Abstract
Objective
To compare the effects of treatments for mild or moderate (that is, non-severe) coronavirus disease 2019 (covid-19).
Design
Systematic review and network meta-analysis.
Data sources
Covid-19 Living Overview of Evidence Repository (covid-19 L-OVE) by the Epistemonikos Foundation, a public, living repository of covid-19 articles, from 1 January 2023 to 19 May 2024. The search also included the WHO covid-19 database (up to 17 February 2023) and six Chinese databases (up to 20 February 2021). The analysis included studies identified between 1 December 2019 and 28 June 2023.
Study selection
Randomised clinical trials in which people with suspected, probable, or confirmed mild or moderate covid-19 were allocated to drug treatment or to standard care or placebo. Pairs of reviewers independently screened potentially eligible articles.
Methods
After duplicate data abstraction, a bayesian network meta-analysis was conducted. Risk of bias was assessed by use of a modification of the Cochrane risk of bias 2.0 tool, and the certainty of the evidence using the grading of recommendations assessment, development, and evaluation (GRADE) approach. For each outcome, following GRADE guidance, drug treatments were classified in groups from the most to the least beneficial or harmful.
Results
Of 259 trials enrolling 166 230 patients, 187 (72%) were included in the analysis. Compared with standard care, two drugs probably reduce hospital admission: nirmatrelvir-ritonavir (25 fewer per 1000 (95% confidence interval 28 fewer to 20 fewer), moderate certainty) and remdesivir (21 fewer per 1000 (28 fewer to 7 fewer), moderate certainty). Molnupiravir and systemic corticosteroids may reduce hospital admission (low certainty). Compared with standard care, azithromycin probably reduces time to symptom resolution (mean difference 4 days fewer (5 fewer to 3 fewer), moderate certainty) and systemic corticosteroids, favipiravir, molnupiravir, and umifenovir probably also reduce duration of symptoms (moderate to high certainty). Compared with standard care, only lopinavir-ritonavir increased adverse effects leading to discontinuation.
Conclusion
Nirmatrelvir-ritonavir and remdesivir probably reduce admission to hospital, and systemic corticosteroids and molnupiravir may reduce admission to hospital. Several medications including systemic corticosteroids and molnupiravir probably reduce time to symptom resolution.
Systematic review registration
This review was not registered. The protocol is publicly available in the supplementary material.
Introduction
As of November 2024, more than seven million people have died with coronavirus disease 2019 (covid-19).1 The evidence on covid-19 continues to evolve as global efforts to identify effective interventions for the prevention and treatment of covid-19 have resulted in more than 8000 completed or ongoing trials.2
Summarising the rapidly growing evidence base has proved challenging.3 Network meta-analysis, rather than pairwise meta-analysis, provides useful information about the comparative effectiveness of treatments that have not been tested head-to-head. The lack of such direct comparisons can otherwise limit our understanding of important comparisons, and the incorporation of indirect evidence can strengthen evidence in comparisons that were tested head-to-head.4
The current evidence supports two stages of covid-19 disease, an early phase marked by viral replication (non-severe disease) and a later inflammatory phase (severe disease).5 The different pathogenesis and resulting severity of symptoms at each of these stages suggest different interventions are most effective at certain stages of the disease.5 6 Consequently, our systematic review and network meta-analysis now addresses patients with mild or moderate covid-19 (also known as non-severe covid-19) separate from those with severe covid-19. In this publication, we compare the effects of drug treatments for mild or moderate covid-19. A separate publication will compare the effects of drug treatments for severe or critical covid-19, and together these two publications replace our previous living systematic review of covid-19 of all severities. Although we used living systematic review methods to conduct this review, no further updates or analyses are planned, and this publication represents our final analysis for mild or moderate covid-19. This systematic review and network meta-analysis will inform the World Health Organization living guidelines on covid-19 treatments.7 These guidelines were initiated and are dynamically updated to provide trustworthy, actionable, and living guidance to clinicians and patients soon after new and potentially practice-changing evidence becomes available (box 1).8 9 Drugs for prophylaxis10 and antibody based treatments11 are addressed separately.
Box 1. Sources of latest evidence from this systematic review.
-
Author website “COVID-19 living network meta-analysis” (https://www.covid19lnma.com)
Interim updates will be available here.
Linked resources in BMJ Rapid Recommendations cluster
-
Agarwal A, Hunt B J, Stegemann M, et al. A living WHO guideline on drugs for covid-19 [update 13]. BMJ 2020;370:m3379, doi:10.1136/bmj.m3379.
Living WHO BMJ Rapid Recommendations guidance on drugs for covid-19.
World Health Organization. Therapeutics and COVID-19. Living guideline. November 2023. (https://www.who.int/teams/health-care-readiness-clinical-unit/covid-19/therapeutics).
-
MAGICapp (https://app.magicapp.org/#/guideline/nBkO1E).
Expanded version of the WHO living guidelines with methods, processes, and results with multi-layered recommendations, evidence summaries, and decision aids for use on all devices.
-
Siemieniuk RAC, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis [update 4]. BMJ 2020;370:m2980, doi:10.1136/bmj.m2980.
Review and network meta-analysis of all available randomised trials that assessed drug treatments for covid-19 of all severities.
Methods
A protocol provides the detailed methods of this systematic review (supplementary material). We report this systematic review following the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist for network meta-analyses.12 The linked BMJ Rapid Recommendations guideline panels approved all decisions relevant to the eligibility of interventions and choice of outcomes.
Eligibility criteria
We included randomised clinical trials in people with suspected, probable, or confirmed mild or moderate covid-19 that compared drugs for treatment against one another or against no treatment, placebo, or standard care. We classified trials as addressing mild or moderate covid-19 based on the description of participants provided in the trials, using the following hierarchy: (1) WHO criteria (oxygen saturation ≥90% on room air, respiratory rate ≤30, and no respiratory distress, acute respiratory distress syndrome (ARDS), sepsis, or septic shock)8; (2) author definition; (3) infection not requiring respiratory support (such as supplemental oxygen, non-invasive ventilation, invasive ventilation). When authors classified participants receiving respiratory support as mild or moderate, we used respiratory support to classify participants instead of author definition.
We included trials regardless of publication status (peer reviewed, in press, preprint, conference abstracts, or unpublished data from authors) or language and included all drugs and Chinese medicines that were deemed of potential interest by a steering committee composed of clinicians (supplementary material).
The scope of this review did not include trials evaluating vaccination, blood products, and antibody-based antiviral therapies (such as virus specific monoclonal antibodies), nutrition, traditional Chinese herbal or alternative medicines that include more than one molecule or a molecule without specific molecular weighted dosing, and non-drug supportive care interventions. Trials that evaluated these interventions were identified and categorised separately.
Information sources
In collaboration with Epistemonikos, since 1 January 2023, we performed weekly searches using their Living Overview of the Evidence COVID-19 Repository (COVID-19 L-OVE).13 The database retrieves articles by conducting systematic searches in 42 databases, trial registries, and preprint servers and has been found to include 100% of covid-19 related randomised trials.14 Previously, and until 17 February 2023, we performed daily searches from Monday to Friday in the World Health Organization (WHO) covid-19 database– a comprehensive multilingual source of global literature on covid-19. Before its merge with the WHO covid-19 database on 9 October 2020, we performed daily searches from Monday to Friday in the US Centers for Disease Control and Prevention (CDC) COVID-19 Research Articles Downloadable Database.15 Previously, when searching the WHO covid-19 database or the CDC database, we filtered results through a validated and highly sensitive machine learning model to identify randomised trials.16 We tracked preprints of randomised trials for updates and through publication.
In addition, we searched six Chinese databases monthly: Wanfang, Chinese Biomedical Literature, China National Knowledge Infrastructure, VIP, Chinese Medical Journal Net (preprints), and ChinaXiv (preprints). Previous iterations describe the search strategies for these databases.17 Because they did not provide studies that meaningfully altered the evidence for any drug treatment, we stopped searching these databases on 20 February 2021.
We searched all English information sources from 1 December 2019 to 19 May 2024. The data synthesis includes articles retrieved by 28 June 2023 while articles found after this date, alongside a summary of their results are listed in the supplementary material.
Study selection
Using a systematic review software, Covidence,18 pairs of reviewers, following training and calibration exercises, independently screened all titles and abstracts, followed by full texts of trials that were identified as potentially eligible. A third reviewer adjudicated conflicts.
Data collection
For each eligible trial, pairs of reviewers, following training and calibration exercises, extracted data independently using a standardised, pilot tested data extraction form. Reviewers collected information on trial characteristics (trial registration, publication status, study status, design), patient characteristics (country, age, sex, smoking habits, comorbidities, setting and type of care, and severity of covid-19 symptoms), and outcomes of interest (means or medians and measures of variability for continuous outcomes and the number of participants analysed and the number of participants who experienced an event for dichotomous outcomes). Reviewers resolved discrepancies by discussion and, when necessary, with adjudication by a third party. Subsequently, a data cleaning team, made up of one to two experienced reviewers, reviewed all the extracted information for accuracy. We updated the data collected from included preprints as soon as the peer review publication became available.
Outcomes of interest were selected based on importance to patients19 and were informed by clinical expertise in the systematic review team and in the guideline panel responsible for the linked WHO living guidelines on covid-19 therapeutics.8 9 20 The panel includes unconflicted clinical and methodology experts, recruited to ensure global representation, and patient partners. We included the outcomes that the panel prioritised for decision making. The process for outcome selection is described in the living guideline.8 Selected outcomes were aimed at limiting the progression of covid-19 disease and included mortality (closest to 90 days), use of mechanical ventilation (closest to 90 days), adverse events leading to discontinuation (within 28 days), admission to hospital, length of hospital stay, and time to symptom resolution or clinical improvement. For studies assessing anticoagulants, we also analysed incidence of venous thromboembolism and clinically important bleeding. In contrast to our previous living systematic review, for this iteration, we did not collect data on several outcomes which the guideline development group did not think were critical to decision making for a those with mild or moderate covid-19: viral clearance, time to viral clearance, intensive care unit length of stay, duration of mechanical ventilation, and days free from mechanical ventilation. While the last two outcomes are patient important outcomes, we did not include them because their impact on decision making for mild or moderate covid-19 is limited by their minimal baseline risk in such a population.
Mechanical ventilation includes both invasive and non-invasive mechanical ventilation. We used a hierarchy for the outcome mechanical ventilation in which we preferentially used the total number of patients who received mechanical ventilation over the study period. We used the number of patients ventilated at the time point that the largest number of the patients were ventilated, if the trial reported the number of patients ventilated at specific time points. We used author definitions for mechanical ventilation; when different forms were reported separately, we considered continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP) to be non-invasive mechanical ventilation. When reported separately, we did not consider high flow nasal cannula (HFNC) oxygen therapy to be non-invasive mechanical ventilation.
Risk of bias within individual studies
For each eligible trial, reviewers, following training and calibration exercises, used a revision of the Cochrane tool for assessing risk of bias in randomised trials (RoB 2.0)21 to rate trials as (1) at low risk of bias, (2) having some concerns—probably at low risk of bias, (3) having some concerns—probably at high risk of bias, or (4) at high risk of bias, across the following domains: bias arising from the randomisation process; bias owing to departures from the intended intervention; bias from missing outcome data; bias in measurement of the outcome; bias in selection of the reported results, including deviations from the registered protocol; and bias due to competing risks. Unlike previous iterations of our systematic reviews, we considered unblinded trials as probably at low risk of bias due to departures from intended interventions instead of probably high risk of bias, so long as there were no important imbalances in co-interventions.22 We judged trials to be at high risk of bias overall if one or more domains were rated as probably high risk of bias or as high risk of bias and as low risk of bias if all domains were rated as probably low risk of bias or low risk of bias. Reviewers resolved discrepancies by discussion and, when not possible, with adjudication by a third party.
Data synthesis
We conducted network meta-analysis using a bayesian framework.23 Any additional data syntheses will be posted on our website (www.covid19lnma.com).
Summary measures
We summarised the effect of drug treatments on dichotomous outcomes using the odds ratio and corresponding 95% credible interval. Owing to the low number of events in most studies, we summarised adverse effects leading to discontinuation using risk differences. Because we expected similar durations across randomised trials for continuous outcomes, we used the mean difference and corresponding 95% credible interval for days for length of hospital stay. Because we expected substantial variation between studies for time to symptom resolution, we performed the analyses using the ratio of means and corresponding 95% credible interval before calculating the mean difference in days.24
Treatment nodes
We grouped treatments into common nodes based on molecule and included different doses and durations in the same node unless there was evidence to suggest that the effects differed. We created a separate node for intervention arms in which participants received a combination of drugs. For anticoagulants, we grouped treatments into nodes based on intensity (eg, full dose anticoagulation, intermediate dose anticoagulation).25 The networkplot command of Stata version 15.1 (StataCorp, College Station, TX) provided the software for drawing network plots with thickness of lines between nodes and size of the nodes weighted based on the number of studies per comparison or treatment.26
Statistical analysis
We conducted pairwise meta-analyses and network meta-analyses using a bayesian framework with random effects models for all outcomes.23 We used a plausible prior for the variance parameter and a uniform prior for the effect parameter suggested in a previous study based on empirical data.27 For all analyses, we used three Markov chains with 100 000 iterations after an initial burn-in of 10 000 and a thinning of 10. We used node splitting models to assess local incoherence and to obtain indirect estimates.28 All network meta-analyses were performed using the gemtc package and all pairwise meta-analyses using the bayesmeta package in R version 3.6.3 (RStudio, Boston, MA).23 29
In our prior living iteration of this network meta-analysis, some treatment nodes with few total participants and few total events resulted in highly implausible and extremely imprecise effect estimates. To maximise the probability of providing useful results, we therefore included only nodes with at least 100 patients or at least 20 events.
Certainty of the evidence
We assessed the certainty of evidence using the grading of recommendations assessment, development and evaluation (GRADE) approach for network meta-analysis using semi-automated spreadsheets.4 30 31 32 For each outcome, two people with experience in using GRADE rated each domain for each comparison independently and resolved discrepancies by consensus. We rated the certainty for each comparison and outcome as high, moderate, low, or very low, based on considerations of risk of bias, inconsistency, indirectness, publication bias, intransitivity, incoherence (difference between direct and indirect effects), and imprecision.31 To minimise value judgments, our target of certainty rating was any non-zero effect, regardless of the magnitude (that is, we used a minimally contextualised approach with the null effect as the threshold of interest).33 When point estimates were close to the null effect (between the threshold for a small but important effect and the null), we changed our target of certainty rating to assess our certainty on little to no effect (that is, the effect is in the range between the threshold for small benefit and the threshold for small harm).34 We decided on the small effect threshold based on a survey of the authors, and present the thresholds for each outcome.
