Abstract
Introduction
The optimal surgical approach for right ventricular outflow tract obstruction in Tetralogy of Fallot aims to preserve the native pulmonary valve annulus, as this is associated with better long-term outcomes. Pediatric humanitarian patients often present with a delayed diagnosis and lack access to preoperative palliative treatments, reducing the likelihood of pulmonary valve annulus preservation and potentially compromising long-term outcomes. This study aims to identify independent predictors of successful pulmonary valve-sparing repair in pediatric humanitarian patients undergoing corrective surgery for Tetralogy of Fallot.
Methods
Between January 2019 and May 2023, pediatric humanitarian patients with Tetralogy of Fallot underwent surgical correction at our center. We performed a comparative analysis of preoperative, intraoperative, and postoperative variables, followed by univariate and multivariate logistic regression to identify independent predictors of pulmonary valve-sparing repair.
Results
A lower body mass index (OR = 0.711; p = 0.021; 95% CI = 0.533–0.949), a larger pulmonary valve annulus measured in centimeters (OR = 28.653; p = 0.008; 95% CI = 2.360-347.890) and a higher Z-score of pulmonary valve annulus (OR = 1.606; p = 0.023; 95% CI = 1.067–2.418) were identified as independent predictors of pulmonary valve-sparing repair.
Conclusion
Successful pulmonary valve-sparing repair was associated with lower BMI and a larger pulmonary valve annulus (both measurements in centimeters and Z-score). These findings may help guide clinical and policy strategies to promote more equitable and effective surgical care in resource-limited settings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-025-03475-x.
Keywords: Tetralogy of Fallot, Pulmonary valve-sparing repair, Pediatric humanitarian patients
Introduction
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect, accounting for approximately 15% of all congenital heart disease cases [1]. It is characterized by four main cardiac abnormalities: right ventricular outflow tract obstruction (RVOTO), ventricular septal defect (VSD), overriding aorta, and right ventricular hypertrophy [1]. These abnormalities result from underdevelopment of the right ventricular infundibulum, leading to misalignment of the infundibular septum and varying degrees of RVOTO, ranging from moderate stenosis to complete atresia [2].
Surgical correction of TOF is essential for long-term survival, typically performed between 3 and 6 months of age [3, 4]. The preferred approach, when anatomically feasible, involves a transatrial-transpulmonary technique that avoids right ventriculotomy and preserves the native pulmonary annulus [5]. Pulmonary valve preservation is particularly important, as it reduces the risk of chronic pulmonary regurgitation and is associated with better long-term outcomes [6].
In cases of severe infundibular stenosis, ventriculotomy may be necessary. However, the use of transannular patches to enlarge the pulmonary annulus can compromise valve function, leading to significant regurgitation and potential long-term complications.
While early surgical repair during the first year of life is the standard in many high-income countries, access to timely diagnosis and treatment remains a challenge in low- and middle-income countries (LMICs). As a result, many children with TOF in these settings present at advanced stages, exhibiting severe right ventricular hypertrophy, hypoplasia of the pulmonary vascular bed, severe cyanosis, and recurrent hypoxic episodes [7]. These conditions are often exacerbated by malnutrition and comorbidities associated with prolonged hypoxemia, increasing perioperative morbidity and mortality [8].
Humanitarian organizations play a crucial role in providing surgical care for pediatric patients from LMICs by facilitating their transfer to specialized centers. However, late diagnosis and the absence of preoperative palliative interventions decrease the likelihood of performing valve-sparing repairs, potentially leading to poorer long-term outcomes [1, 6].
Identifying predictors of successful pulmonary valve-sparing repair (PV-SR) in pediatric humanitarian patients with TOF is essential for optimizing patient selection and improving surgical outcomes. Late surgical repair in children from LMICs presents unique challenges, making it even more critical to predict which patients would benefit most from this surgical approach. This study aims to explore these predictive factors in a cohort of children from LMICs who underwent TOF corrective surgery at our center.
Materials and methods
This retrospective cohort observational study evaluated pediatric patients from LMICs with a confirmed diagnosis of TOF who underwent complete surgical correction at our center between January 2019 and May 2023. We included all pediatric humanitarian patients under 18 years of age, who were referred by a humanitarian organization and had a confirmed diagnosis of TOF requiring complete surgical repair. Patients were classified into two groups based on the surgical technique employed: with or without PV-SR.
Patients who had previously undergone palliative interventions, such as RVOT stenting, prior to the complete repair at our center were excluded.
The decision to perform a PV-SR or a non-PV-SR technique was made intraoperatively, based on real-time anatomical assessment. The surgical team aimed to preserve the native pulmonary valve whenever technically feasible. Criteria considered included the absolute diameter and Z-score of the pulmonary valve annulus, the degree of annular and valvular dysplasia, and the ability to relive RVOTO without annular disruption. There was no predefined preoperative protocol guiding this decision beyond intraoperative anatomical evaluation.
Clinical and surgical data were retrospectively collected from medical records and surgical logs, including demographic characteristics (age at the time of surgery, sex, country of origin), clinical data (weight, height, body mass index (BMI), TOF severity, presence of comorbidities), and surgical details (technique employed, surgical times, complications, durations of invasive ventilation, intensive care unit (ICU) stay and overall hospitalization time).
