Abstract
Kaposi's sarcoma (KS) is a multifocal angiogenic tumor and appears to be a hyperplastic disorder caused, in part, by local production of inflammatory cytokines. The K1 lymphocyte receptor-like protein of KS-associated herpesvirus (KSHV) efficiently transduces extracellular signals to elicit cellular activation events through its cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM). To further delineate K1-mediated signal transduction, we purified K1 signaling complexes and identified its cellular components. Upon stimulation, the K1 ITAM was efficiently tyrosine phosphorylated and subsequently interacted with cellular Src homology 2 (SH2)-containing signaling proteins Lyn, Syk, p85, PLCγ2, RasGAP, Vav, SH2 domain-containing protein tyrosine phosphatase 1/2, and Grab2 through its phosphorylated tyrosine residues. Mutational analysis demonstrated that each tyrosine residue of K1 ITAM contributed to the interactions with cellular signaling proteins in distinctive ways. Consequently, these interactions led to the marked augmentation of cellular signal transduction activity, evidenced by the increase of cellular tyrosine phosphorylation and intracellular calcium mobilization, the activation of NF-AT and AP-1 transcription factor activities, and the production of inflammatory cytokines. These results demonstrate that KSHV K1 effectively recruits a set of cellular SH2-containing signaling molecules to form the K1 signalosome, which elicits downstream signal transduction and induces inflammatory cytokine production.
Kaposi's sarcoma (KS) is a multifocal angiogenic tumor consisting of characteristic spindle cells and infiltrating leukocytes (38). KS occurs in several epidemiologically distinct forms and is the most common AIDS-associated tumor (2, 4, 41, 44). Unlike most cancers, KS does not appear to be the result of clonal expansion of a transformed cell. Instead, it appears to be a hyperplastic disorder caused, in part, by local production of inflammatory cytokines such as interleukin-1 (IL-1), IL-6, gamma interferon, tumor necrosis factor alpha, and growth factors such as basic fibroblast growth factor and vascular endothelial growth factor (VEGF) (13-16). This is supported by the fact that infiltration of inflammatory cells, including CD8+ T cells, monocytes, macrophages, and dendritic cells, precedes transformation of the spindle-shaped endothelial cells (3, 23, 43). Infiltrating cells systematically produce inflammatory cytokines that are likely responsible for activating vessels and endothelial cells, increasing adhesiveness with extravasation, and recruiting lymphocytes and monocytes (12, 14).
Based on strong epidemiological and histopathological evidence, KS-associated herpesvirus (KSHV), also called human herpesvirus 8, is thought to be an etiologic agent of KS (9). KSHV has been consistently identified in KS tumors from human immunodeficiency virus-positive and human immunodeficiency virus-negative patients (4, 5, 37). KSHV has also been identified in primary effusion lymphoma (PEL) and an immunoblast variant of multicentric Castleman's disease (26), which are of B-cell origin (4, 5, 45). The genomic sequence classifies KSHV as a gamma-2 herpesvirus that is closely related to herpesvirus saimiri (41, 46) and rhesus monkey rhadinovirus (1, 10, 48).
At a position equivalent to the saimiri transformation protein (STP) of herpesvirus saimiri (22) and latent membrane protein 1 (LMP1) of Epstein-Barr virus (11), KSHV contains a distinct open reading frame called K1 (28, 35, 53). The K1 gene is expressed at a low level in PEL, and its expression is significantly induced during the lytic phase of the viral life cycle (28). The K1 protein is predicted to have a signal peptide sequence at the amino terminus, an extracellular domain, a transmembrane domain, and a short cytoplasmic tail at the carboxyl terminus (34). The predicted extracellular domain of the K1 protein demonstrates regional homology with the variable region of the lambda chain of the immunoglobulin (Ig) light chain (34). Besides its regional homology with the Ig family, the extracellular domain of K1 also contains conserved regions (C1 and C2) and variable regions (V1 and V2). Particularly, two 40-amino-acid blocks at the extracellular V1 and V2 domains of K1 show as much as 85% divergence at the nucleotide level and 60% divergence at the amino acid level (7, 20, 27, 53). In addition, the amino-terminal region of K1 specifically interacts with the μ chains of B-cell antigen receptor (BCR) complexes, and this interaction inhibits the intracellular transport of BCR, resulting in downregulation of BCR surface expression (31). Furthermore, similar to Igα and Igβ, the cytoplasmic region of K1 contains a functional immunoreceptor tyrosine-based activation motif (ITAM), which transduces extracellular signals to elicit cellular activation events (30, 34). Recent reports have also shown that ITAM-dependent signaling by K1 modestly augments lytic reactivation in KSHV-infected PEL cells (29), whereas it strongly suppresses chemically induced lytic reactivation (33). These indicate that K1 has multiple roles in cellular signal transduction and viral lytic reactivation.
Despite extensive studies of K1 signal transduction, its cellular targets have not been well characterized. Here we demonstrate that K1 protein efficiently interacts with a number of cellular Src homology 2 (SH2)-containing signaling proteins through its ITAM and that each tyrosine residue of K1 ITAM targets different cellular SH2-containing proteins. K1 interactions with cellular signaling proteins consequently augmented cellular signal transduction activity, including tyrosine phosphorylation, intracellular calcium mobilization, transcription factor activation, and inflammatory cytokine production. These results indicate that KSHV K1 assembles its signalosome upon stimulation, which effectively elicits downstream signal transduction and ultimately induces inflammatory cytokine production.
MATERIALS AND METHODS
Cell culture and transfection.
Cultures of 293T cells were grown in Dulbecco's modified Eagle's medium P supplemented with 10% fetal calf serum. BJAB and DT40 cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum. Fugene 6 (Roche, Indianapolis, IN) or calcium phosphate (Clontech, Palo Alto, CA) was used for transient expression of K1 in 293T cells. Electroporation at 260 V and 975 μF was used for transient expression of K1 in BJAB and DT40 cells. A stable BJAB cell line expressing K1 was selected and maintained by the presence of G418 (1 mg/ml).
Antibodies and reagents.
Monoclonal anti-K1 antibody was generated against the extracellular glycosylated K1 protein purified from mammalian cells as described previously (32). The polyclonal antibodies used in this study were anti-Lyn, anti-Syk, anti-PLCγ2, anti-p85, anti-SH2 domain-containing protein tyrosine phosphatase 1 (SH-PTP1), anti-SH-PTP2, anti-GRB2, and anti-Vav1/2 (Santa Cruz Biotechnology, Santa Cruz, CA). Antiphosphotyrosine antibody 4G10 was purchased from UBI (Lake Placid, NY), phosphatase inhibitor cocktail and sodium vanadate were purchased from Sigma Chemicals (St. Louis, MO), and wortmannin, herbimycin, and piceatannol were purchased from Calbiochem (San Diego, CA).
Plasmid construction.
K1 mutants were generated by PCR-based site-directed mutagenesis. DNA fragments containing full-length K1 were subcloned into vector pDEF3 for mammalian expression. DNA fragments containing the K1 cytoplasmic region (amino acids 251 to 289) were PCR amplified and subcloned in frame into bacterial glutathione S-transferase (GST) vector pGEX4T-1. Each K1 construct was completely sequenced to verify the presence of the mutation and the absence of any other changes.
GST pull-down assays.
GST fusion proteins were purified from either Escherichia coli strain Top10 or TKX1, which contains a mammalian elk tyrosine kinase expression vector (Stratagene, La Jolla, CA). Jurkat T-cell lysates were incubated with glutathione beads containing GST fusion protein in binding buffer (20 mM HEPES [pH 7.4], 100 mM NaCl, 1% NP-40, protease inhibitors) at 4°C for 2 h. Glutathione beads were then washed four times with binding buffer, and the proteins associated with the beads were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblot assay using a Fuji phosphorimager (BAS-1500; Fuji Film Co., Tokyo, Japan).
Protein purification and mass spectrometry.
