Abstract
Introduction
Puberty suppression using gonadotropin-releasing hormone agonists (GnRHa) is a reversible medical intervention that halts endogenous puberty, allowing transgender and gender-diverse (TGD) adolescents to avoid the development of secondary sexual characteristics that may cause psychological distress. This pause in pubertal progression provides time to explore gender identity or facilitates alignment with affirmed gender in those with an established identity. While widely used, long-term evidence on the efficacy and safety of GnRHa in this population remains limited. This systematic review aims to synthesize current data on the benefits and potential risks of GnRHa in TGD adolescents.
Methods
We conducted a comprehensive literature search across PubMed, EMBASE, Cochrane Library, and other databases, covering studies published from February 2011 to February 2024. Eligible studies included adolescents under 18 with gender dysphoria or incongruence treated with GnRHa, reporting outcomes related to efficacy or side effects. Fifty-one studies met inclusion criteria, and data on physical health, mental health, bone density, fertility, and adverse events were extracted and assessed using the GRADE approach.
Results
Of the 51 studies, 22 were rated as moderate to high-quality evidence. GnRHa effectively suppressed puberty and secondary sex characteristics. Effects on growth and body composition varied; bone mineral density declined during treatment, particularly in AMAB individuals. Mental health improved significantly, including reduced depression, anxiety, and suicidality—especially when GnRHa was followed by gender-affirming hormone therapy (GAHT). Quality of life improved over time, while body dissatisfaction often persisted during suppression and improved after GAHT or surgery. No moderate- or high-quality evidence was found on fertility, sexual function, or cancer risk.
Conclusion
GnRHa is effective in halting puberty and improving mental health in TGD adolescents. However, key clinical and ethical considerations—such as bone health monitoring, fertility counseling, psychological support, and informed decision-making—must guide treatment. Long-term safety remains uncertain, particularly regarding skeletal health, reproductive outcomes and cancer risk. A precision medicine approach and co-produced longitudinal studies are essential to support safe, individualized care.
Systematic review registration
https://www.crd.york.ac.uk, identifier CRD42024528334.
Keywords: gender dysphoria (GD), gender incongruence, adolescence, GnRH analog (GnRHa), LHRH analog, transgender and gender diverse (TGD), transgender and gender diverse youth
Introduction
Transgender and gender diverse (TGD) individuals are those whose gender identity differs from the sex assigned to them at birth. Within this population, two clinical concepts are commonly used to describe experiences related to gender identity: gender incongruence (GI) and gender dysphoria (GD). GI, as defined in the International Classification of Diseases (ICD-11) (1), refers to a persistent incongruence between a person’s experienced gender and their assigned gender, without necessarily involving distress. Conversely, GD, as outlined in the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (2), is characterized by clinically significant distress or discomfort resulting from this incongruence.
When GD or GI persist or emerge during puberty, TGD adolescents may be eligible for puberty suppression using gonadotropin-releasing hormone analogs (GnRHa) (3). This intervention serves multiple purposes: it provides time for individuals to explore their gender identity, prevents the development of irreversible secondary sex characteristics that may cause distress, and facilitates a smoother transition to gender-affirming hormone therapy (GAHT) if pursued (4). Conversely, GAHT leads to partially irreversible changes in secondary sex characteristics, such as breast development or deepened voice and facial hair (3). Preventing puberty that is incongruent with gender identity is particularly important, as it can positively affect mental health outcomes, and reduce the need for later invasive and expensive surgeries, such as chest surgery or facial feminization surgery (5).
Despite increasing interest in the effects of GnRHa treatment in TGD adolescents, significant gaps remain in the literature. In 2018 Chew et al. (6) conducted a systematic review concluding that low-quality evidence suggests hormonal treatments achieve their intended physical effect; however, evidence regarding psychological and cognitive impact remained insufficient. In 2023 Ludvigsson et al. performed a systematic review regarding hormone treatment in TGD adolescents, analyzing studies published until November 2021 (7). They found no randomized controlled trials and noted that few longitudinal observational studies were limited by small sample sizes. Consequently, they concluded that the long-term effects of hormone therapy on psychosocial health could not be evaluated.
A more recent systematic review synthesized studies assessing puberty suppression outcomes in TGD adolescents (8). The review included 50 studies and found consistent moderate-quality evidence demonstrating the efficacy of GnRHa in reducing gonadotropin and sex steroid levels, with the effects on secondary sex characteristics varying depending on whether treatment is initiated in early or mid-puberty. However, it raised significant concerns regarding bone mineral density (BMD) and linear growth during treatment, with studies reporting reductions in BMD and height increases that did not align with expected growth trajectories. Importantly, the review noted a lack of high-quality research assessing the impact of GnRHa on GD, mental health, psychosocial health, cognitive development, and fertility outcomes.
To address these gaps, this systematic review provides an updated synthesis of evidence on the efficacy and safety of GnRHa in TGD adolescents, with a focus on studies of moderate to high quality, assessed using the GRADE approach. By examining key outcomes—including physical development, mental health, and bone health—this review aims to contribute to a more comprehensive understanding of the role of GnRHa in gender-affirming care, while also identifying areas where further research is needed.
Materials and methods
The protocol of this systematic review was registered in PROSPERO on March 24, 2024, with registration number CRD42024528334.
Search strategy
We searched electronic databases (Pubmed, EMBASE, The Cochrane Library, Web of Science, Clinicaltrial.gov, International Clinical Trials Registry Platform) for studies published between February 1, 2011 (the date the first study was published) and February 1, 2024. Search terms, or “MESH” (MEdical Subject Headings) for this systematic review included different combinations:
“Gender Dysphor*” or “Gender incongruen*” or transgender or nonbinary
AND “Gonadotropin-releasing hormone agonist” or “GnRH Analogue” AND “puberty block*” or “puberty suppress*” or “puberty inhibit*”. The exact terms used to search each database are reported in Supplementary Material S1 .
We also screened the reference list of eligible studies to avoid missing any relevant studies. We used the “free-text search” technique for fertility, sexual function, side effects, and risk of cancer outcomes, to improve the performance of our search, and more documents that matched the search criteria, not selected by the electronic databases, were included.
Criteria for study selection
To minimize the risk of selection bias, two Authors (RF, GTo) independently conducted a systematic literature search according to the PICOS model (Population, Intervention, Comparison, Results, Study design). We formulated five questions related to the GnRHa’s efficacy, and for each one, we established the outcomes listed in Table 1 :
Table 1.
PICOS (Population, Intervention, Comparison, Results and Study design) framework for the systematic review.
| Population | Adolescents (<18 years, Tanner Stage ≥ 2) with GD/GI |
| Intervention | Puberty suppression with or without GAHT |
| Comparison | Adolescents with GD/GI not treated or age-matched individuals |
| Outcomes | Q1) PHYSICAL CHANGES AND HORMONE LEVELS: pubertal stage, anthropometry (height velocity, final height), body composition (% fat mass, % lean mass), secondary sexual characteristics, blood pressure; % of TGD adolescents that proceed to GAHT, voice, breast, menstrual bleeding, hormone levels. Q2) BONE HEALTH: bone age, bone mineral density, bone turnover, bone geometry. Q3) MENTAL HEALTH: psychological well-being, QoL, social life, satisfaction, body image, depression-anxiety, self-harm and suicidal ideation, intelligence quotient (IQ), autism. Q4) FERTILITY AND SEXUAL FUNCTION: data on fertility and satisfaction with sexual function. Q5) SIDE EFFECTS AND RISK OF CANCER: physical, hematological, biochemical, cardiovascular, % of persistence of dysphoria. Prostate cancer, breast cancer, mortality. |
| Study design | Randomized controlled trials (RCTs), observational studies (cohort, case-control), exploratory studies, and a mix of qualitative and quantitative studies. |
Inclusion criteria were: i) study population: adolescents (age <18 years, Tanner Stage ≥ 2) who had GD/GI treated with GnRHa, and their follow-up data were reported; ii) study type: observational studies (cohort, case-control), exploratory studies (that consist in additional preliminary research to determine priorities and problems to be solved), a mix of qualitative (data from surveys, interviews) and quantitative studies; iii) review articles were excluded, but their reference lists were screened to identify potentially eligible studies; iv) only full papers were included, whereas conference abstracts were not included; v) data on the modality and efficacy of the hormonal therapy (puberty suppression with GnRHa, with or without GAHT), and TGD adolescents’ characteristics: age at the start of therapy, pubertal status, duration of treatment, outcomes, rate of discontinuation and side effects; vi) publication date: last 15 years (2011-2024). Exclusion criteria: i) data available only for adults ≥18 years without baseline status (cross-sectional studies); ii) data only at baseline, without follow-up; iii) non-human animal data; iv) case reports; studies with <10 patients who underwent GnRHa therapy; v) full paper not available; vi) study not yet published; vii) studies not reporting the selected outcomes; viii) studies on GAHT without data on GnRHa treatment; studies on Gender Affirming Surgery (GAS).
Case reports were excluded from the review due to their high potential for bias—both in terms of publication and their focus on rare or unusual cases—as well as their limited generalizability.
Data extraction and management
The same two independent investigators (RF, GTo) screened for inclusion in the title and abstract of all the studies identified by the search strategy. Any discrepancies between them were resolved by consensus. After abstract selection, 25 investigators were subdivided into the five research questions (Q1 to Q5) and conducted the full-text review.
