In 1996, in a discussion of adenovirus biology in a standard virology textbook, it was noted that “the identity of the cellular receptor remains a mystery” (78). In the past few years, not just one but multiple adenovirus receptors have been identified, and new information has accumulated about the early events in adenovirus infection. The interest in receptors has been stimulated in large part by interest in using adenovirus vectors for therapeutic gene delivery to specific tissues. In this review, we will summarize our present understanding of adenovirus interaction with receptors and the role of receptors in virus entry and tissue tropism.
Adenovirus structure and classification.
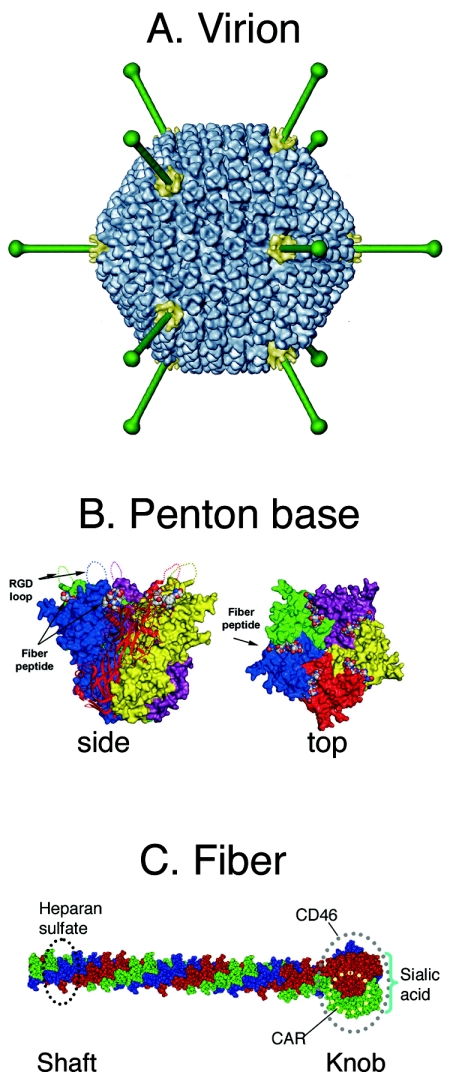
Adenovirus (Ad) is a nonenveloped, double-stranded DNA virus. Viral DNA and associated core proteins are encased in an icosahedral capsid, with 20 triangular faces composed largely of the major capsid protein, hexon (Fig. 1A) (reviewed in reference 68). An elongated fiber projects from each of the 12 fivefold capsid vertices. At its proximal end, the fiber is bound to a pentameric structure, the penton base; at its distal end, the fiber forms a globular “knob” domain (Fig. 1B). In general, the fiber knob functions as the major attachment site for cellular receptors, while the penton base is involved in secondary interactions that are required for virus entry into the cell. The detailed structures of hexon (64), fiber (94, 95), and penton base (105) have been determined by crystallographic methods; the overall virion structure (86) has been determined, at somewhat lower resolution, by cryoelectron microscopy and image reconstruction.
FIG. 1.
Adenovirus structure. (A) Virion structure at 17-Å resolution. Each of the 20 triangular faces of the capsid is composed of 12 copies of the hexon trimer (blue). At each fivefold vertex, a fiber (green) emerges from the pentameric penton base (yellow). The hexon capsid and penton base structures are derived from a cryoelectron microscopic image reconstruction of human adenovirus 5. Fibers are modeled from the atomic structure of the Ad2 fiber (95). Figure provided by Carmen San Martin. (B) Penton base. Side and top views, showing flexible RGD loops projecting from the surface. The insertion sites for fiber are also shown. (The figure is reprinted from reference 68 with permission of the publisher.) (C) Fiber structure and receptor binding sites. The fiber is a trimer whose monomers are indicated in red, blue, and green; the shaft is a tightly wound triple spiral; the knob is a more bulbous trefoil. The figure is modified from reference 95 with permission of the publisher.
To date, 51 human Ad serotypes have been identified (Table 1). These are divided into six subgroups (A to F) based on hemagglutination properties, oncogenicity in rodents, DNA homology, and genomic organization (49); subgroup B is further divided into B1 and B2 (34, 53). There is a correlation, although imperfect, between subgroup and tissue tropism, as follows: viruses in groups B1, C, and E cause respiratory infections; group B2 viruses infect the kidney and urinary tract; group F viruses cause gastroenteritis; and several group D serotypes are associated with epidemic keratoconjunctivitis.
TABLE 1.
Classification of human adenoviruses
| Subgroup | Serotype | Tropism |
|---|---|---|
| A | 12b, 18, 31b | |
| B1 | 3c,e 7, 16c, 21c, 50c | Respiratory |
| B2 | 11c, 14c, 34, 35c | Renal |
| C | 1, 2b,f, 5b,f, 6 | Respiratory |
| D | 8d, 9, 10, 13, 15b, 17, 19pb, 19ad, 20, 22, 23, 24, 25, 26, 27, 28, 29, 30, 32, 33, 36, 37c,d, 38, 39, 42, 43, 44, 45, 46, 47, 48, 49, 51 | Ocular and other |
| E | 4b | Respiratory |
| F | 40, 41b | Intestinal |
Those viruses for which specific receptors have been identified are marked with footnotes; attachment or infection by these viruses has been demonstrated to depend on expression of the indicated receptors. Based on more limited experimental evidence, putative receptors can be assigned for several other serotypes, as discussed in the text.
