Abstract
Plants rely on germline-encoded, innate immune receptors to sense pathogens and initiate the defense response. The exponential increase in quality and quantity of genomes, RNA-seq datasets, and protein structures has underscored the incredible biodiversity of plant immunity. Arabidopsis continues to serve as a valuable model and theoretical foundation of our understanding of wild plant diversity of immune receptors, while expansion of study into agricultural crops has also revealed distinct evolutionary trajectories and challenges. Here, we provide the classical context for study of both intracellular nucleotide-binding, leucine-rich repeat receptors and surface-localized pattern recognition receptors at the levels of DNA sequences, transcriptional regulation, and protein structures. We then examine how recent technology has shaped our understanding of immune receptor evolution and informed our ability to efficiently engineer resistance. We summarize current literature and provide an outlook on how researchers take inspiration from natural diversity in bioengineering efforts for disease resistance from Arabidopsis and other model systems to crops.
Plants are natural bioengineers, and we illustrate how the stunning diversity in plant immune receptors can be translated into engineering efforts from Arabidopsis to crops.
Introduction
Like all organisms, plants can sense the presence of pathogens and induce immune responses. In the absence of adaptive immunity, plant innate immune receptors provide sufficient diversity at a population level to recognize rapidly evolving pathogens, including viruses, bacteria, nematodes, fungi, oomycetes, insects, and parasitic plants (Hamilton et al. 1990; Dangl and Jones 2001; Chisholm et al. 2006). Understanding natural diversity remains essential for uncovering how plants evolve detection of new pathogens and for successful deployment of disease resistance in crops (Barragan and Weigel 2021).
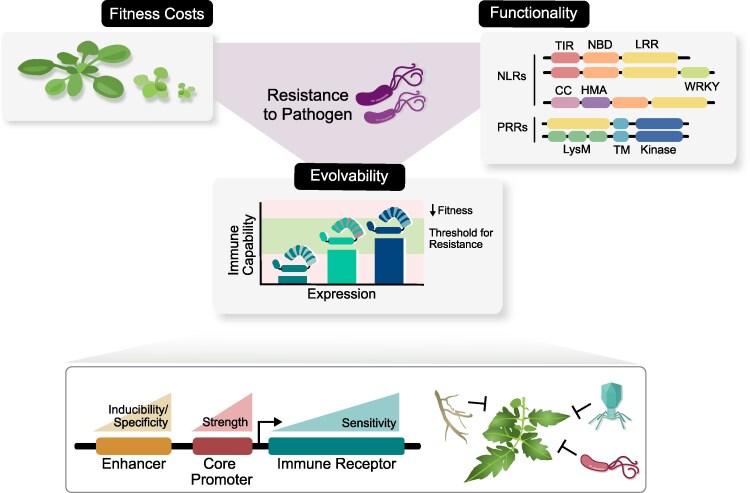
Plant immune receptors come in 2 main forms based on their domain architectures and cellular localization: surface-localized pattern recognition receptors (PRRs) and intracellular nucleotide-binding, leucine-rich repeat receptors (NLRs) (Jones and Dangl 2006; Ngou et al. 2022a). PRRs include receptor-like kinases (RLKs) and receptor-like proteins (RLPs) that bind to host-derived molecules, microbe-derived molecules, or pathogen-derived effector proteins present outside of the plant cell (Shiu and Bleecker 2003). Both RLKs and RLPs recognize diverse ligands with a variety of ectodomains, including leucine-rich repeats (LRRs), lysin motifs, malectin-like domains, and epidermal growth factor-like domains (Boutrot and Zipfel 2017; Albert et al. 2020). Upon ligand binding, a protein complex is formed between primary receptor, ligand, and RLK co-receptor(s), leading to kinase activation and downstream immune activation (Li et al. 2024b).
NLRs are functionally conserved across plants, animals, fungi, and bacteria in that they are able to perceive pathogen-derived effector proteins and induce immune signaling (Jones et al. 2016; Wojciechowski et al. 2022; Kibby et al. 2023). Plant NLRs are defined by their regulatory central NB-ARC (nucleotide-binding adaptor shared by Apaf-1, R proteins, and Ced-4) domain and series of C-terminal LRRs mostly implicated in ligand binding (Baggs et al. 2017). N-terminal signaling domains include coiled-coils (CC), Resistance to Powdery Mildew 8 (RPW8) domains, or Toll-Interleukin receptor (TIR) domains. Upon activation, NLRs form oligomeric structures bringing their signaling domains into activation (Jones et al. 2016; Lolle et al. 2020). Both CC-NLRs and RPW8-NLRs assemble into ion channel pores (Bi et al. 2021; Jacob et al. 2021), while tetrameric TIR-NLRs enable NADase enzymatic activities (Huang et al. 2022; Jia et al. 2022; Yu et al. 2022).
Functionally, NLRs and PRRs could be classified as sensors and helpers. Sensor NLRs can either directly bind pathogen effector proteins translocated into the host, guard host proteins targeted by effectors, or “bait” for the effectors with integrated domains (IDs) (Baggs et al. 2017; Bailey et al. 2018). Helper NLRs aid the activation of immune responses for a subset of sensor NLRs that belong to their regulatory networks or for their specific pairs (Adachi et al. 2019). Similarly, some PRRs act as sensors by binding to a particular epitope or ligand, whereas co-receptors from the SERK (somatic embryogenesis receptor kinase) family as well as SOBIR1 act as helpers, activating subsequent immune signaling (Snoeck et al. 2023). Immune signaling after PRR and NLR activation includes mitogen activated protein kinase signaling, Ca2+ burst, production of reactive oxygen species (ROS), transcriptional reprogramming, and, in case of most NLRs, programmed cell death called hypersensitive response (HR), allowing propagation of responses (Lolle et al. 2020; Jacob et al. 2023; Wang et al. 2023). Pathways activated by PRRs and NLRs work synergistically, with PRRs typically having a broader pathogen recognition range while NLRs have a more narrow, strain-specific but faster evolving response (Tian et al. 2021; Yuan et al. 2021; Ngou et al. 2022a).
Identifying functional PRRs and NLRs, defining their recognition range, and quantifying their diversity across evolutionary scales have led to multiple strategies of rational crop improvement (Jones et al. 2024). In this review, we focus on the “central dogma” as it relates to plant immune receptors and their associated diversity at the DNA, RNA, and protein level. We describe how available data have expanded from single genes to pangenomes and from individual gene expression studies to multi-tissue RNA-seq datasets. We propose that extreme combinatorial amino acid diversity in innate immune receptor repertoires on the plant pan-genome level fits the model of “anticipatory immunity,” in which diversity is rapidly generated on a population level in anticipation of new pathogen challenge, leading to new recognition specificities and selection of functional alleles across generations. Accumulation of expression datasets together with advances in synthetic biology allow for new possibilities in engineering inducible disease resistance. Evolutionary, experimental, and computational structural analyses have facilitated the creation of designer receptors with resurrected, broader binding capabilities, or fully synthetic recognition specificities. With accumulation of training data, the future of incorporating generative machine learning could greatly expedite engineering efforts. Modern technologies have fueled basic research in Arabidopsis thaliana (hereafter Arabidopsis) for over 30 years and now have accelerated translation from model species into diverse crops, providing practical solutions for sustainable agriculture.
Episode 1. Hope for tomorrow: DNA diversity and anticipatory immunity
(By Chandler A. Sutherland and Ksenia V. Krasileva)
“A long time ago, in 119 million nucleotides far, far away…” we start our journey with DNA sequences. Most mutations are deleterious (Eyre-Walker and Keightley 2007; Chen et al. 2022), but immune systems present an evolutionary edge case in which high mutation is beneficial and even required to keep pace with rapidly evolving pathogens (Martincorena and Luscombe 2013; Müller et al. 2018). Across the tree of life, this has led to several radical evolutionary innovations, including emergence of molecular mechanisms of self-mutation and clonal selection on antibodies in vertebrates and CRISPR/Cas-based incorporation of extragenomic DNA in bacteria (Fig. 1) (Müller et al. 2018). The plant immune system is no exception in the need for rapid evolution. The stunning genetic diversity of plant immune receptors has long been appreciated and inspired multiple hypotheses and questions about its generation and maintenance.
Figure 1.
Immune receptors generate and maintain high diversity to manage diverse pathogenic threats. Across the tree of life, the pressure for diversity in immune receptors has resulted in multiple mechanisms for its generation including CRISPR/Cas-based systems in bacteria, adaptive immunity in vertebrates, and anticipatory immunity in invertebrates, plants, and fungi. Innate immune receptors include NLRs, PRRs, and toll-like receptors (TLRs). Within or across species, receptor number (copy number variation [CNV]) and allelic variation (single nucleotide polymorphisms and insertions and deletions) can be measured using genomic sequencing. Long-read sequencing and de novo reference assembly enables capture of diverse immune receptor content that was previously missed in reference-based genome assemblies using short-read sequencing. Anchor genes act as conserved, syntenic loci or blocks across related genomes that aid to compare variable gene content.
Mystery is irresistible: early clues toward immune receptor diversity generation
The molecular study of NLRs and PRRs began with single gene cloning and diversity analysis in Arabidopsis (Bent et al. 1994; Grant et al. 1995; Gómez-Gómez and Boller 2000; Zipfel et al. 2006), tobacco (Whitham et al. 1994), and crops (Martin et al. 1993; Jones et al. 1994; Song et al. 1995; Meyers et al. 1998; Dodds et al. 2001). These studies established systems of immune receptors and their pathogen complements and provided molecular evidence for the gene-for-gene theory, where each functional plant immune gene is paired with a corresponding gene in the pathogen (Flor 1971). In this framework, the presence and activity of both gene partners is required for immunity. Even in this early work, the importance of population-level polymorphism in immune receptors to mediate coevolution of plants and their pathogens was recognized (Mode 1958; Flor 1971; Dodds 2023).
Genetic maps based on molecular markers revealed that many NLRs and PRRs in Arabidopsis (Kunkel 1996; Botella et al. 1997) and crops (Farrara et al. 1987; Islam and Shepherd 1991; Jones et al. 1993; Kesseli et al. 1994) are organized in genomic clusters. This clustered organization was hypothesized to increase the likelihood of tandem duplication, unequal crossing over, and gene conversion, driving structural and copy number variations (Parker et al. 1997; Parniske et al. 1997; Dixon et al. 1998; Michelmore and Meyers 1998; Thomas et al. 1998; Fritz-Laylin et al. 2005). However, clustered status is not a requirement for diversity, with singleton Arabidopsis NLR RPP13 maintaining extremely high amino acid diversity (Bittner-Eddy et al. 2000; Rose et al. 2004). Generally high amino acid diversity of the LRR domain of both NLRs and PRRs was highlighted and hypothesized to be maintained by diversifying and/or balancing selection (Hamilton et al. 1990; Parniske et al. 1997; Michelmore and Meyers 1998).
The first plant genome sequenced was Arabidopsis, allowing for genome-wide quantification of NLR and PRR copy numbers, types, and organization (The Arabidopsis Genome Initiative 2000; Dangl and Jones 2001; Shiu and Bleecker 2001). Characterization of NLRs and PRRs within and between species revealed high nucleotide diversity in the LRR region primarily driven by nonsynonymous mutations (Nordborg et al. 2005; Bakker et al. 2006; Li et al. 2024b). The NLR gene family in Arabidopsis includes the most polymorphic loci and contains the highest frequency of major effect mutations across the genome (Clark et al. 2007; Gan et al. 2011). For many immune-associated PRRs, diversity analysis has lagged behind genome generation due in part to the lack of known targets, making it difficult to functionally group receptors across species (Shiu and Bleecker 2001; Li et al. 2024b). However, mutational frequency is higher in PRRs associated with immunity than those involved in development (Ngou et al. 2024). It was proposed that the high copy number and clustered organization of immune receptors allowed for maintenance of functional recognition despite accumulation of the observed high polymorphism (Fig. 1) (Dangl and Jones 2001).
Non-model and crop reference genomes have become increasingly available with over 4,000 reference plant genomes currently deposited, allowing for interspecies quantification and comparison of PRR and NLRs across plants (Gao et al. 2018; Baggs et al. 2020; Man et al. 2020; Ngou et al. 2022b, 2024). The interspecies perspective on the evolution of immune receptors has shown lineage-specific expansions and contractions potentially mediated by domestication, pathogen pressure, and genome organization (Baggs et al. 2020; Giolai and Laine 2024; Gladieux et al. 2024). The expansion of genomic resources will facilitate identification of receptors with novel functional domains, likely leading to the innovation of new approaches for plant disease resistance.
Change is the essential process of all existence: pangenomic dimensions of diversity
The clustered organization, high incidence of structural variation, and polymorphic nature of immune receptors make them challenging to anchor to reference genomes (Fig. 1) (Jiao and Schneeberger 2020; Barragan and Weigel 2021). To overcome this, Resistance Gene Enrichment Sequencing (RenSeq) uses a combination of hybrid probe-based enrichment of NLR loci and long read sequencing to allow for examination of NLR diversity across and within species (Jupe et al. 2013). Application of RenSeq across 64 Arabidopsis accessions enabled a comprehensive, intraspecies view of NLR diversity (Van de Weyer et al. 2019). The portability of this technique has quickly translated to crop species and their wild relatives, enabling rapid cloning of resistance genes (Witek et al. 2016; Arora et al. 2019; Vendelbo et al. 2022), investigation of selective pressures related to domestication (Seong et al. 2020; Gladieux et al. 2024), and study of the relationship between ecological pressures and NLR gene content (Keepers et al. 2024).
If RenSeq unlocked the door to comprehensive immune receptor diversity characterization, recent long-read, de novo assembled pan-genomes have thrown it wide open. Long read pan-genomes of Arabidopsis now cover 167 distinct accessions (Jiao and Schneeberger 2020; Kang et al. 2023; Wlodzimierz et al. 2023; Lian et al. 2024; Teasdale et al. 2024), and pan-genome scale long-read assemblies are now available for many crops, including maize (Hufford et al. 2021) and rice (Shang et al. 2022), and their availability has recently been reviewed (Pucker et al. 2022; Shi et al. 2023). Long-read genomes provide the opportunity to investigate intraspecies PRR evolution, an opportunity unavailable with RenSeq data. Researchers are no longer limited in the availability and accuracy of sequences of plant immune receptors but in the ability to meaningfully analyze and draw conclusions from them in the pangenomic dimension (Barragan and Weigel 2021).
Phylogenetic grouping of pangenome sequences of PRRs and NLRs has shown high diversity at the solvent-exposed residues of the LRR domains, confirming single gene studies on a genomic level (Man et al. 2020; Prigozhin and Krasileva 2021; Trinh et al. 2023; Li et al. 2024b). Quantification of amino acid diversity by Shannon entropy in the model species of Arabidopsis, Brachypodium (Prigozhin and Krasileva 2021), and maize (Prigozhin et al. 2025) reveal in each a subset of NLRs evolving quite rapidly. Pangenomic, gene-family level analysis of NLRs has shown evidence of balancing and directional selection in rice landraces (Gladieux et al. 2024) and positive, diversifying selection in Arabidopsis driving NLR diversity (Van de Weyer et al. 2019; Sutherland et al. 2024). In Arabidopsis, this high diversity is associated with distinct genomic features and a predicted higher likelihood of mutation (Sutherland et al. 2024). Use of graph-based methods to define the genomic regions surrounding NLRs in 17 Arabidopsis accessions allowed for a holistic view of NLR evolution, reaffirming previous emphasis on local copy number variation, association of variable NLRs with transposable elements (TEs), and high polymorphism (Teasdale et al. 2024). Application of these tools developed in Arabidopsis to other crop species will reveal the extent to which these observations are conserved.
While mechanistic questions remain, long-read, pangenome approaches have supported and unified many early hypotheses on the maintenance and generation of immune receptor diversity. In Arabidopsis, a “diversity in diversification” explanation helps to combine the contributions of large structural variations, fusions, deletions, gene conversion, point mutation, and TE insertions (Teasdale et al. 2024). Understanding the mechanisms of diversity generation, the sequence space of receptors, and the associated genomic contexts will allow us to boost the efficiency and success rate of receptor engineering, inspired by plants' natural genome engineering efforts.
Your focus determines your reality: exploiting de novo mutation events for the study of immune receptor evolution
Plant immune receptor diversity creates population-level resistance against pathogens, with variation in recognition between receptors preventing ubiquitous colonization by single pathogen strains (Hamilton et al. 1990; Dangl and Jones 2001; Bakker et al. 2006; Teasdale et al. 2024). We propose that plant immune systems, placed in the context of innate immune system evolution across the tree of life, follow a pattern of anticipatory immunity (Müller et al. 2018), where immune receptors are both more likely to mutate and are selected for high diversity. Mutation here is used in the broadest sense, encompassing structural variation and sequence polymorphism, reflecting all possible outcomes of DNA damage and repair. This immune system is anticipatory as opposed to adaptive because it includes higher mutational likelihood at immune receptors in anticipation of pathogen attack with classical Darwinian, population-scale selection as opposed to clonal, individual-scale selection.
High diversity generation specific to innate immune genes in anticipation of pathogens has been described in several invertebrates (Ghosh et al. 2011; Müller et al. 2018; Buckley and Dooley 2022) but has not been explicitly extended to plants. While the receptors are distinct in function and protein domain composition, the mechanisms of diversity generation and selective pressures are similar. For example, the SpTransformer (SpTrf) immune effector genes in sea urchins show extensive structural and single nucleotide sequence diversity (Buckley and Smith 2007; Oren et al. 2016a, 2019) and are often in clustered, repetitive gene families that have been compared with NLR clusters (Oren et al. 2016b). These repeat rich regions create genomic instability that promotes unequal crossovers, gene conversion, duplications and deletions, and mispairings that drive SpTrf diversity (Oren et al. 2016a; Barela Hudgell et al. 2024). There is evidence of both somatic recombination and mutation of the SpTrf family (Buckley et al. 2008; Oren et al. 2019) independent of exposure to immune elicitors, consistent with continuous diversity generation in anticipation of pathogen pressure. Though questions around targeting and mechanism remain specifically regarding somatic single nucleotide polymorphisms, we propose that the described “diversity in diversification” in plants fits into an anticipatory innate immunity model where diversity is generated through elevated mutation in selected immune receptors on a population level in anticipation of emergence of new pathogen strains.
