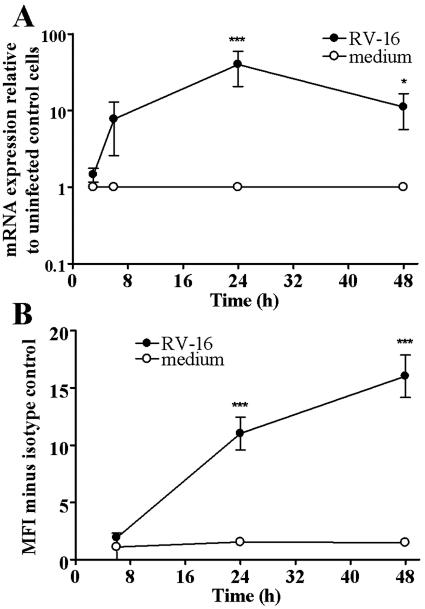
FIG. 1.
RV infection leads to time-dependent increase in TLR3 mRNA and surface protein expression. (A) Cells were infected for 1 h with RV-16 (MOI of 1) and RNA was extracted at 3, 6, 24, and 48 h postinfection. TLR3 mRNA expression was quantified by TaqMan. Data are presented as mRNA expression relative to uninfected cells (n = 5). (B) Cells were infected as described for panel A and harvested for flow cytometry at the same time points. Data are presented as MFI minus the MFI of isotype control-stained cells (n = 3). Data are considered significantly different between infected cells and uninfected control cells at P < 0.05 (*) and P < 0.001 (***).
For protein expression, no increase was observed until 24 h postinfection (Fig. 1B). At 24 h postinfection, very little protein was detected on the surface of uninfected cells (1.54 MFI more than isotype control), but significantly more was detected on the surface of cells infected with RV-16 (11.02 MFI more than isotype control [P < 0.001]). This was further increased after 48 h, with low levels of protein on uninfected cells (1.48 MFI more than isotype control), and was significantly greater in RV-16 infected cells (16.03 MFI more than isotype control [P < 0.001]).
These data demonstrated that infection with RV-16 led to an increase in TLR3 expression.
The induction of TLR3 surface protein expression is dose dependent, RV serotype and receptor independent, and requires active viral replication.
Having demonstrated that infection of bronchial epithelial cells with RV-16 caused a significant increase in TLR3 mRNA and protein expression, we next investigated whether RV infection induced TLR3 in a dose-responsive manner and whether there was a requirement for active viral replication. Also as there are at least 100 distinct RV serotypes, the major group of which bind to cells via intercellular adhesion molecule 1 (14, 42, 45) and the minor group of which use the very-low-density lipoprotein receptor (18, 43), we wished to determine whether RV induction of TLR3 was serotype-specific or receptor restricted.
BEAS-2B cells were therefore infected with RV-16 for 1 h at three different MOIs (1, 0.5 and 0.2). At 24 h postinfection, cells were harvested for flow cytometry (Fig. 2A). RV-16 infection at an MOI of either 1 or 0.5 resulted in a significant increase in the surface expression of TLR3 protein (9.33 and 5.55 MFI more than isotype control, respectively [P < 0.01]) compared to uninfected cells (1.40 MFI more than isotype control), while an MOI of 0.2 had no effect. These data demonstrate that the induction of TLR3 is RV dose dependent.
FIG. 2.
Induction of TLR3 surface protein expression is dose responsive and serotype independent and requires actively replicating virus. (A) Cells were infected for 1 h with three doses of RV-16 at MOIs of 1, 0.5, and 0.2. Cells were harvested for flow cytometry 24 h postinfection. (B) Cells were infected for 1 h with RV-16 (MOI of 1), RV-1B (MOI of 1), RV-9 (MOI of 0.5), UV-inactivated RV-16 (MOI of 1), or the same volume of filtered RV-9. Cells were harvested for flow cytometry 24 h postinfection. Data are presented as MFI minus the MFI of isotype control stained cells (n = 3). Data are considered significantly different between infected and uninfected control cells at P < 0.05 (*) and P < 0.01 (**). ns, data not significantly different at P > 0.05.
