Abstract
Background
Existing studies have explored the association between immune-inflammatory indices and inflammatory bowel disease (IBD), but there is a lack of comprehensive evidence. This meta-analysis and systematic review seeks to synthesize the data of available clinical research and offer the latest and comprehensive evidence-based conclusions regarding whether these immune-inflammatory indices can effectively predict the severity, activity, and prognosis of IBD.
Methods
Seven databases were comprehensively retrieved from their establishment to March 23, 2025. The combined results were described through standardized mean differences (SMD) or odds ratios (OR) with 95% confidence intervals (CI). Review Manager 5.4 and STATA 15.0 were leveraged for data analysis.
Results
Our analysis included 35 studies involving 5,870 patients. The aggregated data revealed that the neutrophil-to-lymphocyte ratio (NLR) (OR = 1.18, 95% CI:1.04 to 1.34; P = 0.001) (SMD = 1.01, 95%CI = 0.73 to 1.29, P < 0.001), platelet-to-lymphocyte ratio (PLR) (SMD = 0.60, 95%CI = 0.46 to 0.74, P < 0.001), neutrophil-to-platelet ratio (NPR) (OR = 1.20, 95% CI:1.08 to 1.32, P < 0.001), and C-reactive protein to albumin ratio (CRP/ALB) (OR = 1.50, 95% CI:1.38 to 1.65, P < 0.001) were potentially linked to disease activity in IBD patients. PLR (SMD = 1.08, 95%CI = 0.60 to 1.55, P < 0.001) showed potential associations with disease severity in IBD patients. Additionally, NLR (SMD = 0.43, 95%CI = 0.15 to 0.70, P = 0.002) and eosinophil-to-lymphocyte ratio (ELR) (SMD = 0.63, 95%CI = 0.26 to 1.00, P < 0.001) had potential associations with endoscopic response in IBD patients. Moreover, NLR was potentially associated with disease relapse(OR = 1.35, 95% CI:1.09 to 1.68; P = 0.006) and steroid responsiveness (SMD = 0.50, 95%CI = 0.15 to 0.85, P = 0.005).
Conclusion
NLR, PLR, NPR, and CRP/ALB are potential predictors of disease activity in IBD patients. PLR shows the potential to predict disease severity, while NLR and ELR are potential indicators of endoscopic response. Furthermore, NLR is also a potential predictor of relapse and steroid responsiveness. Currently, there is insufficient evidence to support an association between NLR and the severity of IBD, whereas lymphocyte-to-monocyte ratio (LMR) appears to be associated with both the severity and activity of IBD and PLR and eosinophil*neutrophil-to-lymphocytes ratio (ENLR) are associated with endoscopic response in IBD.
PROSPERO registration
CRD 42024609659.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-025-04033-4.
Keywords: Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Immunoinflammatory index, Clinical prognostic value, Meta-analysis
Introduction
Inflammatory bowel disease (IBD) is a group of chronic, nonspecific intestinal inflammatory disorders, and its etiology has not been elucidated. Its pathogenesis involves an intricate interplay of multiple factors, including environmental influence, genetic predisposition, and disturbances in intestinal microbiota, which results in an aberrant immune response in the gut [1]. IBD primarily manifests as ulcerative colitis (UC) and Crohn’s disease (CD) [2]. Currently, its diagnosis is mainly based on a comprehensive assessment involving laboratory tests, patient symptoms, endoscopic examination, and tissue biopsy, after excluding other infectious and non-infectious gastrointestinal disorders [3]. The common clinical symptoms are abdominal pain, diarrhea, and mucopurulent bloody stools. In severe cases, there exist complications like intestinal perforation, bowel obstruction, malignancy, thromboembolism, sepsis, as well as loss of intestinal barrier function [4]. The incidence and prevalence of IBD continue to rise [5], making the disease a global healthcare issue. High-income countries such as those in Western Europe and North America have the highest proportion of IBD patients worldwide, with the highest age-standardized prevalence rate found in the United States [6]. In Europe, the prevalence of IBD in the United Kingdom is the highest, reaching 373 cases per 100,000 individuals [7]. In IBD treatment, up to 90% of CD patients may opt for surgical intervention. Currently, surgical procedures remain a key therapeutic approach in the management of CD [8]. In current clinical practice, mucosal healing, as the therapeutic goal for the UC cohort, is shown to reduce the risk of surgery and hospitalization and possibly alter the natural course of the disease [9].
In comparison to colonoscopy and pathology, serological biomarkers are widely accepted, and they are low-cost, convenient, and non-invasive. They have garnered widespread attention for their potential to forecast the activity and severity of IBD [10]. The inflammatory indices typically include the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), neutrophil-to-platelet ratio (NPR), C-reactive protein-to-albumin ratio (CRP/ALB), eosinophil-to-lymphocyte ratio (ELR), and eosinophil*neutrophil-to-lymphocytes ratio (ENLR) [11–13]. These indicators can be readily obtained through routine blood tests, which are economical [14].
PLR and NLR have emerged as novel, non-invasive systemic inflammatory biomarkers. These ratios are associated with prognosis across various disease states. They have garnered widespread attention in the fields of malignancies, inflammatory conditions, coronary heart disease, thrombosis, and autoimmune diseases such as rheumatoid arthritis [15]. Acarturk et al. [16] collected data from 44 UC patients as the observation group and 41 healthy people as the control group. They assessed the NLR, CRP, erythrocyte sedimentation rate (ESR), and white blood cell count (WBC), and found that it was notably higher in the active phase group than that in the remission and control groups. Yao et al. [14] studied 67 patients with CD, categorizing them into active and remission groups. Their findings proved NLR as an independent predictor of activity and severity in CD. Furthermore, significant differences are noted in NLR across the mild, moderate, and severe subgroups of the active phase, which proved its role in assessing disease severity.
Anas Elgenidy et al. [17] published a meta-analysis in 2024 that examined the correlation between the systemic immune-inflammatory index (SII) and disease activity in patients with UC. The objective of our current study is to synthesize the existing clinical data through a meta-analysis and systematic review and update the previous meta-analysis to provide the latest and most comprehensive evidence-based medical data. This will help confirm whether such immune-inflammatory indices can effectively predict the severity, activity, and prognosis of IBD.
Methods
Literature search
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines. The research protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO: CRD 42024609659). The search strategy was developed by two investigators (LPJ and WYL). They independently searched subject headings and keywords across PubMed, Embase, Web of Science, Cochrane Library, Scopus, CNKI, and Wanfang from the creation of the databases to March 23, 2025. A comprehensive set of terms was used, such as “lymphocytes”, “lymphoid cells”, “ratio”, “inflammatory bowel disease”, “IBD”, “cohort”, “case–control” and “clinical study”. The search strategy is detailed in Supplementary Text S1.