The following characteristics were evaluated for each study in the full paper: i) reference details: authorship(s); published or unpublished; year of publication; period in which the study was conducted; other relevant cited papers; ii) study characteristics: study design, topic, treatment period, follow up duration, region; iii) population characteristics: number of participants (assigned male at birth, AMAB; assigned female at birth, AFAB) who underwent puberty suppression or GAHT, age and demographic data; comparator characteristics; iv) methodology: measures to assess the outcomes; v) main results: outcome measures. Data extraction was completed in duplicate.
Assessment of the certainty of the evidence
We used the GRADE approach to rank the quality of evidence (www.gradeworkinggroup.org) for the included studies. Two authors (RF, GTo) independently assessed the certainty of the evidence for each of the outcomes, and any differences between them were resolved by consensus. The two authors used ROBINS-I (“Risk Of Bias In Non-Randomized Studies - of Interventions”) as a tool to ensure consistency and accuracy (9). In the case of risk bias in the study design, imprecision of estimates, inconsistency across studies, indirectness of the evidence, and publication bias, the recommended option of decreasing the level of certainty by one or two levels according to the GRADE guidelines was applied. Using the ROBINS-I tool, the GRADE framework assigns an initial certainty rating of high for outcome data from RCTs and low for observational studies. Certainty can be downgraded based on five domains: limitations in study design and execution (risk of bias), inconsistency (heterogeneity), indirectness (PICO and applicability), imprecision, and publication bias. Conversely, three factors can increase certainty: a large magnitude of effect, opposing plausible residual bias or confounding, and a dose-response gradient. We have reported the cut-off values (median values) used to downgrade the level of certainty by one or two levels.
The GRADE approach results in an assessment of the certainty of a body of evidence and allocation to one of four grades, reported in Table 2 :
Table 2.
GRADE classification of evidence certainty levels.
| High | Further research is very unlikely to change confidence in the estimate of the effect |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of the effect and may change the estimate |
| Low | Further research is very likely to have an important impact |
| Very low | Any estimate of the effect is very uncertain. |
Data synthesis
Starting from Tables that reported the details of each study ( Supplementary Tables ), relevant information reported in the ones with a moderate-high quality level of evidence, were categorized into separate “tables of evidence”, one for each outcome ( Tables 3A-C ).
Table 3A.
Summary of findings on physical changes and hormone levels on GnRHa treatment in TGD adolescents.
| Reference | Study design/ Population | Intervention (duration) | Height | Sexual characteristics | Gonadotropins and sexual steroids | Body composition | Lipids /glucose |
|---|---|---|---|---|---|---|---|
| Boogers 2022 (10) | R/161 (161 AMAB, 0 AFAB) |
GnRHa (2.4 ± 0.8 y) GAHT |
↓ (3m) during GnRH ↓ (adult) High dose E and EE ↓ Bone age during GnRH |
N/A | N/A | N/A | N/A |
| Fisher 2024 (4) | P/36 (14 AMAB, 22 AFAB) |
GnRHa (3-12 m) | ↓ HT centile in AMAB | ↓ Tanner stage | ↓ FSH/LH and T in AMAB/E in AFAB | ↑ BMI in AFAB | ↓ HDL in AMAB = |
| Klaver 2018 (11) | R/192 (71 AMAB, 121 AFAB) |
GnRHa AMAB: 2.1 y (1.0-2.8) AFAB: 1.0 years (0.5-2.9) GAHT |
N/A | N/A | N/A | WHR and body composition changed toward the affirmed sex | N/A |
| Klaver 2020 (12) | R/192 (71 AMAB, 121 AFAB) |
GnRHa AMAB: 2.1 y (1.0-2.8); AFAB: 1.0 y (0.5-2.9) GAHT |
N/A | N/A | N/A | > % of obesity vs controls | = Changes in lipids, insulin, HOMA IR, BP and BMI similar to controls |
| Navabi 2021 (13) | R/172 (51 AMAB, 119 AFAB) |
GnRHa (N/A) | N/A | N/A | N/A |
↑ total body fat % increase in gynoid (%fat), and android (%fat) in AFAB and AMAB was detected without changes in BMI z score |
N/A |
| Schagen 2016 (14) | P/116 (49 AMAB, 67 AFAB) |
GnRHa (N/A) | ↓ (3m) during GnRH | ↓ (3m) during GnRH |
↓ (3m) during GnRH |
↓ Lean body mass % and ↑ fat at 1y | N/A |
| Schagen 2018 (15) | P/127 (73 AMAB, 54 AFAB) |
GnRHa (2y) GAHT |
N/A | N/A | ↑ DHEAS / ↑ A | N/A | N/A |
| Schulmeister 2022 (16) | P/55 (26 AMAB, 29 AFAB) |
GnRHa (1y) | No differences vs controls in early PS Lower HV in later Tanner stage PS initiation |
N/A | N/A | N/A | N/A |
| Valentine 2022 (17) | R-C/4172 (1407 AMAB, 2766 AFAB) |
GnRHa (N/A) GAHT | N/A | N/A | N/A | ↑ overweight/obesity | ↑ dyslipidemia, liver dysfunction and hypertension |
| Van de Grift 2020 (18) | R/200 (66 AMAB, 134 AFAB) |
GnRHa (3y) GAHT |
No difference in FH among early, later, or no GnRHa | ↓ during GnRH | N/A | No difference in BMI SDS among early, later, or no GnRHa | N/A |
| Willemsen 2023 (19) | R/146 (0 AMAB, 146 AFAB) |
GnRHa (3.1±0.9 y) + Testosteron | ↓ HV (during GnRHa) ↑ HV (during GAHT) FH exceeded PAH by 3.0 ± 3.6 cm and MPH by 3.9 ± 6.0 cm | N/A | N/A | N/A | N/A |
A, androstenedione; AFAB, assigned female at birth; AMAB, assigned male at birth; AST, aspartate aminotransferase; BMI, body mass index; BMIz, BMI z-score; BP, blood pressure; C, cross-sectional; DBP, diastolic blood pressure; E, estrogen; EE, ethinyl estradiol; FSH, follicular hormone; f/up, follow-up; FH, final height; GAHT, gender-affirming hormone therapy; GD, gender dysphoria; GnRHa, gonadotropin-releasing hormone agonist; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; HT, height; HTz, height z-score; im, intramuscularly; LBW, lean body weight; LDL, low-density lipoprotein; LH, luteinizing hormone; N/A, not available; NS, not significant; P, prospective; PAH, predicted adult height; pc, percentile; PS, pubertal suppression; R, retrospective; SBP, systolic blood pressure; sc, subcutaneously; T, testosterone; TBF, total body fat; TGD, transgender and gender-diverse; TV, testicular volume; WT, weight; WTz, weight z-score; WHR, waist-hip ratio; y, year(s).
Only studies with moderate-high level quality of evidence are included.Arrow down: reduced; Arrow up: increased.
Table 3B.
Summary of findings on bone outcomes of GnRHa treatment in TGD adolescents. Only studies with moderate-high level quality of evidence are included.
| Reference | Design/Population | Intervention (duration) | Bone mineral density | Bone turnover |
|---|---|---|---|---|
| Carmichael 2021 (20) | P/44 (25 AMAB, 19 AFAB) |
GnRHa (29 m AFAB - 37 m AMAB) | No change from baseline in LS BMD at 12 m nor in hip BMD at 24 and 36 m | N/A |
| Schagen 2020 (21) | P/121 (51 AMAB, 70 AFAB) |
GnRHa (1.8 AFAB - 2.0 AMAB) + GAHT (3 y) |
BMAD =/ ↓ during GnRHa ↑ BMAD during GAHT Z-scores normalized in AFAB but remained <0 in AMAB |
P1NP↓/=, P3NP↓, osteocalcin↓, 1CTP↓ After 3 y of GAHT: P1NP↓, P3NP↓/=, osteocalcin↓, 1CTP↓ |
| Van der Loos 2023 (22) | R/75 (25 AMAB, 50 AFAB) |
GnRHa (1.5 y both AFAB and AMAB) + GAHT (5.7 y AFAB - 5.3 y AMAB) |
BMD Z-scores returned to pretreatment levels except LS Z-scores in AMAB | N/A |
| Vlot 2017 (23) | R/70 (28 AMAB, 42 AFAB) |
GnRHa (1.5 y AFAB – 1.3 y AMAB) + GAHT (5.4 y AFAB – 5.8 y AMAB) |
BMD Z-scores ↓ during GnRHa ↑after 24 m of GAHT |
↓P1NP and 1CTP during GnRHa |
1CTP, cross-linked telopeptide of type 1 collagen; AFAB, assigned female at birth; AMAB, assigned male at birth; BMAD, bone mineral apparent density; BMD, bone mineral density; GAHT, gender-affirming hormone therapy; GnRHa, gonadotropin-releasing hormone analogues; LS, lumbar spine; m, months; N/A, not available; P, prospective; P1NP: procollagen type 1 N propeptide; P3NP, procollagen type 3 N propeptide; R, retrospective; TGD, transgender and gender-diverse; y, years.Arrow down: reduced; Arrow up: increased.
Data synthesis was then reported in the Result section in the form of a narrative summary, as meta-analysis was not possible. Evidence statements were drawn whether evidence were available.
All data included in this review derive from published peer-reviewed studies. No unpublished or proprietary data were used. The authors confirm that all data are appropriately cited.