An opportunity to test this hypothesis is highly accurate quantification of low frequency, de novo mutational events, as new mutations have minimal opportunity for selection to act. This allows for analyses of the underlying mutation distribution. Mutations are rare events on the population scale but occur constantly throughout plant development and growth. Previous applications of somatic mutation sequencing in Arabidopsis were used to compare mutations between tissues and characterize the mutational rate and spectrum of DNA repair knockouts (Wang et al. 2019c; Wu et al. 2020; Quiroz et al. 2024). However, improvements in ultra-accurate sequencing technology have decreased the variant calling error rate such that de novo mutations can be confidently quantified to the nanoscale (Abascal et al. 2021; Bae et al. 2023). Application of this technique to animal tissue has revealed several distinct signatures and rates of mutation associated with tissue types and age (Abascal et al. 2021; Moore et al. 2021; Cagan et al. 2022), but it has yet to be applied to plant nuclear DNA or broadly to innate immune gene evolution. Ultra-accurate mutation sequencing can be applied to both somatic and gametophytic tissues, with an opportunity to use pollen as an accessible representative of germline mutations. Structural variation can additionally be quantified de novo, posing the opportunity to observe the birth and death of immune receptors by copy number variation (Liu et al. 2024b). Arabidopsis is a natural starting point for adaptation of this technology to plants due to its small genome size and corresponding low cost per mutation, but future applications to crops will provide comparative insight. These improvements in sequencing technology will allow for direct quantification of mutation in immune receptors and help identify the mechanisms driving this diversity.
Episode 2. Temptation of response: expression variation and its regulation
(By Wei Wei and Chandler A. Sutherland)
Constitutively activated defense responses can cause severe cell damage and reduce plant growth, making precise regulation of immune receptor expression critical for balancing immunity and growth. While lineage-specific patterns in expression are observable even within species, common pathways for evolution of regulation can be surmised from studies across model plants and crops. Editing transcriptional regulation provides a promising opportunity for engineering broad-spectrum resistance.
Logic is the beginning of wisdom: immune receptor expression is induced by stimuli and can be tightly regulated
The first observed expression pattern for NLRs was induction in response to pathogens or other stress stimuli. In Arabidopsis, 74 out of 124 NLRs surveyed using microarray exhibited significant inductions by at least 1 pathogen, pathogen-related defense elicitors, or hormone treatment (Mohr et al. 2010). The 74 NLR genes varied in the types of pathogens or treatments they responded to, suggesting diverse regulatory pathways mediating NLR induction. Similarly, characterization of the NLR gene family in tomatoes revealed that 15 and 74 NLRs out of 321 were differentially expressed in early and late blight-infected leaves, respectively (Bashir et al. 2022). NLRs in monocot crop species such as maize and rice also exhibited expression changes induced by biotic and abiotic stresses (Ding et al. 2020; Hayford et al. 2024). When responding to pathogens, some NLRs showed differential expression between resistant and susceptible genotypes, pointing to the relevance of expression regulation to immunity (Bashir et al. 2022; Fick et al. 2022a). Pathogen perception triggered by specific NLRs can induce global NLR expression, suggesting a potential positive feedback loop to boost immunity (Mohr et al. 2010; Jacob et al. 2018). In both NLR-based hybrid incompatibilities (Bomblies et al. 2007; Atanasov et al. 2018; Barragan et al. 2021) and autoactive immune mutants (Yang et al. 2020, 2021), a large proportion of noncausal NLRs are induced.
Gene expression is in part regulated by the cis-regulatory elements located in promoters. A motif search of all Arabidopsis NLR promoters revealed enrichment of W-boxes, the binding site for WRKY transcription factors implicated in defense responses (Mohr et al. 2010). Mutagenesis of 3 W-boxes in the RPP8 promoter greatly diminished its inducibility and resistance to pathogens, indicating their importance in regulating NLR expression (Mohr et al. 2010). NLR gene expression regulation has also been widely reported on the pre-transcription level (histone modification and DNA methylation) and post-transcription level (alternative splicing, premature transcription termination and small RNAs), which were recently reviewed (Lai and Eulgem 2018; Fick et al. 2022b).
Most immune associated PRRs appear to be developmentally and spatially regulated, particularly in the roots and shoots. In Arabidopsis, the flagellin receptor FLS2 and elongation factor Tu receptor EFR are expressed in the shoots as early as 3 weeks of age. In roots, only FLS2 was induced in the endodermis of the differentiated root, whereas EFR was not (Beck et al. 2014; Wyrsch et al. 2015; Emonet et al. 2021). In tomato, the flagellin receptor FLS3 and cold shock protein receptor CORE are expressed in foliar tissue as early as 4 and 6 weeks of age, respectively (Clarke et al. 2013; Wang et al. 2016). Conversely in the roots, FLS2, FLS3, and CORE displayed enhanced expression in seedlings during the early, but not late, differentiation state (Leuschen-Kohl et al. 2024). We hypothesize receptor sequences—and, more likely, expression variations—would explain the differential immune response across plant tissues or species previously observed (Clarke et al. 2013; Wei et al. 2018; Trinh et al. 2023).
The force is a balance between extremes: immune receptors exist across axes of regulation
With the increase of RNA-seq datasets across species and tissue types, the precise dynamics of immune receptor expression can be examined at scale. It was recently observed that high NLR expression in the absence of a pathogen is more common than previously recognized in Arabidopsis, tomato, barley, and wild wheat progenitor leaf tissues (Fig. 2) (Brabham et al. 2024b). High steady-state expression of rapidly evolving NLRs has been observed across tissues in Arabidopsis Col-0 (Sutherland et al. 2024), and several NLRs across the pangenome of maize show constitutive expression (Prigozhin et al. 2025). RLK expression in Arabidopsis and tomato has shown constitutive, tissue-specific, and developmentally regulated expression, whereas RLPs in Arabidopsis show constitutive and pathogen-inducible expression (Chuberre et al. 2018; Emonet et al. 2021; Steidele and Stam 2021). Therefore, while some immune receptors may be under tight transcriptional control to prevent autoimmunity or more general reductions in fitness, there are many NLRs and some PRRs that are expressed highly in the absence of pathogens, and this expression is in some cases required for their function as sensors of effectors (Bieri et al. 2004; Brabham et al. 2024b).
Figure 2.
Different selective pressures on immune receptors can lead to diverse expression patterns and inform engineering efforts for resistance to pathogens. Each receptor has a minimal threshold of expression to effectively monitor for the pathogen and an upper threshold of expression associated with fitness costs. Different receptors have diverse modularity that can alter functionality and tolerable range of expression. Precise control of expression by fine-tuning cis-elements in enhancers and core promoters provides a platform for engineering broad-spectrum resistance against pathogenic threats. For functionality, domains are labeled as the following: CC, coiled-coil; NB, nucleotide-binding; LRR, leucine-rich repeat; HMA, heavy-metal-associated; TIR, Toll-Interleukin receptor; TM, transmembrane.
Restricting expression of NLRs to the tissues with the highest pathogen pressure would reduce fitness costs associated with high receptor expression (Contreras et al. 2023; Lüdke et al. 2023). Two examples in tomato support this hypothesis: the I-2 gene provides immunity against vascular wilt and shows vascular tissue-specific expression (Mes et al. 2000), and the NRC6 gene cluster that confers resistance to cyst and root-knot nematodes has root-exclusive expression (Lüdke et al. 2023). Spatial regulation of expression can also be driven by developmental tradeoffs: altering of FLS2-expressing cell types in Arabidopsis caused collapsed meristem cells and inhibited root growth (Emonet et al. 2021). At the gene family level, NLRs in Brassicaceae including Arabidopsis show shoot-skewed NLR expression, while tomato, maize, rice, and several legume species show root-skewed NLR expression (Munch et al. 2018; Lüdke et al. 2023).
Immune receptor expression level is shaped by downward selective pressure driven by fitness costs associated with excessive expression and upward selective pressure of the minimum dosage required for functionality (Fig. 2). Depending on the mode of action and likelihood of autoactivity, individual receptors have different upper and lower bounds of tolerable expression. The NLRs Sr33 and Sr50 are moderately autoactive on the protein level and constitutively high gene expression exacerbates the autoactivity (Tamborski et al. 2023). In this case, pathogen-specific induction of NLRs, in combination with low basal expression, would prevent autoimmunity while maintaining their functionality for defense activation upon pathogen attack. NLRs in sensor-helper pairs are in tight co-evolutionary relationships, as unbalanced expression of the 2 NLRs can spuriously activate the immune system. These NLR pairs are usually found to be co-localized in the genome, with head-to-head orientation, supposedly being co-regulated by the same promoter (Shimizu et al. 2022; Yang 2024). However, multiple transgene copies of the direct recognition NLR Mla7 in barley are required for complementation of a susceptible background (Brabham et al. 2024b), indicating a requirement of high expression for functionality. Analysis of the pan-transcriptome of maize showed orthologous NLRs with high variation in expression: RppM and RppC-like clade members showed silent, tissue specific, or constitutive expression across lines (Prigozhin et al. 2025). Understanding the relationship between expression, mechanism, and functionality of NLRs will greatly assist engineering efforts and successful implementation in crops (Fig. 2).
Two theories have emerged for the relationship between expression and evolvability of immune receptors. For an immune receptor under diversifying selection to evolve new recognition specificity, it can either be lowly expressed while accumulating mutations and then be de-repressed to higher expression once a functional allele evolves (Shivaprasad et al. 2012; Zhang et al. 2016; Brabham et al. 2024b), or it can begin constitutively expressed and evolve regulation after functionality (Prigozhin and Krasileva 2021; Sutherland et al. 2024). Low expression of a rapidly evolving receptor would allow for a higher tolerance of mutations with slightly deleterious effects until functionality is achieved, as supported by the presence of microRNA families capable of silencing multiple NLRs simultaneously in pathogen-free tissues (Shivaprasad et al. 2012; Zhang et al. 2016) and the enrichment of functional, presumably not deleterious NLRs in constitutive expression states (Brabham et al. 2024b). Alternatively, high expression levels observed across highly variable NLRs (Brabham et al. 2024b; Sutherland et al. 2024) could compensate imperfect recognition or low activity of actively evolving receptors and may increase evolvability through increased likelihood of mutation associated with transcription (Staunton et al. 2023) and strengthened effect of individual mutations on fitness, increasing the efficacy of selection (Bódi et al. 2017; Payne and Wagner 2019). However, it is likely the variation in expression drives evolvability of immune receptors (Capp 2021; Prigozhin et al. 2025), with both theories acting in parallel on different receptors depending on the initial expression state and the strength of selection (Fig. 2). High throughput analysis of the relationship between expression levels and fitness of immune receptors of multiple types will provide the evidence required to resolve this thorny evolutionary trajectory.
NLR abundances are also highly regulated at the post-translational level. NLR proteins can be tagged by ubiquitin molecules, marking them for degradation by proteasome. The turnover of NLR proteins SNC1 (SUPPRESSOR of NPR1-1, CONSTITUTIVE 1) and RPS2 (RESISTANT TO P. SYRINGAE 2) are controlled by the E3 ubiquitin ligase complex SKP1-CULLIN1-F-box SCFCPR1 and loss of function of the CPR1 led to higher accumulation of NLR proteins and autoimmune responses (Cheng et al. 2011; Huang et al. 2016). Several studies in Arabidopsis showed that molecular chaperones play essential roles in stabilizing NLR proteins. Chaperone complex formed by HSP90 (heat shock protein 90kD), SGT1 (Suppressor of G2 allele of skp1), and RAR1 (Required for Mla12 resistance) could associate with NLR proteins such as RPM1, N, and Rx, and were required for proper steady-state NLR levels (Kadota et al. 2010). These chaperones and co-chaperones are conserved across eukaryotic organisms and might serve as a conserved role for maintaining NLR homeostasis in both plants and mammals (Kadota et al. 2010). Although components regulating NLR protein abundance mentioned above have been identified and their functions elucidated, many remain unknown. Moreover, it is still unclear how universally applicable these regulatory mechanisms are across different NLR proteins and organisms.
I am one with the force, and the force is with me: opportunities and challenges in immune receptor expression engineering
Recent advancements in understanding the evolution of spatial and temporal expression can assist in the identification and engineering of functional immune receptors. For identification, constitutive expression can be used as a selection criteria to enrich for functional NLRs genome-wide or in map-based NLR cloning (Kawashima et al. 2016; Brabham et al. 2024b), and tissue specificity can be related to pathogen specificity, narrowing the search space for resistance (Contreras et al. 2023; Lüdke et al. 2023). For example, shoot-specific expression of an engineered defense-related gene in rice largely mitigated the fitness cost of constitutive defense, as it concentrated the immune responses to tissues threatened by their target pathogens (Molla et al. 2016). The development of spatial and single cell RNA-seq will provide high-resolution expression atlases (Zhu et al. 2023) and will help identify the underlying regulatory DNA sequences, bringing immune receptor engineering to unprecedented levels of precision. While the idea of engineering inducible expression of immunity has been explored for over 2 decades, it faced challenges in deployment as most approaches required generation of transgenic plants and many of the first promoters identified had fitness effects (Honee et al. 1998; Stuiver and Custers 2001; Li et al. 2020). With the expansion of expression datasets and advancements in genome editing and synthetic biology, there is renewed interest in deploying inducible expression for engineering broad-spectrum resistance (Wei et al. 2024).
The specificity of interaction between NLRs and effectors makes it challenging to engineer receptors for broad spectrum resistance. As an alternative strategy to protein engineering, several studies have explored control of transcription. For example, autoactive NLRs or elicitors of NLRs can be deployed in plants under pathogen-inducible (PI) promoters (Fig. 2). This strategy has been successful against pathogens harboring transcription activator-like (TAL) effectors, such as Xanthomonas and Ralstonia (Zeng et al. 2015; Gallas et al. 2024). Stacking effector binding elements in the promoter drives the expression of a cell death-inducing NLR gene Xa10 in rice, enabling induction of resistance by multiple different Xanthomonas oryzae strains (Zeng et al. 2015). However, it is challenging to apply these synthetic cassettes against other pathogens because the transcription induction depends on TAL effectors. The tobacco promoter hsr203j shows conserved induction by pathogens of several classes and was harnessed to regulate an HR-inducing elicitor in tobacco, producing broad-spectrum resistance (Keller et al. 1999). Recently, an autoactive mutant of Sr33 and the intrinsically autoactive Sr50 were fused with PI promoters in tomato for conferring broad-spectrum resistance (Wei et al. 2024). Critical to the success of this effort was pairing autoactive NLRs of varying sensitivities with PI promoters optimized for strength and inducibility to balance immunity and fitness costs (Fig. 2).
Using PI promoters to drive HR-inducing components is an actionable strategy for conferring broad-spectrum resistance but remains challenging. Widely adopting a transcriptional engineering strategy in crops requires precise transcriptional regulation specifically responsive to pathogens and not other stressors, particularly abiotic stress. Unintended induction of HR in response to other stimuli than pathogens will impose tremendous fitness costs on crops. Some PI promoters are also developmentally regulated, leading to unintended induction of NLR expression and autoimmunity at specific developmental stages (Honee et al. 1998; Wei et al. 2024). Comprehensive examination of transcriptomic resources across different tissues, developmental stages, and stress responses is necessary to mine for promoters specific to pathogen response. In addition, the advancement of synthetic biology tools has empowered engineering of transcriptional regulation. For example, promoter engineering at different scales could fine tune the expression precisely to balance leaky expression and induced immunity (Jores et al. 2021; Wei et al. 2024). Designing transcription circuits that display both pathogen inducibility and tissue specificity would further reduce the fitness cost of defense (Brophy et al. 2022). Several successful cases of engineering induced resistance also suggest adding post-transcriptional or translational regulations in addition to inducible promoters might be required to minimize undesired expression (Gonzalez et al. 2015; Xu et al. 2017). Manipulation of spatial, temporal, and inducibility of immune receptor expression, aided by characterized promoter libraries, represents an exciting opportunity for the future of plant immune engineering.
Episode 3. The rise of ultimate power: immune receptor engineering
(By Kyungyong Seong and Danielle M. Stevens)
Both genetic and regulatory changes are reflected in protein structure and function. In an endless arms race, pathogens evolve effectors to disarm plants, often adapting more rapidly and divergently than plants can respond. In monocultural fields, the breakdown of resistance can destroy entire populations. The ultimate power to overcome threats to food security rises through precise introduction of desired changes in immune receptors and rapid deployment of diverse germplasm.
A little more knowledge lights our way: paving the path to receptor engineering
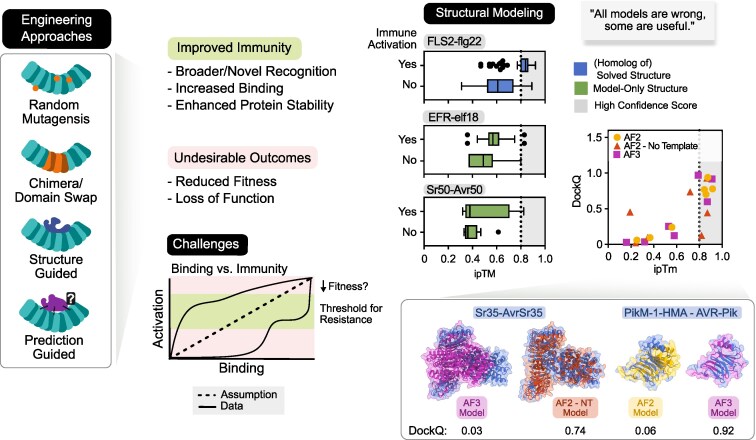
Since the discovery of the first immune receptors, a central goal of our community has been to engineer receptors for improved, altered, or broadened ligand specificity. Early engineering endeavors, often using Arabidopsis and other model organisms, relied primarily on classical techniques including random mutagenesis, chimeras, and domain swaps leading to altered ligand perception (Wulff et al. 2001; Mueller et al. 2012; Steinbrenner et al. 2015; Helft et al. 2016), enhanced immune responses (Harris et al. 2013; Sueldo et al. 2015), and gain of effector recognition (Segretin et al. 2014; Huang et al. 2021). Allelic variation studies further clarified receptor function, residues required for ligand binding, and protein complex formation and stability (Dunning et al. 2007). The overarching aim remains to amplify intended immune outcomes while minimizing unintended consequences (Fig. 3). Achieving this requires precise and effective engineering strategies, together with continued accumulation of knowledge in protein stability during complex formation for PRRs (Dunning et al. 2007; Li et al. 2024a) and addressing challenges posed by intricate interactions of NLRs.