Although this study was not designed with predefined primary or secondary endpoints, the main outcome of interest was the performance of PV-SR. Other operative variables (e.g., residual pulmonary regurgitation, invasive ventilation time, ICU time, hospitalization time, postoperative complications) were assessed to characterize clinical differences between groups. Postoperative complications were defined as clinically relevant adverse events occurring within the first 30 days after surgery, including ECMO support, neurological deficits, delayed chest closure, surgical reintervention, acute renal failure without dialysis, prolonged cardiovascular pharmacological support, chylothorax, respiratory infection requiring re-intubation, and reversible arrhythmias after electrolyte correction.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software, version 27. Descriptive statistics were used to characterize the sample. Continuous variables were summarized using measures of central tendency and dispersion: mean ± standard deviation (SD) for normally distributed variables, or median (interquartile range) (IQR), for non-normally distributed variables. Categorical variables were expressed as absolute (n) and relative frequencies (%).
To compare qualitative variables between the two groups, Pearson’s chi-square test (χ²) or Fisher’s exact test was applied when the conditions for the χ² test were not met.
For quantitative variables, normality was assessed using the Shapiro-Wilk (SW) test. Depending on normality and variance homogeneity (assessed by Levene’s test), either the Student’s t-test for independent samples or the Mann-Whitney U test was applied.
Univariate binary logistic regression was performed to assess associations between independent variables and the dependent variable (PV-SR). Significant variables were then included in a multivariate logistic regression model to adjust for confounders and identify independent predictors of PV-SR.
To ensure the absence of multicollinearity among explanatory variables, tolerance and Variance Inflation Factor (VIF) tests were applied, retaining only variables with tolerance > 0.1 and VIF < 10. The “Forward Stepwise” method, guided by clinical relevance, was used to identify significant independent predictors. Interaction terms were not included to avoid unnecessary model complexity. Model performance was evaluated using − 2 Log Likelihood, Nagelkerke R2, and the Hosmer-Lemeshow test.
Odds Ratios (OR) with 95% confidence intervals (95% CI) were reported. Statistical significance was interpreted at an α = 0.05 level with 95% CI.
This study was approved by the regional ethics committee (Commission Cantonale d’Ethique de la Recherche sur l’Etre Humain) (CCER 2024 − 00407) and conducted in accordance with the ethical principles outlined in the Declaration of Helsinki [9].
Results
Patient characteristics and comparative analysis
A total of 115 pediatric patients (69 males) who underwent corrective TOF surgery were included in the study. Among them, 85 patients (74%) underwent the PV-SR technique for RVOTO relief.
A summary of patient characteristics is presented in Table 1.
Table 1.
Characteristics of the patients
| Total | PV-SR | p | ||
|---|---|---|---|---|
| No | Yes | |||
| (n = 115) | (n = 30) | (n = 85) | ||
| Gendera | ||||
| Male | 69 (60.0%) | 15(50.0%) | 54(63.5%) | .28c |
| Female | 46 (40.0%) | 15(50.0%) | 31(36.5%) | |
| Country of origina | ||||
| Algeria | 6 (5.2%) | 5 (16.7%) | 1 (1.2%) | 0.002 d |
| Burkina Faso | 1 (0.9%) | 1 (3.3%) | 0 (0.0%) | |
| Benin | 19 (16.5%) | 2 (6.7%) | 17 (20.0%) | |
| Cameroun | 1 (0.9%) | 0 (0.0%) | 1 (1.2%) | |
| Chad | 1 (0.9%) | 0 (0.0%) | 1 (1.2%) | |
| Côte d’Ivoire | 1 (0.9%) | 1 (3.3%) | 0 (0.0%) | |
| Djibouti | 1 (0.9%) | 0 (0.0%) | 1 (1.2%) | |
| Georgia | 1 (0.9%) | 1 (3.3%) | 0 (0.0%) | |
| Guinea | 12 (10.4%) | 5 (16.7%) | 7 (8.2%) | |
| Maroc | 15 (13.0%) | 4 (13.3%) | 11 (12.9%) | |
| Mali | 13 (11.3%) | 2 (1.7%) | 11 (12.9%) | |
| Mauritania | 15 (13.0%) | 1 (3.3%) | 14 (16.5%) | |
