Abstract
Heavy metal contamination in agricultural soil poses significant threats to ecosystem sustainability and human health. An outdoor box experiment was conducted as factorial abased on randomized complete block design, with three replications, during 2017 cropping season to evaluate the effects of biofertilizers on Vigna radiata L. growth and yield under different Cu concentrations. The first factor was fertilizer treatment including plant growth-promoting bacterium Sinorhizobium meliloti (PGP), arbuscular mycorrhizal-like fungus Piriformospora indica (AM), and chemical fertilizer (CF) and the second factor was Cu concentrations consisted of 0, 50, 100, and 200 mg Cu/kg soil. The greatest plant height (30.20 and 30.17 cm) and leaf area index (LAI) (1.64 and 1.55) were observed at 0 and 50 mg Cu/kg soil, particularly in CF and AM treatments. The highest Cu concentrations were found in the shoots (74.42 mg/kg) and grains (75.92 mg/kg) when using CF at 200 mg Cu/kg soil. The highest Cu concentration in the roots was obtained in PGP biofertilizer treatment (160.7 mg Cu/kg root). In all Cu concentrations, the shoot bioconcentration factors (BCF) in CF and control treatments were higher than those in PGP and AM treatments. The root BCF improved with the use of PGP and AM treatments, compared to the control. Except the CF, the translocation factor (TF) in other treatments were ˂ 1 and the highest TF was obtained in 200 mg Cu/kg soil (0.842) and CF (1.050) treatment. Based on the results, we concluded that high Cu concentrations reduced the mung bean yield and productivity. However, applying AM in Cu-contaminated soil showed significant potential for improving mung bean yield, reducing Cu availability, and minimizing plant uptake. Generally, compared with chemical fertilizer (CF), P. indica and S. meliloti inoculation effectively increased Cu accumulation in the roots of mung bean grown in Cu-contaminated soil.
Subject terms: Plant ecology, Agroecology
Introduction
Mung bean (Vigna radiata L.) is belonging to the Fabaceae family and plays an important role in providing protein for human due to the richness of protein and essential amino acids1. The short growth period, tasty forage production with high digestibility, and the ability to silage are the important points of mung bean for selecting at different rotations2. Not only it is drought-resistant crop, but also it can fix nitrogen symbiotically and could be used in rotation with cereals and summer crops.
Today, the introduction of heavy metals into agricultural soils is one of the world's most important environmental concerns. Due to their high toxicity and durability, heavy metal-contaminated soil has grown with the fast development of industry and urbanization4. Soil pollution with heavy metals is different from water or air pollution, because heavy metals in soil are more stable than in other parts of the biosphere 5. Heavy metal ions could be absorbed by the roots, transferred to the shoot and disrupting plant metabolism and reduce growth6. The consumption of agricultural products grown in contaminated soils can lead to the accumulation of heavy metals along the food chain, enhancing the potential risks to human health7. Copper (Cu) is an essential micronutrient that plays a crucial role in plant growth. However, when its concentration exceeds critical limits, it can become toxic8. While naturally occurring, anthropogenic sources like mining, refinery operations, fossil fuel combustion, waste incineration, traffic, fertilizers, and soil amendments contribute to elevated Cu levels. A suggested permissible limit for Cu in soil samples is 20 mg/kg9. When Cu concentrations exceed this limit, various adverse effects can occur, including leaf chlorosis and cytotoxicity10. The uptake and transport of Cu within plants are significantly affected by both the Cu levels and the surrounding growth environment10. It is essential to maintain low intracellular Cu concentrations, as excessive amounts can disrupt DNA integrity, photosynthesis, cell membrane stability, enzyme activity, and respiration, ultimately leading to reduced growth and threatening plant survival9.
Heavy metal-contaminated soils have been modified using physicochemical and biological techniques11. Heavy metals could be removed by several techniques including oxidation or reduction, ion exchange, membrane technology, reverse osmosis, filtration and evaporation. These methods eliminate biodiversity and convert the soil into a useless culture medium and non-fertile for plant growth12. Also, these processes sometimes are limited due to technical and economic barriers. Hence, it is necessary to find alternative methods that can clean the soils which are contaminated with heavy metals at low cost, easy, and safe for the environment13. Bioremediation is one of these alternative methods, which is defined as the action of plants and microorganisms in detoxifying, reducing the concentration of pollutants, degrading, or transforming pollutants into a safe state14. The modern bioremediation methods are proposed for environmental protection that use micro-organisms that can act alone or support the action of hyperaccumulators15.
Today, soil refinement is considered based on the use of plants with the name of phytoremediation; in this method, pollutants are removed or rendered harmless from contaminated sites by harvesting plants16. For the success of phytoremediation, increasing the bioavailability of heavy metals in the soil is essential. Biomediators are biological agents with the ability to resist heavy metals and stimulate plant growth, which are important for bioremediation to clean up contaminated sites and increase plant growth13. Bacteria, archaea, and fungi are typically prime bioremediators17. According to Haroun et al.18, biofertilizers, beyond their effects on nitrogen-fixing mechanisms and plant growth, have a potential to reduce heavy metal contents in both polluted soil and plant parts. Piriformospora indica, currently accepted as Serendipita indica, is an arbuscular mycorrhizal-like fungus and a root endophytic basidiomycete belonging to the order Sebacinales19. The P. indica fungus, establish symbiotic relationships with a wide variety of host plants, increase the absorption of food elements by the root, and also improve the host plant tolerance to many biological and non-biological tensions20. However, there are few studies about the effect of this endophyte on heavy metals remediation in soil. Sinorhizobium meliloti is a gram-negative N2-fixing plant growth-promoting (PGP) bacterium that providing nitrogen to the legume plant through symbiotic relationship; also increase the resistance to stress in host plant21. Sinorhizobium meliloti increase the tolerance to copper ions by producing extracellular polymeric compounds22. Inoculation of Rhizobium is an eco-friendly and effective strategy for improving the legume growth, phytoremediation, and bio-modification of areas infected with heavy metals as well as other organic pollutants23.
A few studies have focused on the influence of inoculation with P. indica and S. meliloti on heavy metal bioremediation, despite extensive research on their interactions with plants. However, this study focused on different aspects of this topic: (1) investigating the tolerance of mung bean to copper stress after inoculation of P. indica and S. meliloti; (2) evaluating the effect of these inoculations on the phytoremediation efficiency of mung bean in Cu-contaminated soil; (3) determining how both bio- and chemical fertilizers influence copper accumulation in the shoots, roots, and grains of mung bean; and (4) evaluating the impact of these biofertilizers on the growth of mung bean under varying concentrations of copper.
Results
Plant height
The plant height was affected significantly (p ≤ 0.05) by Cu concentration and fertilizer treatment (Table 1). With increasing Cu concentration in soil, plant height decreased, so that the lowest plant height (18.33 cm) was obtained in 200 mg Cu/kg soil), but there was not a significant difference between the control (0 mg Cu/kg soil) and 50 mg Cu/kg soil (Table 2). Among the fertilizer treatments, the highest plant height (28.51 cm) was observed in CF treatment that was not significantly different with AM treatment (Table 2). The lowest plant height was obtained in PGP treatment (25.97 cm).