BJAB cells were labeled with [35S]methionine-cysteine for 16 h, and cell lysates were used for GST pull down with tyrosine-phosphorylated and nonphosphorylated GST/K1-C protein. To identify K1-binding proteins, 10 liters of BJAB B cells was lysed with lysis buffer (20 mM HEPES [pH 7.4], 100 mM NaCl, 1% NP-40) containing protease and phosphatase inhibitors. After centrifugation, the supernatant was filtered through a 0.45-μm-pore-size syringe filter and precleared by mixing with GST-bound glutathione beads twice. Precleared lysates were mixed with glutathione beads containing GST, GST/K1-C fusion protein, or tyrosine-phosphorylated GST/K1-C(P) fusion protein for 4 h. Afterwards, the beads were extensively washed with lysis buffer. Proteins bound to the beads were eluted, separated by SDS-PAGE, and subjected to peptide sequencing at the Harvard mass spectrometry facility.
Cell stimulation, immunoprecipitation, and immunoblotting.
BJAB cells (5 × 106) expressing wild-type (WT) K1 or its mutant forms were stimulated with an anti-K1 antibody at 37°C for the indicated time. Stimulation was stopped by the addition of an equal volume of ice-cold 2× 1% NP-40 lysis buffer. Cell lysates were then precleared by rocking at 4°C for 1 to 2 h in the presence of protein A/G agarose (Santa Cruz Biotechnology). Precleared cell lysates were incubated with the relevant antibodies for 2 h, followed by protein A/G agarose for 2 h. Immunoprecipitates were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Chicago, IL). Membranes were reacted with appropriate antibodies in 5% skim milk-phosphate-buffered saline (PBS)-Tween 20 (or 3% bovine serum albumin-PBS-Tween 20 for phosphotyrosine blots). Protein was visualized with chemiluminescent detection reagents (Pierce, Rockford, IL) and detected by a Fuji phosphorimager.
Calcium mobilization analysis.
Cells (2 × 106) were loaded with 1 μM indo-1 in 200 μl of RPMI complete medium for 30 min at 37°C, washed once with complete medium, resuspended in 1 ml of RPMI complete medium, and then put on ice until analyzed. Baseline calcium levels were established for 1 min prior to addition of the antibody. Cells were stimulated with 10 μg/ml of anti-K1 2H1 antibody. Baseline absolute intracellular calcium levels were determined by using ionophore and EGTA. Data were collected and analyzed on a FACS Vantage (Becton Dickinson, Mountain View, CA).
Flow cytometry analysis.
Cells (5 × 105) were washed with RPMI medium containing 10% fetal calf serum and incubated for 30 min with mouse monoclonal K1 antibodies, preimmunized mouse serum, or anti-Flag antibody (Sigma, St. Louis, MO). Cells were then incubated for 20 min at 4°C with phycoerythrin-conjugated anti-mouse antibodies (PharMingen, San Diego, CA). After washing, each sample was fixed with 2% paraformaldehyde solution, and fluorescence-activated cell sorter analysis was performed with a FACScan (Becton Dickinson, Mountain View, CA).
Reporter assay.
Cells (107) were transfected with 2 μg of pGK-β-Gal plasmid, 10 μg of luciferase reporter plasmid, and 20 μg of or WT or mutant K1 plasmid. At 24 to 36 h posttransfection, cells were harvested and lysed in 200 μl of reporter lysis buffer. A luciferase assay was carried out according to the manufacturer's instructions (Promega, Madison, WI) and measured by luminometer (Victor). Luciferase activity was normalized to β-galactosidase activity and presented as n-fold induction compared with the control.
Cytokine array.
Cytokine production was performed by sandwich enzyme-linked immunosorbent assay (ELISA) using human cytokine antibody arrays (Panomics, Redwood City, CA). According to the manufacturer's recommendation, cytokine antibody-coated membranes were incubated with blocking solution, followed by incubation with culture supernatants for 1 to 2 h. After extensive washings, membranes were incubated with streptavidin-conjugated anticytokine mixtures for 1 to 2 h and then reacted with horseradish peroxidase-conjugated avidin for 1 h. Cytokines bound to membranes were measured by chemiluminescence assay using a Fuji phosphorimager. Signal intensity was calculated by subtracting the background signal.
RNA preparation and RNase protection assay (RPA).
Cells were transfected with 20 μg of K1 expression vector and harvested at 0, 6, 12, 24, and 36 h posttransfection. RNA was isolated from 2.0 × 107 cells by conventional phenol-chloroform extraction. The template was custom ordered from BD PharMingen based on cytokine array data. At each time point, 30 μg of total RNA was hybridized to the 32P-labeled RNA template at 90°C for 3 min and subsequently hybridized at 56°C for 16 h. RNase protection reactions were carried out using an RPA kit (BD PharMingen) according to the manufacturer's instructions. Following precipitation of the digested RNA, the samples were separated on 5% denaturing acrylamide gels. The gels were analyzed using a phosphorimager (BAS 2000; Fuji Photo Film Co., Tokyo, Japan).
RESULTS
Interaction of tyrosine-phosphorylated K1 with cellular SH2-containing proteins.
To identify cellular proteins interacting with K1, a GST pull-down assay was performed using bacterial GST fusion proteins. Unphosphorylated GST/K1-C containing the cytoplasmic region of K1 was produced from the E. coli strain Top10, and tyrosine-phosphorylated GSTK1-C(P) was produced from E. coli strain TKX1, which contains the elk tyrosine kinase. The elk tyrosine kinase has broad specificity and efficiently phosphorylates many mammalian proteins in E. coli. We found that over 60% of the GST/K1-C protein purified from E. coli TKX1 was tyrosine phosphorylated by elk kinase (Fig. 1A).
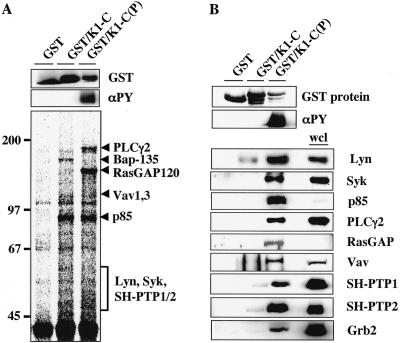
FIG. 1.
Identification of cellular proteins binding to the phosphorylated or nonphosphorylated cytoplasmic domain of K1. (A) K1-C binding protein purification and mass spectrometry analysis. GST and the unphosphorylated GST/K1-C and phosphorylated GST/K1-C(P) fusion proteins are shown at the top, and their tyrosine phosphorylation is shown in the middle. [35S]Met-Cys-labeled lysates of BJAB cells were mixed with bacterially purified Sepharose beads containing GST or the unphosphorylated GST/K1-C or phosphorylated GST/K1-C(P) fusion protein. After 4 h of incubation, beads were washed with lysis buffer and subjected to SDS-PAGE, followed by autoradiography (bottom). Individual proteins were purified from 10 liters of BJAB cells, sliced out of SDS-PAGE gels, and subjected to mass spectrometry. The values on the left are molecular sizes in kilodaltons. (B) In vitro GST pull-down assay. BJAB cell lysates were mixed with GST, GST/K1-C, or GST/K1-C(P) and washed with lysis buffer, followed by immunoblotting with specific antibodies (bottom). GST and the unphosphorylated GST/K1-C and phosphorylated GST/K1-C(P) fusion proteins are shown at the top, and their tyrosine phosphorylation is shown in the middle. αPY and wcl indicate the phosphotyrosine antibody and whole cell lysates, respectively.
To identify cellular proteins interacting with unphosphorylated or phosphorylated K1, the bacterially purified GST/K1-C and GST/K1-C(P) fusion proteins were used as an affinity column for 35S-labeled lysates of BJAB B cells. Polypeptides with apparent molecular masses of 85, 100, 110, and 140 kDa specifically interacted with the GST/K1-C fusion protein, whereas they did not interact with the GST protein (Fig. 1A). Furthermore, polypeptides with apparent molecular masses of 50 to 55, 70, 85, 100, 120, and 160 kDa interacted specifically with the GST/K1-C(P) fusion protein but did not interact with the GST and GST/K-1C fusion proteins (Fig. 1A). In an effort to identify cellular proteins interacting with K1, we performed bulk purification with over 10 liters of BJAB cell pellets with GST or with GST/K1-C or GST/K1-C(P) fusion protein. After identification of the purified proteins by mass spectrometry analysis, the interaction of unphosphorylated or phosphorylated K1 protein with most of these cellular proteins was further confirmed in GST pull-down and immunoblot assays using BJAB B-cell lysates (Fig. 1B). These assays showed that the cellular protein that interacted with unphosphorylated GST/K1-C but poorly with tyrosine-phosphorylated GST/K1-C(P) protein was BTK-associated protein 135 (Bap-135) (Fig. 1A). Conversely, the cellular proteins that interacted only with the tyrosine-phosphorylated GST/K1-C(P) protein but not with the unphosphorylated GST/K1-C protein were Grb2, SH-PTP1/2, the p85α regulatory subunit of phosphatidylinositol 3-kinase (PI3-kinase), Syk, Vav1/3, RasGAP120, and PLCγ2 (Fig. 1A and B). The cellular Lyn, SH-PTP1, and SH-PTP2 proteins showed low levels of interaction with unphosphorylated GST/K1-C, and these interactions were significantly enhanced with the phosphorylation of K1 ITAM (Fig. 1).