| Niger | 1 (0.9%) | 0 (0.0%) | 1 (1.2%) | |
| Polonia | 2 (1.7%) | 2 (6.7%) | 0 (0.0%) | |
| Senegal | 13 (11.3%) | 4 (13.3%) | 9 (10.6%) | |
| Syria | 1 (0.9%) | 0 (0.0%) | 1 (1.2%) | |
| Togo | 5 (4.3%) | 0 (0.0%) | 5 (5.9%) | |
| Tunisia | 7 (6.1%) | 2(6.7%) | 5 (5.9%) | |
| Age at surgical repair (days)b | 1444.00 (942.00 to 2337.00) | 1022.50(248.25 to 2287.50) | 1487.00 (1052.00 to 2417.50) | 0.032 e |
| Weight (Kg)b | 12.20 (10.00 to 16.50) | 10.65 (7.23 to 20.15) | 13.00 (10.00 to 16.15) | .076e |
| Height (cm)b | 97.00 (85.00 to 115.00) | 88.50 (67.75 to 121.00) | 99.00 (89.00 to 109.50) | .069e |
| BMI (Kg/m2)b | 13.45 (12.49 to 14.71) | 13.91 (12.83 to 15.77) | 13.21 (12.38 to 14.34) | 0.014 e |
| TOF Gravitya | ||||
| Mild | 38 (33.0%) | 4(13.3%) | 34(40.0%) | 0.004 d |
| Moderate | 22 (19.1%) | 4(13.3%) | 18(21.2%) | |
| Severe | 55 (47.8%) | 22(73.3%) | 33(38.8%) | |
| Cardiac Associated malformations a | ||||
| Mitral Regurgitation | ||||
| No | 107 (93.0%) | 28 (93.3%) | 79 (92.9%) | 1.000d |
| Yes | 8 (7.0%) | 2 (6.7%) | 6 (7.1%) | |
| Tricuspid Regurgitation | ||||
| No | 95 (82.6%) | 26 (86.7%) | 69 (81.2%) | 0.586d |
| Yes | 20 (17.4%) | 4 (13.3%) | 16 (18.8%) | |
| Aberrant CA | ||||
| No | 109 (95.6%) | 28 (93.3%) | 81 (96.4%) | 0.606d |
| Yes | 5 (4.4%) | 2 (6.7%) | 3 (3.6%) | |
| Interatrial Shunt | ||||
| No | 26 (22.6%) | 5 (16.7%) | 21 (24.7%) | 0.452c |
| Yes | 89 (77.4%) | 25 (83.3%) | 64 (75.3%) | |
| PDA | ||||
| No | 63 (55.3%) | 14 (46.7%) | 49 (58.3%) | 0.292c |
| Yes | 51 (44.7%) | 16 (53.3%) | 35 (41.7%) | |
| MAPCAs | ||||
| No | 90 (86.5%) | 24 (82.8%) | 66 (88.0%) | 0.527d |
| Yes | 14 (13.5%) | 5 (17.2%) | 9 (12.0%) | |
| ASVR | ||||
| No | 108 (93.9%) | 28 (93.3%) | 80 (94.1%) | 1.000d |
| Yes | 7 (6.1%) | 2 (6.7%) | 5 (5.9%) | |
| RAA | ||||
| No | 97 (85.1%) | 25 (86.2%) | 72 (84.7%) | 1.000d |
| Yes | 17 (14.9%) | 4 (13.8%) | 13 (15.3%) | |
Abbreviations: PV-SR Pulmonary Valve - Sparing Repair; BMI Body Mass Index; TOF Tetralogy of Fallot; CA Coronary Artery; PDA Persistent Ductus Arteriosus; MAPCAs Major Aortopulmonary Collateral Arteries; ASVR Anomaly of the Systemic Venous Return; RAA Right Aortic Arch; Kg kilograms; cm centimeter; m2 meters quarter; % percent; g grams; p p-value for general statistical tests
a count (%)
b median (interquartile range)
c Pearson’s chi-square test
d Fisher’s exact test
e Mann-Whitney test
The median age at the time of surgery was 1444 days (approximately 48.1 months; IQR: 942.00 to 2337.00 days), and the median BMI was 13.45 (IQR: 12.49 to 14.71) kg/m2. Most patients presented with severe forms of TOF (n = 55; 47.8%) and were predominantly from North African countries (n = 43; 37.3%).
The clinical and preoperative echocardiographic characteristics are summarized in Table 2.
Table 2.
Preoperative echocardiographic characteristics
| Total | PV-SR | p | ||
|---|---|---|---|---|
| No | Yes | |||
| (n = 115) | (n = 30) | (n = 85) | ||
| Preoperative echocardiography | ||||
| Maximal instantaneous RVOT gradient (mmHg)a | 72.00 (70.00 to 83.00) | 80.00 (70.00 to 92.00) | 71.00 (67.25 to 80.00) | .069c |
| Pulmonary valve annulus (cm)a | 1.00 (0.90 to 1.30) | 0.80 (0.70 to 1.02 | 1.10 (0.90 to 1.30) | < 0.001 c |
| Pulmonary valve annulus Z-scorea | -1.72 (-2.60 to -0.59) | -3.00 (-4.80 to -1.75) | -1.64 (-2.31 to -0.37) | < 0.001 c |
| Peripheral pulse oximetry (%)a | 80.00 (70.00 to 87.00) | 76.00 (71.50 to 88.00) | 82.00 (70.00 to 87.00) | .782c |
| Hemoglobin (g/dl)b | 17.00 ± 4.07 | 16.84 ± 4.12 | 17.34 ± 4.16 | .571d |
Abbreviations: PV-SR Pulmonary Valve - Sparing Repair; RVOT Right ventricular outflow tract; mmHg millimeters of mercury; cm centimeter; % percent; p p-value for general statistical tests
a median (interquartile range)
b mean ± standard deviation
c Mann-Whitney test
d Independent samples t-test
Significant differences were observed between groups regarding the measurements of the native pulmonary valve annulus, both in centimeters (p < 0.001) and Z-score (p < 0.001), with higher values in the PV-SR group.
