Abstract
Objective
Small mammals such as the Julia Creek dunnart (Sminthopsis douglasi) may be difficult to detect using traditional trapping methods. Here, we conducted a pilot study to determine whether eDNA collected from soil and/or air could detect the presence of terrestrial vertebrates, including S. douglasi, in a semi-arid, open grassland environment.
Results
Airborne eDNA analysis returned vertebrate DNA from five sample sites (n = 7), whereas soil eDNA analysis returned vertebrate DNA from a single site (n = 7). The Julia Creek dunnart was not detected in any of the experimental samples. However, several airborne eDNA samples did return strong matches to three terrestrial vertebrates, the long-haired rat (Rattus villosissimus), red kangaroo (Osphranter rufus) and brown quail (Synoicus ypsilophorus), all native species known to occur commonly in the study area. Overall, our preliminary findings suggest that the effectiveness of airborne and soil-derived eDNA in detecting terrestrial vertebrates was constrained by high human signal and low sampling intensity. For future studies, we recommend a number of field and lab-based refinements to increase the likelihood of detecting more taxa, particularly those that occur at low density.
Clinical trial number
Not applicable
Supplementary Information
The online version contains supplementary material available at 10.1186/s13104-025-07302-3.
Keywords: Detection, Environmental, Airborne, Soil, Threatened, Julia creek dunnart
Introduction
Small, cryptic mammals such as the Julia Creek dunnart (Sminthopsis douglasi) present a challenge for detection [1, 2]. This threatened species has been successfully live trapped and detected using camera traps [1, 3]. However, the grasslands where they occur are vast and open, spanning hundreds of kilometres, and populations of the mammal are known to fluctuate markedly, leading to variable outcomes using traditional detection methods [1]. The development of a rapid and more reliable presence/absence detection tool is thus critical to better understand the distribution and prioritise conservation management of the species.
The emergence of environmental DNA (eDNA) collection techniques, capable of surveying entire vertebrate communities, is a major scientific breakthrough of the last few decades [4–6]. However, the best techniques for collecting eDNA of terrestrial vertebrates is still an active and rapidly growing field of research [7]. eDNA has been collected from indirect sources, such as spider webs [8], owl pellets [9] and by swabbing vegetation [10, 11]. But eDNA collected from soil and the air are likely the most promising techniques for detecting a broad range of terrestrial vertebrate taxa. These techniques have been investigated under laboratory and enclosed environmental conditions [12–14] and have more recently shown promise in field trials targeting terrestrial vertebrates in natural habitats [15–19]. However, if the techniques are to be broadly adopted, they must be further tested in a range of natural environments and under different conditions.
Here, we conducted a pilot study to determine whether eDNA collected from soil and/or air could detect the presence of terrestrial vertebrates, including S. douglasi, in a semi-arid, open grassland environment. To our knowledge, this is the first trial of the airborne eDNA technique in semi-arid Australia and one of the first applications to target a threatened terrestrial species.
Methods
Sampling design
To investigate the utility of eDNA as a detection method for vertebrates, including S. douglasi, we collected soil and air filter samples at two sites where S. douglasi individuals had been captured and released as part of a parallel live-trapping program (known sites) and at three sites proximate to known sites in suitable habitat (potential sites) where S. douglasi had been recorded in previous years (see Table 1). We took two negative control samples in unsuitable S. douglasi habitat, where the species was assumed to be absent (Table 1). See Additional File 1 for a detailed description of sample sites and parallel live-trapping. We also collected two positive experimental control samples to validate the accuracy of the DNA extraction, sequencing and bioinformatics processes. We collected the positive experimental controls by placing a sterilised piece of filter paper into a calico bag containing an individual S. douglasi for 3–3.5 h. We acknowledge that the positive experimental control does not test the efficacy of the active eDNA sampler. However, the sampler has already proven effective at collecting airborne eDNA under controlled conditions [14].
Table 1.