Role of each tyrosine residue of K1 ITAM in the interaction with cellular proteins.
The first SH2 binding motif (YY273SL) in K1 ITAM bears an additional tyrosine Y273 residue (Fig. 2A). Interestingly, despite the extensive sequence variation of K1, residue Y273 has been shown to be completely conserved among over 300 K1 isolates (53), suggesting its potential role in signal transduction. Thus, we designated this tyrosine residue Y2 to comply with Y1 and Y3 of the ITAM sequence (Fig. 2A). To investigate the roles of tyrosine residues of K1 ITAM in the interaction with cellular proteins, each tyrosine residue of K1 ITAM was mutated to phenylalanine to generate Y1F, Y2F, Y3F, Y1,2F, Y2,3F, Y1,3F, and Y1,2,3F (TYF). GST/K1-C mutant fusion proteins containing a replacement of the tyrosine residue with phenylalanine were purified from the TKX1 bacterial strains. An immunoblot assay with an antiphosphotyrosine antibody showed that, as seen in mammalian cells (34), the Y1 and Y3 residues but not the Y2 residue of K1 ITAM were phosphorylated in E. coli strain TKX1 (Fig. 2B).
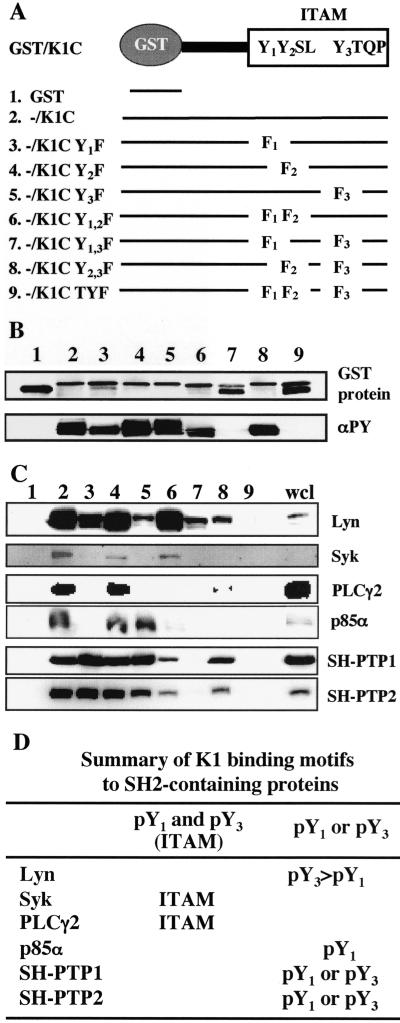
FIG. 2.
Mapping the binding sites in K1 ITAM. (A) Summary of mutant constructs. Three tyrosine residues in K1 ITAM were singly, doubly, or triply mutated to phenylalanine and fused to a pGEX4T-1 fusion construct. (B) GST/K1-C fusion proteins and their tyrosine phosphorylation. GST/K1-C constructs were transformed into E<. coli DH5α or TKX. After purification, GST, GST/K1-C, and GST/K1-C mutant proteins were detected by Coomassie blue staining (top) and their tyrosine phosphorylation was detected by immunoblotting with antiphosphotyrosine (αPY) antibody 4G10 (bottom). (C) Binding of GST/K1-C(P) fusion protein to cellular SH2-containing proteins. Phosphorylated GST/K1-C(P) fusion proteins were mixed with precleared BJAB cell lysates and subjected to immunoblotting with specific antibodies against SH2-containing signaling proteins. wcl, whole cell lysate. (D) Summary of K1 WT and mutant binding to cellular SH2-containing proteins.
Tyrosine-phosphorylated GST/K1-C(P) or GST/K1-C(P) mutant fusion protein was used for in vitro binding assays with BJAB cell lysates. The tyrosine-phosphorylated GST/K1-C, K1-C Y1F, K1-C Y2F, and K1-C Y1,2F fusion proteins bound to Lyn at similar levels; the tyrosine-phosphorylated K1-C Y3F, K1-C Y1,3F, and K1-C Y2,3F GST fusion proteins showed a significant reduction in Lyn interaction; and the K1-C TYF fusion showed no Lyn interaction (Fig. 2C and D). This indicates that the Y3 residue of K1 ITAM is important for the tyrosine phosphorylation-dependent interaction with Lyn and that the Y2 residue of K1 ITAM also contributes to this interaction (Fig. 2C and D). In addition, tyrosine-phosphorylated K1-C, K1-C Y2F, and K1-C Y1,2F GST fusions efficiently bound to Syk, while the other K1-C mutant fusion proteins were not able to bind to Syk (Fig. 2C and D). PLCγ2 interaction required the intact ITAM sequence of K1, whereas p85α interaction was primarily dependent on the phosphorylated Y1 residue of K1 ITAM (Fig. 2C and D). In contrast, the phosphorylated Y1 or Y3 residue of K1 ITAM was sufficient to bind to SH-PTP1/2 (Fig. 2C and D). Finally, K1-C binding activity to cellular SH2-containing proteins appeared to be dependent on tyrosine phosphorylation, because these interactions were detected effectively with the tyrosine-phosphorylated GST/K1-C proteins but poorly with the unphosphorylated GST/K1-C proteins (data not shown). These results indicate that while K1 cytoplasmic ITAM efficiently binds to cellular SH2-containing Lyn, Syk, PLCγ2, p85α, and SH-PTP1/2 signaling molecules through its phosphorylated tyrosine residues, each binding requires the specific phosphorylated SH2 binding motifs of K1.
Distinct role of each tyrosine residue of K1 ITAM in the elevation of intracellular calcium concentration.
Upon engagement with the antigen, BCRs initiate an array of signal transduction events: rapid tyrosine phosphorylation of cellular proteins, increase of intracellular free Ca2+, activation of cellular transcription factor activity, and ultimately, production of cytokine (36). To further examine K1-mediated signal transduction, we constructed KSHV-negative BJAB cells stably expressing WT full-length K1 or its mutants. K1 mutants showed equivalent levels of intracellular and surface expression compared to WT K1 (Fig. 3A and B).
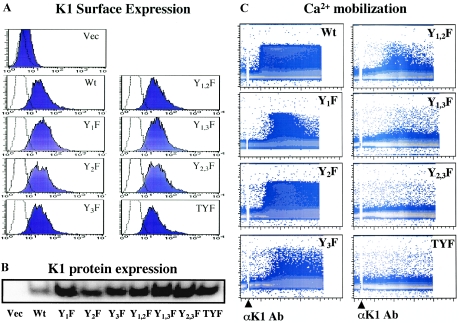
FIG. 3.
K1-mediated intracellular Ca2+ mobilization. (A) Surface expression of WT K1 and its mutant forms. BJAB cells were transfected with the pcDEF vector (Vec), pcDEF/K1, and pcDEF/K1 mutant constructs, and live cells were sorted by Ficoll-Hypaque centrifugation at 24 h posttransfection. These cells were then incubated with 1 mg/ml of G418 for 6 to 8 weeks and tested for K1 surface expression with an anti-K1 antibody. A histogram of each cell line (dark blue shaded) is overlaid with a dotted-line histogram of the anti-mouse total IgG antibody used as an isotype control. (B) WT and mutant K1 expression. WT and mutant K1 expression in BJAB cells was detected by immunoblotting with an anti-K1 antibody. (C) K1-mediated intracellular Ca2+ mobilization. Calcium mobilization was monitored over time by changes in the ratio of violet to blue (405 nm to 485 nm) fluorescence of cells loaded with indo-1 and analyzed by flow cytometry. Data are presented as a histogram of the number of cells with a particular fluorescence ratio (y axis) versus time (x axis). Arrowheads indicate the addition of anti-K1 antibody (Ab) 2H1. Ionomycin was added as a control for intracellular calcium mobilization. The breaks in the graphs indicate the time intervals during addition of antibodies. Data were similar in three independent experiments.
