Abstract
The development of melt-quenched organic-inorganic metal halide (OIMH) glasses is hampered by the scarcity of suitable organic molten salts and low luminescence efficiency. Herein, we developed a series of two-component OIMH amorphous glasses consisting of (TPG)2MnBr4 (TPG+, triphenylguanidium) and A2MnBr4 (A, organic molten cation), named αG(AxTPGy). The high glass-formation ability (GFA) in (TPG)2MnBr4 provides a platform to modulate the crystallization of another molten A2MnBr4 by homogeneous melting. Moreover, the GFA modulation allows controlled in situ crystallization of αG(AxTPGy) and the formation of transparent glass-crystal composites with higher luminescence efficiency. For instance, the light yield of αG(PTP99TPG1) (PTP+, propyltriphenylphosphonium) is improved from 18,800 to 35,140 photons per mega–electron volt after annealing at 55°C, showing huge application potentials in radiation detection and high-resolution x-ray imaging. The present research would inspire further exploration of high-performance OIMH glasses and facilitate multiple applications in advanced photonics such as scintillators, photoconductive fibers, light-emitting diodes, and laser crystals.
A universal co-melting strategy broadens the exploration of highly luminescent organic-inorganic metal halide glasses.
INTRODUCTION
Scintillation-based x-ray detectors with high sensitivity and efficiency play an important role in various fields such as medical diagnosis, security screening, and non-destructive inspection (1–3). Currently, most commercial radiation detectors are based on all-inorganic materials including Bi4Ge3O12 (BGO), cerium-doped Lu3Al5O12 (LuAG: Ce), and thallium-activated CsI (CsI: TI), which have bright scintillation and high radiation hardness (4, 5). However, their sustainability is hampered by energy-intensive production processes and the high demand for toxic or rare-earth metals. Toward these issues, organic-inorganic hybrid metal halides (OIMHs) are considered a promising category of scintillators (6–8). The presence of metal-halogen polyhedrons (e.g., Sb-Cl, Mn-Br, and Cu-I) gives them strong x-ray absorption capabilities. The versatility in assembling organic and inorganic modules allows the physical and chemical properties to be tailored to practical requirements. Specifically, the eco-friendly Mn(II)-based OIMHs have been consistently reported as high-performance scintillators (9). For instance, (C38H34P2)MnBr4, (C24H20P)2MnBr4, and (C8H20N)2MnBr4 crystals all exhibit high photoluminescence quantum yields (PLQYs) of >90% and excellent light yields of >30,000 photons per mega–electron volt (MeV−1) when exposed to x-ray irradiation (10–12).
The fabrication of large-area scintillation screens is a crucial step in practical applications (13). The prevailing approach for OIMH scintillation screens is to incorporate crystalline powders into organic polymers, whose thickness must be on the order of a few hundred micrometers or even millimeters to ensure sufficient radiation attenuation and scintillation brightness (14, 15). However, such kind of scintillation screens often exhibits poor imaging spatial resolutions below 5 line pairs per millimeter (lp mm−1) due to insufficient phase compatibility, particle agglomeration, or uneven crystal distribution (15–17). Large-area transparent single crystals are another option to achieve high-quality x-ray imaging. Some centimeter-sized OIMH single crystals, (C8H20N)2MnBr4 and (C7H10N2)2MnBr4, have shown higher spatial resolutions than 25 lp mm−1, surpassing the commercial standard (2 to 8 lp mm−1) (18, 19). Although achieving effective light management, the growth of large-size transparent single crystals remains a challenge (20). The discovery of melt-quenched OIMH glass allows the mass production of large-area transparent scintillation screens (21). For now, Mn(II)-based OIMH glasses have achieved high spatial resolutions of 6 to 18 lp mm−1, demonstrating promising potentials in the scintillation field (22–25). However, compared to the tens of thousands of single crystals published in the Cambridge Crystallographic Data Center (CCDC), glassy OIMH scintillators are rare and confined to triphenylphosphonium-based and guanidinium-based materials. The presence of rigid organic cations with larger steric hindrance can effectively suppress crystallization in supercooled melts, but further development of OIMH glasses is hampered by the limitation of suitable organic molten salts, which act as the organic cation in OIMHs (22, 26). Therefore, it is important to develop a straightforward, efficient, and universal method for the preparation of OIMH glasses.
The challenge of preparing OIMH glasses can be divided into two distinct areas. The first is to achieve a stable melt, and this can be solved by using a low melting temperature (Tm) organic salt to construct OIMHs. The second is to ensure a successful phase transition from melt to glass, whereas most OIMH melts tend to crystallize upon quenching, hindering the expansion of novel OIMH glasses (27, 28). Previous studies on amorphous alloys have provided a suitable strategy to improve crystallization resistance of supercooled metal melts, that is, the introduction of a second component (or multiple components) (29, 30). The crystallization in the melt can be modulated by regulating the structural “order” or “entropy,” which helps to maintain the long-range disorder upon quenching. The “Confusion principle” proposed by Greer (31) also suggests that the melt comprising multiple metals tends to be highly disordered, facilitating the formation of amorphous alloys with a large mixing entropy. Recently, Bennett et al. first synthesized the zeolitic imidazolate framework (ZIF) glass comprising ZIF-4 and ZIF-62. The two-component ZIF glass has a single glass transition temperature (Tg) of 306°C that falls between the Tg values of ZIF-4 and ZIF-62, indicating the homogeneous melting of parent materials by melt-quenching (32). Also, another two-component ZIF glass comprising ZIF-8 and ZIF-62 was prepared by flux melting (33). It is conceivable that if this strategy to modulate crystallization is applied to the OIMH materials, it would offer rich opportunities to tailor their physical and chemical properties, thereby stimulating the potential applications in advanced photonics (34).
In this work, we developed a universal strategy toward two-component OIMH glass, which can expand the material systems of OIMH glasses. Specifically, the (TPG)2MnBr4 (TPG+, triphenylguanidium) with a high Tg/Tm ratio of 0.80 and a large viscosity at Tm of 1820 mPa s was firstly synthesized, showing the good glass-forming ability (GFA) and being suitable to modulate the crystallization of two-component OIMH melts. To illustrate the universality and scalability of this strategy, a series of A2MnBr4 crystals (A represents the organic molten cation, including triphenylphosphonium, guanidinium, imidazolium, pyridinium, and quaternary phosphonium/ammonium) were co-melted with the (TPG)2MnBr4, respectively, resulting in the formation of stable two-component amorphous glasses, named αG(AxTPGy). Systematic analysis including thermodynamics, rheology, and structure confirmed the homogeneous melting and structural characteristics of two-component glasses. Meanwhile, the tunable GFA enables the amorphous αG(AxTPGy) glass to be annealed into a transparent glass-crystal composite, named αG–C(AxTPGy), with an improved luminescence efficiency. As an example, the light yield of αG–C(PTP99TPG1) (PTP+, propyltriphenylphosphonium) is increased from 18,800 to 35,140 photons MeV−1 after being annealed at 55°C for 1 day, as well as a low limit of detection (LoD) of 43.6 nGy s−1.