Figure 3.
Combination of techniques including structural modeling enable engineering of immune receptors. Engineering approaches aim to improve immune responses but could lead to undesirable outcomes. In particular, our understanding of the relationship between ligand binding and immune activation is yet incomplete, making it challenging to establish strategies to overcome the unintended consequences. Structural prediction enables hypothesis generation of receptor-ligand interactions and may guide engineering endeavors. The interface predicted template modeling (ipTM) score above 0.8 often indicates reliable AlphaFold prediction and correlates with immune responses for the FLS2-flg22 interactions in Arabidopsis. However, this correlation is not strong in other assessed receptor-ligand interactions. The performance of AlphaFold 2 and 3 vary significantly in predicting NLR/ID-effector pairs (AF2, AlphaFold 2; AF2-NT, AlphaFold 2-No effector Template; AF3, AlphaFold 3). When AlphaFold models are compared with experimental structures, their accuracy, measured by DockQ scores (0: inaccurate, and 1: accurate), shows that selection based on high ipTM scores often indicate reliability but does not always guarantee reliable models. Predicted structures are good hypotheses but should be interpreted with caution.
The debut of AlphaFold 1 in 2018 marked an important leap in protein structure prediction, demonstrating the transformative potential of deep learning in biology (AlQuraishi 2019). With the release of AlphaFold 2 in 2021, our field witnessed a paradigm shift in our understanding of immune receptors, effectors, ligands, and their interactions (Evans et al. 2021; Jumper et al. 2021; Abramson et al. 2024; Li et al. 2024b). Recent studies used AlphaFold 2 to explore the structural diversity of N-terminal domains of NLRs within Plantae (Chia et al. 2024) and LRR domains in maize (Prigozhin et al. 2025). AlphaFold 3 further enabled the construction of putative CC-NLR resistosome structures (Madhuprakash et al. 2024). In parallel, AlphaFold has expanded effector biology to the structural universe, notably improving insights in effector diversity, regulation, evolution, and interaction, previously difficult to decipher from primary sequences alone (Seong and Krasileva 2021, 2023; Rocafort et al. 2022; Derbyshire and Raffaele 2023; Homma et al. 2023; Yan et al. 2023; Asghar et al. 2024; Mukhopadhyay et al. 2024; Yu et al. 2024a). With its growing integration to receptor engineering, AlphaFold has begun to reshape our approach to develop precise and effective engineering strategies.
We are what they grew beyond: pushing the boundaries of precision LRR engineering
The structural determination of immune receptors in Arabidopsis has shaped our understanding of receptor function and engineering potential. The first structurally resolved RLKs include the flagellin receptor FLS2 and chitin receptor CERK1, revealing critical ligand-binding residues and receptor-ligand interactions (Table 1) (Liu et al. 2012; Sun et al. 2013). Nearly a decade later, the resistosome structures of NLRs ZAR1 and RPP1 in Arabidopsis demonstrated the mechanisms underlying NLR activation and effector recognition by the LRR domain (Table 1) (Wang et al. 2019a, 2019b; Ma et al. 2020). Only recently, structural determinations of NLRs from crop species have expanded these insights, enabling rational engineering of immune receptors (Table 1).
Table 1.
Representative structures of NLRs and PRRs
| PDB Accessiona | NLR/ PRR |
Type | Organism | Effector/ Ligand |
Organism | References |
|---|---|---|---|---|---|---|
| 4EBZ | CERK1 | LysM-RLK | Arabidopsis thaliana | chitin | (Liu et al. 2012) | |
| 4MN8 | FLS2 | LRR-RLK | Arabidopsis thaliana | flg22 | (Sun et al. 2013) | |
| 6J5T | ZAR1 | CC-NLR | Arabidopsis thaliana | (Wang et al. 2019a, 2019b) | ||
| 7CRC | RPP1 | TIR-NLR | Arabidopsis thaliana | ATR1 | Hyaloperonospora arabidopsidis | (Ma et al. 2020) |
| 7JLU | Roq1 | TIR-NLR | Nicotiana benthamiana | XopQ | Xanthomonas euvesicatoria | (Martin et al. 2020) |
| 7DRC | RXEG1 | LRR-RLP | Nicotiana benthamiana | XEG1 | (Sun et al. 2022) | |
| 7XE0 | Sr35 | CC-NLR | Triticum monococcum | AvrSr35 | Puccinia graminis f. sp. tritici | (Zhao et al. 2022) |
| 7XC2 | Sr35 | CC-NLR | Triticum monococcum | AvrSr35 | Puccinia graminis f. sp. tritici | (Förderer et al. 2022) |
| 9FYC | MLA13 | CC-NLR | Hordeum vulgare | AVRA13-1 | Blumeria hordei | (Lawson et al. 2025) |
| 8RFH | NRC2 | CC-NLR | Nicotiana benthamiana | (Selvaraj et al. 2024) | ||
| 9FP6 | NRC2 | CC-NLR | Nicotiana benthamiana | (Madhuprakash et al. 2024) | ||
| 8XUV | NRC2 | CC-NLR | Solanum lycopersicum | (Ma et al. 2024) | ||
| 9CC9 | NRC4 | CC-NLR | Nicotiana benthamiana | (Liu et al. 2024a) | ||
| 8ZF0 | ADR1 | RPW8-NLR | Oryza sativa | (Wu et al. 2024) | ||
| 8ZW9 | ADR1 | RPW8-NLR | Arabidopsis thaliana | (Yu et al. 2024b) | ||
| 8YN0 | NRG1 | RPW8-NLR | Arabidopsis thaliana | (Huang et al. 2025) | ||
| 8YL7 | NRG1 | RPW8-NLR | Arabidopsis thaliana | (Xiao et al. 2025) |
aOnly 1 structure is associated with each study in this table. Additional structures can be identified within each entry in the Protein Data Bank (PDB).
Availability of receptor complex structures has been instrumental to narrow the enormous mutational landscapes for precise engineering. Consider the NLR Sr50 as an example (Mago et al. 2015): it directly recognizes its effector through its LRR domain, which contains approximately 450 residues. Rational engineering begins with identifying key effector-binding residues within this extensive sequence space. After selecting 12 interacting residues (Tamborski et al. 2023), modifications could still allow up to 2012 allelic variants, comparable to antibody diversity (Briney et al. 2019). Structural insights significantly contribute to addressing these challenges by identifying key residues and informing rational introduction of mutations. For instance, the structure of Arabidopsis FLS2 bound to its ligand flg22 revealed the ligand-binding interface and residues within the LRR domain, enabling modifications that improved flg22 binding affinity and gain of perception of previously evaded variants (Sun et al. 2013; Fürst et al. 2020; Wei et al. 2020; Li et al. 2024a; Zhang et al. 2024a). Similarly, the solved structures of NLRs, such as Sr35 from einkorn wheat and MLA13 from barley (Table 1), in complex with their effectors allowed precise inference of effector-binding residues and facilitated direct transfer or expansion of recognition specificity to other homologs (Förderer et al. 2022; Zhao et al. 2022; Lawson et al. 2025). These examples demonstrate how experimentally determined PRR-ligand and NLR-effector complex structures are driving precision engineering.
Recent advances in computational modeling, particularly AlphaFold, are further advancing receptor engineering. Two independent studies used AlphaFold models to assist the selection and design of altered ligand recognition in FLS2, where a small number of substitutions resulted in gain of recognition of flg22 epitopes (Li et al. 2024a; Zhang et al. 2024a). For NLRs, AlphaFold accurately predicted the effector binding site of MLA3 in barley, subsequently enabling its precise transfer to Sr50 (Gómez De La Cruz et al. 2024). Similarly, AlphaFold reliably predicted the complex structures of Sr50 variants bound to their effector AvrSr50, aiding in the design of novel recognition for the escape effector mutant AvrSr50QCMJC (Ortiz et al. 2022; Seong et al. 2025). These examples suggest that structure prediction has the potential to accelerate LRR engineering, particularly for receptor-ligand pairs lacking experimentally determined structures.
Despite technical advancements in experimental and predictive structural determination, not all receptor-ligand structures can be accurately characterized. An alternative approach is to leverage the evolutionary information that plants, as bioengineers, have naturally accumulated. Quantifying intraspecies receptor sequence diversity, as demonstrated for Arabidopsis, can aid in inferring residues rapidly changing for ligand binding (Van de Weyer et al. 2019; Prigozhin and Krasileva 2021). In wheat, this framework aided in the transfer of recognition specificity of NLR Sr50 to closely related Sr33 (Tamborski et al. 2023), highlighting its applicability in receptor engineering in crops. Similarly, phylogenetic comparisons and ancestral reconstruction of the bean receptor INR prioritized residues that could alter perception of the caterpillar-associated molecule inceptin (Snoeck et al. 2022). In practice, sequence- and structure-based approaches are not independent but complementary. The effective integration of computational modeling, evolutionary insights, and high-throughput screening will continue to push the boundaries of LRR engineering in the near future.
Things are only impossible until they’re not: expanding ingenuity of decoy engineering approaches
The sequencing and annotation of the Arabidopsis genome facilitated early studies and analyses of Arabidopsis NLRs, revealing atypical fusion of domains into NLRs, such as RRS1 (The Arabidopsis Genome Initiative 2000; Deslandes et al. 2002, 2003; Meyers et al. 2003). In parallel, accumulated studies in Arabidopsis NLRs RPS2, RPM1, RPS5 and ZAR1 supported indirect NLR recognition mechanism in which NLRs monitor modifications of host proteins by effectors instead of directly recognizing them (Axtell and Staskawicz 2003; Mackey et al. 2003; Shao et al. 2003; Wang et al. 2015). These early observations became foundations to guardee, decoy, and ID models (Jones and Dangl 2006; van der Hoorn and Kamoun 2008; Cesari et al. 2014), in which pathogen target proteins are re-purposed as effector baits to effectively trigger immune responses, as either individual proteins or integrated into NLRs.
Some of the most advanced engineering of NLR systems with guardees and decoys began with well-characterized Arabidopsis NLR and receptor-like cytoplasmic kinase systems, specifically RPS5/PBS1-like (DeYoung et al. 2012) and ZAR1/ZED1-like (Lewis et al. 2013). Upon cleavage by bacterial effector AvrPphB, PBS1 undergoes a conformational change that is recognized by RPS5 leading to the activation of immune responses (DeYoung et al. 2012). This activation mechanism has been deployed for engineering recognition of diverse pathogen proteases: substituting the proteolytic site of PBS1 with that of another effector AvrRpt2 from bacterial pathogen Pseudomonas syringae or the NIa viral protease of Tobacco etch virus led to partial or full resistance to pathogens (Kim et al. 2016). Most recently, it was shown that the partial resistance can be rescued by overexpressing engineered PBS1 and RPS5, and this approach has been successfully extended to soybean (Helm et al. 2019; Pottinger et al. 2020).
Similar to RPS5, Arabidopsis NLR ZAR1 monitors a diverse set of receptor-like cytoplasmic kinases like ZED1 targeted by the effector HopZ1a (Lewis et al. 2013; Seto et al. 2017). Recent natural diversity analyses across 1,001 Arabidopsis ecotypes revealed key residues governing ZAR1-ZED1 interactions (Baudin et al. 2021). Extending these analyses to other kinases revealed that ZAR1 can interact with the majority of ZED1-like kinases in Arabidopsis and key residues contributing to binding specificity (Diplock et al. 2023). In combination with mutational screens, natural diversity data from Arabidopsis was leveraged to allow tomato ZAR1 to recognize HopZ1a (Diplock et al. 2024). As ZAR1 is an ancient and highly conserved NLR across angiosperms (Gong et al. 2022), the engineering application can expand to other crops.
The majority of NLR-ID engineering efforts have focused on RRS1 in Arabidopsis (Narusaka et al. 2014; Le Roux et al. 2015; Sarris et al. 2015; Zhang et al. 2017; Mukhi et al. 2021) and RGA5 and Pik-1 in rice (Ashikawa et al. 2008; Cesari et al. 2013; Maqbool et al. 2015; Ortiz et al. 2017; Guo et al. 2018; Sugihara et al. 2023), but continued computational and experimental characterization of NLR-IDs in crops will allow for expansion of engineering efforts (Sarris et al. 2015; Kroj et al. 2016; Bailey et al. 2018; Marchal et al. 2018, 2022; Wang et al. 2020). Conceptually, NLR-ID engineering parallels LRR engineering, in that experimentally determined structures of ID-effector complexes, complemented by sequence analyses, have largely informed the selection and modification of effector binding residues (De la Concepcion et al. 2018, 2019; Bentham et al. 2021, 2023; Liu et al. 2021b; Cesari et al. 2022; Saado et al. 2024; Zhang et al. 2024b). Advances in structure-guided precision engineering and diverse tactics in NLR-IDs are well highlighted in recent reviews (Marchal et al. 2022; Cadiou et al. 2023; Zdrzałek et al. 2023).
Distinct from LRR engineering strategies, the homologous relationships between IDs and effector-targeted host proteins provides an effective nature-guided engineering approach. Identifying host targets and elucidating their interactions with effectors allows the transfer of specific effector-binding residues to NLR-IDs. For example, the crystal structure of the effector AVR-Pik bound to its rice host protein HIPP19 informed mutations expanding the recognition specificity of Pik-1 against stealthy effector variants (Maidment et al. 2021, 2023). Similarly, structural insights from the interaction between the effector Pwl2 and the rice protein HIPP43 enabled engineering the Pikm-1 receptor with broad pathogen recognition capabilities (Zdrzałek et al. 2024). Alternatively, IDs in NLRs can be replaced by their homologs targeted by effectors (Zhu et al. 2025). As such, identifying additional effector-targeted host proteins, such as OsHIPP20 (Oikawa et al. 2024), provide further avenues for engineering opportunities.
Modularity and compactness of IDs also facilitated scalable binder design strategies. Yeast cell surface display screening, a technique frequently used for mammalian nanobody evolution (McMahon et al. 2018), has been adapted for directly evolving higher-affinity IDs in vivo (Rim et al. 2024). In this technique, mutagenized IDs are displayed on the yeast cell surface and screened against targeted effectors, allowing selection of improved binders via fluorescence-activated cell sorting. Additionally, the pikobody approach expanded the plant immune engineering toolkit by leveraging the mammalian immune systems (Kourelis et al. 2023). In this method, nanobodies capable of binding to fluorescent proteins were integrated into Pik-1, replacing its original ID. The resulting pikobody (Pik-nanobody fusion) successfully induced immune responses in plants in the presence of fluorescent proteins, demonstrating that modular IDs capable of effector binding could be derived from the mammalian immune system. Ensuring the proper integration of engineered or designed domains without triggering autoimmunity and compromising immune responses remains a key challenge in NLR-ID engineering. Nevertheless, these strategies broaden the toolkit available to engineers, enabling the development of novel synthetic solutions.
May the fold be with you: new structural quests for immune receptors and effectors
The rapid expansion of predicted structure databases, coupled with the advance in structural similarity search algorithms presents new opportunities to explore the structural universe (Varadi et al. 2022; van Kempen et al. 2024). Molecular mimicry has emerged as viable tactics in pathogen evolution, where pathogen effectors structurally mimic host proteins and hijack host signaling pathways (Gao et al. 2021; Fu et al. 2025). Likewise, certain NLRs’ LRR domain mimics the structure of effector-targeted host proteins, effectively trapping effectors to trigger immune responses (Chen et al. 2023; Gómez De La Cruz et al. 2024). Examples include the pepper NLR Tsw, which possesses an extended LRR domain resembling hormone receptors (Chen et al. 2023), and the barely NLR MLA3 that mimics the host protein HIPP43 to bait its effector (Brabham et al. 2024a; Gómez De La Cruz et al. 2024; Zdrzałek et al. 2024). With millions of predicted structures now available, large-scale structural similarity comparisons can further illuminate this molecular arms race between plant immunity and pathogen effectors.
The direct applicability of AlphaFold for modeling and understanding LRR-ligand or LRR-effector interactions is an active area of exploration (Fig. 3). For PRRs, the analysis of FLS2-flg22 interactions indicated that a prediction confidence (ipTM) threshold of 0.8 could reliably distinguish the FLS2-flg22 variant pairs that could induce immune responses in Arabidopsis from the pairs that could not (Fig. 3) (Li et al. 2024a). However, when assessing recognition prediction for EFR-elf18 that do not have experimentally determined structure using the same threshold, accuracy was very low (Fig. 3) (Colaianni et al. 2021; Parys et al. 2021; Stevens et al. 2024; Trinh et al. 2024). Similarly, an ipTM threshold of 0.8 could differentiate reliable from unreliable AlphaFold predictions for the NLR Sr50, yet reliability of models did not always guarantee immune recognition in plants (Seong et al. 2025). Here, we present our own assessments comparing predicted LRR/ID-effector models against experimentally determined structures. Our analyses reinforced that high ipTM thresholds typically, but not always, indicate reliability, as reported previously (Fig. 3) (Supplementary Table S1) (Evans et al. 2021; Wee and Wei 2024). Therefore, while AlphaFold has enabled structural modeling attempts for many known NLR-effector pairs lacking experimental structures (Fick et al. 2024; Wang et al. 2024), these predictions require careful validation through additional experimental data and may not alone be considered as definitive evidence of immune recognition.
