Abstract
The Ki-67 index, which is a proliferative index, has become more important in making treatment decisions for patients with breast cancer (BC) and plays both a predictive role and a prognostic role. However, a few factors limit its use in clinical practice, particularly the assessment of the percentage of Ki-67-positive cells and the cutoff value of Ki-67. In this study, we examined the expression of Ki-67 via immunohistochemistry and systematically evaluated the value of the Ki-67 index in patients with BC. This was a retrospective study including 280 patients diagnosed with BC. There were marked differences in overall survival (OS) between patients with BC when the Ki-67 index ranged from 46 to 68% (χ2 = 5.87, P = 0.0154; χ2 = 7.64, P = 0.0057, respectively), and the same results were also found when the staining density was added to the Ki-67 index; however, the staining density alone has limited value in assessing the value of Ki-67. There were marked differences in disease-free survival (DFS) among BC patients when the Ki-67 index ranged from 50 to 58% (χ2 = 7.31, P = 0.0069; χ2 = 7.88, P = 0.005). When 14% was used as a cutoff point to classify the molecular type of BC, the luminal A-type patients were significantly different from patients with HER2-overexpressing subtype BC in terms of OS (χ2 = 5.33, P = 0.021). There was a significant difference in the OS of patients with human epidermal growth factor receptor 2 (HER-2)-overexpressing subtype BC when the Ki-67 index fell within the range of 49-60% (χ2 = 4.86, P = 0.0275; χ2 = 5.50, P = 0.019, respectively). There were significant differences between luminal A-type BC and HER2-overexpressing subtype BC in terms of OS (χ2 = 5.53, P = 0.019), according to suggestions of the 2019 CSCO consensus. There were significant differences between the two groups of luminal B HER-2(-) BC when the Ki-67 index was 52% (χ2 = 6.61, P = 0.0101). The differentiated Ki-67 index can be used to assess the OS and DFS of patients with BC, and the staining density of Ki-67 has little value in assessing prognosis in these patients. Different molecular classification methods may influence the assessment of prognosis and the results of molecular subtype in patients with BC. To predict the prognosis of BC patients, it is more scientifically feasible to use the interval values of Ki-67 than a specific value.
Keywords: Ki-67, Breast Cancer, Survive, Molecular Type, Prognosis
Introduction
According to the latest national statistical data on cancer from the China National Cancer Center in 2019, the incidence rate of BC in China was 45.29/100,000. BC rank second among all cancers in terms of incidence rate and rank first in terms of the incidence rate of cancers in women. BC constitute the fifth leading cause of cancer-related death among all cancers in women, accounting for approximately 70,000 cases/year [1, 2].
Gerdes and colleagues first identified the Ki-67 antigen in Hodgkin’s lymphoma in the early 1980s, its name was based on the initials of the laboratory name, Ki for Kiel University, and the 67 label referring to the clone number on the 96-well plate [3]. Subsequently, it was found that the Ki-67 antigen was expressed in a variety of tumors and was helpful in judging the proliferative activity of tumor cells [4, 5]. Ki-67 is also a key biomarker for the prognosis and molecular classification of patients with breast cancers (BC) [6, 7]. In 2011, a consensus was reached at the St Gallen Conference, which required the use of 14% as the critical value of Ki-67 to distinguish luminal A-type BC from luminal B-type BC [8, 9]. However, the cutoff value of Ki-67 was revised to 20% at the 2013 St Gallen Conference because the “significant mean value” was extracted from an “in-house” dataset [10]. Moreover, the cutoff value recommended by the international Ki-67-in-breast-cancer working group was unable to achieve universal effects [11]. Even 28.35% was used as a cutoff value by Shet for distinguishing different molecular subtypes of BC [12]. Some guidelines also provided ambiguous expressions of the critical value of Ki-67, such as “high expression’’ and “low expression” [13–15]. Consequently, a few experts have suggested using ≥ 20% as the critical value of a “high expression” state of Ki-67 [16, 17]. The 2019 CSCO Guidelines suggested that the critical value of Ki-67 should be determined according to the practical situations of various laboratories. Most Chinese experts agreed that < 15% indicated low expression and > 30% indicated high expression. When the Ki-67 index is 15%~30%, it is suggested that patients make clinical decisions according to secondary pathology consultations or other indices [18]. Therefore, the importance of Ki-67 in prognosis and different subtypes of BC has been highlighted by pathologists and clinicians.
In practical pathological diagnosis, the cutoff point of Ki-67 is difficult to determine and has poor reproducibility [19–22]. Different laboratories, technicians with different degrees of familiarity and pathologists with or without specialist training might obtain different results. In particular, it was more difficult to perform standard operations after the cutoff point of Ki-67 was fixed. Moreover, whether the intensity of Ki-67 staining is significant for prognosis and molecular classification is still a controversial topic. The question remains: Does a cutoff value interval, not a single number, have greater practicability in daily pathological diagnosis? Moreover, pathologists have difficulty determining an accurate cutoff value by using any method, such as manual counting or artificial intelligence-assisted counting, and different staining intensities are also considered. Given this complexity, it is worth exploring the method of determining the percentage of Ki-67-positive cells simply and conveniently, and the necessity of using a value interval of Ki-67 and not a single number to determine the prognosis of patients with BC, as well as the validity of the interval of Ki-67 in practice, is also herein discussed.
Materials and methods
Clinical data
Paraffin samples from 280 patients with BC treated from January 2005 to July 2013 at the Joint Logistic Support Force 989th Hospital were collected for a retrospective study. All patients had complete follow-up data. In addition, three patients had metastatic lesions, and 6 patients had not undergone radical surgery. None of the patients had received any therapy before the operation. The inclusion criteria for the respondents were as follows: (1) All patients were diagnosed through breast puncture or postoperative examination after radical surgery. (2) none of the patients received any therapy before the operation, such as neoadjuvant chemotherapy, radiotherapy, neoadjuvant endocrine therapy or HER2-targeted therapy, etc. (3) all patients had complete follow-up data. (4) if the patients died, the cause of death was due to metastasis or relapse of BC. Patients who met one of the following conditions were excluded: (1) did not have complete follow-up data, (2) received any treatment for breast cancer before surgery, (3) died for reasons other than BC metastasis or relapse, or (4) had poor quality of life caused by other reasons or died from other tumors. This study was approved by the ethics committee of the 989th hospital, and all included respondents signed informed consent forms. This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from every participant or from their relatives.