RESULTS
Synthesis of high-GFA (TPG)2MnBr4 glass
The (TPG)2MnBr4 single crystals were grown by dissolving TPG·HBr and MnBr2·4H2O in ethanol at a molar ratio of 2:1 and then evaporating the solvent at 80°C for 2 days. As illustrated in Fig. 1A and table S1, the (TPG)2MnBr4 crystallizes in the monoclinic space group of C2/c, in which isolated [MnBr4]2− tetrahedrons are surrounded by TPG+ cations, forming a zero-dimensional crystal structure. Thermogravimetric analysis (TGA) gives the decomposition temperature (Td) of 241°C. Differential scanning calorimetry (DSC) proves the stable melting with a Tm of 158.5°C, and the glass formation with a Tg of 72.6°C through melt-quenching (Fig. 1B). The structural difference between crystalline and glassy states was declared through powder x-ray diffraction (PXRD), where the pattern of long-range disordered glass shows a diffuse feature (Fig. 1C). Meanwhile, crystallographic measurements including single-crystal XRD (SCXRD) and PXRD were performed to support the successful synthesis of TPG·HBr (fig. S1). The Tg/Tm ratio (assessing the GFA) of (TPG)2MnBr4 is 0.80, which is larger than those of reported Mn-based OIMH glasses, indicating the strong resistance to crystallization (Fig. 1D and table S2) (21–24, 35, 36). Rheological properties including a large viscosity of 1820 mPa s and a high flow activation energy (Ea) of 83.6 kJ mol−1 further suggest its remarkable GFA (fig. S2). As illustrated in fig. S3, the glassy (TPG)2MnBr4 remains amorphous without crystallization even after being annealed at temperatures of 80° to 140°C (between Tg and Tm) for 100 hours. The excellent GFA of (TPG)2MnBr4 glass makes it an ideal parental material for preparing two-component OIMH glass, which can be used to modulate the crystallization of another parent OIMH material during annealing. Meanwhile, the identical Fourier transform infrared (FTIR) spectra between (TPG)2MnBr4 crystal and glass indicate the complete retention of TPG+ cations upon melt-quenching (fig. S4). The local electronic and coordination features around Mn ions were studied using x-ray absorption fine structure (XAFS) analysis. The identical normalized x-ray absorption near edge structure (XANES) spectra at Mn K-edge show similar local structures around Mn ions for (TPG)2MnBr4 crystal and glass. Compared with the Mn foil, their absorption edges shift to higher energy regions and approach the MnO, indicating that the Mn valence state in the glassy state is still +2 (fig. S5). Also, as shown in Fig. 1E, the extended XAFS (EXAFS) data were fitted by Fourier transform in Artemis, in which the main peak is attributed to the local coordination environment of one Mn ion to four Br ions (37). The detailed fitting results are summarized in table S3. The presence of a small amount of Mn-O coordination is probably ascribed to the slight hygroscopicity of some powder samples (38).
Fig. 1. Structural, thermodynamic, and photophysical properties of (TPG)2MnBr4 crystal and glass.
(A) The single crystal structure of (TPG)2MnBr4. (B) TGA and DSC plots of (TPG)2MnBr4 crystal. Inset images are photographs of (TPG)2MnBr4 crystal and glass under natural light and ultraviolet light excitation. (C) PXRD patterns of (TPG)2MnBr4 crystal and glass and the simulated one derived from SCXRD result. (D) Tg/Tm ratios of (TPG)2MnBr4 and other reported Mn-based OIMH glasses [P4444+, tetrahexylphosphonium; MTP+, methyltriphenylphosphonium; ETP+, ethyltriphenylphosphonium; BuTP+, (n-butyl)triphenylphosphonium; HTPP+, hexyltriphenylphosphonium; BTP+, benzyltriphenylphosphonium; DPG+, N,N′-diphenylguanidinium; DOTG+, 1,3-di-o-tolylguanidine]. (E) Fitting Fourier-transformed EXAFS spectra at Mn K-edge of (TPG)2MnBr4 crystal and glass. (F) PLE and PL spectra of (TPG)2MnBr4 crystal and glass. a.u., arbitrary units.
Crystalline and glassy (TPG)2MnBr4 both exhibit the green emission and photoluminescent (PL) decay lifetimes of hundreds of microseconds under 365-nm excitation, originating from the 4T1(G)–6A1(S) transition of tetra-coordinated Mn2+ ions (Fig. 1F and fig. S6A) (39). However, because of the long-range disorder feature, there is severe nonradiative recombination in the (TPG)2MnBr4 glass compared with the crystal, resulting in an emission redshift to 540 nm, a shortened decay lifetime to 193.19 μs, and a photoluminescence quantum yield (PLQY) of 5.1% (fig. S6B). Temperature-dependent PL spectra showed the emission behaviors during a whole melt-quenching process. The complete melting of (TPG)2MnBr4 crystals occurs within the temperature range of 420 to 440 K, resulting in severe PL quenching. Then, the quenched glass shows a weaker PL intensity compared with the crystalline state due to the higher disorder degree (fig. S7). In addition, the (TPG)2MnBr4 glass exhibits green emission under x-ray irradiation with a light yield of 6400 photons MeV−1 and an LoD of 247.1 nGy s−1 (fig. S8). Combined with high transmittance of up to 82% and a strong attenuation efficiency of 92.15% (1 mm thick), the (TPG)2MnBr4 glass displays a high x-ray imaging spatial resolution of 18 lp mm−1 and huge potential for practical scintillation application (figs. S9 and S10).
Preparation of single-component OIMH glasses
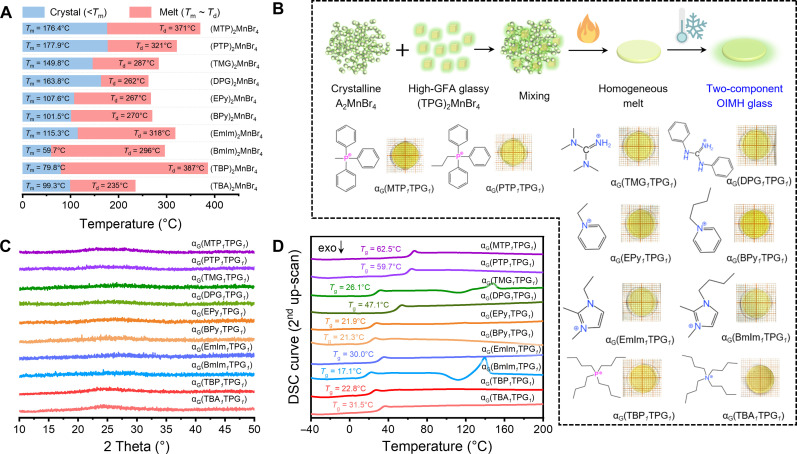
Despite the important application of glassy materials in various scenarios, their exploration is deficient compared with the crystalline materials. Considering that most reported OIMHs decompose at 250° to 400°C, halogenated ionic liquids with low Tm are a good choice. On the basis of the above conditions, as depicted in fig. S11, five classes of low-Tm organic salts (triphenylphosphonium, guanidinium, imidazolium, pyridinium, and quaternary phosphonium/ammonium) were chosen to synthesize A2MnBr4 single crystals. Detail preparation conditions and crystallographic information are summarized in tables S4 and S5 (22, 24, 35, 40, 41). As shown in Fig. 2A and table S6, these crystals exhibit a Tm of <200°C and a ∆T of >98°C (ΔT = Td − Tm), thus achieving a stable melt (TGA and DSC results are illustrated in fig. S12). Also, all of them can be synthesized by the solid-phase melting method, which avoids the use of organic solvents and has the advantages of economy, environmental friendliness, and being suitable for mass production. Specifically, the brominated organic salt and MnBr2·4H2O are mixed in a 2:1 molar ratio and then heated in a muffle furnace at 200°C for 10 min. The crystals are grown by cooling and stirring the melt. As shown in fig. S13, the identical PXRD pattern with the simulated one supports the phase purity.
Fig. 2. Preparation, structure, and thermodynamics of single-component and two-component OIMH glass.