Regarding intraoperative and postoperative data, cardiopulmonary bypass (CPB) and aortic cross-clamp (ACC) times were significantly shorter in the PV-SR group (p < 0.001 and p = 0.002, respectively). The rate of complications within the first 30 days post-surgery differed significantly between the groups with most patients undergoing the PV-SR technique experiencing no complications (n = 71; 83.5%).
Residual pulmonary regurgitation was observed in the majority of patients in our cohort (n = 65; 59.1%), showing significant differences between groups. Although the absolute number of patients with residual pulmonary regurgitation was higher in the PV-SR group (n = 44, representing 53.0% of this group), 77.8% of all patients who underwent alternative techniques (no PV-SR) experienced residual pulmonary regurgitation.
Invasive ventilation duration, ICU stay, and overall hospitalization times were significantly shorter in the PV-SR group.
These findings are detailed in Table 3.
Table 3.
Intraoperative details and postoperative outcomes
| Total | PV-SR | p | ||
|---|---|---|---|---|
| No | Yes | |||
| (n = 115) | (n = 30) | (n = 85) | ||
| Intraoperative data | ||||
| CPB time (min)a | 71.00 (56.00 to 90.00) | 93.50 (81.50 to 105.25) | 62.00 (54.00 to 81.50) | < 0.001 c |
| ACC time (min)a | 41.00 (30.00 to 52.25) | 48.50 (38.00 to 74.25) | 37.00 (30.00 to 49.00) | 0.002 c |
| Complicationsb | ||||
| Yes | 28 (24.3%) | 14(46.7%) | 14(16.5%) | 0.002 d |
| No | 87 (75.7%) | 16(53.3%) | 71(83.5%) | |
| Postoperative echocardiography | ||||
| Residual Maximal instantaneous RVOT gradient (mmHg)a | 22.00 (15.00 to 28.00) | 22.00 (14.10 to 30.00) | 20.00 (15.00 to 28.00) | .069c |
| Residual Pulmonary Regurgitationb, f | ||||
| No | 45 (40.9%) | 6(22.2%) | 39(47.0%) | 0.026 d |
| Yes | 65 (59.1%) | 21(77.8%) | 44(53.0%) | |
| Degree of Residual pulmonary Regurgitationb | ||||
| Minimal | 23 (35.4%) | 3(14.3%) | 20(45.5%) | < 0.001 e |
| Mild | 30 (46.2%) | 8(38.1%) | 22(50.0%) | |
| Moderate | 12 (18.5%) | 10(47.6%) | 2(4.5%) | |
| Invasive ventilation time (hours)a | 16.00 (8.00 to 24.00) | 24.00 (16.00 to 102.00) | 16.00 (8.00 to 24.00) | 0.005 c |
| ICU time (days)a | 3.00 (2.00 to 4.00) | 3.50 (3.00 to 8.75) | 2.00 (2.00 to 3.00) | < 0.001 c |
| Hospitalization time (days)a | 6.00 (5.00 to 9.00) | 9.00 (6.00 to 23.00) | 6.00 (5.00 to 7.00) | < 0.001 c |
Abbreviations: CPB cardiopulmonary bypass; ACC Aortic Cross-Cl min minutes; RVOTO Right ventricular outflow tract obstruction; p p-value for general statistical tests
a median (interquartile range)
b count (%)
c Mann-Whitney test
d Pearson’s chi-square test
e Fisher’s exact Test
f Missing data: Residual pulmonary regurgitation missing in 5 patients
All variables with a significance level below 5% and no multicollinearity were selected. Through univariate binary logistic regression (Table 4), multiple parameters were identified as being associated with the PV-SR technique: TOF gravity, BMI, pulmonary valve annulus (measured in cm and Z-score), complications, residual pulmonary regurgitation and degrees, invasive ventilation time, ICU stay duration, and overall hospitalization time. OR with 95% CI were calculated for the variables that demonstrated significant associations and differences between the groups in the univariate analysis.
Table 4.