Terminology
The classification of GD is considered by some to be stigmatizing due to its pathologizing nature, whereas GI is viewed as a less medicalized term. However, the majority of published studies still refer to GD, and in some countries, including Italy, access to treatment is only possible with a formal GD diagnosis. To maintain consistency and inclusivity, we have aimed to minimize the use of both terms and have instead prioritized the use of “TGD adolescents” throughout the manuscript wherever possible.
Results
Search results
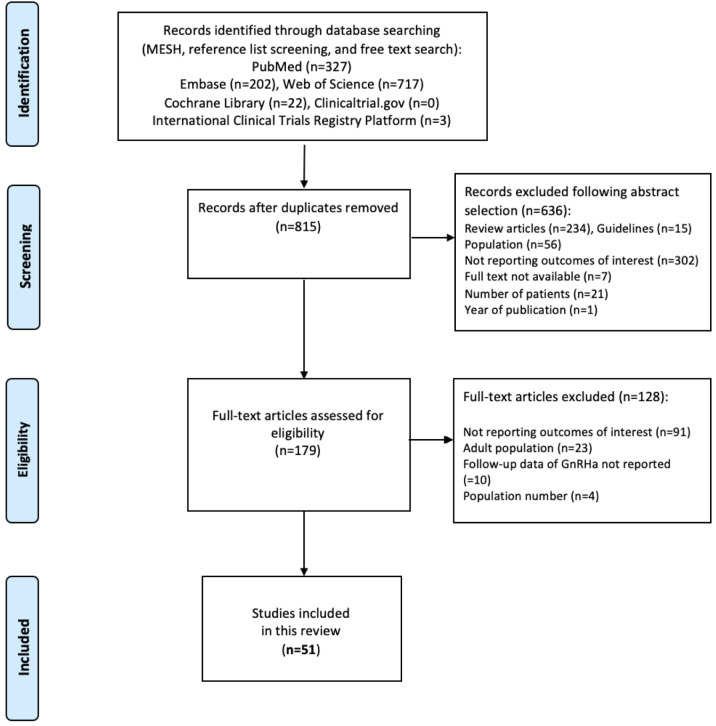
A total of 815 studies were identified following the literature search after duplicates were removed. After reviewing titles and abstracts, 636 additional records were excluded: 234 review articles, 15 guidelines, 56 studies including only participants older than 18 years, 302 studies reporting outcomes different from those of interest, 7 studies not available as full papers, 21 studies with the number of TGD adolescents <10, 1 study with publication period before 2011.
A total of 179 full-text manuscripts were assessed for eligibility. After full-text examination, 128 studies were excluded: 91 not reporting outcomes of interest (most not including data on GnRHa), 23 regarding only the adult population, 10 not presenting follow-up data of GnRHa treatment, and 4 reporting <10 TGD adolescents treated with GnRHa. The PRISMA flow diagram ( Figure 1 ) summarizes the publication screening process. The list of excluded papers and the reasons for exclusion are reported in Supplementary Material S2 .
Figure 1.
PRISMA flow diagram.
Fifty-one studies (4, 10–59) were finally included in this systematic review. The summary and grade of the evidence of each study are reported in Supplementary Tables 1A–D . Among the selected studies, 17 analyzed physical and hormone levels ( Supplementary Table 1A ), 10 bone outcomes ( Supplementary Table 1B ), 20 mental health outcomes ( Supplementary Table 1C ), 3 about fertility and sexual function outcomes, and 7 focused on side effects and risk of cancer ( Supplementary Table 1D ). All the studies included were in English. Because of the heterogeneity of study populations and outcomes, results could not be combined in a meta-analysis.
Among the selected studies, none were RCTs, 19 were prospective longitudinal, 27 were retrospective, and 5 were cross-sectional in design. The number of TGD adolescents treated with GnRH in the studies varied between 13 and 410 (33, 34). The duration of follow-up in GnRHa-treated individuals ranged between 6 months to 22 years (31, 35).
There has been a steady increase in publications over the years: 1 article in 2011; 2 in 2014, 2 in 2015, 1 in 2016, 1 in 2017, 3 in 2018, 1 in 2019, 12 in 2020, 7 in 2021, 6 in 2022, 12 in 2023 and 3 in 2024 until February 1, 2024.
The level of certainty was decreased by one or two levels for these reasons:
- sample size (median values as cut-off): studies including less than 121 individuals treated with GnRHa for the physical-hormonal outcomes, 50 for the mental health outcomes, 72.5 for the bone outcome, 78 for the fertility and sexual function outcome, 214 for side effects and risk of cancer outcome;
- possible bias due to the study design: cross-sectional or retrospective, p-values not shown, short follow-up (median values as cut-off: 3 years for the physical-hormonal outcomes, 1.5 years for the mental health outcomes, 2 years for the bone outcome, 1.45 years for side effects and risk of cancer outcome), no control group;
- imprecision of estimates or inconsistent results across different cohorts included in multicenter studies.
Tables 3A - C summarize the 22 studies with moderate-high quality levels. Below, we report the main results according to moderate-high-level divided by outcome (4, 10–30). No comments on fertility, sexual function, side effects, or cancer risk will be provided, as none of the studies met a moderate or high level of evidence. We explored heterogeneity by conducting subgroup analyses, based on population characteristics (age, Tanner stage), modality of treatment (GnRHa, GAHT), and treatment duration (3 months to 3 years). This analysis led to results for the outcomes height, Body Mass Index (BMI), BMD and Global Function, Cognition, and Behavioral/Emotional Problems, while for others only a few studies were available for each subgroup. Table 4 further summarizes the main findings of the review.
Table 3C.
Summary of findings on mental health outcomes of GnRHa treatment in TGD adolescents. Only studies with moderate-high level quality of evidence are reported.
| Reference | Design/Population | Intervention (duration) | Global function/Cognition behavioral and emotional problems | Suicide ideation | Depression/Anxiety | QoL/wellbeing | Body Dissatisfaction |
|---|---|---|---|---|---|---|---|
| Achille 2020 (24) | P/50 (17 AMAB, 33 AFAB) |
GnRHa (18 m) and/or GAHT |
N/A | ↓ | ↓ | ↑ QoL | N/A |
| Arnoldussen 2022 (25) | R/72 (27 AMAB, 45 AFAB) |
GnRHa (2,4 y) and/or GAHT |
The IQ scores and educational achievement were not significantly different between AMAB and AFAB | N/A | N/A | N/A | N/A |
| Costa 2015 (26) | P/201 (77 AMAB; 124 AFAB) |
GnRHa (18m) + psychological support |
GD adolescents receiving also GnRHa had > psychosocial functioning (+12m) | N/A | N/A | ↑ well-being | N/A |
| De Vries 2011 (27) | P/70 (33 AMAB, 37 AFAB) |
GnRHa (2 y) | ↓behavioral and emotional problems, depressive symptoms ↑ psychological functioning |
N/A | ↓/= | N/A | = Body image and gender dysphoria (> natal female) |
| De Vries 2014 (28) | P/55 (22 AMAB, 33 AFAB) |
GnRHa (N/A) | ↑ psychological functioning | N/A | ↓ | ↑ QoL | ↓ |
| Fisher 2024 (4) | P/36 (14 AMAB, 22 AFAB) | GnRHa (3-12 m) | ↑ psychological functioning | ↓ | ↓ | N/A | ↓ |
| Tordoff 2022 (29) | P/104 (27 AMAB, 63 AFAB, 10 NB, 4 unknown) |
GnRHa (N/A) + GAHT |
N/A | ↓ (PS and GAH) | ↓ (PS and GAH) | N/A | N/A |
| Van der Miesen 2020 (30) | P/178 (68 AMAB, 110 AFAB) |
GnRHa (N/A) vs no treatment |
N/A | ↓ | ↓ | N/A | N/A |
AFAB, assigned female at birth; AMAB, assigned male at birth; NB, non-binary; P, prospective; QoL, quality of life; R, retrospective; TGD, transgender and gender-diverse.Arrow down: reduced; Arrow up: increased.
Table 4.
Summary of findings.
| Outcome Category | Key Findings | Study Limitations | Clinical Implications |
|---|---|---|---|
| Physical Changes & Metabolic Outcomes | GnRHa effectively suppresses puberty. Height velocity decreases during treatment, but final height aligns with target height. Changes in body composition observed: increase in fat mass, reduction in lean body mass, and a transient BMI increase in AFAB individuals. Some adolescents report dissatisfaction with final height when it does not align with the average for their affirmed gender. | Variability in treatment protocols, GnRHa monotherapy duration, and age at initiation complicates predictions on final height and body composition. | Regular monitoring of growth and body composition is necessary. Patients should be counseled about possible height outcomes. Nutritional and physical activity recommendations should be integrated to manage changes in fat and lean body mass. |
| Bone Health | BMD decreases during GnRHa treatment, particularly in AMAB individuals. Recovery during GAHT is partial, and concerns remain about long-term skeletal health. No increased osteoporosis incidence has been observed. Bone turnover markers indicate suppressed bone activity. | Lack of standardized long-term follow-up studies limits understanding of adulthood skeletal outcomes. | Bone health monitoring should be a priority. Clinicians should encourage lifestyle measures to support bone health, including weight-bearing exercise and vitamin D supplementation. Long-term follow-up is needed to assess osteoporosis risk. |
| Mental Health | Significant improvements in global functioning, reduction in emotional and behavioral problems, and decrease in suicidality among adolescents receiving GnRHa, especially when followed by GAHT. Depression and anxiety levels drop significantly post-treatment, but body dissatisfaction and anxiety persist in some cases, particularly among AFAB individuals. | Limited data on long-term psychiatric outcomes. | Mental health support should be integrated throughout the transition process. While GnRHa shows clear benefits for suicidality and depression, continued psychological care is essential to address body image concerns and residual anxiety. |
AFAB, assigned female at birth; AMAB, assigned male at birth; BMI, body mass index; MBD, bone mineral density; GAHT, gender-affirming hormone therapy; GnRHa, gonadotrophin releasing-hormone agonist; TGD, transgender and gender-diverse.