(A) Td and Tm values of single-component A2MnBr4 materials. (B) Preparation process and photographs for two-component OIMH glasses. (C) PXRD patterns of two-component OIMH glasses. (D) The second up-scan heating DSC curves of two-component OIMH glasses.
Notably, most of these melts tend to crystallize upon quenching, while only the (MTP)2MnBr4, (PTP)2MnBr4, and (DPG)2MnBr4 melts could form amorphous glasses with diffuse PXRD patterns (fig. S14). The difference in glass formation can be attributed to the presence of organic cations with large rigid groups, leading to an elevation in melting enthalpy and viscosity, as well as hindering diffusion and rearrangement of atoms in supercooled melts (42). To further investigate the relationship between structure and glass formation, we calculated the radius of guanidinium cations including TMG+ (4.307 Å), DPG+ (6.575 Å), and TPG+ (7.148 Å), which increases with the number of benzene groups (43, 44). The larger DPG+ and TPG+ favor the formation of OIMH glasses, while the molten (TMG)2MnBr4 crystallizes during cooling (fig. S15, A to C). Meanwhile, although the radius of TBP+ (6.709 Å) is larger than that of MTP+ (5.902 Å) and PTP+ (6.042 Å), it is prone to crystallize during quenching because of the lack of a rigid group, which results in a Tg below the room temperature (fig. S15, D to F). The DSC curves confirm the above phenomenon (fig. S16). The Tg values of these glasses are affected by the rigidity of organic cations, where (MTP)2MnBr4 and (TPG)2MnBr4 glasses have a high Tg value of 57.7° and 72.6°C, respectively. A relatively high Tg allows glassy OIMH to be processed and used over a wider temperature interval. Nevertheless, the synthesis and modification of suitable low-Tm rigid organic salts involve complex organic reactions and experimental trials, rendering it challenging to screen a suitable candidate for preparing novel OIMH glasses with optimal Td, Tm, and Tg.
Preparation of two-component OIMH glasses
The preparation of two-component OIMH glasses is considered a potential solution to the above challenge. The introduction of high-GFA (TPG)2MnBr4 melt can effectively regulate the crystallization tendency of A2MnBr4 melt, thereby forming a stable two-component OIMH glass. To demonstrate the universality of this method, as shown in Fig. 2B, all A2MnBr4 crystals were co-melted with (TPG)2MnBr4 at 220°C at a mass ratio of 1:1, respectively, and then cooled to room temperature to obtain transparent two-component OIMH glasses, named αG(AxTPGy) (x:y is the mass ratio of A2MnBr4 and (TPG)2MnBr4). PXRD patterns verify their amorphous state and further demonstrate the feasibility of this method (Fig. 2C). The DSC plots in Fig. 2D exhibit that all amorphous αG(A1TPG1) have a singlet Tg located between A2MnBr4 and (TPG)2MnBr4, suggesting that the parental materials underwent a homogeneous melting process, instead of forming the separate domains (32). Such kind of phenomena is also observed in the fields of metallic glass, organic polymer, or metal-organic framework glasses (45–47). Notably, the different thermodynamic performances between A2MnBr4 and αG(A1TPG1) highlight the GFA modulation effect of high-GFA (TPG)2MnBr4 on the two-component OIMH glass. As shown in Fig. 3A, the increased Tg value of αG(A1TPG1) affords an increased Tg/Tm ratio and enhanced GFA compared with single-component A2MnBr4, thereby forming the amorphous glass. The Tg values of low-GFA A2MnBr4 are predicted in table S7 using a simplified Fox model
where φ1, φ2 is the weight fraction of the parent materials. It can be used to predict the vitrification tendency of binary polymer mixtures, especially for those mixtures where the Tg is linearly dependent on the composition (45, 48). Notably, although there are currently no reported examples demonstrating the applicability of the Fox model to OIMH materials, the simulated Tg values of A2MnBr4 using the Fox model (fig. S17) are very close to the DSC test results (an error is less than 5%).
Fig. 3. Thermodynamic, rheological, and structural analysis of two-component OIMH glasses.
(A) Tg/Tm ratios of A2MnBr4 and αG(A1TPG1). (B) Viscosities and flow activation energies of A2MnBr4 and αG(A1TPG1). (C) Fitting Fourier-transformed Mn K-edge EXAFS spectra of αG(PTP1TPG1) glass. (D) PDFs for two-component αG(PTP1TPG1) and αG(PTP9TPG1) glasses.
Viscosity (η), as an indicator describing the migration ability of liquids, is closely related to the intermolecular forces and microstructure of the melt. According to the classical nucleation theory, the nucleation and growth of crystals are inversely proportional to the viscosity, where migration of atoms in high-viscosity melts is effectively inhibited and benefits the formation of glass. The η(Tm) value of molten A2MnBr4 markedly increases after co-melting with (TPG)2MnBr4, indicating stronger resistance to crystallization (Fig. 3B). At the same time, the η values of all A2MnBr4 are close to that of (TPG)2MnBr4 at the preparation temperature (220°C), which improves the flow and diffusion of melts and allows for homogeneous dispersion of two OIMH-based parent materials (fig. S18A). The smooth viscosity curves of αG(A1TPG1) as a function of temperature also prove the uniformity of the co-melting process (fig. S18B). Further analysis of the temperature-dependent viscosity data provides insight into the melt dynamics. As shown in fig. S19, the flow activation energy (Ea), which represents the energy barrier encountered by each component in the melt to diffuse at a specific temperature, can be calculated using the Arrhenius model (0.9 < Tm/T < 1.0)
where A is a constant, R is the molar gas constant, and Ea is correlated with the evolution of melt structure in the cooling process (49). The high Ea value of two-component OIMH melt displays a rapid increase in the η value as the temperature decreases, which hampers the migration of molecules and then facilitates the formation of amorphous αG(A1TPG1) (Fig. 3B and table S8). Although the Ea values are temperature-dependent because of the limitations of the Arrhenius model when extrapolated over a broader temperature range, their comparative evolution still provides valuable insights. The temperature range of 0.9 < Tm/T < 1.0 was carefully selected for fitting, which corresponds to the duration of the complete flowing homogeneous melt. At this condition, the structure of the material is relatively stable, and the molecular movement is mainly affected by temperature and the intrinsic properties of the molecular chains, thereby minimizing the impact of structural heterogeneity on the measurements. To further understand the dynamics of supercooled melts, we adopted the improved Mauro-Yue-Ellison-Gupta-Allan model as shown below (0.7 < Tg/T < 1.0)
where η∞ is the η value at an infinitely high temperature, and m is the fragility index, which describes changes in the viscosity of a melt as a function of temperature (50). As shown in fig. S20, the m values of αG (A1TPG1) glasses span from 85.2 to142.1, exceeding those of commercial inorganic glasses such as soda-lime-silica glass (15–25), borosilicate glass (10–21), and lead glass (18–28). Relatively high m values observed in αG(A1TPG1) are attributed to weaker intermolecular interactions compared with inorganic glass, indicating a smoother transition in the η value from the high-temperature flow state to the supercooled state. This property facilitates the processing of glasses over a wide temperature range and prevents the formation of irregularities, defects, or stress concentrations.