Immunohistochemical analysis
Paraffin-embedded tumor tissues were cut into 2–5 μm thick sections for next step staining. For immunohistochemical analyses on a Benchmark XT system (Ventana, AZ, USA), deparaffinized sections were immersed in 0.3% H2O2 in methanol for 30 min to eliminate endogenous peroxidase activity, followed by incubation with PBS containing 1% normal serum corresponding to the secondary IgG and 1% bovine serum albumin (BSA) to reduce nonspecific reactions. The sections were incubated with rabbit anti-human Ki-67 (MIB-1) (dilution 1:200; Maxim, Fuzhou, China), rabbit anti-human ready-to-use monoclonal estrogen receptor (ER, SP1, Maxim, Fuzhou, China), progesterone receptor (PR, SP2, Maxim, Fuzhou, China), or human epidermal growth factor receptor 2 (HER-2, MXR001, Maxim, Fuzhou, China). After incubation with biotinylated secondary Abs at room temperature (RT) for 1 h, immune complexes were amplified with streptavidin-alkaline phosphatase (SAP) immunohistochemical staining kits (Maxim, Fuzhou, China) following the manufacturer’s instructions. A negative control was generated by omitting the primary antibodies and substituting them with the corresponding normal isotype-matched immunoglobulin IgG.
IHC scoring for ER, PR, Ki-67
ER, PR and Ki-67 protein positivity involves the cell nucleus. Cells were assessed as positive with the occurrence of yellow nuclei or brown particles. The interpretation of the Ki-67 index was as follows: the whole section was previewed under a low-power lens before interpretation to judge whether the staining was uniform. If there was even staining, samples were observed under a high-power lens (40×) to count more than 500 cancer cells. If there was uneven staining, the hot-spot region with a concentrated positive cell number was selected to count more than 500 cancer cells. The samples that approached to the cutoff point were evaluated again by 2 other independent pathologists, and the average Ki-67 index was calculated. Finally, the mean value was chosen as the final Ki-67 index value (Fig. 1A-D). Samples for which the staining intensity could not be determined and ambiguous cell profiles were excluded from the count. In addition, the staining intensity of the tumor cells was scored as light yellow (1 point), deep yellow (2 points) or brown (3 points). The staining index was calculated as the percentage of positive cells× the staining intensity. The minimum and maximum staining indices were 1% and 285%, respectively. When brown particles were observed in more than 1% of the BC cell nuclei, the ER and PR were considered positive.
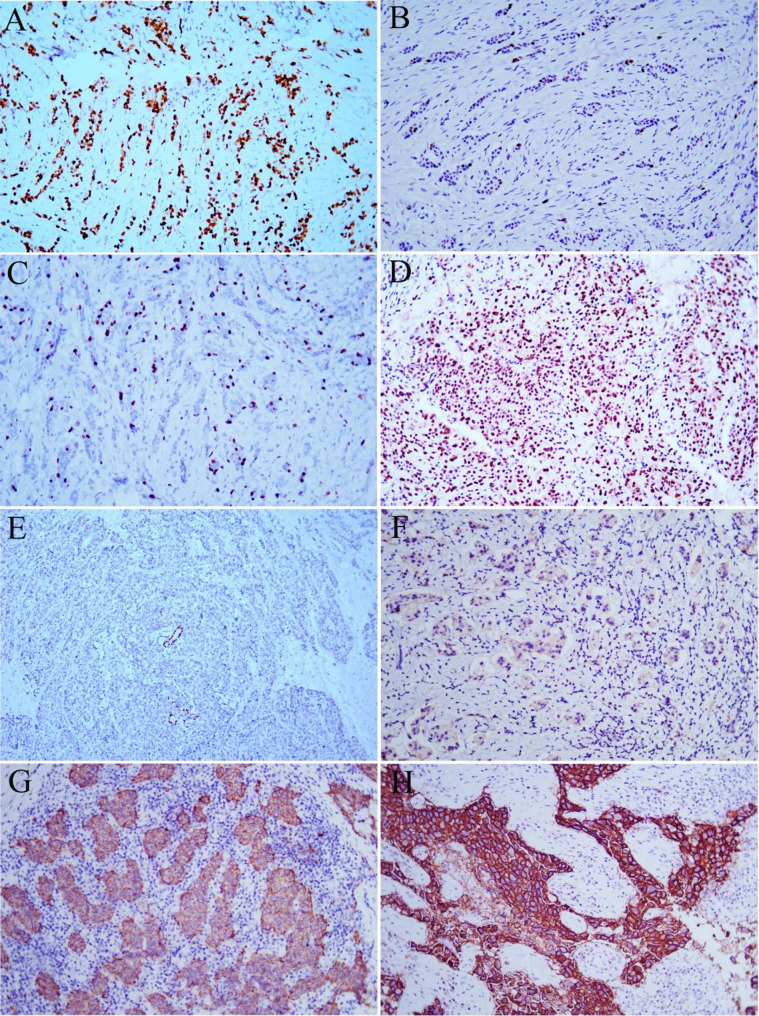
Fig. 1.