Table 1.
Analysis of variance for effect of Cu concentration and fertilizer treatment on growth traits and grain yield of mung bean. ns, * and **: non -significant and significant at p ≤ 0.05 and P ≤ 0.01, respectively.
| Source | df | Mung bean traits | ||
|---|---|---|---|---|
| Plant height | Leaf area index (LAI) | Grain yield per box | ||
| Block | 2 | n.s | n.s | n.s |
| Cu concentration (C) | 3 | ** | ** | ** |
| Fertilizer treatment (F) | 4 | ** | ** | ** |
| C × F | 12 | n.s | n.s | ** |
| Error | 38 | - | - | - |
| Coefficient of variation (%) | 5.91 | 10.92 | 7.83 | |
Table 2.
The mean comparison for effect of Cu concentration and fertilizer treatment on mung bean height, LAI, and TF (translocation factor). AM: Piriformospora indica, CF: chemical fertilizer, PGP: Sinorhizobium meliloti, AM + PGP: Piriformospora indica + Sinorhizobium meliloti, and control: no fertilizer (Different letters indicate significant difference at p ≤ 0.05).
| Effect | Levels | Plant height (cm) | Leaf area index (LAI) | TF |
|---|---|---|---|---|
| Cu concentration (mg Cu/kg soil) | 0 | 30.20 ± 0.70 a | 1.64 ± 0.045 a | - |
| 50 | 30.17 ± 0.26 a | 1.55 ± 0.044 ab | 0.727 ± 0.052 b | |
| 100 | 29.37 ± 0.39 b | 1.49 ± 0.053 b | 0.832 ± 0.044 a | |
| 200 | 18.33 ± 0.33 c | 0.75 ± 0.035 c | 0.842 ± 0.049 a | |
| Fertilizer treatment | CF | 28.51 ± 1.64 a | 1.40 ± 0.123 ab | 1.050 ± 0.040 a |
| AM | 27.63 ± 1.51 ab | 1.50 ± 0.134 a | 0.677 ± 0.026 c | |
| PGP | 25.97 ± 1.50 c | 1.26 ± 0.086 c | 0.602 ± 0.030 d | |
| AM + PGP | 26.63 ± 1.63 bc | 1.30 ± 0.103 bc | 0.738 ± 0.019 c | |
| Control | 26.30 ± 1.58 bc | 1.33 ± 0.121 bc | 0.933 ± 0.039 b |
Leaf area index (LAI)
Leaf area index (LAI) was affected significantly (p ≤ 0.01) by Cu concentration and fertilizer treatment (Table 1). The LAI decreased significantly, with increasing Cu concentration in the soil, so that the highest LAI (1.64) was observed in control (0 mg Cu/kg soil) treatment (Table 2) an the lowest one (0.75) in 200 mg Cu/kg soil. Among the fertilizer treatments, the highest LAI (1.50) was obtained in AM treatment that was not significantly different with CF treatment (1.40) and decreased in other fertilizer treatments (control, PGP and AM + PGP) (Table 2).
Grain yield per box
The mung bean grain yield was affected significantly by Cu concentration, fertilizer treatment, and interaction of Cu concentration × fertilizer treatment (Table 1). By increasing the Cu concentration, seed yield per plant decreased. The mean comparison of the interaction effect of Cu concentration × fertilizer treatment (Fig. 1) shows that at 0 and 50 mg Cu/kg soil, the grain yield in CF and AM treatments were higher than other fertilizer treatments. Under 100 and 200 mg Cu/kg soil, the highest grain yields (2.71 and 1.47 g, respectively) were observed in PGP treatment that were not significantly different with AM and AM + PGP treatments. Also, at 50 mg Cu/kg soil the AM treatment had a higher grain yield (3.95 g) than other fertilizer treatments (Fig. 1).
Fig. 1.
Mung bean grain yield per box as influenced by interaction of Cu concentration × fertilizer treatment (Different letters indicate significant differences at p ≤ 0.05).
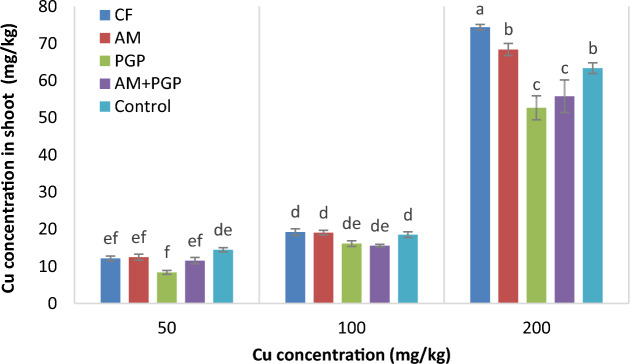
Cu concentration in shoot
The Cu concentration in mung bean shoot was affected by Cu concentration, fertilizer treatment and their interaction effect (p ≤ 0.01) (Table 3). By increasing the Cu concentration in the soil, its accumulation in the shoot increased and the highest value in shoot was obtained under 200 mg Cu/kg soil. The results of mean comparison showed that under 50 and 100 mg Cu/kg soil, the Cu concentrations in shoot were not significantly different at fertilizer treatments. At 200 mg Cu/kg soil, the Cu concentrations in shoot in CF treatment (74.4 mg/kg) increased 14.8% compared with control treatment and decreased in PGP and AM + PGP treatments (Fig. 2).
Table 3.
Analysis of variance for effect of Cu concentration and fertilizer treatment on Cu concentration at different parts of mung bean, BCF (Bioconcentration factor) and TF (Translocation factor).
| Source | df | Cu concentration at different parts of mung bean, BCF and TF | ||||||
|---|---|---|---|---|---|---|---|---|
| Concentration of residual Cu in the soil | Cu concentration in root | Cu concentration in shoot | Cu concentration in grain | Shoot BCF | Root BCF | TF | ||
| Block | 2 | n.s | n.s | n.s | n.s | n.s | n.s | n.s |
| Cu concentration (C) | 2 | ** | ** | ** | ** | ** | ** | ** |
| Fertilizer treatment (F) | 4 | * | ** | ** | ** | ** | ** | ** |
| C × F | 8 | ** | ** | ** | ** | ** | ** | n.s |
| Error | 28 | – | – | – | – | – | – | – |
| CV (%) | 11.54 | 8.30 | 9.06 | 8.81 | 5.15 | 7.69 | 9.19 | |
ns, * and **: non -significant and significant at p ≤ 0.05 and p ≤ 0.01, respectively.
Fig. 2.
Cu concentration in mung bean shoot as influenced by interaction of Cu concentration × fertilizer treatment (Different letters indicate significant differences at p ≤ 0.05).