Our understanding of AlphaFold's predictive capabilities will continue to evolve through ongoing research. For instance, AlphaFold 2 failed to accurately predict the complex structure of the NLR Sr50 and its effector AvrSr50 but successfully modeled reliable structures for Sr50 variants bound to AvrSr50 (Gómez De La Cruz et al. 2024; Seong et al. 2025). Intriguingly, these receptor variants contained as little as a single amino acid substitution from the wild type, yet this seemingly small change dramatically improved prediction accuracy. This highlights a fascinating yet unexplored aspect of AlphaFold's sensitivity to minor mutations in protein complex structure prediction. Computational mutational screening may uncover underlying principles governing this predictive behavior, and their identification may enable the recovery of receptor-ligand structures that cannot be currently predicted with high accuracy.
Continued algorithmic advancements, such as AlphaFold 3, promise improvements in accurate structural modeling. However, despite the release of AlphaFold 3, AlphaFold 2 remains valuable, particularly when experimentally determined structures are needed as templates to improve prediction accuracy (Fig. 3) (Outram et al. 2024). Moreover, AlphaFold 2, along with the more accessible ColabFold (Mirdita et al. 2022), offers tunable parameters, such as number of random seeds, recycles, and ensembles, which can enhance the predictive power (Jumper et al. 2021; Agarwal and McShan 2024). Therefore, researchers can explore both options to identify the best model suited to the characteristics of their system and specific biological question.
Looking ahead, refining AlphaFold predictions for PRR-ligand and NLR-effector complexes will greatly benefit from broader community collaboration. By standardizing benchmarking protocols and experimentally validating predicted structures, our community can more effectively assess AlphaFold's performance and identify pathways for improvement. Expanding the set of reliably predicted receptor-ligand complexes can rapidly deepen our understanding of molecular interactions and enhance predictive capabilities in protein design, while experimental structures provide essential ground-truth over time. Embracing not only successes but also failures in this collective structural endeavor is essential to complete our structural quests. After all, all models are wrong, but some are useful.
To boldly go where no one has gone before: leveraging deep learning in plant immunity
Beyond protein structure prediction, machine learning—particularly deep learning—has rapidly expanded our toolkit for exploring protein biology. Geometric deep learning enables protein surface analyses to identify protein-protein interaction sites (Gainza et al. 2020; Tubiana et al. 2022; Krapp et al. 2023). Advances in generative algorithms like ProteinMPNN and RFDiffusion have made it possible to accurately design primary sequences and protein structures (Dauparas et al. 2022; Watson et al. 2023). Additionally, protein language models have shown remarkable potential with diverse applications, such as elucidating evolutionary principles underlying viral mutations and aiding antibody design by accurately predicting mutations that enhance affinity (Hie et al. 2021, 2022, 2024; Lin et al. 2023; Ruffolo and Madani 2024). Such applications can potentially extend to NLRs and effectors, as well as PRRs and ligands, where language model-driven predictions could link specific mutations to their corresponding roles, functions, and interactions. Notably, evolutionary variability in LRR residues of immune receptors can be quantified as computationally accessible Shannon entropy (Prigozhin and Krasileva 2021), and recent advances have illuminated functional diversity within closely related receptors (Lu et al. 2016; Saur et al. 2019; Bauer et al. 2021). These insights provide the groundwork to integrate sequence evolution with functional diversity, positioning language models as powerful tools for further exploration, which may empower the rational engineering of plant immune receptors.
One common theme for the future is the need to generate, share, and utilize good underlying experimental data for more accurate hypothesis generation and model training. For antibody structure prediction, over 3,400 antibody structures and 550 million antibody sequences were used for training (Ruffolo et al. 2023). Building such models for PRR-ligand or NLR-effector interactions will require well-organized and accessible datasets. Recent comparative genomic approaches have curated a naturally diverse PRR ligand dataset, much of which has been experimentally tested in Arabidopsis or tomato (Colaianni et al. 2021; Parys et al. 2021; Stevens et al. 2024). Similarly, comprehensive resources cataloging NLRs and detailing plant-pathogen interactions have been developed, including RefPlantNLR, ANNA, NLRscape, PlantNLRatlas, and PHI-base (Urban et al. 2020; Kourelis et al. 2021; Liu et al. 2021a; Li et al. 2023; Martin et al. 2023). However, we may need to broaden these databases to include diverse data types, such as predicted immune receptors, effectors or ligands, and their complex structures, along with quantitative and qualitative interaction data. These resources could facilitate generating models for predicting mutation impacts and support the design of functional assays to capture binding, recognition, and immune outcomes. Standardizing data types and reporting methods will be crucial to make these resources broadly usable and to support machine learning applications. In this new journey, our community stands ready to explore new frontiers, boldly going where no one has gone before in plant immunity.
Conclusions
The growth of the availability and quality of data at the DNA, RNA, and protein levels has provided insights into the diversity generation and maintenance of plant immune receptors and has begun to answer the long-standing question of how strictly innate immune systems cope with rapidly evolving pathogens. Natural diversity uncovered at each layer poses challenges and opportunities for receptor engineering. Further understanding of the mechanisms behind high nucleotide diversity, transcriptional regulation, and immune receptors' ligand binding and activation will not only lead to new engineering strategies but also aid in continued development of computational predictive and generative models.
We posit that there is sufficient evidence to suggest that plant immune systems fit an anticipatory model of immune evolution, with a high likelihood of individual mutation maintained by population level-selection. The expression of immune receptors exists along multiple axes of regulation and is increasingly relevant to functional receptor prioritization and optimized control in engineering efforts. The new hope of protein structure prediction has revolutionized immune receptor design, but poses new opportunities and challenges for ensuring accurate and relevant structures. As we gain additional high throughput datasets through community-coordinated efforts, we can better leverage emerging machine learning approaches. Along with progress in understanding plant disease resistance, we face regulatory challenges, in particular which bioengineering efforts will be allowed in scalable agricultural settings and when they will be accepted by the general public. “Difficult to see. Always in motion is the future”—it is not yet clear how far we can apply synthetic immune receptors in crops. However, we must do our best to communicate that plants are natural engineers, and we have learned and continue to learn from them in our efforts.
Supplementary Material
Acknowledgments
We would like to thank all the members of the Krasileva lab for their feedback on the conceptualization and editing of this manuscript. We would like to acknowledge George Lucas and the Star Wars franchise for inspiration in the thematic organization of the manuscript, and to ChatGPT for assistance in incorporating his work into the headings. ChatGPT was not otherwise used in the conceptualization, drafting, or editing of this manuscript.
Contributor Information
Chandler A Sutherland, Department of Plant and Microbial Biology, University of California Berkeley, Berkeley, CA 94720, USA.
Danielle M Stevens, Department of Plant and Microbial Biology, University of California Berkeley, Berkeley, CA 94720, USA.
Kyungyong Seong, Department of Plant and Microbial Biology, University of California Berkeley, Berkeley, CA 94720, USA.
Wei Wei, Department of Plant and Microbial Biology, University of California Berkeley, Berkeley, CA 94720, USA.
Ksenia V Krasileva, Department of Plant and Microbial Biology, University of California Berkeley, Berkeley, CA 94720, USA.
Author contributions
C.A.S. led conceptualization of the manuscript and drafting of the DNA diversity and contributed to expression variation. D.M.S. added PRR content across each section, led figure generation, and contributed to the immune receptor engineering section. W.W. led expression variation and its regulation. K.S. led immune receptor engineering. K.V.K. wrote the introduction, contributed to conceptualization of the manuscript, and contributed to the DNA section. All authors were involved in reviewing and editing.
Funding
CAS has been supported by the Grace Kase-Tsujimoto Graduate Fellowship. KS has been supported by the Berkeley BioEnginuity Fellowship. All authors have received support from the NIH Director’s Award (1DP2AT011967-01) awarded to KVK.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Table S1. Summary of modeling confidence scores of receptors and their known ligands using different versions of Alphafold. PRR-Prediction holds the pTM and ipTM scores from Alphafold3 across several receptor-epitope combinations. NLR-Prediction holds the pTM and ipTM scores for predicted LRR/ID-effector complexes obtained from different versions and configurations of AlphaFold. DockQ socres were obtained by comparing AlphaFold models to experimentally determined structures. Sr50-AvrSr50 holds the ipTM scores of modeled Sr50 and AvrSr50 variants.
Supplemental Data Description. Description of data. AlphaFold_Models holds the raw outputs from all Alphafold modeling, which are also hosted on Zenodo (https://doi.org/10.5281/zenodo.14207050).
Data availability
All raw data from Fig. 3 is available on Zenodo (doi:10.5281/zenodo.14207051) and in the associated supplementary information.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Abascal F, Harvey LMR, Mitchell E, Lawson ARJ, Lensing SV, Ellis P, Russell AJC, Alcantara RE, Baez-Ortega A, Wang Y, et al. Somatic mutation landscapes at single-molecule resolution. Nature. 2021:593(7859):405–410. 10.1038/s41586-021-03477-4 [DOI] [PubMed] [Google Scholar]
- Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, Ronneberger O, Willmore L, Ballard AJ, Bambrick J, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024:630(8016):493–500. 10.1038/s41586-024-07487-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi H, Derevnina L, Kamoun S. NLR singletons, pairs, and networks: evolution, assembly, and regulation of the intracellular immunoreceptor circuitry of plants. Curr Opin Plant Biol. 2019:50:121–131. 10.1016/j.pbi.2019.04.007 [DOI] [PubMed] [Google Scholar]
- Agarwal V, McShan AC. The power and pitfalls of AlphaFold2 for structure prediction beyond rigid globular proteins. Nat Chem Biol. 2024:20(8):950–959. 10.1038/s41589-024-01638-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert I, Hua C, Nürnberger T, Pruitt RN, Zhang L. Surface sensor systems in plant immunity. Plant Physiol. 2020:182(4):1582–1596. 10.1104/pp.19.01299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlQuraishi M. AlphaFold at CASP13. Bioinformatics. 2019:35(22):4862–4865.. 10.1093/bioinformatics/btz422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Steuernagel B, Gaurav K, Chandramohan S, Long Y, Matny O, Johnson R, Enk J, Periyannan S, Singh N, et al. Resistance gene cloning from a wild crop relative by sequence capture and association genetics. Nat Biotechnol. 2019:37(2):139–143. 10.1038/s41587-018-0007-9 [DOI] [PubMed] [Google Scholar]
- Asghar R, Wu N, Ali N, Wang Y, Akkaya M. Computational studies reveal structural characterization and novel families of Puccinia striiformis f. sp. tritici effectors. bioRxiv. 2024. 10.1101/2024.09.12.612600, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, Matsumoto T, Ono K, Yano M. Two adjacent nucleotide-binding site–leucine-rich repeat class genes are required to confer pikm-specific rice blast resistance. Genetics. 2008:180(4):2267–2276. 10.1534/genetics.108.095034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasov KE, Liu C, Erban A, Kopka J, Parker JE, Alcázar R. NLR mutations suppressing immune hybrid incompatibility and their effects on disease resistance. Plant Physiol. 2018:177(3):1152–1169. 10.1104/pp.18.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ, Staskawicz BJ. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell. 2003:112(3):369–377. 10.1016/S0092-8674(03)00036-9 [DOI] [PubMed] [Google Scholar]
- Bae JH, Liu R, Roberts E, Nguyen E, Tabrizi S, Rhoades J, Blewett T, Xiong K, Gydush G, Shea D, et al. Single duplex DNA sequencing with CODEC detects mutations with high sensitivity. Nat Genet. 2023:55(5):871–879. 10.1038/s41588-023-01376-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggs E, Dagdas G, Krasileva K. NLR diversity, helpers and integrated domains: making sense of the NLR IDentity. Curr Opin Plant Biol. 2017:38:59–67. 10.1016/j.pbi.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Baggs EL, Monroe JG, Thanki AS, O’Grady R, Schudoma C, Haerty W, Krasileva KV. Convergent loss of an EDS1/PAD4 signaling pathway in several plant lineages reveals coevolved components of plant immunity and drought response. Plant Cell. 2020:32(7):2158–2177. 10.1105/tpc.19.00903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PC, Schudoma C, Jackson W, Baggs E, Dagdas G, Haerty W, Moscou M, Krasileva KV. Dominant integration locus drives continuous diversification of plant immune receptors with exogenous domain fusions. Genome Biol. 2018:19(1):23. 10.1186/s13059-018-1392-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker EG, Toomajian C, Kreitman M, Bergelson J. A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell. 2006:18(8):1803–1818. 10.1105/tpc.106.042614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barela Hudgell MA, Momtaz F, Jafri A, Alekseyev MA, Smith LC. Local genomic instability of the SpTransformer gene family in the purple sea urchin inferred from BAC insert deletions. Genes (Basel). 2024:15(2):222. 10.3390/genes15020222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan AC, Collenberg M, Wang J, Lee RRQ, Cher WY, Rabanal FA, Ashkenazy H, Weigel D, Chae E. A truncated singleton NLR causes hybrid necrosis in Arabidopsis thaliana. Mol Biol Evol. 2021:38(2):557–574. 10.1093/molbev/msaa245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan AC, Weigel D. Plant NLR diversity: the known unknowns of pan-NLRomes. Plant Cell. 2021:33(4):814–831. 10.1093/plcell/koaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir S, Rehman N, Fakhar Zaman F, Naeem MK, Jamal A, Tellier A, Ilyas M, Silva Arias GA, Khan MR. Genome-wide characterization of the NLR gene family in tomato (Solanum lycopersicum) and their relatedness to disease resistance. Front Genet. 2022:13:931580. 10.3389/fgene.2022.931580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin M, Martin EC, Sass C, Hassan JA, Bendix C, Sauceda R, Diplock N, Specht CD, Petrescu AJ, Lewis JD. A natural diversity screen in Arabidopsis thaliana reveals determinants for HopZ1a recognition in the ZAR1-ZED1 immune complex. Plant Cell Environ. 2021:44(2):629–644. 10.1111/pce.13927 [DOI] [PubMed] [Google Scholar]
- Bauer S, Yu D, Lawson AW, Saur IML, Frantzeskakis L, Kracher B, Logemann E, Chai J, Maekawa T, Schulze-Lefert P. The leucine-rich repeats in allelic barley MLA immune receptors define specificity towards sequence-unrelated powdery mildew avirulence effectors with a predicted common RNase-like fold. PLoS Pathog. 2021:17(2):e1009223. 10.1371/journal.ppat.1009223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Wyrsch I, Strutt J, Wimalasekera R, Webb A, Boller T, Robatzek S. Expression patterns of FLAGELLIN SENSING 2 map to bacterial entry sites in plant shoots and roots. J Exp Bot. 2014:65(22):6487–6498. 10.1093/jxb/eru366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ. RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science. 1994:265(5180):1856–1860. 10.1126/science.8091210 [DOI] [PubMed] [Google Scholar]
- Bentham AR, De La Concepcion JC, Benjumea JV, Kourelis J, Jones S, Mendel M, Stubbs J, Stevenson CEM, Maidment JHR, Youles M, et al. Allelic compatibility in plant immune receptors facilitates engineering of new effector recognition specificities. Plant Cell. 2023:35(10):3809–3827. 10.1093/plcell/koad204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentham AR, Petit-Houdenot Y, Win J, Chuma I, Terauchi R, Banfield MJ, Kamoun S, Langner T. A single amino acid polymorphism in a conserved effector of the multihost blast fungus pathogen expands host-target binding spectrum. PLoS Pathog. 2021:17(11):e1009957. 10.1371/journal.ppat.1009957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Su M, Li N, Liang Y, Dang S, Xu J, Hu M, Wang J, Zou M, Deng Y, et al. The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell. 2021:184(13):3528–3541.e12. 10.1016/j.cell.2021.05.003 [DOI] [PubMed] [Google Scholar]