The XAFS experiment was carried out to explore the structural features of two-component OIMH glass. As shown in fig. S21, the normalized Mn K-edge XANES spectrum of αG(PTP1TPG1) is nearly identical to that of (TPG)2MnBr4, indicating that there are similar local structures around Mn ions. Their absorption edges are close to that of MnO, which proves that the Mn valence of two-component glasses remains +2. The EXAFS data of two-component αG(PTP1TPG1) were fitted by Fourier transform in Artemis, where the main peak demonstrates the local four-coordination environment of Mn-Br (Fig. 3C and table S9). Complementary pair distribution function (PDF) analysis was used to further probe the local atomic arrangements in two-component amorphous OIMH glasses. As shown in Fig. 3D, both αG(PTP1TPG1) and αG(PTP9TPG1) glasses exhibit distinct flat features between 6 and 30 Å, indicating the absence of long-range ordered structure. The preservation of identical PDF oscillation profiles below 6 Å reveals remarkable short-range structural consistency across different compositions. The prominent peaks at 2.5 and 4.1 Å are attributed to Mn-Br and Br-Br atomic pairs due to the larger x-ray scattering strength of Mn and Br relative to lighter elements. In addition, these peaks ranging from 0 to 2 Å are assigned to C/N–H, C–C/N, and C–P atomic pairs within isolated organic cations. These structural characterizations confirm the invariance of organic cations (PTP+ and TPG+) and inorganic [MnBr4]2− units in the glass state, as well as the retention of a zero-dimensional structure at the molecular level.
Energy-dispersive spectroscopy (EDS) elemental mapping verified uniform dispersion of two parent materials during co-melting. For the single-component (TPG)2MnBr4 glass, Mn and N elements are uniformly dispersed (fig. S22). Similarly, in the two-component αG(PTP1TPG1) glass, Mn, N [belong to (TPG)2MnBr4), and P [belongs to (PTP)2MnBr4] elements are homogeneously dispersed, as evidenced by EDS mapping at both micrometer and nanometer scales (fig. S23). As a complementary experiment, we also attempted to extend the two-component glasses to other molten metal halides (e.g. Sb- and Pb-based OIMHs). As shown in fig. S24 (A and B), the introduction of (ETP)2SbCl5 (ETP, ethyltriphenylphosphonium) enhanced the GFA of (Bzmim)2SbCl5 (Bzmim, 1-benzyl-3-methylimidazolium) and increased its Tg value to 34.0°C. In addition, the recrystallization behavior of (BPy)2PbBr4 was also suppressed by co-melting with (ETP)2PbBr4, leading to an increase in Tg to 37.9°C (fig. S24, C and D). Meanwhile, we have further extended this strategy to prepare the three-component Mn-based glass consisting of (TPG)2MnBr4, (DPG)2MnBr4, and (TMG)2MnBr4. In addition, another three-component glass containing different metal-halide units was also prepared by co-melting (TPG)2MnBr4, (ETP)2SbCl5, and (ETP)2PbBr4. Their single Tg values between the parent materials support the occurrence of homogeneous mixing (fig. S25).
On the basis of the above discussion on preparing two-component OIMH glasses, it can be envisaged that parent materials should meet the following requirements. Firstly, they need to have considerable thermal stability to ensure a stable melting process. Second, it is necessary to screen a high-GFA parent material that can regulate the GFA of another one by co-melting. Third, their rheological properties at the preparation temperature must be similar, which helps to achieve homogeneous melting (51). Furthermore, the scintillation performance of two-component OIMH glasses has been evaluated in fig. S26, where αG(MTP1TPG1) and αG(PTP1TPG1) have higher light yields (8810 and 10,240 photons MeV−1) and lower LoD (203.9 and 164.0 nGy s−1) compared with (TPG)2MnBr4 glass, while others exhibit the suboptimal scintillation performance (table S10). This discrepancy is due to the low Tg value (<0°C) of low-GFA A2MnBr4. The absence of large rigid aromatic cations reduces molecular structural rigidity and leads to severe nonradiation recombination at room temperature, resulting in inferior scintillation performance.
In situ crystallization of two-component OIMH glasses
In situ crystallization of the glassy matrix by annealing is an effective post-processing strategy for enhancing the scintillation performance. This method allows for the combination of high optical transparency of the glassy state and efficient luminescence of the crystalline state (52). However, the in situ crystallization in the single-component OIMH glass is challenging to control, which often results in diminished transparency and pronounced light scattering. As shown in fig. S27, both single-component (MTP)2MnBr4 and (PTP)2MnBr4 glasses underwent a glass-crystal transition process upon being heated at 70°C for 20 hours, while the αG(TPG1MTP1) and αG(TPG1PTP1) with better GFA remained amorphous even after being heated for 100 hours. The PXRD patterns before and after heat treatment support the above phenomenon. The two-component OIMH glass discussed above may serve as an appropriate choice. The GFA can be modulated by regulating the mass ratio of parent materials, thus achieving controlled in situ crystallization without notable reduction in light transmittance. Given a high light yield of 10,240 photons MeV−1 and a suitable Tg of 59.7°C for the αG(PTP1TPG1), a series of αG(PTPxTPGy) with varying x:y ratios were annealed to prepare the glass-crystal composite, expressed as αG–C(PTPxTPGy). As shown in Fig. 4A, we first measured the Tg values of αG(PTPxTPGy) and found that there is a strong linear dependence between Tg and the mass ratio of parent materials, which proves the homogeneous mixing and allows us to modulate the Tg value within the range of 47.0° to 72.6°C. In general, the annealing temperature of glass for in situ crystallization is slightly higher than its Tg. At lower temperatures, the diffusion and migration of atoms in melts are impeded, while noticeable softening deformation or excessive crystallization occurs at higher temperatures. Accordingly, we tried to anneal the αG(PTPxTPGy) samples with different x:y ratios (1:1 to 499:1) and (PTP)2MnBr4 glass at 55°C for 1 day (Fig. 4B).
Fig. 4. In situ crystallization and scintillation properties of αG–C(PTPxTPGy).
(A) The Tg values of αG(PTPxTPGy) versus the mass ratio of parent materials. (B) Schematic diagram of the in situ crystallization process. (C) Light transmittance spectra of annealed αG–C(PTPxTPGy) with varying x:y ratios. (D) PXRD patterns of αG–C(PTPxTPGy). (E) Light yields of αG(PTPxTPGy) and αG–C(PTPxTPGy). (F) Fitting signal-to-noise ratio (SNR) of αG–C(PTPxTPGy) under low-dose-rate irradiation.
Photophysical and structural analyses were conducted to characterize these annealed samples. As shown in Fig. 4C, αG–C(PTPxTPGy) samples with an x:y ratio of ≤99:1 exhibit high light transmittance achieving 80% in the visible light range. In contrast, other annealed samples with a higher content of (PTP)2MnBr4 exhibit severe devitrification and poor transparency (fig. S28). The presence of a crystalline phase has been verified through PXRD patterns. As shown in Fig. 4D, αG–C(PTPxTPGy) samples with an x:y ratio of ≤19:1 remained amorphous because of the addition of increased amounts of high-GFA (TPG)2MnBr4, while those with an x:y ratio of ≥39:1 exhibited high-intensity, sharp crystalline peaks. However, the PXRD patterns of annealed αG–C(PTPxTPGy) are different from the simulated pattern of (PTP)2MnBr4 crystals grown by the solution method. A reasonable explanation is that (PTP)2MnBr4 crystals undergo a phase transition before melting, which is supported by the DSC curve with an extra exothermal peak at 120.3°C (fig. S29A). The difference in PXRD patterns of (PTP)2MnBr4 crystals before and after heating at 130°C supports this explanation (fig. S29B). Thermogravimetric-infrared spectroscopy (TG-IR) results proved that the phase transition came from the removal of acetone molecules, where the IR spectrum of gas-phase acetone was observed at 120°C (fig. S30).