The locations of each filter and soil sample, including coordinates, run time and sample type
| Filter ID | Location | Sample type | Latitude | Longitude | Deployment date | Collection date | Total run time | Associated soil sample? | Soil ID |
|---|---|---|---|---|---|---|---|---|---|
| AF01 | Bladensburg National Park | Positive control | -22.5149 | 143.0381 | 17/04/2024 | 17/04/2024 | 3 h 30 min | No | NA |
| AF02 | Bladensburg National Park | Negative control | -22.5740 | 143.1165 | 19/04/2024 | 20/04/2024 | 16 h 20 min | Yes | S02 |
| AF03 | Bladensburg National Park | Known sample | -22.4998 | 143.0612 | 19/04/2024 | 20/04/2024 | 13 h 2 min | Yes | S03 |
| AF04 | Bladensburg National Park | Potential sample | -22.5310 | 143.0486 | 19/04/2024 | 20/04/2024 | 13 h 10 min | Yes | S04 |
| AF05 | Bladensburg National Park | Positive control | -22.5149 | 143.0381 | 21/04/2024 | 21/04/2024 | 3 h | No | NA |
| AF06 | Bladensburg National Park | Potential sample | -22.5500 | 143.0859 | 22/04/2024 | 23/04/2024 | 15 h 45 min | Yes | S06 |
| AF07 | Bladensburg National Park | Known sample | -22.5022 | 143.0640 | 22/04/2024 | 23/04/2024 | 12 h 50 min | Yes | S07 |
| AF08 | Bladensburg National Park | Potential sample | -22.5282 | 143.0466 | 22/04/2024 | 23/04/2024 | 13 h 15 min | Yes | S08 |
| AF09 | Samford Ecological Research Facility (SERF, QUT) | Negative control | -27.3883 | 152.8793 | 28/04/2024 | 29/04/2024 | 12 h 45 min | Yes | S09 |
eDNA collection
We used air sampler design 2 as per Garrett et al. [14]. See Additional File 1 for more information on the air sampler design and specifications, as well as specific cleaning protocols.
We deployed the samplers in the field by hammering a metal star picket into the ground and attaching the frame to the star picket using garden wire with the ‘filter head’ facing down toward the ground/soil cracks at a height of ~ 10 cm above ground. This setup was chosen in an attempt to target small, terrestrial vertebrates, particularly S. douglasi, which are known to shelter in soil cracks [1–3]. We placed the power bank and adapter to run the fan into an airtight plastic container and strapped the container to the star picket. We then turned the sampler on and left it to run overnight (~ 12–16 h). The next day, we collected the filter paper using sterilized forceps and placed it into a vial of RNAlater solution.
At each air sampler site, we also collected topsoil with a sterilised spoon and placed the sample into a 15 ml vial with RNAlater solution. Where possible, we took samples from the outside edge of soil cracks, locations we considered more likely to be used by S. douglasi.
DNA extraction and metabarcoding
Please see Additional File 1 for details regarding DNA extraction and metabarcoding methodology, contamination mitigation, and bioinformatics parameters. We tested two DNA extraction methods for the air filters: Qiagen Blood and Tissue Kit as per Garrett et al. [14] and Qiagen Powersoil Pro Kit (Qiagen, Valencia, CA, USA). Soil samples were only processed with the latter kit. We included a single DNA extraction blank for each method.
We processed all samples in Polymerase Chain Reaction (PCR) duplicate using the MiMammal-U primer set, targeting 12S rRNA [20]. Amplifying a ~ 171 bp insert, this primer set was developed to primarily detect mammals [20], but it also detects a broad range of vertebrate taxa. We modified the primers with a 5’ universal tail as part of the 2-step PCR library preparation method described in Colman et al. [21] for paired-end Illumina sequencing (MiSeq v2 500 cycle kit).
Following quality filtering, error correction, paired-end merging, chimera removal, and post-clustering into Operational Taxonomic Units (OTUs) [22–24], we classified taxa using Lowest Common Ancestor (LCA) from Basic Local Alignment Search Tool (BLAST) searches against Genbank’s core_nt database [25–27]. We then visually inspected the BLAST results of each OTU to verify LCA classifications, correcting for any over- or under-splitting of the taxonomic labels. We removed from consideration sequences deriving from non-vertebrates, any that did not yield BLAST hits, and any deriving from known or suspected pseudogenes, or any non-mtDNA source.