Physical changes and hormone levels
- Height: during GnRHa treatment, significant decreases in height Standard Deviation Score (SDS) were observed in both AMAB and AFAB individuals, particularly in the first 1 to 2 years (14). In one study, only AMAB adolescents showed a progressive reduction in height percentiles (4). Height velocity (HV) also decelerated during treatment (10, 16), with no significant difference between AMAB and AFAB individuals (16). However, the Tanner stage at the start of treatment played a crucial role, as starting GnRHa later was associated with significantly lower HV (16). Final height (FH) outcomes were mixed. Van de Grift et al. found no statistical difference in FH among those treated early, later, or not with GnRHa (18). Boogers et al. reported that in AMAB adolescent FH was slightly lower than predicted at the start of GnRHa but not significantly different from the target height (TH) (10). High doses of estradiol, particularly ethinylestradiol, further reduced FH below predicted values and TH. However, Willemsen et al. noted that, after a period of catch-up growth during GAHT, FH in AFAB individuals exceeded mid-parental height (MPH) by an average of 3.9± 6 cm (19). Thus, the start of GnRHa in early-mid puberty followed by GAHT alters growth velocity in TGD youth, and the individual height gain after the start of GAHT is difficult to predict. However, as shown by Ciancia et al. the medical trajectory does not seem to alter FH in both AMAB and AFAB, for whom FH is in line with TH calculated for the sex at birth (32).
- Sexual characteristics and gonadotropins/sexual steroids: GnRHa reduces the development of sex characteristics (breast or testicular volume) in TGD adolescents (4, 14, 18), and this data is confirmed by gonadotropin and sex steroid hormone suppression (4, 14). As a result, AFAB may not need to undergo mastectomy, while AMAB may require an alternative to penile inversion vaginoplasty (18). Dehydroepiandrosterone sulfate (DHEAS) levels remained stable during treatment, while androstenedione decreased during GnRHa but rose during GAHT in AFAB, with no changes in AMAB (15).
- Body composition and serum hormone profile: during GnRHa treatment, increases in total body fat and gynoid fat and reduction in lean mass without significant changes in BMI SDS were observed in AMAB adolescents, whereas in AFAB adolescents, no significant changes in BMI, lean body mass or total body fat Z-score were observed (13). In another study, a significant but transient increase in BMI percentiles was observed in AFAB individuals, beginning in the third month of therapy and lasting only a few months (4). When considering subsequent GAHT, body composition shifted toward the affirmed sex (11), with a decrease in lean body mass and an increase in fat mass (14). Obesity prevalence was higher in TGD individuals compared to cisgender controls, but lipid profiles and insulin sensitivity remained comparable to the general population (12). TGD adolescents had higher odds of overweight/obesity; however, GnRHa alone and estradiol were not associated with greater odds of cardiometabolic-related diagnoses, while those treated with testosterone, had a higher risk of dyslipidemia and liver dysfunction (17).
Bone health
- Bone mineral density: in a few studies BMD Z-scores at lumbar spine (LS) and femoral neck (FN) decreased during GnRHa treatment and increased during GAHT (22, 23). This finding was not confirmed by another study which did not find any changes in BMD Z-scores at the LS and FN during GnRHa (20), probably due to the relatively short follow-up duration. Of note, AMAB often presents with low BMD z-scores already before the start of any gender-affirming treatment and the low BMD Z-scores (mainly at LS) can persist also after 3 years of GAHT (21).
- Bone turnover markers: the added value of evaluating bone turnover markers seems to be limited. However, decreased levels of Procollagen type 1 N-terminal propeptide (P1NP), as a marker of bone formation, and of cross-linked telopeptide of type 1 collagen (1CTP), as a marker of bone resorption, indicate decreased bone turnover during GnRHa treatment (21, 23).
Mental health
- Global function, cognition, and behavioral/emotional problems: adolescents undergoing GnRHa treatment, especially when followed by GAHT, showed improvements in global functioning and reductions in emotional and behavioral problems over time; these results are more evident for AMAB adolescents (4, 27). For instance, improvements in psychosocial functioning were noted, with those receiving both psychological support and GnRHa showing better and improved outcomes steadily over time, than those receiving psychological support alone (26). Cognitive outcomes, including IQ, were not significantly affected by gender-affirming treatment, and post-treatment educational achievement was largely linked to pre-treatment cognitive scores (25). While behavioral and emotional issues persisted in some cases, the overall trajectory indicated improvement, particularly in global functioning scores (27, 28).
- Suicide ideation: GnRHa treatment has been shown to significantly reduce suicidality in transgender adolescents. In a study comparing transgender adolescents at referral and those using GnRHa, the endorsement of self-harm/suicidality in those who started GnRHa (12.4%) was lower than in those at referral (27.2%) and aligned closely with the rate in cisgender peers (11.9%) (30). Adolescents who received either GnRHa or GAHT experienced a 73% lower risk of suicidality compared to those who did not undergo hormone treatment (29). The reduction in suicidal risk was positively associated with the physical effects and the hormone changes (4). Additionally, Achille et al. reported that suicidal ideation decreased significantly over time across all groups receiving GnRHa or GAHT compared to baseline (from 10% to 6%) (24).
- Depression/anxiety: while at referral, 31.3% of transgender adolescents exhibited clinical levels of internalizing problems, including depression and anxiety, with higher rates in AMAB (35.3%) compared to AFAB (28.2%), after initiating GnRHa these rates dropped to 16.3%, bringing them closer to those of cisgender peers (30). Across multiple studies, both depression and anxiety levels decreased significantly over time with affirmative treatment, with transgender youth showing marked improvements (24, 27, 28); adolescents receiving GnRHa or GAHT had a 60% lower risk of depression compared to those who did not undergo treatment (29). Unlike psychological assessment alone, GnRHa therapy correlated strongly with decreases in anxiety, alongside observed hormonal and physical changes (4).
- Quality of life/well-being: Quality of life (QoL) improved significantly in adolescents who underwent GnRHa treatment, particularly when followed by GAHT and surgery. Emotional, social, and physical well-being scores improved over time, aligning with those of cisgender controls post-treatment (28). Adolescents reported better overall life satisfaction and well-being, though some studies noted that improvements were gradual, particularly in the early stages of treatment (24). While some studies demonstrated gradual improvements in well-being, particularly following surgery, others showed substantial enhancement in overall life satisfaction (26, 28).
- Body dissatisfaction: Gender dysphoria and body image satisfaction remained unchanged during GnRHa, with AFAB individuals reporting higher levels of dissatisfaction with their primary and secondary sex characteristics, compared to AMAB individuals (27). Body dissatisfaction persisted during GnRHa treatment but significantly improved after GAHT and surgery (28). Recently, Fisher et al. reported that adolescents were less worried about body changes once they started GnRHa, and the reduction in body uneasiness was more evident in AMAB adolescents (4).
Discussion
The findings of this systematic review contribute to the growing body of literature on the efficacy and safety of GnRHa for puberty suppression in TGD adolescents with or without GAHT. The review synthesizes data from 51 studies, highlighting both the physical, psychological, and metabolic outcomes of GnRHa in TGD adolescents, incorporating evidence from 22 studies with moderate to high-quality levels.
While previous systematic reviews have identified gaps in the literature, including limited evidence on long-term psychosocial outcomes and inconsistencies in study designs (6, 7), this review adds clarity by focusing on more recent studies and categorizing the outcomes based on their level of evidence, as well as exploring their clinical significance ( Table 4 ). Furthermore, the increasing number of publications in recent years highlights a growing body of research, aiming at high-quality and evidence-based medicine also in this specific field.
However, the impact of GnRHa treatment on physical development, particularly height, body composition, and bone health, remains not completely understood, although increasing evidence supports the safety of this treatment as part of gender-affirming care. As mentioned by previous studies (6), GnRHa effectively suppresses the further development of secondary sexual characteristics, contributing to the reduction of dysphoria. Nevertheless, this treatment alters the linear growth pattern, especially when started during early or mid-puberty. However, the combination with subsequent GAHT compensates for the initial decrease in height velocity (10, 14, 16) and TGD youth tend to reach a FH in line with their TH calculated for the sex at birth (32). From a clinical perspective, these findings suggest that while GnRHa supports gender-affirming care, it is crucial to set realistic expectations regarding final height, particularly for adolescents who desire an FH more aligned with the average for their experienced gender.
Body composition changes observed during GnRHa treatment, such as increases in fat mass and reductions in lean body mass, were consistent among studies (11, 14). While body composition shifts towards the affirmed sex during GAHT, there is a higher prevalence of obesity among TGD adolescents compared to cisgender controls (12). Interestingly, lipid profiles and insulin sensitivity remained comparable to the general population, suggesting that the cardiometabolic risks may be more closely associated with testosterone use rather than GnRHa alone (17). This distinction was not highlighted in earlier reviews and suggests a need for more tailored metabolic monitoring in TGD individuals, particularly those on testosterone therapy.