Different staining intensities of Ki-67 and HER-2 by immunohistochemical staining. A: Ki-67 hot spots, Magnification, ×200. B: Ki-67 index < 14%, Magnification, ×200. C: Ki-67 index = 14%, Magnification, ×200. D: Ki-67 index > 30%, Magnification, ×200. E: HER-2 (0+), Magnification, ×100. F: HER-2 (1+), Magnification, ×200. G: HER-2 (2+), Magnification, ×200. H: HER-2 (3+). Magnification, ×200
IHC scoring and FISH detection for HER-2
The interpretation of HER-2-positive criteria was as follows: 0+: no staining or ≤ 10% of invasive cancer cells showing incomplete and weak membrane staining; 1+: >10% of invasive cancer cells presenting incomplete and weak cell membrane staining; 2+: >10% of invasive cancer cells presenting incomplete and/or moderate cell membrane staining or ≤ 10% of invasive cancer cells presenting strong and complete cell membrane staining; and 3+: >10% of invasive cancer cells presenting strong, complete, and uniform cell membrane staining (Fig. 1E-H). In the presence of HER-2 2 + staining, fluorescence in situ hybridization detection was implemented to further determine the HER-2 status. The probe for FISH detection of HER-2 was purchased from Maxim, Fuzhou, China (Product number: KIT-2001-20), and operation was carried out according to the manufacturer’s instructions.
All immunohistochemistry and FISH results were interpreted independently by 2 senior pathologists.
Molecular classification
Molecular classification was performed on the basis of the consensus at the St Gallen Conference in 2011 (luminal A-type and luminal B-type BC were distinguished by using 14% as the critical value of Ki-67) and the 2019 CSCO guidelines (low expression if the critical value of Ki-67 is less than < 15%). If the critical value of Ki-67 was greater than 30%, this indicated high expression. When the expression of ER (+), HER-2 (-) and Ki-67 was between 15% and 30%, the molecular classification of BC was performed according to the percentage of cells with positive PR expression. Under these circumstances, PR = 20% was used as the critical value according to the 2019 CSCO guidelines. If the PR expression was > 20%, the samples were considered luminal A-type BC. If the PR expression was < 20%, the samples were determined to be luminal B-type BC.
Statistical analysis
The statistical analyses were completed by using SPSS version18.0 (SPSS Inc., Chicago, IL, USA). Survival data (overall survival (OS) and disease-free survival (DFS)) were analyzed via Kaplan‒Meier survival curves and compared by using log-rank tests among different groups. Statistical significance was 2-sided, and P values < 0.05 were considered to indicate statistical significance. In practice, we performed statistical analysis with a cut-off value of 14% according to the recommendations of St. Gallon guideline, and if there was no difference between difference molecular subtypes, we increased the gradient by 5% until a statistically significant interval value of Ki-67 was obtained.
Results
Clinicopathological characteristics of patients with BC
Among the patients with BC enrolled in this study, there were 4 males (1.43%) and 276 females (98.57%). All the respondents were between 25 and 84 years old, with an average age of 51 years. Specifically, 2 patients were younger than 30 years old, 222 patients were between 30 and 60 years old, and 56 patients were over 60 years old. Among these patients, invasive ductal carcinoma not otherwise specified (DCS, NOS) was found in 93.57% of patients, invasive lobular carcinoma (ILC) was found in 2.14% of patients, invasive micropapillary carcinoma was found in 1.07% of patients, mucinous carcinoma was found in 2.5% of patients, medullary carcinoma was found in 0.36% of patients, and apocrine carcinoma was found in 0.36% of patients. With respect to lymphatic metastasis, there were 146 patients without lymphatic metastasis (N0), 66 patients with metastasis to 1–3 nodes (N1), 39 patients with lymphatic metastasis to 4–9 nodes (N2), and 20 patients with lymphatic metastasis to ≥ 10 nodes (N3). In a word, there were 46.13% (125/271) of the patients had positive lymph nodes, and 53.87% (146/271) did not have lymph node metastasis. According to the pathological stages of AJCC (pTNM), there were 42 cases in Stage IA, 104 cases in Stage IIA, 60 cases in Stage IIB, 43 cases in Stage IIIA, 1 case in Stage IIIB, 20 cases in Stage IIIC, and 1 case in Stage IV. There were 15.50% (42/271) of patients had stage I disease, 60.52% (164/271) of patients had stage II disease, 23.62% (64/271) of patients had stage III disease, and 0.37% (1/271) of patients had stage IV disease. Other pathological characteristics of the patients are shown in Table 1. The median follow-up time was 80.2 months (range from 2 to 157 months).
Table 1.
Pathological characteristics of 280 patients with BC
| Factors | N | % |
|---|---|---|
| Sex (N = 280) | ||
| M | 4 | 1.43% |
| FM | 276 | 98.57% |
| Age (year) (N = 280) | ||
| ≤ 30 | 2 | 0.71% |
| 30–60 | 222 | 79.29% |
| >60 | 56 | 20.00% |
| Histological type (N = 280) | ||
| DCS | 262 | 93.57% |
| ILC | 6 | 2.14% |
| invasive micropapillary carcinoma | 3 | 1.07% |
| mucinous carcinoma | 7 | 2.50% |
| medullary carcinoma | 1 | 0.36% |
| apocrine carcinoma | 1 | 0.36% |
| T stage (N = 271)* | ||
| T1b | 15 | 5.54% |
| T1c | 49 | 18.08% |
| T2 | 183 | 67.53% |
| T3 | 22 | 8.12% |
| T4 | 2 | 0.74% |
| N stage (N = 271) * | ||
| 0 | 146 | 53.87% |
| N1 | 66 | 24.35% |
| N2 | 39 | 14.39% |
| N3 | 20 | 7.38% |
| AJCC stage (N = 271) * | ||
| IA | 42 | 15.50% |
| IIA | 104 | 38.38% |
| IIB | 60 | 22.14% |
| IIIA | 43 | 15.87% |
| IIIB | 1 | 0.37% |
| IIIC | 20 | 7.38% |
| IV | 1 | 0.37% |
Abbreviations: M, male; FM, female; DCS, invasive ductal carcinoma; ILC, invasive lobular carcinoma; AJCC, American Joint Committee on Cancer
*There were 3 cases had metastatic lesions and 6 cases had not undergone radical operation, there were no complete T stage, N stage and AJCC stage data
Differences in the different Ki-67 indices and overall survival (OS) rates of patients with BC
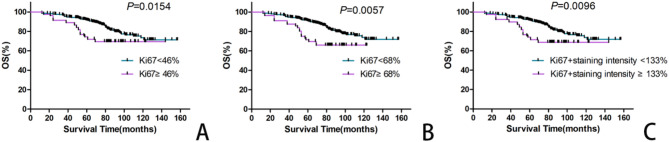
After staining and interpretation, more than 500 tumor cells were counted in 277 samples. Therefore, the actual statistical group included 277 BC samples. The median Ki-67 value was 24.3% (ranging from 1 to 95%), Ki-67 ≤ 1% accounted for 7.22% (20/277) of the patients, > 1% Ki-67 < 90% accounted for 89.17% (247/277) of the patients, and Ki-67 ≥ 90% accounted for 3.61% (10/277) of the patients. According to the statistical analysis, the OS of patients with BC decreased significantly when the Ki-67 index fell within 46 − 68% (χ2 = 5.87, P = 0.0154; χ2 = 7.64, P = 0.0057, respectively, Table 2; Fig. 2A and B). The results indicated that a Ki-67 index interval between 46% and 68% can be used to determine the OS of patients with BC.