- Bieri S, Mauch S, Shen Q-H, Peart J, Devoto A, Casais C, Ceron F, Schulze S, Steinbiß H-H, Shirasu K, et al. RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. Plant Cell. 2004:16(12):3480–3495. 10.1105/tpc.104.026682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner-Eddy PD, Crute IR, Holub EB, Beynon JL. RPP13 is a simple locus in Arabidopsis thaliana for alleles that specify downy mildew resistance to different avirulence determinants in Peronospora parasitica. Plant J. 2000:21(2):177–188. 10.1046/j.1365-313x.2000.00664.x [DOI] [PubMed] [Google Scholar]
- Bódi Z, Farkas Z, Nevozhay D, Kalapis D, Lázár V, Csörgő B, Nyerges Á, Szamecz B, Fekete G, Papp B, et al. Phenotypic heterogeneity promotes adaptive evolution. PLoS Biol. 2017:15(5):e2000644. 10.1371/journal.pbio.2000644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies K, Lempe J, Epple P, Warthmann N, Lanz C, Dangl JL, Weigel D. Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol. 2007:5(9):e236. 10.1371/journal.pbio.0050236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella MA, Coleman MJ, Hughes DE, Nishimura MT, Jones JDG, Somerville SC. Map positions of 47 Arabidopsis sequences with sequence similarity to disease resistance genes. Plant J. 1997:12(5):1197–1211. 10.1046/j.1365-313X.1997.12051197.x [DOI] [PubMed] [Google Scholar]
- Boutrot F, Zipfel C. Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu Rev Phytopathol. 2017:55(1):257–286. 10.1146/annurev-phyto-080614-120106 [DOI] [PubMed] [Google Scholar]
- Brabham HJ, Gómez De La Cruz D, Were V, Shimizu M, Saitoh H, Hernández-Pinzón I, Green P, Lorang J, Fujisaki K, Sato K, et al. Barley MLA3 recognizes the host-specificity effector Pwl2 from Magnaporthe oryzae. Plant Cell. 2024a:36(2):447–470. 10.1093/plcell/koad266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabham HJ, Hernández-Pinzón I, Yanagihara C, Ishikawa N, Komori T, Matny ON, Hubbard A, Witek K, Feist A, Numazawa H, et al. Discovery of functional NLRs using expression level, high-throughput transformation, and large-scale phenotyping. bioRxiv. 2024b. 10.1101/2024.06.25.599845, preprint: not peer reviewed. [DOI]
- Briney B, Inderbitzin A, Joyce C, Burton DR. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature. 2019:566(7744):393–397. 10.1038/s41586-019-0879-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy JAN, Magallon KJ, Duan L, Zhong V, Ramachandran P, Kniazev K, Dinneny JR. Synthetic genetic circuits as a means of reprogramming plant roots. Science. 2022:377(6607):747–751. 10.1126/science.abo4326 [DOI] [PubMed] [Google Scholar]
- Buckley KM, Dooley H. Immunological diversity is a cornerstone of organismal defense and allorecognition across Metazoa. J Immunol. 2022:208(2):203–211. 10.4049/jimmunol.2100754 [DOI] [PubMed] [Google Scholar]
- Buckley KM, Smith LC. Extraordinary diversity among members of the large gene family, 185/333, from the purple sea urchin, Strongylocentrotus purpuratus. BMC Mol Biol. 2007:8(1):68. 10.1186/1471-2199-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley KM, Terwilliger DP, Smith LC. Sequence variations in 185/333 messages from the purple sea urchin suggest posttranscriptional modifications to increase immune diversity. J Immunol. 2008:181(12):8585–8594. 10.4049/jimmunol.181.12.8585 [DOI] [PubMed] [Google Scholar]
- Cadiou L, Brunisholz F, Cesari S, Kroj T. Molecular engineering of plant immune receptors for tailored crop disease resistance. Curr Opin Plant Biol. 2023:74:102381. 10.1016/j.pbi.2023.102381 [DOI] [PubMed] [Google Scholar]
- Cagan A, Baez-Ortega A, Brzozowska N, Abascal F, Coorens THH, Sanders MA, Lawson ARJ, Harvey LMR, Bhosle S, Jones D, et al. Somatic mutation rates scale with lifespan across mammals. Nature. 2022:604(7906):517–524. 10.1038/s41586-022-04618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capp J-P. Interplay between genetic, epigenetic, and gene expression variability: considering complexity in evolvability. Evol Appl. 2021:14(4):893–901. 10.1111/eva.13204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari S, Bernoux M, Moncuquet P, Kroj T, Dodds PN. A novel conserved mechanism for plant NLR protein pairs: the ‘integrated decoy’ hypothesis. Front Plant Sci. 2014:5:606. 10.3389/fpls.2014.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari S, Thilliez G, Ribot C, Chalvon V, Michel C, Jauneau A, Rivas S, Alaux L, Kanzaki H, Okuyama Y, et al. The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell. 2013:25(4):1463–1481. 10.1105/tpc.112.107201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari S, Xi Y, Declerck N, Chalvon V, Mammri L, Pugnière M, Henriquet C, De Guillen K, Chochois V, Padilla A, et al. New recognition specificity in a plant immune receptor by molecular engineering of its integrated domain. Nat Commun. 2022:13(1):1524. 10.1038/s41467-022-29196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Bataillon T, Glémin S, Lascoux M. What does the distribution of fitness effects of new mutations reflect? Insights from plants. New Phytol. 2022:233(4):1613–1619. 10.1111/nph.17826 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhao Y, Luo X, Hong H, Yang T, Huang S, Wang C, Chen H, Qian X, Feng M, et al. NLR surveillance of pathogen interference with hormone receptors induces immunity. Nature. 2023:613(7942):145–152. 10.1038/s41586-022-05529-9 [DOI] [PubMed] [Google Scholar]
- Cheng YT, Li Y, Huang S, Huang Y, Dong X, Zhang Y, Li X. Stability of plant immune-receptor resistance proteins is controlled by SKP1-CULLIN1-F-box (SCF)-mediated protein degradation. Proc Natl Acad Sci U S A. 2011:108(35):14694–14699. 10.1073/pnas.1105685108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia K-S, Kourelis J, Teulet A, Vickers M, Sakai T, Walker JF, Schornack S, Kamoun S, Carella P. The N-terminal domains of NLR immune receptors exhibit structural and functional similarities across divergent plant lineages. Plant Cell. 2024:36(7):2491–2511. 10.1093/plcell/koae113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006:124(4):803–814. 10.1016/j.cell.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Chuberre C, Plancot B, Driouich A, Moore JP, Bardor M, Gügi B, Vicré M. Plant immunity is compartmentalized and specialized in roots. Front Plant Sci. 2018:9:1692. 10.3389/fpls.2018.01692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RM, Schweikert G, Toomajian C, Ossowski S, Zeller G, Shinn P, Warthmann N, Hu TT, Fu G, Hinds DA, et al. Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science. 2007:317(5836):338–342. 10.1126/science.1138632 [DOI] [PubMed] [Google Scholar]
- Clarke CR, Chinchilla D, Hind SR, Taguchi F, Miki R, Ichinose Y, Martin GB, Leman S, Felix G, Vinatzer BA. Allelic variation in two distinct Pseudomonas syringae flagellin epitopes modulates the strength of plant immune responses but not bacterial motility. New Phytol. 2013:200(3):847–860. 10.1111/nph.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni NR, Parys K, Lee H-S, Conway JM, Kim NH, Edelbacher N, Mucyn TS, Madalinski M, Law TF, Jones CD, et al. A complex immune response to flagellin epitope variation in commensal communities. Cell Host Microbe. 2021:29(4):635–649.e9. 10.1016/j.chom.2021.02.006 [DOI] [PubMed] [Google Scholar]
- Contreras MP, Lüdke D, Pai H, Toghani A, Kamoun S. NLR receptors in plant immunity: making sense of the alphabet soup. EMBO Rep. 2023:24(10):e57495. 10.15252/embr.202357495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Jones JDG. Plant pathogens and integrated defence responses to infection. Nature. 2001:411(6839):826–833. 10.1038/35081161 [DOI] [PubMed] [Google Scholar]
- Dauparas J, Anishchenko I, Bennett N, Bai H, Ragotte RJ, Milles LF, Wicky BIM, Courbet A, De Haas RJ, Bethel N, et al. Robust deep learning–based protein sequence design using ProteinMPNN. Science. 2022:378(6615):49–56. 10.1126/science.add2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Concepcion JC, Franceschetti M, MacLean D, Terauchi R, Kamoun S, Banfield MJ. Protein engineering expands the effector recognition profile of a rice NLR immune receptor. eLife. 2019:8:e47713. 10.7554/eLife.47713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Concepcion JC, Franceschetti M, Maqbool A, Saitoh H, Terauchi R, Kamoun S, Banfield MJ. Polymorphic residues in rice NLRs expand binding and response to effectors of the blast pathogen. Nat Plants. 2018:4(8):576–585. 10.1038/s41477-018-0194-x [DOI] [PubMed] [Google Scholar]
- Derbyshire MC, Raffaele S. Surface frustration re-patterning underlies the structural landscape and evolvability of fungal orphan candidate effectors. Nat Commun. 2023:14(1):5244. 10.1038/s41467-023-40949-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes L, Olivier J, Peeters N, Feng DX, Khounlotham M, Boucher C, Somssich I, Genin S, Marco Y. Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl Acad Sci U S A. 2003:100(13):8024–8029. 10.1073/pnas.1230660100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes L, Olivier J, Theulières F, Hirsch J, Feng DX, Bittner-Eddy P, Beynon J, Marco Y. Resistance to Ralstonia solanacearum in Arabidopsis thaliana is conferred by the recessive RRS1-R gene, a member of a novel family of resistance genes. Proc Natl Acad Sci U S A. 2002:99(4):2404–2409. 10.1073/pnas.032485099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung BJ, Qi D, Kim S-H, Burke TP, Innes RW. Activation of a plant nucleotide binding-leucine rich repeat disease resistance protein by a modified self protein. Cell Microbiol. 2012:14(7):1071–1084. 10.1111/j.1462-5822.2012.01779.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Xu X, Kong W, Xia X, Zhang S, Liu L-W, Liu A, Zou L. Genome-wide identification and expression analysis of rice NLR genes responsive to the infections of Xanthomonas oryzae pv. oryzae and Magnaporthe oryzae. Physiol Mol Plant Pathol. 2020:111:101488. 10.1016/j.pmpp.2020.101488 [DOI] [Google Scholar]
- Diplock N, Baudin M, Derek Xiang X, Liang L-Y, Dai W, Murphy JM, Lucet IS, Hassan JA, Lewis JD. Molecular dissection of the pseudokinase ZED1 expands effector recognition to the tomato immune receptor ZAR1. Plant Physiol. 2024:196(1):651–666. 10.1093/plphys/kiae268 [DOI] [PubMed] [Google Scholar]
- Diplock N, Baudin M, Harden L, Silva CJ, Erickson-Beltran ML, Hassan JA, Lewis JD. Utilising natural diversity of kinases to rationally engineer interactions with the angiosperm immune receptor ZAR1. Plant Cell Environ. 2023:46(7):2238–2254. 10.1111/pce.14603 [DOI] [PubMed] [Google Scholar]
- Dixon MS, Hatzixanthis K, Jones DA, Harrison K, Jones JD. The tomato Cf-5 disease resistance gene and six homologs show pronounced allelic variation in leucine-rich repeat copy number. Plant Cell. 1998:10(11):1915–1925. 10.1105/tpc.10.11.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN. From gene-for-gene to resistosomes: flor's enduring legacy. Mol Plant-Microbe Interactions®. 2023:36(8):461–467. 10.1094/MPMI-06-23-0081-HH [DOI] [PubMed] [Google Scholar]
- Dodds PN, Lawrence GJ, Ellis JG. Six amino acid changes confined to the leucine-rich repeat β-strand/β-turn motif determine the difference between the P and P2 rust resistance specificities in flax. Plant Cell. 2001:13(1):163–178. 10.1105/tpc.13.1.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning FM, Sun W, Jansen KL, Helft L, Bent AF. Identification and mutational analysis of Arabidopsis FLS2 leucine-rich repeat domain residues that contribute to flagellin perception. Plant Cell. 2007:19(10):3297–3313. 10.1105/tpc.106.048801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet A, Zhou F, Vacheron J, Heiman CM, Dénervaud Tendon V, Ma K-W, Schulze-Lefert P, Keel C, Geldner N. Spatially restricted immune responses are required for maintaining root meristematic activity upon detection of bacteria. Curr Biol. 2021:31(5):1012–1028.e7. 10.1016/j.cub.2020.12.048 [DOI] [PubMed] [Google Scholar]
- Evans R, O’Neill M, Pritzel A, Antropova N, Senior A, Green T, Žídek A, Bates R, Blackwell S, Yim J, et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv 2021. 10.1101/2021.10.04.463034, preprint: not peer reviewed. [DOI]
- Eyre-Walker A, Keightley PD. The distribution of fitness effects of new mutations. Nat Rev Genet. 2007:8(8):610–618. 10.1038/nrg2146 [DOI] [PubMed] [Google Scholar]
- Farrara BF, Ilott TW, Michelmore RW. Genetic analysis of factors for resistance to downy mildew (Bremia lactucae) in species of lettuce (Lactuca sativa and L. serriola). Plant Pathol. 1987:36(4):499–514. 10.1111/j.1365-3059.1987.tb02267.x [DOI] [Google Scholar]
- Fick A, Fick JLM, Swart V, and Van Den Berg N. What NLR you recognizing? Predicted binding affinities- and energies may be used to identify novel NLR-effector interactions. bioRxiv 2024. 10.1101/2024.07.26.605369, preprint: not peer reviewed. [DOI]
- Fick A, Swart V, Backer R, Bombarely A, Engelbrecht J, van den Berg N. Partially resistant avocado rootstock Dusa® shows prolonged upregulation of nucleotide binding-leucine rich repeat genes in response to Phytophthora cinnamomi infection. Front Plant Sci. 2022a:13:793644. 10.3389/fpls.2022.793644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick A, Swart V, van den Berg N. The ups and downs of plant NLR expression during pathogen infection. Front Plant Sci. 2022b:13:921148. 10.3389/fpls.2022.921148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor HH. Current status of the gene-for-gene concept. Annu Rev Phytopathol. 1971:9(1):275–296. 10.1146/annurev.py.09.090171.001423 [DOI] [Google Scholar]
- Förderer A, Li E, Lawson AW, Deng Y, Sun Y, Logemann E, Zhang X, Wen J, Han Z, Chang J, et al. A wheat resistosome defines common principles of immune receptor channels. Nature. 2022:610(7932):532–539. 10.1038/s41586-022-05231-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz-Laylin LK, Krishnamurthy N, Tör M, Sjölander KV, Jones JDG. Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis. Plant Physiol. 2005:138(2):611–623. 10.1104/pp.104.054452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Li S, Li J, Zhao Z, Li J, Tan X, Yu S, Jing M, Zhu-Salzman K, Fang J, et al. An insect effector mimics its host immune regulator to undermine plant immunity. Adv Sci. 2025:12(11):e2409186. 10.1002/advs.202409186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst U, Zeng Y, Albert M, Witte AK, Fliegmann J, Felix G. Perception of Agrobacterium tumefaciens flagellin by FLS2XL confers resistance to crown gall disease. Nat Plants. 2020:6(1):22–27. 10.1038/s41477-019-0578-6 [DOI] [PubMed] [Google Scholar]
- Gainza P, Sverrisson F, Monti F, Rodolà E, Boscaini D, Bronstein MM, Correia BE. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat Methods. 2020:17(2):184–192. 10.1038/s41592-019-0666-6 [DOI] [PubMed] [Google Scholar]
- Gallas N, Li X, von Roepenack-Lahaye E, Schandry N, Jiang Y, Wu D, Lahaye T. An ancient cis-element targeted by Ralstonia solanacearum TALE-like effectors facilitates the development of a promoter trap that could confer broad-spectrum wilt resistance. Plant Biotechnol J. 2024:22(3):602–616. 10.1111/pbi.14208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Stegle O, Behr J, Steffen JG, Drewe P, Hildebrand KL, Lyngsoe R, Schultheiss SJ, Osborne EJ, Sreedharan VT, et al. Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature. 2011:477(7365):419–423. 10.1038/nature10414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, He Y, Yin X, Zhong X, Yan B, Wu Y, Chen J, Li X, Zhai K, Huang Y, et al. Ca2 + sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell. 2021:184(21):5391–5404.e17. 10.1016/j.cell.2021.09.009 [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang W, Zhang T, Gong Z, Zhao H, Han G-Z. Out of water: the origin and early diversification of plant R-genes. Plant Physiol. 2018:177(1):82–89. 10.1104/pp.18.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J, Lun CM, Majeske AJ, Sacchi S, Schrankel CS, Smith LC. Invertebrate immune diversity. Dev Comp Immunol. 2011:35(9):959–974. 10.1016/j.dci.2010.12.009 [DOI] [PubMed] [Google Scholar]
- Giolai M, Laine A-L. A trade-off between investment in molecular defense repertoires and growth in plants. Science. 2024:386(6722):677–680. 10.1126/science.adn2779 [DOI] [PubMed] [Google Scholar]
- Gladieux P, van Oosterhout C, Fairhead S, Jouet A, Ortiz D, Ravel S, Shrestha R-K, Frouin J, He X, Zhu Y, et al. Extensive immune receptor repertoire diversity in disease-resistant rice landraces. Curr Biol. 2024:34(17):3983–3995.e6. 10.1016/j.cub.2024.07.061 [DOI] [PubMed] [Google Scholar]
- Gómez De La Cruz D, Zdrzałek R, Banfield MJ, Talbot NJ, Moscou MJ. Molecular mimicry of a pathogen virulence target by a plant immune receptor. bioRxiv. 2024. 10.1101/2024.07.26.605320, preprint: not peer reviewed. [DOI]
- Gómez-Gómez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000:5(6):1003–1011. 10.1016/S1097-2765(00)80265-8 [DOI] [PubMed] [Google Scholar]
- Gong Z, Qi J, Hu M, Bi G, Zhou J-M, Han G-Z. The origin and evolution of a plant resistosome. Plant Cell. 2022:34(5):1600–1620. 10.1093/plcell/koac053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez TL, Liang Y, Nguyen BN, Staskawicz BJ, Loqué D, Hammond MC. Tight regulation of plant immune responses by combining promoter and suicide exon elements. Nucleic Acids Res. 2015:43(14):7152–7161. 10.1093/nar/gkv655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, Innes RW, Dangl JL. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science. 1995:269(5225):843–846. 10.1126/science.7638602 [DOI] [PubMed] [Google Scholar]