Univariate regression analysis
| OR | (95% CI) | p | |
|---|---|---|---|
| Country of origin | 1.099 | (0.979–1.233) | 0.109 |
| Age at surgical repair (days) | 1.000 | (1.000-1.001) | 0.296 |
| BMI (Kg/m2) | 0.812 | (0.672–0.982) | 0.032 |
| Preoperative echocardiography | |||
| Pulmonary valve annulus (cm) | 54.406 | (5.542-534.064) | 0.001 |
| Pulmonary valve annulus (Z-score) | 1.929 | (1.348–2.758) | < 0.001 |
| TOF Gravity | |||
| Mild | 5.667 | (1.762–18.223) | 0.004 |
| Moderate | 3.00 | (0.894–10.063) | 0.075 |
| Severe | 0.176 | (0.005–0.567) | 0.004 |
| CPB time (min) | 0.955 | (0.933–0.977) | < 0.001 |
| ACC time (min) | 0.956 | (0.932–0.98) | 0.001 |
| Complications | |||
| No | 4.437 | (1.772–11.114) | 0.001 |
| Yes | 0.225 | (0.090–0.564) | 0.001 |
| Residual Pulmonary Regurgitation | |||
| No | 3.102 | (1.136–8.471 | 0.027 |
| Yes | 0.322 | (0.118–0.880) | 0.027 |
| Degree of Residual pulmonary Regurgitation | |||
| Minimal | 50.000 | (6.112-409.055) | < 0.001 |
| Mild | 13.750 | (2.461–76.816) | 0.003 |
| Moderate | 0.02 | (0.002–0.164) | < 0.001 |
| Invasive Ventilation time (hours) | 0.981 | (0.968–0.995) | 0.008 |
| ICU time (days) | 0.714 | (0.577–0.883) | 0.002 |
| Hospitalization time (days) | 0.915 | (0.861–0.971) | 0.004 |
Abbreviations: OR Odds Ratio; 95%CI 95% confidence interval; BMI Body Mass Index; Kg Kilograms; m2 meters quarter; cm centimeter; min minutes; ICU Intensive care unit; p p-value for general statistical tests
Given the significant associations observed in the univariate analysis, we developed a multivariate logistic regression model for the PV-SR technique (Table 5). Only clinically relevant and independent variables were included in this model. Intraoperative and postoperative variables, although associated, were considered consequences of adopting the PV-SR technique and thus excluded as predictors.
Table 5.
Multivariate regression analysis
| OR | (95% CI) | p | |
|---|---|---|---|
| BMI (Kg/m2) | 0.711 | (0.533–0.949) | 0.021 |
| Preoperative echocardiography | |||
| Pulmonary valve annulus (cm) | 28.653 | (2.360-347.890) | 0.008 |
| Pulmonary valve annulus (Z-score) | 1.606 | (1.067–2.418) | 0.023 |
Abbreviations: OR Odds Ratio; 95%CI 95% confidence interval; BMI Body Mass Index; Kg Kilograms; m2 meters quarter; cm centimeter; p p-value for general statistical tests
The multivariate analysis identified BMI and pulmonary valve annulus measurement (both in centimeters and Z-score), as the only significant independent predictors of the PV-SR technique.
The model was statistically significant [χ²=30.148; df = 3; p < 0.001; -2 Log Likelihood = 71.689; Nagelkerke R²=0.410]. The Hosmer-Lemeshow test ([χ²=8.889; p = 0.352]) confirmed a good fit of the model to the data.
The results showed that for each unit decrease in BMI, the likelihood of undergoing the PV-SR technique increased by 1.41 times (OR = 0.711; p = 0.021; 95%CI = 0.533–0.949). For each unit increase in the pulmonary valve annulus in centimeters, the likelihood of undergoing the PV-SR technique increased by 28.65 times (OR = 28.653; p = 0.008; 95%CI = 2.360-347.890). In line with these findings, an increment of one unit in the pulmonary valve annulus Z-score, increased the probability of performing the PV-SR by 1.6 times (OR = 1.606; p = 0.023; 95% CI = 1.067–2.418).
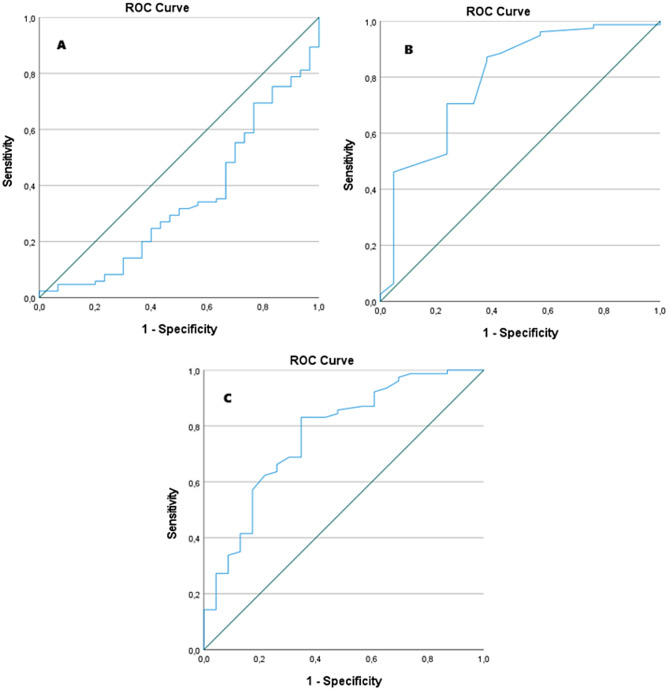
To further explore the clinical utility of these predictors, receiver operating characteristic (ROC) curve analyses were performed to determine optimal cutoff values for each continuous variable.
The ROC curve for BMI yielded an area under the curve (AUC) of 0.348 (95% CI:0.230–0.463; p = 0.014), indicating a statistically significant but inverse association with PV-SR. The optimal cutoff was 13.66 kg/m2, with a sensitivity of 35.3% and specificity of 66.7% (Fig. 1A).