Results and discussion
After processing, we retained 444,605 reads that were classified to at least the order level (median = 12,809 reads per library) with OTU richness consistently plateauing at a sequencing depth of 8000 reads in rarefaction curves (Additional File 1). None of the DNA extraction blanks (n = 3) or PCR-negative controls (n = 5) prepared with the samples yielded mergeable, paired-end reads in the DADA2 pipeline (Additional File 2), and therefore, they did not yield taxonomic data. The PCR positive control yielded 2 out of 4 expected OTUs, which we determined to be an issue with the mock community itself and not due to processing or sequencing depth (see Additional file 1 for verification). None of the sequences in the PCR positive controls were detected in any other sample.
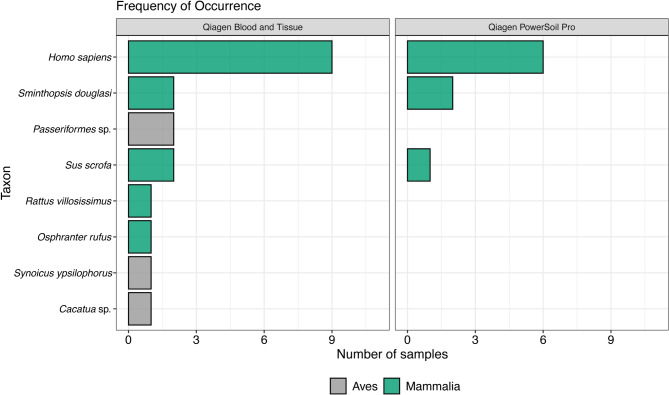
For DNA extraction from air filters, the Qiagen Blood and Tissue Kit was the most successful in terms of amplification success and taxonomic richness (Fig. 1). The Qiagen Blood and Tissue Kit detected eight taxa (present in 1–9 samples), and the Qiagen Powersoil pro kit detected three taxa (present in 1–6 samples).
Fig. 1.
Frequency of occurrence of the bird and mammal species detected using two DNA extraction protocols
We obtained taxonomic data from all nine air filters but only one sediment sample (Fig. 2). The Julia Creek dunnart was not detected in any soil or airborne eDNA samples, despite two samplers being deployed at S. douglasi release locations. Based on mark-recapture data from previous years, S. douglasi are frequently recaptured at the same trap locations on successive nights and have a median trap movement distance of ~ 61 m [1]. Therefore, we assumed these locations would have a higher chance of detecting the target species. However, the species was only detected from the two experimental positive controls, where a filter was placed directly into a clean calico bag with a live Julia Creek dunnart. As highlighted by Leempoel et al. [17], there is still much we do not know about how frequently, closely and/or recently an animal must move through an area before being detectable by an eDNA sampler. Therefore, in future studies, we would recommend monitoring airborne eDNA samplers with unbaited camera traps to identify vertebrates that move in close vicinity to the samplers in order to assess their accuracy. We would also collect at least one positive control sample, by holding a live S. douglasi underneath an active airborne eDNA sampler.
Fig. 2.
Vertebrate taxa from each sample that had a minimum 95% identity match via BLAST search
The airborne eDNA samples at Bladensburg National Park did detect red kangaroo (Osphranter rufus), long-haired rat (Rattus villosissimus), brown quail (Synoicus ypsilophorus) and one sequence variant that could only be classified to order Passeriformes (Sequence Read Archive BioProject PRJNA1173596; Fig. 2). This represents just three terrestrial vertebrates of the 24 species recorded via camera trapping (30 cameras deployed for four weeks) at the same locations [28]. Our low number of eDNA species detections may be due to the small number of samples collected and relatively short deployment timeframes in comparison to other methods. A similar study by Lynggaard et al. [18] in a natural environment, using a pair of two different custom-made air samplers per site, detected 57 ‘wild’ taxa across six, 12 h sampling events (over three days). Therefore, for future studies in this system, we recommend deploying samplers at (at least) ten sites for 3–4 consecutive nights to better account for variability in weather conditions and animal movements/behaviour. We would also recommend deploying two active samplers per site at differing heights and/or orientation (horizontal and vertical) to the ground to determine whether sampler placement influences the detection of S. douglasi and/or the overall number of taxa recorded.
Overall, the airborne eDNA samplers did detect the most commonly recorded species at Bladensburg National park, as previously determined via cameras, live trapping and/or opportunistic observations [1, 3, 28]. It is noteworthy that during the eDNA collection period, the long-haired rat was undergoing a population irruption (E.L. Gray and A.M. Baker, pers. obs.), and only two taxa were detected via the concurrent live trapping (see Additional File 1). Other studies of airborne eDNA have observed that more common species are often more frequently detected [16] or generally have higher read counts [14] than rare species.