Table 2.
Relationship between Ki-67 index and OS of patients with BC
| Ki-67 index | Death (n) | Survive (n) | χ2 | P |
|---|---|---|---|---|
| < 46% | 45 | 186 | 5.87 | 0.0154 |
| ≧ 46% | 14 | 32 | ||
| < 68% | 51 | 206 | 7.64 | 0.0057 |
| ≧ 68% | 8 | 12 |
Fig. 2.
Kaplan-Meier curves of the patients with BCs according to the different Ki-67 index. A: Patients with Ki-67 labeling index ( ≧ 46%) had significantly inferior OS rate compared with Ki-67 labeling index (< 46%). B: Patients with Ki-67 labeling index ( ≧ 68%) had significantly inferior OS rate compared with Ki-67 labeling index (< 68%). C: Kaplan-Meier curves of the BC patients with Ki-67 labeling index plus different stained intensity
Does the staining intensity of Ki-67 in tumor cells significantly influence the OS of patients with BC? The staining intensity was determined on the basis of the Ki-67 index. The results revealed significant differences in OS between the two groups when the critical value was 133% after accumulation of the Ki-67 index and the staining intensity, and patients with high expression of the Ki-67 index had a relatively poor prognosis (χ2 = 6.71, P = 0.0096; Table 3; Fig. 2C). When staining intensity is not considered, the cut-off value of 46% should be used to judge the OS of BC patients. However, there was no significant difference in OS between the group with staining intensity (cut-off value 133%) and the group without staining intensity (cut-off value 46%) for other indices (χ2 = 2.90, P = 0.0888; Table 3). These findings indicated that the staining intensity of Ki-67 was not valuable for OS prediction in patients with BC.
Table 3.
Relationship between staining intensity of Ki-67 and OS of patients with BC
| Ki-67 index + staining intensity | Death (n) | Survive (n) | Death rate (%) | χ2 | P |
|---|---|---|---|---|---|
| < 46% | 45 | 188 | 19.31% | ||
| ≧ 46% | 14 | 30 | 34.09% | ||
| < 133% | 46 | 189 | 19.57% | 2.90 | 0.0888※ |
| ≧ 133% | 13 | 29 | 30.95% | 6.71 | 0.0096* |
※46% vs. 133% group; * <133% vs. ≧ 133% group
Relationship between the expression of Ki-67 in BC and the DFS of patients
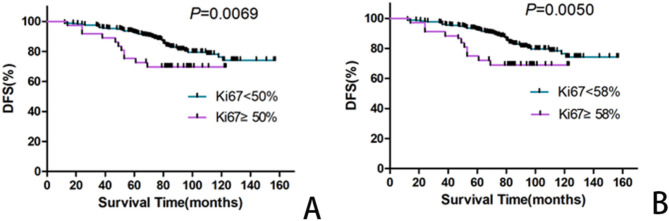
At the end of the follow-up period, 36 of the 280 patients with BC developed metastasis or in situ relapse at different positions. According to the statistical analysis, the DFS of patients with BC gradually decreased as the Ki-67 index increased from 50 to 58% (χ2 = 7.31, P = 0.0069; χ2 = 7.88, P = 0.005, respectively). This revealed that the Ki-67 index was useful for determining the DFS of patients with BC when it was in the range of 50 − 58% (Table 4; Fig. 3).
Table 4.
Relationship between ki-67 index and DFS of patients with BC
| Ki-67 index | Death (n) | Survive (n) | χ2 | P |
|---|---|---|---|---|
| < 50% | 33 | 174 | 7.31 | 0.0069 |
| ≧ 50% | 11 | 26 | ||
| < 58% | 34 | 178 | 7.88 | 0.0050 |
| ≧ 58% | 10 | 22 |
Fig. 3.
Kaplan-Meier curves (DFS) of the patients with BCs according to the different Ki-67 cutoff point. A: Patients with labeling index ( ≧ 50%) had significantly inferior DFS rate compared withKi-67 labeling index (< 50%). B: Patients with Ki-67 labeling index ( ≧ 58%) had significantly inferior DFS rate compared with Ki-67 labeling index (< 58%)
Relationship between the expression of Ki-67 in different molecular subtypes of BC and the OS of patients
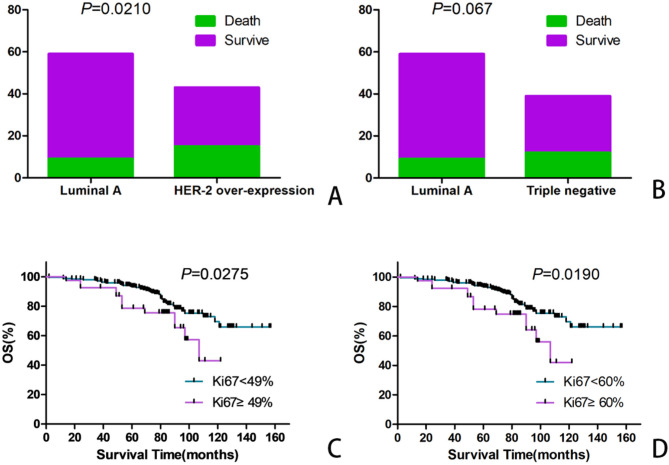
Among 280 patients with BC, 254 patients with invasive ductal carcinoma were classified on the basis of the critical Ki-67 index value (14%) according to the consensus of the St. Gallen Conference in 2011. Sixty cases of luminal A-type BC, 112 cases of luminal B-type BC, 43 cases of HER2-overexpressing subtype BC, and 39 cases of triple-negative-type BC were distinguished. According to the statistical analysis, luminal A-type BC patients were significantly different from patients with HER2-overexpressing subtype in terms of OS (χ2 = 5.33, P = 0.0210; Table 5; Fig. 4A), and no difference with triple negative-type BC patients (χ2 = 3.36, P = 0.067. Table 5; Fig. 4B).