Study selection
The following studies were eligible: (1) patients diagnosed with IBD via pathological observation, (2) studies with immune-inflammatory indices like NLR, lymphocyte-to-monocyte ratio (LMR), PLR, NPR ELR, ENLR, and CRP/ALB, and (3) studies specifically evaluating the predictive value of immune-inflammatory indices for incidence, severity, and prognosis of IBD, UC, or CD, (4) and studies that provided odds ratio (OR), risk ratio (RR), or hazard ratio (HR) with 95% confidence intervals (CI) for the predictive accuracy of immune-inflammatory indices in IBD, or the means and standard deviations of continuous variables. These data should either be directly extractable from the studies or calculable from the available data; (5) the study must be formally published.
The excluded studies were (1) reviews, commentaries, conference abstracts, case reports, letters, or animal studies; (2) studies without adequate information for the calculations of OR, RR, HR with 95% CI, means and standard deviations; (3) those without detailed baseline data; (4) those with duplicated data or insufficient data.
Two researchers (LPJ and XW) independently reviewed the titles and abstracts of retrieved studies, assessed the full-text articles, and identified eligible studies.
Data extraction
Data were extracted by two researchers (LPJ and CJH).In cases of disagreement, the final decision was made by a third researcher, Bing Yang. Extracted data encompassed the first author, publication year, country (study location), design, sample size, patient age, study duration, BMI, disease duration, cut-off values, and data on immune-inflammatory indices in various outcome measures of IBD (UC and CD).
Quality assessment
The quality of eligible studies was examined via the Newcastle–Ottawa Scale (NOS). Cohort studies were assessed based on three parameters: selection, comparability, and outcome, while case–control studies were evaluated in terms of selection, comparability, and exposure. The maximum possible score was 9 [18]. High-quality studies were defined as those scoring 7—9 [19].
Statistical analysis
For continuous variables, due to significant differences in the measurement methods and units of the indices used in the original studies, the standardized mean difference (SMD) with a 95% CI was employed for the data synthesis of continuous variables. For categorical variables, OR, RR, or HR, along with 95% CIs, were employed. Heterogeneity was examined through Cochran’s Q test and the Higgins I2 statistic. Significant heterogeneity was considered when I2 > 50% or P < 0.1. When heterogeneity was not significant (I2 < 50%), a fixed-effect model was employed; when heterogeneity was significant, a random-effect model was applied. Furthermore, the stability of the metrics and potential sources of heterogeneity were explored through subgroup and sensitivity analyses. Publication bias was evaluated via Egger’s test. P < 0.05 signified statistical significance. STATA 15.0 and Review Manager 5.4 were applied for all statistical analyses. Moreover, in accordance with the GRADE system, the evidence for each outcome was assessed and rated as “high”, “moderate”, “low”, or “very low” quality to derive conclusions [20].
Results
Study characteristics
Initially, 1,139 articles were retrieved. 340 records were first excluded due to duplicate publications. After a title and abstract review of the rest records, 754 studies were excluded. Subsequently, the full texts of 45 studies were assessed and 10 were removed due to insufficient data on outcomes. Ultimately, 35 studies (comprising a total of 93 comparative groups) [1, 2, 10–14, 16, 21–47]were selected, with a total of 5,870 patients (Fig. 1).
Fig. 1.
Flow chart of the literature search and selection of the main research process. By searching PubMed, Embase, Web of Science, Cochrane Library, and Chinese databases from inception to 23 February 2024, a total of 942 relevant records were retrieved and 35 full-text documents were included
Among the eligible studies, one was carried out in Israel, one in Vienna, one in Spain, one in Italy, two in Japan, and four in Turkey, with the remaining 25 studies conducted in China. Notably, four of the eligible studies were cohort studies, all of which were retrospective, while the remaining studies were case–control studies. Five studies investigated the predictive performance of NLR for the severity of CD, six studies for disease activity in CD, two studies for complications in CD, eight studies for the severity of UC, and twelve studies for disease activity in UC. In terms of LMR, three studies examined its predictive performance for the severity of CD, three studies for disease activity in CD, six studies for the severity of UC, and four studies for disease activity in UC. Regarding PLR, four studies investigated its predictive value for the severity of CD, five studies for disease activity in CD, four studies for the severity of UC, and six studies for disease activity in UC. For NPR, one study investigated its predictive value for CD and one study for UC. Concerning CRP/ALB, three studies assessed its predictive value for disease activity in CD, and three studies for disease activity in UC. One study assessed the prognostic significance of the ELR in CD, and one study investigated the prognostic significance of the ENLR. Supplementary Table S1 presents additional characteristics of the included studies.
Study quality
The NOS scores for the included studies were from 7 to 9, which indicated high quality (Supplementary Table S2).
Meta-analysis results
Correlation between NLR and IBD
Forty-five comparative groups involving 5,173 participants were included to analyze the associations between NLR and IBD. In the categorical variable analysis of NLR in the prediction of disease severity in IBD patients, NLR could not predict the severity of IBD (OR = 1.81, 95% CI: 0.79 to 4.13; P = 0.16, Fig. 2A). Nevertheless, in the continuous variable analysis of NLR in the prediction of IBD severity, the severe IBD group exhibited a marked elevation in NLR levels compared with the mild IBD group (SMD = 0.90, 95% CI: 0.60 to 1.21, P = 0.02, Fig. 2A).
Fig. 2.

A Forest plot of the association between NLR and severity in patients with IBD; (B) Forest plot of the association between NLR and disease activity in patients with IBD; (C) Forest plot of association between NLR and recurrence, endoscopic response and steroid response in IBD patients
In the categorical variable analysis of NLR in the prediction of disease activity in IBD patients, NLR was predictive of disease activity, with higher NLR levels indicating greater disease activity (OR = 1.18, 95% CI: 1.04 to 1.34; P = 0.001, Fig. 2B). In the continuous variable analysis, NLR levels in the active disease group were notably elevated in comparison to the remission cohort (SMD = 1.01, 95% CI: 0.73 to 1.29, P < 0.001, Fig. 2B).
In the analysis of NLR for relapse, endoscopic response, and steroid response in IBD patients, NLR was found to be a predictor for all three outcomes (Relapse: OR = 1.35, 95% CI: 1.09 to 1.68; P = 0.006; Endoscopic Response: SMD = 0.43, 95% CI: 0.15 to 0.70, P = 0.002; Steroid Response: SMD = 0.50, 95% CI: 0.15 to 0.85, P = 0.005, Fig. 2C).