- Guo L, Cesari S, De Guillen K, Chalvon V, Mammri L, Ma M, Meusnier I, Bonnot F, Padilla A, Peng Y-L, et al. Specific recognition of two MAX effectors by integrated HMA domains in plant immune receptors involves distinct binding surfaces. Proc Natl Acad Sci U S A. 2018:115(45):11637–11642. 10.1073/pnas.1810705115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites (a review). Proc Natl Acad Sci U S A. 1990:87(9):3566–3573. 10.1073/pnas.87.9.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CJ, Slootweg EJ, Goverse A, Baulcombe DC. Stepwise artificial evolution of a plant disease resistance gene. Proc Natl Acad Sci U S A. 2013:110(52):21189–21194. 10.1073/pnas.1311134110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayford RK, Haley OC, Cannon EK, Portwood JL, Gardiner JM, Andorf CM, Woodhouse MR. Functional annotation and meta-analysis of maize transcriptomes reveal genes involved in biotic and abiotic stress. BMC Genomics. 2024:25(1):533. 10.1186/s12864-024-10443-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft L, Thompson M, Bent AF. Directed evolution of FLS2 towards novel flagellin peptide recognition. PLoS One. 2016:11(6):e0157155. 10.1371/journal.pone.0157155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm M, Qi M, Sarkar S, Yu H, Whitham SA, Innes RW. Engineering a decoy substrate in soybean to enable recognition of the soybean mosaic virus NIa protease. Mol Plant-Microbe Interactions. 2019:32(6):760–769. 10.1094/MPMI-12-18-0324-R [DOI] [PubMed] [Google Scholar]
- Hie B, Zhong ED, Berger B, Bryson B. Learning the language of viral evolution and escape. Science. 2021:371(6526):284–288. 10.1126/science.abd7331 [DOI] [PubMed] [Google Scholar]
- Hie BL, Shanker VR, Xu D, Bruun TUJ, Weidenbacher PA, Tang S, Wu W, Pak JE, Kim PS. Efficient evolution of human antibodies from general protein language models. Nat Biotechnol. 2024:42(2):275–283. 10.1038/s41587-023-01763-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hie BL, Yang KK, Kim PS. Evolutionary velocity with protein language models predicts evolutionary dynamics of diverse proteins. Cell Syst. 2022:13(4):274–285.e6. 10.1016/j.cels.2022.01.003 [DOI] [PubMed] [Google Scholar]
- Homma F, Huang J, Van Der Hoorn RAL. AlphaFold-multimer predicts cross-kingdom interactions at the plant-pathogen interface. Nat Commun. 2023:14(1):6040. 10.1038/s41467-023-41721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honee G, Buitink J, Jabs T, De Kloe J, Sijbolts F, Apotheker M, Weide R, Sijen T, Stuiver M, De Wit PJ. Induction of defense-related responses in Cf9 tomato cells by the AVR9 elicitor peptide of Cladosporium fulvum is developmentally regulated. Plant Physiol. 1998:117(3):809–820. 10.1104/pp.117.3.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Huang S, Li J, Wang H, Zhao Y, Feng M, Dai J, Wang T, Zhu M, Tao X. Stepwise artificial evolution of an Sw-5b immune receptor extends its resistance spectrum against resistance-breaking isolates of tomato spotted wilt virus. Plant Biotechnol J. 2021:19(11):2164–2176. 10.1111/pbi.13641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Chen X, Zhong X, Li M, Ao K, Huang J, Li X. Plant TRAF proteins regulate NLR immune receptor turnover. Cell Host Microbe. 2016:19(2):204–215. 10.1016/j.chom.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Huang S, Jia A, Song W, Hessler G, Meng Y, Sun Y, Xu L, Laessle H, Jirschitzka J, Ma S, et al. Identification and receptor mechanism of TIR-catalyzed small molecules in plant immunity. Science. 2022:377(6605):eabq3297. 10.1126/science.abq3297 [DOI] [PubMed] [Google Scholar]
- Huang S, Wang J, Song R, Jia A, Xiao Y, Sun Y, Wang L, Mahr D, Wu Z, Han Z, et al. Balanced plant helper NLR activation by a modified host protein complex. Nature. 2025:639(8054):447–455. 10.1038/s41586-024-08521-7 [DOI] [PubMed] [Google Scholar]
- Hufford MB, Seetharam AS, Woodhouse MR, Chougule KM, Ou S, Liu J, Ricci WA, Guo T, Olson A, Qiu Y, et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science. 2021:373(6555):655–662. 10.1126/science.abg5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Shepherd KW. Present status of genetics of rust resistance in flax. Euphytica. 1991:55(3):255–267. 10.1007/BF00021246 [DOI] [Google Scholar]
- Jacob F, Kracher B, Mine A, Seyfferth C, Blanvillain-Baufumé S, Parker JE, Tsuda K, Schulze-Lefert P, Maekawa T. A dominant-interfering camta3 mutation compromises primary transcriptional outputs mediated by both cell surface and intracellular immune receptors in Arabidopsis thaliana. New Phytol. 2018:217(4):1667–1680. 10.1111/nph.14943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Hige J, Dangl JL. Is localized acquired resistance the mechanism for effector-triggered disease resistance in plants? Nat Plants. 2023:9(8):1184–1190. 10.1038/s41477-023-01466-1 [DOI] [PubMed] [Google Scholar]
- Jacob P, Kim NH, Wu F, El-Kasmi F, Chi Y, Walton WG, Furzer OJ, Lietzan AD, Sunil S, Kempthorn K, et al. Plant “helper” immune receptors are Ca2+-permeable nonselective cation channels. Science. 2021:373(6553):420–425. 10.1126/science.abg7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia A, Huang S, Song W, Wang J, Meng Y, Sun Y, Xu L, Laessle H, Jirschitzka J, Hou J, et al. TIR-catalyzed ADP-ribosylation reactions produce signaling molecules for plant immunity. Science. 2022:377(6605):eabq8180. 10.1126/science.abq8180 [DOI] [PubMed] [Google Scholar]
- Jiao W-B, Schneeberger K. Chromosome-level assemblies of multiple Arabidopsis genomes reveal hotspots of rearrangements with altered evolutionary dynamics. Nat Commun. 2020:11(1):989. 10.1038/s41467-020-14779-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Dickinson MJ, Balint-Kurti PJ, Dixon MS, Jones JDG. Two complex resistance loci revealed in tomato by classical and RFLP mapping of the Cf-2, Cf-4, Cf-5, and Cf-9 genes for resistance to Cladosporium fulvum. Mol Plant Microbe Interact. 1993:6(3):348–348. 10.1094/MPMI-6-348 [DOI] [Google Scholar]
- Jones DA, Thomas CM, Hammond-Kosack KE, Balint-Kurti PJ, Jones JDG. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science. 1994:266(5186):789–793. 10.1126/science.7973631 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006:444(7117):323–329. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Staskawicz BJ, Dangl JL. The plant immune system: from discovery to deployment. Cell. 2024:187(9):2095–2116. 10.1016/j.cell.2024.03.045 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science. 2016:354(6316):aaf6395. 10.1126/science.aaf6395 [DOI] [PubMed] [Google Scholar]
- Jores T, Tonnies J, Wrightsman T, Buckler ES, Cuperus JT, Fields S, Queitsch C. Synthetic promoter designs enabled by a comprehensive analysis of plant core promoters. Nat Plants. 2021:7(6):842–855. 10.1038/s41477-021-00932-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021:596(7873):583–589. 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupe F, Witek K, Verweij W, Sliwka J, Pritchard L, Etherington GJ, Maclean D, Cock PJ, Leggett RM, Bryan GJ, et al. Resistance gene enrichment sequencing (RenSeq) enables reannotation of the NB-LRR gene family from sequenced plant genomes and rapid mapping of resistance loci in segregating populations. Plant J Cell Mol Biol. 2013:76(3):530–544. 10.1111/tpj.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Shirasu K, Guerois R. NLR sensors meet at the SGT1–HSP90 crossroad. Trends Biochem Sci. 2010:35(4):199–207. 10.1016/j.tibs.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Kang M, Wu H, Liu H, Liu W, Zhu M, Han Y, Liu W, Chen C, Song Y, Tan L, et al. The pan-genome and local adaptation of Arabidopsis thaliana. Nat Commun. 2023:14(1):6259. 10.1038/s41467-023-42029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima CG, Guimarães GA, Nogueira SR, MacLean D, Cook DR, Steuernagel B, Baek J, Bouyioukos C, Melo B do V, Tristão G. A pigeonpea gene confers resistance to Asian soybean rust in soybean. Nat Biotechnol. 2016:34(6):661–665. 10.1038/nbt.3554 [DOI] [PubMed] [Google Scholar]
- Keepers K, Peterson K, Raduski A, Turner KM, Van Tassel D, Smith K, Harkess A, Bever JD, Brandvain Y. Disease resistance gene count increases with rainfall in Silphium integrifolium. Ecol Evol. 2024:14(9):e11143. 10.1002/ece3.11143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H, Pamboukdjian N, Ponchet M, Poupet A, Delon R, Verrier J-L, Roby D, Ricci P. Pathogen-induced elicitin production in transgenic tobacco generates a hypersensitive response and nonspecific disease resistance. Plant Cell. 1999:11(2):223. 10.2307/3870852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesseli RV, Paran I, Michelmore RW. Analysis of a detailed genetic linkage map of Lactuca sativa (lettuce) constructed from RFLP and RAPD markers. Genetics. 1994:136(4):1435–1446. 10.1093/genetics/136.4.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby EM, Conte AN, Burroughs AM, Nagy TA, Vargas JA, Whalen LA, Aravind L, Whiteley AT. Bacterial NLR-related proteins protect against phage. Cell. 2023:186(11):2410–2424.e18. 10.1016/j.cell.2023.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Qi D, Ashfield T, Helm M, Innes RW. Using decoys to expand the recognition specificity of a plant disease resistance protein. Science. 2016:351(6274):684–687. 10.1126/science.aad3436 [DOI] [PubMed] [Google Scholar]
- Kourelis J, Marchal C, Posbeyikian A, Harant A, Kamoun S. NLR immune receptor–nanobody fusions confer plant disease resistance. Science. 2023:379(6635):934–939. 10.1126/science.abn4116 [DOI] [PubMed] [Google Scholar]
- Kourelis J, Sakai T, Adachi H, Kamoun S. RefPlantNLR is a comprehensive collection of experimentally validated plant disease resistance proteins from the NLR family. PLoS Biol. 2021:19(10):e3001124. 10.1371/journal.pbio.3001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp LF, Abriata LA, Cortés Rodriguez F, Dal Peraro M. PeSTo: parameter-free geometric deep learning for accurate prediction of protein binding interfaces. Nat Commun. 2023:14(1):2175. 10.1038/s41467-023-37701-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroj T, Chanclud E, Michel-Romiti C, Grand X, Morel J-B. Integration of decoy domains derived from protein targets of pathogen effectors into plant immune receptors is widespread. New Phytol. 2016:210(2):618–626. 10.1111/nph.13869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN. A useful weed put to work: genetic analysis of disease resistance in Arabidopsis thaliana. Trends Genet. 1996:12(2):63–69. 10.1016/0168-9525(96)81402-8 [DOI] [PubMed] [Google Scholar]
- Lai Y, Eulgem T. Transcript-level expression control of plant NLR genes. Mol Plant Pathol. 2018:19(5):1267–1281. 10.1111/mpp.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson AW, Flores-Ibarra A, Cao Y, An C, Neumann U, Gunkel M, Saur IML, Chai J, Behrmann E, Schulze-Lefert P. The barley MLA13-AVRA13 heterodimer reveals principles for immunoreceptor recognition of RNase-like powdery mildew effectors. EMBO J. 2025:38(2):213–225. 10.1038/s44318-025-00373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux C, Huet G, Jauneau A, Camborde L, Trémousaygue D, Kraut A, Zhou B, Levaillant M, Adachi H, Yoshioka H, et al. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell. 2015:161(5):1074–1088. 10.1016/j.cell.2015.04.025 [DOI] [PubMed] [Google Scholar]
- Leuschen-Kohl R, Roberts R, Stevens DM, Zhang N, Buchanan S, Pilkey B, Coaker G, Iyer-Pascuzzi AS. Tomato roots exhibit distinct, development-specific responses to bacterial-derived peptides. bioRxiv. 2024. 10.1101/2024.11.04.621969, preprint: not peer reviewed. [DOI]
- Lewis JD, Lee AH-Y, Hassan JA, Wan J, Hurley B, Jhingree JR, Wang PW, Lo T, Youn J-Y, Guttman DS, et al. The Arabidopsis ZED1 pseudokinase is required for ZAR1-mediated immunity induced by the Pseudomonas syringae type III effector HopZ1a. Proc Natl Acad Sci U S A. 2013:110(46):18722–18727. 10.1073/pnas.1315520110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Bolaños EJ, Stevens DM, Sha H, Prigozhin DM, Coaker G. Unlocking expanded flagellin perception through rational receptor engineering. bioRxiv. 2024a. 10.1101/2024.09.09.612155, preprint: not peer reviewed. [DOI]
- Li T, Moreno-Pérez A, Coaker G. Plant pattern recognition receptors: exploring their evolution, diversification, and spatiotemporal regulation. Curr Opin Plant Biol. 2024b:82:102631. 10.1016/j.pbi.2024.102631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Deng Y, Ning Y, He Z, Wang G-L. Exploiting broad-spectrum disease resistance in crops: from molecular dissection to breeding. Annu Rev Plant Biol. 2020:71(1):575–603. 10.1146/annurev-arplant-010720-022215 [DOI] [PubMed] [Google Scholar]
- Li X, Ma L, Wang Y, Ye C, Guo C, Li Y, Mei X, Du F, Huang H. PlantNLRatlas: a comprehensive dataset of full- and partial-length NLR resistance genes across 100 chromosome-level plant genomes. Front Plant Sci. 2023:14:1178069. 10.3389/fpls.2023.1178069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Q, Huettel B, Walkemeier B, Mayjonade B, Lopez-Roques C, Gil L, Roux F, Schneeberger K, Mercier R. A pan-genome of 69 Arabidopsis thaliana accessions reveals a conserved genome structure throughout the global species range. Nat Genet. 2024:56(5):982–991. 10.1038/s41588-024-01715-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Akin H, Rao R, Hie B, Zhu Z, Lu W, Smetanin N, Verkuil R, Kabeli O, Shmueli Y, et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science. 2023:379(6637):1123–1130. 10.1126/science.ade2574 [DOI] [PubMed] [Google Scholar]
- Liu F, Yang Z, Wang C, You Z, Martin R, Qiao W, Huang J, Jacob P, Dangl JL, Carette JE, et al. Activation of the helper NRC4 immune receptor forms a hexameric resistosome. Cell. 2024a:187(18):4877–4889.e15. 10.1016/j.cell.2024.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MH, Costa BM, Bianchini EC, Choi U, Bandler RC, Lassen E, Grońska-Pęski M, Schwing A, Murphy ZR, Rosenkjær D, et al. DNA mismatch and damage patterns revealed by single-molecule sequencing. Nature. 2024b:630(8017):752–761. 10.1038/s41586-024-07532-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu Z, Song C, Hu Y, Han Z, She J, Fan F, Wang J, Jin C, Chang J, et al. Chitin-induced dimerization activates a plant immune receptor. Science. 2012:336(6085):1160–1164. 10.1126/science.1218867 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zeng Z, Zhang Y-M, Li Q, Jiang X-M, Jiang Z, Tang J-H, Chen D, Wang Q, Chen J-Q, et al. An angiosperm NLR atlas reveals that NLR gene reduction is associated with ecological specialization and signal transduction component deletion. Mol Plant. 2021a:14(12):2015–2031. 10.1016/j.molp.2021.08.001 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang X, Yuan G, Wang D, Zheng Y, Ma M, Guo L, Bhadauria V, Peng Y-L, Liu J. A designer rice NLR immune receptor confers resistance to the rice blast fungus carrying noncorresponding avirulence effectors. Proc Natl Acad Sci U S A. 2021b:118(44):e2110751118. 10.1073/pnas.2110751118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolle S, Stevens D, Coaker G. Plant NLR-triggered immunity: from receptor activation to downstream signaling. Curr Opin Immunol. 2020:62:99–105. 10.1016/j.coi.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kracher B, Saur IML, Bauer S, Ellwood SR, Wise R, Yaeno T, Maekawa T, Schulze-Lefert P. Allelic barley MLA immune receptors recognize sequence-unrelated avirulence effectors of the powdery mildew pathogen. Proc Natl Acad Sci U S A. 2016:113(42):E6486–E6495. 10.1073/pnas.1612947113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdke D, Sakai T, Kourelis J, Toghani A, Adachi H, Posbeyikian A, Frijters R, Pai H, Harant A, Ernst K, et al. A root-specific NLR network confers resistance to plant parasitic nematodes. bioRxiv. 2023. 10.1101/2023.12.14.571630, preprint: not peer reviewed. [DOI]
- Ma S, An C, Lawson AW, Cao Y, Sun Y, Tan EYJ, Pan J, Jirschitzka J, Kümmel F, Mukhi N, et al. Oligomerization-mediated autoinhibition and cofactor binding of a plant NLR. Nature. 2024:632(8026):869–876. 10.1038/s41586-024-07668-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Lapin D, Liu L, Sun Y, Song W, Zhang X, Logemann E, Yu D, Wang J, Jirschitzka J, et al. Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science. 2020:370(6521):eabe3069. 10.1126/science.abe3069 [DOI] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell. 2003:112(3):379–389. 10.1016/S0092-8674(03)00040-0 [DOI] [PubMed] [Google Scholar]
- Madhuprakash J, Toghani A, Contreras MP, Posbeyikian A, Richardson J, Kourelis J, Bozkurt TO, Webster MW, Kamoun S. A disease resistance protein triggers oligomerization of its NLR helper into a hexameric resistosome to mediate innate immunity. Sci Adv. 2024:10(45):eadr2594. 10.1126/sciadv.adr2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mago R, Zhang P, Vautrin S, Šimková H, Bansal U, Luo M-C, Rouse M, Karaoglu H, Periyannan S, Kolmer J, et al. The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus. Nat Plants. 2015:1(12):1–3. 10.1038/nplants.2015.186 [DOI] [PubMed] [Google Scholar]