Table 5.
Molecular type of patients with BC according to St. Gallen conference in 2011
| Molecular type | Death (n) | Survive (n) | Total (n) | median Ki-67 index (%) | χ2 | P |
|---|---|---|---|---|---|---|
| Luminal A | 9 | 51 | 60 | 7.52% | ||
| Luminal B HER-2 (-) | 13 | 57 | 70 | 27.41% | ||
| Luminal B HER-2 (+) | 9 | 33 | 42 | 23.84% | ||
| Over-expression HER-2 | 15 | 28 | 43 | 31.10% | 5.33 | 0.021※ |
| Triple-negative | 12 | 27 | 39 | 35.32% | 3.36 | 0.067* |
※Luminal A type vs.HER-2 over-expression type; * Luminal A type vs. triple negative type
Fig. 4.
According to the 11th St. Gallen International Consensus Meeting, the difference was seen between Luminal A type and over-expression of HER-2 type, triple-negative type. A: Luminal A type vs. over-expression of HER-2 type. B: Luminal A type vs. triple-negative type. C: Patients withHER-2 over-expression BC with labeling index ( ≧ 49%) had significantly inferior OS rate compared with Ki-67 labeling index (< 49%). D: Patients withHER-2 over-expression BC with Ki-67 labeling index ( ≧ 60%) had significantly inferior OS rate compared with Ki-67 labeling index (< 60%)
To further investigate the relationship between the Ki-67 index and each molecular type of BC, a statistical analysis of the relationship between the percentage of Ki-67-positive cells and the molecular type of patients with BC was carried out. There was a significant difference in the OS of patients with HER2-overexpressing subtype BC when the Ki-67 index ranged from 49 − 60% (χ2 = 4.86, P = 0.0275; χ2 = 5.50, P = 0.0190, respectively; Fig. 4C and D), but there were no significant differences among the luminal A-type group, luminal B-type group and triple negative-type group.
Molecular classification of patients with BC on the basis of the molecular classification proposed by the Chinese society of clinical oncology (CSCO) in 2019
Among 280 patients with BC, 250 patients with invasive ductal carcinoma underwent molecular classification according to the CSCO standards set forth in 2019. According to the molecular classification results, there were 90 cases of luminal A-type BC, 78 cases of luminal B-type BC, 43 cases of HER2-overexpressing subtype BC, and 39 cases of triple-negative BC. According to the statistical analysis results, there were significant differences between luminal A-type BC and HER2-overexpressing subtype BC in terms of OS (χ2 = 5.53, P = 0.019). Patients with HER2-overexpressing subtype BC had a higher death rate and poorer prognosis, but there was no significant difference in OS between patients with luminal B-type BC and those with triple-negative BC (Table 6; Fig. 5A).
Table 6.
Molecular type of patients with BC according to CSCO consensus in 2019
| Molecular type | Death (n) | Survive (n) | Total (n) | Death rate (%) | median Ki-67 index (%) | χ2 | P |
|---|---|---|---|---|---|---|---|
| Luminal A | 15 | 75 | 90 | 16.67% | 11.23% | ||
| Luminal B HER-2 (-) | 7 | 29 | 36 | 19.44% | 37.7% | ||
| Luminal B HER-2 (+) | 9 | 33 | 42 | 21.43% | 23.84% | ||
| Over-expression HER-2 type※ | 15 | 28 | 43 | 34.88% | 31.10% | 5.53 | 0.019※ |
| Triple-negative type | 12 | 27 | 39 | 30.77% | 35.32% |
※ Luminal A type vs. HER-2 over-expression type
Fig. 5.
A: According to the 2019 CSCO meeting, the difference was seen between Luminal A type and over-expression of HER-2 type of BC. B: Luminal B HER-2 (-) type patients with Ki-67 labeling index ( ≧ 52%) had significantly inferior OS rate compared with Ki-67 labeling index (< 52%). C: the HER-2 over-expression patients with labeling index ( ≧ 47%) had significantly inferior OS rate compared with Ki-67 labeling index (< 47%). D: the HER-2 over-expression patients with Ki-67 labeling index ( ≧ 60%) had significantly inferior OS rate compared with Ki-67 labeling index (< 60%)
The relationship between the Ki-67 index and the prognosis of patients with specific molecular classifications based on CSCO in 2019 was analyzed. According to the statistical analysis, there were significant differences between the two groups in terms of the luminal B HER-2 (-) type when the cutoff value of the Ki-67 index was 52% (χ2 = 6.61, P = 0.0101) (Fig. 5B). Moreover, there were significant differences between the two groups in terms of the HER2-overexpressing subtype when the Ki-67 index fell within the critical interval of 47-60% (χ2 = 4.86, P = 0.0275; χ2 = 5.0, P = 0.0190, respectively) (Fig. 5C and D), but these two groups showed no significant difference in terms of other molecular subtypes. Interestingly, all 19 patients with luminal A-type BC survived when the Ki-67 index was ≤ 4% (during the follow-up visit period), and the results indicated that BC patients with very low Ki-67 indices had high survival rates.