Since the poor transparency of αG–C(PTPxTPGy) samples with an x:y ratio of ≥149:1 is detrimental to practical application in radiation detection and x-ray imaging, we focus on the scintillation performance of transparent αG–C(PTPxTPGy) samples with an x:y ratio of ≤99:1. The presence of crystalline (PTP)2MnBr4 enhances the luminescence efficiency of annealed samples due to its higher PLQY of 86.8% than that of glassy state (44.0%) (fig. S31). As shown in Fig. 4E, the light yield of annealed samples with an x:y ratio ranging from 39:1 to 99:1 is 86 to 95% higher than that of amorphous αG(PTPxTPGy). For instance, after being annealed at 55°C for 1 day, the light yield of αG–C(PTP99TPG1) increased from 18,800 photons MeV−1 to 35,140 photons MeV−1, as well as an LoD value as low as 43.6 nGy s−1, which is about 126 times below the medical x-ray diagnostic dose of 5.5 μGy s−1 (Fig. 4F and table S11) (53). The high light transmittance of up to 80% also makes it promising for a variety of photonics applications such as scintillators, photoconductive fibers, light-emitting diodes, and laser crystals. The scintillation properties of annealed samples with an x:y ratio ≤19:1 were not improved because of the stronger resistance to crystallization, remaining in an amorphous state after annealing treatment. Overall, the combination of the preparation and in situ crystallization of two-component OIMH glass provides a robust approach for fabricating high-performance scintillation screens.
Application in the x-ray imaging and mechanical properties
To assess the feasibility of αG–C(PTPxTPGy) scintillators for practical x-ray imaging, a self-built x-ray imaging system was constructed, comprising an x-ray tube, a shading film, a high-reflection mirror, and a digital camera (Fig. 5A). Firstly, these scintillators with varying x:y ratios were used to inspect the internal structure of a chip. As illustrated in Fig. 5B, there are discernible disparities in the image quality. The imaging brightness and contrast improve as the x:y ratio increases from 1:1 to 99:1. The annealed samples with a larger x:y ratio of 149:1, 299:1, 499:1, and even pure (PTP)2MnBr4, suffer an obvious loss in optical transparency and lead to a notable decline of x-ray imaging quality (fig. S32A). The imaging quality of annealed (TPG)2MnBr4 is close to the αG–C(PTP1TPG1) due to the strong resistance to crystallization (fig. S32B). The extracted gray value contours along the lines of the red box highlight the advantages of αG–C(PTP99TPG1), which provide the highest brightness and contrast because of its high light yield of 35140 photons MeV−1 (Fig. 5C). At the same time, the spatial resolution of αG–C(PTP99TPG1) was determined to be 20 lp∙mm−1 using a standard line pair card, outperforming most of the reported OIMH scintillation, which is also proved by corresponding gray value contours (Fig. 5D, E). Moreover, we demonstrated the feasibility of the αG–C(PTP99TPG1) in dynamic x-ray imaging. As shown in Fig. 5F and movie S1, the x-ray images of the rotating lead fan blade were monitored. The x-ray images at 0.1-s intervals show clear outlines of the lead blade without ghosting effects. Considering the inspiring scintillation performance, the αG–C(PTP99TPG1) distinguishes itself as a potential candidate for dynamic x-ray imaging.
Fig. 5. Scintillation application of αG–C(PTPxTPGy).
(A) Schematic representation of the home-built x-ray imaging system, the target subject was placed between the x-ray source and shading film. (B) X-ray images of a chip using αG–C(PTPxTPGy) with varying x:y ratios (total dose of one image is 0.4 mGy). (C) Gray value contours along the lines of the red box extracted from Fig. 4B. (D) Spatial resolution of the αG–C(PTP99TPG1) determined by a lead-made line pair card. (E) Gray value contours extracted from Fig. 4D. (F) Dynamic x-ray images of rotating lead fan blade using the αG–C(PTP99TPG1) (total dose of one image is 22.6 μGy).
Nanoindentation technology has been used to investigate the mechanical properties of the scintillation screens, including a single-component (TPG)2MnBr4 glass, a two-component αG(PTP99TPG1) glass, and an annealed αG–C(PTP99TPG1) sample. As shown in Fig. 6A, the load-displacement curves obtained from five parallel tests are smooth with no breaks or turning points, indicating that these screens have relatively uniform and stable microstructures, and will not experience crack propagation or local collapse due to local stress concentration. The Young’s moduli (E) and hardness (H) were illustrated in Fig. 6B and fig. S33. Within the displacement range from 600 to 1000 nm, the E and H values tend to be stable and are less affected by the depth of the indentation. The three OIMH-based screens exhibit similar mechanical properties, with E values ranging from 5.52 to 5.79 GPa and H values ranging from 0.229 to 0.269 GPa. In addition, we compared the E and H values between OIMH-based screens and other reported or normal materials. As shown in Fig. 6C and table S12, these values of OIMH glasses are approximately located between those of organic polymers and inorganic or metallic glasses and are close to those reported organic-inorganic hybrid glasses, such as ZIF glasses (54–56).
Fig. 6. Mechanical properties.
(A) Load-displacement curves recorded in five parallel tests. (B) Young’s modulus (E) as a function of indentation depth for (TPG)2MnBr4 glass, αG (PTP99TPG1), and annealed αG–C(PTP99TPG1). (C) The summary of E and H values for polymers, OIMH glasses, ZIF glasses, inorganic glasses, and metallic glasses.
DISCUSSION
In conclusion, we have developed a universal strategy for preparing two-component OIMH glasses and transparent glass-crystal composites. The introduction of (TPG)2MnBr4 with high-GFA provides a platform to modulate the crystallization of A2MnBr4 melts by homogeneous melting. The idea of two-component OIMH melting allows those that are easy to crystallize to remain amorphous glassy state, which is supported by related thermodynamic and rheological analysis. The stable formation of multiple two-component OIMH glasses demonstrates the generality and scalability of the present strategy, which will greatly expand the scope of the glassy OIMH system. Moreover, the GFA modulation enables controlled in situ crystallization of two-component OIMH glasses, leading to the formation of transparent glass-crystal composites with higher luminescence efficiency. The facile implementation of high-performance glass-crystal composites will greatly stimulate the promising applications of OIMH materials in various photonics and electronics fields.
MATERIALS AND METHODS
Materials
The chemicals used are as follows: TPG (TCI, 97%), MTP·HBr (Aladdin, 99%), PTP·HBr (Aladdin, 99%), TMG (Bidepharm, 97%), DPG·HBr (Bidepharm, 98%), EPy·HBr (Bidepharm, 99%), BPy·HBr (Bidepharm, 95%), EmIm·HBr (Aladdin, 99%), BmIm·HBr (Aladdin, 98%), TBP·HBr (Aladdin, 98%), TBA·HBr (Aladdin, 99%), MnBr2·4H2O (Aladdin, 98%), hydrobromic acid (Aladdin, 48 weight% in water,), anhydrous methanol (Guangzhou Chemical Reagent, ≥99.5%), anhydrous ethanol (Guangzhou Chemical Reagent, ≥99.5%), isobutanol (Macklin, ≥99.0%), dichloromethane (Guangzhou Chemical Reagent, ≥99.9%), acetone (Guangzhou Chemical Reagent, ≥99.9%), acetonitrile (Guangzhou Chemical Reagent, ≥99%), and ethyl acetate (General-reagent, ≥99.5%). All the chemicals were used without further purification.
Synthesis of TPG·HBr single crystals
TPG was dissolved in a mixed solution of methanol and hydrobromic acid. The precursor solution was stirred and then slowly evaporated at 80°C for several hours. The crystals were precipitated and washed with ethyl acetate.