Bone health remains an area of concern, as GnRHa treatment is associated with reductions in BMD (21, 22). These findings are consistent with earlier reviews (6), but the evidence provided here indicates that BMD improves during GAHT, albeit not always to pre-treatment levels, especially in AMAB individuals (notably, AMAB adolescents often present with low BMD Z-scores even before initiating any gender-affirming treatment, indicating a possible baseline vulnerability that may influence long-term bone outcomes) (21). This raises concerns about the potential long-term impact on skeletal health, especially in those who undergo prolonged periods of puberty suppression. However, so far there is no evidence of increased osteoporosis incidence during childhood, defined as BMD below normal values for age and gender (Z-score ≤ −2) accompanied by a history of repeated fractures (60). If the treatment would increase the risk of osteoporosis later in life is also still to be understood. The determination of bone turnover markers (P1NP, 1CTP) has been proposed to better characterize bone remodeling during GnRHa treatment, but it has shown limited utility, although a decrease in these markers has been registered, indicating suppressed bone activity during the treatment (21, 23). This findings reinforce the need for a multifaceted approach to monitor bone health, especially in cases with multiple risk factors, but above all to increase measures aimed at promoting the maintenance of good bone health (e.g. physical activity, balanced diet, vitamin D supplementation).
Previous reviews reported that the impact of GnRHa on mental health remains a central focus of debate (6–8), as a few studies of low or moderate quality of evidence regarding psychological and cognitive outcomes were included (8). Compared to earlier reviews, this study incorporates more recent data, demonstrating significant improvements in global functioning, depression, and anxiety, with studies reporting a reduction in suicidal ideation and self-harm in TGD adolescents also receiving GAHT (24, 29). These improvements are critical, given that TGD adolescents often experience elevated rates of mental health issues prior to treatment (61–63). The substantial decrease in self-harm ideation aligns with reports that suggest affirmative treatments can mitigate mental health challenges faced by transgender youth (4, 30). Importantly, the reduction in suicidality observed in adolescents using GnRHa alone compared to those at referral (30) and also to psychological assessment alone (4) provides stronger evidence supporting the protective role of puberty suppression in transgender youth, a point that had been largely hypothesized but not robustly evidenced in earlier reviews.
While the reduction in depressive symptoms was consistently reported across multiple studies (24, 28), this review also highlights that anxiety and body dissatisfaction persist during GnRHa treatment (4), particularly in AFAB individuals (27). Additionally, the association between improved mental health outcomes and the initiation of GAHT or surgery suggests that while GnRHa is effective in halting the distressing progression of puberty, the long-term benefits on mental health may be realized more fully with comprehensive gender-affirming treatment.
Furthermore, our results show that QoL improves significantly following GnRHa treatment, particularly when followed by GAHT. This finding supports earlier studies indicating enhanced psychosocial functioning among adolescents who receive comprehensive gender-affirming care (28).
The strengths of this review include its focus on moderate to high-quality evidence, providing a clearer understanding of the outcomes associated with GnRHa treatment in TGD adolescents. By excluding studies with low levels of evidence, this review presents more reliable conclusions than earlier reviews that included studies with significant methodological limitations. Overall, this study builds upon previous systematic reviews by providing a more comprehensive analysis of the current evidence on GnRHa treatment for TGD adolescents, highlighting both its benefits and potential risks. Unlike earlier reviews (6, 7), which primarily focused on physical outcomes with limited discussion of psychosocial aspects, this study offers a broader examination of both efficacy and adverse effects, including physical changes, mental health, bone health, and psychosocial outcomes. Furthermore, this study employs a rigorous GRADE assessment to evaluate the quality of evidence for each outcome across included studies, enhancing its reliability compared to previous reviews. While earlier reviews pointed to insufficient data on mental health impacts, this study emphasizes significant improvements in mental health outcomes, quality of life, and reductions in suicidality among adolescents receiving GnRHa treatment. Additionally, this study addresses concerns regarding bone health more thoroughly than prior reviews, specifically highlighting findings related to decreased BMD during treatment. Lastly, this review includes a substantial amount of longitudinal data, which was either absent in Chew et al. or only partially covered in Ludvigsson’s analysis (6, 7).
However, the review has some limitations. One key limitation is that a meta-analysis was not conducted. This decision was made due to the significant heterogeneity in study designs, treatment protocols, follow-up durations, and outcome measures across the included studies. The variation in how outcomes were assessed and reported made it challenging to synthesize the data into a meaningful pooled estimate. Additionally, differences in the populations studied, including variations in age at treatment initiation and duration of GnRHa use, further limited the feasibility of conducting a meta-analysis. This heterogeneity may impact the generalizability of findings and clinicians must be aware of the diverse results and factors that could influence treatment decisions. Moreover, the lack of RCTs and the reliance on observational and retrospective studies introduce potential biases that could affect the robustness of the conclusions. Additionally, publication bias may be a factor, as studies reporting positive outcomes may be more likely to be published, potentially skewing the overall interpretation of results.
Given the existence of 22 studies with moderate to high levels of evidence so far, it seems inaccurate to assert that there is still insufficient and/or inconsistent evidence about the effects of puberty suppression in TGD adolescents (8) or that GnRH treatment should only be offered under a research protocol due to a lack of solid evidence on long-term outcomes for managing gender-related distress (64). GnRHa has been safely and effectively used to treat central precocious puberty (CPP) since 1981, despite their approval being based on relatively short, open-label studies involving small patient populations. A recent systematic review (65) found only 98 studies (with no RCTs), 98.5% of which involved girls (meaning that there is very limited literature supporting male cases): 18 were comparative studies (with only 13 comparing against no treatment), while the remaining 81 were single-arm studies. Conducting more rigorous studies, such as RCTs, would be considered at this point neither feasible nor ethical in CPP as in TGD adolescents (66). While medicine constantly strives for more robust evidence, it would be unethical to deny or discontinue the use of GnRHa in TGD adolescents when substantial evidence demonstrates its benefits.
Clinical decisions, particularly in pediatrics, where the diseases can be rare and drugs can be not yet approved, are rarely based solely on RCTs. A recent study found that over 90% of healthcare interventions reviewed in Cochrane Reports lack high-quality evidence (67). This highlights the broader challenge in medicine where decisions often must be made in the face of imperfect evidence, relying on a combination of clinical experience, patient preferences, and available data. In cases where strong evidence is still lacking, it becomes essential to outline the appropriate approach for therapeutic choices and monitoring, based on regularly updated systematic reviews that weigh the significance of available publications.
The use of hormone therapy in TGD adolescents continues to be a topic of discussion; however, prioritizing the mental and physical health of TGD youth, who are more vulnerable to psychological challenges, remains crucial. Healthcare professionals should base treatment decisions on the specific needs and context of each individual, with a foundation in scientific research rather than personal beliefs or societal biases. As in the Italian experience, GnRHa therapy is administered only in carefully selected cases at specialized centers, following thorough psychological assessment and reserved for those experiencing significant and enduring distress (68).
Clinical implications and ethical considerations
Considering the increased importance of GnRHa treatment in this area, it is crucial to highlight several key clinical and ethical considerations related to the use of GnRHa in transgender and gender-diverse adolescents.
Given the observed decrease in bone mineral density during GnRHa treatment, especially in AMAB individuals, long-term monitoring of skeletal health is essential. Although no cases of osteoporosis have been reported during adolescence, the potential long-term risks remain uncertain, requiring continued vigilance.
While improvements in mental health are well documented, persistent anxiety and body dissatisfaction—particularly in AFAB individuals—highlight the need for ongoing psychological support throughout GnRHa treatment, a recommendation emphasized in earlier reviews but not always reflected in clinical practice.
Moreover, the long-term effects of GnRHa on fertility preservation, sexual function, and cancer risk remain understudied, as no studies with moderate or high evidence addressed these outcomes in this review. These remain important research priorities to inform future clinical guidelines.
Due to the sensitive nature of GnRHa treatment, a rigorous informed consent process involving both adolescents and their families is essential. This should include a thorough explanation of known or potential benefits, risks, uncertainties, and long-term consequences, allowing for shared decision-making (68, 69).
Finally, a precision medicine approach is crucial in managing GnRHa treatment, taking into account factors such as pubertal stage, psychological health, individual goals, and social context. Personalized care plans optimize outcomes while minimizing potential risks. In this context, we propose a structured framework for clinical monitoring to guide individualized care and ensure comprehensive, evidence-informed follow-up throughout the course of treatment (Table 5).
Table 5.