Discussion
The applications of Ki-67 in BC can be divided into two main parts: (1) Molecular classification is particularly necessary when distinguishing luminal A-type BC from luminal B-type BC because the former requires only endocrine therapy, whereas the latter necessitates systematic chemotherapy based on endocrine therapy to achieve good clinical effects [10]. Hence, distinguishing these two types of BC in clinical practice is necessary. (2) Prognosis of patients with BC. Many studies have demonstrated that patients with higher Ki-67 indices have poorer prognoses and that they more easily relapse [9, 23, 24]. However, the opposite results have also been reported: patients with a higher Ki-67 index achieve better pathological complete response (pCR), without considering the HR state, HER-2 state or triple-negative type of BC [25]. Some studies have even reported that the Ki-67 index is unrelated to the prognosis of patients with BC [19, 26]. Moreover, the prognosis is poorer when the Ki-67 index is lower [27]. Researchers have demonstrated that other clinical pathological features, including histological grade, lymphatic metastasis, tumor volume, vascular invasion, etc., should be considered when assessing the influence of the Ki-67 index on patients with BC [28]. Therefore, the value of Ki-67 in the management of BC is controversial, and how to use the cutoff value of Ki-67 correctly and precisely is a clinical conundrum.
Moreover, Ki-67 has not been widely applied in the clinic because of differences among observers, which has resulted in different molecular classifications and different treatment options. Additionally, some clinicians depend more on the Ki-67 division between the “high Ki-67 group” and the “low Ki-67 group” when determining treatment. However, the critical value of the “low Ki-67 group” ranged between 0% and 28.6% [29, 30], indicating significant differences. In a word, the cut-off value of Ki-67 index was significant difference between the different pathologists. Therefore, precisely identifying the critical value of the Ki-67 index and the values of the Ki-67 index for determining the prognosis and molecular classification of patients with BC, as well as making reasonable and efficient use of Ki-67 in BC, are highly important.
Immunohistochemical staining is the most common analytical method for detecting Ki-67. This method is not only economic and simple but also requires no complicated equipment. However, Ki-67 has limited practical value in clinical practice because there are many interference factors before, during and after the experiment. The main interference factors before the experiment are the selection of stationary liquid, the fixed time and the cold ischemia time. Interference during the experiment generally include the type of antibody, antigen repair method, staining development time and proficiency of technicians. The main postexperimental influencing factors include the method for counting positive cells, professional training of observers, and accurate recognition of positive cells. Influencing factors before and during the experiment can be avoided through standardized operation as much as possible, including choosing 10% neutral formalin as the fixed liquid, controlling the time of samples in the fixed liquid after separation within 30 min, ensuring at least 24 h of fixation time, and using the primary antibody of the same batch from the same factory, the same antigen repair method after optimization, and the same technician. The counting of positive cells after the experiment was the most difficult work.
To optimize the counting method and decrease differences among observers as much as possible, the International Ki-67 in Breast Cancer Working Group offered the following suggestions to promote applications of Ki-67 in BC: counting at least 3 high-power fields (×40 objectives) or counting 500–1000 tumor cells, including invasion edges of tumors and hot spot regions [31]. However, the artificial intelligence (AI)-assisted Ki-67 digital image analysis was reported recently, and the results showed a high degree of concordance with manual counting of Ki-67, at the same time the results of Ki-67 index have prognostic potential [32]. Ki-67 index was also calculated by using image analysis software, the results showed that the staining intensity of the tumor cells to be counted can be adjusted, and the efficiency was much higher than manual counting [33]. Even a replacement for Ki-67 had been fully evaluated. A head-to-head comparison of Ki-67 index and phosphohistone H3 to assess its prognostic value in breast cancer, the results displayed that phosphohistone H3 was a stronger and more robust prognostic indicator than Ki-67 in patients with BC [34]. A published paper evaluated the Ki-67 index in luminal BC by using 3 methods: (1) the number of Ki-67 expression in 1000 tumor cells in the hot spot area was counted, (2) the average expression of Ki-67 in tumor cells in the defined hot spot area, (3) the expression of Ki-67 in tumor cells throughout the whole section, the results showed that the first method was an optimal method for its high reliability and reproducibility [35]. Thus, the puzzle of whether to count the hot spot or average count of the Ki-67 index has been perfectly solved. At the same time, the same observer was assigned three counts in 2 days for patients whose Ki-67 index ranged between 10% and 20% to decrease the discrepancies among different observers as much as possible in our study. Next, the mean value was used as the final Ki-67 index.
In this study, the percentage of Ki-67-positive cells in 277 patients with BC was calculated, and statistically significant differences were detected among patients with BC in terms of OS when the critical value of the Ki-67 index fell within the interval of 46 − 68%. In other words, the death rate of patients gradually increased, and the prognosis deteriorated with an increasing Ki-67 index. Since the interval of the Ki-67 index is large, it is easy for pathologists to promote this index, and it has relatively high repeatability. The staining intensity of Ki-67 was included in the evaluation index, which can also influence the OS prediction of patients with BC (cut-off value 133% ). However, the group for with the staining intensity was considered (cut-off value 133% ) and the group without such consideration of the staining intensity (cut-off value 46%) showed no statistically significant differences in OS prediction. These findings indicate that the intensity of Ki-67 staining has no significant value in the prediction of OS in patients with BC. In other words, if the staining intensity of Ki-67 was considered, different cut-off values should be used to determine the OS of BC patients.