Correlation between PLR and IBD
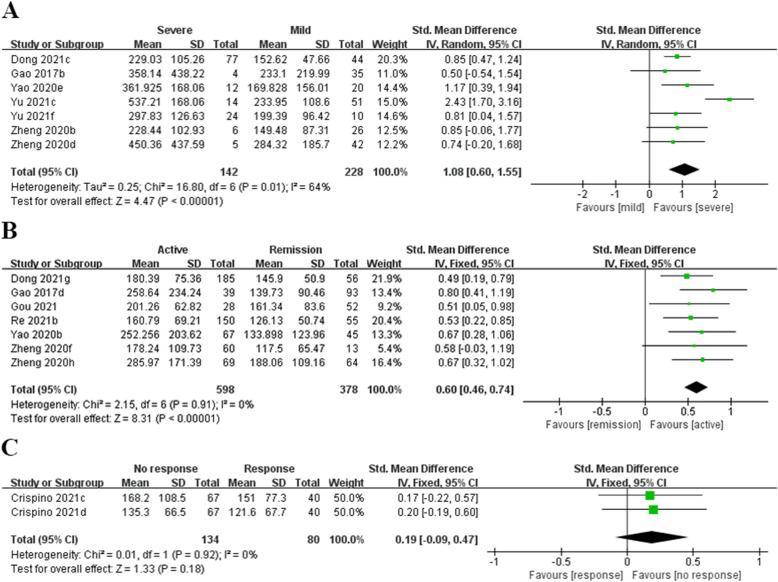
Sixteen comparative groups, including 2,734 participants, were analyzed to investigate the link of PLR to IBD. In the continuous variable analysis of PLR in the prediction of disease severity in IBD patients, PLR levels in the severe IBD group were notably increased in contrast to the mild IBD cohort(SMD = 1.08, 95% CI: 0.60 to 1.55, P < 0.001, Fig. 3A). In the continuous variable analysis of PLR in the prediction of disease activity, the active disease group had higher PLR levels than the remission group (SMD = 0.60, 95% CI: 0.46 to 0.74, P < 0.001, Fig. 3B). PLR was not a significant predictor of endoscopic response in IBD patients (SMD = 0.19, 95% CI: −0.09 to 0.47, P = 0.18, Fig. 3C).
Fig. 3.
A Forest plot of the association between PLR and severity in patients with IBD; (B) Forest plot of the association between PLR and disease activity in patients with IBD; (C) Forest plot of the association between PLR and endoscopic response in patients with IBD
Correlation between LMR and IBD
Eighteen comparative groups, including 2,698 participants, were selected for the analysis of the connection of LMR with IBD. As for the analysis of LMR as a categorical variable in the prediction of disease severity in IBD patients, LMR was not a significant predictor of disease severity (OR = 0.41, 95% CI: 0.15 to 1.18, P = 0.10, Fig. 4A), but in the continuous variable analysis, LMR levels in the severe IBD group were markedly decreased compared to the mild IBD group (SMD = −0.66, 95% CI: −0.89 to −0.44, P < 0.001, Fig. 4A).
Fig. 4.
A Forest plot of the association between LMR and severity in patients with IBD; (B) Forest plot of the association between LMR and disease activity in patients with IBD
In the categorical variable analysis of LMR in the prediction of disease activity in IBD individuals, elevated LMR levels were linked to decreased disease activity (OR = 0.88, 95% CI: 0.80 to 0.96, P = 0.004, Fig. 4B). Concerning the continuous variables, in comparison to the remission group, the active disease group had significantly lower LMR levels (SMD = −0.43, 95% CI: −0.85 to −0.01, P = 0.04, Fig. 4B).
Correlation between NPR, CRP/ALB, ELR, ENLR and IBD
Two comparative groups involving 172 individuals were selected for exploring the relationship between NPR and IBD, with six comparative groups involving 1,782 participants on the relationship between CRP/ALB and IBD, two comparative groups involving 107 participants on the relation of ELR to IBD, and four comparative groups involving 107 participants on the relationship between ENLR and IBD. In the categorical variable analysis of NPR and CRP/ALB in the prediction of disease activity, both NPR and CRP/ALB were predictive of disease activity, with increased levels correlated with greater disease activity (NPR: OR = 1.20, 95% CI: 1.08 to 1.32, P < 0.001, Fig. 5A; CRP/ALB: OR = 1.50, 95% CI: 1.38 to 1.65, P < 0.001, Fig. 5B).
Fig. 5.
A Forest plot of the association between NPR and disease activity in patients with IBD; (B) Forest plot of the association between CRP/ALB and disease activity in patients with IBD; (C) Forest plot of the association between ELR and endoscopic response in IBD patients; (D) Forest plot of the association between ENLR and endoscopic response in patients with IBD
In the continuous variable analysis of ELR and ENLR in the prediction of endoscopic response, the active group exhibited increased ELR and ENLR levels in contrast to the remission cohort(ELR: SMD = 0.63, 95% CI: 0.26 to 1.00, P < 0.001, Fig. 5C; ENLR: SMD = 0.60, 95% CI: 0.32 to 0.88, P < 0.001, Fig. 5D). However, in the categorical variable analysis of ENLR in the prediction of endoscopic response, ENLR was not a marked predictor (OR = 0.11, 95% CI: 0.01 to 1.12, P = 0.06, Fig. 5D).
Subgroup analysis of peripheral blood NLR, PLR, and LMR across groups
Subgroup analyses were conducted to detect potential heterogeneity based on different IBD types (CD and UC), sample size, regions and patient age. The results of these analyses are presented in Table 1.
Table 1.
Subgroup analysis on predictive value of NLR, PLR and LMR for clinical outcome in patients with inflammatory bowel disease
| Subgroup | Severity (continuous) | Disease activity (continuous) | Disease activity (classification) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | SMD [95%CI] | P value | I2 | Study | SMD [95%CI] | P value | I2 | Study | OR [95%CI] | P value | I2 | |
| NLR | ||||||||||||
| Total | 12 | 0.90 [0.60, 1.21] | < 0.00001 | 50% | 14 | 1.01 [0.73, 1.29] | < 0.00001 | 84% | 9 | 1.18 [1.04, 1.34] | 0.009 | 69% |
| Population | ||||||||||||
| UC | 7 | 0.91 [0.54, 1.27] | < 0.00001 | 46% | 10 | 1.03 [0.70, 1.36] | < 0.00001 | 84% | 7 | 1.21 [1.00, 1.46] | 0.05 | 76% |
| CD | 5 | 0.86 [0.27, 1.46] | 0.004 | 63% | 4 | 0.98 [0.40, 1.57] | 0.0009 | 86% | 2 | 1.17 [1.06, 1.30] | 0.002 | 0% |
| Sample size | ||||||||||||
| ≥ 100 | 4 | 0.81 [0.21, 1.41] | 0.009 | 73% | 7 | 0.83 [0.59, 1.07] | < 0.00001 | 68% | 8 | 1.18 [1.03, 1.35] | 0.02 | 73% |
| < 100 | 8 | 0.95 [0.59, 1.32] | < 0.00001 | 34% | 7 | 1.26 [0.66, 1.85] | < 0.00001 | 90% | 1 | 1.25 [0.96, 1.63] | 0.09 | NA |
| Mean/median age | ||||||||||||
| ≥ 40 years | 7 | 0.85 [0.47, 1.23] | < 0.00001 | 50% | 10 | 0.85 [0.60, 1.09] | < 0.00001 | 73% | 7 | 1.21 [1.00, 1.46] | 0.05 | 76% |
| < 40 years | 5 | 0.97 [0.42, 1.51] | 0.0005 | 55% | 4 | 1.55 [0.62, 2.47] | 0.001 | 93% | 2 | 1.17 [1.06, 1.30] | 0.002 | 0% |
| Region | ||||||||||||
| Asia | / | / | / | / | 10 | 0.83 [0.62, 1.05] | < 0.00001 | 62% | / | / | / | / |
| Non-Asia | / | / | / | / | 5 | 1.40 [0.64, 2.15] | 0.0003 | 92% | / | / | / | / |
| PLR | ||||||||||||
| Total | 7 | 1.08 [0.60, 1.55] | < 0.00001 | 64% | 7 | 0.60 [0.46, 0.74] | < 0.00001 | 0% | ||||