CAR.
CD46.
SA.
CD80/86.
Heparan sulfate.
Overview of virus entry.
Adenovirus entry into cells, as defined by experiments with cultured cells, generally involves attachment to a primary receptor, followed by interaction with a secondary receptor responsible for internalization. The virus enters the cell in a clathrin-coated vesicle and is transported to endosomes, where acidification results in partial disassembly of the capsid; the altered virion escapes into the cytoplasm and is transported to the nucleus, where replication occurs (54). The route of intracellular trafficking is influenced by the fiber knob and, thus, by interaction with a specific primary receptor. Ad5, whose fiber binds to the coxsackievirus and adenovirus receptor (CAR; see below) escapes rapidly from endosomes into the cytoplasm; however, when it is pseudotyped with fibers that bind to another primary receptor, Ad5 is retained in the endosomal pathway (55, 56, 76).
Integrins as entry receptors.
Many adenovirus serotypes display an RGD peptide within the penton base, which serves as a recognition site for several cellular integrins, members of a large family of heterodimeric (αβ) adhesion receptors. The engagement of integrins by the penton base induces signals, including activation of PI3 kinase (46), p130CAS (47), and Rho GTPases (45), that are important for rearrangements in the actin cytoskeleton and initiation of virus internalization. Interaction with integrins is also important for virus escape from the endosome (99).
Integrins known to facilitate adenovirus entry include the vitronectin receptors αvβ3 and αvβ5 (100), as well as αvβ1 (44), α3β1 (70), and α5β1 (19), all of which recognize RGD ligands. The role of integrins has been studied most extensively for Ads 2 and 5, whose primary receptor is CAR. However, integrin-RGD interactions are also involved in entry by viruses that bind to other primary receptors (52, 75), and—with the exception of the subgroup F viruses Ads 40 and 41 (1, 18)—all of the adenoviruses whose sequences have been determined have an RGD sequence within the penton base. It is important to note that mutation of the penton base RGD sequence slows, but does not prevent, virus internalization and infection (6). It is not clear whether this indicates that entry occurs by integrin-independent routes or whether some virus interactions with integrins are independent of the RGD motif. Internalization of RGD-deficient virus is more rapid in cells that express high levels of fiber receptor, suggesting that recruitment of multiple fiber receptors may compensate for the loss of penton-integrin interaction (27).
One consequence of the two-step entry mechanism is that adenoviruses can be quite flexible in their use of primary receptors. The major function of the fiber receptor is to hold the virion in close proximity to the cell surface, permitting interaction with an integrin molecule; a variety of cell surface molecules can serve this function.
ATTACHMENT RECEPTORS
CAR.
The best-studied adenovirus receptor is CAR, a 46-kDa protein that also mediates infection by group B coxsackieviruses (9, 92). CAR is an immunoglobulin superfamily member with two immunoglobulin-like extracellular domains. It mediates homotypic cell adhesion (35) and is present in specialized intracellular junctions, including the cardiac intercalated disk (60) and the tight junction of polarized epithelial cells (14). Although CAR's tissue distribution in humans is not well defined, mRNA is present in a number of organs, including the heart, brain, pancreas, and intestine (92) as well as the lung, liver, and kidney (26). CAR-mediated signals have been proposed to suppress the proliferation of tumor cells (62), and CAR knockout animals have developmental abnormalities, including ventricular hyperplasia (12).
CAR expression on target cells permits virus attachment by selected viruses belonging to subgroups A (Ad12), C (Ad2, Ad5), E (Ad4), and F (Ad41); in addition, soluble CAR interacts with Ad31 (subgroup A) and Ads 9 and 19 (subgroup D) or with their isolated fibers (66). CAR also serves as a receptor for several animal Ads (15, 84, 89). These observations have sometimes been interpreted to mean that CAR is a receptor for all the viruses outside group B, but this need not be the case; subgroups may contain heterogeneous viruses, and even viruses within a serotype may differ in their receptor interactions.
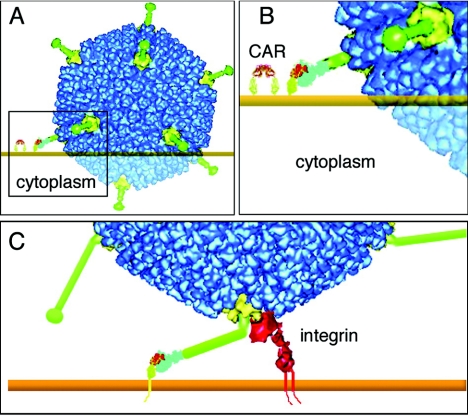
Furthermore, results obtained with isolated fibers or soluble CAR may not reflect what happens when intact virus interacts with receptors on the cell surface. The crystal structure of fiber bound to CAR's N-terminal domain (D1) (28) reveals that CAR interacts with a site on the outer surface of the trimeric fiber knob (67). Alignments of knob sequences from CAR-binding viruses indicate that the contact residues are only partially conserved; the capacity to bind receptors is somehow maintained despite sequence variation resulting from immunologic pressures (37). The need for virus to engage CAR and integrins at the same time appears to impose geometric constraints on receptor interactions—evident in structural models (Fig. 2) —so that CAR-binding viruses require fibers that are both long (66, 77) and flexible (101). Although the very short fibers of Ad9 can bind to CAR, Ad9 interaction with cells is not CAR dependent—the penton base may interact directly with an integrin molecule (65). Although the Ad37 fiber binds to isolated CAR protein, Ad37 attachment to cells depends on other receptors (see below). It has been proposed that free adenovirus fibers may facilitate virus spread by disrupting CAR-dependent intercellular junctions (96); this could conceivably occur even with fibers whose length or rigidity does not permit CAR-dependent virus infection.