Synthesis of (TPG)2MnBr4 single crystals
TPG·HBr (2 mmol) and MnBr2·4H2O (1 mmol) were added to ethanol and then stirred at 80°C to form a clear solution. The precursor solution was slowly evaporated at 80°C for 2 days. Transparent crystals were precipitated and washed several times with ethyl acetate.
Synthesis of A2MnBr4 single crystals (A, organic cations)
The A2MnBr4 single crystals were synthesized through solvent evaporation or cooling-induced crystallization methods. Specifically, the organic salt and MnBr2·4H2O at a molar ratio of 2:1 were dissolved in the organic solvent under magnetic stirring. Then, the clear precursor solution was evaporated at 40° to 80°C or was slowly cooled to room temperature to obtain transparent crystals.
Preparation of A2MnBr4 and (TPG)2MnBr4 glasses
All OIMH glasses mentioned in the manuscript were prepared using the melt-quenching method. First, the hybrid manganese bromide crystals were heated in a muffle furnace for 10 to 20 min to form a clear melt. Then, the bubbles in the melt were removed using a vacuum-drying oven. The transparent glass was formed upon cooling to room temperature naturally.
Preparation of two-component αG(AxTPGy) and αG–C(AxTPGy)
The amorphous αG(AxTPGy) sample was prepared by the melt-quenching process. The (TPG)2MnBr4 glass was mixed with A2MnBr4 crystals in a special mass ratio. The mixture was then heated in a vacuum muffle furnace at 220°C to form a homogenous melt. Last, the melt was cooled to room temperature to form a transparent αG(PTPxTPGy). The αG–C(PTPxTPGy) samples were obtained by annealing the αG(PTPxTPGy) at 55°C for 1 day.
Crystallography measurements
SCXRD was performed on a Bruker D8 VENTURE diffractometer with Mo-Kα radiation (λ = 0.71073 Å). The data were solved by the intrinsic phasing method (SHELXT) and refined by the SHELXL package included in the Olex2 software (57, 58). Deposited CCDC numbers are 2,378,331 [for (TPG)2MnBr4], 2,380,213 [for (PTP)2MnBr4], 2,382,136 [for (TMG)2MnBr4], 2,408,182 [for (BmIm)2MnBr4], and 2,408,056 [for TPG·HBr]. PXRD patterns were collected using Cu-Kα radiation (λ = 1.54184 Å) on a Rigaku Mini-flex600 diffractometer.
Thermodynamics and rheological measurements
TGA and DSC data were obtained using NETZSCH TG 209F1 Libra system and NETZSCH DSC 214, respectively. Synchronous TG-IR spectra were carried out on a NETZSCH STA449F3/Nicolet 6700 system. All measurements were performed in a nitrogen atmosphere with a heating rate of 10 K min−1. Temperature-dependent viscosity data were collected on a HAAKE MARS40 rotary rheometer. The samples were preheated to form melt and then cooled down at a rate of 10 K min−1, and the viscosity values were recorded. A shear rate of 10 s−1 was used for the measurements.
Spectroscopic and mechanical measurements
Relevant photoluminescence spectra were obtained using an Edinburgh FLS980 spectrometer equipped with the Oxford cryostat. PLQY values were measured by using the Hamamatsu C9920 instrument. FTIR spectra were performed on the PerkinElmer Frontier spectrophotometer. The light transmittance spectra were recorded by a Shimadzu UV-3600 spectrophotometer. The XAFS spectra on (TPG)2MnBr4 crystals and glass were obtained using the RapidXAFS (Anhui Absorption Spectroscopy Analysis Instrument Co. Ltd.) by transmission mode at 10 kV and 20 mA, and a Si (440) spherically bent crystal analyzer was used for Mn K-edge. The XAFS experiments for αG(PTP1TPG1) were carried out on the sample BL11 second hatch x-ray absorption beamline of the SAGA Light Source. The energy resolution of the station ranges from 10−4 to 10−3 ΔE/E, with a photon flux ranging from 2 × 109 to 1 × 108 photons s−1, corresponding to x-ray energies in the range of 2.1 to 14 keV. Nanoindentation experiments were performed using the KLA iMicro equipped with a Berkovich diamond indenter tip. All tests were conducted under the continuous stiffness measurement. The EDS mapping was carried out on the GeminiSEM 500.
PDF analysis
The high-resolution synchrotron x-ray total scattering measurements were performed at beamline ID31 of the European Synchrotron Radiation Facility. The sample powders were loaded into cylindrical slots (approx. 1-mm thickness) held between Kapton windows in a high-throughput sample holder. Each sample was measured in transmission with an incident x-ray energy of 75.051 keV (λ = 0.16520 Å). Measured intensities were collected using a Pilatus CdTe 2M detector (1679 × 1475 pixels, 172 μm by 172 μm each) positioned with the incident beam in the corner of the detector. The sample-to-detector distance was approximately 0.3 m for the total scattering measurement. Background measurements for the empty windows were measured and subtracted. National Institute of Standards and Technology (NIST) SRM 660b (LaB6) was used for geometry calibration. The integration of the 2D XRD pattern was performed with the software pyFAI, followed by image integration, including flat-field, geometry, solid-angle, and polarization corrections. The PDF data obtained from the experiment were processed using PDFgetX3 software (59–61).
Scintillation and x-ray imaging
The x-ray attenuation efficiency (AE) is calculated using the following equation
where t is the total attenuation coefficient obtained from the XCOM database of the NIST. ρ and d denote the density(gram per cubic centimeter) and thickness (centimeter) of scintillators. The commercial LuAG: Ce single crystal was used as a reference to estimate the light yield of these samples. An Amptek Mini-X2 x-ray tube (target: Ag) and an Ocean Optics portable spectrometer (QEpro) equipped with an integrating sphere were used to construct the measurement system. The size of these samples is consistent with the LuAG: Ce (diameter = 13 mm, thickness = 1 mm). The measured photon counts are normalized via the equation below
where Pmeasured denotes the absolute number of photons and AE(d) is the attenuation efficiency (%) of the sample at a certain thickness. The LoD is defined as the dose rate when the signal-to-noise ratio equals 3. An H10721-210 photomultiplier tube was used to collect the photons produced by the sample under different dose rates and convert them to signal currents. The noise data were obtained in the absence of the x-ray radiation. The dose rate was calibrated by the Radical x-ray dosimeter.
Acknowledgments
We acknowledge the help in SEM, DSC, and ICP-MS analysis from Instrumental Analysis & Research Center, Sun Yat-sen University.
Funding: We acknowledge the financial support from the National Natural Science Foundation of China (22375220 and U2001214).