| Population | ||||||||||||
| UC | 3 | 0.85 [0.52, 1.17] | < 0.00001 | 0% | 4 | 0.52 [0.33, 0.70] | < 0.00001 | 0% | ||||
| CD | 4 | 1.25 [0.37, 2.14] | 0.006 | 76% | 3 | 0.71 [0.49, 0.93] | < 0.00001 | 0% | ||||
| Sample size | ||||||||||||
| ≥ 100 | 3 | 1.34 [0.29, 2.39] | 0.01 | 86% | 4 | 0.58 [0.41, 0.74] | < 0.00001 | 0% | ||||
| < 100 | 4 | 0.87 [0.45, 1.30] | < 0.0001 | 0% | 3 | 0.66 [0.40, 0.93] | < 0.00001 | 0% | ||||
| Mean/median age | ||||||||||||
| ≥ 40 years | 3 | 0.91 [0.58, 1.23] | < 0.00001 | 0% | 5 | 0.55 [0.38, 0.71] | < 0.00001 | 0% | ||||
| < 40 years | 4 | 1.15 [0.22, 2.09] | 0.02 | 79% | 2 | 0.73 [0.47, 0.99] | < 0.00001 | 0% | ||||
| LMR | ||||||||||||
| Total | 7 | −0.66 [−0.89, −0.44] | < 0.00001 | 0% | ||||||||
| Population | ||||||||||||
| UC | 4 | −0.70 [−0.99, −0.41] | < 0.00001 | 13% | ||||||||
| CD | 3 | −0.53 [−0.96, −0.09] | 0.02 | 0% | ||||||||
| Sample size | ||||||||||||
| ≥ 100 | 4 | −0.77 [−1.02, −0.51] | < 0.00001 | 0% | ||||||||
| < 100 | 3 | −0.30 [−0.78, 0.18] | 0.22 | 0% | ||||||||
| Mean/median age | ||||||||||||
| ≥ 40 years | 3 | −0.73 [−1.10, −0.37] | < 0.00001 | 34% | ||||||||
| < 40 years | 4 | −0.51 [−0.88, −0.13] | 0.008 | 0% | ||||||||
Abbreviations: SMD Standard mean difference, CI Confidence interval, NA Not available
First, concerning the NLR as a classification variable for disease activity in IBD patients, high NLR corresponded to greater disease activity in CD individuals (OR: 1.17; 95% CI: 1.06 to 1.30; P = 0.002). In contrast, no discernible prognostic effect of NLR was noted in UC patients (OR: 1.21; 95% CI: 1.00 to 1.46; P = 0.05). In studies with a sample size greater than 100, high NLR was associated with greater disease activity compared to those with a sample size of less than 100 (OR = 1.18; 95% CI: 1.03 to 1.35; P = 0.02). Furthermore, high NLR was related to greater disease activity in the group with an average/median age below 40 (OR: 1.17; 95% CI: 1.06 to 1.30; P = 0.002). Conversely, a marked prognostic effect of NLR was not observed in patients with an average/median age over 40 (OR: 1.21; 95% CI: 1.00 to 1.46; P = 0.05). Additionally, when LMR was regarded as a continuous variable for disease severity in IBD patients, no significant prognostic effect was found in studies with a sample size of less than 100 (P = 0.22). However, in studies with a sample size greater than 100, LMR was proved as a predictive factor for disease severity in IBD patients (SMD = −0.77; 95% CI: −1.02 to −0.51; P = 0.0006). Subgroup analysis of PLR revealed that its predictive value for disease severity and activity in IBD patients remained consistent across all subgroups.
Subgroup analyses indicated that for NLR in predicting IBD, population, sample size, patient age and regions were major contributors to the heterogeneity of disease severity and activity. In the case of PLR, population, sample size, patient age were the primary factors for the heterogeneity of disease severity and activity (Table 1).
NLR and postoperative fistula in CD patients
Given that only one study reported on postoperative fistulas, no data pooling was performed; instead, a descriptive analysis was conducted. In a 2022 study by Liu Zhongcheng et al. [44], data from 240 patients who received surgery for CD from September 2017 to September 2019 were analyzed. The study found that a high NLR value (OR = 1.082; 95% CI: 1.023 to 1.144; P = 0.006) was a risk factor for postoperative fistulas in CD patients.
Sensitivity analysis
Sensitivity analysis revealed that LMR was an unstable predictor for disease activity in the IBD cohort as a continuous variable. The primary factors contributing to this instability were the studies by Dong (2021) [28] and Li (2020) [31]. Upon sequentially removing these two studies, the effect size no longer aligned with the original range. Therefore, evidence is inadequate to prove the predictive value of LMR for disease activity at this time (Fig. 6). In contrast, for the remaining studies, after each study was sequentially removed, the effect size remained consistent within the original range, which shows the reliability of the analysis results (Supplementary Text S2).
Fig. 6.
Sensitivity analysis of continuous variables for LMR and disease activity in IBD
Publication bias
Publication bias was assessed through Egger’s test. No significant publication bias was detected in the associations between NLR and the severity (P = 0.291, P = 0.309), activity (P = 0.059, P = 0.230), and endoscopic response (P = 0.244) in IBD patients, between PLR, LMR, and the severity (P = 0.849, P = 0.115, P = 0.136) and activity (P = 0.630, P = 0.458, P = 0.384) of IBD patients, and between CRP/ALB and disease activity (P = 0.425) in IBD patients. P > 0.05 indicates the absence of publication bias. However, since the number of studies (< 3) was small, publication bias analysis could not be performed for other outcomes. Detailed results are presented in Supplementary Text S2.
GRADE classification
The PLR with continuous variables of disease activity, LMR with continuous variables of disease severity and disease activity classification, NPR and CRP/ALB with disease activity classification, as well as ELR and ENLR with endoscopic response classification, were assessed as low. The NLR with classification variables of disease severity, disease activity, and relapse, NLR with continuous variables of disease severity, disease activity, endoscopic response, and steroid response, PLR with continuous variables of disease severity and endoscopic response, LMR with classification variables of disease severity and continuous variables of disease activity, and ENLR with endoscopic response classification were evaluated as very low. Detailed GRADE classification can be found in Supplementary Table S3.