FIG. 2.
Geometric constraints on receptor interaction. In panels A and B, attachment of a short fiber (green) to CAR on the cell surface does not permit the penton base protein to interact with a cell surface integrin molecule. In panel C, a virus with a long flexible fiber is able to interact with CAR and integrin at the same time. The figure is modified from Wu et al. (101) with permission.
Heparan sulfate glycosaminoglycans.
Heparan sulfate glycosaminoglycans (HS-GAGs) are long, heterogeneous, heavily sulfated carbohydrates, abundant within the extracellular matrix and the cellular glycocalyx; in many cases, the glycosaminoglycan is bound to a protein core, forming a proteoglycan (33). HS-GAGs mediate CAR-independent attachment and infection by Ads 2 and 5 (21, 22). Basic amino acid motifs are believed to permit protein recognition of HS-GAG (11); mutation of a KKTK motif within the proximal fiber shaft significantly modifies Ad5 tropism in vivo (82, 83), although it has not been confirmed that the effect results specifically from altered interaction with HS-GAG. It is not known whether other Ads also bind to these ubiquitous molecules, but the putative HS binding site is conserved in all group C adenoviruses and absent from other fibers whose sequences are available.
CD46.
Before any specific receptors had been identified, it was observed that Ad3, a group B virus, did not compete for an attachment site with Ads 2 or 5, suggesting that it bound to a different receptor from that used by these group C viruses (23, 85); consistent with this idea, none of the group B adenoviruses tested thus far—Ads 3, 7, 11, and 35—interacts with CAR (9, 66, 73). Further competition experiments suggest that group B viruses interact with at least two receptors. One of these appears to permit attachment by viruses from both groups B1 and B2; a second receptor is postulated to bind only those in group B2 (73).
In the past 2 years, many group B viruses have been shown to attach to CD46 (membrane cofactor protein), a complement regulatory protein expressed on all nucleated human cells (16). The evidence is strongest for Ads 11 (73, 74) and 35 (29), which have been shown to infect cells after attaching to CD46; for Ad3 (80) and the subgroup D virus Ad37 (93, 102), evidence for CD46-dependent infection comes from experiments with Ad5 vectors pseudotyped with Ad3 and Ad37 fibers. Ads 14, 16, 21, 35, and 50 bind to CD46, but infection has not been tested. Because their fibers are virtually identical to those of other CD46 binders, Ads 19a and 34 are also likely to interact with CD46. Both B1 and B2 viruses thus appear to bind to CD46, which may represent the common group B receptor postulated from competition experiments. It is interesting that CD46 functions as a receptor for a number of other pathogens, including measles virus (50), human herpesvirus 6 (71), Streptococcus pyogenes (61), and pathogenic Neisseria spp. (41).
Isolated knob domains block virus attachment to CD46, indicating that the attachment site resides within the knob rather than the fiber shaft (29, 74, 80). Ad35 fiber binds to a membrane-distal fragment of CD46 (30). However, the binding site is not well defined, and it is possible that different viruses attach to different sites within the molecule (74, 102).
CD80 and CD86.
Ad3, a virus that can interact with CD46, also enters cells after binding to either CD80 or CD86 (B7-1 and B7-2) (79), distantly related immunoglobulin superfamily members that are expressed on antigen-presenting cells and are best known for their important function in T-cell activation (32). Ad3-mediated transduction of isolated dendritic cells depends on interaction with CD80 and CD86 (79). Replication-defective adenoviruses are being developed as vaccine vectors, and viruses that target receptors expressed on dendritic cells (90) (including CD80/86 and CD46) may prove useful in eliciting strong immune responses. It is not yet known whether other Ad serotypes also interact with CD80 or CD86. Since Ad3 is a member of the B1 subgroup, CD80 and CD86 are unlikely to represent the putative B2-specific receptor, which remains to be identified.
Sialic acid.
Several subgroup D viruses (Ads 37, 8, and 19a) (3-5) have been shown to infect cells after attachment to α(2-3)-linked sialic acid (SA), a common carbohydrate component of glycoproteins and glycolipids. Crystallographic studies demonstrate that SA binds to a site at the very top of the fiber knob (10); residues potentially involved in SA interaction are conserved in the fibers of all group D viruses. The three viruses known to bind SA all cause conjunctivitis, but it is not clear how attachment to a ubiquitous cell surface molecule could account for a specific tropism for the conjunctiva. As mentioned above, Ad37 also interacts with CD46 (103), and there remains some controversy about whether SA, CD46, or both SA and CD46 contribute to attachment and infection.