To investigate whether the increases observed in TLR3 expression were unique to RV-16, experiments were carried out using MOIs of 1 for RV-1B (a minor group RV) and 0.5 for RV-9 (an alternative major group RV) alongside RV-16. In addition, to investigate the requirement for virus replication, filtered RV-9 was included to act as a negative control, and parallel cells were infected with UV-inactivated RV-16 (MOI of 1). At 24 h postinfection, cells were harvested and assayed by flow cytometry (Fig. 2B). Infection with either RV-1B or RV-9 led to a significant increase in TLR3 protein expression (10.82 [P < 0.01] and 4.64 [P < 0.05] MFI more than isotype control, respectively) compared to uninfected cells (1.36 MFI more than isotype control). However, infection with filtered RV-9 did not increase TLR3 expression. Finally, infection with UV-inactivated RV-16 did not lead to an increase in TLR3 protein expression (1.36 MFI over isotype control, compared to 1.40 in uninfected cells). These data demonstrate that the up-regulation in TLR3 expression is independent of RV serotype, is not virus receptor restricted, and requires actively replicating virus.
Functional inhibition of TLR3 during RV infection leads to an increase in proinflammatory mediators.
Several previous studies demonstrated that both poly(IC) stimulation and RV infection of bronchial epithelial cells lead to induction of proinflammatory mediators such as IL-6, CXCL8, and CCL5 (13, 21, 24, 39, 41, 52, 53) whose function is to recruit and activate cells required for host defense. However, it is not known how much of this induction results from activation of TLR3. Initially, we confirmed the requirement for actively replicating RV to induce proinflammatory mediators (at an MOI of 1 for UV-inactivated RV-16, as described above) and, having demonstrated that RV infection led to an increase in TLR3 expression, we then investigated the functional role of TLR3 activation in recognition of and response to RV infection in terms of the same proinflammatory mediators. We also compared the studies with live virus with those using poly(IC), as this is a stimulus frequently used to mimic the effects of virus infection in laboratory studies. BEAS-2B cells were therefore pretreated for 1 h with an anti-TLR3 antibody, previously shown to inhibit poly(IC) induction of IFN-β in MRC-5 cells by approximately 50% (28), or with an isotype control antibody. Cells were then infected for 1 h with RV-16 before the antibody was replaced. Additionally, cells were blocked with the same dose and timing of antibody and then stimulated with poly(IC). At 24 h postinfection and stimulation, supernatants were harvested for cytokine analysis.
Initially, we confirmed the requirement for actively replicating RV in the induction of proinflammatory mediators by infecting BEAS-2B cells with UV-inactivated RV-16. At 24 h postinfection, it was observed that UV-inactivated RV-16 induced significantly less IL-6 (P < 0.001), CXCL8 (P < 0.001), and CCL5 (P < 0.001) compared to active RV-16, indicating that replicating RV was required for induction of cytokines (Fig. 3A) as well as TLR3 expression (Fig. 2B).
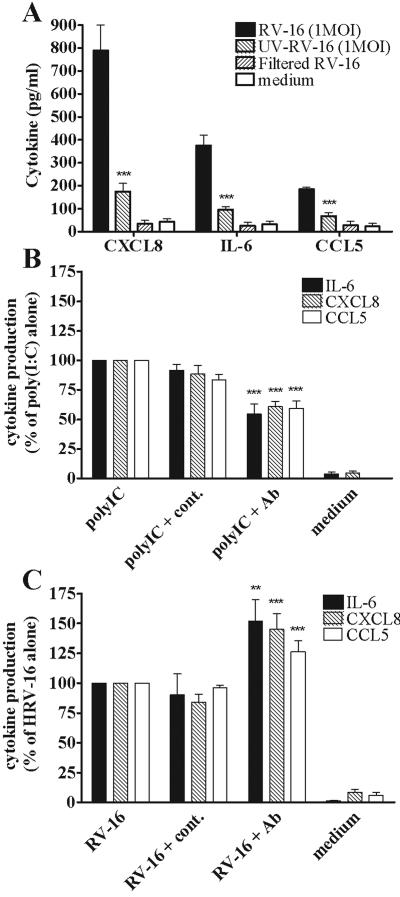
FIG. 3.