Discussion
Biomarkers could be employed to predict BD [45]. Bertani et al. [48] proved that for UC patients receiving anti-TNF therapy, elevated baseline and eight-week post-treatment NLR and PLR could be employed to predict clinical remission, mucosal healing, and overall mucosal improvement. Crispino et al. [13] included a cohort of 107 CD patients and performed multivariate analysis to evaluate whether baseline and week-12 NLR, PLR, ELR, and ENLR could predict endoscopic remission (ER) at 52 weeks in patients initiating biologic therapy. The results indicated that low NLR, ELR, and ENLR were predictive of ER, providing valuable support for clinical decision-making in CD management. Additionally, NLR, ELR, and ENLR are simple, cost-effective, and rapid biomarkers to forecast CD treatment outcomes, thereby assisting clinicians in making informed decisions. Argeny et al. [46] demonstrated that preoperative high NLR was linked to acute surgical indications and fewer postoperative complications.
In the clinical decision-making process, predictive serum biomarkers are becoming increasingly important [49]. In this study, seven simple inflammatory biomarkers, encompassing NLR, PLR, LMR, NPR, CRP/ALB, ELR, and ENLR, were evaluated. They can be easily calculated from routine blood cell counts and serve as non-invasive tests for predicting prognostic indicators such as disease activity, severity, and other clinical outcomes in patients with IBD. NLR was found to be predictive of disease activity, disease relapse, endoscopic response, and steroid responsiveness in IBD patients. PLR was identified as a predictor of disease severity and activity in IBD patients. Additionally, NPR and CRP/ALB were indicative of disease activity in IBD. The ELR was found to predict endoscopic response in individuals with IBD. However, when LMR was evaluated as a predictor of disease activity in IBD, sensitivity analysis revealed significant instability in the results of the analysis. Therefore, there is insufficient evidence to confirm that LMR can reliably predict disease activity in these patients. Significant publication bias was not identified across all outcome measures in IBD patients in Egger’s test. In 2021, Wei et al. [50] published a meta-analysis on the correlation of NLR with the disease activity of IBD. Their analysis included 16 clinical studies, and the results demonstrated that peripheral blood NLR was a valuable biomarker for IBD severity, which aligns with the findings of the present study. The meta-analysis by Anas Elgenidy et al. [17], published in 2024, investigated the correlation between SII and disease activity in patients with UC. Their analysis included seven clinical studies, and the results demonstrated the significant potential of SII as a non-invasive biomarker for assessing UC activity and severity. While both our study and theirs evaluated the predictive value of inflammatory indices for UC activity and severity, our research takes a broader approach by assessing immune-inflammatory indices similar to SII in relation to the activity, severity, and prognosis of IBD. However, this study expanded on their work by incorporating a greater number of the most recently published clinical studies and analyzing six additional inflammatory markers beyond NLR, thereby providing more comprehensive evidence. This is a key strength of our study. Furthermore, through subgroup analysis, this study explored the optimal patient population and conditions for the application of immune-inflammatory indices in predicting the severity of IBD, which holds critical significance for the clinical application of these indices in risk stratification and treatment guidance.
It is noteworthy that subgroup analysis revealed a significant prognostic impact of the NLR, as a categorical variable, on disease activity in CD patients (P = 0.002), whereas no significant prognostic effect of NLR was found in UC patients (P = 0.05). This discrepancy may be attributed to the limited research on the prognostic value of NLR in UC, resulting in insufficient reliability of the findings. One study indicated that while NLR may serve as a reference for assessing UC disease activity, it could not be an independent clinical marker for determining UC disease activity [51]. Additionally, a study by Akpinar et al. [52] has suggested that the relation of NLR to disease activity in UC is weak, and there is no association between NLR and disease progression. Their study has indicated that although peripheral blood neutrophil counts (NeuC) are elevated in UC patients with active disease, lymphocyte counts (LymC) do not significantly change, which limits the accuracy of NLR in assessing the severity of UC. In subgroup analysis, it was further observed that NLR could predict IBD in younger individuals (< 40) (P = 0.002), but not in the older cohort(> 40) (P = 0.05). This difference may be due to the inflammatory levels, immune system function, and presence of chronic diseases. Research suggests that as people age, the levels of inflammatory markers increase, which gives rise to various age-related pathological conditions like cardiovascular disease, neurodegenerative disorders, cancer, and frailty. These inflammatory markers may serve as indicators of inflammaging [53]. With advancing age, immune functionality may progressively decline, potentially leading to a diminished response to inflammation and an increased risk of chronic inflammation. This condition is associated with cellular senescence, immunosenescence, organ dysfunction, as well as age-related diseases. Chronic inflammation, referred to as inflammaging, is linked to many diseases related to age, such as cardiovascular diseases, neurodegenerative disorders, cancer, and frailty [54].
NLR and PLR are simple biomarkers of subclinical inflammation and can be easily obtained through a complete differential blood count. They are shown to be connected with the prognosis and clinical outcomes of various pathological conditions, including infections, immune-mediated diseases, and malignancies [55]. NLR and PLR are derived from the counts of neutrophils, platelets, and lymphocytes. These ratios are more reliable in reflecting disease changes compared to the individual components. The underlying mechanisms of this association were not elucidated; however, a high NLR is thought to be the consequence of intricate interactions between neutrophils and lymphocytes, which raise the levels of several cytokines in the blood and finally cause inflammation [56]. Neutrophils, as important infiltrating and regulatory cells of innate immunity, are critical in the immune response [57]. Their accumulation in the gut may enhance the recruitment of neutrophils and promote apoptosis of damaged cells. This response can be mediated by the secretion of cytokines like IL-6 and IL-1, which contribute to the activation of nonspecific inflammatory reactions [47]. Furthermore, impaired neutrophil function may lead to a reduced capacity to eliminate intestinal bacteria, thereby promoting the development of chronic inflammation [58]. Lymphocytes and their subgroups are essential in the pathogenesis of IBD. In CD, this is associated with an increase and persistence of interferon-γ-induced Th1 cells, which, through the activation of dendritic cells by Th1, trigger localized chronic inflammation [59]. Therefore, lymphocyte count can indicate how well the immune system reacts to inflammatory events. Furthermore, studies on BD patients have demonstrated that lymphocyte function is abnormal at mucosal and peripheral levels [60]. A study has suggested that peripheral lymphocytes in UC and CD patients exhibit reduced lymphocyte responses to the mitogen phytohemagglutinin [61].
In IBD patients, there is often a hypercoagulable and thrombophilic state. Platelets may be vital in the pathophysiology of IBD. Research has demonstrated that, in addition to their traditional hemostatic function, platelets act as pro-inflammatory cells. Upon activation, they release a variety of bioactive molecules that induce and amplify the inflammatory response [62]. Additionally, Erademir et al. [63] have demonstrated that increased NLR and PLR in CD patients are linked to elevated malondialdehyde (MDA) and nitric oxide (NO) levels. Our study further reveals that the levels of oxidative stress in the body are positively correlated with MDA and NO, suggesting that NLR may reflect the level of oxidative stress. Studies by Luceri and Bourgonje et al. [64, 65] indicate that oxidative stress levels are correlated with the activity of IBD. It is hypothesized that NLR and PLR may be associated with IBD activity. LMR, an emerging inflammatory marker, has been shown to be related to monocytic activation and innate immune dysfunction in IBD pathogenesis and progression. The increase in monocyte count has been closely linked to the disease activity of UC [66].
