Abstract
Herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 stimulates lytic infection and the reactivation of quiescent viral genomes. These roles of ICP0 require its RING finger E3 ubiquitin ligase domain, which induces the degradation of several cellular proteins, including components of promyelocytic leukemia nuclear bodies and centromeres. ICP0 also interacts very strongly with the cellular ubiquitin-specific protease USP7 (also known as HAUSP). We have shown previously that ICP0 induces its own ubiquitination and degradation in a RING finger-dependent manner, and that its interaction with USP7 regulates this process. In the course of these studies we found and report here that ICP0 also targets USP7 for ubiquitination and proteasome-dependent degradation. The reciprocal activities of the two proteins reveal an intriguing situation that poses the question of the balance of the two processes during productive HSV-1 infection. Based on a thorough analysis of the properties of an HSV-1 mutant virus that expresses forms of ICP0 that are unable to bind to USP7, we conclude that USP7-mediated stabilization of ICP0 is dominant over ICP0-induced degradation of USP7 during productive HSV-1 infection. We propose that the biological significance of the ICP0-USP7 interaction may be most pronounced in natural infection situations, in which limited amounts of ICP0 are expressed.
An important biological and clinical aspect of herpes simplex virus type 1 (HSV-1) is its ability to establish a latent state in neuronal cell nuclei following an initial epithelial infection (for general reviews, see references 31 and 32). Periodically the latent virus will reactivate and cause an episode of lytic replication at the site of the original epithelial infection. While in most cases this is not serious, in immunocompromised patients the infections can become severe and even life-threatening. Since latent virus is accessible to neither the immune system of the host nor current antiviral treatments, an understanding of the molecular mechanisms that govern the establishment, maintenance, and particularly the reactivation of latent genomes is important for the future development of effective therapies.
ICP0 is a member of the immediate-early class of HSV-1 gene products, and it is required for the efficient initiation of viral lytic infection and reactivation of latent virus in animal models. ICP0 also stimulates reactivation of quiescent viral genomes in cultured cells (for reviews, see references 12, 13, 19, and 31). A major biochemical function of ICP0 is its E3 ubiquitin ligase activity, conferred by a RING finger domain near its N terminus (4, 20). This activity leads to the proteasome-dependent degradation of several cellular proteins during infection. These include the promyelocytic leukemia protein PML and small ubiquitin-like modifier (SUMO)-modified forms of Sp100 (both of which are major components of nuclear substructures known as ND10 or PML nuclear bodies) (3, 7, 15, 28, 29), centromere proteins CENP-C and CENP-A (14, 24), and the catalytic subunit of DNA-PK (30). Whether ICP0 targets these proteins directly or indirectly for ubiquitination is currently unknown. Apart from ICP0 itself (6), the only other direct target of ICP0-mediated ubiquitination that has been established to date is p53 (1), but this activity is weak in vivo compared to that of Mdm2, the major cellular E3 ubiquitin ligase involved in p53 metabolism (1). Therefore, the direct substrates of the E3 ubiquitin ligase activity of ICP0 in vivo remain poorly understood.
ICP0 interacts very strongly with the cellular ubiquitin-specific protease enzyme USP7 (HAUSP) (17), and we have shown that this interaction protects ICP0 from auto-ubiquitination in vitro and RING finger- and proteasome-dependent degradation during infection of U2OS osteosarcoma cells (6). This observation provides an explanation for the interaction of ICP0 with components of both the ubiquitin conjugation and deconjugation pathways. However, in the course of these previous studies we found and report here that in addition to ubiquitinated ICP0 being a substrate of USP7, the converse is also true: ICP0 ubiquitinates USP7 in vitro, and this activity leads to a reduction in cellular USP7 levels during HSV-1 infection. Protection of ICP0 from auto-ubiquitination and degradation by USP7 is analogous to USP7-mediated stabilization of mdm2 (22), but the concomitant ICP0-induced degradation of USP7 presents a complex situation with the potential for interplay between the stabilities of the two proteins. These findings pose the question of which of the two activities is dominant during productive HSV-1 infection.
In the present study, we have analyzed in detail the phenotype of an HSV-1 mutant virus that expresses a form of ICP0 with amino acid substitution mutations that render the protein unable to bind to USP7. We have conducted these experiments in HFFF-2 human fibroblast cells, in which ICP0 is very important for efficient HSV-1 infection. Under these conditions, we found that the USP7 binding-deficient form of ICP0 is destabilized and the rates of accumulation of other representative HSV-1 proteins are reduced. Reduction in USP7 levels by siRNA treatment prior to wild-type HSV-1 infection caused a defect in the accumulation of ICP0, resulting in lower levels of expression of immediate-early regulatory protein ICP4 and UL42, a member of the early class of viral proteins. These results demonstrate that the stabilization of ICP0 by USP7 is dominant over ICP0-induced USP7 degradation during productive HSV-1 infection under ICP0-dependent conditions. We conclude that USP7 is required for efficient HSV-1 lytic infection, especially during the early stages of a low-multiplicity infection. Our analysis suggests that the biological consequence for HSV-1 infection of the interaction between ICP0 and USP7 may be most pronounced when ICP0 levels and rates of synthesis are low, for example, during the initial stages of reactivation from latency.
MATERIALS AND METHODS
Viruses and cells.
HSV-1 strain 17+ was the wild-type strain used. Other strains used were the ICP0 null mutant dl1403 (35) and viruses FXE, E52X, and M1, which express forms of ICP0 lacking the RING finger (FXE) or the USP7 interaction region (M1 and E52X) (10, 16, 25). Rescuant virus M1R was isolated after cotransfection of M1 viral DNA with the XhoI-SalI fragment of the IE1 gene that includes the locations of the M1 mutation. Progeny viruses were screened for the loss of the FspI site that marks the presence of the M1 mutation. Rescuant viruses were plaque-purified three times, and the genotype of the final isolate was confirmed by Southern blotting and DNA sequencing of fragments derived by PCR from M1 and M1R viral DNAs (data not shown). All viruses were grown in BHK cells and titrated in U2OS cells, in which ICP0 is not required for efficient replication of HSV-1 (41). All viruses were used on the basis of their titers in U2OS cells, regardless of the cell type used (13). U2OS and HEp-2 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Baby hamster kidney (BHK) cells were grown in Glasgow modified Eagle's medium supplemented with 10% newborn calf serum and 10% tryptose phosphate broth. Vero and HeLa cells were grown in Glasgow modified Eagle's medium containing 10% fetal calf serum. Human fetal foreskin fibroblast cells (HFFF-2; European Collection of Cell Cultures) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 1% glutamine. All cell growth media were supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin.
Baculoviruses.
Recombinant baculoviruses Ac.HTa-USP7, Ac.HTa-ICP0, and Ac.HTa-FXE have been described previously (4, 6). For the preparation of baculovirus-infected cell extracts, Sf21 insect cells were grown in suspension in TC100 medium (Gibco BRL) supplemented with 10% fetal calf serum and antibiotics as above. When the cells reached a density of 106 cells/ml, they were infected for 72 h using a multiplicity of infection (MOI) of 2 PFU per cell. The cells were harvested by low-speed centrifugation, washed in phosphate-buffered saline, and stored at −70°C until required.
Baculovirus Ac.CMV.His-ICP0 was constructed by inserting the NcoI-HpaI ICP0 genomic coding region of HSV-1, linked to an N-terminal polyhistidine tag and the human cytomegalovirus promoter region from pCIneo, into the Bac-to-Bac transfer plasmid vector pFastBac-HTa (Life Technologies). The resultant plasmid, pFastBac.CMV.HisICP0, was used to transfer the human cytomegalovirus promoter and tagged ICP0 coding region into a recombinant baculovirus that allows efficient expression of ICP0 after infection of several mammalian cell types (27). Baculovirus Ac.CMV.EYFP was constructed by a similar strategy, except the enhanced yellow fluorescent protein coding region was used in place of that of His-tagged ICP0.
Expression and purification of ICP0 and USP7 and ubiquitin ligase assays.
The expression and purification of ICP0 and USP7 and ubiquitin ligase assays were conducted as previously described (4, 6). Ubiquitin ligase assay products were subjected to electrophoresis on 7.5% or 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels, or 4 to 12% Bis-Tris NuPAGE gels (Invitrogen), and the products were analyzed by Western blotting using anti-ubiquitin monoclonal antibody P4D1 (Santa Cruz Biotechnology), anti-ICP0 or -USP7 antibodies, or, in the case of radiolabeled substrates, by phosphorimaging.
Infections and Western blot analysis.
Cells were seeded into 24-well dishes at 105 cells per well and then infected the following day with HSV-1 as stated. Cell monolayers were washed twice with 1 ml of phosphate-buffered saline before harvesting in SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer. Proteins were resolved on 7.5% SDS gels and then transferred to nitrocellulose membranes by Western blotting. ICP0, ICP4, and UL41 were detected using anti-ICP0 monoclonal antibody 11060, anti-ICP4 monoclonal antibody 10176, and anti-UL42 monoclonal antibody Z1F11 as previously described (6). USP7 was detected using rabbit antibody BL851 (Bethyl Laboratories). Filters were then incubated with horseradish peroxidase-conjugated secondary antibodies, incubated with Western Lightning chemiluminescence detection reagent (Perkin-Elmer Life Sciences), and exposed to film. Where necessary, the relative amounts of proteins were determined by densitometry of appropriate scanned film exposures (neither underexposed nor oversaturated) using Bio-Rad QuantityOne software, which gives a read-out of density volume measurements in arbitrary units.