Interpretation of results
To facilitate interpretation of the results, we calculated absolute effects for outcomes in which the summary measure was an odds ratio or ratio of means. For the outcomes of mortality, admission to hospital, and time to symptom resolution, we inferred baseline risk in the standard care group for each outcome according to the WHO covid-19 living guideline moderate risk group.8 For all other outcomes, we used the median from all studies in which participants received standard of care to calculate the baseline risk for each outcome. We calculated absolute effects using the transitive risks model using the R2jags package in R.35 36
For each outcome, we classified treatments in groups from the most to the least effective using the minimally contextualised framework, which focuses on the treatment effect estimates and the certainty of the evidence.37
We interpreted and communicated the results using standardised language to reflect the certainty of the evidence.38
Subgroup and sensitivity analysis
We performed subgroup analyses for specific drug treatments of interest at the direction of the WHO living guideline panel. Previous iterations included subgroup analyses for ivermectin, interleukin-6 receptor antagonists, corticosteroids, hydroxychloroquine, lopinavir-ritonavir, and remdesivir. To conduct these analyses, we used bayesian hierarchical meta-regression with study as a random effect. Where possible, we performed within rather than between trial analyses. We did not perform any sensitivity analyses.
Patient and public involvement
As part of the WHO living guideline and BMJ Rapid Recommendations initiative, patients were involved in outcome selection and the generation of parallel recommendations.7 8 9
Results
After screening 138 138 titles and abstracts and 3247 full texts, we identified 863 unique randomised trials. Of those, 259 randomised trials evaluated drug treatments of interest in patients with mild or moderate covid-19 as of 28 June 2023 (fig 1). The supplementary material includes a table of excluded studies from full text screening along with their reason of exclusion. Of the 259 trials, 217 (84%) have been published in peer reviewed journals, 24 (9%) are preprints, and 18 (7%) remain unpublished as abstracts, data from meta-analyses, data from authors, or data from presentations (table 1). Most trials were registered (234/259; 90%), and half evaluated treatment in patients admitted to hospital with covid-19 (128/259; 50%). The majority (at least 80%) of patients in 221 of the 259 included studies had confirmed covid-19 infection. Trials were most commonly conducted in the USA, Brazil, India, UK, and China. Researchers evaluated 108 different drug treatments.
Fig 1.
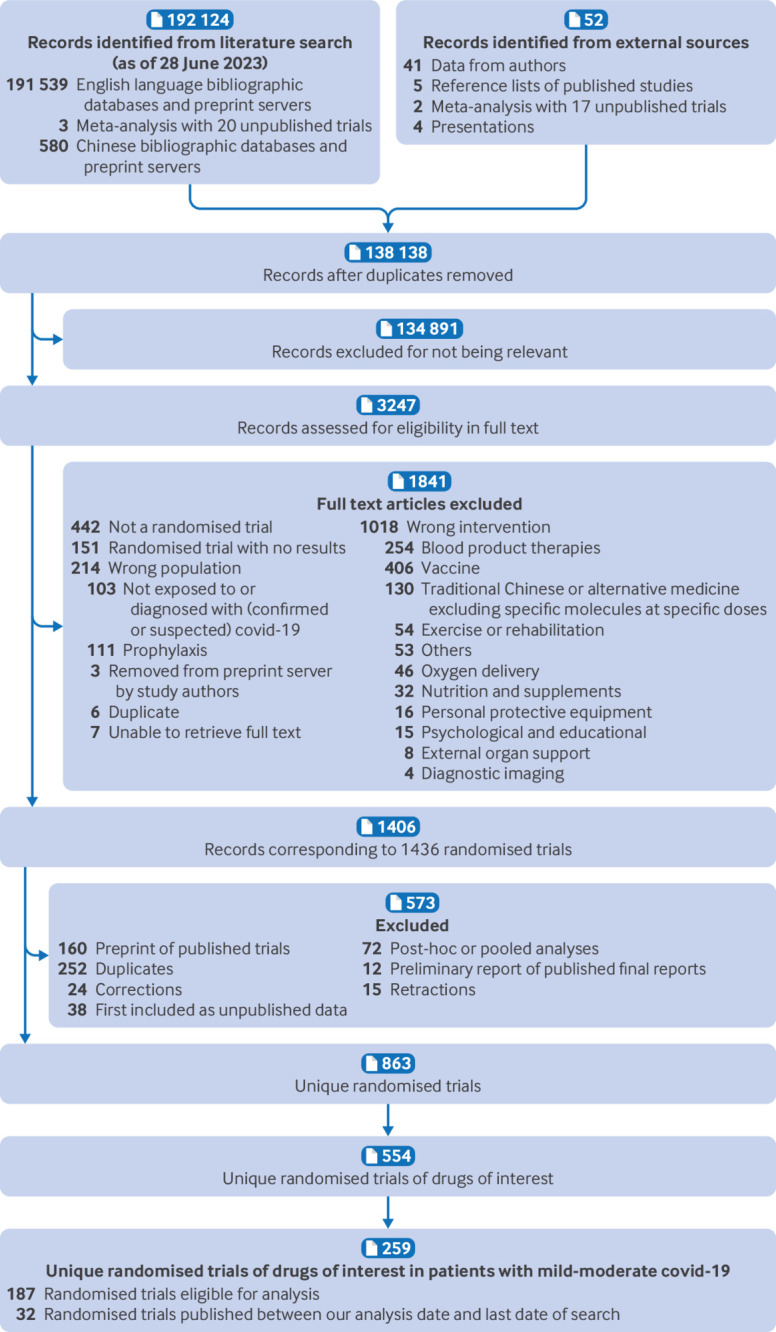
Study selection
Table 1.
Study characteristics. Data are number (%) of studies unless stated otherwise.
| Characteristic | No (%) of studies |
|---|---|
| Registered | 234/259 (90.3) |
| Publication status: | |
| Preprint | 24/259 (9.3) |
| Published | 217/259 (83.8) |
| Unpublished | 18/259 (6.9) |
| Median (IQR) No of patients | 157 (73-470) |
| Country of study: | |
| USA | 48/259 (18.5) |
| Brazil | 33/259 (12.7) |
| India | 32/259 (12.4) |
| UK | 28/259 (10.8) |
| China | 25/259 (9.7) |
| Intensity of care: | |
| Outpatient | 108/259 (41.7) |
| Inpatient | 129/259 (49.8) |
Between 28 June 2023 (data analysis date) and until 19 May 2024, we identified 150 eligible records, of which 51 evaluated treatments of interest in patients with mild or moderate covid-19. Of these 51 records, 12 (24%)39 40 41 42 43 44 45 46 47 48 49 50 were associated with already included studies and provided no additional data, seven (14%)51 52 53 54 55 56 57 would be excluded from analysis because they do not report outcomes of interest or investigated treatments with fewer than 100 patients, while the remaining 32 (63%)58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 reported relevant data. The supplement lists and describes the results of these 32 studies and compares them to our current findings.
Several trials (35/259; 13%) could not be included in the analysis because both arms would have been classified within the same treatment node (7/35; 20%)90 91 92 93 94 95 96 or they reported no outcomes of interest (28/35; 80%).97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 Ultimately, we analysed 187 (187/259; 72%) trials that reported on treatments with at least 100 patients or 20 events. Thirty seven trials reported on treatments that did not meet this threshold and therefore were not included in the analysis.125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 Table 1 presents a summary of the characteristics of the 259 included studies across all included studies. Additional study characteristics and risk of bias assessments for each study are available in the supplementary material.
Of the 187 randomised trials included in the analyses, nine (5%) did not have publicly accessible protocols or registrations. Of those with publicly accessible protocols or registrations, 73 (39%) reported results for one or more of our outcomes of interest that were not prespecified in protocols or registrations. Three studies had discrepancies in the reporting of the time point at which an outcome was assessed.
Sixty five studies were initially posted as preprints and subsequently published after peer review. The supplementary material presents the differences between study preprint and peer reviewed publications. Twenty-eight studies (28/65; 43%) had discrepancies in outcome reporting between the preprint and peer-reviewed publication, 29 studies (29/65; 45%) had discrepancies with patient baseline characteristics, and eight studies (8/65; 12%) had discrepancies in reporting that led to changes in risk of bias ratings. No substantive differences were found for 22 studies (22/65; 34%).
Risk of bias in included studies
The supplementary material presents the assessment of risk of bias of the included studies for each outcome: 114 studies (114/187; 61%) were judged at low risk or probably low risk of bias in all domains for at least one outcome.
Effects of the interventions
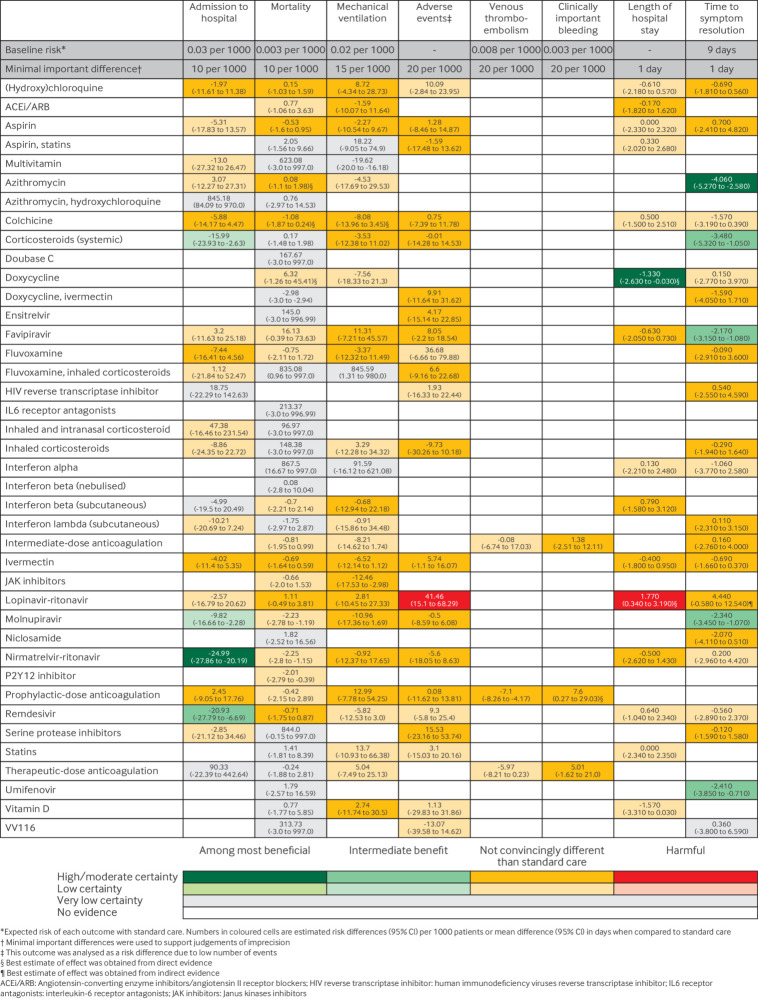
The supplementary material presents the network plots depicting the drug treatments included in the network meta-analysis of each outcome. Figure 2 presents a summary of the effects of the interventions on the outcomes. The supplementary material also presents relative and absolute effect estimates and certainty of the evidence for all comparisons and outcomes. We did not detect statistical incoherence in any of the network meta-analyses. All analyses reached convergence based on trace plots and a Brooks-Gelman-Rubin statistic less than 1.05.
Fig 2.
Summary of effects of drug treatments compared with standard care for mild and moderate covid-19
Admission to hospital
The network meta-analysis included 75 trials162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 with 75 968 participants addressing 24 different drug treatments. The supplementary material contains the network plots and complete network meta-analysis results including the certainty of the evidence. The most common treatments were standard care or placebo (74 trials, 37 321 participants), ivermectin (11 trials, 3821 participants), and hydroxychloroquine (10 trials, 1766 participants).
Based on our classification from most to least effective, nirmatrelvir-ritonavir (odds ratio compared with standard care (ORSC) 0.15 (95% credible interval (95% CI) 0.07 to 0.32), moderate certainty) is probably the best at reducing hospital admission, while remdesivir (ORSC 0.25 (0.07 to 0.77), moderate certainty) probably provides an intermediate benefit for this outcome (fig 2). Systemic corticosteroids (ORSC 0.43 (0.20 to 0.90), low certainty) and molnupiravir (ORSC 0.66 (0.44 to 0.92), low certainty) may also provide an intermediate benefit. Colchicine (ORSC 0.79 (0.52 to 1.15), moderate certainty), fluvoxamine (ORSC 0.73 (0.44 to 1.15), moderate certainty), hydroxychloroquine (ORSC 0.91 (0.61 to 1.39), moderate certainty), ivermectin (ORSC 0.85 (0.61 to 1.18), moderate certainty), and prophylactic-dose anticoagulation (ORSC 1.06 (0.69 to 1.62), moderate certainty) are probably not convincingly different from standard care.
Mortality
The network meta-analysis included 161 trials162 163 164 165 167 168 169 170 171 172 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 210 213 214 215 216 217 218 219 222 223 224 225 226 229 230 231 232 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 with 104 831 participants, addressing 40 different drug treatments (supplementary material). The most common treatments were standard care or placebo (155 trials, 51 298 participants), hydroxychloroquine (22 trials, 3119 participants), and ivermectin (21 trials, 4790 participants).
Based on our classification, aspirin (ORSC 0.8 (0.48 to 1.29), high certainty), azithromycin (ORSC 1.03 (0.63 to 1.66), high certainty), colchicine (ORSC 0.64 (0.38 to 1.08), high certainty), hydroxychloroquine (ORSC 1.06 (0.68 to 1.55), high certainty), ivermectin (ORSC 0.75 (0.48 to 1.18), high certainty), lopinavir-ritonavir (ORSC 1.30 (0.84 to 2.17), high certainty), and remdesivir (ORSC 0.75 (0.43 to 1.28), high certainty) are not convincingly different from standard care (fig 2). Evidence was low or very low certainty for all other drug treatments, and no treatment suggested a benefit.
Mechanical ventilation
The network meta-analysis included 74 trials164 167 168 171 172 175 181 182 183 189 190 194 197 198 199 200 203 204 213 214 216 217 221 223 226 230 232 236 241 242 243 244 247 253 254 256 257 260 263 264 265 269 270 273 278 280 282 283 284 285 290 291 293 297 298 299 302 303 305 306 307 308 311 315 317 320 321 324 325 326 with 43 566 participants, addressing 28 different drug treatments (supplementary material). The most common treatments were standard care or placebo (73 trials, 21 329 participants), ivermectin (13 trials, 2511 participants), and favipiravir (8 trials, 702 participants).
Based on our classification, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEi/ARB) (ORSC 0.87 (0.49 to 1.59), high certainty), aspirin (ORSC 0.86 (0.47 to 1.50), high certainty), colchicine (ORSC 0.59 (0.30 to 1.18), high certainty), and ivermectin (ORSC 0.66 (0.40 to 1.07), high certainty) are not convincingly different from standard care (fig 2). Moderate certainty evidence found that the following interventions were probably not convincingly different from standard care: JAK inhibitors, systemic corticosteroids, favipiravir, fluvoxamine, hydroxychloroquine, subcutaneous interferon β, lopinavir-ritonavir, molnupiravir, nirmatrelvir-ritonavir, prophylactic-dose anticoagulation, and vitamin D. Evidence was low or very low certainty for all other drug treatments.