Most of our soil samples amplified DNA, but the majority was either non-vertebrate or uninformative. Plausibly, at most sites, there was in fact little vertebrate DNA contained in the soil sample. Substrate selection, frequency of sampling and target animal size are all recognised factors that may limit vertebrate detectability via soil eDNA [29]. The exception was the soil sample from the off-site negative control. The mammal species detected from this site in the soil eDNA sample was the brush-tailed possum (Trichosurus vulpecula) (99.41% match), a species known to occur in high density at the location.
Human DNA was detected most frequently in the airborne samples, accounting for 98.1% of reads (excluding the positive experimental controls). Two air filter samples also returned only human DNA. This likely represents both human airborne eDNA in the environment and also contamination, the latter a known issue with airborne eDNA [19]. High levels of contamination have been known to result in false negative results in other eDNA studies due to the competitive amplification of contaminant DNA over low quantity DNA during the PCR process [30]. In future studies, the risk of human contamination may be reduced by wearing surgical half or full facepiece respirators (with no exhalation valve) when in the field and refining other field collection protocols. Blocking the amplification of human DNA by developing blocking primers using MiMammal-U (12 S), may also be useful. Although the DNA of other vertebrate species was detected despite the contamination, with less human contaminant present, the chance of detecting DNA of target taxa will increase, especially if the latter occur at relatively low density in the environment and/or the sample [30–32].
Limitations
We have shown in this pilot study that a small number of active air samplers left running overnight were able to detect common vertebrate species within an open tussock grassland ecosystem. However, our study failed to detect S. douglasi, the focal species, and we detected fewer species at Bladensburg National Park compared to traditional methods such as camera trapping and live trapping. Our study was limited by the small sample size and short deployment of the airborne eDNA samplers. Therefore, we have recommended practical field and lab-based refinements to the sampling design to increase the likelihood of detecting more taxa, particularly those that occur at low density.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Additional file 1. Additional information on the methodology employed in the study.
Supplementary Material 2: Additional file 2. Read retention table from the DADA2 pipeline for each sequenced library.
Acknowledgements
We thank the Guwa Koa Aboriginal Corporation for providing approval to work on the traditional lands of the Koa people. We are grateful to Kate Moffatt, Michael Daddow and Jules Moffatt for assisting us in the field. We thank Beth Clare for providing the air samplers and DETSI personnel for their support and advice in conducting the research at Bladensburg National Park, especially Shane Hume (and his partner, Mary) for on-ground assistance during the trapping periods. We thank QUT and the caretakers of the Samford Ecological Research Facility (SERF), Marcus Yates and Lorrelle Allen, for permitting us access to the property to collect eDNA samples. We also thank the anonymous reviewers for their thoughtful feedback, which helped us improve the manuscript.
Abbreviations
- eDNA
Environmental DNA
- QUT
Queensland University of Technology
- PCR
Polymerase chain reaction
- OTUs
Operational taxonomic units
- BLAST
Basic local alignment search tool
- LCA
Lowest common ancestor
Author contributions
ELG, FMW and AMB contributed to study conceptualisation and design; ELG and AMB collected the data; FMW, DES and SM analysed the data; ELG, FMW, DES, SM and AMB interpreted the results; ELG and AMB wrote the manuscript; FMW, DES and SM reviewed the manuscript; AMB acquired the funding.
Funding
This research was principally funded by Multicom Resources as part of an Offset Management Plan conceptualised by Multicom Resources and Epic Environmental and approved under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) for the Saint Elmo Vanadium project (EPBC 2017/8007). Additional funding and resources were provided by Queensland University of Technology.
Data availability
The data supporting the findings of this study are available within the article, its additional files and via the NCBI Sequence Read Archive, BioProject ID: PRJNA1173596.
Declarations
Ethics approval and consent to participate
All animal trapping, handling and eDNA collection was conducted under the auspices of Queensland Department of Environment, Tourism, Science and Innovation (DETSI) Permit P-PTUKI-100171210 and QUT Research Ethics Permit AE 2024-5154-17968. No additional permissions were required to collect the specimens in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bakker AH, Patterson CR, Mifsud G, Reside AE, Fuller S, Baker AM. Density of a cryptic Australian small Mammal: the threatened Julia creek Dunnart (Sminthopsis douglasi). Ecol Evol. 2024;14. 10.1002/ece3.11674 [DOI] [PMC free article] [PubMed]
- 2.Mifsud G. Ecology of the Julia Creek dunnart, Sminthopsis douglasi, (Marsupialia: Dasyuridae). Master’s thesis. La Trobe University, 1999.