Fig. 1.
The ROC Curves for the three continuous predictors of successful pulmonary valve-sparing repair. A- ROC Curve for BMI; B- ROC Curve for pulmonary valve annulus diameter in centimeters; C- ROC Curve for pulmonary valve annulus Z-Score
For the pulmonary valve annulus in centimeters, the ROC curve identified an optimal cutoff of 0.855 cm, with a sensitivity of 87.2% and specificity of 61.9% (AUC = 0.793; 95% CI: 0.677–0.909; p < 0.001) (Fig. 1B).
And, for pulmonary valve annulus Z-score, the optimal cutoff was– 2.65, with an AUC of 0.769 (95% CI:0.655–0.883;p < 0.001), sensitivity of 83.1%, and specificity of 65.2%. (Fig. 1C).
Additional visual comparisons between groups are provided in Supplemental Figs. 1–11 S.
Discussion
Due to pediatric cardiac surgery programs implemented in developed countries and the protocols established by non-governmental organizations (NGOs), only a small proportion of children diagnosed with TOF in LMICs have access to corrective surgical interventions [10]. However, delayed surgical correction poses technical challenges, particularly concerning the preservation of the native pulmonary valve, a technique associated with a reduced chronic pulmonary regurgitation and improved long-term outcomes [1, 5].
In this study, we investigated predictors for the PV-SR in 115 pediatric humanitarian patients undergoing complete TOF repair, with a median age of 1444 (IQR: 942.00 to 2337.00) days. Among these, 85 patients (74%) had their native pulmonary valve annulus preserved during the repair of RVOTO, demonstrating the feasibility of PV-SR even in delayed repair settings. Comparative analysis between the two groups was followed by a multivariate logistic regression to identify independent predictors for the PV-SR technique.
Through multivariate analysis, BMI and pulmonary valve annulus size (measured in centimeters and Z-score) emerged as significant predictors. For each unit decrease in BMI, the likelihood of performing PV-SR increased by 1.41 times (OR = 0.711; p = 0.021; 95%CI = 0.533–0.949). Similarly, a one-unit increase in the pulmonary valve annulus size (in centimeters) increased the likelihood by 28.65 times (OR = 28.653; p = 0.008; 95%CI = 2.360-347.890), while an increment in Z-score raised the probability by 1.6 times (OR = 1.606; p = 0.023; 95%CI = 1.067–2.418).
This finding aligns with existing literature, which identifies the Z-score as a crucial criterion for surgical decision-making [11].
Previous studies suggest that a Z-score of -2 or higher is often used as threshold for attempting pulmonary valve preservation [5, 12]. Additionally, other authors report a Z-score of pulmonary valve annulus between − 3 and − 4 a technical limit for performing PV-SR [4, 13]. In our study, the median Z-score in the PV-SR group was − 1.64 (IQR: -2.61 to -0.37), consistent with findings from other studies involving delayed TOF correction [14].
Pulmonary annulus hypoplasia emerged as an independent factor predicting PV-SR success, highlighting the importance of detailed preoperative anatomical assessments [11]. While the Z-score contextualizes this measurement by accounting for body surface area, the absolute size in centimeters offers additional clarity, particularly in older or larger patients where indexed values might be less intuitive for intraoperative planning.
BMI also emerged as an independent predictor, inversely correlated with the likelihood of PV-SR. This finding aligns with evidence suggesting that PV-SR are more feasible in patients with an adequate pulmonary annulus diameter relative to their BMI [15]. In patients with higher BMI, the annulus may be proportionally smaller, posing technical challenges to valve preservation. Some authors have shown that childhood obesity is associated with alterations in cardiac geometry, which may impact the proportionality between valve annulus size and body size [16]. This reinforces the importance of considering BMI as a relevant preoperative factor when evaluating suitability for PV-SR.
While other factors, such as CPB time and residual pulmonary regurgitation, demonstrated significant associations in univariate analysis, they were not retained as independent predictors in the multivariate model. Their significance likely reflects the downstream effects of the surgical technique itself, rather than inherent determinants of its feasibility. This interpretation aligns with previous studies reporting increased surgical complexity in cases where PV-SR is not feasible, as evidenced by significantly longer CPB times in patients undergoing alternative techniques, such as transannular patching or RV-PA conduits [17, 18]. The longer CPB times observed in these cases likely reflect the additional technical demands associated with these less conservative approaches [18].
Pulmonary regurgitation, a common sequela following RVOTO relief, poses significant long-term risks, including right ventricular remodeling, progressive dysfunction, and fatal arrhythmias [19]. Notably, up to 40% of TOF patients require reoperation for pulmonary valve replacement within 30 years [14]. In this context, our findings highlight the importance of PV-SR in mitigating these adverse effects, particularly in vulnerable populations with limited access to follow-up care and reoperations.
Additionally, our univariate analysis demonstrated significant associations between PV-SR and postoperative outcomes, including shorter invasive ventilation time, ICU time, and overall hospitalization duration. This result aligns with studies demonstrating the association between preserved pulmonary function and reduced ventilation time, ICU stay, and hospital length of stay, underscoring both clinical and economic benefits [17, 20].