Adverse events leading to discontinuation
The network meta-analysis included 81 trials164 169 170 173 176 178 179 181 184 187 188 189 191 192 194 196 198 199 200 201 204 208 209 210 211 212 213 215 221 222 223 224 227 229 230 231 232 238 240 242 246 248 250 251 253 256 259 260 261 264 269 271 276 277 278 279 280 281 282 283 287 295 296 297 299 305 307 309 310 312 313 317 319 325 327 328 329 330 331 332 with 34 727 participants, addressing 23 different drug treatments (supplementary material). The most common treatments were standard care or placebo (78 trials, 15 995 participants), favipiravir (13 trials, 1626 participants), and ivermectin (12 trials, 1529 participants). Based on our classification, high certainty evidence found lopinavir-ritonavir to be harmful compared with standard care (41 more per 1000 (15 more to 68 more)) and ivermectin to be not convincingly different from standard care (6 more per 1000 (1 fewer to 16 more)) (fig 2). Moderate certainty evidence found that the following intervention were probably not convincingly different from standard care: aspirin, aspirin combined with statins, colchicine, systemic corticosteroids, doxycycline combined with ivermectin, ensitrelvir, favipiravir, fluvoxamine combined with inhaled corticosteroids, inhaled corticosteroids, molnupiravir, nirmatrelvir-ritonavir, prophylactic-dose anticoagulation, and serine protease inhibitors.
Venous thromboembolism
Ten trials164 168 169 180 181 210 289 290 292 308 with 5386 participants allocated to four different drug treatments reported venous thromboembolism (supplementary material). Based on our classification, prophylactic-dose anticoagulation is probably not convincingly different from standard care (ORSC 0.12 (0.02 to 0.50), moderate certainty) (fig 2). Evidence was low certainty for all other drug treatments, and no treatment suggested a benefit.
Clinically important bleeding
Ten trials164 168 169 180 181 210 289 290 292 308 with 5298 participants allocated to four different drug treatments reported clinically important bleeding (supplementary material). Based on our classification, prophylactic-dose anticoagulation (ORSC 3.28 (1.08 to 9.90), moderate certainty), intermediate-dose anticoagulation (ORSC 1.08 (0.25 to 4.67), moderate certainty), and therapeutic-dose anticoagulation (ORSC 1.98 (0.53 to 7.49), moderate certainty) are probably not convincingly different from standard care (fig 2).
Length of hospital stay
The network meta-analysis included 29 trials189 193 196 217 236 238 243 244 247 251 253 254 255 260 270 279 282 283 284 297 299 306 311 317 320 321 324 with 11 642 participants, addressing 16 different drug treatments (supplementary material). The most common treatments were standard care or placebo (29 trials, 5592 participants), hydroxychloroquine (5 trials, 656 participants), ACEi/ARB (4 trials, 463 participants), and ivermectin (4 trials, 424 participants).
Based on our classification, doxycycline probably reduces length of hospital stay (mean difference compared with standard care (MDSC) −1.33 days (95% CI −2.63 to −0.03), moderate certainty) (fig 2). ACEi/ARB (MDSC −0.17 (−1.82 to 1.62), moderate certainty), favipiravir (MDSC −0.63 (−2.05 to 0.73), moderate certainty), subcutaneous interferon β (MDSC 0.79 (−1.58 to 3.12), moderate certainty), ivermectin (MDSC −0.40 (−1.80 to 0.95), moderate certainty), and nirmatrelvir-ritonavir (MDSC −0.50 (−2.62 to 1.43), moderate certainty) are probably not convincingly different from standard care. Lopinavir-ritonavir increases duration of hospital admission (MDSC 1.77 days (0.34 to 3.19), high certainty).
Time to symptom resolution
The network meta-analysis included 70 trials162 163 167 170 171 173 174 175 178 179 191 192 193 195 196 197 200 202 204 205 206 211 216 217 218 221 223 224 228 231 232 234 237 241 242 243 244 245 246 248 250 251 252 258 261 262 264 269 277 285 287 290 293 297 305 309 311 312 317 318 326 328 329 332 333 334 335 336 337 338 with 48 497 participants, addressing 24 different drug treatments (supplementary material). A description of how symptom resolution was defined in each trial can be found in the supplementary material. The most common treatments were standard care or placebo (67 trials, 23 063 participants), ivermectin (11 trials, 3144 participants), and hydroxychloroquine (11 trials, 756 participants).
Based on our classification from most to least effective, azithromycin is probably the best at reducing time to symptom resolution (MDSC −4.06 days (−5.27 to −2.58), moderate certainty) (fig 2). Several interventions also suggested an intermediate benefit: molnupiravir (MDSC −2.34 (−3.45 to −1.07), high certainty), systemic corticosteroids (MDSC −3.48 (−5.32 to −1.05), moderate certainty), favipiravir (MDSC −2.17 (−3.15 to −1.08), moderate certainty), and umifenovir (MDSC −2.41 (−3.85 to −0.71), moderate certainty). The results suggest that the remaining interventions may be not convincingly different from standard care (fig 2).
Subgroups and sensitivity analyses
We have conducted subgroup analyses in previous iterations which did not yield important results.17 Consequently, as this analysis includes a narrower population for which we did not observe consistent, serious heterogeneity, and as the guideline panel did not request any subgroup analyses, we did not conduct any for this iteration. We did not perform any sensitivity analyses.
Discussion
Summary of findings
This systematic review and network meta-analysis provides a comprehensive overview of the evidence for drug treatments for mild or moderate covid-19 up to 28 June 2023. This includes more than 250 randomised trials specific to non-severe patients and analyses results from 40 different interventions for treating covid-19. We have also identified 32 records58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 (with relevant data) that would be eligible as of 19 May 2024 (supplementary material). These 32 records would inform 75 outcomes across 25 comparisons. These comparisons include five new treatments: leritrelvir, simnotrelvir, GST-HG171 combined with ritonavir, fluoexetine, and bemnifosbuvir. The preliminary analysis across these 75 outcomes is largely consistent with our results (supplementary material); however, it is limited by the lack of risk of bias assessments and certainty of the evidence assessments, which are only included for the results of our network meta-analysis.
For patients with non-severe covid-19, two antivirals probably reduce admission to hospital: nirmatrelvir-ritonavir and remdesivir. Low certainty evidence suggests systemic corticosteroids and molnupiravir may also reduce hospital admission. For those admitted to hospital, moderate certainty evidence found doxycycline probably reduces their length of stay. Additionally, several drug treatments probably hasten symptom resolution, including azithromycin, molnupiravir, systemic corticosteroids, favipiravir, and umifenovir. High certainty evidence found lopinavir-ritonavir to increase adverse effects leading to discontinuation and increase duration of hospital stay (perhaps as a result of the side effects).
According to our classification no drug was convincingly different from standard care for the outcomes of mortality, mechanical ventilation, venous thromboembolism, and clinically important bleeding; however, rating the certainty using a different target may provide important insights. For example, some interventions suggested little or no effect. For mortality, low certainty evidence suggested nirmatrelvir-ritonavir, and molnupiravir may provide little or no benefit. For mechanical ventilation, moderate certainty evidence suggested JAK inhibitors probably provide little or no benefit. Moderate certainty evidence suggested prophylactic-dose anticoagulation probably provides little or no benefit for venous thromboembolism and probably results in little or no increase of clinically important bleeding.
Several interventions do not seem to result in a benefit for any patient important outcomes, including ACEi/ARB, aspirin, colchicine, fluvoxamine, hydroxychloroquine, ivermectin, and lopinavir-ritonavir. Lopinavir-ritonavir increases the risk of adverse effects leading to drug discontinuation and increases length of hospital stay.
Strengths and limitations of this review
Our review includes studies from various stages of the pandemic, with many trials being conducted earlier, before the deployment of vaccines and when covid-19 had worse outcomes. To ensure that our results remain applicable, we consulted with the WHO guideline panel to choose baseline risks (non-severe, moderate risk) most reflective of this population.8 Additionally, while our included studies include patients infected with different covid-19 variants, we do not believe this is likely to importantly affect our results as, to our knowledge, there is no substantial evidence that viral subtype is an effect modifier of any of our included drugs.339 Some of the included drugs have downsides that this review did not capture; however, further details can be found in the guideline.8 For example, molnupiravir could be carcinogenic,340 nirmatrelvir-ritonavir has many critical drug-drug interactions, and remdesivir is administered intravenously. Thus, while this review provides an overview of the evidence, individual considerations (such as patient values and preferences, contraindications, drug-drug interactions, and drug-specific adverse events not included in this review) must be taken into account when deciding whether to use any medication.
Our search strategy and eligibility criteria were comprehensive, without restrictions on language of publication or publication status. To ensure expertise in all areas, our team is composed of clinical and methods experts who have undergone training and calibration exercises for all stages of the review process. To minimise problems with counterintuitive results, we anticipated challenges that arise in network meta-analysis when data are sparse.341 Many of the results for comparisons with sparse data were uninformative and sometimes implausible. For that reason, we decided to restrict our analysis to treatments for which at least 100 people were randomised or for which there were at least 20 events. Despite our best effort with this restriction, we observed that the use of a bayesian framework and random effects models for analysis resulted in several estimates of effect having wide confidence intervals, particularly for comparisons in which there were few studies and infrequent outcomes. Although we considered conducting sensitivity analyses using different statistical methods, we judged that the wide confidence intervals appropriately reflected the statistical uncertainty in those cases and decided against switching our analysis methods.
As implied above, the main limitation of the evidence is that there are few events (and thus serious imprecision) for many of the drugs for many outcomes. Partly, the low number of events for some outcomes was because our systematic review was restricted to patients with mild or moderate covid-19, who are less likely to experience outcomes such as admission to hospital, mortality, and mechanical ventilation. As a result (and not only because of the method of analysis), many credible intervals are imprecise, which reduced the certainty of the evidence. Additionally, data for some outcomes, especially time to symptom resolution, are limited by the different measurement and reporting methods between studies.
The inclusion of preprints in our network meta-analysis might limit our results due to errors and the reporting limitations of preprints. Differences between preprints and peer reviewed publications have thus far been limited to additional baseline patient information, clarification on study design, and outcomes reported in the peer reviewed publications (supplementary material). We believe that none of these changes would have resulted in a meaningful change to pooled effect estimates or certainty for any outcome.342
Our systematic review and network meta-analysis has continued to inform the development of the WHO living guidelines and BMJ Rapid Recommendations.8 9 However, these have an important difference in the methods for assessing the certainty of the evidence. In this systematic review and network meta-analysis, we use a minimally contextualised approach for rating the certainty of the evidence, whereas the WHO guideline panels use a fully contextualised approach in which thresholds of importance of magnitudes of effects depend on all other outcomes and factors involved in the decision.33 The contextualisation explains differences in the certainty of the evidence between the two, driven by different judgments on imprecision. One example is molnupiravir, for which the WHO guideline panel concluded with moderate certainty evidence—as opposed to our low certainty evidence—for a reduction in hospital admission in patients with non-severe covid-19.
Comparison with other studies
We are aware of two other similar efforts addressing covid-19.343 344 Our intention is different in that our results fully inform clinical decision making for the associated living WHO guideline.8 We also use different analytical methods, which we believe are best suited for this evidence. It is also important to evaluate the reproducibility and replicability of findings from different scientific approaches. We are aware of three network meta-analyses investigating treatments specific to non-severe covid-19; however, the most recent of these included studies up to April 2022 and each looked at only a specific subset of treatment comparisons.345 346 347
This is the first and only version of this systematic review and network meta-analysis that considered only patients with non-severe covid-19. We will perform separate network meta-analyses for patients with severe covid-19. Although not planned, any updates will be published on www.covid19lnma.com, the primary resource to go to between the intermittent publications in The BMJ (box 1).
Conclusions
Evidence from this systematic review and network meta-analysis of drug treatments for non-severe covid-19 suggests nirmatrelvir-ritonvir and remdesivir probably reduce admission to hospital while systemic corticosteroids and molnupiravir may reduce hospital admissions. Azithromycin, corticosteroids, favipiravir, molnupiravir, and umifenovir probably reduce duration of symptoms. For most other interventions, the evidence remains uncertain regarding their impact on patient-important outcomes or indicates they are probably not beneficial.
What is already known on this topic
The evidence for drug treatments for covid-19 continues to evolve, with evidence suggesting different treatments are most effective at certain disease stages
This article focuses on patients with mild or moderate (non-severe) covid-19 from a network meta-analysis of covid-19 drug treatments
What this study adds
Network meta-analysis of 187 of 259 randomised trials (72%) comparing 40 different drug treatments in people with mild or moderate covid-19
Nirmatrelvir-ritonavir and remdesivir probably reduce hospital admission; molnupiravir and systemic corticosteroids may reduce hospital admission
Several drugs, including molnupiravir and systemic corticosteroids, probably reduce symptom duration, whereas nirmatrelvir-ritonavir and remdesivir may not
Acknowledgments
We thank Donald Arnold, Camilla Avila, Jessica Bartoszko, Kevin Cheung, Ellen Cusano, Andrea Darzi, Zaira Escamilla, Carmen Fang, Fang Fang, Long Ge, Miah Han, Assem Khamis, Elena Kum, Bonnie Lam, Mark Loeb, Sophia Mangala, Maura Marcucci, Kruti Modi, Shahrzad Motaghi, Srinivas Murthy, Amir Reza Farhoud, Yamna Rizwan, Pakeezah Saadat, Charlotte Switzer, Lehana Thabane, George Tomlinson, Robin Vernooij, Catherine Wang, Ying Wang, Zhikang Ye, and Dena Zeraatkar for input and early contributions.
Web Extra.
Extra material supplied by the author
Supplementary material
Contributors: SI, RACS, MJO, NI, JPMD, contributed equally to the systematic review and are joint first authors. SI, RACS, MJO, NI, JPMD, AQ and RB-P were the core team leading the systematic review. MJO, LY, MG, YW, AK, RC, YZ, FMF, YC, MP, DB, CB, and GR identified and selected the studies. SI, YC, KH, LY, YG, SRC, GB, CZ, YR, MA, GB, AA, SLM, MG, MP, and CM collected the data. JPDM analysed the data. RB-P, AI, AM, BR, DKC, FF, FL, HPH, RAM, TD, CZ, and TA assessed the certainty of the evidence. SLM, FL, BR, TA, POV, and GHG provided advice at different stages. SI, RACS, and RB-P drafted the manuscript. All authors approved the final version of the manuscript. RACS is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by the Canadian Institutes of Health Research (grant MM1-174897). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Canadian Institutes of Health Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Transparency: RACS affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The infographic and MAGICapp decision aids (available at www.magicapp.org/) were created to facilitate conversations between healthcare providers and patients or their surrogates. The MAGICapp decision aids were co-created with people who have lived experience of covid-19.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not applicable. All the work was developed using published data.
Data availability statement
No additional data available.
References
- 1.World Health Organization. WHO COVID-19 dashboard. 2023. https://covid19.who.int.
- 2.COVID-19 Studies from the World Health Organization Database - ClinicalTrials.gov. 2023 https://classic.clinicaltrials.gov/ct2/who_table.