Integrins αMβ2 and αLβ2.
In some cases, virus attachment may depend on direct interaction between the penton base and a cell surface integrin, without the need for a primary fiber receptor. Fiber-deficient Ad2 virions can infect CAR-negative monocytic cells by a mechanism that involves a primary attachment to integrins αMβ2 and αLβ2, followed by an interaction with αv integrins that is needed for internalization (38).
Other protein and nonprotein receptors.
The α2 domain of the class I major histocompatibility complex (MHC-I) has been reported to promote high-affinity interaction with Ad5 when expressed on an MHC-deficient human cell line (36). The putative fiber attachment site shows slight sequence similarity to the fiber binding site identified within CAR D1. However, when expressed on hamster cells, MHC-I shows little affinity for Ad5 fiber (20). The role of this ubiquitous protein in Ad infection remains unclear.
Ad5 has also been shown to attach to vascular cell adhesion molecule 1 (VCAM-1), a member of the immunoglobulin gene superfamily expressed on activated endothelial cells (17, 48, 69). Because VCAM-1 is more highly expressed on atherosclerotic endothelium than on normal endothelium, it has been suggested that VCAM-mediated infection may be useful for gene therapy of atherosclerosis (13).
Ad5 hexon interacts with dipalmitoyl phosphatidylcholine (DPPC), a component of pulmonary surfactant, and DPPC liposomes enhance virus uptake by a receptor-independent mechanism (7). It is not known whether interaction with surfactant plays a role in adenovirus infection in vivo.
RECEPTORS AND TROPISM
Anatomic and functional barriers to virus-receptor interaction.
Many factors may prevent virus from encountering a receptor on a target cell in vivo. The immune system is probably most important, because patients with immune deficiencies suffer disseminated infections that are rarely, if ever, seen in those with normal immunity (42). The endothelial junctions that constitute the blood-brain barrier may prevent virus from reaching receptors in the central nervous system. Interaction with heparan sulfate or sialic acid may trap virus within the extracellular matrix or within the cellular glycocalyx (63, 87). The precise subcellular location of the receptor may be critical. CAR, which is localized to intercellular junctions, is inaccessible to virus on the apical surface of polarized epithelial cells (14), so that infection of these cells may require disruption of junctions or attachment to another molecule.
Tropism in vivo.
Clinical information suggests that specific adenoviruses have tropism for particular organ systems. Because human adenoviruses do not replicate in rodent models, most experimental data come from gene therapy experiments with replication-defective Ad2 and Ad5 vectors. In a typical experiment, a large virus dose (1010 to 1012 virus particles) is administered intravenously; this is unlikely to mimic the process of natural infection, which begins when a small virus inoculum attaches to an epithelial surface, replicates locally, and then possibly spreads to the bloodstream.
Transgenic animals in which CAR is broadly overexpressed show increased susceptibility to Ad-mediated transduction of many tissues (88). Tissue-specific CAR expression in transgenic animals markedly increases the susceptibility of skeletal muscle (58) and lymphocytes (40, 72, 97), which are normally CAR deficient and refractory to transduction. Although these results indicate that CAR can influence tropism in vivo, it is certainly not the sole determinant. Experiments with CAR knockout animals (or animals deficient in CD46 or B7) have not been reported, but mice deficient in integrin β5 (39) or MHC-I (104) expression are susceptible to Ad5, indicating that these molecules are not essential for infection.
After intravenous administration, the vast majority of Ad2 or Ad5 is taken up rapidly by the liver (2, 43, 57, 81); Ad5 pseudotyped with fibers of Ad7 (31) or of subgroup D viruses (24) shows somewhat less hepatotropism. Although CAR is highly expressed in rodent liver, it does not appear that hepatotropism results primarily from virus interaction with CAR. Many investigators have observed that mutations in the fiber knob that reduce or ablate virus attachment to CAR, thus preventing transduction of primary hepatocytes in culture (51, 81), do not greatly affect hepatotropism in vivo (2, 43, 57, 81). In only one study was liver tropism of such a “detargeted” vector significantly reduced (25). Similarly, mutation of the penton base RGD sequence, which prevents interaction with integrins, does not prevent liver tropism. In contrast, mutation of the putative site for interaction with HS-GAG significantly reduces virus delivery to the liver; the results suggest that HS-GAG is more important for tropism than might have been expected from in vitro experiments.
Dual mutations, which are aimed at eliminating interactions with two or more of the receptors, may be more effective than single mutations in “detargeting” the liver. In a rat model, the elimination of the CAR and integrin binding sites together greatly reduced liver transduction (59); however, in some, but not all (25), experiments with mice, a similar combination of mutations had no effect (51, 59).
With multiple receptors and tissue-specific variation in receptor expression, the importance of particular receptors may vary as different tissues are examined and may depend on the route by which virus is administered. For example, when virus is injected into the brain, tropism for specific cell populations depends on interaction with CAR; little transduction is seen with vectors that bind neither CAR nor integrins (91). The complex results of gene therapy experiments suggest that each of the receptors is likely to play some role in vivo, but our present understanding of virus-receptor interaction does not permit us to predict with confidence whether a particular tissue will be efficiently transduced.