FACS assays.
Cells in 35-mm dishes were infected with wild-type HSV-1 or M1 mutant virus then harvested for fluorescence-activated cell sorting (FACS) analysis. The cells were processed, stained for either ICP4 or ICP0 expression, and analyzed on a Becton-Dickinson FACSCalibur machine as described previously (6, 13).
siRNA analysis.
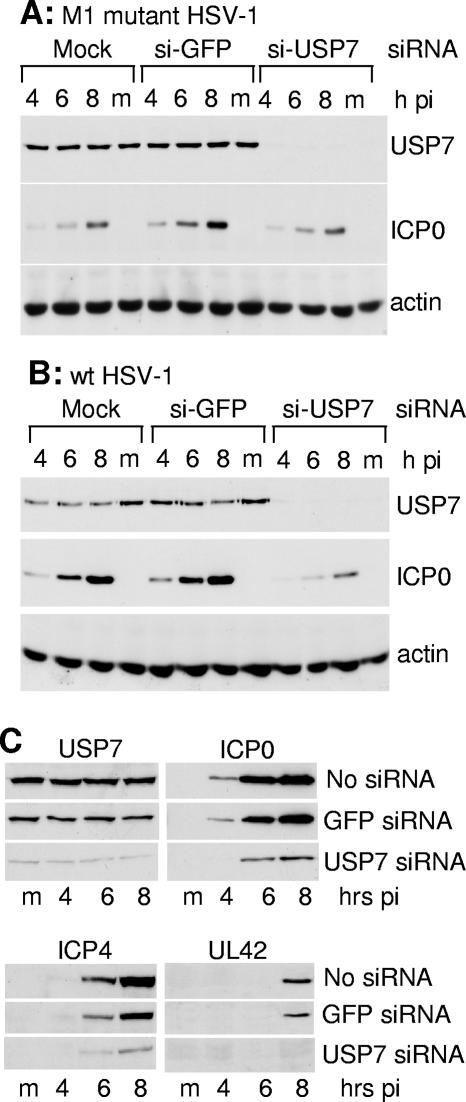
Reduction in levels of USP7 by transfection of anti-USP7 small interfering RNAs (siRNAs) into HeLa cells in 35-mm plates was performed as described previously (6). Two days after transfection, the cells were trypsinized, counted, and reseeded into 24-well dishes at 105 cells per well. The following day the cells were infected with either mutant M1 or wild-type HSV-1 as detailed in the text and the legend to Fig. 11.
FIG. 11.
Reductions in USP7 levels by siRNA treatment reduce HSV-1 gene expression. (A) HeLa cells were mock transfected or transfected with anti-GFP control and anti-USP7 siRNAs. The cells were reseeded into 24-well dishes 2 days later, and the following day the cells were infected with M1 mutant HSV-1 (MOI of 2). Samples were taken at 4, 6, and 8 h after infection and analyzed by Western blotting for USP7, ICP0, and actin. (B) As in A, except wild-type HSV-1 was used (MOI of 2). (C) HeLa cells were mock transfected or transfected with anti-GFP control and anti-USP7 siRNAs. The cells were reseeded into 24-well dishes 2 days later, and then the following day the cells were infected with wild-type HSV-1 (MOI of 5 PFU/cell) and samples harvested at the indicated times postinfection (hours pi) were analyzed by Western blotting for expression of USP7, ICP0, ICP4, and UL42. Actin levels were constant in all samples (not shown).
RESULTS
ICP0 mediates the ubiquitination of USP7 in a RING finger-dependent manner.
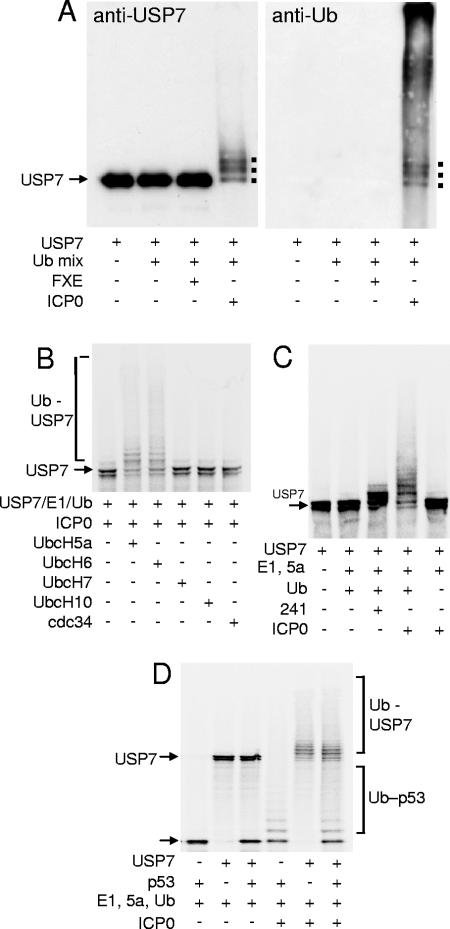
ICP0 interacts with itself (25) and with p53 (1) and both of these proteins are substrates for ICP0-mediated ubiquitination in vitro (1, 6). Since ICP0 also interacts strongly with USP7 (17), we questioned whether USP7 was an in vitro substrate of ICP0. Purified full-length ICP0 was added to in vitro ubiquitination reactions containing purified E1, UbcH5a, ubiquitin, and USP7. We found that USP7 was readily ubiquitinated by ICP0, with the majority of the input USP7 receiving at least one ubiquitin moiety (Fig. 1A, left). Purified mutant ICP0 lacking the RING finger (FXE) was unable to ubiquitinate USP7, illustrating that this activity was RING finger dependent (Fig. 1A, left). Reprobing of the filter for ubiquitin illustrated that the major ubiquitinated forms of USP7 could clearly be distinguished among the high-molecular-weight ubiquitinated products (Fig. 1A, right).
FIG. 1.
ICP0 ubiquitinates USP7 in vitro. (A) Purified USP7 was incubated with E1, UbcH5a, and ubiquitin (Ub mix) in a buffer containing ATP and with purified samples of either wild-type ICP0 or its RING finger deletion derivative FXE. The products were analyzed by Western blotting using either anti-USP7 (left) or antiubiquitin (right) antibodies. Ubiquitinated forms of USP7 form a higher-molecular-weight smear, with distinct bands corresponding to USP7 molecules with small numbers of ubiquitin adducts marked by dots. These bands were also detected by the antiubiquitin antibody. (B) ICP0 ubiquitinates radiolabeled USP7 in conjunction with E2 enzymes UbcH5a and UbcH6, but not UbcH7, UbcH10, or cdc34 (UbcH3). The relevant E2 enzymes were added to mixtures containing the other components as marked. (C) USP7 is not efficiently ubiquitinated by the isolated RING finger domain of ICP0. Radiolabeled USP7 was mixed with E1, UbcH5a (5a), ubiquitin (Ub), and either GST-241 (241; the N-terminal third of ICP0 containing the RING finger linked to GST) or full-length ICP0. (D) ICP0 ubiquitinates both USP7 and p53 in vitro. Radiolabeled USP7 and p53 were incubated with components of ICP0 ubiquitination reactions as marked.
Full-length ICP0 catalyzes the formation of high-molecular-weight polyubiquitin chains in vitro in the presence of E2 enzymes UbcH5a and UbcH6 (4). However, it has also been reported that a segment of ICP0 that overlaps the USP7 binding domain stimulates auto-ubiquitination of cdc34 (UbcH3) when used in high concentration as a glutathione S-transferase (GST) fusion protein (36). Therefore, we tested the specificity of E2 utilization in the ICP0-mediated ubiquitination of USP7. Of the E2 enzymes tested, only UbcH5a and UbcH6 were active in the USP7 ubiquitination assay (Fig. 1B), despite all E2 enzymes being catalytically active in terms of thiolester formation with ubiquitin (data not shown).
As USP7 ubiquitination is dependent on the RING finger domain of ICP0 and specific E2 enzymes, we investigated whether the RING finger domain alone of ICP0 was sufficient to mediate ubiquitination of USP7. The isolated RING finger domain of ICP0 was expressed as a GST fusion protein (GST-241) (4) and used in ubiquitination reactions containing radiolabeled substrate USP7. The ICP0 RING domain is as active as the full-length protein in substrate-independent ubiquitination assays in vitro (4), but it lacks the USP7 binding region of ICP0. Although GST-241 was able to induce limited monoubiquitination of USP7, its ability to produce a ladder of higher-molecular-weight ubiquitinated forms of USP7 was clearly much less than that of full-length ICP0 (Fig. 1C), despite equivalent amounts of high-molecular-weight polyubiquitin chains being produced in the GST-241 and purified ICP0 reactions (data not shown). Assuming that any potential posttranslational modifications of ICP0 that might occur in insect but not bacterial cells do not affect ubiquitination of USP7 in vitro, we conclude that ICP0 sequences in the C-terminal two-thirds of the protein that include the USP7 interaction domain are required for efficient ubiquitination of USP7 in vitro.