- 3. Djulbegovic B, Guyatt G. Evidence-based medicine in times of crisis. J Clin Epidemiol 2020;126:164-6. 10.1016/j.jclinepi.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puhan MA, Schünemann HJ, Murad MH, et al. GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 5. Trougakos IP, Stamatelopoulos K, Terpos E, et al. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J Biomed Sci 2021;28:9. 10.1186/s12929-020-00703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol 2022;20:270-84. 10.1038/s41579-022-00713-0 [DOI] [PubMed] [Google Scholar]
- 7. Siemieniuk R A AT, Agoritsas T, Macdonald H, Guyatt GH, Brandt L, Vandvik PO. Introduction to BMJ Rapid Recommendations. BMJ 2016;354:i5191. 10.1136/bmj.i5191 [DOI] [PubMed] [Google Scholar]
- 8. Lamontagne F, Agarwal A, Rochwerg B, et al. A living WHO guideline on drugs for covid-19. BMJ 2020;370:m3379. [DOI] [PubMed] [Google Scholar]
- 9. Lamontagne F, Stegemann M, Agarwal A, et al. A living WHO guideline on drugs to prevent covid-19. BMJ 2021;372:n526. 10.1136/bmj.n526 [DOI] [PubMed] [Google Scholar]
- 10. Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ 2021;373:n949. 10.1136/bmj.n949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siemieniuk RA, Bartoszko JJ, Díaz Martinez JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ 2021;374:n2231. 10.1136/bmj.n2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 13.Epistemonikos Foundation. Living evidence repository for COVID-19. Epistemonikos Foundation. https://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d.
- 14. Verdugo-Paiva F, Vergara C, Ávila C, et al. COVID-19 Living Overview of Evidence repository is highly comprehensive and can be used as a single source for COVID-19 studies. J Clin Epidemiol 2022;149:195-202. 10.1016/j.jclinepi.2022.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephen B Thacker CDC Library. COVID-19 research articles downloadable database: Centers for Disease Control and Prevention 2020. https://www.cdc.gov/library/researchguides/2019novelcoronavirus/researcharticles.html.
- 16. Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying Randomized Controlled Trials: An evaluation and practitioner’s guide. Res Synth Methods 2018;9:602-14. 10.1002/jrsm.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ 2020;370:m2980. 10.1136/bmj.m2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covidence systematic review software [program]. Melbourne, Australia: Veritas Health Innovation.
- 19. Jimenez-Mora MA, Varela AR, Meneses-Echavez JF, et al. Patient-important outcomes reported in randomized controlled trials of pharmacologic treatments for COVID-19: a protocol of a META-epidemiological study. Syst Rev 2021;10:289. 10.1186/s13643-021-01838-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rochwerg B, Agarwal A, Zeng L, et al. Remdesivir for severe covid-19: a clinical practice guideline. BMJ 2020;370:m2924. 10.1136/bmj.m2924 [DOI] [PubMed] [Google Scholar]
- 21. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 22. Zeraatkar D, Pitre T, Diaz-Martinez JP, et al. Impact of Allocation Concealment and Blinding in Trials Addressing Treatments for COVID-19: A Methods Study. Am J Epidemiol 2023;192:1678-87. 10.1093/aje/kwad131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Röver C. Bayesian random-effects meta-analysis using the bayesmeta R package: arXiv 2017; 2017.. https://arxiv.org/abs/1711.08683.
- 24. Friedrich JOAN, Adhikari NK, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol 2011;64:556-64. 10.1016/j.jclinepi.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 25. Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv 2021;5:872-88. 10.1182/bloodadvances.2020003763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med 2015;34:984-98. 10.1002/sim.6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods 2016;7:80-93. 10.1002/jrsm.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Network meta-analysis using Bayesian methods [program]: R package version 0.8-4 version, 2020.
- 30. Brignardello-Petersen R, Bonner A, Alexander PE, et al. GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018;93:36-44. 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 31. Brignardello-Petersen R, Mustafa RA, Siemieniuk RAC, et al. GRADE Working Group . GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol 2019;108:77-85. 10.1016/j.jclinepi.2018.11.025 [DOI] [PubMed] [Google Scholar]
- 32. Izcovich A, Chu DK, Mustafa RA, Guyatt G, Brignardello-Petersen R. A guide and pragmatic considerations for applying GRADE to network meta-analysis. BMJ 2023;381:e074495. 10.1136/bmj-2022-074495 [DOI] [PubMed] [Google Scholar]
- 33. Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017;87:4-13. 10.1016/j.jclinepi.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng L, Brignardello-Petersen R, Hultcrantz M, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol 2021;137:163-75. 10.1016/j.jclinepi.2021.03.026 [DOI] [PubMed] [Google Scholar]
- 35.Spineli L, Brignardello-Petersen R, Heen A, et al. Obtaining absolute effect estimates to facilitate shared decision making in the context of multiple comparisons. Global Evidence Summit, 2017. https://abstracts.cochrane.org/2017-cape-town-global-evidence-summit/obtaining-absolute-effect-estimates-facilitate-shared.
- 36.Using R to Run ‘JAGS’ [program]: R package version 0.6-1 version, 202, 2020.
- 37. Brignardello-Petersen R, Florez ID, Izcovich A, et al. GRADE working group . GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ 2020;371:m3900. 10.1136/bmj.m3900 [DOI] [PubMed] [Google Scholar]
- 38. Santesso N, Glenton C, Dahm P, et al. GRADE Working Group . GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020;119:126-35. 10.1016/j.jclinepi.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 39. Correction to Lancet Infect Dis 2022; published online Oct 19. https://doi.org/10.1016/S1473-3099(22)00644-2. 2023;23:e1. 10.1016/S1473-3099(22)00740-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Erratum: effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2023;329:178. 10.1001/jama.2022.23315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erratum: effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2023;330:666. 10.1001/jama.2023.13799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boulware DR, Lindsell CJ, Stewart TG, et al. ACTIV-6 Study Group and Investigators . Inhaled fluticasone furoate for outpatient treatment of covid-19. N Engl J Med 2023;389:1085-95. 10.1056/NEJMoa2209421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bramante CT, Beckman KB, Mehta T, et al. Favorable antiviral effect of metformin on severe acute respiratory syndrome coronavirus 2 viral load in a randomized, placebo-controlled clinical trial of coronavirus disease 2019. Clin Infect Dis 2024;79:354-63. 10.1093/cid/ciae159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jittamala P, Schilling WHK, Watson JA, et al. PLATCOV Collaborative Group . Clinical antiviral efficacy of remdesivir in coronavirus disease 2019: an open-label, randomized controlled adaptive platform trial (PLATCOV). J Infect Dis 2023;228:1318-25. 10.1093/infdis/jiad275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Partap U, Sharma KK, Marathe Y, et al. Effect of vitamin D and zinc supplementation on treatment outcomes among COVID-19 patients in India: results from a double-blind randomized placebo-controlled trial. Curr Dev Nutr 2023;7:100441. 10.1016/j.cdnut.2023.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sobngwi E, Zemsi S, Guewo M, et al. Doxycycline vs Hydroxychloroquine + Azithromycin in the Management of COVID-19 Patients: An Open-Label Randomized Clinical Trial in Sub-Saharan Africa (DOXYCOV). Cureus 2023;15:e45619. 10.7759/cureus.45619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Standing JF, Buggiotti L, Guerra-Assuncao JA, et al. PANORAMIC Virology Group . Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients. Nat Commun 2024;15:1652. 10.1038/s41467-024-45641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strizki JM, Grobler JA, Murgolo N, et al. Virologic Outcomes with Molnupiravir in Non-hospitalized Adult Patients with COVID-19 from the Randomized, Placebo-Controlled MOVe-OUT Trial. Infect Dis Ther 2023;12:2725-43. 10.1007/s40121-023-00891-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strizki JM, Grobler JA, Murgolo N, et al. Correction to: Virologic Outcomes with Molnupiravir in Nonhospitalized Adult Patients with COVID-19 from the Randomized, Placebo-Controlled MOVe-OUT Trial. Infect Dis Ther 2024;13:1159-60. 10.1007/s40121-024-00947-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Victoria H, Jane H, Oghenekome G, et al. Molnupiravir Treatment for COVID-19 in People at Higher Risk in the Community: Outcomes from the PANORAMIC Randomised Controlled Trial at Three and Six Months. SSRN 2024.
- 51. Bindu KH, Saritha M, Kondaveti S, et al. Efficacy of montelukast in treating covid-19 a double-blind, randomized, placebo-controlled study. J Cardiovasc Dis Res 2024;15:808-16. 10.48047/jcdr.2024.15.01.89. [DOI] [Google Scholar]
- 52. Iwahori K, Nii T, Yamaguchi N, et al. A randomized phase 2 study on demeclocycline in patients with mild-to-moderate COVID-19. Sci Rep 2023;13:13809. 10.1038/s41598-023-41051-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luvira V, Schilling WHK, Jittamala P, et al. PLATCOV Collaborative Group . Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial. BMC Infect Dis 2024;24:89. 10.1186/s12879-023-08835-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pan Z, Wan Z, Wang Y, et al. An open-label randomized controlled trial of leflunomide in patients with acute SARS-CoV-2 omicron variant infection. Front Med (Lausanne) 2023;10:1218102. 10.3389/fmed.2023.1218102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siripongboonsitti T, Tawinprai K, Avirutnan P, Jitobaom K, Auewarakul P. A randomized trial to assess the acceleration of viral clearance by the combination Favipiravir/Ivermectin/Niclosamide in mild-to-moderate COVID-19 adult patients (FINCOV). J Infect Public Health 2024;17:897-905. 10.1016/j.jiph.2024.03.030 [DOI] [PubMed] [Google Scholar]
- 56. Wang B, Li HJ, Cai MM, et al. Antiviral efficacy of RAY1216 monotherapy and combination therapy with ritonavir in patients with COVID-19: a phase 2, single centre, randomised, double-blind, placebo-controlled trial. EClinicalMedicine 2023;63:102189. 10.1016/j.eclinm.2023.102189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang F, Xiao W, Tang Y, et al. Efficacy and safety of SIM0417 (SSD8432) plus ritonavir for COVID-19 treatment: a randomised, double-blind, placebo-controlled, phase 1b trial. Lancet Reg Health West Pac 2023;38:100835. 10.1016/j.lanwpc.2023.100835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Amra B, Vaezi A, Soltaninejad F, Salahi M, Salmasi M, Haghjooy Javanmard S. Steroid in the Treatment of Outpatient COVID-19: A Multicenter Randomized Controlled Trial. Adv Biomed Res 2023;12:122. 10.4103/abr.abr_72_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Horga A, Saenz R, Yilmaz G, et al. Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY). Future Virol 2023;18:839-53. 10.2217/fvl-2023-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boffito M, Dolan E, Singh K, et al. A Phase 2 Randomized Trial Evaluating the Antiviral Activity and Safety of the Direct-Acting Antiviral Bemnifosbuvir in Ambulatory Patients with Mild or Moderate COVID-19 (MOONSONG Study). Microbiol Spectr 2023;11:e0007723. 10.1128/spectrum.00077-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cao B, Wang Y, Lu H, et al. Oral Simnotrelvir for Adult Patients with Mild-to-Moderate Covid-19. N Engl J Med 2024;390:230-41. 10.1056/NEJMoa2301425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Souza SB, Cabral PGA, da Silva RM, et al. Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients. Front Med (Lausanne) 2023;10:1215916. 10.3389/fmed.2023.1215916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fan X, Dai X, Ling Y, et al. Oral VV116 versus placebo in patients with mild-to-moderate COVID-19 in China: a multicentre, double-blind, phase 3, randomised controlled study. Lancet Infect Dis 2024;24:129-39. 10.1016/S1473-3099(23)00577-7 [DOI] [PubMed] [Google Scholar]
- 64. Guan Y, Puenpatom A, Johnson MG, et al. Impact of Molnupiravir Treatment on Patient-Reported Coronavirus Disease 2019 (COVID-19) Symptoms in the Phase 3 MOVe-OUT Trial: A Randomized, Placebo-Controlled Trial. Clin Infect Dis 2023;77:1521-30. 10.1093/cid/ciad409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hammond J, Fountaine RJ, Yunis C, et al. Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19. N Engl J Med 2024;390:1186-95. 10.1056/NEJMoa2309003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hayward G, Yu L-M, Little P, et al. PRINCIPLE Trial Collaborative Group . Ivermectin for COVID-19 in adults in the community (PRINCIPLE): An open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes. J Infect 2024;88:106130. 10.1016/j.jinf.2024.106130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hejazi S, Jahani Z, Elyasi S, Salarbashi D, Kabiri M. The Efficacy of Colchicine as an Adjunct Therapy in Non-hospitalized COVID-19 Patients: A Randomized Placebo-Controlled Trial. Recent Adv Antiinfect Drug Discov 2024;19:254-63. 10.2174/2772434418666230914113010 [DOI] [PubMed] [Google Scholar]
- 68. Horga A, Kuritzkes DR, Kowalczyk JJ, et al. Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19. Future Virol 2023;18:489-500. 10.2217/fvl-2023-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang DQ, Ajmera V, Tomaszewski C, et al. Ramipril for the Treatment of COVID-19: RAMIC, a Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Adv Ther 2023;40:4805-16. 10.1007/s12325-023-02618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Iwata S, Kobayashi O, Kurashima K, et al. Findings from a discontinued clinical trial of favipiravir in high-risk patients with early-onset COVID-19. J Infect Chemother 2024;30:219-27. 10.1016/j.jiac.2023.10.010 [DOI] [PubMed] [Google Scholar]
- 71. Jagannathan P, Chew KW, Giganti MJ, et al. ACTIV-2/A5401 Study Team . Safety and efficacy of inhaled interferon-β1a (SNG001) in adults with mild-to-moderate COVID-19: a randomized, controlled, phase II trial. EClinicalMedicine 2023;65:102250. 10.1016/j.eclinm.2023.102250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jittamala P, Boyd S, Schilling WH, et al. Antiviral efficacy of fluoxetine in early symptomatic COVID-19: an open-label, randomised, controlled, adaptive platform trial (PLATCOV). medRxiv 2024. 10.1101/2024.01.16.24301337 [DOI] [PMC free article] [PubMed]
- 73. Jittamala P, Schilling WHK, Watson JA, et al. PLATCOV Collaborative Group . Clinical antiviral efficacy of remdesivir in COVID-19: an open label, randomized, controlled adaptive platform trial (PLATCOV). J Infect Dis 2023;228:1318-25. 10.1093/infdis/jiad275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jun C, Danping L, Li L. Preliminary study of hydroxychloroquine sulfate in patients with common 2019 coronavirus disease (COVID-19). J Zhejiang Univ 2020;49:215-9. [Google Scholar]
- 75. Lu H, Zhang G, Mao J, et al. Efficacy and safety of GST-HG171 in adult patients with mild to moderate COVID-19: a randomised, double-blind, placebo-controlled phase 2/3 trial. EClinicalMedicine 2024;71:102582. 10.1016/j.eclinm.2024.102582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mettananda C, Peiris C, Abeyrathna D, et al. Inhaled beclomethasone in the treatment of early COVID-19: a double-blind, placebo-controlled, randomised, hospital-based trial in Sri Lanka. BMJ Open 2023;13:e075803. 10.1136/bmjopen-2023-075803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mikamo H, Takahashi S, Yamagishi Y, et al. IVERMILCO Study Group . Efficacy and safety of ivermectin in patients with mild COVID-19 in Japan and Thailand. J Infect Chemother 2024;30:536-43. 10.1016/j.jiac.2023.12.012 [DOI] [PubMed] [Google Scholar]
- 78. Okugawa S, Ikeda M, Kashiwabara K, et al. Antiviral effect and safety of nafamostat mesilate in patients with mild early-onset COVID-19: An exploratory multicentre randomized controlled clinical trial. Int J Antimicrob Agents 2023;62:106922. 10.1016/j.ijantimicag.2023.106922 [DOI] [PubMed] [Google Scholar]
- 79. Partap U, Sharma KK, Marathe Y, et al. Vitamin D and Zinc Supplementation to Improve Treatment Outcomes among COVID-19 Patients in India: Results from a Double-Blind Randomized Placebo-Controlled Trial. Curr Dev Nutr 2023;7:101971. 10.1016/j.cdnut.2023.101971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reiersen AM, Mattar C, Bender Ignacio RA, et al. The STOP COVID 2 Study: Fluvoxamine vs Placebo for Outpatients With Symptomatic COVID-19, a Fully Remote Randomized Controlled Trial. Open Forum Infect Dis 2023;10:ofad419. 10.1093/ofid/ofad419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salvadori N, Jourdain G, Krittayaphong R, et al. Molnupiravir versus favipiravir in at-risk outpatients with COVID-19: A randomized controlled trial in Thailand. Int J Infect Dis 2024;143:107021. 10.1016/j.ijid.2024.107021 [DOI] [PubMed] [Google Scholar]
- 82. Schilling WHK, Jittamala P, Watson JA, et al. PLATCOV Collaborative Group . Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial. Lancet Infect Dis 2024;24:36-45. 10.1016/S1473-3099(23)00493-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Siripongboonsitti T, Ungtrakul T, Tawinprai K, et al. Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study). Int J Infect Dis 2023;134:211-9. 10.1016/j.ijid.2023.06.018 [DOI] [PubMed] [Google Scholar]