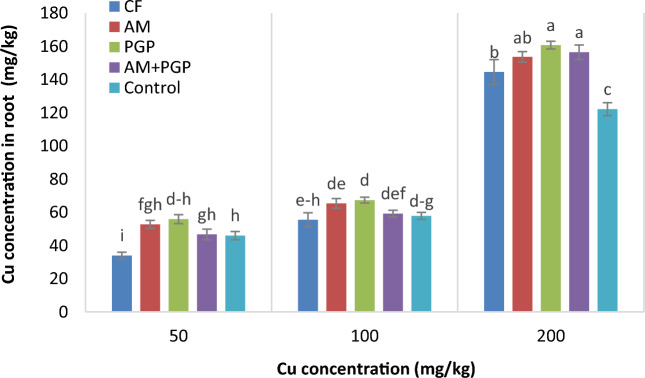
Cu concentration in root
The Cu concentration in mung bean root was affected significantly (p ≤ 0.01) by Cu concentration, fertilizer treatment and interaction effect of Cu concentration × fertilizer treatment (Table 3). By increasing the Cu concentration in soil, the accumulation of Cu in the roots also increased and the highest values were observed in 200 mg/kg Cu concentration. At 50 mg Cu/kg soil, the lowest Cu concentration in root (33.9 mg/kg) was observed in CF treatment and increased significantly at other fertilizer treatments. Under 100 and 200 mg Cu/kg soil, the highest Cu concentrations in root (67.3 and 160.7 mg/kg, respectively) were obtained in PGP treatments that were not significantly different with those in AM and AM + PGP treatments (Fig. 3). Under 200 mg/kg Cu level, the Cu concentration in root increased at all fertilizer treatments compared with control treatment.
Fig. 3.
Cu concentration in mung bean root as influenced by interaction of Cu concentration × fertilizer treatment (Different letters indicate significant differences at p ≤ 0.05).
Cu concentration in grain
The Cu concentration in mung bean grains was affected significantly by Cu concentration, fertilizer treatment and interaction effect of Cu concentration × fertilizer treatments (p ≤ 0.01) (Table 3). By increasing the Cu concentration in soil, its accumulation in the grain also increased. At 50 mg Cu/kg soil, the Cu concentrations in grain were not significantly different at different fertilizer treatments. Under 100 and 200 mg Cu/kg soil, the highest Cu concentrations in grain (39.4 and 75.9 mg/kg, respectively) were obtained in CF treatments and decreased significantly in biofertilizer treatments (AM + PGP, PGP and AM). At 200 mg/kg Cu, among the biofertilizer treatments, the lowest Cu concentrations in grain was observed in AM treatment (43.6 mg/kg) (Fig. 4).
Fig. 4.
Cu concentration in mung bean grain as influenced by interaction of Cu concentration × fertilizer treatment (Different letters indicate significant differences at p ≤ 0.05).
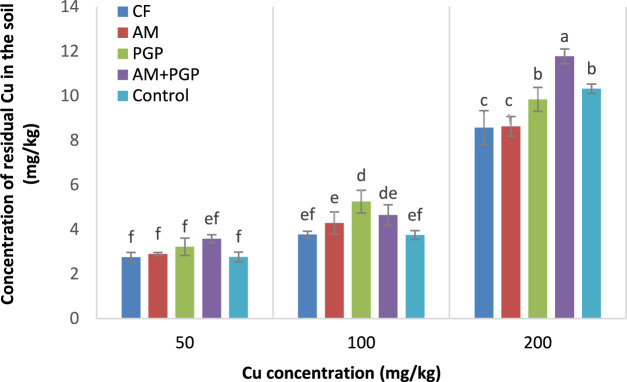
The concentration of residual Cu in the soil
The concentration of residual Cu in the soil was affected significantly (p ≤ 0.01) by Cu concentration, fertilizer treatment, and interaction of Cu concentration × fertilizer treatment (Table 3). By increasing the initial concentration of Cu in the soil, more Cu remained in the soil after harvesting the mung bean. The mean comparison indicated that at 50 mg Cu/kg soil, the residual Cu concentrations in soil were not significantly different at different fertilizer treatments. Under 100 mg Cu/kg soil, the highest residual Cu concentration in soil (5.25 mg/kg) was observed in PGP treatment that was not significantly different with AM + PGP treatment. At 200 mg/kg Cu, the highest residual Cu concentration was observed in AM + PGP treatment (11.77 mg/kg) and the lowest ones were obtained in CF and AM treatments (8.57 and 8.63 mg/kg, respectively) (Fig. 5).
Fig. 5.
Concentration of residual Cu in the soil as influenced by interaction of Cu concentration × fertilizer treatment (Different letters indicate significant differences at p ≤ 0.05.).
Shoot BCF
The results showed that the BCF in the shoot of mung bean was significantly influenced by Cu concentration, fertilizer treatment, and interaction of Cu concentration × fertilizer treatment (Table 3). At 50 and 100 mg Cu/kg soil, the shoot BCF values in CF and control treatments increased compared with those in biofertilizer treatments (AM, PGP and AM + PGP). In 200 mg Cu/kg soil, the highest shoot BCF (0.75) was observed in CF treatment that increased significantly compared with other fertilizer treatments (Fig. 6). In all Cu concentrations, using the biofertilizers reduced the shoot BCF values compared to the control and CF treatments (Fig. 6).
Fig. 6.
Mung bean shoot BCF as influenced by interaction of Cu concentration × fertilizer treatment (Different letters indicate significant differences at p ≤ 0.05).
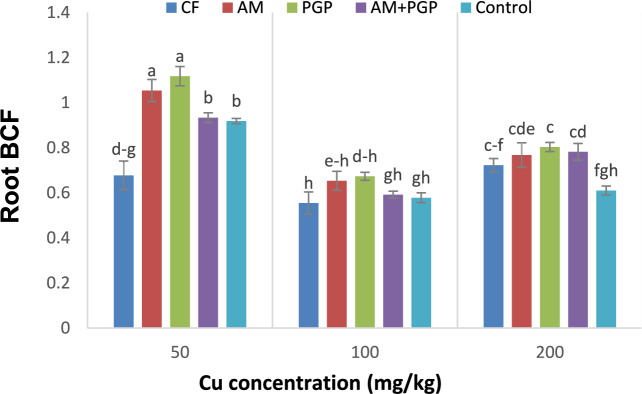
Root BCF
The root BCF of mung bean was also affected by Cu concentration, fertilizer treatment, and the interaction of Cu concentration × fertilizer treatment (p ≤ 0.01) (Table 3). At 50 mg Cu/kg soil, the highest root BCF values were observed in AM and PGP treatments (1.12 and 1.05, respectively) and the lowest one (0.68) in CF treatment. In 100 mg Cu/kg soil, there were no significant differences in root BCF values among the all fertilizer treatments. Under 200 mg Cu/kg soil, the root BCF values for all fertilizer treatments (CF, AM, PGP and AM + PGP) increased compared with control treatment and the lowest value (0.61) was obtained in this treatment (Fig. 7).