We found that ICP0 was able to ubiquitinate both p53 and USP7 when these proteins were present in the same reaction mixture (Fig. 1D). USP7 has been shown to deubiquitinate p53 in vitro (23), and in high concentration USP7 reduces the levels of p53-ubiquitin conjugates produced by ICP0 (6). However, the levels of USP7 substrate used in the experiment of Fig. 1D were insufficient to protect p53 from ICP0-mediated ubiquitination.
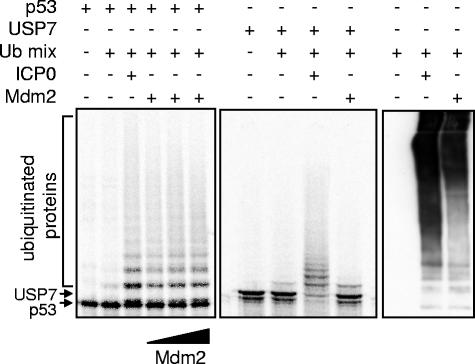
To test the specificity of RING finger-dependent ubiquitination of USP7 by ICP0, similar reactions were carried out to compare the effects of ICP0 and mdm2 on p53 and USP7. Although mdm2 and ICP0 ubiquitinated p53 with similar efficiencies in vitro (Fig. 2, left) only ICP0 caused extensive ubiquitination of USP7 (Fig. 2, center), although ICP0 and mdm2 produced equivalent amounts of high-molecular-weight polyubiquitin chains in these reactions (Fig. 2, right).
FIG. 2.
Mdm2 does not ubiquitinate USP7 in vitro. Radiolabeled substrate p53 (left panel) or USP7 (middle panel) was mixed with E1, ubiquitin, and UbcH5a (Ub mix) and either purified ICP0 or GST-mdm2 (full-length mouse mdm2 linked to GST, purified after expression in bacteria). The right panel shows total polyubiquitin chains produced in parallel reactions by ICPO and Mdm2. The reaction products were analyzed by SDS-PAGE and phosphorimaging.
ICP0 induces the proteasome-dependent degradation of USP7 during HSV-1 infection.
The finding that ICP0 ubiquitinates USP7 in vitro was unexpected because we had previously isolated the USP7 protein and cloned its cDNA after purification of USP7 in complex with ICP0 from infected BHK and HeLa cells (16, 17, 26). We had also observed that USP7 colocalized with ICP0 in infected HEp-2 cells (16, 17). However, in the light of the findings of Fig. 1, we tested whether ICP0 affected the stability of USP7 during HSV-1 infection. Because the requirement for ICP0 for efficient HSV-1 infection varies greatly with cell type, we investigated the fate of USP7 in a range of different HSV-1-infected cells. Expression levels of ICP0, ICP4 (a viral immediate-early protein required for the activation of transcription of viral early and late genes), and UL42 (a viral early protein that is required for DNA replication) were also monitored to control for the efficiency of HSV-1 infection. Expression of UL42 generally indicates that viral gene expression has progressed from the immediate-early stage to the productive phase of infection.
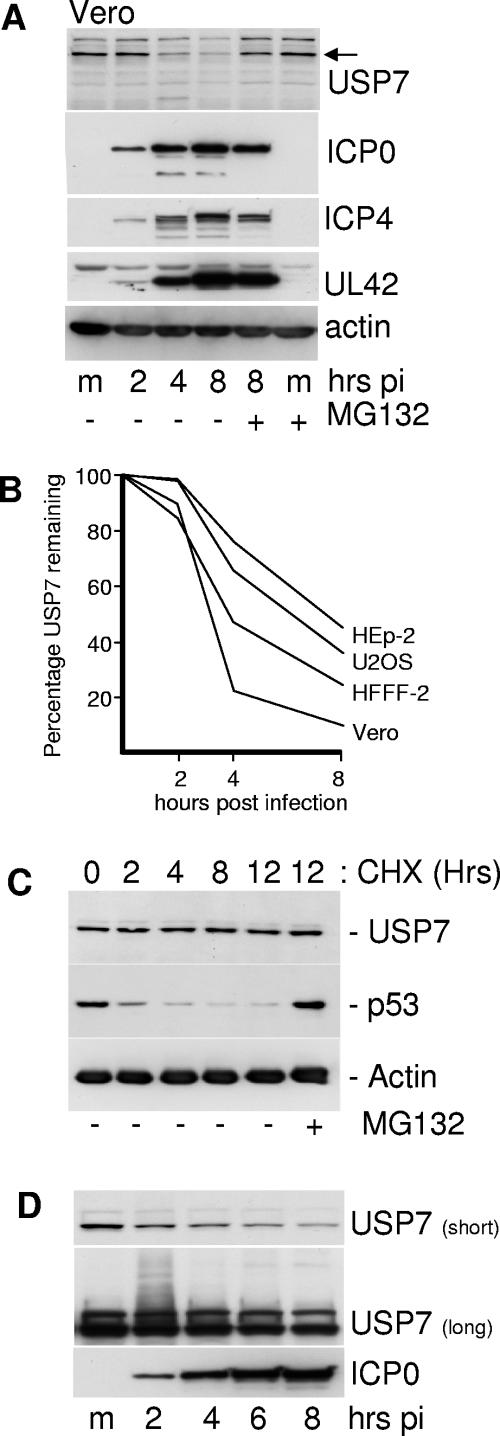
High-multiplicity HSV-1 infection reduced the levels of USP7 in Vero cells by a mechanism that was inhibited by the proteasome inhibitor MG132 (Fig. 3A). This effect was partially cell type dependent, in that USP7 was least sensitive in HEp-2 cells and most sensitive in Vero and HFFF cells (Fig. 3B). We note these cell type variations may be influenced by how rapidly the infection was progressing and may not necessarily reflect intrinsic differences in the ability of ICP0 to reduce USP7 levels. USP7 is normally stable in uninfected HFFF-2 cells, as treatment with cycloheximide over a 12-h period did not result in loss of the protein, in marked contrast to the loss of p53 in the same samples (Fig. 3C). Since USP7 is not normally rapidly turned over in uninfected cells, the loss of USP7 from infected cells cannot be a simple consequence of virus-induced shut-off of host protein synthesis.
FIG. 3.
Levels of USP7 are reduced in a proteasome-dependent manner during HSV-1 infection of several cell types. (A) Vero cells were infected with wild-type HSV-1 (MOI of 20 PFU/cell) and samples were taken at 2, 4, and 8 h after virus adsorption. Whole-cell extracts were analyzed by Western blotting for USP7, immediate-early proteins ICP0 and ICP4, DNA replication protein UL42, and actin as indicated. m, mock-infected control. The two rightmost tracks contain samples from cells that had been treated with MG132 (10 μM final concentration) from the time of infection. (B) Vero, HFFF-2, Hep-2, and U2OS cells were infected with wild-type HSV-1 (MOI of 20 PFU/cell), and the levels of USP7 at 2, 4, and 8 h after infection were determined by Western blot analysis and densitometry as described in the text. (C) USP7 is stable in uninfected cells. HFFF-2 cells were treated with cycloheximide (100 μg/ml) and then samples were taken at the indicated time points and analyzed for USP7, p53, and actin. MG132 was present at 10 μM in the rightmost sample. (D) Detection of likely ubiquitinated forms of USP7 during HSV-1 infection. HFFF-2 cells were infected with wild-type HSV-1 (MOI of 20 PFU/cell) and then samples taken at 2-hour intervals were analyzed by Western blotting. The short exposure (10 seconds) demonstrates the decrease in USP7 levels that occurs during infection; the longer exposure (10 min) detects the smear of lower-mobility forms of USP7 in the 2-h sample.
Prolonged exposure of the Western blots of infected HFFF-2 cell samples at early times of infection revealed a smear of higher-molecular-weight forms of USP7 that are characteristic of ubiquitination (Fig. 3D). Interestingly, the lower-mobility USP7 smear was reproducibly much more prominent at the 2-h time point than at later times. This could imply that there is a pool of USP7 that is particularly sensitive to ubiquitination, or that later events influence the efficiency of USP7 ubiquitination during HSV-1 infection. These later events could include posttranslational modification of USP7 and/or ICP0, or the accumulation of ICP0 in the cytoplasm once DNA replication has commenced.
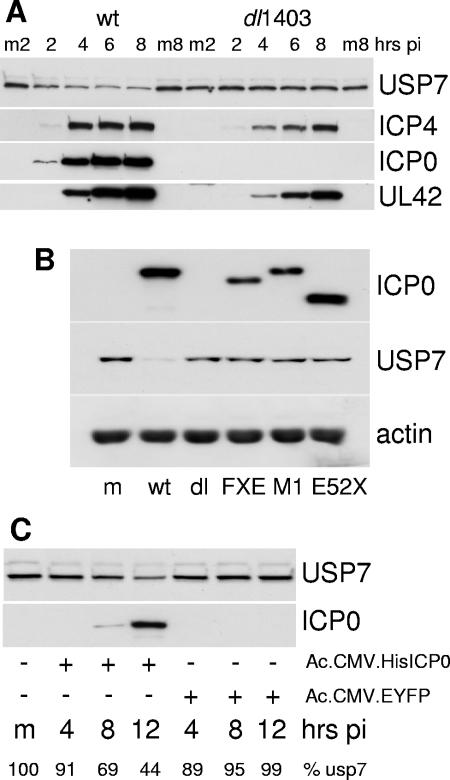
Degradation of USP7 did not occur during infection with ICP0-null mutant virus dl1403 (Fig. 4A) or with virus FXE, which expresses ICP0 lacking the RING finger domain, or with viruses M1 and E52X (Fig. 4B), which express forms of ICP0 that are unable to bind to USP7 (16). Therefore the decrease in the levels of USP7 that occurs during HSV-1 infection is dependent on ICP0, its RING finger E3 ligase activity, and the ability of ICP0 to interact with USP7. Use of a baculovirus vector that had been engineered to allow efficient expression of recombinant proteins in mammalian cells (27) demonstrated that ICP0 alone was sufficient to reduce cellular levels of USP7 (Fig. 4C). A reduction in USP7 levels also occurred during infection of HFFF-2 cells with the HSV-1 mutant in1411 (33), a virus that does not express ICP4 and is therefore restricted to the immediate-early phase of infection (data not shown).