- 84. Stewart TG, Rebolledo PA, Mourad A, et al. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators . Higher-Dose Fluvoxamine and Time to Sustained Recovery in Outpatients With COVID-19: The ACTIV-6 Randomized Clinical Trial. JAMA 2023;330:2354-63. 10.1001/jama.2023.23363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wannigama DL, Hurst C, Phattharapornjaroen P, et al. COVID-EarlyMed Trial Team . Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: a randomized clinical trial. EClinicalMedicine 2024;70:102517. 10.1016/j.eclinm.2024.102517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yang Z, Xu Y, Zheng R, et al. COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment: A Randomized Clinical Trial. JAMA Netw Open 2024;7:e241765. 10.1001/jamanetworkopen.2024.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yotsuyanagi H, Ohmagari N, Doi Y, et al. Efficacy and Safety of 5-Day Oral Ensitrelvir for Mild to Moderate COVID-19. JAMA Netw Open 2024;7. 10.1001/jamanetworkopen.2023.54991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhan Y, Lin Z, Liang J, et al. other Collaborative Institutes . Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial. EClinicalMedicine 2023;67:102359. 10.1016/j.eclinm.2023.102359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yotsuyanagi H, Ohmagari N, Doi Y, et al. Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild-to-Moderate COVID-19: The SCORPIO-SR Randomized Clinical Trial. medRxiv 2023. 10.1101/2023.07.11.23292264 [DOI] [PMC free article] [PubMed]
- 90. Annweiler C, Beaudenon M, Gautier J, et al. COVIT-TRIAL study group . High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med 2022;19:e1003999. 10.1371/journal.pmed.1003999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Doi Y, Hibino M, Hase R, et al. A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19. Antimicrob Agents Chemother 2020;64:e01897-20. 10.1128/AAC.01897-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li C, Luo F, Liu C, et al. Effect of a genetically engineered interferon-alpha versus traditional interferon-alpha in the treatment of moderate-to-severe COVID-19: a randomised clinical trial. Ann Med 2021;53:391-401. 10.1080/07853890.2021.1890329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li Q, Cui C, Xu F, et al. Evaluation of the efficacy and safety of hydroxychloroquine in comparison with chloroquine in moderate and severe patients with COVID-19. Sci China Life Sci 2021;64:660-3. 10.1007/s11427-020-1871-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Arefizadeh R, Moosavi SH, Towfiqie S, Mohsenizadeh SA, Pishgahi M. Effect of Ticagrelor Compared to Clopidogrel on Short-term Outcomes of COVID-19 Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention; a Randomized Clinical Trial. Arch Acad Emerg Med 2023;11:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sabico S, Enani MA, Sheshah E, et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients 2021;13:2170. 10.3390/nu13072170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kumar D, Kaimaparambil V, Chandralekha S, Lalchandani J. Oral Rivaroxaban in the Prophylaxis of COVID-19 Induced Coagulopathy. J Assoc Physicians India 2022;70:11-2. [PubMed] [Google Scholar]
- 97. Abdeen S, Abu-Fanne R, Bdeir K, et al. Divergent impacts of tocilizumab and colchicine in COVID-19-associated coagulopathy: the role of alpha-defensins. Br J Haematol 2022;196:923-7. 10.1111/bjh.17885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Abroug H, Maatouk A, Bennasrallah C, et al. Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial. Trials 2023;24:123. 10.1186/s13063-023-07114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Abulmeaty MMA, Aljuraiban GS, Shaikh SM, et al. The Efficacy of Antioxidant Oral Supplements on the Progression of COVID-19 in Non-Critically Ill Patients: A Randomized Controlled Trial. Antioxidants (Basel) 2021;10:804. 10.3390/antiox10050804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Adhikari P, Koirala J, Shrestha A, et al. Efficacy of Favipiravir in treatment of mild & moderate COVID-19 infection in Nepal: a multi-center, randomized, open-labelled, phase III clinical trial. Int J Infect Dis 2022;116:S45-6. 10.1016/j.ijid.2021.12.109. [DOI] [Google Scholar]
- 101. Ahmad B, Ul Hassan N, Sehar B, Zeb F, E Nayab D, Siddiqui FA. Effect of Chloroquine and Hydroxychloroquine on Cytokine Release Syndrome in Patients with COVID-19. Clin Med Res 2021;19:179-82. 10.3121/cmr.2021.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Akbari N, Ostadrahimi A, Tutunchi H, et al. Possible therapeutic effects of boron citrate and oleoylethanolamide supplementation in patients with COVID-19: A pilot randomized, double-blind, clinical trial. J Trace Elem Med Biol 2022;71:126945. 10.1016/j.jtemb.2022.126945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Amirsavadkouhi A, Shahrami R, Zadeh NM, et al. Effects of Morphine and Fentanyl on Patients with COVID-19. Tanaffos 2021;20:164-71. [PMC free article] [PubMed] [Google Scholar]
- 104. Arefin MK, Rumi SKNF, Uddin AKMN, et al. Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: an open-label randomized clinical trial. Indian J Otolaryngol Head Neck Surg 2022;74(Suppl 2):2963-7. 10.1007/s12070-021-02616-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chaccour C, Casellas A, Blanco-Di Matteo A, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine 2021;32:100720. 10.1016/j.eclinm.2020.100720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fitzgerald R, Dickinson L, Else L, et al. Pharmacokinetics of β-d-N4-hydroxycytidine, the parent nucleoside of prodrug molnupiravir, in non-plasma compartments of patients with SARS-CoV-2 infection. medRxiv 10.1101/2021.12.06.21267342. [DOI] [PMC free article] [PubMed]
- 107. Ghaderkhani S, Khaneshan AS, Salami A, et al. Efficacy and Safety of Arbidol in Treatment of Patients with COVID-19 Infection: A Randomized Clinical Trial. J Iranian Med Council 2022;5:297-307. 10.18502/jimc.v5i2.10468 [DOI] [Google Scholar]
- 108. Ghaznavi H, Mohammadghasemipour Z, Shirvaliloo M, et al. Short-term celecoxib (celebrex) adjuvant therapy: a clinical trial study on COVID-19 patients. Inflammopharmacology 2022;30:1645-57. 10.1007/s10787-022-01029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jahangirifard A, Omidi A, Sharifzadeh K, et al. The Effect of Bromelain (Anaheal) on Clinical and Para-Clinical Parameters in Hospitalized COVID-19 Patients. Acta Med Iran 2021. 10.18502/acta.v59i12.8066. [DOI] [Google Scholar]
- 110. Moreira TG, Matos KTF, De Paula GS, et al. Nasal Administration of Anti-CD3 Monoclonal Antibody (Foralumab) Reduces Lung Inflammation and Blood Inflammatory Biomarkers in Mild to Moderate COVID-19 Patients: A Pilot Study. Front Immunol 2021;12:709861. 10.3389/fimmu.2021.709861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jilg NC, Giganti M, Daar ES, et al. Camostat is not effective for mild-moderate covid-19 in a phase 2 trial of ACTIV-2. Top Antivir Med 2022;30:41. [Google Scholar]
- 112.Ogbuagu O, Tashima KT, GuÌ HF, et al. Acute Kidney Injury In Patients With Moderate COVID-19 treated With RDV Versus SoC. Conference on Retroviruses and Opportunistic Infections. 2021. https://www.natap.org/2021/CROI/croi_196.htm.
- 113. Shah Bukhari KH, Asghar A, Perveen N, et al. Efficacy of Ivermectin in COVID-19 Patients with Mild to Moderate Disease. medRxiv 10.1101/2021.02.02.21250840 [DOI]
- 114. Zeeshan Khan Chachar A, Ahmad Khan K, Asif M, et al. Effectiveness of Ivermectin in SARS-CoV-2/COVID-19 Patients. Int J Sci 2020;9:31-5. 10.18483/ijSci.2378 [DOI] [Google Scholar]
- 115. Kishoria N, Mathur S, Parmar V, et al. Ivermectin as adjuvant to hydroxycholoroquine in patients resistant to standard treatment for SARS-CoV-2: results of an open-label randomized clinical study. Paripex Indian J Res 2020;9:50-3. 10.36106/paripex/4801859. [DOI] [Google Scholar]
- 116. Rashid RA, Zgair A, Al-Ani RM. Effect of nasal corticosteroid in the treatment of anosmia due to COVID-19: A randomised double-blind placebo-controlled study. Am J Otolaryngol 2021;42:103033. 10.1016/j.amjoto.2021.103033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ried K, BinJemain T, Sali A. Therapies to Prevent Progression of COVID-19, Including Hydroxychloroquine, Azithromycin, Zinc, and Vitamin D3 With or Without Intravenous Vitamin C: An International, Multicenter, Randomized Trial. Cureus 2021;13:e19902. 10.7759/cureus.19902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sauša S, Kistkins S, Čirkova O, et al. Rapid elevation of vitamin D level reduces C-reactive protein level in COVID-19 patients with vitamin D insufficiency. 2022.
- 119. Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr 2021;15:102329. 10.1016/j.dsx.2021.102329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Vaira LA, Hopkins C, Petrocelli M, et al. Efficacy of corticosteroid therapy in the treatment of long- lasting olfactory disorders in COVID-19 patients. Rhinology 2021;59:21-5. [DOI] [PubMed] [Google Scholar]
- 121. Prabowo N, Apriningsi H. Colchicine reduces the degree of inflammation in COVID-19 patients. IOP Conf Ser Earth Environ Sci 2021;824:012087. 10.1088/1755-1315/824/1/012087. [DOI] [Google Scholar]
- 122. Biber A, Harmelin G, Lev D, et al. The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19 - a double-blind, randomized placebo-controlled trial. Int J Infect Dis 2022;122:733-40. 10.1016/j.ijid.2022.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rahman SMA, Kabir A, Abdullah ABM, et al. Safety and efficacy of favipiravir for the management of COVID-19 patients: A preliminary randomized control trial. Clin Infect Pract 2022;15:100145. 10.1016/j.clinpr.2022.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fan S, Zhen Q, Chen C, et al. Clinical efficacy of low-dose emetine for patients with COVID-19: a real-world study. J BioX Res 2021;4:53-9. 10.1097/JBR.0000000000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Abd-Elsalam S, Soliman S, Esmail ES, et al. Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine?: a Randomized, Multicenter Trial. Biol Trace Elem Res 2021;199:3642-6. 10.1007/s12011-020-02512-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126. Atipornwanich K, Kongsaengdao S, Harnsomburana P, et al. Various combinations of Favipiravir, Lopinavir-Ritonavir, Darunavir-Ritonavir, high-dose Oseltamivir, and Hydroxychloroquine for the treatment of Covid-19: A randomized controlled trial. (FIGHT-COVID-19 Study). Lancet 2021. 10.2139/ssrn.3936499 [DOI]
- 127. Bishop CW, Ashfaq A, Melnick JZ, et al. REsCue trial: Randomized controlled clinical trial with extended-release calcifediol in symptomatic COVID-19 outpatients. Nutrition 2023;107:111899. 10.1016/j.nut.2022.111899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Crippa JAS, Pacheco JC, Zuardi AW, et al. Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE Study): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cannabis Cannabinoid Res 2022;7:658-69. 10.1089/can.2021.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cruz LR, Baladrón I, Rittoles A, et al. ATENEA-Co-300 Group . Treatment with an Anti-CK2 Synthetic Peptide Improves Clinical Response in COVID-19 Patients with Pneumonia. A Randomized and Controlled Clinical Trial. ACS Pharmacol Transl Sci 2020;4:206-12. 10.1021/acsptsci.0c00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Davoodi L, Abedi SM, Salehifar E, et al. Febuxostat therapy in outpatients with suspected COVID-19: A clinical trial. Int J Clin Pract 2020;74:e13600. 10.1111/ijcp.13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fogleman C, Cohen D, Mercier A, et al. A Pilot of a Randomized Control Trial of Melatonin and Vitamin C for Mild-to-Moderate COVID-19. J Am Board Fam Med 2022;35:695-707. 10.3122/jabfm.2022.04.210529 [DOI] [PubMed] [Google Scholar]
- 132. Fu W, Liu Y, Liu L, et al. An open-label, randomized trial of the combination of IFN-κ plus TFF2 with standard care in the treatment of patients with moderate COVID-19. EClinicalMedicine 2020;27:100547. 10.1016/j.eclinm.2020.100547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Guzman-Esquivel J, Galvan-Salazar HR, Guzman-Solorzano HP, et al. Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID‑19: A randomized double‑blind placebo‑controlled trial. Int J Mol Med 2022;49:29. 10.3892/ijmm.2022.5084 [DOI] [PubMed] [Google Scholar]
- 134. Hu K, Wang M, Zhao Y, et al. A Small-Scale Medication of Leflunomide as a Treatment of COVID-19 in an Open-Label Blank-Controlled Clinical Trial. Virol Sin 2020;35:725-33. 10.1007/s12250-020-00258-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Huang YQ, Tang SQ, Xu XL, et al. No Statistically Apparent Difference in Antiviral Effectiveness Observed Among Ribavirin Plus Interferon-Alpha, Lopinavir/Ritonavir Plus Interferon-Alpha, and Ribavirin Plus Lopinavir/Ritonavir Plus Interferon-Alpha in Patients With Mild to Moderate Coronavirus Disease 2019: Results of a Randomized, Open-Labeled Prospective Study. Front Pharmacol 2020;11:1071. 10.3389/fphar.2020.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020;395:1695-704. 10.1016/S0140-6736(20)31042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Husain P, Ahmed SK, Husain B. Role of doxycycline, oral steroids, and nasal steroid in treatment of anosmia due to COVID-19 with the new insights into the doxycycline activity.: Research Square 2020. 10.21203/rs.3.rs-107710/v1. [DOI]
- 138. Ikeda M, Okugawa S, Kashiwabara K, et al. Multicenter, single-blind, randomized controlled study of the efficacy and safety of favipiravir and nafamostat mesilate in patients with COVID-19 pneumonia. Int J Infect Dis 2023;128:355-63. . 10.1016/j.ijid.2022.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kasiri H, Rouhani N, Salehifar E, Ghazaeian M, Fallah S. Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: A randomized, double blind clinical trial. Int Immunopharmacol 2021;98:107871. 10.1016/j.intimp.2021.107871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kerget F, Kerget B, Aksakal A, et al. Can 2-Aticyto Complex be an Effective Agent for Recovery of The Symptoms and Enhancing Laboratory Parameters in COVID-19 Patients? European Journal of Medical and Health Sciences 2021;3:35-9. 10.24018/ejmed.2021.3.2.745 [DOI] [Google Scholar]
- 141. Nimitvilai S, Suputtamongkol Y, Poolvivatchaikarn U, et al. A Randomized Controlled Trial of Combined Ivermectin and Zinc Sulfate versus Combined Hydroxychloroquine, Darunavir/Ritonavir, and Zinc Sulfate among Adult Patients with Asymptomatic or Mild Coronavirus-19 Infection. J Glob Infect Dis 2022;14:69-74. 10.4103/jgid.jgid_281_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. O’Halloran JA, Kedar E, Anstrom KJ, et al. ACTIV-1 IM study group members . Infliximab for Treatment of Adults Hospitalized with Moderate or Severe Covid-19. medRxiv 2022;19:2022.09.22.22280245. 10.1101/2022.09.22.22280245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Önal H, Arslan B, Üçüncü Ergun N, et al. Treatment of COVID-19 patients with quercetin: a prospective, single center, randomized, controlled trial. Turk J Biol 2021;45(SI-1):518-29. 10.3906/biy-2104-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Panda PK, Singh BO, Moirangthem B, et al. Antiviral Combination Clinically Better Than Standard Therapy in Severe but Not in Non-Severe COVID-19. Clin Pharmacol 2021;13:185-95. 10.2147/CPAA.S325083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pandit A, Bhalani N, Bhushan BLS, et al. Efficacy and safety of pegylated interferon alfa-2b in moderate COVID-19: A phase II, randomized, controlled, open-label study. Int J Infect Dis 2021;105:516-21. 10.1016/j.ijid.2021.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Purwati B, Budiono, Rachman BE, et al. A Randomized, Double-Blind, Multicenter Clinical Study Comparing the Efficacy and Safety of a Drug Combination of Lopinavir/Ritonavir-Azithromycin, Lopinavir/Ritonavir-Doxycycline, and Azithromycin-Hydroxychloroquine for Patients Diagnosed with Mild to Moderate COVID-19 Infections. Biochem Res Int 2021;2021:6685921. 10.1155/2021/6685921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ren Z, Luo H, Yu Z, et al. A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv Sci (Weinh) 2020;7:e2001435. 10.1002/advs.202001435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Shebley M, Wang S, Ali I, et al. Phase 1 study of safety, pharmacokinetics, and antiviral activity of SARS-CoV-2 neutralizing monoclonal antibody ABBV-47D11 in patients with COVID-19. Pharmacol Res Perspect 2023;11:e01036. 10.1002/prp2.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Skendelas JP, Phan D, Caputo V, et al. Novel CCR5 Antagonist for the Treatment of Mild-Moderate COVID-19 Infection after Lung Transplant. J Heart Lung Transplant 2021;40:S315. 10.1016/j.healun.2021.01.891 [DOI] [Google Scholar]
- 150. Sobngwi E, Zemsi S, Guewo-Fokeng M, et al. Doxycycline is a safe alternative to Hydroxychloroquine + Azithromycin to prevent clinical worsening and hospitalization in mild COVID-19 patients: An open label randomized clinical trial (DOXYCOV). medRxiv 10.1101/2021.07.25.21260838 [DOI] [Google Scholar]
- 151. Thomas S, Patel D, Bittel B, et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw Open 2021;4:e210369. 10.1001/jamanetworkopen.2021.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wang M, Zhao Y, Hu W, et al. Treatment of Coronavirus Disease 2019 Patients With Prolonged Postsymptomatic Viral Shedding With Leflunomide: A Single-center Randomized Controlled Clinical Trial. Clin Infect Dis 2021;73:e4012-9. 10.1093/cid/ciaa1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zheng F, Zhou Y, Zhou Z, et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: A randomized, open-label, parallel-group trial. Int J Infect Dis 2020;99:84-91. 10.1016/j.ijid.2020.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.World Health Organization. Unpublished data for RESET (NCT04380519). 2022.