The proinflammatory effect of functional inhibition of surface-expressed TLR3 in response to poly(IC) stimulation and RV infection. (A) Cells were infected for 1 h with RV-16 (MOI of 1), UV-inactivated RV-16 (MOI of 1), or the same volume of filtered RV-16. The amounts of IL-6, CXCL8, and CCL5 secreted into the supernatants were quantified after 24 h. Data for panel A are presented as the concentrations of proinflammatory mediator in the supernatants. (B) Cells were pretreated for 1 h with anti-TLR3 or isotype control antibodies before stimulation with 0.1 μg/ml poly(IC) [antibodies were replaced 1 h after the initial application of poly(IC)]. The amounts of IL-6, CXCL8, and CCL5 secreted into the supernatants were quantified after 24 h. (C) Cells were pretreated with the antibodies as described for panel B before being infected for 1 h with RV-16 (MOI of 1). The antibodies were then replaced, and IL-6, CXCL8, and CCL5 were quantified 24 h postinfection. Data for panels B and C are presented as percentage release compared to stimulated/infected cells without antibody. For panel A data are significantly different (***) between RV-16- and UV-RV-16-infected cells at P < 0.001 (n = 5). For panels B and C, values are significantly different between stimulated/infected cells ± anti-TLR3 antibody at P < 0.01 (*) and P < 0.001 (***) (n = 5).
Functional inhibition of TLR3 activity by blocking antibody demonstrated that the induction of the proinflammatory mediators IL-6, CXCL8, and CCL5 by poly(IC) was, at least partially, TLR3 dependent (Fig. 3B). It was observed that following TLR3 blocking, the ability of poly(IC) to induce IL-6, CXCL8, and CCL5 was reduced by approximately 40 to 50% compared to poly(IC) stimulation alone (P < 0.001), while no effect was observed in cells treated with an isotype control antibody (P was not significant [NS]). However, when TLR3 activity was blocked and cells were then infected with RV-16, a different consequence was observed (Fig. 3C). After TLR3 was blocked, cells produced more mediators than unblocked, infected cells. IL-6, CXCL8, and CCL5 production was increased by 52%, 45%, and 27%, respectively, compared to RV-16 infection alone (P < 0.01 and 0.001, respectively). Again, no significant effect was observed in cells treated with an isotype control antibody (P = NS).
These data confirm that poly(IC) stimulation of IL-6, CXCL8, and CCL5 is regulated at least in part by TLR3 and that blocking TLR3 function inhibits proinflammatory cytokine production. In marked contrast, inhibition of TLR3 increased cellular proinflammatory responses following RV infection.
Blocking of TLR3 during RV infection impairs the antiviral response, resulting in increased RV replication.
Finally, we investigated whether TLR plays a biological role in virus infections, as this has been the subject of recent debate (7). In addition, we wished to determine a cause for the increased proinflammatory cytokine production observed with RV infection when TLR3 was blocked, since one possible explanation was that blocking TLR3 inhibited antiviral responses in the infected cells, leading to an increased viral load within the experiment.