FIG. 4.
Downregulation of USP7 requires ICP0 and its RING finger- and USP7-interacting domains. (A) HFFF-2 cells were infected with wild-type HSV-1 or ICP0-null mutant dl1403 (MOI of 20 PFU/cell) and then samples were harvested 2, 4, 6, and 8 h later. Mock-infected samples were harvested at the 2- and 8-hour time points (m2 and m8, respectively). The samples were analyzed by Western blotting for USP7, ICP4, ICP0, and UL42. (B) HFFF-2 cells were either mock infected (m) or infected at an MOI of 10 PFU/cell with wild-type HSV-1 or ICP0 mutant virus dl1403, FXE, M1, or E52X as indicated. Total cell extracts were harvested at 8 h postinfection and analyzed by Western blotting to detect USP7, ICP0, and actin. (C) Vero cells were infected with baculovirus Ac.CMV.HisICP0 or Ac.CMV.EYFP at an MOI of 100 PFU/cell and samples were harvested at 4, 8, and 12 h postinfection (pi) as indicated. The extracts were analyzed for USP7 and ICP0 expression by Western blotting. The percentage of USP7 compared to the mock-infected control (m) in each sample (% USP7) was determined by densitometry as described in the text.
Virus dose and time dependence of USP7 degradation.
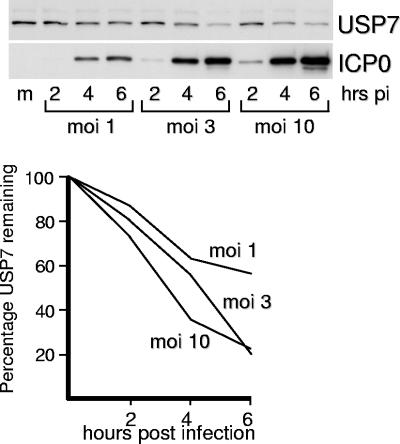
In order to assess the effect of time and virus dose on the rate of USP7 degradation, HFFF-2 cells were infected with wild-type HSV-1 at multiplicities of infection of 1, 3, and 10 PFU per cell, and then samples were harvested at increasing times after infection and analyzed for USP7 (Fig. 5). The extent of loss of USP7 from the culture as a whole increased with virus multiplicity, which could be due to increased proportions of cells being infected, increased levels of ICP0 expression, or both. However, some USP7 remained even at very high levels of ICP0 expression, indicating that a proportion of USP7 molecules may be resistant to ICP0-mediated degradation. As noted above, this could occur as a consequence of posttranslational modification, sequestration through binding to other partners, or spatial separation of pools of the two proteins. This apparently ICP0-resistant pool of USP7 contrasts with the almost complete ICP0-induced degradation of PML that occurs over a similar time course (see Fig. 7E). On the other hand, some USP7 is degraded even when ICP0 levels are low (Fig. 3, 4, and 5), which may indicate that there is a pool of USP7 that is very susceptible to ICP0-mediated degradation. This is consistent with the preferential detection of apparently ubiquitinated forms of USP7 at early times of infection (Fig. 3D).
FIG. 5.
Effect of multiplicity of HSV-1 infection on the degradation of USP7. HFFF-2 cells were infected with wild-type HSV-1 at the indicated MOI and then samples that had been harvested at 2, 4, and 6 h after infection were analyzed for USP7 and ICP0 by Western blotting. The lower panel shows the relative rates of degradation of USP7 determined by densitometry as described in the text.
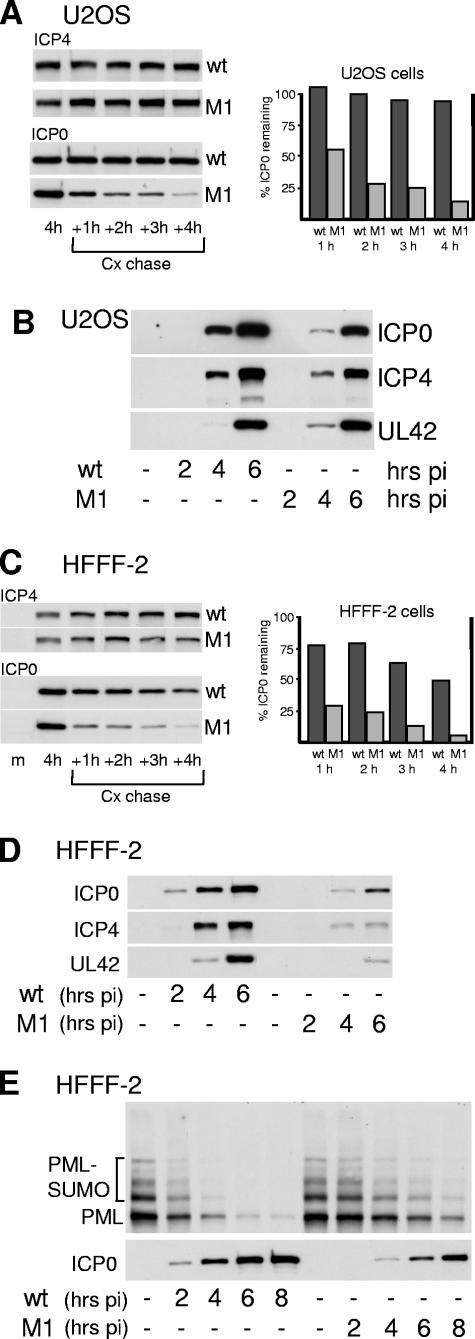
FIG.7.
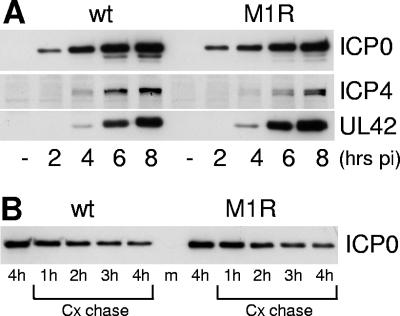
Gene expression phenotype of M1 mutant HSV-1 in U2OS and HFFF-2 cells. (A) U2OS cells were infected with wild-type or M1 HSV-1 at an MOI of 2 PFU per cell. At 4 h postinfection, cycloheximide was added to a final concentration of 100 μg per ml and then replicate samples were harvested immediately and at the indicated hours postinfection (hrs pi) thereafter. After Western blotting, to detect ICP4 and ICP0 (left-hand panels) the intensities of the ICP0 bands were determined by densitometry (right-hand panel). Because M1 ICP0 is expressed at lower levels than the wild-type protein at 4 h (see B), a longer exposure time was used for the M1 samples. The percent ICP0 remaining refers to the amount in the sample harvested at the time of cycloheximide addition in the relevant virus infection. (B) Expression of ICP0, ICP4, and UL42 during wild-type and M1 mutant HSV-1 infection of U2OS cells at a multiplicity of 2 PFU per cell. Samples were harvested at the indicated time points for Western blot analysis of ICP0, ICP4, and UL42. (C) As in panel A, except using HFFF-2 cells. The exposure times in the left-hand panels have been selected to account for lower levels of expression of ICP4 and ICP0 in the mutant M1 compared to wild-type HSV-1 infections (see D). (D) As in panel B, except using HFFF-2 cells. (E) HFFF-2 cells were infected with wild-type or M1 mutant HSV-1 (MOI of 2 PFU/cell) and then samples taken at the indicated time points were analyzed for PML and ICP0 by Western blotting. The SUMO-1-modified and major unmodified forms of PML are indicted. Scanned film exposures were used for analysis by densitometry as described in the text.
Potential consequences for the cell of ICP0-induced degradation of USP7.
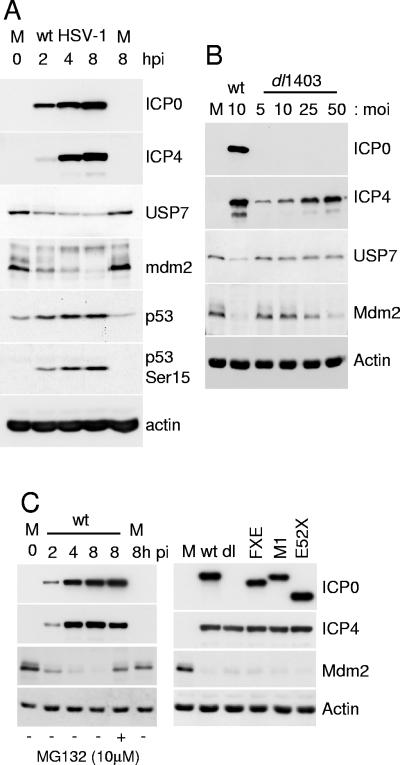
The reports that USP7 can protect p53 and mdm2 from ubiquitination (8, 9, 22, 23) led us to investigate in previous studies the potential relationships between ICP0, USP7, and p53 (1, 2). Despite the potential of ICP0 to ubiquitinate p53 (1) we found that HSV-1 infection induces the stabilization of p53 during HSV-1 infection of HFFF-2 cells by multiple mechanisms that include but do not depend on p53 phosphorylation (2). Therefore, the stabilization of p53 that occurred during HSV-1 infection was dominant over any ICP0-mediated ubiquitination. Since USP7 protects mdm2 from degradation (9, 22) it was possible that ICP0-induced degradation of USP7 could result in destabilization of mdm2 and hence phosphorylation-independent stabilization of p53. To test this hypothesis, we investigated the fate of mdm2 during HSV-1 infection.