WHERE WE ARE NOW
Because adenoviruses use different receptors for entry and internalization, attachment to any of a number of cell surface molecules may lead to virus infection. In the past few years, information about these receptor molecules has accumulated rapidly, but a number of important issues remain to be clarified. For many of the Ad serotypes, no specific receptor has yet been identified, and it is likely that additional receptors remain to be found. The structural details of how viruses interact with a number of the identified receptor molecules remain to be defined. We do not know whether virus interaction with receptors such as CD46 or B7 triggers signals that may be involved in pathogenesis.
A major focus of research in adenovirus-mediated gene therapy is the effort to design vectors that deliver genes to specific tissues or cell types (8, 98). Successful targeting will require reducing the toxicity of the available vectors, eliminating unwanted hepatotropism, and promoting interaction with alternative receptors whose tissue distribution is more limited than that of CAR, CD46, heparan sulfate, or sialic acid.
Ten years ago, it seemed that identification of the adenovirus receptor would put us well on our way toward targeted gene delivery, but the factors governing tissue tropism are quite complex. A virus may use different receptors or combinations of receptors in its interaction with various cell types or under different circumstances. For example, hematogenous spread to a tissue may involve different receptors from those needed for contiguous spread from cell to cell. Experiments using “detargeted” virus mutants, complemented by experiments with genetically modified animals, may be useful in teasing out the contributions of individual receptor molecules. However, given that human adenoviruses replicate poorly in animals, an understanding of the factors that govern tropism during a natural infection is likely to require new experimental approaches.
Acknowledgments
This work was supported by grants from the National Institutes of Health.
We thank Glen Nemerow for helpful comments on the manuscript. We also thank Carmen San Martin and Roger Burnett for providing the unpublished cryo-EM structure shown in Fig. 1A and Stephen Cusack and Glen Nemerow for permission to reproduce published figures.
REFERENCES
- 1.Albinsson, B., and A. H. Kidd. 1999. Adenovirus type 41 lacks an RGD αv-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 64:125-136. [DOI] [PubMed] [Google Scholar]
- 2.Alemany, R., and D. T. Curiel. 2001. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 8:1347-1353. [DOI] [PubMed] [Google Scholar]
- 3.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74:42-48. [PMC free article] [PubMed] [Google Scholar]
- 4.Arnberg, N., A. H. Kidd, K. Edlund, F. Olfat, and G. Wadell. 2000. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus αv integrins. J. Virol. 74:7691-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnberg, N., P. Pring-Akerblom, and G. Wadell. 2002. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J. Virol. 76:8834-8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai, M., B. Harfe, and P. Freimuth. 1993. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J. Virol. 67:5198-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balakireva, L., G. Schoehn, E. Thouvenin, and J. Chroboczek. 2003. Binding of adenovirus capsid to dipalmitoyl phosphatidylcholine provides a novel pathway for virus entry. J. Virol. 77:4858-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett, B. G., C. J. Crews, and J. T. Douglas. 2002. Targeted adenoviral vectors. Biochim. Biophys. Acta 1575:1-14. [DOI] [PubMed] [Google Scholar]
- 9.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 10.Burmeister, W. P., D. Guilligay, S. Cusack, G. Wadell, and N. Arnberg. 2004. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 78:7727-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardin, A. D., and H. J. Weintraub. 1989. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21-32. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J.-W., B. Zhou, S. J. Shin, Q.-C. Yu, K. Jiao, M. D. Schneider, H. S. Baldwin, and J. M. Bergelson. Submitted for publication.
- 13.Chu, Y., D. Heistad, M. I. Cybulsky, and B. L. Davidson. 2001. Vascular cell adhesion molecule-1 augments adenovirus-mediated gene transfer. Arterioscler. Thromb. Vasc. Biol. 21:238-242. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, C. J., J. T. Shieh, R. J. Pickles, T. Okegawa, J. T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen, C. J., Z. Q. Xiang, G. P. Gao, H. C. Ertl, J. M. Wilson, and J. M. Bergelson. 2002. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 83:151-155. [DOI] [PubMed] [Google Scholar]
- 16.Cole, J. L., G. A. Housley, Jr., T. R. Dykman, R. P. MacDermott, and J. P. Atkinson. 1985. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc. Natl. Acad. Sci. USA 82:859-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cybulsky, M. I., and M. A. Gimbrone, Jr. 1991. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251:788-791. [DOI] [PubMed] [Google Scholar]
- 18.Davison, A. J., E. A. Telford, M. S. Watson, K. McBride, and V. Mautner. 1993. The DNA sequence of adenovirus type 40. J. Mol. Biol. 234:1308-1316. [DOI] [PubMed] [Google Scholar]