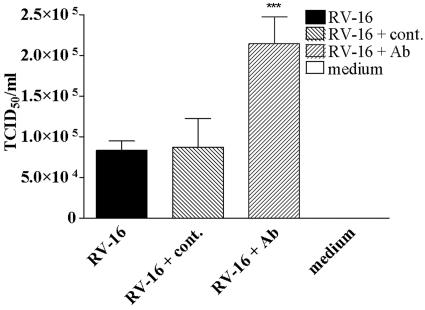
Supernatants from experiments designed identically to those above were therefore assessed for infectious RV. It was observed that, when the function of TLR3 was blocked with the anti-TLR3 antibody, the level of active infectious RV released into the supernatant was increased from 8.3 × 104 TCID50/ml to 21.5 × 104 TCID50/ml (P < 0.001), a greater than twofold increase in virus released (Fig. 4). However, cells treated with an isotype control antibody and infected with RV-16, showed no significant difference to untreated cells (P = NS).
FIG. 4.
The effect of functional inhibition of surface-expressed TLR3 on RV replication. Cells were pretreated with anti-TLR3 or isotype control antibodies and infected with RV-16 as described in the legend of Fig. 3C. The amount of infectious RV virions released into the supernatants was assessed by virus titration 24 h postinfection. Data are presented as TCID50/ml. ***, data are significantly different between infected cells ± anti-TLR3 antibody at P < 0.001 (n = 5).
These data demonstrate that the observed increase in proinflammatory responses following blocking of TLR3 is likely to be due to the increased amounts of infectious virus released. In addition, these data demonstrate an important antiviral effect of TLR3 activation following RV infection of bronchial cells.
DISCUSSION
The vast majority of studies undertaken to examine TLR3-mediated events have used the synthetic dsRNA analogue poly(IC) as a direct ligand for TLR3. While this has certain merits in examining TLR3 function, studies with poly(IC) cannot determine whether TLR3 has a functional role in host response to viral infections; thus, this question remains unresolved. A recently published report has sought to demonstrate a role for TLR3 in response to virus infection. Edelmann et al. used TLR3 knockout mice and infected them with four different model viruses (7). It was observed that knockout mice did not differ from wild-type mice in terms of their ability to mount adaptive responses as quantified by IFN-γ-positive specific T cells. However, TLR3 is primarily involved in innate immunity, and this arm of the immune response was not investigated. Therefore, different responses early in the infection may have been missed in this study. Another recent publication was unable to find evidence that TLR3 played a role in antiviral responses to respiratory syncytial virus infection (37). It was observed that expression of TLR3 in a TLR3−/− cell line (HEK 293) did not suppress virus replication. However, it must be remembered that this system was artificial, and intracellular signaling may have been deficient between the introduced TLR3 expression and any downstream antiviral mediator expression.
It has been reported that exogenously applied dsRNA increases the surface expression of TLR3 and that it is functional since responses can be prevented by antibody blocking of the surface receptor (25, 26, 28). However, none of these studies has investigated the natural host cells infected by human viruses, as they investigated retinal cells, fibroblasts, and mast cells. We therefore wished to investigate the functional role of surface TLR3 activation in respiratory epithelial cells, the target cell type of all respiratory viruses. In addition to studying poly(IC) in this more relevant context, we also wished to carry out studies of an important human virus in the natural host cells of that virus and elected to study the most common respiratory virus type, RV.
We first demonstrated that both poly(IC) (data not shown) and live virus induced TLR3 mRNA and surface protein expression, thereby enhancing the innate immune host defense in the natural host cells of respiratory viruses. This up-regulation was confirmed to be virus induced in that there was a clear dose-response relationship, and filtering of virus from the inoculum removed its ability to induce TLR3 expression. The induction was also RV serotype and receptor independent, indicating that this response is likely to be a general host defense mechanism applying to many virus types, rather than being specific to one virus serotype. Finally, we demonstrated that active viral replication was required for this induction, as no induction was seen with UV-inactivated virus.