We found that mdm2 was rapidly lost from HSV-1-infected HFFF-2 cells, concomitantly with the loss of USP7 and the stabilization and phosphorylation of p53 (Fig. 6A). The reduction in mdm2 levels during HSV-1 infection did not occur in the presence of the proteasome inhibitor MG132 (data not shown and Fig. 6C). However, the loss of mdm2 was not dependent on ICP0, since it occurred in cells infected with the ICP0-null mutant dl1403 at high MOI (Fig. 6B). The degree of degradation of mdm2 during dl1403 infection was proportional to multiplicity and the extent to which viral gene expression in general (exemplified by ICP4) had progressed (Fig. 6B). Although these results demonstrate that ICP0 is not required for mdm2 degradation in high-multiplicity dl1403 infection, this analysis in HFFF-2 cells is complicated by the multiplicity dependence of the ICP0-null phenotype. Therefore we repeated the experiment in U2OS cells, in which ICP0 is not required for efficient HSV-1 gene expression. Analysis of mdm2 levels in U2OS cells infected by the wild-type and a panel of ICP0 mutant viruses confirmed that the loss of mdm2 during HSV-1 infection is independent of ICP0 and its ability to bind to USP7 (Fig. 6C).
FIG. 6.
Degradation of mdm2 during HSV-1 infection. (A) HFFF-1 cells were infected with wild-type HSV-1 (MOI of 10 PFU per cell) and then harvested at the indicated times after infection and analyzed for ICP0, ICP4, USP7, p53, p53 phosphorylated on serine 15, mdm2, and actin by Western blotting. M, mock-infected controls. (B) Extracts from cells infected with wild-type HSV-1 or ICP0-null mutant dl1403 at various MOIs were harvested at 8 h after infection and analyzed by Western blotting for ICP0, ICP4, USP7, mdm2, and actin by Western blotting. (C) The left-hand panel is similar to that of part A except that U2OS cells were infected at an MOI of 10 PFU per cell. The lane second from the right shows a sample from cells that had been treated with 10 μM MG132 from the beginning of the experiment. The right-hand panel shows the results using a panel of ICP0-negative mutant HSV-1 viruses (dl, dl1403), FXE, M1, and E52X. The viruses were used at an MOI of 10 PFU per cell and the cells were harvested at 8 h after infection.
These observations provide a mechanism that may explain the stabilization of p53 that we had observed previously in HFFF-2 cells, and they are consistent with our earlier conclusion that ICP0 was not involved in stabilization of p53 during HSV-1 infection (2). Thus, while the interaction between ICP0 and USP7 could impinge upon the stability of both p53 and mdm2 in principle, in practice we have yet to find evidence that this is the case. However, in that loss of USP7 expression appears to be detrimental to cell viability (8, 9), ICP0-induced reductions in USP7 levels could affect all potential and as yet unidentified substrates of USP7, and this activity could contribute to the reasons why it has not been possible to isolate cell lines that express significant levels of ICP0.
Consequences of the ICP0-USP7 interaction for ICP0 stability and HSV-1 gene expression.
In apparent contrast to the ICP0-induced reduction in the levels of USP7 reported above, we recently demonstrated that USP7 protects ICP0 from auto-ubiquitination in vitro, and that ICP0 mutant proteins that are unable to bind to USP7 are much less stable than the wild-type protein during HSV-1 infection of U2OS cells (6). This creates a complicated situation because ICP0-mediated degradation of USP7 could lead to enhanced auto-ubiquitination and decreased stability of ICP0. The contrasting activities of USP7 deubiquitination of ICP0 and ubiquitination of the former by the latter raise the important question of the relative balance of these events during HSV-1 infection. Accordingly, we investigated the consequences of failure of ICP0 to bind to USP7 for the efficiency of expression of ICP0 and other reference viral proteins during HSV-1 infection.
The accumulation of ICP0 during normal HSV-1 infection may be affected by several factors, including (i) the efficiency of immediate-early gene transcription, stimulated by both the virion transactivator VP16 and ICP0, and potentially repressed by ICP4 (reviewed in references 31 and 38); (ii) the ensuing rate of ICP0 protein synthesis; (iii) the efficiency of ICP0 auto-ubiquitination; (iv) the rate of ICP0-mediated USP7 degradation; and (v) the availability of the components of the ubiquitin conjugation pathway utilized by ICP0. The situation is further complicated by the differential requirement for ICP0 to stimulate the expression of other viral genes in different cell types. Taken with the observation that only low levels of ICP0 are required for its full functionality (21), these considerations suggest that the analysis of HSV-1 mutants expressing forms of ICP0 that do not bind to USP7 is an extremely complicated issue. Nonetheless, we found clear phenotypes that provide intriguing insights into the biological role of the ICP0-USP7 interaction.
Comparison of the stabilities of wild-type and M1 mutant ICP0 proteins and the rates of ICP0, ICP4, and UL42 accumulation during wild-type and M1 virus infections of U2OS and HFFF-2 cells are shown in Fig. 7. These two cell types were selected because ICP0-deficient viruses have the most marked phenotype in limited-passage human fibroblasts such as HFFF-2 cells, while in U2OS cells HSV-1 infection is ICP0 independent (13, 41). For the stability experiments, cells were infected with wild-type and mutant M1 HSV-1 viruses at a multiplicity of 2 PFU per cell, then cycloheximide was added 4 h later to inhibit de novo protein synthesis, and the levels of ICP0 were analyzed at hourly intervals thereafter.
The half-life of wild-type ICP0 at this time point of infection was about 4 h in HFFF-2 cells and considerably longer in U2OS cells. The stability of ICP0-M1 mutant protein at the 4-hour time point was greatly reduced compared to that of the wild-type protein in both U2OS and HFFF-2 cells (Fig. 7, panels A and C). ICP0-M1 was also considerably less stable than the wild-type protein in infected BHK and HEp-2 cells in similar experiments using the same time point and MOI (data not shown), and also in other cell types (see below). The stabilities of the ICP0 proteins expressed by the wild-type HSV-1 and the rescuant M1R virus in HFFF-2 cells were not distinguishable (see Fig. 10).
FIG. 10.
Gene expression phenotype of M1R rescuant virus in HFFF-2 cells. (A) Cells were infected with wild-type or M1R HSV-1 viruses at an MOI of 2 PFU per cell. Samples were harvested at the indicated time points for Western blot analysis of ICP0, ICP4, and UL42. (B) Cells were infected with wild-type or M1R HSV-1 viruses at an MOI of 2 PFU per cell. At 4 h postinfection, cycloheximide was added to a final concentration of 100 μg per ml and then replicate samples were harvested immediately and at the indicated times postinfection (hrs pi) thereafter. The samples were analyzed for ICP0 by Western blotting.
In U2OS cells infected at a multiplicity of 2 PFU per cell, the reduced stability of ICP0-M1 caused a decrease in its accumulation compared to that of the wild-type protein, particularly at the 4-h time point, but this did not affect the rates of accumulation of ICP4 and UL42 (Fig. 7B) because of the lack of a requirement for ICP0 for HSV-1 gene expression in this cell type (41). In contrast, in HFFF-2 cells infected at the same multiplicity, the decreased stability of ICP0-M1 resulted in reduced levels of its accumulation compared to wild-type ICP0, with concomitant decreases in the expression of both ICP4 and UL42 (Fig. 7D). Compared to wild-type HSV-1, the M1 mutant virus also exhibited marked defects in the accumulation of ICP0, ICP4, and UL42 during infection of HeLa and HEp-2 cells at a multiplicity of 2 PFU per cell (data not shown). Unlike in U2OS cells, ICP0 is required for efficient HSV-1 gene expression in these two cell types.
Failure to bind to USP7 has no direct effect on the efficiency of PML degradation induced by ICP0.
In a previous report we had noted that the M1 mutation reduced the rate at which PML was degraded during infection of human fibroblast cells (29). In view of the current results, we investigated whether this was due directly to the failure to bind to USP7, or whether it could be attributed to reduced stability and expression of ICP0. HFFF-2 cells were infected with wild-type and M1 mutant HSV-1 (MOI of 2 PFU per cell) and then samples were analyzed to compare the rates of PML degradation induced by the two viruses (Fig. 7E). Although the rate of degradation of PML was considerably lower in the HSV-1 M1 than in the wild-type virus infection, comparison of the amounts of PML and ICP0 in the 4-h wild-type sample (903 arbitrary units of ICP0; 20% remaining PML) with those in the 8-h M1 sample (1,100 arbitrary units of ICP0; 24% remaining PML) suggested that there was at best only a marginal difference between the intrinsic abilities of wild-type and M1 mutant ICP0 proteins to induce degradation of PML.