These findings collectively emphasize the importance of preoperative planning in optimizing surgical outcomes. Accurate echocardiographic assessments, incorporating both Z-score and absolute dimensions of the pulmonary valve annulus, are essential for identifying candidates most likely to benefit from PV-SR. Furthermore, refining patient selection criteria, particularly with respect to BMI and pulmonary valve annulus dimensions, could reduce perioperative complications and optimize resources, particularly in humanitarian settings. Training surgical teams in PV-SR techniques may potentially decrease reoperation rates and improve long-term outcomes.
While the predictors identified in this study– BMI, absolute pulmonary valve annulus size, and Z-score– have been previously described, there is scarce literature exploring their applicability in humanitarian pediatric populations. To the best of our knowledge, this is among the first studies to confirm their relevance in a setting characterized by delayed diagnosis and absence of preoperative palliation. This underscores the clinical utility of these factors even in resource-limited environments and may help guide surgical planning in humanitarian missions and global cardiac programs.
To support the clinical utility of these predictors, ROC curve analyses were performed. The pulmonary valve annulus Z-score showed an optimal cutoff of– 2.65, with a sensitivity of 83.1% and specificity of 65.2%. We know that previous studies have proposed similar thresholds [11, 21]. However, our cutoff was derived from a humanitarian pediatric population with delayed presentation, enhancing its relevance in resource-limited settings.
The absolute annulus diameter in centimeters also demonstrated strong discriminative performance, with an optimal cutoff of 0.855 cm (sensitivity 87.2%, specificity 61.9%). Although both variables are correlated, they provide complementary perspectives: the Z-score offers a standardized measure adjusted for body size, while the absolute value may be more directly actionable in intraoperative settings.
In contrast, BMI, although statistically associated with PV-SR, demonstrated limited standalone predictive power in the ROC analysis (AUC = 0.348). This suggests that BMI contributes more meaningfully within a multivariable model than as a single clinical criterion.
While this study provides valuable insights, several limitations must be acknowledged. First, the retrospective nature of the analysis limited access to some echocardiographic data and introduced selection bias. The decision to pursue PV-SR was made intraoperatively, based on real-time anatomical assessment, rather than predefined preoperative criteria. Although this reflects real-word surgical practice, it limits the standardization of patient selection. Nonetheless, the comparative approach between PV-SR and non-PV-SR patients remains essential to identify meaningful preoperative predictors. Without contrast between these two groups, it would not be possible to statistically determine which patient characteristics are associated with successful valve preservation. Additionally, due to the humanitarian nature of the cohort, fallow-up data on long-term outcomes such as reintervention or progression of pulmonary regurgitation were not available for the majority of patients. This represents an important limitation and underscores the need for prospective studies capable of capturing these data over time. The single-center design also limits the generalizability of our findings. It is important to acknowledge that patients who had undergone prior palliation were excluded from this analysis. The decision was made to maintain a more homogeneous cohort and avoid confounding effects related to anatomical and physiological alterations induced by staged interventions. However, this exclusion may limit the generalizability of our findings, particularly in clinical settings where palliation remains part of standard care. Future studies that include both palliated and non-palliated patients are necessary to validate the applicability of these predictors across a broader TOF population. Multicenter studies with larger sample sizes and structured follow-up are needed to validate these results and expand our understanding of factors associated with PV-SR in pediatric humanitarian patients with TOF.
Comparative analyses between populations from low-and high-income countries could identify specific intervention areas to improve outcomes in resource-limited settings. Programs enabling echocardiographic screening and technical training in humanitarian contexts are essential for ensuring more equitable and effective practices [22].
Conclusions
This study identified three key predictors for successful PV-SR in pediatric TOF patients from humanitarian contexts: BMI, pulmonary valve annulus size (in centimeters), and its Z-score. These findings underscore the importance of detailed preoperative assessments and provide a framework for clinical and policy strategies that promote more equitable and effective surgical practices, even in challenging settings. Moreover, these findings may assist in defining objective criteria for PV-SR eligibility, optimizing resource allocation, and improving surgical planning in vulnerable populations. By refining patient selection and enhancing surgical training in PV-SR techniques, it may be possible to reduce perioperative complications and long-term reoperation rates, ultimately improving patient outcomes. These findings, derived from a uniquely vulnerable population, may assist in refining patient selection and optimizing surgical strategies within humanitarian and global health initiatives.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
VM contributed to the conception and design of the study, analyzed and interpreted the data, and was a major contributor to the writing of the manuscript. VM and AM contributed to data acquisition and manuscript revision. TP contributed to the data analysis, interpretation, and manuscript revision. JJ, TN, AA, and TS reviewed the manuscript and provided critical revisions. All authors contributed to the final version of the manuscript, approved its content, and agreed to be accountable for all aspects of the work.