Fig. 7.
Mung bean root BCF as influenced by interaction of Cu concentration × fertilizer treatment (Different letters indicate significant difference at p ≤ 0.05).
TF index
The effects of Cu concentration and fertilizer treatment were significant (p ≤ 0.01) on translocation factor (TF) (Table 3). TF index increased with increasing Cu concentration in soil, but this increase was insignificant from 50 to 100 mg Cu/kg soil (Table 2) and the TF indices between 100 and 200 mg Cu/kg soil were not significantly different. Among the fertilizer treatments, the highest TF (1.050) was obtained in CF treatment and after CF, the control treatment had higher TF (0.933) than other treatments, but its value was less than one. TF values were significantly reduced by application of biofertilizer treatments (AM, PGP and AM + PGP) and the lowest TF (0.602) was observed in PGP fertilizer treatment (Table 2).
Discussion
As the concentration of Cu in the soil increased, the mung bean height, LAI and grain yield decreased. Afzal et al.24 reported a similar reduction in plant height with increasing the Cu concentration in the soil. Additionally, Begum et al.25 found that the leaf area of mung bean also decreased with increasing Cu concentration. This inhibitory effect of Cu on plant height and LAI may be attributed to reduced cell division and the harmful impact of Cu on respiration, photosynthesis and protein synthesis25,26. Conversely, CF treatment enhanced plant height, since nitrogen plays a crucial role in plant nutrition, growth, and yield. The observed decrease in plant height at high Cu concentrations could be due to the toxicity associated with elevated Cu levels27. Numerous studies demonstrated the toxic effect of higher Cu concentrations on the growth parameters and yield of crops28,29. Adrees et al.30 reported that Cu toxicity reduced the nutrients availability, biosynthesis of chlorophyll and crop yield. Copper stress can lead to reduced nutrient uptake and accumulation due to several factors 8,9: competition between copper and essential nutrients for transporters, changes in the expression of genes related to nutrient uptake at both transcriptional and post-transcriptional levels, and alterations in plasma membrane permeability. Copper has a notably strong antagonistic relationship with iron compared to other nutrients, and increased levels of copper can lead to a reduction in iron concentrations in plants30. Since iron is crucial for cytochrome complexes, ferredoxin, and intermediates in the thylakoid electron transport chain, the copper-induced limitation of iron significantly hampers chlorophyll biosynthesis and overall photosynthetic performance8.
In 100 and 200 mg Cu/kg soil, the PGP and AM + PGP treatments produced the highest grain yields. This indicates that these treatments are particularly effective in enhancing mung bean yield at high Cu concentrations. Soil microorganisms increase soil quality through different mechanisms and facilitate plant growth in polluted environments31. These microorganisms can decrease heavy metal toxicity through reduction in concentration of metals, and convert toxic heavy metals into non-hazardous forms17, alteration of soil pH, and oxidation/reduction reactions32. Also, heavy metals bioavailability could be decreased by soil microorganisms through synthesis of phytohormones, acidification, precipitation and chelation33. It has been reported that organic acids, such as citrate and malate, secreted by plant roots, reduce metal absorption through chelation and complexation8. Additionally, microorganisms produce chelating agents like siderophores and organic acids that bind to heavy metals, forming stable complexes that help to reduce the mobility and bioavailability of the metals34.
The highest Cu concentrations in the shoot were observed in 200 mg Cu/kg soil and CF treatment. Also, the Cu concentrations in the mung bean shoot were lower than those in root (Fig, 2 and 3). Under high level of Cu in the soil, the accumulation of Cu in the shoot is lower than root, which is probably due to limitation in the transferring potential of plant vessels for this element35. Similar to our results, previous studies have indicated a trend of increasing heavy metal concentrations in plant shoots corresponding to higher rates of heavy metal pollution in the soil36,37. Metal transportation and accumulation in shoot and root are influenced by several factors, including metal supply, growth stage and conditions, plant species, soil characteristics, and the type of heavy metal 13. On the other hand, the uptake of heavy metals by plant roots is affected by factors such as concentration of metal ions in the soil solution, the specific plant species, and the availability of other nutrients. This process is also regulated by soil pH and carbonate content38. Decreases in shoot and root growth, necrosis and chlorosis, are the observed symptoms of Cu stress due to harmful interactions at the cellular level and production of reactive oxygen species39. In this experiment, symptoms of Cu toxicity were observed as red dots on the leaves of plants grown in soil containing 200 mg Cu/kg soil.
At 50 and 200 mg Cu/kg soil, the Cu concentrations in roots for PGP and AM + PGP treatments were significantly higher than CF treatment (Fig. 3). Biofertilizers enhance the availability of heavy metals through solubilization, chelation, and oxidation processes43. Consequently, using biofertilizers for remediation presents a promising approach to address heavy metal contamination. Wang et al.44 reported that inoculation of microorganism can improve the mineral nutrition of plants under heavy metal pollution, and directly affects the accumulation and absorption of heavy metals in plants. The mung bean tolerance to copper concentration could be increased by inoculation of AM and PGP, which lead to an increase in the production of antioxidants, plant hormones and osmotic regulatory substances. Plants experiencing copper toxicity produce increased levels of reactive oxygen species (ROS)8. However, inoculation with arbuscular mycorrhizal fungi (AM) and plant growth-promoting bacteria (PGP) significantly enhances the production of important antioxidant enzymes, such as superoxide dismutase, catalase, and peroxidases45. These enzymes actively scavenge ROS, transforming them into less harmful molecules.
Similar to the root and shoot, the highest Cu concentrations in grain of mung bean were observed in 200 mg Cu/kg soil (Fig. 4). Plant roots can absorb heavy metal ions present in the soil through various mechanisms, including chelation, active transport and ion exchange through ion channels40. Generally, surface area of roots for heavy metal absorption could be increased by root hairs. These ions accumulate in root cells and after crossing the endodermis, they are transferred to the shoot by the symplastic pathway through the xylem and phloem41. However, the movement of heavy metal ions from the root to the shoot is limited, as the endoderm serves as a partial barrier that restricts their entry into the xylem, thereby regulating their transport to the shoots42.
At 200 mg Cu/kg soil level, higher Cu amounts remained in the soil after harvesting the mung bean. Mani et al 46 also reported that an increase in the applied lead (Pb) had a corresponding increase in the residual content in the soil after the plant harvest. It was also found that the highest residual copper concentration in the soil was observed in AM + PGP treatment (Fig. 5). It has been reported that the bacteria found in biofertilizers can immobilize heavy metals, effectively decrease their availability for plant uptake through binding them to their cellular components16. Furthermore, heavy metals degrade in the soil slowly which can lead to their accumulation in soil47.