- 155. Esquivel-Moynelo I, Pérez-Escribano J, Duncan-Roberts Y, et al. Effect of combination of interferon alpha-2b and interferon-gamma or interferon alpha- 2b alone for elimination of SARS-CoV-2 viral RNA. Preliminary results of a randomized controlled clinical trial. medRxiv 2020. 10.1101/2020.07.29.20164251 [DOI]
- 156. Hakamifard A, Soltani R, Maghsoudi A, et al. The effect of vitamin E and vitamin C in patients with COVID-19 pneumonia; a randomized controlled clinical trial. Immunopathologia Persa 2022;8:e80. 10.34172/ipp.2022.08. [DOI] [Google Scholar]
- 157. Abbaspour Kasgari H, Moradi S, Shabani AM, et al. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother 2020;75:3373-8. 10.1093/jac/dkaa332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Khavkina D, Chukhliaev P, Ruzhentsova T. The Role of SARS-CoV-2 Etiotropic Therapy in Controlling the Spread of Infection. Epidemiology International J 2021;5. [Google Scholar]
- 159. Miyazaki T, Hosogaya N, Fukushige Y, et al. A Multicenter Randomized Controlled Trial To Evaluate the Efficacy and Safety of Nelfinavir in Patients with Mild COVID-19. Microbiol Spectr 2023;11:e0431122. 10.1128/spectrum.04311-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Sunil Naik K, Andhalkar N, Pendse S. Effect of colchicine and aspirin given together in patients with moderate COVID-19. Contemp Clin Trials Commun 2023;32:101070. . 10.1016/j.conctc.2023.101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yang X, Wang Y, Liu Y, et al. Traditional Chinese medicine together with high-dose vitamin C improves the therapeutic effect of western medicine against COVID-19. Am J Transl Res 2022;14:501-10. [PMC free article] [PubMed] [Google Scholar]
- 162. Spivak AM, Barney BJ, Greene T, et al. A Randomized Clinical Trial Testing Hydroxychloroquine for Reduction of SARS-CoV-2 Viral Shedding and Hospitalization in Early Outpatient COVID-19 Infection. Microbiol Spectr 2023;11:e0467422. 10.1128/spectrum.04674-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Amaravadi RK, Giles L, Carberry M, et al. Hydroxychloroquine for SARS-CoV-2 positive patients quarantined at home: The first interim analysis of a remotely conducted randomized clinical trial. medRxiv 2021. 10.1101/2021.02.22.21252228. [DOI]
- 164. Ananworanich J, Mogg R, Dunne MW, et al. Randomized Study of Rivaroxaban vs Placebo on Disease Progression and Symptoms Resolution in High-Risk Adults With Mild Coronavirus Disease 2019. Clin Infect Dis 2022;75:e473-81. 10.1093/cid/ciab813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Angkasekwinai N, Rattanaumpawan P, Chayakulkeeree M, et al. Safety and Efficacy of Ivermectin for the Prevention and Treatment of COVID-19: A Double-Blinded Randomized Placebo-Controlled Study. Antibiotics (Basel) 2022;11:796. 10.3390/antibiotics11060796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Arruda EAG, Pires-Neto RJ, Medeiros MS, et al. Clinical Trial of Efficacy and Toxicity of Disoproxil Tenofovir Fumarate and Emtricitabine for Mild to Moderate SARS-CoV-2 Infections. 2021. 10.1101/2021.09.28.21264242 [DOI]
- 167. Avezum Á, Oliveira GBF, Oliveira H, et al. COPE - COALITION COVID-19 Brazil V Investigators . Hydroxychloroquine versus placebo in the treatment of non-hospitalised patients with COVID-19 (COPE - Coalition V): A double-blind, multicentre, randomised, controlled trial. Lancet Reg Health Am 2022;11:100243. 10.1016/j.lana.2022.100243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Avezum Á, Oliveira Junior HA, Neves PDMM, et al. Coalition VIII COVID-19 Brazil Investigators . Rivaroxaban to prevent major clinical outcomes in non-hospitalised patients with COVID-19: the CARE - COALITION VIII randomised clinical trial. EClinicalMedicine 2023;60:102004. 10.1016/j.eclinm.2023.102004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Barco S, Voci D, Held U, et al. OVID investigators . Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet Haematol 2022;9:e585-93. 10.1016/S2352-3026(22)00175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bosaeed M, Alharbi A, Mahmoud E, et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect 2022;28:602-8. 10.1016/j.cmi.2021.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Boulware DR, Lindsell CJ, Stewart TG, et al. ACTIV-6 Study Group and Investigators . Inhaled Fluticasone Furoate for Outpatient Treatment of Covid-19. N Engl J Med 2023;389:1085-95. 10.1056/NEJMoa2209421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bramante CT, Huling JD, Tignanelli CJ, et al. COVID-OUT Trial Team . Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Engl J Med 2022;387:599-610. 10.1056/NEJMoa2201662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Buonfrate D, Chesini F, Martini D, et al. High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial. Int J Antimicrob Agents 2022;59:106516. 10.1016/j.ijantimicag.2021.106516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Butler C, Hobbs FDR, Gbinigie O, et al. Molnupiravir Plus Usual Care Versus Usual Care Alone as Early Treatment for Adults with COVID-19 at Increased Risk of Adverse Outcomes (PANORAMIC): Preliminary Analysis from the United Kingdom Randomised, Controlled Open-Label, Platform Adaptive Trial. SSRN 2022. 10.2139/ssrn.4237902 [DOI] [PMC free article] [PubMed]
- 175. Butler CC, Dorward J, Yu L-M, et al. PRINCIPLE Trial Collaborative Group . Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 2021;397:1063-74. 10.1016/S0140-6736(21)00461-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Caraco Y, Crofoot GE, Moncada PA, et al. Phase 2/3 Trial of Molnupiravir for Treatment of Covid-19 in Nonhospitalized Adults. NEJM Evid 2022;1:a2100043. 10.1056/EVIDoa2100043 [DOI] [PubMed] [Google Scholar]
- 177. Castro-Rodriguez JA, Fish EN, Kollmann T, et al. Interferon Beta-1α ring prophylaxis to reduce household transmission of SARS-CoV-2: the Containing Coronavirus Disease-19 randomized clinical trial. medRxiv 10.1101/2022.06.13.22276369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Choudhary R, Ali O, Singh BK. Study on Hydroxychloroquinine Sulfate Being Given to the Admitted COVID -19 Positive Patients at Institute of JLNMCH, Bhagalpur, Bihar, India. Cureus 2022;14:e26388. 10.7759/cureus.26388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Clemency BM, Varughese R, Gonzalez-Rojas Y, et al. A randomized controlled trial of inhaled ciclesonide for outpatient treatment of symptomatic COVID-19 infections. medRxiv 2021. 10.1101/2021.09.07.21261811. [DOI] [PMC free article] [PubMed]
- 180. Connors JM, Brooks MM, Sciurba FC, et al. ACTIV-4B Investigators . Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. JAMA 2021;326:1703-12. 10.1001/jama.2021.17272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Cools F, Virdone S, Sawhney J, et al. ETHIC investigators . Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial. Lancet Haematol 2022;9:e594-604. 10.1016/S2352-3026(22)00173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Dorward J, Yu L-M, Hayward G, et al. PRINCIPLE Trial Collaborative Group . Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial. Br J Gen Pract 2022;72:e446-55. 10.3399/BJGP.2022.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Duvignaud A, Lhomme E, Onaisi R, et al. Coverage Study Group . Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE). Clin Microbiol Infect 2022;28:1010-6. 10.1016/j.cmi.2022.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Eikelboom JW, Jolly SS, Belley-Cote EP, et al. Colchicine and aspirin in community patients with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial. Lancet Respir Med 2022;10:1160-8. 10.1016/S2213-2600(22)00299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ezer N, Belga S, Daneman N, et al. Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ 2021;375:e068060. 10.1136/bmj-2021-068060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Feld JJ, Kandel C, Biondi MJ, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med 2021;9:498-510. 10.1016/S2213-2600(20)30566-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Fischer WA, 2nd, Eron JJ, Jr, Holman W, et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med 2022;14:eabl7430. 10.1126/scitranslmed.abl7430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Gottlieb RL, Vaca CE, Paredes R, et al. GS-US-540-9012 (PINETREE) Investigators . Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med 2022;386:305-15. 10.1056/NEJMoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hammond J, Leister-Tebbe H, Gardner A, et al. EPIC-HR Investigators . Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022;386:1397-408. 10.1056/NEJMoa2118542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Hinks TSC, Cureton L, Knight R, et al. A randomised clinical trial of azithromycin versus standard care in ambulatory COVID-19 – the ATOMIC2 trial. medRxiv 10.1136/thorax-2021-BTSabstracts.56 [DOI] [Google Scholar]
- 191. Holubar M, Subramanian A, Purington N, et al. Favipiravir for Treatment of Outpatients With Asymptomatic or Uncomplicated Coronavirus Disease 2019: A Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial. Clin Infect Dis 2022;75:1883-92. 10.1093/cid/ciac312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Issak ER, Amin MM, ER I . Timing of corticosteroids in non-severe non-hospitalized COVID-19 patients: open-label, two-center, randomized controlled study (TICS-COV19 study). Korean J Intern Med 2023;38:207-17. 10.3904/kjim.2022.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Jagannathan P, Andrews JR, Bonilla H, et al. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nat Commun 2021;12:1967. 10.1038/s41467-021-22177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. MOVe-OUT Study Group . Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 2022;386:509-20. 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Jilg N, Chew KW, Giganti MJ, et al. ACTIV-2/A5401 Study Team . One Week of Oral Camostat Versus Placebo in Nonhospitalized Adults With Mild-to-Moderate Coronavirus Disease 2019: A Randomized Controlled Phase 2 Trial. Clin Infect Dis 2023;77:941-9. 10.1093/cid/ciad342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Johnston C, Brown ER, Stewart J, et al. COVID-19 Early Treatment Study Team . Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial. EClinicalMedicine 2021;33:100773. 10.1016/j.eclinm.2021.100773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kaizer AM, Shapiro NI, Wild J, et al. Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial. Int J Infect Dis 2023;128:223-9. 10.1016/j.ijid.2022.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Khoo SH, FitzGerald R, Saunders G, et al. AGILE CST-2 Study Group . Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect Dis 2023;23:183-95. 10.1016/S1473-3099(22)00644-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA 2020;324:2292-300. 10.1001/jama.2020.22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. López-Medina E, López P, Hurtado IC, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA 2021;325:1426-35. 10.1001/jama.2021.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lowe DM, Brown LK, Chowdhury K, et al. FLARE Investigators . Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. PLoS Med 2022;19:e1004120. 10.1371/journal.pmed.1004120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. McCarthy MW, Naggie S, Boulware DR, et al. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators . Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2023;329:296-305. 10.1001/jama.2022.24100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. McMahon JH, Lau JSY, Coldham A, et al. Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial. EClinicalMedicine 2022;54:101703. 10.1016/j.eclinm.2022.101703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mirahmadizadeh A, Semati A, Heiran A, et al. Efficacy of single-dose and double-dose ivermectin early treatment in preventing progression to hospitalization in mild COVID-19: A multi-arm, parallel-group randomized, double-blind, placebo-controlled trial. Respirology 2022;27:758-66. 10.1111/resp.14318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Naggie S, Boulware DR, Lindsell CJ, et al. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators . Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2022;328:1595-603. 10.1001/jama.2022.18590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Naggie S, Boulware DR, Lindsell CJ, et al. Accelerating Covid-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators . Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial. JAMA 2023;329:888-97. 10.1001/jama.2023.1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Oldenburg CE, Pinsky BA, Brogdon J, et al. Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA 2021;326:490-8. 10.1001/jama.2021.11517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Omrani AS, Pathan SA, Thomas SA, et al. Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19. EClinicalMedicine 2020;29:100645. 10.1016/j.eclinm.2020.100645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Parienti J-J, Prazuck T, Peyro-Saint-Paul L, et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. EClinicalMedicine 2021;38:100993. 10.1016/j.eclinm.2021.100993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Piazza G, Spyropoulos AC, Hsia J, et al. PREVENT-HD Investigators . Rivaroxaban for Prevention of Thrombotic Events, Hospitalization, and Death in Outpatients With COVID-19: A Randomized Clinical Trial. Circulation 2023;147:1891-901. 10.1161/CIRCULATIONAHA.123.063901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Plasse TF, Delgado B, Potts J, et al. A randomized, placebo-controlled pilot study of upamostat, a host-directed serine protease inhibitor, for outpatient treatment of COVID-19. Int J Infect Dis 2023;128:148-56. 10.1016/j.ijid.2022.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Pourdowlat G, Saghafi F, Mozafari A, et al. Efficacy and safety of colchicine treatment in patients with COVID-19: A prospective, multicenter, randomized clinical trial. Phytother Res 2022;36:891-8. 10.1002/ptr.7319 [DOI] [PubMed] [Google Scholar]