Zhu noted that setting the critical value of the Ki-67 index at 30% was an independent prognostic factor for the OS and DFS of triple-negative BC patients [36]. Patients with triple-negative cancer and a Ki-67 index > 30% had a relatively poor prognosis, and this was especially true for patients with stage I disease. When the critical value of the Ki-67 index was 10%, BC patients with both high expression (≥ 10%) and low expression (< 10%) had significant differences in OS and recurrence-free survival (RFS) when there was low PR expression (< 20%). In particular, patients with high Ki-67 expression and low PR expression had the poorest prognosis [37]. Similarly, the Ki-67 index is important for determining the DFS of patients with BC. According to this study, the DFS of patients with BC decreased gradually when the Ki-67 index value increased from 50 to 58%, but the interval from the Ki-67 index to the determination of DFS was relatively small, and special attention should be given to this interval. However, some studies have shown that when the critical value of the Ki-67 index is 14%, patients with luminal-type BC and high Ki-67 expression (≥ 14%) and low PR expression (< 50%) achieve relatively high clinical complete remission (CR) from neoadjuvant chemotherapy [38].
There is a “gray zone” during molecular classification of patients with BC on the basis of the Ki-67 index, especially when different methods are applied. During the molecular classification of patients with BC based on the consensus of the St. Gallen Conference in 2011, it is relatively difficult to set 14% as the critical value of the Ki-67 index in practical applications. In particular, the repeatability is poorer when the primary count is between 12% and 16%. However, there were still counting errors made by the same technician, and the error was even greater across multiple technicians. In this study, the same observer was assigned three counts in 2 days for patients whose Ki-67 index ranged between 10% and 20%. Next, the mean value was used as the final Ki-67 index. This can decrease errors among different observers as much as possible, and the total number of cells can increase significantly. The biggest defect lies in high workloads, and it is applicable only to situations with small sample sizes. During molecular classification based on the CSCO standards of 2019, repeated counting or decision-making according to other evidence was needed when the Ki-67 index ranged between 15% and 30% [18], which increased the workload and led to poor repeatability as well as increased errors. Decreasing the number of “gray zones” and differences in the molecular classification of BC was the key challenge in this study.
During the molecular classification of patients with BC on the basis of the consensus of the St. Gallen Conference in 2011, the OS of patients with luminal A-type BC was not significantly different from that of patients with HER2-overexpressing subtype and patients with triple-negative-type BC. The OS of patients with HER2-overexpressing subtype BC significantly differed when the Ki-67 index fell within 49 − 60%, but there was no significant difference among the other subtypes. In 2019, the OS of patients with luminal A-type BC significantly differed from that of patients with HER2-overexpressing subtype BC in terms of OS, but it was not significantly different from that of patients with other subtypes. Differences caused by two different molecular classification methods might influence the prognosis of patients. When 52% was used as the Ki-67 index, there was a significant differences in OS among patients with the luminal B HER-2(-) subtype. Moreover, there was a significant difference in OS among patients with HER2-overexpressing subtype BC when the Ki-67 index was 47%. These findings further indicate differences in the Ki-67 index among different molecular classification methods, which deserve special attention.
Conclusions
In summary, a systematic study on the application value of the Ki-67 index in patients with BC was carried out. The results revealed that the Ki-67 index has a large value range that is suitable for determining the OS of patients with BC and that it can decrease the degree of difference in interpretation among different observers. The staining intensity slightly influenced OS predictions made on the basis of the Ki-67 index. The interval of the Ki-67 index for the determination of DFS in patients with BC is relatively small, and the Ki-67 index may impose a high requirement for interpretation in practice. Different molecular classification methods influence the prognostic assessment and classification of patients with BC. Therefore, molecular classification methods should be chosen carefully to provide more effective clinical treatment. Moreover, other clinical pathological features should be considered.
Acknowledgements
None.
Abbreviations
- BC
Breast cancer
- BSA
Bovine serum albumin
- RT
Room temperature
- SAP
Streptavidin-Alkaline Phosphatase
- OS
Overall survival
- DFS
Disease-free survival
- DCS NOS
Invasive ductal carcinoma not otherwise specified
- ER
Estrogen receptor
- PR
Progesterone receptor
- CSCO
Chinese Society of Clinical Oncology
- AJCC
American Joint Committee on Cancer
- PBS
Phosphate buffer saline
Author contributions
Nianlong Meng and Tian Yun: Formal analysis, Methodology, Investigation. Xuexia Lve and Yaxi Wang: Investigation, Visualization. Naijun Fan and Jingchang Chen: Validation, Investigation, Writing-original draft. Fulin Li, Xiaoyue Wang and Yansha Cao: Methodology, Writing-original draft. Changsong Wang, Xiaoyue Wang: Conceptualization, Supervision, Methodology, Writing-review & editing. All of the authors approved the contents and the data in this manuscript, and agreed for its submission for publication.
Funding
This study was supported by the Medical Science and Technique Program of Henan Province (No. LHGJ20210823, LHGJ20240990).
Data availability
The corresponding author takes responsibility to provide the data which support the conclusions of this project, and these data only can be used in present study. At the same time, the data are obtained from the authors upon reasonable request and authorized by corresponding authors.
Declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards, and were approved by the ethical committee of the 989th Hospital of PLA review board.
Consent for publication
Written informed consent was obtained from the family members of the patient or patient for publication of this article and any accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Changsong Wang JingChang Chen and Xuexia Lv contributed equally to this work.
Contributor Information
Changsong Wang, Email: wangtmmu150@163.com.
Xiaoyue Wang, Email: xiaoyuewnormal@163.com.