Author contributions: Conceptualization: D.-B.K. and Z.-L.H. Methodology: D.-B.K., Z.-L.H., J.-B.L., J.-H.C., and J.-H.W. Validation: D.-B.K., Z.-L.H., J.-B.L., J.-H.C., Z.-Z.Z., and Q.-P.P. Formal analysis: D.-B.K., Z.-L.H., J.-B.L., X.-H.M., and X.-X.G. Investigation: Z.-L.H., J.-B.L., J.-H.C., J.-H.W., X.-H.M., Z.-Z.Z., and Q.-P.P. Resources: D.-B.K. and X.-H.M. Data curation: D.-B.K. and Z.-L.H. Writing—original draft: Z.-L.H. Writing—review and editing: D.-B.K., Z.-L.H., J.-B.L., J.-H.W., and X.-H.M. Visualization: D.-B.K., Z.-L.H., J.-H.C., J.-H.W., Z.-Z.Z., and X.-X.G. Supervision, project administration, and funding acquisition: D.-B.K.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
The PDF file includes:
Figs. S1 to S33
Tables S1 to S12
Legend for movie S1
Other Supplementary Material for this manuscript includes the following:
Movie S1
REFERENCES AND NOTES
- 1.Roques-Carmes C., Rivera N., Ghorashi A., Kooi S. E., Yang Y., Lin Z., Beroz J., Massuda A., Sloan J., Romeo N., Yu Y., Joannopoulos J. D., Kaminer I., Johnson S. G., Soljačić M., A framework for scintillation in nanophotonics. Science 375, eabm9293 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Ou X., Qin X., Huang B., Zan J., Wu Q., Hong Z., Xie L., Bian H., Yi Z., Chen X., Wu Y., Song X., Li J., Chen Q., Yang H., Liu X., High-resolution x-ray luminescence extension imaging. Nature 590, 410–415 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Chen Q., Wu J., Ou X., Huang B., Almutlaq J., Zhumekenov A. A., Guan X., Han S., Liang L., Yi Z., Li J., Xie X., Wang Y., Li Y., Fan D., Teh D. B. L., All A. H., Mohammed O. F., Bakr O. M., Wu T., Bettinelli M., Yang H., Huang W., Liu X., All-inorganic perovskite nanocrystal scintillators. Nature 561, 88–93 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Melcher C. L., Thermoluminescence and radiation damage in bismuth germanate. Nature 313, 465–467 (1985). [Google Scholar]
- 5.Lin Z., Lv S., Yang Z., Qiu J., Zhou S., Structured scintillators for efficient radiation detection. Adv. Sci. 9, 2102439 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L.-J., Lin X., He Q., Worku M., Ma B., Highly efficient eco-friendly x-ray scintillators based on an organic manganese halide. Nat. Commun. 11, 4329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., Li M., He Y., Song J., Guo K., Pan W., Wei H., Arising 2D perovskites for ionizing radiation detection. Adv. Mater. 36, e2309588 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Li M., Xia Z., Recent progress of zero-dimensional luminescent metal halides. Chem. Soc. Rev. 50, 2626–2662 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Zhang W., Zheng W., Li L., Huang P., Xu J., Zhang W., Shao Z., Chen X., Unlocking the potential of organic–inorganic hybrid manganese halides for advanced optoelectronic applications. Adv. Mater. 36, e2408777 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Liu L., Hu H., Pan W., Gao H., Song J., Feng X., Qu W., Wei W., Yang B., Wei H., Robust organogel scintillator for self-healing and ultra-flexible x-ray imaging. Adv. Mater. 36, e2311206 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Han K., Sakhatskyi K., Jin J., Zhang Q., Kovalenko M. V., Xia Z., Seed-crystal-induced cold sintering toward metal halide transparent ceramic scintillators. Adv. Mater. 34, e2110420 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Ma W., Liang D., Qian Q., Mo Q., Zhao S., Cai W., Chen J., Zang Z., Near-unity quantum yield in zero-dimensional lead-free manganese-based halides for flexible x-ray imaging with high spatial resolution. eScience 3, 100089 (2023). [Google Scholar]
- 13.Zhou B., Yan D., Glassy inorganic-organic hybrid materials for photonic applications. Matter 7, 1950–1976 (2024). [Google Scholar]
- 14.Shao W., He T., Wang L., Wang J.-X., Zhou Y., Shao B., Ugur E., Wu W., Zhang Z., Liang H., De Wolf S., Bakr O. M., Mohammed O. F., Capillary manganese halide needle-like array scintillator with isolated light crosstalk for micro-X-ray imaging. Adv. Mater. 36, e2312053 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Shonde T. B., Chaaban M., Liu H., Olasupo O. J., Ben-Akacha A., Gonzalez F. G., Julevich K., Lin X., Winfred J. S. R. V., Stand L. M., Zhuravleva M., Ma B., Molecular sensitization enabled high performance organic metal halide hybrid scintillator. Adv. Mater. 35, e2301612 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Feng T., Zhou Z., An Y., Chen L., Fu Y., Zhou S., Wang N., Zheng J., Sun C., Large-Area transparent antimony-based perovskite glass for high-resolution x-ray imaging. ACS Nano 18, 16715–16725 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Wei J.-H., Liao J.-F., Wang X.-D., Zhou L., Jiang Y., Kuang D.-B., All-inorganic lead-free heterometallic Cs4MnBi2Cl12 perovskite single crystal with highly efficient orange emission. Matter 3, 892–903 (2020). [Google Scholar]
- 18.Zhang Z.-Z., Wei J.-H., Luo J.-B., Wang X.-D., He Z.-L., Kuang D.-B., Large-area laminar TEA2MnI4 single-crystal scintillator for x-ray imaging with impressive high resolution. ACS Appl. Mater. Interfaces 14, 47913–47921 (2022). [DOI] [PubMed] [Google Scholar]
- 19.He Z.-L., Wei J.-H., Zhang Z.-Z., Luo J.-B., Kuang D.-B., Manganese-halide single-crystal scintillator toward high-performance x-ray detection and imaging: Influences of halogen and thickness. Adv. Optical Mater. 11, 2300449 (2023). [Google Scholar]
- 20.Xu X., Xie Y.-M., Shi H., Wang Y., Zhu X., Li B.-X., Liu S., Chen B., Zhao Q., Light management of metal halide scintillators for high-resolution x-ray imaging. Adv. Mater. 36, e2303738 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Luo J.-B., Wei J.-H., Zhang Z.-Z., He Z.-L., Kuang D.-B., A melt-quenched luminescent glass of an organic–inorganic manganese halide as a large-area scintillator for radiation detection. Angew. Chem. Int. Ed. Engl. 62, e202216504 (2023). [DOI] [PubMed] [Google Scholar]
- 22.He Z.-L., Wei J.-H., Luo J.-B., Zhang Z.-Z., Chen J.-H., Guo X.-X., Kuang D.-B., Guanidinium-based manganese(II) bromide with high glass-forming ability for thermoplastic curved x-ray imaging. Laser Photonics Rev. 18, 2301249 (2024). [Google Scholar]
- 23.Li B., Jin J., Yin M., Han K., Zhang Y., Zhang X., Zhang A., Xia Z., Xu Y., In situ recrystallization of zero-dimensional hybrid metal halide glass-ceramics toward improved scintillation performance. Chem. Sci. 14, 12238–12245 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z.-Z., He Z.-L., Luo J.-B., Wei J.-H., Guo X.-X., Chen J.-H., Kuang D.-B., Organic–inorganic hybrid Mn-based transparent glass for curved x-ray scintillation imaging. Adv. Optical Mater. 12, 2302434 (2024). [Google Scholar]
- 25.Luo J.-B., Wei J.-H., He Z.-L., Chen J.-H., Peng Q.-P., Zhang Z.-Z., Kuang D.-B., Bisphosphonium cation based metal halide glass scintillators with tunable melting points. Chem. Sci. 15, 16338–16346 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nie F., Yan D., Zero-dimensional halide hybrid bulk glass exhibiting reversible photochromic ultralong phosphorescence. Nat. Commun. 15, 5519 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W., Liu C.-D., Fan C.-C., Fu X.-B., Jing C.-Q., Jin M.-L., You Y.-M., Zhang W., Rational design of 2D metal halide perovskites with low congruent melting temperature and large melt-processable window. J. Am. Chem. Soc. 146, 9272–9284 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Shaw B. K., Hughes A. R., Ducamp M., Moss S., Debnath A., Sapnik A. F., Thorne M. F., McHugh L. N., Pugliese A., Keeble D. S., Chater P., Bermudez-Garcia J. M., Moya X., Saha S. K., Keen D. A., Coudert F.-X., Blanc F., Bennett T. D., Melting of hybrid organic–inorganic perovskites. Nat. Chem. 13, 778–785 (2021). [DOI] [PubMed] [Google Scholar]