Clinical monitoring recommendations for TGD adolescents undergoing GnRHa therapy.
| 1. Physical changes and hormone levels |
|---|
| • Anthropometric Measures: Monitor height, weight, and body mass index (BMI) every 6–12 months, with attention to changes in growth velocity and deviations from expected growth curves based on mid-parental height. • Pubertal Status: Document Tanner stage at baseline and follow-up, assessing suppression of secondary sex characteristics. • Laboratory Evaluation: Regular measurement (every 6–12 months) of gonadotropins (LH/FSH), sex steroids (estradiol or testosterone), and adrenal androgens (e.g., DHEAS, androstenedione) to confirm adequate suppression. • Body Composition: Consider periodic assessment (e.g., annually) of lean/fat mass using tools such as bioimpedance or DEXA, especially if BMI changes significantly. • Transition to GAHT: Assess readiness and timing for initiating gender-affirming hormone therapy, ensuring appropriate informed consent and psychosocial support. |
| 2. Bone health |
| • BMD Monitoring: Perform DEXA scans every 12–24 months, focusing on lumbar spine and femoral neck Z-scores. • Bone Turnover Markers: Optional use of markers such as P1NP and CTX/1CTP to evaluate bone remodeling activity during suppression. • Risk Mitigation: Encourage regular weight-bearing physical activity and ensure adequate intake of calcium and vitamin D. Supplementation should be prescribed when needed. • Watchpoints: Monitor for additional risk factors such as low BMI, nutritional deficits, family history of osteoporosis, or long periods of suppression before GAHT initiation. |
| 3. Mental health and psychosocial support |
| • Baseline Assessment: Conduct a comprehensive psychological evaluation before treatment initiation to identify pre-existing mental health conditions. • Ongoing Support: Provide continuous access to affirming mental health services throughout GnRHa treatment and beyond, including individual or family therapy. • Monitoring Tools: Use standardized tools (e.g., CBCL, YSR) to track emotional and behavioral outcomes over time. • Watchpoints: Pay special attention to persistent body dissatisfaction, anxiety, or depressive symptoms, particularly in AFAB youth. |
| 4. Fertility and sexual function |
| • Fertility Counseling: Discuss potential impact of GnRHa and future GAHT on fertility before starting treatment, even if adolescents are young or undecided. • Preservation Options: Refer to fertility specialists early for options such as sperm or oocyte cryopreservation (depending on Tanner stage and consent). • Follow-Up: Continue to revisit the topic as adolescents age or approach transition to GAHT or adulthood. |
| 5. Cancer risk and other adverse effects |
| • Cancer Surveillance: While direct evidence is lacking, encourage regular clinical follow-up with physical exams and targeted evaluations as clinically indicated. • Long-Term Monitoring: Document any adverse effects, including rare events (e.g., idiopathic intracranial hypertension), and collect data in registries or longitudinal cohorts to support future evidence generation. • Breast/Prostate Considerations: In post-GAHT individuals, tailor cancer screening recommendations according to affirmed gender and anatomy. |
| 6. Cognitive and educational monitoring (optional but recommended) |
| • Cognitive Development: For adolescents with neurodevelopmental conditions or learning differences, consider baseline and follow-up neuropsychological evaluations. • Educational Support: Collaborate with schools to provide appropriate accommodations if cognitive or psychosocial challenges are identified during treatment. |
| 7. Ethical and informed consent |
| • Shared Decision-Making: Ensure informed consent processes are developmentally appropriate, inclusive of both adolescent and caregivers, and revisited regularly. • Documentation: Maintain thorough records of discussions around benefits, risks, unknowns, and the adolescent’s evolving goals. |
Given the current evidence, we recommend a multidisciplinary and personalized approach to the clinical monitoring of adolescents undergoing GnRHa therapy. The following areas should be regularly evaluated: AFAB, Assigned Female at Birth; BMD, Bone Mineral Density; BMI, Body Mass Index; CBCL, Child Behavior Checklist; CTX/1CTP, Cross-linked Telopeptide of Type 1 Collagen; DEXA, Dual-Energy X-ray Absorptiometry; DHEAS, Dehydroepiandrosterone Sulfate; FSH, Follicle-Stimulating Hormone; GAHT, Gender-Affirming Hormone Therapy; GnRHa, Gonadotropin-Releasing Hormone agonists; LH, Luteinizing Hormone; P1NP, Procollagen Type 1 N-Terminal Propeptide; TGD, Transgender and Gender Diverse; YSR, Youth Self Report.
Conclusions
This systematic review provides a comprehensive overview of current evidence on the use of GnRHa in TGD adolescents, confirming its efficacy in suppressing puberty and improving mental health outcomes. While long-term data remain limited, especially regarding bone health, fertility, and cancer risk, available moderate to high-quality evidence supports its clinical use as part of gender-affirming care.
In addition to clinical outcomes, several key considerations must guide practice. Bone health monitoring is essential, particularly in AMAB individuals who are at greater risk of reduced BMD during treatment. Ongoing psychological support should be provided throughout GnRHa use, especially for AFAB adolescents, given the persistence of body dissatisfaction and anxiety in some cases. Fertility preservation should be discussed before treatment initiation, despite the current lack of robust evidence.
A rigorous shared decision-making process involving adolescents and their families is critical, ensuring informed consent and personalized care based on individual needs and goals.
Future research should prioritize co-produced, longitudinal studies that address these gaps and further support ethical, safe, and evidence-based use of GnRHa in this population.
Funding Statement
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Italian Ministry of Health, through the contribution given to the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy (RC 9/16) and by the Italian Society for Pediatric Endocrinology and Diabetes (ISPED).
Abbreviations
1CTP, cross-linked TeloPeptide of type 1 Collagen; AFAB, Assigned Female At Birth; AMAB, Assigned Male At Birth; BMD, Bone Mineral Density; BMI, Body Mass Index; CPP, Central Precocious Puberty; DHEAS, DeHydroEpiAndrosterone Sulfate; DSM-5, Diagnostic and Statistical Manual of Mental Disorders - 5th edition; FH, Final Height; FN, Femoral Neck; GAHT, Gender-Affirming Hormonal Treatment; GAS, Gender Affirming Surgery; GD, Gender Dysphoria; GI, Gender Incongruence; GnRHa, Gonadotropin-Releasing Hormone agonist; HV, Height Velocity; ICD-11, International Classification of Diseases -11th revision; LS, Lumbar Spine; MPH, Mid-Parental Height; P1NP, Procollagen type 1 N Propeptide; QoL, Quality of life; RCT, Randomized Clinical Trial; SDS, Standard Deviation Score; TGD, Transgender and Gender Diverse; TH, Target Height.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material , Further inquiries can be directed to the corresponding author/s.
Author contributions
GTo: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Resources, Validation. RD: Data curation, Formal analysis, Writing – review & editing. JM: Data curation, Formal analysis, Writing – review & editing. SC: Data curation, Formal analysis, Writing – review & editing. FS: Data curation, Formal analysis, Writing – review & editing. DF: Data curation, Formal analysis, Writing – review & editing. EC: Data curation, Formal analysis, Writing – review & editing. DC: Data curation, Formal analysis, Writing – review & editing. CU: Data curation, Formal analysis, Writing – review & editing. NI: Data curation, Formal analysis, Writing – review & editing. PD: Data curation, Formal analysis, Writing – review & editing. PM: Data curation, Formal analysis, Writing – review & editing. LG: Data curation, Formal analysis, Writing – review & editing. TT: Data curation, Formal analysis, Writing – review & editing. VS: Data curation, Formal analysis, Writing – review & editing. GTa: Data curation, Formal analysis, Writing – review & editing. GB: Data curation, Formal analysis, Writing – review & editing. ML: Data curation, Formal analysis, Writing – review & editing. MSt: Data curation, Formal analysis, Writing – review & editing. TA: Data curation, Formal analysis, Writing – review & editing. MD: Data curation, Formal analysis, Writing – review & editing. MF: Data curation, Formal analysis, Writing – review & editing. LI: Data curation, Formal analysis, Writing – review & editing. VC: Data curation, Formal analysis, Writing – review & editing. LD: Data curation, Formal analysis, Writing – review & editing. MSa: Data curation, Formal analysis, Writing – review & editing. RF: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1555186/full#supplementary-material
References
- 1. World Health Organization . International classification of diseases for mortality and morbidity statistics (11th revision) Geneva: World Health Organization. (2019). [Google Scholar]
- 2. American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. 5 ed. Washington, DC: American Psychiatric Publishing; (2013). [Google Scholar]