BCF indicates the concentration of pollutants in plants compared to their concentrations in environment48. The BCF higher than 1 indicates that the plant is effective for phytoextraction, while the BCF less than 1 suggests that the plant is suitable for phytostabilization and functions as an excluder49. In the present study, at all Cu concentrations and fertilizer treatments, the shoot BCF of mung bean did not exceed one. When comparing the shoot BCF with root BCF values (Fig. 6 and 7), we found that the root BCF for all treatments at all Cu concentrations were higher than those of the shoot, except for the CF treatment. This result indicates restricted mobility of Cu within the plants that is in agreement with findings reported by Eben et al.48, as they stated that this situation is the strategy of excluders for tolerating high concentrations of heavy metals. In this way, more values of metals accumulate in the roots, but restrict their transport to upper tissues. As we observed that at all Cu concentrations, the highest root BCF values were obtained in AM and PGP treatments (Fig. 7). Also, the lower shoot BCF values in biofertilizer treatments (AM, PGP and AM + PGP) were likely due to the higher efficiency of biofertilizers in reduction of heavy metal uptake (Fig. 6). Generally, the addition of microorganisms to the soil decreases the heavy metals concentration, lead to decrease in root uptake50. In such a way that metals adsorb on the surfaces of microorganisms, their mobilization and concentrations in the soil, as well as their translocation in plants, will be decreased51. Abdelkrim et al.52 reported that the plant growth-promoting bacteria increase the accumulation and uptake of heavy metal in plant roots by releasing metal chelates, degrading enzymes and organic acids in the rhizosphere. They also observed that microorganisms converted metal into bioavailable forms in the rhizosphere, which increased the heavy metals absorption by plant roots52.
TF provides information about metal translocation within plants53. Considering that a major portion of Cu was contained in the roots (compared to the shoots), it could be concluded that reduction in TF values in biofertilizer (AM, PGP and AM + PGP) treatments (Table 2) could be attributed to metal precipitation in the root tissues (as presented in Fig. 3). Therefore, using S. meliloti and P. indica could be a viable treatment for decrease in TF values. It has been reported that plant-mycorrhiza symbiosis could enable mechanisms of selective transport for both heavy metals and essential minerals 54 and effectively reduce transport of heavy metal from roots to the shoots53. On the other hand, the TF values of < 1 at all Cu concentrations shows that mung bean roots have internal restrictions, lead to decrease in Cu translocation from the roots to the shoots. This may be due to the natural defense mechanisms of the plant, which aim to limit the absorption and translocation of metals to prevent their toxicity55. Plants employ various strategies to manage metal stress, including enhanced metal chelation through metal-binding compounds like phytochelatins and metallothioneins56. In the presence of heavy metals such as cadmium, lead, or copper in the soil, plants initiate the production of phytochelatins in their root tissues. These synthesized phytochelatins form strong bonds with heavy metals, creating stable complexes that are less mobile and less likely to be absorbed by the roots or transported to the shoots57. Additionally, it has been reported that mycorrhizal symbiosis can stimulate the production of phytochelatins and metallothioneins in cereal crops58.
Most copper-tolerant plants are Cu excluders with very limited transfer from root to the shoot42. Excluders indicate high build-up of heavy metals in their roots but have TF less than 1 and are hyper-tolerant59. However, the translocation of heavy metals from root to the shoot tissues and subsequent harvesting of shoot is of primary importance for phytoextraction48. Therefore, the phytoextraction potential of species with TF < 1 can be classified as low.
Conclusions
The mung bean growth and grain yield were significantly reduced at high Cu concentrations (200 mg Cu/kg soil). However, the application of biofertilizers, mitigated the negative effects of high Cu toxicity. The translocation and accumulation of Cu varied in mung bean root and shoot. Higher Cu accumulation in the root than that of shoot, indicates limited Cu transport from the root to the shoot. The use of S. meliloti and P. indica decreased the BCF and TF values. Moreover, these treatments were effective in reduction of Cu transport from the root to the shoot. Decreases in BCF values were likely due to the higher efficiency of S. meliloti and P. indica in reduction of Cu uptake. Overall, this study indicates that the combined inoculation of mung bean with P. indica and S. meliloti can effectively remediate soils with low to moderate copper contamination (50–100 mg Cu/kg soil). Biofertilizers offer a sustainable, specific, and cost-effective approach to treating contaminated soils. Future research should focus on optimizing biofertilizer formulations, evaluating long-term impacts, investigating enzyme mechanisms, and performing field-scale studies to improve our understanding of their role in heavy metal bioremediation.
Materials and methods
Experimental design and treatments
The outdoor box experiment was conducted during 2017 cropping season in Maragheh city, East Azarbaijan province, Iran (latitude 37˚4' N, longitude 46˚26' E, altitude 1478 m above sea level). The soil used in this study was provided from a crop field in Maragheh city, at a depth of 0–30 cm. The physico-chemical properties of the soil were presented in Table 4.
Table 4.
The physico–chemical properties of the soil used in the experiment.
| CEC Cmol(+)/kg |
Cu mg/kg |
TN % |
K mg/kg |
P mg/kg |
OM % |
pH | EC dS/m | Texture |
|---|---|---|---|---|---|---|---|---|
| 3.53 | 0.47 | 0.033 | 620 | 12.5 | 1.1 | 7.28 | 1.76 | Loamy Sand |
This research was arranged as a factorial experiment based on randomized complete block design, with three replications. The first factor was soil contamination with copper, with concentrations of 0, 50, 100, and 200 mg Cu/kg of dry soil. The second factor was included different fertilizer treatments as chemical fertilizer (urea 150 kg/ha) (CF), biofertilizers consisted of Sinorhizobium meliloti (PGP), Piriformospora indica (AM), combination of P. indica and S. meliloti (AM + PGP), and a control treatment (no fertilizer). Copper sulfate (Cu SO₄) was provided from Merck, Germany. Mycorrhiza-like fungi (P. indica) and bacterial strain (S. meliloti) used in this experiment were obtained from the Laboratory of Soil Biology at the University of Tabriz (Tabriz, Iran). The P. indica fungus was propagated on a plate in Kafer medium for two weeks at 24°C. After propagation, the mycelium was mixed with sterilized bagasse: perlite (50:50) carrier. The resulting inoculum contained mycelium and 104 spores of P. indica per gram of carrier, which was applied as a biofertilizer in the experiment. S. meliloti was cultured overnight for 16 h in nutrient broth (NB) and it was used in the experiment once the desired optical density (OD600) was achieved 60.
Experimental procedure
In this experiment, the box with dimensions of 28 × 48 cm and a height of 22 cm containing 22 kg of soil was considered as an experimental unit. Artificially contaminated soil was created using copper sulfate (CuSO4·5H2O) solutions. To achieve the desired contamination levels, 4.4 g of copper sulfate was dissolved in 3 L of water for the 200 mg Cu/kg soil level, 2.2 g for the 100 mg Cu/kg soil level, and 1.1 g for the 50 mg Cu/kg soil level. The resulting solutions were then evenly sprayed onto the soil in their respective boxes and thoroughly mixed to ensure uniform distribution. Then, for 30 days, the boxes were periodically dried and moistened so that the Cu in the soil reached a relative equilibrium 61. Also, to address nutrient deficiencies, 0.67 g of dry granular urea fertilizer (46% N) was added to all boxes as starter N for mung bean growth and symbiosis. Throughout this process, the soil moisture content was maintained at 65% of its field capacity.