We have previously demonstrated that the 2H1 anti-K1 antibody, which recognizes amino acids 92 to 125 of the K1 ectodomain, is capable of inducing an increase in the intracellular Ca2+ concentration (32). BJAB cells expressing WT K1 or its mutant forms were treated with the anti-K1 2H1 antibody, and their intracellular Ca2+ concentration was monitored by flow cytometry. While control BJAB-vector cells showed no alteration of the intracellular Ca2+ concentration, BJAB-K1 and BJAB-K1 Y2F cells exhibited rapid increases in the intracellular Ca2+ concentration immediately after anti-K1 antibody stimulation and this response lasted more than 10 min (Fig. 3C). BJAB-K1 Y1F cells showed a slightly delayed elevation in the intracellular Ca2+ concentration upon stimulation, and this response was much shorter than that observed in BJAB-K1 cells (Fig. 3C). Interestingly, BJAB-K1 Y3F cells showed a significantly reduced level of intracellular Ca2+ elevation but this response lasted for a prolonged period, as seen in BJAB-K1 cells (Fig. 3C). BJAB-K1 Y1,2F cells showed the minimal level of intracellular Ca2+ mobilization upon stimulation (Fig. 3C). Finally, BJAB cells expressing K1 Y1,3F, K1 Y2,3F, or K1 TYF showed little or no response in the elevation of the intracellular Ca2+ concentration under the same conditions (Fig. 3C). These results collectively suggest that the Y1 residue of K1 ITAM is responsible for the duration of signaling activity, whereas the Y3 residue of K1 ITAM contributes to the strength of signaling activity. Furthermore, while the Y2 mutation alone did not affect the intracellular Ca2+ mobilization, its combination with the Y1 or Y3 mutation significantly diminished the level of intracellular Ca2+ mobilization, indicating that the Y2 residue may play an indirect role in K1 ITAM-mediated signal transduction.
Effect of K1 signaling on the activation of transcription factor activity.
To further detail the role of each tyrosine residue in K1 ITAM-mediated signal transduction, we examined whether WT K1 or its mutant expression induced the activation of cellular AP-1 and NF-AT transcription factor activity. At 24 h postelectroporation with each transcription factor luciferase reporter construct together with K1 or its mutant expression vector, lysates of BJAB cells were used to measure luciferase activity. pGK-β-gal expressing β-galactosidase from a phosphoglucokinase promoter was included as a transfection control. WT K1 and K1 Y2F expression drastically activated AP-1 and NF-AT transcription factor activity (Fig. 4A). In contrast, the K1 Y1F, K1 Y3F, K1 Y2,3F, K1 Y1,3F, and K1 TYF mutants poorly activated AP-1 and NF-AT transcription activity, indicating that the Y1 and Y3 residues but not the Y2 residue of K1 ITAM are required for the full strength of NF-AT and AP-1 transcription activation (Fig. 4A). Surprisingly, the additional mutation at the Y2 residue restored the ability of the K1 Y1F mutant to induce NF-AT transcription activation to the level seen with WT K1, but only partially restored its ability to induce AP-1 transcription activation under the same conditions (Fig. 4A). WT K1 and mutant forms of K1 were expressed at equivalent levels (Fig. 4A). Finally, it should be noted that despite their lack of signaling activity, most K1 mutants reproducibly showed a detectable level of NF-AT and AP-1 transcription activation, suggesting that sequences other than that of cytoplasmic ITAM may also play a role in the K1-mediated activation of NF-AT and AP-1 transcription activity (Fig. 4A).
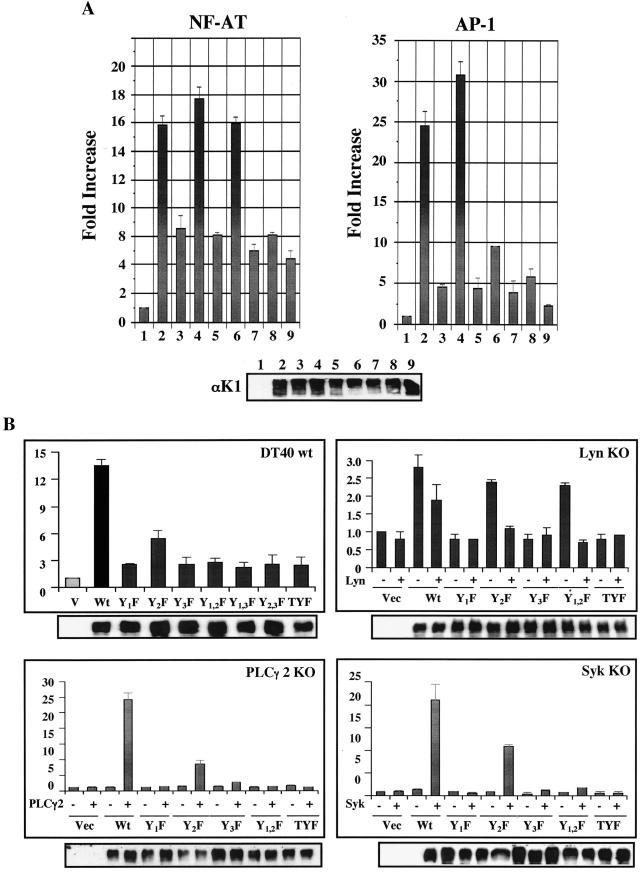
FIG.4.
Activation of NF-AT and AP1 transcription factors activity by K1. (A) Activation of NF-AT and AP1 transcription factor activity by K1 in BJAB cells. WT and mutant forms of K1 were electroporated into BJAB cells with NF-AT- or AP1-luciferase together with pGK-β-gal. At 24 h after transfection, cells were washed once in PBS and assayed for luciferase. Luciferase values were normalized by β-galactosidase activity to control transfection efficiency. The y axis indicates n-fold induction of NF-AT and AP1 activity by K1 expression. The results are averaged from three independent assays. Columns and lanes: 1, vector only; 2, WT K1; 3, Y1F mutant K1; 4, Y2F mutant K1; 5, Y3F mutant K1; 6, Y1,2F mutant K1; 7, Y1,3F mutant K1; 8, Y2,3F mutant K1; 9, TYF mutant K1. (B) K1-mediated NF-AT activation in DT40 WT and KO cell lines. Fifteen milligrams of pcDEF/K1 or mutant constructs, 7 mg of NFAT-luciferase constructs, and 2 mg of β-galactosidase plasmids were electroporated into 107 DT40 or KO cells. To restore the deleted gene expression in KO cells, 15 mg of PLCγ2 or Syk expression vector was included in the transfection mixture. Luciferase activity was evaluated as described for panel A.
To further delineate K1 ITAM function in NF-AT transcription factor activation, we used DT40 chicken B cells. Because of its unusually high homologous recombination efficiency, Lyn knockout (KO), Syk KO, or PLCγ2 KO cells from the DT40 B-cell line were established by specific gene targeting (25). To investigate the effects of cellular gene deficiency on K1 signaling, DT40, DT40 Lyn KO, DT40 Syk KO, and DT40 PLCγ2 KO cells were electroporated with WT K1 or its mutant expression vector together with the NF-AT luciferase reporter construct. At 48 h postelectroporation, cell lysates were used for luciferase assay. WT K1 expression effectively induced NF-AT transcription factor activity in chicken DT40 B cells, whereas the K1 Y2F mutant did so weakly under the same conditions (Fig. 4B). In contrast, WT K1 and its mutant forms were not capable of inducing NF-AT activation in PLCγ2- and Syk-deficient DT40 cells (Fig. 4B). To further confirm that the loss of K1 signaling activity was attributed solely to the specific cellular gene deletion and not to other unexpected alterations, the deleted cellular gene for PLCγ2 or Syk was restored by transfecting an expression vector containing each cDNA into DT40 mutant cell lines. Expression of PLCγ2 or Syk in DT40 PLCγ2 KO cells or DT40 Syk KO cells, respectively, fully restored K1-mediated NF-AT activation but did partially restore K1 Y2F mutant-mediated NF-AT activation (Fig. 4B). In contrast, the expression of PLCγ2 or Syk did not supplement the lack of NF-AT activation activity of other K1 mutants in DT40 PLCγ2 KO cells or DT40 Syk KO cells (Fig. 4B). These results indicate that cellular PLCγ2 and Syk are required for K1-mediated NF-AT activation and that the carboxyl-terminal ITAM sequence is also necessary for PLCγ2- and Syk-mediated recovery of K1 signaling activity, which is consistent with their ability to bind K1, as shown in Fig. 3. These results are consistent with previous results that show the essential role of Syk in K1 signal transduction (30).