- 19.Davison, E., R. M. Diaz, I. R. Hart, G. Santis, and J. F. Marshall. 1997. Integrin α5β1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 71:6204-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davison, E., I. Kirby, T. Elliott, and G. Santis. 1999. The human HLA-A*0201 allele, expressed in hamster cells, is not a high-affinity receptor for adenovirus type 5 fiber. J. Virol. 73:4513-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dechecchi, M. C., P. Melotti, A. Bonizzato, M. Santacatterina, M. Chilosi, and G. Cabrini. 2001. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 75:8772-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268:382-390. [DOI] [PubMed] [Google Scholar]
- 23.Defer, C., M. T. Belin, M. L. Caillet-Boudin, and P. Boulanger. 1990. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J. Virol. 64:3661-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denby, L., L. M. Work, D. Graham, C. Hsu, D. J. von Seggern, S. A. Nicklin, and A. H. Baker. 2004. Adenoviral serotype 5 vectors pseudotyped with fibers from subgroup D show modified tropism in vitro and in vivo. Hum. Gene Ther. 15:1054-1064. [DOI] [PubMed] [Google Scholar]
- 25.Einfeld, D. A., R. Schroeder, P. W. Roelvink, A. Lizonova, C. R. King, I. Kovesdi, and T. J. Wickham. 2001. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 75:11284-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fechner, H., A. Haack, H. Wang, X. Wang, K. Eizema, M. Pauschinger, R. Schoemaker, R. Veghel, A. Houtsmuller, H. P. Schultheiss, J. Lamers, and W. Poller. 1999. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 6:1520-1535. [DOI] [PubMed] [Google Scholar]
- 27.Freimuth, P. 1996. A human cell line selected for resistance to adenovirus infection has reduced levels of the virus receptor. J. Virol. 70:4081-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freimuth, P., K. Springer, C. Berard, J. Hainfeld, M. Bewley, and J. Flanagan. 1999. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J. Virol. 73:1392-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 30.Gaggar, A., D. M. Shayakhmetov, M. K. Liszewski, J. P. Atkinson, and A. Lieber. 2005. Localization of regions in CD46 that interact with adenovirus. J. Virol. 79:7503-7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gall, J., A. Kass-Eisler, L. Leinwand, and E. Falck-Pedersen. 1996. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J. Virol. 70:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2004. The B7 family revisited. Annu. Rev. Immunol. [DOI] [PubMed]
- 33.Hassell, J. R., J. H. Kimura, and V. C. Hascall. 1986. Proteoglycan core protein families. Annu. Rev. Biochem. 55:539-567. [DOI] [PubMed] [Google Scholar]
- 34.Hierholzer, J. C. 1973. Further subgrouping of the human adenoviruses by differential hemagglutination. J. Infect. Dis. 128:541-550. [DOI] [PubMed] [Google Scholar]
- 35.Honda, T., H. Saitoh, M. Masuko, T. Katagiri-Abe, K. Tominaga, I. Kozakai, K. Kobayashi, T. Kumanishi, Y. G. Watanabe, S. Odani, and R. Kuwano. 2000. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res. Mol. Brain Res. 77:19-28. [DOI] [PubMed] [Google Scholar]
- 36.Hong, S. S., L. Karayan, J. Tournier, D. T. Curiel, and P. A. Boulanger. 1997. Adenovirus type 5 fiber knob binds to MHC class I α2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 16:2294-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howitt, J., M. C. Bewley, V. Graziano, J. M. Flanagan, and P. Freimuth. 2003. Structural basis for variation in adenovirus affinity for the cellular coxsackievirus and adenovirus receptor. J. Biol. Chem. 278:26208-26215. [DOI] [PubMed] [Google Scholar]
- 38.Huang, S., T. Kamata, Y. Takada, Z. M. Ruggeri, and G. R. Nemerow. 1996. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 70:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang, X., M. Griffiths, J. Wu, R. V. Farese, Jr., and D. Sheppard. 2000. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol. Cell. Biol 20:755-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurez, V., R. Dzialo-Hatton, J. Oliver, R. J. Matthews, and C. T. Weaver. 2002. Efficient adenovirus-mediated gene transfer into primary T cells and thymocytes in a new coxsackie/adenovirus receptor transgenic model. BMC Immunol. 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kallstrom, H., M. K. Liszewski, J. P. Atkinson, and A. B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639-647. [DOI] [PubMed] [Google Scholar]
- 42.Kojaoghlanian, T., P. Flomenberg, and M. S. Horwitz. 2003. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 13:155-171. [DOI] [PubMed] [Google Scholar]
- 43.Leissner, P., V. Legrand, Y. Schlesinger, D. A. Hadji, M. van Raaij, S. Cusack, A. Pavirani, and M. Mehtali. 2001. Influence of adenoviral fiber mutations on viral encapsidation, infectivity and in vivo tropism. Gene Ther. 8:49-57. [DOI] [PubMed] [Google Scholar]
- 44.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, E., D. Stupack, G. M. Bokoch, and G. R. Nemerow. 1998. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J. Virol. 72:8806-8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, E., D. Stupack, R. Klemke, D. A. Cheresh, and G. R. Nemerow. 1998. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J. Virol. 72:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, E., D. G. Stupack, S. L. Brown, R. Klemke, D. D. Schlaepfer, and G. R. Nemerow. 2000. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J. Biol. Chem. 275:14729-14735. [DOI] [PubMed] [Google Scholar]