Before sowing mung bean (on May 12, 2017), the soil in the biofertilizer treatment boxes was inoculated with P. indica and S. meliloti at rates of 1.3 and 1.6 g/kg soil, respectively 60. To ensure an even distribution of microorganisms, the inoculant was thoroughly mixed with the soil in the boxes. Before planting, 2.0 g of urea was dissolved in the irrigation water and applied to the soil in the designated boxes. No inoculation was performed in the control treatment. The planting density for mung bean cv. Ghohar was 25 plants m-2. Thus, on May 15, 2017, 16 seeds were planted in each box at a depth of 2 cm, and were irrigated with tap water. After emergence of seedling and establishment, the seedlings were thinned and four plants were kept in each box. The soil water content at all boxes were maintained at field capacity level. The experimental boxes were placed outdoor in a field in Maragheh city, East Azarbaijan province, Iran during summer 2017. The mean temperature and monthly total precipitation of the experimental site during growth season of 2017 is presented in Table 5.
Table 5.
Monthly total precipitation and mean temperature during 2017 growing season in experimental area.
| May | June | July | August | September | |
|---|---|---|---|---|---|
| Total precipitation (mm) | 4.31 | 1.27 | 0.25 | 0.0 | 0.0 |
| Mean temperature (˚C) | 20.4 | 25.9 | 30.0 | 30.0 | 25.7 |
To measure the leaf area index (LAI), a Delta-T leaf area meter (Delta-T Devices, Cambridge, England) was used at the flowering stage (on 15 July 2017). The LAI for all treatments was calculated by dividing the leaf area by the ground area (1 m2) 62. After harvesting (on 12 September 2017), the plant height and grain yield per box were measured. After harvesting the shoots and removing the roots from the soil, the shoots and roots of each plant were dried in an oven at 70 °C for 48 h. The dried Shoots, roots, and seeds were then milled to prepare for measuring the concentration of Cu using an atomic absorption spectrophotometer. Each sample was converted into ash through a dry ashing process in an electric furnace and weighed accordingly. Extraction involved adding a specified volume of 2 M hydrochloric acid to the solution, which was then filtered using Whatman filter paper, following the method described by Madanan et al. 59. The atomic absorption device was calibrated for copper based on metal grade standards provided by PerkinElmer. Finally, the concentration of residual Cu in the soil was also measured.
The bioconcentration factor (BCF) 63 of Cu and the translocation factor (TF) 64 were calculated as following:
BCF indicates the accumulation ability of plant for specific metal based on its concentration in the soil; and its values are classified into the following categories: intensive, BCF > 1; medium, BCF = 1– 0.1; weak, BCF = 0.1–0.01; and no accumulation, BCF = 0.01–0.001 65. TF also indicates the ability of the plant to translocate metals from the roots to the aerial parts of the plant. The TF > 1 indicates a good transfer of metal ions from the roots to the upper part of the plant and phytoextraction capability, and the TF ˂ 1 indicates that the metals are largely stored in the roots of plants 66.
Statistical analysis
SAS version 9.0.3 was used for analysis of variance (ANOVA) based on a randomized complete block design with 20 treatments and three replicates. The whole experiment was conducted two times. The interactions of time × treatments were not significant, so the data from both experiments were pooled for data analysis. For comparison of the means, Duncan´s multiple range test was used at p ≤ 0.05.
Acknowledgements
The authors thank the University of Tabriz for supporting this research.
Author contributions
Z.A.C.: conducted the experiment, collected the data, analyzed the data, and wrote the manuscript, prepared figures and tables. R.A.: analyzed the data, reviewed the final data, edited and reviewed the manuscript. A.D.M.N.: edited and reviewed the manuscript. All authors reviewed the manuscript.
Data availability
The necessary information is available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar, S. & Pandey, G. Biofortification of pulses and legumes to enhance nutrition. Heliyon.6(3), 1–6. 10.1016/j.heliyon.2020.e03682 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li, C. et al. Integration of comparative transcriptomics and WGCNA characterizes the regulation of anthocyanin biosynthesis in mung bean (Vigna radiata L.). Front. Plant Sci.14, 1251464. 10.3389/fpls.2023.1251464 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong, X. et al. Boosting proso millet yield by altering canopy light distribution in proso millet/mung bean intercropping systems. Crop J.8(2), 365–377. 10.1016/j.cj.2019.09.009 (2020). [Google Scholar]
- 4.Bai, Y. et al. Distribution of cadmium, copper, lead, and zinc in mudflat salt-soils amended with sewage sludge. Land Degrad. Dev.29(4), 1120–1129. 10.1002/ldr.2914 (2018). [Google Scholar]
- 5.Golia, E. E. The impact of heavy metal contamination on soil quality and plant nutrition Sustainable management of moderate contaminated agricultural and urban soils, using low cost materials and promoting circular economy. Sustainable Chem. Pharm.33, 101046 (2023). [Google Scholar]
- 6.Yaashikaa, P. R., Kumar, P. S., Jeevanantham, S. & Saravanan, R. A review on bioremediation approach for heavy metal detoxification and acCumulation in plants. Environ. Pollut.301, 119035. 10.1016/j.envpol.2022.119035 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Yang, W. et al. Application of rapeseed residue increases soil organic matter, microbial biomass, and enzyme activity and mitigates cadmium pollution risk in paddy fields. Environ. Pollut.264, 114681. 10.1016/j.envpol.2020.114681 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Wairich, A. et al. Throwing copper around: how plants control uptake, distribution, and accumulation of copper. Agronomy12(5), 994. 10.3390/agronomy12050994 (2022). [Google Scholar]
- 9.Kumar, V. et al. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere262, 127810. 10.1016/j.chemosphere.2020.127810 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Saleem, M. H. et al. Copper uptake and acCumulation, ultra-structural alteration, and bast fibre yield and quality of fibrous jute (Corchorus capsularis L.) plants grown under two different soils of China. Plants.9(3), 404. 10.3390/plants9030404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song, P. et al. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ.838, 156417. 10.1016/j.scitotenv.2022.156417 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Wróbel, M., Śliwakowski, W., Kowalczyk, P., Kramkowski, K. & Dobrzyński, J. Bioremediation of heavy metals by the genus Bacillus. Int. J. Environ. Res. Public Health.20(6), 4964. 10.3390/ijerph20064964 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokrani, S. et al. Bioremediation techniques for soil organic pollution: Mechanisms, microorganisms, and technologies-A comprehensive review. Ecol. Eng.207, 107338. 10.1016/j.ecoleng.2024.107338 (2024). [Google Scholar]