Although our study provides a wealth of information, several limitations should be considered. First, most eligible studies are from Asia, particularly China and Japan. Given the variability in publication years, the criteria used to assess IBD outcomes may differ across studies. Therefore, the prognostic relevance of immune-inflammatory indices, such as NLR, in non-Asian IBD populations, should be validated in the future. Second, the included studies predominantly adopted retrospective designs, with few prospective studies. The retrospective nature of these studies may introduce confounding factors that could compromise the reliability of our findings, potentially leading to selection bias. Furthermore, although subgroup analyses were executed to evaluate the sources of heterogeneity, the included studies still exhibited significant heterogeneity. Finally, the relatively limited samples may impact the robustness of the results.
Conclusion
NLR, PLR, NPR, and CRP/ALB are potential predictors of disease activity in patients with IBD. Specifically, PLR may serve as a potential indicator of disease severity, while NLR and ELR show potential in predicting endoscopic response. Furthermore, NLR has been identified as a potential predictor of both relapse and steroid response. However, there is currently insufficient evidence to establish a clear association between NLR and the severity of IBD, nor between LMR and the severity and activity of IBD, as well as between PLR and ENLR and endoscopic response in IBD. Subgroup analyses revealed that the type of IBD and age may influence the predictive accuracy of these immune-inflammatory indices. Given the limitations, including a predominance of research from Asia, a retrospective design, and relatively small sample sizes, larger prospective studies should be conducted to further verify the predictive and prognostic value of immune-inflammatory indices in IBD patients.
Supplementary Information
Supplementary Material 1: Table S1. Additional characteristics of the included studies.
Supplementary Material 2: Table S2. Quality evaluation of the eligible studies with Newcastle–Ottawa scale.
Supplementary Material 3: Table S3. GRADE rating of outcomes.
Supplementary Material 4: Text S1. Literature search strategies.
Supplementary Material 5: Text S2. Sensitivity analysis and publication bias.
Acknowledgements
Not applicable.
Abbreviations
- SMD
Standardized mean differences
- OR
Odds ratios
- CI
Confidence intervals
- NLR
Neutrophil-to-lymphocyte ratio
- LMR
Lymphocyte-to-monocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- IBD
Inflammatory bowel disease
- CD
Crohn’s disease
- UC
Ulcerative colitis
- MLR
Monocyte-to-lymphocyte ratio
- NPR
Neutrophil-to-platelet ratio
- CRP/ALB
C-reactive protein-to-albumin ratio
- ELR
Eosinophil-to-lymphocyte ratio
- ENLR
Eosinophil*neutrophil-to-lymphocytes ratio
- WBC
White blood cell count
- ESR
Erythrocyte sedimentation rate
Authors’ contributions
Peiji Li: Conceptualization, Resources, Formal analysis, Writing—Original Draft, Writing—Review & Editing; Bing Yang: Conceptualization, Supervision, Project administration, Writing—Review & Editing.; Yilin Wu: Formal analysis, Data curation, Investigation, Writing- Reviewing and Editing.; Wei Xiong): Resources, Formal analysis, Data curation, Writing—Review & Editing; Jiahui Cao: Formal analysis, Data Curation, Resources, Writing- Reviewing and Editing.; Mengyun Chen: Formal analysis, Visualization, Writing- Reviewing and Editing.; Zhaowei Yuan: Data Curation, Visualization,Writing- Reviewing and Editing.; Wenxin Guo: Data Curation, Writing- Reviewing and Editing. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that they did not receive any funding from any source.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yilin Wu, Wei Xiong and Jiahui Cao co-second authors.
Mengyun Chen and Zhaowei Yuan co-third authors.
Contributor Information
Peiji Li, Email: 2021016018@stu.gzucm.edu.cn.
Bing Yang, Email: Yangbing6708@163.com.
References
- 1.Zheng YD. Changes and clinical significance of hematologic indices in inflammatory bowel disease. Master. Suzhou University: Suzhou University; 2020. 10.27351/d.cnki.gszhu.2020.000768.
- 2.Lai X. Clinical application value of blood routine indexes lmr, hct and pct for ibd activity. Master. Jilin University: Jilin University; 2020. 10.27162/d.cnki.gjlin.2020.004143.
- 3.Zundler S, Becker E, Weidinger C, Siegmund B. Anti-adhesion therapies in inflammatory bowel disease-molecular and clinical aspects. Front Immunol. 2017;8:891. 10.3389/fimmu.2017.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornton RF, Murphy EC, Kagawa TF, O’Toole PW, Cooney JC. The effect of environmental conditions on expression of bacteroides fragilis and bacteroides thetaiotaomicron c10 protease genes. BMC Microbiol. 2012;12:190. 10.1186/1471-2180-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–34. 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 6.Loftus EV Jr. Update on the incidence and prevalence of inflammatory bowel disease in the united states. Gastroenterol Hepatol (N Y). 2016;12(11):704–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Stone MA, Mayberry JF, Baker R. Prevalence and management of inflammatory bowel disease: a cross-sectional study from central england. Eur J Gastroenterol Hepatol. 2003;15(12):1275–80. 10.1097/00042737-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Mirow L, Hauenschild L, Hildebrand P, Kleemann M, Keller R, Franke C, et al. recurrence of crohn’s disease after surgery–causes and risks. Zentralbl Chir. 2008;133(2):182–7. 10.1055/s-2008-1004743. [DOI] [PubMed] [Google Scholar]
- 9.Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14(9):1245–55.e8. 10.1016/j.cgh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Cao XX. Influence of nlr values in peripheral blood on disease activity in ulcerative colitis. J China Prescription Drug. 2019;17(3):141–2. 10.3969/j.issn.1671-945X.2019.03.097. [Google Scholar]
- 11.Chen JY, Chen Y, Chen CW, Zhang YG, Yu BQ, Lin XR, et al. The relationship between c-reactive protein/albumin ratio and disease activity in patients with inflammatory bowel disease. Chin J Integr Tradit West Med Digestion. 2022;30(2):102–7. 10.3969/j.issn.1671-038X.2022.02.05. [Google Scholar]
- 12.Chen LJ, Ma YY, Zhang HY, Liu TW, Zhang BP, He ZR, et al. The value of neutrophil/platelet pressure ratio, faecal calprotectin and serological indexes in determining the degree of disease activity in crohn’s disease. Chin J Integr Tradit West Med Digestion. 2024;32(02):156–61. [Google Scholar]
- 13.Crispino F, Grova M, Maida M, Renna S, Mocciaro F, Casà A, et al. Blood-based prognostic biomarkers in crohn’s disease patients on biologics: a promising tool to predict endoscopic outcomes. Expert Opin Biol Ther. 2021;21(8):1133–41. 10.1080/14712598.2021.1935857. [DOI] [PubMed] [Google Scholar]
- 14.Yao FL, Fan DD, Qu H, Miao CQ, Mi C, Gao Y. Predictive value of neutrophil to lymphocyte ratio in crohn’s disease. J Guizhou Med Univ. 2020;45(12):1449–54. 10.19367/j.cnki.2096-8388.2020.12.016. [Google Scholar]
- 15.Battat R, Dulai PS, Jairath V, Vande CN. A product review of vedolizumab in inflammatory bowel disease. Hum Vaccin Immunother. 2019;15(10):2482–90. 10.1080/21645515.2019.1591139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acarturk G, Acay A, Demir K, Ulu MS, Ahsen A, Yuksel S. Neutrophil-to-lymphocyte ratio in inflammatory bowel disease - as a new predictor of disease severity. Bratisl Lek Listy. 2015;116(4):213–7. 10.4149/bll_2015_041. [DOI] [PubMed] [Google Scholar]
- 17.Anas E, Omar A, Tasbih E, Sara KK, Islam E, Aya S, et al. Systemic immune-inflammation index: Unveiling the diagnostic potential in ulcerative colitis through a comprehensive systematic review and meta-analysis. Gastroenterol Endosc. 2025;3(1):22–31. 10.1016/j.gande.2024.10.003. [Google Scholar]
- 18.Wells GA, Wells G, Shea B, Shea B, O'Connell D, Peterson J, et al. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses. 2014;2014. 10.1006/bioe.2002.0137.