Effects of cell type, time, and multiplicity of infection on the stability of wild-type and USP7-binding-deficient ICP0.
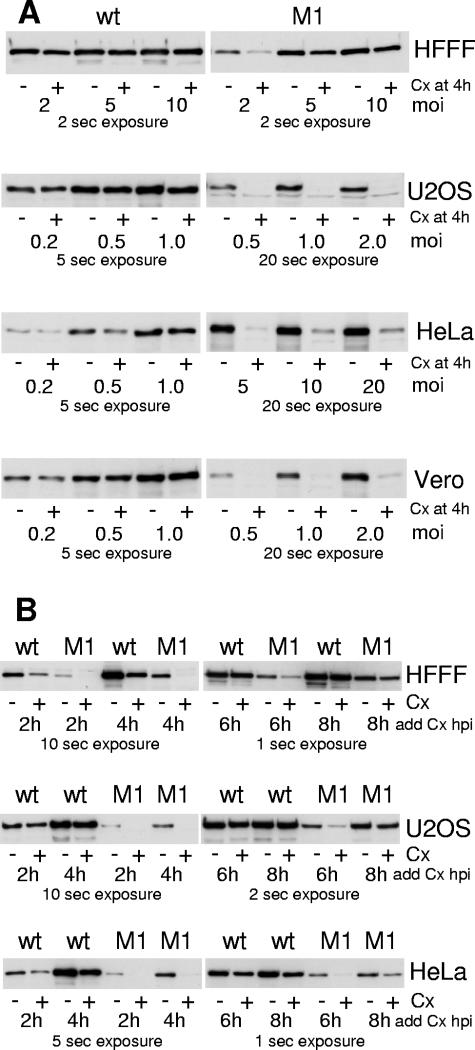
During the course of these experiments we noted that the relative stabilities of the wild-type and the M1 ICP0 proteins could be affected by the multiplicity of infection, the time point at which cycloheximide was added, and the cell type in which the experiments were done. For example, increasing the MOI to 5 and particularly to 10 PFU per cell increased both the level of expression and the stability of ICP0-M1 in HFFF-2 cells, so that the mutant was not distinguishable from the wild-type protein in this assay (Fig. 8A, uppermost panel). Similarly, in Vero and U2OS cells infected at 5 PFU per cell, the differences between the expression levels and stabilities of the wild-type and M1 ICP0 proteins were small if the cycloheximide chase was initiated at 4 h postinfection (data not shown). Given these findings, it could be argued that the stability of ICP0 might be controlled by its level of expression, and that the M1 phenotype might be caused by some other property that reduces its expression levels, distinct from its failure to bind to USP7. Therefore we conducted a thorough analysis of the effects of multiplicity and time of infection on the stability of wild-type and M1 mutant ICP0 in several cell types.
FIG. 8.
Effects of cell type, time, multiplicity of infection, and the M1 mutation on ICP0 stability. (A) Effect of multiplicity. The indicated cell types were infected with wild-type (left-hand panels) or M1 mutant (right-hand panels) viruses in duplicate at the indicated MOIs. At 4 h postinfection, one of each duplicate was harvested, and the other was treated with cycloheximide (100 μg/ml) and harvested 4 h later. Whole-cell extracts were analyzed for ICP0 by Western blotting. Increased exposure times were generally required for the M1 samples because of lower expression levels. The exposure times were selected in order to present data for cells that are neither under- nor overexposed. (B) Effect of time after infection. The indicated cell types were infected with wild-type or M1 mutant viruses (as indicated) in duplicate at an MOI of 2 PFU/cell. At the indicated times, one of each pair was harvested and the other was treated with cycloheximide as above and harvested 4 h later. Whole-cell extracts were analyzed for ICP0 by Western blotting. Increased exposure times were required for the 2-h and 4-h samples, as noted.
In the experiments illustrated in Fig. 8A, the multiplicities of infection were varied while in all cases cycloheximide was added 4 h after infection (at which point one of a duplicate set of samples was harvested), and then the treated samples were harvested 4 h later. The choice of MOI for the wild-type infection in each cell type was selected on the basis of obtaining readily detectable levels of ICP0. The same MOIs of wild-type and M1 mutant HSV-1 were used in all cell types except in HeLa cells, in which expression levels of ICP0-M1 at the multiplicities used for the wild-type infections were too low for analysis.
Wild-type ICP0 was entirely stable in U2OS, HeLa, and Vero cells even at very low multiplicities. ICP0-M1 was unstable at the same multiplicities in U2OS and Vero cells, while in HeLa cells ICP0-M1 was very unstable even at multiplicities as high as 20 PFU per cell. Importantly, this instability of ICP0-M1 in HeLa cells could not be ascribed simply to low levels of expression, because the level of expression of the mutant protein prior to the addition of cycloheximide at an MOI of 20 exceeded that of the wild-type protein at an MOI of 0.2 (even allowing for the differences in exposure time in the examples presented). As noted above, ICP0-M1 became more stable at higher multiplicities of infection in HFFF-2, U2OS, and Vero cells. Since increased multiplicity has the effect of increasing the rate at which infection develops, the basis of the MOI effect is highly likely to be related to the as yet unidentified mechanism by which ICP0-M1 can be stabilized at later times of infection. This is discussed in the final paragraph of this section.
To test the effect of time of infection, HFFF-2 and U2OS cells were infected with the viruses at an MOI of 2 PFU per cell, and HeLa cells at an MOI of 10, then cycloheximide was added to one of a pair duplicate wells at 2, 4, 6, or 8 h postinfection, with the other being harvested at the time of drug addition. The cycloheximide-treated samples were harvested 4 h later. In all three cell types the stability of ICP0-M1 was increased at the 8-h time point, and in HFFF-2 and U2OS cells the mutant protein was almost completely stable by this time (Fig. 8B). While wild-type ICP0 was completely stable in U2OS cells at the earliest time point tested, in HFFF-2 and to a lesser degree in HeLa cells even the wild-type protein was sensitive to the addition of cycloheximide at 2 h postinfection However, ICP0-M1 was again much less stable than the wild-type protein, even when expressed at equivalent or greater levels than the wild-type protein at a time when the latter exhibited a degree of instability (compare M1 at 4 h in HFFF-2 and HeLa cells with the corresponding wild-type samples at 2 h).
These data illustrate that in certain cell types ICP0 can exhibit limited instability at low MOI and at early times of infection despite the presence of USP7. However, failure to bind USP7 causes a substantial reduction in ICP0 stability in equivalent infections. These reductions in stability cannot be explained simply by reduced expression of the mutant protein. This conclusion is consistent with the observation that wild-type ICP0 behaves in a similar manner to the M1 mutant when USP7 levels are depleted using siRNA (6) (see also Fig. 11 below). These data confirm that the ability to bind to USP7, rather than some other potential function of the USP7 binding region of ICP0, is a major factor that regulates ICP0 stability at the early times of infection.
However, since ICP0-M1 becomes more stable at high MOI and as infection progresses, factors other than USP7 contribute to the stability of ICP0 at later times of infection. These could include accumulation of other viral proteins that might affect ICP0 stability; changes in the intranuclear localization or posttranslational modification of ICP0; or because of its tendency to accumulate in the cytoplasm of infected cells once viral replication compartments have begun to develop. Because the rates at which these events occur depend on the cell type, the question of the stability of ICP0 must be analyzed with considerable care. An intriguing possibility is that HSV-1 tolerates the ability of ICP0 to degrade USP7 because at later times of infection, when USP7 levels are reduced, ICP0 can be effectively stabilized by this other, as yet unidentified, mechanism that comes into play as the infection develops.
Consequences of the ICP0-USP7 interaction for HSV-1 gene expression and replication in low-multiplicity infections.
The phenotype of ICP0 mutant HSV-1 viruses is most pronounced during low-multiplicity infections (13). While the infections carried out in HFFF-2 cells at an MOI of 2 PFU per cell clearly show a defect in gene expression by virus M1 (Fig. 7D), this multiplicity is only a few fold below that at which HSV-1 infection becomes ICP0 independent in this cell type (13). Therefore we expected the M1 phenotype to become more pronounced at lower multiplicities. However, viral gene expression becomes increasingly difficult to detect as the multiplicity is lowered, particularly at early times of infection.
Two approaches allow study of viral gene expression and replication at low multiplicity: FACS analysis of virus-infected cells gives a reliable estimation of the proportion of cells infected by wild-type HSV-1 and expressing selected antigens at multiplicities as low as 0.05 PFU per cell, while plaque-forming assays are performed at multiplicities on the order of 0.001 PFU per cell. These two techniques were used to compare wild-type and M1 mutant HSV-1 infections in HFFF-2 cells.