- 14.Sharma, P., Kumar, S. & Pandey, A. Bioremediated techniques for remediation of metal pollutants using metagenomics approaches: a review. J. Environ. Chem. Eng.9(4), 105684. 10.1016/j.jece.2021.105684 (2021). [Google Scholar]
- 15.Pham, V. H. T., Kim, J., Chang, S. & Chung, W. Bacterial biosorbents, an efficient heavy metals green clean-up strategy: prospects, challenges, and opportunities. Microorganisms.10(3), 610. 10.3390/microorganisms10030610 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta, P. K., Ranjan, S. & Gupta, S. K. Phycoremediation of petroleum hydrocarbon-polluted sites: application, challenges, and future prospects. In Application of Microalgae in Wastewater Treatment Domestic and Industrial Wastewater Treatment (eds Kumar, S. & Bux, F.) (Springer international publications, 2019). [Google Scholar]
- 17.Abatenh, E., Gizaw, B., Tsegaye, Z. & Wassie, M. The role of microorganisms in bioremediation-A review. J. Environ. Biol.2(1), 038–046. 10.1735/ojeb.000007 (2017). [Google Scholar]
- 18.Haroun, M., Xie, S., Awadelkareem, W., Wang, J. & Qian, X. Influence of biofertilizer on heavy metal bioremediation and enzyme activities in the soil to revealing the potential for sustainable soil restoration. Sci. Rep.13(1), 20684. 10.1038/s41598-023-44986-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao, A. et al. Piriformospora indica Culture filtrate application adds brilliance to the promoting effects of facility warming on winter jujube fruit ripening. Food Chem X.24, 101986. 10.1016/j.fochx.2024.101986 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, L., Zhu, P., Wang, X. & Zhang, Z. Phytoremediation effect of Medicago sativa colonized by Piriformospora indica in the phenanthrene and cadmium co-contaminated soil. BMC Biotechnol.20, 1–14. 10.1186/s12896-020-00613-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan, C. et al. Rhizobium inoCulation enhances the resistance of alfalfa and microbial characteristics in copper-contaminated soil. Front. Microbiol.12, 781831. 10.3389/fmicb.2021.781831 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirhanov, G. G. & Zhernossekov, D. D. Sinorhizobium meliloti bacterium as a perspective object for biotechnology. Biotechnol. Acta.14, 23 (2021). [Google Scholar]
- 23.Bai, T. et al. Mixed nitrogen form addition facilitates the growth adaptation of legume plant to heavy metal contamination in degraded mining areas. Glob. Ecol. Conserv.24, e01387. 10.1016/j.gecco.2020.e01387 (2020). [Google Scholar]
- 24.Afzal, A. et al. Biosorbents removed copper heavy metal from agriCultural land Cultivated with Vigna radiata (Mung bean). Int. J. Agron.2022(1), 6067181. 10.1155/2022/6067181 (2022). [Google Scholar]
- 25.Begum, M. M. S. F., Geretharan, T. & Srikrishnah, S. Effects of different copper concentration on growth and nodulation of green gram (Vigna radiata L). Asian J. Res. Agric. Forestry.10(4), 28–37. 10.9734/ajraf/2024/v10i4313 (2024). [Google Scholar]
- 26.Mir, A. R., Pichtel, J. & Hayat, S. Copper: uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals34(4), 737–759. 10.1007/s10534-021-00306-z (2021). [DOI] [PubMed] [Google Scholar]
- 27.Sui, X. et al. Nitrate reduces copper toxicity by preventing oxidative stress and inhibiting copper translocation from roots to shoots in Liriodendron Chinense. Environ. Sci. Pollut. Res.31(10), 15946–15957. 10.1007/s11356-024-32053-2 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Alengebawy, A., Abdelkhalek, S. T., Qureshi, S. R. & Wang, M. Q. Heavy metals and pesticides toxicity in agriCultural soil and plants: Ecological risks and human health implications. Toxics.9(3), 42. 10.3390/toxics9030042 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlček, V. & Pohanka, M. Adsorption of copper in soil and its dependence on physical and chemical properties. Acta Univ. Agric. Silvic. Mendelianae Brun.66(1), 219–224. 10.11118/actaun201866010219 (2018). [Google Scholar]
- 30.Adrees, M. et al. The effect of excess copper on growth and physiology of important food crops: a review. Environ. Sci. Pollut. Res.22, 8148–8162. 10.1007/s11356-015-4496-5 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Gupta, R., Khan, F., Alqahtani, F. M., Hashem, M. & Ahmad, F. Plant growth–promoting Rhizobacteria (PGPR) assisted bioremediation of Heavy Metal Toxicity. Appl. Biochem. Biotechnol.196(5), 2928–2956. 10.1007/s12010-023-04545-3 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Tara, N. et al. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J. Cleaner Prod.217, 541–548. 10.1016/j.jclepro.2019.01.258 (2019). [Google Scholar]
- 33.Mishra, S. K. et al. Characterisation of Pseudomonas spp. and Ochrobactrum sp. isolated from volcanic soil. Antonie Van Leeuwenhoek110, 253–270. 10.1007/s10482-016-0796-0 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Sarvepalli, M., Velidandi, A. & Korrapati, N. Optimization of siderophore production in three marine bacterial isolates along with their heavy-metal chelation and seed germination potential determination. Microorganisms11(12), 2873. 10.3390/microorganisms11122873 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souri, M. K., Hatamian, M. & Tesfamariam, T. Plant growth stage influences heavy metal acCumulation in leafy vegetables of garden cress and sweet basil. Chem. Biol. Technol. Agric.6(1), 1–7. 10.1186/s40538-019-0170-3 (2019). [Google Scholar]
- 36.Sharma, V. et al. Screening the potential of different brassica genotypes for phytoremediation of nickel (Ni) spiked soil. Water, Air, Soil Pollut.235(6), 432. 10.1007/s11270-024-07256-5 (2024). [Google Scholar]
- 37.Dhaliwal, S. S., Taneja, P. K., Singh, J., Bhatti, S. S. & Singh, R. Cadmium accumulation potential of Brassica species grown in metal spiked loamy sand soil. Soil. Sediment Contam.29(6), 638–649. 10.1080/15320383.2020.1758031 (2020). [Google Scholar]
- 38.Waterlot, C., Pruvot, C., Marot, F. & Douay, F. Impact of a phosphate amendment on the environmental availability and phytoavailability of Cd and Pb in moderately and highly carbonated kitchen garden soils. Pedosphere27(3), 588–605. 10.1016/S1002-0160(17)60354-0 (2017). [Google Scholar]