- 19.Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose⁻response meta-analysis. Nutrients. 2019;11(4). 10.3390/nu11040826. [DOI] [PMC free article] [PubMed]
- 20.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. Grade guidelines: 1. Introduction-grade evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Mullin G, Zager Y, Anteby R, Jacoby H, Kent I, Ram E, et al. Inflammatory markers may predict post-operative complications and recurrence in crohn’s disease patients undergoing gastrointestinal surgery. ANZ J Surg. 2022;92(10):2538–43. 10.1111/ans.17852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio-Acosta F, Moctezuma-Velázquez P, Castro-Romero E, Uscanga-Domínguez L, Yamamoto-Furusho K, Moctezuma-Velázquez C. The neutrophil-lymphocyte ratio as a predictor of steroid response in patients with severe ulcerative colitis: a retrospective cohort study. Rev Esp Enferm Dig. 2023;115(4):197–9. 10.17235/reed.2022.9006/2022. [DOI] [PubMed] [Google Scholar]
- 23.Nishida Y, Hosomi S, Yamagami H, Sugita N, Itani S, Yukawa T, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts clinical relapse of ulcerative colitis after tacrolimus induction. PLoS One. 2019;14(3):e0213505. 10.1371/journal.pone.0213505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang WM, Zhu CZ, Yang XX, Yu JC, Ma ZQ, Ye X, et al. Application of the onodera prognostic nutrition index and neutrophil-to-lymphocyte ratio in risk evaluation of postoperative complications in crohn’s disease. Sci Rep. 2017;7(1):8481. 10.1038/s41598-017-09265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurimoto N, Nishida Y, Hosomi S, Itani S, Kobayashi Y, Nakata R, et al. Neutrophil-to-lymphocyte ratio may predict clinical relapse in ulcerative colitis patients with mucosal healing. PLoS One. 2023;18(1):e0280252. 10.1371/journal.pone.0280252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JY, Shi YT, Zhou JF, Ma TH, Yan W, Tan YJ, et al. Clinical significance of monocytes to lymphocytes ratio in determining the severity of ulcerative colitis. J Shanxi Med Univ. 2018;49(11):1351–3. 10.13753/j.issn.1007-6611.2018.11.015. [Google Scholar]
- 27.Xie L, Wang S, Wang QM, Yu Y. The predictive value of serum inflammatory markers in severity of ulcerative colitis. Anhui Med J. 2022;43(12):1378–82. 10.3969/j.issn.1000-0399.2022.12.002. [Google Scholar]
- 28.Dong SS. Study on the correlation between serum crp/alb ratio and disease activity of ulcerative colitis. Master. Jilin University: Jilin University; 2021. [Google Scholar]
- 29.Zimanguli R. Study on the predictive value of serum globulin combined with nlr, crp and esr on the activity of ulcerative colitis. Master. Xinjiang Medical University: Xinjiang Medical University; 2023. [Google Scholar]
- 30.Yu J. Correlation analysis of nlr, mlr, plr and inflammatory bowel disease activity. 硕士. Nanchang University: Nanchang University; 2021. 10.27232/d.cnki.gnchu.2021.000917.
- 31.Li CN. The value of nlr, mlr, crp and esr in determining endoscopic activity in ulcerative colitis. Master. Guangxi Medical University: Guangxi Medical University; 2020. 10.27038/d.cnki.ggxyu.2020.000513.
- 32.Wu YY, Zheng Y, Chen XF. The value of crp/alb and lmr in evaluating the severity of ulcerative colitis under endoscopy. Med Sci J Central South China. 2023;51(4):592–4. 10.15972/j.cnki.43-1509/r.2023.04.030. [Google Scholar]
- 33.Gou X, Ye YW, Lliu M, Zhang Y. The value of the ratio of platelets to lymphocytes in the evaluation of patients with ulcerative colitis. Med Inf. 2021;34(08):168–9. 10.3969/j.issn.1006-1959.2021.08.046. [Google Scholar]
- 34.Zhang Q, Fei SJ. Assessment value of neutrophil to lymphocyte ratio in peripheral blood for ulcerative colitis. J China Prescription Drug. 2019;17(5):128–9. 10.3969/j.issn.1671-945X.2019.05.077. [Google Scholar]
- 35.Yang MM, Xia QY, Li HZ, Cao MB. Value of peripheral blood cell count and its ratio in evaluation of ulcerative colitis. J Med Forum. 2021;42(19).