- 3. Coleman E, Radix AE, Bouman WP, Brown GR, de Vries ALC, Deutsch MB, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. (2022) 23:S1–S259. doi: 10.1080/26895269.2022.2100644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher AD, Ristori J, Romani A, Cassioli E, Mazzoli F, Cocchetti C, et al. Back to the future: is gnRHa treatment in transgender and gender diverse adolescents only an extended evaluation phase? J Clin Endocrinol Metab. (2024) 109:1565–79. doi: 10.1210/clinem/dgad729 [DOI] [PubMed] [Google Scholar]
- 5. Panagiotakopoulos L, Chulani V, Koyama A, Childress K, Forcier M, Grimsby G, et al. The effect of early puberty suppression on treatment options and outcomes in transgender patients. Nat Rev Urol. (2020) 17:626–36. doi: 10.1038/s41585-020-0372-2 [DOI] [PubMed] [Google Scholar]
- 6. Chew D, Anderson J, Williams K, May T, Pang K. Hormonal treatment in young people with gender dysphoria: A systematic review. Pediatrics. (2018) 141:e20173742. doi: 10.1542/peds.2017-3742 [DOI] [PubMed] [Google Scholar]
- 7. Ludvigsson JF, Adolfsson J, Höistad M, Rydelius P, Kriström B, Landén M. A systematic review of hormone treatment for children with gender dysphoria and recommendations for research. Acta Paediatr. (2023) 112:2279–92. doi: 10.1111/apa.16791 [DOI] [PubMed] [Google Scholar]
- 8. Taylor J, Mitchell A, Hall R, Heathcote C, Langton T, Fraser L, et al. Interventions to suppress puberty in adolescents experiencing gender dysphoria or incongruence: a systematic review. Arch Dis Child. (2024) 30;109:s33–47. doi: 10.1136/archdischild-2023-326669 [DOI] [PubMed] [Google Scholar]
- 9. Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. (2019) 111:105–14. doi: 10.1016/j.jclinepi.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boogers LS, Wiepjes CM, Klink DT, Hellinga I, Van Trotsenburg ASP, Den Heijer M, et al. Transgender girls grow tall: adult height is unaffected by gnRH analogue and estradiol treatment. J Clin Endocrinol Metab. (2022) 107:e3805–15. doi: 10.1210/clinem/dgac349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klaver M, De Mutsert R, Wiepjes CM, Twisk JWR, Den Heijer M, Rotteveel J, et al. Early hormonal treatment affects body composition and body shape in young transgender adolescents. J Sex Med. (2018) 15:251–60. doi: 10.1016/j.jsxm.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 12. Klaver M, De Mutsert R, van der Loos MATC, Wiepjes CM, Twisk JWR, Den Heijer M, et al. Hormonal treatment and cardiovascular risk profile in transgender adolescents. Pediatrics. (2020) 145:e20190741. doi: 10.1542/peds.2019-0741 [DOI] [PubMed] [Google Scholar]
- 13. Navabi B, Tang K, KhatChadourian K, Lawson ML. Pubertal suppression, bone mass, and body composition in youth with gender dysphoria. Pediatrics. (2021) 148:e2020039339. doi: 10.1542/peds.2020-039339 [DOI] [PubMed] [Google Scholar]
- 14. Schagen SEE, Cohen-Kettenis PT, Delemarre-van De Waal HA, Hannema SE. Efficacy and safety of gonadotropin-releasing hormone agonist treatment to suppress puberty in gender dysphoric adolescents. J Sex Med. (2016) 13:1125–32. doi: 10.1016/j.jsxm.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 15. Schagen SEE, Lustenhouwer P, Cohen-Kettenis PT, Delemarre-van De Waal HA, Hannema SE. Changes in adrenal androgens during puberty suppression and gender-affirming hormone treatment in adolescents with gender dysphoria. J Sex Med. (2018) 15:1357–63. doi: 10.1016/j.jsxm.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 16. Schulmeister C, Millington K, Kaufman M, Finlayson C, Kennedy J--, Garofalo R, et al. Growth in transgender/gender-diverse youth in the first year of treatment with gonadotropin-releasing hormone agonists. J Adolesc Health. (2022) 70:108–13. doi: 10.1016/j.jadohealth.2021.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valentine A, Davis S, Furniss A, Dowshen N, Kazak AE, Lewis C, et al. Multicenter analysis of cardiometabolic-related diagnoses in transgender and gender-diverse youth: A PEDSnet study. J Clin Endocrinol Metab. (2022) 107:e4004–14. doi: 10.1210/clinem/dgac469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van De Grift TC, Van Gelder ZJ, Mullender MG, Steensma TD, De Vries ALC, Bouman M-B. Timing of puberty suppression and surgical options for transgender youth. Pediatrics. (2020) 146:e20193653. doi: 10.1542/peds.2019-3653 [DOI] [PubMed] [Google Scholar]
- 19. Willemsen LA, Boogers LS, Wiepjes CM, Klink DT, van Trotsenburg ASP, den Heijer M, et al. Just as tall on testosterone; a neutral to positive effect on adult height of gnRHa and testosterone in trans boys. J Clin Endocrinol Metab. (2023) 108:414–21. doi: 10.1210/clinem/dgac571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carmichael P, Butler G, Masic U, Cole TJ, De Stavola BL, Davidson S, et al. Short-term outcomes of pubertal suppression in a selected cohort of 12 to 15 year old young people with persistent gender dysphoria in the UK. PloS One. (2021) 16:e0243894. doi: 10.1371/journal.pone.0243894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schagen SEE, Wouters FM, Cohen-Kettenis PT, Gooren LJ, Hannema SE. Bone development in transgender adolescents treated with gnRH analogues and subsequent gender-affirming hormones. J Clin Endocrinol Metab. (2020) 105:e4252–63. doi: 10.1210/clinem/dgaa604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Der Loos MATC, Vlot MC, Klink DT, Hannema SE, Heijer D, Wiepjes CM, et al. Bone mineral density in transgender adolescents treated with puberty suppression and subsequent gender-affirming hormones. JAMA Pediatr. (2023) 177:1332–41. doi: 10.1001/jamapediatrics.2023.4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vlot MC, Klink DT, Den Heijer M, Blankenstein MA, Rotteveel J, Heijboer AC. Effect of pubertal suppression and cross-sex hormone therapy on bone turnover markers and bone mineral apparent density (BMAD) in transgender adolescents. Bone. (2017) 95:11–9. doi: 10.1016/j.bone.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 24. Achille C, Taggart T, Eaton NR, Osipoff J, Tafuri K, Lane A, et al. Longitudinal impact of gender-affirming endocrine intervention on the mental health and well-being of transgender youths: preliminary results. Int J Pediatr Endocrinol. (2020) 2020:8. doi: 10.1186/s13633-020-00078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arnoldussen M, Hooijman EC, Kreukels BP, De Vries AL. Association between pre-treatment IQ and educational achievement after gender-affirming treatment including puberty suppression in transgender adolescents. Clin Child Psychol Psychiatry. (2022) 27:1069–76. doi: 10.1177/13591045221091652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costa R, Dunsford M, Skagerberg E, Holt V, Carmichael P, Colizzi M. Psychological support, puberty suppression, and psychosocial functioning in adolescents with gender dysphoria. J Sex Med. (2015) 12:2206–14. doi: 10.1111/jsm.13034 [DOI] [PubMed] [Google Scholar]
- 27. De Vries ALC, Steensma TD, Doreleijers TAH, Cohen-Kettenis PT. Puberty suppression in adolescents with gender identity disorder: a prospective follow-up study. J Sex Med. (2011) 8:2276–83. doi: 10.1111/j.1743-6109.2010.01943.x [DOI] [PubMed] [Google Scholar]
- 28. De Vries ALC, McGuire JK, Steensma TD, Wagenaar ECF, Doreleijers TAH, Cohen-Kettenis PT. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics. (2014) 134:696–704. doi: 10.1542/peds.2013-2958 [DOI] [PubMed] [Google Scholar]
- 29. Tordoff DM, Wanta JW, Collin A, Stepney C, Inwards-Breland DJ, Ahrens K. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. (2022) 1;5:e2229031. doi: 10.1001/jamanetworkopen.2022.29031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Der Miesen AIR, Steensma TD, De Vries ALC, Bos H, Popma A. Psychological functioning in transgender adolescents before and after gender-affirmative care compared with cisgender general population peers. J Adolesc Health. (2020) 66:699–704. doi: 10.1016/j.jadohealth.2019.12.018 [DOI] [PubMed] [Google Scholar]
- 31. Klink D, Caris M, Heijboer A, Van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab. (2015) 100:E270–5. doi: 10.1210/jc.2014-2439 [DOI] [PubMed] [Google Scholar]
- 32. Ciancia S, Klink D, Craen M, Cools M. Early puberty suppression and gender-affirming hormones do not alter final height in transgender adolescents. Eur J Endocrinol. (2023) 189:396–401. doi: 10.1093/ejendo/lvad125 [DOI] [PubMed] [Google Scholar]
- 33. Becker I, Auer M, Barkmann C, Fuss J, Möller B, Nieder TO, et al. A cross-sectional multicenter study of multidimensional body image in adolescents and adults with gender dysphoria before and after transition-related medical interventions. Arch Sex Behav. (2018) 47:2335–47. doi: 10.1007/s10508-018-1278-4 [DOI] [PubMed] [Google Scholar]
- 34. Karamanis G, Frisell T, Holmberg M, Halldin M, Sylvén S, Skalkidou A, et al. Incidence of idiopathic intracranial hypertension in individuals with gonadotropin-releasing hormone analogue treatment for gender dysphoria in Sweden. JAMA Pediatr. (2023) 177:726–7. doi: 10.1001/jamapediatrics.2023.0656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cantu AL, Moyer DN, Connelly KJ, Holley AL. Changes in anxiety and depression from intake to first follow-up among transgender youth in a pediatric endocrinology clinic. Transgend Health. (2020) 5:196–200. doi: 10.1089/trgh.2019.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Becker-Hebly I, Fahrenkrug S, Campion F, Richter-Appelt H, Schulte-Markwort M, Barkmann C. Psychosocial health in adolescents and young adults with gender dysphoria before and after gender-affirming medical interventions: a descriptive study from the Hamburg Gender Identity Service. Eur Child Adolesc Psychiatry. (2021) 30:1755–67. doi: 10.1007/s00787-020-01640-2 [DOI] [PubMed] [Google Scholar]