DT40 Lyn KO cells displayed marked differences from DT40 Syk KO and DT40 PLCγ2 KO cells in K1-mediated signal transduction. Not only did DT40 Lyn KO cells display a higher background level of NF-AT transcription factor activity than DT40 Syk KO and DT40 PLCγ2 KO cells (data not shown), but they also weakly supported K1-mediated activation of NF-AT transcription factor activity (Fig. 4B). Furthermore, as seen in BJAB cells (Fig. 4A), K1 Y2F and Y1,2F mutants but not Y1F, Y3F and TYF mutants were capable of inducing NF-AT activation in DT40 Lyn KO cells to a level equivalent to WT K1 (Fig. 4B). In contrast to the expression of Syk and PLCγ2, which restored K1 signal transduction in cell lines deficient in Syk and PLCγ2 expression, Lyn expression weakly suppressed the K1-mediated activation of NF-AT transcription factor activity, suggesting that Lyn may play an inhibitory role in K1-meidated signal transduction (Fig. 4B). Immunoblot analysis with an anti-K1 antibody showed equivalent expression of WT and mutant forms of K1 in DT40 cells (Fig. 4B). In summary, K1 interactions with individual SH2-containing proteins play distinctive roles in the alteration of lymphocyte signal transduction.
Inhibition of K1 signal transduction by Syk and PI3-kinase inhibitors.
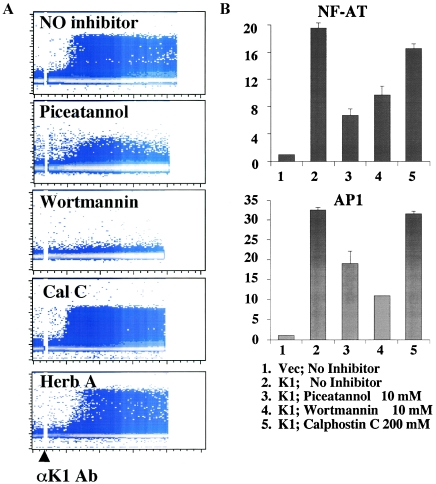
To further delineate the roles of cellular SH2-containing proteins in K1 signal transduction, we measured intracellular Ca2+ mobilization in the presence or absence of the Syk inhibitor piceatannol, the PI3-kinase/p85 inhibitor wortmannin, the protein kinase C (PKC) kinase inhibitor calphostin C, and the Src kinase inhibitor herbimycin A. BJAB cells were electroporated with the pTracer-GFP or pTracer-GFP-K1 expression vector, and live cells were sorted at 6 h posttransfection. After an additional 16 to 18 h of incubation, sorted cells were individually treated with inhibitors for 1 h, gated for the GFP-positive population, and then subjected to measurement of intracellular Ca2+ mobilization upon anti-K1 2H1 antibody stimulation. This showed that treatment with the Syk inhibitor piceatannol markedly suppressed K1-mediated intracellular Ca2+ mobilization and the p85 inhibitor wortmannin completely abolished K1-mediated intracellular Ca2+ mobilization (Fig. 5A). By contrast, treatment with the PKC kinase inhibitor calphostin C showed no effect on K1-mediated intracellular Ca2+ mobilization whereas the Src kinase inhibitor herbimycin slightly delayed and abated K1-mediated intracellular Ca2+ mobilization (Fig. 5A). To further support these findings, we measured the K1-mediated activation of NF-AT and AP-1 transcription factor activities in the presence or absence of inhibitors. Consistent with the results of intracellular Ca2+ mobilization, treatment with piceatannol and wortmannin, but not with calphostin C, considerably suppressed the K1-mediated activation of NF-AT and AP-1 factor activity (Fig. 5B). Thus, these data indicate that Syk and PI3-kinase are critical for the K1-mediated Ca2+ response and transcription factor activation.
FIG. 5.
Effects of specific inhibitors on K1-mediated Ca2+ mobilization and transcription factor activation. (A) Effects of specific inhibitors on K1-mediated Ca2+ mobilization. BJAB cells stably expressing K1 were loaded with Indo-1 dye and treated with or without 10 mM Syk inhibitor piceatannol, 10 mM PI3-kinase inhibitor wortmannin, 200 mM PKC kinase inhibitor calphostin C (Cal C), or 100 mM Src inhibitor herbimycin A (Herb A) for 30 min before analysis. Intracellular Ca2+ mobilization was measured as described in the legend to Fig. 3B. (B) Effects of specific inhibitors on K1-mediated transcription factor activation. BJAB cells were coelectroporated with 20 mg of pcDEF/K1 and 10 mg of NF-AT or AP-1 luciferase constructs. Live cells were separated by Ficoll-Hypaque centrifugation at 6 h posttransfection and aliquoted into six-well plates. Cells were further cultured for an additional 16 to 18 h in the presence or absence of inhibitors, washed once in PBS, and then assayed for luciferase activity. Luciferase values were normalized by β-galactosidase activity to control transfection efficiency. The y axis indicates n-fold induction of NF-AT and AP-1 activity by K1 expression. The results are averaged from three independent assays.
K1 signaling induces inflammatory cytokine production.
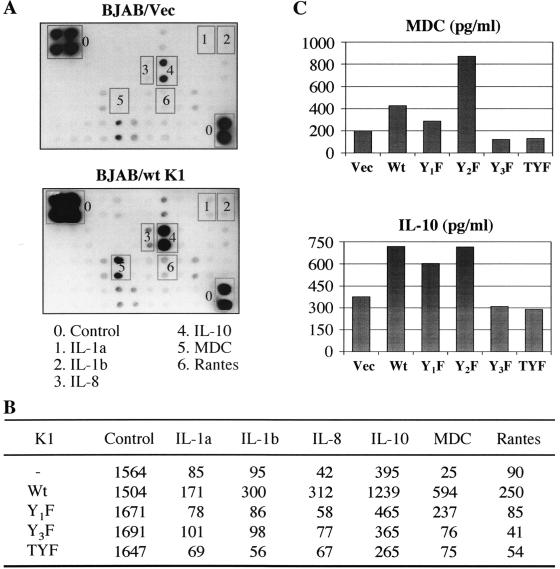
Cytokines or chemokines and angiogenic factors have been proposed to play key roles in the development of KS pathogenesis (8, 19). Since K1 strongly elicits an NF-AT and AP1 transcriptional potential, we tested the effect of K1 on cellular cytokine production using a cytokine antibody array that contains 41 different cytokine antibodies. This procedure uses a sandwich immunoblot assay to profile the expression of cellular cytokines and chemokines. The supernatants of BJAB cells transfected with the vector only or the K1 expression vector were incubated with the array membrane to permit cytokine binding to the immobilized antibodies on the array. The captured cytokines were detected by a mixture of biotin-labeled anticytokine antibodies, followed by streptavidin-horseradish peroxidase and chemiluminescence detection. This assay showed that K1 expression significantly induced the production of IL-1α, IL-1β, IL-8, IL-10, monocyte-derived chemokine (MDC), and RANTES (Fig. 6A). To further investigate the role of each tyrosine residue of the K1 ITAM sequence in cytokine production, we included K1 Y1F, K1 Y3F, and K1 TYF mutants in the cytokine antibody array. This also showed that mutations at the Y1 and/or Y3 residues of K1 ITAM considerably abolished its ability to induce the production of cytokines except IL-10; the K1 Y1F mutant weakly induced IL-10 production (Fig. 6B). Finally, an ELISA was used to determine the specific amounts of MDC and IL-10 from BJAB cells transfected with the vector, WT K1, K1 Y1F, K1 Y2F, K1 Y3F, or K1 TYF. Consistent with its signaling ability, K1 and K1 Y2F expression significantly induced MDC and IL-10 production (Fig. 6C). In addition, as seen in the cytokine antibody array, the K1 Y1F mutant weakly induced IL-10 production (Fig. 6C). These data suggest that K1 ITAM-mediated signal transduction ultimately induces cellular cytokine production and that each tyrosine residue of K1 ITAM may play a distinctive role in this process.