- 29.McLean D., The amorphous alloy. Nature 175, 1083–1083 (1955). [Google Scholar]
- 30.Mattern N., Schöps A., Kühn U., Acker J., Khvostikova O., Eckert J., Structural behavior of CuxZr100−x metallic glass (x=35−70). J. Non Cryst. Solids 354, 1054–1060 (2008). [Google Scholar]
- 31.Greer A. L., Confusion by design. Nature 366, 303–304 (1993). [Google Scholar]
- 32.Longley L., Collins S. M., Zhou C., Smales G. J., Norman S. E., Brownbill N. J., Ashling C. W., Chater P. A., Tovey R., Schönlieb C.-B., Headen T. F., Terrill N. J., Yue Y., Smith A. J., Blanc F., Keen D. A., Midgley P. A., Bennett T. D., Liquid phase blending of metal-organic frameworks. Nat. Commun. 9, 2135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longley L., Collins S. M., Li S., Smales G. J., Erucar I., Qiao A., Hou J., Doherty C. M., Thornton A. W., Hill A. J., Yu X., Terrill N. J., Smith A. J., Cohen S. M., Midgley P. A., Keen D. A., Telfer S. G., Bennett T. D., Flux melting of metal-organic frameworks. Chem. Sci. 10, 3592–3601 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie F., Yan D., Bio-sourced flexible supramolecular glasses for dynamic and full-color phosphorescence. Nat. Commun. 15, 9491 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adranno B., Paterlini V., Smetana V., Bousrez G., Ovchinnikov A., Mudring A.-V., Enhanced stability and complex phase behaviour of organic–inorganic green-emitting ionic manganese halides. Dalton Trans. 52, 6515–6526 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Li B., Xu Y., Zhang X., Han K., Jin J., Xia Z., Zero-Dimensional luminescent metal halide hybrids enabling bulk transparent medium as large-area X-ray scintillators. Adv. Optical Mater. 10, 2102793 (2022). [Google Scholar]
- 37.Ravel B., Newville M., ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for x-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Liu H.-L., Ru H.-Y., Sun M.-E., Wang Z.-Y., Zang S.-Q., Organic−inorganic manganese bromide hybrids with water-triggered luminescence for rewritable paper. Adv Optical Mater 10, 2101700 (2022). [Google Scholar]
- 39.Zhang W., Sui P., Zheng W., Li L., Wang S., Huang P., Zhang W., Zhang Q., Yu Y., Chen X., Pseudo-2D layered organic-inorganic manganese bromide with a near-unity photoluminescence quantum yield for white light-emitting diode and x-ray scintillator. Angew. Chem. Int. Ed. Engl. 62, e202309230 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Ma Y.-Y., Song Y.-R., Xu W.-J., Zhong Q.-Q., Fu H.-Q., Liu X.-L., Yue C.-Y., Lei X.-W., Solvent-free mechanochemical syntheses of microscale lead-free hybrid manganese halides as efficient green light phosphors. J. Mater. Chem. C 9, 9952–9961 (2021). [Google Scholar]
- 41.Jana A., Zhumagali S., Ba Q., Nissimagoudar A. S., Kim K. S., Direct emission from quartet excited states triggered by upconversion phenomena in solid-phase synthesized fluorescent lead-free organic–inorganic hybrid compounds. J. Mater. Chem. A 7, 26504–26512 (2019). [Google Scholar]
- 42.Singh A., Jana M. K., Mitzi D. B., Reversible crystal–glass transition in a metal halide perovskite. Adv. Mater. 33, e2005868 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Lu T., Chen F., Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Lu T., A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 161, 082503 (2024). [DOI] [PubMed] [Google Scholar]
- 45.Kim D. H., Kim W. T., Park E. S., Mattern N., Eckert J., Phase separation in metallic glasses. Prog. Mater. Sci. 58, 1103–1172 (2013). [Google Scholar]
- 46.Kalogeras I. M., Brostow W., Glass transition temperatures in binary polymer blends. J Polym Sci B 47, 80–95 (2009). [Google Scholar]
- 47.Lin R., Chai M., Zhou Y., Chen V., Bennett T. D., Hou J., Metal-organic framework glass composites. Chem. Soc. Rev. 52, 4149–4172 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Chiang W.-J., Woo E. M., Comparison of glass transition and interpretation on miscibility in blends of amorphous poly(vinyl methyl ether) with highly crystallizable versus less-crystallizable polyesters. J Polym Sci B 45, 2899–2911 (2007). [Google Scholar]
- 49.Liu M., McGillicuddy R. D., Vuong H., Tao S., Slavney A. H., Gonzalez M. I., Billinge S. J. L., Mason J. A., Network-forming liquids from metal–bis(acetamide) frameworks with low melting temperatures. J. Am. Chem. Soc. 143, 2801–2811 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Mauro J. C., Yue Y., Ellison A. J., Gupta P. K., Allan D. C., Viscosity of glass-forming liquids. Proc. Nat. Acad. Sci. U.S.A. 106, 19780–19784 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y., Zhang Y., Zhao Y., Jia H., Yang Z., Yin B., Wu Y., Yi Y., Zhang C., Yao J., Crystal-liquid-glass transition and near-unity photoluminescence quantum yield in low melting point hybrid metal halides. J. Am. Chem. Soc. 145, 12360–12369 (2023). [DOI] [PubMed] [Google Scholar]
- 52.Du G., Wen S., Zhao J., Ran P., Wang D., Wei L., Qiao X., Yang Y., Qiu J., Zhou S., Hybridization engineering of oxyfluoride aluminosilicate glass for construction of dual-phase optical ceramics. Adv. Mater. 35, e2205578 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Wei H., Fang Y., Mulligan P., Chuirazzi W., Fang H.-H., Wang C., Ecker B. R., Gao Y., Loi M. A., Cao L., Huang J., Sensitive x-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 10, 333–339 (2016). [Google Scholar]
- 54.Li S., Limbach R., Longley L., Shirzadi A. A., Walmsley J. C., Johnstone D. N., Midgley P. A., Wondraczek L., Bennett T. D., Mechanical properties and processing techniques of bulk metal–organic framework glasses. J. Am. Chem. Soc. 141, 1027–1034 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Hodge A. M., Nieh T. G., Evaluating abrasive wear of amorphous alloys using nanoscratch technique. Intermetallics 12, 741–748 (2004). [Google Scholar]
- 56.C. Anthony, A. C. Fischer-Cripps, “[Appendices 1–7]” in Nanoindentation (Springer, ed. 3, 2011), pp. 257.
- 57.Sheldrick G., SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spek A., Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 36, 7–13 (2003). [Google Scholar]
- 59.Peterson P. F., Bozin E. S., Proffen T., Billinge S. J. L., Improved measures of quality for the atomic pair distribution function. J. Appl. Cryst. 36, 53–64 (2003). [Google Scholar]
- 60.Billinge S. J. L., Farrow C. L., Towards a robust ad hoc data correction approach that yields reliable atomic pair distribution functions from powder diffraction data. J. Phys. Condens. Matter 25, 454202 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Juhas P., Davis T., Farrow C. L., Billinge S. J. L., PDFgetX3: A rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Cryst. 46, 560–566 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S33
Tables S1 to S12
Legend for movie S1
Movie S1