- 48.Li, H., M. I. Cybulsky, M. A. Gimbrone, Jr., and P. Libby. 1993. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler. Thromb. 13:197-204. [DOI] [PubMed] [Google Scholar]
- 49.Lukashok, S. A., and M. S. Horwitz. 1998. New perspectives in adenoviruses. Curr. Clin. Top. Infect. Dis. 18:286-305. [PubMed] [Google Scholar]
- 50.Manchester, M., D. Naniche, and T. Stehle. 2000. CD46 as a measles receptor: form follows function. Virology 274:5-10. [DOI] [PubMed] [Google Scholar]
- 51.Martin, K., A. Brie, P. Saulnier, M. Perricaudet, P. Yeh, and E. Vigne. 2003. Simultaneous CAR- and αv integrin-binding ablation fails to reduce Ad5 liver tropism. Mol. Ther. 8:485-494. [DOI] [PubMed] [Google Scholar]
- 52.Mathias, P., T. Wickham, M. Moore, and G. Nemerow. 1994. Multiple adenovirus serotypes use αv integrins for infection. J. Virol. 68:6811-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mei, Y. F., and G. Wadell. 1996. Epitopes and hemagglutination binding domain on subgenus B:2 adenovirus fibers. J. Virol. 70:3688-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meier, O., and U. F. Greber. 2004. Adenovirus endocytosis. J. Gene Med. 6(Suppl. 1):S152-S163. [DOI] [PubMed] [Google Scholar]
- 55.Miyazawa, N., R. G. Crystal, and P. L. Leopold. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyazawa, N., P. L. Leopold, N. R. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. G. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizuguchi, H., N. Koizumi, T. Hosono, A. Ishii-Watabe, E. Uchida, N. Utoguchi, Y. Watanabe, and T. Hayakawa. 2002. CAR- or αv integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 9:769-776. [DOI] [PubMed] [Google Scholar]
- 58.Nalbantoglu, J., N. Larochelle, E. Wolf, G. Karpati, H. Lochmuller, and P. C. Holland. 2001. Muscle-specific overexpression of the adenovirus primary receptor CAR overcomes low efficiency of gene transfer to mature skeletal muscle. J. Virol. 75:4276-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicol, C. G., D. Graham, W. H. Miller, S. J. White, T. A. Smith, S. A. Nicklin, S. C. Stevenson, and A. H. Baker. 2004. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol. Ther. 10:344-354. [DOI] [PubMed] [Google Scholar]
- 60.Noutsias, M., H. Fechner, H. de Jonge, X. Wang, D. Dekkers, A. B. Houtsmuller, M. Pauschinger, J. Bergelson, R. Warraich, M. Yacoub, R. Hetzer, J. Lamers, H. P. Schultheiss, and W. Poller. 2001. Human coxsackie-adenovirus receptor is colocalized with integrins αvβ3 and αvβ5 on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections. Circulation 104:275-280. [DOI] [PubMed] [Google Scholar]
- 61.Okada, N., M. K. Liszewski, J. P. Atkinson, and M. Caparon. 1995. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc. Natl. Acad. Sci. USA 92:2489-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okegawa, T., R. C. Pong, Y. Li, J. M. Bergelson, A. I. Sagalowsky, and J. T. Hsieh. 2001. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of car protein structure. Cancer Res. 61:6592-6600. [PubMed] [Google Scholar]
- 63.Pickles, R. J., J. A. Fahrner, J. M. Petrella, R. C. Boucher, and J. M. Bergelson. 2000. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J. Virol. 74:6050-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts, M. M., J. L. White, M. G. Grütter, and R. M. Burnett. 1986. Three-dimensional structure of the adenovirus major coat protein hexon. Science 232:1148-1151. [DOI] [PubMed] [Google Scholar]
- 65.Roelvink, P. W., I. Kovesdi, and T. J. Wickham. 1996. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J. Virol. 70:7614-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roelvink, P. W., G. Mi Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 68.Rux, J. J., and R. M. Burnett. 2004. Adenovirus structure. Hum. Gene Ther. 15:1167-1176. [DOI] [PubMed] [Google Scholar]
- 69.Sakai, A., N. Kume, E. Nishi, K. Tanoue, M. Miyasaka, and T. Kita. 1997. P-selectin and vascular cell adhesion molecule-1 are focally expressed in aortas of hypercholesterolemic rabbits before intimal accumulation of macrophages and T lymphocytes. Arterioscler. Thromb. Vasc. Biol. 17:310-316. [DOI] [PubMed] [Google Scholar]
- 70.Salone, B., Y. Martina, S. Piersanti, E. Cundari, G. Cherubini, L. Franqueville, C. M. Failla, P. Boulanger, and I. Saggio. 2003. Integrin α3β1 is an alternative cellular receptor for adenovirus serotype 5. J. Virol. 77:13448-13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt, M. R., B. Piekos, M. S. Cabatingan, and R. T. Woodland. 2000. Expression of a human coxsackie/adenovirus receptor transgene permits adenovirus infection of primary lymphocytes. J. Immunol. 165:4112-4119. [DOI] [PubMed] [Google Scholar]