- 39.Lange, B. et al. Copper and cobalt acCumulation in plants: a critical assessment of the Current state of knowledge. New Phytol.213(2), 537–551. 10.1111/nph.14175 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Wu, D., Saleem, M., He, T. & He, G. The mechanism of metal homeostasis in plants: A new view on the synergistic regulation pathway of membrane proteins, lipids and metal ions. Membranes11(12), 984. 10.3390/membranes11120984 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhalaria, R. et al. Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy10(6), 815. 10.3390/agronomy10060815 (2020). [Google Scholar]
- 42.Ogorek, L. L. P. et al. Outer apoplastic barriers in roots: prospects for abiotic stress tolerance. Funct. Plant Biol.10.1071/FP23133 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Rahman, Z. & Singh, V. P. Bioremediation of toxic heavy metals (THMs) contaminated sites: concepts, applications and challenges. Environ. Sci. Pollut. Res.27(22), 27563–27581. 10.1007/s11356-020-08903-0 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Wang, H. R., Zhao, X. Y., Zhang, J. M., Lu, C. & Feng, F. J. ArbusCular mycorrhizal fungus regulates cadmium acCumulation, migration, transport, and tolerance in Medicago sativa. J. Hazard. Mater.435, 129077. 10.1016/j.jhazmat.2022.129077 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Begum, N. et al. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol.10.1007/s00248-021-01815-7 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Mani, F. A., Talha, I. Z. & Ismaila, M. Phytoremediation potential of Kenaf (HibisCus cannabinus L.) in lead (Pb) contaminated sandy loam soil of Maiduguri Nigeria. J. Global Ecol. Environ.8(1), 43–49. 10.1016/j.envpol.2021.116911 (2018). [Google Scholar]
- 47.Banerjee, A., Barik, S. K. & Joshi, S. R. Bacilli and Sustainable Jhum Agrobiotechnology. In Bacilli in Agrobiotechnology Plant Stress Tolerance, Bioremediation, and Bioprospecting (eds Islam, T. et al.) (Springer International Publishing, 2022). [Google Scholar]
- 48.Eben, P., Mohri, M., Pauleit, S., Duthweiler, S. & Helmreich, B. Phytoextraction potential of herbaceous plant species and the influence of environmental factors–A meta-analytical approach. Ecol. Eng.199, 107169. 10.1016/j.ecoleng.2023.107169 (2024). [Google Scholar]
- 49.Luthansa, U. M., Titah, H. S. & Pratikno, H. The ability of mangrove plant on lead phytoremediation at Wonorejo Estuary, Surabaya. Indonesia. Ecol. Eng.22(6), 253–268. 10.1291/22998993/137675 (2021). [Google Scholar]
- 50.Huang, R. et al. Sediment microbiomes associated with the rhizosphere of emergent macrophytes in a shallow, subtropical lake. Limnol. Oceanograph.10.1002/lno.11325 (2020). [Google Scholar]
- 51.Jiang, H. H., Cai, L. M., Wen, H. H. & Luo, J. Characterizing pollution and source identification of heavy metals in soils using geochemical baseline and PMF approach. Sci. Rep.10(1), 6460. 10.1038/s41598-020-63604-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdelkrim, S. et al. In situ effects of Lathyrus sativus-PGPR to remediate and restore quality and fertility of Pb and Cd polluted soils. Ecotoxicol. Environ. Saf.192, 110260. 10.1016/j.ecoenv.2020.110260 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Ibrahim, E. A., El-Sherbini, M. A. & Selim, E. M. M. Effects of biochar, zeolite and mycorrhiza inoCulation on soil properties, heavy metal availability and cowpea growth in a multi-contaminated soil. Sci. Rep.13(1), 6621. 10.1038/s41598-023-33712-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begum, N. et al. Role of arbusCular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci.10, 1068. 10.3389/fpls.2019.01068 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drzewiecka, K. et al. Drought Differently Modifies Tolerance and Metal Uptake in Zn-or Cu-Treated Male and Female Salix× fragilis L. Forests15(3), 562. 10.3390/f15030562 (2024). [Google Scholar]
- 56.Riyazuddin, R. et al. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules12(1), 43. 10.3390/biom12010043 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasilachi, I. C., Stoleru, V. & Gavrilescu, M. Analysis of heavy metal impacts on cereal crop growth and development in contaminated soils. Agriculture13(10), 1983. 10.3390/agriculture13101983 (2023). [Google Scholar]
- 58.Li, H. et al. Physio-biochemical and transcriptomic features of arbuscular mycorrhizal fungi relieving cadmium stress in wheat. Antioxidants11(12), 2390. 10.3390/antiox11122390 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madanan, M. T., Shah, I. K., Varghese, G. K. & Kaushal, R. K. Application of Aztec Marigold (Tagetes erecta L.) for phytoremediation of heavy metal polluted lateritic soil. Environ. Chem. Ecotoxicol.3, 17–22. 10.1016/j.enceco.2020.10.007 (2021). [Google Scholar]
- 60.Amini, R., Zafarani-Moattar, P., Shakiba, M. R. & Hasanfard, A. Inoculating moldavian balm (Dracocephalum moldavica L.) with mycorrhizal fungi and bacteria may mitigate the adverse effects of water stress. Sci. Rep.13, 16176. 10.1038/s41598-023-43539-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turan, M. & Esringu, A. Phytoremediation based on canola (Brassica napus L.) and Indian mustard (Brassica juncea L.) planted on spiked soil by aliquot amount of Cd. Cu, Pb, and Zn Plant, Soil Environ.53(1), 7 (2007). [Google Scholar]
- 62.Amiriyan Chelan, Z., Amini Dabbagh Mohammadi Nasab, R. & A.,. Essential oil yield and compositions of Dracocephalum moldavica L. in intercropping with fenugreek, inoculation with mycorrhizal fungi and bacteria. Sci Rep.13(1), 8039. 10.1038/s41598-023-35156-x (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naveed, M. et al. Microbiologically modified bioorganic fertilizer and metal-tolerant Bacillus sp. MN54 regulate the nutrient homeostasis and boost phytoextraction efficiency of mustard (Brassica juncea L.) in nickel-contaminated soil. Chem. Biol. Technol. Agric.11(1), 168. 10.1186/s40538-024-00689-4 (2024). [Google Scholar]
- 64.de Oliveira Mesquita, F., Pedrosa, T. D., Batista, R. O. & de Andrade, E. M. Translocation factor of heavy metals by elephant grass grown with varying concentrations of landfill leachate. Environ. Sci. Pollut. Res.28(32), 43831–43841. 10.1007/s11356-021-13765-1 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Nematollahi, M. J., Keshavarzi, B., Zaremoaiedi, F., Rajabzadeh, M. A. & Moore, F. Ecological-health risk assessment and bioavailability of potentially toxic elements (PTEs) in soil and plant around a copper smelter. Environ. Monit. Assess.192(10), 639. 10.1007/s10661-020-08589-4 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Parihar, J. K., Parihar, P. K., Pakade, Y. B. & Katnoria, J. K. BioacCumulation potential of indigenous plants for heavy metal phytoremediation in rural areas of Shaheed Bhagat Singh Nagar, Punjab (India). Environ. Sci. Pollut. Res.28, 2426–2442. 10.1007/s11356-020-10454-3 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The necessary information is available from the corresponding author on reasonable request.