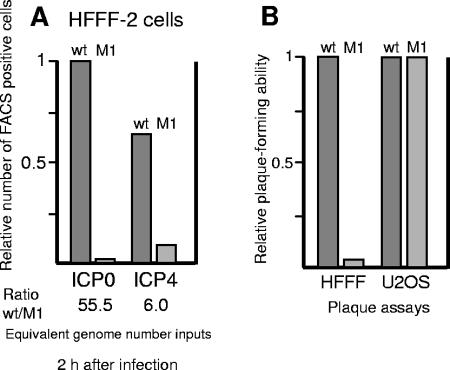
HFFF-2 cells were infected with wild-type HSV-1 and mutant M1 at low multiplicity, and then the relative numbers of cells expressing the immediate-early proteins ICP0 and ICP4 were determined in replicate samples by FACS at 2 h after infection (Fig. 9A). Consistent with previous results (13), in wild-type HSV-1 infection there was a slight excess of ICP0- over ICP4-positive cells. However, at this time point the M1 mutant virus produced over 50-fold fewer ICP0-positive cells than wild-type virus when the results were normalized to the number of input PFU based on titers in U2OS cells (and hence viral genome numbers) (13). The relative number of ICP4-positive cells was decreased only sixfold in mutant M1 compared to wild-type HSV-1 infection (Fig. 9A), indicating that the M1 mutant virus, in contrast to the wild-type virus, produced a considerable excess of ICP4- over ICP0-positive cells at this time point in HFFF-2 cells. This result is analogous to but not as marked as the dramatic decrease in ICP0-positive cell numbers detected by FACS analysis after low-multiplicity infection of U2OS cells by M1 mutant HSV-1 (6).
FIG. 9.
FACS analysis of low-multiplicity wild-type and M1 HSV-1 infections in HFFF-2 cells. Cells in 35-mm plates were infected in duplicate with the wild-type or M1 mutant virus at MOIs of 0.1 and 0.5 PFU/cell, respectively. The dose of M1 virus was greater than that of wild-type HSV-1 to allow for the reduced numbers of antigen-positive cells produced by the M1 virus. At 2 h postinfection, the cells were harvested and prepared for FACS analysis using anti-ICP0 or anti-ICP4 antibodies, with positive cell counts determined by gating the data compared to mock-infected controls (A). After adjustment to take account of the number of input PFU based on U2OS cell titers, the data were normalized in relation to the number of ICP0-positive cells produced by wild-type HSV-1 (B).
This deficit in the ICP0-positive cell population reflects the decreased stability of ICP0 caused by the M1 mutation at early times of infection. As with the Western blot approach described above, increasing the MOI or analyzing samples at later times of infection decreased the differential between the M1 mutant and wild-type viruses in FACS experiments (data not shown). The decrease in the relative numbers of ICP0- and ICP4-positive cells produced by mutant virus M1 was consistent with a 17-fold reduction in the ability of virus M1 to produce plaques in HFFF-2 compared to U2OS cells (Fig. 9B). Similar but slightly less marked reductions in the ability of mutant virus M1 to produce ICP0- and ICP4-positive cells and plaques were observed in BHK cells (data not shown).
As a control for the phenotype of mutant virus M1, we constructed a rescuant virus (M1R) and analyzed ICP0 stability and viral gene expression in comparison with wild-type virus during infection of HFFF-2 cells at an MOI of 2 PFU per cell. As expected, ICP0 expressed by M1R accumulated to similar levels and had a stability similar to that of the wild-type protein (Fig. 10). In addition, the wild-type and M1R viruses expressed similar amounts of UL42 in these infections (Fig. 10A). The rescuant virus M1R and wild-type HSV-1 had identical plaque-forming efficiencies on HFFF-2 and U2OS cells (data not shown).
siRNA depletion of USP7 reduces HSV-1 gene expression.
We had previously demonstrated that treatment of U2OS cells with anti-USP7 siRNAs reduced the expression levels and stability of wild-type ICP0 (6). This experiment could not test whether this reduction in ICP0 levels caused a decrease in the rate of development of HSV-1 early gene expression because ICP0 is not required in U2OS cells. To test the requirement for USP7 for development of HSV-1 early gene expression we conducted similar experiments in HeLa cells, in which ICP0 is required for efficient HSV-1 infection. Although depletion of USP7 is deleterious for long-term cell survival, HeLa cells transfected with anti-USP7 siRNAs showed no phenotypic changes, deleterious effects, or cell loss (as determined by actin levels) over the time course of this assay.
Initial experiments demonstrated that, as in U2OS cells, wild-type ICP0 accumulated to markedly lower levels in HSV-1-infected HeLa cells with depleted USP7 than in controls that were either mock transfected or transfected with an anti-GFP siRNA (Fig. 11B). In contrast, mutant M1 ICP0 accumulation was little affected by a reduction in USP7 levels compared to the GFP siRNA control and not at all compared to the mock control, as expected (Fig. 11A). We found that transfection of the control anti-GFP siRNA had little effect on the efficiency of expression of ICP4 and UL42 during wild-type HSV-1 infection of HeLa cells (Fig. 11C). In contrast, the anti-USP7 siRNA-mediated reduction in ICP0 levels led to a concomitant reduction in ICP4 accumulation and at least a delay in progression into productive infection as measured by reduced levels of UL42 expression (Fig. 11C). Analogous results were obtained in HFFF-2 cells, but we also observed a general moderate reduction in HSV-1 gene expression in such cells transfected with control siRNAs (data not shown), perhaps because of an interferon response induced by transfection of double-stranded RNA. Note that little ICP0-mediated degradation of USP7 was observed in these particular experiments, especially in Fig. 11C, although to a greater extent in Fig. 11B. This may have been due to the choice of a relatively low multiplicity (in order to ensure that the rate of HSV-1 gene expression was ICP0 dependent), or because in HeLa cells, as in HEp-2 cells (Fig. 3B), USP7 may be less sensitive to ICP0.
The apparent paradox that prior reduction of USP7 expression by siRNA impedes HSV-1 infection while ICP0 brings about equivalent reductions in USP7 levels during productive infection can be rationalized by the requirement for USP7 early during infection to stabilize ICP0 and therefore maximize the efficiency of viral immediate-early and early gene expression. Once viral DNA replication has commenced, viral DNA copy number increases and the high rate of ICP0 synthesis and other factors discussed above overcomes any instability caused by reduced levels of USP7. Thus, USP7 is required to stabilize ICP0 at early times of infection when the critical transition from the immediate-early to the early phase of infection occurs, but not at later times of infection. However, it is possible that the reduction in total USP7 levels that occurs at later times of infection could have other effects on the fate of the cell which may contribute in as yet undetected ways to the biology of HSV-1 infection. In addition, we cannot exclude the possibility that the relatively modest reductions in USP7 that occur at early times of wild-type HSV-1 infection reflect degradation of a specific pool of USP7 molecules whose loss also contributes to efficient infection.
DISCUSSION
This paper confirms the role of USP7 in regulating the stability of ICP0 and demonstrates that this process is important for efficient HSV-1 infection. The RING finger-mediated ubiquitination of USP7 by ICP0 both in vitro and in cultured cells and the ability of USP7 to stabilize ICP0 by protecting it from auto-ubiquitination (6) demonstrate that ICP0 and USP7 have complex interacting effects upon each other. The situation is further complicated by factors other than USP7 that appear to affect ICP0 stability at later times of infection, and that sequences downstream of the USP7 binding can influence ICP0 stability in certain situations (manuscript in preparation). The present paper concentrates on the specific issues of (i) USP7-mediated stabilization of ICP0, (ii) ubiquitination of USP7 by ICP0, and (iii) the consequences of abrogation of the USP7-ICP0 interaction and reduction of USP7 levels for the progress of HSV-1 infection.
ICP0 is a member of a growing list of RING finger E3 ubiquitin ligases whose levels are regulated at least in part by auto-ubiquitination. Stabilization of E3 ubiquitin ligase proteins by ubiquitin-specific protease enzymes has also been implicated in the interactions between mdm2 and USP7 (8, 9, 22, 23), Nrdp1 and USP8 (40), and GRAIL and USP8 (34), and in the presence of Ubp12p in a cullin-containing ubiquitin ligase complex (43). This principle may apply to further as yet undocumented examples involving E3 ubiquitin ligase enzymes. For example, certain TRAF E3 ubiquitin ligases that ubiquitinate themselves (5) have been proposed to interact with USP7 (42). Interestingly, the A20 protein, which is a potent repressor of NF-κB signaling, contains both ubiquitin ligase and ubiquitin protease moieties within the same polypeptide (39). In this case, the A20 protease motif was shown to remove K63-linked ubiquitin chains from RIP, a component of the tumor necrosis factor receptor signaling complex, thus enabling K48-linked polyubiquitination and proteasome-mediated degradation of the same protein by the A20 RING finger ubiquitin ligase domain (39). However, it is possible that the A20 protease motif may also be able to protect A20 from auto-ubiquitination.
This paper demonstrates that USP7 can be regulated through ubiquitination and proteasome-mediated degradation during HSV-1 infection. Therefore, in addition to being subject to proteolytic cleavage in response to varied stimuli (37), USP7 has the potential to be regulated by the ubiquitin-proteasome pathway. While ICP0 has been shown to induce the proteasome-dependent degradation of several cellular proteins through a process requiring its RING finger domain, USP7 is the first direct substrate of ICP0 to be defined that is efficiently ubiquitinated in vitro and degraded in cultured cells in response to ICP0 expression during HSV-1 infection. For example, PML is readily degraded in cells expressing ICP0, but neither PML nor its SUMO-modified forms can be ubiquitinated directly in vitro by ICP0 (3). However, intracellular levels of ubiquitinated PML were increased by ICP0, and therefore we speculated that either an additional factor was required for an active ICP0-UbcH5a-PML ubiquitination complex to form or ubiquitination and degradation of PML may be a consequence of the activity of ICP0 on some other as yet unknown substrate (3). Indeed, this is likely to be true for Sp100, another ICP0 target in cultured cells that is not ubiquitinated by ICP0 in vitro (3), especially since it is only the SUMO-modified forms of Sp100 that are particularly sensitive to the effects of ICP0 (29). Direct ubiquitination of other proteins that are sensitive to the presence of ICP0, such as CENP-C, CENP-A, and the catalytic subunit of DNA-PK, has yet to be investigated.