- 3.Bakker AH, Schoenefuss P, Mifsud G, Fuller S, Baker AM. Comparing methods of detecting an elusive dasyurid marsupial, the threatened Julia creek Dunnart (Sminthopsis douglasi), in central Western Queensland, Australia. Ecol Evol. 2024;14:e70507. 10.1002/ece3.70507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofreiter M, Mead JI, Martin P, Poinar HN. Molecular caving. Curr Biol. 2003;13:R693–5. 10.1016/j.cub.2003.08.039 [DOI] [PubMed] [Google Scholar]
- 5.Thomsen PF, Willerslev E. Environmental DNA– An emerging tool in conservation for monitoring past and present biodiversity. Biol Conserv. 2015;183:4–18. 10.1016/j.biocon.2014.11.019 [Google Scholar]
- 6.Willerslev E, Hansen AJ, Binladen J, Brand TB, Gilbert MTP, Shapiro B, et al. Diverse plant and animal genetic records from holocene and pleistocene sediments. (Reports). Science. 2003;300:791–6. [DOI] [PubMed] [Google Scholar]
- 7.Newton JP, Allentoft ME, Bateman PW, Heyde M, Nevill P. Targeting terrestrial vertebrates with eDNA: trends, perspectives, and considerations for sampling. Environ DNA 2025;7. 10.1002/edn3.70056
- 8.Gregorič M, Kutnjak D, Bačnik K, Gostinčar C, Pecman A, Ravnikar M, et al. Spider webs as eDNA samplers: biodiversity assessment across the tree of life. Mol Ecol Resour. 2022;22:2534–45. 10.1111/1755-0998.13629 [DOI] [PubMed] [Google Scholar]
- 9.Schoenefuss P. The efficacy of morphological and genetic owl pellet analysis compared to live-trapping in arid and semi-arid Australian small mammal monitoring systems. Doctoral Thesis. Queensland University of Technology, 2023.
- 10.Allen MC, Kwait R, Vastano A, Kisurin A, Zoccolo I, Jaffe BD, et al. Sampling environmental DNA from trees and soil to detect cryptic arboreal mammals. Sci Rep Nat Publ Group. 2023;13:180. 10.1038/s41598-023-27512-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyman JA, Sanchez DE, Hershauer SN, Sobek CJ, Chambers CL, Zahratka J, et al. Mammalian eDNA on herbaceous vegetation? Validating a qPCR assay for detection of an endangered rodent. Environ DNA. 2022;4:1187–97. 10.1002/edn3.331 [Google Scholar]
- 12.Andersen K, Bird KL, Rasmussen M, Haile J, Breuning-Madsen H, Kjær KH, et al. Meta-barcoding of ‘dirt’ DNA from soil reflects vertebrate biodiversity. Mol Ecol. 2012;21:1966–79. 10.1111/j.1365-294X.2011.05261.x [DOI] [PubMed] [Google Scholar]
- 13.Clare EL, Economou CK, Faulkes CG, Gilbert JD, Bennett F, Drinkwater R, et al. eDNAir: proof of concept that animal DNA can be collected from air sampling. PeerJ. 2021;9:e11030–11030. 10.7717/peerj.11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrett NR, Watkins J, Simmons NB, Fenton B, Maeda-Obregon A, Sanchez DE, et al. Airborne eDNA documents a diverse and ecologically complex tropical Bat and other mammal community. Environ DNA. 2023;5:350–62. 10.1002/edn3.385 [Google Scholar]
- 15.Frere C, Jackson N, Moreno J, Oliveros Sandino A, Ball S, Powell D. Koalas, friends and foes—The application of airborne eDNA for the biomonitoring of threatened species. J Appl Ecol. 2024;61:2837–47. 10.1111/1365-2664.14784 [Google Scholar]
- 16.Johnson MD, Barnes MA, Garrett NR, Clare EL. Answers blowing in the wind: detection of birds, mammals, and amphibians with airborne environmental DNA in a natural environment over a yearlong survey. Environ DNA. 2023;5:375–87. 10.1002/edn3.388 [Google Scholar]
- 17.Leempoel K, Hebert T, Hadly EA. A comparison of eDNA to camera trapping for assessment of terrestrial mammal diversity. Proc R Soc B Biol Sci. 2020;287:20192353. 10.1098/rspb.2019.2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynggaard C, Frøslev TG, Johnson MS, Olsen MT, Bohmann K. Airborne environmental DNA captures terrestrial vertebrate diversity in nature. Mol Ecol Resour. 2024;24:e13840. 10.1111/1755-0998.13840 [DOI] [PubMed] [Google Scholar]