An important aspect of host defense is the release of proinflammatory cytokines by virus-infected respiratory epithelial cells. These cytokines recruit and activate cells of both innate and acquired immune responses and are implicated in viral exacerbations of both asthma (11, 15, 34, 46) and COPD (35, 51). Several previous studies demonstrated that both poly(IC) stimulation and RV infection of bronchial epithelial cells lead to induction of proinflammatory mediators such as IL-6, CXCL8, and CCL5 (13, 21, 24, 39, 41, 52, 53). However, it is not known how much of this induction results from activation of TLR3. Our studies with poly(IC) indicate that around 50% of proinflammatory mediator signaling occurs via TLR3 (Fig. 3B). However, as the first hour of stimulation with poly(IC) was in the absence of the TLR3 blocking antibody, significantly more of the proinflammatory response could be via TLR3. These findings could have important implications for developing strategies for both augmenting antiviral host defense and suppressing unwanted inflammatory responses.
However, while we observed a clear requirement for functional surface TLR3 in the production of the proinflammatory mediators IL-6, CXCL8, and CCL5 on stimulation with poly(I · C) (Fig. 3B), in terms of responses to RV infection this was not the case. In contrast, inhibition of TLR3 potentiated the release of the mediators (Fig. 3C). We also determined that blocking the TLR3-ligand interaction increased replication of RV, with more than twice the amount of infectious RV detected in the cell supernatants (Fig. 4). This led us to hypothesize that there is a balance of responses induced by TLR3 activation in response to RV that is not apparent for poly(IC) (Fig. 5). In the case of poly(IC), TLR3 is activated and causes cells to produce proinflammatory mediators (29, 38, 41) as well as antiviral mediators (IFN-β) (6). However, as poly(IC) does not replicate, the primary effect of blocking the receptor is a reduction in proinflammatory responses, as the concomitant inhibition of the antiviral response is redundant in the absence of live virus. However, while for RV infection the same basic response occurs, the downstream effect is different. By blocking TLR3, the antiviral response is inhibited, leading to an increase in RV replication and release. This increases the amount of RV in the system infecting other cells. Non-TLR3 receptors (for example, protein kinase R [13]) are therefore stimulated to a higher level, increasing the proinflammatory responses and overwhelming any potential reduction observed by blocking TLR3. The fact that TLR3 is an extremely potent inducer of antiviral responses has previously been identified (6) and could explain our observation that it is the more potent arm of its response to RV infection.
FIG. 5.
A scheme for the differential effects mediated by inhibition of surface TLR3. Inhibition of TLR3 produces a differential effect, dependent on the stimuli. The inhibition of antiviral responses is redundant in response to poly(IC); hence, the primary effect of the antibody is to inhibit proinflammatory responses. In the case of RV, inhibition of TLR3-mediated antivirals leads to enhanced RV replication, causing enhanced activation of other non-TLR3-mediated recognition events and resulting in increased proinflammatory responses as a result of increased RV replication.
The role of TLR3 in human disease requires further characterization. However, interesting respiratory disease associations have been made for other TLRs such as the expression of TLR2 in cystic fibrosis cells (30); TLR2, TLR4, and IL-4 expression in tuberculosis granulomas (10); and TLR2 and TLR4 polymorphisms and asthma development (8, 9). In terms of asthma and TLR3, no such associations have been reported. However, it has been demonstrated that asthmatics are more susceptible to RV infections than normal individuals, having longer duration, and increased severity, of lower respiratory tract symptoms when infected with RV (4). Given that we have recently reported that asthmatics have deficient IFN-β responses to RV infection (48), it is interesting to hypothesize that asthmatic patients may be defective in TLR3 expression or responsiveness. This could, at least in part, explain their increased symptoms due to a decreased ability to clear the virus in the early stage of infection.
In conclusion, we have shown that surface-expressed TLR3 does have an important function in response to a common human viral infection of its natural host cell. We have also shown that RVs are able to induce TLR3 mRNA and surface protein expression and that TLR3 plays an important role in innate immune responses against RV infection. We also identified a key difference in responses to RV infection and poly(IC) stimulation that has important implications for the development of TLR3 antagonists or agonist ligands for therapeutic purposes.