An issue that must be considered arises from reports of a second E3 ubiquitin ligase activity of ICP0, distinct from that of the RING finger, conferred by the region of ICP0 that includes the USP7 binding domain (20, 36). It is possible that the activity ascribed to this domain (named HUL1) may affect ICP0 stability and contribute to the phenotype of viruses with lesions in this region of the protein. Previous publications have discussed the controversy regarding the authenticity of the HUL1 activity (4, 11, 18, 20, 36). Of relevance to this paper are the observations that ICP0 proteins containing the HUL1 region but lacking the RING finger are stable (6) and that the gene expression defect of mutant virus M1 (Fig. 7) correlates with reduced gene expression by wild-type HSV-1 in cells with depleted USP7 levels (Fig. 11). These observations demonstrate that the M1 phenotype is due to failure to bind USP7 rather than an effect on any other potential function of ICP0 that involves its USP7 binding region. Taking all the data together, we suggest that the phenotypes of ICP0 proteins with mutations in this region are most easily interpreted in terms of USP7 binding and the concomitant effects on ICP0 stability and function.
Given the potentially competing effects of ICP0 and USP7 on each other, what is the principal biological significance of their interaction? The data suggest that during the initial stages of infection the dominant factor is the stabilization that USP7 confers upon ICP0 rather than ICP0-mediated degradation of USP7. This is particularly true in low-multiplicity infections, in which the rate of USP7 degradation is low. High-multiplicity infections may mask the importance of the ICP0-USP7 interaction. Although high levels of ICP0 will increase the rate of USP7 loss from the cell, high rates of ICP0 synthesis in high-multiplicity infections will compensate for any consequent loss of ICP0: newly synthesized ICP0 will rapidly replace that lost through auto-ubiquitination. In addition, factors other than USP7 contribute to the stability of ICP0 at higher multiplicities and at later times of infection (Fig. 8). In contrast, in low-multiplicity wild-type HSV-1 infections, when the rate of ICP0 synthesis is low, there are no substantial reductions in the levels of USP7 in the first few hours of infection (Fig. 5). In these situations, wild-type ICP0 is stabilized by USP7, but in the case of mutant ICP0 proteins that fail to bind USP7, their rates of synthesis may be insufficient to replace the protein that is lost through auto-ubiquitination and degradation. This problem may be particularly important during reactivation from latency, since VP16, the component of the virus particle that stimulates immediate-early gene expression, is unlikely to be present. This hypothesis predicts that viruses expressing mutant forms of ICP0 that cannot bind USP7 will reactivate from latency inefficiently. This prediction is currently being tested.
Acknowledgments
This work was supported by the Medical Research Council.
REFERENCES
- 1.Boutell, C., and R. D. Everett. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596-36602. [DOI] [PubMed] [Google Scholar]
- 2.Boutell, C., and R. D. Everett. 2004. Herpes simplex virus type 1 infection induces the stabilization of p53 in a USP7- and ATM-independent manner. J. Virol. 78:8068-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutell, C., A. Orr, and R. D. Everett. 2003. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 77:8686-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, K. D., B. S. Hostager, and G. A. Bishop. 2002. Regulation of TRAF2 signaling by self-induced degradation. J. Biol. Chem. 277:19433-19438. [DOI] [PubMed] [Google Scholar]
- 6.Canning, M., C. Boutell, J. Parkinson, and R. D. Everett. 2004. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem. 279:38160-38168. [DOI] [PubMed] [Google Scholar]
- 7.Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 8.Cummins, J. M., C. Rago, M. Kohli, K. W. Kinzler, C. Lengauer, and B. Vogelstein. 2004. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature 428:487. [DOI] [PubMed]
- 9.Cummins, J. M., and B. Vogelstein. 2004. HAUSP is required for p53 destabilization. Cell Cycle 3:689-692. [PubMed]
- 10.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J Gen. Virol. 70:1185-1202. [DOI] [PubMed] [Google Scholar]
- 11.Everett, R. D. 2004. Herpes simplex virus type 1 regulatory protein ICP0 does not protect cyclins D1 and D3 from degradation during infection. J. Virol. 78:9599-9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 13.Everett, R. D., C. Boutell, and A. Orr. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 78:1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett, R. D., M. Meredith, and A. Orr. 1999. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J. Virol. 73:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett, R. D., M. Meredith, A. Orr, A. Cross, M. Kathoria, and J. Parkinson. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagglund, R., and B. Roizman. 2003. Herpes simplex virus 1 mutant in which the ICP0 HUL-1 E3 ubiquitin ligase site is disrupted stabilizes cdc34 but degrades D-type cyclins and exhibits diminished neurotoxicity. J. Virol. 77:13194-13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagglund, R., C. Van Sant, P. Lopez, and B. Roizman. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 99:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs, W. E., D. E. Brough, I. Kovesdi, and N. A. DeLuca. 2001. Efficient activation of viral genomes by levels of herpes simplex virus ICP0 insufficient to affect cellular gene expression or cell survival. J. Virol. 75:3391-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, M., C. L. Brooks, N. Kon, and W. Gu. 2004. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell 13:879-886. [DOI] [PubMed] [Google Scholar]
- 23.Li, M., D. Chen, A. Shiloh, J. Luo, A. Y. Nikolaev, J. Qin, and W. Gu. 2002. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416:648-653. [DOI] [PubMed] [Google Scholar]
- 24.Lomonte, P., K. F. Sullivan, and R. D. Everett. 2001. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 276:5829-5835. [DOI] [PubMed] [Google Scholar]
- 25.Meredith, M., A. Orr, M. Elliott, and R. Everett. 1995. Separation of sequence requirements for HSV-1 Vmw110 multimerisation and interaction with a 135-kDa cellular protein. Virology 209:174-187. [DOI] [PubMed] [Google Scholar]
- 26.Meredith, M., A. Orr, and R. Everett. 1994. Herpes simplex virus type 1 immediate-early protein Vmw110 binds strongly and specifically to a 135-kDa cellular protein. Virology 200:457-469. [DOI] [PubMed] [Google Scholar]
- 27.Merrihew, R. V., T. A. Kost, and J. P. Condreay. 2004. Baculovirus-mediated gene delivery into mammalian cells. Methods Mol. Biol. 246:355-365. [DOI] [PubMed] [Google Scholar]
- 28.Muller, S., and A. Dejean. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkinson, J., and R. D. Everett. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006-10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkinson, J., S. P. Lees-Miller, and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 32.Roizman, B., and P. E. Pellett. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 33.Russell, J., N. D. Stow, E. C. Stow, and C. M. Preston. 1987. Herpes simplex virus genes involved in latency in vitro. J. Gen. Virol. 68:3009-3018. [DOI] [PubMed] [Google Scholar]
- 34.Soares, L., C. Seroogy, H. Skrenta, N. Anandasabapathy, P. Lovelace, C. D. Chung, E. Engleman, and C. G. Fathman. 2004. Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat Immunol. 5:45-54. [DOI] [PubMed] [Google Scholar]
- 35.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 36.Van Sant, C., R. Hagglund, P. Lopez, and B. Roizman. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 98:8815-8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vugmeyster, Y., A. Borodovsky, M. M. Maurice, R. Maehr, M. H. Furman, and H. L. Ploegh. 2002. The ubiquitin-proteasome pathway in thymocyte apoptosis: caspase-dependent processing of the deubiquitinating enzyme USP7 (HAUSP). Mol. Immunol. 39:431-441. [DOI] [PubMed] [Google Scholar]
- 38.Weir, J. P. 2001. Regulation of herpes simplex virus gene expression. Gene 271:117-130. [DOI] [PubMed] [Google Scholar]
- 39.Wertz, I. E., K. M. O'Rourke, H. Zhou, M. Eby, L. Aravind, S. Seshagiri, P. Wu, C. Wiesmann, R. Baker, D. L. Boone, A. Ma, E. V. Koonin, and V. M. Dixit. 2004. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430:694-699. [DOI] [PubMed] [Google Scholar]
- 40.Wu, X., L. Yen, L. Irwin, C. Sweeney, and K. L. Carraway, 3rd. 2004. Stabilization of the E3 ubiquitin ligase Nrdp1 by the deubiquitinating enzyme USP8. Mol. Cell. Biol. 24:7748-7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69:6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zapata, J. M., K. Pawlowski, E. Haas, C. F. Ware, A. Godzik, and J. C. Reed. 2001. A diverse family of proteins containing tumor necrosis factor receptor-associated factor domains. J. Biol. Chem. 276:24242-24252. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, C., S. Wee, E. Rhee, M. Naumann, W. Dubiel, and D. A. Wolf. 2003. Fission yeast COP9/signalosome suppresses cullin activity through recruitment of the deubiquitylating enzyme Ubp12p. Mol. Cell 11:927-938. [DOI] [PubMed] [Google Scholar]