- Maidment JH, Shimizu M, Bentham AR, Vera S, Franceschetti M, Longya A, Stevenson CE, De La Concepcion JC, Białas A, Kamoun S, et al. Effector target-guided engineering of an integrated domain expands the disease resistance profile of a rice NLR immune receptor. eLife. 2023:12:e81123. 10.7554/eLife.81123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment JHR, Franceschetti M, Maqbool A, Saitoh H, Jantasuriyarat C, Kamoun S, Terauchi R, Banfield MJ. Multiple variants of the fungal effector AVR-Pik bind the HMA domain of the rice protein OsHIPP19, providing a foundation to engineer plant defense. J Biol Chem. 2021:296:100371. 10.1016/j.jbc.2021.100371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man J, Gallagher JP, Bartlett M. Structural evolution drives diversification of the large LRR-RLK gene family. New Phytol. 2020:226(5):1492–1505. 10.1111/nph.16455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool A, Saitoh H, Franceschetti M, Stevenson C, Uemura A, Kanzaki H, Kamoun S, Terauchi R, Banfield M. Structural basis of pathogen recognition by an integrated HMA domain in a plant NLR immune receptor. eLife. 2015:4:e08709. 10.7554/eLife.08709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal C, Pai H, Kamoun S, Kourelis J. Emerging principles in the design of bioengineered made-to-order plant immune receptors. Curr Opin Plant Biol. 2022:70:102311. 10.1016/j.pbi.2022.102311 [DOI] [PubMed] [Google Scholar]
- Marchal C, Zhang J, Zhang P, Fenwick P, Steuernagel B, Adamski NM, Boyd L, McIntosh R, Wulff BBH, Berry S, et al. BED-domain-containing immune receptors confer diverse resistance spectra to yellow rust. Nat Plants. 2018:4(9):662–668. 10.1038/s41477-018-0236-4 [DOI] [PubMed] [Google Scholar]
- Martin EC, Ion CF, Ifrimescu F, Spiridon L, Bakker J, Goverse A, Petrescu A-J. NLRscape: an atlas of plant NLR proteins. Nucleic Acids Res. 2023:51(D1):D1470–D1482. 10.1093/nar/gkac1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GB, Brommonschenkel SH, Chunwongse J, Frary A, Ganal MW, Spivey R, Wu T, Earle ED, Tanksley SD. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993:262(5138):1432–1436. 10.1126/science.7902614 [DOI] [PubMed] [Google Scholar]
- Martin R, Qi T, Zhang H, Liu F, King M, Toth C, Nogales E, Staskawicz BJ. Structure of the activated Roq1 resistosome directly recognizing the pathogen effector XopQ. bioRxiv. 2020. 10.1101/2020.08.13.246413, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- Martincorena I, Luscombe NM. Non-random mutation: the evolution of targeted hypermutation and hypomutation. BioEssays. 2013:35(2):123–130. 10.1002/bies.201200150 [DOI] [PubMed] [Google Scholar]
- McMahon C, Baier AS, Pascolutti R, Wegrecki M, Zheng S, Ong JX, Erlandson SC, Hilger D, Rasmussen SGF, Ring AM, et al. Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat Struct Mol Biol. 2018:25(3):289–296. 10.1038/s41594-018-0028-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes JJ, Van Doorn AA, Wijbrandi J, Simons G, Cornelissen BJC, Haring MA. Expression of the Fusarium resistance gene I-2 colocalizes with the site of fungal containment. Plant J. 2000:23(2):183–193. 10.1046/j.1365-313x.2000.00765.x [DOI] [PubMed] [Google Scholar]
- Meyers BC, Chin DB, Shen KA, Sivaramakrishnan S, Lavelle DO, Zhang Z, Michelmore RW. The major resistance gene cluster in lettuce is highly duplicated and spans several megabases. Plant Cell. 1998:10(11):1817–1832. 10.1105/tpc.10.11.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW. Genome-wide analysis of NBS-LRR–encoding genes in Arabidopsis. Plant Cell. 2003:15(4):809–834. 10.1105/tpc.009308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelmore RW, Meyers BC. Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res. 1998:8(11):1113–1130. 10.1101/gr.8.11.1113 [DOI] [PubMed] [Google Scholar]
- Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold: making protein folding accessible to all. Nat Methods. 2022:19(6):679–682. 10.1038/s41592-022-01488-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mode CJ. A mathematical model for the co-evolution of obligate parasites and their hosts. Evolution. 1958:12(2):158–165. 10.2307/2406026 [DOI] [Google Scholar]
- Mohr TJ, Mammarella ND, Hoff T, Woffenden BJ, Jelesko JG, McDowell JM. The Arabidopsis Downy Mildew resistance gene RPP8 is induced by pathogens and salicylic acid and is regulated by W box cis elements. Mol Plant-Microbe Interactions. 2010:23(10):1303–1315. 10.1094/MPMI-01-10-0022 [DOI] [PubMed] [Google Scholar]
- Molla KA, Karmakar S, Chanda PK, Sarkar SN, Datta SK, Datta K. Tissue-specific expression of Arabidopsis NPR1 gene in rice for sheath blight resistance without compromising phenotypic cost. Plant Sci. 2016:250:105–114. 10.1016/j.plantsci.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Moore L, Cagan A, Coorens THH, Neville MDC, Sanghvi R, Sanders MA, Oliver TRW, Leongamornlert D, Ellis P, Noorani A, et al. The mutational landscape of human somatic and germline cells. Nature. 2021:597(7876):381–386. 10.1038/s41586-021-03822-7 [DOI] [PubMed] [Google Scholar]
- Mueller K, Bittel P, Chinchilla D, Jehle AK, Albert M, Boller T, Felix G. Chimeric FLS2 receptors reveal the basis for differential flagellin perception in Arabidopsis and tomato. Plant Cell. 2012:24(5):2213–2224. 10.1105/tpc.112.096073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhi N, Brown H, Gorenkin D, Ding P, Bentham AR, Stevenson CEM, Jones JDG, Banfield MJ. Perception of structurally distinct effectors by the integrated WRKY domain of a plant immune receptor. Proc Natl Acad Sci U S A. 2021:118(50):e2113996118. 10.1073/pnas.2113996118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Javed MA, Wu J, Pérez-López E. Structure-guided secretome analysis of gall-forming microbes offers insights into effector diversity and evolution. bioRxiv. 2024. 10.1101/2024.09.03.609900, preprint: not peer reviewed. [DOI]
- Müller V, de Boer RJ, Bonhoeffer S, Szathmáry E. An evolutionary perspective on the systems of adaptive immunity. Biol Rev Camb Philos Soc. 2018:93(1):505–528. 10.1111/brv.12355 [DOI] [PubMed] [Google Scholar]
- Munch D, Gupta V, Bachmann A, Busch W, Kelly S, Mun T, Andersen SU. The Brassicaceae family displays divergent, shoot-skewed NLR resistance gene expression. Plant Physiol. 2018:176(2):1598–1609. 10.1104/pp.17.01606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusaka M, Hatakeyama K, Shirasu K, Narusaka Y. Arabidopsis dual resistance proteins, both RPS4 and RRS1, are required for resistance to bacterial wilt in transgenic Brassica crops. Plant Signal Behav. 2014:9(7):e29130. 10.4161/psb.29130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngou BPM, Ding P, Jones JDG. Thirty years of resistance: zig-zag through the plant immune system. Plant Cell. 2022a:34(5):1447–1478. 10.1093/plcell/koac041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngou BPM, Heal R, Wyler M, Schmid MW, Jones JDG. Concerted expansion and contraction of immune receptor gene repertoires in plant genomes. Nat Plants. 2022b:8(10):1146–1152. 10.1038/s41477-022-01260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngou BPM, Wyler M, Schmid MW, Kadota Y, Shirasu K. Evolutionary trajectory of pattern recognition receptors in plants. Nat Commun. 2024:15(1):308. 10.1038/s41467-023-44408-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg M, Hu TT, Ishino Y, Jhaveri J, Toomajian C, Zheng H, Bakker E, Calabrese P, Gladstone J, Goyal R, et al. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 2005:3(7):e196. 10.1371/journal.pbio.0030196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa K, Fujisaki K, Shimizu M, Takeda T, Nemoto K, Saitoh H, Hirabuchi A, Hiraka Y, Miyaji N, Białas A, et al. The blast pathogen effector AVR-Pik binds and stabilizes rice heavy metal-associated (HMA) proteins to co-opt their function in immunity. PLoS Pathog. 2024:20(11):e1012647. 10.1371/journal.ppat.1012647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M, Barela Hudgell MA, D’Allura B, Agronin J, Gross A, Podini D, Smith LC. Short tandem repeats, segmental duplications, gene deletion, and genomic instability in a rapidly diversified immune gene family. BMC Genomics. 2016a:17(1):900. 10.1186/s12864-016-3241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M, Barela Hudgell MA, Golconda P, Man Lun C, Smith LC. Genomic instability and shared mechanisms for gene diversification in two distant immune gene families: the plant NBS-LRR genes and the echinoid 185/333 genes. In: The evolution of the immune system, Malagoli D, editor. Academic Press; 2016b, p. 295–310. [Google Scholar]
- Oren M, Rosental B, Hawley TS, Kim G-Y, Agronin J, Reynolds CR, Grayfer L, Smith LC. Individual Sea Urchin coelomocytes undergo somatic immune gene diversification. Front Immunol. 2019:10:1298. 10.3389/fimmu.2019.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, Chen J, Outram MA, Saur IML, Upadhyaya NM, Mago R, Ericsson DJ, Cesari S, Chen C, Williams SJ, et al. The stem rust effector protein AvrSr50 escapes Sr50 recognition by a substitution in a single surface-exposed residue. New Phytol. 2022:234(2):592–606. 10.1111/nph.18011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, De Guillen K, Cesari S, Chalvon V, Gracy J, Padilla A, Kroj T. Recognition of the Magnaporthe oryzae effector AVR-pia by the decoy domain of the rice NLR immune receptor RGA5. Plant Cell. 2017:29(1):156–168. 10.1105/tpc.16.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outram MA, Chen J, Broderick S, Li Z, Aditya S, Tasneem N, Arndell T, Blundell C, Ericsson DJ, Figueroa M, et al. Avrsr27 is a zinc-bound effector with a modular structure important for immune recognition. New Phytol. 2024:243(1):314–329. 10.1111/nph.19801 [DOI] [PubMed] [Google Scholar]
- Parker JE, Coleman MJ, Szabò V, Frost LN, Schmidt R, van der Biezen EA, Moores T, Dean C, Daniels MJ, Jones JD. The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell. 1997:9(6):879–894. 10.1105/tpc.9.6.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M, Hammond-Kosack KE, Golstein C, Thomas CM, Jones DA, Harrison K, Wulff BBH, Jones JDG. Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the cf-4/9 locus of tomato. Cell. 1997:91(6):821–832. 10.1016/S0092-8674(00)80470-5 [DOI] [PubMed] [Google Scholar]
- Parys K, Colaianni NR, Lee H-S, Hohmann U, Edelbacher N, Trgovcevic A, Blahovska Z, Lee D, Mechtler A, Muhari-Portik Z, et al. Signatures of antagonistic pleiotropy in a bacterial flagellin epitope. Cell Host Microbe. 2021:29(4):620–634.e9. 10.1016/j.chom.2021.02.008 [DOI] [PubMed] [Google Scholar]
- Payne JL, Wagner A. The causes of evolvability and their evolution. Nat Rev Genet. 2019:20(1):24–38. 10.1038/s41576-018-0069-z [DOI] [PubMed] [Google Scholar]
- Pottinger SE, Bak A, Margets A, Helm M, Tang L, Casteel C, Innes RW. Optimizing the PBS1 decoy system to confer resistance to potyvirus infection in Arabidopsis and soybean. Mol Plant-Microbe Interactions. 2020:33(7):932–944. 10.1094/MPMI-07-19-0190-R [DOI] [PubMed] [Google Scholar]
- Prigozhin DM, Krasileva KV. Analysis of intraspecies diversity reveals a subset of highly variable plant immune receptors and predicts their binding sites. Plant Cell. 2021:33(4):998–1015. 10.1093/plcell/koab013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozhin DM, Sutherland CA, Rangavajjhala S, Krasileva KV. Majority of the highly variable NLRs in maize share genomic location and contain additional target-binding domains. Mol Plant-Microbe Interactions. 2025:38(2):275–284. 10.1094/MPMI-05-24-0047-FI [DOI] [PubMed] [Google Scholar]
- Pucker B, Irisarri I, de Vries J, Xu B. Plant genome sequence assembly in the era of long reads: progress, challenges and future directions. Quant Plant Biol. 2022:3:e5. 10.1017/qpb.2021.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz D, Oya S, Lopez-Mateos D, Zhao K, Pierce A, Ortega L, Ali A, Carbonell-Bejerano P, Yarov-Yarovoy V, Suzuki S, et al. H3k4me1 recruits DNA repair proteins in plants. Plant Cell. 2024:36(6):2410–2426. 10.1093/plcell/koae089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim EY, Garrett OD, Howard AJ, Shim Y, Li Y, Van Dyke JE, Packer RC, Ho N, Jain RS, Stewart VJ, et al. Directed evolution of a plant immune receptor for broad spectrum recognition of pathogen effectors. bioRxiv. 2024. 10.1101/2024.09.30.614878, preprint: not peer reviewed. [DOI]
- Rocafort M, Bowen JK, Hassing B, Cox MP, McGreal B, De La Rosa S, Plummer KM, Bradshaw RE, Mesarich CH. The Venturia inaequalis effector repertoire is dominated by expanded families with predicted structural similarity, but unrelated sequence, to avirulence proteins from other plant-pathogenic fungi. BMC Biol. 2022:20(1):246. 10.1186/s12915-022-01442-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose LE, Bittner-Eddy PD, Langley CH, Holub EB, Michelmore RW, Beynon JL. The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics. 2004:166(3):1517–1527. 10.1534/genetics.166.3.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo JA, Chu L-S, Mahajan SP, Gray JJ. Fast, accurate antibody structure prediction from deep learning on massive set of natural antibodies. Nat Commun. 2023:14(1):2389. 10.1038/s41467-023-38063-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo JA, Madani A. Designing proteins with language models. Nat Biotechnol. 2024:42(2):200–202. 10.1038/s41587-024-02123-4 [DOI] [PubMed] [Google Scholar]
- Saado I, Brabham HJ, Bennett JW, Lam AHC, Hernández-Pinzón I, Moscou MJ, De la Concepcion JC, Banfield MJ. Engineering an Exo70 integrated domain of a barley NLR for improved blast resistance. bioRxiv. 2024. 10.1101/2024.12.24.630226, preprint: not peer reviewed. [DOI]
- Sarris PF, Duxbury Z, Huh SU, Ma Y, Segonzac C, Sklenar J, Derbyshire P, Cevik V, Rallapalli G, Saucet SB, et al. A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell. 2015:161(5):1089–1100. 10.1016/j.cell.2015.04.024 [DOI] [PubMed] [Google Scholar]
- Saur IM, Bauer S, Kracher B, Lu X, Franzeskakis L, Müller MC, Sabelleck B, Kümmel F, Panstruga R, Maekawa T, et al. Multiple pairs of allelic MLA immune receptor-powdery mildew AVRA effectors argue for a direct recognition mechanism. eLife. 2019:8:e44471. 10.7554/eLife.44471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segretin ME, Pais M, Franceschetti M, Chaparro-Garcia A, Bos JIB, Banfield MJ, Kamoun S. Single amino acid mutations in the potato immune receptor R3a expand response to phytophthora effectors. Mol Plant-Microbe Interactions®. 2014:27(7):624–637. 10.1094/MPMI-02-14-0040-R [DOI] [PubMed] [Google Scholar]
- Selvaraj M, Toghani A, Pai H, Sugihara Y, Kourelis J, Yuen ELH, Ibrahim T, Zhao H, Xie R, Maqbool A, et al. Activation of plant immunity through conversion of a helper NLR homodimer into a resistosome. PLoS Biol. 2024:22(10):e3002868. 10.1371/journal.pbio.3002868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong K, Krasileva KV. Computational structural genomics unravels common folds and novel families in the secretome of fungal phytopathogen Magnaporthe oryzae. Mol Plant-Microbe Interactions®. 2021:34(11):1267–1280. 10.1094/MPMI-03-21-0071-R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong K, Krasileva KV. Prediction of effector protein structures from fungal phytopathogens enables evolutionary analyses. Nat Microbiol. 2023:8(1):174–187. 10.1038/s41564-022-01287-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong K, Seo E, Witek K, Li M, Staskawicz B. Evolution of NLR resistance genes with noncanonical N-terminal domains in wild tomato species. New Phytol. 2020:227(5):1530–1543. 10.1111/nph.16628 [DOI] [PubMed] [Google Scholar]
- Seong K, Wei W, Sent SC, Vega B, Dee A, Ramirez-Bernardino G, Kumar R, Parra L, Saur IM, Krasileva K. Resurrection of the plant immune receptor Sr50 to overcome pathogen immune evasion. bioRxiv. 2025. 10.1101/2024.08.07.607039, preprint: not peer reviewed. [DOI]
- Seto D, Koulena N, Lo T, Menna A, Guttman DS, Desveaux D. Expanded type III effector recognition by the ZAR1 NLR protein using ZED1-related kinases. Nat Plants. 2017:3(4):1–4. 10.1038/nplants.2017.27 [DOI] [PubMed] [Google Scholar]
- Shang L, Li X, He H, Yuan Q, Song Y, Wei Z, Lin H, Hu M, Zhao F, Zhang C, et al. A super pan-genomic landscape of rice. Cell Res. 2022:32(10):878–896. 10.1038/s41422-022-00685-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science. 2003:301(5637):1230–1233. 10.1126/science.1085671 [DOI] [PubMed] [Google Scholar]
- Shi J, Tian Z, Lai J, Huang X. Plant pan-genomics and its applications. Mol Plant. 2023:16(1):168–186. 10.1016/j.molp.2022.12.009 [DOI] [PubMed] [Google Scholar]
- Shimizu M, Hirabuchi A, Sugihara Y, Abe A, Takeda T, Kobayashi M, Hiraka Y, Kanzaki E, Oikawa K, Saitoh H. A genetically linked pair of NLR immune receptors shows contrasting patterns of evolution. Proceedings of the National Academy of Sciences. 2022:119(27). 10.1073/pnas.2116896119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE. 2001:2001(113):re22. 10.1126/stke.2001.113.re22 [DOI] [PubMed] [Google Scholar]
- Shiu S-H, Bleecker AB. Expansion of the receptor-like kinase/pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003:132(2):530–543. 10.1104/pp.103.021964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaprasad PV, Chen H-M, Patel K, Bond DM, Santos BACM, Baulcombe DC. A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell. 2012:24(3):859–874. 10.1105/tpc.111.095380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck S, Abramson BW, Garcia AGK, Egan AN, Michael TP, Steinbrenner AD. Evolutionary gain and loss of a plant pattern-recognition receptor for HAMP recognition. eLife. 2022:11:e81050. 10.7554/eLife.81050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck S, Garcia AGK, Steinbrenner AD. Plant receptor-like proteins (RLPs): structural features enabling versatile immune recognition. Physiol Mol Plant Patholathol. 2023:125:102004. 10.1016/j.pmpp.2023.102004 [DOI] [Google Scholar]