Funding
Open access funding provided by University of Geneva
Not applicable
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Domnina YA, Kerstein J, Johnson J, Sharma MS, Kazmerski TM, Chrysostomou C, et al. Tetralogy of fallot. Critical care of children with heart disease. Cham: Springer International Publishing; 2020. pp. 191–7. [Google Scholar]
- 2.Geva T, Ayres NA, Pac FA, Pignatelli R. Quantitative morphometric analysis of progressive infundibular obstruction in tetralogy of fallot. Circulation. 1995;92(4):886–92. [DOI] [PubMed] [Google Scholar]
- 3.Apitz C, Webb GD, Redington AN. Tetralogy of fallot. Lancet. 2009;374(9699):1462–71. [DOI] [PubMed] [Google Scholar]
- 4.Martins IF, Doles IC, Bravo-Valenzuela NJM, dos Santos AOR, Varella MSP. When is the best time for corrective surgery in patients with tetralogy of fallot between 0 and 12 months of age?? Braz J Cardiovasc Surg. 2018;33(5). [DOI] [PMC free article] [PubMed]
- 5.Bacha E. Valve-Sparing options in tetralogy of fallot surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15(1):24–6. [DOI] [PubMed] [Google Scholar]
- 6.Boni L, García E, Galletti L, Pérez A, Herrera D, Ramos V, et al. Current strategies in tetralogy of fallot repair: pulmonary valve sparing and evolution of right ventricle/left ventricle pressures ratio☆. Eur J Cardiothorac Surg. 2009;35(5):885–90. [DOI] [PubMed] [Google Scholar]
- 7.Kuruvilla S, Balakrishnan K, Parvathy U. Right ventricular myocardium in Fallot’s tetralogy: a light microscopic, morphometric and ultrastructural study. Images Paediatr Cardiol. 2004;6(4):1–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Ross F, Latham G, Joffe D, Richards M, Geiduschek J, Eisses M, et al. Preoperative malnutrition is associated with increased mortality and adverse outcomes after paediatric cardiac surgery. Cardiol Young. 2017;27(9):1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association Declaration of Helsinki. JAMA. 2013;310(20):2191. [DOI] [PubMed] [Google Scholar]
- 10.Dib N, Chauvette V, Diop MS, Bouhout I, Hadid M, Vô C, et al. Tetralogy of fallot in Low- and Middle-Income countries. CJC Pediatr Congenital Heart Disease. 2024;3(2):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awori MN, Mehta NP, Mitema FO, Kebba N. Optimal use of Z -Scores to preserve the pulmonary valve annulus during repair of tetralogy of fallot. World J Pediatr Congenit Heart Surg. 2018;9(3):285–8. [DOI] [PubMed] [Google Scholar]
- 12.Alifu A, Wang H, Chen R. Technical performance scores associate with early prognosis of tetralogy of fallot repair. Front Pediatr. 2024;12. [DOI] [PMC free article] [PubMed]
- 13.Cohen MI, Khairy P, Zeppenfeld K, Van Hare GF, Lakkireddy DR, Triedman JK. Preventing arrhythmic death in patients with tetralogy of fallot. J Am Coll Cardiol. 2021;77(6):761–71. [DOI] [PubMed] [Google Scholar]
- 14.Heinisch PP, Guarino L, Hutter D, Bartkevics M, Erdoes G, Eberle B et al. Late correction of tetralogy of fallot in children. Swiss Med Wkly. 2019. [DOI] [PubMed]
- 15.Aydın S. The impact of pulmonary valve-sparing techniques on postoperative early and midterm results in tetralogy of fallot repair. Turkish J Thorac Cardiovasc Surg. 2018;26(3):370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marciniak M, van Deutekom AW, Toemen L, Lewandowski AJ, Gaillard R, Young AA, et al. A three-dimensional atlas of child’s cardiac anatomy and the unique morphological alterations associated with obesity. Eur Heart J Cardiovasc Imaging. 2022;23(12):1645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaffner D, Maitre G, Lava SAG, Boegli Y, Dolci M, Pfister R, et al. Outcome of humanitarian patients with late complete repair of tetralogy of fallot: A 13-year long single-center experience. Int J Cardiol Congenital Heart Disease. 2022;10:100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei X, Li T, Ling Y, Chai Z, Cao Z, Chen K, et al. Transannular patch repair of tetralogy of fallot with or without monocusp valve reconstruction: a meta-analysis. BMC Surg. 2022;22(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meca Aguirrezabalaga JA, Silva Guisasola J, Díaz Méndez R, Escalera Veizaga AE, Hernández-Vaquero Panizo D. Pulmonary regurgitation after repaired tetralogy of fallot: surgical versus percutaneous treatment. Ann Transl Med. 2020;8(15):967–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touré T, Roubertie F, Bridier T, Foulgoc H, Thambo JB, Ouattara A, et al. Early post-operative benefits of a pulmonary valve-sparing strategy during fallot repair. Int J Cardiol Congenital Heart Disease. 2022;8:100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha R, Gooty V, Jang S, Dodge-Khatami A, Salazar J. Validity of pulmonary valve Z-Scores in predicting valve-Sparing tetralogy Repairs—Systematic review †. Children. 2019;6(5):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vervoort D, Meuris B, Meyns B, Verbrugghe P. Global cardiac surgery: access to cardiac surgical care around the world. J Thorac Cardiovasc Surg. 2020;159(3):987–e9966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.