FIG. 6.
Cytokine antibody array and ELISA. (A) Cytokine antibody array. BJAB cells (107) were electroporated with 20 mg each of pcDEF, pcDEF/K1, pcDEF/K1 Y1F, pcDEF/K1 Y3F, or pcDEF/K1 TYF. Live cells were separated by Ficoll-Hypaque centrifugation at 6 h posttransfection and cultured for additional 30 h. The supernatants were collected, spun down to remove cell debris, and subjected to a cytokine antibody array. The antibody array was carried out in accordance with the manufacturer's recommendations using a cytokine array membrane (Panomics, Redwood City, CA), which contains 42 cytokines and chemokines and six controls. (B) Values from the cytokine array membrane were evaluated by densitometry after subtraction of the background signal and normalized by the control signal. (C) MDC and IL-10 ELISA. Supernatants were prepared as described above. ELISA (BD PharMingen) was carried out in accordance with the manufacturer's recommendations. Vec, vector.
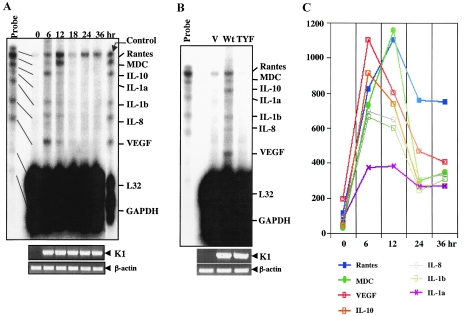
To further confirm K1-mediated cytokine production, we performed an RPA. Based on cytokine array data, we generated a custom-made RPA template set composed of RANTES, MDC, IL-10, IL-1α, IL-1β, IL-8, and VEGF. L32 and glyceraldehyde-3-phosphate dehydrogenase were included as internal controls. Figure 7A shows that the levels of the MDC, IL-10, IL-1α, IL-1β, IL-8, and VEGF mRNAs were markedly induced at 6 h and 12 h posttransfection of the K1 expression vector and rapidly diminished thereafter. Interestingly, the RANTES mRNA level was significantly increased upon K1 expression and stayed high up to 36 h posttransfection (Fig. 7A). However, the K1 TYF mutant showed no ability to induce cytokine mRNA production under the same conditions, indicating that K1 signaling activity is solely required for the induction of cytokine production (Fig. 7B). Finally, cellular L32 and glyceraldehyde-3-phosphate dehydrogenase mRNA levels were not altered by K1 expression (Fig. 7A and B). Densitometry analysis further showed the different kinetics of cytokine mRNA production induced by K1 expression (Fig. 7C). These results demonstrated that K1 signal transduction leads to the robust production of inflammatory cytokines.
FIG. 7.
RPA. (A) Induction of cytokine mRNAs by K1. BJAB cells (107) were electroporated with 20 mg of pcDEF/K1 WT, and live cells were separated by Ficoll-Hypaque centrifugation at 0, 6, 12, 24, and 36 h postelectroporation. Total RNA was isolated by phenol-chloroform extraction, and contaminated DNA was removed by DNase I treatment. Two milligrams of total RNA was then subjected to RPA using an RPA kit (BD PharMingen, San Diego, CA) according to the manufacturer's recommendations. 32P-labeled probes before and after RPA were included at the left and right sides of the gel, respectively, as controls. Reverse transcription-PCR was performed to detect K1 and actin expression (bottom). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Cytokine mRNA production by WT K1 but not by TYF mutant K1. BJAB cells (10 × 106) were electroporated with 20 mg of pcDEF, pcDEF/K1, or pcDEF/K1TYF. Live cells were harvested and separated by Ficoll-Hypaque centrifugation at 18 h. RPA was carried out as described above. 32P-labeled probes before RPA were included at the left side of the gel as controls. Reverse transcription-PCR was performed to detect WT K1, TYF mutant K1, and actin expression (bottom). V, vector. (C) Kinetics of cytokine mRNA production induced by K1. Signal intensities are shown as kinetics. The values shown were obtained by subtraction of the background signal intensity.
DISCUSSION
Here we report on the characterization of the K1 signalosome that contains a variety of cellular SH2-containing proteins. Upon stimulation, K1 ITAM is efficiently tyrosine phosphorylated and subsequently interacts with cellular SH2-containing signaling proteins Lyn, Syk, the p85α regulatory subunit of PI3-kinase, PLCγ2, RasGAP, Vav, SH-PTP1/2, and Grb2. These interactions ultimately lead to the marked augmentation of cellular signal transduction activity including the increase of cellular tyrosine phosphorylation and intracellular calcium mobilization, activation of the NF-AT and AP-1 transcription factors, and production of inflammatory cytokines. These results demonstrate that KSHV K1 effectively forms a signalosome upon stimulation, which elicits downstream signal transduction and thereby induces inflammatory cytokine production.
ITAM, which is responsible for the activation signal, is present in the coreceptor Ig-α and Ig-β molecules of the BCR complex (6, 17, 52). In resting B cells, the Src family kinase Lyn associates weakly with the BCR by virtue of an interaction between the N-terminal domain of Lyn and the unphosphorylated ITAM of Ig-α and Ig-β (6, 17, 52). This weak interaction is further stabilized by Lyn binding to the plasma membrane through its SH4 domain (49). Upon receptor ligation, associated Lyn kinase rapidly phosphorylates ITAM (pITAM) of Ig-α and Ig-β of the BCR complex. pITAM of Ig-α and Ig-β of the BCR complex then interacts with SH2-containing kinases, including Syk, which turns on proximal activation signaling events. Our binding assay showed that the unphosphorylated K1 protein weakly interacted with Lyn and this interaction was dramatically enhanced after K1 tyrosine phosphorylation. This indicates that, as seen with BCR, Lyn loosely associates with unphosphorylated K1 before stimulation and likely initiates K1 phosphorylation upon stimulation. Phosphorylated K1 subsequently interacts with Syk, which elicits a number of signaling events. This hypothesis is supported by the essential role of Syk in the induction of K1-mediated signal transduction. In addition, tyrosine-phosphorylated K1 also interacts with PLCγ2, Vav, and Grb2 as parts of The K1 signalosome that play important roles in K1 signal transduction. However, it was to our surprise that K1 was able to weakly induce NF-AT activity in Lyn-deficient DT40 cells and that Lyn expression led to the slight suppression, rather than activation, of K1-mediated NF-AT activation. This is likely because Lyn plays both activating and inhibitory roles in B-cell function (17). In fact, Lyn has been shown to have the ability to negatively regulate signaling pathways through phosphorylation of inhibitory receptors, enzymes, and adaptors (17). It should be noted that K1-mediated activation of NF-AT activity in DT40 Lyn−/− cells was lower than that in WT, Syk−/−, and PLCg2−/− DT40 cells (Fig. 4B). This was likely because Lyn gene KO resulted in the constitutive activation of B-cell signal transduction, which led to the higher basal level of NF-AT activity and subsequently limited the activation level of NF-AT activity induced by K1. Nevertheless, these results indicate that K1 mimics BCR in the recruitment of the Lyn and Syk kinases. Further study with cellular Lyn and Syk will delineate the detailed mechanism of K1-mediated signal transduction.