- 36.Cui NL, Zhang MH, Zhao MQ, Li WW, Zhang XQ. The value of neutrophil-to-lymphocyte ratio in the assessment of ulcerative colitis disease. Healthy Friends. 2020;16:1. [Google Scholar]
- 37.Yang MQ, Zhang JX, Xie HB, Dong WG. Evaluation value of serum c-reactive protein/albumin ratio on clinical activity of crohn’s disease. Chin J Difficult Complicated Cases. 2023;22(5):489–93. 10.3969/j.issn.1671-6450.2023.05.009. [Google Scholar]
- 38.Rong M, Zhang GM. Application value of the ratio of peripheral blood neutrophil count to lymphocyte count in the assessment of ulcerative colitis. Contemp Med Forum. 2019;17(10):195–6. [Google Scholar]
- 39.Dong Q, Ruan HL. The study of the correlation between peripheral neutrophil-to-platelet ratio and the disease activity in patients with ulcera- tive colitis. Chin J Lab Diagn. 2022;26(5):687–93. 10.3969/j.issn.1007-4287.2022.05.013. [Google Scholar]
- 40.Aizhez R, Li J, Hu AN, Keremu R, Zheng M, Wushouer G, et al. The correlation analysis among nlr, plr and endoscopic disease severity in ulcerative colitis. Chin J Clin Gastroenterology. 2021;33(2):102–6. 10.3870/lcxh.j.issn.1005-541X.2021.02.007. [Google Scholar]
- 41.Celikbilek M, Dogan S, Ozbakır O, Zararsız G, Kücük H, Gürsoy S, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. 2013;27(1):72–6. 10.1002/jcla.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demir AK, Demirtas A, Kaya SU, Tastan I, Butun I, Sagcan M, et al. The relationship between the neutrophil-lymphocyte ratio and disease activity in patients with ulcerative colitis. Kaohsiung J Med Sci. 2015;31(11):585–90. 10.1016/j.kjms.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: A promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. 2012;36(5):491–7. 10.1016/j.clinre.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, Yang QY, Fu X, Long M, Peng B, Xiao Z, et al. A multicenter case-control study on postoperative intestinal fistula in Chinese patients with crohn disease. Medicine (Baltimore). 2023;102(49):6159. 10.1097/md.0000000000036159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian G, Hongxia D, Jin LI. N#eutrophil-lymphocyte ratio at 14th week predicts loss of response to 52-week infliximab therapy in patients with crohn’s disease. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(4):453–8. 10.12122/j.issn.1673-4254.2020.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Argeny S, Stift A, Bergmann M, Mittlböck M, Maschke S, Yang Y, et al. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in crohn’s disease. Wien Klin Wochenschr. 2018;130(11–12):398–403. 10.1007/s00508-018-1322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang YL, Zhuang SH, Zhang SY. Changes and clinical significance of blood cell-related parameters in patients with inflammatory bowel disease. Lab Med Clin. 2016;13(6):837–9. 10.3969/j.issn.1672-9455.2016.06.048. [Google Scholar]
- 48.Bertani L, Rossari F, Barberio B, Demarzo MG, Tapete G, Albano E, et al. Novel prognostic biomarkers of mucosal healing in ulcerative colitis patients treated with anti-tnf: Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Inflamm Bowel Dis. 2020;26(10):1579–87. 10.1093/ibd/izaa062. [DOI] [PubMed] [Google Scholar]
- 49.Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149(5):1275–85.e2. 10.1053/j.gastro.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Fu W, Fu H, Ye W, Han Y, Liu X, Zhu S, et al. Peripheral blood neutrophil-to-lymphocyte ratio in inflammatory bowel disease and disease activity: a meta-analysis. Int Immunopharmacol. 2021;101(Pt B):108235. 10.1016/j.intimp.2021.108235. [DOI] [PubMed] [Google Scholar]
- 51.Lu JJ, Li L, Munila M, Ayinuer A, Gao F. Analysis of the correlation between neutrophil and lymphocyte ratio and disease activity in patients with ulcerative colitis. J Chin Physicians. 2019;18(12):1837–40. 10.3760/cma.j.issn.1008-1372.2016.12.020. [Google Scholar]
- 52.Akpinar MY, Ozin YO, Kaplan M, Ates I, Kalkan IH, Kilic ZMY, et al. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict mucosal disease severity in ulcerative colitis. J Med Biochem. 2018;37(2):155–62. 10.1515/jomb-2017-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh A, Schurman SH, Bektas A, Kaileh M, Roy R, Wilson DM 3rd, et al. Aging and inflammation. Cold Spring Harb Perspect Med. 2024;14(6):a041197. 10.1101/cshperspect.a041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Li C, Zhang W, Wang Y, Qian P, Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8(1):239. 10.1038/s41392-023-01502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim TG, Park W, Kim H, Choi DH, Park HC, Kim SH, et al. Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in rectal cancer patients following neoadjuvant chemoradiotherapy. Tumori. 2019;105(5):434–40. 10.1177/0300891618792476. [DOI] [PubMed] [Google Scholar]
- 56.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 57.Baert T, Van Camp J, Vanbrabant L, Busschaert P, Laenen A, Han S, et al. Influence of ca125, platelet count and neutrophil to lymphocyte ratio on the immune system of ovarian cancer patients. Gynecol Oncol. 2018;150(1):31–7. 10.1016/j.ygyno.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Gao SQ, Huang LD, Dai RJ, Chen DD, Hu WJ, Shan YF. Neutrophil-lymphocyte ratio: a controversial marker in predicting crohn’s disease severity. Int J Clin Exp Pathol. 2015;8(11):14779–85. [PMC free article] [PubMed] [Google Scholar]
- 59.He XH. Immune mechanisms associated with the pathogenesis of inflammatory bowel disease. J Nanchang Univ (Med Sci). 2011;51(10):93–6. 10.3969/j.issn.1000-2294.2011.10.030. [Google Scholar]
- 60.Roche JK, Watkins MH, Cook SL. Inflammatory bowel disease: prevalence and level of activation of circulating t-lymphocyte subpopulations mediating suppressor/cytotoxic and helper function as defined by monoclonal antibodies. Clin Immunol Immunopathol. 1982;25(3):362–73. 10.1016/0090-1229(82)90201-x. [DOI] [PubMed] [Google Scholar]
- 61.Sachar DB, Taub RN, Brown SM, Present DH, Korelitz BI, Janowitz HD. Imparied lymphocyte responsiveness in inflammatory bowel disease. Gastroenterology. 1973;64(2):203–9. [PubMed] [Google Scholar]
- 62.Selby WS, Janossy G, Bofill M, Jewell DP. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984;25(1):32–40. 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eraldemir FC, Musul M, Duman AE, Oztas B, Baydemir C, Hulagu S. The relationship between neutrophil/lymphocyte and platelet/lymphocyte ratios with oxidative stress in active crohn’s disease patients. Hippokratia. 2016;20(4):368–73. [PMC free article] [PubMed] [Google Scholar]
- 64.Luceri C, Bigagli E, Agostiniani S, Giudici F, Zambonin D, Scaringi S, et al. Analysis of oxidative stress-related markers in crohn’s disease patients at surgery and correlations with clinical findings. Antioxidants (Basel). 2019;8(9):378. 10.3390/antiox8090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bourgonje AR, Feelisch M, Faber KN, Pasch A, Dijkstra G, van Goor H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol Med. 2020;26(11):1034–46. 10.1016/j.molmed.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Cherfane CE, Gessel L, Cirillo D, Zimmerman MB, Polyak S. Monocytosis and a low lymphocyte to monocyte ratio are effective biomarkers of ulcerative colitis disease activity. Inflamm Bowel Dis. 2015;21(8):1769–75. 10.1097/mib.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1. Additional characteristics of the included studies.
Supplementary Material 2: Table S2. Quality evaluation of the eligible studies with Newcastle–Ottawa scale.
Supplementary Material 3: Table S3. GRADE rating of outcomes.
Supplementary Material 4: Text S1. Literature search strategies.
Supplementary Material 5: Text S2. Sensitivity analysis and publication bias.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.