- 213. Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. TOGETHER Investigators . Oral Fluvoxamine With Inhaled Budesonide for Treatment of Early-Onset COVID-19 : A Randomized Platform Trial. Ann Intern Med 2023;176:667-75. 10.7326/M22-3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. TOGETHER investigators . Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022;10:e42-51. 10.1016/S2214-109X(21)00448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Reis G, Moreira Silva EADS, Medeiros Silva DC, et al. TOGETHER Investigators . Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER Randomized Clinical Trial. JAMA Netw Open 2021;4:e216468. 10.1001/jamanetworkopen.2021.6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Reis G, Moreira Silva EAS, Medeiros Silva DC, et al. TOGETHER Investigators . Early Treatment with Pegylated Interferon Lambda for Covid-19. N Engl J Med 2023;388:518-28. 10.1056/NEJMoa2209760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Reis G, Silva EASM, Silva DCM, et al. TOGETHER Investigators . Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med 2022;386:1721-31. 10.1056/NEJMoa2115869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Rezai MS, Ahangarkani F, Hill A, et al. Non-effectiveness of Ivermectin on Inpatients and Outpatients With COVID-19; Results of Two Randomized, Double-Blinded, Placebo-Controlled Clinical Trials. Front Med (Lausanne) 2022;9:919708. 10.3389/fmed.2022.919708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Rodrigues C, Freitas-Santos RS, Levi JE, et al. Hydroxychloroquine plus azithromycin early treatment of mild COVID-19 in an outpatient setting: a randomized, double-blinded, placebo-controlled clinical trial evaluating viral clearance. Int J Antimicrob Agents 2021;58:106428. 10.1016/j.ijantimicag.2021.106428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Roy-García IA, Moreno-Noguez M, Rivas-Ruiz R, et al. “Efficacy and Safety of Fixed Combination of Hydroxychloroquine with Azithromycin Versus Hydroxychloroquine and Placebo in Patients with Mild COVID-19: Randomized, double blind, Placebo controlled trial”. 2022. 10.1101/2022.04.06.22273531 [DOI]
- 221. Ruzhentsova TA, Chukhlyaev PV, Khavkina DA, et al. Efficacy and safety of favipiravir in a complex therapy of mild to moderate COVID-19. Infectious diseases: News, Opinions . Training 2020;9:26-38. [Google Scholar]
- 222. Schilling WHK, Jittamala P, Watson JA, et al. PLATCOV Collaborative Group . Pharmacometrics of high-dose ivermectin in early COVID-19 from an open label, randomized, controlled adaptive platform trial (PLATCOV). Elife 2023;12:e83201. 10.7554/eLife.83201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Schwartz I, Boesen ME, Cerchiaro G, et al. ALBERTA HOPE COVID-19 Collaborators . Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID-19: a randomized controlled trial. CMAJ Open 2021;9:E693-702. 10.9778/cmajo.20210069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Sinha S, N K, Suram VK, et al. Efficacy and Safety of Molnupiravir in Mild COVID-19 Patients in India. Cureus 2022;14:e31508. 10.7759/cureus.31508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial. Ann Intern Med 2020;173:623-31. 10.7326/M20-4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Tardif J-C, Bouabdallaoui N, L’Allier PL, et al. Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19. medRxiv 2021. 10.1101/2021.01.26.21250494. [DOI]
- 227. Tehrani S, Yadegarynia D, Bagherzade A, et al. Efficacy of Favipiravir in the Treatment of Moderate COVID-19 Patients: A Randomized, Open-label, Controlled Clinical Trial. Mediterranean Journal of Infection Microbes and Antimicrobials 2022;11. 10.4274/mjima.galenos.2022.2022.30. [DOI] [Google Scholar]
- 228. Tobback E, Degroote S, Buysse S, et al. Efficacy and safety of camostat mesylate in early COVID-19 disease in an ambulatory setting: a randomized placebo-controlled phase II trial. Int J Infect Dis 2022;122:628-35. 10.1016/j.ijid.2022.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Vaezi A, Salmasi M, Soltaninejad F, Salahi M, Javanmard SH, Amra B. Favipiravir in the Treatment of Outpatient COVID-19: A Multicenter, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Adv Respir Med 2023;91:18-25. 10.3390/arm91010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Vallejos J, Zoni R, Bangher M, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis 2021;21:635. 10.1186/s12879-021-06348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.World Health Organization. Unpublished data on Krishnan and Kumar trials on molnupiravir. 2021.
- 232.World Health Organization. Unpublished data on Vyas molnupiravir trial (CTRI/2021/08/035424), 2021.
- 233.World Health Organization. Unpublished data on STOP-COVID-2 trial (NCT04668950), 2021.
- 234.World Health Organization. Unpublished data for EPIC-SR (NCT05011513). 2021.
- 235. Abaleke E, Abbas M, Abbasi S, et al. RECOVERY Collaborative Group . Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:605-12. 10.1016/S0140-6736(21)00149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Abd-Elsalam S, Noor RA, Badawi R, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study. J Med Virol 2021;93:5833-8. 10.1002/jmv.27122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Abdulamir AS, Gorial FI, Saadi SJ, et al. A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management. Ann Med Surg (Lond) 2021;69:102779. 10.1016/j.amsu.2021.102779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. AlQahtani M, Kumar N, Aljawder D, et al. Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease. Sci Rep 2022;12:4925. 10.1038/s41598-022-08794-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun 2021;12:2349. 10.1038/s41467-021-22446-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Babalola OE, Bode CO, Ajayi AA, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: A randomised controlled double blind dose response study in Lagos. medRxiv 10.1101/2021.01.05.21249131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Balykova LA, Pavelkina VF, Shmyreva NV, et al. Efficacy and safety of some etiotropic therapeutic schemes for treating patients with novel coronavirus infection (covid-19). Pharmacy & Pharmacology 2020;8:150-9. 10.19163/2307-9266-2020-8-3-150-159. [DOI] [Google Scholar]
- 242. Beigel JH, Tomashek KM, Dodd LE, et al. ACTT-1 Study Group Members . Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813-26. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Bhushan B L S, Wanve S, Koradia P, et al. Study Investigators Group . Efficacy and safety of pegylated interferon-α2b in moderate COVID-19: a phase 3, randomized, comparator-controlled, open-label study. Int J Infect Dis 2021;111:281-7. 10.1016/j.ijid.2021.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Butler CC, Yu L-M, Dorward J, et al. PRINCIPLE Trial Collaborative Group . Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir Med 2021;9:1010-20. 10.1016/S2213-2600(21)00310-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Cairns DM, Dulko D, Griffiths JK, et al. Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19: A Phase 2 Randomized Clinical Trial. JAMA Netw Open 2022;5:e2144942. 10.1001/jamanetworkopen.2021.44942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Cao Z, Gao W, Bao H, et al. VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19. N Engl J Med 2023;388:406-17. 10.1056/NEJMoa2208822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Coalition Covid-19 Brazil I Investigators . Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med 2020;383:2041-52. 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Chahla RE, Medina Ruiz L, Ortega ES, et al. A randomized trial - intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for covid-19 in healthcare agents. medRxiv 2021. 10.1101/2021.03.26.21254398 [DOI] [PMC free article] [PubMed]
- 249. Chen C-P, Lin Y-C, Chen T-C, et al. Taiwan HCQ Study Group . A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19). PLoS One 2020;15:e0242763. 10.1371/journal.pone.0242763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Chen J, Liu D, Liu L, et al. [A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020;49:215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Chen L, Zhang Z-Y, Fu J-G, et al. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study. medRxiv 10.1101/2020.06.19.20136093 [DOI]
- 252. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv 10.1101/2020.03.22.20040758 [DOI]
- 253. Chuah CH, Chow TS, Hor CP, et al. Malaysian Favipiravir Study Group . Efficacy of Early Treatment With Favipiravir on Disease Progression Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19): A Randomized, Open-Label Clinical Trial. Clin Infect Dis 2022;75:e432-9. 10.1093/cid/ciab962 [DOI] [PubMed] [Google Scholar]
- 254. Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med 2021;9:275-84 10.1016/S2213-2600(20)30558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. De Niet S, Trémège M, Coffiner M, et al. Positive Effects of Vitamin D Supplementation in Patients Hospitalized for COVID-19: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2022;14:3048. 10.3390/nu14153048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Dubée V, Roy P-M, Vielle B, et al. HYCOVID study group . Hydroxychloroquine in mild-to-moderate coronavirus disease 2019: a placebo-controlled double blind trial. Clin Microbiol Infect 2021;27:1124-30. 10.1016/j.cmi.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Ferrarini A, Vacca A, Solimando AG, et al. Early administration of tofacitinib in COVID-19 pneumonitis: An open randomised controlled trial. Eur J Clin Invest 2023;53:e13898. 10.1111/eci.13898 [DOI] [PubMed] [Google Scholar]
- 258. George B, Moorthy M, Kulkarni U, et al. Single Dose of Ivermectin is not Useful in Patients with Hematological Disorders and COVID-19 Illness: A Phase II B Open Labelled Randomized Controlled Trial. Indian J Hematol Blood Transfus 2022;38:615-22. 10.1007/s12288-022-01546-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Criner GJ, Huhn G, Subramanian A, et al. Safety of Remdesivir vs Standard Care in Patients with Moderate Covid-19. Open Forum Infect Dis 2020;7:S345-6 10.1093/ofid/ofaa439.755. [DOI] [Google Scholar]
- 260. Ghati N, Bhatnagar S, Mahendran M, et al. Statin and aspirin as adjuvant therapy in hospitalised patients with SARS-CoV-2 infection: a randomised clinical trial (RESIST trial). BMC Infect Dis 2022;22:606. 10.1186/s12879-022-07570-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Golan Y, Campos JAS, Woolson R, et al. Favipiravir in Patients With Early Mild-to-moderate Coronavirus Disease 2019 (COVID-19): A Randomized Controlled Trial. Clin Infect Dis 2023;76:e10-7. 10.1093/cid/ciac712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Gorial F, Maulood M, Abdulamir A, et al. Randomized controlled trial of colchicine add on to the standard therapy in moderate and severe corona virus Disease-19 infection. Ann Med Surg (Lond) 2012;2022:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Guimarães PO, Quirk D, Furtado RH, et al. STOP-COVID Trial Investigators . Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med 2021;385:406-15. 10.1056/NEJMoa2101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Kasiri H, Ghazaiean M, Rouhani N, Naderi-Behdani F, Ghazaeian M, Ghodssi-Ghassemabadi R. The effects of colchicine on hospitalized COVID-19 patients: A randomized, double-blind, placebo-controlled clinical trial. J Investig Med 2023;71:124-31. 10.1177/10815589221141815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Murugesan H, Cs G, Nasreen HS, et al. An Evaluation of Efficacy and Safety of Tofacitinib, A JAK Inhibitor in the Management of Hospitalized Patients with Mild to Moderate COVID-19 - An Open-Label Randomized Controlled Study. J Assoc Physicians India 2022;69:11-2. [PubMed] [Google Scholar]
- 266. Han MK, Antila M, Ficker JH, et al. Ruxolitinib in addition to standard of care for the treatment of patients admitted to hospital with COVID-19 (RUXCOVID): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Rheumatol 2022;4:e351-61. 10.1016/S2665-9913(22)00044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Horby PW, Emberson JR, Mafham M, et al. RECOVERY Collaborative Group . Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. medRxiv 10.1101/2022.03.02.22271623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Horby PW, Mafham M, Bell JL, et al. RECOVERY Collaborative Group . Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2020;396:1345-52. 10.1016/S0140-6736(20)32013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clin Infect Dis 2021;73:531-4. 10.1093/cid/ciaa1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Mariani J, Antonietti L, Tajer C, et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial. PLoS One 2022;17:e0267918. 10.1371/journal.pone.0267918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Jalal A, Aref S, Albustany D. Effectiveness of colchicine among patients with COVID-19 infection: A randomized, open-labeled, clinical trial. Indian J Rheumatol 2022;17. 10.4103/injr.injr_264_21. [DOI] [Google Scholar]
- 272. Jardine MJ, Kotwal SS, Bassi A, et al. CLARITY trial investigators . Angiotensin receptor blockers for the treatment of covid-19: pragmatic, adaptive, multicentre, phase 3, randomised controlled trial. BMJ 2022;379:e072175. 10.1136/bmj-2022-072175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Ali K, Azher T, Baqi M, et al. Canadian Treatments for COVID-19 (CATCO) Association of Medical Microbiology and Infectious Disease Canada (AMMI) Clinical Research Network and the Canadian Critical Care Trials Group . Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ 2022;194:E242-51. 10.1503/cmaj.211698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Kalil AC, Mehta AK, Patterson TF, et al. ACTT-3 study group members . Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med 2021;9:1365-76. 10.1016/S2213-2600(21)00384-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Kalil AC, Patterson TF, Mehta AK, et al. ACTT-2 Study Group Members . Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med 2021;384:795-807. 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Khoo SH, Fitzgerald R, Fletcher T, et al. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study. J Antimicrob Chemother 2021;76:3286-95. 10.1093/jac/dkab318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Kinoshita T, Shinoda M, Nishizaki Y, et al. Phase 3, multicentre, double-blind, randomised, parallel-group, placebo-controlled study of camostat mesilate (FOY-305) for the treatment of COVID-19 (CANDLE study). medRxiv 10.1101/2022.03.27.22271988 [DOI] [Google Scholar]
- 278. Krolewiecki A, Lifschitz A, Moragas M, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial. EClinicalMedicine 2021;37:100959. 10.1016/j.eclinm.2021.100959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Lakkireddy M, Gadiga SG, Malathi RD, et al. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Sci Rep 2021;11:10641. 10.1038/s41598-021-90189-4 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 280. Les I, Loureiro-Amigo J, Capdevila F, et al. Methylprednisolone Pulses in Hospitalized COVID-19 Patients Without Respiratory Failure: A Randomized Controlled Trial. Front Med (Lausanne) 2022;9:807981. 10.3389/fmed.2022.807981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Li Y, Xie Z, Lin W, et al. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med 2020;1:105-113.e4. 10.1016/j.medj.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Lim SCL, Hor CP, Tay KH, et al. I-TECH Study Group . Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial. JAMA Intern Med 2022;182:426-35. 10.1001/jamainternmed.2022.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Liu J, Pan X, Zhang S, et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study. Lancet Reg Health West Pac 2023;33:100694. 10.1016/j.lanwpc.2023.100694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. BRACE CORONA Investigators . Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA 2021;325:254-64. 10.1001/jama.2020.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Lou Y, Liu L, Yao H, et al. Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial. Eur J Pharm Sci 2021;157:105631. 10.1016/j.ejps.2020.105631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Madioko BM, Rissassi J-RM, Mihigo CB, et al. Incidence of QTc interval prolongation in patients treated for covid-19 with Doubase C or Hydroxychloroquine-azithromycin at University Hospital of Kinshasa. Research Square 2022. 10.21203/rs.3.rs-2168785/v1. [DOI]