- 73.Segerman, A., N. Arnberg, A. Erikson, K. Lindman, and G. Wadell. 2003. There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J. Virol. 77:1157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shayakhmetov, D. M., A. M. Eberly, Z. Y. Li, and A. Lieber. 2005. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J. Virol. 79:1053-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shayakhmetov, D. M., Z. Y. Li, V. Ternovoi, A. Gaggar, H. Gharwan, and A. Lieber. 2003. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J. Virol. 77:3712-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shayakhmetov, D. M., and A. Lieber. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shenk, T. 1996. Adenoviridae: the viruses and their replication, p. 2111-2171. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields Virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 79.Short, J. J., A. V. Pereboev, Y. Kawakami, C. Vasu, M. J. Holterman, and D. T. Curiel. 2004. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology 322:349-359. [DOI] [PubMed] [Google Scholar]
- 80.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kalin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith, T., N. Idamakanti, H. Kylefjord, M. Rollence, L. King, M. Kaloss, M. Kaleko, and S. C. Stevenson. 2002. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Mol. Ther. 5:770-779. [DOI] [PubMed] [Google Scholar]
- 82.Smith, T. A., N. Idamakanti, J. Marshall-Neff, M. L. Rollence, P. Wright, M. Kaloss, L. King, C. Mech, L. Dinges, W. O. Iverson, A. D. Sherer, J. E. Markovits, R. M. Lyons, M. Kaleko, and S. C. Stevenson. 2003. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum. Gene Ther. 14:1595-1604. [DOI] [PubMed] [Google Scholar]
- 83.Smith, T. A., N. Idamakanti, M. L. Rollence, J. Marshall-Neff, J. Kim, K. Mulgrew, G. R. Nemerow, M. Kaleko, and S. C. Stevenson. 2003. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 14:777-787. [DOI] [PubMed] [Google Scholar]
- 84.Soudais, C., S. Boutin, S. S. Hong, M. Chillon, O. Danos, J. M. Bergelson, P. Boulanger, and E. J. Kremer. 2000. Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J. Virol. 74:10639-10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stevenson, S. C., M. Rollence, B. White, L. Weaver, and A. McClelland. 1995. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J. Virol. 69:2850-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stewart, P. L., S. D. Fuller, and R. M. Burnett. 1993. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 12:2589-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stonebraker, J. R., D. Wagner, R. W. Lefensty, K. Burns, S. J. Gendler, J. M. Bergelson, R. C. Boucher, W. K. O'Neal, and R. J. Pickles. 2004. Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection. J. Virol. 78:13755-13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tallone, T., S. Malin, A. Samuelsson, J. Wilbertz, M. Miyahara, K. Okamoto, L. Poellinger, L. Philipson, and S. Pettersson. 2001. A mouse model for adenovirus gene delivery. Proc. Natl. Acad. Sci. USA 98:7910-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan, P. K., A. I. Michou, J. M. Bergelson, and M. Cotten. 2001. Defining CAR as a cellular receptor for the avian adenovirus CELO using a genetic analysis of the two viral fibre proteins. J. Gen. Virol. 82:1465-1472. [DOI] [PubMed] [Google Scholar]
- 90.Tatsis, N., and H. C. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas, C. E., P. Edwards, T. J. Wickham, M. G. Castro, and P. R. Lowenstein. 2002. Adenovirus binding to the coxsackievirus and adenovirus receptor or integrins is not required to elicit brain inflammation but is necessary to transduce specific neural cell types. J. Virol. 76:3452-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trauger, S. A., E. Wu, S. J. Bark, G. R. Nemerow, and G. Siuzdak. 2004. The identification of an adenovirus receptor by using affinity capture and mass spectrometry. ChemBioChem 5:1095-1099. [DOI] [PubMed] [Google Scholar]
- 94.van Raaij, M. J., N. Louis, J. Chroboczek, and S. Cusack. 1999. Structure of the human adenovirus serotype 2 fiber head domain at 1.5 Å resolution. Virology 262:333-343. [DOI] [PubMed] [Google Scholar]
- 95.van Raaij, M. J., A. Mitraki, G. Lavigne, and S. Cusack. 1999. A triple β-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 401:935-938. [DOI] [PubMed] [Google Scholar]
- 96.Walters, R. W., P. Freimuth, T. O. Moninger, I. Ganske, J. Zabner, and M. J. Welsh. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110:789-799. [DOI] [PubMed] [Google Scholar]
- 97.Wan, Y. Y., R. P. Leon, R. Marks, C. M. Cham, J. Schaack, T. F. Gajewski, and J. DeGregori. 2000. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc. Natl. Acad. Sci. USA 97:13784-13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wickham, T. J. 2000. Targeting adenovirus. Gene Ther. 7:110-114. [DOI] [PubMed] [Google Scholar]
- 99.Wickham, T. J., E. J. Filardo, D. A. Cheresh, and G. R. Nemerow. 1994. Integrin αvβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 127:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 101.Wu, E., L. Pache, D. J. Von Seggern, T. M. Mullen, Y. Mikyas, P. L. Stewart, and G. R. Nemerow. 2003. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 77:7225-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu, E., S. A. Trauger, L. Pache, T. M. Mullen, D. J. von Seggern, G. Siuzdak, and G. R. Nemerow. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78:3897-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433-442. [DOI] [PubMed] [Google Scholar]
- 105.Zubieta, C., G. Schoehn, J. Chroboczek, and S. Cusack. 2005. The structure of the human adenovirus 2 penton. Mol. Cell 17:121-135. [DOI] [PubMed] [Google Scholar]