References
- 1.Global Burden of Disease 2019 Cancer Collaboration, Kocarnik JM, Compton K, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 Cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8(3):420–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zeng X, Yin P, Zhu J, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. [DOI] [PubMed] [Google Scholar]
- 4.Fabian CJ, Kimler BF, Zalles CM, et al. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast Cancer Res Treat. 2007;106:75–84. [DOI] [PubMed] [Google Scholar]
- 5.Okumura Y, Yamamoto Y, Zhang Z, et al. Identifi cation of biomarkers in ductal carcinoma in situ of the breast with microinvasion. BMC Cancer. 2008;8:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheri A, Smith IE, Johnston SR, et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol. 2015;26:75–80. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara N, Mukai H, Masumoto M, et al. Survival outcome and reduction rate of Ki-67 between pre- and post-neoadjuvant chemotherapy in breast cancer patients with non-pCR. Breast Cancer Res Treat. 2014;147:95–102. [DOI] [PubMed] [Google Scholar]
- 8.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, et al. Strategies for subtypes - dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast Cancer. Ann Oncol. 2011;22:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann Oncol. 2013;24(9):2206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YP. Interpretation of Ki-67 assessment update of international Ki-67 in breast Cancer working group. Zhonghua Bing Li Xue Za Zhi. 2021;50(7):704–9. [DOI] [PubMed] [Google Scholar]
- 12.Shet T. Ki-67 in breast cancer: simulacra and simulation. Indian J Cancer. 2020;57(3):231–3. [DOI] [PubMed] [Google Scholar]
- 13.Li YZ, Huang YH, Su XY, Gu ZQ, Lai QQ et al. (2022) Breast MRI segmentation and Ki-67 high- and low-expression prediction algorithm based on deep learning. Comput Math Methods Med. 2022:1770531. [DOI] [PMC free article] [PubMed]
- 14.Juan MW, Yu J, Peng GX, Jun LJ, Feng SP, Fang LP. Correlation between DCE-MRI radiomics features and Ki-67 expression in invasive breast cancer. Oncol Lett. 2018;16(4):5084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baisch H. Elevated Ki-67 expression is correlated with TNF alpha- and IFN gamma-induced apoptosis in tumour cells. Cell Prolif. 2002;35(6):333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res. 2014;16:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast Cancer. Ann Oncol. 2015;26:1533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JB, Jiang ZF. Update and interpretation of 2019 guideline of Chinese society of clinical oncology (CSCO): breast cancer. Chin J Surg Oncol. 2019;11(3):155–60. [Google Scholar]
- 19.Polley MY, Leung SC, McShane LM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105(24):1897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimm DL, Leung SCY, McShane LM, et al. An international multicenter study to evaluate reproducibility of automated scoring for assessment of Ki67 in breast cancer. Mod Pathol. 2019;32(1):59–69. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in breast cancer: updated recommendations from the international Ki67 in breast Cancer working group. J Natl Cancer Inst. 2021;113(7):808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YH, Lai CR, Lien HC, Hsu CY. Good staining quality ensuring the reproducibility of Ki67 assessment. J Clin Pathol. 2020;73(7):413–7. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura R, Osako T, Nishiyama Y, Tashima R, Nakano M, et al. Prognostic significance of Ki-67 index value at the primary breast tumor in recurrent breast cancer. Mol Clin Oncol. 2014;2(6):1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathmanathan N, Balleine RL, Jayasinghe UW, Bilinski KL, Provan PJ, et al. The prognostic value of Ki67 in systemically untreated patients with nodenegative breast cancer. J Clin Pathol. 2014;67:222–8. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, He C, Han D, Zhou M, Wang Q, et al. The predictive value of Ki-67 before neoadjuvant chemotherapy for breast cancer: A systematic review and Meta-Analysis. Future Oncol. 2017;13(9):843–57. [DOI] [PubMed] [Google Scholar]
- 26.Hashmi AA, Hashmi KA, Irfan M, Khan SM, Edhi MM, et al. Ki67 index in intrinsic breast cancer subtypes and its association with prognostic parameters. BMC Res Notes. 2019;12(1):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurozumi S, Matsumoto H, Hayashi Y, Tozuka K, Inoue K, et al. Power of PgR expression as a prognostic factor for ER positive /HER-2 negative breast cancer patients at intermediate risk classified by the Ki67 labeling index. BMC Cancer. 2017;17(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadivar M, Aram F. Assessment of Ki67 in breast cancer: A comparison between the Eye-10 method, Stepwise counting strategy, and international system of Ki67 evaluation. Iran J Pathol. 2020;15(1):13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23(28):7212–20. [DOI] [PubMed] [Google Scholar]
- 30.Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17(4):323–34. [DOI] [PubMed] [Google Scholar]
- 31.Fulawka L, Halon A. Ki-67 evaluation in breast cancer: the daily diagnostic practice. Indian J Pathol Microbiol. 2017;60(2):177–84. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto H, Miyata R, Tsuruta Y, et al. Ki-67 evaluation using deep-learning model-assisted digital image analysis in breast cancer. Histopathology. 2025;86(3):460–71. [DOI] [PubMed] [Google Scholar]
- 33.Maeda I, Abe K, Koizumi H, et al. Comparison between Ki67 labeling index determined using image analysis software with virtual slide system and that determined visually in breast cancer. Breast Cancer. 2016;23(5):745–51. [DOI] [PubMed] [Google Scholar]
- 34.Gerring Z, Pearson JF, Morrin HR, Robinson BA, Harris GC, Walker LC. Phosphohistone H3 outperforms Ki67 as a marker of outcome for breast cancer patients. Histopathology. 2015;67(4):538–47. [DOI] [PubMed] [Google Scholar]
- 35.Lashen A, Toss MS, Green AR, Mongan NP, Rakha E. (2022) Ki67 assessment in invasive luminal breast cancer: a comparative study between different scoring methods. Histopathology 81(6):786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X, Chen L, Huang B, Wang Y, Ji L, et al. The prognostic and predictive potential of Ki-67 intriple-negative breast cancer. Sci Rep. 2020;10(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang YJ, Lee HB, Kim YG, Han J, Kim Y et al. (2019) Ki-67 expression is a significant prognostic factor only when progesterone receptor expression is low in estrogen receptor-positive and HER2-negative early breast cancer. J Oncol. 7386734. [DOI] [PMC free article] [PubMed]
- 38.Silva LRD, Vargas RF, Shinzato JY, Derchain SFM, Ramalho S, Zeferino LC. Association of menopausal status, expression of progesterone receptor and Ki67 to the clinical response to neoadjuvant chemotherapy in luminal breast cancer. Rev Bras Ginecol Obstet. 2019;41(2):710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author takes responsibility to provide the data which support the conclusions of this project, and these data only can be used in present study. At the same time, the data are obtained from the authors upon reasonable request and authorized by corresponding authors.