Acknowledgments
This work was supported by an Asthma UK project grant awarded to S.L.J. and C.A.H. (grant number 03/073) and by British Lung Foundation/Severin Wunderman Family Foundation Lung Research Programme grant number P00/2.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Atmar, R. L., E. Guy, K. K. Guntupalli, J. L. Zimmerman, V. D. Bandi, B. D. Baxter, and S. B. Greenberg. 1998. Respiratory tract viral infections in inner-city asthmatic adults. Arch. Intern. Med. 158:2453-2459. [DOI] [PubMed] [Google Scholar]
- 4.Corne, J. M., C. Marshall, S. Smith, J. Schreiber, G. Sanderson, S. T. Holgate, and S. L. Johnston. 2002. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 359:831-834. [DOI] [PubMed] [Google Scholar]
- 5.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and R. E. Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 6.Doyle, S. E., R. O'Connell, S. A. Vaidya, E. K. Chow, K. Yee, and G. Cheng. 2003. Toll-like receptor 3 mediates a more potent antiviral response than Toll-like receptor 4. J. Immunol. 170:3565-3571. [DOI] [PubMed] [Google Scholar]
- 7.Edelmann, K. H., S. Richardson-Burns, L. Alexopoulou, K. L. Tyler, R. A. Flavell, and M. B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231-238. [DOI] [PubMed] [Google Scholar]
- 8.Eder, W., W. Klimecki, L. Yu, E. von Mutius, J. Riedler, C. Braun-Fahrlander, D. Nowak, and F. D. Martinez. 2004. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J. Allergy Clin. Immunol. 113:482-488. [DOI] [PubMed] [Google Scholar]
- 9.Fageras Bottcher, M., M. Hmani-Aifa, A. Lindstrom, M. C. Jenmalm, X.-M. Mai, L. Nilsson, H. A. Zdolsek, B. Bjorksten, P. Soderkvist, and O. Vaarala. 2004. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12(p70) responses in Swedish children. J. Allergy Clin. Immunol. 114:561-567. [DOI] [PubMed] [Google Scholar]
- 10.Fenhalls, G., G. R. Squires, L. Stevens-Muller, J. Bezuidenhout, G. Amphlett, K. Duncan, and P. T. Lukey. 2003. Associations between Toll-Like Receptors and Interleukin-4 in the Lungs of Patients with Tuberculosis. Am. J. Respir. Cell Mol. Biol. 29:28-38. [DOI] [PubMed] [Google Scholar]
- 11.Fleming, H. E., F. F. Little, D. Schnurr, P. C. Avila, H. Wong, J. Liu, S. Yagi, and H. A. Boushey. 1999. Rhinovirus-16 Colds in healthy and in asthmatic subjects. Similar changes in upper and lower airways. Am. J. Respir. Crit. Care Med. 160:100-108. [DOI] [PubMed] [Google Scholar]
- 12.Freymuth, F., A. Vabret, J. Brouard, F. Toutain, R. Verdon, J. Petitjean, S. Gouarin, J. F. Duhamel, and B. Guillois. 1999. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J. Clin. Virol. 13:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gern, J. E., D. A. French, K. A. Grindle, R. A. Brockman-Schneider, S.-I. Konno, and W. W. Busse. 2003. Double-stranded RNA induces the synthesis of specific chemokines by bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 28:731-737. [DOI] [PubMed] [Google Scholar]
- 14.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. McClelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 15.Grunberg, K., H. H. Smits, M. C. Timmers, E. P. de Klerk, R. J. Dolhain, E. C. Dick, P. S. Hiemstra, and P. J. Sterk. 1997. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am. J. Respir. Crit. Care Med. 156:609-616. [DOI] [PubMed] [Google Scholar]
- 16.Guillot, L., R. Le Goffic, S. Bloch, N. Escriou, S. Akira, M. Chignard, and M. Si-Tahar. 2005. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571-5580. [DOI] [PubMed] [Google Scholar]
- 17.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 18.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blass. 1994. Members of the low-density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens, S., and R. Beyaert. 2003. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 16:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, Z., M. Zamanian-Daryoush, H. Nie, A. M. Silva, B. R. G. Williams, and X. Li. 2003. Poly(dI-dC)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFκB and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 278:16713-16719. [DOI] [PubMed] [Google Scholar]
- 21.Johnston, S. L., A. Papi, P. J. Bates, J. G. Mastronarde, M. M. Monick, and G. W. Hunninghake. 1998. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J. Immunol. 160:6172-6181. [PubMed] [Google Scholar]
- 22.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, F. Lampe, L. Josephs, P. Symington, S. O'Toole, S. H. Myint, and D. A. Tyrrell. 1995. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Br. Med. J. 310:1225-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston, S. L., and D. A. Tyrrell. 1995. Rhinoviruses, p. 253-263. In E. H. Lennette and N. J. Schmidt (ed.), Diagnostic procedures for viral, rickettsial and chlamydial infections. American Public Health Association, Washington, D.C.