Receptor-mediated signaling pathways are tightly regulated by a coordinated activity of protein tyrosine kinases and protein tyrosine phosphatases (50). While protein tyrosine kinases are critical for signaling initiation, protein tyrosine phosphatases are involved in terminating activation signals by dephosphorylating the receptors and other components of the signaling cascade. SH-PTP has been shown to be a prominent factor to turn off signals by dephosphorylation of tyrosine residues within ITAM (50). In fact, after a short period of activation, a negative regulatory mechanism mediated by SH-PTP suppresses BCR signal activity back to the inactive status, which is essential for the maintenance of thresholds of BCR activation. Intriguingly, we found that K1 efficiently interacted with SH-PTP1/2. This suggests that, as seen in BCR signal transduction, SH-PTP1/2 may play a feedback-inhibitory role in K1 signal transduction or that K1 may deregulate SH-PTP1/2 to elicit abnormal signal transduction. We found that, unlike BCR signaling, which induced a transient increase of intracellular calcium mobilization upon antigen ligation (less than 2 to 3 min), the K1 signal displayed a considerably prolonged increase of intracellular Ca2+ mobilization upon anti-K1 antibody stimulation he (Fig. 3). This indicates that, unlike BCR, K1 may interact with and deregulate SH-PTP1/2 to induce uncompromised signal transduction upon stimulation, which leads to continuous activation and thereby uncontrolled cell growth. This hypothesis is currently under active investigation.
Detailed mutation analysis indicates that each tyrosine residue of K1 ITAM contributes to the interactions with cellular SH2-containing signaling proteins in a distinctive way. Both the Y1 and Y3 residues of the K1 ITAM sequence were required for the interaction with Syk and PLCγ2, whereas residue Y1 or Y3 was sufficient to interact with Lyn, p85, Grb2, and SH-PTP1/2. Furthermore, the Y3 residue of K1 ITAM played a more significant role in Lyn interaction than the Y1 residue, whereas the Y1 residue of K1 ITAM was essential for binding to p85. Furthermore, the analysis of intracellular Ca2+ mobilization showed that the Y1 residue of K1 ITAM appeared to be responsible for the duration of its signaling activity and the Y3 residue contributed to the strength of its signaling activity. Finally, despite its high conservation among 300 different K1 alleles, the Y2 residue did not undergo tyrosine phosphorylation in vitro (Fig. 2) and in vivo (34), indicating that it may not be directly involved in the interaction with the SH2 domain. In fact, mutation at the Y2 residue did not affect the modulation of intracellular Ca2+ mobilization and the activation of NF-AT and AP-1 factor activity. However, K1 Y1,2F and Y2,3F mutants showed detectably different features from K1 Y1F and Y3F mutants, respectively, in the interaction with SH2-containing proteins, the modulation of intracellular Ca2+ mobilization, and the activation of NF-AT factor activity. This indicates that while it does not play a direct role in K1 signal transduction, the Y2 residue in combination with the Y1 and Y3 residues may modulate the stability and/or strength of physical interaction between K1 pITAM and SH2-containing proteins.
KS is a multifocal angiogenic tumor and appears to be a hyperplastic disorder caused, in part, by local production of inflammatory cytokines such as IL-1, IL-6, gamma interferon, and ανδ tumor necrosis factor alpha and growth factors such as basic fibroblast growth factor and VEGF (13-16). Our cytokine antibody array and RPA revealed that K1-medaited signal transduction markedly induced MDC, IL-8, IL-10, VEGF, IL-1αβ, and RANTES and that this K1 activity required the intact cytoplasmic ITAM sequence. The MDC promoter sequence contains NF-AT, NF-κB, and AP-1 binding sites (40), the RANTES promoter sequence has NF-AT and NF-κB binding sites (18), the IL-8 and IL-10 promoter sequences contain AP-1 and NF-κB binding sites (24, 39), and the VEGF promoter sequence bears SP-1 and AP-1 binding sites (21). The existence of these cis elements in each cytokine gene promoter suggests that K1-mediated activation of cellular transcriptional factor activity results in the up regulation of cytokine expression. Indeed, several reports have demonstrated that K1 efficiently induces IL-8 (47) and VEGF transcription by activating its promoter activity (42, 51). Thus, our results indicate that K1 ITAM forms a unique signalosome where each tyrosine residue has a distinct role in SH2 domain binding and signal transduction activity and that this signalosome ultimately induces the expression of inflammatory cytokine and angiogenic factor genes. This event consequently contributes to efficient virus dissemination and virus-associated angiogenic proliferation.
Acknowledgments
We especially thank S. G. Lee, G. A. Koretzky, and A. Weis for providing reagents; S. Gygi for mass spectrometry analysis; and M. Connole for flow cytometry analysis.
This work was partly supported by U.S. Public Health Service grants CA106156, CA82057, CA91819, and RR00168. Pinghui Feng and J. Jung are a Leukemia and Lymphoma Society Fellow and a Leukemia and Lymphoma Society Scholar, respectively.
REFERENCES
- 1.Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boshoff, C., and R. A. Weiss. 1998. Kaposi's sarcoma-associated herpesvirus. Adv. Cancer Res. 75:57-86. [DOI] [PubMed] [Google Scholar]
- 3.Browning, P. J., J. M. Sechler, M. Kaplan, R. H. Washington, R. Gendelman, R. Yarchoan, B. Ensoli, and R. C. Gallo. 1994. Identification and culture of Kaposi's sarcoma-like spindle cells from the peripheral blood of human immunodeficiency virus-1-infected individuals and normal controls. Blood 84:2711-2720. [PubMed] [Google Scholar]
- 4.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 5.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 6.Clark, M. R., K. S. Campbell, A. Kazlauskas, S. A. Johnson, M. Hertz, T. A. Potter, C. Pleiman, and J. C. Cambier. 1992. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science 258:123-126. [DOI] [PubMed] [Google Scholar]
- 7.Cook, R. D., T. A. Hodgson, A. C. W. Waugh, E. M. Molyneux, E. Borgstein, A. Sherry, C. G. Teo, and S. R. Porter. 2002. Mixed patterns of transmission of human herpesvirus-8 (Kaposi's sarcoma-associated herpesvirus) in Malawian families. J. Gen. Virol. 83:1613-1619. [DOI] [PubMed] [Google Scholar]
- 8.Cornali, E., C. Zietz, R. Benelli, W. Weninger, L. Masiello, G. Breier, E. Tschachler, A. Albini, and M. Sturzl. 1996. Vascular endothelial growth factor regulates angiogenesis and vascular permeability in Kaposi's sarcoma. Am. J. Pathol. 149:1851-1869. [PMC free article] [PubMed] [Google Scholar]
- 9.Damania, B. 2004. Oncogenic gamma-herpesviruses: comparison of viral proteins involved in tumorigenesis. Nat. Rev. Microbiol. 2:656-668. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers, R. C., V. G. Sasseville, S. C. Czajak, X. Zhang, K. G. Mansfield, A. Kaur, R. P. Johnson, A. A. Lackner, and J. U. Jung. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J. Virol. 71:9764-9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliopoulos, A. G., and L. S. Young. 2001. LMP1 structure and signal transduction. Semin. Cancer Biol. 11:435-444. [DOI] [PubMed] [Google Scholar]
- 12.Ensoli, B., C. Sgadari, G. Barillari, M. C. Sirianni, M. Sturzl, and P. Monini. 2001. Biology of Kaposi's sarcoma. Eur. J. Cancer 37:1251-1269. [DOI] [PubMed] [Google Scholar]
- 13.Ensoli, B., and M. Sturzl. 1998. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 9:63-83. [DOI] [PubMed] [Google Scholar]
- 14.Ensoli, B., M. Sturzl, and P. Monini. 2000. Cytokine-mediated growth promotion of Kaposi's sarcoma and primary effusion lymphoma. Semin. Cancer Biol. 10:367-381. [DOI] [PubMed] [Google Scholar]