- 287. Mahmud R, Rahman MM, Alam I, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res 2021;49:3000605211013550. 10.1177/03000605211013550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Marconi VC, Ramanan AV, De Bono S, et al. Efficacy and safety of baricitinib in patients with COVID-19 infection: Results from the randomised, double-blind, placebo-controlled, parallel-group COV-BARRIER phase 3 trial. medRxiv 10.1101/2021.04.30.21255934 [DOI] [Google Scholar]
- 289. Marcos-Jubilar M, Carmona-Torre F, Vidal R, et al. BEMICOP Investigators . Therapeutic versus Prophylactic Bemiparin in Hospitalized Patients with Nonsevere COVID-19 Pneumonia (BEMICOP Study): An Open-Label, Multicenter, Randomized, Controlled Trial. Thromb Haemost 2022;122:295-9. 10.1055/a-1667-7534 [DOI] [PubMed] [Google Scholar]
- 290. McQuilten ZK, Venkatesh B, Jha V, et al. Anticoagulation Strategies in Non-Critically Ill Patients with Covid-19. NEJM Evid 2023;2:a2200293. 10.1056/EVIDoa2200293 [DOI] [PubMed] [Google Scholar]
- 291. Kim MH, Elbaz J, Jilg N, et al. Peginterferon lambda for the treatment of hospitalized patients with mild COVID-19: A pilot phase 2 randomized placebo-controlled trial. Front Med (Lausanne) 2023;10:1095828. 10.3389/fmed.2023.1095828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Mohamed AS, Ahmad HM, Abdul-Raheem ASA, Kamel FMM, Khames A, Mady AF. Thromboprophylaxis and clinical outcomes in moderate COVID-19 patients: A comparative study. Res Social Adm Pharm 2022;18:4048-55. 10.1016/j.sapharm.2022.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Mohan A, Tiwari P, Suri TM, et al. Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial. J Infect Chemother 2021;27:1743-9. 10.1016/j.jiac.2021.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Monk PD, Marsden RJ, Tear VJ, et al. Inhaled Interferon Beta COVID-19 Study Group . Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2021;9:196-206. 10.1016/S2213-2600(20)30511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Mukae H, Yotsuyanagi H, Ohmagari N, et al. A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-Like Protease Inhibitor, in Japanese Patients with Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part. Antimicrob Agents Chemother 2022;66:e0069722. 10.1128/aac.00697-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Mukae H, Yotsuyanagi H, Ohmagari N, et al. Efficacy and Safety of Ensitrelvir in Patients With Mild-to-Moderate Coronavirus Disease 2019: The Phase 2b Part of a Randomized, Placebo-Controlled, Phase 2/3 Study. Clin Infect Dis 2023;76:1403-11. 10.1093/cid/ciac933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Pascual-Figal DA, Roura-Piloto AE, Moral-Escudero E, et al. COL-COVID Investigators . Colchicine in Recently Hospitalized Patients with COVID-19: A Randomized Controlled Trial (COL-COVID). Int J Gen Med 2021;14:5517-26. 10.2147/IJGM.S329810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Puskarich MA, Cummins NW, Ingraham NE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine 2021;37:100957. 10.1016/j.eclinm.2021.100957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Rahman M, Datta PK, Islam K, et al. Efficacy of colchicine in patients with moderate COVID-19: A double-blinded, randomized, placebo-controlled trial. PLoS One 2022;17:e0277790. 10.1371/journal.pone.0277790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Ramachandran R, Bhosale V, Reddy H, et al. Phase III, Randomized, Double-blind, Placebo controlled trial of Efficacy, Safety and Tolerability of Antiviral drug Umifenovir vs Standard care of therapy in non-severe COVID-19 patients. Int J Infect Dis 2022;115:62-9. 10.1016/j.ijid.2021.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Rashad A, Nafady A, Hassan MH, et al. Therapeutic efficacy of macrolides in management of patients with mild COVID-19. Sci Rep 2021;11:16361. 10.1038/s41598-021-95900-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Ravikirti RR, Roy R, Pattadar C, et al. Evaluation of Ivermectin as a Potential Treatment for Mild to Moderate COVID-19: A Double-Blind Randomized Placebo Controlled Trial in Eastern India. J Pharm Pharm Sci 2021;24:343-50. 10.18433/jpps32105 [DOI] [PubMed] [Google Scholar]
- 303. Bradbury CA, Lawler PR, Stanworth SJ, et al. REMAP-CAP Writing Committee for the REMAP-CAP Investigators . Effect of Antiplatelet Therapy on Survival and Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA 2022;327:1247-59. 10.1001/jama.2022.2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Rocha CDL, Cid-Lopez MA, Venegas-Lopez BI, et al. Ivermectin compared with placebo in the clinical evolution of Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial: Research Square Platform, 2022. 10.21203/rs.3.rs-1640339/v1. [DOI] [PMC free article] [PubMed]
- 305. Sarojvisut P, Apisarnthanarak A, Jantarathaneewat K, et al. An Open Label Randomized Controlled Trial of Ivermectin Plus Favipiravir-Based Standard of Care versus Favipiravir-Based Standard of Care for Treatment of Moderate COVID-19 in Thailand. Infect Chemother 2023;55:50-8. 10.3947/ic.2022.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Sharma A, Elharram M, Afilalo J, et al. A randomized controlled trial of renin-angiotensin-aldosterone system inhibitor management in patients admitted in hospital with COVID-19. Am Heart J 2022;247:76-89. 10.1016/j.ahj.2022.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Shinkai M, Tsushima K, Tanaka S, et al. Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial. Infect Dis Ther 2021;10:2489-509. 10.1007/s40121-021-00517-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Sholzberg M, Tang GH, Rahhal H, et al. RAPID trial investigators . Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021;375:n2400. 10.1136/bmj.n2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Sirijatuphat R, Manosuthi W, Niyomnaitham S, et al. Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study. Emerg Microbes Infect 2022;11:2197-206. 10.1080/22221751.2022.2117092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Song J-Y, Yoon J-G, Seo Y-B, et al. Ciclesonide Inhaler Treatment for Mild-to-Moderate COVID-19: A Randomized, Open-Label, Phase 2 Trial. J Clin Med 2021;10:3545. 10.3390/jcm10163545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Spinner CD, Gottlieb RL, Criner GJ, et al. GS-US-540-5774 Investigators . Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020;324:1048-57. 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020;369:m1849. 10.1136/bmj.m1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Terada-Hirashima J, Suzuki M, Tsujimoto Y, et al. Impact of inhaled ciclesonide on asymptomatic or mild COVID-19: A randomized trial. Drug Discov Ther 2022;16:225-32. 10.5582/ddt.2022.01068 [DOI] [PubMed] [Google Scholar]
- 314. Horby P, Mafham M, Linsell L, et al. RECOVERY Collaborative Group . Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020;383:2030-40. 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Horby P, Lim WS, Emberson JR, et al. RECOVERY Collaborative Group . Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693-704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Shankar-Hari M, Vale CL, Godolphin PJ, et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group . Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021;326:499-518. 10.1001/jama.2021.11330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Udwadia ZF, Singh P, Barkate H, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis 2021;103:62-71. 10.1016/j.ijid.2020.11.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Vityala Y, Tugolbai T, Melis Sholpanbai U, et al. Efficacy of umifenovir in the treatment of mild and moderate COVID-19 patients. International Journal of Research in Pharmaceutical Sciences 2020;11:506-9. 10.26452/ijrps.v11iSPL1.2839. [DOI] [Google Scholar]
- 319. Wada T, Hibino M, Aono H, et al. CORVETTE-01 Study Group . Efficacy and safety of single-dose ivermectin in mild-to-moderate COVID-19: the double-blind, randomized, placebo-controlled CORVETTE-01 trial. Front Med (Lausanne) 2023;10:1139046. 10.3389/fmed.2023.1139046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. WHO Solidarity Trial Consortium . Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 2022;399:1941-53. 10.1016/S0140-6736(22)00519-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Lawler PR, Derde LPG, van de Veerdonk FL, et al. Writing Committee for the REMAP-CAP Investigators . Effect of Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Initiation on Organ Support-Free Days in Patients Hospitalized With COVID-19: A Randomized Clinical Trial. JAMA 2023;329:1183-96. 10.1001/jama.2023.4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Yadegarinia D, Tehrani S, Abolghasemi S, et al. Evaluation of the Efficacy of Arbidol in Comparison with the Standard Treatment Regimen of Hospitalized Patients with Covid-19: A Randomized Clinical Trial. Arch Clin Infect Dis 2020;15. 10.5812/archcid.106622. [DOI] [Google Scholar]
- 323. Zhao H, Zhang C, Zhu Q, et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: A multicenter, open-label, randomized trial. Int Immunopharmacol 2021;97:107702. 10.1016/j.intimp.2021.107702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis 2021;103:214-6. 10.1016/j.ijid.2020.11.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Pott-Junior H, Paoliello MMB, Miguel AQC, et al. Use of ivermectin in the treatment of Covid-19: A pilot trial. Toxicol Rep 2021;8:505-10. 10.1016/j.toxrep.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 326. Yu L-M, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial. medRxiv
- 327. Balykova LA, Selezneva NM, Gorshenina EI, et al. Modern directed antiviral covid-19 therapy: results of multicenter clinical effectiveness and safety study of fixed nirmatrelvir+ritonavir combination. Pharmacy & Pharmacology 2022;10:371-86. 10.19163/2307-9266-2022-10-4-371-386. [DOI] [Google Scholar]
- 328. Byakika-Kibwika P, Sekaggya-Wiltshire C, Semakula JR, et al. Safety and Efficacy of Hydroxychloroquine for Treatment of Non-Severe COVID-19 in Adults in Uganda: A Randomized Open Label Phase II Clinical Trial. Research Square 2021. 10.21203/rs.3.rs-506195/v1. [DOI] [PMC free article] [PubMed]
- 329. da Silva Santos PS, da Fonseca Orcina B, Machado RRG, et al. Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomised trial. Sci Rep 2021;11:19937. 10.1038/s41598-021-99013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Rastogi A, Bhansali A, Khare N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J 2022;98:87-90. 10.1136/postgradmedj-2020-139065 [DOI] [PubMed] [Google Scholar]
- 331. Seo H, Kim H, Bae S, et al. Fluvoxamine Treatment of Patients with Symptomatic COVID-19 in a Community Treatment Center: A Preliminary Result of Randomized Controlled Trial. Infect Chemother 2022;54:102-13. 10.3947/ic.2021.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Zou R, Peng L, Shu D, et al. Antiviral Efficacy and Safety of Molnupiravir Against Omicron Variant Infection: A Randomized Controlled Clinical Trial. Front Pharmacol 2022;13:939573. 10.3389/fphar.2022.939573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Chen C, Zhang Y, Huang J, et al. Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial. Front Pharmacol 2021;12:683296. 10.3389/fphar.2021.683296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Karolyi M, Pawelka E, Omid S, et al. Camostat Mesylate Versus Lopinavir/Ritonavir in Hospitalized Patients With COVID-19-Results From a Randomized, Controlled, Open Label, Platform Trial (ACOVACT). Front Pharmacol 2022;13:870493. 10.3389/fphar.2022.870493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Kim MH, Elbaz J, Jilg N, et al. Peginterferon lambda for the treatment of hospitalized patients with mild COVID-19: A pilot phase 2 randomized placebo-controlled trial. Front Med (Lausanne) 2023;10:1095828. 10.3389/fmed.2023.1095828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Ramakrishnan S, Nicolau DV, Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med 2021;9:763-72. 10.1016/S2213-2600(21)00160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Zhao J, Zhang J, Jin Y, et al. A trial of arbidol hydrochloride in adults with COVID-19. Chin Med J (Engl) 2022;135:1531-8. 10.1097/CM9.0000000000002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Zhuravel SV, Khmelnitskiy OK, Burlaka OO, et al. Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial. EClinicalMedicine 2021;41:101169. 10.1016/j.eclinm.2021.101169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Masaki I, Mutsumi I, Maki K, et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N Engl J Med 2022;388:89-91. 10.1056/NEJMc2214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Zhou S, Hill CS, Sarkar S, et al. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J Infect Dis 2021;224:415-9. 10.1093/infdis/jiab247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Brignardello-Petersen R, Murad MH, Walter SD, et al. GRADE Working Group . GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol 2019;105:60-7. 10.1016/j.jclinepi.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 342. Zeraatkar D, Pitre T, Leung G, et al. Consistency of covid-19 trial preprints with published reports and impact for decision making: retrospective review. BMJ Med 2022;1:e000309. 10.1136/bmjmed-2022-000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Boutron I, Chaimani A, Devane D, et al. Interventions for the prevention and treatment of COVID-19: a living mapping of research and a living systematic review. Cochrane Database Syst Rev 2020. 10.1002/14651858.CD013769 [DOI] [Google Scholar]
- 344. Juul S, Nielsen N, Bentzer P, et al. Interventions for treatment of COVID-19: a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING Project). Syst Rev 2020;9:108. 10.1186/s13643-020-01371-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Pitre T, Van Alstine R, Chick G, et al. Antiviral drug treatment for nonsevere COVID-19: a systematic review and network meta-analysis. CMAJ 2022;194:E969-80. 10.1503/cmaj.220471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Cheng Q, Chen J, Jia Q, Fang Z, Zhao G. Efficacy and safety of current medications for treating severe and non-severe COVID-19 patients: an updated network meta-analysis of randomized placebo-controlled trials. Aging (Albany NY) 2021;13:21866-902. 10.18632/aging.203522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Wu X, Li W, Qin Z, et al. Traditional Chinese medicine as an adjunctive therapy for mild and common COVID-19: A systematic review and network meta-analysis. Medicine (Baltimore) 2021;100:e27372. 10.1097/MD.0000000000027372 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
No additional data available.