- Song W-Y, Wang G-L, Chen L-L, Kim H-S, Pi L-Y, Holsten T, Gardner J, Wang B, Zhai W-X, Zhu L-H, et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995:270(5243):1804–1806. 10.1126/science.270.5243.1804 [DOI] [PubMed] [Google Scholar]
- Staunton PM, Peters AJ, Seoighe C. Somatic mutations inferred from RNA-seq data highlight the contribution of replication timing to mutation rate variation in a model plant. Genetics. 2023:225(2):iyad128. 10.1093/genetics/iyad128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidele CE, Stam R. Multi-omics approach highlights differences between RLP classes in Arabidopsis thaliana. BMC Genomics. 2021:22(1):557. 10.1186/s12864-021-07855-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrenner AD, Goritschnig S, Staskawicz BJ. Recognition and activation domains contribute to allele-specific responses of an Arabidopsis NLR receptor to an oomycete effector protein. PLoS Pathog. 2015:11(2):e1004665. 10.1371/journal.ppat.1004665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DM, Moreno-Pérez A, Weisberg AJ, Ramsing C, Fliegmann J, Zhang N, Madrigal M, Martin G, Steinbrenner A, Felix G, et al. Natural variation of immune epitopes reveals intrabacterial antagonism. Proc Natl Acad Sci U S A. 2024:121(23):e2319499121. 10.1073/pnas.2319499121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuiver MH, Custers JH. Engineering disease resistance in plants. Nature. 2001:411(6839):865–868. 10.1038/35081200 [DOI] [PubMed] [Google Scholar]
- Sueldo DJ, Shimels M, Spiridon LN, Caldararu O, Petrescu A-J, Joosten MHAJ, Tameling WIL. Random mutagenesis of the nucleotide-binding domain of NRC1 (NB-LRR required for hypersensitive response-associated cell death-1), a downstream signalling nucleotide-binding, leucine-rich repeat (NB-LRR) protein, identifies gain-of-function mutations in the nucleotide-binding pocket. New Phytol. 2015:208(1):210–223. 10.1111/nph.13459 [DOI] [PubMed] [Google Scholar]
- Sugihara Y, Abe Y, Takagi H, Abe A, Shimizu M, Ito K, Kanzaki E, Oikawa K, Kourelis J, Langner T, et al. Disentangling the complex gene interaction networks between rice and the blast fungus identifies a new pathogen effector. PLoS Biol. 2023:21(1):e3001945. 10.1371/journal.pbio.3001945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou J-M, Chai J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science. 2013:342(6158):624–628. 10.1126/science.1243825 [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang Y, Zhang X, Chen Z, Xia Y, Wang L, Sun Y, Zhang M, Xiao Y, Han Z, et al. Plant receptor-like protein activation by a microbial glycoside hydrolase. Nature. 2022:610(7931):335–342. 10.1038/s41586-022-05214-x [DOI] [PubMed] [Google Scholar]
- Sutherland CA, Prigozhin DM, Monroe JG, Krasileva KV. High allelic diversity in Arabidopsis NLRs is associated with distinct genomic features. EMBO Rep. 2024:25(5):2306–2322. 10.1038/s44319-024-00122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborski J, Seong K, Liu F, Staskawicz BJ, Krasileva KV. Altering specificity and autoactivity of plant immune receptors Sr33 and Sr50 via a rational engineering approach. Mol Plant-Microbe Interactions. 2023:36(7):434–446. 10.1094/MPMI-07-22-0154-R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale LC, Murray KD, Collenberg M, Contreras-Garrido A, Schlegel T, van Ess L, Jüttner J, Lanz C, Deusch O, Fitz J, et al. Pangenomic context reveals the extent of intraspecific plant NLR evolution. bioRxiv. 2024. 10.1101/2024.09.02.610789, preprint: not peer reviewed. [DOI]
- The Arabidopsis Genome Initiative . Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000:408(6814):796–815. 10.1038/35048692 [DOI] [PubMed] [Google Scholar]
- Thomas CM, Dixon MS, Parniske M, Golstein C, Jones JD. Genetic and molecular analysis of tomato Cf genes for resistance to Cladosporium fulvum. Philos Trans R Soc B Biol Sci. 1998:353(1374):1413–1424. 10.1098/rstb.1998.0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Wu Z, Chen S, Ao K, Huang W, Yaghmaiean H, Sun T, Xu F, Zhang Y, Wang S, et al. Activation of TIR signalling boosts pattern-triggered immunity. Nature. 2021:598(7881):500–503. 10.1038/s41586-021-03987-1 [DOI] [PubMed] [Google Scholar]
- Trinh J, Li T, Franco JY, Toruño TY, Stevens DM, Thapa SP, Wong J, Pineda R, de Dios EÁ, Kahn TL, et al. Variation in microbial feature perception in the Rutaceae family with immune receptor conservation in citrus. Plant Physiol. 2023:193(1):689–707. 10.1093/plphys/kiad263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh J, Tran M, Coaker G. The perception and evolution of flagellin, cold shock protein and elongation factor tu from vector-borne bacterial plant pathogens. Mol Plant Pathol. 2024:25(10):e70019. 10.1111/mpp.70019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiana J, Schneidman-Duhovny D, Wolfson HJ. ScanNet: an interpretable geometric deep learning model for structure-based protein binding site prediction. Nat Methods. 2022:19(6):730–739. 10.1038/s41592-022-01490-7 [DOI] [PubMed] [Google Scholar]
- Urban M, Cuzick A, Seager J, Wood V, Rutherford K, Venkatesh SY, De Silva N, Martinez MC, Pedro H, Yates AD, et al. PHI-base: the pathogen-host interactions database. Nucleic Acids Res. 2020:48(D1):D613–D620. 10.1093/nar/gkz904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn RAL, Kamoun S. From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell. 2008:20(8):2009–2017. 10.1105/tpc.108.060194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Weyer A-L, Monteiro F, Furzer OJ, Nishimura MT, Cevik V, Witek K, Jones JDG, Dangl JL, Weigel D, Bemm F. A species-wide inventory of NLR genes and alleles in Arabidopsis thaliana. Cell. 2019:178(5):1260–1272.e14. 10.1016/j.cell.2019.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kempen M, Kim SS, Tumescheit C, Mirdita M, Lee J, Gilchrist CLM, Söding J, Steinegger M. Fast and accurate protein structure search with foldseek. Nat Biotechnol. 2024:42(2):243–246. 10.1038/s41587-023-01773-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022:50(D1):D439–D444. 10.1093/nar/gkab1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendelbo NM, Mahmood K, Steuernagel B, Wulff BBH, Sarup P, Hovmøller MS, Justesen AF, Kristensen PS, Orabi J, Jahoor A. Discovery of resistance genes in rye by targeted long-read sequencing and association genetics. Cells. 2022:11(8):1273. 10.3390/cells11081273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Roux B, Feng F, Guy E, Li L, Li N, Zhang X, Lautier M, Jardinaud M-F, Chabannes M, et al. The decoy substrate of a pathogen effector and a Pseudokinase specify pathogen-induced modified-self recognition and immunity in plants. Cell Host Microbe. 2015:18(3):285–295. 10.1016/j.chom.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Wang H, Zou S, Li Y, Lin F, Tang D. An ankyrin-repeat and WRKY-domain-containing immune receptor confers stripe rust resistance in wheat. Nat Commun. 2020:11(1):1353. 10.1038/s41467-020-15139-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hu M, Wang J, Qi J, Han Z, Wang G, Qi Y, Wang H-W, Zhou J-M, Chai J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science. 2019a:364(6435):eaav5870. 10.1126/science.aav5870 [DOI] [PubMed] [Google Scholar]
- Wang J, Song W, Chai J. Structure, biochemical function, and signaling mechanism of plant NLRs. Mol Plant. 2023:16(1):75–95. 10.1016/j.molp.2022.11.011 [DOI] [PubMed] [Google Scholar]
- Wang J, Wang J, Hu M, Wu S, Qi J, Wang G, Han Z, Qi Y, Gao N, Wang H-W, et al. Ligand-triggered allosteric ADP release primes a plant NLR complex. Science. 2019b:364(6435):eaav5868. 10.1126/science.aav5868 [DOI] [PubMed] [Google Scholar]
- Wang L, Albert M, Einig E, Fürst U, Krust D, Felix G. The pattern-recognition receptor CORE of Solanaceae detects bacterial cold-shock protein. Nat Plants. 2016:2(12):16185. 10.1038/nplants.2016.185 [DOI] [PubMed] [Google Scholar]
- Wang L, Ji Y, Hu Y, Hu H, Jia X, Jiang M, Zhang X, Zhao L, Zhang Y, Jia Y, et al. The architecture of intra-organism mutation rate variation in plants. PLoS Biol. 2019c:17(4):e3000191. 10.1371/journal.pbio.3000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jia Y, Osakina A, Olsen KM, Huang Y, Jia MH, Ponniah S, Pedrozo R, Nicolli C, Edwards JD. Receptor-ligand interactions in plant inmate immunity revealed by AlphaFold protein structure prediction. bioRxiv. 2024. 10.1101/2024.06.12.598632, preprint: not peer reviewed. [DOI]
- Watson JL, Juergens D, Bennett NR, Trippe BL, Yim J, Eisenach HE, Ahern W, Borst AJ, Ragotte RJ, Milles LF, et al. De novo design of protein structure and function with RFdiffusion. Nature. 2023:620(7976):1089–1100. 10.1038/s41586-023-06415-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee J, Wei G-W. Evaluation of AlphaFold 3's protein–protein complexes for predicting binding free energy changes upon mutation. J Chem Inf Model. 2024:64(16):6676–6683. 10.1021/acs.jcim.4c00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Kim D, Koehler N, Bendl A, Cho M-J, Krasileva K. Engineering pathogen-inducible promoters for conferring disease resistance in tomato. bioRxiv. 2024. 10.1101/2024.08.30.610566, preprint: not peer reviewed. [DOI]
- Wei Y, Balaceanu A, Rufian JS, Segonzac C, Zhao A, Morcillo RJL, Macho AP. An immune receptor complex evolved in soybean to perceive a polymorphic bacterial flagellin. Nat Commun. 2020:11(1):3763. 10.1038/s41467-020-17573-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Caceres-Moreno C, Jimenez-Gongora T, Wang K, Sang Y, Lozano-Duran R, Macho AP. The Ralstonia solanacearum csp22 peptide, but not flagellin-derived peptides, is perceived by plants from the Solanaceae family. Plant Biotechnol J. 2018:16(7):1349–1362. 10.1111/pbi.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B. The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell. 1994:78(6):1101–1115. 10.1016/0092-8674(94)90283-6 [DOI] [PubMed] [Google Scholar]
- Witek K, Jupe F, Witek AI, Baker D, Clark MD, Jones JDG. Accelerated cloning of a potato late blight–resistance gene using RenSeq and SMRT sequencing. Nat Biotechnol. 2016:34(6):656–660. 10.1038/nbt.3540 [DOI] [PubMed] [Google Scholar]
- Wlodzimierz P, Rabanal FA, Burns R, Naish M, Primetis E, Scott A, Mandáková T, Gorringe N, Tock AJ, Holland D, et al. Cycles of satellite and transposon evolution in Arabidopsis centromeres. Nature. 2023:618(7965):557–565. 10.1038/s41586-023-06062-z [DOI] [PubMed] [Google Scholar]
- Wojciechowski JW, Tekoglu E, Gąsior-Głogowska M, Coustou V, Szulc N, Szefczyk M, Kopaczyńska M, Saupe SJ, Dyrka W. Exploring a diverse world of effector domains and amyloid signaling motifs in fungal NLR proteins. PLoS Comput Biol. 2022:18(12):e1010787. 10.1371/journal.pcbi.1010787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xu W, Zhao G, Lei Z, Li K, Liu J, Huang S, Wang J, Zhong X, Yin X, et al. A canonical protein complex controls immune homeostasis and multipathogen resistance. Science. 2024:386(6728):1405–1412. 10.1126/science.adr2138 [DOI] [PubMed] [Google Scholar]
- Wu Z, Waneka G, Broz AK, King CR, Sloan DB. MSH1 is required for maintenance of the low mutation rates in plant mitochondrial and plastid genomes. Proc Natl Acad Sci U S A. 2020:117(28):16448–16455. 10.1073/pnas.2001998117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff BBH, Thomas CM, Smoker M, Grant M, Jones JDG. Domain swapping and gene shuffling identify sequences required for induction of an avr-dependent hypersensitive response by the tomato Cf-4 and Cf-9 proteins. Plant Cell. 2001:13(2):255–272. 10.1105/tpc.13.2.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrsch I, Domínguez-Ferreras A, Geldner N, Boller T. Tissue-specific FLAGELLIN-SENSING 2 (FLS2) expression in roots restores immune responses in Arabidopsis fls2 mutants. New Phytol. 2015:206(2):774–784. 10.1111/nph.13280 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Wu X, Wang Z, Ji K, Zhao Y, Zhang Y, Wan L. Activation and inhibition mechanisms of a plant helper NLR. Nature. 2025:639(8054):438–446. 10.1038/s41586-024-08517-3 [DOI] [PubMed] [Google Scholar]
- Xu G, Yuan M, Ai C, Liu L, Zhuang E, Karapetyan S, Wang S, Dong X. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature. 2017:545(7655):491–494. 10.1038/nature22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Tang B, Ryder LS, MacLean D, Were VM, Eseola AB, Cruz-Mireles N, Ma W, Foster AJ, Osés-Ruiz M, et al. The transcriptional landscape of plant infection by the rice blast fungus Magnaporthe oryzae reveals distinct families of temporally co-regulated and structurally conserved effectors. Plant Cell. 2023:35(5):1360–1385. 10.1093/plcell/koad036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chen X, Wang Z, Sun Q, Hong A, Zhang A, Zhong X, Hua J. HOS15 and HDA9 negatively regulate immunity through histone deacetylation of intracellular immune receptor NLR genes in Arabidopsis. New Phytol. 2020:226(2):507–522. 10.1111/nph.16380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Z, Hua J. A meta-analysis reveals opposite effects of biotic and abiotic stresses on transcript levels of Arabidopsis intracellular immune receptor genes. Front Plant Sci. 2021:12:625729. 10.3389/fpls.2021.625729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Furzer OJ, Fensterle EP, Lin S, Zheng Z, Kim NH, Wan L, Dangl JL. Paired plant immune CHS3-CSA1 receptor alleles form distinct hetero-oligomeric complexes. Science. 2024:383(6684). 10.1126/science.adk3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Song W, Tan EYJ, Liu L, Cao Y, Jirschitzka J, Li E, Logemann E, Xu C, Huang S, et al. TIR domains of plant immune receptors are 2′,3′-cAMP/cGMP synthetases mediating cell death. Cell. 2022:185(13):2370–2386.e18. 10.1016/j.cell.2022.04.032 [DOI] [PubMed] [Google Scholar]
- Yu DS, Outram MA, Smith A, McCombe CL, Khambalkar PB, Rima SA, Sun X, Ma L, Ericsson DJ, Jones DA, et al. The structural repertoire of Fusarium oxysporum f. sp. lycopersici effectors revealed by experimental and computational studies. eLife. 2024a:12:RP89280. 10.7554/eLife.89280.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Xu W, Chen S, Wu X, Rao W, Liu X, Xu X, Chen J, Nishimura MT, Zhang Y, et al. Activation of a helper NLR by plant and bacterial TIR immune signaling. Science. 2024b:386(6728):1413–1420. 10.1126/science.adr3150 [DOI] [PubMed] [Google Scholar]
- Yuan M, Jiang Z, Bi G, Nomura K, Liu M, Wang Y, Cai B, Zhou J-M, He SY, Xin X-F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature. 2021:592(7852):105–109. 10.1038/s41586-021-03316-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdrzałek R, Stone C, De La Concepcion JC, Banfield MJ, Bentham AR. Pathways to engineering plant intracellular NLR immune receptors. Curr Opin Plant Biol. 2023:74:102380. 10.1016/j.pbi.2023.102380 [DOI] [PubMed] [Google Scholar]
- Zdrzałek R, Xi Y, Langner T, Bentham AR, Petit-Houdenot Y, De La Concepcion JC, Harant A, Shimizu M, Were V, Talbot NJ, et al. Bioengineering a plant NLR immune receptor with a robust binding interface toward a conserved fungal pathogen effector. Proc Natl Acad Sci U S A. 2024:121(28):e2402872121. 10.1073/pnas.2402872121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tian D, Gu K, Zhou Z, Yang X, Luo Y, White FF, Yin Z. Genetic engineering of the Xa10 promoter for broad-spectrum and durable resistance to Xanthomonas oryzae pv. oryzae. Plant Biotechnol J. 2015:13(7):993–1001. 10.1111/pbi.12342 [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu S, Lai H-F, Caflisch A, Zipfel C. Reverse engineering of the pattern recognition receptor FLS2 reveals key design principles of broader recognition spectra against evading flg22 epitopes. bioRxiv. 2024a. 10.1101/2024.10.10.617594, preprint: not peer reviewed. [DOI]
- Zhang X, Liu Y, Yuan G, Wang S, Wang D, Zhu T, Wu X, Ma M, Guo L, Guo H, et al. The synthetic NLR RGA5HMA5 requires multiple interfaces within and outside the integrated domain for effector recognition. Nat Commun. 2024b:15(1):1104. 10.1038/s41467-024-45380-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xia R, Kuang H, Meyers BC. The diversification of plant NBS-LRR defense genes directs the evolution of MicroRNAs that target them. Mol Biol Evol. 2016:33(10):2692–2705. 10.1093/molbev/msw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-M, Ma K-W, Gao L, Hu Z, Schwizer S, Ma W, Song J. Mechanism of host substrate acetylation by a YopJ family effector. Nat Plants. 2017:3(8):1–10. 10.1038/nplants.2017.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y-B, Liu M-X, Chen T-T, Ma X, Li Z-K, Zheng Z, Zheng S-R, Chen L, Li Y-Z, Tang L-R, et al. Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism. Sci Adv. 2022:8(36):eabq5108. 10.1126/sciadv.abq5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Moreno-Pérez A, Coaker G. Understanding plant pathogen interactions using spatial and single-cell technologies. Commun Biol. 2023:6(1):814. 10.1038/s42003-023-05156-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Wu X, Yuan G, Wang D, Bhadauria V, Peng Y-L, Liu J, Zhang X. A resurfaced sensor NLR confers new recognition specificity to non-MAX effectors. J Integr Plant Biol. 2025:67(1):11–14. 10.1111/jipb.13805 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G. Perception of the bacterial PAMP EF-tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006:125(4):749–760. 10.1016/j.cell.2006.03.037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data from Fig. 3 is available on Zenodo (doi:10.5281/zenodo.14207051) and in the associated supplementary information.