- 24.Kim, J., S. P. Sanders, E. S. Siekierski, V. Casolaro, and D. Proud. 2000. Role of NF-kappa B in cytokine production induced from human airway epithelial cells by rhinovirus infection. J. Immunol. 165:3384-3392. [DOI] [PubMed] [Google Scholar]
- 25.Kulka, M., L. Alexopoulou, R. A. Flavell, and D. D. Metcalfe. 2004. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J. Allergy Clin. Immunol. 114:174-182. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, M. V., C. N. Nagineni, M. S. Chin, J. J. Hooks, and B. Detrick. 2004. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 153:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makela, M. J., T. Puhakka, O. Ruuskanen, M. Leinonen, P. Saikku, M. Kimpimaki, S. Blomqvist, T. Hyypia, and P. Arstila. 1998. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 36:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto, M., S. Kikkawa, M. Kohase, K. Miyake, and T. Seya. 2002. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 293:1364-1369. [DOI] [PubMed] [Google Scholar]
- 29.Matsushima, H., N. Yamada, H. Matsue, and S. Shimada. 2004. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J. Immunol. 173:531-541. [DOI] [PubMed] [Google Scholar]
- 30.Muir, A., G. Soong, S. Sokol, B. Reddy, M. I. Gomez, A. van Heeckeren, and A. Prince. 2004. Toll-Like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30:777-783. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson, K. G., J. Kent, and D. C. Ireland. 1993. Respiratory viruses and exacerbations of asthma in adults. Br. Med. J. 307:982-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshiumi, H., M. Matsumoto, K. Funami, T. Akazawa, and T. Seya. 2003. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4:161-167. [DOI] [PubMed] [Google Scholar]
- 33.Papi, A., and S. L. Johnston. 1999. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kB-mediated transcription. J. Biol. Chem. 274:9707-9720. [DOI] [PubMed] [Google Scholar]
- 34.Pizzichini, M. M., E. Pizzichini, A. Efthimiadis, A. J. Chauhan, S. L. Johnston, P. Hussack, J. Mahony, J. Dolovich, and F. E. Hargreave. 1998. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am. J. Respir. Crit. Care Med. 158:1178-1184. [DOI] [PubMed] [Google Scholar]
- 35.Qiu, Y., J. Zhu, V. Bandi, R. L. Atmar, K. Hattotuwa, K. K. Guntupalli, and P. K. Jeffery. 2003. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 168:968-975. [DOI] [PubMed] [Google Scholar]
- 36.Rohde, G., A. Wiethege, I. Borg, M. Kauth, T. T. Bauer, A. Gillissen, A. Bufe, and G. Schultze-Werninghaus. 2003. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax 58:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudd, B. D., E. Burstein, C. S. Duckett, X. Li, and N. W. Lukacs. 2005. Differential Role for TLR3 in respiratory syncytial virus-induced chemokine expression. J. Virol. 79:3350-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt, K. N., B. Leung, M. Kwong, K. A. Zarember, S. Satyal, T. A. Navas, F. Wang, and P. J. Godowski. 2004. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J. Immunol. 172:138-143. [DOI] [PubMed] [Google Scholar]