- 37. Boogers LS, van der Loos MATC, Wiepjes CM, Van Trotsenburg ASP, Den Heijer M, Hannema SE. The dose-dependent effect of estrogen on bone mineral density in trans girls. Eur J Endocrinol. (2023) 189:290–6. doi: 10.1093/ejendo/lvad116 [DOI] [PubMed] [Google Scholar]
- 38. de Nie I, Mulder CL, Meißner A, Schut Y, Holleman EM, van der Sluis ,WB, et al. Histological study on the influence of puberty suppression and hormonal treatment on developing germ cells in transgender women. Hum Reprod. (2022) 37:297–308. doi: 10.1093/humrep/deab240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eitel KB, Hodax JK, DiVall S, Kidd KM, Salehi P, Sequeira GM. Leuprolide acetate for puberty suppression in transgender and gender diverse youth: A comparison of subcutaneous eligard versus intramuscular lupron. J Adolesc Health. (2023) 72:307–11. doi: 10.1016/j.jadohealth.2022.09.017 [DOI] [PubMed] [Google Scholar]
- 40. Ghelani R, Lim C, Brain C, Fewtrell M, Butler G. Sudden sex hormone withdrawal and the effects on body composition in late pubertal adolescents with gender dysphoria. J Pediatr Endocrinol Metab. (2020) 33:107–12. doi: 10.1515/jpem-2019-0045 [DOI] [PubMed] [Google Scholar]
- 41. Joseph T, Ting J, Butler G. The effect of GnRH analogue treatment on bone mineral density in young adolescents with gender dysphoria: findings from a large national cohort. J Pediatr Endocrinol Metab. (2019) 32:1077–81. doi: 10.1515/jpem-2019-0046 [DOI] [PubMed] [Google Scholar]
- 42. KhatChadourian K, Amed S, Metzger DL. Clinical management of youth with gender dysphoria in Vancouver. J Pediatr. (2014) 164:906–11. doi: 10.1016/j.jpeds.2013.10.068 [DOI] [PubMed] [Google Scholar]
- 43. Kuper LE, Stewart S, Preston S, Lau M, Lopez X. Body dissatisfaction and mental health outcomes of youth on gender-affirming hormone therapy. Pediatrics. (2020) 145:e20193006. doi: 10.1542/peds.2019-3006 [DOI] [PubMed] [Google Scholar]
- 44. Lavender R, Shaw S, Maninger J-K, Butler G, Carruthers P, Carmichael P, et al. Impact of hormone treatment on psychosocial functioning in gender-diverse young people. LGBT Health. (2023) 10:382–90. doi: 10.1089/lgbt.2022.0201 [DOI] [PubMed] [Google Scholar]
- 45. López De Lara D, Pérez Rodríguez O, Cuellar Flores I, Pedreira Masa JL, Campos-Muñoz L, Cuesta Hernández M, et al. Evaluación psicosocial en adolescentes transgénero [Psychosocial assessment in transgender adolescents. Pediatr (Engl Ed). (2020) 93:41–8. doi: 10.1016/j.anpedi.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 46. McGregor K, McKenna JL, Williams CR, Barrera EP, Boskey ER. Association of Pubertal Blockade at Tanner 2/3 With Psychosocial Benefits in Transgender and Gender Diverse Youth at Hormone Readiness Assessment. J Adolesc Health. (2024) 74:801–7. doi: 10.1016/j.jadohealth.2023.10.028 [DOI] [PubMed] [Google Scholar]
- 47. McPherson S, Freedman DEP. Psychological outcomes of 12-15-year-olds with gender dysphoria receiving pubertal suppression in the UK: assessing reliable and clinically significant change. J Sex Marital Ther. (2024) 50:315–25. doi: 10.1080/0092623X.2023.2281986 [DOI] [PubMed] [Google Scholar]
- 48. Nokoff NJ, Scarbro SL, Moreau KL, Zeitler P, Nadeau KJ, Reirden D, et al. Body composition and markers of cardiometabolic health in transgender youth on gonadotropin-releasing hormone agonists. Transgend Health. (2021) 6:111–9. doi: 10.1089/trgh.2020.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Olson-Kennedy J, Streeter LH, Garofalo R, Chan Y-M, Rosenthal SM. Histrelin Implants for Suppression of Puberty in Youth with Gender Dysphoria: A Comparison of 50 mcg/Day (Vantas) and 65 mcg/Day (SupprelinLA). Transgend Health. (2021) 6:36–42. doi: 10.1089/trgh.2020.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pang KC, Peri AJS, Chung HE, Telfer M, Elder CV, Grover S. Rates of fertility preservation use among transgender adolescents. JAMA Pediatr. (2020) 174:890–1. doi: 10.1001/jamapediatrics.2020.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perl L, Segev-Becker A, Israeli G, Elkon-Tamir E, Oren A. Blood pressure dynamics after pubertal suppression with gonadotropin-releasing hormone analogs followed by testosterone treatment in transgender male adolescents: A pilot study. LGBT Health. (2020) 7:340–4. doi: 10.1089/lgbt.2020.0026 [DOI] [PubMed] [Google Scholar]
- 52. Pine-Twaddell E, Newfield RS, Marinkovic M. Extended use of histrelin implant in pediatric patients. Transgend Health. (2023) 8:264–72. doi: 10.1089/trgh.2021.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Russell I, Pearson B, Masic U. A longitudinal study of features associated with autism spectrum in clinic referred, gender diverse adolescents accessing puberty suppression treatment. J Autism Dev Disord. (2021) 51:2068–76. doi: 10.1007/s10803-020-04698-8 [DOI] [PubMed] [Google Scholar]
- 54. Segev-Becker A, Israeli G, Elkon-Tamir E, Perl L, Sekler O, Amir H, et al. Children and adolescents with gender dysphoria in Israel: increasing referral and fertility preservation rates. Endocr Pract. (2020) 26:423–8. doi: 10.4158/EP-2019-0418 [DOI] [PubMed] [Google Scholar]
- 55. Stoffers IE, De Vries MC, Hannema SE. Physical changes, laboratory parameters, and bone mineral density during testosterone treatment in adolescents with gender dysphoria. J Sex Med. (2019) 16:1459–68. doi: 10.1016/j.jsxm.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 56. Turban JL, King D, Carswell JM, Keuroghlian AS. Pubertal suppression for transgender youth and risk of suicidal ideation. Pediatrics. (2020) 145:e20191725. doi: 10.1542/peds.2019-1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Van Der Loos MA, Hellinga I, Vlot MC, Klink DT, Den Heijer M, Wiepjes CM. Development of hip bone geometry during gender-affirming hormone therapy in transgender adolescents resembles that of the experienced gender when pubertal suspension is started in early puberty. J Bone Miner Res. (2020) 36:931–41. doi: 10.1002/jbmr.4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Heesewijk J, Steenwijk MD, Kreukels BPC, Veltman DJ, Bakker J, Burke SM. Alterations in the inferior fronto-occipital fasciculus - a specific neural correlate of gender incongruence? Psychol Med. (2023) 53:3461–70. doi: 10.1017/S0033291721005547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Waldner RC, Doulla M, Atallah J, Rathwell S, Grimbly C. Leuprolide acetate and QTc interval in gender-diverse youth. Transgend Health. (2023) 8:84–8. doi: 10.1089/trgh.2021.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom. (2014) 17:275–80. doi: 10.1016/j.jocd.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 61. Spack NP, Edwards-Leeper L, Feldman HA, Leibowitz S, Mandel F, Diamond DA, et al. Children and adolescents with gender identity disorder referred to a pediatric medical center. Pediatrics. (2012) 129:418–25. doi: 10.1542/peds.2011-0907 [DOI] [PubMed] [Google Scholar]
- 62. Aitken M, VanderLaan DP, Wasserman L, Stojanovski S, Zucker KJ. Self-harm and suicidality in children referred for gender dysphoria. J Am Acad Child Adolesc Psychiatry. (2016) 55:513–20. doi: 10.1016/j.jaac.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 63. Holt V, Skagerberg E, Dunsford M. Young people with features of gender dysphoria: Demographics and associated difficulties. Clin Child Psychol Psychiatry. (2016) 21:108–18. doi: 10.1177/1359104514558431 [DOI] [PubMed] [Google Scholar]
- 64. Cass H. The cass review: Independent review of gender identity services for children and young people – final report. London: NHS England; (2024). [Google Scholar]
- 65. Luo X, Liang Y, Hou L, Wu W, Ying Y, Ye F. Long-term efficacy and safety of gonadotropin-releasing hormone analog treatment in children with idiopathic central precocious puberty: A systematic review and meta-analysis. Clin Endocrinol (Oxf). (2021) 94:786–96. doi: 10.1111/cen.14410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barbi L, Tornese G. The Brexit in adolescent gender dysphoria care? J Paediatr Child Health. (2024) 60:617–8. doi: 10.1111/jpc.16651 [DOI] [PubMed] [Google Scholar]
- 67. Howick J, Koletsi D, Ioannidis JPA, Madigan C, Pandis N, Loef M, et al. Most healthcare interventions tested in Cochrane Reviews are not effective according to high quality evidence: a systematic review and meta-analysis. J Clin Epidemiol. (2022) 148:160–9. doi: 10.1016/j.jclinepi.2022.04.017 [DOI] [PubMed] [Google Scholar]
- 68. Calcaterra V, Tornese G, Zuccotti G, Staiano A, Cherubini V, Gaudino R, et al. Adolescent gender dysphoria management: position paper from the Italian Academy of Pediatrics, the Italian Society of Pediatrics, the Italian Society for Pediatric Endocrinology and Diabetes, the Italian Society of Adolescent Medicine and the Italian Society of Child and Adolescent Neuropsychiatry. Ital J Pediatr. (2024) 50:73. doi: 10.1186/s13052-024-01644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vrouenraets LJJJ, de Vries ALC, de Vries MC, van der Miesen AIR, Hein IM. Assessing medical decision-making competence in transgender youth. Pediatrics. (2021) 148:e2020049643. doi: 10.1542/peds.2020-049643 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material , Further inquiries can be directed to the corresponding author/s.