- 19.Polling M, Buij R, Laros I, Groot GA. Continuous daily sampling of airborne eDNA detects all vertebrate species identified by camera traps. Environ DNA 2024;6:NA-NA. 10.1002/edn3.591
- 20.Ushio M, Fukuda H, Inoue T, Makoto K, Kishida O, Sato K, et al. Environmental DNA enables detection of terrestrial mammals from forest pond water. Mol Ecol Resour. 2017;17:e63–75. 10.1111/1755-0998.12690 [DOI] [PubMed] [Google Scholar]
- 21.Colman RE, Schupp JM, Hicks ND, Smith DE, Buchhagen JL, Valafar F et al. Detection of Low-Level Mixed-Population drug resistance in Mycobacterium tuberculosis using high fidelity amplicon sequencing. PLoS ONE 2015;10. 10.1371/journal.pone.0126626 [DOI] [PMC free article] [PubMed]
- 22.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible Microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–8. 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods. 2016;13:581–8. 10.1038/NMETH.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frøslev TG, Kjøller R, Bruun HH, Ejrnæs R, Brunbjerg AK, Pietroni C, et al. Nat Commun. 2017;8:1–11. 10.1038/s41467-017-01312-x. Algorithm for post-clustering curation of DNA amplicon data yields reliable biodiversity estimates. [DOI] [PMC free article] [PubMed]
- 25.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 26.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank Nucleic Acids Res. 2009;37:D26–31. 10.1093/nar/gkn723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–86. 10.1101/gr.5969107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tighe E. Fine-scale habitat associations of the vertebrate community at Bladensburg National Park, with reference to the Julia Creek dunnart (Sminthopsis douglasi). Honours Thesis. University of Queensland, 2022.
- 29.Ryan E, Bateman P, Fernandes K, van der Heyde M, Nevill P. eDNA metabarcoding of log Hollow sediments and soils highlights the importance of substrate type, frequency of sampling and animal size, for vertebrate species detection. Environ DNA. 2022;4:940–53. 10.1002/edn3.306 [Google Scholar]
- 30.Boessenkool S, Epp LS, Haile J, Bellemain E, Edwards M, Coissac E, et al. Blocking human contaminant DNA during PCR allows amplification of rare mammal species from sedimentary ancient DNA. Mol Ecol. 2012;21:1806–15. 10.1111/j.1365-294X.2011.05306.x [DOI] [PubMed] [Google Scholar]
- 31.Beng KC, Corlett RT. Applications of environmental DNA (eDNA) in ecology and conservation: opportunities, challenges and prospects. Biodivers Conserv. 2020;29:2089–122. 10.1007/s10531-020-01980-0 [Google Scholar]
- 32.Marshall NT, Stepien CA. Macroinvertebrate community diversity and habitat quality relationships along a large river from targeted eDNA metabarcode assays. Environ DNA. 2020;2:572–86. 10.1002/edn3.90 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Frøslev TG, Kjøller R, Bruun HH, Ejrnæs R, Brunbjerg AK, Pietroni C, et al. Nat Commun. 2017;8:1–11. 10.1038/s41467-017-01312-x. Algorithm for post-clustering curation of DNA amplicon data yields reliable biodiversity estimates. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Supplementary Material 1: Additional file 1. Additional information on the methodology employed in the study.
Supplementary Material 2: Additional file 2. Read retention table from the DADA2 pipeline for each sequenced library.
Data Availability Statement
The data supporting the findings of this study are available within the article, its additional files and via the NCBI Sequence Read Archive, BioProject ID: PRJNA1173596.