- 39.Schroth, M. K., E. Grimm, P. Frindt, D. M. Galagan, S. I. Konno, R. Love, and J. E. Gern. 1999. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 20:1220-1228. [DOI] [PubMed] [Google Scholar]
- 40.Seemungal, T., R. Harper-Owen, A. Bhowmik, I. Moric, G. Sanderson, S. Message, P. Maccallum, T. W. Meade, D. J. Jeffries, S. L. Johnston, and J. A. Wedzicha. 2001. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 164:1618-1623. [DOI] [PubMed] [Google Scholar]
- 41.Sha, Q., A. Q. Truong-Tran, J. R. Plitt, L. A. Beck, and R. P. Schleimer. 2004. Activation of airway epithelial cells by Toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 31:358-364. [DOI] [PubMed] [Google Scholar]
- 42.Staunton, D. E., V. J. Merluzzi, R. Rothlein, R. Barton, S. D. Marlin, and T. A. Springer. 1989. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 56:849-853. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, T., M. Yamaya, M. Kamanaka, Y. X. Jia, K. Nakayama, M. Hosoda, N. Yamada, H. Nishimura, K. Sekizawa, and H. Sasaki. 2001. Type 2 rhinovirus infection of cultured human tracheal epithelial cells: role of LDL receptor. Am. J. Physiol. 280:L409-L420. [DOI] [PubMed] [Google Scholar]
- 44.Tanabe, M., M. Kurita-Taniguchi, K. Takeuchi, M. Takeda, M. Ayata, H. Ogura, M. Matsumoto, and T. Seya. 2003. Mechanism of up-regulation of human Toll-like receptor 3 secondary to infection of measles virus-attenuated strains. Biochem. Biophys. Res. Commun. 311:39-48. [DOI] [PubMed] [Google Scholar]
- 45.Tomassini, J. E., D. Graham, C. M. DeWitt, D. W. Lineberger, J. A. Rodkey, and R. J. Colonno. 1989. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 86:4907-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner, R. B., K. W. Weingand, C. H. Yeh, and D. W. Leedy. 1998. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin. Infect. Dis. 26:840-846. [DOI] [PubMed] [Google Scholar]
- 47.Wark, P. A., S. L. Johnston, I. Moric, J. L. Simpson, M. J. Hensley, and P. G. Gibson. 2002. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur. Respir. J. 19:68-75. [DOI] [PubMed] [Google Scholar]
- 48.Wark, P. A. B., S. L. Johnston, F. Bucchieri, R. Powell, S. Puddicombe, V. Laza-Stanca, S. T. Holgate, and D. E. Davies. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169:6668-6672. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, J., Y. S. Qiu, S. Majumdar, E. Gamble, D. Matin, G. Turato, L. M. Fabbri, N. Barnes, M. Saetta, and P. K. Jeffery. 2001. Exacerbations of bronchitis. Bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractants. Am. J. Respir. Crit. Care Med. 164:109-116. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, Z., W. Tang, J. M. Gwaltney, Jr., Y. Wu, and J. A. Elias. 1997. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am. J. Physiol. 273:L814-L824. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, Z., W. Tang, A. Ray, Y. Wu, O. Einarsson, M. Landry, J. Gwaltney, Jr., and J. A. Elias. 1996. Rhinovirus stimulation of interleukin-6 in vivo and in vitro: evidence for nuclear factor κB-dependent transcriptional activation. J. Clin. Investig. 97:421-430. [DOI] [PMC free article] [PubMed] [Google Scholar]