- 15.Fiorelli, V., R. Gendelman, F. Samaniego, P. D. Markham, and B. Ensoli. 1995. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi's sarcoma spindle cells. J. Clin. Investig. 95:1723-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorelli, V., R. Gendelman, M. C. Sirianni, H. K. Chang, S. Colombini, P. D. Markham, P. Monini, J. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Gamma interferon produced by CD8+ T cells infiltrating Kaposi's sarcoma induces spindle cells with angiogenic phenotype and synergy with human immunodeficiency virus-1 Tat protein: an immune response to human herpesvirus-8 infection? Blood 91:956-967. [PubMed] [Google Scholar]
- 17.Gauld, S. B., and J. C. Cambier. 2004. Src-family kinases in B-cell development and signaling. Oncogene 23:8001-8006. [DOI] [PubMed] [Google Scholar]
- 18.Genin, P., M. Algarte, P. Roof, R. Lin, and J. Hiscott. 2000. Regulation of RANTES chemokine gene expression requires cooperativity between NF-κB and IFN-regulatory factor transcription factors. J. Immunol. 164:5352-5361. [DOI] [PubMed] [Google Scholar]
- 19.Gruffat, H., A. Sergeant, and E. Manet. 2000. Kaposi's sarcoma-associated herpesvirus and Kaposi's sarcoma. Microbes Infect. 2:671-680. [DOI] [PubMed] [Google Scholar]
- 20.Hayward, G. S. 1999. KSHV strains: the origins and global spread of the virus. Semin. Cancer Biol. 9:187-199. [DOI] [PubMed] [Google Scholar]
- 21.Josko, J., and M. Mazurek. 2004. Transcription factors having impact on vascular endothelial growth factor (VEGF) gene expression in angiogenesis. Med. Sci. Monit 10:RA89-RA98. [PubMed]
- 22.Jung, J. U., J. K. Choi, A. Ensser, and B. Biesinger. 1999. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin. Cancer Biol. 9:231-239. [DOI] [PubMed] [Google Scholar]
- 23.Kraffert, C., L. Planus, and N. S. Penneys. 1991. Kaposi's sarcoma: further immunohistologic evidence of a vascular endothelial origin. Arch. Dermatol. 127:1734-1735. [PubMed] [Google Scholar]
- 24.Kube, D., C. Platzer, A. von Knethen, H. Straub, H. Bohlen, M. Hafner, and H. Tesch. 1995. Isolation of the human interleukin 10 promoter. Characterization of the promoter activity in Burkitt's lymphoma cell lines. Cytokine 7:1-7. [DOI] [PubMed] [Google Scholar]
- 25.Kurosaki, T., A. Maeda, M. Ishiai, A. Hashimoto, K. Inabe, and M. Takata. 2000. Regulation of the phospholipase C-γ2 pathway in B cells. Immunol. Rev. 176:19-29. [DOI] [PubMed] [Google Scholar]
- 26.Lacey, S. F., K. J. Weinhold, C. H. Chen, C. McDanal, C. Oei, and M. L. Greenberg. 1998. Herpesvirus saimiri transformation of HIV type 1 suppressive CD8+ lymphocytes from an HIV type 1-infected asymptomatic individual. AIDS Res. Hum. Retrovir. 14:521-531. [DOI] [PubMed] [Google Scholar]
- 27.Lacoste, V., J. G. Judde, J. Briere, M. Tulliez, E. Kassa-Kelembho, J. Morvan, P. Couppie, E. Clyti, J. F. Vila, B. Rio, A. Delmer, P. Mauclere, and A. Gessain. 2000. Molecular epidemiology of human herpesvirus 8 in Africa: both B and A5 K1 genotypes, as well as the M and P genotypes of K14.1/K15 loci, are frequent and widespread. Virology 278:60-74. [DOI] [PubMed] [Google Scholar]
- 28.Lagunoff, M., and D. Ganem. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus. Virology 236:147-154. [DOI] [PubMed] [Google Scholar]
- 29.Lagunoff, M., D. M. Lukac, and D. Ganem. 2001. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J. Virol. 75:5891-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagunoff, M., R. Majeti, A. Weiss, and D. Ganem. 1999. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 96:5704-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, B. S., X. Alvarez, S. Ishido, A. A. Lackner, and J. U. Jung. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J. Exp. Med. 192:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, B. S., M. Connole, Z. Tang, N. L. Harris, and J. U. Jung. 2003. Structural analysis of the Kaposi's sarcoma-associated herpesvirus K1 protein. J. Virol. 77:8072-8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, B. S., M. Paulose-Murphy, Y. H. Chung, M. Connole, S. Zeichner, and J. U. Jung. 2002. Suppression of tetradecanoyl phorbol acetate-induced lytic reactivation of Kaposi's sarcoma-associated herpesvirus by K1 signal transduction. J. Virol. 76:12185-12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, H., J. Guo, M. Li, J. K. Choi, M. DeMaria, M. Rosenzweig, and J. U. Jung. 1998. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol. Cell. Biol. 18:5219-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, H., R. Veazey, K. Williams, M. Li, J. Guo, F. Neipel, B. Fleckenstein, A. Lackner, R. C. Desrosiers, and J. U. Jung. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat. Med. 4:435-440. [DOI] [PubMed] [Google Scholar]
- 36.Lin, J., and A. Weiss. 2001. T cell receptor signalling. J. Cell Sci. 114:243-244. [DOI] [PubMed] [Google Scholar]
- 37.Mesri, E. A., E. Cesarman, L. Arvanitakis, S. Rafii, M. A. Moore, D. N. Posnett, D. M. Knowles, and A. S. Asch. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J. Exp. Med. 183:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore, P. S., and Y. Chang. 2003. Kaposi's sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu. Rev. Microbiol. 57:609-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukaida, N., S. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56:554-558. [PubMed] [Google Scholar]
- 40.Nakayama, T., K. Hieshima, D. Nagakubo, E. Sato, M. Nakayama, K. Kawa, and O. Yoshie. 2004. Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein-Barr virus. J. Virol. 78:1665-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prakash, O., O. R. Swamy, X. Peng, Z. Y. Tang, L. Li, J. E. Larson, J. C. Cohen, J. Gill, G. Farr, S. Wang, and F. Samaniego. 2005. Activation of Src kinase Lyn by the Kaposi sarcoma-associated herpesvirus K1 protein: implications for lymphomagenesis. Blood 105:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regezi, J. A., L. A. MacPhail, T. E. Daniels, Y. G. DeSouza, J. S. Greenspan, and D. Greenspan. 1993. Human immunodeficiency virus-associated oral Kaposi's sarcoma. A heterogeneous cell population dominated by spindle-shaped endothelial cells. Am. J. Pathol. 143:240-249. [PMC free article] [PubMed] [Google Scholar]
- 44.Reitz, M. S., Jr., L. S. Nerurkar, and R. C. Gallo. 1999. Perspective on Kaposi's sarcoma: facts, concepts, and conjectures. J. Natl. Cancer Inst. 91:1453-1458. [DOI] [PubMed] [Google Scholar]
- 45.Renne, R., M. Lagunoff, W. Zhong, and D. Ganem. 1996. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J. Virol. 70:8151-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samaniego, F., S. Pati, J. E. Karp, O. Prakash, and D. Bose. 2001. Human herpesvirus 8 K1-associated nuclear factor-kappa B-dependent promoter activity: role in Kaposi's sarcoma inflammation? J. Natl. Cancer Inst. Monogr. 28:15-23. [DOI] [PubMed] [Google Scholar]
- 48.Searles, R. P., E. P. Bergquam, M. K. Axthelm, and S. W. Wong. 1999. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman, L., M. Sudol, and M. D. Resh. 1993. Members of the src family of nonreceptor tyrosine kinases share a common mechanism for membrane binding. Cell Growth Differ. 4:475-482. [PubMed] [Google Scholar]
- 50.Veillette, A., S. Latour, and D. Davidson. 2002. Negative regulation of immunoreceptor signaling. Annu. Rev. Immunol. 20:669-707. [DOI] [PubMed] [Google Scholar]
- 51.Wang, L., N. Wakisaka, C. C. Tomlinson, S. M. DeWire, S. Krall, J. S. Pagano, and B. Damania. 2004. The Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 64:2774-2781. [DOI] [PubMed] [Google Scholar]
- 52.Yamanashi, Y., T. Kakiuchi, J. Mizuguchi, T. Yamamoto, and K. Toyoshima. 1991. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science 251:192-194. [DOI] [PubMed] [Google Scholar]
- 53.Zong, J. C., D. M. Ciufo, D. J. Alcendor, X. Wan, J. Nicholas, P. J. Browning, P. L. Rady, S. K. Tyring, J. M. Orenstein, C. S. Rabkin, I. J. Su, K. F. Powell, M. Croxson, K. E. Foreman, B. J. Nickoloff, S. Alkan, and G. S. Hayward. 1999. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 73:4156-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]